Syllabus (Fourth Edition, 2023)
Topics
i. Understand the pharmacology of inotropes and vasopressors.
ii. Understand the pharmacology of anti-hypertensive drugs.
iii. Understand the pharmacology of anti-arrhythmic drugs.
iv. Understand the pharmacology of anti-anginal drugs.
Topics not covered in previous SAQs
.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
i. Understand the pharmacology of inotropes and vasopressors.
2017B 15 – 2012B 18
List the properties of the ideal inotrope (50% of marks).
How does adrenaline compare with respect to these ideal properties? (50% of marks)
CICMWrecks Answer
An Ideal Inotrope is pharmaceutically suitable, has beneficial pharmacodynamic properties, has an excellent pharmacokientic profile.
PHARMACEUTIC
| IDEAL INOTROPE | ADRENALINE |
|---|---|
| Non-toxic | Y |
| Cost effective | Y |
| Stable preparation | Y |
| Compatible with other drugs | Y |
| Peripherally deliverable | Y |
| Multiple preparations | Y |
| Stable in all solutions | N (Oxidises to adenochrome in alkaline solutions, turns pink) |
PHARMACODYNAMIC
| IDEAL INOTROPE | ADRENALINE |
|---|---|
| Increases contractility | Y |
| Increases mean arterial pressure, Maintenance of diastolic blood pressure | Y |
| Increases cardiac output | Y |
| Improves regional perfusion, without pharmacodynamic costs | N |
| No increase in myocardial oxygen consumption | N (increases myocardial oxygen consumption) |
| Avoidance of tachycardia, Non-arrhythmogenic | N (causes tachyarrhythmias) |
| Suitable in pregnancy and paediatric populations | Y |
| No Adverse effects | N (hyperglycaemia, lactic acid production, worsens pulm HTN, Peripheral necrosis) |
PHARMACOKINETIC
| IDEAL INOTROPE | ADRENALINE |
|---|---|
| Does not develop tolerance | N |
| Titratable | Y |
| Rapid onset, Rapid termination of action | Y |
| Metabolised independent of liver and kidney function | N (some hepatic metabolism) |
| Doesn’t require concentration monitoring | Y |
JC 2019
Examiner Comments
2017B 15: 98% of candidates passed this question.
Many candidates scored very highly on this core topic. It was expected information be included on pharmaceutics, cost, availability and compatibilities. Relevant pharmacokinetics (onset/offset, titratability) and pharmacodynamics (including relevant receptors, nuances of haemodynamic effects e.g. effect on diastolic pressure and regional perfusion) should have been detailed. Adverse effects and safety profile (e.g. use in pregnancy, therapeutic index) should also have been included.
Good answers were structured and highlighted differences with specific facts and data
2012B 18: 19 (86.4%) of candidates passed.
Inotropes are drugs that increase the force and velocity of myocardial contraction resulting in increased contractility and stroke volume and hence cardiac output. Good answers were those that adopted a systematic approach, such as providing a coherent list of ideal properties that included pharmaceutical, pharmacokinetic and pharmacodynamics characteristics, and then contrasted adrenaline against that list. The area less well covered was that of those aspects of adrenaline that made it less than an ideal inotrope, e.g. it increases myocardial oxygen consumption, causes tachyarrhythmias, tolerance may develop, hyperglycaemia, lactic acid production, etc.
2014B 01
Classify commonly used inotropic agents. (40% of marks)
Outline four different mechanisms of action for inotropic agents. (60% of marks)
2010A 07
Classify the commonly used inotropic agents and describe their mechanism of action.
2008A 12
Classify the commonly used inotropic agents and list their mechanisms of action.
CICMWrecks Answer
- Inotrope: Agent that alters the force of contraction of a muscle
- Inotropes can be classified in an number of ways:
- Negative or positive (the examiners did not want a discussion of negative inotropes on previous reports)
- Naturally occurring: Eg Adrenalin, Noradrenalin, Dopamine
vs Synthetic: Dobutamine, dopexamine, isoprenalin, salbutamol
Classification by Mechanism of action is most effective
Postitive Inotropes
Class 1: Increase intracellular Calcium by a variety of mechanisms → ↑force of contraction
- Direct Adrenoceptor agonists (NA, Adren):
- ↑ intracellular cAMP and ↑ Ca by Gs protein coupled mechanism
- Indirect adrenoceptor agonists (Ephedrine):
- displacing NA from vesicles into cytoplasm
- ↑’s carrier-mediated diffusion into synaptic cleft
- ↑NA release at nerve terminal → ↑’d stimulation
- Phosphodiesterase inhibitors (Theophyline, Milrinone):
- inhibit PDE → ↓breakdown of cAMP (cGMP) → effective ↑cAMP
- cAMP effects as per direct adrenoceptor agonists
- Glucagon
- Bypasses adrenergic receptor to stimulate ↑cAMP
- Na/K atpase inhibitors (digoxin):
- Inhibit Na+/K+-ATPase → ↑[Na+]i → Impair Na+/Ca2+ exchange pump → ↑[Ca2+]i
Class 2: Increase sensitivity of actin/myosin complex to calcium by action on troponin C
- Levosimendin
- ↑Ca interaction with troponin C → enhance contractility without ↑[Ca]i
- Activate KATP channels on mitochondrial membrane → protect muscle from ischaemia leading to ischaemic preconditioning
Class 3: Act via metabolic or endocrine pathways (T3, Ca, Mg)
Negative Inotropes
- Beta-blockers
- Usually β1 blocking action
- Inhibits the action of natural stimulation of Class 1 above
- Calcium-channel blockers are used for treating high blood pressure, chest pain, and irregular heart rhythm.
- ↓ intracellular Ca via action on L-type calcium channels
- nifedipine, amlodipine
- Centrally acting sympatholytics are used for treating high blood pressure.
- Act centrally on α2 receptors to decrease sympathetic tone.
- Clonidine
Gladwin 2016
Pharmacopeia Table
Examiner Comments
2014B 01: 65% of candidates passed this question.
This question was generally well answered. The poorer answers suffered for want of a useful classification system that enabled them to separate the various drug classes
2010A 07: 6 (60%) of candidates passed this question
This question required a classification based on chemical structure and class action. Sympathomimetics, phosphodiesterase inhibitors, calcium sensitizers and cardiac glycosides should have been mentioned. Additional detail was expected, subdividing Sympathomimetics into catecholamines (naturally occurring and synthetic), and non-catecholamines (direct and indirect acting). Further classification based on peripheral vasomotor action demonstrated greater understanding.
Better answers included diagrams illustrating the mechanism and point of action on the cardiac myocyte. Discussion of receptors, second messengers, and the role of calcium was essential.
The question was aimed at “commonly used” agents, although some marks were awarded for discussion of calcium, glucagon and other rarely used drugs.
Insufficient detail regarding mechanisms of action was a common observation.
Syllabus: C2d 2
References: Pharmacology and Physiology in Anaesthetic Practice, Stoelting 4th Ed p293-320. Basic and Clinical Pharmacology Katzung 10th Ed p121-198. Pharmacology Rang & Dale 6th Ed p168-187, 290-291
2008A 12: No candidates (0%) passed this question.
Candidates could use a number of different classifications, however, were required to include all of the major groups of agents. Most made some mention of the sympathomimetics, however failed to sub-classify these, or confused catecols versus non-catecols, or naturally occurring versus synthetic agents. Other agents, such as phosphodiesterase inhibitors, calcium sensitisers, cardiac glycosides, or calcium itself received minimal attention.
Mechanisms of action required more than listing adrenergic receptor types. Some listing or discussion of the sub-cellular mechanisms was necessary. Comment about intracellular calcium being the final common end-point would have scored additional marks
Syllabus C2d 2
Reference: Stoelting 4th edition 293-320, Katzung 10th edition 121- 198, Rang and Dale 6th edition 168-187 290-291
2021A 01
Describe the pharmacology of adrenaline.
Examiner Comments
2021A 01: 90% of candidates passed this question.
Adrenaline is a level 1 drug and is commonly used in intensive care. A comprehensive explanation of the drugs MOA, PK, PD and side effect were expected. Candidates who scored well generally provided a factually accurate, detailed and well-structured answer. Overall, the quality of answer provided for this question was of a high standard.
2009B 12
Outline the pharmacology of noradrenaline.
Examiner Comments
2009B 12: 2 (22%) of candidates passed this question.
Candidates should expect that questions relating to “the pharmacology of ……” are likely to be common. Thus candidates should have prepared structured approach for any such question. For example, one that includes predefined major categories such as pharmacodynamics and pharmacokinetics and sub-categories such as mechanism of action, absorption, preparations, bioavailability, volume of distribution, metabolism, elimination, adverse effect, clinical indications, precautions/interactions, etc. and the information relevant to each category. Failure to take a structured approach to such questions, as was observed amongst some candidates within this exam, risks omission of vital facts (and not gaining marks) and errors. Noradrenaline is such a common drug within intensive care practice and so candidates would be expected to know it in great detail. There are many references for it, such as the ones listed below.
Syllabus – G3a, 2b
References – Goodman and Gillman Chp 10 and Katzung.
2017A 20
Describe the pharmacology of vasopressin (70% of marks) and its analogues (30% of marks).
2013A 22
Describe the actions of endogenous vasopressin. (60% of marks)
List the vasopressin analogues and their uses. (40% of marks)
Examiner Comments
2017A 20: 28% of candidates passed this question.
A pharmacology answer template outlining pharmacokinetics and dynamics was required. Candidates failed to score marks for describing the physiology of vasopressin secretion. A number of answers demonstrated limited knowledge about its indications for use and its potential adverse effects.
2013A 22:
Overall the first section was not answered in sufficient detail. Aspects such as the antidiuretic effects of vasopressin were often overlooked. Some candidates spent more time on outlining the clinical contexts in which vasopressin is used (which did not score marks), rather than the physiology (which did score marks). DDAVP was the most common analogue mentioned, with the others often being omitted (e.g. terlipressin, ornipressin, etc.).
2023A 20
Compare and contrast the relevant pharmacology of intravenous adrenaline and vasopressin.
Examiner Comments
2023A 20: 20% of candidates passed this question.
The major emphasis of this question and opportunity to score marks reside in “comparing the two drugs” in various aspects – pharmaceutics, indication, mechanism of action, pharmacodynamics, and pharmacokinetics. Although most of the candidates were able to list pharmaceutics, indications, kinetics and dynamics of both the drugs in reasonably structured and tabulated format, many failed to highlight the important commonalities and differences between the two. In mechanism of action, details of the receptor, their location and second messenger system were expected. In pharmacodynamics, similarities and differences in cardiovascular, respiratory, haematological, renal and metabolic effects were needed. There are additional neurological effects and genito-urinary (tocolysis and sphincter tone) of adrenaline which were rarely mentioned. There were frequent significant omissions or incorrect details in the pharmacokinetics sections of both drugs.
2020A 10 – 2011A 23 – 2007B 07
Compare and contrast the pharmacology of Noradrenaline (Norepinephrine) and Vasopressin.
Examiner Comments
2020A 10: 49% of candidates passed this question.
These are both level 1 drugs regularly used in intensive care. Significant depth and detail of each drug were expected. Overall knowledge was deemed to be superficial and lacked integration. Better answers identified key points of difference and overlap in areas such as structure, pharmaceutics, pharmacokinetics, pharmacodynamics, mechanism of action, adverse effects and contraindications. A tabular list of individual drug pharmacological properties alongside each other did not score as well as answers which highlighted key areas of difference and similarities.
2011A 23: 6 (50%) of candidates passed this question.
A straightforward question that was reasonably answered, with most candidates using a methodical tabular approach to explaining the differences in pharmacology between noradrenaline and vasopressin. The benefits of adhering to a wellorganised system of columns showing direct comparisons of the various relevant drug characteristics was clear, with candidates who chose this approach covering most of the necessary information in a clear and comprehensive manner. Some candidates managed to provide a great deal of relevant detail within the allocated time as would be expected in this relatively uncomplicated question. Simple definitions were often lacking and failing to provide this basic introductory information resulted in lower marks for this question. While most candidates were able to discuss the effects of each drug on the cardiovascular system, not as many were able to give outline other physiological effects in sufficient detail. For example, many candidates made little mention of important renal, metabolic and haematological effects. While some candidates discussed pharmacokinetics well, many provided only a very superficial outline of this aspect. The important area of adverse reactions could also have been covered in greater detail.
Syllabus: C2d, 2a and N2, 2f
Recommended sources: Basic and Clinical Pharmacology, Katzung, Chp 9 and 37
2007B 07: 5 candidates (71%) passed this question.
This was best answered using a table. The main points expected for a pass were
- Both are naturally occurring substances
- Direct acting via receptorrs
- Mechanisms by which both increase mean arterial pressure
- Metabolism
- Uses in Intensive Care, septic shock, vasodilatory shock and diabetes insipidus
- Side effects related to intense vasoconstriction and for vasopressin possible coronary ischaemia and sodium and water retention
2016B 15
Compare and contrast the pharmacology of noradrenaline and dobutamine.
Examiner Comments
2016B 15: 84% of candidates passed this question.
The best answers used tables and key pharmacological headings for comparisons, and avoided long sentences/ paragraphs.
An answer that correctly considered the following sections would be awarded a very good pass: Presentation, pharmacodynamics, mechanism of action, organ effects, side effects and pharmacokinetics.
Many candidates failed to identify agents as natural / synthetic catecholamines. Few answers correctly mentioned the available preparations of these drugs or considered the structure activity relationships. Only 3 candidates commented that dobutamine is a racemic mixture.
Intracellular second messenger pathways were often incorrectly recounted or not mentioned at all. Pharmacodynamic effects on all organ systems, and all CVS parameters (HR, inotropy, PVR, SVR, SBP/DBP/MAP, regional circulations) should be considered. Metabolic fate and clinical dosage ranges were frequently incorrectly quoted.
2019B 18
Compare and contrast the pharmacology of metaraminol and noradrenaline.
Examiner Comments
2019B 18: 71% of candidates passed this question.
Marks were distributed across pharmaceutics, uses, dose & administration, mechanism of action, Pharmacokinetcs and Pharmacodynamics. Common omissions were doses/rates of infusion, effects other than on heart/SVR (e.g. splanchnic, renal blood flow), indirect effect of metaraminol, receptor effect of noradrenaline other than alpha 1 and tachyphylaxis.
2022B 13
Compare and contrast the pharmacology of EPHEDRINE and METARAMINOL.
Examiner Comments
2022B 13: 27% of candidates passed this question.
The ‘compare and contrast’ pharmacology question indicates the use of a standardised structure that incorporates pharmaceutics, pharmacokinetics and pharmacodynamics. The best answers provided excellent detail, ie. precise descriptions of mechanisms of action and emphasised noteworthy areas of contrast between the two drugs. Highlighting opportunities for use and areas of caution/drug limitations.
Overall, most candidates seemed to have a sufficient knowledge of metaraminol but details surrounding ephedrine were often lacking.
2011B 14
Compare and contrast the pharmacology of dobutamine and milrinone
Examiner Comments
2011B 14: 14 (56%) of candidates passed this question.
Many candidates presented their information in a tabular form and this worked well as it allowed direct comparison between the two drugs. Most candidates did not mention that dobutamine was a racemic mixture of [+] and [-] isomers. Also that the [+] isomer was a potent β1 agonist and α1 antagonist, while the [-] isomer was an α1 agonist. The administration of the racemic mixture results in the overall β1 agonism responsible for its activity and also its mild β2 agonist effect. While most candidates stated that milrinone was an inodilator details on its mechanism of action as a selective phosphodiesterase type III inhibitor were on the whole vague. Within the cytoplasm of the cardiac myocyte milrinone inhibits the enzyme PDE 3 which results in the inhibition of the breakdown of cyclic AMP which in turn results in elevated cellular levels of cAMP. These elevated levels of cAMP in turn activate cAMP dependant protein kinases with a resultant increase in the influx of Ca2+ into the cell via the sarcolemma. Also uptake of Ca2+ by the sarcoplasmic reticulum is increased. The overall effect is an increase in intracellular Ca2+ which increases myocardial contractility. Milrinone also has lusitropic action, inducing left ventricular relaxation. This probably occurs as a result of the inhibition of SR membrane bound PDE3.
Milrinone also cause peripheral vasodilatation by inhibiting PDE3 in vascular smooth muscle cells which again results in elevated cAMP levels.In vascular smooth muscle cAMP normally inhibits myosin light chain kinase the enzyme that is responsible for phosphorylating smooth muscle myosin and causing muscle contraction.
Many candidates did not emphasize how very different the pharmacokinetics of these two drugs were. Milrinone has a much longer half life than dobutamine and because of its predominant renal excretion accumulates in renal failure.
Syllabus: C2d 2a
Recommended sources: Goodman & Gillman The pharmacological Basis of Therapeutics Chp 33.
2020B 15
Compare and contrast the pharmacology of dobutamine and levosimendan.
Examiner Comments
2020B 15: 41% of candidates passed this question.
The objective of this question was that candidates relay a detailed knowledge of both drugs with respect to their individual pharmacology highlighting the important clinical aspects of each drug (e.g., mechanism of action, metabolism, duration of effect). Then an integration of this knowledge was in the contrast section where the better candidates highlighted features of the drug that would influence when or why one may use it with respect to the second agent. Tabular answers of the pharmacology of each drug without any integration or comparison scored less well. A detailed knowledge of both agents was expected to score well.
2018A 02
Compare and contrast the pharmacology of adrenaline and milrinone.
Examiner Comments
2018A 02: 45% of candidates passed this question.
This question was best answered using a table. Better answers included: the mechanisms of action, the pharmacokinetics and pharmacodynamics, indications for use and adverse effects. To complete the answer, the two drugs should have been compared and contrasted. There are many areas which could be contrasted e.g. different indications, different mechanisms of action, different half-lives and duration of action, different metabolism and different pharmacodynamic effects, in particular the effects on the cardiovascular system and the pulmonary circulation. Similarities should also have been highlighted.
2012A 08
Compare and contrast adrenaline and levosimendan
Examiner Comments
2012A 08: 6 (60%) of candidates passed.
A basic and fundamental question which required candidates to present their answer in a coherent fashion (a table worked best), as well as demonstrate sufficient knowledge. The majority of candidates did so, and so scored well. Candidates tended to struggle most with levosimendan. Candidates also confused the use of the terms “elimination” and “metabolism”, often using them interchangeably.
2013B 10
Compare and contrast the mechanism of action, pharmacokinetics, pharmacodynamics, and adverse effects of digoxin and levosimendan.
Examiner Comments
2013B 10: 11 candidates passed (40.8%).
This question provided candidates with a clear structure and headings that were often ignored. Candidates wasted time on pharmaceutics, derivation (“foxglove” mentioned often) and dosing – these were not requested and scored no marks. Superficial answers such as “cardiac glycoside” or “calcium sensitiser” were not adequate. Responses such as “modest” for Vd are inadequate – marks could be gained for identifying at least the direction of the difference between the two agents. Likewise “hepatic metabolism and renal excretion” is inadequate. Both agents had quantitative and qualitative differences in outcome of metabolic products and the renal elimination of active drug. Confusing diagrams with inadequate labelling, arrows with two heads and the use of uncommon abbreviations without definition all served to confuse the examiners rather than help the candidate. Candidates should read the questions carefully.
ii. Understand the pharmacology of anti-hypertensive drugs.
2010B 24
Classify antihypertensive agents by their mechanism of action, with a brief outline of each mechanism, and an example of a drug in each class.
CICMWrecks Answer
Classification of Antihypertensive Agents
- ADRENERGIC AGENTS
- Alpha antagonists – prazosin is an alpha 1 antagonist which blocks the Gp protein, reducing phospholipase C, DAG, IP3 and intracellular calcium, causing vasodilatation and thus reducing TPR and BP.
- Beta antagonists – metoprolol is a beta antagonist which blocks the GS protein and thus reduces intracellular cAMP, causing a reduction in heart rate and cardiac output
- Mixed alpha/beta antagonists – labetalol, carvedilol
- Alpha-2 agonists – clonidine, methyldopa. Act on the alpha 2 receptor to reduce central sympathetic outflow
- RAAS AGENTS
- ACE’s and ARBs block the effects of angiotensin II (namely, vasoconstriction, sympathetic stimulation, sodium and water retention via afferent/efferent arteriolar constriction and upregulation of sodium resorption along the length of the renal tubule, stimulation of aldosterone release and ADH).
- Examples include lisinopril (ACEI) and irbesartan or candesartan (ARB)
- CA CHANNEL BLOCKERS
Specifically block the L type calcium channels to reduce cardiac contractility and heart rate. In vascular endothelial cells, calcium channel blockade reduces intracellular calcium and causes vasodilatation, reducing TPR and thus BP. Three classes:- Class 1 phenylalkylamines (verapamil) – reduces HR, contractility and causes vasodilatation
- Class 2 dihydropyridines (amlodipine) – main effects are on vasodilatation
- Class 3 benzothiazepines (diltiazem) – some reduction in HR and contractility, also causes vasodilatation
- DIURETICS
There are multiple diuretics but in general they work by reducing renal sodium and hence water retention, thus reducing blood volume. Some (including frusemide) also have an effect on TPR prior to the diuretic effect occurs.- Acetazolamide works in the proximal tubule to inhibit carbonic anhydrase
- Frusemide acts in the TAL of LOH inhibiting the triple symporter
- Thiazides act in the early distal tubule inhibiting the Na/Cl symporter
- Amiloride acts in the cortical collecting duct by blocking ENaC.
- Spironolactone: Aldosterone antagonist, inhibits Na.K.ATPase activity.
- VASODILATORS
- Nitrate drugs – eg, GTN. NO activates soluble guanylate cyclase to causes vasodilatation
- Hydralazine – mechanism not entirely clear, possibly via guanylate cyclase, causes arteriolar vasodilatation
- Minoxidil – direct arteriolar dilator, possibly via K channels
- Nicorandil – K-ATP channel activator which also contains a nitrate moiety
JC 2019
Pharmacopeia Tables: Antihypertensives
Examiner Comments
2010B 24: 10 (67%) of candidates passed this question.
There are many valid lists that can be used as a template to answer this question. One such list might broadly classify antihypertensive agents into sympatholytic agents, vasodilators, calcium channel antagonists, renin-angiotensin inhibitors and diuretics. Within each of these categories are a variable number of sub classes, for example diuretics might include thiazides, loop diuretics and potassium sparing diuretics.
A good answer would include such a listing with a brief description of the mechanism of action with respect to the antihypertensive effect and the name of a typical drug that acts in the manner described. Most candidates were able to generate such a list and populate it as required by the question, thus being rewarded with good marks. Poorer answers lacked any logical classification system and were merely a random list of antihypertensive drugs and their actions.
Candidates are reminded that organisation within an answer helps in answering the question and achieving marks.
Syllabus: C2b,2e
References: Berne & Levy, Physiology, Ch 2-3
2007B 17
List the potential clinical uses of an alpha 2 adrenoceptor agonist. Outline the limitations of clonidine for each use.
CICMWrecks Answer
Potential Clinical Uses of α2 adrenoreceptor agonist
| α2 Agonist Uses | Clonidine |
|---|---|
| Essential and secondary Hypertension Hypertensive Crisis | • Marked hypertensive response (α1) – Seen in infusion acutely, not common with PO • Hypertensive crisis on withdrawal (2° upregulation of NA with chronic use) following – long infusion – cessation of chronic use PO • Prolonged refractory ↓MAP 2° to prolonged elimination t½ 9-18hrs |
| Migraine | • Hypotension • Sedation • Prolonged offset |
| Chronic Pain | • Ceiling effect (partial agonist) • Dose limited by side-effects (dry-mouth, hypotension, sedation) |
| Opiate Withdrawal | • Dose limited by side-effects (dry-mouth, hypotension, sedation) |
| Spinal anaesthesia / ↓ post-op shivering | • Ceiling effect (partial agonist) • Long time to peak effect • Long elimination t½ 9-18hrs |
| ICU Sedation or Anaesthetic Augmentation (50% MAC sparing) Procedural Sedation | • Long time to peak effect (~90min IV, 3hrs PO). • Prolonged refractory ↓MAP limits use in peri-operative setting • Dexmedetomadine preferred due to shorter t½ |
| Palliation | • Treatment of symptoms of distress (intractable pain, agitation or delirium) at the end of life. • Limited by hypotension and sedation |
| Antiemesis | • Limited use due to side effects of sedation and dry mouth |
Alpha 2 Adrenoceptor Details (include some details if you have time)
- Gi-PCR (↓Adenylyl cyclase activity → ↓ cAMP → ↓ PKA activation → Effects)
- Three main types 2A, 2B and 2C vary by location.
Important Locations:
- Postsynaptic α2 receptors in CNS
- Locus coerrulus (hypnosis)
- Spinal cord (analgesia)
- Lateral Reticular Nucleus (↓SNS tone, ↑ PSNS tone)
- Presynaptic α2 receptors in Sympathetic terminals of blood vessels
- ↓Noradrenalin release (vasodilation)
Clonidine
Class: Imidazole Derivative
Administration: PO 1-2 mcg/kg oral for premed, IV (150mcg in 2mL) 1-2mcg/kg bolus, Relatively long-time to peak effect (~90min IV, 3hrs PO)
Mechanism:
- Relative selectivity α1:α2 = 1:400 (vs dexmedetomadine (1:1600))
- Stimulation of presynaptic alpha 2 receptors
- ↓’s NAd release from sympathetic nerve terminals via negative feedback mechanism.
- Hypnosis
- Hyperpolarisation of Lateral reticular nucleus → ↓ central SNS tone and ↑ central PSNS tone
- Anti-emesis
- CTZ desensitisation, ↓ Intragastric pressure, anti-sialogogue
- Analgesia (dorsal horn of the spinal cord)
- Augment endogenous opiate release
- ↓Aδ- C fibre afferent activity
- Modulates descending noradrenergic pathways (↓’d Norad and Neuropeptide Y release)
- Long term dosing → ↓ responsiveness of peripheral vessels to vasoactive substances
For Further Reading:
Click to Open CICMWrecks Table: Alpha-2 agonist agents
CICMWrecks 2021
Examiner Comments
2007B 17: 2 candidates (29%) passed this question.
Producing a list that included the following was required:
– To trat hypertension and substance withdrawal.
– To provide anxiolysis, sedation, analgesia, and sympatholysis.
A brief discussion of the abilities of clonidine in each of these areas would have rounded off a good answer.
Many candidates listed only a couple of uses of these agents, and then followed this with a comparison of clonidine and dexmedetomidine. Most answers did not include sufficient information to achieve a pass mark.
2023B 07
Describe the mechanism of action, dose, pharmacokinetics and pharmacodynamics of clonidine.
Examiner Comments
2023B 07: 38% of candidates passed this question.
This question was broken down into mechanism of action, dose, PK and PD. The mechanism of action required a detailed description of its alpha agonism 200:1 affinity for alpha 2 over alpha 1 including the classification of these receptors and the downstream effects. Correct dose and/or dose ranges for oral and intravenous formulation particularly for different indications for the prescription of clonidine. Pharmacokinetic details expected the absorption, distribution, metabolism and elimination characteristics, with enough detail to demonstrate an understanding. Pharmacodynamic marking was weighted towards the significant cardiovascular, neurological and the relative absence of respiratory effects that make it desirable for use as a sedative/co-analgesic in ICU practice. A description of the these pharmacodynamic effects and why they occur was required to achieve marks in this section.
2023B 12
List the effects of stimulation of adrenoreceptors on target organs and tissues (60% marks). Describe the mechanism of action and pharmacokinetics of metoprolol (40% marks).
CICMWrecks Answer
Adrenoceptor Effects
| Organ / Tissue | α1 (A, B, D) | α2 (A, B, C) | β1 | β2 | |
|---|---|---|---|---|---|
| Nervous System | CNS | ↑ locomotor activity, neurotransmission | Sedation inhibition of sympathetic flow neurotransmission | ||
| eye | iris Sm.M contracts (pupils dilate) | relaxation of ciliary muscle for far vision | |||
| Sympathetic nerve terminal | inhibit Noradrenaline release | ↑ neurotransmitter release | |||
| Cholinergic neurons and cell bodies of noradrenergic neurons | Inhibition of firing | ||||
| CVS | Myocardium | ↑ force of contraction | SA node: ↑HR Atria: ↑contractility, ↑conduction velocity A-V node, HIS, Purkinge: ↑automaticity, cond vel Ventricles: ↑contractility, conduction, velocity, automaticity, and rade of idioventriculatr pacemakers | similar effect to β1 | |
| Vascular Smooth muscle | contraction | contraction | relax | ||
| Endothelium | release of vasodilator substance | ||||
| SKELETAL MUSCLE | ↑ contractility, glycogenolysis, K+ uptake | ||||
| Smooth Muscle | GIT | relaxation | relax | ||
| sphincters (GI, bladder) | contract | relax | |||
| uterus | contract | relax | |||
| Respiratory | bronchial glands | ↓ secretion | ↑ secretion | ||
| Airway | bronchodilation | ||||
| Haem | Platelets | Aggregation granule release | |||
| GIT | Salivary gland | secretion (K+, H2O) | Amylase secretion | ||
| Jejunum | Inhibition of secretion | ||||
| Liver | Glycogenolysis, gluconeogenesis, ureogenesis | Glycogenolysis, Gluconeogenesis | |||
| motility and tone | decreased stomach, intestine | decreased | decreased | decreased | |
| Pancreas | ↓ secretion | Inhibition of insulin release | Insulin secretion | ||
| splenic capsule | contracts | relaxation | |||
| Kidney | Glucogenesis (proximal tubule) ↑ renin secretion | Inhibition of renin release | ↑ Renin secretion | ||
| Metabolic / Endocrine | Adipose tissue | Glycogenolysis | Inhibition of lipolysis | Lipolysis | |
| Posterior pituitary | ADH secretion | ||||
| Others | Eye | lacrimation | ↓ intra ocular pressure | ||
| Melanocytes | Inhibition of MSH-induced granule dispersion | ||||
β3 effects:
- Fat
- lipolysis and thermogenesis
Metoprolol Pharmacology
Examiner Comments
2023B 12: 74% of candidates passed this question.
This question required a list of effects of the stimulation of adrenoreceptors, thus detailed description of downstream effects and exact mechanisms was not required. Using a systems based structure with a subdivision into each receptor (or vice versa) meant that important GIT, GUT, endocrine and metabolic effects were not omitted. Given the need for little depth, this part of the question required breadth particularly within cardiovascular effects. Venoconstriction, dromotropy and lusitropic effects should also be covered.
The second part of the question required a detailed description of the mechanism of action and pharmacokinetics only, thus dose, pharmaceutics and pharmacodynamic information was not required. Here it would be important to elaborate on the downstream effects of blocking the beta adrenergic receptors as compared with the information required in the first part of the question.
2021B 09 – 2019B 14
Outline the classification and effects of beta-blocking drugs with examples (50% of marks).
Compare and contrast the pharmacokinetics of metoprolol with esmolol (50% of marks).
CICMWrecks Answer
Beta receptor antagonists (Beta blockers)
- bind to beta-adrenoceptors and block the binding of noradrenaline and adrenaline
- Inhibits normal sympathetic effects that act through these receptors (Sympatholytic)
- mostly competitive antagonists, there is some evidence of partial agonist activity (Labetalol)
- Some (partial agonists) show intrinsic sympathomimetic activity (ISA): partially activate receptor + prevent noradrenaline from binding to the receptor.
- Some possess membrane stabilizing activity (MSA) similar to sodium-channels blockers
- variable specificity for beta 1 versus beta 2 receptors
- important clinically due to different effects
- often dose related, may be B1 selective at low doses but non-selective at higher
- lipid solubility determines speed of onset
- most resemble isoproterenol
Effects of β-adrenergic blockade
| Type | Main site of action | Effects of β-adrenergic blockade |
|---|---|---|
| β1 | Heart | Anti-ischemic effect: β1 blockade → ↓ heart rate and ↓ cardiac contractility → ↓ BP and ↓ oxygen consumption by the heart → anti-ischemic effect |
| Antiarrhythmic effect: β1 blockade → ↓ AVN conduction, ↑ AVN refractory time, and ↓ heart rate → anti-arrhythmic effect | ||
| Anti-remodeling effect | ||
| Kidneys | β1 blockade of the juxtaglomerular cells → ↓ renin release → ↓ angiotensin II conversion → ↓ H2O resorption → ↓ BP | |
| β2 | Smooth muscle | Vasculature: vasoconstriction Bronchioles: bronchoconstriction |
| Ciliary body of the eye | ↓ Aqueous humor production → ↓ intraocular pressure | |
| Pancreatic beta cells | ↓ Insulin release → hyperglycemia and new-onset diabetes | |
| Skeletal muscle | ↓ Glucose uptake (↓ insulin sensitivity) | |
| Liver | ↓ Hepatic glycogenolysis → hypoglycemia (esp. in diabetics) | |
| Lipoprotein lipase enzyme | Inhibits lipoprotein lipase → ↑ triglycerides and ↓ HDL → hyperlipidemia | |
| β3 | Adipose tissue | ↓ Lipolysis → weight gain |
Classification
- β1-selective (cardioselective):
- e.g. esmolol, nebivolol, bisoprolol, atenolol, metoprolol
- is used for rate control in tachycardia and hypertension management
- side effects: hypotension, heart block, bronchoconstriction at higher doses
- Non-selective β-blockade
- e.g. propranolol
- used for hypertension, to reduce bleeding risk in oesophageal varices, tremor, and as migraine prophylaxis. It is the treatment of choice in thyrotoxicosis as it stops conversion of T4 to T3, reduce ocular pressure in glaucoma
- side effects: rapid withdrawal may precipitate tachycardia, can cause bronchoconstriction and is not recommended in patients with obstructive respiratory disease, issues with hypoglycaemia
- Non-selective α- and β-blockade
- e.g. labetalol, carvedilol
- Potent vasodilators because of their α-blocking action
- Improve endothelial function and vascular re-modelling
- Others
- e.g. sotalol : also acts on K+ channels as class III antiarrhythmic
METOPROLOL | ESMOLOL
PHARMACOKINETICS
| Metoprolol | Esmolol | |
| relatively selective beta blocker with no intrinsic sympathomimetic activity. | Cardio-selective beta blocker with rapid onset and offset. | |
| PK – A | bioavailabilty Absoption is rapid and complete, however there is extensive first pass metabolism. BA 50% routes of admin PO or IV dose Oral in 12.5mg increments, IV in 1-2mg boluses | bioavailabilty Only available as IV therefore 100% routes of admin IV dose In 10mg increments titrate to effect |
| D | volume of distrib 5.5 L/Kg protien binding 10-20% to albumin lipid solubility is high so it crosses the BBB | volume of distrib 3.5 L/Kg protien binding 60% to albumin lipid solubility is high so it crosses the BBB |
| M | hepatic or renal Extensively hepatic via CYP2D6 | neither hepatic or renal! by red blood cell esterases to a mostly inactive metabolite |
| E | half life 3-8hours excretetion In urine 5-10% unchanged | half life 10 minutes excretion In urine |
Sources: CVPharmacology
JC 2019
Pharmacopeia Table: Adrenoceptor Antagonists
Examiner Comments
2021B 09: 59% of candidates passed this question.
This was a two-part question with marks and thus timing of the answers given as a percentage. There are generally many ways to classify drugs within the same class. These are usually well described in the relevant recommended pharmacological texts. Receptor distribution throughout the body and the effect of the drug-receptor interaction are useful ways to organise systemic pharmacodynamic responses, as opposed to a list of organ systems with associated vague statements of interaction.
2019B 14: 47% of candidates passed this question.
Beta-blocking drugs were generally well classified. Selectivity, membrane stabilising activity and ISA should have been mentioned. Many candidates omitted or poorly answered the ‘effects’ of 6 beta blockers. Candidates who performed well answering the pharmacokinetics of metoprolol and esmolol provided a table of the two drugs. Superficial statements such as “hepatic metabolism and renal excretion” attracted minimal marks. The mechanism of action of beta blockers was not requested.
2022A 05
Write short notes on the pharmacology of labetalol and esmolol, highlighting their differences.
Examiner Comments
2022A 05: 32% of candidates passed this question.
Overall, this question was poorly answered. Most answers demonstrated limited knowledge about the major differences between the two drugs’ including the target receptors and subsequent effects. Antiarrhythmic effects were often omitted in answers, and scant or incorrect details provided about the metabolism and overall pharmacokinetics of the drugs. Generic vague statements about pharmacokinetic properties of medications do not attract marks. Better scoring answers demonstrated a factual knowledge about both individual drugs and specific details related to any differences influencing the potential application of these differences. A table superficially listing aspects of both drugs would not be of a passing standard. Many answers demonstrated significant incorrect facts.
2024B 09
Compare and contrast metoprolol and verapamil using the following headings:
(a) Class and indications for use (20% of marks).
(b) Mechanisms of action (25% of marks).
(c) Pharmacodynamics and adverse effects (55% of marks).
Examiner Comments
2024B 09: 21% of candidates passed this question.
The most effective format for this question was to split information into the headings provided. The higher scoring responses were done as a table, with headings as per the question, concise facts and clear comparisons. Information on pharmaceutics, dose and pharmacokintetics were not required. When effects of the drugs were discussed detail regarding the mechanism of pharmacodynamics and adverse effects was expected. Cardiovascular effects of these drugs required specificity in their description and included their action on the duration and slope of the cardiac action potential thus affecting the HR, as well as effects on SVR and BP. Other adverse effects included respiratory, neurological and gastrointestinal actions.
2022B 08
Classify calcium channel blockers providing examples (15% marks). Describe the pharmacology of verapamil (85% marks).
2017B 08
Classify calcium channel antagonists and give one example of each class (30% of marks).
Describe the pharmacology of Nimodipine including important drug interactions (70% of marks).
2014A 02
Classify calcium channel blockers, and give an example for each classification. (30% of marks)
Describe the pharmacology of verapamil. (70% of marks)
2011B 17
Classify the calcium channel blockers and provide one example of a drug for each class (20% marks).
Compare and contrast the pharmacology of nimodipine and verapamil (80% marks).
CICMWrecks Answer
Calcium Channel Blocker Classification
- Class I – Phenylalkylamines:
- Verapamil
- Reduces HR, contractility and causes vasodilatation, decrease in cardiac contractility, heart rate, and both coronary and peripheral vascular tone.
- Class II – Dihydropyridines:
- main effects are on vasodilatation
- 1st gen = nifedipine
- 2nd gen = felodipine, nimodipine
- 3rd gen = amlodipine
- Class III – Benzothiazepines:
- diltiazem
- some reduction in HR and contractility, also causes vasodilatation, mild to moderate decrease in myocardial contraction, and decreases heart rate by decreasing both the automaticity of the SA node and the rate of conduction in the AV node.
Calcium Channel Blocker Pharmacology
(Include as per the question)
Click here to Open CICMWrecks Table: Calcium Channel Blockers
Class Effects:
- Heart
- V-W Class 4 antiarrhythmic
- Decr SA node automaticity
- Decr AV node conductivity
- Vasodilation
- Negative inotropy
- reflex tachycardia
- 2) Vasculature
- Decr SVR and BP
- Decr Hypoxic pulmonary vasoconstriction
- Incr coronary and cerebral blood flow
- 3) Other
- Tocolytic
- NDMR potentiation.
Gladwin / JC 2021
Examiner Comments
2022B 08: 35% of candidates passed this question.
Most candidates provided an acceptable classification of calcium channel blockers. Candidates are encouraged to read the question as a few failed to provide relevant examples with their classification. A basic understanding of the common features of verapamil (indeed most of these are common to the calcium channel blockers as a group) in clinical practice and toxicology was sufficient to pass. Marks allocated to dosing and presentation were not gained for writing that it is available as tablets or as a clear colourless solution, unless relevant information was given. Many candidates listed neuropsychiatric effects of verapamil, which are not a feature normally associated with calcium channel blockers. Many also listed QT prolongation among its effects, which is not listed in the standard texts and is the opposite to the true effect. The examiners noted that in some papers there appeared to be a contradiction between the effect of Verapamil on the cardiac action potential and the detail provided about the drugs clinical effects, these answers demonstrated a lack of solidity in the understanding of the underlying physiology.
2017B 08: 19% of candidates passed this question.
The classification was done well. Most candidates demonstrated that they had a structure for a “drug” question, but were often challenged to fill in the detail of that structure and failed to deliver enough content to secure a pass. Many candidates wrote a generic answer for calcium channel blockers instead of the specifics of nimodipine.
Frequently the pharmacokinetic data recounted was incorrect. Candidates failed to distinguish between absorption and bioavailability. The difference between oral and intravenous dosing was often omitted. Few answered the section on important drug interactions.
2014A 02: 61% of candidates passed this question.
Most candidates managed to provide a classification of calcium anatagonists. The pharmacology of verapamil was less well understood. The structure for answering a pharmacology question was often poor and there was commonly a lack of precision in pharmacokinetics. We suggest that candidates look at a general pharmacokinetic structure when answering these questions. One approach would be a structure that covers drug name and description, pharmaceutics (chemistry/ ampoule contents), Pharmacokinetics, Pharmacodynamics (Think CNS/CVS/Resp/GIT etc. if relevant), Dose and Side effects then Indications and Contraindications can help organize the information.
2011B 17: 9 (36%) of candidates passed this question.
Most candidates were able to classify the calcium channel blockers well (Type I : Phenylalkylamines eg verapamil, Type II : Dihydropyridines eg nimodipine and Type III : Benzothiazepines eg diltiazem) However, the comparison of the pharmacology of nimodipine and verapamil was in general answered poorly. Few candidates demonstrated an organised approach to this part of the question. The two drugs’ presentation, routes of administration, indications and dosing were poorly answered considering that nimodipine in particular is used frequently in intensive care units. Mode of action was well answered, but important principles relating to pharmacokinetics (such as a basic outline of protein binding, bioavailability, and metabolism) were expected, but common omissions. More knowledge than ‘metabolism in the liver’ is required. Few candidates mentioned interactions, adverse effects, or predictable effects of over dosage of these drugs.
Syllabus: C2b 2d
Recommended sources: Peck Hill and Williams Pharmacology for Anaesthesia and Intensive care 3rd Ed pgs 252-255.
2008B 21
Outline the pathophysiological basis for the use of angiotensin converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARB) in congestive cardiac failure.
CICMWrecks Answer
Angiotensinogen
- Cleaved by renin
- release from juxtaglomerular cells:
- Lowered renal arterial pressure
- Less NaCl (more about Cl–) past macula densa
- Sympathetic nervous system activation
Angiotensin I
- Converted by ACE to Angiotensin II
Angiotensin II
- Actions: Via AT1 receptors (AT2 receptors have unclear significance)
- Renal:
- Afferent and renal artery vasoconstriction (weaker)
- Efferent vasoconstriction (stronger)
- Constricts mesangial cells (decrease GFR)
- Increased sodium and water resorption
- -> Na+/H+ antiporter
- -> Decreased hydrostatic pressure in capillary (Starling force)
- Vascular:
- Widespread vaso and veno constriction
- Promotes hyperplasia
- Cardiac
- Promotes hyperplasia
- Neural:
- Directly increases noradrenaline release from presynaptic membrane
- Stimulates catecholamine release
- Promotes aldosterone release
- Degrades bradykinin
- So less NO, cAMP -> less vasodilation
Aldosterone
- Increases activity of Na+/H+ antiporter, Na+/K+ ATPase in the distal convoluted tubule and collecting duct -> water retention
- There is also an ACE independent pathway to angiotensin II formation
ACE inhibitors:
- Block ACE
- Reduce afterload
- Modulate cardiac sympathetic stimulation
- Inhibit cardiac and vascular hypertrophy
- Cause cardiac remodelling
A2RB:
- Block AT1 receptors
Mooney 2016
Further Reading:
Examiner Comments
2008B 21: 0 (0%) candidates passed this question
The renin-angiotensin system plays a central role in the pathophysiology of heart failure. Thus this question required integration of knowledge of the renin-angiotensin system and how pharmacological agents affect it in the treatment of cardiac failure. Candidates were expected to describe the pathway and the influence of these drug groups on cardiac failure and to recognise underlying basic physiological principles such as the interaction between AT1 and AT2 receptors along with awareness of production of Ang II by ACE-independent enzymes.
A good answer was expected to contain the following points: Angiotensinogen is cleaved by kidney-derived renin to form the decapeptide angiotensin I (Ang I); ACE converts Ang I to Ang II; Ang II is a potent arterial vasoconstrictor and an important mediator of Na + and water retention through its effects on glomerular filtration pressure and aldosterone secretion; Ang II potentiates neural catecholamine release, is a secretagogue for catecholamine release from the adrenal medulla, promotes vascular hyperplasia and pathologic myocardial hypertrophy.
ACE inhibitors suppress Ang II and aldosterone production, decrease sympathetic nervous system activity, and potentiate the effects of diuretics in heart failure. ACE is identical to kininase II, which degrades bradykinin and other kinins that stimulate production of NO, cyclic GMP, and vasoactive eicosanoids; these vasodilator substances seem to oppose the effects of Ang II on the growth of vascular smooth muscle and cardiac fibroblasts and on production of extracellular matrix. Thus, the increased levels of bradykinin that result from ACE inhibition may play a role in the hemodynamic and anti-remodeling effects of ACE inhibitors.
An alternative means of attenuating the haemodynamic and vascular impact of the reninangiotensin system is through inhibition of angiotensin receptors. Most of the known clinical actions of angiotensin II are mediated through the AT1 angiotensin receptor. AT1 receptor antagonists may provide more potent reduction of the effects of angiotensin II than do ACE inhibitors.
2013A 10
Outline the pharmacokinetics, and mechanism of action of carvedilol and spironolactone.
Examiner Comments
2013A 10:
Carvedilol and spironolactone are common drugs used in the management of cardiac failure. They have different mechanisms of action and pharmacokinetics. Both are drugs listed in the Syllabus as Level B and thus candidates are expected to have a general understanding of their pharmacology. Many candidates gave class specific information about beta blockers rather than demonstrating an understanding of carvedilol`s particular properties. Most candidates were able to score marks by commenting upon the results of aldosterone antagonism suggesting an understanding of the physiology of this hormone but appeared to know little more about the pharmacology of spironolactone. Overall there was insufficient information provided by most candidates for both drugs.
iii. Understand the pharmacology of anti-arrhythmic drugs.
2023A 11
Outline the Vaughan Williams classification of anti-arhythmic drugs with examples (30% of Marks). Describe the relevant pharmacology of adenosine (70% of Marks)
2016B 21
Classify anti-arrhythmic drugs by mechanism of action, giving examples of each (75% of marks).
Describe the electrophysiological and ECG effects of sotalol (25% of marks).
2012B 09
Classify the anti-arrhythmic drugs using the Vaughan-Williams classification (30% of marks).
Compare and contrast the electrophysiological effects of Class 1 anti-arrhythmic drugs (70% of marks).
2008B 13
Classify antiarrhythmic drugs, including their mechanisms of action, and give an example of one drug from each group.
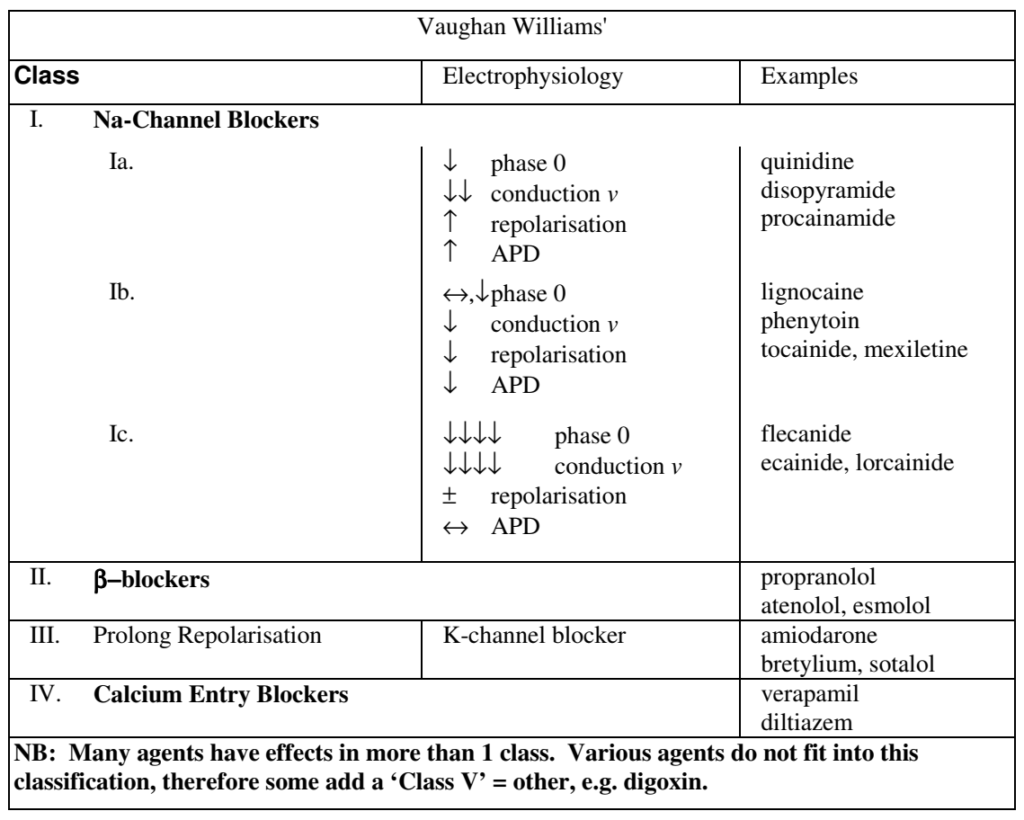
CICMWrecks Answer: Classification of anti-arrhythmic drugs (Vaughan-Williams)
| CLASS | ACTION | ELECTRO- PHYSIOLOGY | DRUGS | THERAPEUTIC INDICATIONS |
|---|---|---|---|---|
| I. Na – Channel Blockers | ||||
| Ia | Intermediate dissociation | ↓ phase 0 ↓↓ conduction v ↑ repolarisation ↑ APD | Quinidine Disopyramide Procainamide | Atrial and ventricular arrhythmias esp. post MI |
| Ib | Fast dissociation | ↔,↓ phase 0 ↓ conduction v ↓ repolarisation ↓ APD | lignocaine phenytoin tocainide, mexilitine | Ventricular arrhythmias post MI, digoxin induced arrhythmias |
| Ic | Slow dissociation | ↓↓↓↓ phase 0 ↓↓↓↓ conduction v ± repolarisation ↔ APD | flecainide ecainide, lorcainide | Refractory arrhythmias |
| II. β – Blockers | ||||
| ↓ SA firing – ↓ rate, conduction | propranalol* atenolol, esmolol | Rate control in AF, AT, Flutter and VT | ||
| III. K – Channel Blockers | ||||
| Delay phase 3 (repol) ↑ APD ↑ ERP | amiodarone bretylium, sotalol^ | AF / Flutter termination | ||
| IV. Ca – Channel Blockers | ||||
| ↓ AV conduction ↑ PR interval ↓ rate/conduction | verapamil diltiazem | SVT and AF or Flutter | ||
| OTHERS (Some call this Class V) | ||||
| Blocks Na+/K+ ATPase → ↑Ca2+, ↓K+, ↑Ach | ↑ contractility ↓ AV conduction | Digoxin | AF rate control Heart Failure | |
| Opens K+ channels via adenosine receptors | Hyperpolarizes myocardium ↓ AV conduction ↓ SA firing | Adenosine# Ibutilide | Terminate SVT or reveal underlying rhythm in tachycardias | |
| Stimulates Na+/K+ATPase | Membrane stabilization | Magnesium | VF / Torsades de pointes | |
| Notes | * Propranalol also has Na blocking activity ^ l-sotalol has beta blocking and class III activities; d-sotalol is a pure class III agent. Commercially available sotalol is a racemic (equal part) mixture. # Amiodarone – blocks Na, Ca, K channels, and exhibits beta blockade. | |||
Gladwin / Sakurai / JC 2020
CICMWrecks Answer: Class Effects
The effect on the myocardial action potential of each class is:
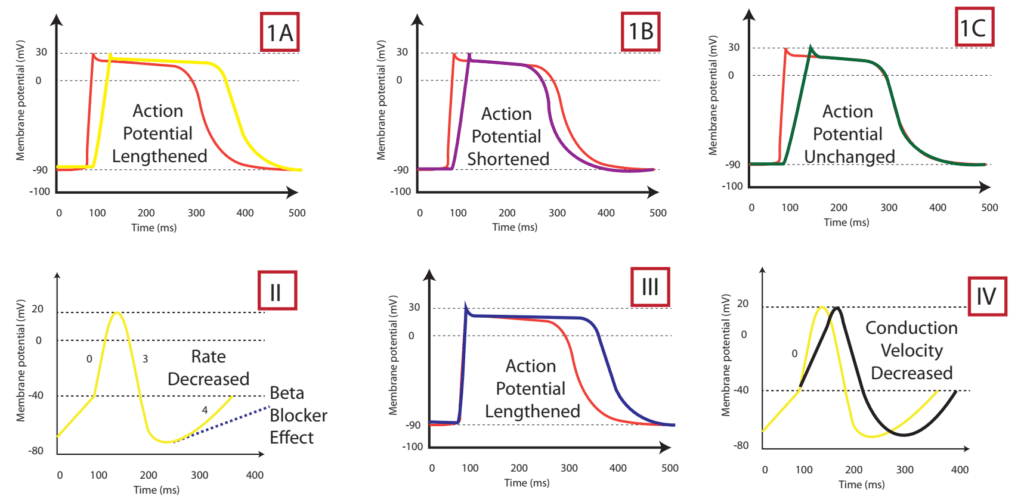
| Class Ia | Class Ib | Class Ic | Class II | Class III | Class IV | |
|---|---|---|---|---|---|---|
| Depolarisation rate (phase 0) | ↓ | ↔ or ↓ | ↓↓↓↓ | ↔ | ↔ | ↓↓↑↔± ↔ |
| Conduction velocity | ↓↓ | ↓ | ↓↓↓↓ | ↓ | ↓ | ↔ |
| Effective Refractory Period | ↑↑↑↑ | ↓ | ↑ | ↓ | ↑↑↑↑ | ↔ |
| Action potential duration | ↑ | ↓ | ↔ or ↑ | ↑ | ↑↑↑↑ | ↓ |
| Automaticity | ↓ | ↓ | ↓ | ↓ | ↓ | ↔ |
| P-R duration | ↔ | ↔ | ↑ | ↔ or ↑ | ↑ | ↔ or ↑ |
| QRS duration | ↑ | ↔ | ↑↑↑↑ | ↔ | ↑ | ↔ |
| QTc duration | ↑ | ↔ or ↓ | ↑ | ↓ | ↑↑↑↑ | ↔ |
Gladwin / Sakurai / JC 2020
CICMWrecks Answer: Sotalol Effects
Sotalol Effects
- l-sotalol has beta blocking and class III activities
- d-sotalol is a pure class III agent
- Commercially available sotalol is a racemic (equal part) mixture.
- Class III Effects (K – channel blocking)
- Blocking of outward K+ channels slows cardiac repolarisation, which increases the cardiac refractory period.
- Delay phase 3 (repol), ↓ AV conduction,
- ↑↑↑↑ APD, ↑↑↑↑ ERP
- ↑↑↑↑ QTc
- ↓ automaticity
- ↓ ectopy
- ↓ defibrillation energy requirement
- May ↑ inotropy
- Class II Effects (β – Blocker)
- Antagonizes β1 & β2 receptors
- ↓ SA firing – ↓ heart rate, conduction
- can prolong the QT interval.
- can cause polymorphic ventricular tachycardia (Torsades de Pointes)
- recommended to be initiated in the inpatient setting to monitor the QT interval after each dose
- can cause severe bradycardia necessitating drug discontinuation
Gladwin / Sakurai / JC 2020
CICMWrecks Answer: Pharmacology
Examiner Comments
2023A 11: 67% of candidates passed this question.
The high pass rate of this question was largely due to the ability of most candidates to reproduce the VW classification, with correct information regarding each class with examples. The pharmacology of adenosine was less consistently covered and whilst most kept to the usual pharmacology structure correct detail was often lacking to achieve high marks for this section.
2016B 21: 88% of candidates passed this question.
Most answers displayed a good knowledge of the Vaughan Williams classification, classes I to IV and the relevant electrophysiological characteristics of the classes.
Answers should also have included mention of other antiarrhythmics, such as digoxin, magnesium and adenosine. The second part of the question required comment about K ion blockade and its effects. It was helpful to mention prolongation of QT and risk of torsade. Most answers omitted reference to its being a racemic mixture, with different actions of the isomers.
2012B 09: 16 (72.7%) of candidates passed.
Most candidates displayed a basic knowledge of the Vaughan-Williams classification and
gave an example of each class. The remainder of the question lent itself very well to a
tabular format. Better answers included the effect on the action potential (diagrams were
useful here), channel dissociation kinetics (this was frequently omitted) and examples from each class of drug. There is an excellent table in Stoelting which answers this question nicely. Marks were not awarded for clinical effects. Overall, this question was generally well.
2008B 13: 3 (60%) candidates passed this question
This question again highlighted the importance of candidates utilising a predetermined format or structure to their questions. Well structured responses were less likely to overlook important details, which was the predominate weakness for some candidates. A table format was one useful way of displaying a good answer, for example –
Syllabus: C2c
Reference Text: Goodman and Gillman Chp 34
2024A 19
Describe the pharmacology of amiodarone using the following headings:
(i) Indications for use (5% of marks)
(ii) Dose (10% of marks)
(iii) Mechanism of Action (20% of marks)
(iv) Pharmacodynamics including adverse effects (25% of marks)
(v) Pharmacokinetics (25% of marks)
(vi) Drug Interactions (15% of marks).
2014B 13 – 2008B 05
Outline the pharmacology of amiodarone.
2021B 11 – 2016A 11
Provide a detailed account of the side effects of Amiodarone.
CICMWrecks Answer
Side Effects of Amiodarone:
Side effects reflect long elimination half-life and significant accumulation in tissues. Side affects increase when maintenance doses are above 400mg daily.
- Respiratory
- Most serious is pneumonitis. Risk is 5-10% at 3 years with a mortality of 10% long term.
- May be due to increased oxygen free radicals and risk increased by high FiO2
- 2 phenotypes- slow insidious and acute onset
- May be reversible if treatment stopped early
- Cardiac
- Prolonged QTc with Torsade’s
- Proarrhythmic (ventricular tachyarrhythmia)
- Bradycardias resistant to atropine
- Peripheral vasodilation leading to hypotension with decreased responsiveness to adrenergic stimuli
- Neurological
- Peripheral neuropathy, tremor, sleep disturbance
- Myopathy
- Endocrine
- Both hyperthyroidism and hypothyroidism may occur (usually reversible)- 2-4% of patients
- Prevents peripheral conversion of T4 to T3 (detected by increased plasma [TSH])
- 37% of weight of Amiodarone is iodine, which may precipitate hyperthyroidism
- Pre-existing thyroid disease increases risk
- GIT
- Hepatitic dysfunction, LFT derangement and cirrhosis has been observed
- Metallic taste during loading
- Ophthalmic
- Corneal microdeposits but visual impairment less likely
- Optic neuropathy (insidious onset, bilateral). Often reversible if discontinued
- Dermatological
- Photosensitivity
- Slate gray pallor of the face, continues after drug discontinuation
- Pregnancy and lactation
- Avoid in from 3 months pre-pregnancy to after breast feeding has finished
- May cause hypothyroidism and bradycardia in the fetus. Foetal hypothyroidism may lead to impairment of myelination
- Contraindicated in porphyria
- Miscellaneous
- IV preparation is irritant and should be administered through a central vessel
Examiner Comments
2021B 11: 17% of candidates passed this question.
The question asked for a detailed account of the side effects of amiodarone, hence those candidates that just provided a list or outline scored less well. It was expected that candidates provide some detail of the side effect. Answers that scored well prioritised those relevant to ICU clinical practice. Many provided disorganised outlines of the side effects and frequently the cardiovascular side effects were poorly explained. Many candidates omitted the important drug interactions of amiodarone use and few candidates related the side effect profile to the duration of treatment.
2016A 11: 26% of candidates passed this question.
The question asked for a detailed account and the expected marks were spread across a range of systemic side effects, not just the cardiovascular and pulmonary side effects. Many candidates provided irrelevant and lengthy descriptions of the mechanisms of action of amiodarone which was not asked for in the question and gained no additional marks. Most successful answers used an organ systems approach to include the many side effects of amiodarone.
Many candidates failed to mention skin side effects, neurological side effects, GI/hepatic side effects, pregnancy and breast feeding considerations, and interactions with other highly protein bound drugs. The predominant mechanism for hypotension with rapid IV administration of amiodarone was incorrectly given in a number of answers.
2014B 13: 77% of candidates passed this question.
This was a repeat question and was generally answered well. Some candidates lost marks for being too approximate on the pharmacokinetics.
2008B 05: 2 (40%) candidates passed this question
Successful candidates applied, a systematic approach/format to answer questions that refer to outlining pharmacology of select drugs. A number of useful mnemonics are suggested in the recommended texts for use when answering such a question. All candidates correctly stated what amiodarone is used for but most were not structured methodically and thus suffered from significant omission. Amiodarone is an important class III anti-arrhythmic (with some characteristics of all 4 Vaughan-Williams classes). For a good pass candidates were expected to actions of amiodarone (eg blocks inactivated Na channels, decreases Ca current, noncompetitive adrenergic blocking effect, blocks myocardial K channels which contributes to slowing of conduction and prolongation of refractory period in AV node, prolongs refractory period in all cardiac tissues, prolongs cardiac action potential duration) and it’s pharmacokinetics (eg bioavailability, large volume of distribution, high protein binding, complex metabolism and long elimination half life – 29 days)
Syllabus: C2c
Reference Text: Goodman and Gillman’s The Pharmacological basis of Therapeutics 11th ed 2006 and Pharmacology and Physiology in Anaesthetic Practice / Stoelting 4th ed 2006
2009B 05
Outline the kinetic characteristics and the mode of action of digoxin. (75% of marks)
List the cardiovascular effects of digoxin (25% of marks).
Examiner Comments
2009B 05: 0 (0%) of candidates passed this question
The Syllabus for the Primary examination describes an outline to be “Provide a summary of the important points.” Thus candidates were expected to briefly mention the fundamental pharmacokinetic characteristics (eg highly lipid soluble, well absorbed from small intestine, oral bioavailability of 60 – 90%, protein binding of 20 – 30%, volume of distribution, half life, etc) and mode of action. This was poorly done and candidates’ answers often lacked structure.
The question outlines the distribution of marks, being 25% for listing cardiovascular effects. Thus candidates were expected to broadly list the important cardiovascular effects relating to mechanical (eg increase intensity of myocardial contraction, direct venous and arteriolar constriction, etc) and electrical ( increase phase 4 slope & automaticity, hyperpolarization, shortening of atrial action potentials, decrease AV conduction velocity and prolong AV refractory period, increase PR & QT intervals, dose and baseline autonomic activity dependent actions, etc).
2018B 02 – 2010B 22
Compare and contrast the pharmacology of digoxin and amiodarone.
Examiner Comments
2018B 02: 82% of candidates passed this question.
Most candidates had a good structure for answering this question; a table was commonly used.
Marks were awarded for indications and an explanation of the mechanism of action of both drugs, which was generally well explained. The pharmacodynamic effects were often listed in a general manner and more detail would have achieved a higher mark, including a list of the ECG effects. Some detail on the pharmacokinetics and adverse effects of the drugs was expected and this section was generally well answered. Better answers noted digoxin levels and potential drug interactions.
2010B 22: 6 (40%) of candidates passed this question.
This question required a structured approach to a comparative description of the pharmacology of two commonly used and encountered drugs in intensive care practice. Candidates who did not gain a sufficient mark, did so because of a poor knowledge of this topic, as well as a critical failure to structure their answer.
Syllabus: C2c,2b
References: Stoelting, Pharmacology and Physiology in Anaesthetic Practice pg 280 and 339,
Peck Hill and Williams, Pharmacology for Anaesthesia and Intensive Care, pgs 224, 232
2019A 14
Compare and contrast the mechanism of action, pharmacokinetics and adverse effects of digoxin and sotalol.
Examiner Comments
2019A 14: 19% of candidates passed this question.
Good answers listed class and the multiple mechanisms of action for both these antiarrhythmics, briefly outlining relevant downstream physiological effects and contrasting effects on inotropy. Knowledge of specific pharmacokinetic parameters of these agents was generally lacking. Clinically relevant adverse effects were frequently omitted (e.g. prolonged QT/Torsades for sotalol, hypokalaemia potentiating toxicity of digoxin).
iv. Understand the pharmacology of anti-anginal drugs.
2020A 02
Describe the pharmacology of glyceryl trinitrate (GTN).
Examiner Comments
2020A 02: 69% of candidates passed this question.
GTN is a commonly used ‘level 1’ drug. The most comprehensive answers included information on available drug preparations, indications, mechanism of action, pharmacodynamics and pharmacokinetics and its side-effect profile. It was expected that significant detail be included in the pharmacodynamic section (e.g. preferential venodilation, reflex tachycardia, effects on myocardial oxygen demand etc). Common omissions included tachyphylaxis, dosing and its metabolism. Many answers didn’t mention the first pass effect.
2020B 20
Describe the pharmacology of intravenous sodium nitroprusside.
2015A 11
Outline the pharmacology of sodium nitroprusside (50% of marks).
Discuss the mechanisms of toxicity and their management (50% of marks).
Examiner Comments
2020B 20: 49% of candidates passed this question.
This was a straightforward pharmacology question relating to a relatively common and archetypal intensive care medication. The structure of the question was well handled by most of the candidates; easily falling into the classic pharmaceutics, pharmacokinetic and pharmacodynamics framework. Many candidates had a superficial knowledge of the presentation and formulation of the drug, aside from its light sensitivity. Better answers detailed the drug according to the above-mentioned framework but also accurately highlighted specific points relevant to the ICU practise such as the metabolic handling of sodium nitroprusside and relating this to the consequences of the various metabolic products.
2015A 11: 38 % of candidates passed this question.
Most candidates presented a structured answer and exhibited a good understanding of the pharmacology of sodium nitroprusside. Few candidates demonstrated an understanding of the mechanisms of SNP toxicity and details on management of cyanide toxicity were lacking.
Cobalt EDTA is no-longer recommended as initial therapy in the management for cyanide toxicity.
More specific detail was expected beyond a generic comment on “mechanisims of toxicity” such as potentially causes of respiratory, renal, hepatic or CNS failure.
Few candidates mentioned adverse effects other than that of cyanide toxicity. Many candidates also failed to outline the management of sodium nitroprusside toxicity.
2016B 02
Compare and contrast the mechanisms of action and toxicity of sodium nitroprusside and glyceryl trinitrate (GTN)
2008B 20
Compare and contrast the pharmacology of sodium nitroprusside and glyceryl trinitrate.
2008A 15
Compare and contrast the pharmacology of sodium nitroprusside and glyceryl trinitrate for the treatment of acute hypertension
Examiner Comments
2016B 02: 55% of candidates passed this question.
Some excellent responses to this question showed a clear understanding of the pharmacology of these agents – the differing mechanisms of action involving both involving nitric oxide. Better answers were able to use this to explain the altered vascular specificity.
Toxicity was similarly well prepared for with a good understanding of the role of cyanide in SNP and the low rates of toxicity with GTN. This question was best handled in a tabular format which minimised omissions.
Some candidates focused on pharmaceutics, indications and side effects which were not allocated any marks.
‘Compare & contrast’ means the similarities; differences & unique features need to be related to each other. Several candidates confused ‘nitrous oxide’ with nitric oxide.
2008B 20: 4 (80%) candidates passed this question.
2008A 15: 2 (66%) candidates passed this question.
It was expected candidates would address specific aspects of pharmacology such as action, mechanism of action, half life and duration of effect, route of administration, potential toxicity and special precautions. These agents lend themselves to comparison and contrast as several distinct similarities and differences exist and credit was given for highlighting these. Specific comments should include that both agents result in blood vessel dilation with extra credit given for detailing the differences in the balance of arterial versus venous effects between them. For both agents the effect is mediated through nitric oxide and it was expected candidates would identify that nitroprusside releases NO spontaneously and GTN requires enzymatic degradation with the resultant effects on smooth muscle mediated via cGMP. They are both short acting agents when used intravenously and require careful titration to measured blood pressure for effect.
Extra credit was given for mentioning that routes other than IV are available for GTN (topical / oral) but not for nitroprusside. Comments on special precautions such as Nitroprusside should be protected from light and GTN given via non PVC giving sets gained additional marks. In addition to the well described adverse effects of each agent, it was expected candidates would mention the potential for cyanide toxicity with nitroprusside and extra marks were awarded for an indication of usual doses.
Syllabus C2b 2e
References Katzung 10th edition, Goodman and Gillman Chp 31 & 32
2010A 16
Define the mechanisms of action and adverse effects of metoprolol and glyceryl trinitrate when used to manage myocardial ischaemia.
CICMWrecks Answer
Myocardial ischaemia
- Occurs when myocardial oxygen demand is not met by oxygen delivery
- Type I
- Due to atherosclerotic disease – plaque rupture, or coronary artery spasm
- Type II
- Due to increased metabolic demand of myocardium (e.g. tachycardia, hypertensive crisis)
- Type I
- Determinants of myocardial oxygen demand
- Basal metabolism of heart
- Heart rate
- Contractility
- External work
- Wall tension
| METOPROLOL | GLYCERYL TRINITRATE | |
|---|---|---|
| β1 selective adrenoceptor antagonist | Nitrate | |
| MoA | β1 adrenoceptor = GsPCR In Myocardium – Inhibition → Decreased activation of adenylyl cyclase → Decreased intracellular [cAMP] → Decreased activation of protein kinase A → Decreased phosphorylation and activation of intracellular enzymes → Decreased intracellular [Ca2+] – Decreased inotropy and decreased chronotropy → Decreased myocardial oxygen consumption → Relief of ischaemia | Nitrate groups activated by thiols to nitric oxide In vascular smooth muscle (veins > arteries) – → Diffuses intracellularly and activated guanylyl cyclase → increased intracellular [cAMP] → Decreased intracellular [Ca2+] → decreased vascular tone → venodilation and vasodilation – → Decreased preload and decreased afterload – → Decreased myocardial O2 consumption → Relief of ischaemia |
| A/E | CVS – Bradycardia – Hypotension – Arrhythmia – Decompensation of acute heart failure → Cardiogenic shock – Rebound myocardial ischaemia on abrupt cessation Resp – Bronchospasm if reactive airways disease CNS – Vivid dreams – Lethargy Other – Dyslipidaemia – Hyperglycaemia | CVS – Hypotension – Reflex tachycardia – If preload dependent cardiac output → decreased cardiac output → shock Resp – Methaemoglobin → decreased O2 delivery – Bronchodilation → V/Q mismatch CNS – Increased cerebral blood flow Other – Tolerance |
Sakurai 2016
Examiner Comments
2010A 16: 8 (80%) of candidates passed this question.
For a good answer candidates were expected to make some mention of the link between myocardial O2 demand/heart rate/contractility/ This was often overlooked, and candidates who did tended to not respond to what the question was asking, that is “when used to manage myocardial ischaemia”
Good answers had a structured response. For a good answer, candidates were expected to mention metoprolol effects of reducing left ventricular wall stress, decreased cAMP/mechanism, decreased heart rate, contractility, resultant decreased O2 demand as well as adverse effects include Bradycardia/ heart block/ hypotension/ bronchoconstriction, etc. In relation to GTN, to mention dilation via nitric oxide, predominantly venodilation, decreased venous return, LVEDP, wall stress, decreased O2 demand and increased supply via coronary vasodilation and adverse effects include hypotension/tachycardia/tolerance/headache. Candidates are reminded that if they are to use non-standard abbreviations, then those abbreviations must be defined somewhere within their answer.

Recent Comments