Syllabus (Fourth Edition, 2023)
Topics
i. Understand the pharmacology of anti-coagulants, anti-platelet drugs, thrombolytic drugs, and anti-fibrinolytic drugs.
Topics not covered in previous SAQs
.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
i. Understand the pharmacology of anti-coagulants, anti-platelet drugs, thrombolytic drugs, and anti-fibrinolytic drugs.
2009A 05
Outline the mechanism of action of drugs used to promote haemostasis.
CICMWrecks Answer
Haemostasis: the physiological processes which arrest bleeding
Drugs which reduce blood flow:
- Adrenaline: causes local vasoconstriction
- Wound glue: physical barrier to reduce bleeding
Drugs which increase the available coagulation factors:
- Desmopressin: increases plasma levels of FVIII and von Willebrand Factor, which promote formation of the platelet plug and participate in the coagulation cascade
- Vitamin K: required for the gamma-carboxylation of coagulation factors II, VII, IX and X to activate them in the clotting cascade. Also activates proteins C and S. Vitamin K may be given to reverse supratherapeutic INR associated with warfarin use; may also be given in liver disease or other situations when the INR is elevated and potential bleeding is a concern.
- Prothrombinex: powder for injection containing 500IU each of FII, IX and X, low levels of FV and VII, 25mg of antithrombin which participate in the coagulation cascade as normal
- Biostate: Human derived FVIII/VWF complex which can participate in the coagulation cascade
- Other factor concentrates
Drugs which reduce clot breakdown:
- Tranexamic acid: acts by inhibiting the binding of plasminogen and plasmin to fibrin, to inhibit fibrinolysis of a formed clot
- Aprotinin: a naturally occurring proteolytic enzyme inhibitor acting on trypsin, plasmin and tissue kallikrein. It inhibits the fibrinolytic activity of the streptokinase-plasminogen complex and decreases activation of the clotting cascade.
- Aminocaproic acid: Binds competitively to plasminogen, blocks the binding of plasminogen to fibrin and subsequent conversion to plasmin, resulting in inhibition of fibrinolysis
- Oestrogen: inhibits Protein C (naturally occurring regulator of haemostasis)
Drugs which reverse anticoagulants:
- Vitamin K: as above
- Protamine sulfate: binds to heparin in circulation to produce a stable inactive complex for removal by reticuloendothelial system. Useful in cases of bleeding associated with heparin use.
- Idarucizumab: specific reversal agent for dabigatran
- Andexanet Alfa: Recombinant protein that inactivates direct factor Xa inhibitors like Rivaroxaban as well as antithrombin activated by LMWH or Fondaparinux
JC 2019
Examiner Comments
2009A 05: Pass rate: 10%
Most candidates mentioned factor VIIa, Vitamin K, and desmopressin in their answers.
Outlining the mechanism of action of the drugs used is essential in order to pass this question. The answers may include which coagulation factors are affected by warfarin and Vitamin K, the mechanism by which desmopressin promotes haemostasis and the multiple effects of Aprotinin. Topical treatment (e.g. adrenaline, glue) and drugs such as protamine, oestrogen, and tranexamic acid were common omissions.
Syllabus J 2a 2
Reference: Stoelting and Hillier 4th edition page 449 and 607.
2013B 03
Outline the mechanisms of action of anti-platelet drugs. (50% of marks) Briefly describe the mechanism of action, and pharmacokinetics of aspirin, in relation to its use as an anti-platelet drug. (50% of marks).
2010B 05
List the antiplatelet agents and outline their mechanisms of action, adverse effects, mode of elimination and duration of action.
CICMWrecks Answer: Anti-platelet drugs
Anti-platelet drugs
| COX Inhibitors | Aspirin | MoA | Cyclooxygenase-1 (COX-1) produces precursor to thromboxane A2 (TxA2) Aspirin irreversibly binds and inactivates |
| A/E | GI bleeding and ulceration Kidney injury Reye’s syndrome Bronchospasm In overdose – metabolic acidosis | ||
| Elimination | CYP2C19, metabolites in urine | ||
| Duration | Irreversible COX-1 inhibition → last until platelet turnover (7-10 days) | ||
