SAQs
1. Describe the pharmacology of lignocaine.
Examiner Comments
2019A 01: 16% of candidates passed this question.
Comprehensive answers included uses (including antiarrhythmic action and a role in analgesia), physical properties and preparations, pharmacodynamics and pharmacokinetics. Its mode of action should also have been described. Many candidates focussed on toxicity and its management but provided little information on pharmacodynamics and pharmacokinetics, commonly omitting factors which affect its systemic absorption. Other common omissions were the dose required for its local anaesthetic effect and for its antiarrhythmic effect.
2. Outline the components required to measure blood pressure from an intra-arterial catheter (75% of marks). What information (other than blood pressure) may be gained from an arterial line trace (25% of marks)?
CICMWrecks Answer

Process
- BP pulsations transmitted from arterial cannula up the tubing to transducer
- Woks by hydraulic coupling between blood and saline
- Transducer converts pressure waves to electrical signals (via strain gauge and wheatstone bridge)
- Signal amplified and displayed on monitor
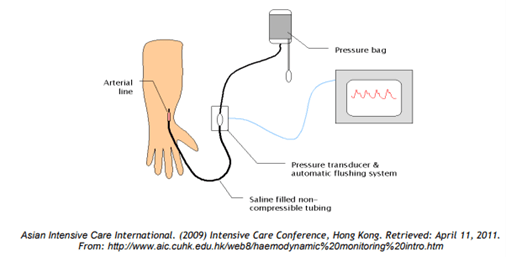
Information which may be gained from an arterial line trace:
- Normal trace
- Pulse rate and rhythm
- Arrhythmias
- Morphology:
- Slow rising in AS
- Steepness of ascending phase:
- Increased: Inc Systemic vascular resistance, vasopressors, inc contractility
- Decreased: Septic shock, vasodilators
- Dicrotic ‘fall-off’ : changes with changes in SVR
- Variation with insertion site: Further from aorta: taller systolic peak, delayed dicrotic notch, lower end diastolic pressure, later arrival of pulse
- Dampened trace
- Kinks/bubbles/clots
- Overly compliant tubing
- Hyperactive trace
- Noncompliant tubing, reverberations, increased vascular resistance
- Arterial line swing
- Reduction in venous return
- Hyperexpansion of lungs
- Pulse pressure variation (~fluid responsiveness)
- Pulsus alternans in tamponade
- Adjuncts: Pulse contour analysis with PICCO / other devices
JC 2019
Examiner Comments
2019A 02: 44% of candidates passed this question.
Most of the marks were allocated to the components of the measuring system (as detailed in the question), hence a level of detail was required. An explanation of how the various components work was required; e.g. hydraulic coupling and transducers. Some candidates forgot to include heart rate as a piece of information derived from the trace.
3. Compare and contrast fresh frozen plasma and prothrombin complex concentrate.
CICMWrecks Answer
| Fresh Frozen Plasma (FFP) | Prothrombin Complex Concentrate (Prothrombinex) | |
| Intro | Human plasma containing all coagulation factors including the labile plasma coagulation Factors VIII and V | Human plasma derivative containing concentrates of factors II, IX and X (500IU PCC has 500IU of II, IX, X each in a vial) The 3 factor prothrombin complex concentrate available in Australia (4 factor PCC in some European countries) |
| Preparation | Prepared either via: Separation from whole blood Apheresis: Removal of a large volume (typically 800ml) of plasma from a single patient, with return of red cells to the donor. Once collected, it is frozen and rethawed in a water bath prior to use | developed through the process of ion-exchange chromatography from the cryoprecipitate supernatant of large plasma pools and after removal of antithrombin and factor XI |
| Indications | reversal of warfarin anticoagulation (in setting of bleeding or need for invasive procedure) Bleeding and multiple coagulation defects (e.g. DIC with significant PT/PTT elevation) Correction of coagulation defects for which no specific factor is available Transfusion of more than one blood volume with evidence of active bleeding + coagulopathy accepted treatment for patients with thrombotic thrombocytopenic purpura often in conjunction with plasma exchange. Where PCCs are not available | Bleeding and warfarinized Bleeding from factor deficiency (either congenital or due to liver disease, and haemophilia) Prophylaxis |
| Contraindications | Allergy to class/drug ABO incompatibility when you can correct coagulopathy effectively with specific therapy in plasma exchange procedures except for treatment in thrombotic thrombocytopenic purpura treatment of immunodeficiency states | Allergy to class/drug DIC known HIT (Heparin induced thrombocytopenia) patients with haemophilia B if a specific factor IX concentrate is available |
| PC | Dose: 10-15ml/kg will increase factors by ~20-30% Transfuse at least 15 mL/kg at a time (4 units in 70-kg adult) INR >1.5 and needs invasive procedure INR >1.5 and actively bleeding (e.g. massive transfusion protocol, post-bypass surgery) INR of FFP is ~1.6; therefore transfusing for INR <1.7 is not advised | Dose: – 25-50 IU\kg (1 IU\kg of Factor IX raises the Factor IX by 1%) – Dose adjusted based on INR |
| PD – MoA | restores factors II, VII, IX and X in the anticoagulated patient | provision of factors II, IX and X |
| Side Effects | Serious Hemolytic transfusion reactions Febrile non-hemolytic reactions Transfusion-associated circulatory overload (TACO) Transfusion-related acute lung injury (TRALI) Transfusion-associated graft-versus-host disease Anaphylaxis Sepsis Common Headache, paraesthesia Nausea, Pruritus, urticaria | Serious Thromboembolic Events Common Allergy, anaphylaxis phlebitis vomiting, fever rash, urticaria SOB pain thrombocytopenia |
| PK | IV Distributed and metabolized in the same way as endogenous coagulation factors | IV Distributed and metabolized in the same way as endogenous coagulation factors |
| Advantages | cheap | small volume readily available reliable reversal avoids complications of FFP – immune reaction, TRALI, fever, infection risk familiarity cheap |
| Disadvantages | ABO compatibilty a must (but crossmatch before transfusing not) Needs to be thawed – time delay Immune reaction, TRALI, fever, infection risk significant fluid load in patients that are elderly and frail, or have heart failure. | More expensive than FFP May cause thromboembolic complications |
Examiner Comments
2019A 03: 10% of candidates passed this question.
Very few answers included details on prothrombin complex concentrate which meant it was difficult to score well. Useful headings included preparation and administration, dose, indications and adverse effects. Not many candidates knew the dose of FFP, and few were able to describe the preparation/production of the product. Few candidates knew the factors available from either product. Commonly missed was the need for ABO typing for FFP and that Prothrombin complex concentrate did not require this.
