1. Describe the pharmacology of adrenaline.
Examiner Comments
2021A 01: 90% of candidates passed this question.
Adrenaline is a level 1 drug and is commonly used in intensive care. A comprehensive explanation of the drugs MOA, PK, PD and side effect were expected. Candidates who scored well generally provided a factually accurate, detailed and well-structured answer. Overall, the quality of answer provided for this question was of a high standard.
2. Describe the work of breathing and its components.
CICMWrecks Answer
Work of Breathing
- WOB = Energy used by the respiratory muscles during ventilation
- Work = Pressure x Volume (measured in Joules)
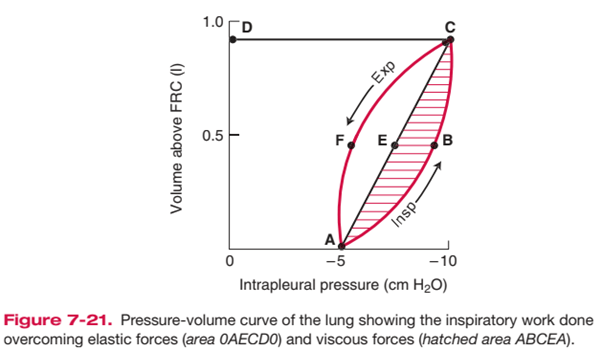
- represented as area on pressure-vol loop (dynamic compliance curve)
- Tidal breathing at rest: <2% BMR; O2 requirement = 3ml/min
Components of Work of Breathing
WOB can be divided into:
- Inspiratory work
- Active
- Elastic + resistive work
- Expiratory work
- Usually passive (uses potential energy stored during inspiration)
- Resistive work
Inspiratory elastic work
- ~65% of total work
- work done on inspiration to overcome:
- surface tension of lung (70%)
- elastic properties of lung/ lung elastic recoil (30%)
- stored as elastic potential energy → used on expiration
Inspiratory + expiratory resistive work
- ~35% of total work; lost as heat
- energy required to overcome frictional forces: includes
- Airway resistance: between gas molecules
- depends on type of flow + airway calibre
- turbulent flow (↑RR; upper airway obstruction, ↑airway density)
→ ↑ airway resistance than laminar flow - ↓ airway calibre (radius) (e.g. bronchoconstriction, dynamic airway compression, ETT)
→ ↓ calibre → ↑ WOB
- Tissue resistance e.g. interstitial lung disease
- Airway resistance: between gas molecules
- Usually potential energy stored from insp work is sufficient to perform expiratory resistive work → expiration can become active in pathological conditions e.g. COPD, asthma
Total work and efficiency of breathing
- O2 cost of breathing:
- During a normal TV breath at rest, the total work of breathing is low – The
inspiratory muscles consumes 3 mL O2/min, which is ~1% of the body’s total
O2 consumption at rest (VO2 = 250 mL O2/min) - O2 consumption of breathing increases by up to 30% with hyperventilation (due to an increase in either minute volume or RR)
- During a normal TV breath at rest, the total work of breathing is low – The
- Total work and efficiency of breathing:
- Difficult to measure – It is calculated by measuring O2 cost of breathing, then
assuming efficiency as:
- Difficult to measure – It is calculated by measuring O2 cost of breathing, then
Normal efficiency is estimated at 5-10%
Increased WoB
- Elastic Work:
- Breathing large lung volumes: increases total work of inspiration by extending the line AEC to a higher volume above FRC, increasing area 0AECD0
- Poorly compliant lungs (e.g. restrictive disease surfactant deficiency): increases total work of inspiration by reducing slope of AEC, increasing area 0AECD0
- Resistive Work
- e.g. turbulent flow with rapid RR, lung disease associated with increased AWR
- Increases total work of inspiration by producing a larger area ABCEA (i.e. it bulges)
- This also causes AECFA to bulge out and increase area, such that when stored elastic potential is insufficient to overcome this tissue resistance force, expiration becomes an active process requiring work as expiratory muscles are recruited
Minimizing WoB
- Elastic work:
- PEEP: keep lung vol at FRC and maximise number of ventilated alveoli
- Positioning: optimise lung volume
- Surfactant: minimise surface tension
- Optimise RR: elastic WOB ↓s with ↑ RR
- Resistive work
- ↓RR: RR directly proportional to resistive work
- ↑laminar flow: more efficient than turbulent flow; can be ↑ by ↓gas density e.g. heliox
- ↑radius: ↑lung vol; bronchodilators
Kerr / Bianca
Examiner Comments
2021A 02: 24% of candidates passed this question.
This is a core topic within respiratory physiology. There was a very low pass rate for this question. Expected components of the answer included: a definition of WOB as a product of pressure and volume or force and distance including the units of measurement; followed by a detailed explanation of the following three broad components – elastic resistance, viscous resistance and airflow resistance. Further marks were awarded to situations where the energy for expiration increases beyond stored potential energy as well as the impact of respiratory rate and tidal volume on different aspects of the WOB. For example, the changes in TV will have relatively greater impact on the elastic component, whereas RR will impact the resistance component. Additional marks were awarded for describing the efficiency of breathing. A common area where candidates missed out on marks was producing a diagram of WOB without a description; many diagrams were often incorrectly drawn or had no axes labelled. There were many incorrect definitions or respiratory equations provided without any link to the written answer. Factual inaccuracy and limited depth of knowledge were also prevalent in poorly performing answers. Marks were not awarded for a description of the control of breathing.
3. Outline the formation, structure, and function of the platelet.
CICMWrecks Answer: Platelet
PLATELET
Formation
- produced during hematopoiesis in a sub-process called thromopoiesis, or production of thrombocytes.
- Bone marrow: Common myeloid progenitor cells → promegakaryocytes → megakaryocytes
- Megakaryocytes produce protoplatelets within their cytoplasm → released in cytoplasmic extensions upon cytokine stimulus
- Protoplatelets break up into hundreds of platelets that circulate throughout the bloodstream
- The remaining nucleus of the ruptured megakaryocyte is consumed by macrophages.
- Megakaryocyte and platelet production is regulated by thrombopoietin (hormone produced by the liver and kidneys)
- Thrombopoietin stimulates differentiation of myeloid progenitor cells into megakaryocytes and causes the release of platelets.
- Thrombopoietin is regulated by a negative feedback mechanism based on platelet levels
- Each megakaryocyte produces between 5,000 and 10,000 platelets
- Altogether, around 1011 platelets are produced each day in a healthy adult
Fate
- Average lifespan of a platelet is 5 to 10 days
- Old platelets are destroyed by macrophage phagocytosis in the spleen and by Kupffer cells in the liver
- Up to 40% of platelets are stored in the spleen as a reserve, released when needed by sympathetically-induced splenic muscle contractions during severe injury.
Structure
- Small anucleated cells derived from megakaryocytes which originate from haematopoietic stem cell
- Normally 150~300 x 103 /μl
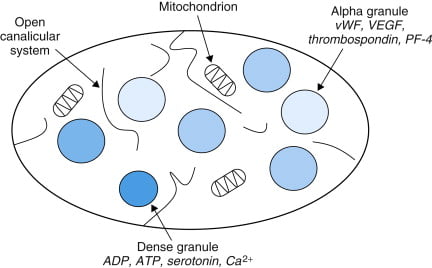
- Actin and myosin
- Remnants of the ER, SR storing calcium
- Mitochondria
- Enzyme systems → production of prostaglandins
- α granules
- Thrombin
- PDGF
- P-selectin
- Fibronectin
- vWF
- Dense granules
- ADP
- ATP
- Ca2+
- Serotonin
- Histamine
Function
- Haemostasis
- Formation of platelet plug
- Interact with collagen exposed at damaged endothelium by GPIa
- Activated by PAF, Thrombin
- Morphological change → irradiating pseudopods
- Degranulation
- Aggregation
- Expression of GPIIb/IIIa → binds fibrin and vWF
- Formation of platelet plug
- Immunomodulation
- Deployed to sites of inflammation and infection and secrete cytokines and chemokines
Sakurai / JC 2019
Examiner Comments
2021A 03: 79% of candidates passed this question.
This question was divided in three sections to help candidates formulate an answer template. The first section required a brief outline of the formation of platelets from pluripotent stem cells via megakaryocytes. The second section required an outline of platelet structure highlighting the special features such as, an absence of a nucleus, the presence of an external glycocalyx layer, specific surface receptors, contractile proteins, dense tubular system and granules. The third section was about platelet function where the expected focus was on the role of platelets in haemostasis. This required outlining the mechanism of platelet plug formation by adhesion-activation-aggregation, interactions with the coagulation cascade and role of platelets in clot contraction as well as fibroblast invasion. Although many candidates were able to answer the first section reasonably well, there was a noticeable knowledge deficit in the latter two sections. A significant proportion of answers had missing information on platelet structure and lack of structure in outlining platelet function.
4. Outline the dose (10% marks), composition (60% marks) and side effects (30% marks) of total parenteral nutrition (TPN).
