Syllabus (Fourth Edition, 2023)
Topics
i. Outline the physiological production of blood and its constituents.
ii. Explain the major blood groups and the principles of cross matching.
iii. Outline the constituents and functions of plasma.
iv. Describe the process and regulation of haemostasis, coagulation, and fibrinolysis.
v. Describe the mechanisms of preventing thrombosis including endothelial factors and natural anticoagulants.
vi. Explain the physiological consequences of acute and chronic anaemia.
Topics not covered in previous SAQs
i. Outline the physiological production of blood and its constituents.
v. Describe the mechanisms of preventing thrombosis including endothelial factors and natural anticoagulants.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
i. Outline the physiological production of blood and its constituents.
2014A 11
Outline the formation, structure and function of the adult red blood cell.
2016B 14
Describe the features of a red blood cell that facilitate oxygen transport.
CICMWrecks Answer
RED BLOOD CELL
Formation
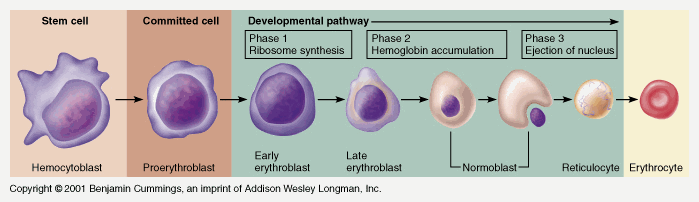
Pluripotent haemopoetic stem cell → Colony Forming unit → Proerythroblast → Reticulocyte → Erythrocyte
Simplified – main steps.
Takes 7 days
- Proerythroblast propagates
- From proerythroblast to reticulocyte
- Concentration of haemoglobin increases
- Nucleus disappears
- Endoplasmic reticulum disappears
- Reticulocyte
- Ribosomes
- Mitochondria
- Reticulocyte released into circulation
- Matures to RBC in 1-2 days
EPO increases rate of differentiation
Produced in the kidney (in response to low PO2)
Structure
- Are 7.5μm in diameter
- Are 2um thick
- biconcave disc, contains haemoglobin A (Hb F in-utero), has a central Fe moiety and demonstrates positive cooperativity in binding oxygen
- Have a lifespan of 120 days
- make up 40-50% of the blood volume, usual value 4-5 x 1012/L
- Carry ~29pg of haemoglobin
| Structure | Function |
|---|---|
Biconcave disc shape | Maximises surface area (optimising gas transfer) |
| Flexible membrane with shape maintained by structural proteins | makes the cells flexible enough to pass through capillary beds (which are narrower than the cell). |
| No nucleus & other organelles | Maximises cell volume available for Hb. |
| No mitochondria (No oxygen utilization) | Cannot perform aerobic metabolism – all ATP is generated via glycolysis. 3 shunts that come off the anaerobic glycolytic pathway (RBC’s only means of ATP generation): i) the production of 2-3 DPG via the Rapoport-Luebering shunt ii) generation of NADPH by the hexose monophosphate shunt (protects RBC from oxidative damage) iii) reduction of metHb back to Hb by way of the NADH. Optimizes Oxygen carriage |
| No ribosomes | Incapable of producing protein |
| Haemoglobin Four globulin chains (2α + 2β) links to heme Heme: Porphyrin ring chelated to iron Formed in the mitochondria | High affinity for oxygen O2 in haemoglobin is Hb x SatO2 x 1.34, compared to 0.003 x PO2 dissolved in blood Haemoglobin is a good buffer |
| Contains carbonic anhydrase | CO2 + H2O → H2CO3 Enables transport of CO2 as bicarbonate |
JC / Mooney 2019
Examiner Comments
2014A 11: 19% of candidates passed this question.
Candidates generally provided detailed description of the cell lineage that led up to the production of the mature red blood cell (RBC), but often omitted to mention those aspects unique to the RBC that were essential to its functions (e.g. biconcave shape gives the RBC a greater surface area and shorter distance to central regions, thus optimising diffusion of gases; the RBC enhanced ability to change shape and travel through narrow capillaries; lack of organelles maximises space for Hb, etc.). Mention of RBC function often lacked detail (e.g. restricted to just mentioning “O2 carriage”) or failed to mention, and describe, the RBC’s role in acid base buffering and HCO3- production.
2016B 14: 33% of candidates passed this question.
This question was best answered by considering form and then function. Detailing red cell size, that it is a biconcave disc, contains haemoglobin A (Hb F in-utero), has a central Fe moiety and demonstrates positive cooperativity in binding oxygen would be a good start. Additionally, noting that the RBC has a flexible membrane with shape maintained by structural proteins and that it lacks a nucleus, organelles and mitochondria, but contains carbonic anhydrase would pass this question.
A complete answer would mention the3 shunts that come off the anaerobic glycolytic pathway (RBC’s only means of ATP generation), namely the production of 2-3 DPG via the RapoportLuebering shunt, generation of NADPH by the hexose monophosphate shunt (protects RBC from oxidative damage) and the reduction of metHb back to Hb by way of the NADH.
Many answers lacked sufficient information to pass this question. Many answers included lengthy discussions about the production of RBC’s, the Oxyhaemoglobin dissociation curve or calculated the oxygen content of blood. RBC metabolic adaptations (e.g. 2, 3-DPG, NADPH production by the HMP shunt/ G6phosphatase to regenerate glutathione and metHb reductase) were rarely mentioned, as were vasodilatory mediators released by RBCs.
2022B 18
Describe the generation of ATP by mitochondria (50% marks) and outline the processes by which ATP is generated in red blood cells (50% marks).
CICMWrecks Answer
ATP Generation by mitochondia
- Mitochondria form ATP via oxidative phosphorylation (Major), via Kreb’s cycle and Electron transport chain (ETC)
- mitochon found in high conc in cells with high metabolic demands eg myocardium (23% of cell), brown fat (neonate)
- exercise ↑s numbers
- OP = production of ATP associated with oxidation by the flavoprotein cytochrome system in mitochondria
- Structure
- The inner and outer membranes of mitochondria define three compartments within the organelle, each with its distinct role and corresponding protein components.
- Outer membrane separates mitochondria from cytoplasm
- The innermost compartment, surrounded by the inner membrane, is the mitochondrial matrix
- The inner membrane of the mitochondrion contains the components of the electron transport chain.
- The high pH of the mitochondrial matrix creates the trans-membrane electrochemical gradient that drives ATP synthesis
- Oxidation/reduction reactions along the components of the electron transport chain generate a proton gradient that is used by ATP synthase to phosphorylate ADP, thereby producing ATP.
- To increase the capacity of the mitochondrion to synthesize ATP, the inner membrane is folded to form cristae. These folds allow a much greater amount of electron transport chain enzymes and ATP synthase to be packed into the mitochondrion.
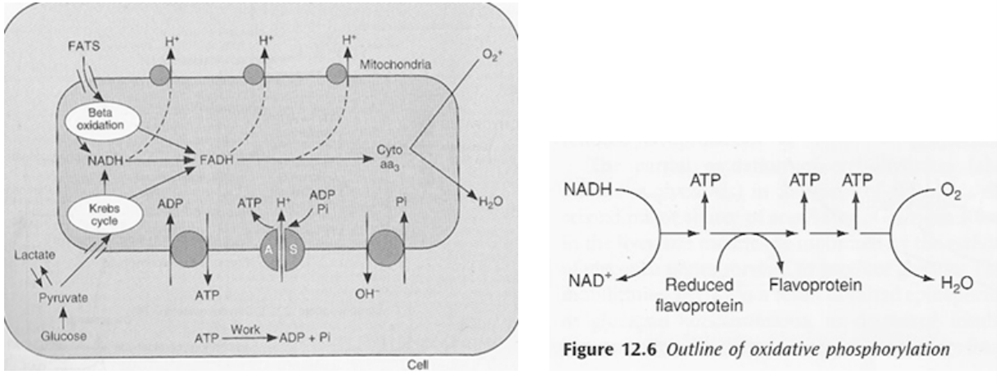
- Kreb’s cycle (TCA or citric acid cycle)
- Carbohydrate / Protein / Lipid ⇒ Acetyl coenzyme A or other intermediates
- Acetyl CoA ⇒ Kreb’s cycle
- Electron Transport Chain (ETC)
- The metabolic pathway through which the electron passes, starting with one transporter and then onto the next, is known as the electron transport framework (ETS).
- The electron transport framework happens in the inward mitochondrial layer.
