1. Outline the abnormalities in the following arterial blood gas (25% of Marks).
Explain the Stewart approach to acid-base interpretation (75% of Marks).
19CICMWrecks Answer
Abnormalities in ABG
ABG from question not available
Stewart approach to acid-base interpretation
(Physico-chemical approach)
- Developed to address the criticism that traditional approach is merely a mathematical description of pH and fails to provide any mechanistic insight into rising and falling [H+]
- Peter Stewart (1978) modeled a solution that contained a complex mixture of ions of constant charge over the physiological pH range (strong ions), non-volatile proton donor/acceptors which transfer H+ within the physiological pH range (weak acid/base), and the volatile bicarbonate–CO2 buffer system.
- Key aspect of Stewart’s concept was the classification of each variable as dependent or independent in determining the H+ concentration of the solution.
- Three independent variables that independently determine the dissociation of water, and consequently the [H+] and [HCO3–] to maintain electrical neutrality:
- partial pCO2 of the solution
- Total concentration of weak acids (ATOT)
- Strong ion difference (SID)
- Thus, in the Stewart’s approach, metabolic disorders are the results of changes in SID or ATOT.
PaCO2
- ↑ = respiratory acidosis
- ↓ = respiratory alkalosis
Apparent SID and effective SID
Apparent SID (SIDa): represents the difference between measured strong cations and strong anions
- Normal plasma SID is 42 mEq/L
- ↑SID = Metabolic Alkalosis
- ↓SID = Metabolic Acidosis
SID can be changed by:
- Concentration change
- ↑ H2O: concentrates alkalinity and ↑ SID
- ↓ H2O: dilutes alkalinity and ↓ SID
- Strong ion change
- ↓ Na+: ↓ SID and acidosis
- ↑ Na+: ↑ SID and alkalosis
- ↑ Cl-: ↓ SID and acidosis (~NAGMA, for e.g with Normal saline)
- ↑ organic acids with pKa <4 (lactate, formate, ketoacids): ↓SID and acidosis (~HAGMA)
- ↑ ↓
Effective SID (SIDe): calculated to account for electrical neutrality. Sum of bicarbonate and weak acids (Albumin and phosphate).
SIG
- Gap between SIDa and SIDe due to failure to measure the concentration of all strong and weak ions in plasma
- quantifies [unmeasured anions] – [unmeasured cations] of both strong and weak ions
- theoretical advantage over AG due to pure representation of unmeasured ions
- Unmeasured anions in AG: Mg,Ca,Alb,phosphate,lactate and other ions
- Unmeasured anions in SIG: just other ions
- normal AG 8 to 12, whereas SIG closer to zero in normal situations
- ↑SIG = ↑ unmeasured anions
- ↓SIG = ↑ unmeasured cations
ATOT
- represents all non-bicarbonate buffers
- is made up of mainly serum albumin and other minor charges such as phosphate and globulins
- ↑ATOT = Metabolic acidosis
- ↓ATOT = Metabolic alkalosis
Advantages and Disadvantages
- Advantages
- provides physico-chemical basis and mechanistic insight into rising and falling [H+]
- Pure representation if unmeasured ions
- Diminishes importance of [HCO3-] which is just a dependent variable
- Better explanation of some phenomenon such as acidosis from Normal Saline
- Disadvantages
- Complex calculation, cannot be used bedside quickly
- Substantially different from well-validated classical approach
- Only reflects plasma (base excess reflects whole body and influence of Hb)
- Unclear clinical correlation, not extensively validated
- Higher chance of error due to numerous variables
Examiner Comments
2023A 01: 21% of candidates passed this question.
High performing answers correctly outlined the ABG findings including consideration of electrolyte abnormalities, A-a gradient, acid-base disturbance (including anion gap and strong ion difference) and whether compensation was appropriate. The best explanations of the Stewart approach described its physicochemical basis, discussed the independent variables (strong ions, total weak acids, and pCO2) in detail, and described their effect on the dependent variables and how they result in acid-base derangements.
The ABG provided depicted an incorrect base excess with an omission of (-) symbol. Candidates were marked accordingly depending on their response to this and all candidates were compensated equally for the confusion that this may have caused.
2. Describe the factors affecting drug absorption throughout the gastrointestinal tract.
CICMWrecks Answer
Drug Factors
- Lipid solubility
◦ ↑ lipid sol → ↑ absorption
◦ Must still have some water soluble component - Molecular size
◦ ↓ size → ↑ rate of absorption
◦ Larger molecules require processes other than simple diffusion - Degree of ionisation
◦ pH-pKa gives the degree of ionisation
◦ ↓’d ionisation favours absorption - Physical forms
◦ Gases faster than liquids faster than solids - Dosage forms
◦ Capsules more rapidly absorbed than tablets - Formulation
◦ The addition of Na to the formulation for stability can reduce the bioabalability via the oral route - Concentration
◦ From Fick
Patient factors
- Area of absorptive surface
◦ intestinal resection or coeliac → ↓ absorptive surface → ↓ absorption from Fic - Vascularity
◦ Shock → ↓ absorption - pH
◦ effect degree of ionisation
▪ ↓ pH favours absorption of acidic drugs
▪ ↑ pH favours absorption of basic drugs - Presence of other substances
◦ Statins should be taken with food
◦ Milk ↓’s and Vit C ↑’s absorption of iron - GI motility
◦ ↑ motility → ↓ absorption - Functional Integrity of the absorptive surface
◦ Coeliac disease → flattened mucosa → ↓ absorption - Coexisting disease state
◦ diarrhoea → ↓ absorption
Gladwin 2016
Examiner Comments
2023A 02: 39% of candidates passed this question.
This question required candidates to draw on knowledge across the syllabus. Many candidates did not appreciate the breadth required to adequately address this question unfortunately providing very detailed information on only aspects of the answer required to pass.
Excellent answers described the following: differences between different GI routes/ sites of absorption, mechanisms of absorption including Fick’s law of diffusion, drug formulations, drug factors (including concentration gradient, lipid solubility, ionisation, and molecular weight), site factors (including surface area, first pass metabolism, GI wall thickness, blood flow, and GI motility/ transit time). Marks were also given for consideration of drug interactions, enterohepatic recycling, and luminally active drugs.
3. Outline the principles of measurement of end-tidal CO2 using infrared radiation (25% of Marks). Describe the potential sources of error when using this modality and how they may be mitigated (75% of Marks).
CICMWrecks Answer
Principles
- Beer Lambert law: At a given wavelength, the amount of infrared radiation absorbed by gas is proportional to the concentration of gas present
- CO2 is a heteronucleic molecule, and so absorbs infra-red light
- Infrared light shone across a sample of gas
- Narrow band light emitted from infrared source
- Band frequency chosen which fits the peak absorption frequency of CO2
- 4.23micrometres
- Shone across gas, and absorbed at a detector
- Detector emits signal to analyser -> screen
- CO2 reading outputted is inversely proportional to CO2 present in sample, as per Beer-Lambert Law
- Narrow band light emitted from infrared source
- Gas can be sampled at the patient within the ventilation circuit (in-line), or in a sample of gas diverted away to a separate analysing chamber (sidestream)
Sources of error and limitations
- Sampling
- Entrainment of atmospheric gas if leak in sidestream line
- Occlusion of sidestream line causes loss of gas sampling
- Water condensation absorbs IR light -> erroneously high ETCO2
- Modern capnography includes a water trap and heater (to reduce condensation)
- Calibration
- Incorrect calibration of analyser
- Interference
- Other gases (notably N2O) have a similar absorption spectrum
- Presence may falsely elevate measured ETCO2 (esp. if infrared frequency band too broad)
- Presence of other gases causes ‘collision broadening’
- Absorption spectrum of CO2 is broadened
- Pressure
- Partial pressure, rather than percentage composition, is measured
- If pressure ↓ (e.g. by suction drawing gas into sampling chamber), erroneously low measured ETCO2
- Sampling chamber
- If too large, mixing of gas between respiratory cycles -> compression of waveform and erroneously low measured ETCO2
Mooney 2016
Examiner Comments
2023A 03: 21% of candidates passed this question.
The principles of infrared (IR) measurement of expired CO2 was answered in acceptable detail by most candidates. Sources of error were rarely identified nor explained in acceptable detail. Many candidates demonstrated errors of understanding when attempting to answer this section. Important concepts not covered by numerous candidates included side-stream vs mainstream techniques; how the Beer-Lambert law is applied and how concentration is used for partial pressure; what is the role of sapphire glass and the difference between collision broadening and additional IR absorption by N2O.
4. Explain the structural features of the alveolus that facilitate its function?
CICMWrecks Answer
Alveolus
- Hollow cavity found in the lung parenchyma, Ends of the respiratory tree.
- There are ~300 million alveoli that are engulfed by a rich capillary network
- cumulative Surface Area of all alveoli is ~85 m2 with a volume of 4000 mL
- Polyhedral-shaped → 0.33 mm in diameter
- Alveolar walls contain dense mesh of capillaries large enough for a red cell to pass through
- Each alveolar wall facing air is lined by Type I cells (one-cell-thick) → parts of alveolar
wall consists of interstitial space that contain pulmonary capillaries - Diffusion barrier that alveolar gas has to travel to pulmonary blood is short (0.3 um only!)
→ consists of (i) Type I cell (epithelium), (ii) Basement membrane of type I cell, (iii)
Basement membrane of pulmonary capillary endothelium, and (iv) Pulmonary capillary
endothelium - In some alveolar walls, there are pores between alveoli called Pores of Kohn
- Alveoli are inherently unstable (Ie. collapse) given their small size and surface tension of
their liquid-lining → this is offset by surfactant produced by Type II cells (round cells
with large nuclei and cytoplasmic granules containing surfactant)
Alveolar cells
Type 1 Pneumocytes
- 90% of alveolar surface area
- Thin walled, Involved in gas exchange
- Unable to replicate, Susceptible to toxic insults,
Type II pneumocytes
- 10% of alveolar surface area
- Secrete surfactant
- Proliferate and differentiate into type I and type II cells
Alveolar Macrophages
- Present in alveolar septae, lung interstitium
- Phagocytosis of foreign particles
JC / Bianca 2019
Examiner Comments
2023A 04: 14% of candidates passed this question.
The question asked for an explanation of the structural features of the alveolus that facilitate its function. Candidates who scored well integrated the specific anatomical and structural elements of the alveolus with the multiple functions of the alveolus, including the relevant explanation regarding mechanisms. No marks were given for simply listing the structural features of an alveolus without an explanation on how this facilitates function, nor for listing functional requirements of an alveolus without explaining whether or how they are met by its structure. For the same reason, this was one question where simply listing equations with no discussion as to how these relate to the structure and function of the alveoli garnered no marks. Equations were not required for full marks but may be an efficient way to represent physical relationships that are hard to write in a few words. Common omissions included a description and the role of collagen and elastin fibres, capillary structure, the filtration function of the membrane, lymphatic drainage, recruitment and distensibility and metabolic functions. Candidates are encouraged to practice model answer templates for these integrative questions in the months leading up to the exam.