CICMWrecks Answer
Overview of parental nutrition
- Parental nutrition is the delivery of nutrients into venous circulation (peripherally or centrally) instead of the enteral route → it can be used to supplement nutrient delivery via enteral route, or supplement nutrient delivery in its entirety (total parental nutrition)
- It is prepared by sterile technique → typically a 3L bag of hyperosmolar solution (containing glucose, amino acids, lipids, H2O, electrolytes, vitamins and trace elements)
- It can only be administered by CVC due to its ↑ tonicity (Nb. issues with irritation and thrombosis if given via PIVC) → infused over 24 hrs
- It also requires multidisciplinary team involvement
Indications for parental nutrition
- Indicated for those unable to ingest or digest nutrients or to absorb them from GI tract for a prolonged period of time (> 3-4 days) → includes those who have:
- A failed trial of enteral feeding
- Contraindications to enteral nutrition:
- GI obstruction
- Upper GI strictures and fistulae (Eg. enterocutaneous fistulae)
- Severe pancreatitis
- Prolonged ileus (Eg. major abdominal surgery where feeding not anticipated for days, paralytic ileus)
- Inflammatory conditions (Eg. IBD or mucositis due to chemotherapy)
- Short gut syndromes (Eg. major SB resection)
Daily nutritional requirements for parental nutrition
| Nutrient | Requirement |
|---|---|
| H2O | 30-40 mL/kg/day |
| Energy | 25-30 kcal/kg/day (can ↑ up to 1.5-1.75x with disease/stress) |
| Nitrogen | 0.5 g/kg/day (can ↑ to 1-1.5 g/kg/day with disease/stress) |
| Glucose | 3 g/kg/day |
| Lipid | 2 g/kg/day |
| Na+ | 1-2 mmol/kg/day |
| K+ | 0.7-1 mmol/kg/day |
| Ca2+ | 0.1 mmol/kg/day |
BUT these vary according to:
- Physiological factors:
- Age, gender, body size
- Pregnancy
- Activity level
- Hydration status
- Pathological factors
- Burns and sepsis → ↑ protein and caloric intake
- Renal failure → ↓ volume, protein and electrolyte (esp K+) content
- CCF → fluid reduction
- Hepatic failure → ↓ a.a/protein content to prevent encephalopathy
- Respiratory failure → ↓ glucose to minimise CO2 production
Components of parental nutrition
- (1) Energy source → glucose:lipid mixture (60:40 or 50:50)
- Glucose (as 50% dextrose)
- Used as an energy substrate → provides 50% of daily caloric needs
- Glucose has ↓ energy density → 3.4 kcal/g only (cf. 4 kcal/g for carbohydrates) → 50% dextrose needs to be given (1 L = 940 kcal) → BUT this is hypertonic (1900 mosm/L) and requires delivery via CVC
- Insulin can be given to aid glucose utilisation (esp in stressed patients)
- Need to avoid excess glucose delivery → risk of ↑ BGL, lipogenesis (liver disease), ↑ MR and ↑ CO2 production (delayed ventilatory wean)
- Lipid (as 10% or 20% intralipid)
- Used as (i) an energy substrate (provides 30-40% daily caloric needs), and (ii) provision of essential FAs (vital for cell membranes and PG synthesis)
- ↑ energy density cf. glucose → 1.1 kcal/mL (10%) and 2 kcal/mL (20%) → thus 1000 kcal can be achieved by 500 mL of 20% or 1L of 10%
- Can be infused separately (Ie. not mixed with glucose/a.a. solution) → can be given 1x/week BUT usually given daily to aid caloric delivery in the smallest volume of solution –
- Glucose (as 50% dextrose)
- Nitrogen-source (as symthamin 17)
- Used for (i) tissue protein synthesis (Eg. enzymes) and (ii) caloric needs
- Includes > 50% essential a.a.’s (isoleucine, leucine, valine, lysine, methionine, phenylalanine, threonine, tryptophane) and > 25% branched chain a.a
- Nb. A.a. are all L-isomers (as the body cannot use D-isomers)
- H2O → to maintain body H2O balance and replace losses (Eg. dehydration, bleeding)
- Electrolytes (Na+ /K+ /Cl- /PO4 3-/Mg2+, Ca2+) → to maintain and replace losses
- Vitamin/trace elements → vital to enzyme systems and metabolic pathways in body
- Vitamin solutions – both H2O and fat-soluble vitamins (esp folic, thiamine, vitamin K)
- Trace elements (Zn, Fe, Cu, Mn, Co, Se, I, Cr, Mb)
Monitoring of parental nutrition
- Needed to assess for (i) progress of nutritional state and (ii) complications of therapy
- Involves:
- Clinical r/v (Eg. fluid balance, weight, nutritional assessment, infections)
- Frequent ward glucose testing (until BGL stabilises)
- Investigations → daily EUC, CMP, BGL; weekly FBC, LFT (incl albumin), Coags, Lipid studies, and plasma/urine osmolality
Complications of parental nutrition
- Catheter-related (MOST common) → PTX, chylothorax, embolism (air/thrombus), infection, Etc.
- Fluid/electrolyte disturbances
- Fluid overload or hyperosmolar dehydration
- Electrolyte disturbances
- Normal AG metabolic acidosis (due to ↑ Cl- content)
- Metabolic disturbances
- Hyperglycaemia (initially) → delay in ↑ endogenous insulin (thus supplemental insulin required)
- Rebound hypoglycaemia (with abrupt cessation of TPN) → due to ↑ levels of endogenous insulin
- Metabolic bone disease
- Others:
- Immune suppression (due to fat component)
- Liver disease → initially deranged LFTs → later fatty liver/steatohepatitis
- ↑ PaCO2 (due to excessive glucose metabolism)
Bianca
Examiner Comments
2021A 04: 59% of candidates passed this question.
The pharmacology of enteral and parenteral nutrition is a level 1 topic in the first part syllabus. The TPN dose in terms of daily calorie and other nutritional requirements were key expectations in first part of the question. A detailed list of all macro and micronutrients was required under TPN composition. Expected information about macronutrients were their forms in the TPN solution (e.g., carbohydrate in the form of glucose, protein in the form amino acids), their relative calorie contributions and their essential components (e.g., the names of the essential amino acids). Identification of potential variability in composition and dose based on specific patient factors scored extra marks. Side effects included metabolic derangements (refeeding syndrome, over or under-feeding, hyperglycaemia, hyperlipemia), biochemical disturbances (fluid and electrolyte imbalances), organ injury (liver, pancreas) and vascular access related complications. Limited breadth and depth of information as well as incorrect facts were prevalent in the answers that scored lower marks.
5. Outline the factors that determine central venous pressure (60% marks) and explain how it is measured (40% marks).
CICMWrecks Answer
CVP
- Central venous pressure (CVP) is mean vena caval or right atrial pressure
- In the absence of tricuspid stenosis, equals right ventricular end-diastolic pressure.
- expressed in mmHg or cmH2O (1.36 cm water = 1.0 mm Hg)
- Normal 0-6mmHg in spontaneously breathing non-ventilated patient
- Venous Return, VR = (MSFP – RAP or CVP) / Resistance to venous return
- CVP is a major determinant of RV filling pressure (RV Preload)
- This regulates stroke volume through Frank-Starling mechanism
- CVP increased in disorders that increase Rt sided diastolic pressures
- left heart disease, lung disease, primary pulmonary hypertension, and pulmonic stenosis
- CVP is determined by an interplay of various factors
- Mainly influenced by volume of blood and compliance of central compartment
Factors affecting the measured CVP:
- Central venous blood volume (Inc volume à inc flow à inc CVP)
- Venous return/cardiac output
- Vena cava compression (Obesity/Valsalva/pregnancy/inc IAbd pressure – dec VR à dec CVP)
- Total blood volume
- Regional vascular tone
- Venous return/cardiac output
- Compliance of central compartment (inc Comp à inc return and venous pressure)
- Vascular tone
- Right ventricular compliance
- Myocardial disease
- Pericardial disease
- Tamponade
- Tricuspid valve disease
- Stenosis (inc mean CVP)
- Regurgitation (transient inc CVP)
- Cardiac rhythm (dec CVP with no atrial contraction, inc CVP when RA contracting against closed TV)
- Junctional rhythm
- AF
- A-V dissociation
- Reference level of transducer (inc or dec)
- Positioning of patient
- Intrathoracic pressure (inc pressure à inc CVP)
- Respiration (Inspiration makes intrathoracic pressure more negativeà increases CVP)
- Intermittent positive pressure ventilation (IPPV)
- Positive end-expiratory pressure (PEEP)
- Tension pneumothorax
Measurement
- Invasive:
- by inserting central venous catheter into Internal Jugular or Subclavian Vein
- Non-Invasive:
- Height of JVP provides visual estimate
- peripheral vein collapse on the dorsum of the hand or antecubital fossae
- Bedside ECHO and doppler techniques: IJV, Hepatic veins, IVC assessment
Invasive Measurement:

Complications of CVP Monitoring
- Mechanical complications:
- Arterial puncture and cannulation, Hematoma, Hemothorax, Surrounding nerve injuries, Pneumothorax, Embolization of broken catheter or guide wire, Air embolus, Arrhythmias, Lymphatic system injury
- Infectious complications:
- Sepsis, Endocarditis
- Thrombotic complications:
- Venous thrombosis, Pulmonary embolism
CVP Waveform
(not required for this answer)
| Mechanical | ECG | Clinical | |
|---|---|---|---|
| a wave | Atrial contraction | Starts just after p wave ends | Closely parallels increase in RVEDP |
| z point | Just before closure of TV (just at onset of c wave) | Coincides with middle of QRS | Good indicator of RVEDP esp when a waves not visible (AF) |
| c wave | Closure of tricuspid valve Crest – bulging of TV | Correlates with end of QRS complex | |
| x descent | Fall in intra atrial pressure during atrial relaxation | Before T wave | |
| v wave | Passive atrial filling Crest – opening of TV | Usually after t wave | |
| y descent | Decreased atrial pressure as RA empties into RV | Before p wave |
Changes in waveform:
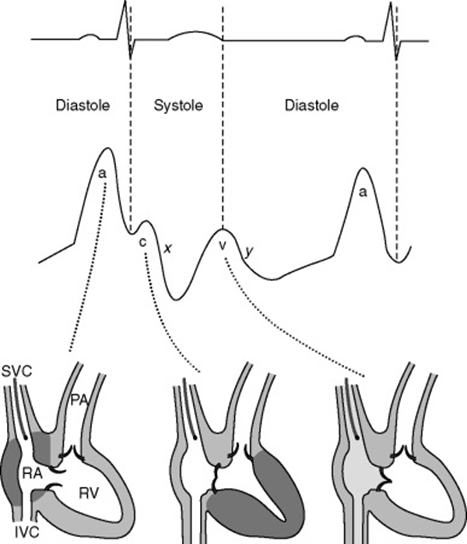
| AF | a wave lost, c wave more prominent. If coarse AF, fibrillation waves may be visible |
| A-V dissociation / Junctional rhythm | tall canon a waves (due to atrial contraction against closed Tricuspid valve) |
| TR | large fused c-v wave (due to blood ejected back during ventricular systole) |
| TS | attenuated a-wave and y-descent (increased pressure required to overcome TS) |
| RV compliance decreased | a wave accentuated |
| Pericardial constriction | short steep y-descent |
| Cardiac tamponade | CVP monophasic with a single x-descent |
Sources:
Daniel Saddawi-Konefka, Critical Care Secrets
Shay McGuinness Cardiothoracic Critical Care 2007
Richard E. Klabunde, CVphysiology.com
T. Smith, Central Venous Pressure: Uses and Limitations
JC 2019
Examiner Comments
2021A 05: 57% of candidates passed this question.
This question examined a core area of cardiac physiology and measurement. Considering this, candidates overall, scored poorly in this section. There was a common misunderstanding around the relationship between cardiac output and CVP. A decrease in cardiac output (e.g. due to either decreased stroke volume or heart rate) will cause an increase in CVP as blood backs up in the venous circulation, increasing venous volume as less blood moves through to the arterial circulation; the resultant increase in thoracic volume increases central venous pressure. Several candidates confused the direction of their arrows, for example “increased right atrial compliance increases CVP”. Double negatives were used by several candidates which then resulted in the incorrect relationship described. (e.g., “arrow down compliance and arrow down CVP”). The measurement section should have included an explanation of the components of an invasive pressure monitoring system relevant to the measurement of CVP.
6. Describe the pharmacology of vecuronium, including factors that prolong its action of neuromuscular blockade.
CICMWrecks Answer: Factors
FACTORS
The factors which alter the response (number of receptors occupied) may be separated into: pharmacodynamic factors, pharmacokinetic factors, pharmaceutical and drug interaction factors
Pharmacodynamic Factors
- decreased pH increases the affi¬nity of non depolarising NMB
- electrolyte disturbances
- Ca disturbances affect the release of ACh from the presynaptic terminal (triggered by Ca)
- hypercalcaemia results in shortened blockade
- hypocalcaemia potentiates blockage
- magnesium
- stabilise membranes and decrease release and sensitivity to ACh
- potassium
- hypokalaemia leads to membrane hyperpolarisation (decreased drug effect)
- hyperkalaemia has the reverse effect
- pathology
- myasthenia gravis patients have defective nACh receptors
- eaton-lambert syndrome patients have decreased ACh release
- burns – leads to receptor mediated resistance to non depolarising NMBD
Pharmacokinetic factors
(affect the amount of drug reaching the receptor)
- absorption
- dose given (higher dose – more receptors occupied)
- distribution
- lipid soluble drugs such as vecuronium may accumulate in lipid rich tissue (esp infusion)
- women have higher lipid content which may lead to reduced effect site conc
- metabolism
- hepatic failure may lead to decreased metabolism of a drug – pancuronium
- excretion
- renally excreted drugs, especially minimally metabolised vecuronium, rocuronium
Pharmaceutical / Drug Interactions
- Increase effect
- aminoglycosides – decreased prejunctional ACh release
- volatile anaesthetics – mechanism not well understood
- frusemide – potassium effect, may interfere with cAMP and ACh release
- decrease effect
- anticonvulsants – may develop resistance to pancuronium, vecuronium
JC 2019
Examiner Comments
2021A 06: 13% of candidates passed this question.
Vecuronium is a commonly available and regularly used amino-steroid neuromuscular blocking agent. It is a level 1 drug in the 2017 syllabus. A simple template utilising the headings; pharmaceutics, PK, PD, uses in ICU and adverse reactions with associated relevant important facts would have scored well. Expected information regarding the factors prolonging neuromuscular blockade included electrolyte abnormalities, drug interactions and patient factors. Overall, the level of understanding and knowledge demonstrated in the answers was below an expected standard for a level 1 drug.
7. Outline the anatomy of the blood supply (arteries and veins) of the gastrointestinal system (oesophagus to anus)
CICMWrecks Answer
Blood supply to the Gastrointestinal system
- Portal circulatory system + arterial blood flow into liver
- 1100ml of portal blood + 400ml from hepatic artery = 1500ml (30% CO)
- Oxygen consumption – 20-35% of total body needs
Arterial Supply
| Aorta | Division / Branches | Organ supplied | |||
|---|---|---|---|---|---|
| Thoracic A. | Subclavian A. | Thyrocervical trunk | Inf. Thyroid A. | Cervical Oesophagus | |
| Bronchial A. | branches | Thoracic Oesophagus | |||
| direct branches | |||||
| Abdominal Aorta / Coeliac | Inf. Phrenic A. | Abdominal Oesophagus | |||
| Anterior branches of Abdominal Aorta | COELIAC TRUNK | Lt. GASTRIC A. | |||
| Foregut (Stomach → major duodenal papilla | |||||
| SPLENIC A. | Short Gastric A.s | ||||
| Lt. Gastroepiploic A. | |||||
| COMMON HEPATIC A. | Hepatic A. Proper | Lt. Hepatic A. | |||
| Rt. Hepatic A. | |||||
| Gastroduodenal A. | Rt. Gastroepiploic A. (gastro-omental) | ||||
| Sup. Pancreatoduodenal A. | |||||
| Supraduodenal A. | |||||
| SUPERIOR MESENTERIC ARTERY | Inf. Pancreaticoduodenal A. | Midgut (Major duodenal papilla → 2/3 of transverse colon) | |||
| Jejunal and Ileal A.s | |||||
| Middle colic A. | |||||
| Rt colic A. | |||||
| Ileocolic A. | |||||
| INFERIOR MESENTERIC ARTERY | Lt colic A. | (2/3 transverse colon → rectum) | |||
| several sigmoid A.s | |||||
| Superior rectal A. | |||||
| Distal Abdominal Aorta | Internal Iliac A. | Middle Rectal A. | Rectum | ||
| Internal Pudendal A. | Inferior Rectal A. | ||||
Venous Drainage
| Organs drained | Branches / Tributaries | IVC | |||
|---|---|---|---|---|---|
| Liver | Hepatic V.s | IVC | |||
| Fundus + L greater curvature Stomach | Short gastric V.s | Splenic V | Portal Vein | Liver parenchyma → Hepatic Veins → IVC | |
| Greater curvature Stomach | Lt. gastro-omental V. | ||||
| Pancreas | Pancreatic V. | ||||
| colon | Lt. colic V. | IMV | |||
| sigmoid | Sigmoid V. | ||||
| rectum | Superior rectal V. | ||||
| Rectum, Colon | direct | ||||
| Rt greater curvature Stomach | Rt gastro-omental V. | SMV | |||
| Pancreas, duodenum | Ant sup pancreatocoduodenal V. | ||||
| Ant and Post inferior pancreaticoduodenal V.s | |||||
| Jejunum | Jejunal V. | ||||
| Ileum | Ileal V. | ||||
| Ileum, colon, cecum | Ileocolic V. | ||||
| ascending colon | Rt. colic V. | ||||
| transverse colon | Middle colic V. | ||||
| pancreas, duodenum | Post Sup pancreaticoduodenal V. | ||||
| stomach | Rt. gastric V. | ||||
| stomach | Lt. gastric V. | ||||
| gall bladder | cystic veins | ||||
| Skin of umbilical region | para-umbilical vein | ||||
Portosystemic Anastomosis
- Areas:
- Lower end of oesophagus
- Upper part of anal canal
- Umbilicus
- Retroperitoneal Bare area of liver
- The gastroesophageal junction around the cardia of the stomach-where the left gastric vein and its tributaries form a portosystemic anastomosis with tributaries to the azygos system of veins of the caval system.
- The anus-the superior rectal vein of the portal system anastomoses with the middle and inferior rectal veins of the systemic venous system.
- The anterior abdominal wall around the umbilicus-the para-umbilical veins anastomose with veins on the anterior abdominal wall.
Examiner Comments
2021A 07: 48% of candidates passed this question.
This question was answered best if the main arteries and veins were discussed first and then their corresponding supply outline in reasonable detail. Very few candidates were able to achieve this. Listing the names of vessels with no context and in a random non-sequential order did not attract many marks. The physiology of the blood supply to the liver also did not attract marks.
8. Describe renal handling of potassium (60% marks),
including factors that may influence it (40% marks).
CICMWrecks Answer
Potassium
- Predominany intracellular cation
- Total Stores: Approx 3200mmol (50mmol/kg)
- Key Functions:
- Main determinant of ICF osmolality and tonicity
- Responsible for RMP of excitable cells via Goldmann-Hodgkin-Katz due to ↑↑gK relative to other species
- Role in action potential → repolarisation phase
- Secretion of insulin and multiple other KATP dependant processes
- Regulation of IC processes (protein/glycogen synthesis)
- Involved in Na+/K+ ATPase in cell membranes
Potassium Elimination
- Faecal – 8mmol/day
- Renal – 92mmol/day (See next heading for details)
- Urinary K+ excretion = [K+ filtered by glomerulus] + [K+ secreted by CCD/LDCT] – [K+ reabsorbed by renal tubules]
- Glomerular filtration: Freely filtered = 756mmol/day (180L x 4.2mmol/L)
- PCT: 65% reabsorption
- LoH: 25~30% reabsorption
- DCT/Collecting Ducts – variable
- Determined by Aldosterone, Plasma [K+], Tubular Flow rate
- Secreted by principal cells
- Reabsorbed by intercalating cells
K+ handling within the tubular system:
- PCT (55% of filtered K+ is reabsorbed):
- K+ is reabsorbed passively via the paracellular route due to:
- Solvent drag → coupled to flow of Na+ and H2O
- [ ] gradient → created by reabsorption of H2O
- Electrical gradient → due to +ve luminal charge in late PCT
- K+ is reabsorbed passively via the paracellular route due to:
- TAL of LoH (30% of filtered K+ is reabsorbed):
- K+ is reabsorbed by 2° active transport via paracellular route → +ve charged lumen generated by ion flux created by NKCCT transporter causes K+ to be reabsorbed by diffusion down its electrical gradient
- LDCT and CCD (0 to > 15% K+ is secreted):
- Principal cells of CCD and LDCT → 2° active secretion K+ → via:
- Basolateral Na+ /K+ ATPase → (i) generates an electrochemical gradient that draws Na+ intracellularly from the tubular lumen (via the ENaC channel), and (ii) pumps K+ from peritubular capillaries into tubular cell
- The –vely charged lumen generated by influx of Na+ across ENaC channel → favours tubular secretion of K+
- o Type A intercalated cells of CCD and LDCT → 2° active reabsorption of K+ :
- H+ is produced within tubular cell by hydration of CO2 (using CA) → H+ is then exchanged for tubular K+
- Principal cells of CCD and LDCT → 2° active secretion K+ → via:
- MCD (5% of filtered K+ is reabsorbed)
Renal K+ regulation:
- Renal K+ regulation occurs MAINLY via control of K+ secretion at the distal nephron (CCD and LDCT) via the following factors:
- Circulating factors:
- Plasma [K+ ]
- ↑ [K+ ] causes ↑ K+ secretion by → (i) Directly stimulating Na+ /K+ ATPase in principal cells, and (ii) Directly stimulating Aldosterone release from adrenal cortex
- Aldosterone
- ↑ aldosterone causes ↑ K+ secretion by → ↑ production of Na+ /K+ ATPase, K+ channels and ENaC Na+ channels in the principal cells
- Plasma pH
- Alkalosis via ↓ plasma [H+ ] causes ↑ K+ secretion by → stimulating Na+ /K+ ATPase in principal cells
- Plasma [K+ ]
- Luminal factors:
- Flow of tubular fluid in DCT and CCD
- ↑ tubular fluid flow causes ↑ K+ secretion by → maintaining ↓ luminal [K+ ] (Ie. continuously washing it away) → permits passive diffusion of K+ ↓ its [ ] gradient into tubular lumen
- ↑ Na+ delivery rate to DCT and CCD → ↑ K+ secretion
- -ve lumen potential difference → ↑ K+ secretion
- Flow of tubular fluid in DCT and CCD
- Circulating factors:
JC / Gladwin / Sakurai / Bianca 2020
Examiner Comments
2021A 08: 33% of candidates passed this question.
This question covers a core physiology topic. The detail required is well described in the recommended reference texts. Generally, this question was poorly answered. From an answer template perspective, a “describe question” in this context involves both the stating the relevant potassium handling mechanism and then giving a description of how it occurs and how this system is regulated. Many answers that scored poorly simply listed sites of potassium handling but excluded the details surrounding the specific receptors and channels involved as well as the processes that exist to perpetuate and regulate these biological processes. Simple identification as to whether the potassium was being secreted or reabsorbed as well as the location as to where this may occur within the nephron, were often not specifically detailed or used interchangeably. Such answers scored poorly.
9. Outline the mechanisms by which normal body temperature is maintained and regulated.
CICMWrecks Answer: Temperature Regulation
Definitions
- Heat – A form of kinetic energy → being a state of “thermal agitation” of molecules in a substance
- Temperature – A measure of a physical property of a substance that determines the tendency for heat to flow from one object to another → heat energy is transferred from a region of higher temperature to a region of lower temperature
- Temperature setpoint is the level at which the body attempts to maintain its temperature.
- Core body temperature:
- Refers to deep body temperature of main internal organs (in head, trunk, abdomen) →
sites where metabolic activity occur (Ie. heat production) - Kept constant at 37 +/- 0.4 °C (“Normothermia”) → displays normal variations:
- Diurnal variation – ↑ in evening (37.3 °C) and ↓ in early morning (35.8 °C)
- Menstrual variation – ↑ 0.5 °C in latter half of cycle
- Refers to deep body temperature of main internal organs (in head, trunk, abdomen) →
- Variations in core body temperature:
- Normothermia → core body temperature 37 +/- 0.4 °C
- Hypothermia → core body temperature < 36 °C
- Hyperthermia → core body temperature > 37.5 °C
- Peripheral body temperature:
- Refers to body temperature peripherally (Ie. skin, arms, legs, superficial tissues of core
sites) → sites where heat loss occur - Temperature varies widely → always LESS than core body temperature
- Refers to body temperature peripherally (Ie. skin, arms, legs, superficial tissues of core
- Regulation of body temperature is done by balancing heat loss and heat production, predominantly through behavioural mechanisms and skin
Thermoneutral Zone
- The range of environmental temperatures in which the metabolic heat production (and oxygen consumption) is minimal and steady and where core temperature is maintained by vasomotor activity alone.
- 25-30 °C for a naked, upright man in still air
Interthreshold Range
- The range of core temprature over which no autonomic thermoregulatory responses occur
- Normally 0.2 -0.4 °C in a non-anaesthetized state
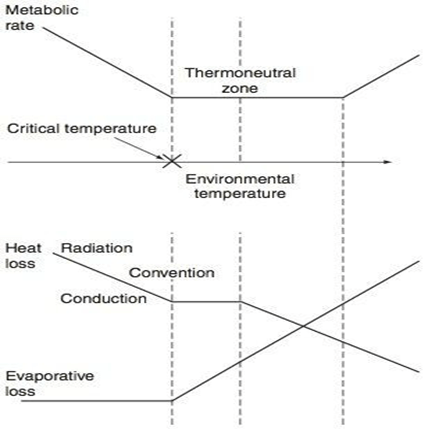
Regulation of Body Temperature
Temperature sensors are central and peripheral, whilst regulation occurs centrally, and has multiple
effectors
| Cold | Warm | |
|---|---|---|
| Receptor | Bulbs of Krause | Bulbs of Ruffini |
| Afferent nerve type | via Aδ and unmyelinated C sensory fibres | Unmyelinated C fibres |
| Spinal synapse location | Rexed lamina 1,5 | Rexed lamina 1, 2 |
| Ascending tract | Lateral spinothalamic tract in anterior spinal cord | Lateral spinothalamic tract in anterior spinal cord |
| Brainstem synapse | Reticular system of medulla | Reticular system of medulla |
| Central Processor | Hypothalamus (posterior) | Hypothalamus (anterior) |
| Thresholds | No activity at 40 degrees ↑ing activity between 10-27 degrees (remember thermoneutral zone) | Activity with increasing between 30-45 °C Upper threshold limit = 46 °C |
Sensors:
- Central
- Deep tissues/viscera (Eg. in intestinal wall)
- Brain (anterior hypothalamus and extra-hypothalamic areas)
- Spinal cord
- Peripheral
- Dermis (main)
- Corneas
Central Controller:
Hypothalamus is the main body temperature regulatory centre
- Posterior hypothalamus responds to cold
- inputs from peripheral afferents
- responsible for the temperature set point
- ACh is the major neurotransmitter here.
- Anterior hypothalamus responds to heat
- both peripheral input and change in blood temperature
- Major neurotransmitters are Nad, 5HT, dopamine and prostaglandins.
Effector Mechanisms:
- Skin
- Sweating (evaporation)
- Secrete plasma-like fluid Na142, Cl- 104 (urea/K, etc v low amounts) osmolality controlled by rate of secretion and aldosterone
- loss via evaporation (latent heat of vapourisation of water)
- 1-2L in extreme exercise up to 10 L max per 25 hours.
- 0.54 kcal/gram of H2O evaporated
- Degree of evaporation determined by ambient temperature and relative humidity
- Blood Flow (radiation)
- 8 (300 mL/min) – 30% CO skin
- Vasodilation up to 30 fold increase
- Vasoconstriction up to 10 fold decrease
- vasoconstriction/dilation controlled by SNS (α1-mediated) vascular SM contraction and hypothalamic feedback
- Fat/Clothing
- Fat conducts heat 1/3 as readily as other tissues (decreased radiation)
- Clothes reduce conductive heat loss via private zone of air adjacent to skin and decreased convection air currents -50% and more if specialised
- Sweating (evaporation)
- Non-Skin
- Non-shivering thermogenesis
- Predominantly in brown fat
- Uncoupling of oxadative phosphorylation
- Increased heat gain without oxygen consumption/ATP production
- Shivering
- Increased heat gain by metabolism
- Behaviour
- For gain (exercise) or loss (submerging in water)
- Voluntary muscle contraction (Ie. ↑ activity with cold stress; ↓ with hot stress) – Affects heat production
- Body posturing (Ie. ↓ BSA with cold stress;↑ with hot stress) – Affects heat loss
- Clothing (Ie. ↑ clothing with cold stress; ↓ with hot stress) – Affects heat loss
- Appetite
- Cold stress stimulates food-induced thermogenesis → ↑ metabolic rate and heat production
- Thyroid hormone secretion
- Cold stress stimulates thyroid hormone secretion → long-term ↑ metabolic rate and heat production
- Non-shivering thermogenesis
Gladwin / JC 2019
Examiner Comments
2021A 09: 59% of candidates passed this question.
This question was relatively well answered by most candidates. There was significant variation in the temperatures expressed as normal and few candidates mentioned CORE temperature as a concept. Several candidates gave a detailed description of thermo-neutrality for which there were no marks.
10. How does warfarin exert its pharmacological effect (40% marks)? Write brief notes on the pharmacology of the agents that can be used to reverse the effects of warfarin (60% marks).
CICMWrecks Answer: Pharmacology of Warfarin
Warfarin Pharmacology
CICMWrecks Answer: Warfarin Reversal
Warfarin Reversal
The following reversal strategies may be either used alone or in combination:
- Stopping warfarin
- S-warfarin is eliminated via oxidation predominantly by CYP2C9 (and to a lesser extent by CYP3A4 and 1A2) → inactive metabolites
- Elimination half-life of S-warfarin is 29 hours
- ∴ in patients with normal hepatic metabolic and synthetic functions take 4 ~ 5
- days for INR to normalise
- Vitamin K
- Can be given either enterally or parenterally (IV, IM) (bioavailability ≈ 100%)
- Requires few hours to work
- Enteral administration Requires presence of bile salts to be absorbed by gut
- Parenteral administration has rare but life threatening complication of hypersensitivity probably related to its preservative, benzyl alcohol
- Low dose vitamin K (1 ~ 2.5 mg) will slowly reduce INR over 24 hours
- but often will not result in complete reversal and would not significantly affect re-establishment of anticoagulation with warfarin
- High dose vitamin K (10 mg) will slowly reduce INR over 12 hours
- ∴ often given together with clotting factors (e.g. FFP)
- Can be given either enterally or parenterally (IV, IM) (bioavailability ≈ 100%)
- Fresh frozen plasma
- Plasma from donated blood, which contains all clotting factors
- ∴ immediately reverses coagulopathy
- However, short duration of action (24 ~ 48 hours) ∴ reserved for active bleeding
- Used when PCC not available
- Dose 2 ~ 4 units (10-15ml/kg) depending on INR and bleeding risk
- Disadvantages = relatively large fluid volume, possible transfusion reactions (e.g. immune reaction, TRALI, infection, etc)
- PCC – Prothrombin complex concentrates (Prothrombinex)
- Human plasma derivative containing concentrates of factors II, IX and X
- 500IU PCC has 500IU of II, IX, X each in a vial
- ∴ immediately reverses coagulopathy
- Dose 25 ~ 50 IU/kg
- Advantages = reliable reversal, smaller fluid volume, avoids transfusion
- complications associated with FFP
- Disadvantages = expensive
- Recombinant activated factor VII (NovoSeven)
- Controversial treatment, present in some guidelines
- Indication = life threatening bleed with significantly elevated INR
- Given together with vitamin K and FFP
- Disadvantage = thromboembolic complications
Examiner Comments
2021A 10: 43% of candidates passed this question.
Warfarin is listed as a level 1 drug in the 2017 syllabus and as such a detailed knowledge of its mechanism of action would be expected from candidates sitting the exam. The reversal agents for warfarin are collectively classed as level 2 drugs and hence the knowledge required would be at a write short notes level. The following topics were expected: what drugs may be used, how they work, in what dose, any common side effects, why/when would one be used in preference to others etc. The use of reversal agents for warfarin is a common practice in ICU. Generally, answers demonstrated a lack of a precise and detailed knowledge with respect to warfarin’s mechanism of action and had a very superficial knowledge with incorrect facts regarding the reversal agents.
11. Describe the buffer systems in the body.
CICMWrecks Answer – Buffer Systems
Buffer
- Weakly ionised acid or base in equilibrium with its full ionised salt
- A buffer can “resist” change in pH by absorbing or releasing H+ ions
- Works best when pKa is closest to the target pH (7.4)
- Isohydric Principle
- All buffer systems which participate in defence of acid-base changes are in equilibrium with each other. There is after all only one value for [H+] at any moment. This is known as the Isohydric Principle.
Effectiveness:
- buffer pKa (most effective if pKa = pH of carrying solution)
- pH of carrying solution
- amount of buffer present
- open (physiological) vs closed (chemical) system
Buffer Systems by site
| Site | Buffer System | Comment |
|---|---|---|
| ISF | Bicarbonate | For metabolic acids |
| Phosphate | Not important because concentration too low | |
| Protein | Not important because concentration too low | |
| Blood | Bicarbonate | Important for metabolic acids |
| Haemoglobin | Important for carbon dioxide | |
| Plasma protein | Minor buffer | |
| Phosphate | Concentration too low | |
| ICF | Proteins | Important buffer |
| Phosphates | Important buffer | |
| Urine | Phosphate | Responsible for most of ‘Titratable Acidity’ |
| Ammonia | Important – formation of NH4+ | |
| Bone | Ca carbonate | Important in prolonged metabolic acidosis |
| CSF | Bicarbonate | Important – (as very low proteins for buffering) free Bicarbonate flow in CSF |
| Phosphate | negligible |
Buffer Systems
| Name | Location |
|---|---|
| Protein | Intra cellular |
| Haemo globin | Blood |
| Bicarb | Plasma, Interstitium, Urine (minimal) |
| Ammonia | Urine |
| Phosphate | Urine, Bone |
| CaCO3 | Bone |
Protein (Intracellular)
- pKa of imidazole group is 6.0
- pKa of the histadine residues themselves = 6.8
- Protein buffer ability is proportional to Histadine residue content.
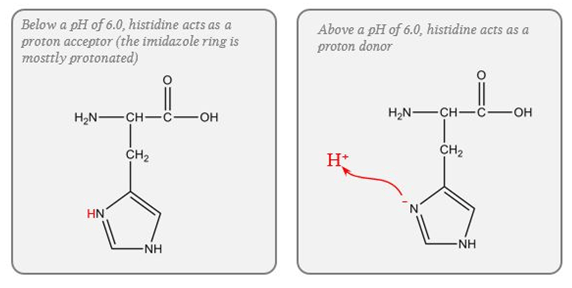
Haemoglobin (Blood)
- Protein buffering system
- Important intracellular buffer in RBC
- Exists as weak acid – HHb and potassium salt KHb.
- Hb a pKa of 8.2 in deoxy form and 6.6 in oxyHb
- Buffering capacity due to imidazole residues on 38 histidine residues on Hb molecule
- pKa of residues 6.8 à close to physiological pH
- Also important in extracellular buffering (following bicarbonate buffer system)
- Due to fast equilibration of HCO3– (Hamburger Shift)
- Band3 Transporter (HCO3–/Cl– antiporter)
- Allows carbonic anhydrase reaction to take place by limiting build-up of HCO3– (la chatelier principle)
- Formed in erythrocytes as a tetramer of 4 subunits
- Hb ~ 6 times the number of histadine (38) residues compared with albumin. (3-6x buffering capacity)
- Hb is in much greater concentrations than any other protein (15 g/dL vs 7 g/dL)
- For each mmol OxyHb, 0.7mmol H+ is buffered and 0.7mmol of CO2 can enter circulation without a change in pH
- Available at high concentrations in RBC
- Isohydric exchange
- the buffer system (HHbO2-HbO2-) is converted to another more effective buffer (HHb-Hb-) exactly at the site where an increased buffering capacity is required
- Deoxyhaemoblobin is a much more effective buffer
- oxygen unloading increases the amount of deoxyhaemoglobin and this better buffer is produced at exactly the place where additional H+ are being produced because of bicarbonate production for CO2 transport in the red cells.
Bicarb (Plasma, Interstitium, Urine (minimal))
- Catalysed by carbonic anhydrase (slow where CA is absent (plasma))
- Open at both ends
- Bicarb regulation at kidneys
- CO₂ regulation at lungs
Ammonia (Urine)
Equlibrium between ammonia and ammonium
Steps:
- Glutamine enters PCT cells
- 20% from filtrate
- 80% from peritubular capillaries
- Ammonia (NH3) produced from glutamine in PCT by glutaminase secreted into lumen
- Reabsorbed in TAL of LoH
- Diffuses into peritubular cells of CD down conc Grad
- H+ secreted into lumen of CD
- Combines with ammonia to form ammonium
- Ammonium positive charge prevents reabsorption
- Thus “excess hydrogen can be secreted with ammonium”
Phosphate (Urine, Bone)
Effect in urine is limited due to:
- Minimal urinary pH is 4.5 → equation B is >99% ionised → of little use.
- Requires filtered PO4 → Normally low levels.
- Depends on Diet and PTH.
- Where H is secreted in distal tubules, there is minimal PO4 excretion
CaCO3 (Bone)
- Important in chronic metabolic acidosis
- Release of calcium carbonate from bone is the most important buffering mechanism involved in chronic metabolic acidosis.
Please Note: This answer is viewed from the Traditional approach to Acid-Base theory. Alternative (physicochemical approaches) deemphasise bicarb due to its equilibrium with water and relatively low concentration compared with the strong ions.
Gladwin / Sakurai / JC 2020
Examiner Comments
2021A 11: 57% of candidates passed this question.
This is a core physiology topic; a detailed knowledge of buffering and the available buffer systems is crucial to ICU practice. A candidate presenting for the first part exam should have a detailed understanding of all aspects of the buffer systems. Higher scoring answers provided both technical details of the buffer systems, the context for their normal function and their relative importance. Efficient answers dealt with the buffers by chemical rather than by site, but many answers categorising buffers by site also scored well. Many low scoring answers simply failed to provide detail, some provided incorrect information. Very few candidates demonstrated an understanding of the isohydric principle.
12. Describe the pharmacology of oxycodone.
Examiner Comments
2021A 12: 54% of candidates passed this question.
There were many exceptional answers which provided extensive detail on the drug. The best of these gave context for the drug characteristics, such as by referring to oxycodone relative to other opioid drugs that might be chosen, or to considerations for safe and effective administration. Some answers, however, provided generic information on opioid drugs, which could not gain all the available marks.
13. List the cell types in the anterior pituitary gland. Outline their secretions, control and target organ effects.
CICMWrecks Answer
Anterior Pituitary Gland
- Anterior lobe of pituitary gland (aka. adenohyophysis) → forms 80% of pituitary gland → consists of 3 parts:
- Pars tuberalis
- Parts intermedius (intermediate lobe)
- Pars distalis (anterior lobe) → produces most anterior pituitary hormones
- Derived from buccal ectoderm → it extends upwards from the epithelial lining of the primitive mouth cavity (as Rathke’s pouch), then fuses with the downward growing infundibulum of the hypothalamus (which forms the posterior pituitary)
- Consists of non-neural secretory epithelial cells connected INDIRECTLY to the hypothalamus via the “hypothalamic-hypophysial portal system”:
- Non-neural secretory epithelium:
- Granular secretory cells (chromophils)
- Acidophils (80%) → red-staining cells → synthesise and store large peptide hormones in secretory granules
- Lactotropes → PRL
- Somatoropes → GH (somatotropins)
- Basophils (20%) → blue-staining cells → synthesise and store glycoprotein hormones in secretory granules
- Gonadotropes → FSH and LH
- Thyrotropes → TSH
- Corticotropes → ACTH (and MSH)
- Acidophils (80%) → red-staining cells → synthesise and store large peptide hormones in secretory granules
- Agranular secretory cells (chromophobes)
- Weakly staining cells → inactive or degranulated secretory cells → does not synthesise any hormones
- Granular secretory cells (chromophils)
- Hypothalamic-hypophysial portal system
- Branches of superior hypophysial artery → forms “1° capillary plexus” near median eminence of hypothalamus → then forms lesser portal veins that gives rise to “2° capillary plexus” in anterior lobe of pituitary gland
- Functions of portal venous system
- Blood supply of 90% of anterior pituitary (and infundibulum)
- Pathway for hypophysiotropic hormones (from median eminence of hypothalamus) to reach the anterior pituitary gland, and
- Allows hormones secreted by anterior pituitary gland to enter systemic circulation
- Non-neural secretory epithelium:
- Blood supply → 90% supplied by hypothalamic-hypophysial portal system (from superior hypophysial artery)
Hypophysiotrophic hormones: Control of anterior pituitary gland
- Hypophysiotrophic hormones are small peptides (EXCEPT for DA) produced by cell bodies (containing “parvocellular neurons”) in the hypothalamus located near the median eminence → small amounts are secreted from the tuberoinfundibular tract into the hypothalamic-hypophysial portal system → reaches anterior pituitary where it exerts either an “excitatory” or “inhibitory” effect on non-neural secretory epithelial cells
Nb. Only trace levels of these hormones are found in systemic circulations b/c → (i) small amounts are secreted, and (ii) they are secreted into the hypothalamic-hypophysial portal system
- Nature of release from hypothalamus:
- Pulsatile manner → can vary with circadian rhythm, but induced in the presence of specific stimuli (Eg. cold, stress, Etc.)
- Regulated by –ve feedback (esp from hormones secreted by peripheral organs) EXCEPT PRL (see below)
- Types of hormones:
- Thyrotrophin-releasing hormone (TRH) → tripeptide (3 a.a.) → stimulates TSH secretion from thyrotropes
- Corticotrophin-releasing hormone (CRH) → 41 a.a. peptide → stimulates ACTH secretion from corticotropes
- Gonadotrophin-releasing hormone (GnRH) → 10 a.a. peptide → stimulates LH and FSH secretion from gonadotropes
- Growth-hormone releasing hormone (GHRH) → 43 a.a. peptide → stimulates GH secretion from somatoropes
- Somatostatin (GHIH) → 14 a.a. peptide → suppresses GH secretion from somatotropes (also ACTH, TSH and PRL release; also inhibits insulin/glucagon)
- Prolactin releasing factor (PRF) → stimulates PRL secretion from lactotropes
- Dopamine (DA) → suppresses PRL secretion from lactotropes
Bianca 2016
Examiner Comments
2021A 13: 40% of candidates passed this question.
Few candidates described cell types as chromophils and chromophobes. There were many errant references to chromaffin cells which are found mainly in the adrenal medulla, and to staining on H&E. Chromophil cells stain by absorbing chromium salts. Few candidates mentioned that the hormones secreted by the anterior pituitary are peptides. Most candidates outlined the hypophyseal-portal system well. Knowledge of TSH and ACTH control and target organ effects were good. Similar knowledge for LH, FSH, PRL and GH was much more sporadic.
14. Describe the pharmacology of sodium bicarbonate.
Examiner Comments
2021A 14: 29% of candidates passed this question.
This question was best answered with a structured approach as per any pharmacology question. It nonetheless required good understanding of various aspects of physiology. Many candidates failed to gain marks by omitting to mention facts which could have been prompted by a defined structure. A good response mentioned the pharmaceutic features including formulation and the hypertonicity of IV bicarbonate, pharmacodynamics including indications for use, mode of action, adverse effects (systemic and local), pharmacokinetics and dose. Pleasingly a few candidates stated that sodium bicarbonate’s mechanism of action to cause alkalosis involved increasing the strong ion difference in plasma. Credit was also given for stating the mechanism of action as providing bicarbonate ions to augment the extracellular buffer system.
15. Explain perfusion limited and diffusion limited transfer of gases in the alveolus.
CICMWrecks Answer
Fick’s law of diffusion
- Describes Diffusion through tissues
- Rate of movement of solute across semi-permiable membrane J is
where
C = concentration (or partial pressure for gasses)
A = cross-sectional area
T = thickness of the membrane or distance over which diffusion takes place.
The rate of diffusion of a gas through a tissue is:
Directly ∝:
- Surface area of the barrier
Affected by:- Parenchyma volume: Changes with Body size and in disease states
- V/Q mismatch: reduced in shunt and dead space
- Pulmonary Blood Volume: changed with vascular distension and recruitment
- Cardiac output: increased recruitment in high output states, decreased recruitment and increased V/Q mismatch in shock states
- Posture: Increased surface area while supine (compared to sitting or standing)
- Solubility of the gas
- CO2 is ~20 times as soluble as O2 in blood
- Concentration gradient (partial pressure difference)
- Rate of blood flow through lungs
- Transit time of blood across capillary (not part of Fick’s Equation) – based on HR
- alters total gas exchange across alveolus
- Can potentially change perfusion limited gas exchange to diffusion limited
- Alveolar partial pressure affected by:
- Atmospheric pressure
- Ventilation: Alveolar hypoventilation → ↑ PACO2 , ↓ PAO2
- Partial pressure in blood affected by:
- Binding of gas to protein:
- Rate of oxygenation of reduced Hb:
- Shift of oxygen dissociation curve (pH, temperature, PCO2, 2,3-DPG)
- Haematocrit
- Abnormalities of haemoglobin
- Formation of carbamino compounds
- Anaesthetic agents to plasma contents, e.g Albumin, cholesterol
- Enzymatic Action
- carbonic anhydrase (conversion of HCO3 to CO2)
- Rate of blood flow through lungs
Inversely ∝:
- Membrane thickness
- Increased thickness impedes gas exchange – Pathological states like pulmonary edema and cardiac failure
- Square root of Molecular Weight
- smaller substances diffuse more quickly
Diffusion vs. perfusion limited
Transfer of gases can be diffusion or perfusion limited dependent on the rate limiting step
Diffusion Limited
- Occurs in gases which do not reach equilibration of Pa and PA
- The rate of gas diffusion across the alveolar membrane limits its transport away from the lung
- Rate limiting step = rate of diffusion
- E.g. CO
- CO binds so avidly to Hb (250x that of O2) → virtually no CO dissolved in plasma → PaCO rises only slightly
- Even when RBC traversed entire length of pulmonary capillary, there is still substantial partial pressure difference across alveolar-capillary barrier → equilibrium of PaCO and PACO never reached
Perfusion Limited
- Characterized by complete equilibration i.e. Pa = PA
- amount of gas transferred between alveolus and capillary = dependent on amount of blood passing through the capillary
- rate limiting step = rate of blood flow
- E.g. N2O
- N2O rapidly diffuses across alveolar-capillary barrier
- Insoluble; does not bind to Hb; only carried in plasma in dissolved form
- PaN2O = PAN2O (<0.07sec); well before RBC has traversed pulmonary capillary
- ↑ diffusion rate will not ↑ blood transport away from the lungs → limiting factor = rate of blood flow / perfusion
- e.g. CO2 (ventilation limited i.e. perfusion limited in reverse)
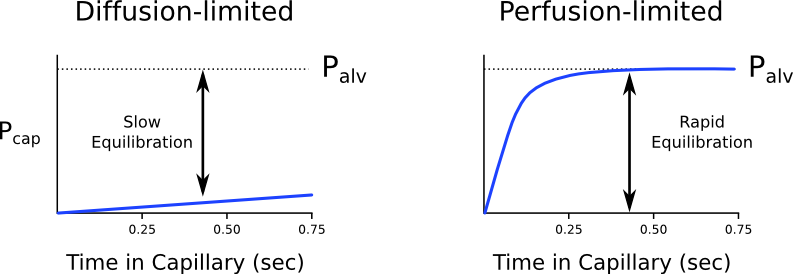
Is the transfer of O2 perfusion or diffusion limited?
- Can behave as both perfusion and diffusion limited
- O2 diffusion takes 0.25s; pulmonary capillary transit time is 0.75s
- Normal conditions
- Transfer of O2 across the alveolar capillary barrier is perfusion limited
- Equilibrium is reached between alveolar and capillary PO2 before the RBC has traversed the pulmonary capillary
- Conditions where transfer of O2 may become diffusion limited
- Disease of the alveolar capillary barrier
- Pulmonary fibrosis: thickening of alveolar-capillary barrier → ↓ rate of diffusion
- Exercise: ↑ CO → ↓ RBC transit time
- Altitude: ↓ PaO2
- Disease of the alveolar capillary barrier
Diffusion of O2 and CO2
Oxygen
- Oxygen diffusion takes ~0.25s
- Pulmonary capillary transit time is 0.75s
- Therefore, under normal conditions oxygen is a perfusion limited gas
- However, oxygen may become diffusion limited in certain circumstances:
- Alveolar-capillary barrier disease
Decreases the rate of diffusion.- Decreased surface area
- Increased thickness
- High cardiac output
Decreases pulmonary transit time. - Altitude
Decreases PAO2
- Alveolar-capillary barrier disease
Carbon Dioxide
- Carbon dioxide is ventilation limited, rather than diffusion or perfusion limited
- This is because it is:
- 20x more soluble in blood than oxygen
- Rapidly produced from bicarbonate and carbamino compounds
- Present in far greater amounts than oxygen
1.8L.kg-1 exist in the body (though 1.6L-1 of this are in bone and other relatively inaccessible compartments).
- Impairment of diffusion capacity causes type 1 respiratory failure as oxygen is affected to a much greater extent than carbon dioxide
Kerr / JC 2020
Examiner Comments
2021A 15: 36% of candidates passed this question.
This question required detail on those factors affecting gas exchange at the level of the alveolus. A description of the components of the Fick equation was expected – and how this related to oxygen and carbon dioxide transfer at the alveolar capillary membrane. The rapid rate of equilibration (developed tension) was the limiting factor in of blood/alveolar exchange that rendered some gases perfusion limited (examples – N2O, O2 under usual conditions but not all) and the slower rate of others diffusion limited (examples CO and O2 under extreme conditions e.g., exercise, altitude). Estimates of time taken for each gas to equilibrate relative to the time taken for the RBC to travel across the interface was also expected for full marks. CO2 despite rapid equilibration and higher solubility was correctly described as perfusion limited (unless in disease states). Better answers described CO2 as ventilation limited. Some answers also correctly included the component of interaction with the RBC and haemoglobin. Ventilation/perfusion inequalities over the whole lung were not asked for and scored no marks.
16. Describe the pharmacology of piperacillin-tazobactam.
Examiner Comments
2021A 16: 62% of candidates passed this question.
Most candidates used a structured approach with the usual major pharmacology headings. Mechanism of action was well described by most, with better answers including mechanisms of resistance. Higher scoring candidates included an explanation as to the combination of the drugs. Likewise, better answers included detailed information on spectrum of activity beyond “gram positive and gram negative”, including relevant groups of organisms which are not covered. There also seemed to be some confusion about coverage for anaerobes, which piperacillin tazobactam covers well. Specific detail about adverse reactions, other than ‘allergy, rash, GI upset, phlebitis, etc’, is expected for commonly used antibiotics.
17. Describe the principles of measurement of arterial haemoglobin O2 saturation using a pulse oximeter (60% marks). Outline the limitations of this technique (40% marks).
CICMWrecks Answer
Principles
Beer-Lambert Law
- Incidence of light is inversely proportional to the path distance and concentration of light absorbing particles within the path.
Hb absorbance at 660nm and 940nm utilized
- 660nm maximally absorbed by deoxyhaemoglobin
- Ratio of absorbance at 660nm and 940nm utilized to calculate SpO2
using healthy volunteers derived R values
Sats 100% R = 0.4
Sats 85% R = 1.0
Sats 50% R = 2
Sats 0% R = 3.4
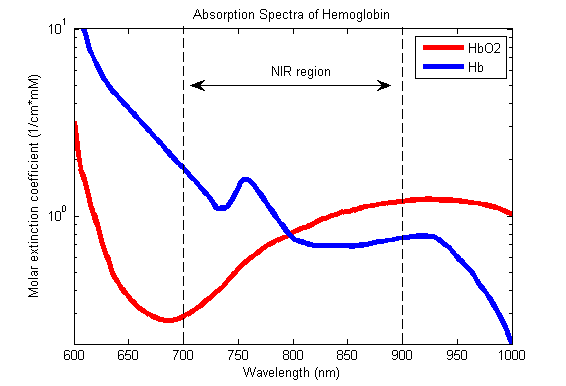
Pulse oximeter
- Light emitter
- Produces infra-red light at 660nm and 950nm
- Light detector
- Detects light at 660nm and 950nm
- Processor
- Calculates SpO2 using the ratio of absorbance at 660nm and 950nm
- Light detected (incidence) represents light passing through both pulsatile (arterial) blood and non-pulsatile elements (venous blood and other tissues)
- Processor differentiates light incidence during maxima and minima of pulse wave and calculates SpO2 from pulsatile blood only giving SaO2
Limitations
| PATIENT FACTORS | ||
| Low or High SpO2 | Low SpO2 | Normal or High SpO2 |
| Met-Hb Sulph-Hb | Poor perfusion of finger Movement artifact Venous pulsations Fingernail polish Intravenous pigmented dyes Haemoglobinopathy Anaemia with co-existing hypoxia | Carbon Monoxide poisoning |
| EQUIPMENT FACTORS | ||
| – Ambient light interference – Poorly fitting probe – Assay calibrated using healthy volunteers only down to SpO2 80%. Unknown significance if tested SpO2 less than 80% | ||
| PHYSIOLOGICAL FACTORS | ||
| – Due to O2 dissociation curve, insensitive to changes above PaO2 80mmHg – Does not measure tissue oxygenation | ||
Absorption spectra confounded by:
- Carboxyhaemoglobin
- Absorbs 660nm, not at 940nm
- R closer to 0.4
- causes the pulse oximeter to read artificially high
- Methemoglobin
- though it absorbs 660nm light, it also absorbs 940nm light to a greater degree
- R closer to 1
- causes the SpO2 to trend towards 85%
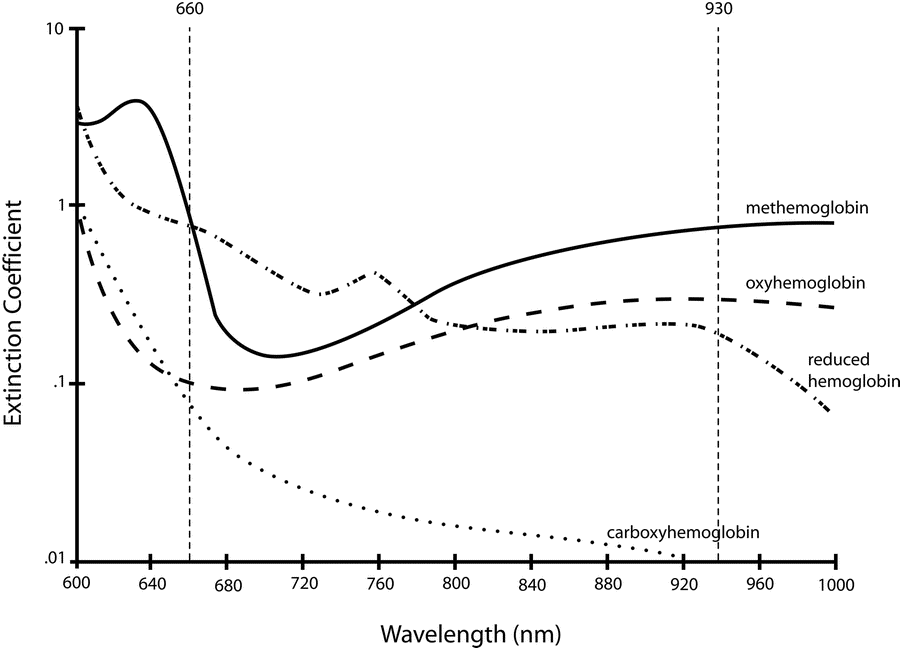
Sakurai 2016
Examiner Comments
2021A 17: 74% of candidates passed this question.
Most candidates provided a reasonable structured sequence of how a pulse oximeter generates a value. Nearly all candidates described the Beer-Lambert laws correctly, but few specifically described the basic principles of absorption spectrophotometry. Most candidates had a reasonable list of extrinsic factors that can interfere with pulse oximeter performance, but few described the intrinsic/inherent limitations of the device that can cause SpO2 to be different to SaO2, such as functional versus fractional saturation.
18. Outline the pharmacology of intravenous magnesium sulphate.
Examiner Comments
2021A 18: 57% of candidates passed this question.
The best answers appropriately addressed the pharmacology of magnesium sulphate, rather than diverting into physiology. They noted that the question concerned intravenous magnesium sulphate and did not discuss other routes. They included pharmaceutics, important examples of the wide-ranging indications, listed potential modes of action and considered the full range of body systems affected including potential adverse effects. Drug interactions, such as potentiation of neuromuscular blocking agents, and pharmacokinetics (including stating that magnesium is not metabolised) were described.
19. Describe the adult coronary circulation (50% marks) and its regulation (50% marks).
CICMWrecks Answer
Arteries
- LCA to
- LCx to
- OM1
- OM2
- LAD has branches
- Diagonal 1 or 2
- LCx to
- RCA
- Right Ventricular
- Acute Marginal
- Posterior descending
Veins
- Great, small, middle
- Drain into the thebesian veins → ventricles directly
- Empty into the coronary sinus on the posterior wall or the RV
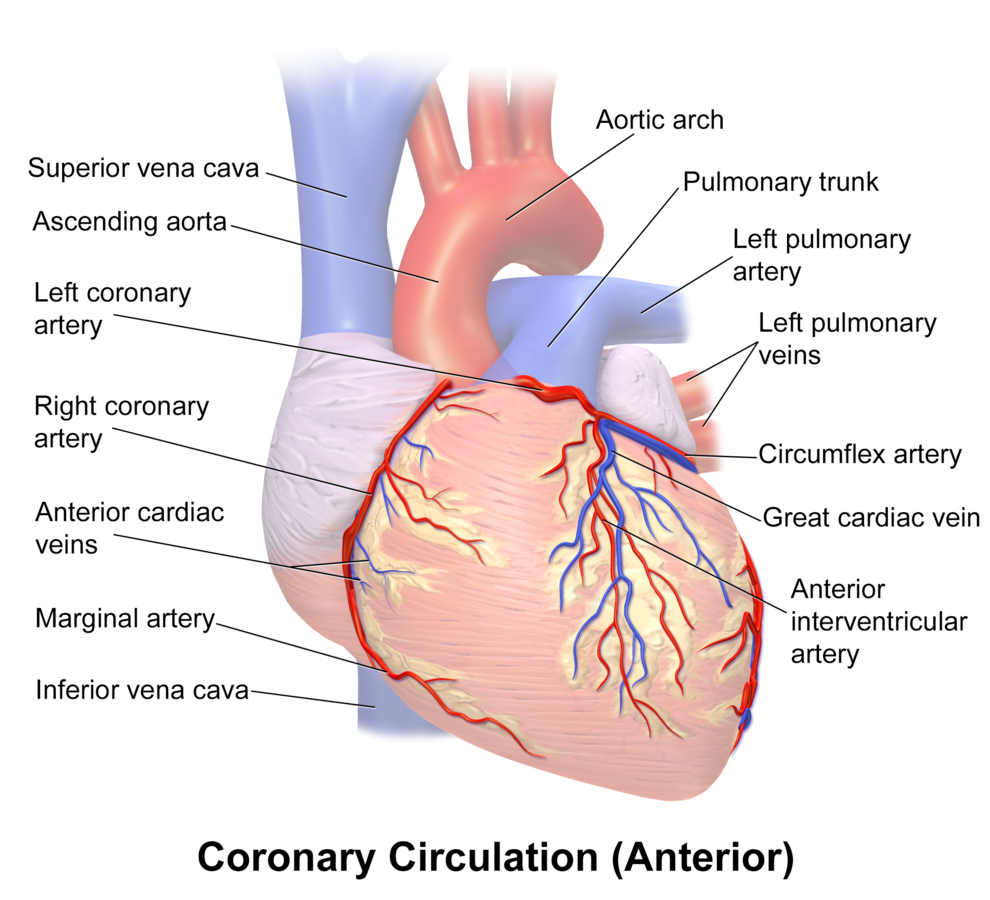
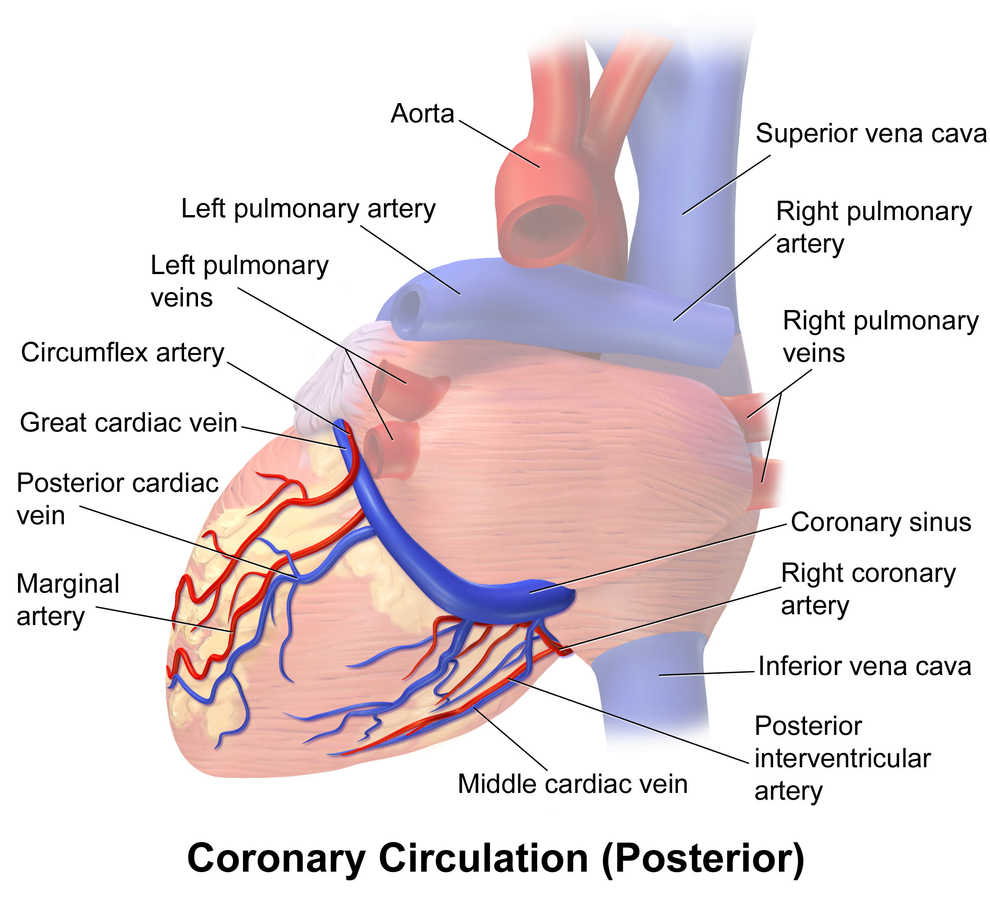
Regional Supply
| Artery | Anatomical region | ECG abnormality on occlusion |
|---|---|---|
| RCA | Down Anterior aspect of the heart, right of the pulmonary trunk Supplies RA SAN and AVN Posterior IVS | RCA occlusion causes → inferior: 2,3 aVF → true posterior: tall R in V1 |
| LCA | Left coronary artery ostia adjacent to left leaflet → Left Main → LCx and LAD Supplies LA, LV IVS AV Bundles | LCA occlusion → Lateral: 1, aVL, V1-V6 |
| LCx | LA and superior LV | anterolateral leads (1, aVL, V5 and V6) |
| LAD | RV, LV Anterior 2/3rds Interventricular Septum | Anteroseptal (V1 and V2) Anteroapical (V3 and V4) with some RCA supply. |
| Left Marginal | The continuation of the LCA after LAD branch Supplies LV laterally | Isolated Abnormalities rare V5, V6 |
| Right Marginal | The anterior branch of the RCA along the inferior border Supplies right ventricle and apex | Isolated Abnormalities rare RCA occlusion causes 2,3 aVF |
Coronary Blood Flow
- 80 mL/min/100 g
- or 200-250 mL/min
- 5% of CO at rest
- Can increase by 3-4 times (up to 400mL/min/100g)
High Extraction Ratio
High at rest (55-65%) body average of 25%
- Extraction ratio can only rise by factor of < 2 to 90%
- AV Δ O₂ = 11 mL/dL
- Coronary venous O₂ content = 5 mL/dL
- Coronary sinus SpO₂= 20mmHg
Coronary perfusion pressure (CPP)
- ADP = Ao Diastolic Prssure
- It is different throughout the cycle and b/w ventricles
Phasic Supply as per diagram.
Determinants of Coro BF
- Physical Factors
- Extravascular compression (CPP factors)
- Neural and Neurohumoral Factors
- ↑ SNS tone →
- α receptor mediated vasoconstriction
- β receptor mediated vasodialtion
- ↑ force and rate of contractions → ↑ vasodialtor metabolite release
- Overall effect is dilation
- ↑ PSNS tone → KACh stimulation → mild ↓ Coronary vascular resistance
- ↑ SNS tone →
- Metabolic Factors (main)
- Vasodilatory
- ↑ Adenosine, H, K, CO2, Lactate
- NO → GTP
- ↑ O2 demand → ↓ ATP → ↑KATP channel activation → hyperpolarisation → vasodilation
- Vasodilatory
- Myogenic autoregulation (keep CPP 60-180 mmHg)
Gladwin 2016
Examiner Comments
2021A 19: 62% of candidates passed this question.
Good candidates described normal blood flow to the coronary circulation, including differences between the right and left ventricles. Coronary artery anatomy was outlined, including the regions of the heart supplied and the concept of dominance. In addition to epicardial vessels, strong answers also outlined penetrating arteries, subendocardial supply and venous drainage. Regulation of coronary blood flow required an explanation of flow-dependence of the heart given its high oxygen extraction rate. Metabolic autoregulation and its mediators needed to be described, along with the physical factors driving coronary blood flow. Less important mechanisms such as the role of the autonomic nervous system were also described, with an emphasis on indirect effects over direct effects.
20. Outline the physiological factors that influence cerebral blood flow.
CICMWrecks Answer: Cerebral Blood Flow
Cerebral Blood Flow
- CBF = CPP / CVR (Cerebral Perfusion Pressure / Cerebral Vascular Resistance)
- CBF 15% resting CO → ~750ml/min or 50ml/100g brain tissue/min
- Gray Matter: 75–80 mL/100 g/min – Significantly higher due to the high metabolic activity and dense synaptic connections
- White Matter: 20–30 mL/100 g/min
- Abnormal CBF
- CBF<50ml/100g/min → cellular acidosis
- CBF<40ml/100g/min → impaired protein synthesis
- CBF <30ml/100g/min → cellular oedema
- CBF <20ml/100g/min → failure of cell membrane ion pumps, loss of transmembrane electrochemical gradients
- CBF <10ml/100g/min → cell death
Determinants of CBF
- CPP
- Net pressure gradient driving blood flow through the cerebral circulation
- CPP = MAP – ICP
- MAP = CO x SVR; CO = HR x SV; SVR = MAP / CO
- ICP dependent on: brain, blood, CSF
- CVR
Regulated by 4 primary factors:
- Cerebral metabolism
- flow-metabolism coupling:
↑ metabolic demand → ↑ CBF + substrate delivery - Controlled by vasoactive metabolic mediators:
H+ ions, K, CO2, adenosine, glycolytic intermediates, NO
- flow-metabolism coupling:
- CO2 and O2
- CO2
- At normotension: relationship between PaCO2 and CBF = almost linear
- ↑ PaCO2 → cerebral arteriolar vasodilation → ↓ CVR + ↑ CBF
- 2~4% increase for every 1mmHg increase in CO2
- ↓ PaCO2 → cerebral arteriolar vasoconstriction → ↑ CVR + ↓ CBF
- Initial stimulus = ↓ brain ECF pH
- Effects regulated by: NO, prostanoids, K channels, intracellular [Ca2+]
- PaO2
- little effect at normal PaO2
- PaO2 <60mmHg → cerebral arteriolar vasodilation → ↑ CBF
- Mechanism: hypoxia acts on →
- cerebral tissue to promote release of adenosine → cerebal vasodilation
- cerebrovascular smooth muscle → hyperpolarisation → ↓ Ca2+ uptake → vasodilation
- CO2
- Autoregulation
- Constant across CPP 50-150mmHg
- CPP >150mmHg: CBF µ CPP
- CPP <50mmHg: CBF <50ml/100g brain tissue/min → ischaemia
- Stimulus to autoregulation = CPP (not MAP)
- autoregulation curve R shifted in HTN; L in neonates
- Mechanism:
- Myogenic mechanism: arterioles vasoconstrict in response to ↑ wall tension + vasodilate in response to ↓ wall tension → ↓ or ↑ CVR
- May involve adenosine
- Can be impaired in SAH, tumour, stroke, head injury
- Constant across CPP 50-150mmHg
- Neurohumeral factors
- Relative lack of humoral + autonomic control on normal cerebrovascular tone
- Main action of SY nerves = vasoconstriction
- Other factors
- Blood viscosity: directly related to HCt; ↓ viscosity → ↑ CBF as per Hagen-Poiseuille law
- Temperature: ↓ CMRO2 by 7% for each ↓ 1°C in temp
- Drugs
- E.g. barbiturates ↓ cerebral metabolism
- Volatile agents → ↓ tension cerebral vascular smooth muscle → vasodilation + CBF
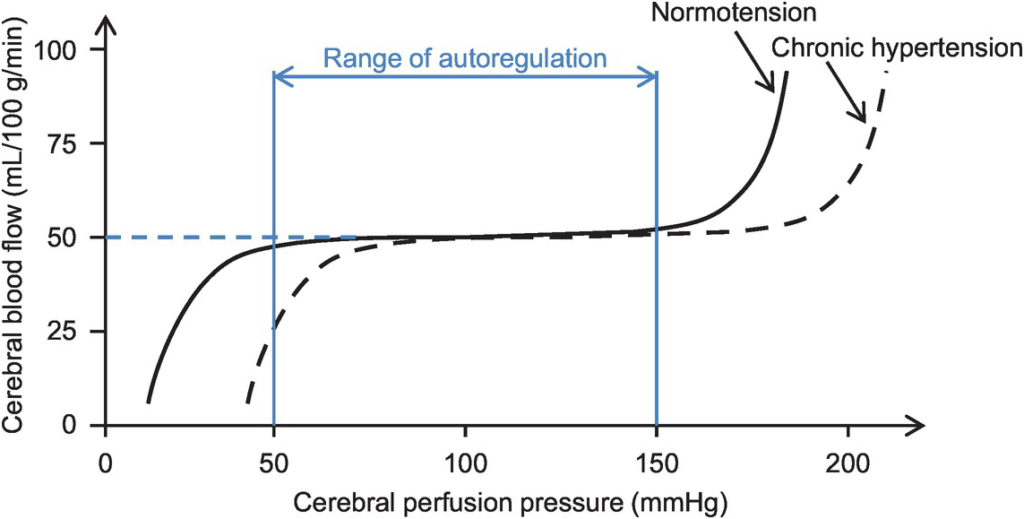
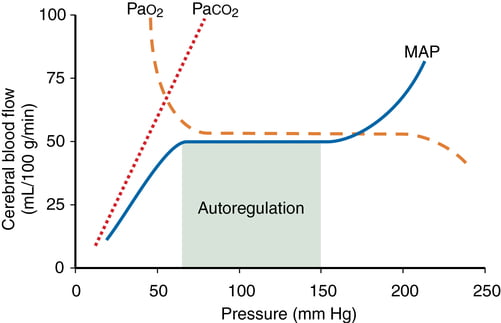
Kerr 2018
Examiner Comments
2021A 20: 19% of candidates passed this question.
Overall, this question was poorly answered with a high failure rate. A good answer gave a normal value, iterated that CBF is held relatively constant by autoregulation, and proceeded to divide factors affecting CBF into categories with an explanation/description of each. Those factors with the greatest influence were expected to have more accompanying information (e.g., pressure/myogenic autoregulation, metabolic). Systemic factors such as MAP, O2, CO2 were expected to be mentioned with detail of the impact (i.e., key values, relationships demonstrated with a description and/or labelled graph). Local factors within the brain such as H+ concentration/pH, metabolic activity (including the impact of temperature, inclusion of mediators, regional variation based on activity & grey versus white matter) were also expected to be mentioned. Few answers mentioned impact of pH change independently of CO2. Few answers mentioned how CO2 changes the pH of CSF and that over time, this impact is buffered/reduces. The role of the sympathetic nervous system was required to be mentioned although not explored in detail (although many answers overstated the importance of the SNS on CBF or gave a simplistic concept such as increased SNS activity increases CBF).
Many answers focussed on descriptions of the Monro-Kelly doctrine and ICP to the exclusion of the aforementioned factors or included detail on factors influencing MAP which were not required (and irrelevant when within the autoregulation range). Many answers were simplistic: e.g., increase MAP increase CPP therefore increase CBF, or by stating CO2/O2 without mentioning a relationship or the limits/patterns of the relationship. Many answers failed to separate the effect of systemic PaO2 and PaCO2 from metabolic autoregulation.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Compliance Measurement: Concentration of oxygen in inspired gas Oxygen cascade: Steps and values Oxygen delivery devices CO2 carriage, physiology |
| G. CVS | Invasive arterial monitoring systems, improve accuracy MeasurementL CO, SVR Baroreceptor reflexes, rapid onset hypovolemia (20% blood volume loss over 10 mins) Arterial waveform: label, what determines shape of waveform |
| H. Renal | Principles of haemodialysis |
| I. Body Fluids and Electrolytes | IVF, effects. Large volume (2-3L) infusion NS Blood plasma and non-protein components |
| J. Acid Base | Acid, how much produced daily ABG interpretation (pH 7.4 BE -20 HCO# 8 Na 145 K 4.4 Cl 113) |
| K. Neuro | Cranial neve anatomy, pupillary reflex Propofol pharmacology. What is in a bottle of propofol Opioid pharmacology, MoA |
| L. Musculoskeletal | End plate potential generation, how is it different from miniature end plate potential, NM transmission and monitoring |
| M. ANS | |
| N. Liver | Functional unit of liver. Label structures, significance of arrangement |
| O. GIT | Nausea and Vomiting. Sequential muscle contractions during vomiting |
| P. Nutrition and Metabolism | |
| Q. Haematology | Blood groups and determination Viscoelastic measurement of coagulation, information obtained. Anti-platelet pharmacology |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | Glucose storage, physiology, pharmacology |
| V. Obstetrics | Maternofoetal physiology. Normal ABG at term. Explain changes. ABG: pH 7.45 pO2 105 pCO2 30 HCO3 20 BE -2 |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments