Syllabus (Fourth Edition, 2023)
Topics
i. Describe the anatomy of cranial nerves relevant to brainstem reflexes.
ii. Explain the determinants of intra-cranial pressure.
iii. Describe the anatomy and regulation of cerebral circulation.
iv. Describe the physiology of cerebrospinal fluid.
v. Describe the major sensory and motor pathways (including anatomy) from the periphery to cortex.
vi. Explain the basic electro-physiology of neural tissue, including conduction of nerve impulses and synaptic function.
vii. Describe the major neurotransmitters and their physiological role, with particular reference to excitatory and inhibitory amino acids (including those acting on the NMDA receptor), GABA, acetylcholine, noradrenaline, dopamine and serotonin.
Topics not covered in previous SAQs
i. Describe the anatomy of cranial nerves relevant to brainstem reflexes.
iii. Describe the anatomy and regulation of cerebral circulation.
v. Describe the major sensory and motor pathways (including anatomy) from the periphery to cortex.
vii. Describe the major neurotransmitters and their physiological role, with particular reference to excitatory and inhibitory amino acids (including those acting on the NMDA receptor), GABA, acetylcholine, noradrenaline, dopamine and serotonin.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
i. Describe the anatomy of cranial nerves relevant to brainstem reflexes.
2021B 18
Outline the neural pathways for the pupillary light, corneal, oculomotor and gag reflexes. The anatomical course of nerves is NOT required.
CICMWrecks Answer
| Reflex | Examination technique | Normal response | Afferent pathway | Brainstem centers | Efferent pathway |
|---|---|---|---|---|---|
| Pupillary light | Response to light | Direct and consensual myosis followed by mydriasis | Retina, optic nerve, chiasma, optic tract | Pupillo-constrictor: midbrain, pretectal olivary nucleus, Edinger-Westphall nucleus Pupillo-dilator: posterior-lateral hypothalamus, cervical ganglion, trigeminal ganglion, abducens | Sympathetic fibers of CN III (Oculomotor) |
| Corneal | Stimulation of cornea with saline drops / cotton wisp | Eyelid closure | CN V (Trigeminal) | Pons, trigeminal and facial nuclei | CN VII (facial) |
| Occulomotor | Oculocephalic: Turn head from side to side | Eyes move conjugately in direction opposite to head | Semicircular canals, CN VIII (oculovestibular) | Pons (parapontine reticular formation), nucleus vestibularis, nucleus abducens | CN III (oculomotor) and VI (abducens) |
| Oculovestibular: Irrigate external auditory meatus with cold water | Nystagmus with fast component beating away with stimulus | ||||
| Gag / Pharyngeal reflex | Stimulation of solft palate | Symmetrical rise of soft palate gag reflex | CN IX (Glossopharyngeal) CN X (vagus) | Medulla, nucleus tractus solitarius | CN IX (Glossopharyngeal) CN X (vagus) |
Sources:
Benghanem, Sarah & Mazeraud, Aurélien & Azabou, Eric & Chhor, Vibol & Shinotsuka, Cássia & Claassen, Jan & Rohaut, Benjamin & Sharshar, Tarek. (2020). Brainstem dysfunction in critically ill patients. Critical Care. 24. 10.1186/s13054-019-2718-9.
Stevens, R., Sharshar, T., & Ely, E. (Eds.). (2013). Brain Disorders in Critical Illness: Mechanisms, Diagnosis, and Treatment. Cambridge: Cambridge University Press. doi:10.1017/CBO9781139248822
Examiner Comments
2021B 18: 43% of candidates passed this question.
This is a fact-based question with little integration of knowledge required. Those candidates who synthesised their knowledge into a succinct and precise description of afferent and efferent pathways with a description of the various sensor and integrator components scored very high marks. A good working knowledge of all the cranial nerve reflex pathways are crucial to the practise of intensive care medicine. Marks were not awarded for any anatomical description related to these pathways.
2023B 20
Outline the anatomy (60% marks) and synaptic physiology (40% marks) of the vagus nerve.
CICMWrecks Answer
Vagus Nerve Anatomy
| Nuclei | Dorsal motor nucleus – sends parasympathetic fibers to the GI tract, lungs Nucleus ambiguus – sends efferent motor and parasympathetic fibers to the heart Solitary nucleus – receives special gustatory afferent from the tongue and visceral afferent fibers from organs Spinal trigeminal nucleus – receives general sensory afferent fibers from outer ear, dura of posterior cranial fossa, mucosa of larynx |
| Course | Exits brain from medulla oblangata – series of rootlets in retro-olivary groove → Travels laterally and exits skull via jugular foramen → Sensory ganglia consist of superior and inferior ganglionic swelling → Joined by cranial root of accessory nerve (CN XI) → Passes down the neck in carotid sheath (between Carotid artery and Internal Jugular Vein) → Enters thorax at base of neck → Left: travels ant to aortic arch, behind Lt main bronchus and into oesophagus → Right: travels behind oesophagus and Rt main bronchus →Both enter abdomen through oesophageal hiatus in diaphragm |
| Branches | In the jugular fossa: meningeal, auricular branches In the neck: pharyngeal, superior laryngeal, recurrent laryngeal nerves; superior cardiac branches In the thorax: inferior cardiac nerve, anterior bronchial branches, posterior bronchial branches, esophageal branches In the abdomen: gastric, celiac and hepatic branches |
| Field of innervation | General sensory afferent fibers – sensory information from larynx, auricle, external acoustic meatus, dura mater of the posterior cranial fossa General visceral afferent – information from the aortic body, esophagus, lungs, bronchi, heart, intestines Special afferent – information about taste General visceral efferent – parasympathetic division that simulates smooth muscle and glands of the pharynx, larynx, thoracic and abdominal organs. – Heart via cardiac plexus: SA node: Rt vagus. AV node, ventricles: Lt vagus. – Lungs via pulmonary plexus – Gut (from stomach to proximal to splenic flexure) via gastric plexus Branchial efferent – innervate muscles of mastication and tensor vali palatini |
| Communications | Trunk and ganglia with CN IX,XI,XII, superior sympathetic ganglion and 1st and 2nd cervical nerves Auricular branch with CN VII Pharyngeal plexus with IX and superior cervical ganglion Cardiac, pulmonary, oesophageal and gastric branches with sympathetic outflow to viscera (Superficial and deep Cardiac plexuses receives branches from cardiac nerves of vagus, recurrent laryngeal nerves and cervical ganglia of sympathetic trunk.) |
Physiology
- 75% of all parasympathetic fibres are in vagus nerve
- Pre-ganglionic fibre
- Long fibre
- Release Acetylcholine to stimulate post-ganglionic neuron at nicotinic ACh receptor
- activation is tonic
- Ganglia located near or within effector organs
- Post-ganglionic fibre
- Short
- Release Acetylcholine to stimulate muscarinic ACh receptor
Effector
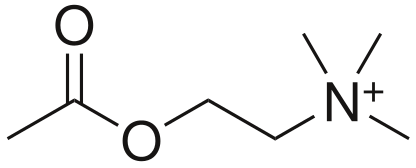
- Acetylcholine (CCOOCCNH3)
- Formed for Choline and Acetyl CoA
- Receptors
- Muscarinic
- G Protein Coupled
- Nicotinic
- Ligand gated ion channels
- Muscarinic
- Metabolism
- Acetylcholinesterase
Muscarinic ACh receptor
- Known as muscarinic because muscarine also agonises this receptor
- Metabotropic, G-protein coupled
- Phosphorylate various second messengers
- Mediate slow metabolic response via second messenger cascades
- 5 subtypes: M1-M5
- M1,M3,M5 – excitatory (Gq: IP3/DAG → ↑Ca++)
- M2, M4 – Inhibitory (Gi: ↓cAMP)
- Receptor at parasympathetic postganglionic terminals
- M1: ANS
- M2: Cardiac
- M3: Lungs
| Ganglion / Target organ | Receptor | Signal | Effect |
|---|---|---|---|
| Respiratory: Pulmonary plexus | M3 (Gq) | IP3/DAG ↑Ca++ | Bronchoconstriction increased mucous production |
| Cardiovascular: – SA node – AV node | M2 (Gi) | ↓cAMP ↑ K+(G) | ↓ inotropy (Atria > Vent) ↓↓↓ Chronotropy ↓ lusitropy (Atria > Vent) ↓↓↓ dromotropy |
| stomach to proximal two-thirds of the transverse colon | M1 (Gq) | IP3/DAG ↑ Ca++ | Increased gastric motility, secretions Relaxation of pyloric sphincter |
Examiner Comments
2023B 20: 25% of candidates passed this question.
The vagus nerve anatomy was best broken down into a description of the fibers it carries (visceral, parasympathetic and somatic sensory fibres) and then origin and course from the parasympathetic, sensory and motor nuclei in the medulla as the tenth cranial nerve to its branches; the pharyngeal, cardiac, pulmonary and laryngeal branches. Pre and post-ganglionic physiology involved a detailed description of the Muscarinic Ach receptor and events. This would also include the 5 subtypes of the muscarinic receptor, with the locations and downstream effects of the M1-M3 locations being the most important to note.
ii. Explain the determinants of intra-cranial pressure.
2018A 15 Describe the physiological regulation of intracranial pressure.
2016A 14 Describe the factors that influence intracranial pressure.
2010A 18 Describe the physiology of intracranial pressure and the physiological mechanisms that limit a rise in intracranial pressure.
