Syllabus (Fourth Edition, 2023)
Topics
i. Describe the concepts of global and regional ventilation and perfusion and the factors that affect these.
ii. Describe West’s zones of the lung and explain the mechanisms responsible for them.
iii. Explain ventilation-perfusion matching and mismatching. Explain the effect of ventilation-perfusion mismatch on oxygen transfer and carbon dioxide elimination.
iv. Define dead space and its components. Explain how these may be measured and describe the physiological impact of increased dead space.
v. Explain the concept of shunt, its physiological effects, and its measurement.
vi. Explain venous admixture, its relationship to shunt and ventilation-perfusion (V/Q) mismatch.
Topics not covered in previous SAQs
(Overall not covered much in SAQs but very important for VIVAs)
i. Describe the concepts of global and regional ventilation and perfusion and the factors that affect these.
iii. Explain ventilation-perfusion matching and mismatching. Explain the effect of ventilation-perfusion mismatch on oxygen transfer and carbon dioxide elimination.
v. Explain the concept of shunt, its physiological effects, and its measurement.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
i. Describe the concepts of global and regional ventilation and perfusion and the factors that affect these.
ii. Describe West’s zones of the lung and explain the mechanisms responsible for them.
2021B 10
Describe the ventilation / perfusion (V/Q) relationships in the upright lung according to West’s zones (40%). Explain the physiological mechanisms responsible for these relationships (60%).
CICMWrecks Answer
West Zones
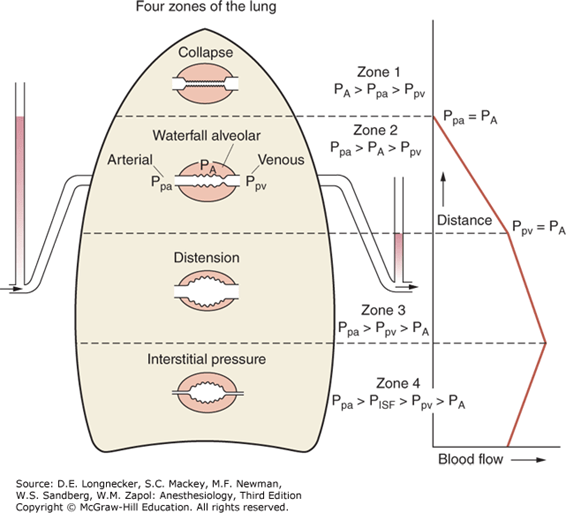
- Divided into zones based on relationship between Alveolar pressure (PA) Arterial pressure (Pa) Venous pressure (Pv) Interstitial pressure (Pi)
- West Zones 1,2,3: Due to changes in hydrostatic pressure when pumping to top of lung vs. bottom of lung
| West Zone | Pressure Relationships | Physiology | Location |
|---|---|---|---|
| Zone 1 | PA > Pa > Pv | No flow of blood, as arterial pressure completely opposed by alveolar pressure | not seen in normal lung |
| Zone 2 | Pa > PA > Pv | Resistance to flow is determined by alveolar pressure (Starling resistor effect) | about 3cm above the heart |
| Zone 3 | Pa > Pv > PA | Resistance to flow is determined by venous pressure Venous pooling causes increased distension of pulmonary capillaries | majority of healthy lung |
| Zone 4 | Pa > Pi > Pv > PA | Low lung volume causes narrowing of extra-alveolar vessels | Lung bases at low lung volume or in pulmonary edema |
Vertical gradient of pleural pressure and alveoli
Inspiration
- Inspiratory muscle contraction → Overcomes resistance to insp flow → negative alveolae pressure -1cm H2O → VT 500ml
