1. Describe the regulation of body water.
CICMWrecks Answer
H2O
Body H2O content (or TBW state) is determined by the body’s H2O balance (daily H2O
intake vs loss) → normally, it is balanced (as per table below):
| Daily H2O Intake | |
|---|---|
| Drinking | 1200 ml |
| Food | 1000 ml |
| Metabolism (Eg. ETC) | 350 ml |
| Total Intake | 2550 ml/day (in 70kg adult) |
| 25-35 ml/kg/day | |
| Daily H2O loss | |
|---|---|
| Urine | 1500 ml (includes obligatory loss ~ 430ml) |
| Insensible losses (skin, lungs) | 900 ml |
| Faecal | 100 ml |
| Sweat | 50 ml |
| Total loss | 2550 ml/day |
- Note: Abnormal TBW states arise when an imbalance in body H2O exists:
- ↓ TBW (“H2O deficit” → due to H2O loss > intake) → results in ↑ plasma osmolality due to a relative ↑ plasma [Na+] → associated with ↓ ECFV (and PV)
- ↑ TBW (“H2O excess” → due to H2O intake > loss) → results in ↓ plasma osmolality due to a relative ↓ plasma [Na+] → associated with ↑ ECFV (and PV)
Control of TBW
TBW state is controlled via –ve feedback system as follows:
Sensors
- Osmoreceptors (anterior hypothalamus)
- Responds to ↑ plasma osmolality. Very sensitive (detects 1% change) → threshold for stimulation is 280 mosm/kg (near lower normal limit) → steep linear rise in response > 290 mosm/kg
- Low-pressure baroreceptors (right atrium and great vessels)
- Responds to ↓ plasma volume indirectly by ↓ CVS PHYDROSTATIC (↓ MAP) → ↓ sensitive cf. osmoreceptors (detects 5-10% ∆ in PV)
- High-pressure baroreceptors (carotid sinus and aortic arch)
- Responds to ↓ plasma volume indirectly by ↓ CVS PHYDROSTATIC (↓ MAP) → Even ↓ sensitive cf. osmoreceptors (detects > 10% ∆ in PV → large H2O deficits) → BUT its response overrides that of the osmoreceptors!
Effectors
Hypothalamus integrates afferent signals from these sensors and modulates an
appropriate effector response that includes:
- Thirst response → triggered by:
- ↑ plasma osmolality
- ↓ plasma volume (or ↓ MAP)
- AT-II (acting on circumventricular organs (SFO/OVLT)
- ADH
- 9 a.a peptide hormone synthesised in hypothalamus (SON/PVN) → transported to posterior pituitary where it is secreted by:
- ↑ plasma osmolarity (main trigger)
- ↓ plasma volume (or ↓ MAP) → note that LARGE ∆ in PV (> 10%) can override response by osmoreceptors (Ie. ADH is secreted irrespective of plasma osmolality)
- Other stimuli: AII, pain, nausea/vomiting, exercise
- 9 a.a peptide hormone synthesised in hypothalamus (SON/PVN) → transported to posterior pituitary where it is secreted by:
- Effects:
- Via V1 receptor (GPCR via Gq → activates PLC to ↑ IP3 → ↑ IC [Ca2+] → SM contraction) → causes ↓ GFR to ↓ glomerular filtration (and loss of) H2O → via:
- Renal afferent arteriolar constriction
- Renal mesangial cell contraction
- Via V2 receptor (GPCR via Gs → activates AC to ↑ cAMP → activates PKA) → this causes:
- Upregulates insertion of luminal AQP2 (stored in vesicles) in all parts of CD → ↑ H2O permeability → ↑ H2O reabsorption into hypertonic medullary interstitium
- Upregulates “urea transporters” in inner MCD → ↑ permeability to urea → ↑ urea absorption to maintain ↑ medullary osmolality (strengthens CCM) → promotes ↑ H2O reabsorption
- ↑ Na+ reabsorption and K+ secretion by principal cells of CCD
- Via V1 receptor (GPCR via Gq → activates PLC to ↑ IP3 → ↑ IC [Ca2+] → SM contraction) → causes ↓ GFR to ↓ glomerular filtration (and loss of) H2O → via:
Bianca 2016
Examiner Comments
2021B 01: 28% of candidates passed this question.
This is a level 1 topic. An understanding as to how the body regulates water is crucial to the daily practice of critical care, this topic is well described in the major texts. This type of question lends itself to the basic template of sensor mechanisms, central processing and integration with effector limbs and feedback loops. However, high scoring answers require a quantification of responses and an introduction into how these processes are integrated and fine-tuned.
2. Describe the pharmacology of lidocaine.
Examiner Comments
2021B 02: 71% of candidates passed this question.
The answers for this question were generally of a good standard. Lidocaine is a core drug in intensive care practice and thus a high level of detail was expected. This question was best structured using a standard pharmacology template (pharmaceutics, pharmacokinetics and pharmacodynamics). A small number of answers omitted any pharmaceutic elements. Another common error was the use of vague and imprecise statements. For example, many answers stated that the maximum dose (without adrenaline) is 3 mg/kg, without specifying that this is subcutaneous.
The concept of the ratio of the dose required to produce cardiovascular collapse to that required to induce seizures (CC/CNS ratio) was often mentioned. However, in many cases this was conveyed simply as an abbreviated statement without any additional explanation leaving the examiner unsure as to whether the candidate understood the concept (and thus unable to award any additional marks). In addition, many candidates confused the order of this ratio (incorrectly referring to it as a CNS/CC ratio of 7). Lastly, few answers made specific mention of the narrow therapeutic index and the associated implications for use in the ICU.
3. Discuss the physiological determinants of cardiac output.
CICMWrecks Answer
Cardiac output is the amount of blood pumped into the systemic circulation per unit time.
Normal Cardiac Output = 5l/min
Normal Cardiac Index = 3l/min/m2
Cardiac Output = SV x HR
Stroke Volume
SV = Amount of blood pumped into the circulation per contraction
Usually 70mls
Influenced by Preload, Afterload, Contractility
- Preload / Venous Return:
- = (MSFP – RAP) / RVR
- MSFP: Mean Systemic Filling Pressure
- Equilibration of pressures in the systemic circulation if cardiac output was abolished
- Describes the filling state of the circulation and tone of capacitance vessels
- RAP: Right Atrial Pressure
- RVR: Resistance to Venous Return
- Calibre of transmitting venous system according to Poisuille-Hagen equation (where resistance ∝ 1/r4)
- Physical obstructions to venous return
- Mechanical obstruction
- Valvular stenosis
- MSFP: Mean Systemic Filling Pressure
- Thoracic pump (negative intrathoracic pressure created during inspiration)
- Skeletal muscle pump
- One way valves
- Pump function of the ventricle
- Venous tone
- = (MSFP – RAP) / RVR
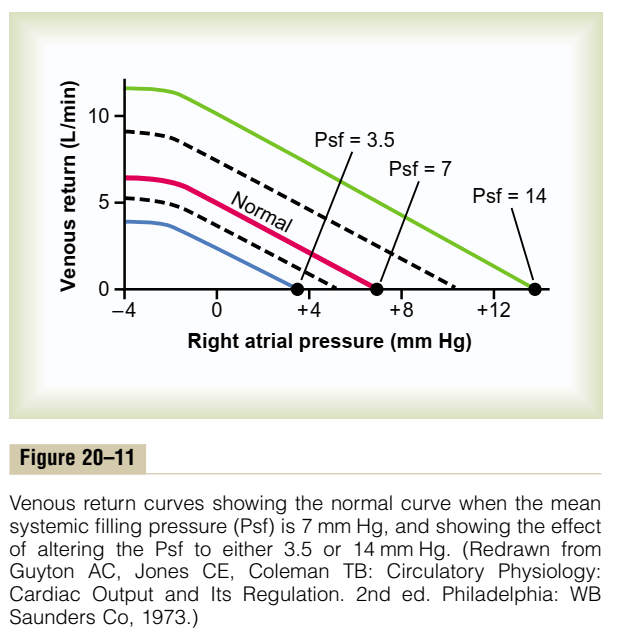
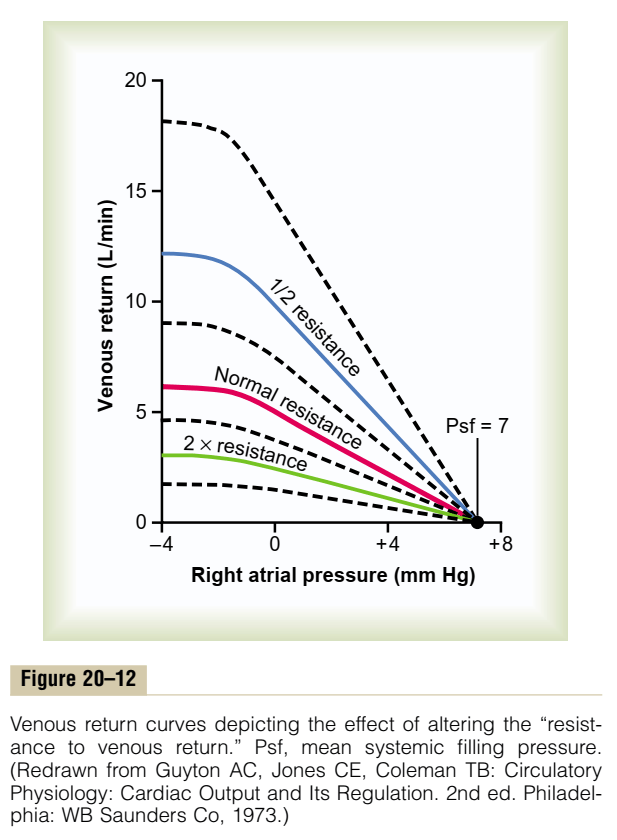
- Afterload
- Proportional to aortic pressure and ventricular size
- Inversely proportional to ventricular wall thickness
- Affected by obstruction to the LVOT
- Contractility
- Affected by adrenergic or muscarinic stimulation
- Availability of Ca
Heart Rate
- Affected by automatic rhythmicity of cell and sympathetic/muscarinic input
Cardiac Output regulated by venous return
- Ventricles have ability to adapt to various degrees of filling according to the Frank-Starling Principal
- Increasing preload increases cardiac output to a degree, before cardiac output falls
- Due to the optimal alignment of actin and myosin filaments at sarcomere length of 2.2μm
- Also due to some stretch related release of Ca2+ increasing contractility
Autonomic Regulation
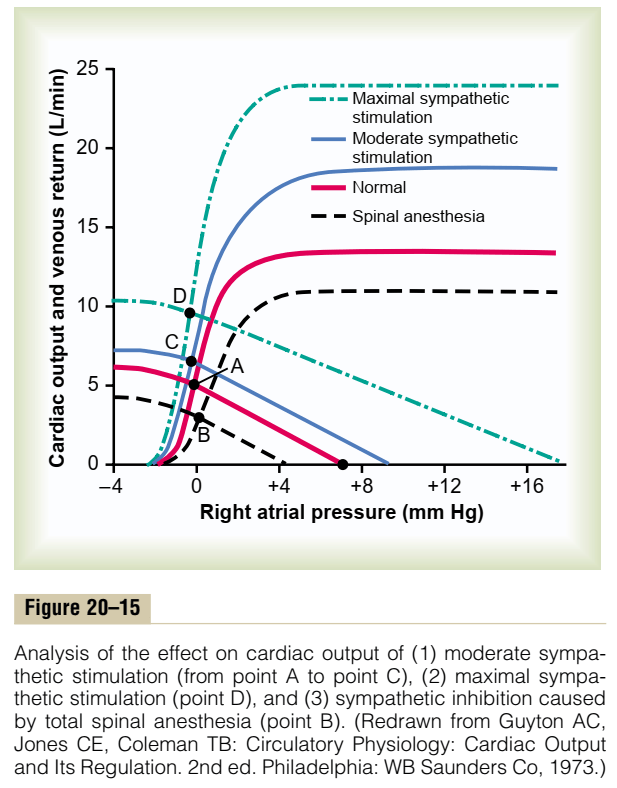
- Medullary vasomotor area
- Inputs to sensory area in NTS
- Vasoconstrictor area in anterolateral upper medulla
- Sympathetic output
- Vasodilator area in anterolateral lower medulla
- Parasympathetic output
- Adrenaline and to a lesser extent noradrenaline stimulates β2 adrenoceptors
- GsPCR
- Increased cAMP due to upregulation of adenylyl cyclase
- Activation of PKA leading to phosphorylation and activation of intracellular enzymes
- Inotropy
- Increased Ca2+ release from and SR and influx from T-tubules
- Increased intracellular [Ca2+]
- Increased Ca2+ – troponin C interaction
- Increased Actin and myosin interaction due to exposure of myosin binding sites
- Increased contractility
- Chronotropy
- Inotropy
- Acetylcholine
- GiPCR → decreased cAMP and reversing the above
- Muscarinic receptor coupled K+ channels hyperpolarize the cell reducing chance for action potential
- At rest
- Parasympathetic and Sympathetic tone
- Parasympathetic > Sympathetic
- HR ~60
- + Propranolol → HR 50
- + Atropine → HR 110
- + Propranolol + Atropine → HR 100
- HR ~60
- Parasympathetic > Sympathetic
- Parasympathetic and Sympathetic tone
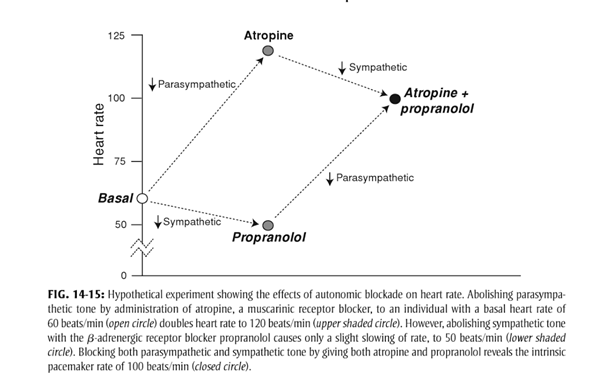
Reflexes
Baroreflex
- Sensor
- Aortic arch and carotid sinus stretch receptors
- Transmit via vagal nerve and glossopharyngeal nerve respectively
- Control centre
- Vasomotor area in medulla
- Increased stretch causes inhibition of sympathetic and promotion of parasympathetic output
- Effector
- Parasympathetic innervation of the heart via vagal nerve upregulated
- Sympathetic innervation via thoracic segments of the sympathetic chain
Bainbridge reflex
- Input
- Increased venous return or obstruction to atrial outflow causes atrial stretch
- Atrial stretch receptors signal to vasomotor area
- Increased sympathetic output to heart à tachycardia
Sakurai 2016
Examiner Comments
2021B 03: 65% of candidates passed this question.
Although the pass rate for this question was reasonably high the examiners commented on a lack of detailed knowledge within most answers for such a core component of our daily practice. Several candidates failed to provide a normal value and only few provided anything other than 5l/min. There was a general lack of detail, and at times, some confusion about the Frank Starling effect. Most candidates outlined the major determinants of stroke volume, although many were light on the
determinants of each or incorporated incorrect facts. Several candidates did not mention HR as a determinant of CO.
4. Compare the pharmacology of fluconazole and amphotericin.
Examiner Comments
2021B 04: 6% of candidates passed this question.
This question exposed an area of the syllabus neglected by the candidates. Answers were generally vague in detail with lots of incorrect facts and generally displayed a very limited knowledge. Antifungal agents are regularly used in critically ill patients either as treatment or prophylaxis. An understanding of the aspects of these drugs with respect to spectrum of activity, mechanism of action, specific PK and PD properties as well as potential side effects would have been the basis for this compare and contrast question. Examiners want to be convinced that the candidates understand the strengths and weaknesses of each drug and in which circumstances one agent might be used in preference to the other.
5. Write detailed notes on angiotensin, including its synthesis, role within the body and regulation.
CICMWrecks Answer
Angiotensinogen is a peptide hormone produced by the liver
- Angiotensin 1 (decapeptide)
- ↑ Renin by JGA → cleavage of Angiotensinogen → AT1
- No biological activity
- Angiotensin 2 (octapeptided)
- ACE in pulmonary capil endothelia
- → ↑AT2
- Angiotensin 3 (septapeptide)
- 40% of the pressor activity AT2
- 100% of the aldosterone-producing activity
- Angiotensin 4 (hexapeptide)
- Minimal biological activity
Actions of AT2 include:
- Acts via GPCR (Gq via PLC to increase IP3 and intracellular Ca)
- CVS
- Vasopressor
- Resets baroreceptor control of HR at higher pressure
- Potent mitogen for smooth muscle and cardiac myocytes
- Direct positive inotrope
- CNS
- ↑ sympathetic outflow
- ↑ thirst
- ↑ ADH release from posterior pituitary.
- Renal
- Negative feedback on renin release
- ↑ aldosterone release
- ↓ RBF and GFR
- Direct renal arteriole constriction (efferent = afferent)
- Mesangial cell contraction thus ↓ Kf and GFR
- ↑ sodium/chloride reabsorption in PCT
- Direct effect and via ↑ aldosterone release
Factors which alter renin release cause a corresponding change in Angiotensin 2
- Stimulation of renin secretion (↑ renin → ↑ angiotensin 2)
- β-1 agonism
- ↓ renal perfusion pressure
- ↓ sodium delivery to DCT
- Prostaglandins
- Inhibition or renin secretion (↓ renin → ↓ angiotensin 2):
- ↑ renal perfusion pressure (via afferent arteriolar dilatation)
- ↑ sodium delivery to DCT
- ↑ by angiotensin II (negative feedback) and vasopressin
Gladwin 2016
Examiner Comments
2021B 05: 24% of candidates passed this question.
This question provided headings for the answer template. Good answers integrated the required facts from the appropriate chapters of the major texts. Most answers lacked detail surrounding the factors that increase or decrease angiotensin activity. Few answers provided any detail as to all the mechanisms through which angiotensin exerts it effects. A lot of answers focussed singularly on the vascular effects of angiotensin. Overall, there was often a paucity of detail, with vague statements and incorrect facts.
6. Describe the functions of the placenta (80% marks). Outline the determinants of placental blood flow (20% marks).
CICMWrecks Answer
Functions of the Placenta
- Organ of foetal + maternal origin; supports developing foetus
- Low pressure, low resistance AV shunt that provides metabolic nutrients necessary for growing foetus
- Functions
- Transfer: gas exchange; nutrient + waste exchange; drugs; heat
- Immunological barrier
- Metabolic
- Endocrine
1. Transfer
a. Gas Exchange
- Diffusion dependent on Fick’s Principle
- Maternal placental flow ~600ml/min at term (2x foetal flow) → ↑diffusion by ↑concentration gradient for solutes
- Molecules <600Da more readily diffuse down concentration gradient
- O2 diffusion
- PO2 entering placenta via uterine artery = 18mmHg (SpO2 45%)
- PO2 leaving placenta via uterine vein 28mmHg (SpO2 70%)
- Foetus able to absorb large enough vol O2 despite low PO2 because:
- Foetal Hb
- 2 y subunits cf β → prevent binding of 2,3-DPG → L shifted OHDC → favours O2 loading at ↓PaO2
- [FHb] 50% > maternal [Hb]
- Double Bohr effect
- Describes Bohr effects happening on either side of placental gas exchange in mother + foetal circulations
- Accounts for 2-8% of O2 transfer
- Bohr effect 1: placenta: foetus unloads CO2 in placenta → ↑placental CO2 → RIGHT shift HbA OHDC → ↓HbA affinity for O2 → ↑placental O2 unloading
- Bohr effect 2: foetus: foetus unloads CO2 → ↓foetal CO2 → LEFT shift HbF OHDC → ↑HbF affinity for O2 → ↑foetal O2 binding/ uptake
- Foetal Hb
- CO2 diffusion
- Foetal PaCO2 50mmHg; intervillous PCO2 37mmHg
- CO2 offloading favoured in foetus by:
- High foetal [Hb] ↑s amount of CO2 that can be carried as carbaminoHb
- Double Haldane effect
- Haldane effect: describes how ∆O2 sat of Hb affect CO2 transport: deoxyHb has ↑affinity for CO2 than oxyHb
- Double Haldane: describes Haldane effects happening across the placenta
- Accounts for 45% of CO2 transfer between maternal and foetal circulation
- Haldane 1: placenta: placenta unloads O2 → ↓placental O2 → deoxyHbA has ↑affinity of CO2 → ↑placental CO2 uptake from foetus
- Haldane 2: foetus: foetus takes up O2 → ↑HbF O2 → ↓HbF affinity for CO2 → ↑HbF CO2 release to placenta
b. Nutrient delivery
- Nutrient diffusion
- High foetal caloric requirements in late pregnancy
- Facilitated diffusion of glucose via carrier molecules in trophoblasts
- Active transport for amino acids, Ca2+, Fe, folate, vit A and C
c. Waste removal – Urea, Uric acid, Creatinine, Br
d. Heat transfer
2. Immunological function
- permeable to IgG via pinocytosis → allows maternal abs to provide passive immunity to foetus
- Trophoblast cells lose many cell surface MHC molecules → making them less immunogenic; also cells cover themselves in mucoprotein which
disguises them from maternal immunie system - Chorionic cells act as immunological barrier – preventing maternal T cells and abs from reaching foetal circulation
- Some bacteria (listeria) and viruses (rubella, parvovirus B19, HIV) can cross into foetal circulation
- Progesterone + alpha-foetoprotein produced by yolk sac act as maternal immunosuppressive agents
3. Metabolic
- synthesis of glycogen, cholesterol, FA, enzymes
4. Endocrine function
- synthesis of 4 main hormones:
- bHCG
- hPL: human placental lactogen (human chorionic somatomammotrophin)
- Oestriol
- Progesterone
- Synthesis of other hormones and growth factors
- Placental corticotrophin
- Human chorionic somatostatin
- Human chorionic thyrotropin
- Epidermal growth factor
- Somatomedin
Placenta
- Placenta:
- temporary fetal organ that begins developing from the blastocyst shortly after implantation
- ~22cm length, 2.5cm thickness. ~500gm
- Connects to fetus by umbillical cord
- “Chorionic villus” is the basic structural unit of the placenta → it is a vascular projection of foetal tissue that is bathed by maternal blood within the “Intervillous space”. It consists of:
- Foetal connective tissue containing foetal capillaries
- Chorion → outermost layer of foetal tissue that is made of 2 layers →
- (a) Syncytiotrophoblast (directly contacts maternal blood in intervillous space) and
- (b) Cytotrophoblast (b/t syncytiotrophoblasts and foetal CT)
Placental Circulation
- Placental circulation = Two circulation systems in parallel – maternal and the fetal
- Maternal circulation system
- Uterine Arteries (600ml/min, 100mm Hg) → arcuate arteries → radial arteries → spiral arteries (70 mm Hg) & basal arteries (myometrium and deciduas)
- Spiral arteries → intervillous spaces (10mm Hg) → uterine veins that are arranged in the periphery of the intervillous space.
- Blood in the intervillous space is exchanged 2-3 times per minute.
- Fetal circulation system:
- Two villus Umbilical Arteries (50mmHg) → finer vessels that cross through the chorionic plate → villus capillaries (30mmHg) → leaves placenta through umbillical vein (20mmHg)
- Their supply amounts to approximately 40% of the fetal heart blood volume per minute.
- The pressure in the fetal vessels and their villus branches always lies over that of the intervillous space. This protects the fetal vessels from collapse.
- Substances traverse between foetal and maternal blood via the following layers:
- Maternal blood (intervillous space) ↔ Chorion (2x layers of trophoblasts) ↔ Foetal connective tissue ↔ Endothelium of foetal capillaries ↔ Foetal blood
Determinants of Placental Blood Flow
Uteroplacental blood flow
- At term → uteroplacental BF is 500-700 mL/min (10% maternal C.O.) of which:
- 70-90% of this BF enters the intervillous space (via the spiral arteries) → NOT autoregulated as blood flow is “pressure-dependent” (see factors below)
- 10-30% of this BF supplies the myometrium/deciduas (via the basal arteries) → autoregulated blood flow
- Blood flow to the intervillous space (which participates in substance exchange with foetal blood) is affected by the following factors:
- Uterine arterial pressure (UAP):
- Maternal arterial BP → ↓ MABP (Ie. due to SNS block 2° neuraxial block,
hypovolaemia, supine hypotension syndrome, Etc.) causes ↓ UAP → ↓ UBF
- Maternal arterial BP → ↓ MABP (Ie. due to SNS block 2° neuraxial block,
- Uterine venous pressure (UVP):
- Uterine tone and contractions → ↑ tone/contractions (Ie. due to contractions,
oxytoxics, ketamine, Etc.) causes ↑ UVP → ↓UBF
- Uterine tone and contractions → ↑ tone/contractions (Ie. due to contractions,
- Uterine vascular resistance (UVR):
- Uterine arteriolar tone → ↑ vasoconstriction (Ie. a/w essential HT and PET, α-adrenoceptor stimulation (by endogenous SNS innervation, catecholamines or sympathomimetics), and vasopressin) causes ↑ UVR → ↓ UBF
Umbilical blood flow
- At term → umbilical BF is 360 mL/min (25-50% of foetal C.O. (≈ 1000 mL/min))
- It is “autoregulated” (cf. uterine BF) → involves vasodilators (PCI-2/NO) derived from vascular endothelium
- BF is ↓ with severe hypoxia, ↑ BGL, catecholamine and cord compression
Kerr / Bianca 2020
Examiner Comments
2021B 06: 49% of candidates passed this question.
There was a wide range of marks for this question with a few candidates scoring excellent marks. Those answers that scored well provided a comprehensive list of functions as well as an explanation as to the what, how and/or why of these functions. Poorer answers omitted some of the functions or failed to elaborate on them by providing only a limited list. The second component of the question was generally well outlined, most candidates provided some estimate of normal values at term and a simple elaboration regarding the factors that affect placental blood flow.
7. Outline how the measurement of the following can be used in the assessment of liver function (25% marks of each):
• Albumin
• Prothrombin time
• Glucose
• Ammonia
CICMWrecks Answer
Table of LFT values and clinical significance
(LIMITED)
| Test | Reference Range | Clinical Significance |
|---|---|---|
| Serum proteins (including albumin, globulin, fibrinogen) | Total protein 60-80 g/L Albumin 35-45 g/L Globulin 25-35 g/L Fibrinogen 2-4 g/L | Liver synthesises nearly all plasma proteins (except Ig’s) |
| ↓ serum total protein, albumin, globulin and fibrinogen levels suggest impaired synthetic function due to hepatocellular damage | ||
| Non-specific → also can suggest nephrotic syndrome, malnutrition states, burns, Etc. | ||
| Note – Albumin is a long-term marker of liver function (as t ½ 20 days) → ↓ albumin means chronic liver dysfunction (as it can be normal with acute liver disease) | ||
| Coagulation studies (INR and PT) | INR >1.2 PT 10-15 s | Hepatocytes synthesise clotting factors (incl vitamin K-dependent CFs) |
| ↑ PT or INR suggests either (i) impaired CF synthesis (hepatocellular damage → acute liver dysfunction), or (ii) vitamin K malabsorption (cholestasis) | ||
| Non-specific → also can suggest warfarin use, DIC, Etc. | ||
| Plasma glucose | BGL 4-8 mmol/L | Liver has vital role in glucostat function (glycolysis, glycogen metabolism, gluconeogenesis) |
| BGL derangements suggest hepatocellular dysfunction | ||
| Non-specific → also can suggest DM, insulin use, Etc. | ||
| Plasma ammonia/urea | NH3 < 35 umol/L Urea 3-7 umol/L | Liver converts toxic NH3 (metabolite of a.a. and pyrimidine) into less toxic urea for renal excretion via the “urea cycle” |
| ↑ NH3 and ↓ urea suggests hepatocellular damage |
Bianca 2020
Full Table
Liver Function Tests
Principles of liver function tests (LFTs):
- They indicate presence of liver injury
- They test hypotheses about types of hepatobiliary pathophysiology → BUT do NOT ascertain precise cause of hepatobiliary disease (Ie. need biopsy, serology, Etc.)
- The tests are very sensitive BUT non-specific (Eg. many other causes of derangements)
Table of LFT values and clinical significance
| Test | Reference Range | Clinical Significance |
|---|---|---|
| Synthetic function | ||
| Coagulation studies (INR and PT) | INR >1.2 PT 10-15 s | Hepatocytes synthesise clotting factors (incl vitamin K-dependent CFs) |
| ↑ PT or INR suggests either (i) impaired CF synthesis (hepatocellular damage → acute liver dysfunction), or (ii) vitamin K malabsorption (cholestasis) | ||
| Non-specific → also can suggest warfarin use, DIC, Etc. | ||
| Serum proteins (including albumin, globulin, fibrinogen) | Total protein 60-80 g/L Albumin 35-45 g/L Globulin 25-35 g/L Fibrinogen 2-4 g/L | Liver synthesises nearly all plasma proteins (except Ig’s) |
| ↓ serum total protein, albumin, globulin and fibrinogen levels suggest impaired synthetic function due to hepatocellular damage | ||
| Non-specific → also can suggest nephrotic syndrome, malnutrition states, burns, Etc. | ||
| Note – Albumin is a long-term marker of liver function (as t ½ 20 days) → ↓ albumin means chronic liver dysfunction (as it can be normal with acute liver disease) | ||
| Serum platelet and Hb | Hb 100-130 g/L Plt 150-450 x 109/L | Liver produces TPO (and 10% of EPO) → ↓ Hb and platelet counts suggest hepatocellular damage |
| Non-specific → other causes of anaemia (Eg. Fe and vitamin B12 deficiencies, haemolysis, Etc.) and → ↓ platelets (Eg. ITP, HITS, Etc.) | ||
| Metabolic function | ||
| Plasma ammonia/urea | NH3 < 35 umol/L Urea 3-7 umol/L | Liver converts toxic NH3 (metabolite of a.a. and pyrimidine) into less toxic urea for renal excretion via the “urea cycle” |
| ↑ NH3 and ↓ urea suggests hepatocellular damage | ||
| Plasma glucose | BGL 4-8 mmol/L | Liver has vital role in glucostat function (glycolysis, glycogen metabolism, gluconeogenesis) |
| BGL derangements suggest hepatocellular dysfunction | ||
| Non-specific → also can suggest DM, insulin use, Etc. | ||
| Serum bilirubin (total and direct bilirubin) | Total biliruin < 20 umol/L Direct bilirubin < 5 umol/L | Unconjugated (indirect) bilirubin is produced in RE system from breakdown of haem from haemproteins (esp Hb) → conjugated in liver before it is excreted in bile |
| Total and direct bilirubin levels are measured → indirect levels are calculated from these figures | ||
| ↑ total bilirubin can be due to: (i) ↑ direct bilirubin → suggests cholestasis due to impaired excretion of bile (ii) ↑ indirect bilirubin → suggests a prehepatic cause (Eg. haemolysis, Gilbert’s syndrome) (iii) ↑ direct and indirect bilirubin (mixed) → suggests hepatic disease a/w impaired hepatic uptake and conjugation of bilirubin | ||
| Hepatocellular injury | ||
| Serum aminotransferases (AST and ALT) | AST < 30 IU/L ALT < 20 IU/L | AST and ALT are enzymes found in hepatocyte mitochondria → ↑ AST and ALT indicates hepatocellular damage → release enzymes in blood |
| ALT is specific to hepatocytes only → so ↑ ALT:AST ratio strongly suggests hepatocellular damage | ||
| AST is less specific to hepatocytes → also found in heart, RBC, skeletal muscle → so ↓ ALT:AST ratio less likely a/w hepatocellular dysfunction | ||
| Serum lactate dehydrogenase (LDH) | LDH 140-280 IU/L | LDH is found in hepatocytes → ↑ LDH with hepatocellular damage → release enzymes in blood |
| Non-specific → also found in heart, pancreas, RBC, skeletal muscle, lungs, placenta → MI, cancer, haemolysis, pancreatitis, Etc. | ||
| Cholestasis | ||
| Serum alkaline phosphatase (ALP) and γ-glutamyltranspeptidase (GGT) | ALP < 100 IU/L GGT < 50 IU/L | ALT and GGT are enzymes found on hepatocytes AND ductal cells along bile duct/canaliculus |
| ↑ ALP and GGT suggest hepatobiliary disease (intra- and extra hepatic cholestasis) | ||
| GGT ↑ alone is non-specific → can be induced by EtOH or drug ingestion | ||
| ALP ↑ alone is non-specific → it is produced elsewhere (Ie. bone → Paget’s disease; placenta) | ||
Bianca 2020
Examiner Comments
2021B 07: 54% of candidates passed this question.
This was a new question and overall, most candidates provided some detail on each component as requested. Those answers that used a simple template for each section generally scored better than those who wrote in a paragraph style for each section. Areas expected to be covered included the following; a definition of the variable to provide context, a normal value and the range of influences that effect the variable both related to liver function and or extrinsic to the liver (attempting to introduce the concepts of sensitivity and specificity for each test). Stronger answers provided some context as to whether the variable was sensitive to acute or chronic changes in liver function and which synthetic/metabolic component of the liver the variable represented.
8. Describe the anatomy of the internal jugular vein including surface anatomy landmarks relevant to central venous line insertion.
CICMWrecks Answer: IJV (Internal Jugular Vein) Anatomy
Origin / Course / Termination
- Originates at the jugular foramen where it continues the sigmoid sinus
- Exits the foramen accompanies by the glossopharyngeal (IX), vagus (X) and accessory (XI) nerves
- runs caudally down the neck – The vein lies most superficially in the upper part of the neck
- The vein lies lateral, first, to the internal carotid artery, separated by the hypoglossal (XII) nerve, and then to the common carotid artery, with the vagus nerve between and posterior to the artery and vein.
- These three structures lie in a common fascial compartment (the carotid sheath) with the cervical sympathetic chain lying posterior to the sheath.
- These four structures, two vessels and two nerves, form a quartet throughout the neck.
- The deep cervical chain of lymph nodes lies close against the carotid sheath
- The vein is initially posterior to, then lateral and then anterolateral to the carotid artery during its descent in the neck
- Terminates behind the sternoclavicular joint, where it joins the subclavian vein to form the brachiocephalic vein
Relations of the IJV
- Anterior –
- Internal carotid artery and vagus nerve
- Superficially, the internal jugular vein is overlapped above and then lower down covered by the sternocleidomastoid muscle and is crossed above by the posterior belly of the digastric and lower down by the inferior belly of the omohyoid
- Posterior –
- C1, sympathetic chain, dome of the pleura. On the left side, the IJV lies anterior to the thoracic duct
- Posteriorly, the vein rests on (from above downwards) the rectus capitis lateralis, the transverse process of the atlas, the scalenus medius, and the scalenus anterior, with the phrenic nerve crossing it; and all are covered by the precervical fascia
- Medial – Carotid arteries, cranial nerves IX-XII
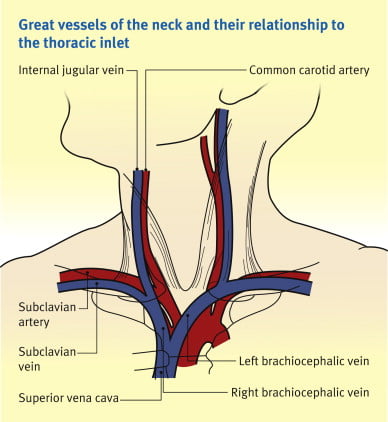
Anaesthesia & Intensive Care Medicine,
https://doi.org/10.1016/j.mpaic.2009.10.005.
Surface Anatomy for Venepuncture
- Lies lateral to the carotid artery within triangle formed by: Two heads of Sternocleidomastoid muscle and clavicle
- On Ultrasound: Non-pulsatile, thin walled, compressible vessel lateral to the carotid artery
Anatomical Variations
- The right IJV (80.5%) was more often larger than the left IJV.
- With reference to the CCA, 85.2% of the IJV were found in the lateral position, 12.5% anteriorly, 1.1% medially and 1.1% posteriorly.
- IJV were sometimes found to be hypoplastic, and in rare cases this was seen bilaterally in both the right and left IJV.
- 5.5% have an IJV that does not correspond to the site predicted by external markings
- Other variations:
- IJV bifurcation
- IJV duplication
- IJV fenestration
- IJV trifurcation
- Posterior tributary
Sources:
Ellis H. The clinical anatomy of the great veins of the neck. Br J Hosp Med (Lond). 2010 Feb;71(2):M20-1. doi: 10.12968/hmed.2010.71.Sup2.46501. PMID: 20220708.
Asari AIA, Barros RAV, Borges MAP. Anatomic variant of the internal jugular vein and its importance in vascular access for hemodialysis. J Vasc Bras. 2019 Oct 23;18:e20190014. doi: 10.1590/1677-5449.190014. PMID: 31692937; PMCID: PMC6822959.
Gladwin / JC 2020
Examiner Comments
2021B 08: 38% of candidates passed this question.
The overall pass rate for this question was poor considering how relevant this area of anatomy is in our daily practice. Better scoring answers used a template including a general description, origin, course and relations, tributaries and as requested in this question, the surface anatomy. Many answers that scored poorly only provided the briefest detail, were vague in their descriptions and incorrect with respect to the facts presented or imprecise with respect to the terminology used.
9. Outline the classification and effects of beta-blocking drugs, including examples (50% marks). Compare and contrast the pharmacokinetics of metoprolol with esmolol (50% marks).
CICMWrecks Answer
Beta receptor antagonists (Beta blockers)
- bind to beta-adrenoceptors and block the binding of noradrenaline and adrenaline
- Inhibits normal sympathetic effects that act through these receptors (Sympatholytic)
- mostly competitive antagonists, there is some evidence of partial agonist activity (Labetalol)
- Some (partial agonists) show intrinsic sympathomimetic activity (ISA): partially activate receptor + prevent noradrenaline from binding to the receptor.
- Some possess membrane stabilizing activity (MSA) similar to sodium-channels blockers
- variable specificity for beta 1 versus beta 2 receptors
- important clinically due to different effects
- often dose related, may be B1 selective at low doses but non-selective at higher
- lipid solubility determines speed of onset
- most resemble isoproterenol
Effects of β-adrenergic blockade
| Type | Main site of action | Effects of β-adrenergic blockade |
|---|---|---|
| β1 | Heart | Anti-ischemic effect: β1 blockade → ↓ heart rate and ↓ cardiac contractility → ↓ BP and ↓ oxygen consumption by the heart → anti-ischemic effect |
| Antiarrhythmic effect: β1 blockade → ↓ AVN conduction, ↑ AVN refractory time, and ↓ heart rate → anti-arrhythmic effect | ||
| Anti-remodeling effect | ||
| Kidneys | β1 blockade of the juxtaglomerular cells → ↓ renin release → ↓ angiotensin II conversion → ↓ H2O resorption → ↓ BP | |
| β2 | Smooth muscle | Vasculature: vasoconstriction Bronchioles: bronchoconstriction |
| Ciliary body of the eye | ↓ Aqueous humor production → ↓ intraocular pressure | |
| Pancreatic beta cells | ↓ Insulin release → hyperglycemia and new-onset diabetes | |
| Skeletal muscle | ↓ Glucose uptake (↓ insulin sensitivity) | |
| Liver | ↓ Hepatic glycogenolysis → hypoglycemia (esp. in diabetics) | |
| Lipoprotein lipase enzyme | Inhibits lipoprotein lipase → ↑ triglycerides and ↓ HDL → hyperlipidemia | |
| β3 | Adipose tissue | ↓ Lipolysis → weight gain |
Classification
- β1-selective (cardioselective):
- e.g. esmolol, nebivolol, bisoprolol, atenolol, metoprolol
- is used for rate control in tachycardia and hypertension management
- side effects: hypotension, heart block, bronchoconstriction at higher doses
- Non-selective β-blockade
- e.g. propranolol
- used for hypertension, to reduce bleeding risk in oesophageal varices, tremor, and as migraine prophylaxis. It is the treatment of choice in thyrotoxicosis as it stops conversion of T4 to T3, reduce ocular pressure in glaucoma
- side effects: rapid withdrawal may precipitate tachycardia, can cause bronchoconstriction and is not recommended in patients with obstructive respiratory disease, issues with hypoglycaemia
- Non-selective α- and β-blockade
- e.g. labetalol, carvedilol
- Potent vasodilators because of their α-blocking action
- Improve endothelial function and vascular re-modelling
- Others
- e.g. sotalol : also acts on K+ channels as class III antiarrhythmic
METOPROLOL | ESMOLOL
PHARMACOKINETICS
| Metoprolol | Esmolol | |
| relatively selective beta blocker with no intrinsic sympathomimetic activity. | Cardio-selective beta blocker with rapid onset and offset. | |
| PK – A | bioavailabilty Absoption is rapid and complete, however there is extensive first pass metabolism. BA 50% routes of admin PO or IV dose Oral in 12.5mg increments, IV in 1-2mg boluses | bioavailabilty Only available as IV therefore 100% routes of admin IV dose In 10mg increments titrate to effect |
| D | volume of distrib 5.5 L/Kg protien binding 10-20% to albumin lipid solubility is high so it crosses the BBB | volume of distrib 3.5 L/Kg protien binding 60% to albumin lipid solubility is high so it crosses the BBB |
| M | hepatic or renal Extensively hepatic via CYP2D6 | neither hepatic or renal! by red blood cell esterases to a mostly inactive metabolite |
| E | half life 3-8hours excretetion In urine 5-10% unchanged | half life 10 minutes excretion In urine |
Sources: CVPharmacology
JC 2019
Examiner Comments
2021B 09: 59% of candidates passed this question.
This was a two-part question with marks and thus timing of the answers given as a percentage. There are generally many ways to classify drugs within the same class. These are usually well described in the relevant recommended pharmacological texts. Receptor distribution throughout the body and the effect of the drug-receptor interaction are useful ways to organise systemic pharmacodynamic responses, as opposed to a list of organ systems with associated vague statements of interaction.
10. Describe the ventilation / perfusion (V/Q) relationships in the upright lung according to West’s zones (40%). Explain the physiological mechanisms responsible for these relationships (60%).
CICMWrecks Answer
West Zones
- Divided into zones based on relationship between Alveolar pressure (PA) Arterial pressure (Pa) Venous pressure (Pv) Interstitial pressure (Pi)
- West Zones 1,2,3: Due to changes in hydrostatic pressure when pumping to top of lung vs. bottom of lung
| West Zone | Pressure Relationships | Physiology | Location |
|---|---|---|---|
| Zone 1 | PA > Pa > Pv | No flow of blood, as arterial pressure completely opposed by alveolar pressure | not seen in normal lung |
| Zone 2 | Pa > PA > Pv | Resistance to flow is determined by alveolar pressure (Starling resistor effect) | about 3cm above the heart |
| Zone 3 | Pa > Pv > PA | Resistance to flow is determined by venous pressure Venous pooling causes increased distension of pulmonary capillaries | majority of healthy lung |
| Zone 4 | Pa > Pi > Pv > PA | Low lung volume causes narrowing of extra-alveolar vessels | Lung bases at low lung volume or in pulmonary edema |
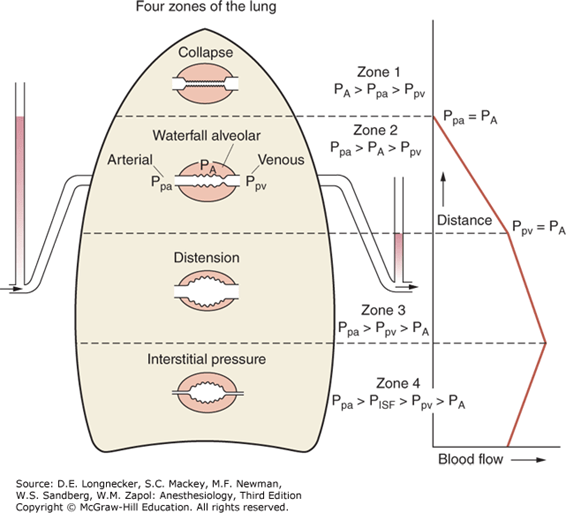
Vertical gradient of pleural pressure and alveoli
Inspiration
- Inspiratory muscle contraction → Overcomes resistance to insp flow → negative alveolae pressure -1cm H2O → VT 500ml
- Weight of lung on alveoli and pleura above:
- causes gradient of pleural pressure: Apex (-10cmH2O) Base (-2.5cm H2O)
- Apical alveoli larger
- But compliance lesser (due to baseline distension)
- Lesser ventilation for same pressure
- Apex and base on different part of compliance curve
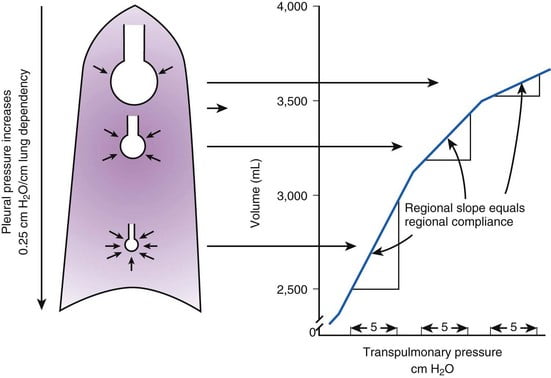
Expiration:
- Gradient of pleural pressure smaller
- Apex: -4cm H2O → on steeper part of compliance curve → ventilation better
- Base +3.5cm H2O →airway closure → gas trapping & shunt
Ventilation, perfusion, V/Q matching
Vertical segments based on V and Q
- Both V and Q ↓ as you move from base to apex.
- ↓ total number of alveoli → ↓ diffusive area → ↓ V
- ↑ compression of intra-alveolar vessels → ↑ west zone 1 → ↓ Q
- V ↓’s slower than Q
| Level | V/Q | Ventilation | Perfusion | PO2 | PCO2 |
|---|---|---|---|---|---|
| Apex | ~3 High | Lower Alveoli largest (no wt of lung) | Very Low Blood hydrostatic pressure low (high gravity) | High 132 | Low 28 |
| At level of heart | ~1 | Slightly more ventilated (Optimized) | Optimized (less effect of gravity) | 108 | 39 |
| Base | ~0.67 towards mixed venous point | Well ventilated (in normal lung): small alveoli, more compliance (effect of wt of lung) | Highly perfused Maximal Hydrostatic pressure | relativly hypoxic 89 | relatively hypercapnoeic 42 |
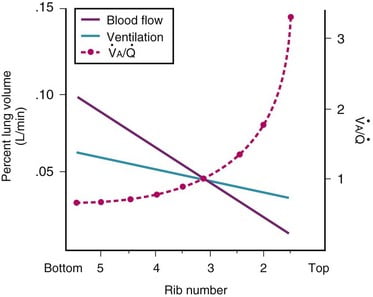
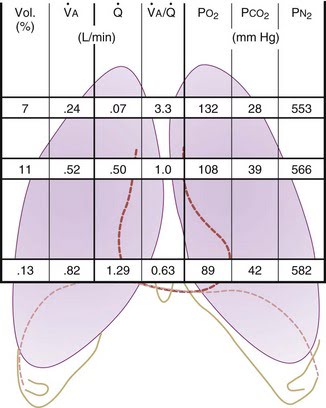
JC 2019
Examiner Comments
2021B 10: 47% of candidates passed this question.
This is a core aspect of respiratory physiology, and a detailed understanding of this topic is crucial to the daily practise of intensive care. As such the answers were expected to be detailed. Strong answers included precise descriptions of the zones of the lung as described by West and related these to the V/Q relationship in the upright lung. Generally, most candidates scored well in this section. Diagrams were of varying value. However, an impression from the examiners was that candidates spent too much time on this first section and ran out of time for a detailed answer in the second section. The answers to the second section seemed rushed and were often lacking in detail with many incorrect facts. This question highlights the importance of exam technique preparation in the lead up to the written paper.
11. Provide a detailed account of the side-effects of amiodarone.
CICMWrecks Answer
Side Effects of Amiodarone:
Side effects reflect long elimination half-life and significant accumulation in tissues. Side affects increase when maintenance doses are above 400mg daily.
- Respiratory
- Most serious is pneumonitis. Risk is 5-10% at 3 years with a mortality of 10% long term.
- May be due to increased oxygen free radicals and risk increased by high FiO2
- 2 phenotypes- slow insidious and acute onset
- May be reversible if treatment stopped early
- Cardiac
- Prolonged QTc with Torsade’s
- Proarrhythmic (ventricular tachyarrhythmia)
- Bradycardias resistant to atropine
- Peripheral vasodilation leading to hypotension with decreased responsiveness to adrenergic stimuli
- Neurological
- Peripheral neuropathy, tremor, sleep disturbance
- Myopathy
- Endocrine
- Both hyperthyroidism and hypothyroidism may occur (usually reversible)- 2-4% of patients
- Prevents peripheral conversion of T4 to T3 (detected by increased plasma [TSH])
- 37% of weight of Amiodarone is iodine, which may precipitate hyperthyroidism
- Pre-existing thyroid disease increases risk
- GIT
- Hepatitic dysfunction, LFT derangement and cirrhosis has been observed
- Metallic taste during loading
- Ophthalmic
- Corneal microdeposits but visual impairment less likely
- Optic neuropathy (insidious onset, bilateral). Often reversible if discontinued
- Dermatological
- Photosensitivity
- Slate gray pallor of the face, continues after drug discontinuation
- Pregnancy and lactation
- Avoid in from 3 months pre-pregnancy to after breast feeding has finished
- May cause hypothyroidism and bradycardia in the fetus. Foetal hypothyroidism may lead to impairment of myelination
- Contraindicated in porphyria
- Miscellaneous
- IV preparation is irritant and should be administered through a central vessel
Examiner Comments
2021B 11: 17% of candidates passed this question.
The question asked for a detailed account of the side effects of amiodarone, hence those candidates that just provided a list or outline scored less well. It was expected that candidates provide some detail of the side effect. Answers that scored well prioritised those relevant to ICU clinical practice. Many provided disorganised outlines of the side effects and frequently the cardiovascular side effects were poorly explained. Many candidates omitted the important drug interactions of amiodarone use and few candidates related the side effect profile to the duration of treatment.
12. Explain the physiological factors that affect airway resistance.
CICMWrecks Answer
Airway Resistance
- Resistance is the impedence to flow.
- Normal Airways Resistance (AWR):
- Adult: ~2 cmH2O/L/s
- Newborn: ~20 cmH2O/L/s – declines markedly
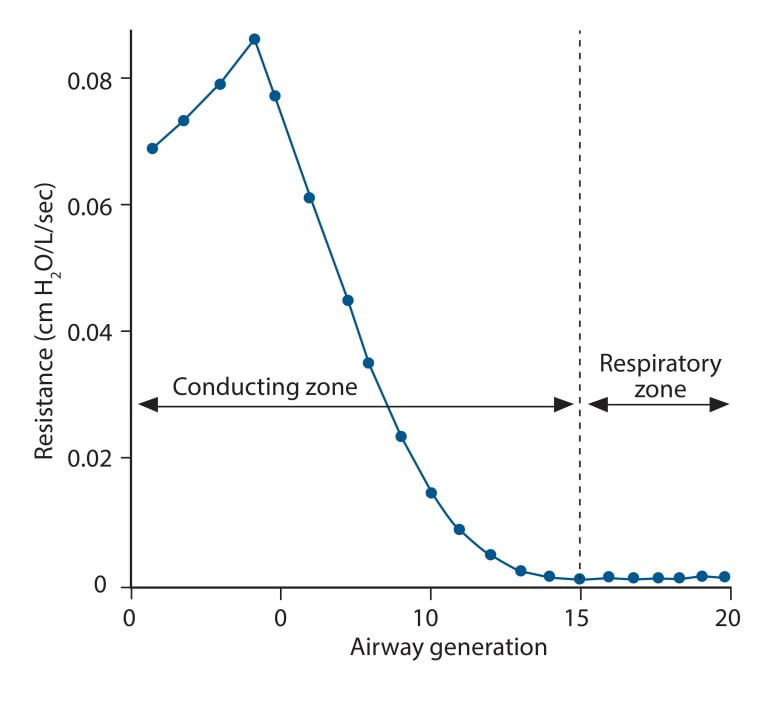
- Main Site of AWR:
- Mid-sized bronchi 7th/8th generation
- comparatively smaller cross-sectional area
- (note that in neonates, a greater proportion of Raw comes from the smaller peripheral airways)
- In the airway flow can be laminar or turbulent
- Flow depends on Depends on Reynolds number
where
Re is Reynold’s number
r is radius
ρ is density
v is velocity
η is viscosity
Laminar Flow
- Ordered flow occuring in concentric layers within a tube
- Flow in the center is fastest and flow in the most peripheral layer is the slowest
- Resistance to laminar flow obeys the Poisuille-Hagen Equation
where
R is vessel resistance
η is viscosity
L is length of vessel
r is radius of vessel
- Increases in viscosity of gas, or length of the tube increase resistance
- Increases in radius of the tube, decreases resistance by a power of 4
Turbulent Flow
- In turbulent flow, due to the disorganized flow and increased likelihood of friction
with the static airway wall, resistance is markedly increased. - Resistance to turbulent flow obeys the following equation
where
R is Resistance to flow
ρ is density
l is length of vessel
r is radius of vessel
- Increases in density of gas, or length of the tube increase resistance
- Increases in radius of the tube, decreases resistance by a power of 5
Factors affecting Airway Resistance
- Physics factors (see above):
- Location in the airway (see above): Mid-sized bronchi are the location of greatest airway resistance, resistance progressively declines with successive airway generations
- Flow:
- Laminar Flow vs turbulent flow
- Depends on Reynolds number factors
- density more important than dynamic viscosity, velocity (flow rate) as Main Site is Turbulent.
- Flow rate:
- Flow related airway collapse
- Airways beyond generation 11 have no structural rigidity
- High flows can reverse transmural pressure gradient and cause airway collapse
- Flow related airway collapse
- Newborn: ~20 cmH2O/L/s – declines markedly with age to ~2 cmH2O/L/s
- Radius changes
- Muscular control of airway diameter
- Neural:
- Parasympathetics important in bronchomotor tone → airway constriction via ACh and muscarinic receptors
- Sympathetic system virtually absent in lung
- Hormonal:
- Although no symppathetic innervation, abundance of β2 adrenoceptors → airway dilation
- Neural:
- Smooth mm tone:
- (↓r) bronchospasm, Musc antag (PSNS), LTs, PGF2-alpha
- (↑r) β2-agonists, adrenaline neb, SNS
- ↓intramural radius:
- oedema, ↑mucous, wall hypertrophy
- External compression:
- tumour, haemorrhage, PTX
- dynamic airways compression with forced expiration
- Muscular control of airway diameter
- Lung Volume
- ↑ lung vol →
- ↑ radial traction → ↓ AWR
- ↑ -ve intrapleural → ↑ patency of small airways
- ↓ lung vol → ↑ AWR
- CPAP or PEEP → ↑ FRC → reduces flow-related airway collapse at low lung volumes
- ↑ lung vol →
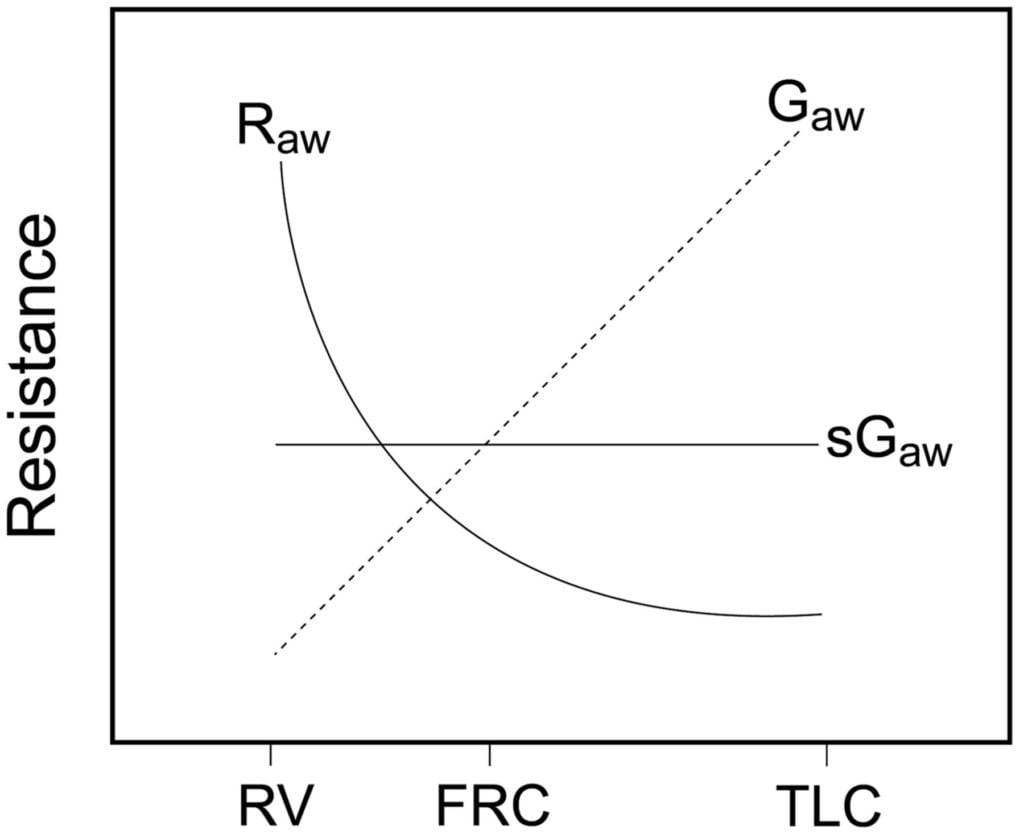
Relationship between Lung volume, Airway Resistance (R) , reciprocal of resistance (Conductance G, specific conductance sG)
Notice that only sGaw is independent of lung volume
Measurement of Airway Resistance
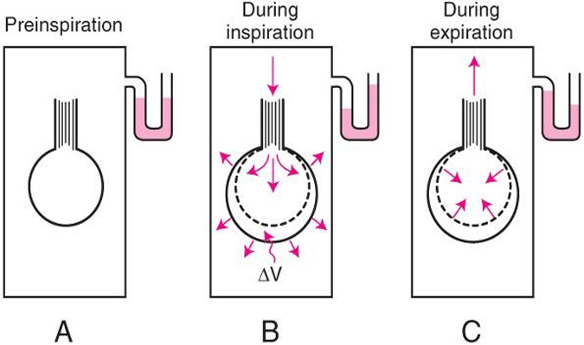
Body Plethysmography:
- Q measured with flow meter
- Lung Volume is measured with Plethysmography
- ∆P via Plethysmography and Boyle’s Law
- (A) box pressure is atmospheric
- (B) inspiration
- ∆V allows for ∆P measurement for given Q since
- PV = constant
- Then AWR can be calculated from 1
Interrupter Technique (direct from AWR eqn)
- Method
- Manometer distal to shutter
- Used to measure mouth and alveolar pressure
- Flow during inspiration or expiration in interrupted for 50–100 ms repeatedly throughout the respiratory cycle.
- Measure
- Q – flow rate (measured before interruption)
- P2 – mouth pressure (measured before interruption)
- P1 – pressure in alveoli (measured at the end of the interruption at te level of the mouth after equilibration)
- Apply Ohm’s Law (AWR equation above).
- Adequate for normal lungs not diseased.
Gladwin / Sakurai 2016
Examiner Comments
2021B 12: 31% of candidates passed this question.
It was expected candidates cover the breadth of the factors that affect airway resistance. Generally, as a concept the type of flow (laminar vs turbulent) was answered well by most candidates, however many failed to mention the other factors that affect airway resistance. Airway diameter as a primary determinant of airway resistance was commonly omitted. Better answers which covered the factors affecting airway diameter classified them broadly and included examples such as physical compression/external obstruction, broncho-motor tone and local cellular mechanisms. Some answers did not explain these factors in enough detail and often with incorrect facts.
13. Describe the factors that affect mixed venous oxygen saturation.
CICMWrecks Answer
Mixed Venous Blood
- Mixed Venous Blood = blood taken from the pulmonary artery
- Normal mixed venous partial pressure of oxygen = 40mmHg -> 75% saturation
- Majority of oxygen in blood bound to haemoglobin
- Hb has 4 haem molecules, each can bind one oxygen molecule
- Affinity of Hb for oxygen changes with oxygen saturation, due to conformational change in the haem group on oxygen binding
- Affinity for oxygen decreased by: low pH, high CO2, high temperature, 1-3-DPG
Factors
- Complex interplay of factors which affect Venous Oxygen Partial pressure of oxygen and hence content
- Modified Fick principle: Mixed venous oxygen content = oxygen delivery – oxygen extraction
Oxygen Content
- = 20ml/100ml in arterial blood, 15ml/100ml in venous blood
- Vast majority of oxygen carried by haemoglobin, tiny dissolved portion
- Non-linear relationship between dissolved O2 and O2 content
- PO2 is the fraction of dissolved O2
- PO2 determines Sats based on Oxygen-Haemoglobin Dissociation Curve
Oxygen Delivery
= Cardiac output x Arterial oxygen content
Oxygen Demand
= Cardiac output x Arteriovenous O2 content difference
Oxygen flux
Hence SvO2 and pvO2 are surrogate markers for Oxygen flux
- O2 Delivery
- 1000ml/min in health
- Decrease in cardiac output: ↓HR, ↓SV (↓preload, ↓contractility, ↑afterload)
- Decrease in functional Hb: anaemia, carbon monoxide, congenital abnormalities of haemoglobin
- Decrease in oxygen saturation: V/Q mismatch, anatomical shunt, hypoventilation, low FiO2 (e.g. at altitude), right-shift of oxygen dissociation curve (low pH etc. – see above)
- Oxygen extraction:
- 250ml/min
- Increased by:
- increased tissue metabolism (e.g. sepsis, pregnancy, malignancy, post-operative catabolic state etc.)
- right shift of oxygen dissociation curve (see above)
Mooney / JC 2020
Examiner Comments
2021B 13: 49% of candidates passed this question
Mixed venous oxygen saturation is used as a surrogate marker for the overall balance between oxygen delivery and oxygen consumption. A good answer stated this, described the importance of where it is measured and went on to describe the various factors that affect oxygen delivery and consumption.
Descriptions of the factors that affect oxygen saturation of haemoglobin, partial pressure of oxygen in the blood and position of oxygen-haemoglobin dissociation curve were necessary to score well. Important omissions were factors that increased and decreased oxygen consumption. While many candidates were able to correctly write the equations for oxygen content and oxygen flux, they then failed to describe how the variables within these equations were related to mixed venous oxygen saturation.
14. Describe the production, action and regulation of thyroid hormones.
CICMWrecks Answer
Thyroid Hormones
- 3 main thyroid hormones
- thyroxine (T4): 95%; ½ life 7 days; less active
- tri-iodothyronine (T3): 7%; ½ life 24hrs; 3-5x activity of T4
- reverse T3 (rT3): inactive
- T3 + T4 formed from iodination of aa tyrosine
- iodine obtained from diet in form of iodide + actively taken up into thyroid follicular cells (req 120-150ug/day)
- Release
- TRH from hypothalamus → stimulates TSH release → binds to R on cell membrane of follicular cells, GPCR → ↑cAMP → ↑AC →
- ↑iodine uptake into follicular cells
- ↑synthesis of T3 + T4 via ↑iodination + ↑rate coupling reactions
- ↑proteolysis of thyroglobulin within follicular cells → liberate T3 + T4
- MoA
- T3 + T4 highly protein bound (>99%) predominantly to thyroxine binding globulin, albumin, thyroxin binding pre-albumin
- Thyroid hormones enter cell → T3 binds to intracellular thyroid receptors (TR) → hormone receptor complex = transcription factors (bind to DNA via zinc fingers) → alter gene transcription → clinical effects
- T4 de-iodinated to T3
Production and Regulation
Synthesis
- Steps:
- dietary iodine converted to iodide for absorption
- iodide actively transported into follicular cells in thyroid (trapping) → oxidised to iodine (via thyroid peroxidase)
- iodine binds tyrosine in thyroglobulin molecule (iodinase) → forms mono-iodotyrosine then di-iodotyrosine (peroxidase)
- di-iodotyrosine + di-iodotyrosine = thyroxine (T4) (perixodase) → binds thyroxine binding globulin + thyroxine binding pre-albumin
- ↑activity with TSH of:
- iodide pump activity
- thyroid peroxidase
- iodinase
- peroxidase
- vesicular lysosomal activity
- thyroid hormones formed within thyroglobulin; synthesised in golgi apparatus
- thyroglobulin stored in follicular colloid → vesicular lysosomal activity breaks down thyroglobulin to release T3 + T4 which diffuse out of follicular cells and into circulation
Metabolism of thyroid homrones
- T4 deiodinated to T3/ rT3 (inactive compound 1:1) → deiodinated in liver, kidney, skeletal muscle to inactive compounds
Negative feedback
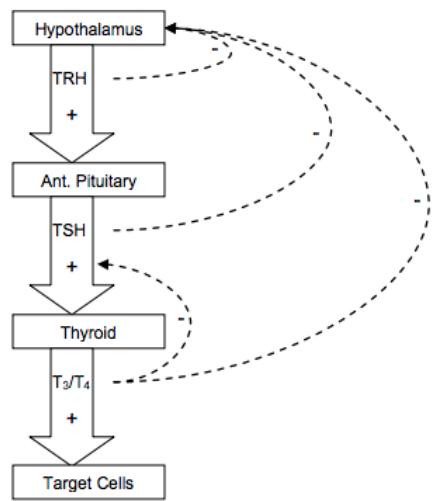
Physiological Action
| System | Action | Physiological + pathological effects |
|---|---|---|
| CNS | Development | – normal CNS development – ↓T → ↓CNS development → retardation, rigidity, deaf-mutism – sexual function |
| CVS | Chronotrope Inotrope Vasodilation | – ↑number β-adrenoceptors → ↑HR – ↑circulating catecholamines → ↑contractility + ↑CO – ↑T → ↑body temp → vasodilation → ↓SVR |
| Resp | Metabolic | – ↑T → ↑metabolic rate → ↑MV |
| Bone | Anabolic | – essential for normal bone growth |
| ANS | Stimulatory | – ↑T → synergy with circulating catecholamines → ↑SNS |
| Metabolic / endocrine | Cellular effect Feedback Anabolic/ catabolic | – ↑Na/K ATPase activity → ↑MR of cells + calorigenic – feedback inhibition → ↓TRH + ↓TSH ; ↑T → ↑GH release – CHO: ↑CHO absorption – Fat: ↑lipolysis; ↑LDL Rs → ↑ liver uptake circulating cholesterol – Protein: physiological amounts: ↑protein synthesis; excess amounts: protein breakdown (thyrotoxic myopathy) |
Kerr 2016
Examiner Comments
2021B 14: 81% of candidates passed this question.
This question was divided in three sections to help candidates formulate an answer template, which for the most part was answered well. Most answers included a detailed description of the production and regulation of thyroid hormones, including the importance of negative feedback. A brief description of the action of thyroid hormones on intracellular receptors, and a system-based description of physiological effects, including CHO, protein and fat metabolism was expected.
15. Classify and describe the mechanisms of drug interactions with examples.
CICMWrecks Answer
Pharmaceutical interactions:
- Physicochemical incompatibility b/t drugs
- causes precipitation of drug (Eg. STP (alkaline) + SCh (acidic))
- Absorption or binding to containers (Eg. GTN + PVC lines)
- Degradation of drug (Eg. insulin denatures in solution of dextrose)
Pharmacodynamic interactions:
- One drug alters the body’s response to another at a given plasma [drug] and can be either
- antagonistic
- Direct (morphine and naloxone) or indirect
- additive
- synergistic
- Inhibition of enzymatic inactivation e.g. clavulanic acid and amoxicillin
- Enhanced agent uptake e.g. gentamicin and penicillin
- antagonistic
- Direct interaction
- Drugs act at same receptor site for effect
- naloxone + opioids → direct antagonism
- N2O + volatiles → direct additivity
- Drugs act at same receptor site for effect
- Indirect interaction
- Drugs act at different receptor sites for same effect
- Opioids + volatiles → indirect synergism
- atropine + neostigmine → indirect antagonism
- Drugs act at different receptor sites for same effect
Pharmacokinetic interactions:
- Absorption
- Mainly due to altered oral absorption a/w:
- Complex formation: tetracycline + Ca in milk/antacids
- Altered gastric emptying/intestinal motility
- opiates ↓ intestinal motility → ↓ absorption of drugs absorbed in small intestine (paracetamol)
- metoclopramide ↑ intestinal motility → ↓ absorption of drugs absorbed in stomach (Eg. cimetidine)
- Altered gastric and intestinal pH
- ↑ gastric pH by antacids impairs absorption of weakly acidic drugs
- Complex formation: tetracycline + Ca in milk/antacids
- Altered parental absorption a/w localised vasoconstriction (Eg. adrenaline + LA)
- Mainly due to altered oral absorption a/w:
- Distribution
- Competition by drugs for plasma protein binding site → affects drugs that:
- Are highly protein-bound drugs (Eg. warfarin, diazepam, phenytoin)
- → ↑ unbound % in plasma
- Have enzyme system close to saturation or zero-order kinetics (Eg. phenytoin)
- →↑ displacement of drug and ↑ unbound % cannot be cleared effectively
- → large ↑ unbound % in plasma
- Are highly protein-bound drugs (Eg. warfarin, diazepam, phenytoin)
- Drugs that alter C.O. impact on distribution of drugs to target, peripheral tissues
- β-blockers ↓ C.O. → slow onset/offset times of drugs reliant on distribution
- Competition by drugs for plasma protein binding site → affects drugs that:
- Metabolism
- Inhibition/induction of microsomal enzymes (Eg. CYP450)
- Enzyme induction → resulting in ↓ plasma [drug]
- Enzyme inhibition → resulting in ↑ plasma [drug]
- Inhibitors of non-microsomal enzymes (Eg. MAOi, COMTi)
- Inhibition/induction of microsomal enzymes (Eg. CYP450)
- Elimination
- ↓ urinary excretion
- Competition for tubular transport system occurs with weak organic acids (Eg. probenecid + penicillin)
- Changes in urine pH
- Alkalinising agents (Eg. NaHCO3/acetazolamide) → ↑ excretion of weak acids
- Changes in urine volume
- Changes in biliary excretion
- Phenobarbital ↑ bile flow and biliary conjugation of drugs
- ↓ urinary excretion
Gladwin 2016
Examiner Comments
2021B 15: 54% of candidates passed this question.
This question has been asked previously, the answer template expected some description rather than a list of drug interactions. Generally, examples were provided for each type of interaction. The examiners reported too many vague, factually incorrect descriptions of the mechanisms and in some cases a very limited classification.
16. Classify the anti-psychotic drugs (25% marks). Outline the pharmacology of haloperidol (75% marks).
CICMWrecks Answer
ANTI-PSYCHOTIC DRUGS
CLASSIFICATION
| TYPICAL Antipsychotic Agents | ATYPICAL Antipsychotic Agents | |
|---|---|---|
| (D2-R) | (D2-R<5-HT2a) | |
| Includes | (1) Phenothiazines (Eg. Chlorpromazine) (2) Butyrophenone (Eg. Haloperidol) (3) Thioxanthenes (Eg. Flupenthixole) | (1) Diazepines (Eg. Clozapine, Olanzapine) (2) Dibenzothiazepines (Eg. Quetiapine) (3) Benzamides (Eg. Sulpiride) (4) Benzisoxazols (Eg. Respirdone) |
| Mechanism of Action | (i) Primarily potent D2R antagonists (> 80% of receptors must be antagonised for therapeutic effect) (ii) Moderate antagonism of 5-HT2 receptors and α-adrenoceptor (iii) Weakly inhibit mAChR and H1R | (i) Highly selective D2R antagonists (> 80% of receptors must be antagonised for therapeutic effect) (ii) High affinity for 5-HT2R and moderate affinity for α-adrenoceptors (Except benzamides) (iii) Diazepines have D4R antagonistic effect also |
| Effect | Ameliorate +ve symptoms only (little effect on –ve symptoms) | Indicated for treatment resistance or side-effects with typical agents (esp extrapyramidal symptoms) Have greater therapeutic efficacy (ameliorate both +ve and –ve symptoms) |
| Side Effects | Very narrow TI – Substantial side-effects at therapeutic doses (i) Movement disorders: – Extrapyramidal symptoms, such as dystonias, akathisias, pseudoparkinsonism – These are acute and reversible – Tardive dyskinesia (involuntary movements of tongue, lips, face, trunk and extremities) – Gradual onset and irreversible (ii) Endocrine – Sexual dysfunction, gynaecomastia (due to PRL release), menstrual disorders, weight gain (iii) Hypotension (α-adrenoceptor-related) (iv) Sedation (H1R related) (v) Seizures (vi) Jaundice and agranulocytosis (esp with phenothiazines) (vii) Anticholinergic symptoms (viii) Neuroleptic malignant syndrome | fewer side effects than typical agents (esp movement disorders and gynaecomastia) (i) Cardiac arrhythmias (ii) seizures (iii) clozapine-induced agranulocytosis (requires blood screening) (iv) myocarditis and cardiomyopathy (requires cardiac enzyme and TTE monitoring) |
PHARMACOLOGY OF HALOPERIDOL
Examiner Comments
2021B 16: 28% of candidates passed this question.
Excellent answers were able to provide a classification of antipsychotics based on either typical/atypical or first/second generation categories, provide examples of each and identify key differences in mechanism and effects. They also distinguished between butyrophenones and phenothiazines within the typical antipsychotic group. Haloperidol was identified as a butyrophenone, with description of pharmaceutics, dose and route, as well as pharmacodynamics and pharmacokinetics. Key adverse effects were detailed, focusing on those specific to haloperidol, including a description of different types of extrapyramidal symptoms and QT prolongation/ torsades de pointes.
17. Explain the components of an ECG (electrocardiogram) monitor (70% marks). Outline the methods employed to reduce artefact (30% marks).
CICMWrecks Answer
ECG
- 12 electrodes on the body surface to detect small potentials (0.5-2 mV) generated by the heart.
- Electrodes made of Ag/AgCl
- ECG signals detected by these electrodes
- Sent to electronic device that filters out noise and boosts the signal
- Displayed on oscilloscope
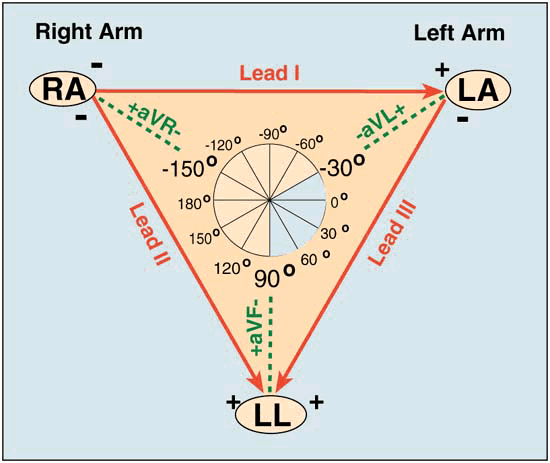
Signal Interpretation from oscilloscope readings via Enthoven’s triangle
- For orientation of the Bipolar and unipolar limb leads synthesised from the 12-lead ECG.
- Bipolar limb leads
- Lead I: RA (-) to LA (+) at 0°
- Lead II: RA (-) to LL (+) at 60°
- Lead III: LA (-) to LL (+) at 120°
- Unipolar limb leads
- aVR: RA (+) at -150°
- aVL: LA (+) at -30°
- aVF: LL (+) at 90°
- Positive deflection in a lead indicates depolarisation wave moving towards lead’s positive terminal
Calibration
- Vertical calibration
- 1 mV signal → vertical deflection of 10 mm (2x large square or 10x small squares)
- 0.1 mV per mm (or small square)
- Horizontal calibration
- paper speed is 25 mm/s → 0.04 s/mm (or small square)
Errors:
Noise and interference caused by:
- Electrical interference by any device using AC current (esp high-frequency diathermy)
- → Minimise by ECG filters, shielding of leads/cables, differential amplifers, Etc.
- Movement or shivering
- → minimised by placement over bony prominences and use of ECG filters
- High skin impedance
- → minimised by degreasing skin with EtOH and use of conducting gel with electrodes
- Incorrect electrode placement relative to heart
Gladwin 2016
Examiner Comments
2021B 17: 46% of candidates passed this question.
Excellent answers described the function of the ECG device and its components. Components include electrodes, which form leads (unipolar and bipolar), the amplifier and an output device. The process of amplification and filtering (e.g., high and low pass filters), as well as monitoring and diagnostic ECG modes were described. A comprehensive list of ways to reduce artefacts, including strategies to address both patient and equipment factors was generally provided.
18. Outline the neural pathways for the pupillary light, corneal, oculomotor and gag reflexes. The anatomical course of nerves is NOT required.
CICMWrecks Answer
| Reflex | Examination technique | Normal response | Afferent pathway | Brainstem centers | Efferent pathway |
|---|---|---|---|---|---|
| Pupillary light | Response to light | Direct and consensual myosis followed by mydriasis | Retina, optic nerve, chiasma, optic tract | Pupillo-constrictor: midbrain, pretectal olivary nucleus, Edinger-Westphall nucleus Pupillo-dilator: posterior-lateral hypothalamus, cervical ganglion, trigeminal ganglion, abducens | Sympathetic fibers of CN III (Oculomotor) |
| Corneal | Stimulation of cornea with saline drops / cotton wisp | Eyelid closure | CN V (Trigeminal) | Pons, trigeminal and facial nuclei | CN VII (facial) |
| Occulomotor | Oculocephalic: Turn head from side to side | Eyes move conjugately in direction opposite to head | Semicircular canals, CN VIII (oculovestibular) | Pons (parapontine reticular formation), nucleus vestibularis, nucleus abducens | CN III (oculomotor) and VI (abducens) |
| Oculovestibular: Irrigate external auditory meatus with cold water | Nystagmus with fast component beating away with stimulus | ||||
| Gag / Pharyngeal reflex | Stimulation of solft palate | Symmetrical rise of soft palate gag reflex | CN IX (Glossopharyngeal) CN X (vagus) | Medulla, nucleus tractus solitarius | CN IX (Glossopharyngeal) CN X (vagus) |
Sources:
Benghanem, Sarah & Mazeraud, Aurélien & Azabou, Eric & Chhor, Vibol & Shinotsuka, Cássia & Claassen, Jan & Rohaut, Benjamin & Sharshar, Tarek. (2020). Brainstem dysfunction in critically ill patients. Critical Care. 24. 10.1186/s13054-019-2718-9.
Stevens, R., Sharshar, T., & Ely, E. (Eds.). (2013). Brain Disorders in Critical Illness: Mechanisms, Diagnosis, and Treatment. Cambridge: Cambridge University Press. doi:10.1017/CBO9781139248822
Examiner Comments
2021B 18: 43% of candidates passed this question.
This is a fact-based question with little integration of knowledge required. Those candidates who synthesised their knowledge into a succinct and precise description of afferent and efferent pathways with a description of the various sensor and integrator components scored very high marks. A good working knowledge of all the cranial nerve reflex pathways are crucial to the practise of intensive care medicine. Marks were not awarded for any anatomical description related to these pathways.
19. Outline the process of fibrinolysis (40% marks). Write short notes on the indications, mechanism of action, pharmacokinetics and side effects of tranexamic acid (60% marks).
CICMWrecks Answer: Fibrinolysis
Fibrinolysis
- Process by which fibrin clot is degraded to prevent excessive clot formation
- Plasminogen incorporated into clot
- Injured tissues and vascular endothelium gradually produce Tissue Plasminogen Activator (t-PA) in a delayed manner.
- Urokinase is produced by monocytes, macrophages and urinary epithelium
- Factor XII (Hageman factor) acts as a weak activator of plasminogen
- Plasminogen activated by t-PA, u-PA and factor XII to plasmin, a serine protease
- Plasmin cleaves fibrin into fibrin degradation products → reducing clot stability → Clot breakdown
Regulation of fibrinolysis
- Plasmin Activator Inhibitor (PAI) 1 & 2 – produced by vascular endothelium and inhibits tPA and uPA
- Protein C inactivates PAI, TAFI
- Thrombomodulin – expressed on in tact endothelium – binds thrombin and subsequently activates protein C
- Thrombin Activatable Fibrinolysis Inhibitor – produced by liver and cleaves plasmin binding sites on fibrin → decreased degradation
- α2 anti-plasmin – produced by liver, inhibits circulating plasmin, not fibrin bound plasmin
Sakurai 2016
CICMWrecks Answer: Pharmacology of Tranexamic Acid
Examiner Comments
2021B 19: 30% of candidates passed this question.
The relative allocation of marks and thus time to be spent on each component was delineated by the relative percentages in the question. The first part of the question required a step-by-step outline of the fibrinolytic pathway with mention of the regulatory processes. Tranexamic acid is an important drug in the practice of intensive care and the question provided the headings under which to answer the question. The detail surrounding the keys aspects of this drug with respect to its use in critical care were often vague and underappreciated.
20. Describe the physical principles of haemodialysis and haemofiltration, including the factors affecting clearance (80% marks). Outline the key components of renal replacement fluids (20% marks).
CICMWrecks Answer
Physical Principles and factors affecting clearance
- Haemodialysis:
- Uses diffusion: “the movement of solutes from a high to a low solute concentration across a semipermeable membrane”
- Convection (see below) occurs during diffusion
- Blood is pumped through an extracorporeal circuit that contains a dialyser
- Dialysate flow is countercurrent, with maximizes the gradient for diffusion
- Solutes move across a membrane between blood and dialysate as per Fick’s Law
- Flow across the circuit is determined by:
- Set flow rate of centrifugal dialysis pump
- parts of the circuit:
- access port of vascular access (catheter / fistula / graft)
- from patient (pre-membrane)
- across membrane (trans-membrane)
- to patient (post-membrane)
- return port of vascular access (catheter / fistula / graft)
- Assuming that machine delivers the flow which is set and the flow remains laminar and constant, the pressure across the various components varies based on the resistance.
- The pressure gradient across the circuit depends upon:
- characteristics of patient:
- Blood viscosity
- hematocrit
- Blood protein and lipid content
- Temperature changes changes flow and viscosity
- Fåhræus–Lindqvist effect: Viscosity reduces in smaller diameter – because erythrocytes move over to the centre of the vessel/circuit, leaving only plasma near the wall of the vessel.
- variable resistance during breathing or movement
- collapsing vessels due to decreased vessel size / hypovolemia
- antivoagulation used
- Blood viscosity
- characteristics of vascular access:
- gauge and length of access
- resistance from physical obstruction
- resistance position
- resistance from clots, fibrin
- characetristics of circuit:
- Age of circuit, presence of clots, fibrin
- radius
- length (usually constant ~3.5m)
- surface coating of circuit
- anticoagulation used
- characteristics of membrane:
- resistance worsens with clot, fibrin, lipid deposition
- characteristics of patient:
- Uses diffusion: “the movement of solutes from a high to a low solute concentration across a semipermeable membrane”
- Haemofiltration:
- Uses ultrafiltration: “the movement of fluid through a membrane caused by a pressure gradient (hydrostatic or osmotic pressure)”
- Convection (see below) occurs during ultrafiltration
- Positive hydrostatic pressure in blood and a negative hydrostatic pressure in dialysate is generated → causing ultrafiltration and removal of solutes via solvent drag
- Elimination via bulk flow is independent of solute concentration gradients across the membrane
- Transport is dependent on starling forces
- The transmembrane pressure generated: blood flow to the membrane; oncotic pressure gradient
- Porosity of the membrane
- Additionally, a high filtration fraction will cause excessive haemoconcentration, and clotting of the filter
- The filtered fluid (ultrafiltrate) is discarded, and replaced with another fluid depending on the desired fluid balance
- Uses ultrafiltration: “the movement of fluid through a membrane caused by a pressure gradient (hydrostatic or osmotic pressure)”
Other Mechanisms involved:
- Osmosis:
- the movement of a pure solvent such as water, through a differentially permeable membrane, from a solution that has a lower solute (particle) concentration to one that has a higher solute concentration
- The rate of osmosis depends on the concentration of solute, the temperature of the solution, the electrical charge of the solute and the difference between the osmotic pressures exerted by the solutions.
- Movement across the membrane continues until the concentrations of the solutions equalise.
- Convection:
- The movement of solutes with a water-flow “solvent drag” across a membrane during osmosis or ultrafiltration
- Important for movement of small solute (urea, creatinine).
- Adsorption:
- when the molecules (solutes) adhere to the surface or interior of the membrane.
- with the movement of fluid across the membrane, if no fluid is moving then adsorption can not occur.
- in 2 manners:
- surface adsorption where the molecules are too large to permeate and migrate through the membrane; however they can adhere to the membrane.
- bulk adsorption occurs within the whole membrane where molecules can permeate it.
- Molecules that can be effectively adsorbed include:
- B2 microglobulin
- Cytokines
- Coagulation factors
- Anaphylatoxins
Renal Replacement Fluids
- Typically pre-packaged in 5L bags of sterile water
- contain sterile water, electrolytes and buffer
- Vary slightly in composition, but all are balanced salt solutions with either a lactate, bicarbonate or citrate buffer
- Bicarbonate-based solutions: most commonly used two-compartment systems. 25-35mmol/L and used as both dialysate and replacement fluids
- Lactate-based solutions: stable, cheaper, practical. But buffering capacity depends on conversion of lactate to bicarbonate. So not commonly used in CRRT
- Citrate-based solutions: new generation, administered prefilter. Advantage that additional anticoagulation is not required.
- Calcium-free solutions: When citrate-based fluids are used prefilter, these are used as dialysate and replacement fluids
- At present, there is no evidence to suggest that the choice of replacement fluid has an impact on survival or renal recovery.
- Replacement fluid can be added pre- or postfilter in varying ratios.
- The benefit of adding some of the replacement fluid prefilter is that it lowers the hematocrit of the blood, which reduces the likelihood of the filter clotting.
- The downside is that predilution reduces solute clearance and a compensatory increase in flow rates should be considered (15% for ultrafiltration rates of 2 L/h and up to 40% for rates of 4.5 L/h).
| Composition (mmol/L) | PrismOcitrate 18/0 (Baxter) | PrismOcal (Baxter) | PrismOcal B22 (Baxter) | Prismasol (Baxter) | Hemosol B0 (Baxter) |
|---|---|---|---|---|---|
| Sodium | 140 | 140 | 140 | 140 | 140 |
| Potassium | 0 | 0 | 4 | 0 | 0 |
| Calcium | 0 | 0 | 0 | 1.75 | 1.75 |
| Magnesium | 0 | 0.5 | 0.75 | 1 | 0.5 |
| Chloride | 86 | 106 | 120 | 110 | 109.5 |
| Bicarbonate | 0 | 32 | 22 | 32 | 32 |
| Phosphate | 0 | 0 | 0 | 0 | 0 |
| Citrate | 18 (=54mmol bicarb post metabolism) | – | – | – | – |
| Lactate | 0 | 3 | 3 | – | 3 |
| Osmolality (mOsmol/L) | 244 (280 post metabolism) | 282 | 282 | 287 | 287 |
Source: Mishra, Rajesh Chandra. ISCCM Manual Of RRT And ECMO In ICU A Reference Book For Practicing Intensivists (p. 25). Jaypee Brothers Medical Publishers.
Kerr’s notes
JC / Kerr 2022
Examiner Comments
2021B 20: 28% of candidates passed this question.
A brief description of the underlying mechanisms of dialysis and hemofiltration was required. Diffusion, the predominant mechanism in haemodialysis, involves movement of solute down the concentration gradient across the semipermeable membrane. This concentration gradient is generated and maintained by counter current movement of dialysate and blood. In hemofiltration the predominant mechanism is convection and solvent drag of the solute across the semipermeable membrane by application of transmembrane pressure. The filtrate is then replaced by replacement fluid. Small molecules are effectively removed by dialysis whereas hemofiltration can remove small and middle molecules. Various factors that impact clearance in haemodialysis and haemofiltration were expected separately.
Constituents of replacement fluid should have included three broad headings of electrolytes, buffer and sterile water. Many answers lacked the details of how counter current mechanisms help, the difference in the two modalities in regard to clearance of molecules, how clearance is impacted by protein binding and volume distribution, sieving coefficient of the membrane and flow rates of blood and dialysate (or effluent) flow. The constituents of replacement fluid lacked details of various types of electrolytes, the common buffers and the strong ion difference.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Components of Work of breathing PaCO2 vs PETCO2, pulmonary vasculature Closing capacity, measurement |
| G. CVS | CVP, Cardiac events Cardiac muscle structure, function Left and Right Coronary arteries Cardiac action potential, anti-arrhythmics |
| H. Renal | Renal physiology, ABG interpretation (Temp 37.5 pH 7.31 pCO2 39 pO2 84 fiO2 30% HCO3 18 BE -4 Ba 136 K 3 Cl 101 Lactate 3.4) Renal water handing, concentrates urine |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | pH, measurement |
| K. Neuro | Sedatives, Propofol Thiopentone pharmacology, why does thio have a rapid onset of action Opioid receptors, pharmacology Phenytoin, antiepileptics, anti-depressants Sensory and motor pathways, pathway from periphery to cortex |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | Functional unit of liver, lactate physiology |
| O. GIT | Functions of stomach. PPI action |
| P. Nutrition and Metabolism | Carbohydrate breakdown, absorption, glucose metabolism |
| Q. Haematology | Coagulation, viscoelastic haemostatic assays (TEG, ROTEM) Thromboelastography Formation of RBC |
| R. Thermoregulation | Temperature, measurement |
| S. Immunology | |
| T. Microbiology | Bacteria gram stain, antibiotics |
| U. Endocrine | Adrenal gland physiology, pharmacology |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |


Recent Comments