- The electron transport chain contains the accompanying:
- Complex I: NADH dehydrogenase
- Complex II: succinate dehydrogenase
- Complex III: cytochromes bc 1
- Complex IV: cytochromes a-a3
- Complex V: ATP synthase
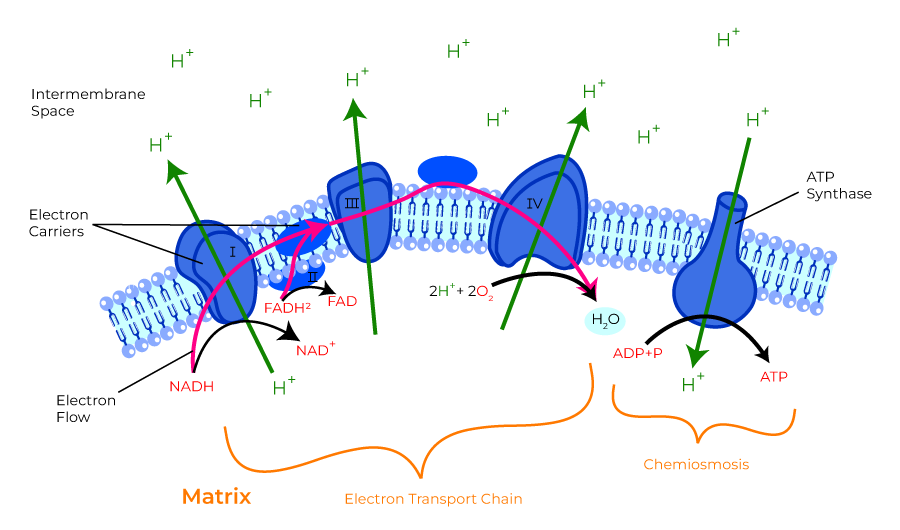
- ATP formed in electron transfer chain:
- Substrate diffuses into mitochon cytoplasm
- Hydrogen removed by a dehydrogenase
- NAD carries hydrogen to respiratory chain
- Hydrogen ionises and protons pass along series of carrier molecules across insulating membrane (inner membrane of mitochondria – forms cristae)
- Movement of protons creates an electrochemical gradient for transport of protons from intermediate space back into matrix ⇒ this drives a reversible ATPase in inner membrane (ATP synthase)
- ATP synthase: ADP + Pi ⇒ ATP
- @end:
- ATP produced
- Reduction of O2 to water – catalysed by cytochrome oxidase
- cyanide inhibits this oxidase ∴ inhibits OP in mitochon
- O2 required to oxidise NADH
- Eg’s of carrier molecules in electron transfer chain
- Flavoprotein
- Cytochromes A, A3, B, C, C1
- Ubiquinone
- Several iron sulphide proteins
- OP depends on:
- Adequate supply of ADP: +ve feedback loop e.g. ↑ATP utilisation ⇒ ↑ADP ⇒ ↑OP
- Rate of delivery of fats, lactate, glucose to interior of mitochon
- Availability of O2: Pasteur point = 1-2mmHg i.e. point below which OP cannot occur
- ∴ cardioresp works in harmony to ensure o2 reaches cells
- defined by oxygen flux equation:
ATP Generation by RBCs
- RBC have no Mitochondia – Cannot perform aerobic metabolism
- All ATP is generated via anaerobic glycolysis (Embden-Meyerhof pathway / EMP) (90% glycolysis)
- 10-step Catabolic pathway in cytosol
- by degradation of glucose molecule
- produces 2x ATP + pyruvate/lactate per glucose → ATP is used by Na+/K+ATPase, which is implicated in maintaining RBC shape, volume and flexibility
- Steps
| Step | Substrate | Enzyme | Product | ATP |
|---|---|---|---|---|
| 1 | Glucose | hexokinase | Glucose-6-phosphate | ATP consumed |
| 2 | Glucose-6-phosphate | glucose phosphate isomerase | Fructose-6-phosphate | |
| 3 | Fructose-6-phosphate | phosphofructo kinase | fructose-1,6-diphosphate | ATP consumed |
| 4 | fructose-1,6-diphosphate | aldolase | 2x glyceraldehyde-3-phosphate | |
| 5 | 2x glyceraldehyde-3-phosphate | glyceraldehyde-3-phosphate dehydrogenase | 2x 1,3-bisphosphoglycerate | |
| 6 | 2x 1,3-bisphosphoglycerate | phosphoglycerate kinase | 2x 3- phosphoglycerate | 2x ATP produced |
| 7 | 2x 3- phosphoglycerate | 3-phosphoglycerate mutase | 2x 2-phosphogylcerate | |
| 8 | 2x 2-phosphogylcerate | enolase | 2x phosphoenolpyruvate | |
| 9 | 2x phosphoenolpyruvate | pyruvate kinase | 2x pyruvate | 2x ATP produced |
| 10 | pyruvate | lactate dehydrogenase | lactate |
- 3 shunts that come off the anaerobic glycolytic pathway (produce no ATP)
- the Rapoport-Luebering shunt (BPG Shunt): 2,3-DPG is interconverted from 1,3-DPG (glycolytic intermediate)
- Hexose monophosphate shunt (HMP shunt or pentose phosphate pathway) – generates NADPH – protects RBC from oxidative damage (10% glycosis)
- NADH generated – used by MetHb reductase to reduce oxidised Hb (MetHb) to Hb
Examiner Comments
2022B 18: 15% of candidates passed this question.
Excellent answers focused on oxidative phosphorylation and the chemiosmotic mechanism in their description of ATP production by mitochondria. This required a description of the structure of the mitochondrial components involved in ATP production, the establishment of an electrochemical gradient of protons across the inner membrane, how the electron transport chain works and its components, and the roles of cytochrome oxidase and ATP synthase. Excellent answers describing ATP generation in red blood cells focused on anaerobic glycolysis in the absence of mitochondria, with an emphasis on the key steps that consume or produce ATP. The role of lactate dehydrogenase in regenerating NAD+ was also emphasised. Some candidates described other pathways of metabolism that do not generate ATP, for which no marks were awarded.
2012A 15
Briefly outline the production and fate of Red Blood Cells (RBC) (40% of marks).
Describe the breakdown of haemoglobin (Hb) (60% of marks).
CICMWrecks Answer
RBC
Role
- Oxygen transport
- Carbon dioxide transport
- Bound to N-terminal of globin → carbamino-Hb
- Buffering function
- Imidazole group on Hb Histadine residues
- Iron storage
- 65-70% of total iron stores
Normal Values
- 40-50% of the blood volume
- Usual value 4-5 x 1012/L
RBC Production
- 3-4w embryionic → Mesenchymal stem cells in yolk sac
- 6w-7mo
- → Liver (and spleen to lesser extent)
- continues until birth
- 7mo onwards:
- Bone marrow
- Proximal ends of femur/humerus
- fat replacement of long bone marrow occurs by age 18-20 y.o.
- Central skeleton (vertebrae, pelvis, ribs, sternum and skull)

Takes 7 days
- Proerythroblast propagates
- From proerythroblast to reticulocyte
- Concentration of haemoglobin increases
- Nucleus disappears
- Endoplasmic reticulum disappears
- Reticulocyte
- Ribosomes
- Mitochondria
- Reticulocyte released into circulation
- Matures to RBC in 1-2 days
EPO increases rate of differentiation
Produced in the kidney (in response to low PO2)
RBC Destruction
Globin chains → aa’s
Haem → Biliverdin + Fe + CO
- Biliverdin
- Haeme + Haem oxygenase → biliverdin
- Biliverdin + biliverdin reductase → bilirubin
- Free bilirubin + albumin → transported to hepatocytes
- Conjugated with glucuronide to ↑ water solubility
- Excreted to bile canniliculus
- Secreted to gut at D2 (duodenum)
- Intestinal flora hydrolyse and reduce → urobilinogen
- 3 fates:
- 1) Gut bacteria form the dark pigment stercobilin, which is egested in the faeces
- 2) Urobilinogen reabsorbed unchanged by the portal system and recycled by the liver
- 3) Remainder is reabsorbed by the portal system and then excreted in the urine
- Fe2+ → oxidised to Fe3+ → recycled
- CO (only endogenous source of CO)
Gladwin 2016
Examiner Comments
2012A 15: 6 (60%) of candidates passed.
The production and fate of red blood cells was well known to most candidates. Marrow production and its change with development, the sequence of haematogeny and RBC lifespan were well known. Some candidates failed to mention the role of erythropoietin and its stimulus by oxygen tension. The breakdown of haemoglobin caused much confusion. Globin (protein), Fe and haem (porphyrin ring) were all expected to be considered separately – many candidates omitted one or all. The steps were often confused as was the nature of transfer in blood, conjugation and release into bile. Conversion to stercobilinogen or urobilinogen (with reabsorption from the gut and excretion in urine) caused similar confusion. No marks were awarded for discussion of bile salt metabolism from cholesterol (not haem) or the differences between direct and indirect acting bilirubin.
2023A 13 – 2020A 11
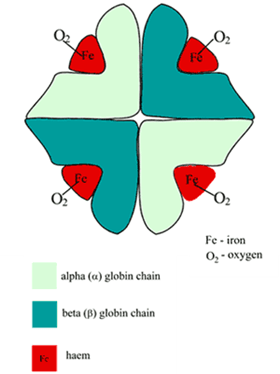
Describe the structure and function of adult haemoglobin.
CICMWrecks Answer
STRUCTURE
Structure
- Globular proteins which contain a haem moiety which binds O₂.
- Haem is an protoporphryin ring derivative with a central Fe2+ molecule that binds O₂
- Haemoglobin: MW ≈ 65000 daltons
- Hb contains 65-70 % of total body iron
- Globular molecule made up of four subunits, each containing a haem moiety conjugated to a polypeptide.
- Polypeptides collectively = globin → two pairs 2α + 2β → 4 haem moieties (Tetramer)
- Can bind a total of 4 O2 and also exhibits cooperative affinity (each subsequent O₂ binding takes less energy → sigmoid shaped OHDC
Location
- Hb is located in large concentrations (≈15g/L) in red blood cells that circulate throughout the blood stream.