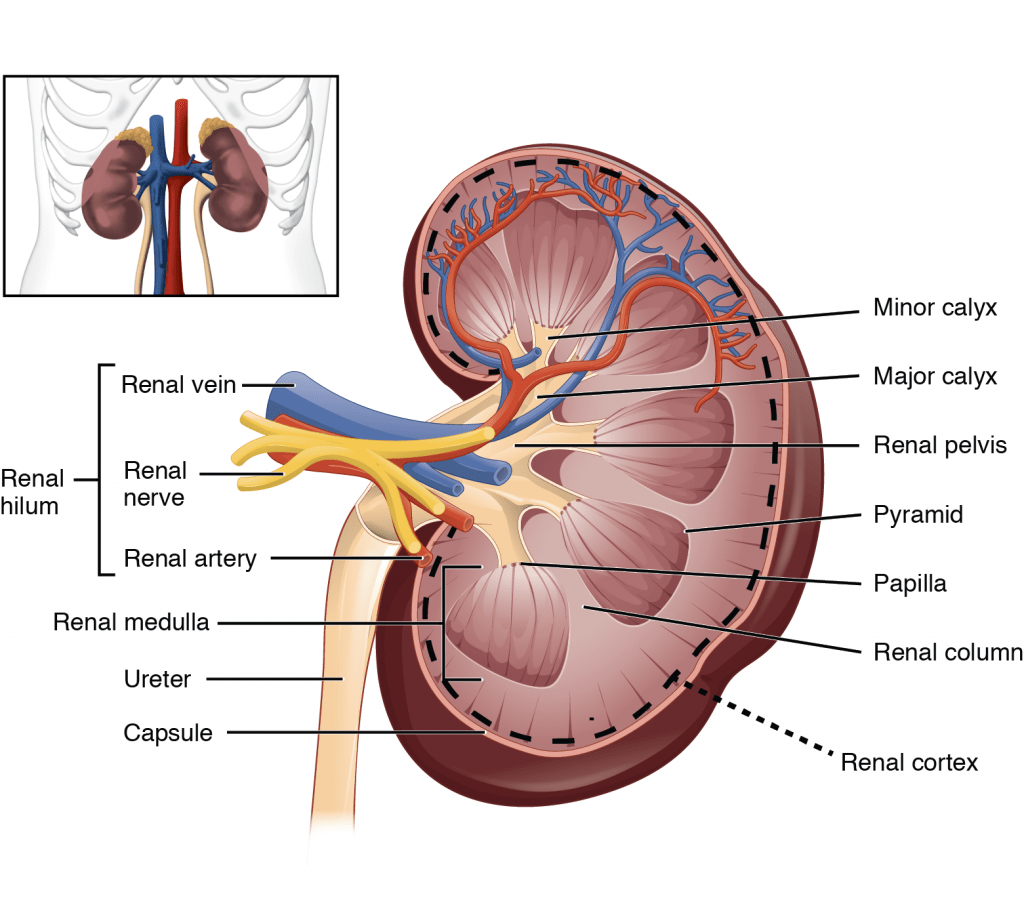
4. Outline the functional anatomy of the kidney (40% of marks). Outline the regulation of renal blood flow (60% of marks).
CICMWrecks Answer – Anatomy
Kidney
- Lie in the posterior wall of the abdomen – outside peritoneum (retroperitoneal)
- Each kidney approx 150g
- Medial side of each kidney = hilum
- Renal artery and vein
- Lymphatic supply
- Nerves
- Ureter
- Kidney surrounded by tough fibrous capsule
- Kidney organized into outer cortex and inner medulla
- Medulla
- Organised into mutliple pyramids with the base at the corticomedullary junction and apices – the papilla – at the hilum draining into the ureters
- Nephrons = functional unit of the kidney (1 million / kidney)
Nephron
The functional unit of the kidney (1 million / kidney)
Parts:
- Afferent Arteriole: Brings blood to glomerulus. Regulate BP and supply blood to kidneys. Diameter bigger than Efferent Arterioles
- Efferent arteriole: Carries blood away. Regulates GFR
- Glomerulus: Provides driving force for solutes and water
- Bowman’s Capsule: Inner visceral layer, outer parietal layer. Filters bloods
- Renal Tubule: Filled with tubular fluid: aids in excretion and reabsorption
- Proximal convoluted tubule
- Loop of Henle:
- Descending Limb
- Lower thin Ascending limb
- Thick Ascending Limb
- Distal convoluted tubule
- Connecting tubule
Types by length:
- Cortical nephrons
- High in cortex, short loops of Henle.
- Juxtamedullary nephrons
- Low in cortex near medulla, long loops of Henle which penetrate medulla
- Only nephrons with vasa recta
Renal Blood Supply
- Renal blood flow ~1.25L/min (24% of cardiac output or 500ml/100g tissue/min) via renal artery
- 95% blood flow to cortex, ~5% to Medulla
- Renal artery enters through hilum it divides
- → interlobar arteries
- → arcuate arteries
- → interlobular arteries
- → afferent arterioles
- → glomerular capillaries
- → efferent arterioles
- → peritubular capillaries
- Peritubular capillaries supply blood to the renal tubules
- Juxtaglomerular capillaries have specialized vasa recta which supply the LoH and is important in renal concentration of urine
- → venous system → interlobular veins → arcuate veins → interlobar veins → renal vein
Juxtaglomerular Apparatus
Formed where the TAL of LoH and DCT passes between the afferent and efferent
arterioles at the vascular pole of the glomerulus.