| P2Y12 receptor antagonists | Clopidogrel Ticagrelor | MoA | P2Y12 is an ADP receptor, which causes platelet recruitment and activation (by inducing activation of GPIIb/IIIa) |
| A/E | Bleeding, itch | ||
| Elimination | Clopidogrel is a prodrug, requiring activation by CYP450 Prasugrel is inactivated by CYP450 | ||
| Duration | Irreversible receptor inactivation → 7-10 days | ||
| Glycoprotein IIb/IIIa inhibitors | Abciximab Tirofiban | MoA | glycoprotein IIb/IIIa binds von Willebrand factor on damaged vascular wall, and fibrinogen to create a pro-clot with other platelets |
| AE | Higher rate of bleeding than aspirin and ADP receptor antagonists | ||
| Elimination | Variable | ||
| Duration | 6-12 hours after cessation of infusion | ||
| Phosphodiesterase inhibitors | Dipyridamole | MoA | Inhibits phosphodiesterase → increased platelet cAMP Also causes coronary vasodilation → useful in angiograms |
| AE | Bleeding, hypotension | ||
| Elimination | Mainly glucuronides in bile 5% in urine | ||
| Duration | 3 hours |
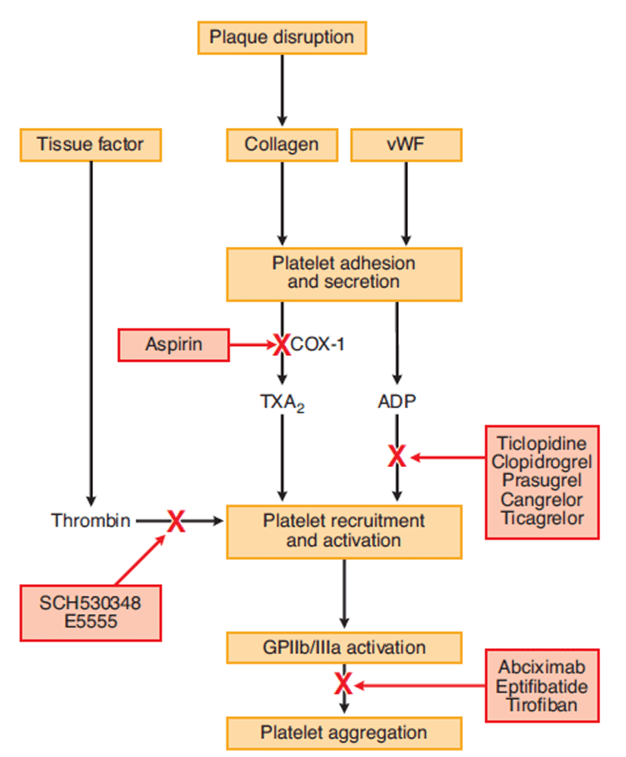
CICMWrecks Answer: Pharmacology of Aspirin
Pharmacology of Aspirin
Examiner Comments
2013B 03: 3 candidates passed (11.1%).
Candidates should take note of how marks are apportioned to multi part questions and to avoid rewriting the same point more than twice. Generally there was a lack of sufficient breadth in knowledge in responses given for mechanism of action of anti-platelet drugs and sufficient depth of knowledge in relation to aspirin, in particular aspirin pharmacokinetics.
2010B 05: 10 (67%) of candidates passed this question.
Most candidates did reasonably well by including aspirin, ADP receptor blockade and glycoprotein 2b/3a blockade in their answers. The best approach to answer this type of question was to use a table with each anti-platelet agent within a column and headings for the rows such as mechanisms of action, adverse effects, mode of elimination and duration of action. Common omissions included the irreversibility of the blockade of the platelet function by many of these agents, renal toxicity and bronchospasm as side effects of aspirin, bone marrow toxicity of ADP receptor blockers, and dipyridamole as an anti-platelet agent. Some candidates classified clopidogrel as a glycoprotein 2b/3a blocker incorrectly and thought clopidogrel has a relative short duration of action on platelet function because of its half-life. Clopidogrel as a prodrug requiring activation by cytochrome P450 and hence significant potential drug interactions were not mentioned by any candidates.
Syllabus: J2a,2d
References: Goodman and Gillman, The Pharmacological basis of therapeutics Chp 55
2017B 12 – 2015B 20
Compare and contrast aspirin and clopidogrel
Examiner Comments
2017B 12: 68% of candidates passed this question.
Both of these commonly used agents are level A in the syllabus and thus a high level of detail was expected. Marks were awarded in the following areas – pharmaceutics, mechanism of action, pharmacokinetics (PK) and side effects. For the PK parameters a general description rather than exact values was sufficient (i.e. ‘high protein binding’ rather than ‘98% protein bound’). It was expected that candidates would mention the fact that clopidogrel is a pro-drug and the factors which influence its conversion to the active form. Additional marks were awarded for well-structured answers which attempted a comparison between the two drugs (helped by the use of a table).
2015B 20: 46% of candidates passed this question.
Both agents are principally used as anti-platelet agents. Aspirin however has wider clinical applications. Mechanism of action of both agents involves irreversible inhibitions of enzymes and/or receptors. The inability of platelets to regenerate these means that physiological effects can not be fully explained by pharmacodynamics or pharmacokinetics alone.
Candidates who followed a traditional template for pharmacology answers scored better, providing answers that covered the breath of the topic.
2011A 17
Outline the physiological processes that occur in a blood vessel after venepuncture (80% of marks).
How are these altered by the administration of aspirin (20% of marks)?