Synthesis
- Haeme is synthesised in mitochondria and cytosol of immature RBCs, Globin is synthesized by ribosomes in cytosol.
- Production continues till RBCs lose their RNA after entering vasculature
Degradation
- RBCs at the end of their life cycle get phagocytosed by macrophages in liver or spleen, or get hemolysed in circulation.
- Haemoglobin is then broken up
- Haeme gets degraded into bilirubin
- iron gets recycled
FUNCTION
- O2 carrier:
- O2 loading exhibits positive cooperativity:
- α1ß1 & α2ß2 contacts stabilise Hb molecule as O2 reacts with it
- reaction of O2 with each subunit occurs sequentially with each facilitating the next
- ∴ ↑ing affinity as O2 loads ⇒ sigmoid OHDC
- O2 loading exhibits positive cooperativity:
- O2 unloading – vice versa:
- ß chains pulled apart
- 2,3-DPG enters molecule ⇒ ↓affinity of Hb for O2
- Protein buffer in RBC:
- Haemoglobin exists as a weak acid (HHb) as well as its potassium salt (KHb)
- In acidosis:
- Additional H+ ions are bound to Hb molecules
- HCO3– diffuses down its concentration gradient into plasma
- Electroneutrality is maintained through the inwards movement of Cl–.
- Dissolved CO2 will also form carbamino compounds by binding to the terminal amino groups
- Ligand Binding:
- Competitive inhibitors such as carbon monoxide (CO) and allosteric ligands such as carbon dioxide (CO2) and nitric oxide (NO).
- The carbon dioxide is bound to amino groups of the globin proteins to form carbaminohemoglobin; this mechanism is thought to account for about 10% of carbon dioxide transport in mammals.
- Nitric oxide can also be transported by haemoglobin; it is bound to specific thiol groups in the globin protein to form an S-nitrosothiol, which dissociates into free nitric oxide and thiol again, as the hemoglobin releases oxygen from its heme site. This nitric oxide transport to peripheral tissues is hypothesized to assist oxygen transport in tissues, by releasing vasodilatory nitric oxide to tissues in which oxygen levels are low.
Examiner Comments
2023A 13: 25% of candidates passed this question.
Good answers provided detail about the specific composition of haemoglobin and related the pertinent features of the molecule to its functions in the carriage of oxygen, carbon dioxide and its role in acid-base balance, including the appropriate mechanistic description. Many candidates provided information on the production and breakdown of haemoglobin which was not required. Many candidates provided an unnecessary amount of detail and diagrams of the oxygen haemoglobin dissociation curve which did not score additional marks.
2020A 11: 57% of candidates passed this question.
Marks were awarded for the two components of this question – structure and function. The structure component was often only briefly described with a cursory overview provided; however, this component contributed around half of the available marks. Many candidates were unable to accurately describe the structural components of the haemoglobin molecule. The functional component was handled better – however much time was wasted with detailed drawings of the oxyhemoglobin curve (not many marks awarded for this). The basic function of haemoglobin carriage of oxygen and carbon dioxide was known, but detail was often missing about its role as a buffer or its role in the metabolism of nitric oxide
2014B 18
Explain the similarities and differences between myoglobin and adult haemoglobin (60% of marks) and their physiologic relevance (40% of marks).
CICMWrecks Answer
The similarities and differences mean that haemoglobin is the primary means of O2 transport from the lungs to the tissues and myoglobin is the primary O2 carrying pigment of skeletal muscle and acts as local O2 reserve for times of intense muscle activity.
| Haemoglobin | Myoglobin |
|---|---|
| Function | |
| Oxygen carriage → lungs to tissues, CO₂ carriage, Acid-base buffer | Oxygen Store → for exercising muscle |
| Binds CO2, CO, NO, O2, H+ | Binds O2, tightly and firmly |
| Location | |
| in large concentrations (≈15g/L) in red blood cells that circulate throughout the blood stream. | haem containing pigment protein found in skeletal and cardiac muscle. |
| Haemoglobin is only found in blood stream following intravascular haemolysis | Myoglobin is only found in the blood stream when it is released following muscle injury → abnormal finding (↑ in AMI or rhabdomyolysis) |
| Structure | |
| structurally related. Both are globular proteins and both contain a haem moiety which binds O₂. | |
| Haem is an protoporphryin ring derivative with a central Fe2+ molecule that binds O₂ | |
| MW ≈ 65000 daltons | MW ≈ 17,700 daltons |
| Hb contains 65-70 % of total body iron | Myoglobin contains 4-5 % of total body iron |
| Globular molecule made up of four subunits, each containing a haem moiety conjugated to a polypeptide. Polypeptides collectively = globin → two pairs 2α + 2β → 4 haem moieties (Tetramer) | Myoglobin is a single-chain globular protein containing a single haem moiety (Monomer) |
| Can bind a total of 4 O2 and also exhibits cooperative affinity (each subsequent O₂ binding takes less energy → sigmoid shaped OHDC | Unlike haemoglobin it does not exhibit cooperative affinity when binding oxygen since it does not exist in a tetramer formation → its dissociation curve is a rectangular hyperbola rather than a sigmoid curve. |
| Carriage of O2 (See figure below) | |
| Sigmoid shaped dissociation curve | Rectangular hyperbole dissociation curve |
| P50 26.6mmHg, operating range 100-20mmHg | P50 2.75mmHg, operating range 5-1mmHg |
Hb has to carry oxygen from the lungs (PaO2 100) down the ‘oxygen cascade’ to the tissues. P50 suits this operating range and enables appropriate loading and unloading of O2. | Myoglobin needs to have a P50 less than Hb so it can take up O₂ from it. Myoglobin needs to be able to load and unload O₂ in the range of pO2 values that occur within the cell → it’s p50 of 2.75mmHg is well matched to the intracellular operating range of pO2 (1↔5mmHg) The myoglobin content is greatest in muscles specialized for sustained contraction → the muscle blood supply is often compressed during such contractions and myoglobin may provide O₂ when blood flow is cut off. |
| Bohr and Haldane Effect | Nil |
| Synthesis | |
| Haeme is synthesised in mitochondria and cytosol of immature RBCs, Globin is synthesized by ribosomes in cytosol. Production continues till RBCs lose their RNA after entering vasculature | Expressed solely in cardiac myocytes and oxidative skeletal muscle fibres |
| Degradation | |
| RBCs at the end of their life cycle get phagocytosed by macrophages in liver or spleen, or get hemolysed in circulation. Haemoglobin is then broken up – Haeme gets degraded into bilirubin, and the iron gets recycled. | It is released from muscle tissue by cell destruction and alteratins in permeability of muscle cell membrane. It is filtered by glomerulus and rapidly excreted by kidney. |
| Nephrotoxicity | |
| Released upon intravascular haemolysis | Released upon rhabdomyolysis Myoglobin is toxic to renal tubular epithelium and large amounts of myoglobin (eg in rhabdomyolysis) can cause renal failure. |
Examiner Comments
2014B 18: 23% of candidates passed this question.
Both are globular proteins that bind and deliver O2. Due to myoglobin containing a single globin chain its dissociation curve is hyperbolic in shape. Haemoglobin contains 4 globin chains and is a quaternary structure which exhibits cooperatively resulting in a sigmoid shaped dissociation curve. The differing dissociation curves mean that when the PO2 is high, as in the lungs, both myoglobin and haemoglobin are saturated with oxygen. However, at the lower levels of PO2 in the tissues, haemoglobin cannot bind oxygen as well as myoglobin. Myoglobin can bind the O2 released by haemoglobin, which it stores to meet the demands of muscle contraction. This means haemoglobin (with its higher p50) can offload O2 to myoglobin. Comments on the synthesis and degradation gained additional marks but were a common omission.
The physiological relevance was poorly explained. The similarities and differences mean that haemoglobin is the primary means of O2 transport from the lungs to the tissues and myoglobin is the primary O2 carrying pigment of skeletal muscle and acts as local O2 reserve for times of intense muscle activity.
2021A 03 – 2016A 06
Outline the formation, structure and function of the platelet.
CICMWrecks Answer
PLATELET
Formation
- produced during hematopoiesis in a sub-process called thromopoiesis, or production of thrombocytes.
- Bone marrow: Common myeloid progenitor cells → promegakaryocytes → megakaryocytes
- Megakaryocytes produce protoplatelets within their cytoplasm → released in cytoplasmic extensions upon cytokine stimulus
- Protoplatelets break up into hundreds of platelets that circulate throughout the bloodstream
- The remaining nucleus of the ruptured megakaryocyte is consumed by macrophages.
- Megakaryocyte and platelet production is regulated by thrombopoietin (hormone produced by the liver and kidneys)
- Thrombopoietin stimulates differentiation of myeloid progenitor cells into megakaryocytes and causes the release of platelets.
- Thrombopoietin is regulated by a negative feedback mechanism based on platelet levels
- Each megakaryocyte produces between 5,000 and 10,000 platelets
- Altogether, around 1011 platelets are produced each day in a healthy adult
Fate
- Average lifespan of a platelet is 5 to 10 days
- Old platelets are destroyed by macrophage phagocytosis in the spleen and by Kupffer cells in the liver
- Up to 40% of platelets are stored in the spleen as a reserve, released when needed by sympathetically-induced splenic muscle contractions during severe injury.
Structure
- Small anucleated cells derived from megakaryocytes which originate from haematopoietic stem cell
- Normally 150~300 x 103 /μl
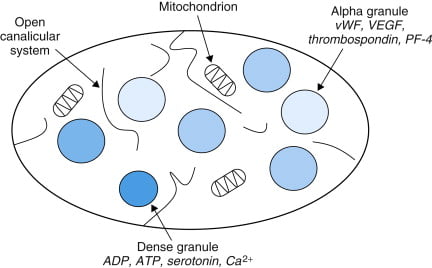
- Actin and myosin
- Remnants of the ER, SR storing calcium
- Mitochondria
- Enzyme systems → production of prostaglandins
- α granules
- Thrombin
- PDGF
- P-selectin
- Fibronectin
- vWF
- Dense granules
- ADP
- ATP
- Ca2+
- Serotonin
- Histamine
Function
- Haemostasis
- Formation of platelet plug
- Interact with collagen exposed at damaged endothelium by GPIa
- Activated by PAF, Thrombin
- Morphological change → irradiating pseudopods
- Degranulation
- Aggregation
- Expression of GPIIb/IIIa → binds fibrin and vWF
- Formation of platelet plug
- Immunomodulation
- Deployed to sites of inflammation and infection and secrete cytokines and chemokines
Sakurai / JC 2019
Examiner Comments
2024B 16: 58% of candidates passed this question.
Good candidates structured their answers into formation, structure, and function of the platelets. The structure should be divided into the surface of the platelets, including the receptors and antigens, and contents of the platelets. The function should be divided into adhesion, activation, and aggregation in that order with an explanation of the triggers and events during these phases followed by a discussion of the interaction between platelets and the coagulation system in haemostasis.
2021A 03: 79% of candidates passed this question.
This question was divided in three sections to help candidates formulate an answer template. The first section required a brief outline of the formation of platelets from pluripotent stem cells via megakaryocytes. The second section required an outline of platelet structure highlighting the special features such as, an absence of a nucleus, the presence of an external glycocalyx layer, specific surface receptors, contractile proteins, dense tubular system and granules. The third section was about platelet function where the expected focus was on the role of platelets in haemostasis. This required outlining the mechanism of platelet plug formation by adhesion-activation-aggregation, interactions with the coagulation cascade and role of platelets in clot contraction as well as fibroblast invasion. Although many candidates were able to answer the first section reasonably well, there was a noticeable knowledge deficit in the latter two sections. A significant proportion of answers had missing information on platelet structure and lack of structure in outlining platelet function.
2016A 06: 50% of candidates passed this question.
The structure of the question outlined exactly what was expected. Platelets are formed in the bone marrow from budding of megakaryocytes. Granulocyte colony stimulating factor and thrombopoeiten play a role in the process and they have a life span of about 10 days. It was expected candidates could describe or draw the structure detailing they have no nucleus, the presence of mitochondria and granules and provide some detail of the important external surface proteins (glycoproteins, ABO, human platelet antigens). Better answers also described the internal microtubule structure and related this to function (allows contraction and shape change). The description of function required detail around the importance of platelet plug formation and the role of adhesion, aggregation and activation in this process.
2011B 20
Describe the structure and function of platelets (50% marks).
Outline the pharmacology of clopidogrel (50% marks).
CICMWrecks Answer: Platelet
PLATELET
Formation
- produced during hematopoiesis in a sub-process called thromopoiesis, or production of thrombocytes.
- Bone marrow: Common myeloid progenitor cells → promegakaryocytes → megakaryocytes
- Megakaryocytes produce protoplatelets within their cytoplasm → released in cytoplasmic extensions upon cytokine stimulus
- Protoplatelets break up into hundreds of platelets that circulate throughout the bloodstream
- The remaining nucleus of the ruptured megakaryocyte is consumed by macrophages.
- Megakaryocyte and platelet production is regulated by thrombopoietin (hormone produced by the liver and kidneys)
- Thrombopoietin stimulates differentiation of myeloid progenitor cells into megakaryocytes and causes the release of platelets.
- Thrombopoietin is regulated by a negative feedback mechanism based on platelet levels
- Each megakaryocyte produces between 5,000 and 10,000 platelets
- Altogether, around 1011 platelets are produced each day in a healthy adult
Fate
- Average lifespan of a platelet is 5 to 10 days
- Old platelets are destroyed by macrophage phagocytosis in the spleen and by Kupffer cells in the liver
- Up to 40% of platelets are stored in the spleen as a reserve, released when needed by sympathetically-induced splenic muscle contractions during severe injury.
Structure
- Small anucleated cells derived from megakaryocytes which originate from haematopoietic stem cell
- Normally 150~300 x 103 /μl

- Actin and myosin
- Remnants of the ER, SR storing calcium
- Mitochondria
- Enzyme systems → production of prostaglandins
- α granules
- Thrombin
- PDGF
- P-selectin
- Fibronectin
- vWF
- Dense granules
- ADP
- ATP
- Ca2+
- Serotonin
- Histamine
Function
- Haemostasis
- Formation of platelet plug
- Interact with collagen exposed at damaged endothelium by GPIa
- Activated by PAF, Thrombin
- Morphological change → irradiating pseudopods
- Degranulation
- Aggregation
- Expression of GPIIb/IIIa → binds fibrin and vWF
- Formation of platelet plug
- Immunomodulation
- Deployed to sites of inflammation and infection and secrete cytokines and chemokines
Sakurai / JC 2019
CICMWrecks Answer: Pharmacology of Clopidogrel
Pharmacology of Clopidogrel
Examiner Comments
2011B 20: 13 (52%) of candidates passed this question.
Platelets are small cells in blood, approximately 150,000 – 300,000 / microliter of blood with a half life of approximately 4 days. They have important membrane receptors (for collagen, vWF and fibrinogen) and intracellular contents (Actin, Myosin, Glycogen, Lysosomes, Dense granules, Alpha granules) and are involved in forming a platelet plug and clotting. Common omissions were to not give a normal platelet count or half life. Diagrams of the clotting cascade were not required.
Clopidogrel is an oral, thienopyridine class antiplatelet agent (a prodrug activated in the liver by cytochrome P450 enzymes), used to inhibit blood clots in coronary artery disease, following stent placement, peripheral vascular disease, and cerebrovascular disease. Mechanism of action is: specifically and irreversibly inhibits the P2Y12 subtype of ADP receptor, which is important in aggregation of platelets and cross-linking by the protein fibrin and thus platelet aggregation, which is inhibited when binding blocks activation of the glycoprotein IIb/IIIa pathway. Elimination half-life of about 8 hours; rapidly absorbed after oral administration; undergoes rapid hydrolysis in liver and renal excretion; 95% protein bound. Important interactions/precautions include proton pump inhibitors, phenytoin, warfarin, heparin, danaparoid, enoxaparin and various thrombolytics. Greatest risk is bleeding. The best answers had a structured approach to describing a drug.
Syllabus: J1 2f, J2 2d
Recommended sources: Ganong Review of Medical Physiology pg 485; Goodman & Gillman. The pharmacological Basis of Therapeutics Chp 54.
ii. Explain the major blood groups and the principles of cross matching.
2015A 14
How is blood typed and cross-matched?
2010B 02
Outline the principles of compatibility testing of blood for transfusion.
2008A 21
Explain the ABO blood groups and how blood group is determined.
How is blood tested for compatibility using the ABO system?
CICMWrecks Answer
Blood Typing
- The purpose of blood typing is the streamlining of blood products on the basis of the presence of antiogenetic surface membrane proteins in order to minimise patient harm.
- Main Types of Antigens:
- ABO
- Rhesus
- Others:
- Kell, P, MN, Lewis
- less antigenic
ABO system:
Red blood cells possess genetically determined antigens on their cell membrane, termed
“agglutinogens”
Agglutinogens: glycoproteins, with differing terminal oligosaccharides
- H gene:
- Forms “H-antigen” by addition of fructose to membrane glycoprotein
- A gene:
- Forms “A-antigen” by extension of H-antigen with “N-acetyl-D-galactosamine”
- B gene:
- Forms “B-antigen” by extension of H-antigen with “D-galactose”
Blood Groups:
| Group | Antigen | Possess Antibody | Incidence |
|---|---|---|---|
| A | A-antigen only | Anti-B antibody | 45% |
| B | B-antigen only | Anti-A antibody | 9% |
| A/B | A and B-antigen | Lack both Anti-A and Anti-B antibodies | 3% |
| O | H-antigen only | possess anti-A and anti-B Ab’s | 43% |
Blood Group antibodies:
- Naturally-occurring Ab to A- and/or B-antigens (as anti-A and anti-B antibodies)
- IgM only
- Develop naturally after 3 months of age due to presence of Ag in bacteria/food that resemble A/B-Ag’s
Typing / Blood Group determination (Coombs)
- Red cells tested using anti-sera (Ab added to sample)
- IgM anti-A and anti-B Abs added to sample
- +ve agglutination indicates blood type
- “Reverse blood grouping” (Sample added to Ab)
- Test individual’s serum using known group A, B, and O red cells
- +ve agglutination indicates blood type
Antibody screen:
- Used to detect if an individual possesses antibodies other than those against agglutinogens A and B
- Individual’s blood is exposed to a number of red cells with known antigens, and if a reaction, an antibody is present
- A common antibody is the D antigen, or Rhesus factor
- Highly antigenic
- Present on RBC only
- Three subtypes
- Cc; Dd; Ee
- d-Ag does not exist
- Any combination of the three with a D-Ag is highly antigenic.
- Rh +ve (if D-Ag present)
Cross Matching
Major Crossmatch: Test in vitro recipient’s serum and donor’s red cell
Minor Crossmatch: In vitro serological compatibility between donor’s serum and recipient’s red cells
Major Crossmatch:
- Saline agglutination test
- To reconfirm ABO grouping
- It tests for presence of IgM (Eg. anti-A and anti-B Ab’s) in recipient serum against donor RBC
- Donor’s RBCs + saline + recipient serum
- +ve agglutination means incompatible ABO
- Indirect Coomb’s test
- To Reconfirm presence of “minor” Ab’s in recipient serum
- Two stages:
- Donor RBCs + recipient serum
- RBC will be coated if Abs present
- AHG test
- RBC washed
- “anti-human globulin” (AHG) added
- agglutination
- → recipient serum contains an Ab against donor RBC
- Donor RBCs + recipient serum
Serological testing:
All donor blood is tested for:
HIV 1 + 2
Hepatitis B
Hepatitis C
HTLV-1
Mooney / Gladwin 2016
Examiner Comments
2015A 14: 38 % of candidates passed this question.
An opening statement of the importance of compatibility testing helped explain the relevance of the process. A brief description/table of agglutinogens (membrane antigens) along with Agglutinins (IgM Antibodies) was helpful.
Typing is the testing of individual red blood cells (donor and recipient) with anti-sera containing anti-A, B and AB antibodies. A positive test results in agglutination. Red cells with known antigens (A, B and O) are then tested with sera (reverse grouping). When discussion antibody screening, a mention of Rhesus antibodies along with testing for minor antibodies (Kell, Duff etc.) was expected.
Cross matching consists of the saline agglutination test andiIndirect Coombs testing. (This involves incubation, washing and testing with antiglobulin serum).
Many answers confused the processes of typing, antibody screening and cross matching.
2010B 02: 8 (53%) of candidates passed this question.
There was a reasonable knowledge of the basics of ABO antigens and antibodies by most candidates. However, many answers seemed to lack perspective of the steps performed in the laboratory to improve immunological safety of transfusion. For a good answer it was expected that candidates also discuss the basis to serological testing and antibody screening.
Syllabus: J2a, J2a2i
References: Guyton Textbook of Physiology Chp 32 and 35, Australian Red cross Transfusion Medicine manual
2008A 21: 1 candidate (33%) passed this question.
The main points candidates were expected to cover included a detailed discussion of the ABO antigens, the evolution of IgM antibodies to these antigens and the prevalence of blood groups in the general population. Answers tabulating blood groups against expected antigens and antibodies present as well as agglutination reactions to anti-sera were most effective.
Discussion of the saline agglutination test was essential and extra marks were awarded for mention of the Coombs test. Candidates were required to demonstrate an understanding of cross-matching, specifically the testing of donor red blood cells against recipient serum.
Syllabus J1 2.a
References: Review of Medical Physiology (Chapter 27), 22nd ed. Ganong, pp. 537-539.
iii. Outline the constituents and functions of plasma.
2019B 11
Outline the composition of plasma (50% of marks). Describe the functions of albumin (50% of marks).
2012B 16
List the constituents of plasma and the functions of plasma proteins.
CICMWrecks Answer
Plasma
- Plasma is the liquid (non-cellular) component of blood, in which the red blood cells, white blood cells, and platelets are suspended.
- 93% water, 6% proteins and 1% other solutes
- Makes up 60% of blood volume
- Makes up 18% of extracellular fluid (or 5% of bodyweight)
Plasma Constituents
- Water
- Proteins
- Albumin (60%)
- Globulins (35%)
- α1 (α1 anti trypsin, α1-fetoprotein, serum amyloid A)
- α2 (haptoglobin, ceruloplasmin, Protein C, thyroxin-binding globulin)
- β (transferrin, plasminogen, β2 microglobulin, C-reactive protein)
- γ (immunoglobulins)
- Fibrinogen (4%)
- Regulatory proteins (<1%)
- Coagulation factors
- Complement proteins
- Other Solutes:
- Nutrients – vitamins, glucose
- Gases – oxygen, CO2, nitrogen
- Hormones
- Electrolytes – Na, K, Cl, Mg
- Lipids and other products of metabolism – urea, creatinine, nitrogenous wastes
Functions of Plasma Proteins
- Maintenance of fluid compartments/oncotic pressure
- starling forces, gibbs-donnan control bulk flow.
- Carrier/transport functions
- transferrin → Fe2+; albumin → free fatty acids.
- Role in acid-base balance and CO2 transport
- buffer e.g. carbamino compounds.
- Immunity
- antibodies e.g. IgG.
- Proteolytic enzymes
- complement, kinins, coagulation, fibrinolytic system.
- Metabolism of xenobiotics
- e.g. plasma cholinesterase.
- Anticoagulant proteins
- vWF; antithrombin III.
Functions of Albumin
- Osmotic pressure
- supplies 80% of total plasma Colloid Osmotic Pressure
- retards fluid efflux from plasma and oedema formation
- Transport and metabolism functions
- Transports thyroid hormones
- Transports other hormones, in particular, ones that are fat-soluble
- Transports fatty acids (“free” fatty acids) to the liver and to myocytes for utilization of energy
- Transports unconjugated bilirubin
- Transports many drugs; serum albumin levels can affect the half-life of drugs
- Extra-cellular acid-base buffer
- Detoxification
- solubilizes bilirubin and neutralizes its toxic effects
- Anti-oxidant effects
- Blocks Cu2+-mediated LDL oxidation
- Blocks Free-radical-mediated haemolysis
- anticoagulant effect
- protein store – for signalling molecules and nitric oxide
- Immunomodulation
- Other
- Competitively binds calcium ions (Ca2+)
- Prevents photodegradation of folic acid
- marker of an inflammatory state
- prevents apoptosis of proximal renal tubular cells
- stimulates proliferation of proximal renal tubular cells
JC / Gladwin 2020
Examiner Comments
2019B 11: 30% of candidates passed this question.
A good answer began with a definition of plasma and then listed the components – water, albumin, globulins, fibrinogen and other proteins before mentioning the lipid content, nutrient content, wastes and electrolytes. Frequently the breakdown of the globulin component was inaccurate. A common omission was dissolved gas components. Descriptions of the calculation of oncotic pressure and GFR were not asked and hence did not attract marks. The functions of albumin may be subdivided into: Osmotic pressure, transport function, acid-base buffer, anti-oxidant, anticoagulant effect, protein store, metabolism and ‘other’.
2012B 16: 15 (68.2%) of candidates passed.
This question was generally well answered. The constituents of plasma include water, electrolytes, glucose, liver enzymes, urea, creatinine, uric acid, dissolved gases and proteins. Plasma does not contain any cells. The proteins in plasma are albumin, globulins and fibrinogen. The globulins include alpha 1 and 2 and beta globulins and gamma globulins. Examples of α1- Globulins are: α1-fetoprotein, α1-protease inhibitor and prothrombin. Examples of α2-Globulins include: ceruloplasmin, haptoglobin, α2-macroglobulin and thyroxin-binding globulin. Examples of β-Globulins are: C-reactive protein, β2-microglobulin and transferrin. Examples of δ-Globulins are the immunoglobulins , IgG, IgA, IgM, etc. There are many more other globulins including the coagulation factors, the complement system and lipoproteins. The functions of plasma proteins include oncotic pressure, transport/carrier function, role in acid base balance (buffering, CO2 transport) and proteolytic systems such as complement, kinins, coagulation and fibrinolysis. More functions include the immune response, enzyme activity eg pseudocholinesterase, metabolism i.e. plasma proteins can be broken down and contribute amino acids to the amino acid pool and a role in thermoregulation. Many answers were deficient in details on the plasma proteins and their functions. The question asked to “list” the constituents, so the level of detail required to score marks reflected this and should have been achievable in the allocated timeframe.
iv. Describe the process and regulation of haemostasis, coagulation, and fibrinolysis.
2010A 03
Outline the major clotting factors and steps in the haemostasis pathway (70% marks).
Outline the mechanism of action of thrombolytics (30% marks).
CICMWrecks Answer: Haemostasis
Haemostasis Pathway
- collective term for the mechanisms that stop blood loss
- balance of pro-coagulant + anticoagulant systems
- procoagulants system: promotes coagulation → bioamplification system involving activation of clotting cascade
- anticoagulant system → regulates or inhibits coagulation
3 main components involved in haemostasis (Virchows triad)
- platelets
- endothelium
- when damaged → rapidly initiates haemostatic response
- normal function = prevent haemostasis + promote blood flow
- inhibition of platelet adhesion:
- NO + prostacyclin (PGI2)
- production of adenosine diphosphate (degrades ADP)
- anticoagulant effects:
- due to 2 endothelial membrane bound proteins:
- heparin sulphate (activates ATIII → inactivates thrombin + FXa)
- thrombomodulin: directly binds thrombin + activates protein C (inactivates FVa + VIIIa)
- Fibrinolytic effects
- Secretes tissue plasminogen activator (t-PA) → cleaves proenzyme plasminogen → form plasmin → degrades fibrin clots from endothelial cell surface (fibrinolysis)
- inhibition of platelet adhesion:
- coagulation proteins
Steps involved in haemostasis
Initiation of haemostasis.
Damaged vessel → plasma exposed to:
- Von willebrand factor (vWF): binds platelets to sub-endothelial collagen fibres
- Collagen fibres: platelets bind to collagen + become activated
- Tissue factor (TF): activates plasma coagulation proteins through extrinsic pathway → thrombin
Clot formation
3 key steps:
- Vasoconstriction
- ↓ blood flow → ↓ platelet plug washed away + ↓ blood loss
- Platelet aggregation
- Adhesion: vessel damage exposes TF, collagen, vWF → platelet GPIb-V-IX binds to subendothelial collagen via vWF
- Activation: metabolic process; adhesion triggers GPIb/IIIa activation → irreversible binding to matrix ligands; change shape + activation. Activation results in:
- Exocytosis of granules: contain: 5-HT, TXA2, ADP, PAF, vWF, fibrinogen, thrombin, Ca2+, PDGF
- Dense granules: release ADP, adrenaline, 5-HT → reinforce platelet activation
- α granules: release fibrinogen, β thromboglobulin, PAF-4, FV, vWF, PDGF, thrombospondin → mediate + reinforce platelet aggregation + adhesion
- Activation of phospholipase A2 to form TXA2
- Deformation from disc to sphere with long projections
- Promotion of coagulation cascade
- Platelet contraction (with clot contraction)
- Exocytosis of granules: contain: 5-HT, TXA2, ADP, PAF, vWF, fibrinogen, thrombin, Ca2+, PDGF
- Aggregation: activated GPIIb/IIIa mediated aggregation via fibrinogen + vWF
- Haemostatic plug: plug of degranulated platelets, fibrin mesh, leukocytes, entrapped RBCs
- Coagulation
- coagulation cascade = biological amplification system involving plasma proteins → formation of thrombin
- Classical model: intrinsic + extrinsic → common pathway
- reflects in vitro lab tests but not in vivo haemostasis
- Exrinsic pathway: activated by TF → FVII → FIIa → activates FX → start of common pathway
- Intrinsic pathway: activated by contact with -vely charged substances e.g. subendothelial collagen
- Final common pathway: FXa → converts prothrombin (FII) to thrombin (FIIa) → thrombin
- New model: cell based
- Better represents in vivo mechanism of coagulation
- Initiation phase: coagulation triggered by vessel damage → exposes plasma to TF → FV + FVII activated → activate other nearby clotting factors → formation of thrombin
- Amplification: further activation of clotting factors + platelets
- Propagation: occurs on surface of activated platelet → catalyses formation of thrombin+++
- Thrombin:
- acts on fibrinogen mesh within platelet plug → hydrolyses soluble fibrinogen → produce insoluble fibrin strands
- Activation of FXIII: forms covalent crossbridges between fibrin strands in platelet plug → stable clot
- +ve feedback loop: activates FV + FVIII → feed into cascade to produce more thrombin
- activation of protein C by thrombin-thrombomodulin complex: inhibitor of coagulation → deactivates FVa and VIIIa
Fibrinolysis
- Physiological mechanism in which the fibrin within blood clots is slowly dissolved
- Normal part of wound healing + important mechanism to keep small vessels patent
- Key points:
- Plasminogen is a b-globulin (proenzyme synthesised by the liver) → becomes interwoven into the fibrin clot as it is formed → converted to plasmin (serum protease)
- Main physiological activator of plasminogen is t-PA, expressed by endothelial cells – helps to keep the endothelial cell surface free of fibrin deposits
- Fibrin cleaved by plasmin → produces fibrin degradation products (FDPs)
- One of the FDPs is the d-dimer – cleavage product of cross-linked fibrin
- Fibrinolysis pathway can be manipulated:
- Thrombolysis eg streptokinase promotes conversion of plasminogen to plasmin → ↑ fibrinolysis
- Inhibition of fibrinolysis: eg tranexamic acid inhibits activation of plasminogen
Kerr / Bianca 2016
CICMWrecks Answer: Thrombolytic Agents
Mechanism of action of Thrombolytics
“Thrombolytic agent” → drug that enhance body’s fibrinolytic system by acting on
plasminogen activators, which ↑ conversion of plasminogen into plasmin
- Streptokinase
- Binds non-covalently to plasminogen to form a “streptokinaseplasminogen activator complex” → ↑ conversion of plasminogen to plasmin → ↑ fibrinolysis
- It is NOT fibrin-specific → causes systemic fibrinolysis
- Urokinase:
- similar to streptokinase
- Alteplase (rt-PA):
- Glycoprotein that is activated when bound to fibrin (clot) only → then selectively converts fibrin-bound plasminogen to plasmin to cause fibrinolysis
- It is fibrin-specific → causes less systemic fibrinolysis (cf. streptokinase)
Kerr / Bianca 2016
Examiner Comments
2010A 03: 8 (80%) of candidates passed this question
This question was also best answered using a structured response and illustrations.
Discussion and/or diagrams of the process of formation of temporary platelet plug and conversion to a definitive haemostatic plug after injury to the vessel wall, showing the Intrinsic, Extrinsic and Common Pathways with note of essential cofactors (tissue thromboplastin, Ca++) and fibrinolysis and clot resolution, inhibitors and controlers that prevent excessive coagulation. The latter would lead into outlining the mechanism of thrombolytics. It was expected candidates would mention such mechanisms as catalysing the formation of plasmin from plasmingoen, activation of endogenous plasminogen and direct conversion of plasminogen to plasmin.
Syllabus: J2. 1, J2. 2e
Reference: Pharmacology and Physiology in Anesthetic Practice, Stoelting pgs 510 – 511, Basic and Clinical Pharmacology, Katzung pg 380 – 383
2022A 15
Describe the sequence of haemostatic events following injury to a blood vessel wall until clot stabilisation.
2019A 10
Outline the sequence of haemostatic events after injury to a blood vessel wall (50% of marks).
Discuss the role of naturally occurring anticoagulants in preventing clot formation in-vivo (50% of marks).
CICMWrecks Answer: Haemostatic events after injury
- Haemostasis is the natural process that stops blood loss when an injury occurs.
- Intact vascular endothelial cells:
- fibrinolytic heparin, Thrombomodulin → prevent clotting
- NO, Prostacyclin → Prevents clotting cascade
Haemostasis following vessel injury
Three steps: Vascular spasm (vasoconstriction), platelet plug formation and coagulation.
- Vasoconstriction:
- Brief reflexive contraction that decreases local blood flow
- Caused by signalling molecules from injured endothelial cells, thromboxane A2 from activated platelets, Nervous system reflexes from local pain receptors
- Vasoconstriction only lasts for a few minutes during haemostasis. During inflammation that follows the injury, it is replaced by vasodilation as the healing process begins.
- Platelet Plug Formation (Primary Haemostasis):
- Within 20 seconds, coagulation is initiated
- Platelet Adherence: vWF released from damaged endothelium → change platelet form → adhere to subendothelial collagen
- Platelet Activation: subendothelial collagen binds to platelet receptors → activates platelets → degranulate → release ADP, vWF, TXA2, PDGF, VEGF, Serotonin, Coagulation factors
- Platelet Aggregation: Platelets bind to vWF and fibrinogen → aggregate over damaged endothelium
- Positive feedback mechanism
- Platelet plug formed in seconds to a few minutes based on extent of injury
- Coagulation Cascade (Secondary Haemostasis):
- Occurs if platelet plug ineffective in controlling bleeding
- Platelets degranulate → release ADP, Serotonin, Thromboxane A2
- Coagulation cascade: Intrinsic, Extrinsic pathway → Common pathway
- Intrinsic (Contact Activation) Pathway: Primary complex (on collage by High molecular weight kininogen, prekallikrenin, factor XII) → XI → IX (which, along with VIII) → Common pathway
- Extrinsic (Tissue factor) Pathway: Tissue factor III → VII → Generates thrombin burst – cleaves fibrinogen to fibrin
- Common pathway: Prothrombin(II) to thrombin (using Factor V) → cleaves fibrinogen to fibrin
- forms mesh that binds and strengthens platelet plug → coagulation → haemostasis
- Also activates factor XIII – covalently bonds to fibrin to strengthen attachment to platelets
- Also activates more factor V which acts as anticoagulant with inhibitor protein C
Fate of the Clot:
One of four outcomes:
- Propagation: Accumulation of additional platelets and fibrin
- Embolization: Thrombus breaks free and becomes mobile
- Dissolution: Fibrinolysis (aided by tissue plasminogen activator, tPA)
- Organization and recanalization: Ingrowth of smooth muscle cells, fibroblasts and endothelium into fibrin-rich thrombus.
Clot lysis and Wound healing:
Over the course of the next few days:
- Clot Retraction: blood clot shrinks.
- dependent on the release of multiple factors, mostly factor XIIIa crosslinks
- Cause contraction, knotting and twisting of fibrin mesh
- Blood clot shrinks
- Fibrinolysis – Plasmin degrades fibrin to Fibrin degradation products, macrophages consume the expended platelets. FDPs inhibit further thrombin and fibrin formation
- Wound Healing:
- Inflammation – Tissue proliferation – Collagen and granulation tissue deposition – angiogenesis – Wound contraction – Epithelialization
Source: https://courses.lumenlearning.com/boundless-ap/chapter/hemostasis/
JC 2019
CICMWrecks Answer: Naturally occurring anticoagulants
Naturally Ocurring Anticoagulants:
Prevent unnecessary coagulation.
| Protein C | a vitamin K-dependent serine protease enzyme that degrades Factor V and factor VIII. |
| Protein S | Cofactor which helps protein C. |
| Heparin | fast-acting anticoagulant expressed on endothelial cells, inhibits activity of thrombin |
| Antithrombin III | a serine protease inhibitor that degrades thrombin, Factor IXa, Factor Xa, Factor XIa, and Factor XIIa |
| Tissue factor pathway inhibitor (TFPI) | limits the action of tissue factor (TF) and the factors it produces. |
| Plasmin | generated by proteolytic cleavage of plasminogen, a potent fibrinolytic that degrades fibrin and destroys clots. |
| Prostacyclin (PGI2) | released by the endothelium and inhibits platelet activation. |
| Thrombomodulin | released by the endothelium and converts thrombin into an inactive form. |
Source: https://courses.lumenlearning.com/boundless-ap/chapter/hemostasis/
JC 2019
Examiner Comments
2022A 15: 46% of candidates passed this question.
A good answer was well structured and covered the areas of vasoconstriction, platelet adhesion, activation and aggregation, coagulation, clot retraction and anticlotting mechanisms. Many answers gave an overview of the haemostatic process but revealed insufficient knowledge of the processes involved. It was acceptable to give a classical view of clotting or to describe the cell-based model; or both. However, in several cases answers became confused by mixing up elements of the classical approach and cell-based model approach. Errors concerning details of the cell-based model were frequent. Many candidates did not include how the clot is limited to just the site of injury which happens in parallel with the formation of clot. Candidates should be aware that writing lengthy introductory statements attracted no marks and wastes time.
2019A 10: 40% of candidates passed this question.
This question was best answered in a chronological manner. Many candidates omitted initial vasoconstriction and its mechanism. The platelet plug and formation of the clot should have then been described followed by the fate of the clot, including in-growth of fibroblasts. Strictly, fibrinolysis is a system for repairing / limiting clot propagation after the fact. Anticoagulants refer to antithrombin III, heparin, thrombomodulin and protein C and S. An explanation of the interaction of these naturally occurring anticoagulants was expected. The clotting factors that are specifically inhibited was expected as part of the discussion. The glycocalyx and vessel wall also plays a role in preventing coagulation.
2011A 17
Outline the physiological processes that occur in a blood vessel after venepuncture (80% of marks).
How are these altered by the administration of aspirin (20% of marks)?
CICMWrecks Answer
Cell-based model of coagulation
Initiation
- Small amounts of Factor VII, X and prothrombin leak into interstitium from intravascular space
- Factor VII interacts with Tissue Factor leading to activation of extrinsic pathway
- Activation of Factor X
- Small amounts of prothrombin converted to thrombin
Amplification
- At onset of tissue damage and vessel disruption
- Platelets exposed to thrombin generated in initiation phase, together with collagen via GPIa
- Platelet activation and release of α and dense granules, and morphological change
- Factor XII activated, leading to consequent activation of factors XI and IX
- Formation of Xase complex on platelet surface
- Amplified activation of factor X
Propagation phase
- Increased activated factor X causes “thrombin burst”
- Fibrinogen activated to fibrin
- Further propagation of platelet activation and aggregation
Modulation of coagulation response
- Circulating proteins C and protein S bind and inactivate factors V and VIII
- Antithrombin 3 binds to thrombin, as well factors IX and X and inhibit these
- Thrombomodulin on intact endothelium binds thrombin → anti-coagulant effect and increased protein C activation
- Plasminogen converted to plasmin → degradation of fibrin and therefore thrombolysis
Effect of aspirin
- Non-selective COX inhibition
- At low doses
- Irreversibly inhibits platelet COX in the portal circulation → inhibits platelet production of thromboxane (TXA)
- TXA stored in α granules and augments the activation of surrounding platelets, therefore aspirin reduces platelet activation in response to coagulation cascade
- At high doses
- Aspirin overcomes hepatic first pass metabolism and escapes to systemic circulation
- Inhibits systemic COX → inhibits endothelial production of prostacyclin
- Prostacyclin inhibits platelet aggregation and adherence, therefore increases clot formation and strength
Sakurai 2016
Examiner Comments
2011A 17: 8 (66%) of candidates passed this question.
The question was answered well overall. Better answers included detail of the platelet receptor and mediator interactions. Discussion of the role of the platelet in providing a phospholipid surface to enable the formation of the activated Xa complex was expected. Modulation of the coagulation cascade and prevention of clot propagation via protein C, nitric oxide, thrombomodulin and fibrinolysis was important to note in a comprehensive answer. The pharmacodynamic action of aspirin was generally understood.
Syllabus: J1,2c and J2, 2d
Recommended sources: Basic and Clinical Pharmacology, Katzung, Chp 34, 36
2009A 16
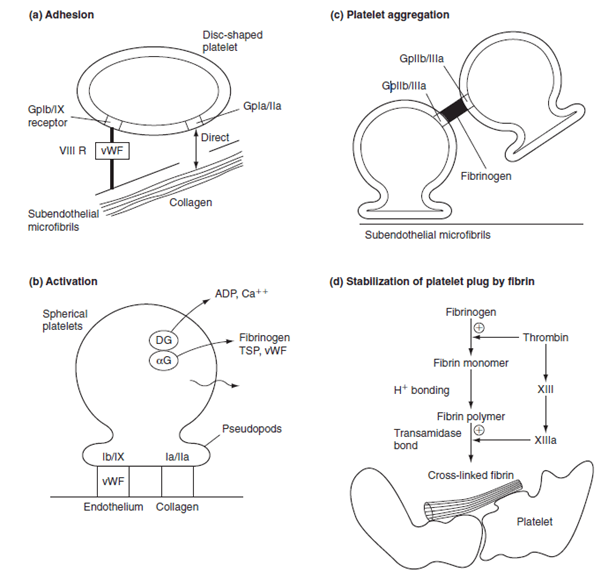
Outline the role of platelets in blood clotting following an injury to a blood vessel.
CICMWrecks Answer
Platelet adhesion:
- Vessel wall subendothelium is exposed
- Platelets bind to subendothelium via
- GpIa directly to collagen
- GpIIa/IX via vWF attached to subendothelial microfibrils
Platelet activation:
- Collagen binding or presence of thrombin
- Thromboxane A2 produced by COX-1
- Granules degranulate
- Dense granules release ADP and serotonin
- ADP binds to ADP receptors on external membrane
- Increased expression of GpIIa-IIIb
- Platelet also changes in morphology → long pseudopods
Platelet aggregation:
- ADP and thromboxane A2 released from the first binding platelets
- Aggregation and activation of further platelets
- ADP → increased expression of GpIIa-IIIb
- Fibrinogen linkage via GpIIaIIIb
- Formation of a platelet plug
- Thrombin (from clotting cascade) converts the fibrinogen into fibrin → stable plug
Factors increasing aggregation: Platelet activating factor
Factors inhibiting aggregation: Nitric oxide (via eNOS), Prostacycline (PGI2)
Mooney 2016
Examiner Comments
2009A 16: Pass rate: 40%
The main points expected in this answer were descriptions of platelet activation following exposure to collagen, platelet adhesion to the endothelium and ADP release and platelet aggregation secondary to activation of the GP11b/111a, COX-1 and other agents e.g. prostaglandin E2
Factors that interacted with platelet receptors e.g. platelet activating factor which increase aggregation and factors that inhibited platelet activation e.g. Prostaglandin I2 and nitric oxide gained marks.
2024A 06
(a) Outline the process of fibrinolysis including how it interacts with the coagulation system (80% of marks).(b) Describe the mechanism of action and adverse effects of alteplase (20% of marks).
2021B 19
Outline the process of fibrinolysis (40% marks). Write short notes on the indications, mechanism of action, pharmacokinetics and side effects of tranexamic acid (60% marks).
2015B 19
Describe the fibrinolytic pathway and identify areas of interaction with the coagulation pathway (80% of marks). List two anti-fibrinolytic agents and state their specific mechanism of action (20% of marks).
2013A 04
Describe the pharmacology of tranexamic acid.
CICMWrecks Answer: Fibrinolysis
Fibrinolysis
- Process by which fibrin clot is degraded to prevent excessive clot formation
- Plasminogen incorporated into clot
- Injured tissues and vascular endothelium gradually produce Tissue Plasminogen Activator (t-PA) in a delayed manner.
- Urokinase is produced by monocytes, macrophages and urinary epithelium
- Factor XII (Hageman factor) acts as a weak activator of plasminogen
- Plasminogen activated by t-PA, u-PA and factor XII to plasmin, a serine protease
- Plasmin cleaves fibrin into fibrin degradation products → reducing clot stability → Clot breakdown
Regulation of fibrinolysis
- Plasmin Activator Inhibitor (PAI) 1 & 2 – produced by vascular endothelium and inhibits tPA and uPA
- Protein C inactivates PAI, TAFI
- Thrombomodulin – expressed on in tact endothelium – binds thrombin and subsequently activates protein C
- Thrombin Activatable Fibrinolysis Inhibitor – produced by liver and cleaves plasmin binding sites on fibrin → decreased degradation
- α2 anti-plasmin – produced by liver, inhibits circulating plasmin, not fibrin bound plasmin
Sakurai 2016
CICMWrecks Answer: Anti-fibrinolytic agents
Anti-Fibrinolytic Agents
- Aprotinin
- Proteolytic enzyme inhibitor that acts on trypsin, plasmin and tissue kallikrein → forms reversible enzyme-inhibitor complex that inactivates free plasmin → ↓ clot lysis
- Tranexamic acid:
- Competitive inhibition of plasminogen conversion to active plasmin → ↓ fibrin (clot) lysis
- Aminocaproic acid
- Forms reversible complex with plasminogen → ↓ conversion to active plasmin → ↓ fibrin (clot) lysis
Sakurai 2016
CICMWrecks Answer: Pharmacology of Tranexamic Acid
CICMWrecks Answer: Pharmacology of Alteplase
Examiner Comments
2024A 06: 3% of candidates passed this question.
(a) This part required candidates to demonstrate their knowledge of the fibrinolytic pathway with details of the relevant mediators and inhibitors. Candidates where then required to apply their knowledge of both the coagulation cascade and fibrinolysis, with detail of how the pathways are simultaneously activated by the same stimuli to ensure balance between bleeding and clotting.
An example would be endothelial damage stimulating both the clotting cascade through collagen exposure and thromboplastin activation (faster response) as well as fibrinolysis through t-PA activation (slower response).
(b) This section of the question required a detailed description of the mechanism of action of alteplase, recombinant t-PA. A comparison to endogenous t-PA helped illustrate this action and the subsequent bleeding and the non-bleeding effects.
2021B 19: 30% of candidates passed this question.
The relative allocation of marks and thus time to be spent on each component was delineated by the relative percentages in the question. The first part of the question required a step-by-step outline of the fibrinolytic pathway with mention of the regulatory processes. Tranexamic acid is an important drug in the practice of intensive care and the question provided the headings under which to answer the question. The detail surrounding the keys aspects of this drug with respect to its use in critical care were often vague and underappreciated.
2015B 19: 8% of candidates passed this question.
The fibrinolytic pathway is a cascade largely made up of proteolytic enzmes and other factors synthesized in the liver that circulate in inactive precursor forms. Marks were awarded for description of the principal members of the cascade and the pathway relations between them. Endothelium is also important in the fibrinolytic pathway.
Regulation of the pathway to localise the site and size of clot as well as delayed onset of action of fibrinolysis is central to any description. Regulation of fibrinolysis by the coagulation cascade and a description of this area of interaction were expected.
Many candidates provided a reasonable description of the fibrinolytic cascade. Marks were not awarded for description of the coagulation cascade that did not have relevance to fibrinolysis. Understanding of regulation of fibrinolysis and it’s interaction with coagulation was poorly answered.
Most candidates were able to name two antifibrinolytic agents. Few were able to describe mechanism of action.
2013A 04:
Tranexamic acid is a drug used to reduce bleeding in trauma or surgery. It is also used for hereditary angioedema and menstrual bleeding. It is being increasingly used in critically ill patients. As a Level B listed drug within the Primary Syllabus candidates would be expected to know it in some depth. Often basic information such as mechanism of action, pharmacokinetics and adverse effects was lacking.
v. Describe the mechanisms of preventing thrombosis including endothelial factors and natural anticoagulants.
vi. Explain the physiological consequences of acute and chronic anaemia.
2013B 24
Outline the physiological responses to anaemia. (The specific physiological responses to hypovolaemia are NOT required.)
2007B 01
Explain how oxygen supply is maintained to the tissues in chronic anemia.
CICMWrecks Answer
Anaemia
- condition (acute or chronic) a/w deficiency of RBC or Hb in blood
- Low haemoglobin
- Males <140g/L
- Females <120g/L
- Eg. due to haemolysis, chronic disease, Fe-deficiency, Vitamin B12/folate deficiency, haemorrhage, etc.
Normal O2 supply to tissues
Approx 1l/min
Consequence of anaemia
- ↓ O2 content of blood (CaO2), and thus ↓ O2 delivery to tissues (DO2)
- Risk of tissue hypoxia – Increase products of anaerobic metabolism
- CO2, Lactate
- Decrease in pH as a consequence
Physiological Responses /
Compensatory Mechanisms that maintain Tissue Oxygenation
Effects on oxyhaemoglobin dissociation
- Increased PaCO2, Decreased pH and Decreased O2 cause right shift in Hb O2 dissociation curve → ↑ Tissue O2 extraction
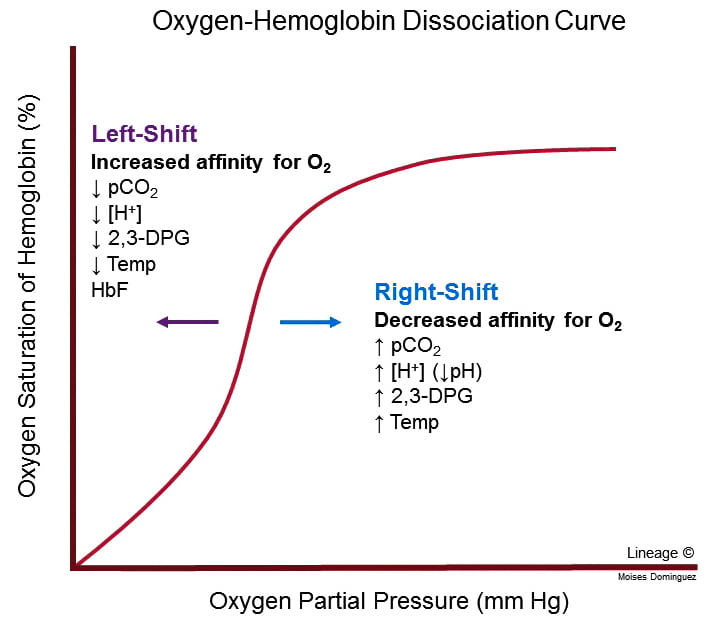
- For given PO2, Hb O2 affinity is reduced → increased offloading of O2 to tissues
- Decreased pH → Production of 2,3-DPG via Leubering-Rapaport Pathway and the enzyme BPG mutase
- Net loss of 1 ATP per 2,3-DPG generated
- Further right shift in the Oxyhaemoglobin dissociation curve
Cardiac Output Increases
- Blood flow and resistance governed by Poisuille-Hagen Equation
- Hb significant factor contributing to blood viscosity
- Anaemia → Decreased viscosity → Increased cardiac output
- Decreased viscosity → decreased resistance to venous return → increased preload → increased cardiac output
- Hb significant factor contributing to blood viscosity
- Stimulation of aortic chemoreceptors by decreased pH and increased CO2
- Increased signalling via vagus nerve NTS vasomotor sensory area → increase sympathetic output by anterolateral upper medulla → increased HR and contractility → Increased CO
Redistribution of blood flow
- To tissues where adequate O2 delivery is critical
- esp heart and brain
Respiratory effects
- Increased CO2 and decreased pH stimulates central and peripheral chemoceptors
- Medullary respiratory centre increases minute ventilation
- Increased respiratory clearance of CO2
- Alveolar CO2 ∝ arterial CO2 → increased PAO2 and hence increased PaO2
Renal compensation
- Decreased O2 delivery to renal interstitium → Hypoxia Inducible Factor (HIF) → Erythropoietin → Stimulates erythropoiesis via EPO-R JAK/STAT signalling.
#Extra:
Techniques to maintain tissue O2 balance in a patient with anaemia:
- ↑ tissue O2 supply (or DO2) by:
- ↑ C.O. → volume load to significantly ↑ C.O. as per Frank-Starling mechanism (preferably with PRBC to replace Hb also, but OK with colloids/crystalloids)
- ↑ [Hb] → replace Hb with PRBC transfusion, and support ongoing haemopoiesis with haemotinic factors (Eg. Fe, vitamin B12, folate)
- ↑ SpO2 and PaO2 → supplemental O2 (FiO2 100%)
- ↓ tissue O2 demand by:
- Sedation, paralysis and artificial ventilation → ↓ muscle MRO2 a/w activity and respiration
- Maintain normal core body temperature → avoid hypothermia and shivering 2° to hypothermia, which ↑ MRO2
- Minimal use of inotropes to maintain C.O. → avoid ↑ cardiac MRO2
CICMWrecks 2016
Examiner Comments
2013B 24: 10 candidates passed (37.0%).
It was expected candidates would expand on the central role haemoglobin has in oxygen delivery and that in the presence of reduced haemoglobin there are various efforts aimed at maintaining oxygen delivery. Cardiac output is increased, systemic vascular resistance is reduced, modifications are seen in regional circulations and as tissue oxygenation begins to falter then the end products of anaerobic metabolism provide a further stimulus to enhance cardiac out and tissue oxygen delivery.
Better answers also included a mention of additional factors that enhance tolerance of chronic anaemia (e.g. angiogenesis).
2007B 01: 1 candidate (14%) passed this question.
Candidates were expected to base their answers around the variables involved in the equations that describe oxygen content in blood and oxygen delivery. Although most candidates mentioned changes in haemoglobin that increase oxygen carriage, a more complete discussion of the changes that influence cardiac output and peripheral circulation was often omitted.





Recent Comments