- Weight of lung on alveoli and pleura above:
- causes gradient of pleural pressure: Apex (-10cmH2O) Base (-2.5cm H2O)
- Apical alveoli larger
- But compliance lesser (due to baseline distension)
- Lesser ventilation for same pressure
- Apex and base on different part of compliance curve
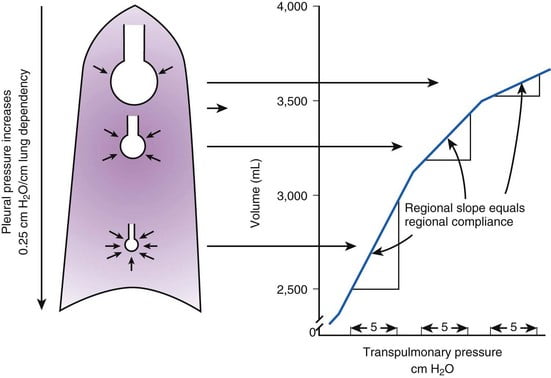
Expiration:
- Gradient of pleural pressure smaller
- Apex: -4cm H2O → on steeper part of compliance curve → ventilation better
- Base +3.5cm H2O →airway closure → gas trapping & shunt
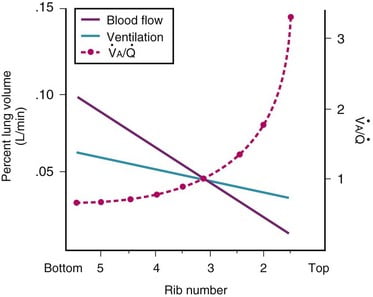
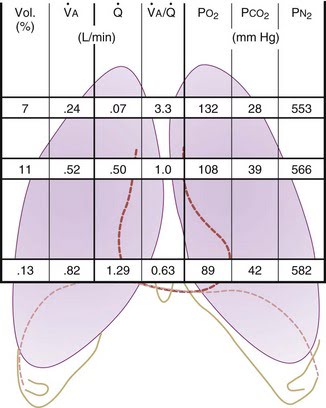
Ventilation, perfusion, V/Q matching
Vertical segments based on V and Q
- Both V and Q ↓ as you move from base to apex.
- ↓ total number of alveoli → ↓ diffusive area → ↓ V
- ↑ compression of intra-alveolar vessels → ↑ west zone 1 → ↓ Q
- V ↓’s slower than Q
| Level | V/Q | Ventilation | Perfusion | PO2 | PCO2 |
|---|---|---|---|---|---|
| Apex | ~3 High | Lower Alveoli largest (no wt of lung) | Very Low Blood hydrostatic pressure low (high gravity) | High 132 | Low 28 |
| At level of heart | ~1 | Slightly more ventilated (Optimized) | Optimized (less effect of gravity) | 108 | 39 |
| Base | ~0.67 towards mixed venous point | Well ventilated (in normal lung): small alveoli, more compliance (effect of wt of lung) | Highly perfused Maximal Hydrostatic pressure | relativly hypoxic 89 | relatively hypercapnoeic 42 |
JC 2019
Examiner Comments
2021B 10: 47% of candidates passed this question.
This is a core aspect of respiratory physiology, and a detailed understanding of this topic is crucial to the daily practise of intensive care. As such the answers were expected to be detailed. Strong answers included precise descriptions of the zones of the lung as described by West and related these to the V/Q relationship in the upright lung. Generally, most candidates scored well in this section. Diagrams were of varying value. However, an impression from the examiners was that candidates spent too much time on this first section and ran out of time for a detailed answer in the second section. The answers to the second section seemed rushed and were often lacking in detail with many incorrect facts. This question highlights the importance of exam technique preparation in the lead up to the written paper.
iii. Explain ventilation-perfusion matching and mismatching. Explain the effect of ventilation-perfusion mismatch on oxygen transfer and carbon dioxide elimination.
2017B 06
Describe the effects of V/Q inequality on the partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2) in arterial blood.
2014B 05 – 2008A 06
Compare the effect on arterial blood carbon dioxide and oxygen levels of ventilation / perfusion inequalities.
CICMWrecks Answer
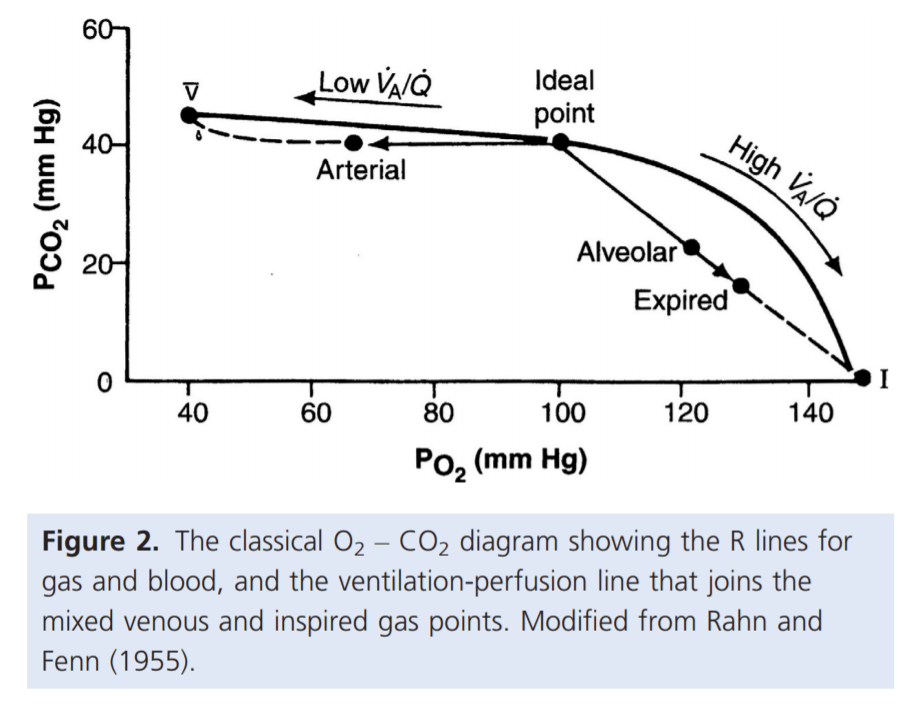
Normal V/Q variation
- Both V and Q ↓ as you move from base to apex.
- ↓ total number of alveoli → ↓ diffusive area → ↓ V
- ↑ compression of intra-alveolar vessels → ↑ west zone 1 → ↓ Q
- V ↓’s slower than Q
- The intersection of V/Q is at rib 3, here V/Q = 1
- V/Q = 3 at the apex
- V/Q = 0.67 at bases

Normal Variation in V/Q causes:
- Shunt
- PACO2 and PAO2 = mixed venous blood
- PACO2 = 46mmHg
- PAO2 = 40mmHg
- Matched ventilation and perfusion
- PACO2 and PAO2 = normal blood gas for healthy patient
- PACO2 = 40mmHg
- PAO2 = 100mmHg
- Dead space
- Alveolar pressures equal that of inspired air
- PACO2 = 0mmHg
- PAO2 = 150mmHg
For PaO₂:
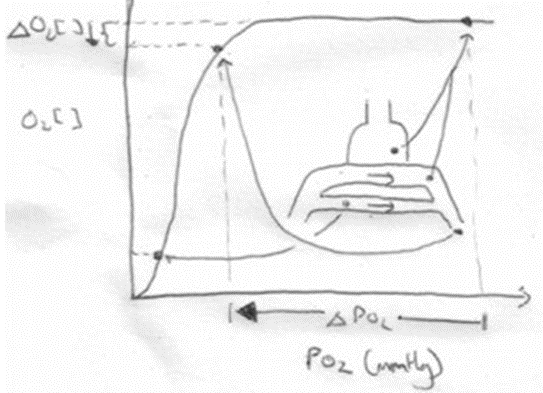
- Increased V/Q inequality causes:
- Mild decrease in PaO2
- At Apex
- decrease in dissolved O2
- Minimal decrease in Hb saturation due to flat part of OHDC
- Thus overall decrease in CaO2
- Base
- PAO2 = PvO2 = 40
- Addition of a small quantity of poorly oxygenated shunted blood flow (PO2 < PcO2) added to a large volume well-oxygenated PECB → small fall in CaO2 BUT a LARGER fall in PaO2
- Because carrying capacity of blood to apex is decreased there is a decrease in PaO2
For PaCO₂:
Remembering that the relationship for the Alveolar gasses:
- Increased V/Q inequality causes:
- Mild increase in PaCO2 which is rapidly compensated for due to Chemoreceptor reflexes
- Apex to base
- ↓PACO2 towards the apices of the lungs
- ↓gas transfer → ↑PaCO2
- Sensed by chemoreceptors (sensitive to ±3mmHg)
- ↑RR and Tv as per the CO2 dissociation curve
- Normal or slightly lower (after compensation) CO2
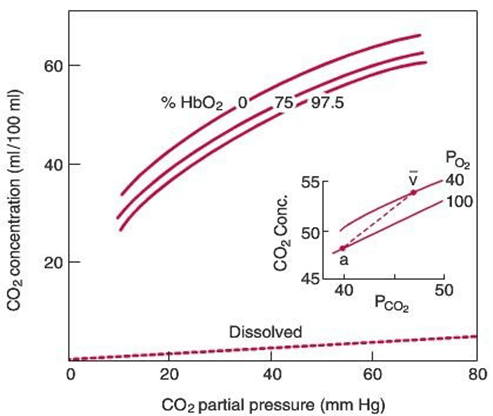
Gladwin 2016
Examiner Comments
2017B 06: 48% of candidates passed this question.
Overall answers lacked sufficient detail on a core area of respiratory physiology. Answers expected included a description of V/Q ratios throughout the lungs and an explanation of how V/Q inequality lowers PaO2
2014B 05: 8% of candidates passed this question.
Very few candidates demonstrated understanding of this core topic. Candidates did not accurately define V/Q inequality and the physiological factors causing this phenomenon. V/Q scatter as well as true shunt (V/Q=0) and dead space (V/Q=∞) needed to be considered. The inability of high V/Q areas to compensate for low V/Q zones owing to the relatively small contribution of blood flow from these high V/Q units was not discussed. The differential effect of FiO2 on true shunt versus V/Q scatter was seldom explained. The shape of the oxy-Hb dissociation curve and CO2-dissociation curve were sometimes mentioned but their effect on arterial gas tensions not well explained.
Often graphs were reproduced inaccurately and contradictory statements made, leaving the impression that candidates did not understand the basic concepts. It is core knowledge for Intensive Care Specialists managing respiratory failure. A sophisticated knowledge based on the chapter in Nunn is a minimum standard expected for this topic.
2008A 06: No candidates (0%) passed this question.
The main points expected for a pass were:
- Range, regional pulmonary differences and gradients of V/Q ratios.
- Definitions of shunt (V/Q = 0) and dead space (V/Q = ∞).
- Explanation of why and how V/Q mismatch lowers arterial PaO2 (majority of pulmonary blood flow being from basal regions, shape of haemoglobin disassociation curve).
- Explanation of why and how V/Q mismatch lowers arterial PaCO2 (majority of pulmonary blood flow being from basal regions, predominately linear shape of CO2 disassociation curve within the physiological range of PaCO2 values).
Again, the use of illustrations would be very useful aids as part of a good answer.
Candidates often failed to frame their answer to the question that was asked and deviated to areas not directly sought after by the question. This resulted in wasted time and opportunities for marks.
Syllabus B1g
Reference Nunn 4th edition page 165-187
iv. Define dead space and its components. Explain how these may be measured and describe the physiological impact of increased dead space.
2018A 03
Define dead space and its components (30% of marks).
Explain how these may be measured (35% of marks)
and describe the physiological impact of increased dead space (35% of marks).
2010A 19
Describe the types of dead space in the respiratory system (50% marks).
Explain the consequences of increased dead space on gas exchange (50% mark)
CICMWrecks Answer
Dead space
- Ventilated lung volume in which no gas exchange occurs
- Anatomical dead space
- Dead space volume of conducting airways
- Oropharynx, nasopharynx and first 16 divisions of the tracheobronchial tree
- Approx 2ml/kg
- Measured by fowlers method (N2 washout)
- Dead space volume of conducting airways
- Alveolar dead space
- Dead space volume of parts of the respiratory airways that are ventilated but not perfused
- West Zone 1 (PA > Pa)
- Low cardiac output
- Positive pressure ventilation
- Posture
- Disease states
- Pulmonary Embolism
- West Zone 1 (PA > Pa)
- Measured by subtracting anatomical deadspace from physiological dead space
- Dead space volume of parts of the respiratory airways that are ventilated but not perfused
- Physiological dead space
- Alveolar + anatomical dead space
- Calculated from Bohr equation
- Normal arterial to end-tidal CO2 gradient is approx 5mmHg
- Increases if increased dead space
- Apparatus dead space
- Dead space contributed by artificial airway devices (endotracheal tubes, LMA, HME filters)
- Usually reduces anatomical deadspace (due to bypass of oropharynx)
Calculation / Measurement of Dead Space
Bohr’s Method
- Bohr’s Equation:
- Based on the principle that all CO2 exhaled must come from ventilated alveoli
- PĒCO2 is mixed-expired CO2 in an expired tidal breath
- Alveolar PCO2 is difficult to measure, so the Enghoff modification is used
- which assumes PACO2 = PaCO2
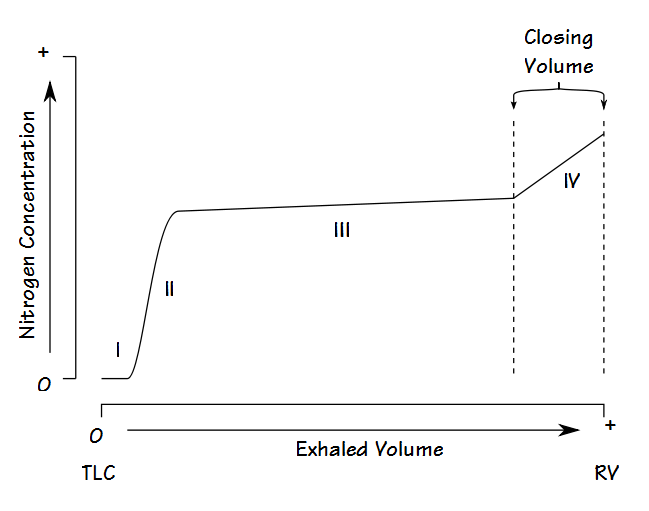
Fowler’s Method
Single-breath nitrogen washout test
- Single Vital Capacity Breath – 100% O2 → Exhales to Residual volume
- Expired Nitrogen concentration and volume is measured
- Plot of concentration by volume generated
- Phase I: Pure dead space. No Nitrogen present.
- Phase II: Midpoint is volume of Anatomical dead space.
- Phase III: Expired N2 plateaus
- Phase IV: Closing capacity. Sudden Increase in Nitrogen.
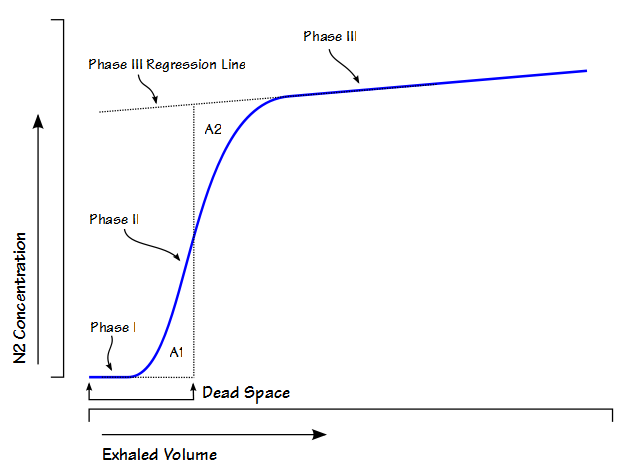
Consequences of increasing dead space
- Alveolar ventilation
- RR x (tidal volume – dead space)
- Therefore, as dead space approaches tidal volume, alveolar ventilation approaches zero, and cannot be compensated by increasing respiratory rate
- PaCO2 is inversely proportional to alveolar ventilation → PaCO2 increases as alveolar ventilation decreases
- Decreased pH
- Increased central and peripheral stimulation of ventilation → Reduction in PaCO2
- Central chemoreceptors activate medullary vasomotor centre → increased sympathetic discharge
- If PaCO2 increases, alveolar CO2 in non-dead space alveoli will increase
- PAO2 will decrease according to alveolar gas equation
Sakurai / JC 2016
Examiner Comments
2018A 03: 59% of candidates passed this question.
Some candidates failed to provide a correct definition of dead space. An outline of anatomical, alveolar and physiological dead space was expected. The Bohr equation was commonly incorrect, and many did not comment on how to measure the components of the Bohr equation. Fowler’s method was generally well described though some plotted the axes incorrectly. The impact of increased dead space was not often well explained. Very few people stated the major impact of increased dead space is reduced minute ventilation and how this would affect CO2.
2010A 19: (50%) of candidates passed this question
A definition of dead space incorporating subtypes (anatomical, apparatus, alveolar, physiological) was expected. Explanation of measurement methods attracted additional marks. Changes to End-tidal CO2 relative to PaCO2 were relevant to the question. The Bohr equation was stated and variables defined in better answers. Causes of an increased dead space should have been described including hypoperfusion and increased alveolar pressure. Increased dead space primarily results in CO2 retention, unless minute ventilation is increased commensurately. The physiological effects of
increased PaCO2, increased respiratory rate and work of breathing are central to the question. Many candidates predicted severe hypoxemia; however the alveolar gas equation was not stated to explain this observation. Hypoxemia is a relatively late effect of significant hypo-ventilation, especially if the patient is breathing supplemental O2.
Syllabus: B1e 2c Reference: Nunn’s Applied Respiratory Physiology p310-311.
Principles of Physiology for the Anaesthetist, Power & Kam p84-87 5
v. Explain the concept of shunt, its physiological effects, and its measurement.
vi. Explain venous admixture, its relationship to shunt and ventilation-perfusion (V/Q) mismatch.
2024A 02
(a) Describe venous admixture and the sources contributing to it in an adult (60% of marks).
(b) Explain the effects of supplemental oxygen on arterial hypoxaemia (40% of marks).
2009B 06
Define venous admixture and list its causes. (50% of marks)
How is it diagnosed and how is it quantified? (50% of marks)
CICMWrecks Answer: Venous Admixture
Venous admixture
“Theoretical volume of mixed venous blood added to pulmonary end-capillary blood to produce the observed CaO2 “
Causes of venous admixture
Shunt
- Physiological
- Thebesian vessels – drain venous blood from coronary circulation directly into cardiac chambers (including L ventricle)
- Bronchial circulation – Fracture of blood from bronchial circulation drains into pulmonary veins
- Pathological
Blood from pulmonary capillaries supplying unventilated alveoli (V/Q = 0)- Obstruction within bronchial tree
- Atelectasis
- Usually mitigated by hypoxic pulmonary vasoconstriction
V/Q mismatch
- Variation in ventilation and perfusion within the lung
- V/Q > 1 – Areas of lung with high relative ventilation to perfusion
- West Zone 1
- Lung apices
- In pathology
- Positive pressure ventilation
- Shock and hypoperfusion
- PE
- V/Q < 1 – Areas of lung with low relative ventilation to perfusion
- Lung bases
- West zone 4
- In pathology: Left heart failure, Pulmonary oedema
- V/Q > 1 – Areas of lung with high relative ventilation to perfusion
- Areas of high V/Q cannot compensate for areas of low V/Q due to the shape of the oxyHb dissociation curve and the sharp desaturation of deoxygenated blood. Also there may be decreased flow through the highly ventilated areas of lung
- V/Q mismatch in pathology
- Obstructive lung diseases: Asthma, COPD
- Restrictive lung diseases: ILD, ARDS
Sakurai 2016
CICMWrecks Answer: Diagnosis and Quantification
Diagnosis of venous admixture
Estimated using A-a gradient using alveolar gas equation
R – Respiratory quotient, usually 0.8
Normal A-a gradient = 2.5 + (0.21 x Age)
Quantification of venous admixture
Shunt equation
Where
- Qt = Total cardiac output
- Qns = Flow through non-shunt areas
- Qs = Flow through shunt
- CaO2 = arterial content of O2
- CcO2 = pulmonary end-capillary O2 (presumed 100% SpO2)
- CmvO2 = mixed venous content of O2
- Blood sampled from R atrium
V/A quantification
- MIGET (Multiple inert gas elimination technique)
- IV injection of a volume of liquid with known quantities of 6 inert gases
- V/Q calculated from the elimination spectra of these gases
- PET
- Xe133 gas and Tc99 labelled RBC
- MRI
Sakurai 2016
CICMWrecks Answer: Effects of supplemental oxygen
Effects of Supplemental Oxygen
| Cause of Hypoxemia | Mechanism | Effect of Supplemental Oxygen | Explanation |
|---|---|---|---|
| Ventilation-Perfusion (V/Q) Mismatch | Uneven distribution of ventilation relative to perfusion, with low V/Q areas contributing to hypoxemia (e.g., COPD, pulmonary embolism). | – Increases PAO₂ in well-ventilated areas, improving overall PaO₂. – Partial compensation for low V/Q areas. | Oxygen improves oxygenation in areas with some ventilation, as increased PAO₂ raises the oxygen gradient in regions with low but functional gas exchange. |
| True Shunt | Blood bypasses ventilated alveoli entirely, leading to no gas exchange in shunted areas (e.g., ARDS, intracardiac right-to-left shunt). | – Minimal improvement in PaO₂, depending on shunt fraction size. – Limited effect in large shunts (>30%). | – Supplemental oxygen cannot oxygenate blood that bypasses ventilated alveoli. – Only oxygenating the non-shunted blood causes a modest rise in PaO₂. |
| Diffusion Abnormalities | Impaired oxygen transfer across thickened alveolar-capillary membranes (e.g., pulmonary fibrosis, interstitial lung disease). | – Increases the oxygen gradient by raising PAO₂, improving oxygen diffusion. – Significant improvement seen unless limitation is extreme. | Higher PAO₂ compensates for the impaired transfer, as oxygen diffusion depends on the alveolar-arterial gradient. |
| Low Inspired Oxygen (FiO₂) | Reduced oxygen concentration in inhaled air (e.g., high altitudes). | Fully corrects hypoxemia by increasing FiO₂ and raising PAO₂. | Supplemental oxygen raises PAO₂ to normal or higher levels, overcoming the reduced oxygen availability in the inspired air. |
| Hypoventilation | Reduced alveolar ventilation decreases PAO₂ and causes a proportional drop in PaO₂ (e.g., opioid overdose, neuromuscular weakness). | – Corrects hypoxemia by increasing PAO₂ and PaO₂. – Does not address underlying hypercapnia or ventilation issues. | – Higher PAO₂ restores oxygenation but does not remove CO₂ buildup. – Treating the underlying cause of hypoventilation is still necessary. |
| V/Q Scatter | Wide distribution of V/Q ratios due to heterogeneity in ventilation and perfusion (e.g., emphysema, interstitial lung disease). | – Improves PaO₂ by raising oxygenation in well-ventilated regions. – Cannot fully normalize due to persistent V/Q mismatch. | Supplemental oxygen increases PAO₂ in high-V/Q units, compensating for low-V/Q areas to some extent, but oxygen delivery remains uneven across the lung. |
Examiner Comments
2024A 02: 43% of candidates passed this question.
(a) This part of the question required candidates to provide a definition of venous admixture and provide detail on the sources. This would include anatomical (including atelectasis/closing capacity) and V/Q Mismatch or Scatter. V/Q Scatter was commonly omitted from answers. The use of the verb “describe” implies that further details are required beyond a simple list of the sources (such as a description of their mechanism and relative importance).
(b) This part required the effect supplemental oxygen on the many causes of arterial hypoxaemia to be explained. This would require the effect that this has on areas of true shunt and different degrees of shunt fraction and also on V/Q scatter. Information for this can be found on Nunn’s Respiratory Physiology.
2009B 06: 5 (56%) of candidates passed this question
This question related to an area of basic respiratory physiology. A good answer necessitated the precise meaning of venous admixture, being that amount of mixed venous blood which would have to be added to ideal pulmonary end-capillary blood to explain the observed pulmonary end-capillary to arterial PO2 difference. Diagnosis required the candidate to mention, a demonstrated increased in the Aa-DO2, what are normal values. For quantification mention and description of the shunt equation was required.
Candidates lacked a definition for venous admixture, were inaccurate with their description of the shunt equation and often overlooked mentioning calculation of arterial and mixed venous blood oxygen content.

Recent Comments