Syllabus (Fourth Edition, 2023)
Topics
I1: Physiology of Body Fluids and Electrolytes
i. Explain the composition, distribution, and movement of body fluids.
ii. Define osmosis, colloid osmotic pressure and reflection coefficients and explain the factors that determine them.
iii. Describe the distribution, regulation and physiological importance of sodium, chloride, potassium, magnesium, calcium, and phosphate ions.
iv. Outline the composition, circulation, and functions of lymph.
I2: Intravenous Fluids
i. Understand the pharmacology of colloids and crystalloids.
I3: Measurement of Body Fluids
i. Describe the regulation of osmolality and outline its measurement.
ii. Describe the principles of estimating body fluid compartments.
Topics not covered in previous SAQs
I1: Physiology of Body Fluids and Electrolytes
ii. Define osmosis, colloid osmotic pressure and reflection coefficients and explain the factors that determine them.
iv. Outline the composition, circulation, and functions of lymph.
I3: Measurement of Body Fluids
i. Describe the regulation of osmolality and outline its measurement.
ii. Describe the principles of estimating body fluid compartments.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
I1. Physiology of Body Fluids and Electrolytes
i. Explain the composition, distribution, and movement of body fluids.
2022B 11
Describe the body fluid compartments.
CICMWrecks Answer
DEFINITION
Body fluid compartment is defined as collections of physiologically significant fluid characterised as →
(i) being easily defined, largely separate from another compartment by some form of physical barrier
(ii) having a similar composition within the compartment
(iii) behaving predictably to certain fluid interventions
DISTRIBUTION OF TOTAL BODY WATER (TBW)
- TBW is 60% of body weight (42 L in 70 kg ♂ adult) → BUT this varies according to:
- Body fat content – ↑ fat content → ↓ %TBW of body weight
- Age – Aging causes ↓ %TBW of body weight (due to ↑ fat content)
- Gender – ♀ TBW is 55% body weight (due to ↑ fat content)
- TBW is broken into various “body fluid compartments” as per the table below:
| COMPARTMENT | % TBW | Volume (L) | % Body Weight | |
|---|---|---|---|---|
| TBW | 100% | 42 L | 60% | |
| ICF | 55 | 23 | 33 | |
| ECF | 45 | 19 | 27 | |
| ECF Components | Interstitial Fluid | 20 | 8.4 | 12 |
| Plasma Volume | 7.5 | 3.2 | 4.5 | |
| Dense CT H2O | 7.5 | 3.2 | 4.5 | |
| Bone H2O | 7.5 | 3.2 | 4.5 | |
| Transcellular fluid | 2.5 | 1 | 1.5 | |
Composition
| ICF | ECF | |
|---|---|---|
| Consists of all fluids within the cell membrane | consists of all fluids outside the cell membrane | |
| % TBW | 55% (23L) | 45% (19L) |
| % Body Weight | 33% | 27% |
| Mainly | Mainly K+ (150 mmol/L), PO43- (100 mmol/L) and proteins (+++) | Mainly Na+ (140 mmol/L) and Cl- (100-105 mmol/L) |
| Smaller amounts | Na+ (10 mmol/L) Cl- (3 mmol/L) HCO3- (10 mmol/L) Mg2+ (0.5 mmol/L) Ca2+ (< 0.01 mmol/L) | HCO3- (27 mmol/L) K+ (3.5-5 mmol/L) Ca2+ (1.1 mmol/L) PO43- (1.1 mmol/L) Mg2+ (0.5 mmol/L) protein (+) |
| Osmolality | 290 mosm/kg H2O | 290 mosm/kg H2O |
| does NOT exist not a single united fluid compartment → exists as 1014 discrete separate packets (cells) of solution |
Extracellular fluid (ECF) Sub-compartments
| Compartment | TBW | %Bdy Wt | |
|---|---|---|---|
| Interstitial fluid | 20% (8.4L) | 12% | Fluid that bathes all cells in body and links their ICF with Plasma volume (PV) |
| role in transfer of metabolic substrates (O2/nutrients), waste products and chemical messengers | |||
| Similar composition to PV → BUT very ↓ protein composition cf. PV | |||
| Includes “lymph” → role in returning excess ISF and protein to circulation | |||
| Plasma fluid | 7.5% (3.2L) | 4.5% | Role in transport function within body (Ie. of metabolic substrates, waste products, chemical messengers) → relies on high “bulk flow” |
| Similar composition to ISF → BUT ↑↑↑ protein content | |||
| Dense Connective tissue (CT) H2O | 7.5% (3.2L) | 4.5% | |
| Bone H2O | 7.5% (3.2L) | 4.5% | |
| Transcellular fluid | 2.5% (1L) | 1.5% | Diverse group of small fluid collections |
| Eg. CSF, bile, joint fluid, aqueous humour, bowel fluid, bladder urine, body cavity fluids, Etc. | |||
| This type of fluid is specially characterised by: – having special physiological roles in body – being in contact with ICF across an epithelial cell membrane (rather than ISF), – and being formed by specific cellular transport activity in epithelial-lined spaces |
There are two groups of ECF:
- “Functional ECF” (PV + ISFV → 27.5% TBW)
- Kinetically active (fast) ECF → vital in determining compartment distribution of acutely infused fluids
- Explains 2:1 ECF:ICF ratio with acute IVF intervention (as functional ECF:ICF ratio, rather than total ECF:ICF ratio which is 55:45)
- “Non-functional ECF” (TCF + bone H2O + dense CT H2O → 17.5% TBW)
- Kinetically inactive (slow) ECF → minor role in determining compartment distribution of acutely infused fluids
Blood volume
- Blood volume (5 L) consists of BOTH ECF and ICF compartments → PV from ECF (3.2 L) and red cell volume from ICF (1.8 L)
- Blood volume = PV (from ECF) + red cell volume (from ICF)
Factors controlling distribution of TBW
- All body fluid compartments are isotonic (or iso-osmotic in terms of “effective”
osmoles) as H2O easily and rapidly moves (via osmosis) across cell membranes - Distribution of TBW in body fluid compartments is due to various factors (see
below) that cause H2O to shift across membranes into a specific compartment
TBW distribution between ICF and ECF
→ determined by ECF content of Na+ because:
- ECF Na+ content is main determinant of ECF osmolality → this is because
Na+ and its associated anion (Cl-) are the main osmotically active solutes in ECF (90% ECF osmolality) → but since ∆ in ECF Cl- content occur 2° to ∆ in ECF
Na+ content, so ECF Na+ content is the true determinant of ECF osmolality- Remember → ECF Na+ is controlled by arterial, venous and cardiac baroreceptors that measure ECFV (which is determined by ECF Na+)
- ECF osmolality determines TBW distribution between ECF and ICF → this is
because cell membranes are H2O-permeable and thus ∆ ECF osmolality cause
H2O movement across it via “osmosis” until both ICF and ECF osmolalities
equalise (Ie. hypertonic ECF draws H2O out from ICF until ICF/ECF osmolality
are equal)- Remember → Semi-permeable membrane (permeable to H2O but impermeable to most solutes) allows osmotic gradients to develop on both sides of the membrane.
“Osmosis” is a process where H2O passively diffuses across the membrane from ↓ to ↑ osmolality until osmolality is same on both sides of the membrane
- Remember → Semi-permeable membrane (permeable to H2O but impermeable to most solutes) allows osmotic gradients to develop on both sides of the membrane.
TBW distribution between ISF and intravascular compartments
→ determined by “Starling forces” → balance of PHYDROSTATIC and PONCOTIC across the capillary membrane determines net ultrafiltration of H2O into ISF:
- Hydrostatic pressure gradient (PIV – PISF) → PIV at arterial end and venous end of capillary is 35 mmHg and 15 mmHg, respectively, while PISF is 0 mmHg → thus, a PHYDROSTATIC gradient exists favouring net H2O filtration into ISF (decreasing from 35 mmHg at start of capillary to 15 mmHg at the end)
- Oncotic pressure gradient (πIV – πISF) → plasma colloids (esp plasma proteins) cannot cross capillary membrane into ISF, so [protein] in capillaries is >> ISF (Ie. 80 vs 20 g/L) → πIV >>. πISF (28 mmHg vs 3 mmHg) → constant but small net oncotic pressure gradient throughout capillary length favouring H2O retention in intravascular space
- KF → = capillary surface area (↑↑↑) and its hydraulic permeability (membrane is semi-permeable → permeable to H2O and most solutes EXCEPT large proteins)
- σ → Reflection coefficient
- Thus → NFP at arterial end of capillary is +10 mmHg (favouring filtration in interstitial space) while at venous end it is – 10 mmHg (favouring reabsorption in capillary) → there is net 2 mL/min fluid filtered into interstitial space
TBW distribution between ECF sub-compartments
→ determined by:
- Starling forces → balance of PHYDROSTATIC and PONCOTIC across the membrane determines ultrafiltration of H2O across compartments (as above)
- Active and passive transport of solute across the membrane (Eg. passive diffusion, facilitated transport, active transport) → influences osmotic gradient across the membrane and causes osmosis of H2O between compartments
- Gibbs-Donnan effect → non-diffusible ion in one compartment causes equal concentration ratios of diffusion ions across the membrane at equilibrium → results in ↑ osmotic pressure in compartment with non-diffusible ion → leads to osmosis of H2O into it
Source: Bianca’s notes
Examiner Comments
2022B 11: 25% of candidates passed this question.
This question was answered poorly. Many answers provided incorrect facts relating to the body fluid compartments, their approximate sizes, the factors that regulate and contribute to those sizes and their constituents. Total body water was often incorrectly calculated and associated with limited explanation as to the factors that may affect it.
2022A 08 – 2021B 01
Describe the regulation of body water.
CICMWrecks Answer
H2O
Body H2O content (or TBW state) is determined by the body’s H2O balance (daily H2O
intake vs loss) → normally, it is balanced (as per table below):
| Daily H2O Intake | |
|---|---|
| Drinking | 1200 ml |
| Food | 1000 ml |
| Metabolism (Eg. ETC) | 350 ml |
| Total Intake | 2550 ml/day (in 70kg adult) |
| 25-35 ml/kg/day | |
| Daily H2O loss | |
|---|---|
| Urine | 1500 ml (includes obligatory loss ~ 430ml) |
| Insensible losses (skin, lungs) | 900 ml |
| Faecal | 100 ml |
| Sweat | 50 ml |
| Total loss | 2550 ml/day |
- Note: Abnormal TBW states arise when an imbalance in body H2O exists:
- ↓ TBW (“H2O deficit” → due to H2O loss > intake) → results in ↑ plasma osmolality due to a relative ↑ plasma [Na+] → associated with ↓ ECFV (and PV)
- ↑ TBW (“H2O excess” → due to H2O intake > loss) → results in ↓ plasma osmolality due to a relative ↓ plasma [Na+] → associated with ↑ ECFV (and PV)
Control of TBW
TBW state is controlled via –ve feedback system as follows:
Sensors
- Osmoreceptors (anterior hypothalamus)
- Responds to ↑ plasma osmolality. Very sensitive (detects 1% change) → threshold for stimulation is 280 mosm/kg (near lower normal limit) → steep linear rise in response > 290 mosm/kg
- Low-pressure baroreceptors (right atrium and great vessels)
- Responds to ↓ plasma volume indirectly by ↓ CVS PHYDROSTATIC (↓ MAP) → ↓ sensitive cf. osmoreceptors (detects 5-10% ∆ in PV)
- High-pressure baroreceptors (carotid sinus and aortic arch)
- Responds to ↓ plasma volume indirectly by ↓ CVS PHYDROSTATIC (↓ MAP) → Even ↓ sensitive cf. osmoreceptors (detects > 10% ∆ in PV → large H2O deficits) → BUT its response overrides that of the osmoreceptors!
Effectors
Hypothalamus integrates afferent signals from these sensors and modulates an
appropriate effector response that includes:
- Thirst response → triggered by:
- ↑ plasma osmolality
- ↓ plasma volume (or ↓ MAP)
- AT-II (acting on circumventricular organs (SFO/OVLT)
- ADH
- 9 a.a peptide hormone synthesised in hypothalamus (SON/PVN) → transported to posterior pituitary where it is secreted by:
- ↑ plasma osmolarity (main trigger)
- ↓ plasma volume (or ↓ MAP) → note that LARGE ∆ in PV (> 10%) can override response by osmoreceptors (Ie. ADH is secreted irrespective of plasma osmolality)
- Other stimuli: AII, pain, nausea/vomiting, exercise
- 9 a.a peptide hormone synthesised in hypothalamus (SON/PVN) → transported to posterior pituitary where it is secreted by:
- Effects:
- Via V1 receptor (GPCR via Gq → activates PLC to ↑ IP3 → ↑ IC [Ca2+] → SM contraction) → causes ↓ GFR to ↓ glomerular filtration (and loss of) H2O → via:
- Renal afferent arteriolar constriction
- Renal mesangial cell contraction
- Via V2 receptor (GPCR via Gs → activates AC to ↑ cAMP → activates PKA) → this causes:
- Upregulates insertion of luminal AQP2 (stored in vesicles) in all parts of CD → ↑ H2O permeability → ↑ H2O reabsorption into hypertonic medullary interstitium
- Upregulates “urea transporters” in inner MCD → ↑ permeability to urea → ↑ urea absorption to maintain ↑ medullary osmolality (strengthens CCM) → promotes ↑ H2O reabsorption
- ↑ Na+ reabsorption and K+ secretion by principal cells of CCD
- Via V1 receptor (GPCR via Gq → activates PLC to ↑ IP3 → ↑ IC [Ca2+] → SM contraction) → causes ↓ GFR to ↓ glomerular filtration (and loss of) H2O → via:
Bianca 2016
Examiner Comments
2022A 08: 43% of candidates passed this question.
Better answers for this question used the “sensor, integrator/controller, effector” structure. They also included appropriate detail relating to the site and mechanism of angiotensin II and the subsequent stimulation of ADH and aldosterone release. A detailed description of ADH was necessary to score well.
Lengthy descriptions of body water distribution or renal handling of water did not attract additional marks.
Answers that scored less well were often disorganised, with limited structure and incorrect facts.
2021B 01: 28% of candidates passed this question.
This is a level 1 topic. An understanding as to how the body regulates water is crucial to the daily practice of critical care, this topic is well described in the major texts. This type of question lends itself to the basic template of sensor mechanisms, central processing and integration with effector limbs and feedback loops. However, high scoring answers require a quantification of responses and an introduction into how these processes are integrated and fine-tuned.
2011A 18
Explain the physiological processes involved in the development of tissue interstitial oedema.
CICMWrecks Answer
Interstitial oedema occurs due to increased permeation of fluid from intravascular to interstitial spaces, and inability of the lymphatics to reabsorb this additional fluid
Starling Forces
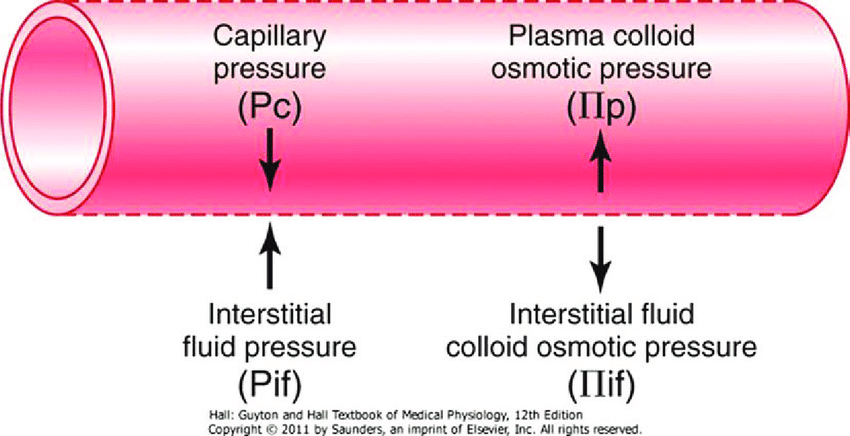
- The NET flux across the membrane is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation:
where
Jv is the trans endothelial solvent filtration volume per second
( [ Pc – Pi ] – σ [ πp – πi ] ) is the net driving force
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
κ = filtration constant = LpS = Hydraulic conductivity x Surface Area
- Typically quoted values for the variables in the classic Starling equation:
| Hydrostatic pressure | Oncotic pressure |
|---|---|
| Pressure moving fluid | pressure exerted by proteins which draw water into and keep it within a compartment |
| Pc ~35 → 15mmHg (Arterial → venous) Capillary hydrostatic pressure Pressure moving fluid out of capillary | πp ~ 20mmHg Plasma oncotic pressure Pressure keeping fluid within capillary |
| Pif = 5mmHg Interstitial hydrostatic pressure Pressure moving fluid into capillary | πif ~ 0mmHg Interstitial fluid oncotic pressure Pressure keeping fluid out of capillary |
- In general,
- at the arterial end of capillary NFP is positive (filtration) +10mmHg
- At the venous end NFP is negative (absorption) -10mmHg
- Approx. 24L fluid filtered / day
- 85% reabsorbed into capillaries
- Rest reabsorbed via lymphatics (~3.5L/day) = Net fluid loss from filtration
Tissue Interstitial Oedema
| Increased κ promotes oedema | – Inflammation |
| Increased Pc promotes oedema | Increased resistance to venous return – Diastolic HF – Increased R atrial pressures – Venous obstruction (DVT, mass) – Loss of one way valves – Loss of veno-muscular pump (in bed-bound patients) |
| Increased intravascular volume – Activation of RAAS in CHF, cirrhosis, nephrotic syndrome – Crystalloid administration in ICU | |
| Gravity and posture – Lower limbs if standing – Sacrum if supine | |
| Decreased πc promotes oedema | – Hepatic failure and decreased plasma proteins – Nephrotic syndrome |
| Endothelial glycocalyx – damage promotes oedema | – Glycoprotein layer on inner surface of capillaries which sequester plasma proteins |
| Decreased lymphatic flow promotes oedema | Obstruction – Compression – Tumour – Infection – filariasis |
| Increased Pi inhibits oedema | – Compression stockings – Deep sea diving, increased atmospheric pressure |
Sakurai / JC 2020
Examiner Comments
2011A 18: 2 (17%) of candidates passed this question.
The question required an accurate statement of Starling’s Equation, including the filtration and reflection co-efficients, and definitions of terms. Marks were awarded for numerical values pertaining to hydrostatic and oncotic pressure gradients and net filtration in a 24 hour period.
A satisfactory answer explained the factors which cause imbalance in Starling’s relationship including; precapillary vasodilation, increased venous pressures, gravity/ posture, fall in plasma protein concentration, changes to capillary permeability and lymphatic obstruction.
Syllabus: E1
Recommended sources: Review of Medical Physiology, Ganong, Chp 23 and other sections
2017B 04
Describe how interstitial fluid recirculates to the vascular system
CICMWrecks Answer
Interstitial fluid:
- Interstitial fluid provides the immediate microenvironment that allows for movement of ions, proteins and nutrients across the cell barrier.
- In the average male (70 kg) human body, the interstitial space has approximately 10.5 litres of fluid.
- This fluid is not static, but is continually being refreshed by the blood capillaries and recollected by lymphatic capillaries.
- Summary of drainage:
- The blood and lymphatic vasculatures constitute two parallel circulatory organs, connected by the emptying of lymph into the venous system.
- Blind-ending lymphatic capillaries collect interstitial fluid by pumping the liquid, which will pass lymphatic valves that close to prevent ‘back-flow’.
- Tissue oedema facilitates the draining of the interstitial fluid through the initial lymphatic vessels by pulling on the vessels through their tissue-anchored filaments
- Approx. 24L fluid filtered / day
- 85% reabsorbed into capillaries
- Rest reabsorbed via lymphatics (~3.5L/day) = Net fluid loss from filtration
Anatomy of Veins:
- Veins are thin-walled, being thinner and larger than the arteries.
- Veins have valves which maintain the unidirectional flow of blood, even against gravity.
- The muscular and elastic tissue content of the venous walls is much less than that of the arteries. This is directly related to the low venous pressure.
- Since the venous pressure is low (7 mm Hg) the valves are of utmost value in the venous return.
- However, the valves are absent in:
- The veins of less than 2 mm diameter.
- The venae cavae.
- The hepatic, renal, uterine, ovarian (not testicular), cerebral, spinal, pulmonary, and umbilical veins.
- Large veins have dead space around them for their dilatation during increased venous return. The dead space commonly contains regional lymph nodes.
Vascular permeability:
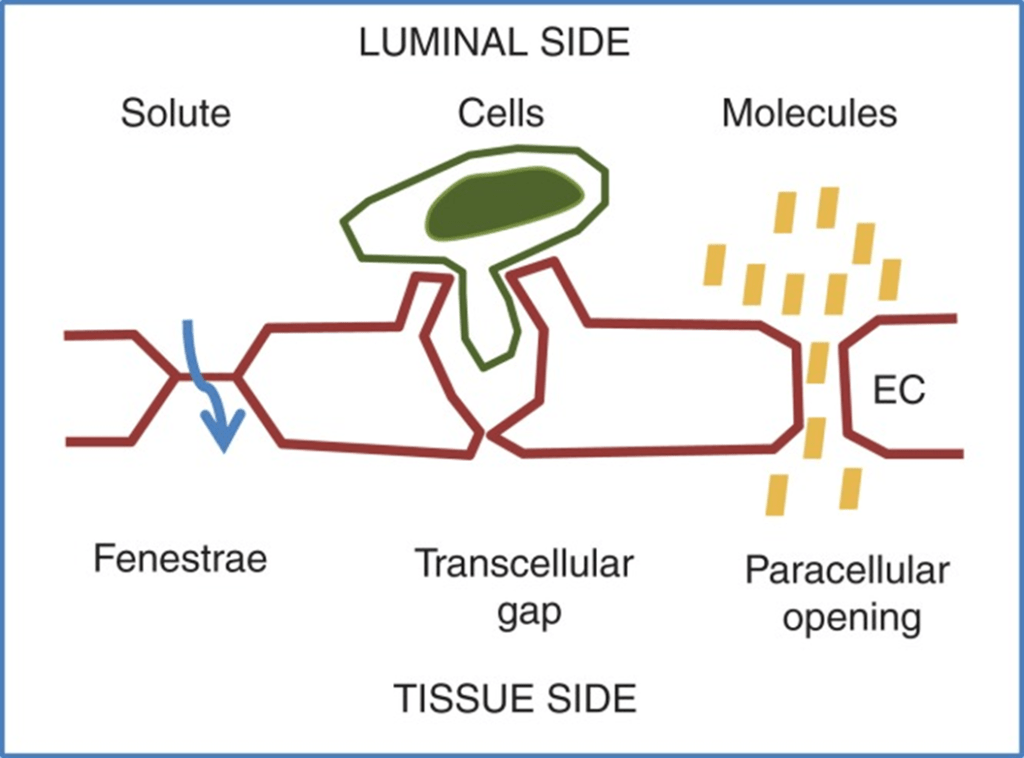
- Vascular permeability, often in the form of capillary permeability or microvascular permeability, characterizes the capacity of a blood vessel wall to allow for the flow of small molecules (drugs, nutrients, water, ions) or even whole cells (lymphocytes on their way to the site of inflammation) in and out of the vessel.
