Syllabus (Fourth Edition, 2023)
Topics
i. Describe the cell membrane, nucleus and cellular organelles and their properties.
ii. Explain mechanisms of transport of substances across cell membranes, including an understanding of the Gibbs-Donnan effect.
iii. Outline the role of cellular receptors and the function of secondary messengers.
iv. Describe the composition and control of intracellular fluid and the mechanisms by which cells maintain homeostasis and integrity.
Topics not covered in previous SAQs
iv. Describe the composition and control of intracellular fluid and the mechanisms by which cells maintain homeostasis and integrity.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
i. Describe the cell membrane, nucleus and cellular organelles and their properties.
2014A 16
Describe, with the aid of a diagram, the structure of the cell membrane, (40% marks) and transmembrane transport processes. (60% marks)
CICMWrecks Answer
Structure of Cell Membrane
Cell Membrane
- Made of
- Phospholipids
- Proteins
- Cholesterol – Found in eukarocytes ie cells with nuclei
- Cell membrane = 7.5nm thick semi permeable structure
Lipid Bilayer
- Fluid rather than solid
- Phospholipids have:
- eg phosphatidyl-choline & phosphatidyl-ethanol-amine
- Hydrophilic head
- Water soluble
- Exposed to aqueous exterior & interior
- Glycerol backbone
- Fatty acid tails –
- Hydrophobic
- Meet in middle of cell membrane
- Proteins can be either:
- Integral – ie pass through bilayer eg ion channels
- Peripheral = straddling
- make up 50% of cell membranes mass
Function of CM Proteins
- Structural
- Carriers for facilitated diffusion (i.e. down electrochemical gradient)
- Pumps for ion active transport
- Ion channels (diffusion down electro- or chemical gradient or both; e.g. K-“leak” channels)
- Receptors for chemical messengers (i.e. hormones , neurotransmitters , autacoids…)
- Enzymes
- Glycoproteins involved in AB processing or anticoagulation (e.g. the mucopolysaccharide glycocalyx of the endothelium which repels clotting factors + PLT’s → helps prevent blood from clotting in intact blood vessels)
Transmembrane Transport Processes
| Mechanism | Energy Expenditure | Electrochemical gradient | Example | ||||
|---|---|---|---|---|---|---|---|
| Diffusion | Passive diffusion | Molecule crosses a membrane to which it is permeable by diffusion | No | With | Carbon dioxide across vascular endothelium | ||
| Facilitated diffusion | Molecule crosses a membrane via a channel, without energy expenditure | No | With | Potassium across excitable cell membranes, via rectifier channels | |||
| Active transport | Primary | Primary active transport | Molecule crosses a membrane via a channel, with energy expenditure (ATP) | Yes | Against | 3Na+ /2K+ ATPase | |
| Secondary | Symport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Co-transported) | Not directly | May be Passive or Active based on gradient of 2nd | sodium and an amino acid | ||
| Antiport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Anti-transported) | Not directly | May be Passive or Active based on gradient of 2nd | Na+ / H+ antiporter in proximal convoluted tubule | |||
| Ligand-gated ion channel | Binding of a ligand causes conformational change in membrane channel, allowing movement of ion across membrane | No | With | Nicotinic acetylcholine receptor. ACh as ligand, Na+ and K + as ions | |||
| Exocytosis | Substance packed in vesicle, moves to cell membrane, two membranes merge -> substance exits cell | Yes | Usually against | Exocytosis of ACh by presynaptic neuron | |||
| Endocytosis | Cell membrane extends to engulf a substance or object, which is then contained in the cell within a vesicle | Yes | Can be with, against or refer to a macroscopic object | Phagocytosis of bacteria by macrophage | |||
Mooney / JC 2019
Examiner Comments
2014A 16: 60% of candidates passed this question.
The structure of the cell membrane was generally well covered by most candidates. Many had difficulties structuring an answer for the transmembrane transport processes. Dividing this section into proteins (some receptors, channels etc.) and carbohydrates (some receptors, immune reactions etc) followed by a very brief discussion of each type of process would have aided candidates towards providing a good answer.
2016A 18
Describe the structure and function of the mitochondrion.
CICMWrecks Answer
Structure of the Mitochondrion
Mitochondria have their own genome & ability to manufacture own RNA and proteins
Their ribosomes = 70S type (30S & 50S) i.e same as bacteria (rest of cell has 80S ribosomes)
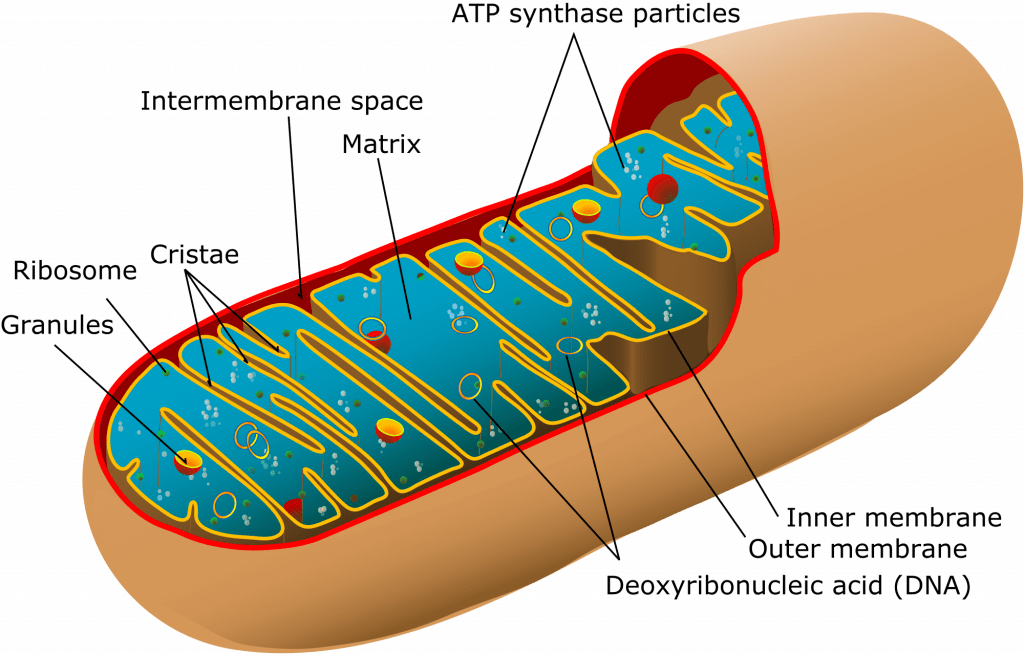
- 1-10µ
- Consist of two membranes (outer and inner)
- Outer membrane
- encloses whole organelle
- contains several integral proteins = porins
- porins form large aqueous channels which allow passage of movement of molecules up to 5000D (pyruvate, amino acids, fatty acids)
- Intermembrane space
- between outer & inner membrane
- chemically equivalent to cells cytosol
- contains cytochrome-c
- Inner membrane:
- No porins
- controlled permeability via transporter proteins
- Different functions
- proteins carrying out oxidative reactions of resp chain
- ATP synthase – makes ATP in matrix
- Transport proteins
- Protein import machinery
- Cristae:
- Formed by folded inner membrane
- Vastly ↑s surface area for ATP production
- Cells which more active e.g myocardium have more cristae
- Inner mitochondrial matrix
- Space enclosed by inner membrane
- Impt in ATP production
- Contains highly conc mixture of
- hundreds of enzymes
- mitochon ribosomes (70S)
- tRNA
- several copies of DNA genome
- Contents important in many metabolic processes:
- Citric acid cycle
- Pyruvate metabolsim
- Fatty acid metabolism
- Urea cycle
- Haeme synthesis
- Outer membrane
- There are several mitochondria are found within a cell – They replicate independently of the cell’s state of division (as they possess their own DNA), and they replicate in response to the metabolic demands of the cell (Ie. number of mitochondria reflects metabolic activity of the cell)
- Mitochondria DNA is unique from nuclear DNA in that it is:
- Contains both double-stranded circular DNA and plasmid DNA, which are both maternally-inherited
- only 1% of mitochondrial proteins (esp enzymes for oxidative phosphorylation) – Remaining 99% of proteins are encoded by nuclear DNA
Function of the Mitochondrion
- Form ATP via oxidative phosphorylation (Major), via Kreb’s cycle and Electron Transport Chain
- Regulation of Cellular proliferation regulation including cell division and differentiation (contributes ATP)
- Regulation of cellular metabolism
- Regulate apoptosis
- Xenobiotic metabolism (esp role of MAO)
- Heat production (esp in brown fat)
- By proton leak or mitochondrial uncoupling
- proton re-enters mitochondrial matrix without contributing to ATP synthesis → heat released
- mediated by therminogenin (proton channel)
- By proton leak or mitochondrial uncoupling
- Sequestration of Ca2+ ions (with swelling/damage post-ischaemia)
- acts as cytosolic buffers for calcium
- Significant interplay with Endoplasmic reticulum
- primary driven by mitochondrial membrane potential
- released back into cell’s interior via Na+-Ca2+ exchange protein or Calcium-induced-Calcium-release pathways
- Calcium also necessary to activate isocitrate dehydrogenace (Kreb’s cycle)
- Signaling through mitochondrial reactive oxygen species
- Cholesterol and steroid synthesis
- Certain haeme synthesis reactions
- Organ specific functions:
- Neuronal: contribute to cellular quality control by reporting neuronal status towards microglia through specialised somatic-junction
- Liver: Detoxify ammonia
Oxidative Phosphorylation – Mitcochondria Energy Production
- mitochon found in high conc in cells with high metabolic demands eg myocardium (23% of cell), brown fat (neonate)
- exercise ↑s numbers
- OP = production of ATP associated with oxidation by the flavoprotein cytochrome system in mitochondria
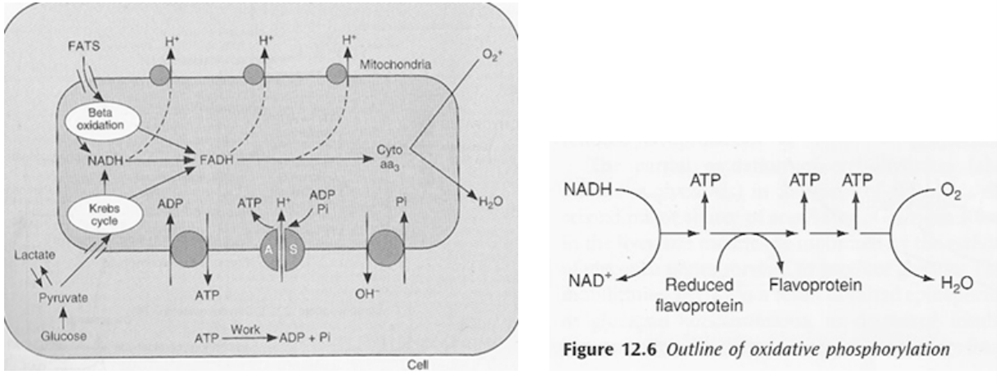
- ATP formed in electron transfer chain:
- Substrate diffuses into mitochon cytoplasm
- Hydrogen removed by a dehydrogenase
- NAD carries hydrogen to respiratory chain
- Hydrogen ionises and protons pass along series of carrier molecules across insulating membrane (inner membrane of mitochondria – forms cristae)
- Movement of protons creates an electrochemical gradient for transport of protons from intermediate space back into matrix ⇒ this drives a reversible ATPase in inner membrane (ATP synthase)
- ATP synthase: ADP + Pi ⇒ ATP
- @end:
- ATP produced
- Reduction of O2 to water – catalysed by cytochrome oxidase
- cyanide inhibits this oxidase ∴ inhibits OP in mitochon
- O2 required to oxidise NADH
- Eg’s of carrier molecules in electron transfer chain
- Flavoprotein
- Cytochromes A, A3, B, C, C1
- Ubiquinone
- Several iron sulphide proteins
- OP depends on:
- Adequate supply of ADP: +ve feedback loop e.g. ↑ATP utilisation ⇒ ↑ADP ⇒ ↑OP
- Rate of delivery of fats, lactate, glucose to interior of mitochon
- Availability of O2: Pasteur point = 1-2mmHg i.e. point below which OP cannot occur
- ∴ cardioresp works in harmony to ensure o2 reaches cells
- defined by oxygen flux equation:
- lack of oxygen causes:
- nothing to scavenge H+ at end of transfer chain
- transfer chain ceases
- build up of reduced compounds ⇒ inhibits TCA cycle ⇒ inhibition of glycolysis
- but glycolysis continues as lactate dehydrogenase removes reduced compounds
Side note: breakdown of 1 glucose molecule:
| Stage | Direct products (net) | Ultimate ATP yield (net) |
| Glycolysis | 2 ATP | 2 ATP |
| 2 NADH | 4 ATP | |
| Pyruvate oxidation | 2 NADH | 6 ATP |
| Citric acid cycle | 2 ATP/GTP | 2 ATP |
| 6 NADH | 18 ATP | |
| 2 FADH2 | 4 ATP | |
| Total | 36 ATP |
JC 2019
Examiner Comments
2016A 18: 19% of candidates passed this question.