CICMWrecks Answer
Definition:
- ICP: hydrostatic pressure within the cranial vault
- Normal value is 5-15 mmHg
- focal ischaemia when ICP > 20 mmHg
- global ischaemia when ICP > 50mmHg
Munro-Kellie Doctrine
- The rigid and closed cranial vault forms a fixed brain volume containing
- Brain parenchyma (80%, 1400 g)
- CSF (10%; 75 mL)
- Cerebral blood and vessels (10%; 75 mL)
- Δ’s in volume of any components → Δ’s in others or and increase in ICP
CSF Production / Absorption
CSF Production:
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
CSF Absorption:
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
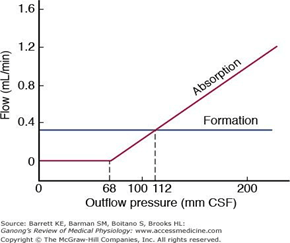
Control of ICP
- ICP is regulated via volume buffering
- i.e. increase in volume of one intracranial component leads to compensatory decrease in volume of other intracranial components
- When volume buffering mechanism is exhausted → rapid increase in ICP (decompensation)
- Movement of cerebral venous blood = rapid compensation, lower capacity
- Movement of CSF = gradual compensation, larger capacity
Determinants of ICP:
- Brain
- Age / Mass
- Space occupying lesions
- Cerebral Oedema
- CSF
- CSF production
- CSF Absorption
- Cerebral Blood Volume
- Cerebral autoregulation: Flow-metabolic coupling
- Cerebral metabolic rate
- Increase in systemic blood pressure / flow
- Venous Outflow obstruction
- Vasoactive agents
- Monro-Kellie Doctrine
- Loss of above – e.g. Fractures, surgery
Compensation for Elevated ICP (Intracranial Pressure)
Early compensation
- Δ CSF distribution and flow
- CSF is displaced to spinal subarachnoid space
- ↑’d resorption rate
Late compensation
- ↑ ICP → ↓ CBF → ↓ in cerebral blood volume → cerebral ischaemia
Decompensation
- ↑ICP → ↓ in cerebral tissue volume → brain herniation
- Cushing Reflex
- Hypertension, bradycardia and abnormal breathing associated with raised ICP
- Mechanism:
- Stage 1:
- ↑ ICP → ↓ blood supply to vasomotor area → Local hypoxia/hypercarbia → ↑ SNS >> ↑ PSPS vasomotor stimulation
- ↑ TPR → ↑ MAP
- ↑ HR → ↑ CO
- → compensatory ↑CBF
- Stage 2:
- ↑ CO → Baroreceptor stimulation → ↑ Vagus nerve stimulation → Bradycardia and ↓ contractility.
- Stage 1:
Gladwin / JC 2020
Examiner Comments
2018A 15: 45% of candidates passed this question.
A definition and a normal value were expected. A description of the Monro-Kellie doctrine was expected. Better answers divided into the various components of the cranium with the answer focussing on cerebral blood volume and CSF volume as the brain tissue as no capacity to change its volume.
2016A 14: 69% of candidates passed this question.
A structure approached works well for “describe the factors …” questions. Better answers provided a definition of ICP, explained the Monro-Kellie doctrine and then detailed the factors which affect the volume of each of the components – cerebro spinal fluid (CSF), cerebral blood flow and brain parenchyma. Some candidates focused only on factors which cause intracranial hypertension and were thus unable to score full marks. Many candidates stated that CSF production was ICP dependant which is incorrect.
2010A 18: 7 (70%) of candidates passed this question
Candidates who did well in this question used graphs to describe the various concepts, described normal physiology and covered the breadth of the topic. A good answer made mention of normal values of ICP, it’s variation with respiration and blood pressure and illustrated a trace of the ICP. An explanation of the Monroe Kelly doctrine was expected, CSF production and absorption and it’s relationship to raised ICP as well other compensatory mechanisms for a high ICP (eg displacement of CSF into spinal canal, displacement of venous blood into the jugular veins, rise in ICP leads to ischaemia if the brain. Critical ischaemia invokes the Cushing reflex. Major omissions by candidates was the use of diagrams, description of normal variation and only a superficial knowledge of compensatory mechanisms.
Syllabus: G1, 2d,g
References: Textbook of Medical Physiology, Guyton, Chp 61
2022A 10 – 2016B 18
Discuss the determinants of intracranial pressure (80% of marks).
Outline how it can be measured (20% of marks).
CICMWrecks Answer: ICP
Definition:
- ICP: hydrostatic pressure within the cranial vault
- Normal value is 5-15 mmHg
- focal ischaemia when ICP > 20 mmHg
- global ischaemia when ICP > 50mmHg
Munro-Kellie Doctrine
- The rigid and closed cranial vault forms a fixed brain volume containing
- Brain parenchyma (80%, 1400 g)
- CSF (10%; 75 mL)
- Cerebral blood and vessels (10%; 75 mL)
- Δ’s in volume of any components → Δ’s in others or and increase in ICP
CSF Production / Absorption
CSF Production:
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
CSF Absorption:
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation

Control of ICP
- ICP is regulated via volume buffering
- i.e. increase in volume of one intracranial component leads to compensatory decrease in volume of other intracranial components
- When volume buffering mechanism is exhausted → rapid increase in ICP (decompensation)
- Movement of cerebral venous blood = rapid compensation, lower capacity
- Movement of CSF = gradual compensation, larger capacity
Determinants of ICP:
- Brain
- Age / Mass
- Space occupying lesions
- Cerebral Oedema
- CSF
- CSF production
- CSF Absorption
- Cerebral Blood Volume
- Cerebral autoregulation: Flow-metabolic coupling
- Cerebral metabolic rate
- Increase in systemic blood pressure / flow
- Venous Outflow obstruction
- Vasoactive agents
- Monro-Kellie Doctrine
- Loss of above – e.g. Fractures, surgery
Compensation for Elevated ICP (Intracranial Pressure)
Early compensation
- Δ CSF distribution and flow
- CSF is displaced to spinal subarachnoid space
- ↑’d resorption rate
Late compensation
- ↑ ICP → ↓ CBF → ↓ in cerebral blood volume → cerebral ischaemia
Decompensation
- ↑ICP → ↓ in cerebral tissue volume → brain herniation
- Cushing Reflex
- Hypertension, bradycardia and abnormal breathing associated with raised ICP
- Mechanism:
- Stage 1:
- ↑ ICP → ↓ blood supply to vasomotor area → Local hypoxia/hypercarbia → ↑ SNS >> ↑ PSPS vasomotor stimulation
- ↑ TPR → ↑ MAP
- ↑ HR → ↑ CO
- → compensatory ↑CBF
- Stage 2:
- ↑ CO → Baroreceptor stimulation → ↑ Vagus nerve stimulation → Bradycardia and ↓ contractility.
- Stage 1:
Gladwin / JC 2020
CICMWrecks Answer: Measurement of ICP
Methods of Measurement of ICP (Outline)
Invasive
| Method | Advantages | Disadvantages |
|---|---|---|
| Intraventricular catheter (EVD) | Provides ‘ture’ global ICP Allows for CSF drainage and administration of drugs In-vivo calibration possible via external pressure transducer | Infection Difficult iinsertion |
| Epidural catheter | Ease of insertion Minimal risk of infection (no penetration of dura) | Low accuracy |
| Lumbar CSF puncture | Extracranial procedure Can be performed ambulatory | May not reflect ICP Dangerous if ICP high |
| Catheter-tip micro transducers (subdural or intra-parenchymal) | Rare complications during procedure Low risk of infections Can be made permanent implants | Drift of transducer output over time In-vivo calibration not possible Inaccurate if intraparenchymal gradient exists |
Non-Invasive
Non-invasive methods like pupillometry, CT, MRI, TCD provide an adjunct to clinical examination of high ICP, but are not a surrogate for invasive ICP measurement.
Gladwin / JC 2020
Examiner Comments
2022A 10: 64% of candidates passed this question.
In the good answers to this question, and there were a number, the candidates included the volumes of the cranium and a correct description of the Monroe Kellie doctrine. A good answer should have included the compensations and consequences of increases in intra-cranial volumes; a discussion of all three components (brain tissue, blood, and CSF) and how they affect intracranial pressure; and then information on intra-ventricular and parenchymal devices in measuring ICP, briefly including their pros and cons. A common issue was writing quite a lot more than was needed on the relationship of cerebral blood flow to cerebral blood volume, and/or on the physiological consequences of raised ICP, which seemed to leave little time for discussion elsewhere. A few candidates did not provide any response for ICP measurement (worth 20% of the marks). Few candidates provided the intra-cranial elastance equation. A significant proportion of candidates missed out a part of the question, either the factors that affect CBV or ICP measurement.
2016B 18: 55% of candidates passed this question.
It was expected answers would include an explanation of the Monro-Kellie Doctrine. Many candidates gave insufficient details of compensatory mechanisms especially regarding decreased total cerebral blood volume (primarily venous) in response to increased intracranial pressure.
Most candidates had all the information but had difficulty synthesising the information to write a cohesive answer. Factors affecting ICP could be divided into factors affecting CBV, factors affecting CSF and factors affecting brain tissue. Under factors affecting CBV the effect of blood gases, autoregulation, temperature, metabolism, drugs and venous obstruction could have been detailed.
2009A 22
Outline the mechanism of action of drugs used to control raised intracranial pressure.
CICMWrecks Answer
Intro
ICP: hydrostatic pressure within the cranial vault
- Normal value is 5-15 mmHg
- focal ischaemia when ICP > 20 mmHg
- global ischaemia when ICP > 50mmHg
Munro-Kellie
- The rigid and closed cranial vault forms a fixed brain volume containing
- Brain parenchyma (80%, 1400 g)
- CSF (10%; 75 mL)
- Cerebral blood and vessels (10%; 75 mL)
- Δ’s in volume of any components → Δ’s in others
Drugs Control:
Volume of Brain Parenchyma
- Osmotic Agents
- Mannitol (Response Augmented by concurrent use of Loop Diuretics
- Hypertonic Saline
Changes in Cerebral Blood Flow (CBF)
- ↑ CBF from ↑
- CO (thus HR and SV)
- Inotropes
- SVR
- Vasopressors
- CO (thus HR and SV)
- ↓ CBF from ↑
- radius (thus peripheral metab (esp adenosine), incr CO2, decreased O2, vagal stimulation)
- Induction agents (thiopental) → ↓ CMRO2 → ↓ CO2 production → ↓ CBF → ↓ Volume
- Other ↓CMRO2 agents
- Anticonvulsants (phenytoin, levitiractam) → ↓CMRO2 and ↓ Temperature
- Analgesic (opiods etc)
- Sedatives (benzodiazepine)
- Antipyretics should be instituted to reduce fever which will increase metabolic demands
- viscosity
- ↑ Free water excretion (frusemide + mannitol)
- radius (thus peripheral metab (esp adenosine), incr CO2, decreased O2, vagal stimulation)
Volume of CSF
- Acetazolamide (Carbonic Anhydrase Inhibitor): ↓ CSF production
Gladwin 2016
Examiner Comments
2009A 22: Pass rate: 20%
The answers to this question were generally not broad enough. Only one or two drugs were discussed rather than the range of drugs used in this situation. Discussion should have included benzodiazepines, intravenous induction agents, opioid narcotics, diuretics including loop diuretics and mannitol. Hypertonic saline also gained marks. Generally the discussions on the drugs mentioned were done well.
Some candidates discussed the physiological control of intracranial pressure which was not required and gained no marks.
Syllabus G2g, G2a2a E2a2a
iii. Describe the anatomy and regulation of cerebral circulation.
2015A 03 Describe the structure and function of the blood brain barrier
2012B 12 – 2009B 08
Describe the blood brain barrier (50% of marks).
What characteristics does a drug need to effectively penetrate the blood brain barrier? (50% of marks)
CICMWrecks Answer
Blood brain barrier
- Anatomical and chemical partition separating the intravascular space from CNS interstitial space
Structure
- Mechanical barrier
- Endothelial cells
- Tight junctions between cells formed by membrain proteins (e.g. occludin) prevents paracellular flow
- Lack fenestrations
- Lack transcellular pathways such as vescicles
- Selective transport proteins (e.g. GLUT, various amino acid transporters)
- Pericytes embedded in basement membrane
- Astrocyte end feet
- Supportive role for endothelium
- Aquaporin regulation
- Endothelial cells
- Physiological barrier
- Enzymatic inactivation
- Enzymtes within endothelial cells metabolise substances absorbed from capillary lumen
- Monoamine oxidase
- Cholinesterase
- Aminopeptidase and endopeptidase
- Enzymtes within endothelial cells metabolise substances absorbed from capillary lumen
- Efflux pumps
- Substances that are absorbed across the luminal capillary membrane may be pumped back into capillary lumen by efflux pumps
- P-Glycoprotein
- ABC-Transporter
- Substances that are absorbed across the luminal capillary membrane may be pumped back into capillary lumen by efflux pumps
- Enzymatic inactivation
- Areas of brain outside BBB
- Subfornical organ
- Organum Vasculosum Lamina Terminalis
- Pituitary
- Area postrema
Function
- Regulate uptake of nutrients and electrolytes into brain
- Regulate migration of leukocytes and inflammatory responses in the brain
- Buffer brain parenchyma and interstitium from fluctuations in blood
- Prevent toxins and pathogens entering brain
- Some substances (such as water) pass BBB readily. These are characterised by:
- Small molecular weight
- Lipophilic (thiopentone)
- Uncharged (e.g. atropine vs. glycopyrollate)
- Poorly protein bound
Characteristics of drugs to penetrate BBB
- Size
- According to Graham’s Law – Diffusion inversely proportional to square root of molecular weight
- Lipophilicity
- Highly lipid soluble compounds can permeate phospholipid bilayers with relative ease (fentanyl > morphine)
- Charge
- Unionized molecules can permeate with relative ease (alfentanil > fentanyl)
- Not metabolized within BBB
- No active efflux mechanism
- Protein binding
- Protein bound drug permeate poorly
Sakurai 2016
Examiner Comments
2015A 03: 33 % of candidates passed this question.
There was general lack of understanding of the conceptual framework of the blood brain barrier (BBB) and its function. To attain a pass, candidates were required to describe the concept of BBB as a physical and a transport barrier, describe the role of tight junctions and glial cells and identify important barrier functions with some examples of things commonly transported across or excluded.
2012B 12: 9 (41%) of candidates passed.
The BBB is the separation of the blood from the brain extracellular fluid and serves to maintain consistent internal environment in the brain and protect the brain from large harmful substances and microorganisms. Most answers displayed some knowledge of the structure of the BBB but many answers did not include its function. Better answers included substances to which the BBB is permeable, how permeability changes with age and a mention of the circumventricular organs and their significance (i.e. are outside the BBB). Most candidates correctly identified the characteristics of drugs that cross the BBB. Marks were also allocated for giving examples.
2009B 08: 2 (22%) of candidates passed this question.
Candidates were expected to state the purpose of the blood brain barrier (BBB), define what constituted the BBB, what its function is, and what parts of the brain lie outside of this barrier (and why). Further candidates should have mentioned what substances cross the BBB, and how this is achieved.
Common omissions included not mentioning the presence of astrocyte foot processes in addition to the tight junctions between the capillary endothelial cells, the presence of active pumps for sugars amines, and some ions, and what parts of the brain lay outside of the BBB.
Characteristics of a drug that will penetrate the BBB included low molecular weight, good lipid solubility, a low volume of distribution, low potency and low protein binding. Also expected was a discussion of Fick’s Law and the drug features that would allow a high concentration gradient in the cerebral blood vessels to be achieved and a high diffusion coefficient and mention of drugs that resemble natural ligands for active transport mechanisms.
Syllabus – G12d G2a
References: Pharmacology, Rang and Dale 6th edition page 476, Principles of physiology for the anaesthetist, Kam page39-40, Pharmacology and Physiology in Anaesthetic Practice, Stoelting, Pg 681, Textbook of medical physiology, Guyton and Hal11th edition page 766. Foundations of Anesthesia. Hemmings and Hopkins. 2nd edition.
2024A 14
(a) List the normal parameters for cerebral blood flow (5% of marks).
(b) Describe the physiological factors that influence cerebral blood flow (95% of marks).
2023B 14 Describe the physiological factors that influence cerebral blood flow?
2021A 20 – 2008A 05 Outline the physiological factors that influence cerebral blood flow.
2009A 11 Describe the control of cerebral blood flow.
2014A 14 – 2011B 16
Using a diagram, explain the effect of PaO2, PaCO2 and MAP (mean arterial pressure) on cerebral blood flow (60% marks).
Outline the effects of propofol and ketamine on cerebral blood flow (CBF), cerebral metabolic requirement for oxygen (CMRO2), and cerebral venous oxygen saturation (40% marks).
CICMWrecks Answer: Cerebral Blood Flow
Cerebral Blood Flow
- CBF = CPP / CVR (Cerebral Perfusion Pressure / Cerebral Vascular Resistance)
- CBF 15% resting CO → ~750ml/min or 50ml/100g brain tissue/min
- Gray Matter: 75–80 mL/100 g/min – Significantly higher due to the high metabolic activity and dense synaptic connections
- White Matter: 20–30 mL/100 g/min
- Abnormal CBF
- CBF<50ml/100g/min → cellular acidosis
- CBF<40ml/100g/min → impaired protein synthesis
- CBF <30ml/100g/min → cellular oedema
- CBF <20ml/100g/min → failure of cell membrane ion pumps, loss of transmembrane electrochemical gradients
- CBF <10ml/100g/min → cell death
Determinants of CBF
- CPP
- Net pressure gradient driving blood flow through the cerebral circulation
- CPP = MAP – ICP
- MAP = CO x SVR; CO = HR x SV; SVR = MAP / CO
- ICP dependent on: brain, blood, CSF
- CVR
Regulated by 4 primary factors:
- Cerebral metabolism
- flow-metabolism coupling:
↑ metabolic demand → ↑ CBF + substrate delivery - Controlled by vasoactive metabolic mediators:
H+ ions, K, CO2, adenosine, glycolytic intermediates, NO
- flow-metabolism coupling:
- CO2 and O2
- CO2
- At normotension: relationship between PaCO2 and CBF = almost linear
- ↑ PaCO2 → cerebral arteriolar vasodilation → ↓ CVR + ↑ CBF
- 2~4% increase for every 1mmHg increase in CO2
- ↓ PaCO2 → cerebral arteriolar vasoconstriction → ↑ CVR + ↓ CBF
- Initial stimulus = ↓ brain ECF pH
- Effects regulated by: NO, prostanoids, K channels, intracellular [Ca2+]
- PaO2
- little effect at normal PaO2
- PaO2 <60mmHg → cerebral arteriolar vasodilation → ↑ CBF
- Mechanism: hypoxia acts on →
- cerebral tissue to promote release of adenosine → cerebal vasodilation
- cerebrovascular smooth muscle → hyperpolarisation → ↓ Ca2+ uptake → vasodilation
- CO2
- Autoregulation
- Constant across CPP 50-150mmHg
- CPP >150mmHg: CBF µ CPP
- CPP <50mmHg: CBF <50ml/100g brain tissue/min → ischaemia
- Stimulus to autoregulation = CPP (not MAP)
- autoregulation curve R shifted in HTN; L in neonates
- Mechanism:
- Myogenic mechanism: arterioles vasoconstrict in response to ↑ wall tension + vasodilate in response to ↓ wall tension → ↓ or ↑ CVR
- May involve adenosine
- Can be impaired in SAH, tumour, stroke, head injury
- Constant across CPP 50-150mmHg
- Neurohumeral factors
- Relative lack of humoral + autonomic control on normal cerebrovascular tone
- Main action of SY nerves = vasoconstriction
- Other factors
- Blood viscosity: directly related to HCt; ↓ viscosity → ↑ CBF as per Hagen-Poiseuille law
- Temperature: ↓ CMRO2 by 7% for each ↓ 1°C in temp
- Drugs
- E.g. barbiturates ↓ cerebral metabolism
- Volatile agents → ↓ tension cerebral vascular smooth muscle → vasodilation + CBF
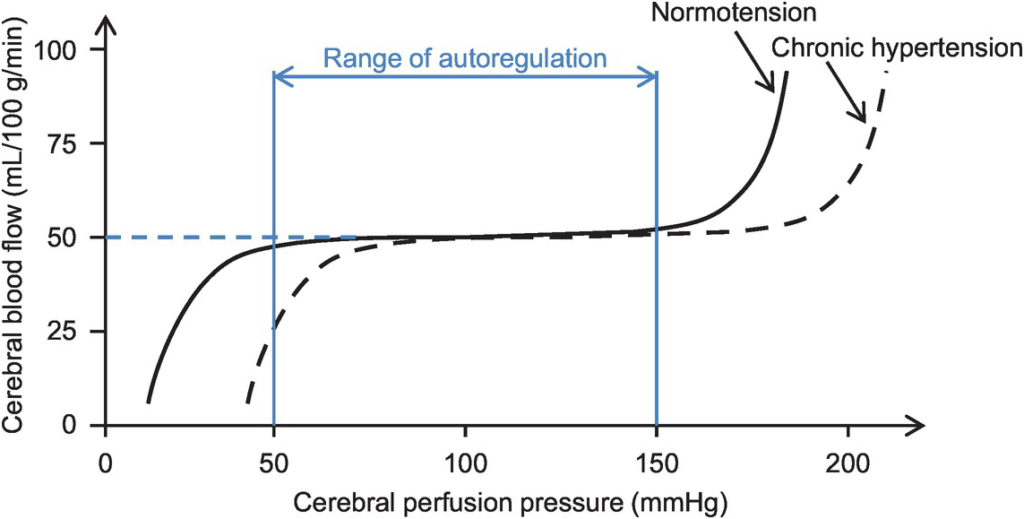
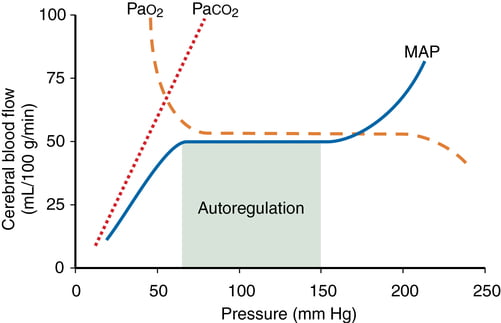
Kerr 2018
CICMWrecks Answer: Effect of Propofol and Ketamine
Effect of Propofol and Ketamine
| Propofol | Ketamine | |
|---|---|---|
| Action | Sedative agent | Dissociative sedative and analgesic agent |
| MoA | Effects on GABAA potentiation and Na channel blockade | Effects on NMDA receptor antagonism |
| CBF | ↓ (dose-dependant) | ↑ |
| CMRO2 | ↓ | ↑ |
| ↓ CBF ∝ ↓ CMRO2 | ↑ CBF > ↑ CMRO2 | |
| SjvO2 | minimal change | ↓ |
| doesn’t affect the autoregulatory curve of CBF and the PaCO2 response |
Kerr 2018
Examiner Comments
2024A 14: 62% of candidates passed this question.
The first part of this question required a list of the normal values for cerebral blood flow including differential flow to grey and white matter. For the second part, an answer structured around the cerebral perfusion/cerebral vascular resistance formula worked well to ensure breadth. Factors influencing flow include autoregulation of pressure (via the myogenic reflex), metabolic autoregulation and local acidity, arterial levels of CO2 and O2, temperature and a minor role for the sympathetic nervous system. Many answers were augmented effectively with graphs of cerebral blood flow versus CPP, PaO2 and PaCO2. A detailed discussed of ICP and factors effecting this was not required.
2023B 14: 56% of candidates passed this question.
Cerebral Blood Flow is result of CPP/CVR. The brain defends a relatively constant high blood flow via multiple auto-regulatory processes that influence cerebral vascular resistance. This question required an in detail description of the physiological factors that alter CBF. Autoregulation via the myogenic and metabolic mechanisms, the difference between grey and white matter due to metabolic variation, the role of pCO2 and O2, sympathetic nervous system and temperature was expected. A “describe” question requires not only the factors that influence CBF but how and why, so detail is required. Whilst the monroe kellie doctrine does describe changes in blood flow this is only important at extremes of intracranial pressure when these normal autoregulatory mechanisms are exceeded, as such it was not part of the answer to this question.
2021A 20: 19% of candidates passed this question.
Overall, this question was poorly answered with a high failure rate. A good answer gave a normal value, iterated that CBF is held relatively constant by autoregulation, and proceeded to divide factors affecting CBF into categories with an explanation/description of each. Those factors with the greatest influence were expected to have more accompanying information (e.g., pressure/myogenic autoregulation, metabolic). Systemic factors such as MAP, O2, CO2 were expected to be mentioned with detail of the impact (i.e., key values, relationships demonstrated with a description and/or labelled graph). Local factors within the brain such as H+ concentration/pH, metabolic activity (including the impact of temperature, inclusion of mediators, regional variation based on activity & grey versus white matter) were also expected to be mentioned. Few answers mentioned impact of pH change independently of CO2. Few answers mentioned how CO2 changes the pH of CSF and that over time, this impact is buffered/reduces. The role of the sympathetic nervous system was required to be mentioned although not explored in detail (although many answers overstated the importance of the SNS on CBF or gave a simplistic concept such as increased SNS activity increases CBF).
Many answers focussed on descriptions of the Monro-Kelly doctrine and ICP to the exclusion of the aforementioned factors or included detail on factors influencing MAP which were not required (and irrelevant when within the autoregulation range). Many answers were simplistic: e.g., increase MAP increase CPP therefore increase CBF, or by stating CO2/O2 without mentioning a relationship or the limits/patterns of the relationship. Many answers failed to separate the effect of systemic PaO2 and PaCO2 from metabolic autoregulation.
2014A 14: 74% of candidates passed this question.
This question was well answered. It is a structured question that guides candidates through exactly what is required. Well drawn graphs were a particularly effective means of scoring marks.
2011B 16: 12 (44%) of candidates passed this question.
Graphical depictions of the effect of Mean Arterial Pressure, oxygen tension and carbon dioxide tension on cerebral blood flow were common and in general accurate. Mention of factors that affected, and regulation of, the MAP vs CBF graph was expected in order to pass this question well.
The effect of propofol and ketamine on the CBF was well answered. Propofol and ketamine have an opposite effect on cerebral haemodynamic and metabolic rate. Propofol produces a dose dependent reduction in CBF with proportionate reduction in CMRO2, and thus a minimal change in cerebral venous O2 Sat. Propofol doesn’t affect the autoregulatory curve of CBF and the PaCO2 response. Ketamine produce a dose dependent increase in CBF and a mild increase in CMRO2.
Syllabus: C1f 2c, G2a,2a.
Recommended sources: Guyton Textbook of Medical Physiology Chp 61; Goodman and Gilman The pharmacological basis of therapeutics 11th edition pgs 350-352
2009A 11: Pass rate: 50%
Good answers included an equation and then explored the various components of the equation. Main points for a pass included pressure and metabolic autoregulation and the various factors that affect cerebral vascular resistance. Graphs were a useful way to answer this question but were generally underutilised. Several candidates wrote about the Monro-Kellie doctrine which was not directly relevant to the question.
Syllabus C1d2a
Reference: Power and Kam 1st edition p 42-43, Guyton and Hall 11th edition p 761-3
2008A 05: 1 candidate (33%) passed this question
The main points expected for a pass were:
• Description of the relationship of CO2; O2; MAP and Cerebral metabolism with cerebral blood flow. The use of graphs, correctly labelled, and associated free text would be an effective means of portraying this information.
• The effect of other factors such as intracranial pressure, cerebral venous pressure, vascular calibre, blood viscosity and regional blood flow differences.
Syllabus C1f2c
iv. Describe the physiology of cerebrospinal fluid.
2023A 12 – 2017A 24 – 2015A 16 Outline / Describe the physiology of cerebrospinal fluid (CSF).
2020A 16 Outline the formation, circulation and functions of cerebrospinal fluid.
2018B 15 Outline the production / absorption (30% of marks), composition (30% of marks) and function of cerebrospinal fluid (CSF) (40% of marks).
2017B 09 Briefly outline the formation, absorption, distribution, role and composition of cerebrospinal fluid
2008B 06 Describe the formation, circulation and functions of cerebrospinal fluid.
2007B 22 Describe the function, flow and absorption of cerebrospinal fluid.
CICMWrecks Answer
Formation / Production of CSF
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
Distribution / Circulation of CSF
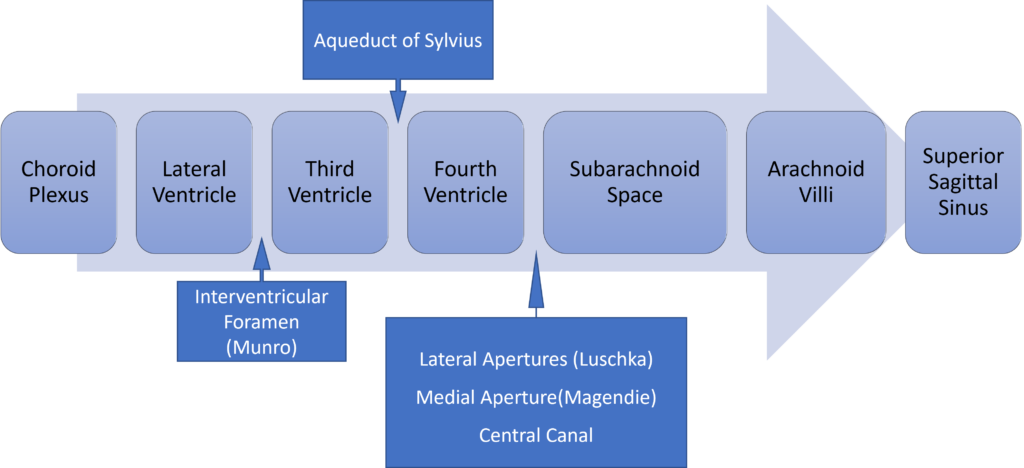
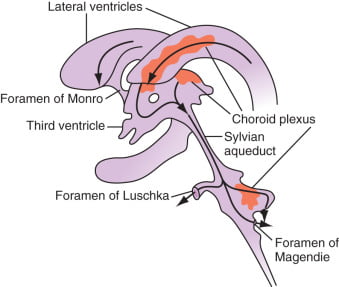
Absorption of CSF
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation

Composition of CSF
IDENTICAL to brain ECF, but differs in several manners from plasma
Compared with plasma:
- ↑ pCO2 (50 mmHg)
- ↓ pH (7.33)
- ↓ protein content
- 0.5% of plasma; 20 mg/dL
- poor acid-base buffering capacity
- ↓ content of glucose (by 60%) and cholesterol
- ↑ [Cl-] (by 7-14%) and ↑ [Mg2+] (by 40%)
- ↓ [K+] (by 40%), ↓ [Ca2+] (by 50%) and ↓ [Pi] (by 20-30%)
- ↑ creatinine (by 25%) but ↓ urea
- IDENTICAL osmolality (295), [Na+] (145), and [HCO3] (25)
Role / Functions of CSF
- Protective role (main function)
- Water bath effect
- Attributed to the low specific gravity of CSF (1.007)
- Causes brain to be buoyant
- ↓ its effective net weight from 1400 g to 50 g
- Mechanical cushion against acceleration/deceleration forces
- Buffer ↑ ICP by CSF translocation to extracranial subarachnoid space
- Abrupt ↑ ICP buffered by translocation of CSF within the vault to extracranial compartments
- Water bath effect
- Maintains constant ionic environment conducive to neuronal electrical activity CSF
- Supply role of nutrients (Eg simple sugars, amino acids) and O2 to brain
- Excretion of toxic substances, metabolic by-products, and CO2 from brain
- “Lymph-type” function → interstitial proteins in brain ECF return to circulation by
- CSF absorption across arachnoid villi
- Acid-base regulation → due to content, CSF allows for tight respiratory control
- Endocrine transport function → transports hormones to other brain regions
Gladwin / JC 2020
Examiner Comments
2023A 12: 88% of candidates passed this question.
This question has been repeated multiple times. Better answers demonstrated a structured approach to discussing the physiology of CSF dividing concepts into; Formation and Composition, Regulation and Circulation, and Functions of CSF with the appropriate level of detail.
2020A 16: 81% of candidates passed this question.
This is a three-part question and was marked as such. The circulation and functions of CSF was generally well answered. Formation of CSF, however, was answered poorly, with many candidates listing its composition instead. The examiners were looking for an understanding of the physiological processes of formation not the composition.
2018B 15: 71% of candidates passed this question.
This question was generally well answered. Better answers noted production including an amount, site and mechanism. Similarly, absorption included the site, the rate and factors which affect the rate. The electrolyte and pH and how they compare to extracellular fluid should have been included in the section on composition.
2017B 09: 44% of candidates passed this question.
The question spelt out very specific areas of CSF physiology to outline and the marks were evenly distributed among these areas. The candidates who did not pass this question usually did not provide enough detailed information.
Details of the production and absorption of CSF were commonly lacking. The majority of candidates correctly described the composition of CSF; indicating whether a particular variable was higher or lower than in plasma, scored less marks than more specific information.
2017A 24: 67% of candidates passed this question.
Better answers included details on CSF production (amount, site), reabsorption and factors which influences CSF and its circulation.
2015A 16: 83 % of candidates passed this question.
Most candidates answered the question well. The most common mistake was incorrect CSF composition. Better answers also discussed raised ICP and CSF’s role in the compensation for raised ICP.
2008B 06: 4 (80%) candidates passed this question
To achieve a pass in this question, candidates needed to state where and how CSF was formed, where it flows to after formation followed by a list of its functions. Additional credit was given for knowledge of rates of production and basic CSF composition. The fact that CSF production is constant whilst its absorption is pressure dependant was often overlooked. Thus candidates were expected to mention that there is ~ 150 ml of CSF in the adult, half within the cranium; about 60-70% of the CSF is formed by the choroid plexuses, the remaining 30-40% by the cerebral vessels lining the ventricular walls; in humans the CSF turns-over ~ 4 times/day; composition is essentially brain ECF; brain ECF normally occupies ~ 15% of brain volume; CSF flows out through the foramina of Magendie and Luschka and is absorbed through the arachnoid villi into the cerebral venous sinuses; absorption, being largely by bulk flow, is proportional to ventricular pressure [at normal pressure ~ 7.0-18.0 cmH2O (mean ~ 11), filtration = absorption, when pressure falls below ~ 7 cmH2O absorption ceases] and CSF Functions [buoyancy, constant metabolic environment, buffers CSF against rapid plasma changes in K+, Ca++, Mg++, transport of chemical messengers, sink for waste disposal].
A number of candidates embarked on long discussions of how CSF pH affects physiology to the exclusion of what was asked for in the question. Many answers did not adequately cover the three components asked for in the question.
Syllabus: CNS 2d
Reference Text: Guyton Chp 61
2007B 22: 2 candidates (29%) passed this question.
The main points expected for a pass were:
- CSF is formed by ultra filtration and secretion
- CSF volumes and turnover
- Flow through the ventricles and subarachnoid spaces
- Absorption through the arachnoid villi
- Relationship between and absorption and pressure
This is not a question about intracranial pressure so no points were given for Munore Kellie doctrine etc, Also no points were given for the functions of CSF. Diagrams need to have the axes labelled correctly.
2019A 11
Describe the physiology of cerebrospinal fluid (CSF) (60% of marks).
Describe the anatomy relevant to performing a lumbar puncture (40% of marks).
2013A 02
Describe the physiology of cerebrospinal fluid (CSF). (70% of marks)
Describe the anatomy relevant to the performance of a lumbar puncture. (30% of marks)
CICMWrecks Answer: Physiology of CSF
Formation / Production of CSF
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
Distribution / Circulation of CSF


Absorption of CSF
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation

Composition of CSF
IDENTICAL to brain ECF, but differs in several manners from plasma
Compared with plasma:
- ↑ pCO2 (50 mmHg)
- ↓ pH (7.33)
- ↓ protein content
- 0.5% of plasma; 20 mg/dL
- poor acid-base buffering capacity
- ↓ content of glucose (by 60%) and cholesterol
- ↑ [Cl-] (by 7-14%) and ↑ [Mg2+] (by 40%)
- ↓ [K+] (by 40%), ↓ [Ca2+] (by 50%) and ↓ [Pi] (by 20-30%)
- ↑ creatinine (by 25%) but ↓ urea
- IDENTICAL osmolality (295), [Na+] (145), and [HCO3] (25)
Role / Functions of CSF
- Protective role (main function)
- Water bath effect
- Attributed to the low specific gravity of CSF (1.007)
- Causes brain to be buoyant
- ↓ its effective net weight from 1400 g to 50 g
- Mechanical cushion against acceleration/deceleration forces
- Buffer ↑ ICP by CSF translocation to extracranial subarachnoid space
- Abrupt ↑ ICP buffered by translocation of CSF within the vault to extracranial compartments
- Water bath effect
- Maintains constant ionic environment conducive to neuronal electrical activity CSF
- Supply role of nutrients (Eg simple sugars, amino acids) and O2 to brain
- Excretion of toxic substances, metabolic by-products, and CO2 from brain
- “Lymph-type” function → interstitial proteins in brain ECF return to circulation by
- CSF absorption across arachnoid villi
- Acid-base regulation → due to content, CSF allows for tight respiratory control
- Endocrine transport function → transports hormones to other brain regions
Gladwin / JC 2020
CICMWrecks Answer: Anatomy of LP
Anatomy:
- Position in sitting or lateral decubitus position
- Level:
- Any of the interspaces between between L2-L5
- L4/5 interspace: Tuffier’s Line: line between iliac crests
- (or) L3/4 interspace: Line joining PSISs (Posterior superior iliac spines)
- Discrepancy between identified and actual intervertebral space in 50% of cases
- Conus medullaris ends at L1 in about 94% of patients
Tissues and target for LP:
- In the subarachnoid space between the arachnoid mater and the pia mater.
- The tissues pierced are (in order):
- skin
- subcutaneous tissue
- supraspinal ligament,
- interspinal ligament,
- ligamentum flavum,
- dura mater,
- the arachnoid mater into the subarachnoid space.
- Lateral/paraspinal approach: Skin – subcutaneous tissue – erector spinae muscles – ligamentum flavum – dura – arachnoid – subarachnoid space
Epidural Space
- Posterolateral Epidural Space
- Posterolateral epidural space extends vertically down the spinal canal and contains arteries, venous plexus, and fat
- Posterolateral epidural space is larger than the anterior epidural space
- Posterolateral epidural space is larger in the sacral region than it is in the cervical region
- Anterior Epidural Space
- Anterior epidural space is a virtual space under normal circumstances (due to adherence of dura to bone of vertebral bodies from the foramen magnum down to L1)
Gladwin / JC 2020
Examiner Comments
2019A 11: 86% of candidates passed this question.
Better answers had a structure with headings such as function, formation, circulation, absorption and composition with dot point facts under each heading. The second part of the question lent itself to a diagram with labelling which scored well. Precise surface anatomy and mentioning all layers from the skin to the sub-arachnoid space scored well.
2013A 02:
Most candidates performed well in this question. The physiology of cerebrospinal fluid (CSF) required candidates to write about CSF formation, circulation and absorption, compare the composition of CSF to plasma and describe normal volumes and pressures. The functions of CSF also need to be listed. Some candidates described the displacement of CSF when intracranial pressure rises as a function of CSF. No marks were given for this.
The best approach to the anatomy of a lumbar puncture was to describe the lumbar intervertebral space at which the lumbar puncture is done and then describe the anatomical structures that the needle would traverse from the skin to the subarachnoid space. Mentioning the indications for a lumbar puncture was not required.
2015B 07
Draw and label a cross section of the lumbar epidural space (50% of marks).
Describe the pharmacology of bupivacaine (50% of marks).
CICMWrecks Answer: Anatomy of LP
Anatomy:
- Position in sitting or lateral decubitus position
- Level:
- Any of the interspaces between between L2-L5
- L4/5 interspace: Tuffier’s Line: line between iliac crests
- (or) L3/4 interspace: Line joining PSISs (Posterior superior iliac spines)
- Discrepancy between identified and actual intervertebral space in 50% of cases
- Conus medullaris ends at L1 in about 94% of patients
Tissues and target for LP:
- In the subarachnoid space between the arachnoid mater and the pia mater.
- The tissues pierced are (in order):
- skin
- subcutaneous tissue
- supraspinal ligament,
- interspinal ligament,
- ligamentum flavum,
- dura mater,
- the arachnoid mater into the subarachnoid space.
- Lateral/paraspinal approach: Skin – subcutaneous tissue – erector spinae muscles – ligamentum flavum – dura – arachnoid – subarachnoid space
Epidural Space
- Posterolateral Epidural Space
- Posterolateral epidural space extends vertically down the spinal canal and contains arteries, venous plexus, and fat
- Posterolateral epidural space is larger than the anterior epidural space
- Posterolateral epidural space is larger in the sacral region than it is in the cervical region
- Anterior Epidural Space
- Anterior epidural space is a virtual space under normal circumstances (due to adherence of dura to bone of vertebral bodies from the foramen magnum down to L1)
Gladwin / JC 2020
CICMWrecks Answer: Pharmacology of Bupivacaine
Examiner Comments
2015B 07: 35% of candidates passed this question.
It was expected answers would include a diagram of a cross section and label the lumbar epidural space and the key landmarks namely dura, subarachnoid space, epidural space. Most candidates were able to give a schematic representation even if not being able to draw. Some candidates confused the subdural space with the epidural space. Pharmacology of bupivacaine needed to cover both pharmacokinetics and pharmacodynamics. Several candidates addressed only one of these components and so missed the opportunity to score marks.
v. Describe the major sensory and motor pathways (including anatomy) from the periphery to cortex.
2022B 03
Briefly outline the major somatosensory pathways of the body (excluding cranial nerves)
CICMWrecks Answer
- Somatic senses: touch, proprioception, pain, temperature
- Somatosensory fibers:
- Non-myelinated fibers (type C): slowest; sense burning pain, hot temperature
- Small myelinated fibers (type Aδ): faster; sense sharp pain, gross touch, cold temperature
- Large myelinated fibers (type A-α; A-β): fastest; sense proprioception, vibration, fine touch
SOMATOSENSORY PATHWAYS
- Carry somatosensory input up spinal cord to brain
- Consist of 4-neuron relay
- 1st order neuron/afferent sensory neuron: has sensory receptors, converts stimuli into impulse
- 2nd order neuron: cell body in spinal cord or brainstem, synapses with 3rd-order neuron
- 3rd order neuron: cell body in thalamus, sends signal to somatosensory cortex
- 4th order neuron/cortical neuron: cell body in sensory cortex
- Includes medial lemniscal/posterior pathway, spinothalamic/anterolateral pathway
MEDIAL LEMNISCAL PATHWAY
- Carries information about fine touch, proprioception
- Large myelinated fibers of 1st order neurons run to spinal cord
- Neurons run through posterior/dorsal funiculus of spinal cord
- Via cuneate fascicle for arms, chest
- Via gracilis fascicle for trunk, legs
- 1st, 2nd order neurons synapse in medulla
- 1st synapse
- 2nd order neurons run to medial lemniscus, decussate; run through pons, midbrain to the thalamus
- 2nd, 3rd order neurons synapse in thalamus
- 2nd synapse
- 3rd order neurons run to sensory cortex in parietal lobe
- 3rd, 4th order neurons synapse in sensory cortex
- 3rd synapse
- Some 1st order neurons synapse with interieurons at posterior horn
- Axons run to anterior horn, synapse directly with motor neuron
- Important for reflexes
SPINOTHALAMIC (ANTEROLATERAL) PATHWAY
- Carries information about pain, temperature, crude touch
- Small/non-myelinated fibers of 1 order neurons run to spinal cord
- Small myelinated fibers: sharp pain, cold temperature
- Non-myelinated fibers: hot temperature, burning pain, crude touch
- 1st, 2nd order neurons synapse in posterior horn of spinal cord/1st synapse
- Small myelinated fibers: enter through dorsal root, bend upwards, travel through two vertebral segments
- Non-myelinated fibers: follow same pathway but synapse with interneurons first, AKA before reaching posterior horn
- 2nd order neurons decussate, cross to anterior horn through central canal
- Neurons then carried through one of two tracts to thalamus
- Lateral tract: carries information for pain, pressure, temperature through lateral funiculus
- Anterior tract: carries information for crude touch through anterior funiculus
- 2nd, 3rd order neurons synapse in thalamus/2nd synapse
- 3rd order neurons run to sensory cortex in parietal lobe
- 3rd, 4th order neurons synapse in sensory cortex /3rd synapse
SOMATOSENSORY RECEPTORS
- Perceive general somatic senses. Includes:
- Mechanoreceptors: for touch
- Proprioceptors: for proprioception
- Thermoreceptors: for temperature
- Nociceptors: for pain
| MECHANOSENSORS | Meissner/ tactile corpuscles | – Sensitive to light touch – Encapsulated; located in dermis of hairless skin – Fast adapting; small receptive fields |
| Merkel (tactile) discs | – Sensitive to pressure – Non-encapsulated; located in epidermis of hairless skin – Slow adapting; small receptive fields | |
| Ruffini (bulbous corpuscles | – Sensitive to skin stretching – Encapsulated; located in dermis of all skin – Slow adaptingl big receptive fields | |
| Pacinian (lamellar) corpuscles | – Sensitive to vibration – Encapsulated; located deep in dermis/ subcutaneous tissue of all skin – Fast adapting; big receptive fields | |
| PROPRIOCEPTORS | Muscle spindle | – detect when muscle stretched – Located throughout perimysium, AKA connective tissue around muscle cells |
| Golgi tendon organ | – Detect when tendon stretched – Located in tendons close to muscle insertion | |
| Joint receptors | – Detect joint position, motion – Located in joint | |
| THERMORECEPTORS | – Transient receptor potential channels mediate sensations – At extremely cold/hot temperaturs, nociceptors take over | |
| NOCICEPTORS | Thermals | sense extremely cold/hot temperatures |
| Mechanical | sense excess pressure/deformation | |
| Polymodal | Sense combination of both |
Examiner Comments
2022B 03: 35% of candidates passed this question.
Many candidates struggled with this question due to poor structure and limited knowledge with incorrect facts. Good answers were able to outline the various pathways from receptor, through the spinal cord to the higher centres with some detail of each aspect of the pathway whilst highlighting some points of difference between the pathways. For example, information expected regarding the types of receptors involved included, vibration, pain, touch, pressure, thermoreceptors, nociceptors and free nerve endings. Information required for the spinal nerve component would include myelinated versus unmyelinated and linked to the specific receptor, eg myelinated A alpha fibre for assessment of proprioception. As the question specifically asked for more than one pathway those answers describing a single somatosensory pathway failed to score well.
2008B 08
Describe the clinical findings you would expect to see in a patient who underwent acute hemi-section of the spinal cord at the upper thoracic level.
CICMWrecks Answer
Spinal Cord Tracts:
(not a comparison table)
Ascending Tracts
SENSORY
Descending Tracts
MOTOR
Convey sensory info from peripheral sensors to higher centres in brain.
From posterior to anterior:
Carry motor information
- Dorsal (posterior) column:
- fine touch + proprioception + vibration
- crosses at the brain stem
- Spinocerebellar tracts (ant + post):
- Carry proprioceptive info from muscles + joints to cerebellum
- Does not cross
- Lateral spinothalamic tracts
- pain + temperature
- Crosses within 2 vertebral segments
- Anterior spinothalamic tracts
- crude touch + pressure
- Crosses within 2 vertebral segments
- Corticospinal tracts (ant + lateral) “pyramidal tracts”:
- motor function – carry axons of UMN
- crosses at the brain stem
- Relay to alpha-motor neurons (LMN) in ventralnhorn of spinal cord
- Extrapyramidal tracts:
- rubrospinal, tectospinal, vestibulospinal, olivospinal, reticulospinal
- Originate in brainstem nuclei and do not pass through medullary pyramids
- Role in control of posture + muscle tone
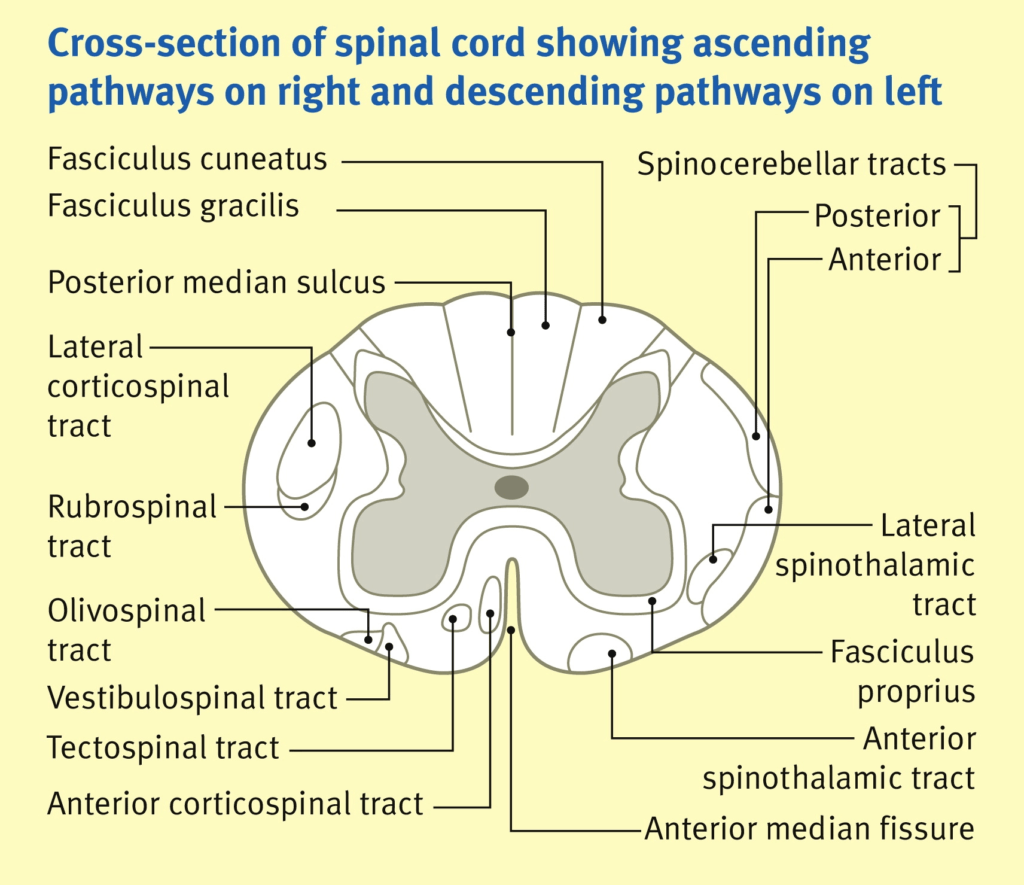
John Craven
Anaesthesia & Intensive Care Medicine.
PAIN. VOLUME 12, ISSUE 1, P26-27, JANUARY 01, 2011
Acute hemi-section of the spinal cord at the upper thoracic level
Brown-sequard syndrome: Hemisection of the cord
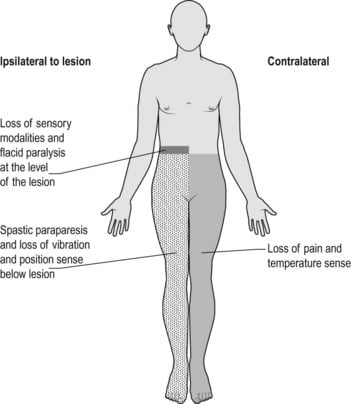
| ipsilateral loss of motor function | below level of lesion | due to interruption of descending fibres in the lateral corticospinal [pyramidal] tracts. Initially the paralysis is flaccid, later it becomes hypertonic and hyperreflexic with extensor plantar response [upper motor neurone lesion]. |
| ipsilateral loss of light touch + proprioception + vibration | below level of lesion | due to interruption of ascending fibres in the posterior [dorsal] columns |
| contralateral loss of pain + temp | below level of lesion | Interruption of ascending fibres in the crossed lateral spinothalamic tract |
| ipsilateral loss of pain + temp | at the level of the lesion | segmental anaesthesia of the dermatome due to damage of the nerve roots and anterior horn cells at this level |
Examiner Comments
2008B 08: 1 (20%) candidate passed this question
The clinical condition that results from this lesion is the so called Brown-Sequard syndrome. However only a very small proportion of points were given to mention of the latter, with the majority of points allocated to knowledge relating to spinal cord anatomy and physiology.
The expected 4 main clinical features that are associated with this lesion are –
1. There is loss of pain and temperature sensation on the contralateral side below the level of the lesion due to interruption of ascending fibres in the crossed lateral spinothalamic tract.
2. There is loss of vibration, joint position and 2 point discrimination on the ipsilateral side below the level of the lesion due to interruption of ascending fibres in the posterior [dorsal] columns.
3. There is paralysis of voluntary movement on the ipsilateral side below the level of the lesion due to interruption of descending fibres in the lateral corticospinal [pyramidal] tracts. Initially the paralysis is flaccid, later it becomes hypertonic and hyperreflexic with extensor plantar response [upper motor neurone lesion].
4. Finally there is segmental anaesthesia of the dermatome at the level of the lesion on the ipsilateral side due to damage of the nerve roots and anterior horn cells at this level.
Some candidates described the clinical features of complete section of the spinal cord which was not asked for.
Syllabus: G1 2b
Reference Text: Guyton Sections IX and XI
2012B 15
Outline the respiratory and cardiovascular consequences of an acute complete spinal cord transection at C6.
CICMWrecks Answer
Cardiovascular
Innervation
- ANS
- Afferents
- Visceral afferent nerves from the heart via the vagal nerve to the NTS
- Baroreceptors via vagal (aortic arch) and glossopharyngeal (carotid sinus)
- Sympathetic system
- Efferents to the heart from the post-ganglionic sympathetic fibres synapsing from T2~5 pre-ganglionic sympathetic fibres
- Positive inotropy, chronotropy, lusitropy and dromotropy
- Efferents to vascular smooth muscle via pre-ganglionic fibres from the thoracolumbar spinal roots synapsing on the sympathetic chain
- Parasympathetic system
- Efferents from the vagal nerve
- Negative inotropy, chronotropy, lusitropy and dromotropy
- Unknown significance of muscarinic innervation of the vasculature
Effects of transection at C6
- Immediate
- Sympathetic stimulation from catecholamine release from adrenal medulla
- Hypertension
- Tachycardia
- Sympathetic stimulation from catecholamine release from adrenal medulla
- Acute
- Afferents from heart and baroreceptors in tact
- Central processing of vasomotor centre in tact
- Efferent sympathetic innervation to heart will be lost
- Resting sympathetic tone of heart lost
- HR will decrease from ~60 to 50bpm
- Baroreceptor response to hypotension will be lost
- No reflex tachycardia or positive inotropy
- Resting sympathetic tone of heart lost
- Efferent sympathetic innervation to vasculature will be lost
- Resting sympathetic tone of blood vessels lost → vaso and venodilatation → decreased MAP and decreased venous return
- Decreased cardiac output
- Decreased baroreflex vasoconstriction in response to hypotension
- Resting sympathetic tone of blood vessels lost → vaso and venodilatation → decreased MAP and decreased venous return
- Efferent parasympathetic innervation in tact (via vagus nerve).
- Parasympathetic tone of resting heart rate will remain
- Reflex negative inotropy and chronotropy in response to hypertension will remain
Respiratory
Innervation
- Afferents from central and peripheral chemoreceptors
- Meduallary respiratory centre
- Sympathetic
- Unknown innervation and significance although β2 adrenoceptors are present within respiratory system
- Postulated response to circulating catecholamine from adrenal medulla
- Parasympathetic
- Vagal innervation of respiratory system causing bronchoconstriction and mucous secretion
- Motor innervation of respiratory muscles
- Phrenic nerve – C3~5
- Diaphragm
- Thoracic spinal nerve roots
- Intercostal muscles
- External (inspiration)
- Internal (expiration)
- Innermost
- Intercostal muscles
- Accessory muscles of respiration
- Accessory nerve innervation of sternocleidomastoid and trapezius
- Brachial plexus
- Pec. major/minor
- Nerve to subclavius
- Serratus anterior
- Thoracic spinal roots
- External, internal, innermost intercostal muscles
- Abdominal wall muscles
- Phrenic nerve – C3~5
Effect of C6 transection
- Sympathetic
- Decreased adrenaline secretion from adrenal medulla → decreased circulating adrenaline → unopposed muscarinic stimulation of lung → increased likelihood of bronchoconstriction
- Vasodilation leading to pooling of blood in pulmonary circulation → decreased lung compliance
- Parasympathetic
- Innervation will remain → bronchoconstriction
- Motor innervation
- Phrenic nerve function in tact
- Diaphragm – Tidal inspiration will largely remain
- Intercostal muscle function lost
- External – Loss of elevation of ribs to increase AP diameter – required in inspiration
- Internal – Loss of lowering of ribs to decrease AP diameter – required in expiration
- Accessory muscles
- Variable effects
- Sternocleidomastoid and trapezius function in tact (forced inspiration)
- Scalenus anterior, medius, posterior in tact (forced inspiration)
- Pec. major and minor in tact (forced inspiration)
- Abdominal muscle function lost
- Required in forced expiration, cough
- Variable effects
- Loss of expiratory reserve volume
- Decreased vital capacity
- Phrenic nerve function in tact
Sakurai 2016
Examiner Comments
2012B 15: 9 (41%) of candidates passed.
The main respiratory consequences of an acute C6 transection include the effects on the inspiratory muscles, the expiratory muscles, lung volumes, effects of changes in posture and effects on gas exchange. Sparing of the phrenic nerve, the main muscle of inspiration (C3 – 5), but paralysis of the external intercostal muscles innervated by thoracic nerve roots results in paradoxical inward movement of the chest wall on inspiration. Paralysis of all the expiratory muscles including the internal intercostal muscles innervated by thoracic nerve roots and the abdominal wall muscles, which are innervated by lower thoric and lumbar nerves. Many candidates did not mention these muscles or their innervation in their answers. While expiration is normally passive these muscles are required for manoeuvres involving forced exhalation like coughing. Forced expiratory lung volumes (FEV1 and FVC) are reduced. Work of breathing is increased. Static lung volumes reveal a restrictive lung defect with most lung volumes decreased but in particular expiratory reserve volume (ERV) is significantly reduced. The reduction in FRC leads to airway closure, atelectasis and pathologic low V/Q and shunt and hence hypoxemia. These mechanisms can result in significant hypoxemia but were not described by many candidates. The second part of the question concerning the cardiovascular consequences of C6 transection was better answered. Areas that required mention in this section included the early massive sympathetic outflow and hypertension via the release of catecholamines from the adrenal medulla. Neurogenic shock is also seen due to interruption of the sympathetic outflow and impaired reflex vasoconstriction secondary to hypotension of any cause. Finally the loss of sympathetic innervation of the heart (T1-T4) results in unopposed parasympathetic cardiac stimulation and bradycardia and bradyarrthymias.
vi. Explain the basic electro-physiology of neural tissue, including conduction of nerve impulses and synaptic function.
2024B 15
(a) Outline the general classification of nerve fibres including details on their function, size and conduction speed (30% of marks).
(b) Describe the mechanisms of action potential generation and propagation along a myelinated peripheral nerve fibre (70% of marks).
CICMWrecks Answer
Classification of Nerve Fibres
(Erlanger and Gasser Classification)
| Type | Appearance | Function | Diameter | Conduction velocity (m/s) |
|---|---|---|---|---|
| A – α | Large peripheral myelinated fibres | Motor | 10-20 µm | 60-120 |
| A – β | Touch, pressure | 5-10 µm | 40-70 | |
| A – γ | Proprioception | 3-6 µm | 15-30 | |
| A – δ | Pain, temperature, touch | 2-5 µm | 10-30 | |
| B | Small myelinated preganglionic ANS fibres in visceral nerves | Preganglionic ANS | 1-3 µm | 3-15 |
| C | Small unmyelinated motor and sensory fibres | Pain/temperature | 0.5-1 µm | 0.5-2 |
Action potential generation and propagation
“Action Potential” (AP) → large rapid change in membrane potential that occurs in excitable cells
- AP = electrical response of neurons and other excitable tissues during which membrane potential rapidly ↑ and ↓
- All or nothing phenomenon
- Allow rapid signalling within excitable cells over long distances
- AP results from brief ↑ in membrane conductance to Na+, followed by slower ↑ in membrane conductance to K+
- Key parameters
- RMP -70mV
- Threshold potential -55mV
- Peak potential (depolarisation) +20-40mV
- Duration of AP 1-2ms
Events of an AP:
- Phase 1 – threshold potential: depolarisation stimulus reaches neuron → CM reaches -55mV → activation of voltage gated Na+ channels → Na+ influx > K+ efflux
- Phase 2 – AP: rapid influx of Na → further depolarisation → +ve feedback → rapid upstroke → drives membrane potential to Nernst potential for Na of ~+50mV → peak potential +30mV
- Phase 3 – repolarisation: AP never reaches theoretical max (+50mV) due to 2 events:
- Inactivation of voltage gated Na+
channels → ↓ membrane permeability to Na+ - Delayed activation of voltage gated K+
channels: ↑ membrane K+ permeability → K+
efflux → membrane potential driven back towards Nernst for K+ of ~-90mV - Membrane potential briefly more –ve than RMP = “after hyperpolarisation” – due to gradual closure of voltage-gated K+
channels
- Inactivation of voltage gated Na+
- Phase 4 – restoration of RMP
- -70mV maintained by: Na/K ATPase (EC gradient) + Na/K pump
Refractory period
- Time following an AP
- 1. Absolute Refractory Period:
- AP cannot be triggered whatever the size of the stimulus
- starts from when voltage-gated Na+ channels open → continues until repolarisation 1/3 complete
- 2. Relative Refractory Period:
- Repolarisation: K leak channels + voltage gated K channels open = K permeability is highest
- AP only with ↑↑stimulus to counteract ↑K+ efflux
- Important for 2 reasons:
- Ensure unidirectional propagation of APs
- Limiting frequency of APs
Saltatory conduction
- Saltatory conduction: propagation of AP along myelinated axons, whereby wave of depolarisation “jumps” from one rode of Ranvier to the next
- Mechanism of saltatory conduction
- Depolarisation of a node → influx of Na ions → creating a sink (area of –ve charge at the surface)
- +ve charge on nodes ahead flows into sink → ↓ polarity inside the membrane → AP → propagating current activates fast Na channels → wave of
depolarisation down axon - minimal electrical signal degradation as axon is insulated by myelin sheath
- AP reaches next node of Ranvier → continues down myelinated fibre
- Nerve impulses appear to rapidly jump from one node to the next
Bianca / Kerr 2016
Examiner Comments
2024B 15: 47% of candidates passed this question.
The first component of this question required a list of the different nerve fibre types including the breakdown of A, B and C fibres along with their primary function, for example motor/sensory/parasympathetic/sympathetic, presence of myelin, size and velocity of conduction.The second component of this question required the mechanism by which an action potential arises and is propagated in a myelinated nerve. This required a detailed overview of electrolyte movement across the neuronal membrane during depolarisation, timing and explanation of the refractory period, the basis for unidirectional movement and the mechanism of saltatory conduction which occurs in a myelinated nerve.
2009A 15
Outline the physiology of excitation and conduction in nerve axons (60% of marks).
List the factors which delay axonal conduction (40% of the marks).
CICMWrecks Answer
Resting Membrane Potential
- Defined as the steady state potential difference (measured in mV) that exists across the cell membrane when the cell is in an unexcited state
- RMP of different excitable tissues varies → due to differing ionic permeability of the
membrane (Ie. opening states of membrane ion channels) in the respective tissues at rest
Myelinated axon: -70 mV
Skeletal muscle cell: – 80 mV
Ventricular myocyte: – 90 mV
Cardiac pacemaker cell: – 60 mV
How is the membrane potential produced?
RMP is generated by uneven distribution of charged particles (i.e. ions and proteins) across the cell membrane 2o to:
- Semi permeable membrane / selective membrane permeability to different ions
At rest CM is:- Slightly permeable to Na: Na channels closed
- Very permeable to K: open K+ leak channels → K down conc gradient from ICP to ECF
- Variable permeability to Cl based on cell type
- Different ionic concentrations of ICF and ECF
- Na+: 140mmol/L ECF; 20mmol/L ICF
- K+: 150mmol/L ICF; 5mmol/L ECF
- Na/K ATPase: 3Na+ out for 2K+ in. Consequences:
- Osmotic effect: ↑ ECF [Na+] balances osmotic effect of ↑intracellular conc of –vely charged protein
- Electrogenic effect: cell interior hyperpolarised
- Gibbs Donnan effect
- Minor contribution to RMP
- unequal distribution of large –vely charged protein impermeable to CM → affects distribution of other diffusible ions (K, Cl) and hence RMP by ~-10mV
Principles
Principles
- Nernst equation
- Nerst potential: voltage difference generated by EC gradient of an ion across CM (assuming complete permeability) i.e. contribution that a single ion makes to RMP
- Calculated from valency, conc difference across membrane, and temp
- The ion with ↑ membrane permeability → Nerst potential has ↑ contribution to total RMP
- Nernst applied:
- RMP has ↑ K permeability → net efflux of +vely charged K down conc gradient
- drives membrane potential towards Nernst potential for K+
- RMP ↓ permeability to Na+ ions
- Therefore: measured neuronal RMP (-70mM) = close to Nernst potential for K+
- Goldman –Hodgkin-Katz equation
- considers all ionic permeabilities and concentrations → RMP more precisely quantified
- Gibbs Donnan effect
Action Potentials
“Action Potential” (AP) → large rapid change in membrane potential that occurs in excitable cells
- AP = electrical response of neurons and other excitable tissues during which membrane potential rapidly ↑ and ↓
- All or nothing phenomenon
- Allow rapid signalling within excitable cells over long distances
- AP results from brief ↑ in membrane conductance to Na+, followed by slower ↑ in membrane conductance to K+
- Key parameters
- RMP -70mV
- Threshold potential -55mV
- Peak potential (depolarisation) +20-40mV
- Duration of AP 1-2ms
Events of an AP:
- Phase 1 – threshold potential: depolarisation stimulus reaches neuron → CM reaches -55mV → activation of voltage gated Na+ channels → Na+ influx > K+ efflux
- Phase 2 – AP: rapid influx of Na → further depolarisation → +ve feedback → rapid upstroke → drives membrane potential to Nernst potential for Na of ~+50mV → peak potential +30mV
- Phase 3 – repolarisation: AP never reaches theoretical max (+50mV) due to 2 events:
- Inactivation of voltage gated Na+
channels → ↓ membrane permeability to Na+ - Delayed activation of voltage gated K+
channels: ↑ membrane K+ permeability → K+
efflux → membrane potential driven back towards Nernst for K+ of ~-90mV - Membrane potential briefly more –ve than RMP = “after hyperpolarisation” – due to gradual closure of voltage-gated K+
channels
- Inactivation of voltage gated Na+
- Phase 4 – restoration of RMP
- -70mV maintained by: Na/K ATPase (EC gradient) + Na/K pump
Saltatory conduction
- Saltatory conduction: propagation of AP along myelinated axons, whereby wave of depolarisation “jumps” from one rode of Ranvier to the next
- Mechanism of saltatory conduction
- Depolarisation of a node → influx of Na ions → creating a sink (area of –ve charge at the surface)
- +ve charge on nodes ahead flows into sink → ↓ polarity inside the membrane → AP → propagating current activates fast Na channels → wave of
depolarisation down axon - minimal electrical signal degradation as axon is insulated by myelin sheath
- AP reaches next node of Ranvier → continues down myelinated fibre
- Nerve impulses appear to rapidly jump from one node to the next
Factors affecting Velocity of conduction
Velocity of conduction dependent on several factors:
- Nerve fibre myelination: AP propogation slow in Unmyelinated fibres
- Axon diameter: ↓ diameter → ↑ resistance to flow → ↓ conduction velocity
- Transmembrane resistance: ↓ resistance → ↑ loss of current flow → ↓ conduction
- Membrane capacitance: ↑ capacitance → longer to alter polarity → ↓ speed of propagation.
- Temperature: ↓ temp → ↓ rate Na+ channel opening → ↓ velocity
- Hyponatremia: ↓ Na+ conc gradient → ↓ velocity
- Hypermagnesemia: → ↓ ACh release → ↓ velocity
- acidosis → ↓ velocity
Bianca / Kerr 2016
Examiner Comments
2009A 15: Pass rate: 30%
The following points were expected to be outlined in this question
1. The resting membrane potential (RMP) and its physiological basis
2. How the RMP changes rapidly after a stimulus e.g. electrical or chemical and reaches a threshold potential and an all or none action potential results
3. The ionic basis of the action potential
4. How the action potential is propagated
5. Factors that delay axonal conduction such as fibre size, myelination and electrolyte abnormalities e.g. hypermagnesaemia.
Syllabus G2a
Reference: Power and Kam 1st edition p 6-9
2015B 18
Describe the stages of sleep (50% of marks).
Describe the respiratory physiological changes that occur in sleep (50% of marks)
CICMWrecks Answer
Stages of sleep
- Slow-wave
- Deep, restful sleep occuring during first hour
- Stage I
- As person drifts to sleep
- α waves replaced by theta waves of low frequency (4~8Hz)
- Stage II
- Occasional bursts of high frequency waves (sleep spindles)
- Stage III
- High voltage, low frequency (1~2Hz) δwaves with bursts of high frequency K complexes
- Stage IV
- Deepest sleep
- High voltage δ waves
- REM
- Rapid Eye Movements
- Occurs every 90 minutes and lasts 30 minutes
- β brain waves resembling alert wakefulness
- Rapid Eye Movements
Respiratory changes during sleep
- Tidal volume
- Declines progressively as slow wave sleep progresses → minimal in REM sleep
- Vt 25% of awake volume during REM sleep
- Respiratory Rate
- Remains relatively unchanged
- Irregular pattern during REM sleep
- Response to pO2 and pCO2 dampened
- PaCO2 increases 3mmHg
- PaO2 decreases 3mmHg
- Upper airway resistance
- Increased
- Especially in REM sleep
- At hypopharynx and soft palate
- Loss of tonic activity of tensor palati
- Increased
Sakurai 2016
Examiner Comments
2015B 18: 29% of candidates passed this question.
Few candidates demonstrated a good knowledge of this topic. Few answers described the EEG changes associated with the stages of sleep. Respiratory changes in sleep were more commonly known though many candidates made no reference to the change in resistance associated with reduction in upper airway tone.
Confusion existed about the tidal volume changes in sleep. The question asked specifically for respiratory changes and marks were not awarded for discussion about cardiovascular or metabolic responses.




Recent Comments