- Blood vessel walls are lined by a single layer of endothelial cells. The gaps between endothelial cells (cell junctions) are strictly regulated depending on the type and physiological state of the tissue.
- Vascular permeability is Regulated by Angiogenic growth factors (VEGF) and Inflammatory cytokines (histamine, bradykinin).
- Vascular permeability is affected in disease states like cancer, MI, Sepsis.
Anatomy of Lymph:
- Lymph is the name given to interstitial fluid which enters the lymphatic vessels.
- Lymphatic capillaries are present in nearly all tissues.
- Significant exceptions are the central nervous system and bone.
- Small interstitial channels are present in the brain and the fluid flows into the CSF and then passes back into the circulation via the arachnoid villi.
- The lymph capillaries are blind-ending and possess flap valves between adjacent lymphatic endothelial cells.
- These functional valves permit entry of ISF but prevent its return to the interstitium.
- The pressure inside the lymph capillary is about 1 mmHg at rest and the flap valves are closed.
- The lymph capillaries interconnect and join together to form lymph venules, and then large lymph veins which drain via lymph nodes into the thoracic duct (on the left) and the right lymphatic duct.
- By these two final pathways, lymph returns into the circulation.
Role of Forces:
Starling Forces (Osmotic and Hydrostatic)

- The NET flux across the membrane is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation:
where
Jv is the trans endothelial solvent filtration volume per second
( [ Pc – Pi ] – σ [ πp – πi ] ) is the net driving force
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
κ = filtration constant = LpS = Hydraulic conductivity x Surface Area
- Typically quoted values for the variables in the classic Starling equation:
| Hydrostatic pressure | Oncotic pressure |
|---|---|
| Pressure moving fluid | pressure exerted by proteins which draw water into and keep it within a compartment |
| Pc ~35 → 15mmHg (Arterial → venous) Capillary hydrostatic pressure Pressure moving fluid out of capillary | πp ~ 20mmHg Plasma oncotic pressure Pressure keeping fluid within capillary |
| Pif = 5mmHg Interstitial hydrostatic pressure Pressure moving fluid into capillary | πif ~ 0mmHg Interstitial fluid oncotic pressure Pressure keeping fluid out of capillary |
- In general,
- at the arterial end of capillary NFP is positive (filtration) +10mmHg
- At the venous end NFP is negative (absorption) -10mmHg
- Approx. 24L fluid filtered / day
- 85% reabsorbed into capillaries
- Rest reabsorbed via lymphatics (~3.5L/day) = Net fluid loss from filtration
Electric forces:
- The ionic composition of the interstitial fluid and blood plasma vary due to the Gibbs–Donnan effect [“Opposing osmotic and electro-chemical gradients in the presence of a non–diffusable ion resulting in unequal distribution of the diffusable ions”]
- This causes a slight difference in the concentration of cations and anions between the two fluid compartments.
JC 2019
Examiner Comments
2017B 04: 10% of candidates passed this question.
Candidates had a limited understanding of this area of the syllabus. It was expected that answers would describe important concepts including the anatomy of venous structures, valves and lymphatics, permeability and factors which influence permeability. A description of hydrostatic forces, other pressures involved, and the role of osmotic and electric forces were required.
ii. Define osmosis, colloid osmotic pressure and reflection coefficients and explain the factors that determine them.
iii. Describe the distribution, regulation and physiological importance of sodium, chloride, potassium, magnesium, calcium, and phosphate ions.
Sodium
2018A 04
Describe the renal handling of sodium.
2014B 23
Describe the regulation of sodium in the body.
2009B 22
Describe how the kidney handles sodium. (50 marks).
What factors influence urinary sodium excretion (50 marks)
CICMWrecks Answer
NORMAL SODIUM
- Total Sodium (Na) = 4000 mmol (60 mmol/kg)
- Distributed:
- Bone (45%)
- ECF (50%)
- 1°ly found in ECF (major EC cation)
- ICF (5%)
- 10-15 mmol/L maintained by
- Na+/K+ ATPase
- low gNa → prevents influx of Na
- Role of Na
- Main determinant of ECF osmolality and tonicityNa/Cl ~ 90% ECF osmotic solute load
- Main determinant of ECFV
- Depolarisation in action potential 2° to ↑Na conductance
- Co-transport of substances across membranes (Eg. Glucose)
- Involved in Na+/K+ ATPase in cell membranes
- Total output =Total input
- 1-1.4 mmol/kg/day
- ~100-300 mmol/day in 70 kg adult
- 10.5 g/day
- Sodium Control – Lost via:
- Kidneys (main) ~ lose 150 mmol/day
- Filters 25000 mmol Na+/day
- 99.5% reabsorbed
- 65% PCT, 25% TAL of LoH, 5% EDCT, 4-5% LDCT and CD
- Sweat and GIT loss in faeces ~ lose 10 mmol/day (0.25 g/day each)
- Kidneys (main) ~ lose 150 mmol/day
Sodium Reabsorption in Kidney
| Location | Contribution | Mechanism |
|---|---|---|
| PCT | 65% | Secondary active transport: – Luminal Na/organic cotransporters with glucose and AAs – Luminal Na/K (NHE-3) exchanger with H from Henderson-Hasselbach intracellularly Passive transcellular – Via solvent drag passively – Down electrical gradient from positive lumenal charge |
| TAL of LoH | 25% | Secondary Active transport: – NKCCT on luminal surface – Dominant mechanism Small amount continues via Secondary active transport as per PCT Paracellular movement driven by net positive charge in lumen |
| Early DCT | 6-10% | 2° active means – Apical Na/K ATPase generates Na gradient – Basal Na/Cl symporter – No alteration in the luminal charge as electrically neutral |
| Late DCT and CD | 5-10% | – Facilitated diffusion across principle cells – Basolateral Na/K ATPase → intracellular Na deficit – Na reabsorbed from lumen via ENaC channels in principle cells up regulated by Aldosterone |
Overview of renal Na+ regulation:
- Thus, renal Na+ regulation depends on:
- Degree of glomerular filtration of Na+ → GFR (minor)
- Changes in GFR due to hyper or hypovolaemia will (indirectly) adjust sodium elimination. Increased plasma volume increases GFR, and vice versa.
- Degree of tubular reabsorption of Na+ (major)
This is the main mechanism for controlling sodium in euvolaemia.
In terms of long term Na excretion; Na reabsorbed is more impt than GFR because:- (i) GFR is heavily autoregulated
- (ii) Glomerulotubular balance blunts any major changes in Na+ excretion that would have resulted from minor changes in GFR changes that actually occurs
- Na reabsorption through GIT/sweat/salivary glands:
- Varies with diet and exercise
- Mainly action of Aldosterone via Na/K ATPase
- Degree of glomerular filtration of Na+ → GFR (minor)
- Normal values
- Na+ filtration: 140mmol/L x 180L/day = 25000mmol Na/day
- Na+ excretion: 140mmol excreted
- rest reabsorbed
- Fractional excretion = 0.5%
Na+ regulation: Control of GFR
- Intrinsic autoregulatory factors (tubuloglomerular feedback and myogenic mechanism)
- MAP has minor effect on GFR over MAP range 70-175 mmHg → BUT changes
in BP that invoke baroreceptor reflexes (BRR) can override these autoregulatory
mechanisms → alter GFR and amount of Na+ filtered
- MAP has minor effect on GFR over MAP range 70-175 mmHg → BUT changes
- Extrinsic factors: Body Na+ content (via ECFV)
- Direct renal effects – ↓ [Na+] (or ↓ ECFV) → results in ↓ GFR due to a ↓ glomerular capillary P(HYDROSTATIC) and ↑ glomerular capillary P(ONCOTIC) → ↓ GFR and Na+ filtered
- Indirect renal effects – ↓ [Na+] (or ↓ ECFV) → stimulates arterial, venous and cardiac BRR → neurohormonal response → to ↓ GFR and Na+ filtered via:
- (i) ↑ SNS and RAAS activity → cause afferent and efferent arteriolar constriction and mesangial cell contraction
- (ii) ↑ ADH → cause afferent arteriolar constriction and mesangial cell contraction
- (iii) ↓ ANP → inhibit afferent arteriolar dilation and mesangial cell relaxation
Na+ Regulation: Control of Reabsorption
- Glomerulotubular balance:
- Intrinsic autoregulatory mechanism that minimises the effect of changes in GFR on Na+ and H2O excretion
- It functions on the basis that the PCT reabsorbs a constant proportion of glomerular filtrate (65% of filtered Na+ /H2O), rather than a constant amount
- In effect – ↑ GFR = ↑ filtration of Na+/H2O = ↑ Na+/H2O reabsorption
- Mechanism:
- With ↑ GFR → large amount of plasma is filtered at the glomerulus → leads to ↑ π(ONCOTIC) of plasma in peritubular capillaries
- This results in an ↑ gradient that –
- (i) Favours tubular reabsorption, and
- (ii) Counteracts the effect of ↑ GFR on fluid leaving the PCT
- Renal interstitial hydrostatic pressure (Intrarenal physical factors)
- ↓ ECFV (and ↓ Na+) results in ↓ MAP → leads to (i) ↓ PHYDROSTATIC and (ii) ↑ πONCOTIC of peritubular capillaries → thus, ↑ Na+ (and ↑ H2O) reabsorption from tubular interstitium into peritubular capillaries
- Hormonal Influences:
- Renin
- Released by ↓ Na delevery to macula densa or β1 stimulation secondary to volume underload
- Tubular effects:
- increased PCT Na/Cl reabsorption, increased tubular K secretion
- Direct PCT effect
- Aldosterone release
- Aldosterone
- Most important regulator of Na+ reabsorption
- Alters protein translation (inducing production of tubular basolateral Na+/K+ATPase and luminal ENaC and K+channels) → causes ↑ Na+ reabsorption by DCT and Principal cells of CCD
- Increased Na reabsorption throughout the GIT/sweat and salivary glands via Na/K ATPase
- Increased H2O reabsorption and increased Na via solvent drag
- Angiotensin II
- Negative feedback on renin release
- Increased aldosterone release
- Decreased RBF and GFR
- Direct renal arteriole constriction (efferent = afferent)
- Mesangial cell contraction thus decreased Kf and GFR
- Direct stimulation of Na+ reabsorption at PCT, and
- Indirect stimulation of Na+ reabsorption via SNS, AII, and aldosterone
- SNS
- Direct stimulation of Na+ reabsorption at the PCT (α1 and β1 receptors), and
- Indirect stimulation of Na+ reabsorption via RAAS
- ADH
- → ↑ Na+ reabsorption at the CCD (principal cells) → acts synergistically with aldosterone here
- ANP
- Inhibition of Na+ reabsorption (blockage of ENaC) in the CDs
- ↓ RAAS and ↓ ADH activity
- Renin
- Other causes ↑Na reabsorb:
- Cortisol
- Oestrogen
- GH
- Thyroid hormone
- Insulin
- Dopamine
- Other cause ↓Na reabsorb:
- PGE2 inhibits NaK ATPase to reduce Na reabsorption
- Glucagon
- Progesterone
- PTH
- Renal vasoDilators:
- PGs
- Kinins
- Pressure natriuresis & diuretics
- renal compensatory mechanism that maintains long-term regulation of arterial BP by controlling the kidney’s excretory ability of Na+and H2O
- Pharmacological agents:
- Ouabain (a cardiac glycoside) inhibits NaK ATPase decreasing excretion
- Loop Diuretics → ↑ Na loss
Gladwin / Bianca / JC 2019
Examiner Comments
2018A 04: 6% of candidates passed this question.
A description of filtration and reabsorption, including amounts was required. Better answers described sodium handling in a logical sequence as it progressed through the nephron including the percentages reabsorbed in each segment. In addition to the amounts reabsorbed, the mechanisms of transport across the tubular luminal and basolateral membranes into interstitial space should have been described.
2014B 23: 19% of candidates passed this question.
This question was generally poorly answered. Total body sodium is regulated within 2% in normal individuals. The vast majority is contained in the extracellular compartment. While any physiological regulation involves a balance of input and output, sodium intake is essentially unregulated in humans. Output is regulated via renal, gastrointestinal and skin losses. Candidates needed to present the renal handling of Na including hormonal control and present factual knowledge about the level of absorption and GFR effects to attain a pass mark. Many candidates focused on osmolality and tonicity and some on the use of diuretics thereby not gaining marks
on regulation of sodium. Most candidates didn’t mention either the skin and GIT role in sodium balance.
2009B 22: 2 (22%) of candidates passed this question.
Candidates were essentially expected to describe the fate of sodium as t passes through the kidney from filtration at the glomerulus to ending up in the urine. Essentially, most Na+ is reabsorbed at the proximal tubule (65%), reabsorption being down an electrochemical gradient (inside cell negative and low Na+ concentration) which is maintained by active Na+/K+ ATPase activity at the basolateral membrane. Then Loop of Henle reabsorbs a further 15% of filtered Na+. At descending limb – no Na+ reabsorption. At ascending limb, thick segment – active process as per proximal tubule, but mostly coupled to K+ and Cl- and paracellular diffusion through tight junctions. Finally the distal convoluted tubule and collecting ducts, a further almost 20% reabsorbed – leaving < 1%
Factors influencing loss are
- aldosterone = stimulates Na+ reabsorption at the collecting tubules
- intra renal factors such as interstitial pressure which is lowered during hypovolaemia and reduced renal perfusion, thus promoting Na+ reabsorption gradient
- sympathetic nervous system – influences interstitial pressure and increases renin production
- angiotensin II – stimulates reabsorption at proximal tubule
- Atrial Naturetic Peptide/Factor – inhibits Na+ reabsorption
- Others – dopamine, cortisol, insulin => increase Na+ reabsorption, but minor factors
Syllabus – D1, 2f
Reference – Textbook of medical Physiology, Guyton, Chp 28
2021A 14
Describe the pharmacology of sodium bicarbonate.
Examiner Comments
2021A 14: 29% of candidates passed this question.
This question was best answered with a structured approach as per any pharmacology question. It nonetheless required good understanding of various aspects of physiology. Many candidates failed to gain marks by omitting to mention facts which could have been prompted by a defined structure. A good response mentioned the pharmaceutic features including formulation and the hypertonicity of IV bicarbonate, pharmacodynamics including indications for use, mode of action, adverse effects (systemic and local), pharmacokinetics and dose. Pleasingly a few candidates stated that sodium bicarbonate’s mechanism of action to cause alkalosis involved increasing the strong ion difference in plasma. Credit was also given for stating the mechanism of action as providing bicarbonate ions to augment the extracellular buffer system.
Potassium
2022B 14 – 2013A 12
Describe the physiological role, distribution and regulation of potassium (K+)
2013B 20
Describe the electrocardiographic (ECG) changes seen with hyperkalaemia. (30% of marks)
Outline the pharmacologic principles of drugs used in the management of severe hyperkalemia. (70% of marks)
2009B 15
Describe the mechanism of actions and duration of effect of drugs used to lower potassium in hyperkalaemia.
2007B 20
Describe the determinants of serum potassium. Outline the consequences of acute hyperkalaemia.
CICMWrecks Answer: Potassium, K+ balance (Determinants of Serum Potassium)
Potassium
- Predominany intracellular cation
- Total Stores: Approx 3200mmol (50mmol/kg)
- Key Functions:
- Main determinant of ICF osmolality and tonicity
- Responsible for RMP of excitable cells via Goldmann-Hodgkin-Katz due to ↑↑gK relative to other species
- Role in action potential → repolarisation phase
- Secretion of insulin and multiple other KATP dependant processes
- Regulation of IC processes (protein/glycogen synthesis)
- Involved in Na+/K+ ATPase in cell membranes
Potassium balance / Determinants of Serum Potassium
- Intake
- Oral – very variable
- Approx. 50-200mmol/day
- Distribution
- Exchangable Pool
- ICF (90%): [K+] 150 mmol/L (1° ICF cation)
- ECF (2%): [K+] 3.5-5 mmol/L
- Non-exchangable Pool
- Bone (8%)
- Exchangable Pool
- Transcellular balance
| Factor | Mechanism | Effect on Serum K+ |
|---|---|---|
| Insulin | Causes intracellular shift of K+ | ↓ |
| β2 adrenergic agonism | Causes intracellular shift of K+ | ↓ |
| Aldosterone | Upregulate Na/K ATPase → Intracellular shift of K+ | ↓ |
| pH | Increased H+ (decreased pH) causes extracellular shift of K+ | ↑ |
| Plasma osmolarity | Hyperosmolar plasma initiall causes osmosis of water out of cells increasing intracellular osmolarity and [K+] → causes extracellular shift of K+ | ↑ |
| Skeletal muscle activity | Causes K+ leakage into serum | ↑ |
| Cell death | Causes K+ leakage into serum | ↑ |
- Elimination
- Faecal – 8mmol/day
- Renal – 92mmol/day (See next heading for details)
- Urinary K+ excretion = [K+ filtered by glomerulus] + [K+ secreted by CCD/LDCT] – [K+ reabsorbed by renal tubules]
- Glomerular filtration: Freely filtered = 756mmol/day (180L x 4.2mmol/L)
- PCT: 65% reabsorption
- LoH: 25~30% reabsorption
- DCT/Collecting Ducts – variable
- Determined by Aldosterone, Plasma [K+], Tubular Flow rate
- Secreted by principal cells
- Reabsorbed by intercalating cells
Renal K+ regulation:
- Renal K+ regulation occurs MAINLY via control of K+ secretion at the distal nephron (CCD and LDCT) via the following factors:
- Circulating factors:
- Plasma [K+ ]
- ↑ [K+ ] causes ↑ K+ secretion by → (i) Directly stimulating Na+ /K+ ATPase in principal cells, and (ii) Directly stimulating Aldosterone release from adrenal cortex
- Aldosterone
- ↑ aldosterone causes ↑ K+ secretion by → ↑ production of Na+ /K+ ATPase, K+ channels and ENaC Na+ channels in the principal cells
- Plasma pH
- Alkalosis via ↓ plasma [H+ ] causes ↑ K+ secretion by → stimulating Na+ /K+ ATPase in principal cells
- Plasma [K+ ]
- Luminal factors:
- Flow of tubular fluid in DCT and CCD
- ↑ tubular fluid flow causes ↑ K+ secretion by → maintaining ↓ luminal [K+ ] (Ie. continuously washing it away) → permits passive diffusion of K+ ↓ its [ ] gradient into tubular lumen
- ↑ Na+ delivery rate to DCT and CCD → ↑ K+ secretion
- -ve lumen potential difference → ↑ K+ secretion
- Flow of tubular fluid in DCT and CCD
- Circulating factors:
JC / Gladwin / Sakurai 2020
CICMWrecks Answer: Hyperkalaemia
Serum Potassium
- Normally 3.5~5mmol/L
- Hyperkalaemia [K+] >5mmol/L
- Severe hyperkalaemia [K+] >7mmol/L
ECG Changes
- Peaked T waves at [K+] > 5.5mmol/L
- Loss of P wave at [K+] > 6.5 mmol/L
- QRS widening at [K+] > 7.5mmol/L
- VF or asystole
Consequences of Acute hyperkalaemia
- CVS
- ECG changes
- Tenting of T wave
- Flattening of P wave and increase PR interval
- Widening of QRS
- VF
- Arrythmia
- VF
- ECG changes
- CNS
- Lethargy
- Other
- Flaccid paraylis
- Muscle weakness
- Respiratory failure
Management
Drugs that eliminate body K+
- Loop diuretics e.g. frusemide
- 40mg IV
- Inhibition of the Na+/K+/2Cl- cotransporter on luminal membrane of the thick ascending Loop of Henle
- This causes loss of normal positive charge in lumen, leading to the loss of paracellular resorption of K+
- Net result is K+ excretion
- Elimination half-life 45-9 mins
- Cation exchange resins e.g. sodium polystyrene sulfonate
- 15~45gm
- Not absorbed in GI tract
- Prevents potassium reabsorption in the GI tract (predominantly in the colon where K+ secretion occurs)
- Onset of action: 1 hr PR, 4-6hrs PO
- Duration: variable
- No effect acutely
Drugs causing increased intracellular uptake of K+
- Insulin/glucose
- 10 units IV (with 50ml 50% dextrose)
- Insulin receptor agonism → transcellular shift of K from extracellular to intracellular space via upregulation of Na/K ATPase → transient reduction in serum [K+]
- Onset: 20-30 mins
- Duration: 2-6 hours (prolonged if i.v. infusion)
- Sodium bicarbonate
- Alkalosis increases the activity of the Na+/K+ ATPase pump, increasing K+ uptake
- Onset: 30-60 mins
- Duration: 2-3 hours
- β2 agonists e.g. salbutamol
- 5~20mg via nebulizer, repeated (or 6-12 puffs via MDI, repeated)
- β2 agonism → increased intracellular [cAMP] → transcellular shift of K from extracellular to intracellular space → transient reduction in serum [K+]
- Onset: 30 mins
- Duration: 2-3 hours
- I.V. fluid
- Causes haemodilution
- Can increase renal excretion of K+ by increasing renal perfusion and increasing urine output
Other Treatments in Hyperkalaemia:
- Calcium (No effect on K+ level)
- 10ml 10% CaCl (6.8mmol) or 10% CaGluconate (2.2mmol)
- Immediate onset
- Stabilizes cardiac membrane by reducing threshold potential
- Dialysis
- via continous Veno-Venous Dialysis or intermittent haemodialysis
- Dialysis fluid adjusted to create large concentration gradient for potassium
extraction via dialysis of filtration
JC / Sakurai 2019
Examiner Comments
2022B 14: 51% of candidates passed this question.
The best answers demonstrated an appreciation of the multiple roles of potassium in normal physiology and described the integrated regulation of potassium concentration/distribution as opposed to many answers that seemed to focus purely on the renal handling of a filtered potassium load.
2013B 20: 23 candidates passed (85.2%).
In general, knowledge of the ECG changes of hyperkalaemia was lacking. Most candidates could list the drugs used in hyperkalaemia, but few gave adequate detail of their mechanism of lowering potassium, dosing, time to onset and duration of action. Many candidates mentioned dialysis or renal replacement therapy which is not a drug therapy for hyperkalaemia; therefore no points were awarded for this.
2013A 12
Potassium is the second most common cation in the body and the main intracellular cation. It is widely distributed and has many important roles. Maintenance of potassium balance depends mainly on secretion by the kidneys in the distal and collecting tubules. Candidates were expected to mention the influence of aldosterone, and other hormones such as glucocorticoids, catecholamines and vasopressin have as well as factors such as acidosis/alkalosis. Candidates who had a systematic and structured approach performed better.
2009B 15: 5 (55%) of candidates passed this question.
A good answer to this question required the collation of knowledge from broad range of areas, ie drug activity. Marks were divided between each of the following: 8.4%NaHCO3-, insulin/glucose, K+ exchange resin, frusemide/loop diuretics, Beta2 agonists and K+ free fluid rehydration/dilution. Candidates often overlooked the fact that only loop diuretics and K+ exchange resins lower total body K+ content, whilst the others induce an intracellular K+ shift which is not sustained and do not directly result in body elimination of K+. Intravenous fluid rehydration lowers total body K+ if there is a resulting diuresis.
Most candidates just passed this question. Common omissions included NaHCO3, loop diuretics and beta 2 agonists. No candidate mentioned rehydration/dilution. Calcium does not lower serum potassium.
2007B 20: 3 candidates (43%) passed this question.
Most candidates did not appreciate that serum potassium is a function of two variables:
1. Total body potassium
2. Distribution between the extracellular and intracellular fluid compartments.
Approximately 98% of total body potassium is intracellular due to the action of Na+/K+ ATPase. Potassium is important in the electrophysiology of excitable cells and changes in serum potassium can affect their function. Hence the importance of keeping the serium potassium within a narrow normal range.
Again most candidates did not provide the overview that serum potassium levels reflect a balance between intake, output and transcellular distribution. Normal dietary intake is highly variable. Transcellular distribution by the mechanisms of insulin and glucagol, catecholamines and Beta2 activity and acid base changes all work to rapidly restore changes in details on the long term renal regulation of serum potssium involving distal tubult potassium secretion and aldosterone and also the effect of distal tubular flow and sodium excretion.
The effects of hyperkalaemia were better described than the first part of the question. Most candidates concentrated on the cardiac effects where most marks were awarded. The effects of an increased potassium on the cardiac action potential earned extra marks. The correlation between actual serum potassium level and ECG changes is variable and depends on many factors including how acute or chronic the hyperkalaemia is.
Tratment of hyperkalaemia was mentioned by a few candidates but attracted no extra marks.
2012A 04
Describe the factors that affect the flux of potassium across the cell membrane
CICMWrecks Answer
Potassium
- Predominany intracellular cation
- Total Stores: Approx 3200mmol (50mmol/kg)
- Key Functions:
- Main determinant of ICF osmolality and tonicity
- Responsible for RMP of excitable cells via Goldmann-Hodgkin-Katz due to ↑↑gK relative to other species
- Role in action potential → repolarisation phase
- Secretion of insulin and multiple other KATP dependant processes
- Regulation of IC processes (protein/glycogen synthesis)
- Involved in Na+/K+ ATPase in cell membranes
Potassium flux is dictated by
- Fick’s law of diffusion
- D proportional to Δ [K] → ↑/↓ ECF K → ↑/↓ diffusion
2. Nernst Equation
where
E is the equilibrium potential for the ion
R is the gas constant (8.314 J.K-1.mol-1 )
T is the temperature in Kelvin
F is Faraday’s Constant
z is the ionic valency (e.g. +2 for Mg2+, -1 for Cl–)
EK = -90 mV
ENa = +55mV
ECl = -65mV
- Temperature via the nernst equation → ↑ temperature → more negative V → increase potassium permeability → ↑ intracellular potassium
3. Gibbs-donnan
- In the presence of non-diffusable species the relative osmolarity drives flow of diffusable species.
- ↑ intracellular osmolarity or ↓ extracellular osmolarity → potassium efflux
4. Homeostatic processess
- Na/K ATPase
- ↑ intracellular K
- β adrenergic stimulation → ↑ Na/K ATPase activity
- Insulin release → ↑ Na/K ATPase activity
- ↑ intracellular K
- Acidosis:
- ↑ H/K antiporter activity →
- ↑ extracellular potassium
- Utilisation of intracellular protein buffer
- ↑ H/K antiporter activity →
- Hormonal factors
- Insulin → ↑ Na/K ATPase activity → ↑ intracellular K
- Aldosterone → ↑ Na/K ATPase activity → ↑ intracellular K
- Activity of ATP sensitive potassium channels
- Glucose (eg in pancreatic cells) → Diffuses into cells → ↑ ATP/ADP ratio → closure of ATP sensitive K channels → ↓ ECF K
- Gluagon → direct increase of cAMP production → ↓ ATP → ↑ gK → ↓ intracellular K
- Burns and UMN lesions
- upregulation of foetal/extrajunctional form of nAChRs
- Prolonged opening relative to foetal form → ↑ potassium efflux on depolarisation
Gladwin 2016
Examiner Comments
2012A 04: 0 (0%) of candidates passed.
Candidates were required to synthesize knowledge across a number of areas and have a good overview of the topic. This included the following – Insulin (acts to up-regulate Na/K ATPase activity promoting intracellular shift of potassium in adipose and muscle tissue); catecholamines (beta2 stimulation up-regulates Na/K ATPase activity promoting intracellular shift of potassium); aldosterone; pH (acidosis promotes H+/K+ exchange (via H+/K+ antiport), and reduces the activity of the Na K ATPase pump); osmolality (cellular dehydration increases intracellular K+ concentration promoting diffusion of potassium out of the cells); exercise; plasma potassium; temperature.
2023A 05
Describe renal handling of potassium (60% of Marks), including physiological factors that may influence it (40% of Marks).
2021A 08
Describe renal handling of potassium (60% marks),including factors that may influence it (40% marks).
2010B 16
Discuss the regulation of body potassium by the kidney.
CICMWrecks Answer
Potassium
- Predominany intracellular cation
- Total Stores: Approx 3200mmol (50mmol/kg)
- Key Functions:
- Main determinant of ICF osmolality and tonicity
- Responsible for RMP of excitable cells via Goldmann-Hodgkin-Katz due to ↑↑gK relative to other species
- Role in action potential → repolarisation phase
- Secretion of insulin and multiple other KATP dependant processes
- Regulation of IC processes (protein/glycogen synthesis)
- Involved in Na+/K+ ATPase in cell membranes
Potassium Elimination
- Faecal – 8mmol/day
- Renal – 92mmol/day (See next heading for details)
- Urinary K+ excretion = [K+ filtered by glomerulus] + [K+ secreted by CCD/LDCT] – [K+ reabsorbed by renal tubules]
- Glomerular filtration: Freely filtered = 756mmol/day (180L x 4.2mmol/L)
- PCT: 65% reabsorption
- LoH: 25~30% reabsorption
- DCT/Collecting Ducts – variable
- Determined by Aldosterone, Plasma [K+], Tubular Flow rate
- Secreted by principal cells
- Reabsorbed by intercalating cells
K+ handling within the tubular system:
- PCT (55% of filtered K+ is reabsorbed):
- K+ is reabsorbed passively via the paracellular route due to:
- Solvent drag → coupled to flow of Na+ and H2O
- [ ] gradient → created by reabsorption of H2O
- Electrical gradient → due to +ve luminal charge in late PCT
- K+ is reabsorbed passively via the paracellular route due to:
- TAL of LoH (30% of filtered K+ is reabsorbed):
- K+ is reabsorbed by 2° active transport via paracellular route → +ve charged lumen generated by ion flux created by NKCCT transporter causes K+ to be reabsorbed by diffusion down its electrical gradient
- LDCT and CCD (0 to > 15% K+ is secreted):
- Principal cells of CCD and LDCT → 2° active secretion K+ → via:
- Basolateral Na+ /K+ ATPase → (i) generates an electrochemical gradient that draws Na+ intracellularly from the tubular lumen (via the ENaC channel), and (ii) pumps K+ from peritubular capillaries into tubular cell
- The –vely charged lumen generated by influx of Na+ across ENaC channel → favours tubular secretion of K+
- o Type A intercalated cells of CCD and LDCT → 2° active reabsorption of K+ :
- H+ is produced within tubular cell by hydration of CO2 (using CA) → H+ is then exchanged for tubular K+
- Principal cells of CCD and LDCT → 2° active secretion K+ → via:
- MCD (5% of filtered K+ is reabsorbed)
Renal K+ regulation:
- Renal K+ regulation occurs MAINLY via control of K+ secretion at the distal nephron (CCD and LDCT) via the following factors:
- Circulating factors:
- Plasma [K+ ]
- ↑ [K+ ] causes ↑ K+ secretion by → (i) Directly stimulating Na+ /K+ ATPase in principal cells, and (ii) Directly stimulating Aldosterone release from adrenal cortex
- Aldosterone
- ↑ aldosterone causes ↑ K+ secretion by → ↑ production of Na+ /K+ ATPase, K+ channels and ENaC Na+ channels in the principal cells
- Plasma pH
- Alkalosis via ↓ plasma [H+ ] causes ↑ K+ secretion by → stimulating Na+ /K+ ATPase in principal cells
- Plasma [K+ ]
- Luminal factors:
- Flow of tubular fluid in DCT and CCD
- ↑ tubular fluid flow causes ↑ K+ secretion by → maintaining ↓ luminal [K+ ] (Ie. continuously washing it away) → permits passive diffusion of K+ ↓ its [ ] gradient into tubular lumen
- ↑ Na+ delivery rate to DCT and CCD → ↑ K+ secretion
- -ve lumen potential difference → ↑ K+ secretion
- Flow of tubular fluid in DCT and CCD
- Circulating factors:
JC / Gladwin / Sakurai / Bianca 2020
Examiner Comments
2023A 05: 33% of candidates passed this question.
This question related to the renal handling of potassium, the physiology of potassium in the rest of the body was not relevant. Ultimately net potassium flux is a function of filtration, reabsorption, secretion and excretion. Good candidates divided the nephron into relevant sections and described how potassium was handled in each section. They correctly described the percentage reabsorption along each section, as well as the relevant active and passive pathways for reabsorption and/or secretion including the cells and channels/pumps involved. The physiological factors regulating each of these mechanisms were then described in correct detail.
2013A 12
Potassium is the second most common cation in the body and the main intracellular cation. It is widely distributed and has many important roles. Maintenance of potassium balance depends mainly on secretion by the kidneys in the distal and collecting tubules. Candidates were expected to mention the influence of aldosterone, and other hormones such as glucocorticoids, catecholamines and vasopressin have as well as factors such as acidosis/alkalosis. Candidates who had a systematic and structured approach performed better.
2010B 16: 4 (27%) of candidates passed this question
A number of candidates discussed the distribution of potassium in the body and its role in membrane potentials. This was not asked for. Common omissions were a lack of comment on glomerular filtration, a lack of detail regards mechanisms of potassium transport in various parts of the glomeruli and failing to discuss control mechanisms other than aldosterone.
Syllabus: E1,2b, D1,2f
References: Guyton and Hall Textbook of Medical Physiology, Chp 29
Magnesium
2021A 18
Outline the pharmacology of intravenous magnesium sulphate.
2019B 04
Outline the pharmacology of intravenously administered magnesium sulphate.
2015B 10 – 2014B 20 – 2011B 10
Describe the pharmacology of magnesium sulphate
Examiner Comments
2021A 18: 57% of candidates passed this question.
The best answers appropriately addressed the pharmacology of magnesium sulphate, rather than diverting into physiology. They noted that the question concerned intravenous magnesium sulphate and did not discuss other routes. They included pharmaceutics, important examples of the wide-ranging indications, listed potential modes of action and considered the full range of body systems affected including potential adverse effects. Drug interactions, such as potentiation of neuromuscular blocking agents, and pharmacokinetics (including stating that magnesium is not metabolised) were described.
2019B 04: 55% of candidates passed this question.
Overall answers were well structured. However, a lack of detail and inaccurate pharmacokinetics was common. Better answers included a discussion of the mechanism of action of Mg++ including Ca++ antagonism, presynaptic cholinergic effects and NMDA receptor antagonism. Adverse effects were not discussed in detail by many candidates and contraindications were commonly omitted.
2015B 10: 27% of candidates passed this question.
The standard “pharmacology template” approach would have served well to cover this question. Answers were generally lacking in detail and focussed on extraneous physiology rather than pharmacology. Toxicity and side-effects were important to emphasise, especially in the context of infusions for treatment of asthma and/or pre-eclampsia
2014B 20: 4% of candidates passed this question.
This was a repeat question and very poorly answered for such a commonly used agent. A structured approach to describing any drugs pharmacology was often not used.
Most answers were lacking depth and detail. The questions asked the pharmacology NOT physiology of magnesium sulphate. This was best answered with a standard template addressing: Presentation, Uses, Main actions, Pharmacodynamics, Pharmacokinetics, Mode of Action, Toxicity, and any Special Points
2011B 10: 1 (4%) of candidates passed this question.
Magnesium is a commonly administered agent within Intensive Care practice. Candidates performed poorly because of a lack of sufficient knowledge and a failure to structure their answer. For a good answer candidates were expected to mention that Magnesium comes as an inorganic sulphate, acts as a cofactor for a vast number of reactions, can be given orally (poor absorption) as well as intravenously, renal excretion with a low threshold.
Syllabus: O2 2c
Recommended sources: Rang and Dale Pharmacology Pg 388; Katzung Basic and Clinical Pharmacology, Pg 244.
2023B 13 – 2012A 19
Outline the distribution, clearance and physiologic functions of magnesium in the body
CICMWrecks Answer
Normal Magnesium:
1000mmol (12mmol/kg)
Normal Distribution of magnesium
- Bone (60%)
- ICF (40% esp organs/muscles)
- Mg2+ is mainly an IC cation
- Total IC [ ] 15 mmol/L cf. 0.5 mmol/L free/ionised inECF
- ECF (< 1%)
- Plasma [ ] 0.5-1 mmol/L
- 60% free/ionised
- 33% protein-bound (esp albumin)
- 7% complexed (citrate/PO4)
- Plasma [ ] 0.5-1 mmol/L
Normal Intake:
8-20 mmol/day (min 0.5 mmol/day)
Normal Losses
- Glomerular filtration of 100 mmol/day
- 95% reabsorbed in tubules (PCT 15%, 70% LoH, 10% distal nephron)
- 5% excreted → 2.5 to 8 mmol/day
- Minimal neurohormonal regulation of Mg2+
- TMAX of Mg transporter ~ plasma [Mg]
- → ↓reabsorption with ↑ Mg
Normal Functions:
- Co-factor in enzyme reactions (as a metallo-coenzyme) eg Na+/K+ ATPase activity
- Energy storage, utilisation, transfer (via role in formation/utilisation of ATP) eg BSL homeostasis
- Protein and nucleic acid synthesis (stabilises DNA and RNA structure)
- Ca2+/K+ metabolism
- ↓ membrane excitability (eg. muscles/nerves)
- ↓ NT release at cholinergic and adrenergic synapses
- Antagonises Ca2+ activity
Gladwin 2016
Examiner Comments
2023B 13: 36% of candidates passed this question.
This question was best answered under the headings distribution, clearance and physiologic functions. Distribution involved intracellular vs extracellular concentrations, the spread amongst organ systems and state of ionisation and protein binding. Clearance of magnesium required an accurate description of its renal filtration and sites and proportion of reabsorption and secretion along the nephron. The regulatory factors and factors that influence this clearance should also be outlined. This included; Mg plasma concentrations, other cations, ECF volume and PTH. Physiologic functions should cover its role as a cofactor of metabolism and enzyme systems with some examples, the role and mechanism in the musculoskeletal system as a calcium antagonist and inhibitory action in the nervous system including the action against Ach, nerves and NMDA activity.
2012A 19: 2 (20%) of candidates passed.
Insufficient breadth and depth of knowledge limited candidates’ performance to this question. Candidates were expected to mention normal plasma (0.7 – 1.1 mmol/l) and intracellular (20mmol/l) levels, distribution (approximately 50% of total body magnesium is in bone & 20% in skeletal muscles), clearance (almost solely renal, approaches GFR, renal threshold set at just above normal serum Mg concentration, below which get almost complete reabsorption), activity (e.g. co-factor in metabolism), effects on muscles (reduces muscle excitability, inhibits excitation-contraction coupling, reduced contractility / weakness / depressed reflexes), effects on nerves (e.g. reduces nerve excitability, blocks NMDA receptors), systemic and coronary vasodilation and inhibits platelet function.
Calcium
2011A 04
Outline the role of calcium in the body (70% of marks). Outline the differences between calcium chloride and calcium gluconate solutions (30% of marks).
CICMWrecks Answer
Calcium
- Total body stores of calcium = 25,000mmol (400mmol/kg)
- Distribution
- 99% in poorly exchangeable pool (bone, teeth)
- 1% in readily exchangeable pool, majority of this is plasma
- 2.15-2.65mmol/l in plasma
- Absorbed from gut (increased with calcitriol)
- Homeostasis maintained
- Short-term by distal tubular resorption, under control of PTH and calcitriol
- Long-term by bone osteoclast activity, influenced by PTH, calcitriol and calcitonin
Role of Calcium
- Intracellular:
- Myocyte excitation-contraction coupling
- Required for cross bridge cycling
- Regulates mitotic function
- Intracellular second messenger for enzyme activation
- Cell membrane:
- Flow of calcium is responsible for automaticity in SA and AV nodes
- Calcium channels are involved in membrane excitation
- Extracellular:
- Forms part of the mineral matrix giving bone and teeth strength
- Involved in fibrinolysis
- Factor IV in the clotting cascade, required for binding of other clotting factors to phospholipids
- Required for the complement cascade
Ca Gluconate vs Ca Chloride
| Calcium gluconate | Calcium chloride | |
|---|---|---|
| Chemical structure | 2 gluconate ions 1 Ca2+ ions | 2 chloride ions 1 Ca2+ ions |
| Dose | 10ml of 10% | 10ml of 10% |
| Potency | 2.2mmol in 10ml | 6.8mmol in 10ml |
| pH | 6.0-8.2 | 5.5-7.5 |
| Difference in adverse effects | Somewhat irritant to veins | Very irritable to veins |
| Administration | Can be safely given through peripheral IV cannulae | Best through CVC Can use in PIVC in resuscitation |
Mooney 2016
Examiner Comments
2011A 04: 4 (33%) of candidates passed this question
The question sought an understanding of the diverse roles of calcium. Some candidates spent considerable time in details of one or two roles. Limited marks were awarded for demonstrating knowledge of calcium distribution & homeostasis. Few candidates had a good understanding of the differences between calcium chloride and gluconate.
2017A 05
Describe the regulation of plasma calcium concentration.
2016B 01
Outline the distribution of calcium in normal plasma (20% of marks).
Describe the hormonal control of the calcium concentration in the plasma (80% of marks)
2008A 07
Outline the regulation of plasma calcium concentration.
Outline the mechanism of action of biphosphonates for the management of hypercalcaemia.
CICMWrecks Answer
Normal Calcium: 25,000 mmol (400 mmol/kg)
Distribution:
- Readily exchangeable pool (1%) (ECF esp plasma)
- Total plasma [Ca2+] = 2.12-2.65 mmol/L
- Ionised [Ca2+] = 1.2 mmol/L
- Only the plasma free Ca is physiologically active and regulated by homeostatic mechanisms
- Two pools
- Diffusible (55%)
- 45% free/ionised
- Active form (above)
- 10% complexed
- 45% free/ionised
- Non-diffusible (45%)
- protein bound (esp to albumin)
- pH-dependent (↑ binding with ↑ pH)
- Diffusible (55%)
- Poorly exchangeable pool (99%)
- Bone/teeth (as hydroxyapatite, phosphates, carbonates)
Calcium balance
ECF and hence plasma Ca is the result of a balance between dietary intake, gastrointestinal absorption and excretion, renal excretion and exchange with bone Ca.
Normal Losses:
- Kidneys (40%) → 2.5-7.5 mmol/day
- Filtration of 250 mmol/day
- 95% reabsorbed by tubules
- PCT 65% with Na
- TAL of LoH 20%
- distal nephron 10%
- 5% excreted
- ↑ reabsorption at LoH/distal nephron
- PTH
- 1,25-dihydroxy-vitamin D
- GIT in faeces (60%) → 6-14 mmol/day
Calcium Regulation
- Calcitonin
- 32 AA peptide with 1 disulfide bond it is the hormone of Procalcitonin
- Released from Parafollicular cells (C-cells) of thyroid
- Release Stimuli: Hypercalcaemia, gastrin, beta-agonists, dopamine, oestrogen, CCK, glucagon and secretin
- Effect:
- Increases osteoblast function/Inhibits osteoclast function
- ↓intestinal calcium reabsorption
- inhibition of renal Ca and PO4 reabsorption
- Vitamin D
- Fat soluble sercosteroid from either
- diet or
- synthesis in skin of cholecalciferol from cholesterol then processed in liver and activated in PCT of the kidney
- Release stimuli
- ↑ concentration of PTH causes ↑ 1-alpha-hydroxylase activity in kidney or ↓ Ca or PO4
- Effect:
- ↑ bone release of Ca/PO4
- ↑ intestinal and renal reabsoption of Ca and PO4
- negative feedback on PTH release
- Fat soluble sercosteroid from either
- Parathyroid hormone
- Polypeptide hormone secreted from the parathyroid gland
- Release stimuli:
- ↓ Ca (primary or due to ↑ PO4) sensed by CaSR (Ca sensing receptor) in PT chief cell
- ↓ vitamin D levels
- Effect:
- ↑ Ca/PO4 reabsorption due to ↑ osteoclast activity
- ↑ Vit-D production by kidney (effect on 25 alpha hydroxylase)
- ↑ illeal Ca reabsorption
- ↑ renal reabsoption of Ca/Mg from DCT and TAL loop of henle
- ↓ PCT phosphate reabsorption
Bisphosphonates
- Indication: Hypercalcaemia
- Action:
- Analogues of pyrophosphate in which P-O-P bond is altered to P-C-P bond that is unhydralysable.
- Deposition of the analogue to the bone prevents osteoclast liberation of Calcium
Gladwin 2016
Examiner Comments
2017A 05: 51% of candidates passed this question.
High scoring answers discussed the three major hormones involved in calcium regulation – parathyroid hormone, vitamin D and calcitonin. For each of these it was expected that candidates include: site of production, stimulus for release, inhibitory factors and actions. In the case of renin it was expected that candidates also include the actions of angiotensin and aldosterone. Very few answers discussed inhibitory factors or negative feedback loops.
2016B 01: 53 % of candidates passed this question.
As stated in the question, the distribution in plasma (not body) was expected. Candidates are reminded to include units [mmol/l].
Many candidates spent considerable effort describing the roles of calcium and particularly its role in excitation contraction coupling – which was not asked and hence scored no marks. It was expected that candidates were able to identify the roles of parathormone (PTH), 1,25 OH vitamin D and calcitonin as the major hormonal regulators. Better answers were able to describe the physiology and integration of these hormones at the gut, kidney and bone. Calcitonin and its transient and (probable) minor opposite role were also identified in these answers.
Better answers were also able to identify the permissive roles of growth hormone, cortisol and thyroxine.
2008A 07: 1 candidate (33%) passed this question.
The main points expected for a pass were:
• The components of plasma calcium are diffusible Ca ( free and complexed ) and nondiffusible Ca (protein bound ). Only the plasma free Ca is physiologically active and regulated by homeostatic mechanisms. Plasma free Ca is also affected by plasma pH and albumin concentration.
• The distribution of Ca in the body and the fact that ECF Ca is less than 0.1% of total body Ca. ECF and hence plasma Ca is the result of a balance between dietary intake, gastrointestinal absorption and excretion, renal excretion and exchange with bone Ca.
• Tight hormonal regulation of GIT absorption, bone exchange and renal excretion mainly by parathyroid hormone and calcitriol.
Also expected were details of the actions of PTH on bone and the kidney and the actions of calcitriol on the gut and bone. No candidates described the feedback control mechanisms involving PTH and calcitriol. Additional marks were given for mention of other hormones that have a lesser effect on plasma Ca concentration.
The second part of the question on the mechanism of action of biphosphonates was poorly
answered.
Syllabus N 2i
Phosphate
2020A 20
Outline the distribution, absorption, elimination, regulation and physiological role of phosphate.
CICMWrecks Answer
Phosphate
- Most abundant anion, approx. 1% of total body weight
- Normal serum level 0.8 to 1.3 mmol/L in adults
- daily requirement = 0.4mmol/kg/day
Distribution
- Mostly intracellular.
- Bone & teeth (85%), tissue (14%), ECF (1%)
- In skeleton with calcium, as hydroxyapatite crystals or amorphous calcium phosphate
- In tissue and ECF:
- 2/3 is tied up in molecules like ATP
- 1/3 in inorganic phosphate
- pH dependent
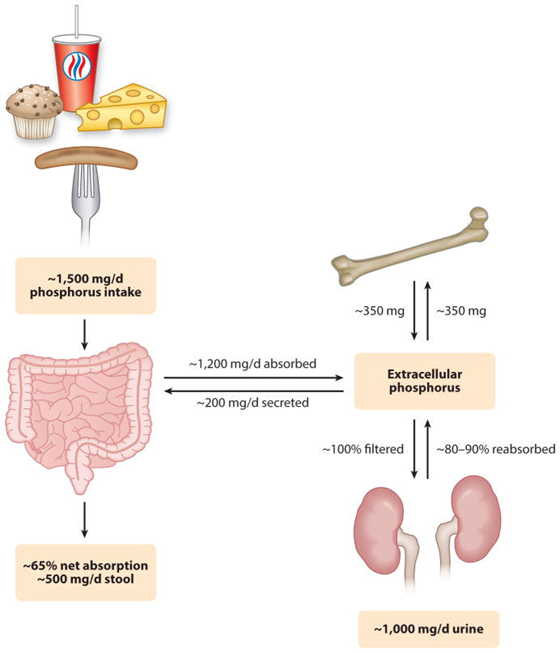
Absorption
Dietary intake 20mg/kg/day → Proximal intestine absorbs 16mg/kg/day
Elimination
- Feces:
- 3mg/kg/day secreted into intestine via pancreatic, bile, intestinal secretion
- unabsorbed 4mg/kg/day from dietary intake
- Kidney:
- 100mg/kg/day in UF.
- 80% reabsorbed in proximal tubule
- 5% in DCT
- rest excreted (~15mg/kg/day)
- 100mg/kg/day in UF.
- Net Intake-Excretion of Phosphate
- modulated based on physiological needs such as bone remodelling, bone growth, etc
- bone resorption ~300mg/day
- absorption ~300mg/day
- modulated based on physiological needs such as bone remodelling, bone growth, etc
Regulation
GI – Bone – Renal axis
Regulators:
- Dietary phosphate intake and absorption
- Calcitriol
- which increases phosphate absorption from the gut and bone
- Parathyroid hormone (PTH)
- directly causes phosphate resorption from bone and decreases its reabsorption in the proximal tubule
- indirectly by stimulating the production of calcitriol.
- Phosphatonins, such as fibroblast growth factor-23 (FGF-23):
- Inhibit renal phosphate reabsorption
- inhibit synthesis of calcitriol
Factors that alter renal regulation of phosphate:
| Increase Phosphate Absorption | Decrease Phosphate Absorption |
|---|---|
| Low-phosphate diet 1,25-Vitamin D3 Thyroid hormone | Parathyroid hormone Phosphatonins (e.g., FGF23) High-phosphate diet Metabolic acidosis Potassium deficiency Glucocorticoids Dopamine Hypertension Estrogen |
Physiological Role
- Bone and teeth formation
- Cell wall structure (phospholipids)
- Nucleic acid generation
- Glucose metabolism
- High energy bonds
- O2 transport (2,3 DPG)
- Phosphate Buffer
- intracellular signalling
- Sources
JC 2020
Examiner Comments
2020A 20: 29% of candidates passed this question.
The answer structure should have utilized the headings provided in the question. Many candidates described the physiology of calcium, which while related, did not attract marks. The distribution section required not only the sites of distribution but also the percentages found in each. The regulation should have included both primary and secondary mechanisms and an outline on the factors affecting renal excretion, intestinal absorption and release from bone etc. An outline of the physiological role of phosphate required a broad knowledge of physiological processes.
iv. Outline the composition, circulation, and functions of lymph.
I2. Intravenous Fluids
i. Understand the pharmacology of colloids and crystalloids.
2018A 17
Define the osmolality and tonicity of an intravenous fluid (20% of marks).
Compare and contrast the pharmacology of intravenous Normal Saline 0.9% and 5% Dextrose (80% of marks).
CICMWrecks Answer
Definitions
Osmolality is a measure of solute concentration as defined as the number of osmotically active particles per kilogram water.
Tonicity is the effective osmolality and is equal to the sum of the concentrations of the solutes which have the capacity to exert an osmotic force across the membrane.
Pharmacology
Examiner Comments
2018A 17: 29% of candidates passed this question.
Most candidates gave an adequate definition of osmolality and tonicity. A single concise sentence for each attracted full marks. Some candidates drew diagrams & equations, which added few marks. Some candidates confused osmolarity (mOsm/L) and osmolality (mOsm/kg).
Tonicity was best defined as the number of ‘effective’ osmols (those that cannot cross the cell membrane) in a solution relative to plasma. The use of a table greatly facilitated the comparison of 0.9% saline and 5% dextrose solutions. Values for composition, osmolarity and osmolality were poorly done. Some manufacturers state calculated values and some approximate values on the bags – both were accepted.
No candidate correctly pointed out the fluids respectively have 9g NaCl and 50g dextrose per litre.
2023B 18
Compare and contrast the pharmacology of Hartmann’s solution and 0.9% saline?
Examiner Comments
2023B 18: 17% of candidates passed this question.
This question asked for a comprehensive description of the components and chemical properties of each solution (including pH and calculated and measured osmolarity). A mechanistic description of the different acid base effects was expected. Marks were also allocated for the advantages and disadvantages of each fluid (for example the calcium in Hartmann’s risks causing precipitation when mixed with certain drugs and blood products). Lastly, it was expected that answers would provide situations where one fluid might be preferred over the other (for example saline to treat dehydration and metabolic alkalosis secondary to gastric losses – as in a pyloric obstruction). Descriptions of the physiological handling of each fluid after bolus or infusion was not required.
2015B 01
Compare and contrast 0.9% saline and 4% albumin.
Examiner Comments
2015B 01: 21% of candidates passed this question.
It was expected answers would include a comparison of the composition, physicochemical features and relevant physiology. Many candidates failed to adequately describe the differences in distribution across the body compartments or differences in physical properties of both fluids. In particular, how albumin is manufactured did not appear to be well understood by candidates.
2011B 01 – 2008B 09
Describe the physiological consequences that follow an intravenous bolus of 50mls of 50% glucose.
CICMWrecks Answer
Immediate
50mls of 50% Glucose = bolus of 25g Glucose
- Hyperglycaemia – normal blood glucose is 4-7 when fasted, <11.1 when not fasted
- Glycosuria – resorption capacity of the renal PCT is exceeded when serum glucose >10-12mmol/l
- resorption of glucose is via co-transport with Na+
Insulin
- Secreted from
- Pancreatic beta cells in islets of Langerhans
- Mechanism of secretion
- Glucose moves into beta cells via GLUT-2 transporters
- Glucose –-(glucokinase)–> Glucose-6-phosphate –-(glycolysis)–> ATP
- ATP inhibits ATP sensitive K+ channels
- Cell depolarises → calcium influx
- Calcium stimulates exocytosis of insulin-containing vesicles
- Response
- Insulin release
- Inhibits gluconeogenesis
- Promotes muscular, adipose and peripheral cell glucose uptake
- GLUT-4 transporter
- Promotes hepatic glycogenesis
- Glucose trapping
- Hexokinase phosphorylates glucose (glucose-6-phosphate)
- Negatively charged G6P cannot cross membrane, so is trapped in hepatocyte
- Stimulates glycogen synthase
- Glucose trapping
- Inhibits glycogenolyis
- Inhibits liver phosphorylase
- Promotes FFA synthesis, fat deposition
- Inhibits lipolysis
- Promotes protein anabolism, inhibits catabolism in muscle
- Insulin release
- Fate of glucose
- Glycolysis -> pyruvate
- Pyruvate and CoA -> acetyl CoA -> enters citrate cycle
- FADH and NADH from citrate cycle -> electron transport chain
- Yield from 1 glucose is around 38 ATP
- Pattern
- Biphasic release
- Phase 1: Dumping of preformed insulin
- Phase 2: Drop-off in insulin secretion, then rise up towards a higher peak when new insulin is made
- Biphasic release
Mooney 2016
Examiner Comments
2011B 01: 14 (56%) of candidates passed this question.
It was expected answers would include a comment this would transiently increase the blood glucose and result in the stimulation of insulin production. Some detail was then required on the mechanism by which insulin production is increased and time frames over which this occurred, as well as mechanism by which glucose passes through to the urine. Other important points expected were the fate of glucose, once taken up by the cells, the inhibitory effects (and mechanisms) of insulin (ie inhibits glycogenolysis, triglyceride and protein breakdown, etc). A description of the physiological consequences of increased insulin production was required. This question has been asked before, in it’s current form. The pass rate on this occasion was higher. Candidates who did poorly did so due to a lack of detail and understanding of the topic.
2008B 09: 1 (20%) candidate passed this question
This question was mainly about the metabolic consequences of an intravenous load of 25 grams of glucose. For a good pass the main areas that required description included: The transient glycosuria. The mechanism and time course of the biphasic release of insulin from pancreatic cells. The mechanism of the trapping of glucose within liver cells. The multiple actions of insulin which include inhibiting glycogenolysis and promoting glycgogen synthesis in the liver, promoting the uptake of glucose by muscle, fat and other cells, increasing the metabolism of glucose to fatty acids and triglycerides, inhibiting triglyceride breakdown and promoting protein synthesis. Additional marks were awarded for details of where these actions of insulin occurred and the enzymes that were stimulated or inhibited by insulin. Some candidates stated that this bolus of hypertonic glucose would produce a significant and prolonged increase in plasma osmolarity and then went on to produce detailed descriptions of the thirst and ADH mechanisms. In fact with a normal insulin response glucose is rapidly cleared from the blood
2015A 07
Outline the physiological responses to the rapid intravenous administration of 1 litre of 0.9 % saline to a 70 kg euvolaemic person.
CICMWrecks Answer
Assumptions
- TBW is one-third ECF & two-thirds ICF
- ECF is one-quarter plasma & three-quarters ISF
- The threshold of the volume receptors is 7-10% change in blood volume
- The osmoreceptors are sensitive to a 1-2% change in osmolality.
- Plasma osmolality is normal prior to the transfusion (ie 287-290 mOsm/kg)
Key
- TBW = Total Body Water
- ECF = Extracellular Fluid
- ICF = Intracellular Fluid
- ISF = Interstitial Fluid
1L NS has 9gm NaCl – It is an ECF replacement fluid
| Volume Litre | [Na+] mmol/L | [Cl–] mmol/L | Osmolarity mOsml/L | |
|---|---|---|---|---|
| NS | 1 | 154 | 154 | 308 |
| Plasma | 5 | 140 | 100 | 290 |
Response to 1L Normal Saline Bolus
| INITIAL PHASE | ||
| Brief Hypervolemia | → ↑ arterial BP | → Reflex response (baroceptors, atrial stretch receptors etc) |
| ↑ Renal perfusion → Stimulation of intra renal apparatus (juxtaglomerular apparatus) | ||
| [Na+] | 154/L into 140/L | very negligible rise |
| [Cl–] | 154/L into 100/L | rises 1L NS unlikely to cause hyperchloraemic metabolic acidosis |
| REDISTRIBUTION PHASE (~15 minutes) | ||
| [Na+] similar to ECF → Distributes | ISF (3) | ↑750ml |
| Plasma (1) | ↑250ml | |
| EXCRETION PHASE (~6 hours) | ||
| Plasma osmolality and tonicity mostly unchanged | no osmoreceptor stimulation | |
| Plasma volume ↑ to 5250mls (5% increase) | below sensitivity of volume receptor → no volume receptor stimulation | |
| No protein → oncotic pressure slightly lowered | Fluid moves into ISF (Starling’s hypothesis) | |
| Glomerulo-tubular Imbalance → ↑GFR → ↓ reabsorption of water in proximal tubule → ↑ Urine flow | ||
| Fluid moves back into intravascular compartment till all infused fluid is excreted (~6hrs) | ||
Examiner Comments
2015A 07: 25 % of candidates passed this question.
Answering this question required the integration of information from areas of cardiovascular, body fluid and renal physiology which proved difficult for most candidates. Both breadth and depth was expected so as to score well.
This question is best answered using a time-based approach. For example, upon the rapid infusion of a litre of normal saline there will be a brief period of hypervolemia, increase in arterial blood pressure and an associated physiological reflex response to these changes (e.g. baroreceptors, atrial stretch receptors, etc.). There will also be an associated increase in renal perfusion and stimulation of intrarenal receptors (e.g. juxtaglomerular apparatus).
Candidates were expected to outline these changes, their effector responses (e.g. autonomic nervous system reflexes and humoral changes) and their physiological consequences.
A more prolonged redistribution phase of the administered saline then occurs. This saline redistributes throughout the extracellular fluid space. Candidates were expected to briefly describe this effect as well as the subsequent management of the sodium and water load by the kidney.
Most candidates spoke about the pressure effects, and only some compared these with the volume effects. The effect of redistribution and other effects were not considered by the majority of the candidates.
2019B 01
Describe the physiological consequences of the oral ingestion of 1 litre of water in a young adult.
CICMWrecks Answer
Water
- Water is distributed across all body compartments
- It is hypotonic compared to all body compartments
- It theoretically gets distributed to ECF (Plasma and ISF) + ICF
- Absorption of ingested water occurs in proximal small intestine.
- Absorption rate depends on rate of gastric emptying and creation of suitable osmotic gradient across the intestinal mucosa.
- 1L Oral ingestion of water is rapidly absorbed in the small intestine.
Mechanism of Absorption of water
- Sodium is absorbed from the intestinal lumen by several mechanisms, most prominently by cotransport with glucose and amino acids, and by Na+/H+ exchange, both of which move sodium from the lumen into the enterocyte.
- Absorbed sodium is rapidly exported from the cell via sodium pumps – when a lot of sodium is entering the cell, a lot of sodium is pumped out of the cell, which establishes a high osmolarity in the small intercellular spaces between adjacent enterocytes.
- Water diffuses in response to the osmotic gradient established by sodium – in this case into the intercellular space. It seems that the bulk of the water absorption is transcellular, but some also diffuses through the tight junctions.
- Water, as well as sodium, then diffuses into capillary blood within the villus.
Water compartments and Responses
- TBW is one-third ECF & two-thirds ICF
- ECF is one-quarter plasma & three-quarters ISF
- The threshold of the volume receptors is 7-10% change in blood volume
- The osmoreceptors are sensitive to a 1-2% change in osmolality.
- Plasma osmolality is normal prior to the transfusion (ie 287-290 mOsm/kg)
Effect of 1L oral intake
- Absorption of water causes a drop in plasma osmolarity without much effect on plasma volume.
- There is a brief period of hypervolemia, increase in arterial blood pressure and an associated physiological reflex response mainly by osmoreceptors
- There will also be an associated increase in renal perfusion and stimulation of intrarenal receptors (e.g. juxtaglomerular apparatus).
- Sensor: Osmoceptors in hypothalamus
- CPU: Hypothalamus
- Effector: ADH (and thirst, not significant in current scenario)
- Anti-diuretic hormone (ADH)
- peptide hormone synthesised in the hypothalamus
- released from the posterior pituitary
- acts on the kidney via GPC V2 receptors
- increases the synthesis of aquaporins
- ADH secretion is decreased,
- ➔ water permeability in the nephrons is increased
- ➔ leads to less water reabsorption
- ➔ larger amount of dilute urine excretion
- Consequence is homeostasis by excretion of the 1L of water quite rapidly.
JC 2019
Examiner Comments
2019B 01: 28% of candidates passed this question.
It was expected candidates would provide details the consequences of water ingestion from its rapid absorption in the small intestine to the resultant impact on plasma osmolarity and the minimal impact of plasma volume of this volume. Some detail on the mechanisms of absorption (transcellular vs osmosis) was expected and the distribution of water across body fluid spaces.
Many candidates accurately described the small drop in plasma osmolarity that is sufficient to trigger osmoreceptors with better answers providing details of the locations and mechanisms involved. The physiological consequences of inhibition of ADH, including the renal effects of decreased water permeability in distal renal tubules and collecting ducts. The volume load after distribution would be lower than the plasma volume triggers for the circulatory reflex responses.
2015A 10
Compare and contrast the pharmacology of mannitol and hypertonic saline.
Examiner Comments
2015A 10: 8 % of candidates passed this question.
A structured approach is important and a table worked best for most candidates, although a few attempted this in free text. Despite attempting a structured answer very few candidates provided information in regards to preparation, dose, monitoring of osmolarity, adverse effects or contraindications. Understanding of the action of these drugs was expected and factual inaccuracies were common with many candidates suggesting hypertonic saline acts as an osmotic diuretic. Better answers mentioned other potential mechanisms of action of mannitol. Many candidates failed to appreciate the impact on raised intracranial pressure.
2020B 12
Describe the physiology (50% marks) and pharmacology (50% marks) of albumin.
CICMWrecks Answer
Physiology of Albumin
| ALBUMIN | |
| DEFINITION | A naturally occurring protein found in blood plasma. Recombinant human albumin is a product that is manufactured to be structurally equivalent to native human serum albumin. |
| CLASSIFICATION | 6 subfamilies – vit D binding proteins occupy family 1-2 |
| SYNTHESIS | In liver |
| Synthesized as preproalbumin → N-terminal peptide removed → proalbumin → cleared in Golgi vesicle → albumin | |
| FACTORS AFFECTING SYNTHESIS | Nutrition |
| Liver function | |
| infection /sepsis/inflammation | |
| DISTRIBUTION | 40% intravascular space 60% extravascular space |
| The balance between plasma and interstitial space is established at varying rates with respect to the two subcompartments of the extravascular albumin pool | |
| Factors affecting distribution: HTN, CCF, trauma, exercise, DM, Burns, CPB | |
| BREAKDOWN | Broken down by cysteine protease |
| Elimination half life – 16hrs | |
| FUNCTIONS OF ALBUMIN | |
| Osmotic pressure | supplies 80% of total plasma Colloid Osmotic Pressure |
| retards fluid efflux from plasma and oedema formation | |
| Transport and metabolism functions | Transports thyroid hormones |
| Transports other hormones, in particular, ones that are fat-soluble | |
| Transports fatty acids (“free”) to the liver and to myocytes for utilization of energy | |
| Transports unconjugated bilirubin | |
| Transports many drugs; serum albumin levels can affect the half-life of drugs | |
| Buffer | Extra-cellular acid-base buffer |
| Detoxification | solubilizes bilirubin and neutralizes its toxic effects |
| Anti-oxidant effects | Blocks Cu2+-mediated LDL oxidation |
| Blocks Free-radical-mediated haemolysis | |
| Anticoagulant effect | |
| Protein store | for signalling molecules and nitric oxide |
| Immunomodulation | |
| Other | Competitively binds calcium ions (Ca2+) |
| Prevents photodegradation of folic acid | |
| marker of an inflammatory state | |
| prevents apoptosis of proximal renal tubular cells | |
| stimulates proliferation of proximal renal tubular cells | |
| MANUFACTURING PROCESS | |
| Source | From Aus-sourced plasma Pooled from thousands of donations (minimises the infection risk) |
| Whole plasma is fractionated to prepare Albumin | |
| Fractionation | Physical separation (by precipitation) |
| Chromatography (separates molecules based on chemical and physical properties | |
| Removal of pathogens by: – Pasteurisation (vapour heat 60C for 10hrs) – Use of solvents and detergents – Exposure to low pH conditions – Nanofiltration | |
| Partitioning | allows purification of immunoglobulins, clotting factors and albumin from plasma |
| Storage | stored in secure & monitored environments |
Pharmacology of Albumin
Guo / JC 2021
Examiner Comments
2020B 12: 19% of candidates passed this question.
The question required an equal treatment of the physiology and pharmacology of albumin. The physiology discussion needed to include synthesis, factors affecting synthesis, distribution in the body (including the proportion divided between the plasma and interstitial space), functions, breakdown, and elimination half-life. Discussion of the pharmacology should have included available preparations (4% and 20% Albumin) and pharmaceutics, distribution, elimination (both the protein and crystalloid components), mechanism of action to expand the plasma compartment, longevity in the plasma compartment, indications, and adverse effects. Oedema, circulatory overload, immunological reactions, and relative contraindication in brain injury were important to mention. There was some confusion regarding the infectious risks of albumin. An outline of the manufacturing process from donated plasma and pasteurisation was expected.
2022A 09
Describe the pharmacology of 4% albumin.
Examiner Comments
2022A 09: 41% of candidates passed this question.
This question required the candidate to consider 4% albumin from a pharmacological perspective. The examiners were therefore after a description that included presentation, pharmaceutics (including correct content description and osmolality), indications, pharmacodynamics, pharmacokinetics, adverse effects, special precautions and dosing.
I3. Measurement of Body Fluids
i. Describe the regulation of osmolality and outline its measurement.
ii. Describe the principles of estimating body fluid compartments.
VIVAs
| 2023B | Body water distribution, measurement of compartments |
| 2023A | |
| 2022B | |
| 2022A | |
| 2021B | |
| 2021A | Blood plasma and non-protein components IVF, effects. Large volume (2-3L) infusion NS |
| 2020B | 1L blood loss over 10 minutes Water content 70kg male, distribution, regulation |
| 2019B | Total body potassium regulation 5L NS on acid base |
| 2019A | |
| 2018B | |
| 2018A | |
| 2017B | intravenous fluids and buffers. Diagram – dluid distribution healthy 70kg male |
| 2017A | |
| 2016B | Physical principles around flow and viscosity, Venous anatomy of UL+chest, in vivo blood clotting |
| 2016A | fluid flow and related physical principles around turbulent and laminar flow, invasive BP monitoring |
| 2015B | |
| 2015A | |
| 2014B | IVF, colligative properties, anion gap, frusemide |
| 2014A | |
| 2013B | |
| 2013A | |
| 2012B | |
| 2012A | NS, osmol, colligative properties, anion gap, diuretics |
| 2011B | |
| 2011A | Hartmann’s, colloid vs crystalloid, semipermeable membrane |
| 2010B | |
| 2010A | |
| 2009B | |
| 2009A | |
| 2008B | |
| 2008A | Outline the distribution of total body water |
| 2007B |

Recent Comments