Comprises of:
- Macula Densa
- Modified tubular epithelial wall of the DCT (located at the angle between
afferent and efferent arterioles) - Contains specialised cells that sense changes in the filtrate NaCl levels →
involved in intrinsic regulation of GFR and RBF by “Tubuloglomerular
feedback” and control of renin secretion
- Modified tubular epithelial wall of the DCT (located at the angle between
- Juxtaglomerular (Granular) cells
- Specialised smooth muscle cells found in the afferent arteriolar wall (esp
“media”) that possess:- Renin-containing granules → secreted to invoke RAAS
- Intrarenal-baroreceptors → involved in intrinsic autoregulation of RBF/GFR by a myogenic mechanism
- Specialised smooth muscle cells found in the afferent arteriolar wall (esp
- Extraglomerular mesangial (Lacis) cells
- Participate in a signalling pathway of the Tubuloglomerular feedback
- Possess contractile elements (actin and myosin) → contract in response to SNS stimulation → thereby ↓ glomerular surface area and GFR
JC / Sakurai 2019
CICMWrecks Answer – Physiology of Blood flow
Regulation of Renal Blood Flow
- Renal blood flow Autoregulated between 80~170mmHg
- Blood flow maintained by modulating resistance based on pressure
- Renal vascular resistance maintained by interlobular arteries, afferent arterioles and efferent arterioles, amenable to external regulation
- GFR approx 180l/day – Autoregulated by tubuloglomerular feedback – relatively constant in response to fluctuating renal blood flow
INTRINSIC Regulation (Autoregulation) of GFR and RBF:
- Renal blood flow (and consequently GFR) Autoregulated between MAP range of 75~170mmHg
- Blood flow maintained by modulating resistance of AFFERENT based on pressure
- Efferent arteriole is NOT involved in autoregulation!
- Autoregulation of GFR and RBF can be overridden by external influences (Eg. hormones and SNS neurons), even when renal perfusion pressure is between MAP 75-170 mmHg!
Mechanisms of Autoregulation:
- Myogenic autoregulation (Myogenic stretch response):
- In response to vascular wall stretch (due to increased intraluminal pressures), stretch dependent Ca influx occurs causing vasoconstricion of arterioles → increased resistance according to Poisuille-Hagen Equation → Decreased flow
- In response to shear stress (due to increased flow), Endothelial derived relaxation factors released (such as NO) → NO acts on guanylyl cyclase → increased cGMP → smooth muscle relaxation → arteriolar vasodilation
- Tubuloglomerular feedback (TGF)
- Negative feedback – Links the rate of GFR to concentration of salt in tubular fluid at macula densa
- Macula densa in wall of Ascending limb of loop of Henle Detects change in tubular flow (by the changing salt concentrations)
- As a consequence of decreased blood flow → GFR decreases → Tubular flow rate decreases → Increased uptake of [Na] in Ascending LoH → Reduced [Na+] and [Cl-] reaching the DCT and macula densa → Juxtaglomerular apparatus releases prostaglandins (PGE2) → vasodilation of afferent arteriole → increased resistance to glomerular blood flow
- With increased GFR → Increased tubular flow rate → Decreased [Na] uptake by the LoH → Increased [Na+] in macula densa → Juxtaglomerular apparatus secretes adenosine → Vasoconstriction of the afferent arteriole → Decreased blood flow
Note: Flow to Juxtamedullary nephrons is not autoregulated. High blood pressure increases juxtamedullary flow, increasing GFR and impairing renal concentration, resulting in a pressure diuresis.
Extrinsic Control of GFR and RBF:
- Hormonal regulation of blood flow
- Afferent arterioles:
- Dilation → PGE-2, PGI-2, DA, ANP, NO, kinins
- Constriction → High dose AII, NAd, ET-1, Adenosine, ADH, Thirst
- Efferent arterioles:
- Dilation → Inhibition of AT-II
- Constriction → Low dose AT-II
- Mesangial cell → Contracts due to AT-II, ADH and NAd (contraction response inhibited by ANP, DA, PGE2, PGI2)
- Note: Angiotensin II:
- At physiological (low) doses → it maintains GFR by efferent arteriolar vasoconstriction (at expense of RBF)
- With ↑ AT-II levels → causes:
- BOTH afferent and efferent arterioles constriction→ ↓ GFR
and RBF - Mesangial cell contraction in renal corpuscle → ↓ KF → ↓
GFR
- BOTH afferent and efferent arterioles constriction→ ↓ GFR
- Afferent arterioles:
- Neural regulation of renal blood flow (SNS, noradrenergic)
- All renal vescles richly innervated by sympathetic nerves
- 2 Mechanisms:
- Constricts BOTH afferent and efferent arterioles → ↓ RBF >>> GFR
- Stimulates renin secretion (via β1 receptors on JG cells) → ↑ Angiotensin II production → afferent and efferent arteriolar vasoconstriction → ↓ RBF and GFR
- Starling resistors
- Increased intra-abdominal pressure will decrease blood flow in starling resistor model
- Increased intra-capsular pressure will decrease renal blood flow
- High blood amino acid/ glucose level
- High filtered AA/ glucose load → reabsorption in PT with Na → ↓NaCl reaches distal tubules → macula densa → ↓adenosine → vasodilation → ↑RBF
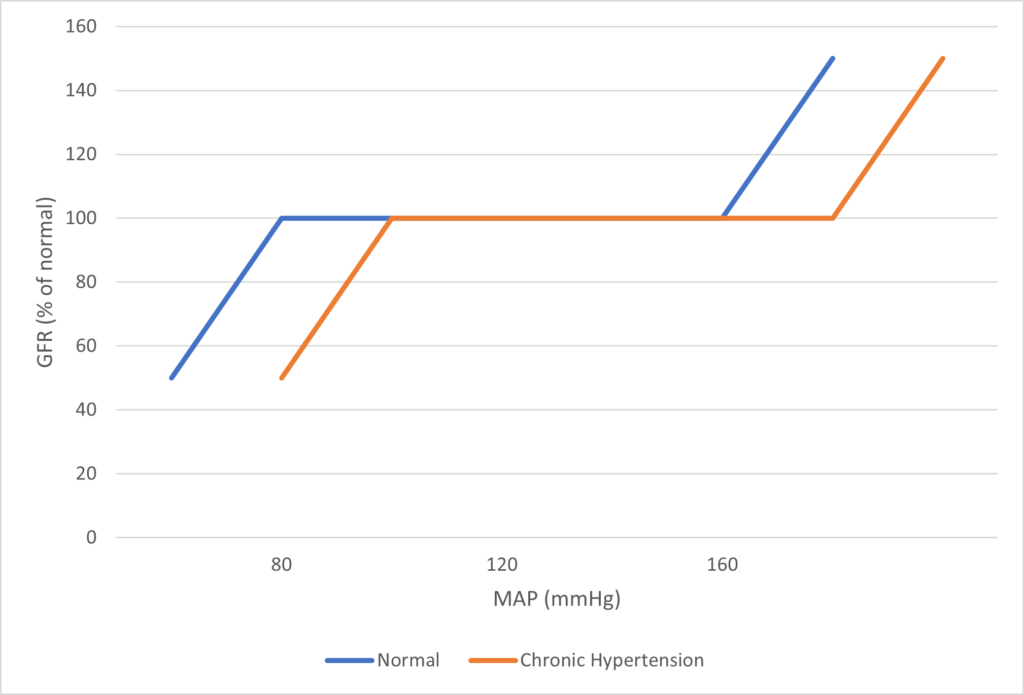
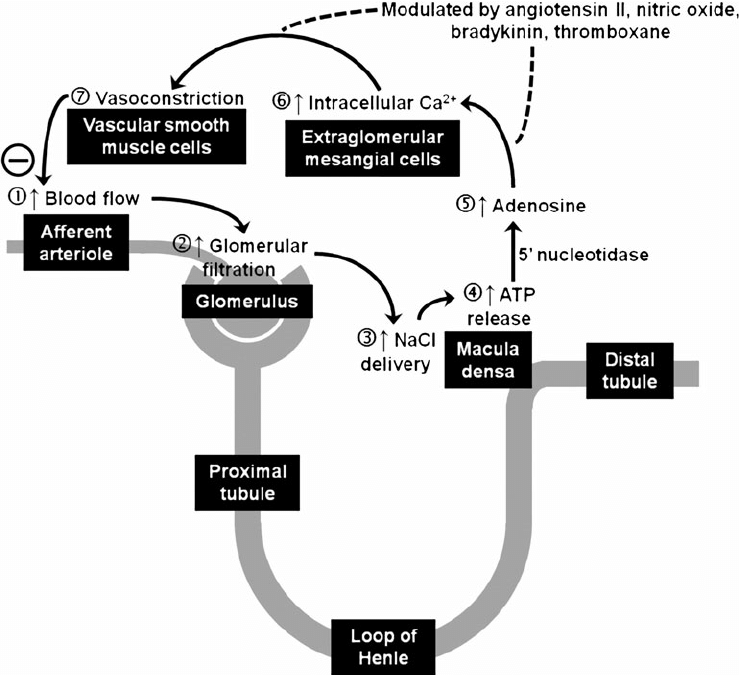
Image Source
JC / Mooney / Sakurai 2019
Examiner Comments
2019A 04: 71% of candidates passed this question.
It was expected that answers include sections on the blood supply, the nephron (including the difference between the cortical and juxta-medullary nephrons) and innervation. A number of candidates failed to quantify renal blood flow and to define autoregulation. The concept that it’s the flow that’s regulated was not described by some. Tubuloglomerular feedback was generally described correctly but a reasonable number had the blood flow increasing when an increased sodium was sensed at the macula densa.
5. Define volume of distribution (15% of marks).
Outline the factors affecting volume of distribution (60% of marks)
and explain how it may be measured (25% of marks).
CICMWrecks Answer
Definition
Volume of Distribution (Vd) = The theoretical volume of bodily fluid in which a drug would need to be distributed following its administration to produce the observed / desired plasma concentration
Mathematically, at time t=0:
Factors Influencing Volume of Distribution
Drug factors
- ↑ Plasma protein binding
- ↓’s Vd
- ↑ Tissue binding
- Binds to tissues → ↓ circulating drug → apparent ↑ Vd
- Physicochemical properties of drug
- ↑ Lipophilicity → ↑ Vd
- ↑ Hydrophilicity → ↓Vd
- ↓ Size → ↑ Vd
- ↓ Charge → ↑ Vd
- pKa
- Basic drug → lower pKa = ↑ unionised portion → ↑ Vd
- Acidic drug → higher pKa = ↑ unionised portion → ↑ Vd
Patient factors
- ↓ pH → ↑’d ionisation of basic drugs → ↓ diffusion → ↓ Vd
- Blood flow
- ↑ blood flow → ↓d concentration → ↑ Vd
- Age
- ↑ Vd in neonates
- Total body water ↑ with
- ↓ age (neonates highest)
- Male gender
- ↑ volume of distribution for water soluble drugs.
- total body fat
- ↓’d in children and men → ↓ Vd of lipophillic drugs
- skeletal muscle bulk
- ↑’d in men → ↑ Vd
- disease
- Sepsis: alterations in pH alter ionised and bound fractions of drugs, increased permeability, organ failure and acute phase reactants all alter protein binding and Vd.
Measurement of Volume of Distribution
It is calculated based on the formula:
Since it is impossible to measure plasma concentration at time zero, an extrapolated plasma concentration is used.
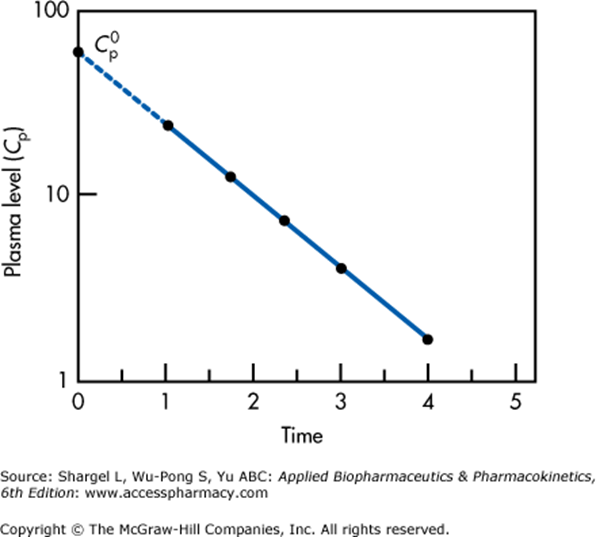
Semilogarithmic plot above illustrates extrapolation of plasma level to time 0 required to determine the volume of distribution
This graph applied for a single compartment model only.
For multiple compartments which will appear as a non-linear relationship, extrapolation back to t = 0 must be performed for each compartment separately.
Gladwin / JC 2019
Examiner Comments
2019A 05: 51% of candidates passed this question.
The first two parts of the question were reasonably done. Most candidates had well-structured answers which included drug factors and patient factors. In addition to listing the factors it was expected candidates state how these factors affect volume of distribution. Explaining how volume of distribution is determined was not so well done.
6. Outline the physiology of the adrenal gland (70% of marks).
Describe the actions of aldosterone (30% of marks).
CICMWrecks Answer
Adrenal Gland
- Wedges of glandular and neuroendocrine tissue adhering to the top of the kidneys by a fibrous capsule.
- Blood flow:
- have one of the greatest blood supply rates per gram of tissue of any organ: up to 60 small arteries may enter each gland
- Artery → Cortex → Medulla → Veins
- Three arteries:
- Superior Suprarenal A. (Br. Of Inferior Phrenic)
- Middle Suprarenal A. (Br. Of Aorta)
- Inferior Suprarenal A. (Br. Of Renal Artery)
- One Suprarenal on each side:
- Rt Suprarenal drains into IVC
- Lt Suprarenal drains into Lt renal V.or Lt Inferior Phrenic V.
The gland can be divided into:
- Adrenal cortex
- component of the hypothalamic-pituitary-adrenal (HPA) axis
- secretes steroid hormones important for the regulation of the long-term stress response, blood pressure and blood volume, nutrient uptake and storage, fluid and electrolyte balance, and inflammation.
- Adrenal medulla
- neuroendocrine tissue composed of postganglionic sympathetic (SNS) neurons
- The sympathomedullary (SAM) pathway involves the stimulation of the medulla by impulses from the hypothalamus via neurons from the thoracic spinal cord.
- The medulla is stimulated to secrete the catecholamine hormones epinephrine and norepinephrine.
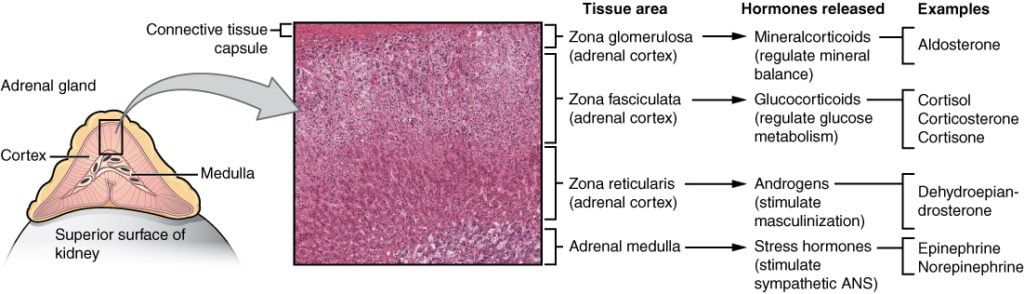
Mineralocorticoids (Aldosterone)
Aldosterone is the key mineralocorticoid hormone, accounting for 95% of mineralocorticoid activity:
- Release is stimulated by:
- Increased serum K+
- Increased Angiotensin II
- Hypovolaemia
- Decreased osmolarity
- Increased ACTH
- Decreased serum pH
- Acts to increase sodium and water retention (and removal of potassium), via:
- Increased expression and activation of Na+/K+ pumps on the basolateral membrane of DCT and CT cells, causing increased Na+ (and water) reabsorption and K+ elimination
- Stimulation of the Na+/H+ pump in intercalated cells on the DCT
- upregulates epithelial sodium channels (ENaCs) in the collecting duct and the colon, increasing apical membrane permeability for Na+ and thus absorption.
- increases Na+ (and water) reabsorption in exchange for K+ (kidney, gut, salivary, sweat)
Sources:
https://opentextbc.ca/anatomyandphysiology/chapter/17-6-the-adrenal-glands/
https://cnx.org/contents/FPtK1zmh@7.28:kaX2y2XZ@3/The-Adrenal-Glands
JC 2019
Examiner Comments
2019A 06: 43% of candidates passed this question.
Lack of breadth and detail were in many of the answers. Physiology of the adrenal gland includes an outline of the adrenal medulla, the types of chromaffin cells, hormones secreted and how secretion is stimulated. The three zones of the adrenal cortex should have been outlined including substances secreted, their function and again how their secretion is stimulated. The actions of aldosterone should have been described; a comment on sodium and water excretion was insufficient to attain many marks for this section. The extra-renal actions of aldosterone were missing from most answers.
7. Compare and contrast the pharmacokinetics and pharmacodynamics of midazolam and dexmedetomidine.
Examiner Comments
2019A 07: 27% of candidates passed this question.
Most candidates used the effective tabular format presenting pharmacokinetics and pharmacodynamics of each drug side by side. Many answers demonstrated a lack of correct detail with respect to the pharmacokinetics and pharmacodynamics of these two level 1 drugs.
Many included pharmaceutics which attracted no marks as it was not asked.
8. Compare and contrast the measurement (40% of marks)
and interpretation (60% of marks)
of both central venous and mixed venous oxygen saturations.
CICMWrecks Answer
Physiology
Oxygen Delivery
= Cardiac output x Arterial oxygen content
Oxygen Demand
= Cardiac output x Arteriovenous O2 content difference
Oxygen flux
Hence SvO2 is a surrogate marker for Oxygen flux
| Decrease in ScvO 2 or SvO 2 | Increase in ScvO 2 or SvO 2 |
|---|---|
| O2 consumption ↑ Stress, Pain Hyperthermia Shivering | O2 delivery ↑ CaO2 ↑ Cardiac output ↑ |
| O2 delivery ↑ CaO2 ↓ (anemia, hypoxia) Cardiac output ↓ | O2 consumption ↓ Analgesia, Sedation Mechanical ventilation Hypothermia |
Measurement: Co-oximetry
Co-oximetry:
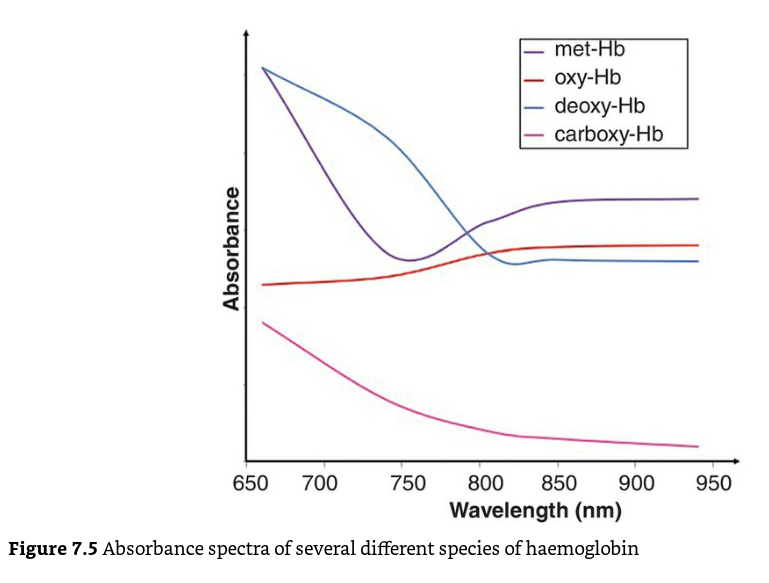
- A co-oximeter is a blood gas analyzer that, in addition to the status of gas tensions provided by traditional blood gas measurements, measures concentrations of oxygenated hemoglobin (oxyHb), deoxygenated hemoglobin (deoxyHb or reduced Hb), carboxyhemoglobin (COHb), and methemoglobin (MetHb) as a percentage of the total hemoglobin concentration in the blood sample
- Different haemoglobin species have different absorption spectra.
Mechanism / Measurement
- Uses Beer-Lambert law to detect different Hb species.
- “Incidence of light is inversely proportional to the path distance and concentration of light absorbing particles within the path”
- multi-wavelength spectrophotometry (measures the absorption of light passing through blood from several dozens of wavelengths)
- Complex, but straightforward internal computations
- enables the instrument to distinguish between oxy-Hb, deoxy-Hb, and carboxyhemoglobin,-COHb, methemoglobin -metHb, other hemoglobin moieties and ‘background’ light-absorbing species
- This is reported as the fractional oxyhaemoglobin content (FO2Hb), which is defined below
- The FO2Hb measure provides a more accurate picture of the availability of oxygen to the tissues in the presence of haemoglobin variants.
- If the original blood sample contained no carboxy-Hb or met-Hb, then the values for FO2Hb and SaO2 would be identical.
Mixed venous vs Central venous O2 Sats
| Mixed Venous Oxygen Sats SvO2 or SmvO2 | Central Venous oxygen sats ScvO2 | |
|---|---|---|
| Measure of Oxygen flux [matching between delivery (DO2), and consumption (VO2)] | Surrogate for SvO2 | |
| Blood drawn from | Proximal Pulmonary Artery More invasive Intermittent only | SVC Less Invasive Can be continuous or intermittent |
| Clinical picture | Average venous oxygenation Balance between systemic delivery and consumption | Venous oxygen saturations of blood from upper body |
| Physiology | SvO2 > ScvO2 as it contains blood from both SVC and IVC Normally 65-70% <65% – impaired tissue oxygenation >80% – hyperoxic or cytotoxic states, shunting | ScvO2 < SvO2 as it contains predominantly SVC blood (which has lower sats than IVC) Normally ~65% <50% tissue hypoxia >75% high flow states |
| Advantages | Systemic picture | No PA catheter required Easily performed in ICU through CVC |
| Disadvantages | Arrhythmias Catheter knotting Perforation of PA Infection | Unreliable correlation with systemic oxygenation in advance sepsis/septic shock Complications of CVC |
| Situations where this value is higher than the other | Normal states | a) Anaesthesia – because of increase in CBF & depression of metabolism b) Patients with head injury where cerebral metab is depressed c) Shock: because of diversion of blood from splanchnic circulation, there is increased O2 extraction and therefore IVC saturation decreases. |
| Other Data Generated | Qt, PA pressures, derived indices and body temperature measurements | CVP |
| Evidence | Study by Gattinoni – only RCT as far as SvO2 is concerned showed no benefit from SVO2 monitoring Study by Jones – showed trending lactate clearance was non-inferior | Study by Rivers- early goal directed therapy improved outcome in septic shock. Newer studies – ProMISE, ProCESS, ARISE have shown no benefit (or harm) from EGDT. |
Sources/Further Reading:
Bloos, F., Reinhart, K. Venous oximetry. Intensive Care Med 31, 911–913 (2005). https://doi.org/10.1007/s00134-005-2670-9
CICM Examiner’s Report Fellowship Paper 2 2006
JC 2020
Examiner Comments
2019A 08: 8% of candidates passed this question.
Many candidates did not appreciate that ScvO2 refers to SVC / RA junction venous oximetry and not femoral or peripheral venous oximetry. Methods of measurement such as co-oximetry and reflectance spectrophotometry needed to be explained. Marks were awarded for the normal values. Discussion of the relationship between ScvO2 and SmvO2 and changes during shock attracted marks. Better answers quoted the modified Fick equation and related this to cardiac output and factors affecting oxygen consumption versus delivery.
9. Classify antibiotics with respect to their mechanism of action (50% of marks).
Outline the mechanisms of antimicrobial resistance (50% of marks).
Give specific examples of each.
CICMWrecks Answer: Antibiotic Classification by MoA
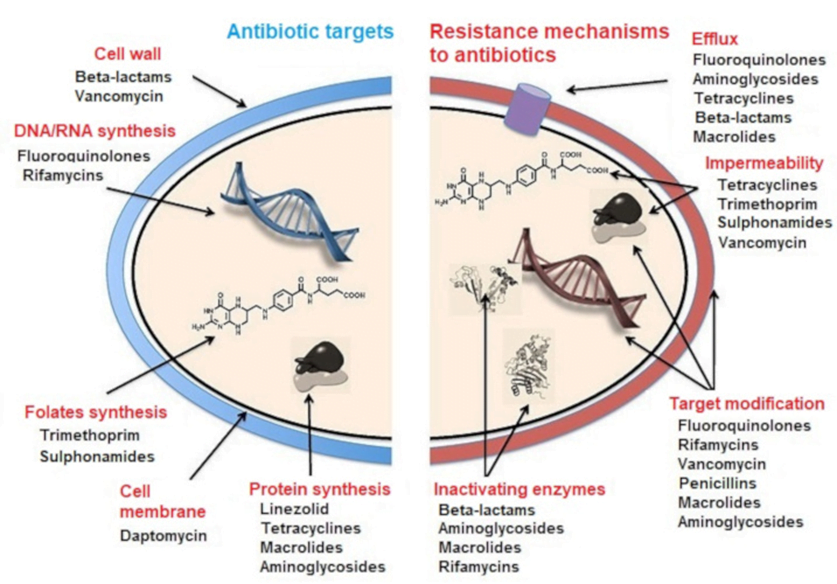
CLASSIFICATION OF ANTIBIOTICS
| 1. Inhibitor of cell wall synthesis/ Peptidoglycan Inhibitors: | · Beta-lactams: Penicillin · Cephalosporins: Ceftriaxone · Carbapenems: Meropenem · Glycopeptides: Vancomycin |
| 2. Inhibitor of Nucleic acid synthesis: | · Quinolones: Ciprofloxacin · Rifamycins: Rifampicin · Nitroimidazoles: Metronidazole · Nitrofurantoin |
| 3. Inhibitor of folic acid synthesis (Folate antagonistic) | · Sulfonamides: Sulfamethoxazole · Aminopyrimidines: Trimethoprim |
| 4. Inhibitor of cytoplasmic membrane: | · Lipopeptide: Daptomycin · Polymyxins: Colistin |
| 5. Inhibitor of protein synthesis: | · Aminoglycosides: Gentamicin · Lincosamides: Clindamycin · Macrolides: Erythromycin · Tetracyclines: Tetracycline |
Sources:
Microbiology Lippincott Williams & Wilkins
https://courses.lumenlearning.com/microbiology/chapter/drug-resistance/
https://www.encyclopedie-environnement.org/en/health/antibiotics-antibiotic-resistance-and-environment/
Gladwin / Sakurai / JC 2019
CICMWrecks Answer: Antibiotic resistance

MECHANISMS OF ANTIBIOTIC RESISTANCE
- Mechanisms of Antibiotic Resistance can be classified broadly:
- Efflux Pumps
- Blocked Penetration / Alteration in access to target site
- Target Modification
- Modification of Drug or pathways
- There might be multiple resistance mechanisms at play in the same organism
| Efflux Pumps: Increased efficiency or expression of efflux pumps (inner membrane proteins). | |
| Removed from cell | Active transport of ciprofloxacin out of the bacterial cell |
| E.Coli to tetracycline. | |
| Trapped between cell wall layers | glycopeptide resistance in VRSA. |
| Blocked Penetration / Alteration in access to target site | |
| Narrowing of porin channels | Streptococcal resistance to penicillins typically occurs by reducing access to PBPs. |
| Alteration of porins in gram negative outer membranes | downregulation of Outer Membrane Proteins (eg enterobacteriaceae, pseudomonas vs penems/cephs) |
| increased selectivity of Outer Membrane Proteins (eg klebsiella outbreaks) | |
| Loss of non-essential transporter channels | Anaerobes have no oxygen-transport channel which prevents penetration by aminoglycosides (Gentamicin) |
| Reduced binding of the antibiotic | VanA and VanB vancomycin resistance involves a gene mutation leading to decreased affinity of vancomycin for the binding sites of peptidoglycan precursors |
| Changes to the DNA-binding surface of DNA supergyrase infers resistance against ciprofloxacin | |
| Target Modification | |
| Modifying the enzyme that the antibiotic inhibits | Rifampicin resistance by point mutations, insertions, or deletions in RNA polymerase gene |
| Production of an alternative enzyme for that which the antibiotic inhibits | MRSA develop or acquire the gene mecA which synthesizes an additional penicillin binding protein that enables it to continue cell wall synthesis in the presence of a beta lactam drug |
| Overproduction of the target of the antibiotic | Trimethoprim DHFR enzyme overproduction in Escherichia coli and Haemophilus influenzae. |
| Synthesis of target-protecting proteins | Ribosomal protection proteins against Tetracyclines in multiple Gram positive and gram negative bacteria |
| Modification of Drug or pathways | |
| Development of metabolic pathways to bypass site of action of antibiotic | Resistance to TMP-SMX by allowing bacteria to synthesize or absorb folic acid. |
| Enzymes produced to Metabolize the drug | β-lactamases and cephalosporinases hydrolyse β-lactam rings |
| Enzymes produced that add a chemical group to antibiotic to inhibit its activity | Aminoglycoside resistance by Staphylococcus aureus or Pseudomonas (Multiple enzymes acetyltransferase, adenyltransferase, and phosphotransferase) |
Spread of Bacterial Resistance
- Selective pressure selects for favourable mutations of resistance
- mosaic genes (from other bacteria eg strep pneumo from strep mitus) or uptake of DNA from environment
- transfer of resistant bacteria from person to person
- horizontal gene transfer;
- transduction – acquisition of bacterial DNA from a phage (a virus that propagates in bacteria);
- transformation – uptake and incorporation of free DNA released into the environment by other bacterial cells;
- conjugation – gene transfer (usually on plasmids), by direct cell-to-cell contact through a bridge.
Sources:
Microbiology Lippincott Williams & Wilkins
https://courses.lumenlearning.com/microbiology/chapter/drug-resistance/
https://www.encyclopedie-environnement.org/en/health/antibiotics-antibiotic-resistance-and-environment/
Gladwin / Sakurai / JC 2019
Examiner Comments
2019A 09: 70% of candidates passed this question.
This question was well answered. Marks were awarded for correct pairing of mechanism of action and resistance with examples of drug class. Few mentioned the mechanisms by which resistance is present; acquired or generated.
10. Outline the sequence of haemostatic events after injury to a blood vessel wall (50% of marks).
Discuss the role of naturally occurring anticoagulants in preventing clot formation in-vivo (50% of marks).
CICMWrecks Answer: Haemostatic events after injury
- Haemostasis is the natural process that stops blood loss when an injury occurs.
- Intact vascular endothelial cells:
- fibrinolytic heparin, Thrombomodulin → prevent clotting
- NO, Prostacyclin → Prevents clotting cascade
Haemostasis following vessel injury
Three steps: Vascular spasm (vasoconstriction), platelet plug formation and coagulation.
- Vasoconstriction:
- Brief reflexive contraction that decreases local blood flow
- Caused by signalling molecules from injured endothelial cells, thromboxane A2 from activated platelets, Nervous system reflexes from local pain receptors
- Vasoconstriction only lasts for a few minutes during haemostasis. During inflammation that follows the injury, it is replaced by vasodilation as the healing process begins.
- Platelet Plug Formation (Primary Haemostasis):
- Within 20 seconds, coagulation is initiated
- Platelet Adherence: vWF released from damaged endothelium → change platelet form → adhere to subendothelial collagen
- Platelet Activation: subendothelial collagen binds to platelet receptors → activates platelets → degranulate → release ADP, vWF, TXA2, PDGF, VEGF, Serotonin, Coagulation factors
- Platelet Aggregation: Platelets bind to vWF and fibrinogen → aggregate over damaged endothelium
- Positive feedback mechanism
- Platelet plug formed in seconds to a few minutes based on extent of injury
- Coagulation Cascade (Secondary Haemostasis):
- Occurs if platelet plug ineffective in controlling bleeding
- Platelets degranulate → release ADP, Serotonin, Thromboxane A2
- Coagulation cascade: Intrinsic, Extrinsic pathway → Common pathway
- Intrinsic (Contact Activation) Pathway: Primary complex (on collage by High molecular weight kininogen, prekallikrenin, factor XII) → XI → IX (which, along with VIII) → Common pathway
- Extrinsic (Tissue factor) Pathway: Tissue factor III → VII → Generates thrombin burst – cleaves fibrinogen to fibrin
- Common pathway: Prothrombin(II) to thrombin (using Factor V) → cleaves fibrinogen to fibrin
- forms mesh that binds and strengthens platelet plug → coagulation → haemostasis
- Also activates factor XIII – covalently bonds to fibrin to strengthen attachment to platelets
- Also activates more factor V which acts as anticoagulant with inhibitor protein C
Fate of the Clot:
One of four outcomes:
- Propagation: Accumulation of additional platelets and fibrin
- Embolization: Thrombus breaks free and becomes mobile
- Dissolution: Fibrinolysis (aided by tissue plasminogen activator, tPA)
- Organization and recanalization: Ingrowth of smooth muscle cells, fibroblasts and endothelium into fibrin-rich thrombus.
Clot lysis and Wound healing:
Over the course of the next few days:
- Clot Retraction: blood clot shrinks.
- dependent on the release of multiple factors, mostly factor XIIIa crosslinks
- Cause contraction, knotting and twisting of fibrin mesh
- Blood clot shrinks
- Fibrinolysis – Plasmin degrades fibrin to Fibrin degradation products, macrophages consume the expended platelets. FDPs inhibit further thrombin and fibrin formation
- Wound Healing:
- Inflammation – Tissue proliferation – Collagen and granulation tissue deposition – angiogenesis – Wound contraction – Epithelialization
Source: https://courses.lumenlearning.com/boundless-ap/chapter/hemostasis/
JC 2019
CICMWrecks Answer: Naturally occurring anticoagulants
Naturally Ocurring Anticoagulants:
Prevent unnecessary coagulation.
| Protein C | a vitamin K-dependent serine protease enzyme that degrades Factor V and factor VIII. |
| Protein S | Cofactor which helps protein C. |
| Heparin | fast-acting anticoagulant expressed on endothelial cells, inhibits activity of thrombin |
| Antithrombin III | a serine protease inhibitor that degrades thrombin, Factor IXa, Factor Xa, Factor XIa, and Factor XIIa |
| Tissue factor pathway inhibitor (TFPI) | limits the action of tissue factor (TF) and the factors it produces. |
| Plasmin | generated by proteolytic cleavage of plasminogen, a potent fibrinolytic that degrades fibrin and destroys clots. |
| Prostacyclin (PGI2) | released by the endothelium and inhibits platelet activation. |
| Thrombomodulin | released by the endothelium and converts thrombin into an inactive form. |
Source: https://courses.lumenlearning.com/boundless-ap/chapter/hemostasis/
JC 2019
Examiner Comments
2019A 10: 40% of candidates passed this question.
This question was best answered in a chronological manner. Many candidates omitted initial vasoconstriction and its mechanism. The platelet plug and formation of the clot should have then been described followed by the fate of the clot, including in-growth of fibroblasts. Strictly, fibrinolysis is a system for repairing / limiting clot propagation after the fact. Anticoagulants refer to antithrombin III, heparin, thrombomodulin and protein C and S. An explanation of the interaction of these naturally occurring anticoagulants was expected. The clotting factors that are specifically inhibited was expected as part of the discussion. The glycocalyx and vessel wall also plays a role in preventing coagulation.
11. Describe the physiology of cerebrospinal fluid (CSF) (60% of marks).
Describe the anatomy relevant to performing a lumbar puncture (40% of marks).
CICMWrecks Answer: Physiology of CSF
Formation / Production of CSF
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
Distribution / Circulation of CSF
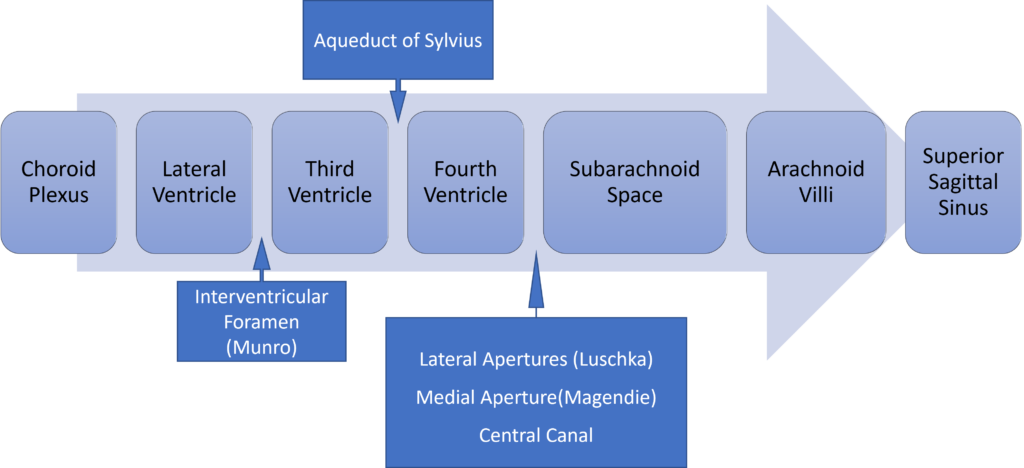
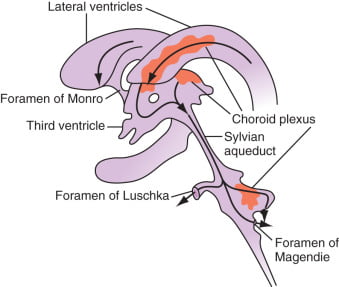
Absorption of CSF
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
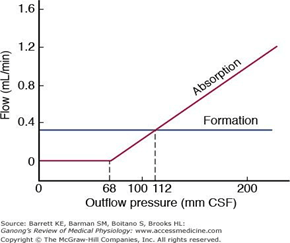
Composition of CSF
IDENTICAL to brain ECF, but differs in several manners from plasma
Compared with plasma:
- ↑ pCO2 (50 mmHg)
- ↓ pH (7.33)
- ↓ protein content
- 0.5% of plasma; 20 mg/dL
- poor acid-base buffering capacity
- ↓ content of glucose (by 60%) and cholesterol
- ↑ [Cl-] (by 7-14%) and ↑ [Mg2+] (by 40%)
- ↓ [K+] (by 40%), ↓ [Ca2+] (by 50%) and ↓ [Pi] (by 20-30%)
- ↑ creatinine (by 25%) but ↓ urea
- IDENTICAL osmolality (295), [Na+] (145), and [HCO3] (25)
Role / Functions of CSF
- Protective role (main function)
- Water bath effect
- Attributed to the low specific gravity of CSF (1.007)
- Causes brain to be buoyant
- ↓ its effective net weight from 1400 g to 50 g
- Mechanical cushion against acceleration/deceleration forces
- Buffer ