Syllabus (Fourth Edition, 2023)
Topics
i. Describe the control of breathing.
Topics not covered in previous SAQs
.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
i. Describe the control of breathing.
2020A 13
Explain the control of breathing.
2015B 13
Describe the control of alveolar ventilation.
2015A 01
Explain the control of breathing.
2013A 21
How is alveolar ventilation regulated?
CICMWrecks Answer
Respiration
- Normal RR ~ 10 breaths per minute
- Normal tidal volume ~ 500ml/kg (in 70kg male)
- Therefore normal minute ventilation approx 5l/min
Afferents / Sensors
Chemoreceptors
- Central
Located in retrotrapezoidal nucleus- Sensitive to changes in CSF [H+]
- CO2 readily diffuses across BBB and converted to H+ and HCO3– via carbonic anhydrase
- Increased [H+] (and therefore CO2) stimulated respiration
- Sensitive to changes in CSF [H+]
- Peripheral
Located in aortic bodies (innervated by vagus nerve) and carotid bodies (innervated by glossopharyngeal nerve)- Sensitive to PO2, [H+], PCO2 and blood flow
- O2 dependent K+ channels
- Respiration increases as O2 drops below 50mmHg
- O2 dependent K+ channels
- CO2
- Linear increase in respiration as CO2 increases
- Sensitive to PO2, [H+], PCO2 and blood flow
Baroreceptors:
- Located in aortic arch and carotid sinuses
- Responds to stretch
- As MAP drops → less stretch on vessel walls → increased sympathetic outflow from medullary vasomotor centre → triggers increase in respiration
- Responds to stretch
Pulmonary receptors
- Stretch receptors
- Increased stretch of pulmonary parenchyma triggers inflation reflex → inhibits inspiration to prevent overdistention
- Collapse of pulmonary parenchyma triggers deflation reflex → inhibits expiration to prevent atelectasis and loss of FRC
- J fibres
- Nociceptive mechano-chemoreceptors
- On stimulation → Bronchospasm, apnoea, bradycardia and hypotension
- Nociceptive mechano-chemoreceptors
Others
- Joint and muscle receptors stimulate ventilation
- Pain and temperature sensation can alter ventilation via the cortex and limbic system
Central control of breathing
Central input
- There is input from the hypothalamus and cortex, with the ability of the cortex to override the medulla and bring ventilation under voluntary control
Controller
- Medullary Respiratory Centre
- Dorsal respiratory group (DRG)
- Located in and adjacent to Nucleus Tractus Solitarus
- Associated with inspiration and timing
- Works as an ‘integrating centre’ with VRG
- Ventral respiratory group (VRG)
- Including Pre-Botzinger and Botzinger complex → Central Pattern Generator
- Associated with control of expiration, airway dilation and central pattern generation
- sends inhibitory impulses to Apneustic centre
- Dorsal respiratory group (DRG)
- Pontine respiratory group (PRG)
- Pneumotaxic centre
- controls both the rate and the pattern of breathing
- Sends inhibitory impulses to the inspiratory area
- Antagonist to apneustic center
- decreases tidal volume
- Apneustic centre:
- sends signals for inspiration for long and deep breaths
- controls the intensity of breathing and is inhibited by the stretch receptors of the pulmonary muscles at maximum depth of inspiration, or by signals from the pnuemotaxic center
- increases tidal volume.
- Pneumotaxic centre
- Inspiratory phase:
- Gradual ramping up of nerve activity – ↑muscle contraction
- Expiratory phase I:
- Gradual reduction of nerve activity – ↓muscle contraction
- Expiratory phase II:
- Inspiratory muscles inactive
- If increased respiratory drive, expiratory muscles are activated
Efferents
- Phrenic nerve (C3, 4, 5) → Innervates diaphragm – Main inspiratory muscle
- Spinal nerves to intercostal muscles (external intercostal → inspiration, internal intercostal → expiration)
- Brachial plexus → Pectoralis major (forced breathing inspiration)
- Accessory muscle → Sternocleidomastoid (forced breathing inspiration)
- Spinal nerves to abdominal muscles (forced expiration)
Mooney / Sakurai / JC 2020
Examiner Comments
2020A 13: 53% of candidates passed this question.
Most candidates provided a structured answer based around a sensor / central integration / effector model with appropriate weighting towards the sensor / integration component. Better answers provided an understanding of details of receptor function, roles of the medullary and pontine nuclei and how these are thought to integrate input from sensors. Marks were awarded to PaCO2 ventilation and PaO2 ventilation response when accurate, correctly labelled diagrams or descriptions were provided.
2015B 13: 42% of candidates passed this question.
The most comprehensive answers were those structured as sensor-controller-effector with an explanation of each part and how homeostasis was maintained. Insufficient detail was generally provided as to how central and peripheral chemoreceptors were stimulated. A description of central control was required, rather than listing nuclei or areas. Many failed to address all three components of a control question and focused primarily on the sensors. Many answers were just too brief and did not present enough information to demonstrate understanding.
2015A 01: 71 % of candidates passed this question.
This question was generally well done. It was expected answers would include discussion of the three core elements of sensors, a central controller and effectors. Central control involves three main groups of neurones in the brainstem with some cortical voluntary control also possible. More in depth answers included graphs of the ventilatory response to oxygen and carbon dioxide tensions.
2013A 21:
This is a core topic (syllabus Level 1) and a high level of understanding was expected. Overall candidates failed to demonstrate sufficient depth and breadth in their knowledge. A structured response considering the three basic elements underpinning the control of alveolar ventilation (the Sensors, Central integration and control and the Effectors) was core material. A detailed description of each was expected.
2019A 16
Describe the role of carbon dioxide in the control of alveolar ventilation.
CICMWrecks Answer
Role of CO2 in control of alveolar ventilation
Sensors
Central chemoreceptors
Located in the medulla, just below the central surface
Stimulated by increased pH of CSF. Not stimulated by low PO2
CO2 crosses the blood brain barrier easily
-> combines with water to make carbonic acid
-> dissociates to produce hydrogen ions lowering pH
Peripheral chemoreceptors
Carotid bodies at bifurcation of common carotid artery (supplied by glossopharyngeal nerve)
Aortic bodies (supplied by the vagus nerve)
Sense changes in PO2, PCO2 and pH (not in aortic bodies)
Globus cells release neurotransmitters (dopamine, noradrenaline, acetylcholine)
-> stimulates afferent nerves -> medulla -> increased ventilation
Lung receptors, Baroreceptors
No role of CO2
Central input
There is input from the hypothalamus and cortex, with the ability of the cortex to override the medulla and bring ventilation under voluntary control
Controller
- Medullary respiratory centre
- Dorsal group
- Part of nucleus tractus solitarius
- Timing of ventilation
- Ventral group
- In nucleus ambiguous and nucleus retroambigualis
- Controls inspiration
- Botzinger complex
- Rostral to nucleus ambuguus
- Controls expiration (inactive at quiet breathing)
- Dorsal group
- Inspiratory phase:
- Gradual ramping up of nerve activity – ↑muscle contraction
- Expiratory phase I:
- Gradual reduction of nerve activity – ↓muscle contraction
- Expiratory phase II:
- Inspiratory muscles inactive
- If increased respiratory drive, expiratory muscles are activated
Effectors
Mainly diaphragm (via phrenic nerves)
Also intercostal muscles and abdominal wall (forced expiration)
Effect of CO2 on Alveolar Ventilation
- ↑ CO2
- ↑ RR + ↑ Depth of breathing → Steady state hyperventilation in few mins
- Linear response in usual range ( MV ↑ 2L/min for 1mmHg rise on PaCO2)
- Max Ventilatory stimulation at ~100mmHg → Respiratory fatigue, CO2 narcosis
- ↓ CO2
- ↓ Alveolar ventilation
- Once PaCO2 <30mmHg
- Some reduce ventilation to point of apnoea
- Some continue to breath (Due to cortical control of respiration especially when awake)
- Responses may be blunted by partial neuromuscular blockade, Restrictive or obstructive lung disease, airway obstruction
- Prolonged periods of CO2 retention:
- → Active secretion of bicarb into CSF → CSF pH normalized → central chemoreceptor stimulation ceases
- → renal reabsorption of bicarb → normalizes arterial pH normalized → ↓ peripheral chemoreceptor stimulation
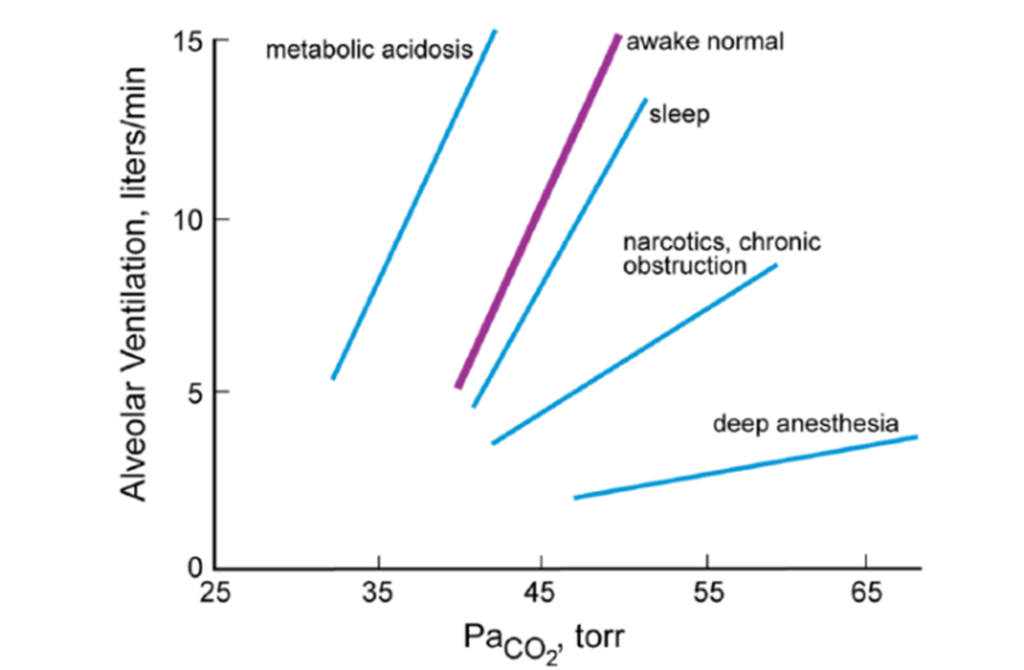
Source: Pulmonary Physiology, 9e. Michael G. Levitzky
References: Nunn’s Applied Respiratory Physiology, Pulmonary Physiology.
Mooney / JC 2019
Examiner Comments
2019A 16: 57% of candidates passed this question.
Better answers considered the role of CO2 in the control of alveolar ventilation in terms of sensors, central processing and effectors – with an emphasis on sensors. Features of central and peripheral chemoreceptors should have been described in detail. The PCO2/ventilation response curve is best described using a graph, with key features of the curve identified (including gradient and axes). Various factors affecting the gradient of this curve and how CO2 affects the response to hypoxic drive should be described.
2022B 04
List the physiological factors which increase respiratory rate and explain their mechanism
2010A 06 – 2008A 11
List the physiological factors that increase respiratory rate.
Include an explanation of the mechanism by which each achieves this increase.
CICMWrecks Answer
Central control of ventilation
- Brainstem
- Medulla (DRG, VRG)
- Pons (Pneumotaxic, Apneustic)
- External inputs
- Sensors from lungs/airways/chemoreceptors
- Cortex
- Can override brainstem function and alter breathing patterns
- Limbic/Hypothalamic Systems
- Alters breathing patters depending on affective states (fear/anxiety/pain)
Peripheral Receptor Inputs alter respiratory rate/depth (ventilation)
- URT receptors (in nose, NP, larynx, trachea)
- mechanical/chemical stimuli
- bronchoconstriction, sneezing/coughing reflexes, and laryngeal spasms
- Joint/Muscle receptors
- limb movement (Eg. exercise) can stimulate ventilation
- Gamma-system (in muscle spindles)
- muscle spindles sense muscle elongation,
- can cause sensation of dyspnoea
- Arterial baroreceptors (in AB and CB)
- ↓ BP causes ↑ ventilation
- ↑ BP can cause ↓ ventilation (and even apnoea)
- Nociceptors
- pain causes apnoea initially, then stimulates ventilation
- Thermoreceptors
- ↑ temperature stimulates ventilation
- ↓ short deep inspiration
Hypocaponea/Hypoxia sensed by Chemoreceptors
- Central CR response
- ↓’d perfusion → ↑[H]/↓PaO2 → CR stimulation
- Afferent signal → Sinus nerve (of herring) / Vagus Nerve → Chemosensitive area of medulla
- Vasomotor area → ↑ peripheral vasoconstriction
- Respiratroy center → ↑rate and depth of respiration → ↑ venous return
- ↓’d perfusion → ↑[H]/↓PaO2 → CR stimulation
- Peripheral CR response
- PCRs found in “Glomus cells”
- ↓ PaO2 – via inhibition of O2 sensitive K-channels
- ↑ PaCO2 and/or ↓pH – via effect on pH sensitive K-channels
- Type I cells (rich in NAd, DA, ACh)
- Hypoxia causes release of NTs
- NAd/ACh – ↑ AP firing rate of AB or CB afferent fibres
- DA – Damping of type 2 cell responses
- Type II cells (rich in capillary supply)
- ↓ PaO2, ↑ PaCO2 and/or ↓pH
- ↓ IC [ATP] which leads to ↑ NT production and release
- ↑ AP firing rate of AB or CB afferent fibres
- PCRs found in “Glomus cells”
Response to:
- ↓ PaO2
- Gradual ↑ MV PaO2 < 500mmHg,
- Rapin ↑MV PaO2 < 50 mmHg
- ↑ PaCO2
- Central CRs (CCRs)
- Most (80%) of response to ↑ PaCO2 by CCRs
- Peripheral CRs
- Response to ↑ PaCO2 is < 20% of total response
- Much FASTER cf. CCR response
- Role is to match ventilation to sudden ∆ in PaCO2
- ↓ blood pH
- Sensed by PCRs in CB only
- Central CRs (CCRs)
CO₂ most important factor

- Minute ventilation is directly proportional to PaCO2 MV ↑ by 2-3 L/min per mmHg PaCO2
- Hypoxaemia has a synergistic effect on hypercapnoeic-ventilatory drive↓ PaO2 causes↑ MV for a given PaCO2,
- ↑ ∆ MV per ∆ PaCO2
O₂ Less important in normal ranges
- PaO2 plays a SMALL role in ventilatory control
- Effect of PaO2 on ventilation:At normal PaCO2PaO2 < 500 mmHg MV ↑ slowly as ↓ PaO2 decrease
- PaO2 < 50 mmHg MV ↑ drastically
- There is synergistic ↑ in ventilatory response in the presence of hypercapnoea and/or acidosis
- MV ↑ drastically when PaO2 is < 100 mmHg

Gladwin 2016
Examiner Comments
2010A 06: 6 (60%) of candidates passed this question
Good candidates had a structured approach to this questions. Submitted question structures took the form of key headings (eg, PaCO2, PaO2, pH, etc) with an accompanying explanation, which included diagrams, which were often underutilised. Candidate answers that lacked any structure were more likely to have omissions and lacked sufficient depth and as a result scored fewer marks. For a good answers candidates where expected to list and explain (preferably by including diagrams) physiological factors such as PaCO2, PaO2, pH, Exercise, temperature, pregnancy and the associated receptors for each mechanism.
Syllabus: B1c 1
Reference: Nunn’s Applied Respiratory Physiology, Lumb, 6th edition 60-68
Principles of Physiology for the Anaesthetist, Power & Kam, 1st edition 92-98
2008A 11: No candidates (0%) passed this question
The main points candidates were expected to cover included:
• A description of the central and peripheral chemoreceptors, their predominant stimuli and effect on ventilation.
• PaCO2 as the main influence on normal ventilation, the near-linear relationship to minute ventilation around the normal range, and how CO2 produces this effect.
• PaO2 and pH and their sites of action.
• Other stimuli to ventilation – exercise, pregnancy, temperature, baroreceptors.
Candidates frequently confused central and peripheral receptor activities and failed to provide any relative significance to the major factor(s). The use of a graph relating the main factors to minute ventilation would have been helpful.
Syllabus B1c 1
Reference: Nunn 6th edition 60-68, Kam 1st edition 92-98

Recent Comments