1. Outline the effects of critical illness on drug pharmacokinetics, including examples.
CICMWrecks Answer
Absorption
- IV
- Unchanged
- Oral
- If GI inflammation → increased GI absorption (aminoglycosides)
- Concomittant drug administration may inhibit intestinal transporters (amiodarone inhibits p-glycoprotein) or hepatic enzymes (valproate inhibits P450 enzymes) à Variable effect on bioavailability
- If hepatic failure (i.e. ischaemic hepatopathy) → Decreased hepatic first pass effect → Increased oral bioavailability (aspirin)
- Decreased splanchnic bloodflow (due to vasopressor agents, shock) → Decreased drug absorption → Decreased bioavailability
- IM/Subcut
- Decreased peripheral blood flow due to shock, vasopressors → Delayed absorption (IM suxamethonium will delay onset)
Distribution
- Protein binding → albumin decreases as an acute phase reactant → Increased free drug fraction of protein bound drugs (propofol)
- pKa
- Acids are ionized when pH >pKa, bases ionized when pH < pKa → acid base disturbances will alter drug ionization → permeability through cell membranes (Na channel blockers are less effective in acid environments)
- Volume of distribution
- Renal failure, hepatic failure, heart failure + overzealous fluid resuscitation → increased total body water → Increased volume of distribution of drugs distributed to extracellular compartment
Metabolism
- Hepatic phase I (zone III) susceptible to ischaemia
- Decreased phase I metabolism in shock (fentanyl metabolized by CYP450 3A4)
- More global reduction of hepatic function in global hepatic failure
- Butylcholinesterase produced by liver → decreased levels in hepatic failure → decreased metabolism (suxamethonium → more drug delivery to NMJ)
Excretion
- Renal
- Shock → decreased renal perfusion pressure (although renal blood flow may actually increase in distributive shock) → decreased glomerular filtration of drugs → Decreased clearance
- Decreased plasma protein will promote glomerular filtration and filtration of free drug
- Acute tubular necrosis → decreased tubular secretion of drugs → Decreased clearance
- Biliary
- Hepatic failure → decreased biliary secretion
Mooney 2016
Examiner Comments
2022A 01: 47% of candidates passed this question.
The effects of critical illness on the physiological factors that influence drug pharmacokinetics was used to analyse the candidates understanding of this core pharmacological principle. Better responses were able to identify the key elements perturbed by critical illness as well as outlining outline the potential cause and effect on the specific PK aspect being discussed. Stronger answers also included specific drug examples. The failure to utilise a structure (absorption, distribution, metabolism, and elimination) with very superficial detail and limited examples limited marks and was common in poorly scoring answers.
2. Explain the mechanisms of transport of substances across cell membranes including appropriate examples (75% marks). Outline the structure and function of the Na+/K+-ATPase pump (25% marks).
CICMWrecks Answer
Transmembrane Transport Mechanisms
| Mechanism | Energy Expenditure | Electrochemical gradient | Example | ||||
|---|---|---|---|---|---|---|---|
| Diffusion | Passive diffusion | Molecule crosses a membrane to which it is permeable by diffusion | No | With | Carbon dioxide across vascular endothelium | ||
| Facilitated diffusion | Molecule crosses a membrane via a channel, without energy expenditure | No | With | Potassium across excitable cell membranes, via rectifier channels | |||
| Active transport | Primary | Primary active transport | Molecule crosses a membrane via a channel, with energy expenditure (ATP) | Yes | Against | 3Na+ /2K+ ATPase | |
| Secondary | Symport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Co-transported) | Not directly | May be Passive or Active based on gradient of 2nd | sodium and an amino acid | ||
| Antiport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Anti-transported) | Not directly | May be Passive or Active based on gradient of 2nd | Na+ / H+ antiporter in proximal convoluted tubule | |||
| Ligand-gated ion channel | Binding of a ligand causes conformational change in membrane channel, allowing movement of ion across membrane | No | With | Nicotinic acetylcholine receptor. ACh as ligand, Na+ and K + as ions | |||
| Exocytosis | Substance packed in vesicle, moves to cell membrane, two membranes merge -> substance exits cell | Yes | Usually against | Exocytosis of ACh by presynaptic neuron | |||
| Endocytosis | Cell membrane extends to engulf a substance or object, which is then contained in the cell within a vesicle | Yes | Can be with, against or refer to a macroscopic object | Phagocytosis of bacteria by macrophage | |||
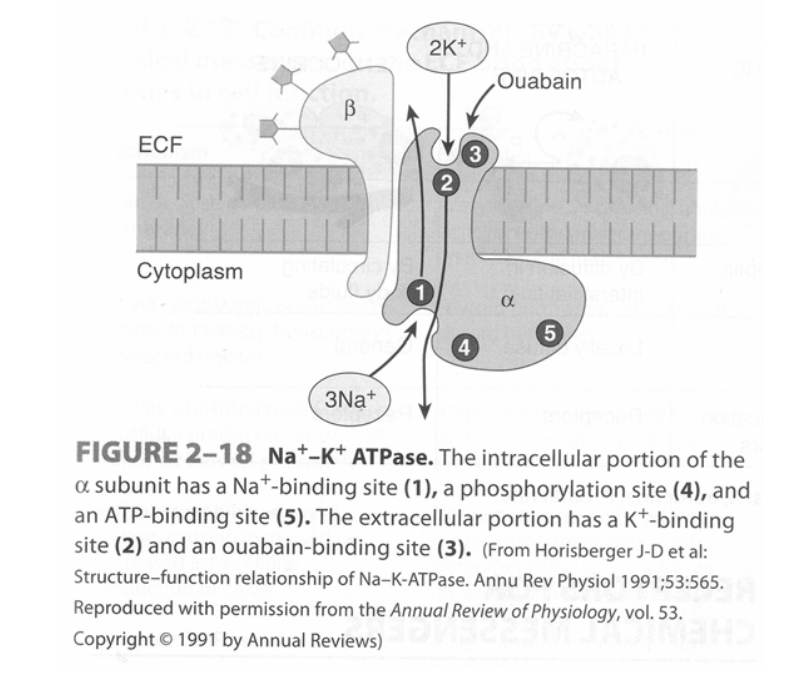
Na+/K+ – ATPase pump
- Found in virtually all cells of the body – It is a heterodimer integral membrane protein that spans the entire membrane:
- α-subunit (larger subunit) – Binds Na+, ATP and PO43- intracellularly, and K+ and ouabain/glycosides extracellularly
- β-subunit (smaller subunit) – A glycoprotein
- Functions as an “electrogenic pump” as it extrudes 3 Na+ from the cell and takes in 2 K+ per ATP hydrolysed
- Process of pump function:
- Na+ and ATP bind to α-subunit intracellularly
- ATP is hydrolysed to ADP and the phosphate is transferred to an Asp-phosphorylation site
- Conformational change of the protein causes Na+ to be extruded into ECF
- K+ binds to the α-subunit extracellularly
- Dephosphorylation of the Asp-phosphorylation site causes the protein to return to its resting conformation, which then transports K+ intracellularly
- Pump function is inhibited by ouabain and digitalis glycosides
JC / Mooney / Bianca 2019
Examiner Comments
2022A 02: 61% of candidates passed this question.
This question examined core cellular physiology knowledge. This knowledge is crucial as it underpins much of the electrochemical responses within the syllabus. Mechanisms of diffusion and the role of individual pathways were well presented in many responses. Answers that scored well generally classified the mechanisms of transport into active and passive processes which ensured an appropriate breadth of answer. Many answers failed to provide any examples which was requested. The structure of the Na+/K+ ATPase pump was less well described, however pleasingly most candidates were able to accurately articulate its role and function.
3. Define respiratory compliance, include its components and their normal values (25% marks).
Explain the factors that affect respiratory compliance (75% marks).
CICMWrecks Answer

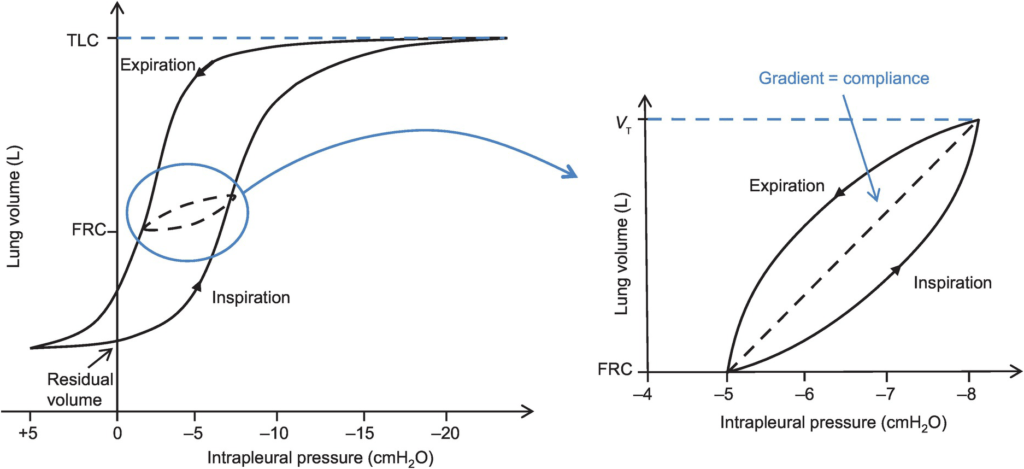
Lung compliance
- Change in lung volume per unit change in transmural pressure
- Normal lung compliance ~200ml/cmH2O
- Static compliance (Cs)
- ” Compliance of lung measured when lung held at constant lung volume”
- no pressure componet due to resistance
- is a function of: Elastic recoil of the lung, Surface tension of alveoli
- Dynamic compliance (Cd)
- ” Compliance of lung measured during cycles of inspiration and expiration”
- includes the pressure required to generate flow by overcoming resistance forces
- is a function of: respiratory rate
- Dynamic Compliance usually < Static Compliance due to the degree of time dependence in the elastic behaviour of a particular lung
- Specific compliance
- Compliance per unit volume lung
- Used to compare different lungs
- Hysteresis
- any process where the future state of a system is dependent on its current and previous state
- Compliance of the lung is different in inspiration and expiration
- In dynamic compliance curves:
- Airways resistance is a function of flow rate. Flow rate (therefore resistance) is maximal at the beginning of inspiration and end-expiration.
- In static compliance curves:
- There is no resistive component. Hysteresis is due to viscous resistance of surfactant and the lung.
Factors Affecting Compliance
Lung Compliance
- Surfactant
- increases lung compliance
- decreases surface tension at alveolar air – water interface
- prevents small alveoli from collapsing
- accounts for most of hysteresis in intact lungs
- Lung volume
- Lung Compliance decreases at higher lung volumes
- Specific compliance (Compliance/FRC) remains constant
- Elephants have greater lung compliance than mice!
- Pulmonary blood volume
- Increased PBV decreases lung compliance
- Pulmonary venous congestion from L heart failure or mitral regurgitation
decreases lung compliance
- Bronchial smooth muscle tone
- Increased bronchial smooth muscle tone decreases compliance
- Decreased dynamic lung compliance by 50% in animal models of methacoline
challenge
- Disease
- ARDS, pneumonia decreases lung compliance
- pulmonary fibrosis → Impraired elasticity → decreases lung compliance
- Asthma, Emphysema increases lung compliance
Chest Wall Compliance
- Chest wall restriction – reduced chest wall compliance
- Obesity
- Spastic paralysis of chest wall musculature
- Ossification of costal cartilages
- Kyphosis/scoliosis
- Scarring/constriction (e.g. circumferential burns)
- Position: Prone (60% reduced compliance)
- Collagen Disorders: Increased Chest Wall Compliance
Measurement of Compliance
Dynamic Compliance
- ” Compliance of lung measured during cycles of inspiration and expiration”
- Measure:
- Measure with spirometer
- Using Volume/Pressure curve during normal rhythmic breathing at points of no flow
- Equation above then gives dynamic compliance
Static Compliance
- ” Compliance of lung measured when lung held at constant lung volume”
- Volume vs Pressure loops during inspiration and expiration demonstrate Hysteresis
- Measure:
- @ no flow in or out of the lung, AND time for pressure to equlibrate across the lung (>10sec).
- Patient exhales in steps holding the volume with open glottis
- Intrapleural pressure measured as oesophageal pressure
- Measured compliance secondary to viscoelastic properties and accounts for both short and long time-constant alveoli.
Specific Compliance
- Measure of the average compliance across all lung units
- Children = Adults ~ 0.05 cmH2O-1
Gladwin / Sakurai / JC 2020
Examiner Comments
2022A 03: 27% of candidates passed this question.
This question covers a core principle of respiratory physiology and would be expected to have a high pass rate. Most candidates were able to provide a concise definition and distinguish between the different types of compliance. The imprecise use of terminology often created the impression of a lack of fundamental understanding of this key concept. Candidates are encouraged to be accurate and concise in their definitions. A lack of detail in describing the relevant components of compliance and the factors that influence it, immediately limited the capacity of some candidates to achieve an adequate score.
Most candidates provided less than half of these factors and only provided a list rather than explaining how compliance was impacted. Marks were maximised by dividing the impacts into those that altered lung compliance versus those that impacted on chest wall compliance, and the better candidates explained how and why compliance was affected. Confusion often arose from the imprecise use of arrows with the result that candidates frequently demonstrated an incorrect fact in relation to the direction of the arrow. Candidates are reminded to take care when using abbreviations or arrows to ensure they are not relying on the examiner to interpret a cause and effect relationship.
4. Describe the mechanisms of action and potential adverse effects of inhaled nitric oxide and prostacyclin.
Examiner Comments
2022A 04: 15% of candidates passed this question.
Most candidates were able to describe the mechanism of action of inhaled nitric oxide (iNO), however many demonstrated very little knowledge about prostacyclin and the adverse effects of both commonly used drugs. General statements about NO, it’s delivery and pharmacological effects did not attract marks candidates are encouraged to read the question and provide information specific to the question.
Methaemoglobin and its effects were reasonably described with many understanding the rational for restricting the concentration of iNO (ppm) because of the risk of N02 formation. The knowledge related to prostacyclin was very limited. Such limited detail as to its mechanism of action prevented any discussion regarding any differences from iNO. Many reasonable answers to the iNO component were limited overall due to a paucity of knowledge and incorrect facts in the prostacyclin section.
5. Write short notes on the pharmacology of labetalol and esmolol, highlighting their differences.
Examiner Comments
2022A 05: 32% of candidates passed this question.
Overall, this question was poorly answered. Most answers demonstrated limited knowledge about the major differences between the two drugs’ including the target receptors and subsequent effects. Antiarrhythmic effects were often omitted in answers, and scant or incorrect details provided about the metabolism and overall pharmacokinetics of the drugs. Generic vague statements about pharmacokinetic properties of medications do not attract marks. Better scoring answers demonstrated a factual knowledge about both individual drugs and specific details related to any differences influencing the potential application of these differences. A table superficially listing aspects of both drugs would not be of a passing standard. Many answers demonstrated significant incorrect facts.
6. Describe the cardiovascular changes seen throughout pregnancy.
CICMWrecks Answer
- Pregnancy is a time of increased metabolic demand, which cardiovascular changes reflect.
- Changes begin from week 8 and ↑ to plateau at 32 weeks → return to normal 2-8 weeks post delivery
- Changes depend on stage of pregnancy
- Hormonal changes: ↑ circulating concentrations of oestrogen, progesterone, hCG
- ↑ metabolic demand esp. during labour: ~↑60% O2 consumption/ CO2 production during labour
- Mechanical effects from gravid uterus
Characteristics
- Mechanical Effects:
- Thoracic changes:
- Anatomical compression of chest
- Diaphragm pushed upwards by 4cm
- ↑ AP + transverse diameter of chest wall (2-3cm)
- placental circulation: ↓pressure, ↓resistance AV shunt
- Aortocaval compression
- Collateral blood flow via collateral paravertebral epidural veins
- Thoracic changes:
- Hormonal Changes:
- ↑ circulating concentrations of oestrogen, progesterone, hCG
- Oestrogen stimulation of RAAS
- Increased plasma volume (40% or 1~1.5L positive)
- Erythropoietin secretion
- Increased erythropoiesis and red blood cell volume (20%)
Changes in CVS
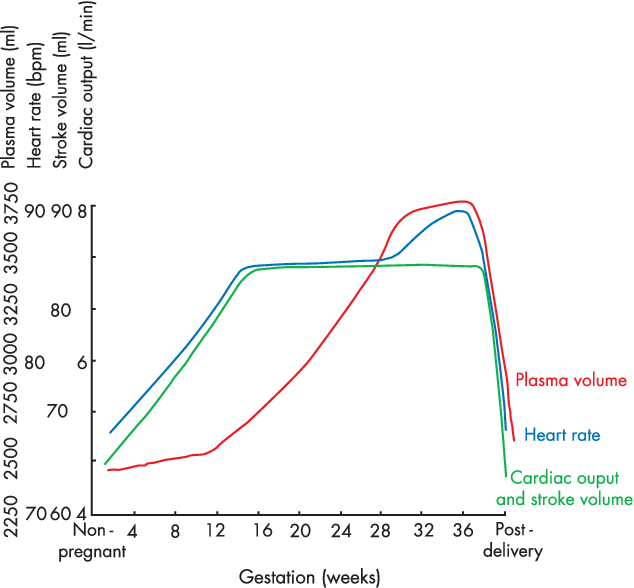
- Anaemia of pregnancy
- Disproportionate plasma volume expansion relative to erythropoiesis
- Increased cardiac output (40%)
- Increased uterine blood flow (750ml/min)
- Increased renal blood flow
- Increased HR (25% by second trimester)
- Increased SV (25% in first trimester)
- Decreased peripheral vascular resistance (30%)
- Progesterone
- Prostaglandins
- Down-regulation of α receptors
- Decreased plasma oncotic pressure (15%) → peripheral oedema
During labour
- Contraction → 300ml return to central maternal circulation
- CO increases 15%, 30% and 45% in latent, active and expulsive phases of labour respectively
- Immediately after labour CO 80% pre-labour levels due to autotransfusion due to uterine involution
- Return to non-pregnant levels 2 weeks after delivery
Sakurai / Kerr / JC 2020
Examiner Comments
2022A 06: 51% of candidates passed this question.
It was expected that candidates would give a detailed description of the changes that occur throughout pregnancy, labour and post-delivery (a timeline). This should include but not be limited to, cardiac output, total peripheral resistance, blood flow distribution, uterine blood flow and blood volume changes. Better answers were able to relate these changes to the underlying mechanisms (such as progesterone induced vasodilatation etc). A detailed description of aortocaval compression and its importance was also required. Vague and imprecise statements attracted fewer marks (for example simply stating that heart rate increases without discussing the magnitude, time course and influences). This topic is well covered in some of the recommend texts.
7. Write notes comparing the use of serum creatinine and creatinine clearance in the assessment of renal function in the critically ill.
CICMWrecks Answer
Clearance:
- Renal clearance = vol of plasma completely cleared of a given substance by the kidneys per unit time (ml/min)
- Involves: glomerular filtration, secretion, reabsorption, and rarely tubular metabolism
- Renal clearance = V x [U]/[P]
- V = volume of urine or urine flow rate in ml/min
- [U] = urinary concentration of substance in mg/ml
- [P] = plasma concentration of substance in mg/ml
Estimating GFR:
- GFR = renal clearance of a substance if it is:
- Freely filtered at glomerulus
- Not secreted
- Not reabsorbed
- Not synthesized
- Not metabolised
- The amount excreted in the urine = amount filtered
- i.e. [plasma] x GFR = [urine] x urine vol
- Rearrange: GFR = urine vol x [urine] / [plasma]
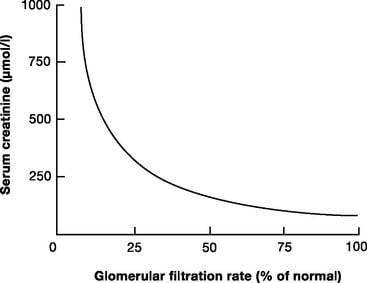
Creatinine
- used to approximate GFR as is more practical
- Released at a steady state from skeletal muscle cells (phosphocreatine)
- Freely filtered + not reabsorbed.
- *Small amount secreted → overestimates GFR by small amount
- Serum creatinine levels can be used as a surrogate marker of GFR
- Creatinine clearance calculation better than creatinine levels
- most accurate to collect urine and use the formula to assess creatinine clearance
- because serum creatinine it is at steady state, eGFR can be calculated by Cockroft-Gault, MDRD or CKD-Epi
- During acute changes in GFR, serum creatinine will underestimate GFR until a new steady state is reached.
Creatinine Clearance Estimation from Serum Creatinine
- CG (Cockcroft-Gault Equation): common method which has a correlation of ~0.83 with CrCl:
- CrCl = [(140−A) × W x S)] / (72 × Cr) , where:
- Cl = Clearance (mL/min), A = Age, W = Lean body Wt (kg)
- S = Sex coefficient (Male = 1, Female = 0.85), Cr = Creatinine in µmol.L-1
- CrCl = [(140−A) × W x S)] / (72 × Cr) , where:
- Alternative formulas are MDRD and CKD-EPI. These equations have two advantages over Cockcroft-Gault:
- They are better predictors of GFR
- They do not require weight, and so can be calculated by the laboratory automatically, Other required data (gender, race, age, creatinine) can be taken from hospital records.
- MDRD (Modification of Diet in Renal Disease Study): useful in estimating glomerular filtration rate (GFR) in stable chronic kidney disease
- CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): more precise formula to estimate glomerular filtrate rate (GFR) from serum creatinine and other readily available clinical parameters, especially at when actual GFR is >60 mL/min per 1.73m2
Limitations
- General limitations
- Dependent on serum creatinine, which can be highly variable. Formulas are derived from average values of dependent variables, and so will be unreliable at extremes of:
- Age
- Muscle mass
- Critically ill
- Malignancy
- Diet (High production with red meat)
- the relationship between creatinine clearance and serum creatinine is non-linear
- filtration is only one component of a complex kidney, although GFR is used as a surrogate of function
- Small amount of creatinine is secreted by proximal tubule – so Creatinine clearance is ~10-20% higher than GFR
- More severely overestimates GFR in patients with very low GFR
- Dependent on serum creatinine, which can be highly variable. Formulas are derived from average values of dependent variables, and so will be unreliable at extremes of:
- Critically ill patients
- the amount of creatinine produced varies with muscle mass, nutrition, steroid use, muscle injury
- there can be a decline of almost 50% of function before serum creatinine levels rise
- they do not indicate dynamic changes in renal function
- are modified by aggressive fluid resuscitation
JC 2019
Examiner Comments
2022A 07: 11% of candidates passed this question.
It was expected that candidates would define both components of the question, discuss the factors which affect serum creatinine and creatinine clearance and demonstrate their interrelationship. More complete answers described the advantages, disadvantages and limitations of their use in critical illness. In many cases candidates failed to correctly define creatinine clearance. Answers that scored well clearly utilised the above-mentioned breadth of knowledge and linked these to pertinent specific changes that may be associated with critical illness.
8. Describe the regulation of body water.
CICMWrecks Answer
H2O
Body H2O content (or TBW state) is determined by the body’s H2O balance (daily H2O
intake vs loss) → normally, it is balanced (as per table below):
| Daily H2O Intake | |
|---|---|
| Drinking | 1200 ml |
| Food | 1000 ml |
| Metabolism (Eg. ETC) | 350 ml |
| Total Intake | 2550 ml/day (in 70kg adult) |
| 25-35 ml/kg/day | |
| Daily H2O loss | |
|---|---|
| Urine | 1500 ml (includes obligatory loss ~ 430ml) |
| Insensible losses (skin, lungs) | 900 ml |
| Faecal | 100 ml |
| Sweat | 50 ml |
| Total loss | 2550 ml/day |
- Note: Abnormal TBW states arise when an imbalance in body H2O exists:
- ↓ TBW (“H2O deficit” → due to H2O loss > intake) → results in ↑ plasma osmolality due to a relative ↑ plasma [Na+] → associated with ↓ ECFV (and PV)
- ↑ TBW (“H2O excess” → due to H2O intake > loss) → results in ↓ plasma osmolality due to a relative ↓ plasma [Na+] → associated with ↑ ECFV (and PV)
Control of TBW
TBW state is controlled via –ve feedback system as follows:
Sensors
- Osmoreceptors (anterior hypothalamus)
- Responds to ↑ plasma osmolality. Very sensitive (detects 1% change) → threshold for stimulation is 280 mosm/kg (near lower normal limit) → steep linear rise in response > 290 mosm/kg
- Low-pressure baroreceptors (right atrium and great vessels)
- Responds to ↓ plasma volume indirectly by ↓ CVS PHYDROSTATIC (↓ MAP) → ↓ sensitive cf. osmoreceptors (detects 5-10% ∆ in PV)
- High-pressure baroreceptors (carotid sinus and aortic arch)
- Responds to ↓ plasma volume indirectly by ↓ CVS PHYDROSTATIC (↓ MAP) → Even ↓ sensitive cf. osmoreceptors (detects > 10% ∆ in PV → large H2O deficits) → BUT its response overrides that of the osmoreceptors!
Effectors
Hypothalamus integrates afferent signals from these sensors and modulates an
appropriate effector response that includes:
- Thirst response → triggered by:
- ↑ plasma osmolality
- ↓ plasma volume (or ↓ MAP)
- AT-II (acting on circumventricular organs (SFO/OVLT)
- ADH
- 9 a.a peptide hormone synthesised in hypothalamus (SON/PVN) → transported to posterior pituitary where it is secreted by:
- ↑ plasma osmolarity (main trigger)
- ↓ plasma volume (or ↓ MAP) → note that LARGE ∆ in PV (> 10%) can override response by osmoreceptors (Ie. ADH is secreted irrespective of plasma osmolality)
- Other stimuli: AII, pain, nausea/vomiting, exercise
- 9 a.a peptide hormone synthesised in hypothalamus (SON/PVN) → transported to posterior pituitary where it is secreted by:
- Effects:
- Via V1 receptor (GPCR via Gq → activates PLC to ↑ IP3 → ↑ IC [Ca2+] → SM contraction) → causes ↓ GFR to ↓ glomerular filtration (and loss of) H2O → via:
- Renal afferent arteriolar constriction
- Renal mesangial cell contraction
- Via V2 receptor (GPCR via Gs → activates AC to ↑ cAMP → activates PKA) → this causes:
- Upregulates insertion of luminal AQP2 (stored in vesicles) in all parts of CD → ↑ H2O permeability → ↑ H2O reabsorption into hypertonic medullary interstitium
- Upregulates “urea transporters” in inner MCD → ↑ permeability to urea → ↑ urea absorption to maintain ↑ medullary osmolality (strengthens CCM) → promotes ↑ H2O reabsorption
- ↑ Na+ reabsorption and K+ secretion by principal cells of CCD
- Via V1 receptor (GPCR via Gq → activates PLC to ↑ IP3 → ↑ IC [Ca2+] → SM contraction) → causes ↓ GFR to ↓ glomerular filtration (and loss of) H2O → via:
Bianca 2016
Examiner Comments
2022A 08: 43% of candidates passed this question.
Better answers for this question used the “sensor, integrator/controller, effector” structure. They also included appropriate detail relating to the site and mechanism of angiotensin II and the subsequent stimulation of ADH and aldosterone release. A detailed description of ADH was necessary to score well.
Lengthy descriptions of body water distribution or renal handling of water did not attract additional marks.
Answers that scored less well were often disorganised, with limited structure and incorrect facts.
9. Describe the pharmacology of 4% albumin.
Examiner Comments
2022A 09: 41% of candidates passed this question.
This question required the candidate to consider 4% albumin from a pharmacological perspective. The examiners were therefore after a description that included presentation, pharmaceutics (including correct content description and osmolality), indications, pharmacodynamics, pharmacokinetics, adverse effects, special precautions and dosing.
10. Discuss the determinants of intracranial pressure (80% marks) and outline how it can be measured (20% marks).
CICMWrecks Answer: ICP
Definition:
- ICP: hydrostatic pressure within the cranial vault
- Normal value is 5-15 mmHg
- focal ischaemia when ICP > 20 mmHg
- global ischaemia when ICP > 50mmHg
Munro-Kellie Doctrine
- The rigid and closed cranial vault forms a fixed brain volume containing
- Brain parenchyma (80%, 1400 g)
- CSF (10%; 75 mL)
- Cerebral blood and vessels (10%; 75 mL)
- Δ’s in volume of any components → Δ’s in others or and increase in ICP
CSF Production / Absorption
CSF Production:
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
CSF Absorption:
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
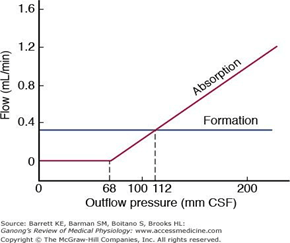
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
Control of ICP
- ICP is regulated via volume buffering
- i.e. increase in volume of one intracranial component leads to compensatory decrease in volume of other intracranial components
- When volume buffering mechanism is exhausted → rapid increase in ICP (decompensation)
- Movement of cerebral venous blood = rapid compensation, lower capacity
- Movement of CSF = gradual compensation, larger capacity
Determinants of ICP:
- Brain
- Age / Mass
- Space occupying lesions
- Cerebral Oedema
- CSF
- CSF production
- CSF Absorption
- Cerebral Blood Volume
- Cerebral autoregulation: Flow-metabolic coupling
- Cerebral metabolic rate
- Increase in systemic blood pressure / flow
- Venous Outflow obstruction
- Vasoactive agents
- Monro-Kellie Doctrine
- Loss of above – e.g. Fractures, surgery
Compensation for Elevated ICP (Intracranial Pressure)
Early compensation
- Δ CSF distribution and flow
- CSF is displaced to spinal subarachnoid space
- ↑’d resorption rate
Late compensation
- ↑ ICP → ↓ CBF → ↓ in cerebral blood volume → cerebral ischaemia
Decompensation
- ↑ICP → ↓ in cerebral tissue volume → brain herniation
- Cushing Reflex
- Hypertension, bradycardia and abnormal breathing associated with raised ICP
- Mechanism:
- Stage 1:
- ↑ ICP → ↓ blood supply to vasomotor area → Local hypoxia/hypercarbia → ↑ SNS >> ↑ PSPS vasomotor stimulation
- ↑ TPR → ↑ MAP
- ↑ HR → ↑ CO
- → compensatory ↑CBF
- Stage 2:
- ↑ CO → Baroreceptor stimulation → ↑ Vagus nerve stimulation → Bradycardia and ↓ contractility.
- Stage 1:
Gladwin / JC 2020
CICMWrecks Answer: Measurement of ICP
Methods of Measurement of ICP (Outline)
Invasive
| Method | Advantages | Disadvantages |
|---|---|---|
| Intraventricular catheter (EVD) | Provides ‘ture’ global ICP Allows for CSF drainage and administration of drugs In-vivo calibration possible via external pressure transducer | Infection Difficult iinsertion |
| Epidural catheter | Ease of insertion Minimal risk of infection (no penetration of dura) | Low accuracy |
| Lumbar CSF puncture | Extracranial procedure Can be performed ambulatory | May not reflect ICP Dangerous if ICP high |
| Catheter-tip micro transducers (subdural or intra-parenchymal) | Rare complications during procedure Low risk of infections Can be made permanent implants | Drift of transducer output over time In-vivo calibration not possible Inaccurate if intraparenchymal gradient exists |
Non-Invasive
Non-invasive methods like pupillometry, CT, MRI, TCD provide an adjunct to clinical examination of high ICP, but are not a surrogate for invasive ICP measurement.
Gladwin / JC 2020
Examiner Comments
2022A 10: 64% of candidates passed this question.
In the good answers to this question, and there were a number, the candidates included the volumes of the cranium and a correct description of the Monroe Kellie doctrine. A good answer should have included the compensations and consequences of increases in intra-cranial volumes; a discussion of all three components (brain tissue, blood, and CSF) and how they affect intracranial pressure; and then information on intra-ventricular and parenchymal devices in measuring ICP, briefly including their pros and cons. A common issue was writing quite a lot more than was needed on the relationship of cerebral blood flow to cerebral blood volume, and/or on the physiological consequences of raised ICP, which seemed to leave little time for discussion elsewhere. A few candidates did not provide any response for ICP measurement (worth 20% of the marks). Few candidates provided the intra-cranial elastance equation. A significant proportion of candidates missed out a part of the question, either the factors that affect CBV or ICP measurement.
11. Outline the structure and function of the N-methyl-D-aspartate (NMDA) receptor (25% marks). Discuss the pharmacology of ketamine (75% marks).
CICMWrecks Answer: NMDA Receptor
N-Methyl D-Aspartate (NMDA) Receptor
- ligand-gated voltage dependent ion channel
- Natural ligand = glutamate
- Associated with the family of glutamate receptors (AMPA, kainite and neurokinin)
Structure:
- transmembrane receptor
- 5 subunits forming central cation ionophore
- at baseline Mg2+ plugs and inhibits central cation pore
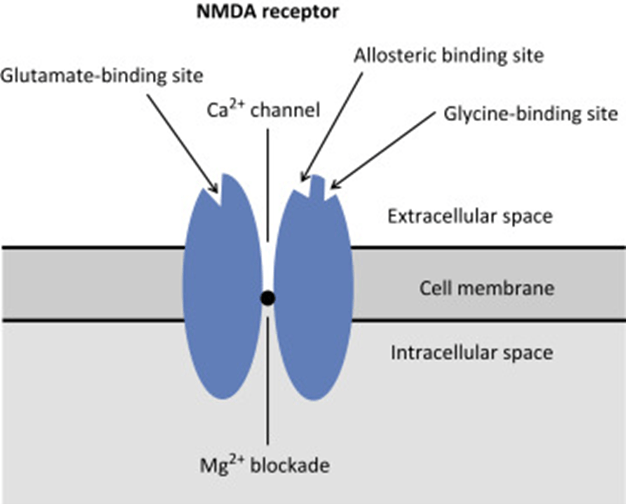
- Location: abundant throughout brain (esp. hippocampus) and spinal cord (esp. dorsal horn) on post-synaptic membranes
- Activation: NMDA receptor contains central Mg2+ plug, which block channel at rest
- Glycine binding + voltage stimulus are required (via activation of adjacent AMPA and neurokinin receptors) → remove Mg plug → primes NMDA receptor for activation by glutamate
- When channel activated→ opens cation channel → ↑cation conductance (Ca2+ and Na+ in; K+ out) → excitatory postsynaptic potential
Physiological Roles of NMDA Receptors in the CNS
- Wind up phenomenon
- Wind up = repeated stimuli of same strength cause increase in pain response
- Proposed mechanism: C fibres synapse in lamina II of dorsal horn → repeated stimulation → glutamate release → NMDA activation → ↑response of dorsal horn neurons to excitatory neurotransmitter input àhyperalgesia and allodynia
- Long term potentiation
- LTP = strengthening of synaptic transmission that occurs following ↑activity across that synapse
- i.e. recurrent painful stimuli → neuroplasticity → chronic pain
- NMDA receptor stimulation leads to various intracellular changes:
- production of NO
- activate second messengers (IP3, DAG, cGMP, PKC)
- PKC → increases NMDA activity → vicious cycle
- second messengers induce oncogenes (e.g. c-fos)
- Memory
- NMDA receptors are highly expressed in hippocampus and throughout cortex Important roles in neuroplasticity and memory formation
- Apoptosis
- Post cerebrovascular accident → significant NMDA activation → ↑↑Ca influx → excitotoxicity → triggers neuronal apoptosis
CICMWrecks Answer: Ketamine Pharmacology
Pharmacology of Ketamine
Examiner Comments
2022A 11: 74% of candidates passed this question.
The first part of this question required a description of both the receptor structure and its function. This includes, but is not limited to, its location, the natural ligand, how the channel may be regulated and the results of receptor stimulation. The second part of this question related to ketamine. Marks lost here often related to vague statements and incorrect facts. The examiners also commented that some candidates got confused between the R and S enantiomers. Few candidates commented on the nature of the metabolites and generally the PD section was vaguely answered.
12. Describe the process of excitation-contraction coupling and relaxation in smooth muscle.
CICMWrecks Answer: Smooth Muscle
Excitation Contraction Coupling in SM
- Motor neuron depolarisation
- ACh vesicular release
- Multiple MEPs → End plate potential
- Muscle AP propagates via T-tubles
- Opens L-type Ca channels
- Influx of EC Ca → ↑ IC [Ca]
- Variable amount of Sarcoplamic reticula in SM
- Can have some ↑ IC [Ca] via Ca induced Ca release.
- Ca binds Calmodulin → Ca-Calmodulin complex
- Ca-Calmod → ↑ MLCK activity
- → Phosphorylation of Myosin Light Chain (MLC)
- → Activation of Myosin ATPase
- Cross-bridge cycling.
Cross Bridge Cycling
- “Flexed” Myosin without ATP binds to Actin.
- Binding of ATP to Myosin causes release of Actin/Myosin complex and extension of the head
- In the presence of Ca-Calmod complex
- Actin binding sites are open and binding of myosin/Actin/ATP complex is formed
- Hydrolysis of ATP to ADP + P in myosin head by Myosin ATPase
- → conformational change “initial flexing” the myosin head and releasing phosphate
- Release of ADP further flexes Myosin head
- In presence of Ca return to step 2.
Relaxation
- Ca removed from cell
- Ca ATPase
- Ca2+/Na+ antiport
- Myosin Light Chain Phosphatase
- dephosphorylates MLC → inhibition of myosin ATPase
Gladwin 2016
Examiner Comments
2022A 12: 36% of candidates passed this question.
This is a straightforward fact-based and process related question. It is important that candidates take the time to read the entire question prior to starting to write an answer. Unfortunately, many candidates wrote about skeletal muscle contraction which scored no marks. A good answer template included a description of the contractile elements of smooth muscle, the regulatory proteins and the role of calcium, and highlighted how these elements interact. They included descriptions of both the contractile and relaxation processes.
13. Compare and contrast the pharmacology of suxamethonium and rocuronium.
Examiner Comments
2022A 13: 75% of candidates passed this question
Suxamethonium is a level 1 drug and therefore requires a detailed knowledge of the drug from a PK and PD perspective as well as consideration of important side effects and considerations when used. The examiners commented that a table structure with the structured pharmacology headings and clear concise facts was the best way to approach this question. Answers that scored poorly often displayed incorrect facts, limited appreciation of side effects and vague statements on the pharmacological particularities of this drug. For example, muscle relaxants are major culprits for anaphylaxis in hospitals, and nuanced facts about this were generally missing from candidate’s answers.
14. Describe the neural integration of vomiting, highlighting the site and mechanism of action of antiemetics.
CICMWrecks Answer: Physiology of Vomiting
Physiology of Vomiting
Vomiting
- Involuntary, forceful and rapid expulsion of gastric contents through the mouth
Triggers
- Excessive GI tract distension
- Stimulation of CTZ
- Directly by certain drugs eg apomorphine, morphin
- Rhythmic motion of the body stimulating the vestibular labyrinth of the inner ear
- Cerebral excitation secondary to odours, pain, stress
Central control of vomiting
- Vomiting center (5HT3, NK1, Muscarinic and Histamine), near NTS
- Inputs
- Chemoreceptor trigger zone (NK1, 5HT3, Dopamine), area postrema outside BBB
- Vestibular system (Histamine and Muscarinic) via CNVIII
- GI Tract (5HT3, stretch and chemoreceptors) via vagal
- Higher centres
- Efferent arc
- via CN V,VII,IX,X,XII and spinal nerves to abdominal wall musles and diaphragm
- Inputs
| Afferent signal | Sensor | Efferent signal | Effect |
|---|---|---|---|
| Chemo/ baroreceptor input Drugs Stim from NTS Stim from GIT (5HT3) | CTZ (area postrema) | 5HT3, D2 and Opioid neurons | Direct stimulation of vomit centre in lateral reticular formation |
| Surgery/ Rhythmic motions | Labyrinths | ACh and H1 neurons | |
| Memory/ Emotions Sensory (sight, smell, taste) | Cortex | ||
| Severe painful stimuli | Pain | H1 neurones | |
| Irritation Manipulation during surgery Distension | GIT | NK1, NAdr, ACh neurons | |
| Via CNX, to NTS | Stimulation of CTZ then Vomit centre | ||
| Via 5HT3 to CTZ | CTZ to vomit centre | ||
| Manipulation of pharynx via CN9 to.. | NTS | Direct to CTZ | To vomit centre |
Vomiting act
- Antiperistalsis as the prelude to vomiting
- At the onset of vomiting, strong intrinsic contractions occur in both the duodenum and the stomach
- Partial relaxation of the lower oesophageal sphincter (LOS)
- Deep inspiration
- Raising of the hyoid bone and larynx to open the upper oesophageal sphincter
- Glottic closure
- Lifting of the soft palate to close the posterior nares
- Strong down ward contraction of diaphragm and simultaneous contraction of all the abdominal wall muscles
- Complete relaxation of the LOS
Gladwin / Sakurai 2016
CICMWrecks Answer: Anti-emetics
Anti-emetic Agents
| CLASS | EXAMPLES | MECHANISM OF ACTION |
|---|---|---|
| Anticholinergics | Hyoscine Atropine | – M1-Ach-R antagonism (NTS, CTZ, VC) – Small antihistamine and D2 antagonist effects |
| Antihistamines (H1) | Cyclizine Promethazine | – H1 antagonism- VC, vestibular nucleus and CTZ – Anti-muscarinic effects- NTS, CTZ, vomit center – D2 antagonism (GIT, CTZ) |
| 5-HT3 Antagonists | Ondansetron | – Peripheral in GIT – Central at VC and CTZ |
| Dopamine Antagonists | 1. Phenothiazines – Prochlorperazine (stemetil) 2. Butyrophenones – Droperidol, domperidone 3. Benzamides – Metoclopramide | – Decreased sensitivity of visceral afferents to vomit center – Central D2 blockade increased threshold at CTZ Other effects: – Inhibition of 5-HT3 – Anti-H1 effects |
| Steroids | Dexamethasone | – Proposed to act centrally to inhibit prostaglandin synthesis and inhibit endorphin receptors |
| Miscellaneous | 1. Propofol 2. Benzos 3. Cannabinoids 4. NK1- Receptor antagonists (aprepitant) | – Propofol and BZD- GABAergic inhibition of VC – Cannabinoids- Direct CTZ and VC inhibition – NK1 antagonists inhibit VC |
Examiner Comments
2022A 14: 60% of candidates passed this question.
The examiners commented that a well-drawn and labelled diagram was a very useful adjunct to answering this question. Consideration of stimulus, sensors, integrators/processors, and effectors was also useful to ensure that all components of the question were covered by a candidate’s answer.
Incorrect facts or a lack of detail about the various receptors and their locations was a common theme in answers that scored poorly. Classes of antiemetics, with specific drugs given as examples, were expected to gain marks.
15. Describe the sequence of haemostatic events following injury to a blood vessel wall until clot stabilisation.
CICMWrecks Answer: Haemostatic events after injury
- Haemostasis is the natural process that stops blood loss when an injury occurs.
- Intact vascular endothelial cells:
- fibrinolytic heparin, Thrombomodulin → prevent clotting
- NO, Prostacyclin → Prevents clotting cascade
Haemostasis following vessel injury
Three steps: Vascular spasm (vasoconstriction), platelet plug formation and coagulation.
- Vasoconstriction:
- Brief reflexive contraction that decreases local blood flow
- Caused by signalling molecules from injured endothelial cells, thromboxane A2 from activated platelets, Nervous system reflexes from local pain receptors
- Vasoconstriction only lasts for a few minutes during haemostasis. During inflammation that follows the injury, it is replaced by vasodilation as the healing process begins.
- Platelet Plug Formation (Primary Haemostasis):
- Within 20 seconds, coagulation is initiated
- Platelet Adherence: vWF released from damaged endothelium → change platelet form → adhere to subendothelial collagen
- Platelet Activation: subendothelial collagen binds to platelet receptors → activates platelets → degranulate → release ADP, vWF, TXA2, PDGF, VEGF, Serotonin, Coagulation factors
- Platelet Aggregation: Platelets bind to vWF and fibrinogen → aggregate over damaged endothelium
- Positive feedback mechanism
- Platelet plug formed in seconds to a few minutes based on extent of injury
- Coagulation Cascade (Secondary Haemostasis):
- Occurs if platelet plug ineffective in controlling bleeding
- Platelets degranulate → release ADP, Serotonin, Thromboxane A2
- Coagulation cascade: Intrinsic, Extrinsic pathway → Common pathway
- Intrinsic (Contact Activation) Pathway: Primary complex (on collage by High molecular weight kininogen, prekallikrenin, factor XII) → XI → IX (which, along with VIII) → Common pathway
- Extrinsic (Tissue factor) Pathway: Tissue factor III → VII → Generates thrombin burst – cleaves fibrinogen to fibrin
- Common pathway: Prothrombin(II) to thrombin (using Factor V) → cleaves fibrinogen to fibrin
- forms mesh that binds and strengthens platelet plug → coagulation → haemostasis
- Also activates factor XIII – covalently bonds to fibrin to strengthen attachment to platelets
- Also activates more factor V which acts as anticoagulant with inhibitor protein C
Fate of the Clot:
One of four outcomes:
- Propagation: Accumulation of additional platelets and fibrin
- Embolization: Thrombus breaks free and becomes mobile
- Dissolution: Fibrinolysis (aided by tissue plasminogen activator, tPA)
- Organization and recanalization: Ingrowth of smooth muscle cells, fibroblasts and endothelium into fibrin-rich thrombus.
Clot lysis and Wound healing:
Over the course of the next few days:
- Clot Retraction: blood clot shrinks.
- dependent on the release of multiple factors, mostly factor XIIIa crosslinks
- Cause contraction, knotting and twisting of fibrin mesh
- Blood clot shrinks
- Fibrinolysis – Plasmin degrades fibrin to Fibrin degradation products, macrophages consume the expended platelets. FDPs inhibit further thrombin and fibrin formation
- Wound Healing:
- Inflammation – Tissue proliferation – Collagen and granulation tissue deposition – angiogenesis – Wound contraction – Epithelialization
Source: https://courses.lumenlearning.com/boundless-ap/chapter/hemostasis/
JC 2019
Examiner Comments
2022A 15: 46% of candidates passed this question.
A good answer was well structured and covered the areas of vasoconstriction, platelet adhesion, activation and aggregation, coagulation, clot retraction and anticlotting mechanisms. Many answers gave an overview of the haemostatic process but revealed insufficient knowledge of the processes involved. It was acceptable to give a classical view of clotting or to describe the cell-based model; or both. However, in several cases answers became confused by mixing up elements of the classical approach and cell-based model approach. Errors concerning details of the cell-based model were frequent. Many candidates did not include how the clot is limited to just the site of injury which happens in parallel with the formation of clot. Candidates should be aware that writing lengthy introductory statements attracted no marks and wastes time.
16. Outline the impact of sedative agents on thermoregulation (40% marks) and describe the physiological effects of a low body temperature (60% marks).
CICMWrecks Answer
Impact of sedative agents on Thermoregulation
3 phases:
- Phase I – 1st hour – Redistribution of heat from core → periphery
- significant decrease in core temperature from 0.5 to 1.5 C
- thermoregulatory center is depressed by anaesthesia
- Contributors:
- undressed
- skin prep
- evaporative loss
- induction → cutaneous vasodilation
- cool IVF
- cool dry ventilation of lungs
- temp drop in spinal > epidural
- Phase II – Hour 2 to 3 – Slow linear decrease in core temperature
- Increased heat loss
- anaethetic induced peripheral vasodilation
- → increased radiant & evaporative loss.
- → redistribution of heat from core to periphery
- Decreased heat production
- NMBD mean cannot shiver
- anaesthesia means cannot get clothes or eat or curl up.
- Increased heat loss
- Phase III – 3+ hours – Plateau in core temperature
- core temperature plateaus
- peripheral temperature continues to decrease.
Mechanisms of ↓ in core body temperature:
- Resetting of interthreshold range (phase II)
- ↑ width of range to 4 °C → ↓ threshold to cold by 3 °C and ↑ threshold to heat by 1° C → ↓ thermoregulatory responses to Δ in body temperature
- Caused by a central effect → GA agent interferes with normal hypothalamic function to maintain a narrow threshold range
- GA agent-induced vasodilation → redistribution of heat from central to peripheral compartments (phase I)
- Muscle paralysis → loss of shivering response and muscle activity (phase II)
- LOC and paralysis → loss of behavioural responses (phase II)
- GA-induced ↓ BMR/heat production (phase II)
- Cold gases and IVF (phase II) → temp. generally ↓ 0.25-0.5 °C/L of IVF
Physiological effects of low body temperature
| CVS | – Tachycardia initially, then progressive bradycardia with ↑ cold – ↑ cardiac arrhythmias and myocardial ischaemia due to catecholamines released from stress response – ↑ SVR and MAP due to peripheral vasoconstriction – ↓ C.O. due to direct –ve inotropic effect of cold and ↑ afterload/SVR |
| Respiratory | – Left-shift in Hb O2 dissociation curve – V/Q mismatching 2° to inhibition of hypoxic pulmonary vasoconstriction – Bronchospasms – ↓ MV (and in severe cases apnoea) – ↑ solubility of gases (incl volatiles) |
| CNS | – Altered mental state (esp ↑ drowsiness, unconsciousness and delayed awakening from GA) – ↓ CBF |
| Haematological and immunological | – Coagulopathy (due to platelet dysfunction and loss of CF enzyme function) → ↑ transfusion requirements due to bleeding – ↓ WBC activity → ↑ incidence of infections |
| Hepatic / renal | – Impaired renal function (oliguria) – Impaired hepatic metabolic function (esp ↓ drug metabolism, such as muscle relaxants) |
| Metabolic and endocrine | – Impaired wound healing (due to catabolic state and wound vasoconstriction) – ↑ protein catabolic state – ↑ stress response (steroids and catecholamine released) – Shivering → 5x ↑ general MRO2 → causes hypoxaemia (risk of myocardial and cerebral ischaemia) – In absence of shivering → general ↓ MRO2 (by up to 50%) and ↓ BMR |
| Others | – ↑ morbidity and mortality rate (due to above reasons) – Hypothermia may be beneficial during cerebral or cardiac ischaemia as it ↓ metabolic O2 requirements (provided shivering response is blunted) – ↓ anaesthetic requirements (MAC-sparing) – ↓ triggering and severity of MH |
JF / Bianca 2016
Examiner Comments
2022A 16: 33% of candidates passed this question.
Sedation reduces body temperature by interfering with heat production and increasing heat loss, along with widening of hypothalamic inter-threshold range. This portion of the question was generally well answered. The question asked to “outline” the answer. Many candidates actually “described” the thermoregulation process in general but were unable to relate those with the impact of sedation. The second part of the question (physiological effect of low body temperature) was answered by most of the candidates with the structure of organ-system wise description. A few candidates scored extra marks by relating these effects with degree of hypothermia and by describing how thermogenesis responses (including shivering) can influence those effects. Some candidates restricted their answers to the effect of thermogenesis in response hypothermia and did not include the overall physiological consequences of low body temperature. Better answers displayed an understanding of core temperature regulation, inter threshold range and the effects of sedatives on thresholds for thermogenic responses, although only a few mentioned gain and maximal response. Better answers included specific detail (mentioned bradyarrhythmia, slow AF, VF, prolonged PR/QRS / J waves rather than just stating arrhythmia) across several organ systems. Marks were not awarded for generic statements such as ‘decreased liver function’ without some additional detail. Inadequate depth of knowledge was main reason behind overall poor scores.
17. Write notes on:
• The principles of ultrasound
• Transducer properties and image resolution
• The Doppler effect
CICMWrecks Answer
Ultrasonography Principles
- Definition
- A sound wave with a frequency > 20 kHz → higher than frequency range audible by human ear
- Used medically → typically involves frequency range of 2-15 MHz
- Piezoelectric and converse piezoelectric effect
- Change of polarization of molecules in a quartz crystal in response to
mechanical stress – Interconverts electrical and sound energy - Application of electrical field creates mechanical deformation in a crystal
- Change of polarization of molecules in a quartz crystal in response to
- US Generation: Piezoelectric crystal within probe is stimulated by electrical current to vibrate → produce sound wave
- US Detection: Sound wave reflected by medium causes same crystals to vibrate → produce electrical signal
- Piezoelectric transducers in US
- Electrical current converted into precise sound waves (1~20mHz)
- Sound waves reaches interface of two mediums of differing density (or acoustic impedance).
- Acoustic impedance = tissue density x acoustic velocity
- unique to tissue type (e.g. fat, bone, etc.)
- At interface:
- Reflection
- sound wave reflected directly back to transducer
- Amount of reflection depends of ratio of acoustic impedance (or density)
- Increased density ~ increased reflection
- Refraction
- sound wave is deflected within the medium
- based on Snell’s law
- Reflection
- Attenuation: Loss of energy or strength of an ultrasound wave as it travels through a medium. Occurs through:
- Absorption: The ultrasound wave energy is converted into heat as it interacts with the tissue.
- Reflection: The sound waves bounce off the boundaries between tissues with different acoustic impedances.
- sound wave reflected back to transducer
- Amount of reflection depends of ratio of acoustic impedance
- Increased density ~ increased reflection
- Scattering: The sound waves are deflected in various directions by irregularities in the tissue and do not reach transducer
- Refraction: bending of the sound wave as it travels from one tissue to another
- results in a change in the wave’s direction
- relationship between the angle of incidence (θi), the angle of refraction (θt), and the speeds of sound (c1 and c2) in the two media is described by Snell’s Law: sin(θi) / c1 = sin(θt) / c2
- Central Processor
- Electrical current generated by piezoelectric crystal signaled to CPU
- CPU calculates the distance between transducer and object according to
- Speed of sound (1540m/sec)
- Delay in echo return
- Information relayed to display for visualization
- Gain
- Sensitivity of CPU to signals received from transducer
- Time-Gain Compensation – selective sensitivity of CPU to different interval of sound delay
Resolution and Penetration
- Ultrasound Resolution:
- Defined as the ability to differentiate b/t structures that are closely related
- Resolution is ↑ with either:
- (i) ↑ frequency (or ↓ wavelength) of sound wave → but this ↓ tissue penetration
- (ii) ↑ amplitude of sound wave → but this ↑ artefact
- (iii) ↑ gain → but this ↑ noise
- Types of Resolution: Spatial (Axial, Lateral, Elevational), Temporal
- Spatial Resolution:
- Axial Resolution: The ability to distinguish two structures that are side-by-side and parallel to the ultrasound beam.
- Achieved with a higher frequency and shorter pulse length.
- Mathematically, it’s half the spatial pulse length.
- Lateral Resolution: The ability to distinguish two structures that are side-by-side to the ultrasound beam.
- Achieved with a narrower ultrasound beam, which is related to the width of the beam.
- Higher frequencies generally lead to narrower beams and better lateral resolution.
- Lateral resolution is roughly three times worse than axial resolution at the focal region of the beam.
- Elevational resolution (aka slice thickness resolution)
- refers to the ability to distinguish structures that are close together in the direction perpendicular to the imaging plane. (similar to lateral resolution, but in perpendicular plane)
- crucial for accurately visualizing the depth and thickness of tissues
- Temporal Resolution:
- The ability to distinguish between instantaneous events of rapidly moving structures.
- Achieved with a high frame rate.
- A higher frame rate means the ultrasound machine can capture and display more images per second, allowing for better visualization of movement.
- Axial Resolution: The ability to distinguish two structures that are side-by-side and parallel to the ultrasound beam.
- Ultrasound penetration:
- defined as the depth to which ultrasound waves can travel into tissue
- Primarily determined by the frequency of the ultrasound waves, with lower frequencies generally penetrating deeper than higher frequencies.
- Trade-off between Resolution and Penetration:
- There’s an inherent trade-off between image resolution and penetration depth.
- Higher resolution requires higher frequencies, which means less penetration, and vice versa.
Modes of Ultrasound
- A (amplitude scan): Amplitude of U/S signal plotted against time → provides information about tissue depth (BUT is no longer used)
- B (brightness): Depth recorded as bright spot (rather than a spike as in A-mode) →
amplitude of U/S signal is proportional to brightness - M (motion): B-mode plotted against time (Ie. assess heart valve movement over time)
- 2-D: Sequential B-mode across 90° (most commonly used) → requires an array of crystals
- Doppler: Uses “Doppler shift” to establish velocity of moving object which is reflecting sound waves → superimposed on 2D mode with colours representing direction of movement (red = towards, blue = away)
Doppler effect
- Change in apparent frequency of sound for an observer moving relative to its source
- Use in ultrasound
- Sound waves reflected off objects moving toward or away from transducer
(usually blood)- If object moving toward transducer, frequency appears increased – displayed as red (however this is not standardized across machines)
- If object moving away from transducer, frequency appears decreased –
displayed as blue
- Can be used to measure velocity of flow, as well as direction
- Sound waves reflected off objects moving toward or away from transducer
Sakurai 2016
Examiner Comments
2022A 17: 23% of candidates passed this question.
This question was taken from core syllabus that requires level one (L1) understanding. Physical principles of ultrasound can be illustrated by outlining how ultrasound waves are generated from piezoelectric crystals, how they travel through the tissues, how they interact with different tissue planes and how the reflected waves return to the transducer and create images. Properties of ultrasound transducers include different geometric configurations of transducer probes and frequency-wavelength bandwidth properties of the crystals used in diagnostic ultrasound. Understanding of physical concepts of image resolution including its various aspects (e.g., spatial, temporal, contrast resolution) is required to address the next portion of the question. “Doppler effect” can be illustrated by a definition and equation along with some practical implications. This question was not answered well by majority of the candidates. Lack of knowledge and limited understanding resulted in poor average mark.
18. Describe the anatomy of the left subclavian vein.
CICMWrecks Answer:
Origin
- Continuation of axillary vein
- Lateral border of 1st rib
Course
- Follows subclavian artery
- Deep to clavicle
- Superior to 1st rib
Termination
- Deep to sternoclavicular joint at medial border of scalenus anterior
- Joints internal jugular vein to form bilateral brachiocephalic veins vein on left
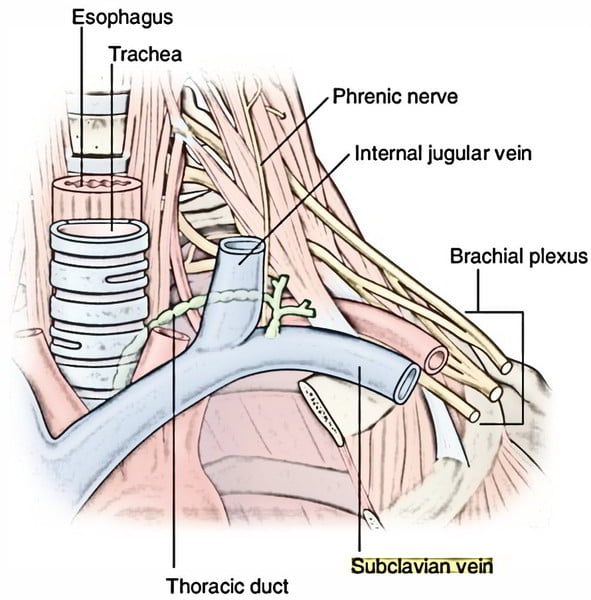
Relations
- Anterior
- Clavicle, subclavius
- Posterior
- Subclavian artery runs deep/posterior (separated by scalenus anterior)
- Internal mammary artery is posterior medially
- Phrenic nerve is posterior
- Superior
- Skin, superficial aponeurosis
- Inferior
- Apex of lung and 1st rib
- Medial
- Brachiocephalic trunk, thoracic duct and trachea and vagal trunks
- Lateral
- Inferior trunk of brachial plexus
Surface Anatomy
- Clavicle
- Deltopectoral groove
- 2 heads of sternocleidomastoid
- Suprasternal notch
Sakurai 2016
Examiner Comments
2022A 18: 14% of candidates passed this question.
An ideal answer includes origin of subclavian vein, its tributaries, course in relation to mediastinal structures and surface anatomy for central line insertion. Vague comments like: “it follows the subclavian artery”, or “it passes between 1st rib and clavicle”, attract minimal marks. Similarly, no marks were awarded for describing technique of central line insertion and complications of procedure. Answers scored poorly due to a combination of the following, a lack of depth to their answers or inaccurate facts and limited structure/approach to an anatomical SAQ. Good answers described the course of the subclavian vein from its origin at the lateral border of the first rib, along the subclavian groove on the upper surface of the first rib, medially to its termination posterior to the sternoclavicular joint at the medial border of the scalenus anterior, where it joined the IJV to form the bracheoceaphalic vein. In addition, high scoring candidates described tributaries (e.g., the thoracic duct, external jugular) where they joined and went on to describe relations in reasonable detail, specifying whether patient is supine or erect.
19. Describe the physiological factors that affect PaCO2.
CICMWrecks Answer
PaCO2: measure of carbon dioxide within arterial or venous blood
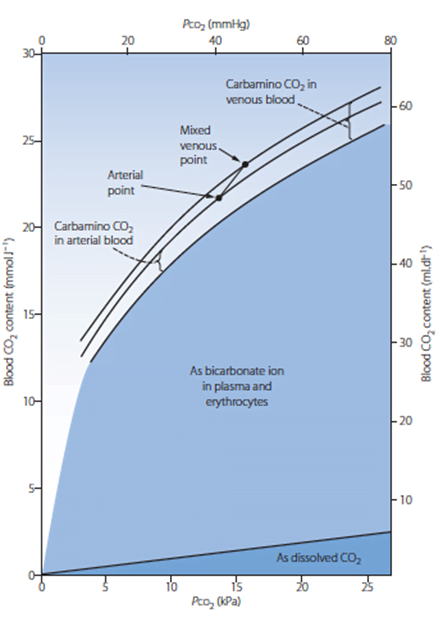
PCO2
- Small portion of CO2 carriage in blood – approx. 5%
- The rest being HCO3 (approx. 90%), Carbamino compounds (approx. 5%), and carbonic acid (negligable)
- Constitutes 10% of A-V difference in CO2 carriage
- The rest being HCO3 (approx. 60%) and Carbamino compounds (approx. 30%)
- Obeys Henry’s Law
- Mass of dissolved gas is proportional to its partial pressure
- Solubility coefficient of CO2 ~0.54
- Obeys Dalton’s Law
- Sum of all partial pressures equals the environmental atmospheric pressure
Factors affecting PaCO2
- Factors affecting PaCO2
- Rate of metabolism and CO2 production
- FiCO2 (usually negligible)
- Alveolar ventilation
- Increased alveolar ventilation decreased PaCO2
- PaCO2
- In ideal system equal to PACO2 – 10% shunt only increases CO2 by 0.7mmHg
- PACO2 = (CO2 delivery to lung) / (Alveolar ventilation)
- Alveolar ventilation = (Vt – Vd) x RR
- Vt = Tidal volume – Approx. 7ml/kg
- Vd = Dead space volume
- Anatomical deadspace = first 16 divisions of airway – approx 2ml/kg
- Alveolar deadspace – Volume of non-perfused lung
- Increases with
- West zone 1
- PE
- Decreased lung perfusion
- Decreased cardiac output
- Positive pressure ventilation
- Upright posture
- Increases with
- RR – Regulated by medullary respiratory centre
- Chemoreceptor
- Peripheral
- Sensitive to decreased pO2, decreased pH, decreased blood flow and increased pCO2
- Increases respiratory rate
- Central
- Sensitive to CO2 via conversion to [H+]
- Increases respiratory rate
- Peripheral
- Baroreceptor
- Decreased stretch of aortic and carotid baroreceptors increases respiratory rate
- Pulmonary receptors
- J fibres
- Stimulation causes apnoea, bronchoconstriction, bradycardia and hypotention
- Stretch receptors
- Inflation reflex – Inhibits inspiration on lung inflation
- Deflation reflex – Inhibits expiration on lung deflation
- J fibres
- Chemoreceptor
- CO2 production and metabolism
- production occurs predominantly in mitochondria
- From metabolism of glucose
- Glucose + 6O2 → 6CO2 + 6H2O
- From
- BMR – 40kcal/hr/m2
- Increased muscle activity
- Post-prandial metabolism
- Thyroid hormones increase metabolism
- Catecholamines increase metabolism
- Disease states
- Fever
- Malignant hyperthermia
- Tourniquet
- Relative perfusion of tissues
- Blood from highly metabolic tissues contributes relatively greater amounts of CO2 per weight
- Blood from less metabolic tissues contributes relatively less CO2 per weight
- From metabolism of glucose
- production occurs predominantly in mitochondria
Sakurai 2016
Examiner Comments
2022A 19: 33% of candidates passed this question.
Candidates who scored well generally defined PaCO2 and proceeded to describe factors in terms of those related to production and elimination. Good answers described the key production factor as being rate of production through aerobic metabolism which is in turn influenced by substrate and BMR. Those who scored well described elimination as being dependent upon minute ventilation, which in turn is influenced by CO2 detection by chemoreceptors, specifically detailing the difference between peripheral and central. Many candidates detailed pathophysiological factors which unfortunately did not gain any marks.
20. Describe the physiological control of systemic vascular resistance (SVR).
CICMWrecks Answer
Systemic Vascular Resistance
(Total Peripheral Resistance)
Systemic vascular resistance (peripheral vascular resistance, SVR) is the resistance in the circulatory system that is used to create blood pressure, the flow of blood and is also a component of cardiac function.
- Majority of resistance in systemic system results from arterioles – state of contraction and relaxation of smooth muscle cells of arterioles which determines distribution of blood to organs
- The % of each organ blood flow is dependent on the organ vascular resistance
- Systemic vascular resistance is the resistance of several circuits in parallel, which have both common and independent factors in their regulation.
- As they are in parallel the sum of reciprocals is used to determine the overall value.
Control
- As per Hagen Poiseuille Equation:
where: η = blood viscosity
L = length of vessel
r = radius of vessel
- radius is the most important factor
Extrinsic Control
- Extrinsic SNS control
- Arterioles have profuse SNS supply → NAd from nerve endings act on α1-adrenoceptors to cause vasoconstriction (and β2-adrenoceptors to cause vasodilation – but effect is weaker!)
- Due to the tonic SNS outflow from medullary vasomotor centres, arterioles have a basal level of vasoconstriction → the degree of basal vasoconstriction (and arteriolar resistance) can be varied by altering SNS outflow
- Extrinsic PNS control
- PNS control of arterioles is less important → vessels of external genitalia have dual ANS supply with PNS dilator nerves and SNS constrictor supply; PNS activation in heart, brain and lungs have an uncertain role
- Extrinsic hormonal control
- Adrenaline (from adrenal medulla) → effect on organ blood flow depends on the relative % of α1- and β2-adrenoceptors present in the arteriole
- AII (from RAAS) → vasoconstriction (via AT2R)
- ADH (from posterior pituitary) → vasoconstriction (via V1R)
- ANP (from RA) → vasodilation (via ANPR)
Intrinsic Control
- Autoregulation
- Ability of an organ to maintain relatively constant blood flow across variations in perfusion pressure
- flow = pressure/ resistance → as the P changes, the R also changes to maintain flow
- Outside limits of autoregulation: flow = dependent on driving pressure
- Kidneys, brain, heart
- Autoregulation is dependent on 2 mechanisms:
- Pressure autoregulation: myogenic stretch response to ↑ and ↓ in pressure → vasoconstriction, and vasodilation
- Metabolic or vasoactive autoregulation: direct action of locally derived metabolites and vasoactive substances e.g. platelets release thromboxane A2 → constriction in damage
Bianca / Kerr 2016
Examiner Comments
2022A 20: 22% of candidates passed this question.
A definition or description of SVR that recognised the importance of radius in small arteries/arterioles as the major determinant attracted marks. Resistance is ΔP/flow; where ΔP is not only MAP and flow is volume/time. The systemic vascular resistance is the resistance of several circuits in parallel, which have both common and independent factors in their regulation. As they are in parallel the sum of reciprocals is used, 1/SVR = 1/R1 + 1/R2 to determine the overall value. A detailed explanation of the Hagen-Pouiselle law was not required, attracted few marks and wasted writing time. The remainder of the answer focus was on the factors that control the radius of these vessels. As a question regarding control, an approach that included sensors, integrators and effectors tended to yield a more comprehensive answer with resultant higher marks. Other useful structures included divisions into intrinsic/local factors (including endothelial input and autoregulation), neural control (reflexes and central controller) and hormonal control. As the question was regarding physiological control, no marks were awarded to pharmacological manipulation of SVR. Given the potential scope of the question, detailed descriptions of how noradrenaline exerts its effect were not required beyond receptor level although stating ‘sympathetic nervous system activation results in vasoconstriction’ were too simplistic to attract full marks.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | FRC change with preoxygenation (100% O2 in supine) O2 physiology and pharmacology. Calculate total O2 content when sats 90 to 95% and pO2 from 60 to 75mmHg) PO2 changes in oxygen cascade V/Q ratios in erect |
| G. CVS | Stroke volume change with heart rate Distribution of blood in different parts of circulation CVP components |
| H. Renal | Principles of haemodialysis Renal blood flow regulation Urea production, effective osmole, renal tubular function |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | ABG Interpretation (fiO2 0.5, pH 7.1, pCO2 25, pO2 100, HCO3 7 BE -19.9 Na 133 K 4.5 Cl 105 AG 25 Lactate 7) |
| K. Neuro | GABA-A receptors: structure and function GABA-A receptors: structure and function Consequence of interrupting vagal transmission at C2, PNS, Cranial nerve reflexes How is pain sensed following a skin incision |
| L. Musculoskeletal | Neuromuscular monitoring, physiology and pharmacology Ach receptor at NMJ |
| M. ANS | |
| N. Liver | Hepatic lobule, label structures |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | Viscoelastic haemostatic assays (TEG, ROTEM) |
| R. Thermoregulation | |
| S. Immunology | innate immunity IgE mediated anaphylaxis |
| T. Microbiology | Gentamicin pharmacology Pharmacological properties to consider when chosing alternative option to meropenem with examples |
| U. Endocrine | how are fluctuations in blood sugar minimized between meals, Blood sugar, metabolic responses to insulin |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |


Recent Comments