Syllabus (Fourth Edition, 2023)
Topics
J1: Acid Base Physiology
i. Explain the principles underlying acid-base chemistry.
ii. Explain the physiological basis to clinical acid-base disturbances.
iii. Describe the chemistry of buffer mechanisms and explain their roles in the body.
iv. Explain the Henderson-Hasselbach (traditional) and the Stewart (physico-chemical) approach to acid-base.
J2: Acid Base Measurement
i. Interpret normal and abnormal arterial blood gases and differentiate arterial from venous blood gases.
ii. Describe the methods of measurement of pH in blood.
Topics not covered in previous SAQs
J1: Acid Base Physiology
i. Explain the principles underlying acid-base chemistry.
ii. Explain the physiological basis to clinical acid-base disturbances.
iv. Explain the Henderson-Hasselbach (traditional) and the Stewart (physico-chemical) approach to acid-base.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
J1: Acid Base Physiology
i. Explain the principles underlying acid-base chemistry.
2024B 06
Describe the following with respect to lactate: (a) production (40% of marks). (b) metabolism (30% of marks). (c) and its role (30% of marks).
2020A 08
Describe the production, metabolism and role of lactate.
2015B 06
Describe the formation and the metabolic fate of lactate. Outline its role in energy production.
2010A 05
Describe the production and metabolism of lactate.
CICMWrecks Answer
Lactate is the conjugate base of lactic acid (an organic 3-C acid)
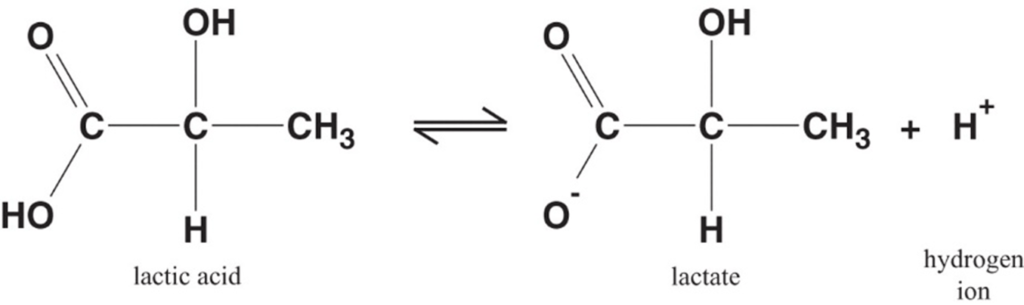
Production:
- It is produced by anaerobic metabolism of pyruvate either:
- Physiologically → in RBC (no mitochondria), renal medulla (↓ PO2), cornea/ lens (↓ PO2) → hence, normal plasma [lactate] is 0.5-2 mmol/L (and NOT zero!)
- Pathologically → reduced tissue perfusion and/or O2 delivery (Eg. shock, hypoxaemia) → thus, plasma [lactate] ↑↑↑ (> 2 mmol/L)
- Plasma [lactate] is 0.5 – 2 mM (and NOT zero) due to physiological production → it can be measured clinically as an indicator of anaerobic metabolism (Ie. ↑ anaerobic metabolism due to pathological situations lead to ↑ [lactate])
Fate of lactate:
- Persistent anaerobic metabolism (Ie. ongoing hypoxia) causes an accumulation of cellular lactate → this diffuses out of the cell into plasma along its [ ] gradient → lactate can then be:
- Used as a fuel source by the heart and brain
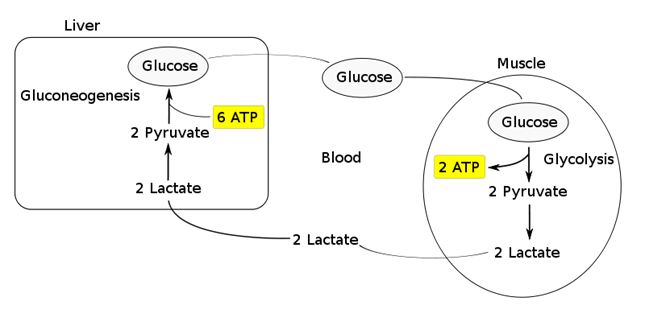
- Transported to the liver where it is:
- Converted back to glucose via gluconeogenesis (requires 6x ATP), which is then transported back peripherally for use → “Cori cycle”
- Converted to pyruvate intermediate → utilised locally in TCA cycle for ATP production via oxidative phosphorylation
- Resolution of hypoxia (Ie. tissue O2 tension restored) → intracellular lactate can be oxidised back to pyruvate for use in local tissue aerobic metabolism (Ie. fed into TCA cycle)
Functions of Lactate:
Lactate Sink:
- Lactate acts as a sink in heart, liver, muscle etc, allowing a period of ongoing ATP production from glycolysis when:
- cells become oxygen deplete
- Kreb’s cycle is inhibited
- Other causes of pyruvate accumulation: circulating catecholamines, exercise, sepsis or lack of mitochondria (RBCs)
Lactate Shuttle:
- Intracellular shuttle:
- Lactate may be shuttle out of:
- mitochoncrial membrane
- peroxisomes
- into cytoplasm of myocytes, neurons, astrocytes
- Ixydised by cytoplasmic LDH to pyruvate, generating NADH for energy use
- Lactate may be shuttle out of:
- Intercellular shuttle:
- Excess lactate, formed within fast-twitch fibres, is transported to other cells within the body with the oxidative capability to metabolize lactate, such as type I (slow-twitch) muscle cells, enhancing their excitability and limiting fatigue
- Furthermore, once in circulation, lactate attaches to red blood cells (RBCs) and is disassociated in the liver where inter-conversion via gluconeogenesis facilitates glucose formation, providing an alternative aerobic energy source.