5. Describe renal handling of potassium (60% of Marks), including physiological factors that may influence it (40% of Marks).
CICMWrecks Answer
Potassium
- Predominany intracellular cation
- Total Stores: Approx 3200mmol (50mmol/kg)
- Key Functions:
- Main determinant of ICF osmolality and tonicity
- Responsible for RMP of excitable cells via Goldmann-Hodgkin-Katz due to ↑↑gK relative to other species
- Role in action potential → repolarisation phase
- Secretion of insulin and multiple other KATP dependant processes
- Regulation of IC processes (protein/glycogen synthesis)
- Involved in Na+/K+ ATPase in cell membranes
Potassium Elimination
- Faecal – 8mmol/day
- Renal – 92mmol/day (See next heading for details)
- Urinary K+ excretion = [K+ filtered by glomerulus] + [K+ secreted by CCD/LDCT] – [K+ reabsorbed by renal tubules]
- Glomerular filtration: Freely filtered = 756mmol/day (180L x 4.2mmol/L)
- PCT: 65% reabsorption
- LoH: 25~30% reabsorption
- DCT/Collecting Ducts – variable
- Determined by Aldosterone, Plasma [K+], Tubular Flow rate
- Secreted by principal cells
- Reabsorbed by intercalating cells
K+ handling within the tubular system:
- PCT (55% of filtered K+ is reabsorbed):
- K+ is reabsorbed passively via the paracellular route due to:
- Solvent drag → coupled to flow of Na+ and H2O
- [ ] gradient → created by reabsorption of H2O
- Electrical gradient → due to +ve luminal charge in late PCT
- K+ is reabsorbed passively via the paracellular route due to:
- TAL of LoH (30% of filtered K+ is reabsorbed):
- K+ is reabsorbed by 2° active transport via paracellular route → +ve charged lumen generated by ion flux created by NKCCT transporter causes K+ to be reabsorbed by diffusion down its electrical gradient
- LDCT and CCD (0 to > 15% K+ is secreted):
- Principal cells of CCD and LDCT → 2° active secretion K+ → via:
- Basolateral Na+ /K+ ATPase → (i) generates an electrochemical gradient that draws Na+ intracellularly from the tubular lumen (via the ENaC channel), and (ii) pumps K+ from peritubular capillaries into tubular cell
- The –vely charged lumen generated by influx of Na+ across ENaC channel → favours tubular secretion of K+
- o Type A intercalated cells of CCD and LDCT → 2° active reabsorption of K+ :
- H+ is produced within tubular cell by hydration of CO2 (using CA) → H+ is then exchanged for tubular K+
- Principal cells of CCD and LDCT → 2° active secretion K+ → via:
- MCD (5% of filtered K+ is reabsorbed)
Renal K+ regulation:
- Renal K+ regulation occurs MAINLY via control of K+ secretion at the distal nephron (CCD and LDCT) via the following factors:
- Circulating factors:
- Plasma [K+ ]
- ↑ [K+ ] causes ↑ K+ secretion by → (i) Directly stimulating Na+ /K+ ATPase in principal cells, and (ii) Directly stimulating Aldosterone release from adrenal cortex
- Aldosterone
- ↑ aldosterone causes ↑ K+ secretion by → ↑ production of Na+ /K+ ATPase, K+ channels and ENaC Na+ channels in the principal cells
- Plasma pH
- Alkalosis via ↓ plasma [H+ ] causes ↑ K+ secretion by → stimulating Na+ /K+ ATPase in principal cells
- Plasma [K+ ]
- Luminal factors:
- Flow of tubular fluid in DCT and CCD
- ↑ tubular fluid flow causes ↑ K+ secretion by → maintaining ↓ luminal [K+ ] (Ie. continuously washing it away) → permits passive diffusion of K+ ↓ its [ ] gradient into tubular lumen
- ↑ Na+ delivery rate to DCT and CCD → ↑ K+ secretion
- -ve lumen potential difference → ↑ K+ secretion
- Flow of tubular fluid in DCT and CCD
- Circulating factors:
JC / Gladwin / Sakurai / Bianca 2020
Examiner Comments
2023A 05: 33% of candidates passed this question.
This question related to the renal handling of potassium, the physiology of potassium in the rest of the body was not relevant. Ultimately net potassium flux is a function of filtration, reabsorption, secretion and excretion. Good candidates divided the nephron into relevant sections and described how potassium was handled in each section. They correctly described the percentage reabsorption along each section, as well as the relevant active and passive pathways for reabsorption and/or secretion including the cells and channels/pumps involved. The physiological factors regulating each of these mechanisms were then described in correct detail.
6. Describe pancreatic secretions and their regulation.
CICMWrecks Answer
Pancreatic Secretions
| EXOCRINE SECRETIONS | HCO3– | |
| Water | ||
| Enzymes | Proteases | |
| Amylase | ||
| Lipase | ||
| Others | ||
| ENDOCRINE SECRETIONS | Insulin | |
| Glucagon | ||
| Somatostatin | ||
Pancreas: Exocrine Secretions
Contents
- Digestive Enzymes:
- Proteases: trypsin and chymotrypsin: proteolysis
- Pancreatic Lipase: Hydrolysis of triglycerides
- Amylase: Hydrolysis of carbohydrates
- Other Pancreatic Enzymes: Ribonuclease, deoxyribonuclease, gelatinase and elastase
- Bicarbonate and water:
- Bicarbonate neutralizes acid coming into small intestine from stomach
- Produced by carbonic anhydrase → secreted into the lumen of the duct → pancreatic juice
Control of pancreatic exocrine secretions
- Vagus nerve:
- Low level stimulus via Ach in response to anticipation of meal
- Enteric endocrine system:
- Cholecystokinin:
- secreted in duodenum
- Stimulated by partially digested proteins and fats
- CCK released into blood → binds to pancr acinar cells
- secreted in duodenum
- Secretin:
- Secreted in proximal small intestine
- In response to acid in duodenum
- Stimulates duct cells to secrete water and bicarb
- Secreted in proximal small intestine
- Gastrin:
- Secreted by stomach
- in response to gastric distension and irritation
- Stimulates acinar cells to secrete digestive enzymes
- Secreted by stomach
- Cholecystokinin:
Occurs in three phases: cephalic → Gastric → Intestinal
Pancreas: Endocrine Secretions
Insulin
- Peptide hormone produced in beta cells of Islets of Langerhans in pancreas
- Proinsulin: insulin precursor; A + B chain joined by 2 disulphide bridges + C peptide
- Insulin: formed when C peptide is cleaved by endopeptidases → insulin + free C peptide are packaged in vesicles
- Exocytosis:
- Primary trigger = ↑BSL
o ↑BSL → ↑facilitated diffusion of glucose through GLUT2 transmembrane channels into B-islet cells → ↑metabolic activity of cell → ↑formation of ATP - ATP-gated K channels on beta islet cell membrane are closed by ↑ATP levels → ↓ K flux → membrane depolarisation → triggers opening of voltage gated Ca2+ channels → Ca2+ influx triggers insulin containing vesicle exocytosis
- Primary trigger = ↑BSL
- Plasma insulin secretion occurs in 2 phases
- ↑BSL → rapid ↑insulin concentration as vesicles with pre-formed insulin empty contents
- When all vesicles have emptied → B islet cells release insulin as it is synthesised
- Physiological effects:
- Facilitation of glucose uptake: GLUT4 in adipose tissue, skeletal muscle, heart – require insulin to facilitate cellular glucose uptake. NB brain has GLUT-1 and liver has GLUT-2 which are not insulin dependent
- Storage of metabolic substrates: hepatic glycogenesis, FA synthesis in liver, ↑esterification of FAs (to make TGs) in adipose tissue
- Inhibition of endogenous glucose production: insulin inhibits lipolysis + glycogenolysis + gluconeogenesis
- Cellular uptake of amino acids and K
- ↑amino acid uptake → promotes protein synthesis
- ↑K uptake: prevents hyperkalaemia following meal
Glucagon
- peptide hormone produced by alpha cells of islets of Langerhans
- unlike insulin, the have no glucose sensing apparatus
- Secretion:
- stimulated by hypoglycaemia: hypoglycaemia-induced ↑ANS activity (i.e. indirect) + ↑adrenaline
- inhibited by: insulin, somatostatin, ↑freeFA and ketone body concentrations
- Actions: ↑plasma glucose conc by: promoting gluconeogenesis+glycogenolysis, inhibits glycolysis in liver. Important during starvation
Somatostatin
- secreted by delta cells in pancreatic islets (and pyloric antrum, duodenum)
- inhibitory hormone
- Stimuli for release: (i) ↑ plasma levels of glucose, a.a. and FA, and (ii) Acidic gastric environment
- inhibited by vagus nerve
- Effects:
- ↓ secretion of pancreatic hormones (insulin, glucagon, pancreatic polypeptide)
- ↓ GH release from pituitary
- ↓ gastric, duodenal and GB motility
- ↓ secretion and absorption in GIT (Eg. gastric acid secretion)
Pancreatic polypeptide
- Polypeptide secreted by F-cells of Islets of Langerhans
- influence GIT function (Ie. ↑enzyme secretion, ↓ intestinal motility, Etc.)
Examiner Comments
2023A 06: 21% of candidates passed this question.
It was vital to recognise and describe both the exocrine and endocrine secretions of the pancreas and their regulation. Insulin, glucagon, and somatostatin are all secreted from the pancreas and their omission was the most common reason for not passing this question. Many candidates unfortunately wrote solely on exocrine secretions. Satisfactory answers provided a moderate amount of detail on both the endocrine (insulin, glucagon and somatostatin) and exocrine (bicarbonate and digestive enzymes; trypsin/chymotrypsin, pancreatic amylase and lipase) secretions of the pancreas including what determines their secretion.
7. Define basal metabolic rate and outline the factors that affect it (60% of marks).
Outline the ways it may be measured (40% of Marks).