Most candidates had at least a basic understanding of mitochondral function although some detail was required for a pass and many did not provide this. A well labelled diagram was used by many candidates and scored marks. Repetition of the same information illustrated on a labelled diagram in subsequent text was not required and did not score additional marks.
It was expected answers would cover basic structure (double membrane structure with cristae and enzymes lining the membrane and within the matrix), details of the electron transport chain, the citric acid cycle and beta-oxidation of long chain fatty acids and mention the maternal origin of DNA. Better Answers provided some information on other functions such as production of reactive oxygen species, role in calcium homeostasis and apoptosis, urea cycle, haem synthesis and heat production
2022B 18
Describe the generation of ATP by mitochondria (50% marks) and outline the processes by which ATP is generated in red blood cells (50% marks).
CICMWrecks Answer
ATP Generation by mitochondia
- Mitochondria form ATP via oxidative phosphorylation (Major), via Kreb’s cycle and Electron transport chain (ETC)
- mitochon found in high conc in cells with high metabolic demands eg myocardium (23% of cell), brown fat (neonate)
- exercise ↑s numbers
- OP = production of ATP associated with oxidation by the flavoprotein cytochrome system in mitochondria
- Structure
- The inner and outer membranes of mitochondria define three compartments within the organelle, each with its distinct role and corresponding protein components.
- Outer membrane separates mitochondria from cytoplasm
- The innermost compartment, surrounded by the inner membrane, is the mitochondrial matrix
- The inner membrane of the mitochondrion contains the components of the electron transport chain.
- The high pH of the mitochondrial matrix creates the trans-membrane electrochemical gradient that drives ATP synthesis
- Oxidation/reduction reactions along the components of the electron transport chain generate a proton gradient that is used by ATP synthase to phosphorylate ADP, thereby producing ATP.
- To increase the capacity of the mitochondrion to synthesize ATP, the inner membrane is folded to form cristae. These folds allow a much greater amount of electron transport chain enzymes and ATP synthase to be packed into the mitochondrion.

- Kreb’s cycle (TCA or citric acid cycle)
- Carbohydrate / Protein / Lipid ⇒ Acetyl coenzyme A or other intermediates
- Acetyl CoA ⇒ Kreb’s cycle
- Electron Transport Chain (ETC)
- The metabolic pathway through which the electron passes, starting with one transporter and then onto the next, is known as the electron transport framework (ETS).
- The electron transport framework happens in the inward mitochondrial layer.
- The electron transport chain contains the accompanying:
- Complex I: NADH dehydrogenase
- Complex II: succinate dehydrogenase
- Complex III: cytochromes bc 1
- Complex IV: cytochromes a-a3
- Complex V: ATP synthase
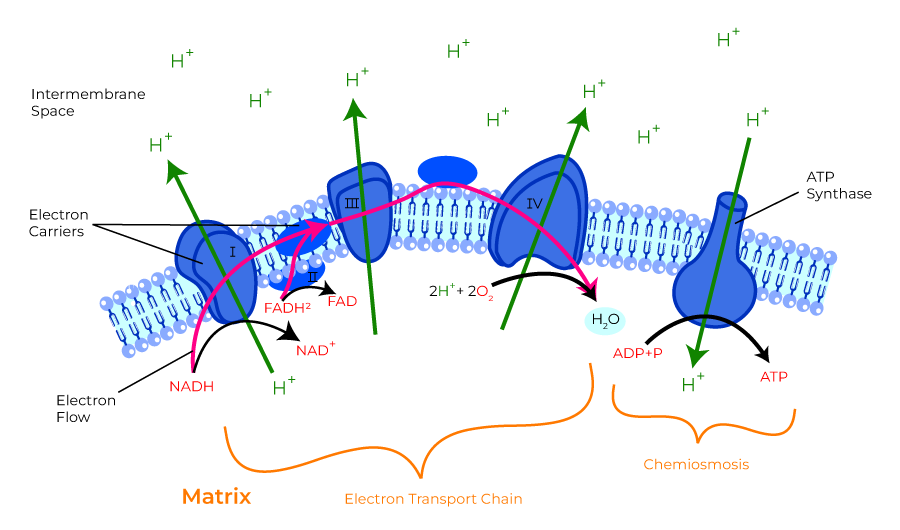
- ATP formed in electron transfer chain:
- Substrate diffuses into mitochon cytoplasm
- Hydrogen removed by a dehydrogenase
- NAD carries hydrogen to respiratory chain
- Hydrogen ionises and protons pass along series of carrier molecules across insulating membrane (inner membrane of mitochondria – forms cristae)
- Movement of protons creates an electrochemical gradient for transport of protons from intermediate space back into matrix ⇒ this drives a reversible ATPase in inner membrane (ATP synthase)
- ATP synthase: ADP + Pi ⇒ ATP
- @end:
- ATP produced
- Reduction of O2 to water – catalysed by cytochrome oxidase
- cyanide inhibits this oxidase ∴ inhibits OP in mitochon
- O2 required to oxidise NADH
- Eg’s of carrier molecules in electron transfer chain
- Flavoprotein
- Cytochromes A, A3, B, C, C1
- Ubiquinone
- Several iron sulphide proteins
- OP depends on:
- Adequate supply of ADP: +ve feedback loop e.g. ↑ATP utilisation ⇒ ↑ADP ⇒ ↑OP
- Rate of delivery of fats, lactate, glucose to interior of mitochon
- Availability of O2: Pasteur point = 1-2mmHg i.e. point below which OP cannot occur
- ∴ cardioresp works in harmony to ensure o2 reaches cells
- defined by oxygen flux equation:
ATP Generation by RBCs
- RBC have no Mitochondia – Cannot perform aerobic metabolism
- All ATP is generated via anaerobic glycolysis (Embden-Meyerhof pathway / EMP) (90% glycolysis)