Lactate as a signaling molecule:
- Redox signaling by intracellular shuttles
- Gene expression
- Increased intracellular levels of lactate can act as a signalling hormone, inducing changes in gene expression that will upregulate genes involved in lactate removal, and stimulates mitochondrial biogenesis.
- Control of lipolysis
- the shuttle regulates FFA mobilization by controlling plasma lactate levels.
- lactate functions to inhibit lipolysis in fat cells through activation of an orphan G-protein couple receptor (GPR81) that acts as a lactate sensor, inhibiting lipolysis in response to lactate
Bianca / JC 2021
Examiner Comments
2024B 06: 36 % of candidates passed this question.
A good description of lactate production should include the sites of production with some estimate of daily production. In addition, it was expected that answers would cover synthesis via glycolysis, pentosephosphate pathway (HMP shunt), the chemical reaction catalysed by LDH along with conversion of NADH to NAD. Lastly an explanation of the mechanisms other than anaerobic conditions that lead to an increased lactate production was required.The expected information for metabolism included an overview of hepatic uptake and the Cori cycle, the role of renal metabolism and excretion and mitochondrial tissue handling.With regards to its role, marks were awarded for a discussion of its use as a fuel for cells (including the myocardium, erythrocytes, astrocytes and skeletal muscle). Lactate’s role as a neurotransmitter and in autoregulation including cerebral vasculature rounded out this section.
2020A 08: 16% of candidates passed this question.
Better answers used the categorisation in the question as a structure for their answer. Many candidates gave a good description of lactate production from glycolysis, increasing with accumulation of NADH and pyruvate, when these are unable to enter Krebs cycle. There were however, many vague and incorrect descriptions as to what lactate is and its physiological role. Many candidates suggested that its presence is abnormal or pathological. Most answers demonstrated a superficial understanding and physiological detail of lactate’s role as an energy currency in times of oxygen debt. Higher scoring candidates often mentioned non-hypoxic causes of pyruvate accumulation which include; circulating catecholamines, exercise, sepsis or lack or mitochondria (RBCs). Mention of the relative ATP production of the two fates of pyruvate was also noted in more complete answers. The Cori cycle was generally superficially described. A key role of lactate is the ‘lactate sink’, allowing a period of ongoing ATP production from glycolysis when cells become oxygen deplete or the Kreb’s cycle is inhibited; few candidates detailed or highlighted this.
2015B 06: 21% of candidates passed this question.
It was expected that the answer would include comments on lactate generation from glucose via pyruvate and the metabolic linkage of nicotinamide adenine dinucleotide (NAD). Lactate regenerates NAD+ (pyruvate is reduced to lactate while NADH is oxidized to NAD+ ). The citric acid cycle and the electron transport chain occur in the mitochondria of cells, and will only proceed in the presence of oxygen.
One molecule of glucose produces 2 ATP anaerobically (pyruvate to lactate) vs 26 aerobically (pyruvate enters TCA cycle) .Total production is about 1500 mmols/day with blood levels resting value of 1–1.5 mmol/L to a peak of 10–15 mmol/L.
Lactate can be used in 3 ways:
1. Conversion to glucose via gluconeogenesis in the liver and release back into circulation (Cori cycle). This is the fate of 80 % circulating lactate from tissues low in oxygen (e.g. exercising muscle with low pO2) or red blood cells (no mitochondria). The production from glucose in RBC’s is the Embden-Meyerhoff pathway.
2. Consumed as a fuel e.g. heart (20% of circulating lactate)
3. Mitochondria and oxygen Oxidation back to pyruvate by well-oxygenated muscle cells, heart cells, and brain cells pyruvate is then directly used to fuel the Krebs cycle (generating 28 mmols ATP)
Lactate generation from muscle is increased with B1 mediated stimulation e.g. from adrenalin. This topic is well covered in Power and Kam Principles of Physiology for the Anaesthetist, 3rd Edition, although some of the details are in several different sections.
Most candidates showed some understanding of the role of glucose in the production of pyruvate to lactate. However, the differential ATP production, the role of NADH availability and how oxygen and the role of mitochondria were involved was less well handled. Better answers described the normal generation of lactate in some tissues (e.g. RBC) and role of muscle and liver in metabolism back to glucose (Cori cycle) and the role of lactate as a metabolic substrate in some organs. Marks were awarded for normal production values and blood levels.
2010A 05: 5 (50%) of candidates passed this question
Lactate is constantly produced from pyruvate via the enzyme lactate dehydrogenase (LDH). Lactate is produced during normal metabolism and in increased quantities during anaerobic metabolism. Candidates were expected to further describe this physiological processes.
A good answered required quantification of lactate production during normal aerobic metabolism, during anaeorobic metabolism, the pathways involved (Glucose to Pyruvate, Pyruvate to Citric Acid cycle in presence of Oxygen, Pyruvate + NADH to Lactate + NAD+ without Oxygen) associated ATP production, site of intracellular production, why red blood cells differ and lactate metabolism (eg oxidation to pyruvate by well-oxygenated muscle cells which is then directly used to fuel the citric
acid cycle conversion to glucose via the Cori cycle in the liver through the process of gluconeogenesis). Good answers illustrated the loss of energy potential with the production of lactate and discussed the situations that would lead to an inbalance between production and metabolism of lactate.
Candidates who did poorly in this question did so due to a lack of depth and breadth for this topic. For example, even though the Cori Cycle was often mentioned, it was poorly described in relation to lactate metabolism.
Syllabus: K2g
References: Textbook of Medical Physiology, Guyton Chp 67
ii. Explain the physiological basis to clinical acid-base disturbances.
2009B 09
A six (6) month old child is diagnosed with a gastric outlet obstruction. Investigations reveal a metabolic alkalosis and a urine pH of 5. Describe the physiological basis of these results.
CICMWrecks Answer
Paradoxical aciduria – Formation of acidic urine in face of alkalosis
Gastric outlet obstruction
- Large volume projectile vomiting
- Hypovolaemia → Contraction alkalosis
- Cl– loss → increased SID → metabolic alkalosis
Compensation
- Respiratory compensation of metabolic alkalosis
- Decreased plasma [H+] causes hypoventilation → CO2 increases
- PaCO2 = 0.7 [HCO3–] + 20
- Renal compensation – Renal handling of HCO3
- PCT
- 85% reabsorbed in PCT via H2O and CO2 (due to carbonic anhydrase)
- In PCT cell, H2O and CO2 reform HCO3– and H+ (via CA)
- H+ secreted into tubular lumen via H+ ATPase
- Increased plasma CO2 due to repiratory compensation of alkalosis, increases intracellular CO2 and causes increased absorption of HCO3 → inhibits HCO3 elimination
- LoH
- 10% reabsorbed
- DCT
- Type B intercalated cells secrete HCO3–
- Via Pendrin antiporter (Secretes HCO3– in exchange for Cl-) on luminal membrane. H+ ATPase on basolateral membrane
- HCO3– eliminated and Cl– reabsorbed leading to decreased SID andcompensation
- However, due to hypochloridaemia from hyperemesis, this mechanism is retarded
- PCT
- Effects due to volume contraction, stress
- RAAS system activated by decreased Na+ and Cl– reaching DCT and macula densa.
- JGA secretes renin → angiotensin → aldosterone
- Aldosterone effect on kidney
- DCT
- Increased Na reabsorption due to upregulation of basolateral Na/K ATPase and luminal ENaC
- Increased Na/K ATPase activity causes increased K+ allowing K secretion → This leads to hypokalaemia
- DCT
- Defense of total body K+
- Total body K+ approx 50mmol/kg.
- K+ in glomerular filtrate reabsorbed in the PCT (65%) and LoH (30%) with remaining DCT accounting for secretion or reabsorption depending on body balance
- With hypokalaemia, K+ reabsorbed by intercalated cells of DCT via H+/K+ antiporter
- Leads to H+ secretion into DCT/Collecting ducts
Sakurai 2016
Examiner Comments
2009B 09: 3 (33%) of candidates passed this question.
Candidates were expected to identify what compounds are lost during vomiting associated with gastric outlet obstruction and that intravascular volume depletion is likely. Furthermore the candidate was then expected to explain that overall the child’s body will defend volume – then tonicity – then acid base status, in this order. An outline of the major physiological defences of volume status (e.g. the renin-angiotensin-aldosterone system), and how these will perpetuate a metabolic alkalosis (prevent the kidney from clearing the excess bicarbonate in the form of an
alkaline urine) was then expected.
A common omission was to not appreciate that the preservation of volume (sodium or chloride) by the kidneys conflicts with the physiological mechanisms that would allow bicarbonate loss through the kidneys’ tubules. Restoration of normovolaemia would allow an ‘alkaline tide’ or bicarbonate loss by the kidneys (alkaline urine production).
Syllabus – G12d G2a
References: Power and Kam. P247-266
iii. Describe the chemistry of buffer mechanisms and explain their roles in the body.
2021A 11
Describe the buffer systems in the body.
2018A 06
Define a buffer (25% of marks). Describe how acid and base shifts in the blood are buffered (75% of marks).
2014A 22
What is a buffer? (10% of marks) Discuss the body’s buffer systems and how they work. (90% of marks)
CICMWrecks Answer
Buffer
- Weakly ionised acid or base in equilibrium with its full ionised salt
- A buffer can “resist” change in pH by absorbing or releasing H+ ions
- Works best when pKa is closest to the target pH (7.4)
- Isohydric Principle
- All buffer systems which participate in defence of acid-base changes are in equilibrium with each other. There is after all only one value for [H+] at any moment. This is known as the Isohydric Principle.
Effectiveness:
- buffer pKa (most effective if pKa = pH of carrying solution)
- pH of carrying solution
- amount of buffer present
- open (physiological) vs closed (chemical) system
Buffer Systems by site
| Site | Buffer System | Comment |
|---|---|---|
| ISF | Bicarbonate | For metabolic acids |
| Phosphate | Not important because concentration too low | |
| Protein | Not important because concentration too low | |
| Blood | Bicarbonate | Important for metabolic acids |
| Haemoglobin | Important for carbon dioxide | |
| Plasma protein | Minor buffer | |
| Phosphate | Concentration too low | |
| ICF | Proteins | Important buffer |
| Phosphates | Important buffer | |
| Urine | Phosphate | Responsible for most of ‘Titratable Acidity’ |
| Ammonia | Important – formation of NH4+ | |
| Bone | Ca carbonate | Important in prolonged metabolic acidosis |
| CSF | Bicarbonate | Important – (as very low proteins for buffering) free Bicarbonate flow in CSF |
| Phosphate | negligible |
Buffer Systems
| Name | Location |
|---|---|
| Protein | Intra cellular |
| Haemo globin | Blood |
| Bicarb | Plasma, Interstitium, Urine (minimal) |
| Ammonia | Urine |
| Phosphate | Urine, Bone |
| CaCO3 | Bone |
Protein (Intracellular)
- pKa of imidazole group is 6.0
- pKa of the histadine residues themselves = 6.8
- Protein buffer ability is proportional to Histadine residue content.
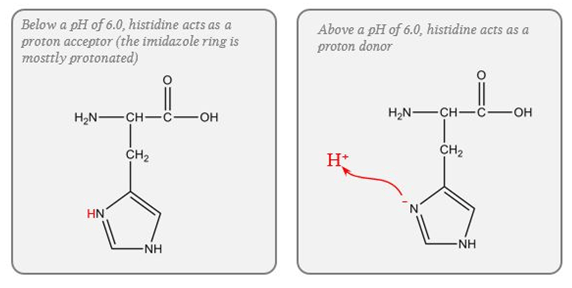
Haemoglobin (Blood)
- Protein buffering system
- Important intracellular buffer in RBC
- Exists as weak acid – HHb and potassium salt KHb.
- Hb a pKa of 8.2 in deoxy form and 6.6 in oxyHb
- Buffering capacity due to imidazole residues on 38 histidine residues on Hb molecule
- pKa of residues 6.8 à close to physiological pH
- Also important in extracellular buffering (following bicarbonate buffer system)
- Due to fast equilibration of HCO3– (Hamburger Shift)
- Band3 Transporter (HCO3–/Cl– antiporter)
- Allows carbonic anhydrase reaction to take place by limiting build-up of HCO3– (la chatelier principle)
- Formed in erythrocytes as a tetramer of 4 subunits
- Hb ~ 6 times the number of histadine (38) residues compared with albumin. (3-6x buffering capacity)
- Hb is in much greater concentrations than any other protein (15 g/dL vs 7 g/dL)
- For each mmol OxyHb, 0.7mmol H+ is buffered and 0.7mmol of CO2 can enter circulation without a change in pH
- Available at high concentrations in RBC
- Isohydric exchange
- the buffer system (HHbO2-HbO2-) is converted to another more effective buffer (HHb-Hb-) exactly at the site where an increased buffering capacity is required
- Deoxyhaemoblobin is a much more effective buffer
- oxygen unloading increases the amount of deoxyhaemoglobin and this better buffer is produced at exactly the place where additional H+ are being produced because of bicarbonate production for CO2 transport in the red cells.
Bicarb (Plasma, Interstitium, Urine (minimal))
- Catalysed by carbonic anhydrase (slow where CA is absent (plasma))
- Open at both ends
- Bicarb regulation at kidneys
- CO₂ regulation at lungs
Ammonia (Urine)
Equlibrium between ammonia and ammonium
Steps:
- Glutamine enters PCT cells
- 20% from filtrate
- 80% from peritubular capillaries
- Ammonia (NH3) produced from glutamine in PCT by glutaminase secreted into lumen
- Reabsorbed in TAL of LoH
- Diffuses into peritubular cells of CD down conc Grad
- H+ secreted into lumen of CD
- Combines with ammonia to form ammonium
- Ammonium positive charge prevents reabsorption
- Thus “excess hydrogen can be secreted with ammonium”
Phosphate (Urine, Bone)
Effect in urine is limited due to:
- Minimal urinary pH is 4.5 → equation B is >99% ionised → of little use.
- Requires filtered PO4 → Normally low levels.
- Depends on Diet and PTH.
- Where H is secreted in distal tubules, there is minimal PO4 excretion
CaCO3 (Bone)
- Important in chronic metabolic acidosis
- Release of calcium carbonate from bone is the most important buffering mechanism involved in chronic metabolic acidosis.
Please Note: This answer is viewed from the Traditional approach to Acid-Base theory. Alternative (physicochemical approaches) deemphasise bicarb due to its equilibrium with water and relatively low concentration compared with the strong ions.
Gladwin / Sakurai / JC 2020
Examiner Comments
2021A 11: 57% of candidates passed this question.
This is a core physiology topic; a detailed knowledge of buffering and the available buffer systems is crucial to ICU practice. A candidate presenting for the first part exam should have a detailed understanding of all aspects of the buffer systems. Higher scoring answers provided both technical details of the buffer systems, the context for their normal function and their relative importance. Efficient answers dealt with the buffers by chemical rather than by site, but many answers categorising buffers by site also scored well. Many low scoring answers simply failed to provide detail, some provided incorrect information. Very few candidates demonstrated an understanding of the isohydric principle.
2018A 16: 45% of candidates passed this question.
Few candidates defined a buffer making it difficult to award 25% of the marks for this question.
The three main buffers in blood should have been described: bicarbonate system, haemoglobin and proteins. The pKa, the buffering mechanism and the capacity of the system should have been described. The Henderson Hasselbach equation was sometimes incorrect. Marks were only awarded for buffers in blood and unfortunately some candidates described non-blood buffers.
2014A: 37% of candidates passed this question.
Candidates who described an overall view of the body’s buffer systems scored well.
Most candidates could define buffer and could discuss the bicarbonate buffer system;
however this was not sufficient to pass the question. To pass there had to also be a discussion of the other buffer systems including phosphate, protein (haemoglobin, plasma proteins and intracellular proteins), ammonia and bone.
Best answers included discussion of open versus closed systems and the relative contribution of the differing buffer systems in blood, intracellular fluid, extracellular fluid and urine.
2011A 19
Explain the role of haemoglobin as a buffer
CICMWrecks Answer
Buffer
- Weakly ionised acid or base in equilibrium with its full ionised salt
- A buffer can “resist” change in pH by absorbing or releasing H+ ions
- Works best when pKa is closest to the target pH (7.4)
- Isohydric Principle
- All buffer systems which participate in defence of acid-base changes are in equilibrium with each other. There is after all only one value for [H+] at any moment. This is known as the Isohydric Principle.
Haemoglobin (Blood)
- Protein buffering system
- Important intracellular buffer in RBC
- Exists as weak acid – HHb and potassium salt KHb.
- Hb a pKa of 8.2 in deoxy form and 6.6 in oxyHb
- Buffering capacity due to imidazole residues on 38 histidine residues on Hb molecule
- pKa of residues 6.8 à close to physiological pH
- Also important in extracellular buffering (following bicarbonate buffer system)
- Due to fast equilibration of HCO3– (Hamburger Shift)
- Band3 Transporter (HCO3–/Cl– antiporter)
- Allows carbonic anhydrase reaction to take place by limiting build-up of HCO3– (la chatelier principle)
- Formed in erythrocytes as a tetramer of 4 subunits
- Hb ~ 6 times the number of histadine (38) residues compared with albumin. (3-6x buffering capacity)
- Hb is in much greater concentrations than any other protein (15 g/dL vs 7 g/dL)
- For each mmol OxyHb, 0.7mmol H+ is buffered and 0.7mmol of CO2 can enter circulation without a change in pH
- Available at high concentrations in RBC
- Isohydric exchange
- the buffer system (HHbO2-HbO2-) is converted to another more effective buffer (HHb-Hb-) exactly at the site where an increased buffering capacity is required
- Deoxyhaemoblobin is a much more effective buffer
- oxygen unloading increases the amount of deoxyhaemoglobin and this better buffer is produced at exactly the place where additional H+ are being produced because of bicarbonate production for CO2 transport in the red cells.
Gladwin / Sakurai / JC 2020
Examiner Comments
2011A 19: 4 (33%) of candidates passed this question.
To pass this question, the candidate only needed to define a buffer (weakly ionised acid or base in equilibrium with its full ionised salt), what it does, then discuss how Haemoglobin functions in this capacity. In that regard, brief review of how CO2 is buffered, the role of haemoglobin histidine residues, buffering capacity of oxy haemoglobin and deoxy haemoglobin and how this contributes to the Haldane effect would have rounded out a very good answer.
Additional credit was given for an understanding that histidine contains an imidazole group and how these groups are effective as a buffer.
Few candidates mentioned that haemoglobin was quantitatively significant and no candidate mentioned that it is the primary buffer for CO2. Many answers were quite brief and did not explore the subject matter asked. Syllabus: B1h, 2c, 2b and Section F
Recommended sources: Nunn’s Applied Respiratory Physiology, Lumb, page 228 to 230
2008B 03
Compare and contrast the body’s bicarbonate, phosphate and protein buffer systems.
CICMWrecks Answer
Buffer
- Weakly ionised acid or base in equilibrium with its full ionised salt
- A buffer can “resist” change in pH by absorbing or releasing H+ ions
- Works best when pKa is closest to the target pH (7.4)
- Isohydric Principle
- All buffer systems which participate in defence of acid-base changes are in equilibrium with each other. There is after all only one value for [H+] at any moment. This is known as the Isohydric Principle.
Effectiveness:
- buffer pKa (most effective if pKa = pH of carrying solution)
- pH of carrying solution
- amount of buffer present
- open (physiological) vs closed (chemical) system
Buffer Systems
| Name | Location |
|---|---|
| Protein | Intra cellular |
| Haemo globin | Blood |
| Bicarb | Plasma, Interstitium, Urine (minimal) |
| Ammonia | Urine |
| Phosphate | Urine, Bone |
| CaCO3 | Bone |
Compare and Contrast:
Bicarbonate, Phosphate, Protein buffer systems
| Bicarbonate | Phosphate | Protein |
|---|---|---|
| H+ + HCO3– ⇌ H2CO3 ⇌ H2O + CO2 | H+ + HPO42- ⇌ H2PO4– | H+ + Prot– ⇌ HProt |
| Extracellular (80% of plasma buffer capacity) | Nil extracellular. Important in tubular fluid | Extracellular (20% of plasma buffer capacity) |
| poor intracellular buffers (poor membrane diffusion of H+, HCO3– (except RBC)) | Some Intracellular buffering | Major Intracellular (60~70% of total body buffering capacity occurs intracellularly and most of this is due to proteins) |
| pKa 6.1 | pKa 6.8 | pKa of histidine residues 6.8 |
| pKa far from physiological pH (7.4) However Good buffer due to abundance of substrates | pKa close to physiological pH However extracellular concentration 1.8 of bicarbonate | pKa close to physiological pH |
| Open ended system – CO2 eliminated by lungs – HCO3 eliminated renally Requires carbonic anhydrase | Becomes important buffer in renal tubules due to H2O reabsorption and consequent concetration of phosphate. | Hb important buffer in blood due to 38 histidine residues per tetramer Deoxyhaemoglobin further increases buffering capacity by increasing pKa to 7.8 |
Gladwin / Sakurai / JC 2020
Examiner Comments
2008B 03: 3 (60%) candidates passed this question
This question was generally well answered. Successful candidates illustrated their answer through the use of a table. For a good pass candidates were expected to include a definition of a buffer; mention the buffering capabilities of the differing buffering systems (bicarbonate, phosphate and protein buffers) in relation to the pKa; the area in the body where they are most effective and whether it was an open or closed system.
The protein buffer system was the least well conveyed by candidates, despite approximately 60 to 70 per cent of the total chemical buffering of the body fluids being inside the cells, and most of this from the intracellular proteins. For a good answer it was expected that candidates also mention that, except for the red blood cells, the slowness with which H+ and HCO3- move through the cell membranes often delays for several hours the maximum ability of the intracellular proteins to buffer extracellular acid-base abnormalities. In addition to the high concentration of proteins in the cells, another factor that contributes to their buffering power is the fact that the pKas of many of these protein systems are fairly close to 7.4.
Syllabus: F2b
Reference Text: Guyton Chp 30
iv. Explain the Henderson-Hasselbach (traditional) and the Stewart (physico-chemical) approach to acid-base.
2023A 01
Outline the abnormalities in the following arterial blood gas (25% of Marks).
Explain the Stewart approach to acid-base interpretation (75% of Marks).
CICMWrecks Answer
Abnormalities in ABG
ABG from question not available
Stewart approach to acid-base interpretation
(Physico-chemical approach)
- Developed to address the criticism that traditional approach is merely a mathematical description of pH and fails to provide any mechanistic insight into rising and falling [H+]
- Peter Stewart (1978) modeled a solution that contained a complex mixture of ions of constant charge over the physiological pH range (strong ions), non-volatile proton donor/acceptors which transfer H+ within the physiological pH range (weak acid/base), and the volatile bicarbonate–CO2 buffer system.
- Key aspect of Stewart’s concept was the classification of each variable as dependent or independent in determining the H+ concentration of the solution.
- Three independent variables that independently determine the dissociation of water, and consequently the [H+] and [HCO3–] to maintain electrical neutrality:
- partial pCO2 of the solution
- Total concentration of weak acids (ATOT)
- Strong ion difference (SID)
- Thus, in the Stewart’s approach, metabolic disorders are the results of changes in SID or ATOT.
PaCO2
- ↑ = respiratory acidosis
- ↓ = respiratory alkalosis
Apparent SID and effective SID
Apparent SID (SIDa): represents the difference between measured strong cations and strong anions
- Normal plasma SID is 42 mEq/L
- ↑SID = Metabolic Alkalosis
- ↓SID = Metabolic Acidosis
SID can be changed by:
- Concentration change
- ↑ H2O: concentrates alkalinity and ↑ SID
- ↓ H2O: dilutes alkalinity and ↓ SID
- Strong ion change
- ↓ Na+: ↓ SID and acidosis
- ↑ Na+: ↑ SID and alkalosis
- ↑ Cl-: ↓ SID and acidosis (~NAGMA, for e.g with Normal saline)
- ↑ organic acids with pKa <4 (lactate, formate, ketoacids): ↓SID and acidosis (~HAGMA)
- ↑ ↓
Effective SID (SIDe): calculated to account for electrical neutrality. Sum of bicarbonate and weak acids (Albumin and phosphate).
SIG
- Gap between SIDa and SIDe due to failure to measure the concentration of all strong and weak ions in plasma
- quantifies [unmeasured anions] – [unmeasured cations] of both strong and weak ions
- theoretical advantage over AG due to pure representation of unmeasured ions
- Unmeasured anions in AG: Mg,Ca,Alb,phosphate,lactate and other ions
- Unmeasured anions in SIG: just other ions
- normal AG 8 to 12, whereas SIG closer to zero in normal situations
- ↑SIG = ↑ unmeasured anions
- ↓SIG = ↑ unmeasured cations
ATOT
- represents all non-bicarbonate buffers
- is made up of mainly serum albumin and other minor charges such as phosphate and globulins
- ↑ATOT = Metabolic acidosis
- ↓ATOT = Metabolic alkalosis
Advantages and Disadvantages
- Advantages
- provides physico-chemical basis and mechanistic insight into rising and falling [H+]
- Pure representation if unmeasured ions
- Diminishes importance of [HCO3-] which is just a dependent variable
- Better explanation of some phenomenon such as acidosis from Normal Saline
- Disadvantages
- Complex calculation, cannot be used bedside quickly
- Substantially different from well-validated classical approach
- Only reflects plasma (base excess reflects whole body and influence of Hb)
- Unclear clinical correlation, not extensively validated
- Higher chance of error due to numerous variables
Examiner Comments
2023A 01: 21% of candidates passed this question.
High performing answers correctly outlined the ABG findings including consideration of electrolyte abnormalities, A-a gradient, acid-base disturbance (including anion gap and strong ion difference) and whether compensation was appropriate. The best explanations of the Stewart approach described its physicochemical basis, discussed the independent variables (strong ions, total weak acids, and pCO2) in detail, and described their effect on the dependent variables and how they result in acid-base derangements.
The ABG provided depicted an incorrect base excess with an omission of (-) symbol. Candidates were marked accordingly depending on their response to this and all candidates were compensated equally for the confusion that this may have caused.
J2: Acid Base Measurement
i. Interpret normal and abnormal arterial blood gases and differentiate arterial from venous blood gases.
ii. Describe the methods of measurement of pH in blood.
2022B 16 – 2014B 22
Describe how the values for PaO2, PaCO2, pH and bicarbonate are determined on a blood gas sample.
CICMWrecks Answer
Blood Gas: pO2
- Measured using Clark Electrode
- Platinum cathode
- Coated in gas permeable membrane à permeable to O2
- Membrane impermeable to other oxidizing molecules
- O2 + 4e– → 2O–
2O– + 2H2O → 4OH–
- Silver chloride anode
- 4Ag → 4Ag+ + 4e–
4Ag+ + 4Cl– → 4AgCl
- 4Ag → 4Ag+ + 4e–
- Phosphate buffer with KCl
- Platinum cathode
- Cathode voltage potential to -0.65v
- In absence of O2 current = 0v
- In presence of O2, O2 diffuses through gas-permeable membrane and is reduced, absorbing 4e–
- Micro-ampmeter measures movement of electrones between anode and cathode
- The current produced is proportional to the pO2 of the test solution
- Sensitivity – related to thickness of membrane and size of cathode area
Blood Gas: pCO2
- Measured using Stowe-Severinghaus Electrode
- Modified pH electrode with outer semi-permeable membrane (Teflon or Silicon Elastic)
- CO2 diffuses into electrolyte layer
- CO2 + H2O → H2CO3 → H+ + HCO3+
- H+ diffuses across glass electrode and alter pH
- Change in pH measured
- Henderson-Hasselbach Equation used to determine pCO2
Blood Gas: pH
- Measured using Sanz Electrode
- 2 half-cells connected by KCl bridge
- Measurement half-cell contain glass membrane with layers of hydrated and non-hydrated glass, permeable or sensitive to H+
- Measurement electrode consists of Silver-Silver chloride bathed in phosphate buffer at known pH 6.84
- Reference half cell contains mercury-mercury chloride in KCl saturated solution
- Measuring electrode responds to the H+ in the sample and the difference between reference and measurement cells measured by a voltmeter or pH meter
- At 37 degrees – change in one pH unit alters voltage by 61.5mV
Blood Gas: Bicarbonate
- Calculated from pH and CO2 using Henderson-Hasselbach Equation
- Actual bicarbonate
- Aerobically drawn arterial sample
- Standard bicarbonate
- Bicarbonate level in an oxygenated plasma specimen at 37 degrees and a pCO2 of 40mmHg
Sakurai 2016
Examiner Comments
2022B 16: 36% of candidates passed this question.
This question about how PaO2, PaCO2, pH, and HCO3 are obtained was not well answered by most candidates. Arterial blood gasses are routinely performed in most ICU on a daily basis. This question relates to a Level 1 (L1) topic in the CICM First Part Syllabus. Most answers simply lacked enough information. Details of how the Clark, Severinghaus, and Sanz electrode’s function was expected. Many candidates confused the pH and PaCO2 electrodes and confused the Clark (Polarographic) electrode with a Fuel Cell. Some knowledge about the types of electrodes and chemical reactions (e.g. reduction of O2 at the Platinum cathode in the Clark electrode) occurring in these devices was expected.
2014B 22: 23 % of candidates passed this question
A correct description of the Clarke electrode, Severinghaus and the pH electrode was expected to attain a pass. Candidates who used correct depictions of these electrodes with annotated description attracted higher marks. Most candidates didn’t comment on the temperature correction and standardization to 37 degrees. There was partial understanding on the calculation of HCO3 by most candidates. In general the question was poorly answered considering the wide spread use of blood gas analysis.
VIVAs
| 2023B | pH, importance, measurement |
| 2023A | |
| 2022B | ABG Interpretation (pH 7.3 pO2 400 pCO2 30 HCO3 14 BE -8 Na 140 K 4 Cl 120) ABG Interpretation (pH 7,3 pCO2 19 pO2 79 HCO3 9 BE -16.4 Na 133 K 4.8 Cl 104 Lactate 7.5, FiO2 30% Temp 37.5) |
| 2022A | ABG Interpretation (fiO2 0.5, pH 7.1, pCO2 25, pO2 100, HCO3 7 BE -19.9 Na 133 K 4.5 Cl 105 AG 25 Lactate 7) |
| 2021B | pH, measurement |
| 2021A | ABG interpretation (pH 7.4 BE -20 HCO# 8 Na 145 K 4.4 Cl 113) Acid, how much produced daily |
| 2020B | Henderson Hasselbach equation, Albumin ABG Interpretation (pH 7.3 pO2 400 pCO2 30 HCO3 14 BE -8 Na 140 K 4 Cl 120) |
| 2019B | ABG interpretation (pH 7.50 pCO2 30 pO2 55 HCO3 22, BE -2), on 6L HM ABG interpretation (pH 7.27 pCO2 55 pO2 144 HCO3 24, BE 1), CO2 Buffer systems Acid base balance, anion gap |
| 2019A | Acids and bases |
| 2018B | |
| 2018A | |
| 2017B | Arterial blood gases and oxygen measurement. Hypoxia vs hypoxaemia |
| 2017A | acid/base physiology, H-h equation |
| 2016B | |
| 2016A | buffer systems in the body and the renal handling of acid. Pharmacology, LA. |
| 2015B | knowledge of blood gas interpretation and insulin physiology |
| 2015A | |
| 2014B | |
| 2014A | |
| 2013B | |
| 2013A | physiology of acid/base and the role of the kidney in acid/base balance, H-h equation |
| 2012B | |
| 2012A | |
| 2011B | |
| 2011A | |
| 2010B | |
| 2010A | |
| 2009B | |
| 2009A | |
| 2008B | |
| 2008A | |
| 2007B |

Recent Comments