CICMWrecks Answer
Basal Metabolic Rate
- Energy expenditure required to maintain the body’s basic homeostatic mechanisms
- Normal BMR = 40kcal/m2/hr or approx. 70kcal/hr for 70kg male
- Relative organ contribution to BMR
- Liver 30%
- Brain 20%
- Muscle 20%
- Kidney 10%
- Heart 10%
- Other 10%
Factors that affect BMR
- Prandial effects
- Metabolic rate increases post-prandially for the digestion and absorption of nutrients
- After prolonged fasting metabolic rate decreases
- Thermoregulatory effects
- Temperatures above and below the thermoneutral zone cause metabolic rate to increase to support extra thermoregulatory mechanisms
- Response to heat
- Sweating
- Vasodilatation
- Response to cold
- Shivering
- Non-shivering thermogenesis
- Vasoconstriction
- Response to heat
- Temperatures above and below the thermoneutral zone cause metabolic rate to increase to support extra thermoregulatory mechanisms
- Physical activity
- Increased muscle activity
- Exercise increases metabolic rate in order to supply ATP to sarcomeres for contraction
- Increased muscle activity
- Inflammation
- Metabolic rate increases
- Chemotaxis
- Increased oxidative bursts
- Cell proliferation and clonal expansion
- Metabolic rate increases
- Psychological stress
- Hormonal factors
- Thyroid hormones → increase metabolic rate
- Maximal thyroid hormone activity increases MR 100%
- Thyroidectomy decreases MR 50%
- Catecholamines → increase metabolic rate
- Growth hormones → increase metabolic rate ~15~20%
- Testosterone → increase metabolic rate ~10~15%
- Thyroid hormones → increase metabolic rate
- Age
- Decreases with age >20
- Sex
- Male BMR > Female BMR possibly due to testosterone effect
- Body mass
- Muscle mass accounts for significant proportion of BMR, therefore increases BMR
- Sleep
- Decreases BMR 10~20%
Measurement of BMR
- Direct Calorimetry
- Atwater Chamber
- Subject placed in insulated chamber surrounded by H2O
- Change in temperature of water proprotional to metabolic rate
- Atwater Chamber
- Indirect calorimetry
- Metabolic carts
- Measurement of O2 consumption (and CO2, Urea production) to determine Metabolic rate
- Respiratory quotient
- Carbohydrates = 1
- Fats = 0.7
- Proteins = 0.8
- Metabolic carts
Conditions required to measure BMR
- Thermoneutral zone
- Free from psychological or physical stimulus
- After a night of restful sleep
- 12 hours after last meal
- 2 hours after last exercise
Sakurai 2016
Examiner Comments
2023A 07: 64% of candidates passed this question.
The first part of the question required candidates to define basal metabolic rate (BMR), identify the standardised conditions under which it is estimated and then elaborate on those conditions which affect it. Most candidates provided reasonable definitions but lost marks for not describing the standard conditions under which it is defined. Many were able to give a comprehensive list of factors that affected BMR however lacked the additional pertinent facts required in an outline question to achieve full marks. Candidates are again referred to the glossary to understand the difference between a list and outline question.
The second part of the question pertained to the measurement of BMR which was not as well answered and understood. Overall, there was a limited ability to discuss direct and indirect calorimetry clearly and some responses confused the two.
8. Describe the cardiovascular and respiratory effects of positive pressure ventilation.
CICMWrecks Answer
Positive Pressure Ventilation
- PEEP = Positive End Expiratory Pressure.
- Equivalent to a constant pressure applied throughout the respiratory cycle.
- Intrinsic PEEP = unintentional or un-measured end-expiratory hyperinflation
- Physiological effects of Positive Pressure Ventilation mostly related to increased mean airway pressure
Cardiovascular Effects
- Causes constant ↑ intrathoracic pressure (ITP) throughout respiratory cycle
- Left Heart
- On Preload
- Initially: → ↑ LV PL → ↑ CO
- Secondarily: → ↓RV output → ↓ LV PL → ↓ CO
- On Afterload
- → ↓myocardial transmural pressure → ↓LV AL → ↑CO
- PEEP > Ao diastolic pressure
- → Collapse of intrathoracic aorta → Starling resistor mechanism → ↑LV AL → ↓ CO
- PEEP < Ao diastolic pressure
- ↑ pressure gradient for flow to systemic circulation
- ↓ AL → ↓ myocardial work
- On Compliance
- diastolic buldging of septum → ↓ LV compliance → ↓LV CO
- On Preload
- Right Heart
- → ↑ Pulmonary vascular resistance → ↑ RV AL → ↓ RV output
- → ↓ Venous return → ↓ RV PL → ↓ RV output
- In the failing LV → ↑CO
- ↓ LV afterload
- ↑ pressure gradient thorax to abdomen
- ↓ transmural pressure → ↓ LV wall tension = ↓afterload
- ↓ LV preload (ie more favourable position on compliance curve)
- ↓ LV afterload
Respiratory Effects
Beneficial Effects:
- ↓ atelectasis and gas trapping
- ↑’s FRC > “closing capacity”
- Shifts position on P-V curve right, above the “closing point”
- ↑ lung compliance → shifts back to steep/compliant part of P-V curve
- ↓ intrapulmonary shunt thus improved V/Q match and ↑ PaO2
- ↓ atelectasis and gas trapping
- ↓ extravascular lung water → ↓interstitial/alveolar oedema
- ↓ AWR
- ↑ lung volume → ↑ radial traction by parenchyma → ↑ airway calibre
- ↓ work of breathing
- ↑ lung compliance = ↓ elastic work
- ↓ AWR = ↓resistance work
Negative Effects:
- ↑ V/Q mismatch
- ↑ west zone 1
- ↓ lung compliance
- shift to flat part of P-V curve
- ↑ Work of breathing (due to compliance change)
- ↑ PVR and RV afterload
- extrinsic compression of pulmonary vessels
- Barotrauma
End-Organ Effects
- Renal:
- ↓CO & ↑renal venous pressure
- → Reduced renal blood flow → Reduced GFR → Reduced urine output
- → Reduced atrial stretch and ANP release → Increased ADH → Fluid retention → Oedema
- ↓CO & ↑renal venous pressure
- Hepatic:
- Reduced hepatic blood flow due to:
- Increased CVP and decreased CO lowering the pressure gradient for hepatic flow
- May result in circulation only intermittently throughout the cardiac cycle
- Hepatocyte dysfunction
- Haematological:
- Neutrophil sequestration in the compressed pulmonary vasculature
- CNS:
- ↓VR ⇒ ↑CVP ⇒ ↑ICP
Gladwin / Mooney / JC 2020
Examiner Comments
2023A 08: 21% of candidates passed this question.
This question required a breadth of response that was largely unrepresented in the answers provided by candidates. Better answers divided responses into cardiovascular effects and respiratory effects however many did not provide sufficient detail or provided only detail on some of the effects and missed the breadth of the question. Cardiovascular effects could be grouped into effects on the; right heart, left heart, ventricular interdependence, cardiac output, other circulations, baroreceptor and hormonal reflexes. Respiratory effects could be grouped into lung volumes, dead space, V/Q matching, compliance, airway resistance and the effect of excessive pressures on the lung. Despite this division in the question many candidates abandoned a structure which made their answers and explanations lack clarity. The examiners commented that most candidates who wrote an answer in paragraph format were likely to provide long vague answers rather than those more factual and succinct answers presented in a format with headings as above and pertinent associated dot point descriptions underneath these headings.
9. Define pain (10% of Marks). Describe how pain is detected and modulated in response to a peripheral noxious stimulus? (90% of Marks)
CICMWrecks Answer
Pain
Pain is an unpleasant sensory and emotional experience, associated with, or resembling that associated with, actual or potential tissue damage. (IASP, Revised 2020)
Anatomical and physiological components of the response to pain
- Primary afferent nociceptors
- Pseudo-Unipolar
- Cell body in dorsal root ganglion
- Free nerve endings or specialized terminal structures
- Pacinian corpuscles
- Location
- Skin
- Connective tissue
- Muscle
- Blood vessels
- Viscera
- Responds to simuli
- Mechanical
- Chemical
- Thermal
- Pseudo-Unipolar
Nociception
- Transient Receptor Potential (TRP) Ion Channels
- Capsaicin
- Heat
- Acid
- Inflammation
- Ischaemia
- Acid-Sensing Ion Channel (ASIC)
- 5-HT3 receptors
- On activation of nociceptors
- Na channels open
- Depolarization from < -60mV to -40mV
- Rapid membrane depolarization
- Voltage Dependent Calcium Channels (VDCC) open and increase intracellular Ca
- Substance P and Calcitonin Gene-Related Peptide (CGRP), Neurokinin A released in response to Ca
- Mediate inflammation → macrophages, neutrophils, mast cells activated
- Increase excitability of sensory and sympathetic fibres
- Vasodilation, extravasation of plasma proteins
- Inflammatory cells release substances
- Histamine
- Bradykinin
- Serotonin
- Nitric Oxide
- Peripheral sensitization → Primary hyperalgesia
- to dampen pain response
- Silent nociceptors – Do not respond to external stimulus, however activated by inflammatory mediators
- Voltage-gated Potassium Channels stabilize the membrane potential
Signal Pathway
- Signal transduced via C fibres (0.5~2m/sec) or Aδ fibres (6~30m/sec) to dorsal horn
- Aδ activation → short, pricking pain
- C activation → burning, aching pain
- Primary afferent enters spinal cord at the spinal level, or above or below the spinal level via lissauer tracts and synapse with interneurons
- Via C fibres or Aδ fibres
- Signal relay via spinal interneurons
- Signalling via Glutamate and NMDA or AMPA receptors
- Nociceptor-specific
- Respond only to pain
- Wide Dynamic Range (WDR)
- Respond to nociceptors and other stimulus
- Central sensitization → allodynia
- Inhibitory interneurons and descending pathways modulate pain
- “Gate control theory of pain” → Aβ fibres carrying signals from benign stimuli inhibit noxious interneuron transmission
- Signal transduction to thalamus via anterolateral spinothalamic tract
- Small but significant proportion of signal transduction also occurs ipsilaterally
- Signal distributed to cerebral cortex via thalamus
- Putamen
- Hypothalamus
- Amygdala
- Periaquaductal grey matter
- Hippocampus
- Cerebellum
- Somatosensory cortex
Descending inhibitory pathways
- Noradrenaline
- Serotonin
- Substance P
- CCK
- GABA
Sakurai 2016
Examiner Comments
2023A 09: 26% of candidates passed this question.
Candidates were expected to give a reasonable definition of pain incorporating the experience and tissue damage. Most candidates only partially incorporated both the actual or perceived harm and the sensory and emotional experience that is included in its formal definition. This question was then best answered by breaking down pain transmission and modulation into; peripheral, spinal cord, cortex, and central downregulation pathways. Good answers provided detailed and specific descriptions of the sensors, neural pathways, synapses, receptors, and neurotransmitters. Whilst pain transmission at the level of the spinal cord is complex, breaking this down into 1st and 2nd order neurons, main neurotransmitters and accessory neurotransmitters from interneurons and descending pathways was helpful. Whilst many covered some of this conceptually most answers did not provide sufficient detail to be considered a pass level answer. Many candidates described the withdrawal reflex to pain in detail which was not asked for and therefore did not attract marks.
10. Describe the pharmacology of salbutamol (70% of Marks), including the principles and efficacy of methods of delivery (30% of Marks).
