Syllabus (Fourth Edition, 2023)
Topics
i. Explain the cardiovascular responses to changes in posture, hypovolaemia, a fluid bolus, anaemia, exercise, and aging.
ii. Explain the physiological consequences of intermittent positive pressure ventilation, positive end-expiratory pressure (see also F10 i.) and the Valsalva manoeuvre.
Topics not covered in previous SAQs
i. Explain the cardiovascular responses to changes in posture, hypovolaemia, a fluid bolus, anaemia, exercise, and aging.
ii. Explain the physiological consequences of intermittent positive pressure ventilation, positive end-expiratory pressure (see also F10 i.) and the Valsalva manoeuvre.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
i. Explain the cardiovascular responses to changes in posture, hypovolaemia, a fluid bolus, anaemia, exercise, and aging.
2017B 19
Describe the physiology of a vasovagal syncope
CICMWrecks Answer
Vasovagal Syncope
- Loss of consciousness from excessive autonomic reflex activity
- typically triggered by seeing blood, pain, emotional stress, or prolonged standing, Time varying magnetic field (i.e. transcranial magnetic stimulation)
- Underlying mechanism involves the nervous system slowing the heart rate and dilating blood vessels resulting in low blood pressure and therefore not enough blood flow to the brain
- Recovery happens without specific treatment. Prevention involves avoiding the triggers.
- Drinking sufficient fluids, salt, and exercise may also be useful.
Mechanism
Regardless of the trigger, the mechanism of syncope is similar
- The nucleus tractus solitarii of the brainstem is activated directly or indirectly by the triggering stimulus
- Results in simultaneous enhancement of parasympathetic nervous system (vagal) tone and withdrawal of sympathetic nervous system tone.
This results in a spectrum of hemodynamic responses:
- On one end of the spectrum is the cardioinhibitory response
- ↓ HR, ↓ contractility → ↓ CO → LOC
- This response results primarily from enhancement in parasympathetic tone.
- On the other end of the spectrum is the vasodepressor response
- ↓ BP without change in HR.
- This occurs due to dilation of the blood vessels, probably as a result of withdrawal of sympathetic nervous system tone.
The majority of people with vasovagal syncope have a mixed response somewhere between these two ends of the spectrum.
One account for these physiological responses is the Bezold-Jarisch reflex.
CVS Challenges
- ↓ in MAP: = due to ↓ CO
- Hydrostatic effects on CPP:
- ↓MAP at level of brain
- effect = immediate.
- NB : ↓MAP at brain level is offset by a similar:
- ↑ CVP venous side (brain circulation is like an inverted U-tube) as well as on the ↓CSF pressure.
- CPP is further augmented by an increase in VR from the brain to the heart in the erect position
- Summary: the main challenge to the CVS (and the brain circulation) is ↓MAP
The CVS response
- baroreceptor reflex mechanism:
- ↓ MAP ⇒ sensed by carotid (mainly) and aortic baroreceptors ⇒ ↓ traffic up to NTS ⇒ via medullary control centre ⇒ ↑ SNS outflow and ↓ PNS outflow.
- The ↑ SNS outflow causes: [ remember: MAP (minus RAP) = CO x SVR ]
- [↑preload] peripheral venoC ⇒ ↑ VR ⇒ ↑ CO ⇒ ↑ MAP
- [↑afterload] peripheral vasoC ⇒ ↑ SVR ⇒ ↑ MAP (slight ↓ in SV due to afterload increase, but net effect = ⇑ MAP)
- ↑ cardiac contractility ⇒ ↑ CO ⇒ ↑ MAP
- ↑ Heart rate ⇒ ↑ CO ⇒ ↑ MAP
NB: Baroreflex ⇒ vasoconstriction = more effective than venoconstriction to restore MAP
! (not to be confused with the vascular function curves where venoconstriction shifts the curve more up than what vasoconstriction rotates it downwards)
- Cerebral pressure autoregulation: a.k.a. the myogenic mechanism:
- effective at maintaining constant cerebral blood flow in a MAP range of 50–150 mmHg
- It effects this by changing the CVR.
- Onset is not immediate though.
- Activity: Mm pump further augments VR
- in conjunction with the one-way valves in the veins to prevents further venous pooling
JC 2019
Examiner Comments
2017B 19: 41% of candidates passed this question.
Generally, there was a lack of knowledge about this topic with many candidates confusing vasovagal syncope with a Valsalva or orthostatic hypotension. A “vasovagal” is from excessive autonomic reflex activity in contrast to orthostatic hypotension which is a failure of the autonomic reflex response.
A good place to start was with a description of vasovagal syncope, also known as neurocardiogenic syncope. It is benign, self-limiting and caused by an abnormal or exaggerated autonomic response to various stimuli (which should have been listed). The mechanism should have been described including the various receptors involved.
2019A 13
Classify circulatory shock and provide examples (40% of marks).
Outline the cardiovascular responses (60% of marks).
CICMWrecks Answer
Shock
Shock is defined as a state of cellular and tissue hypoxia due to reduced oxygen delivery and/or increased oxygen consumption or inadequate oxygen utilization. This most commonly occurs when there is circulatory failure manifested as hypotension (ie, reduced tissue perfusion).
Distributive:
Distributive shock is characterized by severe peripheral vasodilatation (vasodilatory shock)
e.g: Sepsis with shock
Cardiogenic:
Cardiogenic shock is due to intracardiac causes of cardiac pump failure that result in reduced cardiac output
e.g: Cardiomyopathy from MI
Hypovolemic:
Hypovolemic shock is due to reduced intravascular volume (ie, reduced preload), which, in turn, reduces CO.
e.g: Haemorrhage
Obstructive:
Obstructive shock is mostly due to extracardiac causes of cardiac pump failure and often associated with poor right ventricle output.
e.g: Pulmonary Embolism
Mixed / Unknown:
Unknown or more than one cause of shock contributing.
e.g: Pancreatitis with Septic (Distributive) and Hypovolemic shock
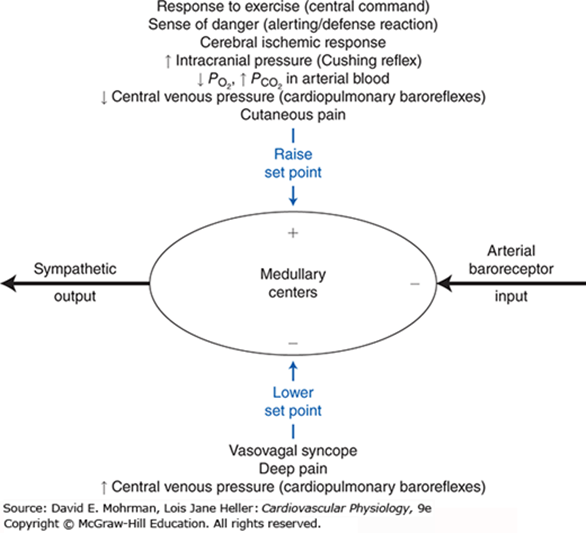
Cardiovascular Responses
- Arterial Baroreceptor Reflex:
- Short term regulation of arterial blood pressure
- Sensor: Stretch sensitive baroreceptor nerve endings in walls of arteries, nonarterial baroceptor units
- Senses: pressure (stretch)
- Integrator: Medullary cardiovascular centres
- Efferent pathways: Cardiovascular sympathetic and cardiac parasympathetic
- Effector organs: Heart, peripheral blood vessels
- Effect: Operates such that an increase in arterial pressure leads to an essentially immediate decrease in sympathetic nerve activity and a simultaneous increase in parasympathetic nerve activity (and vice versa).
- Reflexes from Receptors in Heart and Lungs:
- Mechanoreceptors and chemo receptors in atria, ventricles, coronaries, lungs
- Neurohumoral control of CVS
- Regulate blood volume and body fluid balance
- Chemoreceptor reflexes:
- Hypoxia (low Po2), hypercapnia (high Pco2), and/or acidosis (low pH) levels in the arterial blood cause reflex increases in respiratory rate and mean arterial pressure
- Arterial Chemoreceptors in carotid bodies at bifurcation of CCAs, and aortic bodies in arch of aorta
- Central Chemoreceptors in CNS respond to hypercapnia and acidification of CSF to regulate resp patterns and influence autonomic output
- Exercise responses:
- Reflexes from Receptors in Exercising Skeletal Muscle
- Chemoreceptors respond to muscle ischaemia
- Increased blood pressure
- Central command
- Input from cerebral cortex to lower brain centres during voluntary exercise simultaneous to cortical drives initiating exercise
- Cause reflex tachycardia and increased arterial pressure
- Reflexes from Receptors in Exercising Skeletal Muscle
- The Dive Reflex
- Bradycardia produced by enhanced cardiac parasympathetic activity and peripheral vasoconstriction caused by sympathetic activity
- Cardiovascular Responses associated with Emotion and Stress
- Originate in cerebral cortex – corticohypothalamic pathways – medullary cardiovascular centers
- Response is complex and depends on multiple factors
- E.g blushing, excitement, vasovagal syncope
- Reflex responses to Pain
- Superficial pain: rise in BP
- Deep pain: response similar to vasovagal syncope with dec sympathetic tone, inc parasympathetic tone, dec BP/shock
- Temperature Regulation Reflexes
- Controlled by hypothalamus, operate through cardiovascular centers, discretely control sympathetic activity
JC 2019
Examiner Comments
2019A 13: 83% of candidates passed this question.
Answers should have included the various types of shock and provided clear examples.
Cardiovascular responses including sensor, integrator, effector mechanisms were necessary to pass.
2009B 02
Describe the physiological consequences and responses after an acute haemorrhage of 2.0 litres in a healthy 70kg adult if there is no immediate fluid resuscitation.
CICMWrecks Answer
Normal circulating Volume ~ 5L
2L Haemorrhage ~ 40% loss without resuscitation – Major Haemorrhage
Immediate:
Baroreceptor Response
- HP BR
- Stretch receptor: ↑ distension of vessel → ↑ discharge rate
- Threshold > 60mmHg → normally has baseline tone.
- Located at Carotid Sinus and Aortic Arch
- Carotid sinus and aortic arch receptors
- Detects > 5-10 % change in plasma volume
- ↓ Plasma volume → Increased central SNS tone and decreased PSNS tone
- ↑’d distension → ↓’d HR/Contractility/SVR
- Provides strict negative feedback to Δ’s in CO
- LP BR
- Located at junction of return vessels and atria, vetricular walls, pulmonary vessles
- Throughout the peripheral vasculature (esp kidney)
- Detect > 10% decrease in plasma volume as decreased atrial stretch
- Reduced ANP release
- Increase SNS output
- 2 types
- A receptors → fire at atrial contraction (a wave)
- B type → fire at atrial filling (v wave)
- 1. Medulla afferents cause
- ↓ SNS (NA) and ↑PSNS (RVLM) outflow → peripheral vascular vaso and venodilation
- ↓ SNS activity to kidney → ↓ Na/H2O conservation
- ↑ SNS activity to sinus node
- 2. Hypothalamic afferents cause
- → ↓ ADH release and ↓ Thirst
- Outcome
- ↓ PL → ↓ CO → ↓BR discharge rate
- → ↓ PSNS medullary outflow
- → ↑ RVLM outflow
- ↑ contractility/HR
- ↑ SVR → autotransfusion and ↑ perfusing pressure (but ↑AL)
- ↓ PL → ↓ CO → ↓BR discharge rate
Chemoreceptor response
- Peripheral CR
- “Carotid bodies” (more important)
- at bifurcation point of common carotid arteries
- supplied by CN IX
- “Aortic bodies” (less important)
- above and below the aortic arch
- supplied by CN X
- PCRs found in “Glomus cells”
- ↓ PaO2 – via inhibition of O2 sensitive K-channels
- ↑ PaCO2 and/or ↓pH – via effect on pH sensitive K-channels
- Type I cells (rich in NAd, DA, ACh)
- Hypoxia causes release of NTs
- NAd/ACh – ↑ AP firing rate of AB or CB afferent fibres
- DA – Damping of type 2 cell responses
- Type II cells (rich in capillary supply)
- ↓ PaO2, ↑ PaCO2 and/or ↓pH
- ↓ IC [ATP] which leads to ↑ NT production and release
- ↑ AP firing rate of AB or CB afferent fibres
- CCRs located Below the ventral surface of the medulla
- ↑ ECF [H+] stimulates ↑ ventilation
- CO2 rapidly diffuses into CSF
- Low CSF protein and HCO3 (c.f. plasma)
- Poor buffering capacity
- ΔCSF pH per ΔPaCO2 is GREATER than that of blood
- Cause
- ↑’s SVR due to
- ↓ hypothalamic perfusion pressure
- ↓ Hct
- ↑’d RR → ↑ venous return
- ↑ ADH secretion (intermediate term response)
- ↑’s SVR due to
Intermediate term response
- Chemoreceptor stimulation (↓’d perfusion → ↑pCO₂:pO₂)
- ↑ ADH secretion
- ↑’d RAAS activation
- ↓ renal perfusion → ↑ renin → ↑AT2 and Aldosterone
- ↑ADH → ↑Na/H2O retention and vasoconstriction
- ↓Hydrostatic pressure
- altered starlings forces
- autotransfusion of 0.25mL/kg/min ~ 1L/hr
Long Term Response
- ↑ RAAS → ↑ Na/H2O retention
- Osmoreceptor response
- Anterior hypothalamic receptors
- Detect 1% decrease from normal (280-300mOsm/kg)
- Threshold 280mOsm/kg
- Promotes release of ADH from posterior pituitary
- ↑thirst
- EPO release
- From adrenal medulla → ↑ RBC mass
- ↑ hepatic plasma protein synthesis
Gladwin 2016
Examiner Comments
2009B 02: 5 (56%) of candidates passed this question.
To adequately answer this question, candidates must be able to demonstrate that they recognised this to be a major haemorrhage. When a weight and a volume are supplied it is expected the percentage blood loss would be calculated and the shock graded or the haemorrhage at least described as severe. Often the consequences were omitted.
Consequences were best described in organ systems e.g. CV, renal, metabolic. Many candidates failed to mention the patient would be hypotensive and tachycardic.
A good answer should include mention, and provide explanations, of the mechanisms for the
following compensatory responses:
• Activation of both baroreceptors and chemoreceptors and their consequences
• The sympathetic nervous system response
• Fluid shifts
• Renal effects – most candidates mentioned the urine output would be decreased but did not provide a mechanism for this
Endocrine effects, eg secretion and actions of ADH, ACTH/Cortisol
Syllabus – C1e1
Reference: Textbook of Medical Physiology, Guyton and Hall, Foundations of Anaesthesia:
Basic Clinical Science, Hemmings and Hopkins
2010A 01
Describe the cardiovascular changes that occur following the loss of 1000ml of blood in an adult.
CICMWrecks Answer
Distribution of water
- TBW = 60% body weight ~ 42L
- Intracellular water = 66% of TBW ~ 28L
- Extracellular water = 33% of TBW ~ 14L
- Plasma volume = 25% Extracellular water ~3.5L
- Blood volume (plasma + RBC intracellular water) = 7% of total body water ~5L
1000ml blood loss = approx 20% blood loss = Stage II shock
Cardiovascular changes
Immediate
- Decreased venous return due to loss of blood volume
- Frank-Starling Curve à Cardiac output decreased
- Systolic and mean arterial blood pressure decreases (MAP = CO x SVR)
- Baroreflex
- Decreased MAP
- Decreased stretch of aortic arch and carotid sinus baroreceptors
- Decrease firing of baroreceptors
- Decreased signalling to sensory area of medullary vasomotor centre in NTS
- Decreased inhibition of vasomotor area
- Increased sympathetic and decreased parasympathetic output
- Heart
- Increased heart rate and contractility
- Vasculature
- Vasoconstriction
- Blood diverted from peripheries to essential organ function (coronary and cerebral circulation)
- Increased diastolic blood pressure
- Vasoconstriction
- Leads to increased MAP
- Decreased MAP
- Barcroft-Edholm reflex
- If venous return severely impaired
- Mechanoreceptors in R atrium/ventricle sense collapsing of chambers in systole
- Increased vagal signal to NTS
- Bradycardia (and hypotension) in order to allow greater diastolic filling time
- If venous return severely impaired
- As all blood constituents lost → [Hb] and haematocrit unaltered
- At >10% blood volume loss, low pressure (cardiopulmonary) baroreceptors cause secretion of vasopressin from posterior pituitary
- Vasoconstriction via V1 receptor
- Renal water retention via V2 receptor (also assists in coagulation via vWF release)
Acute Changes
- Decreased intravascular hydrostatic pressure à transcellular shift of extracellular fluid into intravascular space and intracellular water into extracellular space
- Decrease in plasma osmolality (usually 275~290mOsm/L)
- Sensed by Organum Vascularis of the Lamina Terminalis (OVLT)
- Increased secretion of vasopressin from posterior pituitary
- Decreased [Hb] due to transcellular shift of water decreases blood viscosity
- Decreased resistance to laminal flow
- Promotes cardiac output
- Decrease in plasma osmolality (usually 275~290mOsm/L)
- Decreased MAP → Decreased renal perfusion pressure → Decreased glomerular filtrate (although some increase in filtration fraction to compensate)
- Decreased [Na] and [Cl] in DCT
- Activation of RAAS
- Angiotensin II
- Potent vasoconstriction
- Stimulates secretion
- ACTH
- Vasopressin
- Aldosterone
- Potentiates secretion of noradrenaline from post-ganglionic sympathetic fibres
- Stimulates thirst
- Aldosterone
- Increases cellular Na/K ATPase
- Inserts ENaC into Collecting Duct tubular cells
- Increased Na reabsorption
- Water reabsorption
- Inserts UT-A1 into Medullary Collecting Duct
- Increased Urea reabsorption
- Increased osmolarity of medullary interstitium
- Increased water reabsorption
- Decreased [Na] and [Cl] in DCT
Chronic
- Decreased DO2 to renal interstitium → activation of hypoxia inducible factors (HIF)
- Synthesis of secretion of erythropoietin (EPO)
- Stimulation of erythropoiesis by haematopoietic stem cell
- Decreased DO2 to tissues → angiogenesis
Sakurai 2016
Examiner Comments
2010A 01: 10 (100%) of candidates passed this question.
A structured approach that included mentioning that 1000mls of blood was substantial – being approximately 20% of the blood volume of a 70 kg person was required for a good answer. Candidates were expected to also include changes in systolic and diastolic blood pressure, pulse pressure, heart rate, cardiac output and the neuronal (eg sympathetic nervous system response on the various circulations) and hormonal responses (eg rennin aldosterone, Anti-Diuretic Hormone,
catecholamines, etc). Candidates were also expected to discuss differences in responses according to rate of blood loss. Flow diagram could have been used to illustrate some of these concepts.
Syllabus: C1e
References: Textbook of Medical Physiology, Guyton pg 278 – 282, Principles of Physiology for the Anaesthetist, Power & Kam pg 154
2008A 23
Describe the hormonal response to hypovolaemia following the acute loss of one litre of blood in an adult. Include changes that occur in the first 24 hours following the blood loss.
CICMWrecks Answer
Reduced venous return
- Reduced right atrial stretch
- Reduced atrial natriuretic peptide
ANP
- Dilates afferent arteriole, constricts efferent arteriole
- Increased glomerular capillary hydrostatic pressure
- Increased glomerular filtration
- Increased glomerular capillary hydrostatic pressure
- Inhibits glomerular capillary mesangial cell constriction
- Increases filtration coefficient by increasing surface area available for diffusion and osmosis
ADH
Reduced CO → reduced MAP → increased signalling of carotid sinus and aortic arch baroreceptors
- Stimulates release of vasopressin (ADH)
ADH
- Induces systemic vasoconstriction
- Vascular smooth muscle cell V1 receptors → IP3 second messenger → increased intracellular calcium
- Increased renal water resorption
- Distal tubule and collecting duct V2 receptors
- Via cAMP
- Expression of aquaporin 2
- Water channels
- Distal tubule and collecting duct V2 receptors
RAAS
Renin
- Release stimulated (from juxtaglomerular cells) by:
- Sympathetic nerve stimulation (β1-adrenoreceptors)
- Reduced afferent arteriolar pressure
- Reduced flow of Na+ and Cl– in macula densa
Angiotensinogen —renin–> angiotensin I —ACE–> angiotensin II —secreted from zona glomerulosa of adrenal gland —> aldosterone
Angiotensin II
- Systemic vasoconstriction
- Constrict afferent and efferent renal arterioles, constricts mesangial cells -> reduces GFR
Aldosterone
- Stimulates collecting principal cell Na/K ATPase -> sodium resorption, potassium secretion (and so indirectly causes water retention)
Others
- Stimulation of aortic and carotid baroreceptors, cardiopulmonary mechanoreceptors, chemoreceptors
- Sympathetic activation
- Release of adrenaline from adrenal medulla
- Peripheral vasodilation
- Increased inotropy, chronotropy
- Cortisol release increased
- Erythropoetin released within 24 hours after haemorrhage
Sakurai 2016
Examiner Comments
2008A 23: 2 candidates (66%) passed this question.
Candidates were expected to know the different hormonal responses to hypovolaemia The possible approach to this question can be either by explaining the hormonal response in terms of time sequence or by different hormonal systems.
Good answers to this question included how different hormonal responses are activated and mediated.
The common omissions were secretion of erythropoietin within 24 hours of haemorrhage, role of macula densa and juxtaglomercular apparatus, interactions between baroreceptors and sympathetic nervous system with the secretion of ADH, cortisol, glucagon and catecholamines.
Syllabus: C1g 2b
Reference: Kam 1st edition 156, 212, Guyton 11th edition 342,287-280
2015A 07
Outline the physiological responses to the rapid intravenous administration of 1 litre of 0.9 % saline to a 70 kg euvolaemic person.
CICMWrecks Answer
Assumptions
- TBW is one-third ECF & two-thirds ICF
- ECF is one-quarter plasma & three-quarters ISF
- The threshold of the volume receptors is 7-10% change in blood volume
- The osmoreceptors are sensitive to a 1-2% change in osmolality.
- Plasma osmolality is normal prior to the transfusion (ie 287-290 mOsm/kg)
Key
- TBW = Total Body Water
- ECF = Extracellular Fluid
- ICF = Intracellular Fluid
- ISF = Interstitial Fluid
1L NS has 9gm NaCl – It is an ECF replacement fluid
| Volume Litre | [Na+] mmol/L | [Cl–] mmol/L | Osmolarity mOsml/L | |
|---|---|---|---|---|
| NS | 1 | 154 | 154 | 308 |
| Plasma | 5 | 140 | 100 | 290 |
Response to 1L Normal Saline Bolus
| INITIAL PHASE | ||
| Brief Hypervolemia | → ↑ arterial BP | → Reflex response (baroceptors, atrial stretch receptors etc) |
| ↑ Renal perfusion → Stimulation of intra renal apparatus (juxtaglomerular apparatus) | ||
| [Na+] | 154/L into 140/L | very negligible rise |
| [Cl–] | 154/L into 100/L | rises 1L NS unlikely to cause hyperchloraemic metabolic acidosis |
| REDISTRIBUTION PHASE (~15 minutes) | ||
| [Na+] similar to ECF → Distributes | ISF (3) | ↑750ml |
| Plasma (1) | ↑250ml | |
| EXCRETION PHASE (~6 hours) | ||
| Plasma osmolality and tonicity mostly unchanged | no osmoreceptor stimulation | |
| Plasma volume ↑ to 5250mls (5% increase) | below sensitivity of volume receptor → no volume receptor stimulation | |
| No protein → oncotic pressure slightly lowered | Fluid moves into ISF (Starling’s hypothesis) | |
| Glomerulo-tubular Imbalance → ↑GFR → ↓ reabsorption of water in proximal tubule → ↑ Urine flow | ||
| Fluid moves back into intravascular compartment till all infused fluid is excreted (~6hrs) | ||
Examiner Comments
2015A 07: 25 % of candidates passed this question.
Answering this question required the integration of information from areas of cardiovascular, body fluid and renal physiology which proved difficult for most candidates. Both breadth and depth was expected so as to score well.
This question is best answered using a time-based approach. For example, upon the rapid infusion of a litre of normal saline there will be a brief period of hypervolemia, increase in arterial blood pressure and an associated physiological reflex response to these changes (e.g. baroreceptors, atrial stretch receptors, etc.). There will also be an associated increase in renal perfusion and stimulation of intrarenal receptors (e.g. juxtaglomerular apparatus).
Candidates were expected to outline these changes, their effector responses (e.g. autonomic nervous system reflexes and humoral changes) and their physiological consequences.
A more prolonged redistribution phase of the administered saline then occurs. This saline redistributes throughout the extracellular fluid space. Candidates were expected to briefly describe this effect as well as the subsequent management of the sodium and water load by the kidney.
Most candidates spoke about the pressure effects, and only some compared these with the volume effects. The effect of redistribution and other effects were not considered by the majority of the candidates.
2019B 01
Describe the physiological consequences of the oral ingestion of 1 litre of water in a young adult.
CICMWrecks Answer
Water
- Water is distributed across all body compartments
- It is hypotonic compared to all body compartments
- It theoretically gets distributed to ECF (Plasma and ISF) + ICF
- Absorption of ingested water occurs in proximal small intestine.
- Absorption rate depends on rate of gastric emptying and creation of suitable osmotic gradient across the intestinal mucosa.
- 1L Oral ingestion of water is rapidly absorbed in the small intestine.
Mechanism of Absorption of water
- Sodium is absorbed from the intestinal lumen by several mechanisms, most prominently by cotransport with glucose and amino acids, and by Na+/H+ exchange, both of which move sodium from the lumen into the enterocyte.
- Absorbed sodium is rapidly exported from the cell via sodium pumps – when a lot of sodium is entering the cell, a lot of sodium is pumped out of the cell, which establishes a high osmolarity in the small intercellular spaces between adjacent enterocytes.
- Water diffuses in response to the osmotic gradient established by sodium – in this case into the intercellular space. It seems that the bulk of the water absorption is transcellular, but some also diffuses through the tight junctions.
- Water, as well as sodium, then diffuses into capillary blood within the villus.
Water compartments and Responses
- TBW is one-third ECF & two-thirds ICF
- ECF is one-quarter plasma & three-quarters ISF
- The threshold of the volume receptors is 7-10% change in blood volume
- The osmoreceptors are sensitive to a 1-2% change in osmolality.
- Plasma osmolality is normal prior to the transfusion (ie 287-290 mOsm/kg)
Effect of 1L oral intake
- Absorption of water causes a drop in plasma osmolarity without much effect on plasma volume.
- There is a brief period of hypervolemia, increase in arterial blood pressure and an associated physiological reflex response mainly by osmoreceptors
- There will also be an associated increase in renal perfusion and stimulation of intrarenal receptors (e.g. juxtaglomerular apparatus).
- Sensor: Osmoceptors in hypothalamus
- CPU: Hypothalamus
- Effector: ADH (and thirst, not significant in current scenario)
- Anti-diuretic hormone (ADH)
- peptide hormone synthesised in the hypothalamus
- released from the posterior pituitary
- acts on the kidney via GPC V2 receptors
- increases the synthesis of aquaporins
- ADH secretion is decreased,
- ➔ water permeability in the nephrons is increased
- ➔ leads to less water reabsorption
- ➔ larger amount of dilute urine excretion
- Consequence is homeostasis by excretion of the 1L of water quite rapidly.
JC 2019
Examiner Comments
2019B 01: 28% of candidates passed this question.
It was expected candidates would provide details the consequences of water ingestion from its rapid absorption in the small intestine to the resultant impact on plasma osmolarity and the minimal impact of plasma volume of this volume. Some detail on the mechanisms of absorption (transcellular vs osmosis) was expected and the distribution of water across body fluid spaces.
Many candidates accurately described the small drop in plasma osmolarity that is sufficient to trigger osmoreceptors with better answers providing details of the locations and mechanisms involved. The physiological consequences of inhibition of ADH, including the renal effects of decreased water permeability in distal renal tubules and collecting ducts. The volume load after distribution would be lower than the plasma volume triggers for the circulatory reflex responses.
2022B 12 – 2015A 19
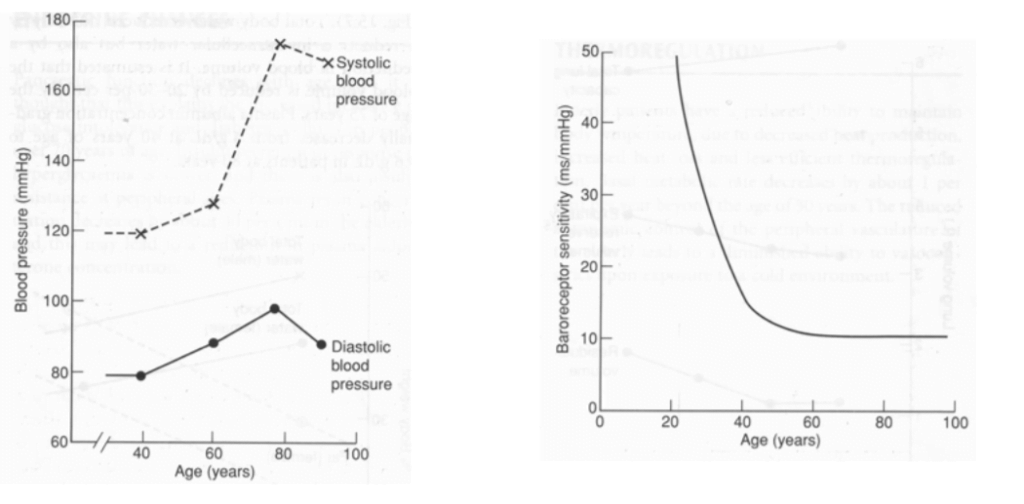
Describe the effects of ageing on the cardiovascular system.
2012A 09
Describe the changes to cardiovascular physiology in a healthy elderly person.
CICMWrecks Answer
Ageing = physiological time-dependent process which results in ↓ cellular function + ↓ reserve
- Heart
- ↑ fibrous infiltration of myocardium → ↓ ventricular compliance (esp LV > RV)
- ↓ # myocytes but compensated by hypertrophy → ↑ myocardial wall thickness (concentric) → ↓ ventricular compliance (esp LV > RV)
- ↑ fibrous infiltration of endocardium → ↓ ventricular compliaince (esp LV > RV)
- Calcification of heart valves → valvular incompetence (stenosis/regurgitation)
- Conducting system (Ie. SAN, AVN, bundle of His, Purkinje):
- ↑ fibrous/fatty infiltration
- ↑ loss of pacemaker cells
- ↓ # and sensitivity of β receptors within pacemaker cells
- Peripheral vasculature:
- ↓ elasticity of large arteries due to thickening and calcification of vessel wall
| Change with ageing | Reason | |
|---|---|---|
| HR | Resting HR → unchanged ↓ maximum HR (Max. HR = 220 – age) ↑ arrhythmias (AF, AV heart block, VEBs, BBB) | Conduction system (SAN, AVN, bundle of His, Purkinje) → fibrous/fatty infiltration and loss of pacemaker cells ↓ # and sensitivity of β receptor of pacemaker cells |
| SV | ↓ resting SV ↓ maximum SV | ↓ ventricular compliance (2° to fibrous infiltration of myocardium/endocardium, and ventricular hypertrophy) Valvular disease |
| CO | ↓ due to preconditioning (Ie. sedentary lifestyle) or age-related disease Unchanged in “healthy” subjects Nb. ↑ C.O. occurs mainly by ↑ SV (preload) via Frank-Starling mechanism (rather than ↑ HR) | ↓ HR – see above ↓ preload (↓ SV) – see above ↑ afterload (↑ SVR) – see below ↑ reliance on “atrial kick” (30% of C.O. vs 5% in adults) → AF/↑HR not well tolerated |
| BP | ↑ MAP and SBP DBP ↑ slightly (until age 60), then ↓ ↑ pulse pressure (due to ↑ SBP > DBP) | ↓ elasticity of large arteries due to thickening and calcification of vessel wall |
| Pulmonary circulation | ↑ PAP a/w ↑ PVR | |
| Control of CVS | ↓ maximum exercise tolerance (due to age-related ↓ maximum HR, SV, C.O.) Impaired BRR → postural hypotension ↓ β receptor response of CVS to catecholamines (↓ # or affinity of receptor, ↓post-receptor signalling, ↓ myocardial contractile response with stimulation) ↑ vagal tone |
Bianca 2016
Examiner Comments
2022B 12: 46% of candidates passed this question.