- 10-step Catabolic pathway in cytosol
- by degradation of glucose molecule
- produces 2x ATP + pyruvate/lactate per glucose → ATP is used by Na+/K+ATPase, which is implicated in maintaining RBC shape, volume and flexibility
- Steps
| Step | Substrate | Enzyme | Product | ATP |
|---|---|---|---|---|
| 1 | Glucose | hexokinase | Glucose-6-phosphate | ATP consumed |
| 2 | Glucose-6-phosphate | glucose phosphate isomerase | Fructose-6-phosphate | |
| 3 | Fructose-6-phosphate | phosphofructo kinase | fructose-1,6-diphosphate | ATP consumed |
| 4 | fructose-1,6-diphosphate | aldolase | 2x glyceraldehyde-3-phosphate | |
| 5 | 2x glyceraldehyde-3-phosphate | glyceraldehyde-3-phosphate dehydrogenase | 2x 1,3-bisphosphoglycerate | |
| 6 | 2x 1,3-bisphosphoglycerate | phosphoglycerate kinase | 2x 3- phosphoglycerate | 2x ATP produced |
| 7 | 2x 3- phosphoglycerate | 3-phosphoglycerate mutase | 2x 2-phosphogylcerate | |
| 8 | 2x 2-phosphogylcerate | enolase | 2x phosphoenolpyruvate | |
| 9 | 2x phosphoenolpyruvate | pyruvate kinase | 2x pyruvate | 2x ATP produced |
| 10 | pyruvate | lactate dehydrogenase | lactate |
- 3 shunts that come off the anaerobic glycolytic pathway (produce no ATP)
- the Rapoport-Luebering shunt (BPG Shunt): 2,3-DPG is interconverted from 1,3-DPG (glycolytic intermediate)
- Hexose monophosphate shunt (HMP shunt or pentose phosphate pathway) – generates NADPH – protects RBC from oxidative damage (10% glycosis)
- NADH generated – used by MetHb reductase to reduce oxidised Hb (MetHb) to Hb
Examiner Comments
2022B 18: 15% of candidates passed this question.
Excellent answers focused on oxidative phosphorylation and the chemiosmotic mechanism in their description of ATP production by mitochondria. This required a description of the structure of the mitochondrial components involved in ATP production, the establishment of an electrochemical gradient of protons across the inner membrane, how the electron transport chain works and its components, and the roles of cytochrome oxidase and ATP synthase. Excellent answers describing ATP generation in red blood cells focused on anaerobic glycolysis in the absence of mitochondria, with an emphasis on the key steps that consume or produce ATP. The role of lactate dehydrogenase in regenerating NAD+ was also emphasised. Some candidates described other pathways of metabolism that do not generate ATP, for which no marks were awarded.
ii. Explain mechanisms of transport of substances across cell membranes, including an understanding of the Gibbs-Donnan effect.
2012B 10
Describe transport mechanisms across cell membranes. Give an example of each.
CICMWrecks Answer
Transmembrane Transport Mechanisms
| Mechanism | Energy Expenditure | Electrochemical gradient | Example | ||||
|---|---|---|---|---|---|---|---|
| Diffusion | Passive diffusion | Molecule crosses a membrane to which it is permeable by diffusion | No | With | Carbon dioxide across vascular endothelium | ||
| Facilitated diffusion | Molecule crosses a membrane via a channel, without energy expenditure | No | With | Potassium across excitable cell membranes, via rectifier channels | |||
| Active transport | Primary | Primary active transport | Molecule crosses a membrane via a channel, with energy expenditure (ATP) | Yes | Against | 3Na+ /2K+ ATPase | |
| Secondary | Symport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Co-transported) | Not directly | May be Passive or Active based on gradient of 2nd | sodium and an amino acid | ||
| Antiport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Anti-transported) | Not directly | May be Passive or Active based on gradient of 2nd | Na+ / H+ antiporter in proximal convoluted tubule | |||
| Ligand-gated ion channel | Binding of a ligand causes conformational change in membrane channel, allowing movement of ion across membrane | No | With | Nicotinic acetylcholine receptor. ACh as ligand, Na+ and K + as ions | |||
| Exocytosis | Substance packed in vesicle, moves to cell membrane, two membranes merge -> substance exits cell | Yes | Usually against | Exocytosis of ACh by presynaptic neuron | |||
| Endocytosis | Cell membrane extends to engulf a substance or object, which is then contained in the cell within a vesicle | Yes | Can be with, against or refer to a macroscopic object | Phagocytosis of bacteria by macrophage | |||
JC / Mooney 2019
Examiner Comments
2012B 10: 11 (50%) of candidates passed.
Candidates were able to list types of transport across cell membranes but frequently described them incorrectly or gave an incorrect example. In a number of answers, there was confusion between facilitated diffusion and secondary active transport. Though diagrams were not required, several Candidates used a diagram of the cell very effectively to illustrate the mechanisms of transport across the membrane. For a good answer, some mention and description of exocytosis, endocytosis, ion channels, facilitated diffusion, passive diffusion, primary and secondary active transport was expected.
2022A 02
Explain the mechanisms of transport of substances across cell membranes including appropriate examples (75% marks).
Outline the structure and function of the Na+/K+-ATPase pump (25% marks).
CICMWrecks Answer
Transmembrane Transport Mechanisms
| Mechanism | Energy Expenditure | Electrochemical gradient | Example | ||||
|---|---|---|---|---|---|---|---|
| Diffusion | Passive diffusion | Molecule crosses a membrane to which it is permeable by diffusion | No | With | Carbon dioxide across vascular endothelium | ||
| Facilitated diffusion | Molecule crosses a membrane via a channel, without energy expenditure | No | With | Potassium across excitable cell membranes, via rectifier channels | |||
| Active transport | Primary | Primary active transport | Molecule crosses a membrane via a channel, with energy expenditure (ATP) | Yes | Against | 3Na+ /2K+ ATPase | |
| Secondary | Symport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Co-transported) | Not directly | May be Passive or Active based on gradient of 2nd | sodium and an amino acid | ||
| Antiport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Anti-transported) | Not directly | May be Passive or Active based on gradient of 2nd | Na+ / H+ antiporter in proximal convoluted tubule | |||
| Ligand-gated ion channel | Binding of a ligand causes conformational change in membrane channel, allowing movement of ion across membrane | No | With | Nicotinic acetylcholine receptor. ACh as ligand, Na+ and K + as ions | |||
| Exocytosis | Substance packed in vesicle, moves to cell membrane, two membranes merge -> substance exits cell | Yes | Usually against | Exocytosis of ACh by presynaptic neuron | |||
| Endocytosis | Cell membrane extends to engulf a substance or object, which is then contained in the cell within a vesicle | Yes | Can be with, against or refer to a macroscopic object | Phagocytosis of bacteria by macrophage | |||
Na+/K+ – ATPase pump
- Found in virtually all cells of the body – It is a heterodimer integral membrane protein that spans the entire membrane:
- α-subunit (larger subunit) – Binds Na+, ATP and PO43- intracellularly, and K+ and ouabain/glycosides extracellularly
- β-subunit (smaller subunit) – A glycoprotein
- Functions as an “electrogenic pump” as it extrudes 3 Na+ from the cell and takes in 2 K+ per ATP hydrolysed
- Process of pump function:
- Na+ and ATP bind to α-subunit intracellularly
- ATP is hydrolysed to ADP and the phosphate is transferred to an Asp-phosphorylation site
- Conformational change of the protein causes Na+ to be extruded into ECF
- K+ binds to the α-subunit extracellularly
- Dephosphorylation of the Asp-phosphorylation site causes the protein to return to its resting conformation, which then transports K+ intracellularly
- Pump function is inhibited by ouabain and digitalis glycosides
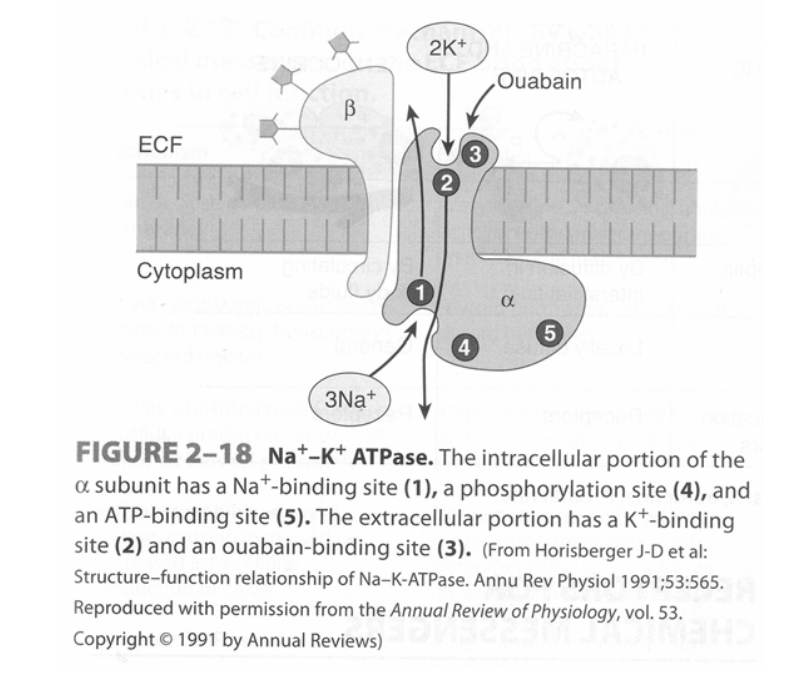
JC / Mooney / Bianca 2019
Examiner Comments
2022A 02: 61% of candidates passed this question.
This question examined core cellular physiology knowledge. This knowledge is crucial as it underpins much of the electrochemical responses within the syllabus. Mechanisms of diffusion and the role of individual pathways were well presented in many responses. Answers that scored well generally classified the mechanisms of transport into active and passive processes which ensured an appropriate breadth of answer. Many answers failed to provide any examples which was requested. The structure of the Na+/K+ ATPase pump was less well described, however pleasingly most candidates were able to accurately articulate its role and function.
2010A 15
Discuss the important factors in exchange of gases and substrates between capillaries and tissue cells.
CICMWrecks Answer
Based on 3 main physical principles
Diffusion via Fick’s Law
- Rate of movement of solute across semi-permiable membrane J is
where
C = concentration (or partial pressure for gasses)
A = cross-sectional area
T = thickness of the membrane or distance over which diffusion takes place.
- These factors alter the rate of transfer as per the equation above.
- Valid for Most medium – small substances
- Water soluble (H2O, electrolytes, glucose, urea) via intercelular clefts
- Lipid soluble substances (O2, CO2) via endothelial cells themselves.
Starling Forces
The NET flux across the membrane is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation:
where
Jv is the trans endothelial solvent filtration volume per second
( [ Pc – Pi ] – σ [ πp – πi ] ) is the net driving force
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
κ = filtration constant = LpS = Hydraulic conductivity x Surface Area
Typically quoted values for the variables in the classic Starling equation:
| Hydrostatic pressure | Oncotic pressure |
|---|---|
| Pressure moving fluid | pressure exerted by proteins which draw water into and keep it within a compartment |
| Pc ~35 → 15mmHg (Arterial → venous) Capillary hydrostatic pressure Pressure moving fluid out of capillary | πp ~ 20mmHg Plasma oncotic pressure Pressure keeping fluid within capillary |
| Pif = 5mmHg Interstitial hydrostatic pressure Pressure moving fluid into capillary | πif ~ 0mmHg Interstitial fluid oncotic pressure Pressure keeping fluid out of capillary |
Gibbs-Donnan Effect:
“Opposing osmotic and electro-chemical gradients in the presence of a nondiffusable ion resulting in unequal distribution of the diffusable ions”
- Diffusible ions move ↓ [ ] gradient
- Because of non-diffusable ion, significant opposing electrical potential develops.
- This prevents further movement of ions, and an electrochemical equilibrium is reached.
- Because of the presence of non-diffusable ions, there is an osmotic disequilibrium.
Osmotic pressure itself can be determined from the Vant Hoff Equation
where
n = # of particles into which substance dissociates
c = [ ] (in g/L)
T = Absolute temperature
R = Universal Gas Constant (8.314 J⋅K−1⋅mol−1)
M = Molecular weight of molecules
It depends on:
- # of particles
- [ ] or molarity of the solution
- Temperature
- Inversely to the molecular weight
Active Processes
Serve to establish Starlings forces and G-D equlibrium
- Facilitated diffusion
◦ Diffusion through the membrane using a specific carrier protein to help - Active transport
◦ Movement of ions or other substances across the membrane in combination with a carrier protein
against an energy gradient.
◦ May be primary (energy derived directly from ATP)
◦ or secondary (occurs via co-transport or counter transport) - Endocytosis/Exocytosis
◦ Vesicular transport by engulfment/extrusion of particle by cellular contents
Gladwin 2016
Examiner Comments
2010A 15: 4 (40%) of candidates passed this question
Good answers were based around Fick’s Law, Starling forces and the Gibb’s Donnan effect.
It was expected that candidates would give Fick’s equation and describe the components :
Fick’s Law J = -DA dc/dx
Candidates were also expected to describe Starlings equation and the equation for Osmotic Pressure. Starling Equation:
Fluid movement = k[(Pc-Pi) – s(πp – πi)]
Osmotic pressure : sRT(Ci-Co).
Gibb’s Donnan effect and, other mechanisms of transport (filtration and pinocytosis) was also expected for a good answer.
Syllabus: A combination C1c2.d, C21 2.e, C2b2.c, C2b2.e
References: Pharmacology and Physiology in Anesthetic Practice, Stoelting pgs
294-300, 322- 325
2018B 16
Describe the forces that result in fluid exchange across capillary membranes.
CICMWrecks Answer
Introduction
Capillaries contain semipermeable membranes to allow the movement of fluid and solutes.
- it is normally impermeable to large protein
- Plasma ultrafiltrate is filtered by bulk flow through the capillary wall by the action of opposing hydrostatic and oncotic pressures
Starling Forces
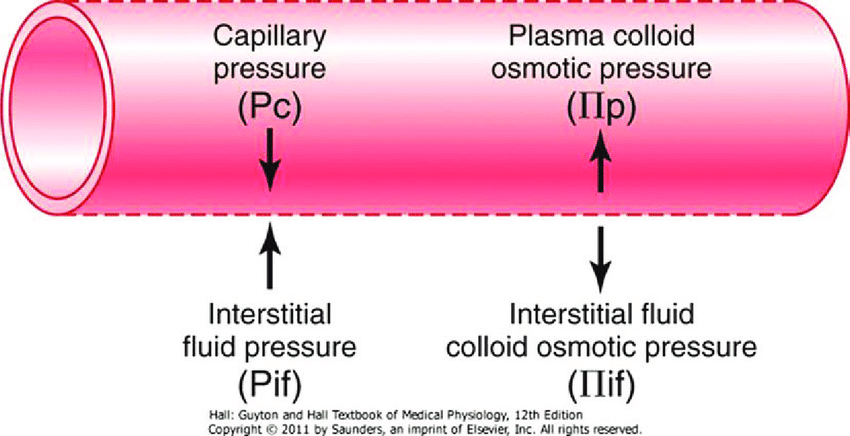
- The NET flux across the membrane is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation:
where
Jv is the trans endothelial solvent filtration volume per second
( [ Pc – Pi ] – σ [ πp – πi ] ) is the net driving force
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
κ = filtration constant = LpS = Hydraulic conductivity x Surface Area
- Typically quoted values for the variables in the classic Starling equation:
| Hydrostatic pressure | Oncotic pressure |
|---|---|
| Pressure moving fluid | pressure exerted by proteins which draw water into and keep it within a compartment |
| Pc ~35 → 15mmHg (Arterial → venous) Capillary hydrostatic pressure Pressure moving fluid out of capillary | πp ~ 20mmHg Plasma oncotic pressure Pressure keeping fluid within capillary |
| Pif = 5mmHg Interstitial hydrostatic pressure Pressure moving fluid into capillary | πif ~ 0mmHg Interstitial fluid oncotic pressure Pressure keeping fluid out of capillary |
- In general, at the arterial end of capillary NFP is positive (filtration) +10mmHg
- At the venous end NFP is negative (absorption) -10mmHg
- Approx. 24L fluid filtered / day
- 85% reabsorbed into capillaries
- Rest reabsorbed via lymphatics (~3.5L/day) = Net fluid loss from filtration
Role of plasma oncotic pressure
- largely fixed as capillary is impermeable to oncoproteins (1° albumin)
- If ↓πc, ↑net movement of fluid into interstitium
- Once this exceeds the drainage capacity of lymphatics → oedema occurs
- Especially dangerous in areas where minimal interstitial distance is required b/n capillary and cell to continue normal functioning (e.g. lung)
JC 2019
Examiner Comments
2018B 16: 57% of candidates passed this question.
The expected answer included a clear explanation of Starling’s forces, including an understanding of the importance of the relative difference along the length of the capillary, with approximate values and examples of factors that influence them. Some explanation of what contributed to the hydrostatic or osmotic pressure gained more marks than merely stating there was a pressure. Several candidates digressed to Fick’s law of diffusion or intracellular flow of ions which was not directly relevant to capillary flow.
2024B 11
(a) Explain the mechanisms responsible for the cell resting membrane potential (70% of marks).
(b) Describe the Gibbs Donnan effect (30% of marks).
2017B 14
Explain the mechanisms responsible for the cell resting membrane potential (60% of marks)
and describe the Gibbs Donnan effect (40% of marks)
CICMWrecks Answer
Equilibrium Potential
Nernst Equation
The potential difference generated by a permeable ion in electrochemical equilibrium when there are different concentrations on either side of the cell can be calculated via the Nernst Equation:
where
E is the equilibrium potential for the ion
R is the gas constant (8.314 J.K-1.mol-1 )
T is the temperature in Kelvin
F is Faraday’s Constant
z is the ionic valency (e.g. +2 for Mg2+, -1 for Cl–)
EK = -90 mV
ENa = +55mV
ECl = -65mV
Goldman-Hodgkin-Katz Equation
The Nernst equation describes the equilibrium potential for a single ion, and assumes that the membrane is completely permeable to that ion.
However, calculation of membrane potential requires examining the effects of many different ions with different permeability. This can be performed with the Goldman-Hodgkin-Katz equation:
where,
Px is the permeability constant for the ion, x
If the membrane is impermeable to x, then Px = 0
Note that:
This model does not consider valency
The concentrations of negative ions are reversed relative to positive ions
Resting Membrane Potential
- Definition: The potential difference (units volts) that exists across the cell membrane (i.e. between the intracellular and extracellular environment) when the cell is in a unexcited state, where the reference voltage is the extracellular environment.
- Resting Membrane Potential (RMP) is the result of two key properties of cells:
- the cell membrane is semi-permeable, that is it has variable permeability to different species in solution.
- differing concentration gradients exist across the semi-permeable cell membrane for different ions in solution, the most important determinant of these gradients being the Na+-K+-ATPase.
- At rest, membranes are:
- Permeable to potassium
- Impermeable to other ions
- Generation of membrane potential:
- Intracellular potassium concentration is much higher than extracellular potassium concentration – Due to the action of the Na+-K+pump.
- As the membrane is permeable to potassium, potassium will attempt to diffuse down this gradient, generating a negative intracellular charge which opposes further diffusion
- At some point, an electrochemical equilibrium is reached between:
- The concentration gradient dragging potassium out of the cell
- Negative electrical charge pulling it in
Determinants of RMP
- K+ Diffusion Potential
- From the Nernst equation above we can see that the normal RMP of most tissue is relatively close to the K+ equilibrium potential (-94mV). From this (and the Goldman equation) we can infer that the membrane is likely to be most permeable to K+ at rest. Indeed the primary determinant of the RMP is K+. Relative permeability of the membrane to K+ vs Na+ is 100:1.
- This has the corollary that changes in K+ concentration will have the most major effect on RMP. This is particularly the case with [K+]o due to its low value – small absolute changes in [K+]o are a relatively large proportion of [K+]o.
- Note that while the RMP is as close (or closer) to the equilibrium potential for Cl–, Cl– is not the primary determinant of the RMP as the concentration gradient for Cl– is largely the passive result of the electrochemical gradient created by the Na+-K+-ATPase.
- Na+ Influx
- While the resting membrane is very impermeable to Na+ the electrochemical gradient for its movement into the cell is so large that there is a small ‘leak current’ of Na+ into the cell.
- This Na+ leak is the single factor responsible for most of the deviation of the RMP from the equilibrium potential for K+ (contributes roughly +8mV to the RMP).
- Na+-K+-ATPase
- As noted there is a constant slow leak of Na+ into the cell. Because of the deviation away from the K+ equilibrium potential that this causes, there is also an electrochemical gradient to cause a slow leak of K+ out of the cell. As such the Na+-K+-ATPase is essential to maintain the relative concentration gradients of these ions and thus the RMP.
- In addition the Na+-K+-ATPase itself is electrogenic, transferring as it does 3 Na+ out for every 2 K+ pumped in, leaving a net negative charge balance on the inside of the cell membrane. This contributes roughly -4mV to the RMP.
- Gibbs-Donnan Effect
- The Gibbs-Donnan effect accounts for the effect of non-diffusible ions on the RMP. In vivo the high concentration of negatively charged intracellular proteins has a small but significant effect on RMP. The presence of this net fixed negative charge on the inside of the cell effects the distribution of permeable ions across the membrane.
Resting Membrane Potential in Different Tissues
Typical values of RMPs:
- Ventricular Myocyte: -90mV
- Cardiac Pacemaker cell: -60mV
- Skeletal Muscle cell: -80mV
- Myelinated Axon: -70mV
Gibbs – Donnan Effect
- Describes the tendency of diffusable ions to distribute themselves such that the ratios of the concentrations are equal when they are in the presence of non-diffusable ions.
- Occurs when:
- A semi-permeable membrane separates two solutions
- At least one of those solutions contains a non-diffusable ion
- The distribution of permeable charged ions will be influenced by both their valence and the distribution of uncharged ions, such that at equilibrium the products of the concentrations of paired ions on each side of the membrane will be equal:
- The two main contributors to the Gibbs-Donnan effect in the body are sodium and protein. This occurs because cell membranes:
- Are impermeable to protein
Intracellular protein concentration is high. - Effectively impermeable to sodium
Due to the Na+-K+ ATP-ase pump.
- Are impermeable to protein
- Changing Gibbs-Donnan equilibriums also change the tonicity on each side of the cell membrane, causing movement of water which then upsets the Gibbs-Donnan effect – therefore there is no stable state.
- The Gibbs-Donnan Effect is important for:
- Maintenance of cell volume
Na+ acts as an effective osmole, reducing cellular swelling. - Plasma oncotic pressure
Increased plasma ion concentration increases oncotic pressure. - Resting Membrane Potential
- Maintenance of cell volume
JC 2019
Examiner Comments
2024B 11: 57% of candidates passed this question.
This question required a description of the separation of charge across the cell membrane due to selective ion permeabilities and concentrations. The equilibrium voltage of an individual ion was best expressed using the Nernst Equation and of multiple ions by the Goldman-Hodgkin-Katz equation. Better responses were able to use the correct eponym, constants and/or logarithmic transformation (from the ln to log10). The fundamental role of potassium conductance and the Na/K ATPase was also expected. The Gibbs-Donnan relationship was allocated 30% of marks so an explanation of the role of negatively charged intracellular proteins and the effect on ionic flux was expected.
2017B 14: 35% of candidates passed this question.
A good answer included a definition of the resting membrane potential and a clear description of the factors that determine it. Explanation of these factors should have included a detailed description of the selective permeability of the membrane, electrochemical gradients and active transport mechanisms. Answers should demonstrate awareness of the Nernst equation and the Goldman-Hodgkin-Katz equation. These were often confused, sometimes with the GibbsDonnan effect. Descriptions of the Gibbs-Donnan effect generally lacked detail and understanding. The better answers included a definition and discussed in detail the influence of non-diffusible ions (intracellular proteins) on the distribution of diffusible ions.
iii. Outline the role of cellular receptors and the function of secondary messengers.
2013A 23
How do chemical messengers in the extracellular fluid bring about changes in cell function? Give an example of a chemical messenger for each mechanism noted.
CICMWrecks Answer
A receptor is a protein, often integral to a membrane, containing a region to which a ligand (chemical messenger) binds specifically to elicit a response.
They may be grouped into three classes based on mechanism of action:
1. Altered ion permeability (ion channels / ionotropic receptors)
Membrane spanning complexes with the potential to form a channel through the membrane.
There are three families:
- Pentameric → contain 5 membrane spanning units (eg, nicotinic Ach receptor at the NMJ which allows a Na channel to form, GABA A receptor which allows a Cl channel to form, 5HT3 receptor)
- Ionotropic glutamate → NMDA, AMPA and kainate ionotropic ligand gated ion channels. They form Na, K and (NMDA only) Ca channels when glutamate binds
- Purinergic receptors → PX1 and PX2 are activated by ATP, permeable to Na, K and Ca, and are associated with mechanosensation and pain.
2. Production of intermediate messengers (Metabotropic receptors)
Membrane bound systems that transduce a ligand gated signal presented on one side of the cell membrane into an intracellular signal transmitted by intermediate messengers.
These messengers may be:
- G proteins (most common) → binding of a chemical messenger to a G-protein coupled receptor activates the G protein, which in turn amplifies and transmits the signal to the appropriate target molecules. This can be done in several ways:
- Activation of phosplipase C (Gq), with intracellular production of DAG, IP3 and other inositol phosphates (eg; angiotensin II, noradrenaline on alpha 1 receptors, vasopressin on V1 receptors)
- Activation (Gs) or inhibition (Gi) of adenylyl cyclase, causing increased or decreased levels of intracellular cAMP (eg, noradrenaline increases cAMP via beta1 receptors, noradrenaline decreases cAMP via alpha 2 receptors)
- Increase in cGMP (eg, atrial natriuretic peptide, nitric oxide)
- Tyrosine kinase → eg, insulin activates tyrosine kinase resulting in the phosphorylation of various proteins
- Guanylyl cyclase → eg, NO, atrial natriuretic peptide
3. Regulation of gene transcription (Nuclear receptors)
- Steroids and thyroid hormones act through intracellular receptors to alter the expression of DNA and RNA, and indirectly alter the production of intracellular proteins.
JC 2019
Examiner Comments
2013A 23:
Overall answers lacked structure and depth, to what is a very fundamental topic. This topic is generally covered within the opening chapters of most physiology texts. Common errors were not answering the question, writing lists rather than describing and explaining, and poor categorisation. Candidates were expected to mention and give example for mechanisms such as hormones binding to cytoplasmic or intra-nuclear receptors, binding to transmembrane receptors coupled to G proteins, cAMP, cGMP, tyrosine kinase, etc.
iv. Describe the composition and control of intracellular fluid and the mechanisms by which cells maintain homeostasis and integrity.
VIVAs
| 2009A | Discuss cell membrane physiology and electrolyte disturbances. |



Recent Comments