CICMWrecks Answer
Pharmacology of Salbutamol
Delivery systems (Too much information)
- Dosage forms available
- Aerosol powder – pMDI (DPI not available)
- Aerosol solution, Inhalation
- Nebulization solution, Inhalation
- Solution, Intravenous (Also IM/Subcut)
- Syrup and Tablets, Oral
- Inhaled Salbutamol is commonly administered via pressured metered dose inhalors and nebulizers (see below for details)
- Soft mist inhalers and Dry Powder Inhalers are not available for Salbutamol
- Infused intravenously
- Requires no patient effort or breath syncrony
- Maximal systemic toxicity
- Ingested orally
- Excellent patient compliance
- Slower response of a lower magnitude
- Gastrointestinal absorption is erratic and unreliable
Pressured Metered Dose Inhalers (pMDI)
- A pMDI consists of a pressurized canister, a metering valve and stem, and a mouthpiece actuator.
- Mechanism:
- The canister contains the drug suspended in a pressurized mixture of propellants, surfactants, preservatives, flavoring agents, and dispersal agents. The propellent is hydrofluoroalkane (HFA)
- The medication-propellant mixture is released from the pMDI canister through the metering valve and stem into an actuator boot. Lung deposition ranges between 10 and 40 percent of the nominal dose in adults and is very technique-dependent
- Difficulty precisely coordinating device actuation with inhalation leads to poor drug delivery, suboptimal disease control, and increased inhaler use
- Devices to optimize usage:
- Spacer or valved holding chamber:
- three basic designs: the open tube, the reservoir or VHC, and the reverse-flow design, in which the pMDI, placed close to the mouth, is fired in the direction away from the patient; adding a one-way valve creates a VHC. They can be used with a mouthpiece or mask, and anti-static devices reduce aerosol losses within the device
- Breath-actuated inhalers
- better in patients with incoordination of inhalation with device actuation
- not available for Salbutamol yet
- Spacer or valved holding chamber:
Nebulizers
- Jet (pneumatic), ultrasonic, mesh
- Factors affecting aerosol delivery by nebulizer
- Technical factors: Mechanism and manufacturer, Flow rate, Fill volume, Solution characteristics, Characteristics of driving gas, Designs to enhance output, Continuous versus intermittent delivery
- Patient factors: Breathing pattern, Nose versus mouth breathing, Artificial airway, Airway obstruction, Positive pressure level
Jet Nebulizers
- Mechanism:
- Air compressor or pressurized gas supply (compressed air or O2) acts as driving force for liquid atomizaiton
- Compressed gas is delivered as a jet through a small orifice, generating a region of negative pressure above medication reservoid
- Solution is first entrained, or pulled into the gas stream (Venturi effect), then sheared into a liquid film
- Film is unstable, and rapidly breaks into droplets due to surface tension forces
- Factors affecting drug delivery
- Respirable dose – function of mass output of nebulizer and size of droplets (mass median aerodynamic diameter) – should be 2 to 5 µm for bronchodilators
- Nebulization time – determined by volume of drug and flow of driving gas – lesser the better for patient compliance and need for clinical supervision
- Dead volume – medication trapped inside nebulizer – typically 1-2ml
- Driving gas – density of gas (improved with heliox)
- Breathing pattern – best with slow breathing pattern with a normal tidal volume and occasional deep breaths
- Nebulizer/compressor combination – more important in portable settings
- Continuous nebulizations
- HEART Nebulizer (Westmed) and the AirLife Misty Finity (Vyaire), Flo-Mist (Smiths Medical), and Hope (B&B Medical Technologies)
- Issue with need for frequent refilling ~
10-15mins- avoided by infusion pump and large volume nebulizer
- drug delivery over time similar to intermittent
- better with mesh than jet
- can be delivered via High Flow Nasal Cannula (not available in most centres)
Mesh Nebulizers
- eFlow (Pari), Aeroneb Solo and Aeroneb Go (Aerogen), MicroAIR/NE-U22 (OMRON), InnoSpire Go (Philips) and the I-neb (Respironics)
- use a mesh or plate with multiple apertures to produce a liquid aerosol
- Solution or suspension of medication is forced through the mesh to produce an aerosol, without need for an internal baffling system or compressed air source
- Able to generate aerosols with a high fine-particle fraction, which results in more efficient drug delivery compared to conventional nebulizers.
- Portable, battery-operated, minimal residual medication volume
- Precise dosing, minimal wastage
- May not give additional benefit for use with Salbutamol (cost-ineffecient)
- May be incorporated with Adaptive Aerosol delivery
- Blockage with particles may impair drug delivery
- More maintenance – disinfection and sterilization
Ultrasonic Nebulizers
- consist of a power unit and transducer, with or without an electric fan.
- Power unit converts electrical energy to high-frequency ultrasonic waves. A piezoelectric element in the transducer vibrates at the same frequency as the applied wave.
- Ultrasonic waves are transmitted to the surface of the solution to create an aerosol.
- The droplets produced by these devices have a slightly higher MMAD than droplets from a jet nebulizer.
- A fan is used to deliver the aerosol to the patient, or the aerosol is evacuated from the nebulization chamber by the inspiratory flow of the patient.
- Advantages: quieter medication delivery and shorter treatment time than the jet nebulizers.
- Problems include poor battery life and overheating.
- Drug inactivation by increased temperature a potential issue (but not with bronchodilators)
- With suspensions, the drug particles tend to settle and ultrasonic nebulizers are inefficient
ICU patient populations:
- Not on ventilation
- Mouthpieces and facemasks
- mouthpiece interface is generally preferred
- Bronchodilator response similar with either interface
- may be based on patient preference
- Face mask – Significant facial and eye deposition of aerosol, important to instruct the patient to inhale through the mouth to minimize nasopharyngeal deposition of medication.
- Patients with tracheostomy
- Nebulize either via a mask over the tracheostomy opening or using a T-piece
- T-piece preferred because more aerosol medication is directed into the tracheostomy tube
- Special T-shaped connectors for T-piece available for pMDI administation. (Not available for direct administration into tracheostomy)
- Mechanically ventilated patients
- using either a pMDI or a nebulizer
- Factors affecting aerosol delivery:
- Nebulizer: position in circuit, type of nebulizer and volume, treatment time, Duty cycle (I:E), Ventilator brand
- pMDI: Type of actuator, Timing of actuation
- Nebulizer and pMDI: ETT size, Humidifaction of inspired gas (deposition, Major factor), use of HME (can filter out aerosol)
- pMDI: special actuator needed, better with a chamber than in-line, should be synchronized with with inspiratory airflow to optimize drug delivery, more consistent dose than nebulizer. HME is removed, or bypassed using commercially available devices
- Nebulizer
- affected by large tidal volume, use of an end-inspiratory pause, and use of a slow inspiratory flow
- Optimized by placing the nebulizer 30 cm from ETT, rather than at the Y-piece, because the inspiratory ventilator tubing acts as a spacer.
- Breath-actuated delivery: Operates the nebulizer only during inspiration – 5x more efficient than continuous – available in some ventilators
- When the humidifier is bypassed the delivered dose increases by a factor of nearly four
- Jet Nebulizer:
- circuit contamination due to opening the ventilator tubing circuit (Valved T-piece devices available to avoid this)
- decreased ability of the patient to trigger the ventilator
- associated increases in tidal volume and airway pressure due to nebulizer flow
- A filter in the expiratory limb needed to protect the expiratory valve and flow/pressure monitors.
- Mesh Nebulizer:
- can be used effectively – placed between the ventilator outlet and the heated humidifier.
- Does not interfere with ventilator function (eg, no additional gas flow, no effect on triggering).
- Patients receiving noninvasive ventilation
- during NIV using devices adapted for inline administration
- the aerosol generator should be placed between the leak port and the interface.
Examiner Comments
2023A 10: 20% of candidates passed this question.
Good candidates answered this question in 4 parts; pharmaceutics, pharmacokinetics, pharmacodynamics and delivery devices. The most common reason for not passing this question was providing vague statements without explanation or the specific information required. Candidates were expected to comment on importance of particle size and drug delivery, patient compliance, the effect of a spacer and systemic effects of different routes of administration. Whilst some candidates did make reference to this it lacked the detail required to demonstrate that they understood the concepts.
11. Outline the Vaughan Williams classification of anti-arhythmic drugs with examples (30% of Marks). Describe the relevant pharmacology of adenosine (70% of Marks)
CICMWrecks Answer: Classification of anti-arrhythmic drugs (Vaughan-Williams)
| CLASS | ACTION | ELECTRO- PHYSIOLOGY | DRUGS | THERAPEUTIC INDICATIONS |
|---|---|---|---|---|
| I. Na – Channel Blockers | ||||
| Ia | Intermediate dissociation | ↓ phase 0 ↓↓ conduction v ↑ repolarisation ↑ APD | Quinidine Disopyramide Procainamide | Atrial and ventricular arrhythmias esp. post MI |
| Ib | Fast dissociation | ↔,↓ phase 0 ↓ conduction v ↓ repolarisation ↓ APD | lignocaine phenytoin tocainide, mexilitine | Ventricular arrhythmias post MI, digoxin induced arrhythmias |
| Ic | Slow dissociation | ↓↓↓↓ phase 0 ↓↓↓↓ conduction v ± repolarisation ↔ APD | flecainide ecainide, lorcainide | Refractory arrhythmias |
| II. β – Blockers | ||||
| ↓ SA firing – ↓ rate, conduction | propranalol* atenolol, esmolol | Rate control in AF, AT, Flutter and VT | ||
| III. K – Channel Blockers | ||||
| Delay phase 3 (repol) ↑ APD ↑ ERP | amiodarone bretylium, sotalol^ | AF / Flutter termination | ||
| IV. Ca – Channel Blockers | ||||
| ↓ AV conduction ↑ PR interval ↓ rate/conduction | verapamil diltiazem | SVT and AF or Flutter | ||
| OTHERS (Some call this Class V) | ||||
| Blocks Na+/K+ ATPase → ↑Ca2+, ↓K+, ↑Ach | ↑ contractility ↓ AV conduction | Digoxin | AF rate control Heart Failure | |
| Opens K+ channels via adenosine receptors | Hyperpolarizes myocardium ↓ AV conduction ↓ SA firing | Adenosine# Ibutilide | Terminate SVT or reveal underlying rhythm in tachycardias | |
| Stimulates Na+/K+ATPase | Membrane stabilization | Magnesium | VF / Torsades de pointes | |
| Notes | * Propranalol also has Na blocking activity ^ l-sotalol has beta blocking and class III activities; d-sotalol is a pure class III agent. Commercially available sotalol is a racemic (equal part) mixture. # Amiodarone – blocks Na, Ca, K channels, and exhibits beta blockade. | |||
Gladwin / Sakurai / JC 2020
CICMWrecks Answer: Pharmacology of Adenosine
Examiner Comments
2023A 11: 67% of candidates passed this question.
The high pass rate of this question was largely due to the ability of most candidates to reproduce the VW classification, with correct information regarding each class with examples. The pharmacology of adenosine was less consistently covered and whilst most kept to the usual pharmacology structure correct detail was often lacking to achieve high marks for this section.
12. Describe the physiology of cerebrospinal fluid (CSF).
CICMWrecks Answer
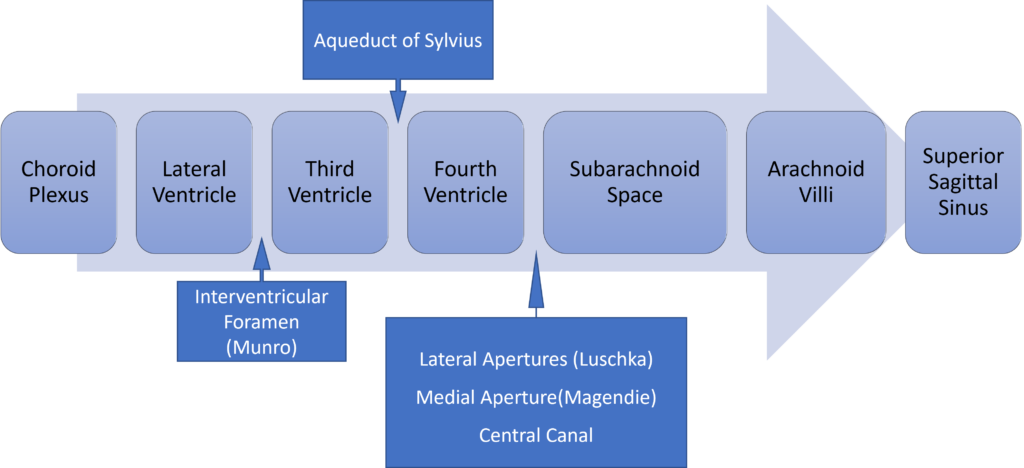
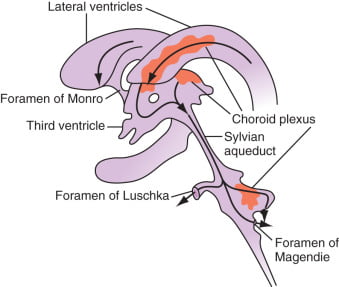
Formation / Production of CSF
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
Distribution / Circulation of CSF
Absorption of CSF
- Absorbed through the arachnoid villi into the cerebral venous sinuses
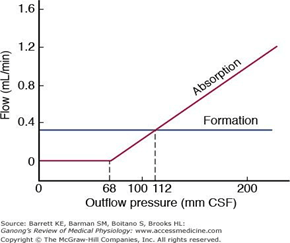
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
Composition of CSF
IDENTICAL to brain ECF, but differs in several manners from plasma
Compared with plasma:
- ↑ pCO2 (50 mmHg)
- ↓ pH (7.33)
- ↓ protein content
- 0.5% of plasma; 20 mg/dL
- poor acid-base buffering capacity
- ↓ content of glucose (by 60%) and cholesterol
- ↑ [Cl-] (by 7-14%) and ↑ [Mg2+] (by 40%)
- ↓ [K+] (by 40%), ↓ [Ca2+] (by 50%) and ↓ [Pi] (by 20-30%)
- ↑ creatinine (by 25%) but ↓ urea
- IDENTICAL osmolality (295), [Na+] (145), and [HCO3] (25)
Role / Functions of CSF
- Protective role (main function)
- Water bath effect
- Attributed to the low specific gravity of CSF (1.007)
- Causes brain to be buoyant
- ↓ its effective net weight from 1400 g to 50 g
- Mechanical cushion against acceleration/deceleration forces
- Buffer ↑ ICP by CSF translocation to extracranial subarachnoid space
- Abrupt ↑ ICP buffered by translocation of CSF within the vault to extracranial compartments
- Water bath effect
- Maintains constant ionic environment conducive to neuronal electrical activity CSF
- Supply role of nutrients (Eg simple sugars, amino acids) and O2 to brain
- Excretion of toxic substances, metabolic by-products, and CO2 from brain
- “Lymph-type” function → interstitial proteins in brain ECF return to circulation by
- CSF absorption across arachnoid villi
- Acid-base regulation → due to content, CSF allows for tight respiratory control
- Endocrine transport function → transports hormones to other brain regions
Gladwin / JC 2020
Examiner Comments
2023A 12: 88% of candidates passed this question.
This question has been repeated multiple times. Better answers demonstrated a structured approach to discussing the physiology of CSF dividing concepts into; Formation and Composition, Regulation and Circulation, and Functions of CSF with the appropriate level of detail.
13. Describe the structure and function of adult hemoglobin.
CICMWrecks Answer
STRUCTURE
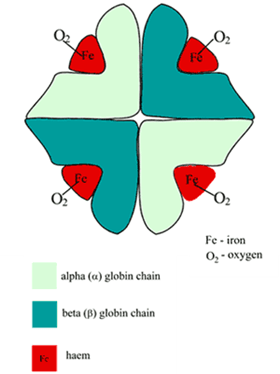
Structure
- Globular proteins which contain a haem moiety which binds O₂.
- Haem is an protoporphryin ring derivative with a central Fe2+ molecule that binds O₂
- Haemoglobin: MW ≈ 65000 daltons
- Hb contains 65-70 % of total body iron
- Globular molecule made up of four subunits, each containing a haem moiety conjugated to a polypeptide.
- Polypeptides collectively = globin → two pairs 2α + 2β → 4 haem moieties (Tetramer)
- Can bind a total of 4 O2 and also exhibits cooperative affinity (each subsequent O₂ binding takes less energy → sigmoid shaped OHDC
Location
- Hb is located in large concentrations (≈15g/L) in red blood cells that circulate throughout the blood stream.
Synthesis
- Haeme is synthesised in mitochondria and cytosol of immature RBCs, Globin is synthesized by ribosomes in cytosol.
- Production continues till RBCs lose their RNA after entering vasculature
Degradation
- RBCs at the end of their life cycle get phagocytosed by macrophages in liver or spleen, or get hemolysed in circulation.
- Haemoglobin is then broken up
- Haeme gets degraded into bilirubin
- iron gets recycled
FUNCTION
- O2 carrier:
- O2 loading exhibits positive cooperativity:
- α1ß1 & α2ß2 contacts stabilise Hb molecule as O2 reacts with it
- reaction of O2 with each subunit occurs sequentially with each facilitating the next
- ∴ ↑ing affinity as O2 loads ⇒ sigmoid OHDC
- O2 loading exhibits positive cooperativity:
- O2 unloading – vice versa:
- ß chains pulled apart
- 2,3-DPG enters molecule ⇒ ↓affinity of Hb for O2
- Protein buffer in RBC:
- Haemoglobin exists as a weak acid (HHb) as well as its potassium salt (KHb)
- In acidosis:
- Additional H+ ions are bound to Hb molecules
- HCO3– diffuses down its concentration gradient into plasma
- Electroneutrality is maintained through the inwards movement of Cl–.
- Dissolved CO2 will also form carbamino compounds by binding to the terminal amino groups
- Ligand Binding:
- Competitive inhibitors such as carbon monoxide (CO) and allosteric ligands such as carbon dioxide (CO2) and nitric oxide (NO).
- The carbon dioxide is bound to amino groups of the globin proteins to form carbaminohemoglobin; this mechanism is thought to account for about 10% of carbon dioxide transport in mammals.
- Nitric oxide can also be transported by haemoglobin; it is bound to specific thiol groups in the globin protein to form an S-nitrosothiol, which dissociates into free nitric oxide and thiol again, as the hemoglobin releases oxygen from its heme site. This nitric oxide transport to peripheral tissues is hypothesized to assist oxygen transport in tissues, by releasing vasodilatory nitric oxide to tissues in which oxygen levels are low.
Examiner Comments
2023A 13: 25% of candidates passed this question.
Good answers provided detail about the specific composition of haemoglobin and related the pertinent features of the molecule to its functions in the carriage of oxygen, carbon dioxide and its role in acid-base balance, including the appropriate mechanistic description. Many candidates provided information on the production and breakdown of haemoglobin which was not required. Many candidates provided an unnecessary amount of detail and diagrams of the oxygen haemoglobin dissociation curve which did not score additional marks.
14. Describe the pharmacology of oxygen.
Examiner Comments
2023A 14: 51% of candidates passed this question.
Candidates who approached this question in a structured pharmacology approach were able to score well, outlining the pharmaceutics, pharmacodynamics, and pharmacokinetics. Many candidates limited their pharmacodynamic discussion to adverse effects of oxygen only or listed effects without demonstrating understanding of their mechanism or consequences which limited their ability to score marks.