CICMWrecks Answer
Cell-based model of coagulation
Initiation
- Small amounts of Factor VII, X and prothrombin leak into interstitium from intravascular space
- Factor VII interacts with Tissue Factor leading to activation of extrinsic pathway
- Activation of Factor X
- Small amounts of prothrombin converted to thrombin
Amplification
- At onset of tissue damage and vessel disruption
- Platelets exposed to thrombin generated in initiation phase, together with collagen via GPIa
- Platelet activation and release of α and dense granules, and morphological change
- Factor XII activated, leading to consequent activation of factors XI and IX
- Formation of Xase complex on platelet surface
- Amplified activation of factor X
Propagation phase
- Increased activated factor X causes “thrombin burst”
- Fibrinogen activated to fibrin
- Further propagation of platelet activation and aggregation
Modulation of coagulation response
- Circulating proteins C and protein S bind and inactivate factors V and VIII
- Antithrombin 3 binds to thrombin, as well factors IX and X and inhibit these
- Thrombomodulin on intact endothelium binds thrombin → anti-coagulant effect and increased protein C activation
- Plasminogen converted to plasmin → degradation of fibrin and therefore thrombolysis
Effect of aspirin
- Non-selective COX inhibition
- At low doses
- Irreversibly inhibits platelet COX in the portal circulation → inhibits platelet production of thromboxane (TXA)
- TXA stored in α granules and augments the activation of surrounding platelets, therefore aspirin reduces platelet activation in response to coagulation cascade
- At high doses
- Aspirin overcomes hepatic first pass metabolism and escapes to systemic circulation
- Inhibits systemic COX → inhibits endothelial production of prostacyclin
- Prostacyclin inhibits platelet aggregation and adherence, therefore increases clot formation and strength
Sakurai 2016
Examiner Comments
2011A 17: 8 (66%) of candidates passed this question.
The question was answered well overall. Better answers included detail of the platelet receptor and mediator interactions. Discussion of the role of the platelet in providing a phospholipid surface to enable the formation of the activated Xa complex was expected. Modulation of the coagulation cascade and prevention of clot propagation via protein C, nitric oxide, thrombomodulin and fibrinolysis was important to note in a comprehensive answer. The pharmacodynamic action of aspirin was generally understood.
Syllabus: J1,2c and J2, 2d
Recommended sources: Basic and Clinical Pharmacology, Katzung, Chp 34, 36
2011B 20
Describe the structure and function of platelets (50% marks).
Outline the pharmacology of clopidogrel (50% marks).
CICMWrecks Answer: Platelet
PLATELET
Formation
- produced during hematopoiesis in a sub-process called thromopoiesis, or production of thrombocytes.
- Bone marrow: Common myeloid progenitor cells → promegakaryocytes → megakaryocytes
- Megakaryocytes produce protoplatelets within their cytoplasm → released in cytoplasmic extensions upon cytokine stimulus
- Protoplatelets break up into hundreds of platelets that circulate throughout the bloodstream
- The remaining nucleus of the ruptured megakaryocyte is consumed by macrophages.
- Megakaryocyte and platelet production is regulated by thrombopoietin (hormone produced by the liver and kidneys)
- Thrombopoietin stimulates differentiation of myeloid progenitor cells into megakaryocytes and causes the release of platelets.
- Thrombopoietin is regulated by a negative feedback mechanism based on platelet levels
- Each megakaryocyte produces between 5,000 and 10,000 platelets
- Altogether, around 1011 platelets are produced each day in a healthy adult
Fate
- Average lifespan of a platelet is 5 to 10 days
- Old platelets are destroyed by macrophage phagocytosis in the spleen and by Kupffer cells in the liver
- Up to 40% of platelets are stored in the spleen as a reserve, released when needed by sympathetically-induced splenic muscle contractions during severe injury.
Structure
- Small anucleated cells derived from megakaryocytes which originate from haematopoietic stem cell
- Normally 150~300 x 103 /μl
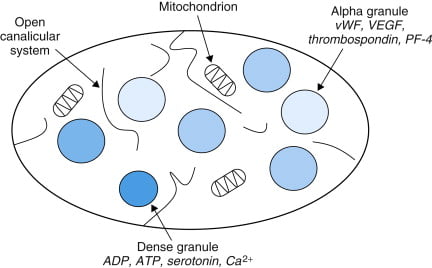
- Actin and myosin
- Remnants of the ER, SR storing calcium
- Mitochondria
- Enzyme systems → production of prostaglandins
- α granules
- Thrombin
- PDGF
- P-selectin
- Fibronectin
- vWF
- Dense granules
- ADP
- ATP
- Ca2+
- Serotonin
- Histamine
Function
- Haemostasis
- Formation of platelet plug
- Interact with collagen exposed at damaged endothelium by GPIa
- Activated by PAF, Thrombin
- Morphological change → irradiating pseudopods
- Degranulation
- Aggregation
- Expression of GPIIb/IIIa → binds fibrin and vWF
- Formation of platelet plug
- Immunomodulation
- Deployed to sites of inflammation and infection and secrete cytokines and chemokines
Sakurai / JC 2019
CICMWrecks Answer: Pharmacology of Clopidogrel
Pharmacology of Clopidogrel
Examiner Comments
2011B 20: 13 (52%) of candidates passed this question.
Platelets are small cells in blood, approximately 150,000 – 300,000 / microliter of blood with a half life of approximately 4 days. They have important membrane receptors (for collagen, vWF and fibrinogen) and intracellular contents (Actin, Myosin, Glycogen, Lysosomes, Dense granules, Alpha granules) and are involved in forming a platelet plug and clotting. Common omissions were to not give a normal platelet count or half life. Diagrams of the clotting cascade were not required.
Clopidogrel is an oral, thienopyridine class antiplatelet agent (a prodrug activated in the liver by cytochrome P450 enzymes), used to inhibit blood clots in coronary artery disease, following stent placement, peripheral vascular disease, and cerebrovascular disease. Mechanism of action is: specifically and irreversibly inhibits the P2Y12 subtype of ADP receptor, which is important in aggregation of platelets and cross-linking by the protein fibrin and thus platelet aggregation, which is inhibited when binding blocks activation of the glycoprotein IIb/IIIa pathway. Elimination half-life of about 8 hours; rapidly absorbed after oral administration; undergoes rapid hydrolysis in liver and renal excretion; 95% protein bound. Important interactions/precautions include proton pump inhibitors, phenytoin, warfarin, heparin, danaparoid, enoxaparin and various thrombolytics. Greatest risk is bleeding. The best answers had a structured approach to describing a drug.
Syllabus: J1 2f, J2 2d
Recommended sources: Ganong Review of Medical Physiology pg 485; Goodman & Gillman. The pharmacological Basis of Therapeutics Chp 54.
2024A 20
Outline the important similarities and differences between unfractionated heparin and enoxaparin using the following headings (20% of marks each):
(i) Mechanism of action
(ii) Pharmacokinetics
(iii) Adverse effects
(iv) Dosing
(v) Monitoring and reversal
2018B 20
Describe the pharmacology of heparin highlighting important differences between unfractionated and fractionated (low molecular weight) heparin.
2017B 05
Compare and contrast unfractionated heparin with low molecular weight heparin
2009B 20
Compare and contrast the pharmacology of heparin and enoxaparin.
Examiner Comments
2024A 20: 27% of candidates passed this question.
These are commonly used and important drugs in intensive care practice and a high level of detail was expected. Unfractionated heparin and enoxaparin have specific differences that influence how we use them in practice and this information was required for full marks. This question was answered well by providing the detail on each domain for both heparin and enoxaparin and then highlighting if this factors into how they are used.
2018B 20: 71% of candidates passed this question.
Better answers were tabulated and included sections on pharmaceutics, indications and an explanation on how the difference in molecular weight influenced pharmacodynamics and pharmacokinetics. Knowledge of adverse effects was limited to bleeding and HITTS, often without consideration of relative risk from LMWH. Many candidates did not know the t1/2 of UFH or LMWH.
2017B 05: 68% of candidates passed this question.
This question was generally well answered and lent itself well to a tabular format. Expected information included an approximation of the molecular weights / significance of the differences in size and therefore its mechanism of action.
Other pertinent areas to mention included pharmacokinetic differences and its use in renal failure, side effect profiles, monitoring, predictability of response and reversibility for the two agents.
2009B 20: 8 (89%) of candidates passed this question.
The vast majority of candidates chose to answer this question in tabular format and in doing so were easily able to consolidate a high scoring answer. This also allowed clear
identification of important differences, such as molecular weight, mechanism of action, halflife, dose-interval, monitoring, elimination, reversal of effect, the influence of renal
impairment and potential side-effects.
One exception to this approach would be clinical indications, where these show marked similarity. Candidates should consider writing similarities once whilst incorporating both drugs so as use the time allocated more efficiently.
Syllabus – J2 2a
Reference: Pharmacology and Physiology in Anaesthetic practice, Stoelting 505-511 Basic
and Clinical Pharmacology, Katzung 546-548
2008A 02
Outline the differences between heparin and enoxaparin with respect to:
a. Phamacokinetics b. Monitoring of effect c. Adverse effects d. Reversal of effect
CICMWrecks Answer
Heparin
- Binds to, and stabilizes anti-thrombin 3-thrombin complex
- → Potentiates the degradation of thrombin (does not occur with LMW heparins)
- Also aids antithrombin 3 degradation of factors IX and X (major function of LMW heparins)
| HEPARIN | ENOXAPARIN | ||
|---|---|---|---|
| PK | A | Subcut or IV administration | Subcut administration |
| ~90% bioavailability SC due to tissue binding | >95% bioavailability SC – NOT bound in tissues | ||
| D | 5% protein bound | 5% protein bound | |
| Vd – 0.3l/kg | Vd – 0.3l/kg | ||
| M | Heparinases | Minimal metabolism | |
| E | Renal elimination of inactive metabolites | Renal elimination of unchanged drug Dosage adjustment required in renal failure | |
| T1/2b ~2 hours | T1/2b 4~6 hours | ||
| Monitoring | APTT, activated clotting time | Anti-Xa assay (expensive, time-consuming) | |
| Adverse Effects | Bleeding | Bleeding | |
| Heparin induced thrombocytopoenia | Less likely to develop HITS | ||
| Osteoporosis | Less likely to develop Osteoporosis | ||
| Reversal | Reversal with protamine sulfate | Partial (40%) reversal with protamine sulfate | |
Sakurai 2016
Examiner Comments
2008A 02: 1 candidate (33%) passed this question.
Candidates were expected to mention the differences between heparin and enoxaparin and to explain why these differences existed and their implications.
For example:
heparin Pharmacokinetics –
MW3000-30000. Large variability has significant effects on:
• mechanism of action (IIa, IXa, Xa)
• dosing( 2/3 molecules in dose have no active binding sites)
• bioavailability poor,T1/2 short (rapid uptake by PF4/endo cells/macrophages and
proteins of high MW components) = poor predictability and dose response both IV and SC, mandates monitoring IV
T1/2 1-2 hours: S/C 2-3 daily doses. Continuous IV – advantage is fast offset.
enoxaparin –
MW3000-5000. Inhibits Xa only.
• bioavailability (100%),longer T1/2 no uptake/protein binding
• predictable activity and dose response = decreased need for monitoring, reliable SC
• No need for IV use
T1/2 4-5 hours, 1-2 daily dosing, but no fast offset.
Better answers were in tabular format under each heading. Higher marks were awarded for mentioning of monitoring pitfalls such as heparin resistance, measuring Xa levels in a timely fashion, and the efficacy and pitfalls of protamine with each agent.
Syallabus J2 2a
Reference: Stoelting 505-511
Katzung 546-548
2011B 04
Describe the pharmacology of low molecular weight heparin (70% marks).
Outline the pharmacology of hirudin (30% marks).
Examiner Comments
2011B 04: 10 (40%) of candidates passed this question.
A question that asks for information of the pharmacology should mention pharmaceutical (only briefly), pharmacokinetics and pharmacodynamics of the drug or class of drugs requested. The term “describe” requests of the candidate a greater depth of information than “outline” and both are defined in the “Notes to Candidates” document. Candidates who did well, did so because they had sufficient knowledge of this commonly used class of drugs, and could structure a well organised answer.
Syllabus: J2a 2a
Recommended sources: Rang and Dale Pharmacology, Chp 21; Goodman & Gillman The pharmacological Basis of Therapeutics Chp 54.
2021A 10
How does warfarin exert its pharmacological effect (40% marks)? Write brief notes on the pharmacology of the agents that can be used to reverse the effects of warfarin (60% marks).
2016A 10
Outline the pharmacology of warfarin.
2015A 08
How does warfarin exert its anti-coagulant effect (50% of marks)? Outline the pharmacology of the agents that can be used to reverse the effects of warfarin, giving examples (50% of marks).
CICMWrecks Answer: Pharmacology of Warfarin
Warfarin Pharmacology
CICMWrecks Answer: Warfarin Reversal
Warfarin Reversal
The following reversal strategies may be either used alone or in combination:
- Stopping warfarin
- S-warfarin is eliminated via oxidation predominantly by CYP2C9 (and to a lesser extent by CYP3A4 and 1A2) → inactive metabolites
- Elimination half-life of S-warfarin is 29 hours
- ∴ in patients with normal hepatic metabolic and synthetic functions take 4 ~ 5
- days for INR to normalise
- Vitamin K
- Can be given either enterally or parenterally (IV, IM) (bioavailability ≈ 100%)
- Requires few hours to work
- Enteral administration Requires presence of bile salts to be absorbed by gut
- Parenteral administration has rare but life threatening complication of hypersensitivity probably related to its preservative, benzyl alcohol
- Low dose vitamin K (1 ~ 2.5 mg) will slowly reduce INR over 24 hours
- but often will not result in complete reversal and would not significantly affect re-establishment of anticoagulation with warfarin
- High dose vitamin K (10 mg) will slowly reduce INR over 12 hours
- ∴ often given together with clotting factors (e.g. FFP)
- Can be given either enterally or parenterally (IV, IM) (bioavailability ≈ 100%)
- Fresh frozen plasma
- Plasma from donated blood, which contains all clotting factors
- ∴ immediately reverses coagulopathy
- However, short duration of action (24 ~ 48 hours) ∴ reserved for active bleeding
- Used when PCC not available
- Dose 2 ~ 4 units (10-15ml/kg) depending on INR and bleeding risk
- Disadvantages = relatively large fluid volume, possible transfusion reactions (e.g. immune reaction, TRALI, infection, etc)
- PCC – Prothrombin complex concentrates (Prothrombinex)
- Human plasma derivative containing concentrates of factors II, IX and X
- 500IU PCC has 500IU of II, IX, X each in a vial
- ∴ immediately reverses coagulopathy
- Dose 25 ~ 50 IU/kg
- Advantages = reliable reversal, smaller fluid volume, avoids transfusion
- complications associated with FFP
- Disadvantages = expensive
- Recombinant activated factor VII (NovoSeven)
- Controversial treatment, present in some guidelines
- Indication = life threatening bleed with significantly elevated INR
- Given together with vitamin K and FFP
- Disadvantage = thromboembolic complications
Examiner Comments
2021A 10: 43% of candidates passed this question.
Warfarin is listed as a level 1 drug in the 2017 syllabus and as such a detailed knowledge of its mechanism of action would be expected from candidates sitting the exam. The reversal agents for warfarin are collectively classed as level 2 drugs and hence the knowledge required would be at a write short notes level. The following topics were expected: what drugs may be used, how they work, in what dose, any common side effects, why/when would one be used in preference to others etc. The use of reversal agents for warfarin is a common practice in ICU. Generally, answers demonstrated a lack of a precise and detailed knowledge with respect to warfarin’s mechanism of action and had a very superficial knowledge with incorrect facts regarding the reversal agents.
2016A 10: 61% of candidates passed this question.
The “traditional” pharmacology answer structure was useful to avoid omitting key details.
Warfarin is a synthetic coumarin derivative presented in tablet form for oral use . It is a racemic mixture. S-enantiomer is 2-5 times more potent than the R-enantiomer. It is used for anticoagulation and the usual dosing involves a loading dose ( 3 to 5 mg for 1 to 3 days) then maintenance dose titrated to INR. It was expected answers would then detail mechanism of action, absorption (commenting on bioavailability), distribution, elimination, excretion and adverse effects. Warfarin has contraindications in pregnancy being teratogenic in first trimester and increasing the risk of fetal haemorrhage in third trimester.
Better answers provided increased detail on mechanism of action including the initial procoagulant effect due to protein C and S inhibition and some details about monitoring effect with INR / PT.
Warfarin has several important drug interactions and detailing these gained additional marks.
Additional credit was given for discussion of reversal options, which includes 1) Stop administration – days 2) Prothrombinex – hours 3) FFP – hours 4) Vitamin K depends on dose given.
2015A 08: 38 % of candidates passed this question.
Warfarin is a competitive inhibitor of the enzyme vitamin K epoxide reductase which converts oxidised or inactive vitamin K to reduced or active vitamin K Reduced vitamin K is required for the gamma carboxylation of the glutamate residues in the vitamin K dependant factors (II, VII, IX and X) and proteins C and S. This gamma carboxylation converts these clotting factors from their inactive to their active form resulting in coagulation. The presence of warfarin inhibits this conversion process resulting in anticoagulation. The presence of inactive protein C and S explains the initial hypercoaguable effect of warfarin.
The three main agents used to reverse the effects of warfarin are vitamin K, prothrombinex and fresh frozen plasma (FFP). It was expected answers would provide a brief overview of all three agents. Most candidates did not highlight the fact that parenteral vitamin K requires a few hours to work whereas prothombinex and FFP work immediately.
Better answers noted additional facts such as oral vitamin K because it is fat soluble requires the presence of bile salts to be absorbed from the gut or the rare but life threatening hypersensitivity reaction caused by intravenous vitamin K possibly related to its preservative benzyl alcohol.
A common omission was the amount of coagulation factors in international units (IU) in an ampoule of prothormbinex or the dose required to reverse warfarin anticoagulation. A description of the clinical pros-cons of the various agents was not required to answer the question.
2014A 21
Compare and contrast the mechanism of action, pharmacokinetics, adverse effects and monitoring of effect of dabigatran and warfarin.
Examiner Comments
2014A 21: 29% of candidates passed this question.
Most candidates were able to provide some details about warfarin however dabigatran was less well known. The syllabus provides a guide to depth of knowledge required for listed drugs and so more detail was expected for Warfarin as a class “A” drug than was expected for dabigatran (a class “C” drug). It was however expected that candidates would provide more than just generalisations regarding “hepatic metabolism and renal excretion” when applied to both agents that actually have different modes of elimination.
2010A 03
Outline the major clotting factors and steps in the haemostasis pathway (70% marks).
Outline the mechanism of action of thrombolytics (30% marks).
CICMWrecks Answer: Haemostasis
Haemostasis Pathway
- collective term for the mechanisms that stop blood loss
- balance of pro-coagulant + anticoagulant systems
- procoagulants system: promotes coagulation → bioamplification system involving activation of clotting cascade
- anticoagulant system → regulates or inhibits coagulation
3 main components involved in haemostasis (Virchows triad)
- platelets
- endothelium
- when damaged → rapidly initiates haemostatic response
- normal function = prevent haemostasis + promote blood flow
- inhibition of platelet adhesion:
- NO + prostacyclin (PGI2)
- production of adenosine diphosphate (degrades ADP)
- anticoagulant effects:
- due to 2 endothelial membrane bound proteins:
- heparin sulphate (activates ATIII → inactivates thrombin + FXa)
- thrombomodulin: directly binds thrombin + activates protein C (inactivates FVa + VIIIa)
- Fibrinolytic effects
- Secretes tissue plasminogen activator (t-PA) → cleaves proenzyme plasminogen → form plasmin → degrades fibrin clots from endothelial cell surface (fibrinolysis)
- inhibition of platelet adhesion:
- coagulation proteins
Steps involved in haemostasis
Initiation of haemostasis.
Damaged vessel → plasma exposed to:
- Von willebrand factor (vWF): binds platelets to sub-endothelial collagen fibres
- Collagen fibres: platelets bind to collagen + become activated
- Tissue factor (TF): activates plasma coagulation proteins through extrinsic pathway → thrombin
Clot formation
3 key steps:
- Vasoconstriction
- ↓ blood flow → ↓ platelet plug washed away + ↓ blood loss
- Platelet aggregation
- Adhesion: vessel damage exposes TF, collagen, vWF → platelet GPIb-V-IX binds to subendothelial collagen via vWF
- Activation: metabolic process; adhesion triggers GPIb/IIIa activation → irreversible binding to matrix ligands; change shape + activation. Activation results in:
- Exocytosis of granules: contain: 5-HT, TXA2, ADP, PAF, vWF, fibrinogen, thrombin, Ca2+, PDGF
- Dense granules: release ADP, adrenaline, 5-HT → reinforce platelet activation
- α granules: release fibrinogen, β thromboglobulin, PAF-4, FV, vWF, PDGF, thrombospondin → mediate + reinforce platelet aggregation + adhesion
- Activation of phospholipase A2 to form TXA2
- Deformation from disc to sphere with long projections
- Promotion of coagulation cascade
- Platelet contraction (with clot contraction)
- Exocytosis of granules: contain: 5-HT, TXA2, ADP, PAF, vWF, fibrinogen, thrombin, Ca2+, PDGF
- Aggregation: activated GPIIb/IIIa mediated aggregation via fibrinogen + vWF
- Haemostatic plug: plug of degranulated platelets, fibrin mesh, leukocytes, entrapped RBCs
- Coagulation
- coagulation cascade = biological amplification system involving plasma proteins → formation of thrombin
- Classical model: intrinsic + extrinsic → common pathway
- reflects in vitro lab tests but not in vivo haemostasis
- Exrinsic pathway: activated by TF → FVII → FIIa → activates FX → start of common pathway
- Intrinsic pathway: activated by contact with -vely charged substances e.g. subendothelial collagen
- Final common pathway: FXa → converts prothrombin (FII) to thrombin (FIIa) → thrombin
- New model: cell based
- Better represents in vivo mechanism of coagulation
- Initiation phase: coagulation triggered by vessel damage → exposes plasma to TF → FV + FVII activated → activate other nearby clotting factors → formation of thrombin
- Amplification: further activation of clotting factors + platelets
- Propagation: occurs on surface of activated platelet → catalyses formation of thrombin+++
- Thrombin:
- acts on fibrinogen mesh within platelet plug → hydrolyses soluble fibrinogen → produce insoluble fibrin strands
- Activation of FXIII: forms covalent crossbridges between fibrin strands in platelet plug → stable clot
- +ve feedback loop: activates FV + FVIII → feed into cascade to produce more thrombin
- activation of protein C by thrombin-thrombomodulin complex: inhibitor of coagulation → deactivates FVa and VIIIa
Fibrinolysis
- Physiological mechanism in which the fibrin within blood clots is slowly dissolved
- Normal part of wound healing + important mechanism to keep small vessels patent
- Key points:
- Plasminogen is a b-globulin (proenzyme synthesised by the liver) → becomes interwoven into the fibrin clot as it is formed → converted to plasmin (serum protease)
- Main physiological activator of plasminogen is t-PA, expressed by endothelial cells – helps to keep the endothelial cell surface free of fibrin deposits
- Fibrin cleaved by plasmin → produces fibrin degradation products (FDPs)
- One of the FDPs is the d-dimer – cleavage product of cross-linked fibrin
- Fibrinolysis pathway can be manipulated:
- Thrombolysis eg streptokinase promotes conversion of plasminogen to plasmin → ↑ fibrinolysis
- Inhibition of fibrinolysis: eg tranexamic acid inhibits activation of plasminogen
Kerr / Bianca 2016
CICMWrecks Answer: Thrombolytic Agents
Mechanism of action of Thrombolytics
“Thrombolytic agent” → drug that enhance body’s fibrinolytic system by acting on
plasminogen activators, which ↑ conversion of plasminogen into plasmin
- Streptokinase
- Binds non-covalently to plasminogen to form a “streptokinaseplasminogen activator complex” → ↑ conversion of plasminogen to plasmin → ↑ fibrinolysis
- It is NOT fibrin-specific → causes systemic fibrinolysis
- Urokinase:
- similar to streptokinase
- Alteplase (rt-PA):
- Glycoprotein that is activated when bound to fibrin (clot) only → then selectively converts fibrin-bound plasminogen to plasmin to cause fibrinolysis
- It is fibrin-specific → causes less systemic fibrinolysis (cf. streptokinase)
Kerr / Bianca 2016
Examiner Comments
2010A 03: 8 (80%) of candidates passed this question
This question was also best answered using a structured response and illustrations.
Discussion and/or diagrams of the process of formation of temporary platelet plug and conversion to a definitive haemostatic plug after injury to the vessel wall, showing the Intrinsic, Extrinsic and Common Pathways with note of essential cofactors (tissue thromboplastin, Ca++) and fibrinolysis and clot resolution, inhibitors and controlers that prevent excessive coagulation. The latter would lead into outlining the mechanism of thrombolytics. It was expected candidates would mention such mechanisms as catalysing the formation of plasmin from plasmingoen, activation of endogenous plasminogen and direct conversion of plasminogen to plasmin.
Syllabus: J2. 1, J2. 2e
Reference: Pharmacology and Physiology in Anesthetic Practice, Stoelting pgs 510 – 511, Basic and Clinical Pharmacology, Katzung pg 380 – 383
2024A 06
(a) Outline the process of fibrinolysis including how it interacts with the coagulation system (80% of marks).(b) Describe the mechanism of action and adverse effects of alteplase (20% of marks).
2021B 19
Outline the process of fibrinolysis (40% marks). Write short notes on the indications, mechanism of action, pharmacokinetics and side effects of tranexamic acid (60% marks).
2015B 19
Describe the fibrinolytic pathway and identify areas of interaction with the coagulation pathway (80% of marks). List two anti-fibrinolytic agents and state their specific mechanism of action (20% of marks).
2013A 04
Describe the pharmacology of tranexamic acid.
CICMWrecks Answer: Fibrinolysis
Fibrinolysis
- Process by which fibrin clot is degraded to prevent excessive clot formation
- Plasminogen incorporated into clot
- Injured tissues and vascular endothelium gradually produce Tissue Plasminogen Activator (t-PA) in a delayed manner.
- Urokinase is produced by monocytes, macrophages and urinary epithelium
- Factor XII (Hageman factor) acts as a weak activator of plasminogen
- Plasminogen activated by t-PA, u-PA and factor XII to plasmin, a serine protease
- Plasmin cleaves fibrin into fibrin degradation products → reducing clot stability → Clot breakdown
Regulation of fibrinolysis
- Plasmin Activator Inhibitor (PAI) 1 & 2 – produced by vascular endothelium and inhibits tPA and uPA
- Protein C inactivates PAI, TAFI
- Thrombomodulin – expressed on in tact endothelium – binds thrombin and subsequently activates protein C
- Thrombin Activatable Fibrinolysis Inhibitor – produced by liver and cleaves plasmin binding sites on fibrin → decreased degradation
- α2 anti-plasmin – produced by liver, inhibits circulating plasmin, not fibrin bound plasmin
Sakurai 2016
CICMWrecks Answer: Anti-fibrinolytic agents
Anti-Fibrinolytic Agents
- Aprotinin
- Proteolytic enzyme inhibitor that acts on trypsin, plasmin and tissue kallikrein → forms reversible enzyme-inhibitor complex that inactivates free plasmin → ↓ clot lysis
- Tranexamic acid:
- Competitive inhibition of plasminogen conversion to active plasmin → ↓ fibrin (clot) lysis
- Aminocaproic acid
- Forms reversible complex with plasminogen → ↓ conversion to active plasmin → ↓ fibrin (clot) lysis
Sakurai 2016
CICMWrecks Answer: Pharmacology of Tranexamic Acid
CICMWrecks Answer: Pharmacology of Alteplase
Examiner Comments
2024A 06: 3% of candidates passed this question.
(a) This part required candidates to demonstrate their knowledge of the fibrinolytic pathway with details of the relevant mediators and inhibitors. Candidates where then required to apply their knowledge of both the coagulation cascade and fibrinolysis, with detail of how the pathways are simultaneously activated by the same stimuli to ensure balance between bleeding and clotting.
An example would be endothelial damage stimulating both the clotting cascade through collagen exposure and thromboplastin activation (faster response) as well as fibrinolysis through t-PA activation (slower response).
(b) This section of the question required a detailed description of the mechanism of action of alteplase, recombinant t-PA. A comparison to endogenous t-PA helped illustrate this action and the subsequent bleeding and the non-bleeding effects.
2021B 19: 30% of candidates passed this question.
The relative allocation of marks and thus time to be spent on each component was delineated by the relative percentages in the question. The first part of the question required a step-by-step outline of the fibrinolytic pathway with mention of the regulatory processes. Tranexamic acid is an important drug in the practice of intensive care and the question provided the headings under which to answer the question. The detail surrounding the keys aspects of this drug with respect to its use in critical care were often vague and underappreciated.
2015B 19: 8% of candidates passed this question.
The fibrinolytic pathway is a cascade largely made up of proteolytic enzmes and other factors synthesized in the liver that circulate in inactive precursor forms. Marks were awarded for description of the principal members of the cascade and the pathway relations between them. Endothelium is also important in the fibrinolytic pathway.
Regulation of the pathway to localise the site and size of clot as well as delayed onset of action of fibrinolysis is central to any description. Regulation of fibrinolysis by the coagulation cascade and a description of this area of interaction were expected.
Many candidates provided a reasonable description of the fibrinolytic cascade. Marks were not awarded for description of the coagulation cascade that did not have relevance to fibrinolysis. Understanding of regulation of fibrinolysis and it’s interaction with coagulation was poorly answered.
Most candidates were able to name two antifibrinolytic agents. Few were able to describe mechanism of action.
2013A 04:
Tranexamic acid is a drug used to reduce bleeding in trauma or surgery. It is also used for hereditary angioedema and menstrual bleeding. It is being increasingly used in critically ill patients. As a Level B listed drug within the Primary Syllabus candidates would be expected to know it in some depth. Often basic information such as mechanism of action, pharmacokinetics and adverse effects was lacking.


Recent Comments