SAQs
1. Describe the carriage of carbon dioxide in blood.
CICMWrecks Answer
CO2
- End-product of aerobic metabolism
- Produced almost entirely in mitochondria
- Normal production approx. 200ml/min
- Total CO2 in blood
- Venous 52mL/dL
- Arterial 48mL/dL
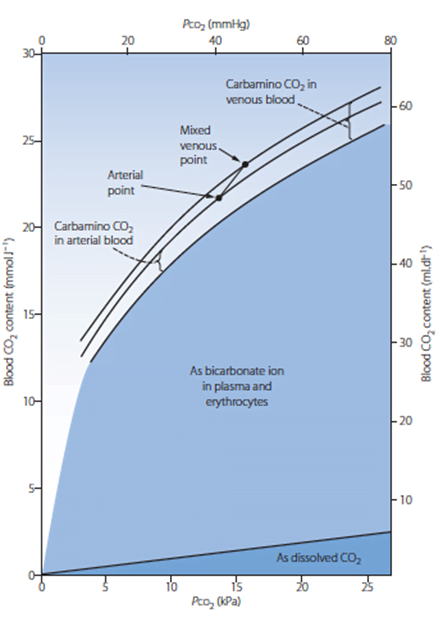
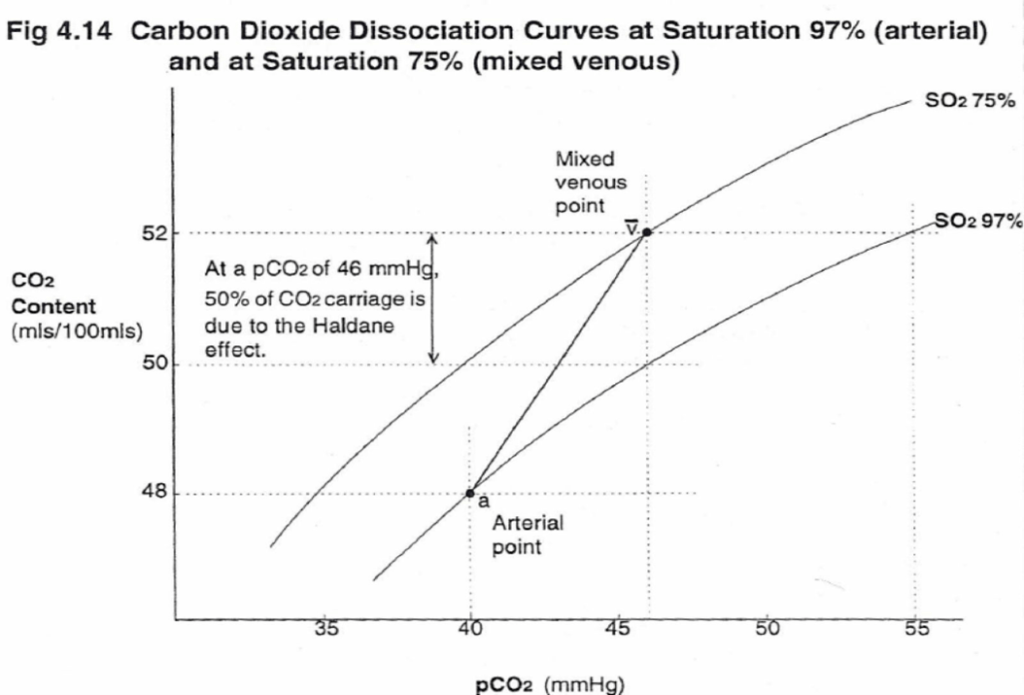
CO2 Carriage in 4 forms:
| Arterial % | % of A-V diff | Details | |
|---|---|---|---|
| 1. Bicarbonate | 90 | 60 | Main form of CO2 delivery in blood Via Hendersen-hasselbach: CO2 + H2O ⇔ H2CO3 ⇔ H+ + HCO3– Process 1. CO2 diffuses into RBCs 2. Carbonic anhydrase converts it to Bicarb 3. Hydrogen ions are buffered by binding to Hb molecules inside RBCs (30% Haldane.) Higher percentage in venous blood as: • Deoxygenated pKa 8.2 • Oxygenated pKa 6.6 • decreased pH = greater increase in [A-]/[HA] of DeoxyHb rel to OxyHb thus greater ability to accept protons 4. Bicarb is transferred out of cell in exchange for Cl ion by an exchange transporter called Band 3. (Hamburger Shift) |
| 2. Carbamino compounds | 5 | 30 | CO2 able to bind amino end of proteins Hb largely responsible as in much greater concentrations than any other protein (15 g/dL vs 7 g/dL) in serum CO2 combines with terminal amine to form carbamic acid dissosciates → at physiological pH to carbamate Affinity of Hb for CO2 increases in venous blood via Conformational change (70% Haldane): • Unbinding of O2 causes a conformational change in the beta chain • CO2 binding site at the N-terminus moves 1nm • Affinity for CO2 is increased. • Deoxy-Hb is 2-3x better at forming carbamino compounds than oxy-Hb |
| 3. Dissolved | 5 | 10 | CO2 ~20 x more soluble than O2 thus via Henry’s Law more CO2 is dissolved PvCO2 ~46mmHg (PaCO2 40mmHg) |
| 4. Carbonic Acid | <1% | <1% | Carried as dissolved H2CO3 Conversion to CO2 via Hendersen-Hasselbach equation above has pKa 6.1 At physiological pH Carbonic acid is ~96% dissociated Reaction is catalysed by Carbonic Anhydrase Not present in plasma, present in high concentrations in erythrocytes and pulmonary capilaries |
Gladwin / Sakurai 2016
Examiner Comments
2020A 01: 68% of candidates passed this question.
A detailed understanding of the carriage of carbon dioxide (CO2) in the blood is essential to the practice of intensive care medicine. Comprehensive answers classified and quantified the mechanisms of CO2 carriage in the blood and highlighted the differences between the arterial and venous systems. An explanation of the physiological principles surrounding these differences and the factors which may affect them was expected. The changes that occur at the alveolar and peripheral tissue interfaces with a similar explanation of process was also required. Candidate answers were often at the depth of knowledge required for an ‘outline question’ and a more detailed explanation was required to score well.
2. Describe the pharmacology of Glyceryl trinitrate (GTN).
Examiner Comments
2020A 02: 69% of candidates passed this question.
GTN is a commonly used ‘level 1’ drug. The most comprehensive answers included information on available drug preparations, indications, mechanism of action, pharmacodynamics and pharmacokinetics and its side-effect profile. It was expected that significant detail be included in the pharmacodynamic section (e.g. preferential venodilation, reflex tachycardia, effects on myocardial oxygen demand etc). Common omissions included tachyphylaxis, dosing and its metabolism. Many answers didn’t mention the first pass effect.
3. Outline the potential adverse consequences of blood transfusion.
CICMWrecks Answer
Adverse Consequences of Blood Transfusion
1. Acute (<24 hours)
1a) Immune-mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Allergic reaction to plasma proteins | mild (urticarial) or severe (anaphylaxis) | Pre-treatment in high risk patients |
| Acute Haemolytic transfusion reaction | incompatibility of donor and recipient blood leads to widespread haemolysis and circulatory collapse | Group and screening |
| Febrile non-haemolytic transfusion reaction (FNHTR) | due to stored cytokines and/or the presence of recipient alloantibodies | Careful monitoring and early cessation of transfusion |
| Transfusion related acute lung injury (TRALI) | noncardiogenic pulmonary oedema caused by HLA antibodies in donor plasma directed against recipient leukocytes or bioactive lipids which accumulate during storage | Careful monitoring and early cessation of transfusion |
1b) Non-immune mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Sepsis | bacterial infections are most common with platelets as they are stored at room temperature | Safe and sterile methods of storage |
| Transfusion Related Circulatory overload (TACO) | fluid overload usually due to rapid or massive transfusion | Careful monitoring and early cessation of transfusion |
| Non-immune mediated haemolysis | Careful monitoring and early cessation of transfusion | |
| Hypothermia | Careful monitoring and early cessation of transfusion | |
| Dilutional coagulopathy | Careful monitoring and early cessation of transfusion |
2. Delayed (>24 hours)
2a) Immune-mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Delayed haemolytic transfusion reaction | Production of anti-donor antibodies post-transfusion May be associated with transfused malaria | Careful monitoring and early cessation of transfusion |
| Transfusion-related immunomodulation (TRIM) | transient immunosuppression in blood recipients which may be due to release of cytokines from donor lymphocytes | Careful monitoring and early cessation of transfusion |
| Alloimmunisation | development of antibodies during exposure to blood products, resulting in an amplified reaction on subsequent exposure Can cause post transfusion thrombocytopenia and purpura | Re-screening every 48-72 hours |
| Transfusion Associated Graft vs. Host Disease | Profound bone marrow aplasia >90% mortality Viable donor T cells implant and attack recipient tissues | Careful monitoring. Re-screening |
2b) Non-immune mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Iron overload | most common in chronically transfused patients | Monitoring of Iron levels |
| Transfusion-related infection | Viral – the risk of HIV, HTLV 1&2 and HCV is <1/1 million. The risk of contracting HBV is slightly higher at 1/500,000. Other – malaria, vCJD, Dengue Fever, West Nile Virus | Pre-screening of donors, screening of collected blood as per local guidelines (different for different areas) |
3. Storage Lesions
A storage lesion refers to the changes that occur to a sample of blood during storage. (Note: Australian Red cross anti-coagulates Whole blood with CPD, but washes and stores Red Cells in SAGM).
| Problem | Methods to Minimize | |
|---|---|---|
| Physical changes | Reduction in the viability of RBCs due to shape changes and reduced deformability Formation of microaggregates | Careful monitoring and usage of products within a specified period |
| Hyperkalaemia | plasma K can be >20 at 28 days in stored blood due to inactivation of the red cell Na/K ATPase pump. | Careful monitoring and usage of products within a specified period |
| High citrate load | can lead to hypocalcaemia and alkalosis (less or nil in SAGM) | Use of SAGM for storage |
| Reduction in 2,3-BPG | causes left shift of the oxygen/haemoglobin dissociation curve (less in CPDA1) | Use of CPDA1 |
| Renal impairment | Due to Increase in free haemoglobin from cell lysis | Routine monitoring of renal function and careful monitoring during transfusion |
Massive Transfusion
- Replacement of >1 blood volume in 24 hours
- 50% of blood volume in 4 hours
Adverse consequences of massive transfusion
| Problem | Methods to Minimize | |
|---|---|---|
| Hypothermia | Cooled products | Appropriately re-warm products. Use of blood warmers |
| Poor O2 delivery | Depletion of 2,3 DPG in PRBC | |
Haemostatic abnormalities | Dilutional coagulopathy | Monitor coagulation profile at appropriate intervals during massive transfusions. Use of appropriate factors like FFPs, Cryoprecipitate during massive transfusions |
| Hypocalcaemia | Consumption with coagulopathy and bound to citrate added to transfused units | Monitor and replace calcium as necessary |
| Hypomagnesaemia | Bound to citrate in transfused units | Monitor and replace magnesium as necessary |
| Citrate toxicity | Citrate is added to stored units as an anticoagulant | Monitor for acidosis during massive transfusions |
| Lactic acidosis | Hyperlactataemia due to anaerobic metabolism in stored units | Monitor for acidosis during massive transfusions |
| Hyperkalaemia | Potassium migrates from stored erythrocytes into plasma whilst in storage | Careful monitoring and usage of products within a specified period |
| Air embolism | Inadvertent infusion | Careful monitoring, use of transfusion sets with air vents, filters |
JC / Sakurai 2019
Examiner Comments
2020A 03: 43% of candidates passed this question.
As only an outline was asked for, a brief statement about each complication was sufficient. Better answers were structured using a classification of: Acute Immunological, Acute Non Immunological, Delayed Immunological and Delayed Non-immunological. Examples of expected detail would include the following:
E.g. Bacterial infection – a statement outlining the incidence of bacterial infection, a common causative organism or why bacterial infections are more commonly associated with platelet transfusions than red cells would have scored the marks allocated to ‘bacterial infection’.
E.g. Acute Haemolytic Transfusion Reaction – a statement about red cells being destroyed due to incompatibility of antigen on transfused cells with antibody of the recipient and an approximate incidence scored the marks allocated to AHTR.
An excellent resource is the Australian Red Cross transfusion website as listed in the suggested reading section of the syllabus
4. Explain the counter-current mechanism in the kidney.
CICMWrecks Answer
Medullary concentrating gradient
- physiological process which sets up a concentration gradient from cortex through to medulla
- allows formation of concentrated urine
- Mechanisms:
- Counter current Multiplier: creates concentrated medullary interstitium
- Counter current Exchange (vasa recta): maintains intersisital osmotic gradient
- Recycling of urea: contributes to high osmolarity of medullary interstitium
- Normal cortico-medullary gradient 300-1400mOsmol.
Counter-current Mechanism in Kidney
Counter Current Multiplier:
Set-up
- Descending limb LoH Permeable to water only
- Ascending limb LoH Permeable to NaCl only
- Thin passive NaCl reabs
- Thick NaCl via active NKCCT cotransport and paracellular
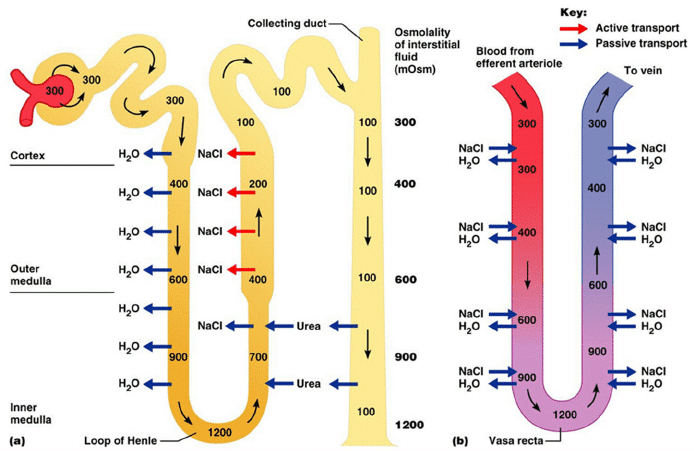
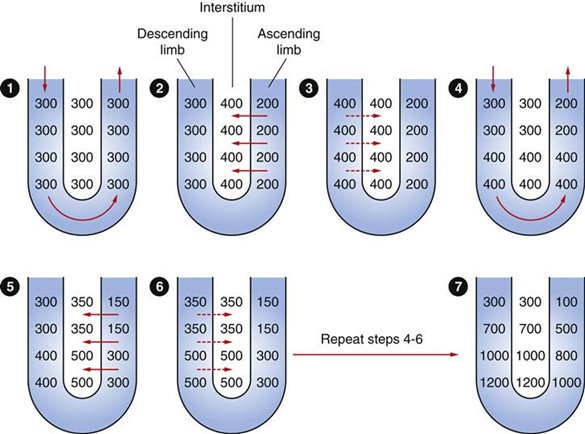
Generation
- Enters LoH osmolarity throughout = 300mOsm
- Max effort of NKCCT = 100mOsm/kg
- Tubular OSM = 200/kg
- Interstitial Osm = 400/kg
- Descending limb diffuses water down gradient
- Descend limb OSM now 400
- Process repeats (flow, pump salt, equilibrate water)
Counter Current Exchange:
- Hairpin loop arrangement of the vasa recta in the juxta-medullary nephrons.
- Provides blood flow to medullary tissue without impact of the cortico-medullary gradient.
- Relies on slow flow of blood.
- Mechanism
- On descent, water is lost from capillaries and NaCl is absorbed into capillaries increasing osmolarity
- On ascent water is reabsorbed and solute is lost
Role of Urea in Medullary Concentration Gradient
- 900mmol/day filtered
- 350-550mmol/day reabsorbed.
- Comprises 50% of osmolality (300-650 mOsm/kg)
- Freely filtered at the glomerulus
- Secreted by thin limbs of LoH down concentration gradient from medullary interstitium to tubular fluid.
- As water and Na are reabsorbed, tubular [Urea] increases
- [Urea] = >500mmol/L at collecting ducts
- Equilibrates with interstium due to slow flow rates.
- ↑ADH
- urea transporters UT1 and UT3 insertion → ↑ CD permeability to urea
- → ↑ medullary gradient (further 10% reabsorption if required)
Gladwin / JC 2020
Examiner Comments
2020A 04: 63% of candidates passed this question.
Higher scoring candidates described the counter-current multiplier mechanism, the countercurrent exchanger and the contribution of urea cycling to the medullary osmotic gradient. Detailing the mechanisms as to how they may be established, maintained and or regulated. Descriptions of the multiplier (LOH) alone did not constitute a passing score. Values for osmolality at the cortex & medulla and within the different parts of the LOH was required.
A description of the countercurrent exchanger system where inflow runs parallel to, counter to and in close proximity to the outflow was expected. This could have been achieved by describing the anatomical layout of the loop of Henle and the vasa recta.
5. Outline the mechanisms of antimicrobial resistance (50% of marks).
Briefly outline the pharmacology of ciprofloxacin (50% of marks).
CICMWrecks Answer: Antibiotic resistance
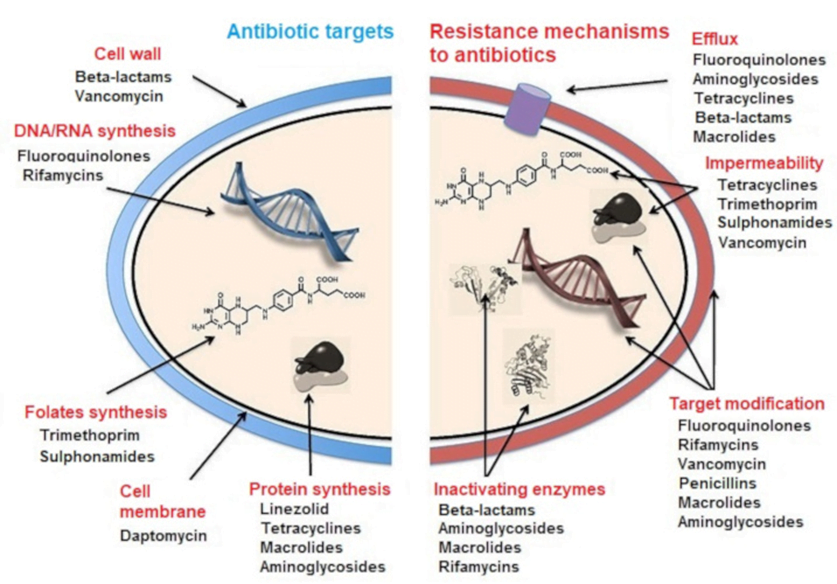
MECHANISMS OF ANTIBIOTIC RESISTANCE
- Mechanisms of Antibiotic Resistance can be classified broadly:
- Efflux Pumps
- Blocked Penetration / Alteration in access to target site
- Target Modification
- Modification of Drug or pathways
- There might be multiple resistance mechanisms at play in the same organism
| Efflux Pumps: Increased efficiency or expression of efflux pumps (inner membrane proteins). | |
| Removed from cell | Active transport of ciprofloxacin out of the bacterial cell |
| E.Coli to tetracycline. | |
| Trapped between cell wall layers | glycopeptide resistance in VRSA. |
| Blocked Penetration / Alteration in access to target site | |
| Narrowing of porin channels | Streptococcal resistance to penicillins typically occurs by reducing access to PBPs. |
| Alteration of porins in gram negative outer membranes | downregulation of Outer Membrane Proteins (eg enterobacteriaceae, pseudomonas vs penems/cephs) |
| increased selectivity of Outer Membrane Proteins (eg klebsiella outbreaks) | |
| Loss of non-essential transporter channels | Anaerobes have no oxygen-transport channel which prevents penetration by aminoglycosides (Gentamicin) |
| Reduced binding of the antibiotic | VanA and VanB vancomycin resistance involves a gene mutation leading to decreased affinity of vancomycin for the binding sites of peptidoglycan precursors |
| Changes to the DNA-binding surface of DNA supergyrase infers resistance against ciprofloxacin | |
| Target Modification | |
| Modifying the enzyme that the antibiotic inhibits | Rifampicin resistance by point mutations, insertions, or deletions in RNA polymerase gene |
| Production of an alternative enzyme for that which the antibiotic inhibits | MRSA develop or acquire the gene mecA which synthesizes an additional penicillin binding protein that enables it to continue cell wall synthesis in the presence of a beta lactam drug |
| Overproduction of the target of the antibiotic | Trimethoprim DHFR enzyme overproduction in Escherichia coli and Haemophilus influenzae. |
| Synthesis of target-protecting proteins | Ribosomal protection proteins against Tetracyclines in multiple Gram positive and gram negative bacteria |
| Modification of Drug or pathways | |
| Development of metabolic pathways to bypass site of action of antibiotic | Resistance to TMP-SMX by allowing bacteria to synthesize or absorb folic acid. |
| Enzymes produced to Metabolize the drug | β-lactamases and cephalosporinases hydrolyse β-lactam rings |
| Enzymes produced that add a chemical group to antibiotic to inhibit its activity | Aminoglycoside resistance by Staphylococcus aureus or Pseudomonas (Multiple enzymes acetyltransferase, adenyltransferase, and phosphotransferase) |
Spread of Bacterial Resistance
- Selective pressure selects for favourable mutations of resistance
- mosaic genes (from other bacteria eg strep pneumo from strep mitus) or uptake of DNA from environment
- transfer of resistant bacteria from person to person
- horizontal gene transfer;
- transduction – acquisition of bacterial DNA from a phage (a virus that propagates in bacteria);
- transformation – uptake and incorporation of free DNA released into the environment by other bacterial cells;
- conjugation – gene transfer (usually on plasmids), by direct cell-to-cell contact through a bridge.
Sources:
Microbiology Lippincott Williams & Wilkins
https://courses.lumenlearning.com/microbiology/chapter/drug-resistance/
https://www.encyclopedie-environnement.org/en/health/antibiotics-antibiotic-resistance-and-environment/
Gladwin / Sakurai / JC 2019
CICMWrecks Answer: Pharmacology: Ciprofloxacin
PHARMACOLOGY OF CIPROFLOXACIN
Examiner Comments
2020A 05: 71% of candidates passed this question.
Most candidates had a structured answer to mechanisms of resistance that covered the major categories (alter target protein, prevent entry, efflux, degrade drug) and provided an example of a bacteria and the affected antibiotic, as was required to answer the question in full. Ciprofloxacin, whilst perhaps not a first line drug in the ICU, was not well known by many candidates. Better answers included a brief outline of class, mechanism of action (action on DNA gyrase to inhibit replication), spectrum (Gram negatives particularly mentioning Pseudomonas, lesser Grampositive cover, not anaerobes, some atypical), PK (with correct dose, wide penetration into tissues including bone/prostate etc., predominantly renal excretion), side effects/toxicity (common or specific to cipro e.g. QT, tendinitis, arthropathy) and an example of resistance.
6. Outline how the respiratory system of a neonate differs from that of an adult.
CICMWrecks Answer
Anatomical and Mechanical Differences
- Relatively larger head
- Prominent occiput
- Short neck
- Neonates do not have dentition
- Relatively larger tongue
- Higher larynx (C3-4 in infant, C4-5 in adult)
- Larynx lies more cephalad and anteriorly
- Epiglottis is narrower, omega shaped, more floppy and relatively larger
- The narrowest part of the airway is the subglottic area at the level of the cricoid cartilage
- Infants are obligatory nasal breathers until 3-5 months of age, the narrow nasal passages can easily be obstructed by secretions
- Shorter compressible trachea
- Increased risk of laryngospasm with inappropriate instrumentation.
- The smaller the patient the harder it is to position a LMA appropriately and maintain the correct position
- More compliant tissues and reduced muscle tone
- Main carina also lies more cephalad
- Small peripheral Airways (50% are < 2 mm diameter)
- ↓↓↓ bronchial muscle → so bronchospasms uncommon and bronchodilator drugs have minimal response
- Alveoli immature, and number <10% of adult total
- Reduced mucociliary clearance
- ↓ % type I muscle fibres (highly oxidative/slow contraction) in diaphragm (25% in neonate vs 55% in adults) and IC muscles (45% vs 65%) → ↑ risk of muscle fatigue
Lung Volumes
- FRC (30 mL/kg) → same as adult (as FRC remains 40% of TLC) BUT ↓ stable and ↑ risk of atelectasis due to following reasons:
- Significant ↓ in outward recoil of CW (as rib is cartilaginous and contains very little respiratory muscles)
- Mild ↓ inward recoil
- TLC (75-80 mL/kg) → same as adult
- VC (45 mL/kg) → lower cf. adult (60 mL/kg)
- TV same as adult (7 mL/kg)
- ↑ CC due to low elastic recoil of lungs → in fact, CC > FRC such that there is small AW closure during tidal ventilation → results in gas trapping and hypoxaemia
Lung Physiology
| Variable | Neonate vs Adult |
| Oxygen consumption | 6mL/min vs 3mL/min in adult |
| O2 flux to tissues | ↑ due to: ↑ O2 carrying capacity(↑Hb, HbF, ↑MV → ↑pO2) ↑ Cardiac output |
| Minute Ventilation | 2x adult (to cope with ↑MRO2) Generated by ↑↑RR → ↑ work of breathing |
| Control of ventilation | Respiratory control less developed CO2 ventilatory response curve shifted to left O2/CO2 ventilatory response easily depressed by hypothermia Peripheral chemoreceptors immature Hering-Breuer reflex (transient apnoea lasting 5 secs evoked by gradual lung inflation) Head’s paradoxical inflation reflex (↑ inspiratory effort evoked by partial inflation of lungs) Periodic respirational (pauses lasting 5-10 sec (up to 6 x per hr during sleep) |
| Chest Wall Compliance | Neonates have ↑ chest wall compliance relative to adult → Prone to collapse on inspiration → ↓ Tv for same respiratory effort → ↑ WOB |
| Tissue Compliance | Initially neonates ↓ relative to adults until a few days of life when surfactant production is fully initiated, then → ↑ WOB in the first few days |
| V/Q matching | Significant V/Q mismatch (V/Q = 0.4) due to shunting from gas trapping/small AW closure |
| Physiological dead space | ↑ physiological dead space further necessitating ↑ MV |
| Work of Breathing | ↑ WOB overall |
Gladwin / JC 2019
Examiner Comments
2020A 06: 20% of candidates passed this question.
This question required an outline of the anatomical, mechanical and functional differences. It was expected that factors leading to an increased work of breathing and oxygen cost would be mentioned. The mechanics of expiration were not often included in candidates’ answers. Immaturity of the alveoli and peripheral chemoreceptors were common omissions. Inaccuracies regarding upper airway anatomy and compliance of the chest wall cost some candidates marks. The question did not call for an explanation of the relative difficulty of intubation. Discussion of pathophysiology due to airway obstruction, causes of central apnoea or sensitivity to drugs was not required. Many answers included inaccurate information. Points which were often missed were difference in bronchial angles, number of alveoli, number of type 1 fibres in diaphragm, ciliary function and peripheral chemoreceptors.
7. Describe the physiological control of systemic vascular resistance (SVR).
CICMWrecks Answer
Systemic Vascular Resistance
(Total Peripheral Resistance)
Systemic vascular resistance (peripheral vascular resistance, SVR) is the resistance in the circulatory system that is used to create blood pressure, the flow of blood and is also a component of cardiac function.
- Majority of resistance in systemic system results from arterioles – state of contraction and relaxation of smooth muscle cells of arterioles which determines distribution of blood to organs
- The % of each organ blood flow is dependent on the organ vascular resistance
- Systemic vascular resistance is the resistance of several circuits in parallel, which have both common and independent factors in their regulation.
- As they are in parallel the sum of reciprocals is used to determine the overall value.
Control
- As per Hagen Poiseuille Equation:
where: η = blood viscosity
L = length of vessel
r = radius of vessel
- radius is the most important factor
Extrinsic Control
- Extrinsic SNS control
- Arterioles have profuse SNS supply → NAd from nerve endings act on α1-adrenoceptors to cause vasoconstriction (and β2-adrenoceptors to cause vasodilation – but effect is weaker!)
- Due to the tonic SNS outflow from medullary vasomotor centres, arterioles have a basal level of vasoconstriction → the degree of basal vasoconstriction (and arteriolar resistance) can be varied by altering SNS outflow
- Extrinsic PNS control
- PNS control of arterioles is less important → vessels of external genitalia have dual ANS supply with PNS dilator nerves and SNS constrictor supply; PNS activation in heart, brain and lungs have an uncertain role
- Extrinsic hormonal control
- Adrenaline (from adrenal medulla) → effect on organ blood flow depends on the relative % of α1- and β2-adrenoceptors present in the arteriole
- AII (from RAAS) → vasoconstriction (via AT2R)
- ADH (from posterior pituitary) → vasoconstriction (via V1R)
- ANP (from RA) → vasodilation (via ANPR)
Intrinsic Control
- Autoregulation
- Ability of an organ to maintain relatively constant blood flow across variations in perfusion pressure
- flow = pressure/ resistance → as the P changes, the R also changes to maintain flow
- Outside limits of autoregulation: flow = dependent on driving pressure
- Kidneys, brain, heart
- Autoregulation is dependent on 2 mechanisms:
- Pressure autoregulation: myogenic stretch response to ↑ and ↓ in pressure → vasoconstriction, and vasodilation
- Metabolic or vasoactive autoregulation: direct action of locally derived metabolites and vasoactive substances e.g. platelets release thromboxane A2 → constriction in damage
Bianca / Kerr 2016
Examiner Comments
2020A 07: 21% of candidates passed this question.
This question invited a detailed discussion of the physiological control mechanisms in health, not pathophysiology nor drug-mediated effects. The central and reflex control mechanisms that regulate SVR over time are distinct from the local determinants of SVR. There was often confusion between dependent and independent variables. Cardiac output is generally depended upon SVR, not vice versa, even though SVR can be mathematically calculated from CO and driving pressures. The question asked about systemic vascular resistance and did not require a discussion of individual organs except for a general understanding that local autoregulation versus central neurogenic control predominates in different tissues. Emotional state, temperature, pain and pulmonary reflexes were frequently omitted. Peripheral and central chemoreceptors and low-pressure baroreceptors were relevant to include along with high pressure baroreceptors.
8. Describe the production, metabolism and role of lactate.
CICMWrecks Answer
Lactate is the conjugate base of lactic acid (an organic 3-C acid)
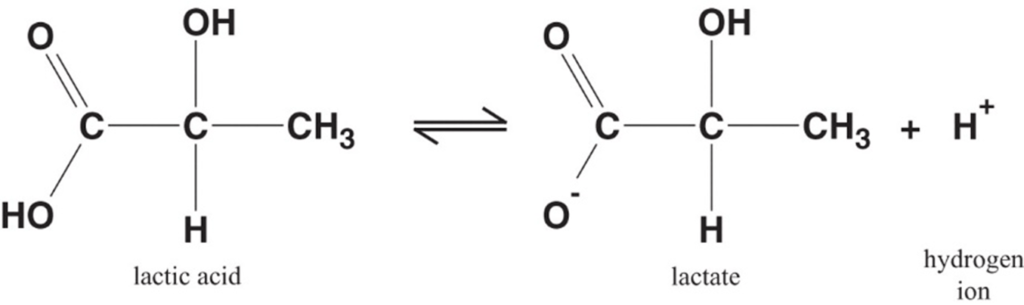
Production:
- It is produced by anaerobic metabolism of pyruvate either:
- Physiologically → in RBC (no mitochondria), renal medulla (↓ PO2), cornea/ lens (↓ PO2) → hence, normal plasma [lactate] is 0.5-2 mmol/L (and NOT zero!)
- Pathologically → reduced tissue perfusion and/or O2 delivery (Eg. shock, hypoxaemia) → thus, plasma [lactate] ↑↑↑ (> 2 mmol/L)
- Plasma [lactate] is 0.5 – 2 mM (and NOT zero) due to physiological production → it can be measured clinically as an indicator of anaerobic metabolism (Ie. ↑ anaerobic metabolism due to pathological situations lead to ↑ [lactate])
Fate of lactate:
- Persistent anaerobic metabolism (Ie. ongoing hypoxia) causes an accumulation of cellular lactate → this diffuses out of the cell into plasma along its [ ] gradient → lactate can then be:
- Used as a fuel source by the heart and brain
- Transported to the liver where it is:
- Converted back to glucose via gluconeogenesis (requires 6x ATP), which is then transported back peripherally for use → “Cori cycle”
- Converted to pyruvate intermediate → utilised locally in TCA cycle for ATP production via oxidative phosphorylation
- Resolution of hypoxia (Ie. tissue O2 tension restored) → intracellular lactate can be oxidised back to pyruvate for use in local tissue aerobic metabolism (Ie. fed into TCA cycle)
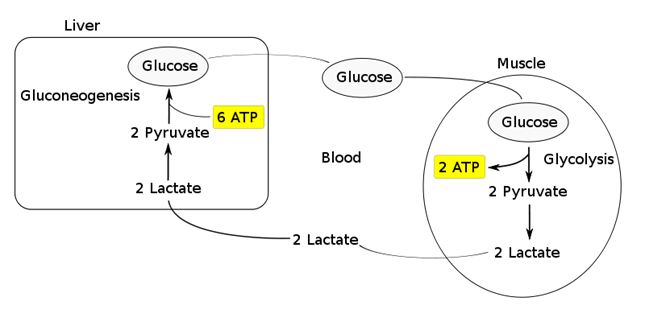
Functions of Lactate:
Lactate Sink:
- Lactate acts as a sink in heart, liver, muscle etc, allowing a period of ongoing ATP production from glycolysis when:
- cells become oxygen deplete
- Kreb’s cycle is inhibited
- Other causes of pyruvate accumulation: circulating catecholamines, exercise, sepsis or lack of mitochondria (RBCs)
Lactate Shuttle:
- Intracellular shuttle:
- Lactate may be shuttle out of:
- mitochoncrial membrane
- peroxisomes
- into cytoplasm of myocytes, neurons, astrocytes
- Ixydised by cytoplasmic LDH to pyruvate, generating NADH for energy use
- Lactate may be shuttle out of:
- Intercellular shuttle:
- Excess lactate, formed within fast-twitch fibres, is transported to other cells within the body with the oxidative capability to metabolize lactate, such as type I (slow-twitch) muscle cells, enhancing their excitability and limiting fatigue
- Furthermore, once in circulation, lactate attaches to red blood cells (RBCs) and is disassociated in the liver where inter-conversion via gluconeogenesis facilitates glucose formation, providing an alternative aerobic energy source.
Lactate as a signaling molecule:
- Redox signaling by intracellular shuttles
- Gene expression
- Increased intracellular levels of lactate can act as a signalling hormone, inducing changes in gene expression that will upregulate genes involved in lactate removal, and stimulates mitochondrial biogenesis.
- Control of lipolysis
- the shuttle regulates FFA mobilization by controlling plasma lactate levels.
- lactate functions to inhibit lipolysis in fat cells through activation of an orphan G-protein couple receptor (GPR81) that acts as a lactate sensor, inhibiting lipolysis in response to lactate
Bianca / JC 2021
Examiner Comments
2020A 08: 16% of candidates passed this question.
Better answers used the categorisation in the question as a structure for their answer. Many candidates gave a good description of lactate production from glycolysis, increasing with accumulation of NADH and pyruvate, when these are unable to enter Krebs cycle. There were however, many vague and incorrect descriptions as to what lactate is and its physiological role. Many candidates suggested that its presence is abnormal or pathological. Most answers demonstrated a superficial understanding and physiological detail of lactate’s role as an energy currency in times of oxygen debt. Higher scoring candidates often mentioned non-hypoxic causes of pyruvate accumulation which include; circulating catecholamines, exercise, sepsis or lack or mitochondria (RBCs). Mention of the relative ATP production of the two fates of pyruvate was also noted in more complete answers. The Cori cycle was generally superficially described. A key role of lactate is the ‘lactate sink’, allowing a period of ongoing ATP production from glycolysis when cells become oxygen deplete or the Kreb’s cycle is inhibited; few candidates detailed or highlighted this.
9. Outline the changes to drug pharmacokinetics and pharmacodynamics that occur at term in pregnancy.
CICMWrecks Answer
PHARMACOKINETICS
Absorption
- ↓ Oral absorption
- ↑ N/V
- ↓ gastric emptying during labour
- ↓ gastric motility 2° to intestinal compression
- ↑ gastric absorption
- ↓intestinal absorption due to ↓ intestinal blood flow
- ↑ IM/SC/transdermal absorption
- ↑ skin blood flow
- ↑ C.O. (by 30-40%)
- ↓ SVR
- ↑ skin blood flow
- IV (↑ onset)
- Neuraxial
- ↓ epidural space 2° to EDVs
- ↓ spinal and epidural doses
- Inhalational
- Progesterone-mediated ↑ MV (by 50-70%)
- ↑ FA/FI ratio (= uptake
Distribution
- ↑ VD (↑ TBW/ECF and fat)
- ↑ TBW/ECF (by 50%) (important for polar/ionized drugs)
- ↑ body fat % (important for lipid soluble drugs)
- ↓ plasma protein 2° to dilutional effect
- ↓ albumin →
- ↑ free % of acidic drugs (Eg. STP, propofol)
- ↓ dose required
- ↑ transplacental transfer of drug.
- ↓ A1AGP (by 30%) →
- ↑ free % of basic drugs (Eg. LA, β blockers)
- ↓ dose required
- ↑ transplacental transfer of drug
- ↓ albumin →
- Ionisation (mild ↑pH alters ionisation based on pKa)
- ↑ MV = mild respiratory alkalosis
- ↑ transplacental transfer of basic drugs as they will have ↑ % in unionized form
- (Base in base is less ionised)
- ↑ ion trapping in more acidotic foetal circulation
- ↑ transplacental transfer of basic drugs as they will have ↑ % in unionized form
- ↑ MV = mild respiratory alkalosis
Metabolism
- Progesterone:oestrogen ratio
- Progesterone → induces hepatic enzymes
- Oestrogen → inhibits hepatic enzymes
- ↓ plasma cholinesterase (30%)
- Placenta metabolises some drugs
- Foetal liver has functioning CYP450
- Can metabolise drugs
- But requires transfer back to maternal circ for conjugation
Excretion
- ↑ RBF/GFR (50%)
- ↑ clearance/↓ elimination t1⁄2 of water-soluble drugs
- ↑ MV/↓FRC
- ↑ washout of volatile agents
PHARMACODYNAMICS
- Decreased MAC – Increased sensitivity to volatile anaesthetics
- Increased LA sensitivity due to decreased α1-glycoprotein
- Increased sensitivity to IV anaesthetics
Gladwin 2016
Examiner Comments
2020A 09: 7% of candidates passed this question.
Answers framed around absorption, distribution, metabolism and excretion performed better. Some brief comments on physiology are required as the basis for pharmacokinetic change, but discussion of physiology that was not then specifically related to pharmacology did not score marks. Specific ‘real life’ examples necessitating change in practice or prescribing were well regarded e.g. reduction in spinal/epidural local anaesthetic dosing. Vague statements about possible or theoretical changes were less well regarded.
10. Compare and contrast the pharmacology of noradrenaline and vasopressin.
Examiner Comments
2020A 10: 49% of candidates passed this question.
These are both level 1 drugs regularly used in intensive care. Significant depth and detail of each drug were expected. Overall knowledge was deemed to be superficial and lacked integration. Better answers identified key points of difference and overlap in areas such as structure, pharmaceutics, pharmacokinetics, pharmacodynamics, mechanism of action, adverse effects and contraindications. A tabular list of individual drug pharmacological properties alongside each other did not score as well as answers which highlighted key areas of difference and similarities.
11. Describe the structure and function of adult haemoglobin.
CICMWrecks Answer
STRUCTURE
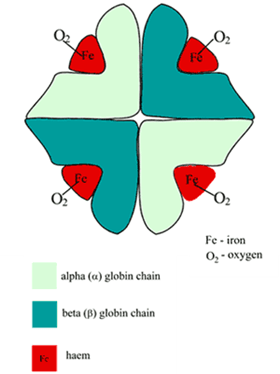
Structure
- Globular proteins which contain a haem moiety which binds O₂.
- Haem is an protoporphryin ring derivative with a central Fe2+ molecule that binds O₂
- Haemoglobin: MW ≈ 65000 daltons
- Hb contains 65-70 % of total body iron
- Globular molecule made up of four subunits, each containing a haem moiety conjugated to a polypeptide.
- Polypeptides collectively = globin → two pairs 2α + 2β → 4 haem moieties (Tetramer)
- Can bind a total of 4 O2 and also exhibits cooperative affinity (each subsequent O₂ binding takes less energy → sigmoid shaped OHDC
Location
- Hb is located in large concentrations (≈15g/L) in red blood cells that circulate throughout the blood stream.
Synthesis
- Haeme is synthesised in mitochondria and cytosol of immature RBCs, Globin is synthesized by ribosomes in cytosol.
- Production continues till RBCs lose their RNA after entering vasculature
Degradation
- RBCs at the end of their life cycle get phagocytosed by macrophages in liver or spleen, or get hemolysed in circulation.
- Haemoglobin is then broken up
- Haeme gets degraded into bilirubin
- iron gets recycled
FUNCTION
- O2 carrier:
- O2 loading exhibits positive cooperativity:
- α1ß1 & α2ß2 contacts stabilise Hb molecule as O2 reacts with it
- reaction of O2 with each subunit occurs sequentially with each facilitating the next
- ∴ ↑ing affinity as O2 loads ⇒ sigmoid OHDC
- O2 loading exhibits positive cooperativity:
- O2 unloading – vice versa:
- ß chains pulled apart
- 2,3-DPG enters molecule ⇒ ↓affinity of Hb for O2
- Protein buffer in RBC:
- Haemoglobin exists as a weak acid (HHb) as well as its potassium salt (KHb)
- In acidosis:
- Additional H+ ions are bound to Hb molecules
- HCO3– diffuses down its concentration gradient into plasma
- Electroneutrality is maintained through the inwards movement of Cl–.
- Dissolved CO2 will also form carbamino compounds by binding to the terminal amino groups
- Ligand Binding:
- Competitive inhibitors such as carbon monoxide (CO) and allosteric ligands such as carbon dioxide (CO2) and nitric oxide (NO).
- The carbon dioxide is bound to amino groups of the globin proteins to form carbaminohemoglobin; this mechanism is thought to account for about 10% of carbon dioxide transport in mammals.
- Nitric oxide can also be transported by haemoglobin; it is bound to specific thiol groups in the globin protein to form an S-nitrosothiol, which dissociates into free nitric oxide and thiol again, as the hemoglobin releases oxygen from its heme site. This nitric oxide transport to peripheral tissues is hypothesized to assist oxygen transport in tissues, by releasing vasodilatory nitric oxide to tissues in which oxygen levels are low.
Examiner Comments
2020A 11: 57% of candidates passed this question.
Marks were awarded for the two components of this question – structure and function. The structure component was often only briefly described with a cursory overview provided; however, this component contributed around half of the available marks. Many candidates were unable to accurately describe the structural components of the haemoglobin molecule. The functional component was handled better – however much time was wasted with detailed drawings of the oxyhemoglobin curve (not many marks awarded for this). The basic function of haemoglobin carriage of oxygen and carbon dioxide was known, but detail was often missing about its role as a buffer or its role in the metabolism of nitric oxide
12. Explain resonance and its significance and the effects of damping on invasive arterial blood pressure measurement.
CICMWrecks Answer
Definitions
Natural frequency: the frequency at which a system oscillates when not subjected to a continuous or repeated external force.
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of a periodically applied force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts.
Resonant frequency: The frequency of a dynamic system at which the amplitude of response to an oscillating force is a relative maximum is known as a resonant frequency (OR) A natural frequency of vibration determined by the physical parameters of the vibrating object.
Damping: the property of a system that diminishes resonance.
Critical Damping: Occurs when the damping coefficient (DC or γ gamma) is equal to the undamped resonant frequency of the oscillator.
Optimum Damping: Level of damping which provides a compromise between the speed of the system with its accuracy
Undamped simple harmonic oscillator
- obeys hooke’s law
- motion is sinusoidal in time
- demonstrates a single resonant frequency.
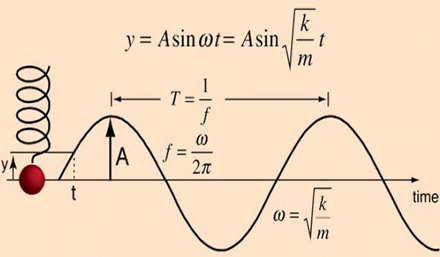
Resonance
Invasive arterial blood pressure is a driven harmonic oscillator
- An external force (pulsations of the heart) adds to the oscillations of the system (the tubing/transducer etc).
- If the frequency of the driving force is similar to, or a harmonic of, the natural frequency of the system then the result is amplification
Arterial waveform is a complex summation of multiple simple sinusoidal waveforms (a fourier series)
- The frequency of the arterial wave (i.e., the pulse rate) is known as the natural or fundamental frequency
- The sine waves used to reproduce it must have a frequency that is a multiple (or harmonic) of the fundamental frequency
- Increasing the number of harmonics allows better reproduction of high-frequency components, such as a steep systolic upstroke
In most clinical systems
- Natural resonant frequency is 10 to 15 Hz
- Primary frequency of the arterial waveform (the heart rate is 60 to 120 bpm or 1 to 2 Hz)
- However the higher frequency components of the more complex arterial waveform are closer to the natural resonant frequency and are amplified to a larger degree.
To minimize the potential of amplification and improve the dynamic accuracy of the real arterial pressure, system should have resonant frequency >> than 6-8 times frequency of the system being measured:
- Should have noncompliant (i.e. stiff) tubing
- Total mass of liquid in the system should also be minimized (shorter/thinner tubes)
- air bubbles or blood clots or occlusion should be eliminated
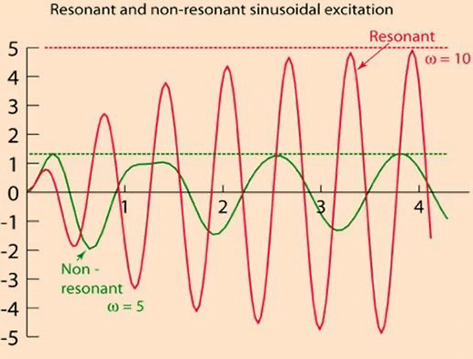
Damping
the property of a system that diminishes resonance.
- Damping:
- decreases the magnitude of the oscillations
- allows the system to come to rest at a new value.
- Degree of damping determines the rate at which the system achieves that new value.
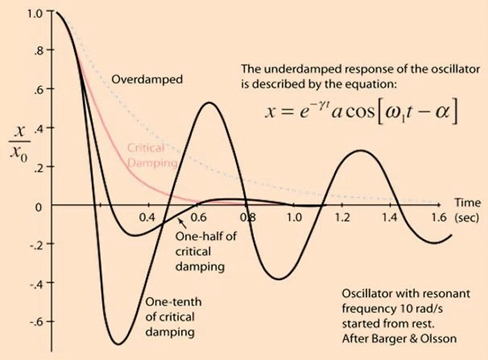
System can be:
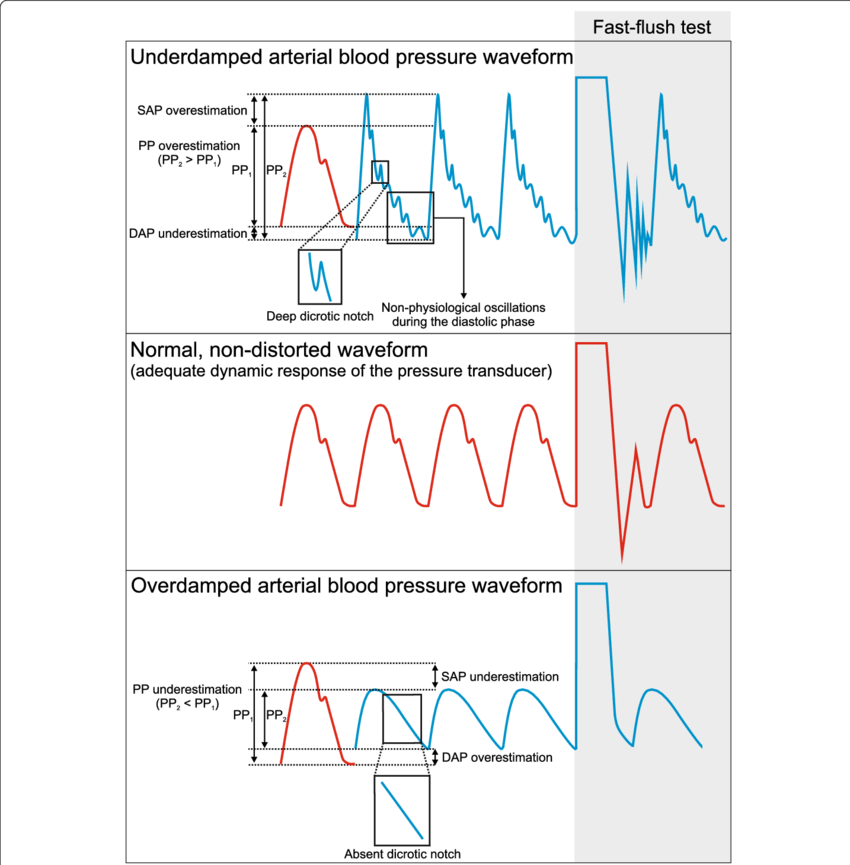
- Underdamped (DC < 0.7):
- Reaches the zero position quickly
- Oscillates around it
- Causes falsely ↑ SBP and falsely ↓ DBP
- Critically damped (DC = 1):
- Quickest approach to zero amplitude for a damped oscillator without overshoot.
- Occurs when the damping coefficient (DC or γ gamma) is equal to the undamped resonant frequency of the oscillator.
- Optimal damping (DC = 0.64):
- Provides a compromise between the speed of the system with its accuracy
- Minimises overshoot of oscillations, phase and amplitude distortion, and provides maximal frequency response
- Overdamped (DC > 1):
- The approach to zero is slower
- Very slow to respond
- Causes falsely ↓ SBP, falsely ↑ DBP and loss of fine details of waveform (Eg. dichrotic notch)
Bedside Flush Test:
- The degree of damping in an invasive arterial line system can be determined by the bedside flush test
- Briefly open the continuous flush device to produce a square wave
Gladwin / Sakurai / JC 2020
Examiner Comments
2020A 12: 23% of candidates passed this question.
Many candidates gave detailed answers that involved the set up and components of the arterial line system that was not asked for in the question and did not attract marks. There was confusion around the correct use of the terms natural frequency, resonance frequency and harmonics – candidates that were able to describe these frequencies correctly went on to achieve a good mark – the graphs and discussion around optimal dampening, over and underdamped traces were often drawn poorly or without sufficient detail, and at times were not used within in the context of the answer. Descriptions of the clinical effect seen with over / under dampened traces on blood pressure was well described.
13. Explain the control of breathing.
CICMWrecks Answer
Respiration
- Normal RR ~ 10 breaths per minute
- Normal tidal volume ~ 500ml/kg (in 70kg male)
- Therefore normal minute ventilation approx 5l/min
Afferents / Sensors
Chemoreceptors
- Central
Located in retrotrapezoidal nucleus- Sensitive to changes in CSF [H+]
- CO2 readily diffuses across BBB and converted to H+ and HCO3– via carbonic anhydrase
- Increased [H+] (and therefore CO2) stimulated respiration
- Sensitive to changes in CSF [H+]
- Peripheral
Located in aortic bodies (innervated by vagus nerve) and carotid bodies (innervated by glossopharyngeal nerve)- Sensitive to PO2, [H+], PCO2 and blood flow
- O2 dependent K+ channels
- Respiration increases as O2 drops below 50mmHg
- O2 dependent K+ channels
- CO2
- Linear increase in respiration as CO2 increases
- Sensitive to PO2, [H+], PCO2 and blood flow
Baroreceptors:
- Located in aortic arch and carotid sinuses
- Responds to stretch
- As MAP drops → less stretch on vessel walls → increased sympathetic outflow from medullary vasomotor centre → triggers increase in respiration
- Responds to stretch
Pulmonary receptors
- Stretch receptors
- Increased stretch of pulmonary parenchyma triggers inflation reflex → inhibits inspiration to prevent overdistention
- Collapse of pulmonary parenchyma triggers deflation reflex → inhibits expiration to prevent atelectasis and loss of FRC
- J fibres
- Nociceptive mechano-chemoreceptors
- On stimulation → Bronchospasm, apnoea, bradycardia and hypotension
- Nociceptive mechano-chemoreceptors
Others
- Joint and muscle receptors stimulate ventilation
- Pain and temperature sensation can alter ventilation via the cortex and limbic system
Central control of breathing
Central input
- There is input from the hypothalamus and cortex, with the ability of the cortex to override the medulla and bring ventilation under voluntary control
Controller
- Medullary Respiratory Centre
- Dorsal respiratory group (DRG)
- Located in and adjacent to Nucleus Tractus Solitarus
- Associated with inspiration and timing
- Works as an ‘integrating centre’ with VRG
- Ventral respiratory group (VRG)
- Including Pre-Botzinger and Botzinger complex → Central Pattern Generator
- Associated with control of expiration, airway dilation and central pattern generation
- sends inhibitory impulses to Apneustic centre
- Dorsal respiratory group (DRG)
- Pontine respiratory group (PRG)
- Pneumotaxic centre
- controls both the rate and the pattern of breathing
- Sends inhibitory impulses to the inspiratory area
- Antagonist to apneustic center
- decreases tidal volume
- Apneustic centre:
- sends signals for inspiration for long and deep breaths
- controls the intensity of breathing and is inhibited by the stretch receptors of the pulmonary muscles at maximum depth of inspiration, or by signals from the pnuemotaxic center
- increases tidal volume.
- Pneumotaxic centre
- Inspiratory phase:
- Gradual ramping up of nerve activity – ↑muscle contraction
- Expiratory phase I:
- Gradual reduction of nerve activity – ↓muscle contraction
- Expiratory phase II:
- Inspiratory muscles inactive
- If increased respiratory drive, expiratory muscles are activated
Efferents
- Phrenic nerve (C3, 4, 5) → Innervates diaphragm – Main inspiratory muscle
- Spinal nerves to intercostal muscles (external intercostal → inspiration, internal intercostal → expiration)
- Brachial plexus → Pectoralis major (forced breathing inspiration)
- Accessory muscle → Sternocleidomastoid (forced breathing inspiration)
- Spinal nerves to abdominal muscles (forced expiration)
Mooney / Sakurai / JC 2020
Examiner Comments
2020A 13: 53% of candidates passed this question.
Most candidates provided a structured answer based around a sensor / central integration / effector model with appropriate weighting towards the sensor / integration component. Better answers provided an understanding of details of receptor function, roles of the medullary and pontine nuclei and how these are thought to integrate input from sensors. Marks were awarded to PaCO2 ventilation and PaO2 ventilation response when accurate, correctly labelled diagrams or descriptions were provided.
14. Describe the pharmacology of frusemide.
Examiner Comments
2020A 14: 51% of candidates passed this question.
Most candidates presented a well-structured answer and provided a basic understanding. Answers that provided accurate indications and details of the mechanism underlying the actions of frusemide attracted more marks. Those recognising the increased delivery of sodium and chloride to the distal tubule (exceeding resorptive capacity) were awarded more marks that those answers that attributed the diuretic action solely to reduction in the medullary gradient. Frusemide has many potential adverse effects and a reasonable list was expected. Conflicting information was common (e.g. highly bound to albumin – Vd 4 L/kg) and better answers avoided this.
15. Define bioavailability (10% of marks).
Outline the factors which affect it (90% of marks).
CICMWrecks Answer
DEFINITION
The fraction of drug that reaches the circulation compared with the same dose given intravenously. (%)
(or)
The ratio of the area under the stated concentration–time curve (AUC) divided by the area under the i.v. concentration–time curve. (%)
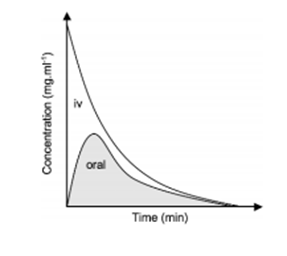
FACTORS AFFECTING BIOAVAILABILITY
- Pharmaceutical factors
- Preparation: Decreasing order: Solutions > Suspensions > Capsule > Tablet > Coated tablet
- Particle Size
- Salt form
- Crystal forms have better availability compared to amorphous forms
- Degree of ionization: – Non-ionised, lipid soluble drugs – higher bioavailability
- Pharmacological factors
- Gastric Emptying and GI Motility
- GI Diseases: Coeliac, Crohn’s
- Timing in relation to food intake
- First pass metabolism: The degree of metabolic breakdown of an orally administered drug that occurs in the intestine or liver before it reaches the systemic circulation.
- Drug-Drug interactions
- Pharmacogenetic factors
- Other factors:
- Area of absorptive surface
- State of circulation (shock, tissue perfusion)
- Hepatic Insufficiency, Poor renal function
- Route of administration
Sources: Cross and Plunkett. Physics, Pharmacology, Physiology for Anaesthetists. Goodman & Gilman’s Pharmacological Basis of Therapeutics. Katzung Clinical Pharmacology
JC 2020
Examiner Comments
49% of candidates passed this question.
Many candidates spent time defining and describing aspects of pharmacokinetics which were not relevant to the question. E.g. clearance, volume of distribution and half-life. Candidates who scored well utilised a structure which incorporated the headings of the factors which affect the bioavailability of medications with a simple description as to the nature of the effect. These factors included: the physical properties of the drug, the preparation, patient factors, the route of administration and metabolism amongst others.
16. Outline the formation, circulation and functions of cerebrospinal fluid.
CICMWrecks Answer
Formation / Production of CSF
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
Distribution / Circulation of CSF
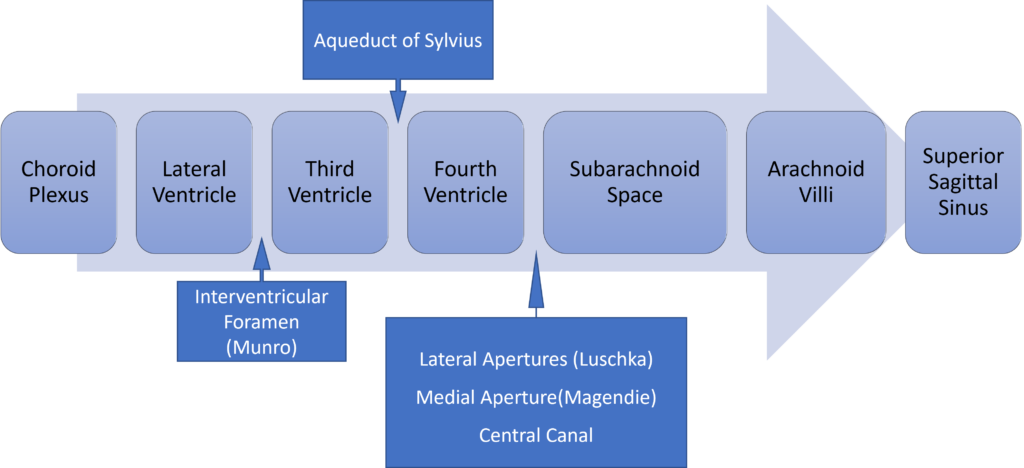
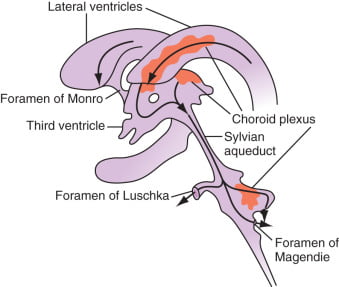
Absorption of CSF
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
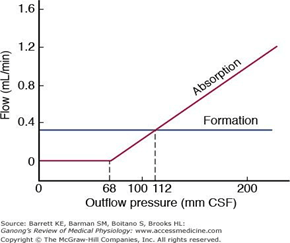
Composition of CSF
IDENTICAL to brain ECF, but differs in several manners from plasma
Compared with plasma:
- ↑ pCO2 (50 mmHg)
- ↓ pH (7.33)
- ↓ protein content
- 0.5% of plasma; 20 mg/dL
- poor acid-base buffering capacity
- ↓ content of glucose (by 60%) and cholesterol
- ↑ [Cl-] (by 7-14%) and ↑ [Mg2+] (by 40%)
- ↓ [K+] (by 40%), ↓ [Ca2+] (by 50%) and ↓ [Pi] (by 20-30%)
- ↑ creatinine (by 25%) but ↓ urea
- IDENTICAL osmolality (295), [Na+] (145), and [HCO3] (25)
Role / Functions of CSF
- Protective role (main function)
- Water bath effect
- Attributed to the low specific gravity of CSF (1.007)
- Causes brain to be buoyant
- ↓ its effective net weight from 1400 g to 50 g
- Mechanical cushion against acceleration/deceleration forces
- Buffer ↑ ICP by CSF translocation to extracranial subarachnoid space
- Abrupt ↑ ICP buffered by translocation of CSF within the vault to extracranial compartments
- Water bath effect
- Maintains constant ionic environment conducive to neuronal electrical activity CSF
- Supply role of nutrients (Eg simple sugars, amino acids) and O2 to brain
- Excretion of toxic substances, metabolic by-products, and CO2 from brain
- “Lymph-type” function → interstitial proteins in brain ECF return to circulation by
- CSF absorption across arachnoid villi
- Acid-base regulation → due to content, CSF allows for tight respiratory control
- Endocrine transport function → transports hormones to other brain regions
Gladwin / JC 2020
Examiner Comments
2020A 16: 81% of candidates passed this question.
This is a three-part question and was marked as such. The circulation and functions of CSF was generally well answered. Formation of CSF, however, was answered poorly, with many candidates listing its composition instead. The examiners were looking for an understanding of the physiological processes of formation not the composition.
17. Discuss the advantages and disadvantages of the use of an intravenous infusion of fentanyl in comparison to morphine.
CICMWrecks Answer
| Fentanyl Infusion | Morphine Infusion | |
|---|---|---|
| Cost | slightly less | |
| Potency | 50 to 100 times more potent highly lipophilic, which allows rapid penetration of the blood-brain barrier and rapid onset of action (four to six minutes), although maximal analgesic and respiratory depressant effects of fentanyl may not be evident for several minutes | |
| Effective analgesia for surgical trauma causing severe pain during the intraoperative or immediate postoperative period, due to a prolonged duration of action. | ||
| PD | a more rapid onset of action than morphine, which reflects its greater lipid solubility and consequent increased ability to cross the blood–brain barrier | Slower onset of analgesia (within 20 minutes) and slower time to peak analgesic effect compared with fentanyl due to lower lipid solubility and a longer lag time for penetration of the blood-brain barrier. |
| redistribution to inactive tissue sites such as fat and skeletal muscle, with an associated decrease in plasma concentration Furthermore, fentanyl has been reported to have 75% first-pass uptake into the lungs, thus the amount of fentanyl that reaches the systemic circulation is limited | morphine’s first-pass pulmonary uptake is extremely small | |
| Despite fentanyl being a more attractive option than morphine for analgo-sedation, because of its potentially shorter duration of action, other pharmacokinetic properties may limit its usefulness. After initial redistribution, fentanyl’s plasma concentrations are maintained by slow reuptake from the tissues across a concentration gradient | ||
| Effects | no significant difference in amount of pain relief | |
| Minimal effect on myocardial or hemodynamic function. | Unsuitable for patients with hemodynamic instability due to possible exacerbation of hypotension by histamine release, as well as persistence of its effects due to a long context-sensitive half time and the mu-receptor-stimulating properties of its morphine-6-glucuronide metabolite. | |
| Metabolism | Metabolised by N-demethylation in the liver, catalysed by the cytochrome p450 system; major metabolite is norfentanyl and other metabolites are hydroxyproprionyl-fentanyl and hydroxyproprionyl-norfentanyl (all these metabolites have minimal pharmacologic activity) | Metabolised by conjugation with glucuronic acid in hepatic and extrahepatic sites, especially the kidneys; major metabolites are morphine-3-glucuronide (75–85%), morphine-6-glucuronide (5–10%); other metabolites are normorphine and a small amount to codeine |
| Active metabolites | Minimal pharmacological effect | Morphine-6-glucuronide – pharmacologically active undergoes renal clearance and accumulates in patients with renal failure, with clinical effects including prolonged sedation and respiratory depression elimination half-life of about 1.4 hours, but this is increased in patients with renal failure |
| Fentanyl has a high hepatic extraction ratio with clearance approaching liver blood flow | ||
| Excretion | Metabolites are renally excreted and < 10% of fentanyl is excreted unchanged in the urine | Metabolites are renally excreted with 7–10% undergoing biliary excretion |
| Elimination half life | 3.1–6.6 hours | 1.7–2.3 hours |
| fentanyl’s effects may be as prolonged as morphine, even when allowing for potential accumulation of metabolically active metabolites of morphine in renal failure | Cautious use in patients with a history of seizures if renal insufficiency is present, due to neuroexcitation caused by the morphine-6-glucuronide metabolite, with possible occurrence of myoclonus or exacerbation of seizure activity. | |
| Context-sensitive half-time | Consequently, following infusion, the clinical effects of fentanyl become increasingly prolonged owing to its long “context-sensitive half-time” | |
CSHT 4hr inf | 200 minutes | Not applicable |
| CSHT 8hr inf | 300 min | Not applicable |
| longer inf | unpredictably long | Not applicable |
| Adverse effects | Higher incidence of most opioid-related adverse side effects (eg, pruritus, urinary retention, constipation, and nausea), compared with other opioids. | |
| Absence of histamine-releasing properties; thus, fentanyl is appropriate for patients with bronchospasm. | headedness, sedation, drowsiness and euphoria). In addition, morphine contains a tertiary amine group, which causes non-immune-mediated release of histamine and can lead to skin rashes, itchiness and hypotension | |
| Drug-drug interactions | Similar interactions – may enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome | |
Examiner Comments
2020A 17: 27% of candidates passed this question.
These are both level 1 drugs commonly used as an infusion in daily practice. This question specifically asked the candidates to frame their answers around an intravenous infusion of fentanyl in comparison to morphine. A tabular listing of general properties of the two drugs highlighting the differences between the drugs would not score well. The question asks for a considered response that should focus on context sensitive half-life, compartments and metabolism, instead many focused on the speed of onset and potency, which are minor considerations when drugs are given for long periods by infusion. Candidates often demonstrated a superficial knowledge of key pharmacokinetic concepts with limited application of these principles in the context of an intravenous infusion. Better answers also related the above to various relevant pharmacodynamic influences such as age, liver and renal impairment.
618. Describe the respiratory changes that occur throughout pregnancy.
CICMWrecks Answer
Respiratory Changes In Pregnancy
Anatomical
- Diaphragm ascends ~4cm due to foetus
- AP and lateral diameters of thorax increase 2~3cm due to relaxin effect on chest
wall - Thoracic circumference increases 5~7cm (AP and lateral diameters increased 2~3cm)
- Airway dilatation → decreased resistance (35%) and increased deadspace (45%)
- Increased cardiac output, intravascular volume, and decreased oncotic pressure
- Increased plasma volume → increased pulmonary blood volume → decreased compliance
- Venous engorgement → Upper airway oedema
Mechanical
- Lung volume changes occur from 20 weeks
- TLV decreases 5% from baseline
- FRC decreased 20% due to both ERV and RV
- Significant postural change in FRC of 70%
- Progesterone → increased sensitivity to CO2 → Minute ventilation increases 50%
- TV increases (25%)
- RR increases
- Results in hypocarbia and compensated respiratory alkalosis
- pCO2 ~ 26-32mmHg
- HCO3 ~ 18-20mmol/l
- Further increase in MV during contractions
- Inspiratory capacity increased by 10%
- Closing capacity unchanged
- Increased O2 consumption during labour (60%)
After birth
- FRC and RV return to normal within 48 hours
- TV returns to normal within 5 days
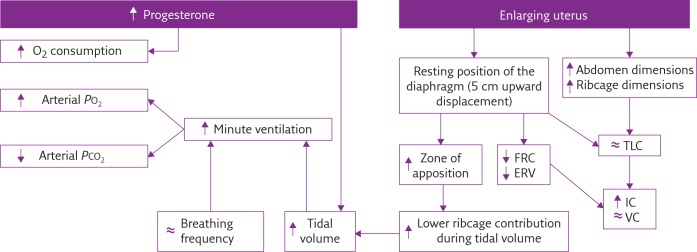
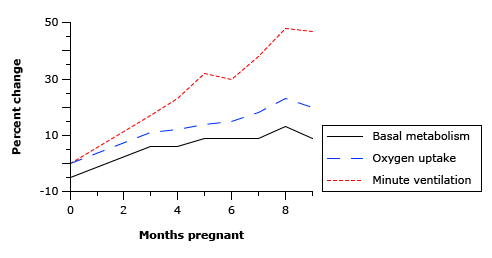
Sakurai 2016
Examiner Comments
2020A 18: 31% of candidates passed this question.
The question asked for a description of the respiratory changes throughout pregnancy, which includes labour. Simple lists of changes did not score highly. A straightforward structure including; first, second and third trimester delineation would have elevated many answers from below par to a pass. Many good answers gave succinct detail on both mechanical respiratory changes and the hormonal mechanisms behind them. Higher scoring answers also described the overall effect of individual changes to spirometry, geometry or respiratory control.
19. Discuss the determinants of venous return to the heart.
CICMWrecks Answer
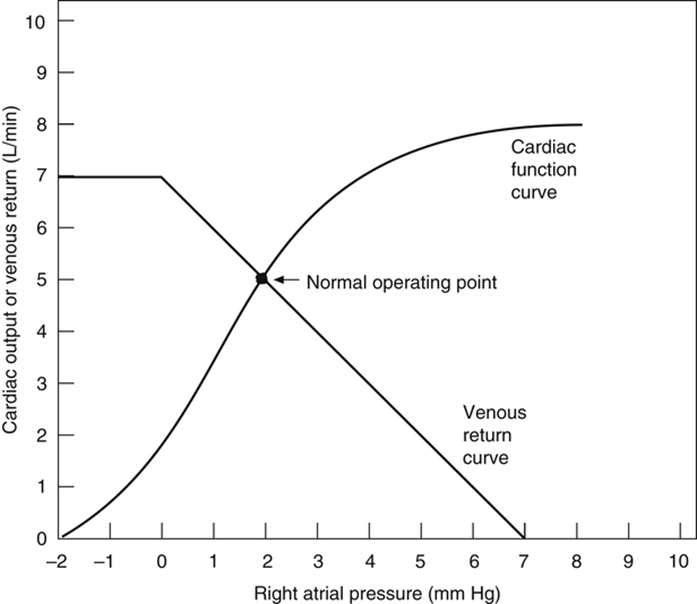
Vascular function (venous return) curve shows the effect of increases in venous return or CO (independent variable) on RA
pressure (dependent variable)
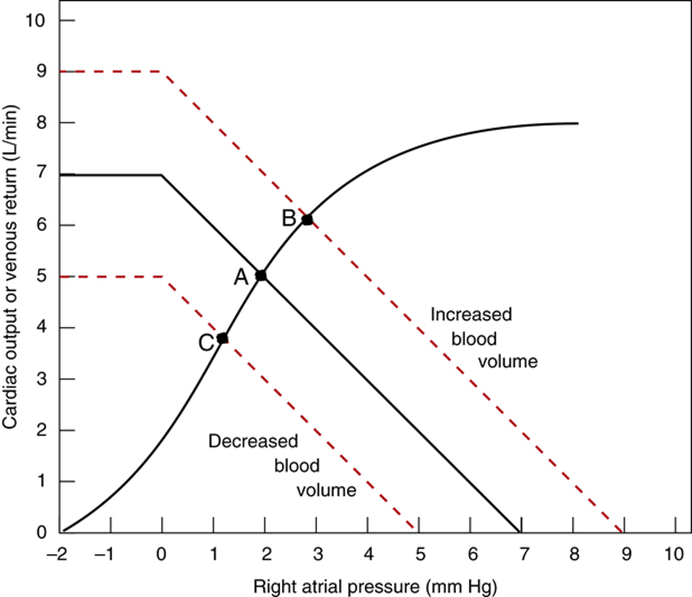
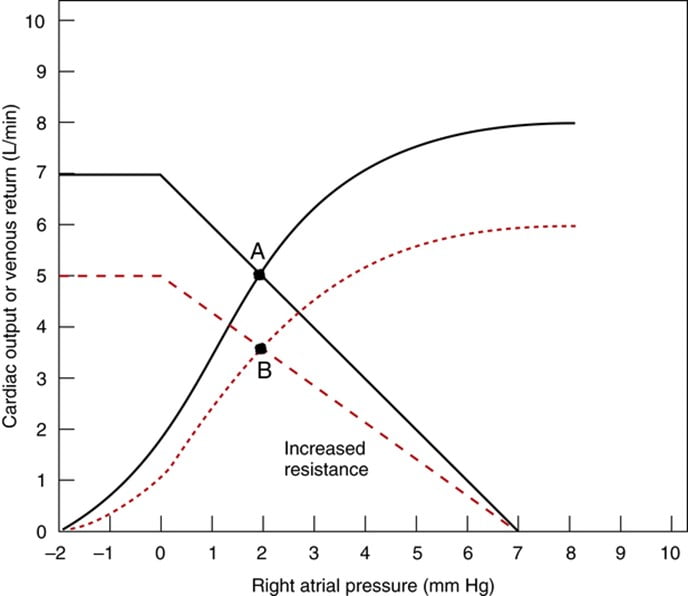
Factors that determine venous return back to the heart
The factors that influence VR are captured in 2 formulae:
The three variables that influence venous return are:
- Mean Systemic Filling pressure (MSFP)
- MSFP is the theoretical pressure present in the systemic circulation at equilibrium
- MFSP is the main driving pressure moving blood back towards the RA – It is the MAIN factor determining venous return (and thus CO)
- MSFP can be used to assess the degree of filling of systemic circulation: It is normally 7 mmHg (0-20 mmHg)
- Two factors influence MSFP:
- Blood volume ↑ → ↑ MSFP
- Venomotor tone (venous capacitance) ↑ → ↑ MSFP
- RA pressure (RAP)
- Resistance to venous return (RVR)
Therefore, the factors that affect venous return influence one or more of the aforementioned variables:
- Blood volume
- Blood volume is proportional to MSFP and thus venous return
- Venomotor tone
- ↑ venomotor tone (Eg. due to SNS activity) ↓ vein compliance and capacity → ↑ MSFP → ↑ venous return
- ↓ venomotor tone (Eg. due to SAB) ↑ vein compliance and capacity → ↓ MSFP → ↓ venous return
- This has a greater effect on venous return when
- (i) venous pressures are normal, and
- (ii) veins are circular (not collapsed and contain large volumes of blood)
- Venous valves
- Veins have one-way valves that prevent retrograde flow
- Skeletal muscle pump
- Alternating contraction and relaxation of limb skeletal muscle forces blood out of the veins towards the heart (thus, increasing MSFP)
- During contraction, veins compress to expel blood towards the heart, then during relaxation, veins distend and fill with blood
- With exercise, this pump function enhances net venous return
- Respiratory pump
- During inspiration, venous return is ↑ due to
- (i) ↓ RAP (associated with fall in PINTRAPLEURAL), and
- (ii) ↑ IAP (due to diaphragmatic contraction)
- During expiration, the effects are reversed
- Note that if RAP is < 0 mmHg, the respiratory pump will NOT have any effect on venous return as the thoracic veins would have collapsed at subatmospheric pressures
- During inspiration, venous return is ↑ due to
- Posture
- When going from supine to erect, there is ↓ venous return due to venous pooling in the lower extremities
- Normally, there is reflex vasoconstriction to prevent this BUT this reflex is delayed and less effective in the elderly
- Effect of ventricular contraction and relaxation
- During rapid ejection phase of ventricular systole, atrial pressure falls sharply (to zero or –ve values) as the ventricle contraction pulls the atrioventricular fibrous ring downwards and increases the atrial volume – This causes net blood flow into atria and increases venous return
- During early diastole, ventricles fill rapidly causing both ventricular and atrial pressures to decline – This facilitate blood flow into atria and increases venous return
- Intrapericardial pressure
- ↑ intrapericardial pressure (Eg. tamponade) can ↑ RAP, thus impeding venous return
- Afterload
- Changes in the dimension of the resistance vessels (arterioles) has a small effect on MSFP as only 2% of blood volume is in arterioles (cf. venous capacitance vessels) – Instead, it has an impact on “Resistance to venous return”
- ↓ afterload (such as decreased SVR due to arteriolar vasodilation) → ↓ RVR → ↑ VR
- ↑ afterload (such as increased SVR due to arteriolar vasoconstriction) ↑ RVR → ↓ VR
Bianca 2020
Examiner Comments
2020A 19: 67% of candidates passed this question.
The factors that influence VR are captured in 2 formulae; VR = CO, and VR = (MSFP-RAP) / Venous Resistance. Candidates that used these as the backbone structure of their answer scored well. Quite a few candidates failed to consider factors that affect left heart CO also effect VR.
Recognising that CO does = VR appeared to elude some candidates.
20. Outline the distribution, absorption, elimination, regulation and physiological role of phosphate.
CICMWrecks Answer
Phosphate
- Most abundant anion, approx. 1% of total body weight
- Normal serum level 0.8 to 1.3 mmol/L in adults
- daily requirement = 0.4mmol/kg/day
Distribution
- Mostly intracellular.
- Bone & teeth (85%), tissue (14%), ECF (1%)
- In skeleton with calcium, as hydroxyapatite crystals or amorphous calcium phosphate
- In tissue and ECF:
- 2/3 is tied up in molecules like ATP
- 1/3 in inorganic phosphate
- pH dependent
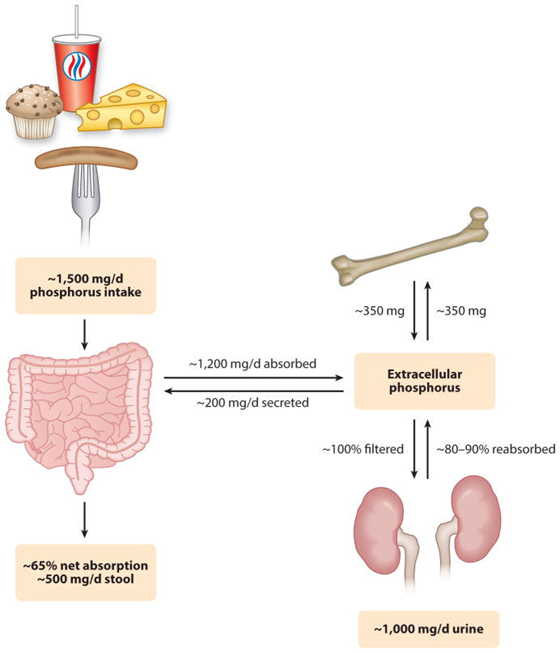
Absorption
Dietary intake 20mg/kg/day → Proximal intestine absorbs 16mg/kg/day
Elimination
- Feces:
- 3mg/kg/day secreted into intestine via pancreatic, bile, intestinal secretion
- unabsorbed 4mg/kg/day from dietary intake
- Kidney:
- 100mg/kg/day in UF.
- 80% reabsorbed in proximal tubule
- 5% in DCT
- rest excreted (~15mg/kg/day)
- 100mg/kg/day in UF.
- Net Intake-Excretion of Phosphate
- modulated based on physiological needs such as bone remodelling, bone growth, etc
- bone resorption ~300mg/day
- absorption ~300mg/day
- modulated based on physiological needs such as bone remodelling, bone growth, etc
Regulation
GI – Bone – Renal axis
Regulators:
- Dietary phosphate intake and absorption
- Calcitriol
- which increases phosphate absorption from the gut and bone
- Parathyroid hormone (PTH)
- directly causes phosphate resorption from bone and decreases its reabsorption in the proximal tubule
- indirectly by stimulating the production of calcitriol.
- Phosphatonins, such as fibroblast growth factor-23 (FGF-23):
- Inhibit renal phosphate reabsorption
- inhibit synthesis of calcitriol
Factors that alter renal regulation of phosphate:
| Increase Phosphate Absorption | Decrease Phosphate Absorption |
|---|---|
| Low-phosphate diet 1,25-Vitamin D3 Thyroid hormone | Parathyroid hormone Phosphatonins (e.g., FGF23) High-phosphate diet Metabolic acidosis Potassium deficiency Glucocorticoids Dopamine Hypertension Estrogen |
Physiological Role
- Bone and teeth formation
- Cell wall structure (phospholipids)
- Nucleic acid generation
- Glucose metabolism
- High energy bonds
- O2 transport (2,3 DPG)
- Phosphate Buffer
- intracellular signalling
- Sources
JC 2020
Examiner Comments
2020A 20: 29% of candidates passed this question.
The answer structure should have utilized the headings provided in the question. Many candidates described the physiology of calcium, which while related, did not attract marks. The distribution section required not only the sites of distribution but also the percentages found in each. The regulation should have included both primary and secondary mechanisms and an outline on the factors affecting renal excretion, intestinal absorption and release from bone etc. An outline of the physiological role of phosphate required a broad knowledge of physiological processes.
VIVAs
| Postponed and conducted during 2020 Second Sitting (due to COVID-19). |

Recent Comments