15. Describe the adult coronary circulation (50% of Marks) and its regulation (50% of Marks).
CICMWrecks Answer
Arteries
- LCA to
- LCx to
- OM1
- OM2
- LAD has branches
- Diagonal 1 or 2
- LCx to
- RCA
- Right Ventricular
- Acute Marginal
- Posterior descending
Veins
- Great, small, middle
- Drain into the thebesian veins → ventricles directly
- Empty into the coronary sinus on the posterior wall or the RV
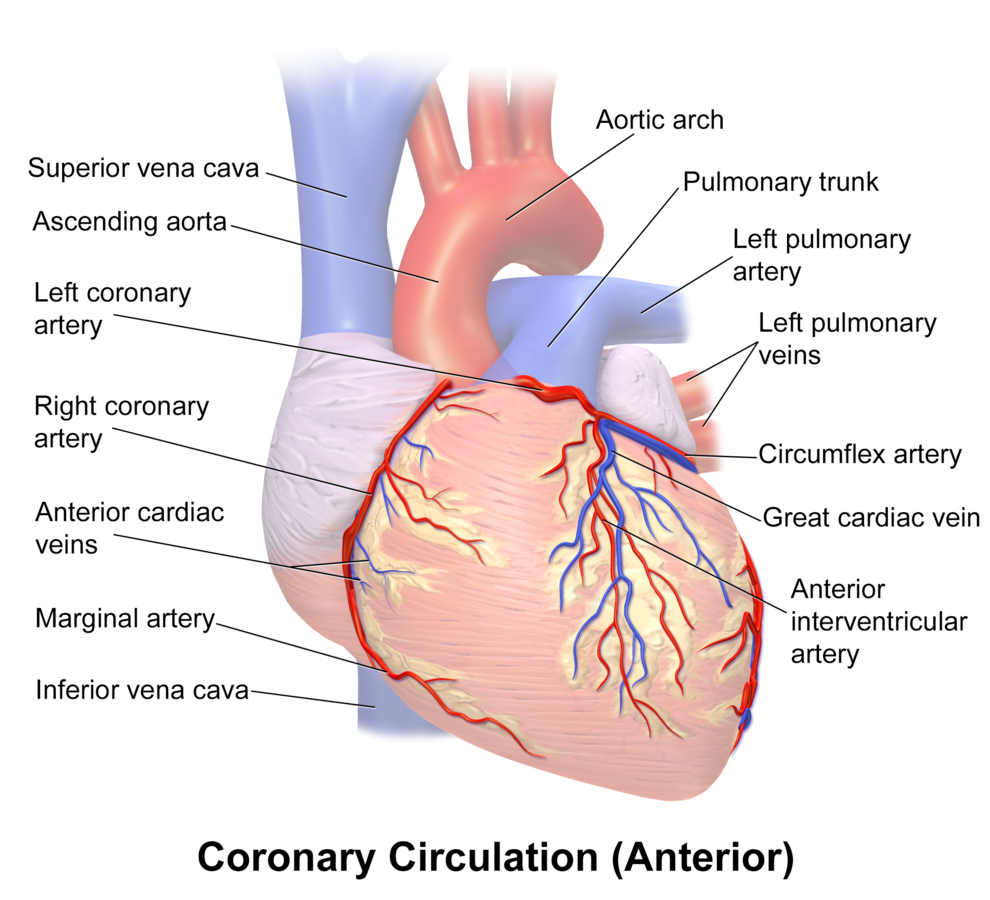
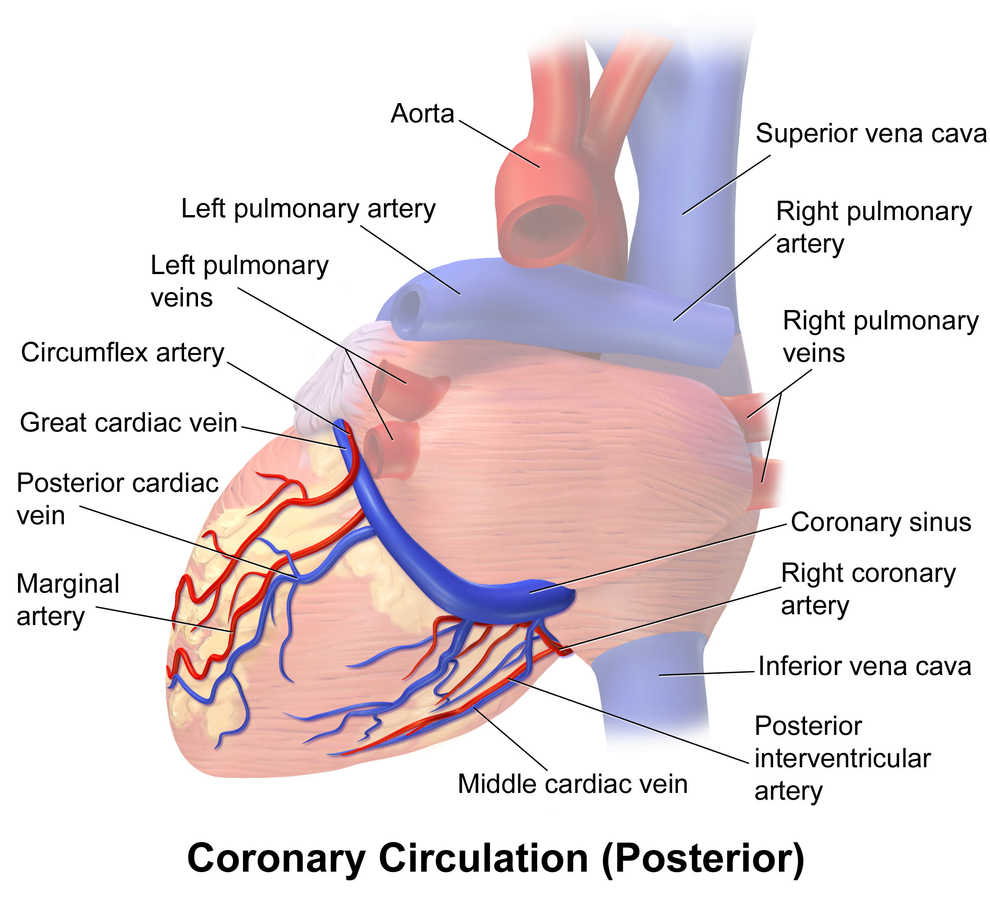
Regional Supply
| Artery | Anatomical region | ECG abnormality on occlusion |
|---|---|---|
| RCA | Down Anterior aspect of the heart, right of the pulmonary trunk Supplies RA SAN and AVN Posterior IVS | RCA occlusion causes → inferior: 2,3 aVF → true posterior: tall R in V1 |
| LCA | Left coronary artery ostia adjacent to left leaflet → Left Main → LCx and LAD Supplies LA, LV IVS AV Bundles | LCA occlusion → Lateral: 1, aVL, V1-V6 |
| LCx | LA and superior LV | anterolateral leads (1, aVL, V5 and V6) |
| LAD | RV, LV Anterior 2/3rds Interventricular Septum | Anteroseptal (V1 and V2) Anteroapical (V3 and V4) with some RCA supply. |
| Left Marginal | The continuation of the LCA after LAD branch Supplies LV laterally | Isolated Abnormalities rare V5, V6 |
| Right Marginal | The anterior branch of the RCA along the inferior border Supplies right ventricle and apex | Isolated Abnormalities rare RCA occlusion causes 2,3 aVF |
Coronary Blood Flow
- 80 mL/min/100 g
- or 200-250 mL/min
- 5% of CO at rest
- Can increase by 3-4 times (up to 400mL/min/100g)
High Extraction Ratio
High at rest (55-65%) body average of 25%
- Extraction ratio can only rise by factor of < 2 to 90%
- AV Δ O₂ = 11 mL/dL
- Coronary venous O₂ content = 5 mL/dL
- Coronary sinus SpO₂= 20mmHg
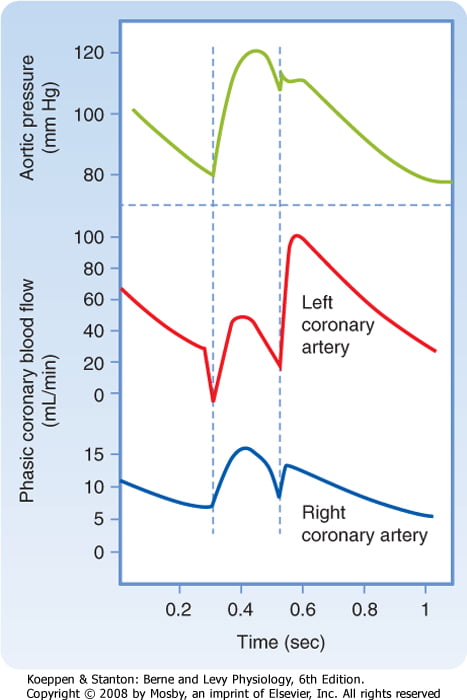
Coronary perfusion pressure (CPP)
- ADP = Ao Diastolic Prssure
- It is different throughout the cycle and b/w ventricles
Phasic Supply as per diagram.
Determinants of Coro BF
- Physical Factors
- Extravascular compression (CPP factors)
- Neural and Neurohumoral Factors
- ↑ SNS tone →
- α receptor mediated vasoconstriction
- β receptor mediated vasodialtion
- ↑ force and rate of contractions → ↑ vasodialtor metabolite release
- Overall effect is dilation
- ↑ PSNS tone → KACh stimulation → mild ↓ Coronary vascular resistance
- ↑ SNS tone →
- Metabolic Factors (main)
- Vasodilatory
- ↑ Adenosine, H, K, CO2, Lactate
- NO → GTP
- ↑ O2 demand → ↓ ATP → ↑KATP channel activation → hyperpolarisation → vasodilation
- Vasodilatory
- Myogenic autoregulation (keep CPP 60-180 mmHg)
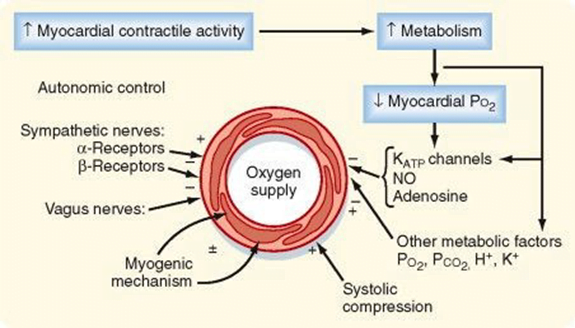
Gladwin 2016
Examiner Comments
2023A 15: 66% of candidates passed this question.
For this question a good answer should have encompassed a description of the following components; the arterial supply (epicardial, endocardial supplies), venous supply (major and minor venous drainage), the phasic ventricular blood supply, a description of and the relevance of the high oxygen extraction ratio, an emphasis on metabolic autoregulation whilst including any other mechanisms of physiological regulation.
Most candidates provided reasonable detail surrounding the arterial-circulation and the area of the heart these vessels supply. Less well described were the perforating branches, the capillary network and the venous drainage, these components were often incorrectly described or left out of the candidate answers. Most candidates correctly identified the phasic flow and the difference in flow that occurs between the right and left ventricle. Those candidates that used diagrams to explain this were rewarded when these diagrams were labelled and accurate with respect to their systolic and diastolic distinction. The control of regulation was less well answered with generic statements not specifically identifying the local metabolic controls importance over any other mechanism. Those answers that scored well coupled the metabolic control to the flow dependence highlighting the high oxygen extraction within the coronary circulation.
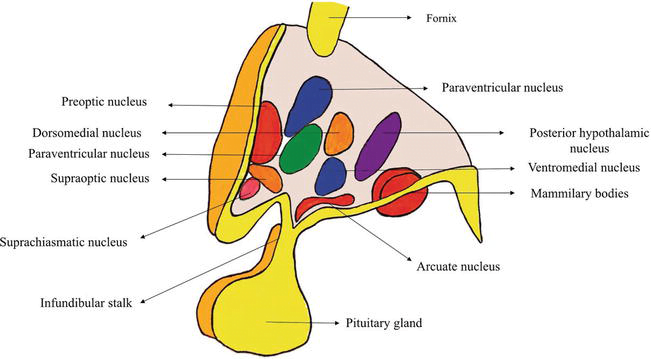
16. Outline the role of the hypothalamus.
CICMWrecks Answer
Hypothalamus
- Hypothalamus
- organ that regulates large number of autonomic/ endocrine processes
- acts as control centre
- Cells
- magnocellular neurosecretory cells in the paraventricular nucleus and the supraoptic nucleus of the hypothalamus produce neurohypophysial hormones, oxytocin and vasopressin. These hormones are released into the blood in the posterior pituitary.
