Syllabus (Fourth Edition, 2023)
Topics
L1: Musculoskeletal System Physiology
i. Describe the anatomy and physiology of skeletal, smooth, and cardiac muscle.
ii. Describe the physiology of the neuromuscular junction and its receptors.
iii. Describe the mechanism of excitation-contraction coupling.
iv. Describe the relationship between muscle length and tension.
v. Explain the concept of motor units.
vi. Describe the monosynaptic stretch reflex, single twitch, and tetanus.
L2: Musculoskeletal System Pharmacology
i. Understand the pharmacology of neuromuscular blocking drugs and reversal agents.
L3: Neuromuscular Measurement and Monitoring
i. Describe the monitoring of neuromuscular blockade.
Topics not covered in previous SAQs
L1: Musculoskeletal System Physiology
v. Explain the concept of motor units.
vi. Describe the monosynaptic stretch reflex, single twitch, and tetanus.
L3: Neuromuscular Measurement and Monitoring
i. Describe the monitoring of neuromuscular blockade.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
L1. Musculoskeletal System Physiology
i. Describe the anatomy and physiology of skeletal, smooth, and cardiac muscle.
2024B 04 – 2015B 11
Compare and contrast the anatomy and physiology of skeletal and smooth muscle.
CICMWrecks Answer
Anatomy
| Skeletal Muscle | Smooth Muscle |
|---|---|
| Long, cylindrical, multi-nucleated cells | Spindle shaped single-nucleated cells |
| 10 μm diameter 100 μm long | 2 μm diameter 400 μm long |
| Striated | Not striated |
| Actin and Myosin arranged in sarcomeres | More actin than myosin Actin inserts into dense bodies and cell membrane |
| Well developed sarcoplasmic reticulum and transverse tubules | Poorly developed sarcoplasmic reticulum No Transverse tubules |
| Containes troponin in the thin filaments | Contains calmodulin → binds to Ca2+ → activates myosin light-chain kinase |
| Ca2+ released from cytoplasm from sarcoplasmic reticulum | Ca2+ enters cytoplasm from extracellulr fluid, sarcoplasmic reticulum and mitochondria |
| Cannot contract without nerve stimulation | Maintains tone in absence of nerve stimulation Visceral smooth muscle produces pacemaker potentials |
| Denervation results in muscle atrophy | denervation results in hypersensitivity to stimulation |
| Muscle fibers stimulated independently No gap junctions | Gap junctions usually present |
| Two Types 1. Extrafusal (α-motor neuron supply) a) Slow-type (tonic) b) Fast-type (twitch) – Slow fatigue (type 1/red – slow oxidative) – Fast fatigue-resistant (type 2B/red – fast oxidative) – Fast fatiguable (type 2B/white) 2. Intrafusal (γ-motor neuron supply) – Non-contractile sensory fibers associated with muscle spindles | Two types 1. Visceral – located in hollow viscera – linked in sheets by gap junctions – contractile elements extend across multiple cells – Spontaneous depolarisation 2. Multiunit – located in eye (iris and ciliary body), large blood vessels, small airways, hair follicles – No gap junctions – Under ANS control without spontanaetiy |
Physiology
| Skeletal Muscle | Smooth Muscle |
|---|---|
| Excitation | |
| – Motor neuron depolarisation – ACh vesicular release – Multiple MEPs → End plate potential – Muscle AP propagates via T-tubles – Opens L-type Ca channels – Calcium induced calcium release from SR – Ca-troponin C interaction – Alteration and movement of Trop-tropomyocin complex – Exposure of Actin – Removal of Troponin I inhibition of myosin ATPase – Cross bridge cycling | – Motor neuron depolarisation – ACh vesicular release – Multiple MEPs → End plate potential – Muscle AP propagates – Opens L-type Ca channels |
| Contraction | |
| Sliding filament cross bridge cycling – “Flexed” Myosin without ATP binds to Actin. – Binding of ATP to Myosin causes release of Actin/Myosin complex and extension of the head – In the presence of Ca, Actin binding sites are open and binding of myosin/Actin/ATP complex is formed – Hydrolysis of ATP to ADP + P in myosin head causes a conformational change “initial flexing” the myosin head and releasing phosphate – Release of ADP further flexes Myosin head – In presence of Ca return to step 2. | – Influx of EC Ca → ↑ IC [Ca] – Ca binds Calmodulin → Ca-Calmodulin complex – Ca-Calmod → ↑ MLCK activity → – Phosphorylation of Myosin Light chain (MLC) → Activation of Myosin ATPase – Normal Cross-bridge cycling as per Skeletal muscle cross bridge cycling – Latch-bridging (Sustained SM contraction with minimal O₂ or ATP use) ◦ ↓ IC [Ca] + dephosphorylation of MLC does not cause unbinding of actin/myosin ◦ Unbinding occurs slowly |
| Relaxation | |
| – AChE metabolises ACh in the NMJ ◦ ↓ nAChR activation at MEP → ↓ EPP and muscle AP generation – SR actively sequesters Ca (via Ca-Mg ATPase) ◦ ↓ IC [Ca] → Ca removed from troponin C ◦ tropomyosin mediated inhibition of actin-myosin – Titin ◦ Elastic component of sarcomere → ↓ to normal length | – Ca removed from cell ◦ Ca ATPase ◦ Ca2+/Na+ antiport – Myosin Light Chain Phosphatase ◦ dephosphorylates MLC → inhibition of myosin ATPase |
Gladwin / JC 2020
Examiner Comments
2024B 04: 53% of candidates passed this question.
The anatomy component of this question required a detailed description of both the macro and microstructure of skeletal and smooth muscle, noting the differences and similarities between them. For skeletal muscle specific detail on the fibre arrangement, subtypes (I/IIA/IIB) and their innervation as well as the structure of the sarcomere, thick and thin bands and associated proteins was required. It was then expected that candidates would provide a description of how these components are similar or different in smooth muscle including details of unitary and multi-unit smooth muscle arrangements.When comparing the physiology of skeletal and smooth muscle, better candidates discussed not only the process of AP generation, propagation and stimulus, but also detailed sources of ATP, and highlighted the differences in requirements for energy of each muscle type. Higher scoring candidates discussed specific membrane potentials, noting the unstable membrane potential of smooth muscle which does not exhibit a resting membrane potential.
2015B 11: 23% of candidates passed this question.
It was expected answers would describe in detail the role of troponin, tropomyosin and calmodulin in mediating muscle contraction. Detail on the structure (histology) of the skeletal and smooth muscle cells was often lacking. Many answers omitted the mechanism of muscle relaxation.
2014A 08
Describe the physiology of skeletal muscle cell contraction.
CICMWrecks Answer
Sarcomere
“Sarcomere” → basic unit of muscle contraction within myofibril
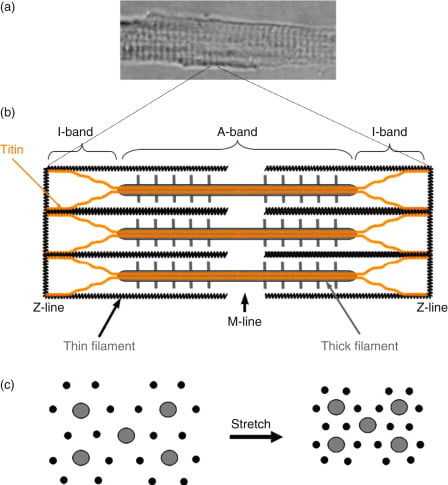
I band – Has actin filaments only
A band – Contains myosin and some actin
H band – Band of myosin only
M line – Joins myosin filaments together
Components of the sarcomere:
- Myosin
- Large protein that consists of:
- 1x long tail – 2 interwound α-helices with a flexible hinge (S2 segment)
- 2x globular heads (S1 segment) – Connected to long tail via S2 segment. Each head has 1x heavy chain that binds 1x G-actin, and 2x short chains that bind ATP
- 2x myosins fuse together at M-line via their long tails → forms “thick filament”
- Large protein that consists of:
- Actin (thin filament)
- Consists of 2x chains of F-actin (≈ double-strand cord) formed from the polymerisation of G-actin
- Tropomyosin
- 2x α-helical chains that lie between 2x chains of actin polymers → under the influence of Troponin, it can acts to inhibit or permit myosin-actin interaction
- Troponin
- Present with tropomyosin on thin filament at regular intervals (every 7 actin monomers)
- Consists of 3 subunits – (a) T (binds tropomyosin), (b) I (inhibits myosin ATPase) and (c) C (binds Ca2+)
- Binding to Ca2+ causes a conformation change in troponin-tropomyosin complex → allows actin to interact with myosin
- Titin
- Large elastic molecule that attaches myosin to Z-line → acts to return muscle to
resting length
- Large elastic molecule that attaches myosin to Z-line → acts to return muscle to
Process of Muscle Contraction and Relaxation:
(1) Excitation-contraction coupling:
- Motor neuron depolarisation
- ACh vesicular release
- Multiple MEPs → End plate potential
- Muscle AP propagates via T-tubles
- Opens L-type Ca channels
- Calcium induced calcium release from SR
- Ca-troponin C interaction
- Alteration and movement of Trop-tropomyocin complex
- Exposure of Actin
- Removal of Troponin I inhibition of myosin ATPase
- Cross bridge cycling
2) Contraction (Sliding filament cross bridge cycling):
- “Flexed” Myosin without ATP binds to Actin.
- Binding of ATP to Myosin causes release of Actin/Myosin complex and extension of the head
- In the presence of Ca, Actin binding sites are open and binding of myosin/Actin/ATP complex is formed
- Hydrolysis of ATP to ADP + P in myosin head causes a conformational change “initial flexing” the myosin head and releasing phosphate
- Release of ADP further flexes Myosin head
- In presence of Ca return to step 2
(3) Muscle relaxation:
- AChE metabolises ACh in the NMJ → ↓ nAChR activation at MEP → ↓ EPP and muscle AP generation
- SR actively sequesters Ca2+ (via Ca2+-Mg2+ ATPase) → ↓ IC [Ca2+] → Ca2+ removed from troponin C → tropomyosin mediated inhibition of actin-myosin interaction returns
- Elastic components of the sarcomere causing the muscle to return to its normal length
Energy Source for Muscle Contraction
- Energy (in the form of ATP) is needed for muscle contraction AND relaxation
- The method of ATP generation depends on the level of activity present:
- Creatinine phosphate store
- ATP generated from Creatine phosphate during intense exercise
- ADP + Creatine phosphate → ATP + creatinine (enzyme: Creatine Phosphokinase)
- Replenished at rest by reversing the above reaction by aerobic/anaerobic generation of ATP
- Aerobic generation of ATP:
- Glucose (and Glycogen stores) – ATP is produced during exercise/activity by substrate phosphorylation (via glycolysis) and oxidative phosphorylation (via Kreb’s cycle and the ETC)
- Fat – β-oxidation of fat produces ATP during light exercise and at rest
- Anaerobic generation of ATP:
- When O2 supplies are insufficient, ATP is produced in smaller amounts from the anaerobic glycolysis of glucose → produces lactic acid
- Creatinine phosphate store
JC / Gladwin 2020
Examiner Comments
2014A 08: 34% of candidates passed this question.
This question required a description of excitation- contraction coupling. Marks were gained for a brief outline of the structure of a sarcomere and how it facilitates shortening. An explanation of membrane processes, receptor interactions and the contraction processes was required. Mention of the role of ATP was also required and marks were gained for commenting on the mechanism of return to the relaxed state.
Most candidates wrote extensively on the nerve action potential and neuromuscular junction transmission, with minimal reference to events occurring within the skeletal muscle cell membrane. They could not gain marks for this. Few candidates demonstrated knowledge of the ATP dependent walk along processes of myosin heads during contraction.
2012B 23
Outline the mechanisms that control regional skeletal muscle blood flow.
CICMWrecks Answer
- Normal blood flow to skeletal muscle ~1l/min
- Can increase 20-fold during exercise
Local factors
Acute (Autoregulation)
- Myogenic
- Stretch of vessel walls causes constriction due to stretch mediated Ca2+ release
- Prominent in small arterioles throughout body
- Prevents excessive increases in blood flow in response to pressure
- Sheer stress on vessels due to increased flow causes release of Endothelial Derived Relaxing Factors such as Nitric Oxide which dilates the vessel, increasing vessel diameter and reducing sheer stress
- Stretch of vessel walls causes constriction due to stretch mediated Ca2+ release
- Metabolic
- Increased concentration of metabolic byproducts such as CO2, lactate, adenosine and H+, K+, cause upstream vasodilation
- This diffuse through the interstitium to upstream pre-capillary sphincters, arterioles and meta-arterioles
- Due to alteration of vessel radius, flow increases, according to the Poisuille-Hagen Equation
- Conversely, presence of excess nutrients (and O2) leads to vasoconstriction and decreased flow
- Increased concentration of metabolic byproducts such as CO2, lactate, adenosine and H+, K+, cause upstream vasodilation
- Reactive hyperaemia
- During muscle contraction, interstitial pressure increases compressing blood vessels and decreasing flow
- On restoration of flow to the tissue, flow can increase 4~7 fold due to buildup of metabolites and autoregulatory mechanisms
- During muscle contraction, interstitial pressure increases compressing blood vessels and decreasing flow
- Active hyperaemia
- During exercise, due to the consumption of nutrients and production of metabolites, blood flow increases due to autoregulation
Long term
- Arterioles and vessels increase in size and number due to increased metabolic requirements of a tissue and chronic hypoxia
- Important factors
- VEGF
- Angiogenin
- Important factors
Neural factors
- Sympathetic
- Sympathetic α adrenergic stimulation causes vasoconstriction (integral in baroreceptor response)
- Resting sympathetic tone contributes significantly to peripheral vascular resistance
- Sympathetic cholinergic vasodilator supply under cortical control
- Sympathetic α adrenergic stimulation causes vasoconstriction (integral in baroreceptor response)
Hormonal factors
- β2 mediated vasodilation in response to adrenaline
- α1 mediated vasocontriction at higher concentrations
Sakurai 2016
Examiner Comments
2012B 23: 4 (18.2%) of candidates passed.
Candidates were expected to present the cellular mechanisms underlying the control of skeletal muscle blood flow. Many candidates correctly identified a role for sympathetic nervous system, metabolic (e.g. vasodilator metabolites such as CO2, H+, K+, lactate and adenosine), vasoactive substances released by endothelium (nitric oxide, prostacyclin, endothelin 1, etc.) and autoregulatory control but failed to present any details of direction and magnitude of control. Better answers also mentioned humeral (e.g. catecholamines, vasopressin, ANP, angiotensin II, histamine, serotonin, etc.) or myogenic control (i.e. when the pressure within a smooth muscle blood vessel is suddenly increased, the vascular smooth muscle is stretched.
ii. Describe the physiology of the neuromuscular junction and its receptors.
2019A 17
Explain the physiology of neuromuscular transmission.
CICMWrecks Answer
Neuromuscular Transmission
- In nerve cells, a reduction in cell membrane potential above RMP (ie. – 70mV) results in a voltage dependent increase in Na+ permeability.
- the wave of excitation moves along the axon unidirectionally at a constant amplitude & velocity.
- At the neuromuscular junction presynaptic motor axons terminate 30 nanometers from the cell membrane or sarcolemma of a muscle fiber.
- The sarcolemma at the junction has invaginations called postjunctional folds, which increase its surface area facing the synaptic cleft.
- These postjunctional folds form the motor endplate, which is studded with nicotinic acetylcholine receptors (nAChRs)
Neuromuscular Junction
Acetyl Choline:
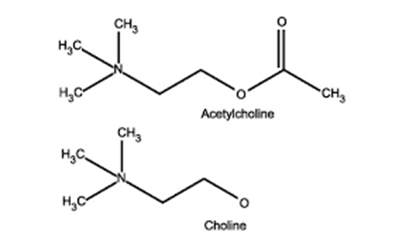
- stored in vesicles within the terminal button
- approx. 10,000 molecules in each vesicle
- 3 pools (1% immediately releasable, 80% readily releasable, rest stationary store)
- Ach inhibits Choline Acetyltransferase
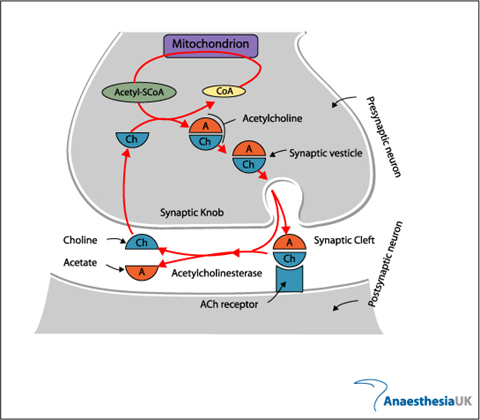
- Vesicles exocytose in response to depolarization of presynaptic terminal and influx of calcium
- Acetylcholine released into the synaptic cleft
- Pyruvate in Mitochondria → Acetyl-CoA
- Choline – 50% from extracellular (diet) + 50% recycled from Ach
ACh Receptor
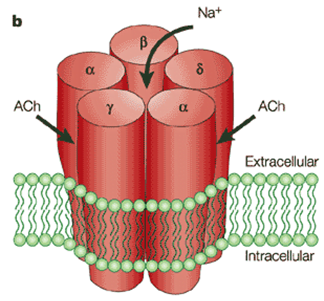
- Nicotinic subtype
- 5 polypeptide subunits – ring structure with central pore
- Two α (Bind ACh), One β, One g, One δ
- Locations:
- Post junctional membrane receptors on motor end plate
- Extra-junctional
- Pre-junctional
Activation:
- Ach bind to alpha subunits → opens the ion gated channed
- Movement of all cations, but mainly sodium → depolarizes membrane
- Once reaches threshold of -50mV (from resting of -80) → voltage gated sodium channels open → rapid depolarization → end plate potential (EPP) of 50-100mV
- This triggers muscle action potential → muscle contraction
- Muscle AP propogates along muscle membrane to cause contraction
- Pre-junctional receptors → positive feedback → increased Ach by second messenges system
- Play role in “fade” seen in non-depolarizing muscle blockers
Fate:
- Acetyl choline hydrolysed to choline and acetate by AChE (Acetyl Choline Esterase)
- Breakdown is very rapid, so the inward current through receptor is transient, followed by rapid repolarisation to resting state
Sources:
AnaesthesiaUK
Arthur Karlin, Nicotinic Acetylcholine receptors, Nature reviews Neuroscience
JC 2019
Examiner Comments
2019A 17: 60% of candidates passed this question.
Description of sequential events from axon conduction to detail at the neuromuscular junction was required. Well-constructed answers defined neuromuscular transmission, elucidated the structure of the neuromuscular junction (best done with a detailed diagram), described the central importance of acetylcholine, including synthesis, storage, receptors, and degradation. An ideal answer also described both pre-synaptic (e.g. voltage-gated calcium channels, exocytosis of vesicles) and post-synaptic events (acetylcholine receptors, end plate potentials, and the events that lead to excitation-contraction coupling in skeletal muscle).
iii. Describe the mechanism of excitation-contraction coupling.
2022A 12
Describe the process of excitation-contraction coupling and relaxation in smooth muscle.
2012A 18
Describe the processes of excitation and contraction within a smooth muscle cell (60% of marks).
Briefly outline the mechanism by which nitric oxide affects smooth muscle cell activity (40% of marks)
CICMWrecks Answer: Smooth Muscle
Excitation Contraction Coupling in SM
- Motor neuron depolarisation
- ACh vesicular release
- Multiple MEPs → End plate potential
- Muscle AP propagates via T-tubles
- Opens L-type Ca channels
- Influx of EC Ca → ↑ IC [Ca]
- Variable amount of Sarcoplamic reticula in SM
- Can have some ↑ IC [Ca] via Ca induced Ca release.
- Ca binds Calmodulin → Ca-Calmodulin complex
- Ca-Calmod → ↑ MLCK activity
- → Phosphorylation of Myosin Light Chain (MLC)
- → Activation of Myosin ATPase
- Cross-bridge cycling.
Cross Bridge Cycling
- “Flexed” Myosin without ATP binds to Actin.
- Binding of ATP to Myosin causes release of Actin/Myosin complex and extension of the head
- In the presence of Ca-Calmod complex
- Actin binding sites are open and binding of myosin/Actin/ATP complex is formed
- Hydrolysis of ATP to ADP + P in myosin head by Myosin ATPase
- → conformational change “initial flexing” the myosin head and releasing phosphate
- Release of ADP further flexes Myosin head
- In presence of Ca return to step 2.
Relaxation
- Ca removed from cell
- Ca ATPase
- Ca2+/Na+ antiport
- Myosin Light Chain Phosphatase
- dephosphorylates MLC → inhibition of myosin ATPase
Gladwin 2016
CICMWrecks Answer: Effect of NO on Smooth Muscle
Action of Nitric Oxide
- Produced:
- Endothelium
- L-arginine via reaction with the enzyme nitric oxide synthase
- 3 forms (iNOS, nNOS and eNOS)
- Action:
- Diffuses into vascular SMCs
- Binds to Guanylate Cyclase
- ↑ intracellular cGMP
- ↓’s Ca entry into the cell
- Activates K+ channels → hyperpolarization and relaxation
- ↑ cGMP-dependent protein kinase action
- Activates myosin light chain phosphatase
- Dephosphorylates myosin light chains
- Smooth muscle relaxation.
Gladwin 2016
Examiner Comments
2012A 18: 2 (20%) of candidates passed.
Insufficient breadth and depth of knowledge limited candidates’ performance to this question. Candidates were expected to mention mechanisms of muscle cell membrane activation (e.g. Ca2+ channel mediated action potential, Pacemaker potential, etc), sources of rise in intracellular Ca2+, intracellular Ca2+ binding to calmodulin in cytoplasm Ca2+calmodulin complex binding to, and activation of myosin light chain kinase, energy dependent myosin cross bridges and cycling and mechanism of smooth muscle relaxation.
Nitric Oxide (NO) activates guanylyl cyclise which in turn catalyzes the dephosphorylation of GTP to cyclicGMP which in turn induces smooth muscle relaxation, and the various mechanisms by which this occurs.
2024A 10
(a) Explain the excitation contraction mechanism as it relates to the smooth muscle of the myometrium including a gravid uterus (50% of marks).
(b) For each tocolytic agent: salbutamol, nifedipine, magnesium sulphate, provide the following information:
(i) List the class (10% of marks)
(ii) Provide a dose (10% of marks)
(iii) Describe the mechanism of action (20% of marks)
(iv) Outline the adverse effects (10% of marks)
CICMWrecks Answer: Smooth Muscle in Uterus
Excitation Contraction Coupling in SM
- Motor neuron depolarisation
- ACh vesicular release
- Multiple MEPs → End plate potential
- Muscle AP propagates via T-tubles
- Opens L-type Ca channels
- Influx of EC Ca → ↑ IC [Ca]
- Variable amount of Sarcoplamic reticula in SM
- Can have some ↑ IC [Ca] via Ca induced Ca release.
- Ca binds Calmodulin → Ca-Calmodulin complex
- Ca-Calmod → ↑ MLCK activity
- → Phosphorylation of Myosin Light Chain (MLC)
- → Activation of Myosin ATPase
- Cross-bridge cycling.
Cross Bridge Cycling
- “Flexed” Myosin without ATP binds to Actin.
- Binding of ATP to Myosin causes release of Actin/Myosin complex and extension of the head
- In the presence of Ca-Calmod complex
- Actin binding sites are open and binding of myosin/Actin/ATP complex is formed
- Hydrolysis of ATP to ADP + P in myosin head by Myosin ATPase
- → conformational change “initial flexing” the myosin head and releasing phosphate
- Release of ADP further flexes Myosin head
- In presence of Ca return to step 2.
Relaxation
- Ca removed from cell
- Ca ATPase
- Ca2+/Na+ antiport
- Myosin Light Chain Phosphatase
- dephosphorylates MLC → inhibition of myosin ATPase
Differences in Gravid Uterus
These physiological adaptations in the gravid uterus maintain quiescence throughout most of pregnancy, ensuring fetal development, while preparing it to transition into a highly excitable and contractile state essential for effective labor.
| Feature | Gravid Uterus | Other Smooth Muscle |
|---|---|---|
| Resting Membrane Potential | Less negative near term, increasing excitability | Stable, less variation in response to hormones |
| Hormonal Regulation | Progesterone dominance (quiescence early), estrogen dominance (contractility late) | Task-specific, less pronounced |
| Gap Junctions | Increased (connexin-43) near term for synchronized contractions | Sparse, localized contraction |
| Calcium Handling | Increased L-type calcium channel activity and intracellular calcium recruitment near term | Stable calcium influx and release mechanisms |
| Oxytocin Sensitivity | High near term, upregulated oxytocin receptor density | Minimal, plays minor or no role |
| Relaxation Mechanisms | Progesterone/NO-mediated quiescence during pregnancy, reduced near term | Nitric oxide, prostaglandins, or sympathetic input |
| Action Potentials | Long, frequent, propagating bursts near term | Short, localized |
| Structural Adaptations | Hypertrophy and hyperplasia of myocytes | Minimal structural changes |
| Functional Synchrony | Whole-organ synchronization with gap junctions, pacemaker cells, and oxytocin | Localized function, no whole-organ synchronization |
Gladwin / JC 2024
CICMWrecks Answer: Tocolytic Agents (Limited)
Examiner Comments
2024A 10: 42% of candidates passed this question.
(a) This question required an explanation of the process of contraction in smooth muscle, as well as specific information pertaining to the smooth muscle of the uterus. Specific information included: stimuli for contraction (i.e., hormones such as oxytocin, oestrogen, and prostaglandins and stretch); possessing an unstable membrane potential which plays a greater role than nervous input; as well as the uterus smooth muscle functioning as a syncytia and having baseline tone.
The key steps of contraction (influx of calcium, formation of calcium-calmodulin formation, activation of MLCK, creation of myosin-actin cross bridges with utilisation of ATP) are those generic to any smooth muscle and marks were awarded accordingly. Descriptions of striated muscle did not attract marks. A description or explanation was required rather than a list of steps (e.g., the sources of calcium rather than a simple statement of calcium influx occurring).
(b) Use of these drugs as tocolytics is acknowledged as a less common reason for their utilisation – the fractionation of this question was to breakdown into more achievable parcels of information. This allowed candidates to score more marks for relating the mechanism of action specifically to the uterus. Higher marks in this section were awarded for specific unwanted effects relevant to use as a tocolytic e.g., foetal tachycardia for salbutamol as result of crossing the placenta. Marks were awarded to doses for these drugs when used as a tocolytic, as opposed to for other indications (i.e., salbutamol infusion rather than nebulised).
iv. Describe the relationship between muscle length and tension.
2019B 03
Describe the relationship between muscle length and tension (50% of marks).
Outline the physiologic significance of this relationship in cardiac muscle (50% of marks).
CICMWrecks Answer
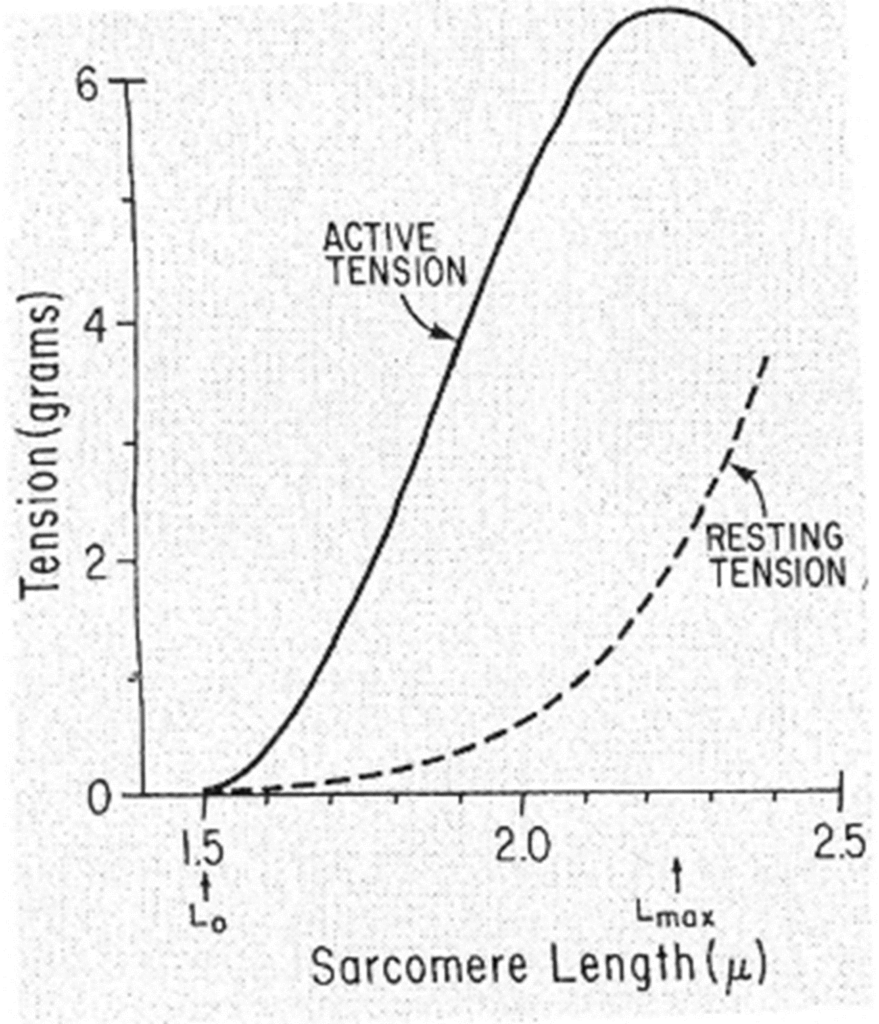
Muscle length and tension
- during isometric stimulation the total tension can vary with muscle length (total tension)
- also when unstimulated, tension can vary with muscle length (passive tension)
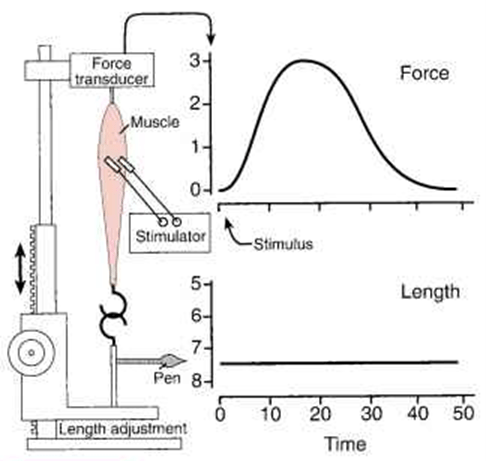
- this relationship can be studied in a muscle preparation study
- muscle length can be varied by changing the distance between its two attachments -> passive tension measured.
- muscle then electrically stimulated -> total tension measured.
- the difference between the two values at any length is the amount of tension generated by the contractile process -> the active tension.
Passive tension curve = tension exerted by this skeletal muscle at each length when not stimulated.
Total tension curve = tension developed when the muscle contracts isometrically in response to a maximal stimulus.
Active tension = difference between the two.
- the length at which the active tension is maximal is usually = resting length
- when muscle fibers contract isometrically -> tension developed is proportionate to the number of cross-linkages between the actin & myosin molecules.
- when muscle stretched -> overlap reduced -> potential tension reduced
- when muscle shorter -> distance filaments have to move is reduced -> potential tension reduced.
- Optimal length = resting length
Frank-Starling Law
“energy of contraction is proportional to the initial length of the cardiac muscle fiber.”
- For the heart, the length of the muscle fibers (ie, the extent of the preload) is proportionate to the end- diastolic volume.
- Increased venous return increases the end-diastolic volume and therefore preload, which is the initial stretching of the cardiac myocytes prior to contraction.
- Myocyte stretching increases the sarcomere length, which causes an increase in force generation. Increasing the sarcomere length increases troponin C calcium sensitivity, which increases the rate of cross-bridge attachment and detachment, and the amount of tension developed by the muscle fiber. The effect of increased sarcomere length on the contractile proteins is termed length-dependent activation.
- This ability of the heart to change its force of contraction and therefore stroke volume in response to changes in venous return is called the Frank-Starling mechanism.
- The mechanism is very important for:
- Rapidly responding to acute changes in venous return
- Keeping the right and left ventricle outputs exactly equal
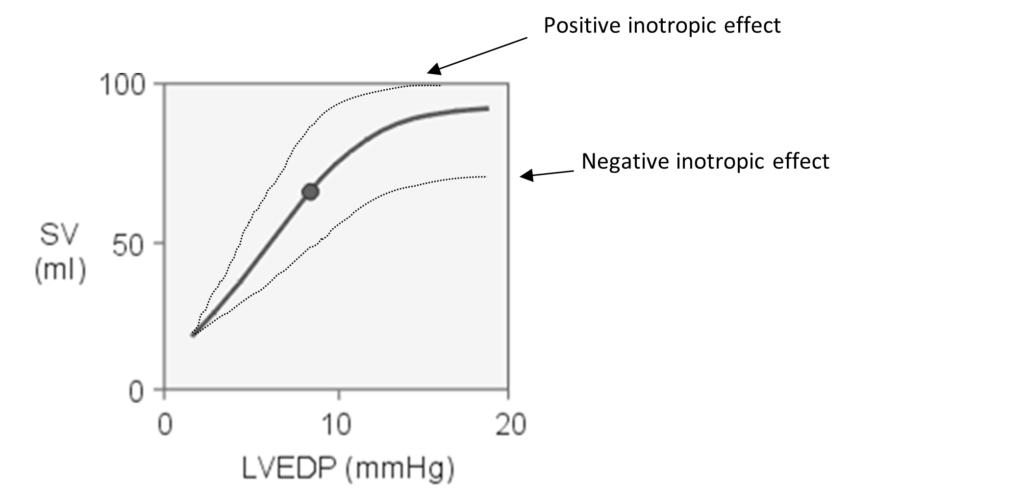
Frank-Starling curve: Relationship between ventricular stroke volume and end-diastolic volume
JC 2019
Examiner Comments
2019B 03: 41% of candidates passed this question.
Some detail was expected on a general description that tension is variable with the length of muscle. It was expected answers would describe that there is a resting length at which tension developed on stimulation is maximal.
Many candidates omitted that differences exist between muscle types with smooth muscle behaving differently. Additional credit was given for the distinction about active tension vs resting tension. It was expected a description of the potential mechanism would be included with discussion of sliding filament theory, overlapping fibres and optimal sarcomere length. Some candidates utilised a diagram effectively to convey understanding and more detail was rewarded with additional marks.
The second half of the question involved describing how this relationship is particularly important in cardiac muscle and underpins the Frank Starling relationship and all the cardiac physiology that follows. Initial length of fibres is determined by the diastolic filling of the heart, so pressure developed is proportionate to the total tension developed. The developed tension increases as diastolic volume increases to a maximum (the concept of Heterometric regulation). Better answers appreciated that the physiology may be different for a while heart rather than isolated muscle fibres.
v. Explain the concept of motor units.
vi. Describe the monosynaptic stretch reflex, single twitch, and tetanus.
L2. Musculoskeletal System Pharmacology
i. Understand the pharmacology of neuromuscular blocking drugs and reversal agents.
2008B 01
Outline three (3) factors that alter the pharmacodynamic response of non-depolarising neuro-muscular blocking drugs and describe the mechanism by which they may occur.
CICMWrecks Answer
Pharmacodynamics
Prejunctional Factors:
- Electrolyte abnormalities
- Hypo/Hypercalcaemia
- ↓/↑ Prejunctional Ca entry on depoarisation → ↓/↑ ACh release
- ↓ Ca → prolongation of blockade by NDMB due to ↓ Competitive antagonism
- ↓/↑ Prejunctional Ca entry on depoarisation → ↓/↑ ACh release
- Hypo/Hyperkalaemia
- Hyper/Hypopolarisation of pre-junctional membrane
- ↓ [K] → Hyperpolarisation → prolonged effect of NDMB
- Hyper/Hypopolarisation of pre-junctional membrane
- Hypo/Hypercalcaemia
Junctional Factors:
- Presence of co-administered anticholinesterase drugs (eg neostigmine
- ↑ [Ach] in NMJ → ↑competitive antagonism of NDMB → ↓ duration of effect/delayed onset
- Effect of NDMB relates to receptor occupancy
- 95% occupancy → TOF ¼
- 90% occupancy → TOF ¾
Post-Junctional Factors:
- Thermal injury/UMN lesion/Burns
- Upregulation of foetal type junctional and extra-junctional nAChRs
- ↑ receptor numbers → greater dose required to get the same percentage of receptors occupied
- Prolonged opening compared with adult receptors → predisposition to hyperkalameia → ↑’s RMP
- ↓ Plasma cholinesterase → ↓ metabolism of mivacurium
- Upregulation of foetal type junctional and extra-junctional nAChRs
- Co-administered local anaesthetic
- Na-channel blockade →
- pre-junctional membrane stabilisation → ↓ ACh release → ↑ NDMB effect duration
- post-junctional membrane stabilisation → ↑’s receptor occupancy required to achieve excitatory post synaptic potential (EPSP) → prolonged effect of NDMB
- Na-channel blockade →
CICMWrecks 2016
Examiner Comments
2008B 01: 2 (40%) candidates passed this questions
There were a number of possible factors that candidates could have selected. Examples include drug interactions (anticholinesterases, aminoglycoside antibiotics, local anaesthetics, steroids, antiarrhythmic drugs, anticonvulsants (phenytoin), diuretics, magnesium, lithium), hypothermia / hyperthermia, acidosis, [k+], burn injury, allergic reactions, gender, altered elimination due to renal or hepatic dysfunction and disease states (adrenocortical dysfunction, myasthenia, myopathies, denervation injury). Also extremes of age and pregnancy. Areas of weakness for the candidates were failure to include sufficient factual knowledge for their selected factors, and as a result, a failure to
illustrate sufficiently the mechanisms by which the pharmacodynamic response of nondepolarising neuro-muscular blocking drugs may be affected.
Syllabus: H2a 2 (c)
Reference Text: Pharmacology and Physiology in Anaesthetic Practice / R K Stoelting
2014B 07
Outline the mechanisms that potentiate the action of non-depolarising muscle relaxants and give examples.
CICMWrecks Answer
Non depolarising neuromuscular blockers
- act by non-competitive binding to the nicotinic ACh receptor on the motor end plate
- this prevents the action of ACh at the motor end plate and stops muscle excitation
- aminosteroids – rocuronium, pancuronium, vecuronium
- isoquinolones – atracurium, cisatracurium
- the pharmacodynamic response is dependent on the number of receptors occupied
- dose dependent according to the laws of mass action
- >70% to elicit a response
The factors which alter the response (number of receptors occupied) may be separated into
- pharmacodynamic factors
- pharmacokinetic factors
- pharmaceutical and drug interaction factors
Pharmacodynamic factors
- Decreased pH increases the affinity of non depolarising NMB
- Electrolyte disturbances
- Ca disturbances affect the release of ACh from the presynaptic terminal (triggered by Ca)
- hypocalcaemia potentiates blockage
- magnesium
- hypomagnesemia
- potassium
- hyperkalaemia leads to increased drug effect
- Ca disturbances affect the release of ACh from the presynaptic terminal (triggered by Ca)
Pharmacokinetic factors (affect the amount of drug reaching the receptor)
- Absorption
- dose given (higher dose – more receptors occupied)
- Distribution
- lipid soluble drugs such as vecuronium may accumulate in lipid rich tissue (esp infusion)
- Patients with decreased lipid content may lead to increased effect site conc
- Metabolism
- hepatic failure may lead to decreased metabolism of a drug – pancuronium
- deranged pH changes the metabolism of the isoquinolones which use Hoffmann elim
- Excretion
- renally excreted drugs, especially minimally metabolised vecuronium, rocuronium
Pharmaceutical/ drug interactions
- storage of the drug (isoquinolones must be stored at 4 degrees to maintain potency)
- aminoglycosides – decreased prejunctional ACh release
- volatile anaesthetics – mechanism not well understood
- frusemide – potassium effect, may interfere with cAMP and ACh release
JC 2019
Examiner Comments
2014B 07: 15% of candidates passed this question.
A structured approach would work well for this question but was often lacking. Many answers were superficial providing short lists of factors affecting NDMRs without reference to mechanism. Candidates failed to differentiate factors which potentiate NDMRs from those which inhibit the action of the drugs. Some confusion also existed confusing speed of onset kinetics with potentiation kinetics. Candidates who structured their answer into pharmacokinetic and pharmacodynamic factors generally scored better marks. Candidates who used pre-post synaptic type structure tended to omit kinetics completely from their answers. Incorrect facts were common.
2016A 12
In relation to neuromuscular blocking drugs – Discuss the factors that influence the speed of ONSET of neuromuscular block.
2014A 09
Discuss the factors that may potentially influence the speed of onset of neuromuscular blockade.
2009B 03
Describe the factors that influence the speed of ONSET of neuromuscular blockade.
CICMWrecks Answer
Drug Factors
- Dose: Higher dose (eg. multiples of ED95) = faster onset.
- Plasma clearance: Postulated that drugs with faster clearance (eg. sux) have faster onset.
- Potency:
- Inverse log relationship between potency and speed of onset (non-depolarisers).
- Low potency = bigger dose needed (more molecules), so higher conc gradient between plasma and site of action = more rapid onset.
- Depolarisers vs non-depolarisers.
Patient Factors
- Rate of injection: faster rate = faster onset.
- Site of injection: Central vein (more rapid distribution)> peripheral vein > IM
- Cardiac output and muscle blood flow: Higher CO and muscle flow = faster delivery to site of action.
- Priming: small dose of non-depolariser as a premed can partially block NMJ, followed by intubating dose. This decreases time from intubating dose to intubation conditions.
- Disease states: eg. myasthenia gravis (fewer ACh receptors, so needs more sux, but about 10% dose of non-depolariser).
Other factors
- Muscle group: Fastest affected = small, rapidly moving muscle groups (eye, digits), with trunk/abdo muscles last affected.
- Recovery is in reverse order: diaphragm/intercostals recover first.
- Onset more rapid in muscle groups relevant to intubation: laryngeal, masseters. Slower in monitored muscles (eg.adductor pollicus).
- Probably due to muscle blood flow.
- Smaller volume of distribution due to blood loss can augment potency ie: speed of onset(Stoelting)
Drugs:
eg. ephedrine can increase speed of rocuronium onset. Inhalationals/aminoglycosides.
JC 2019
Examiner Comments
2016A 12: 38% of candidates passed this question.
The question specifically asked for factors that influenced the speed of onset of neuromuscular block. This information is different to the factors that influence neuromuscular blockade in general which was what many candidates focussed on. It was expected candidates would address the main factors known to influence the speed of onset of neuromuscular block: – potency of the agent used (inverse relationship to speed of onset); rate of delivery of the agent to the NMJ (blood flow / muscle group); and mechanism of the neuromuscular block (nondepolarising vs depolarising).
2014A 09: 16% of candidates passed this question.
Speed of onset is related to how quickly an effective dose reaches the neuromuscular junction, the type of interaction with the receptor and the margin of safety of the receptors. Examples of parameters that increase speed of onset include a high drug rate of delivery (high CO, high muscle
group blood flow, and fast injection rate), high drug concentration (higher dose, low potency, higher ED 95, lower protein binding) or a depolarising block. A good answer would include a list of these factors, with a brief explanation. Mention of other factors such as electrolyte disturbances gained additional marks. It was expected that the direction of an effect would be clearly indicated (e.g. “potency” would not score a mark unless the candidate wrote – “low potency increases speed of onset”, etc.). These drugs are charged molecules which do not cross cell membranes and have a low volume of distribution. Absorption from GIT, Lipid solubility, pKa, metabolism and clearance have minimal relevance to speed of onset.
2009B 03: 0 (0%) of candidates passed this question.
The better candidates had an organised approach to their answer. For example factors could be broadly classified as pharmacokinetic and pharmacodynamic factors or patient and drug factors. For example, pharmacokinetic factors could include dose, the use of a priming dose, patient’s volume status, cardiac output, muscle group and skeletal muscle blood flow. Pharmacodynamic factors expected were those of mechanism of blockade, receptor affinity, agent potency, neuromuscular disorders, age, drug interactions and electrolyte disorders. Many answers listed factors which affect the duration of neuromuscular blockade instead of the onset. Marks were not allocated for information provided by candidates that did not address the question asked. It is important that candidates provide an answer specific to the question asked. Many candidates applied Fick’s Law inappropriately. Lipid solubility and pKa are not relevant as these drugs do not cross the nerve membrane.
Syllabus – H2a, 2c
Reference: Pharmacology and Physiology in clinical practice, Stoelting Pg 186, Foundations
of Anaesthesia: Basic clinical Science, Hemmings and Hopkins ( 2nd ed)
2023A 19
Describe factors that prolong the action of neuromuscular blocking agents (60% of Marks) Outline the pharmacokinetics and pharmacodynamics of vecuronium (40% of Marks).
2021A 06
Describe the pharmacology of vecuronium, including factors that prolong its action of neuromuscular blockade.
CICMWrecks Answer: Factors
FACTORS
The factors which alter the response (number of receptors occupied) may be separated into: pharmacodynamic factors, pharmacokinetic factors, pharmaceutical and drug interaction factors
Pharmacodynamic Factors
- decreased pH increases the affi¬nity of non depolarising NMB
- electrolyte disturbances
- Ca disturbances affect the release of ACh from the presynaptic terminal (triggered by Ca)
- hypercalcaemia results in shortened blockade
- hypocalcaemia potentiates blockage
- magnesium
- stabilise membranes and decrease release and sensitivity to ACh
- potassium
- hypokalaemia leads to membrane hyperpolarisation (decreased drug effect)
- hyperkalaemia has the reverse effect
- pathology
- myasthenia gravis patients have defective nACh receptors
- eaton-lambert syndrome patients have decreased ACh release
- burns – leads to receptor mediated resistance to non depolarising NMBD
Pharmacokinetic factors
(affect the amount of drug reaching the receptor)
- absorption
- dose given (higher dose – more receptors occupied)
- distribution
- lipid soluble drugs such as vecuronium may accumulate in lipid rich tissue (esp infusion)
- women have higher lipid content which may lead to reduced effect site conc
- metabolism
- hepatic failure may lead to decreased metabolism of a drug – pancuronium
- excretion
- renally excreted drugs, especially minimally metabolised vecuronium, rocuronium
Pharmaceutical / Drug Interactions
- Increase effect
- aminoglycosides – decreased prejunctional ACh release
- volatile anaesthetics – mechanism not well understood
- frusemide – potassium effect, may interfere with cAMP and ACh release
- decrease effect
- anticonvulsants – may develop resistance to pancuronium, vecuronium
JC 2019
Examiner Comments
2023A 19: 20% of candidates passed this question.
The first part of the question required a description of the factors prolonging neuromuscular blockade which would include a description of the reasoning behind the prolongation. Many candidates missed the important factors and most did not include any explanation. Some candidates confused the causes of “delayed onset” and “prolonged action”. For vecuronium mechanism of action all the details were expected, superficial explanations did not score well. The following key words were expected to be included in the mechanism description; competitive binding/ Nicotinic ACH (N2)/alpha subunit/post synaptic. In pharmacokinetics, details on hepatic metabolism to active metabolites, renal and biliary excretion along with accurate values of the volume of distribution, protein binding and half-life were expected to score full marks. These facts were frequently lacking in candidate answers.
2021A 06: 13% of candidates passed this question.
Vecuronium is a commonly available and regularly used amino-steroid neuromuscular blocking agent. It is a level 1 drug in the 2017 syllabus. A simple template utilising the headings; pharmaceutics, PK, PD, uses in ICU and adverse reactions with associated relevant important facts would have scored well. Expected information regarding the factors prolonging neuromuscular blockade included electrolyte abnormalities, drug interactions and patient factors. Overall, the level of understanding and knowledge demonstrated in the answers was below an expected standard for a level 1 drug.
2018B 13
Compare and contrast rocuronium and cisatracurium.
Examiner Comments
2018B 13: 32% of candidates passed this question.
This question was best answered using a tabular format outlining class of drug, pharmaceutics, pharmacokinetics, reversibility and side effects. Better answers commented on the significance of the differences between the two agents and its relevance to ICU practice. Many candidates confused these muscle relaxants with each other and with depolarising muscle relaxants.
2020B 10 – 2013B 01 – 2011A 06
Describe the pharmacology of suxamethonium
Examiner Comments
2020B 10: 63% of candidates passed this question.
This was a level 1 pharmacology question, and it represents core knowledge. The mechanism of action of suxamethonium and the interactions at the neuromuscular junction as well as pharmaceutics were areas that often required further detail. Few candidates mentioned the effects of suxamethonium on the autonomic nervous system. Another common omission related to the factors that reduce plasma cholinesterase activity beyond genetic deficiency (such as liver disease, renal failure, thyrotoxicosis). Pleasingly, there was generally a good understanding of role, dosing, side effect profile, pharmacokinetics and of special situations and limitations of use pertinent to this drug.
2013B 01: 16 candidates passed (59.3 %).
This question was generally well answered. A structured approach that included headings such as pharmaceutics, mechanism of action, pharmacodynamics, kinetics, dose and side effects was associated with a good answer.
2011A 06: 7 (58%) of candidates passed this question.
Most candidates presented a structured answer and demonstrated reasonable understanding of the pharmacology of suxamethonium. This is however a core subject and candidates should be able to answer this question in depth.
Syllabus: H2a, 2c
Recommended sources: Basic and Clinical Pharmacology, Katzung, Chp 27
2022A 13
Compare and contrast the pharmacology of suxamethonium and rocuronium.
Examiner Comments
2022A 13: 75% of candidates passed this question
Suxamethonium is a level 1 drug and therefore requires a detailed knowledge of the drug from a PK and PD perspective as well as consideration of important side effects and considerations when used. The examiners commented that a table structure with the structured pharmacology headings and clear concise facts was the best way to approach this question. Answers that scored poorly often displayed incorrect facts, limited appreciation of side effects and vague statements on the pharmacological particularities of this drug. For example, muscle relaxants are major culprits for anaphylaxis in hospitals, and nuanced facts about this were generally missing from candidate’s answers.
2018A 10
Outline the advantages (15% of marks) and disadvantages (85% of marks) of the clinical use of suxamethonium.
2012B 02
Suxamethonium is a non-competitive partial agonist.
Explain what is meant by this statement using definitions of the underlined terms (50% of marks).
List the advantages and disadvantages of suxamethonium within Intensive Care practice (50% of marks).
CICMWrecks Answer
- Suxamethonium is the dicholine ester of succinic acid which acts as an ultrashort acting depolarising muscle relaxant.
- It is used in rapid sequence induction and to modify seizures caused by ECT.
- Mechanism of Action:
- mimics the actions of Ach by binding to the nicotinic Ach receptor and causing membrane depolarization.
- However, because its hydrolyzing enzyme is not present at the NMJ, the effect lasts longer than for Ach.
- The persistent depolarization renders the voltage sensitive Na channels inactive within 1-2 mins.
- This prevents the transmission of further APs. Initially causes fasciculations, then muscle relaxation.
- Partial agonists are drugs that bind to and activate a given receptor, but have only partial efficacy at the receptor relative to a full agonist
- A partial agonist is unable to achieve a maximal biological response following active site binding, despite increasing doses
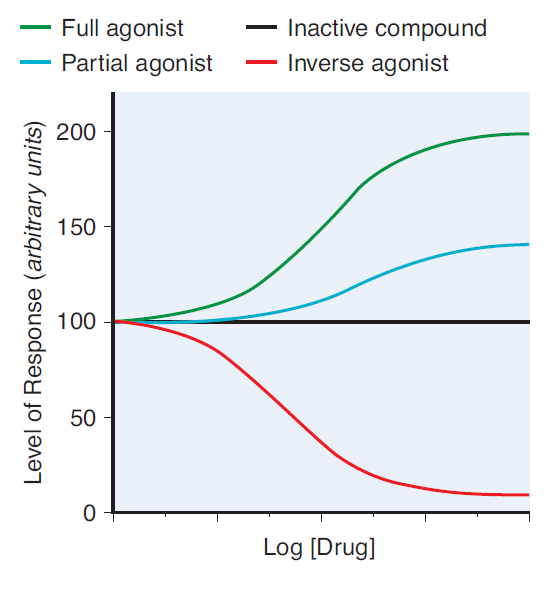
- A non-competitive antagonist binds to an allosteric (non-agonist) site on the receptor to prevent activation of the receptor
- Since it induces a lower biological response on active site binding than acetylcholine, it is a non-competitive antagonist of acetylcholine at the nicotinic receptor
- No matter how high levels of acetylcholine are at the neuromuscular junction, maximal response will not be reached
Advantages and Disadvantages of Sux in ICU
| Effect | Advantage | Disadvantage |
|---|---|---|
| Pharmaceutics | Inexpensive | Needs to be stored at 4 degrees -Shelf life only 7 days when kept in resuscitation trolley |
| Can be given peripherally | ||
| Excretion of 10% of drug unchanged in urine means active metabolites may accumulate and prolong the effects. | ||
| 0.03% of population are abnormal metabolisers resulting in prolonged apnoea | ||
| Causes fasiculations | Offset of fasiculations indicates paralysis | Muscle pains |
| Causes malignant hyperthermia | ||
| Prolonged suxemethonium apnoea | If intubation fails, prolonged apnoea | |
| Some agonism at muscarinic receptor | Second dose of suxemethonium commonly causes bradycardia | |
| Widespread neural depolarisation | Hyperkalaemia Raised intraocular pressure Raised GIT sphincter pressures | |
| Rapid onset (30-60 seconds) | Short period of time before intubating conditions achieved – Less risk of desaturation / apnoea when intubating | |
| Rapid offset (5-10 minuts) | If intubation fails, apnoea will not be prolonged | Muscle paralysis may disappear before airway secured or patient made safe |
| Metabolised by plasma cholinesterase | Metabolism not dependant on organ function | Plasma cholinesterase may be lower in some patients – myriad reasons |
Mooney / JC 2020
Examiner Comments
2018A 10: 46% of candidates passed this question.
This commonly used drug should be very well-known. The question asked for an outline, hence long explanations of various aspects of pharmacology (e.g. pseudocholinesterase deficiency) were unnecessary.
Headings should have included: advantages (e.g. rapid onset, rapid offset, short acting, IV or IM administration, not end organ dependent for metabolism, premixed, safe in pregnancy and neonates). The disadvantages section should have included the following headings: pharmaceutical, adverse drug reactions (including several potentially fatal ones), numerous contraindications, unpleasant side-effects and potential problems with repeat dosing.
2012B 02: 13 (59.1%) of candidates passed.
For a good answer candidates were expected to mention that an agonist is a drug that elicits a maximal response on binding to a receptor. A partial agonist has intrinsic affinity with only partial efficacy and hence is unable to elicit a maximal response. A competitive drug acts at the same binding site of a receptor as an endogenous ligand (e.g. acetylcholine at the neuromuscular junction) and its action therefore is surmountable with increasing concentrations of drug and how this concept relates to suxemethonium. For the remainder of the question, Candidates were expected to mention the advantages and disadvantages of suxamethonium within Intensive Care practice. Good answers included a systematic approach and use of tables and/or well organised lists.
L3. Neuromuscular Measurement and Monitoring
i. Describe the monitoring of neuromuscular blockade.
VIVAs
| 2023B | Skeletal myocyte: Extra and intracellular conc of Na, K, Cl Events that lead to skeletal muscle contraction in reponse to motor neurone AP |
| 2023A | |
| 2022B | Motor unit, skeletal contraction and relaxation |
| 2022A | Ach receptor at NMJ Neuromuscular monitoring, physiology and pharmacology |
| 2021B | |
| 2021A | End plate potential generation, how is it different from miniature end plate potential, NM transmission and monitoring |
| 2020B | Smooth muscle, G proteins |
| 2019B | Pharmacology: NMBDs, LA |
| 2019A | |
| 2018B | NM function, NM blockers, Peripheral nerve stimulator |
| 2018A | |
| 2017B | NM physio, NM blockers |
| 2017A | NMJ, NM blockers |
| 2016B | NM blockers, physio of Deep tendon reflex |
| 2016A | NMJ, NM blockers Calcium, cardiac myocyte, pharm, regulation |
| 2015B | |
| 2015A | neuromuscular physiology and pharmacology |
| 2014B | |
| 2014A | |
| 2013B | |
| 2013A | |
| 2012B | |
| 2012A | |
| 2011B | Diagram of NMJ, NM blockers, MoAction, pharmokinetic properties |
| 2011A | |
| 2010B | |
| 2010A | Sacromere, length-tension relationship, muscle relaxants, malignant hyperthermia, dantrolene |
| 2009B | |
| 2009A | |
| 2008B | |
| 2008A | |
| 2007B | Asthma in extremis – NMJ, NMJ blocking drugs |

Recent Comments