The physiological changes associated with ageing are well described in the major texts although candidates do need to assimilate this information from a number of sources. Core components of the answer templates that were frequently missed included those of; concentric hypertrophy and its effects on afterload, as well as the difference between compliance, elastance, elasticity, elastin and collagen and how ageing affects these elements. A template using broad headings such as the effects on the heart/myocardium, the vasculature, the autonomic nervous system, the conduction system and perhaps epithelial function would be a good starting point when constructing an answer to this question. The examiners noted that several candidates wrote at length about various pathologies that increase in incidence with ageing which does not adequately address the core of the question.
2015A 19: 29 % of candidates passed this question.
Many candidates described the pathological processes which might affect the aging heart rather than the physiological ones.
Recognition that aging reduces cardiovascular reserve followed up with an outline of the effects of aging on the heart, the vasculature, endothelial function and the conducting system would be rewarded with a good mark.
Few answers quantified the decrease of cardiac output with age and only even fewer ventured into the contribution of ventricular filling by atrial systole. No answer discussed
endothelial changes with aging.
Some answers were repetitious. Some answers included a significant discussion of information that was not asked for (Laplace law/Poiseuille’s law).
2012A 09: 1 (10%) of candidates passed.
It is clearly stipulated in the syllabus that candidates would be expected to understand physiology as it applies at the extremes of age. In the past, questions have been asked relating to foetal and neonatal physiology as well as for the elderly. This question was poorly answered as candidates lacked a detailed and coherent knowledge of this topic. For a good answer candidates were expected to at least mention the effects on the heart, e.g. increases in size due to concentric ventricular hypertrophy (LVH), hypertrophy of myocytes but a decrease in the number of myocytes, cardiac output decreases, the increase in cardiac output in response to severe exertion is attenuated, ventricular filling is particularly dependent on diastolic relaxation which is impaired, greater contribution of atrial contraction, increase in left ventricular afterload, effects on vasculature, e.g. intima and media thickening result in less distensibility, effects on endothelial function, e.g. nitric oxide release is decreased, effects upon autonomic and integrated responses, e.g. decline in receptor numbers, down regulation of post-receptor signalling and decreased receptor density and impaired baroreceptor reflexes.
2020B 11
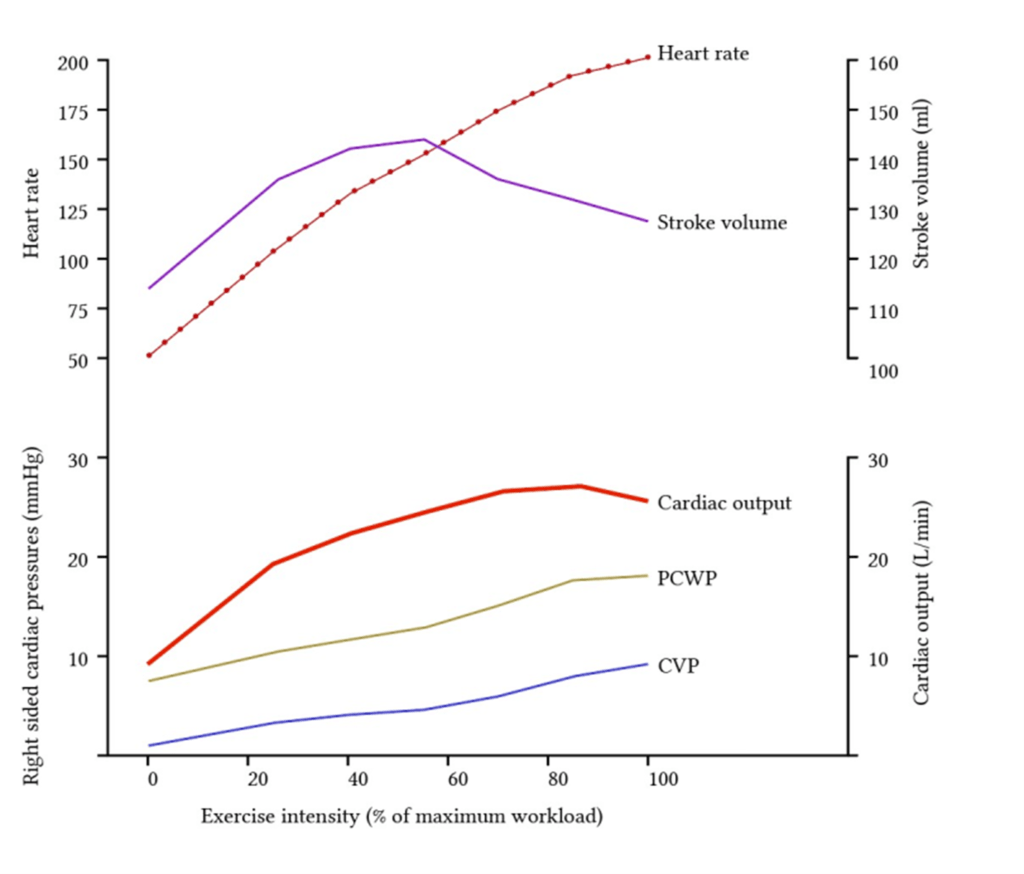
Describe the changes in the circulatory system that occur during exercise.
CICMWrecks Answer
Definition:
Exercise = Strenuous physical activity where there is increased metabolic activity leading to increased requirement for energy and oxygen
CVS changes to accommodate and compensate for such increase in metabolism
Normal parameters at rest:
| Cardiac output | C.O. | 5L/min |
| Heart rate | HR | 60-100 bpm |
| Systemic vascular resistance | SVR | 1600 dynes.s.cm-5 |
| Stroke volume | SV | 70mls |
| Blood pressure | BP | 120/80 |
| Central venous pressure | CVP | 0-5mmHg |
Changes to CVS parameters:
| Cardiac output | ↑ | Can increase up to 4-5 folds CO plateaus at near-maximal workload |
| HR | ↑ | Max HR = 220-age Increases and plateaus at max |
| SV | ↑→↓ | Initially increases, then decreases due to diminishing diastolic time |
| Contractility | ↑ | Increased HR → increased contractility (treppe effect)Activation of sympathetic nervous system → increased contractility |
| CVP | ↑ | Increases |
| Systolic BP | ↑ | Increases |
| Diastolic BP | ↓ | Decreases |
| MAP | ↑ | Increases slightly |
| Pulse pressure | ↑ | Widens |
| PVR | ↑ | Decreases overall |
Redistribution of blood flow
- Increased muscle activity -> increased o2 demand
- Regional vasodilation
- Increased local concentration of metabolites ie Co2, lactate, K+
- Vasoactive mediator released by endothelium ie NO, ATP
- Sympathetic stimulation -> B2 adrenoceptor activation
- Overall: decrease in vascular resistance to muscles -> increased blood flow
- Corresponding vasoconstriction of viscera and skin
Increase in cardiac output
- Cardiac output can increase up to 0L/min
- Due to both increase in HR and SV and decreased afterload
- With increasing workload HR increases but SV decreases due to dec diastolic time
Guo 2021
Examiner Comments
2020B 11: 22% of candidates passed this question.
This is an applied physiology question. Better answers categorised the changes in some manner and included a measure of the degree of change as applicable (e.g., what increases, what decreases and what may stay the same). The question was to describe the changes so that the detail behind the mechanisms enabling these changes to occur was expected (e.g., neurohumoral, local factors). Marks were also awarded for any regional variation that occurs.
2017A 15
Outline the cardiovascular changes associated with morbid obesity.
CICMWrecks Answer
Obesity
- excessive fat accumulation in adipose tissue
- WHO classification based on BMI (weight in kg/ height in m2)
| BMI | |
|---|---|
| Underweight | <18.5 kg/m2 |
| Normal weight | ≥18.5 to 24.9 kg/m2 |
| Overweight | ≥25 to 29.9 kg/m2 |
| Obesity | ≥30 kg/m2 |
| Obesity class 1 | 30 to 34.9 kg/m2 |
| Obesity class 2 | 35 to 39.9 kg/m2 |
| Obesity class 3 | BMI ≥40 kg/m2 |
*Alternate names from different classifications:
| BMI | |
|---|---|
| Severe Obesity | ≥ 35 or 40 kg/m2 |
| Morbid Obesity | ≥ 35 kg/m2 + obesity-related health conditions or ≥ 40 kg/m2 |
| Super Obesity | ≥ 45 or 50 kg/m2 |
Characteristics:
- Changes depend on extent + duration of obesity
- Complex genetic and environmental causes
- Increased caloric intake
- Increased metabolic rate (normal for BSA)
- associated with HTN, HF, IHD, cardiomyopathy, sudden cardiac death, arrhythmias, PVD, DVT, CVD
- Hormonal changes:
- ↑ SNS
- ↑ HR + ↑ SV
- ↑ RAAS → Na+ retention → ↑ blood vol → ↑ MAP (Systemic HTN)
- ↑ MAP → LVH → LV dilation → LV failure
- LV diastolic failure + ↑ PVR → RV hypertrophy
- ↑ Leptin → Cardiac remodelling + LVH
- Plasminogen Activator Inhibitor-1 → ↓ fibrinolysis → predisposes to VTE
- Inflammatory Adipokines → Impairs endothelial function → ↑ SVR
- insulin resistance + hyperlipidaemia → inflammatory mediator upregulation → disrupt endothelial function → IHD + cerebrovascular disease + PVD
- ↑ SNS
- Mechanical Effect: Compression of abdominal + leg vessels
- ↓ VR → supine hypotension + ↑ risk DVTs
Changes in CVS:
- Increased VO2 : Due to increased LBM and fat mass.
- Increased Blood Volume : Due to increased angiotensin II and aldosterone.
- Increased Stroke Volume: Due to:
- Increased preload (major factor)
- Increased contractility (minor factor) due to increased circulating adrenal hormones.
- Increased Cardiac Output: To maintain DO2.
- Initially with preserved ejection fraction
- ↑ Peripheral vascular resistance
- Inflammatory Adipokines → Impairs endothelial function
- ↑ SNS
- Diastolic dysfunction: Due to myocardial fibrosis impairing relaxation.
- direct deposition of fat in myocardium → conduction disease (& predisposition to arrhythmias) + cardiomyopathy
- OSA
- Pulmonary HTN → cor pulmonale
- Polycythaemia → ↑ viscosity
Kerr / JC 2020
Examiner Comments
2017A 15: 42% of candidates passed this question.
Many candidates did not include enough detail in their answers. Higher scoring answers included more depth such as the following: blood volume, left ventricular changes, arterial blood pressure, pulmonary artery pressures, risks of ischaemia, arrhythmias etc.
2015B 08
Compare and contrast the physiological changes in the cardiovascular system in pregnancy at term and morbid obesity (BMI > 30).
CICMWrecks Answer
Pregnancy at Term
Morbid Obesity
- Pregnancy is a time of increased metabolic demand, which cardiovascular changes reflect.
- Changes begin from week 8 and ↑ to plateau at 32 weeks → return to normal 2-8 weeks post delivery
- excessive fat accumulation in adipose tissue
- WHO/NIH classification based on BMI:
- Overweight: BMI 25 – 30
- Obesity: BMI ≥ 30
- Obesity Class 1: 30 to 34.9 kg/m2
- Obesity Class 2: 35 to 39.9 kg/m2
- Obesity Class 3: BMI ≥40 kg/m2
- Alternate classifications:
- Severe Obesity: ≥ 35 or 40 kg/m2
- Morbidly Obese: BMI ≥ 40 (or) Obesity related disease and a BMI ≥ 35
- Super Obesity: ≥ 45 or 50 kg/m2
- Changes depend on stage of pregnancy
- Hormonal changes: ↑ circulating concentrations of oestrogen, progesterone, hCG
- ↑ metabolic demand esp. during labour: ~↑60% O2 consumption/ CO2 production during labour
- Mechanical effects from gravid uterus
- Changes depend on extent + duration of obesity
- Complex genetic and environmental causes
- Increased caloric intake
- Increased metabolic rate (normal for BSA)
- associated with HTN, HF, IHD, cardiomyopathy, sudden cardiac death, arrhythmias, PVD, DVT, CVD
- Thoracic changes:
- Anatomical compression of chest
- Diaphragm pushed upwards by 4cm
- ↑ AP + transverse diameter of chest wall (2-3cm)
- placental circulation: ↓pressure, ↓resistance AV shunt
- Aortocaval compression
- Collateral blood flow via collateral paravertebral epidural veins
- Compression of abdominal + leg vessels
- ↓ VR → supine hypotension + ↑ risk DVTs
- ↑ circulating concentrations of oestrogen, progesterone, hCG
- Oestrogen stimulation of RAAS
- Increased plasma volume (40% or 1~1.5L positive)
- Erythropoietin secretion
- Increased erythropoiesis and red blood cell volume (20%)
- ↑ SNS
- ↑ HR + ↑ SV
- ↑ RAAS → Na+ retention → ↑ blood vol → ↑ MAP (Systemic HTN)
- ↑ MAP → LVH → LV dilation → LV failure
- LV diastolic failure + ↑ PVR → RV hypertrophy
- ↑ Leptin → Cardiac remodelling + LVH
- Plasminogen Activator Inhibitor-1 → ↓ fibrinolysis → predisposes to VTE
- Inflammatory Adipokines → Impairs endothelial function → ↑ SVR
- insulin resistance + hyperlipidaemia → inflammatory mediator upregulation → disrupt endothelial function → IHD + cerebrovascular disease + PVD
- Anaemia of pregnancy
- Disproportionate plasma volume expansion relative to erythropoiesis
- Increased cardiac output (40%)
- Increased uterine blood flow (750ml/min)
- Increased renal blood flow
- Increased HR (25% by second trimester)
- Increased SV (25% in first trimester)
- Increased VO2 : Due to increased LBM and fat mass.
- Increased Cardiac Output: To maintain DO2.Initially with preserved ejection fraction
- Increased Stroke Volume: Due to:
- Increased preload (major factor)
- Increased contractility (minor factor) due to increased circulating adrenal hormones.
- Decreased peripheral vascular resistance (30%)
- Progesterone
- Prostaglandins
- Down-regulation of α receptors
- Decreased plasma oncotic pressure (15%) → peripheral oedema
- ↑ Peripheral vascular resistance
- Inflammatory Adipokines → Impairs endothelial function
- ↑ SNS
- Diastolic dysfunction: Due to myocardial fibrosis impairing relaxation.
- direct deposition of fat in myocardium → conduction disease (& predisposition to arrhythmias) + cardiomyopathy
- OSA
- Pulmonary HTN → cor pulmonale
- Polycythaemia → ↑ viscosity
Sakurai / Kerr / JC 2020
Examiner Comments
2015B 08: 2% of candidates passed this question.
The question was very specific for the cardiovascular system and therefore answers that
described respiratory changes and airway modulation failed to score marks. This answer leant itself to a tabular format. Candidates are reminded to ensure they document the facts in the correct column i.e. obesity facts in the obesity column. The cardiovascular changes associated with term pregnancy are well described in various texts. Those associated with morbid obesity required some integration from various sources and would include a structured series of comments such as heart rate (unchanged), blood pressure (tendency for hypertension), stroke volume (increased), cardiac output (increased), blood volume (increased – although perhaps decreased on a ml/kg basis), systolic function (preserved or increased), LV wall thickness increased. The pathological changes seen with the diseases associated with obesity are difficult to tease out and better answers identified this. Morbid obesity has a specific definition and
stating this aided focus of the answers.
2022A 06
Describe the cardiovascular changes seen throughout pregnancy.
2016A 07
Describe the cardiovascular changes of pregnancy including parturition.
2013B 19
Describe the cardiovascular changes during pregnancy.
2010B 04
Describe the cardiovascular changes that occur in pregnancy
CICMWrecks Answer
- Pregnancy is a time of increased metabolic demand, which cardiovascular changes reflect.
- Changes begin from week 8 and ↑ to plateau at 32 weeks → return to normal 2-8 weeks post delivery
- Changes depend on stage of pregnancy
- Hormonal changes: ↑ circulating concentrations of oestrogen, progesterone, hCG
- ↑ metabolic demand esp. during labour: ~↑60% O2 consumption/ CO2 production during labour
- Mechanical effects from gravid uterus
Characteristics
- Mechanical Effects:
- Thoracic changes:
- Anatomical compression of chest
- Diaphragm pushed upwards by 4cm
- ↑ AP + transverse diameter of chest wall (2-3cm)
- placental circulation: ↓pressure, ↓resistance AV shunt
- Aortocaval compression
- Collateral blood flow via collateral paravertebral epidural veins
- Thoracic changes:
- Hormonal Changes:
- ↑ circulating concentrations of oestrogen, progesterone, hCG
- Oestrogen stimulation of RAAS
- Increased plasma volume (40% or 1~1.5L positive)
- Erythropoietin secretion
- Increased erythropoiesis and red blood cell volume (20%)
Changes in CVS
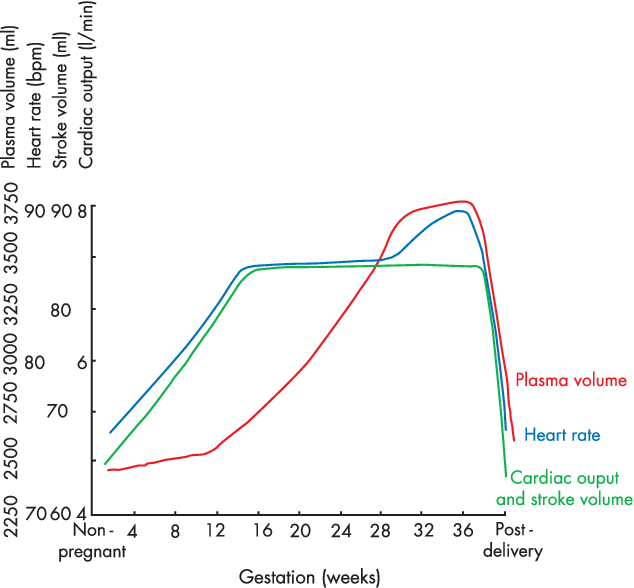
- Anaemia of pregnancy
- Disproportionate plasma volume expansion relative to erythropoiesis
- Increased cardiac output (40%)
- Increased uterine blood flow (750ml/min)
- Increased renal blood flow
- Increased HR (25% by second trimester)
- Increased SV (25% in first trimester)
- Decreased peripheral vascular resistance (30%)
- Progesterone
- Prostaglandins
- Down-regulation of α receptors
- Decreased plasma oncotic pressure (15%) → peripheral oedema
During labour
- Contraction → 300ml return to central maternal circulation
- CO increases 15%, 30% and 45% in latent, active and expulsive phases of labour respectively
- Immediately after labour CO 80% pre-labour levels due to autotransfusion due to uterine involution
- Return to non-pregnant levels 2 weeks after delivery
Sakurai / Kerr / JC 2020
Examiner Comments
2022A 06: 51% of candidates passed this question.
It was expected that candidates would give a detailed description of the changes that occur throughout pregnancy, labour and post-delivery (a timeline). This should include but not be limited to, cardiac output, total peripheral resistance, blood flow distribution, uterine blood flow and blood volume changes. Better answers were able to relate these changes to the underlying mechanisms (such as progesterone induced vasodilatation etc). A detailed description of aortocaval compression and its importance was also required. Vague and imprecise statements attracted fewer marks (for example simply stating that heart rate increases without discussing the magnitude, time course and influences). This topic is well covered in some of the recommend texts.
2016A 07: 62% of candidates passed this question.
Significant CVS changes can occur by eight weeks and then progressively over the term of the pregnancy. Structured answers helped candidates avoid missing important areas of the answer. It was expected candidates could detail the major changes such as a 40 – 50% increase in blood volume, a 30 – 50% increase in cardiac output, a slight decrease in blood pressure, the heart size and position changes, the impact of aortocaval compression and alterations in colloid osmotic pressure. Some mention of the changes during labour and delivery was expected noting uterine contraction squeezes blood to maternal circulation (auto transfusion), cardiac output increases (immediately after delivery up by about 60 – 80%) and blood pressure increases (both systolic and diastolic) during labour.
Hormones, particularly the effects of foetoplacental production or transformation of hormones, and their cardiovascular effects, especially on total body composition / filling pressures were under explained. The cardiovascular changes at parturition were not well explained.
2013B 19: 4 candidates passed (14.8%).
Many candidates’ answers included respiratory and other systems (e.g. endocrine) when only cardiovascular changes were asked for. Candidates are reminded to read the questions carefully. Poor candidates lacked detail of the progressive changes through the trimesters. Changes during (and immediately post) labour and delivery were often overlooked. This is listed as a core topic within the syllabus, that is readily covered by most physiology texts and candidates are expected to have a very sound knowledge of it.
2010B 04: 4 (27%) of candidates passed this question.
A structured approach to answering this question was important. The principal topics to discuss included the increased cardiac output, increased blood volume, changes in red blood cell mass and protein concentrations, decrease in blood pressure and gravitational effects of the gravid uterus on major blood vessels. Answers benefited by specific details of percentage change and reference to timing of occurrence.
Syllabus: O1,2a
References: Hemmings Foundation of Anaesthesia: Basic and Clinical Science Pg 823 – 825, Miller Anaesthesia pgs2308-2309
ii. Explain the physiological consequences of intermittent positive pressure ventilation, positive end-expiratory pressure (see also F10 i.) and the Valsalva manoeuvre.
2023A 08
Describe the cardiovascular and respiratory effects of positive pressure ventilation.
2019A 20
Describe the cardiovascular effects of positive pressure ventilation on a patient who has received a long acting muscle relaxant.
2016A 02
Describe the respiratory and cardiovascular effects of applying 10 cm of PEEP (positive end-expiratory pressure) to a healthy mechanically ventilated adult.
2014B 03 – 2009A 17
Describe the physiological consequences of Positive End-Expiratory Pressure (PEEP).
CICMWrecks Answer
Positive Pressure Ventilation
- PEEP = Positive End Expiratory Pressure.
- Equivalent to a constant pressure applied throughout the respiratory cycle.
- Intrinsic PEEP = unintentional or un-measured end-expiratory hyperinflation
- Physiological effects of Positive Pressure Ventilation mostly related to increased mean airway pressure
Cardiovascular Effects
- Causes constant ↑ intrathoracic pressure (ITP) throughout respiratory cycle
- Left Heart
- On Preload
- Initially: → ↑ LV PL → ↑ CO
- Secondarily: → ↓RV output → ↓ LV PL → ↓ CO
- On Afterload
- → ↓myocardial transmural pressure → ↓LV AL → ↑CO
- PEEP > Ao diastolic pressure
- → Collapse of intrathoracic aorta → Starling resistor mechanism → ↑LV AL → ↓ CO
- PEEP < Ao diastolic pressure
- ↑ pressure gradient for flow to systemic circulation
- ↓ AL → ↓ myocardial work
- On Compliance
- diastolic buldging of septum → ↓ LV compliance → ↓LV CO
- On Preload
- Right Heart
- → ↑ Pulmonary vascular resistance → ↑ RV AL → ↓ RV output
- → ↓ Venous return → ↓ RV PL → ↓ RV output
- In the failing LV → ↑CO
- ↓ LV afterload
- ↑ pressure gradient thorax to abdomen
- ↓ transmural pressure → ↓ LV wall tension = ↓afterload
- ↓ LV preload (ie more favourable position on compliance curve)
- ↓ LV afterload
Respiratory Effects
Beneficial Effects:
- ↓ atelectasis and gas trapping
- ↑’s FRC > “closing capacity”
- Shifts position on P-V curve right, above the “closing point”
- ↑ lung compliance → shifts back to steep/compliant part of P-V curve
- ↓ intrapulmonary shunt thus improved V/Q match and ↑ PaO2
- ↓ atelectasis and gas trapping
- ↓ extravascular lung water → ↓interstitial/alveolar oedema
- ↓ AWR
- ↑ lung volume → ↑ radial traction by parenchyma → ↑ airway calibre
- ↓ work of breathing
- ↑ lung compliance = ↓ elastic work
- ↓ AWR = ↓resistance work
Negative Effects:
- ↑ V/Q mismatch
- ↑ west zone 1
- ↓ lung compliance
- shift to flat part of P-V curve
- ↑ Work of breathing (due to compliance change)
- ↑ PVR and RV afterload
- extrinsic compression of pulmonary vessels
- Barotrauma
End-Organ Effects
- Renal:
- ↓CO & ↑renal venous pressure
- → Reduced renal blood flow → Reduced GFR → Reduced urine output
- → Reduced atrial stretch and ANP release → Increased ADH → Fluid retention → Oedema
- ↓CO & ↑renal venous pressure
- Hepatic:
- Reduced hepatic blood flow due to:
- Increased CVP and decreased CO lowering the pressure gradient for hepatic flow
- May result in circulation only intermittently throughout the cardiac cycle
- Hepatocyte dysfunction
- Haematological:
- Neutrophil sequestration in the compressed pulmonary vasculature
- CNS:
- ↓VR ⇒ ↑CVP ⇒ ↑ICP
Gladwin / Mooney / JC 2020
Examiner Comments
2019A 20: 33% of candidates passed this question.
Structured answers separating effects of positive pressure on right and left ventricle, on preload and on afterload were expected. Overall there was a lack of depth and many candidates referred to pathological states such as the failing heart. Simply stating that positive pressure ventilation reduced right ventricular venous return and/or left ventricular afterload, without some additional explanation was not sufficient to achieve a pass level.
2016A 02: 29% of candidates passed this question.
This topic has been asked previously. It was expected candidates could detail the impact of PEEP on a variety of respiratory parameters such as lung volume, dead space, arterial pO2 and intrapleural pressure. The cardiovascular consequences are well described including the effect on cardiac output, blood pressure and oxygen delivery. The physiological impact of lower levels PEEP in a young healthy person is different to that often seen in the critically ill and this was not appreciated by most candidates.
2014B 03: 27% of candidates passed this question.
Most answers were quite brief and superficial. They simply did not cover enough of the required knowledge base to gain a pass mark. A definition of PEEP is a useful way to start this answer and this was missing in more than half the answers. Deficiencies in knowledge included even the primary respiratory and cardiovascular effects of PEEP. Many candidates incorrectly concluded that PEEP would increase afterload and decrease pulmonary vascular resistance. Some candidates provided
description of the cardiovascular effects of Valsalva, which was not part of the question. It was expected candidates would also mention physiological effects on other organ systems such as potential cerebral and renal effects.
This topic (Level 1) requires a detailed knowledge and candidates should read widely to gain the depth of understanding required. The core material is covered in texts such as Nunn’s’ Applied Respiratory Physiology and additional applied information can be found in a variety of texts such as Textbook of Critical Care by Fink et al, Irwin and Rippe’s Intensive Care Medicine or Miller’s Anaesthesia.
2009A 17: Pass rate: 40%
Points required included a definition of PEEP, both intrinsic and extrinsic.
The important physiological consequences that need to be discussed are respiratory including
increased FRC, increased compliance and decreased work of breathing.
Cardiovascular consequences include decreased venous return and subsequently decreased
cardiac output and an increased pulmonary vascular resistance.
Renal consequences include decreased renal blood flow and increased ADH
Effects on intra-abdominal pressure, hepatic blood flow and the beneficial effects in cardiac
failure earned marks.
Syllabus B1k.2a
Reference: Nunn 6th edition p. 431.9
2013A 03
What is the Valsalva manoeuvre? Explain the cardiovascular response and include graphs in your answer.
CICMWrecks Answer
Valsalva manoeuvre
- Forced expiration against closed glottis
- Requires airway pressures of 40mmHg for 15 seconds
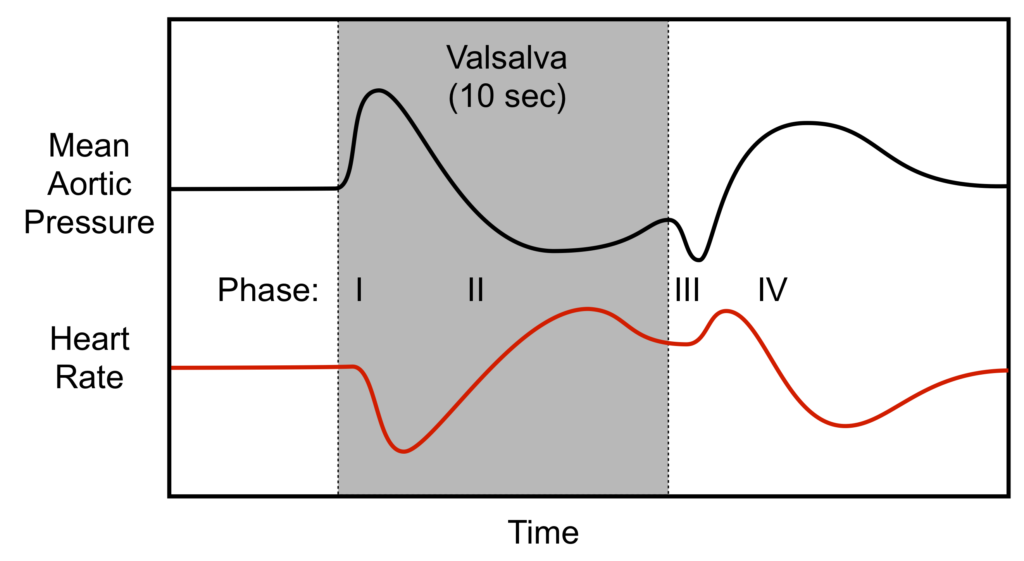
Phase I
- Onset of manoeuvre
- Transient increase in blood pressure with a baroreflex bradycardia
- Intrathoracic pressure transmitted to LV and aorta → Increased ventricular transmural pressure and decreased afterload → Increased cardiac output
- Increased CO causing hypertension and stretch of aortic and carotid baroreceptors → inhibits sympathetic output and promotes vagal output from the medullary vasomotor centre → Bradycardia
- Transient increase in blood pressure with a baroreflex bradycardia
Phase II
- Decreased venous return
- Decreased blood pressure and baroreflex tachycardia
- Continual increase in intrathoracic pressure decreases venous return to RV
- Decreased Venous return → Decreased preload
- Decreased RV CO secondary to Frank-Starling Mechanism
- Decreased RV CO → Decreased LV Preload → Decreased LV Cardiac Output
- Decreased output causes decreased blood pressure → carotid and aortic baroreceptor not stretched → release of sympathetic inhibition and decreased vagal output from medullary vasomotor centre → Tachycardia
- Reflex tachycardia and sympathetic stimulation tends to restore BP
- Decreased blood pressure and baroreflex tachycardia
Phase III
- Release of intrathoracic pressure
- Transient further decrease in blood pressure and further tachycardia
- Decreased transmural pressure on LV and aorta leading to increased afterload → Decreased cardiac output → Decreased stretch of baroreceptors → Tachycardia
- Transient further decrease in blood pressure and further tachycardia
Phase IV
- Normalization of venous return
- Venous return to RV promoted by negative intrathoracic pressure on normal respiration → Increased VR → increased RV CO → increased LV VR → Increased LV Cardiac output
- Increased LV CO → stretch of baroreceptors → Reflex bradycardia
Sakurai 2016
Examiner Comments
2013A 03:
A good answer to this question required attention to detain and an ability to describe changes in many variables at each stage e.g. intrathoracic pressure, blood volumes, baroreceptor firing and the subsequent cardiovascular response (e.g. heart rate and blood pressure). Using graph(s) is a useful way to assist in the explanation and was requires as part of the answer. Dividing the response into four stages makes answering the question much easier. Overall there was a deficiency in a deep understanding of the integrated physiology associated with the Valsalva manouvre. The most common mistakes were describing a change but not saying why it happened, not considering each element at each stage and confusing terms e.g. saying increased cardiac output when the response was increased mean arterial pressure. Very few candidates drew accurate grahs. Graphs required were those of the changes in intrathoracic pressure, the pulse pressure response and the heart rate response


Recent Comments