- Much smaller parvocellular neurosecretory cells, neurons of the paraventricular nucleus, release corticotropin-releasing hormone and other hormones into the hypophyseal portal system, where these hormones diffuse to the anterior pituitary.
- Location
- Part of the fore brain. Considered part of the diencephalon
- Located below the thalamus and forms the floor and lower part of the lateral walls of the third ventricle
- Anteriorly extends up to the optic chiasma
- Posteriorly continuous with the tegmentum of midbrain
- Structure
- formed by gray matter conglomeration of neurons that organize in nuclei and also by white-matter substance formed by myelinated nervous fibers.
- Nuclei send and receive fibers to other parts of brain
- divided by the anterior horns of the fornix in a lateral, medial, and periventricular (median) region and by a coronal plane passing through the infundibulum in an anterior and posterior region.
- Regions
- Anterior / Prechiasmatic / Supraoptic
- located above the optic chiasm
- contains: supraoptic, preoptic and medial preoptic, the suprachiasmatic and the anterior hypothalamic nucleus, alongside with the paraventricular nucleus
- Infundibular / Tuberal
- located above tuber cinereum (between anterior and posterior)
- composed of two parts, anterior and lateral
- contains: dorsomedial, ventromedial, paraventricular, supraoptic, and arcuate nucleui
- Posterior / Mamillary
- formed by medial and lateral area
- medial area contains mamillary nuclei and posterior nucleus
- lateral area contains lateral nucleus and tuberomammillary nucleus
- Anterior / Prechiasmatic / Supraoptic
- Connections: Nervous, Endocrine, CSF, Direct to pituitary
- Blood supply
- mainly hypophyseal artery (branch of anterior cerebral artery)
- Detailed arterial supply:
- Anterior: branches of ACA and ACOM
- Tuberal: branches of PCOM and superior hypophyseal artery
- Posterior: branches of PCA
- Infundibulum: superior hypophyseal arteries from the ophthalmic branch of ICA (C6)
- drains into hypothalamohypophyseal system of veins to anterior pituitary → hypophyseal vein
Function of Hypothalamus
Autonomic nervous system activity
- CVS:
- Ant hypothalamic stimulation → ↓BP + ↓HR
- Post hypothalamic stimulation → ↑BP + ↑HR
- Thermoregulatory: integrates thermoreceptor input + controls activity of heat loss + heat gain mechanisms
- Satiety: hunger modulated by glucose, CCK, glucagon, and leptin
- Water balance:
- Osmoreceptors: control ADH release from posterior pituitary
- ATII: stimulates thirst + ADH release via subfornical organ + organum vasculosum
- Circadian rhythm
- Behaviour
- Sexual function
Endocrine/ hormonal activity
- Direct neural control of posterior pituitary gland
- Pituitary neurosecretory neurons
- Magnocellular neurons:
- consists of SON + PVN
- synthesise and secrete ADH and oxytocin
- Parvocellular neurons:
- form tuberoinfundibular tract → secrete hypophysiotropic hormones
- release controlled by Nad, dopamine, 5-HT
- Magnocellular neurons:
- Hypothalamic hormones → action in pituitary
- Anterior pituitary by hormone secretion into long portal vein
- GnRH → stimulates FSH + LH release
- CRH → stimulates ACTH release
- GHRH → stimulates GH release
- TRH → stimulates TSH release
- Somatostatin (GH inhibiting hormone) → inhibits GH, TSH, ACTH, and PRL release
- PRL releasing hormone (PRH) → stimulates PRL release
- Dopamine → inhibits PRL release
- Posterior pituitary by neuronal innervation
- ACh → stimulates release of ADH + oxytoxin
- NAd → inhibits ADH + oxytocin secretion
- Anterior pituitary by hormone secretion into long portal vein
(See next tab for detailed table of nuclei with functions, and communications of hypothalamus)
Sources: https://www.intechopen.com/chapters/63258
https://human-memory.net/hypothalamus/#Structure
JC / Kerr 2022
Additional: Nuclei and functions Table, Communications
Nuclei and functions
| Region | Area | Nucleus | Function |
|---|---|---|---|
| Anterior (Supraoptic / prechiasmatic) | Preoptic | Preoptic nucleus | – Thermoregulation |
| Medial | Medial preoptic nucleus | – Regulates the release of gonadotropic hormones from the adenohypophysis – Contains the sexually dimorphic nucleus, which releases GnRH, differential development between sexes is based upon in utero testosterone levels – Thermoregulation | |
| Supraoptioc nucleus | – Vasopressin release – Oxytocin release | ||
| Paraventricular nucleus | – thyrotropin-releasing hormone release – corticotropin-releasing hormone release – oxytocin release – vasopressin release – somatostatin round | ||
| Anterior hypothalamic nucleus | – thermoregulation – panting – sweating – thyrotropin inhibition | ||
| Suprachiasmatic nucleus | – Circadian rhythms | ||
| Lateral | Lateral nucleus | primary source of orexin neurons that project throughout the brain and spinal cord – Mainly promotion of feeding behaviour and arousal | |
| Infundibular (Middle / tuberal) | Medial | Dorsomedial hypothalamic nuclei | – blood pressure – heart rate – GI stimulation |
| Ventromedial nucleus | – satiety – neuroendocrine control | ||
| Arcuate nucleus | – Growth hormone-releasing hormone (GHRH) – feeding – Dopamine-mediated prolactin inhibition | ||
| Lateral | Lateral nucleus | primary source of orexin neurons that project throughout the brain and spinal cord – Mainly promotion of feeding behaviour and arousal | |
| Lateral tuberal nuclei | – regulation of feeding. Its absence or destruction has been implicated in extremes of starvation such as anorexia nervosa. | ||
| Posterior (mamillary) | Medial | Mamillary nuclei (part of mamillary bodies) | – memory |
| Posterior nucleus | – Increase blood pressure – pupillary dilation – shivering – vasopressin release | ||
| Lateral | Lateral nucleus | primary source of orexin neurons that project throughout the brain and spinal cord – Mainly promotion of feeding behaviour and arousal | |
| Tuberomammillary nucleus | – arousal (wakefulness and attention) – feeding and energy balance – learning – memory – sleep |
Communications of Hypothalamus
- Nervous connections: divided into afferent and efferent fibers
- Afferent fibres: carry somatic and visceral sensations as well as from special senses
- Somatic and visceral afferents via lemniscal afferent fibers and nucleus of tractus solitarius, that reach the hypothalamus via reticular formation
- Visual afferents from the optic chiasma reach the suprachiasmatic nucleus
- Olfactory afferents are received through medial forebrain bundle
- Auditory afferents though not identified completely but are influenced by the hypothalamus
- Hippocampo-hypothalamic afferents reach via fornix to mamillary bodies
- Tegmental fibers from midbrain
- Thalamo-hypothalamic fibers from the midline and dorsomedial nuclei of the thalamus
- Amygdalo-hypothalamic fibers from the amygdaloid complex reach the hypothalamus via stria terminalis
- Efferent fibers: complex and numerous
- To brain stem and spinal cord: The hypothalamic nuclei send efferent fibers to nuclei present in the brainstem and spinal cord. In this way, they control the autonomic nervous system.
- Mammillothalamic Tract: This tract consists of fibers arising in the mamillary body and terminating in the anterior nucleus of thalamus.
- Mammillotegmental Tract: These fibers terminate in the reticular formation, present in the tegmentum of the midbrain.
- Limbic System: The nuclei in the hypothalamus also send efferent fibers to the various nuclei of the limbic system.
- Afferent fibres: carry somatic and visceral sensations as well as from special senses
- Endocrine:
- regulating factors or hormones released by hypothalamus → reach pituitary via the hypophyseal portal system of veins → cells of pituitary release hormones
- Direct:
- Cell bodies present in hypothalamus → axonal terminals in posterior pituitary gland
- Bodies synthesise oxytocin and vasopressin, stored in axonal terminals in posterior pituitary and released on demand
- CSF:
- clear metabolic waste
- distribute glucose, amino acids, lipids, and neurotransmitters
Examiner Comments
2023A 16: 29% of candidates passed this question.
This question required candidates to integrate parts of the syllabus that are often not presented together in the reference texts. Many candidates provided only an incomplete list of the roles of the hypothalamus as their answer which did not score enough marks to pass. Additionally, presenting information succinctly and in a format that demonstrated an understanding of priority of function with respect to the CICM syllabus, was critical for time management and ensuring that more the important/major roles were presented in more detail. This question required candidates to cover roles including autonomic control, thermoregulation, hormonal regulation, body rhythms and regulation of drives with feedback control mechanisms where appropriate. Those who scored well demonstrated a comprehensive breadth of knowledge of these areas as well as giving the pertinent points around areas of positive and negative feedback and the sensor areas and effector responses required to answer an outline question.
17. Explain the following components of an electrocardiogram (ECG) machine; electrodes, leads, amplifiers and filters. (70% of Marks) Outline methods employed to reduce artefact. (30% of Marks)
CICMWrecks Answer
ECG
- 12 electrodes on the body surface to detect small potentials (0.5-2 mV) generated by the heart.
- Electrodes made of Ag/AgCl
- ECG signals detected by these electrodes
- Sent to electronic device that filters out noise and boosts the signal
- Displayed on oscilloscope
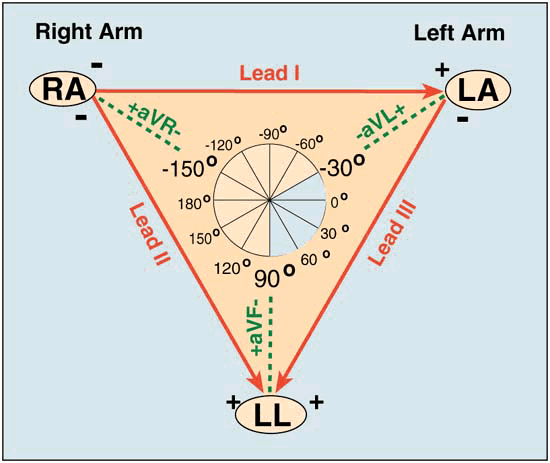
Signal Interpretation from oscilloscope readings via Enthoven’s triangle
- For orientation of the Bipolar and unipolar limb leads synthesised from the 12-lead ECG.
- Bipolar limb leads
- Lead I: RA (-) to LA (+) at 0°
- Lead II: RA (-) to LL (+) at 60°
- Lead III: LA (-) to LL (+) at 120°
- Unipolar limb leads
- aVR: RA (+) at -150°
- aVL: LA (+) at -30°
- aVF: LL (+) at 90°
- Positive deflection in a lead indicates depolarisation wave moving towards lead’s positive terminal
Calibration
- Vertical calibration
- 1 mV signal → vertical deflection of 10 mm (2x large square or 10x small squares)
- 0.1 mV per mm (or small square)
- Horizontal calibration
- paper speed is 25 mm/s → 0.04 s/mm (or small square)
Errors:
Noise and interference caused by:
- Electrical interference by any device using AC current (esp high-frequency diathermy)
- → Minimise by ECG filters, shielding of leads/cables, differential amplifers, Etc.
- Movement or shivering
- → minimised by placement over bony prominences and use of ECG filters
- High skin impedance
- → minimised by degreasing skin with EtOH and use of conducting gel with electrodes
- Incorrect electrode placement relative to heart
It looks like this Examiner reads Hemmings.
Gladwin 2016
Examiner Comments
2023A 17: 39% of candidates passed this question.
Good answers described electrode components, intensity of signals measured (requirement for and levels of amplification), lead position and differences between unipolar and bipolar leads. Rationale and mechanisms of filtering were less well described. Description of highand low-pass filtering along with detail on monitoring and diagnostic modes scored marks. Patient-related, electrical and environmental methods of reducing artefact attracted marks.
18. Explain the respiratory changes that occur at term in pregnancy.
CICMWrecks Answer
Normal Spirometry trace
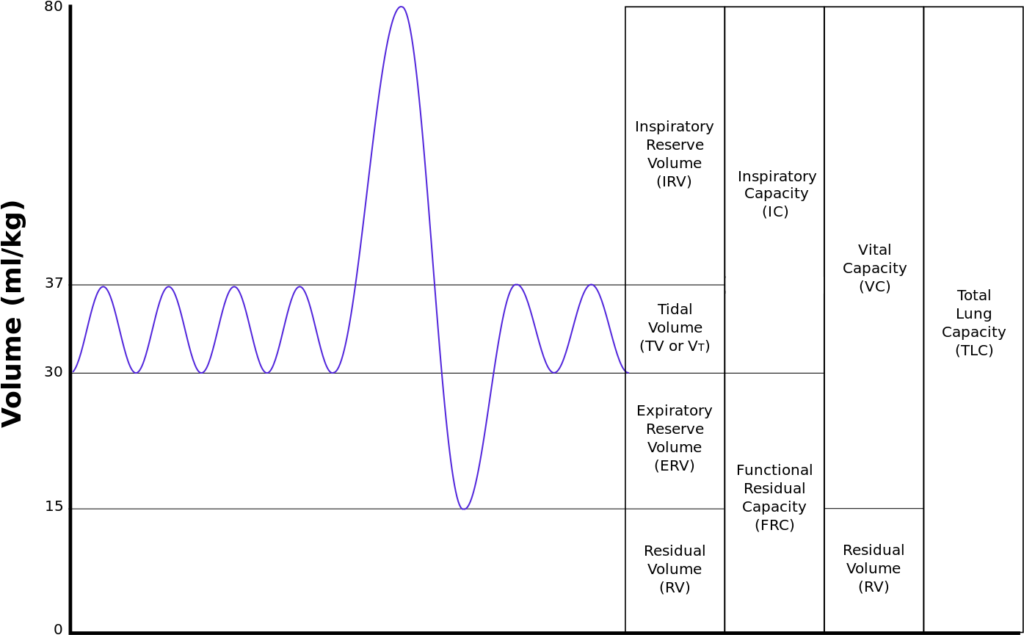
| Inspiratory reserve volume | 45ml/kg | 3 L |
| Tidal volume | 7ml/kg | 500 ml |
| Expiratory reserve volume | 15ml/kg | 1.1 L |
| Residual volume | 15ml/kg | 1.1 L |
| Total Lung Capacity | 82ml/kg | 5.7 L |
| Vital Capacity | 67ml/kg | 4.6 L |
| Functional Residual Capacity | 30ml/kg | 2.2 L |
In Pregnancy
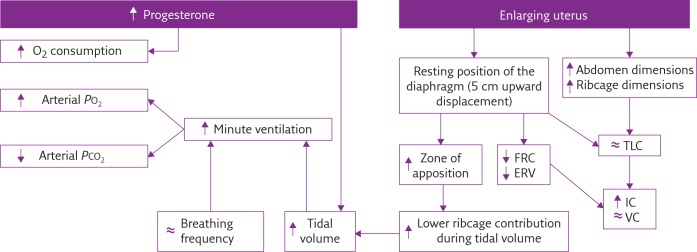
Anatomical
- Diaphragm ascends ~4cm due to foetus
- AP and lateral diameters of thorax increase 2~3cm due to relaxin effect on chest
wall - Thoracic circumference increases 5~7cm (AP and lateral diameters increased 2~3cm)
- Airway dilatation → decreased resistance (35%) and increased deadspace (45%)
- Increased cardiac output, intravascular volume, and decreased oncotic pressure
- Increased plasma volume → increased pulmonary blood volume → decreased compliance
- Venous engorgement → Upper airway oedema
Mechanical
- Lung volume changes occur from 20 weeks
- TLV decreases 5% from baseline
- FRC decreased 20% due to both ERV and RV
- Significant postural change in FRC of 70%
- Progesterone → increased sensitivity to CO2 → Minute ventilation increases 50%
- TV increases (25%)
- RR increases
- Results in hypocarbia and compensated respiratory alkalosis
- pCO2 ~ 26-32mmHg
- HCO3 ~ 18-20mmol/l
- Further increase in MV during contractions
- Inspiratory capacity increased by 10%
- Closing capacity unchanged
- Increased O2 consumption during labour (60%)
After birth
- FRC and RV return to normal within 48 hours
- TV returns to normal within 5 days
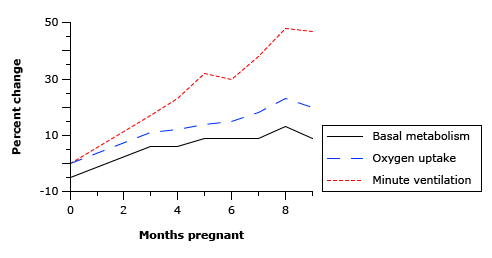
Sakurai 2016
Examiner Comments
2023A 18: 43% of candidates passed this question.
Good answers addressed the following in an ordered way: anatomic changes (including to airway, thoracic dimensions, dead space, airways resistance); what changes there are to respiratory volumes, capacities, and compliance; what happens to oxygen consumption and oxygen tension; and the acid-base changes that occur. Good answers didn’t just list these changes, but also provided an explanation for them. Few candidates mentioned the changes in oxygen tension and oxygen consumption, and why these occurred.
19. Describe factors that prolong the action of neuromuscular blocking agents (60% of Marks) Outline the pharmacokinetics and pharmacodynamics of vecuronium (40% of Marks).
CICMWrecks Answer
FACTORS
The factors which alter the response (number of receptors occupied) may be separated into: pharmacodynamic factors, pharmacokinetic factors, pharmaceutical and drug interaction factors
Pharmacodynamic Factors
- decreased pH increases the affinity of non depolarising NMB
- electrolyte disturbances
- Ca disturbances affect the release of ACh from the presynaptic terminal (triggered by Ca)
- hypercalcaemia results in shortened blockade
- hypocalcaemia potentiates blockage
- magnesium
- stabilise membranes and decrease release and sensitivity to ACh
- potassium
- hypokalaemia leads to membrane hyperpolarisation (decreased drug effect)
- hyperkalaemia has the reverse effect
- pathology
- myasthenia gravis patients have defective nACh receptors
- eaton-lambert syndrome patients have decreased ACh release
- burns – leads to receptor mediated resistance to non depolarising NMBD
Pharmacokinetic factors
(affect the amount of drug reaching the receptor)
- absorption
- dose given (higher dose – more receptors occupied)
- distribution
- lipid soluble drugs such as vecuronium may accumulate in lipid rich tissue (esp infusion)
- women have higher lipid content which may lead to reduced effect site conc
- metabolism
- hepatic failure may lead to decreased metabolism of a drug – pancuronium
- excretion
- renally excreted drugs, especially minimally metabolised vecuronium, rocuronium
Pharmaceutical / Drug Interactions
- Increase effect
- aminoglycosides – decreased prejunctional ACh release
- volatile anaesthetics – mechanism not well understood
- frusemide – potassium effect, may interfere with cAMP and ACh release
- decrease effect
- anticonvulsants – may develop resistance to pancuronium, vecuronium
JC 2019
Examiner Comments
2023A 19: 20% of candidates passed this question.
The first part of the question required a description of the factors prolonging neuromuscular blockade which would include a description of the reasoning behind the prolongation. Many candidates missed the important factors and most did not include any explanation. Some candidates confused the causes of “delayed onset” and “prolonged action”. For vecuronium mechanism of action all the details were expected, superficial explanations did not score well. The following key words were expected to be included in the mechanism description; competitive binding/ Nicotinic ACH (N2)/alpha subunit/post synaptic. In pharmacokinetics, details on hepatic metabolism to active metabolites, renal and biliary excretion along with accurate values of the volume of distribution, protein binding and half-life were expected to score full marks. These facts were frequently lacking in candidate answers.
20. Compare and contrast the relevant pharmacology of intravenous adrenaline and vasopressin.
Examiner Comments
2023A 20: 20% of candidates passed this question.
The major emphasis of this question and opportunity to score marks reside in “comparing the two drugs” in various aspects – pharmaceutics, indication, mechanism of action, pharmacodynamics, and pharmacokinetics. Although most of the candidates were able to list pharmaceutics, indications, kinetics and dynamics of both the drugs in reasonably structured and tabulated format, many failed to highlight the important commonalities and differences between the two. In mechanism of action, details of the receptor, their location and second messenger system were expected. In pharmacodynamics, similarities and differences in cardiovascular, respiratory, haematological, renal and metabolic effects were needed. There are additional neurological effects and genito-urinary (tocolysis and sphincter tone) of adrenaline which were rarely mentioned. There were frequent significant omissions or incorrect details in the pharmacokinetics sections of both drugs.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Oxygen measurement, sodium channel physiology View of larynx during intubation Work of breathing and components, diagram |
| G. CVS | Compare pulmonary and bronchial circulations Cardiovascular physiology, graph, factors that affect venous return to heart CVS in elderly Beta adrenergic receptors and signal transduction, adrenergic and steroid pharmacology Cardiovascular physiology, graph, factors that affect venous return to heart |
| H. Renal | Name and describe the components of nephron |
| I. Body Fluids and Electrolytes | Body water distribution, measurement of compartments |
| J. Acid Base | |
| K. Neuro | Opioid receptors, pharmacology NMDA receptor |
| L. Musculoskeletal | |
| M. ANS | Anatomy of sympathetic nervous system |
| N. Liver | Hepatic lobule, label structures |
| O. GIT | Packed Red blood cells preparation |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | Factors that determine ability of IV Antibacterial to treat localized infection |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments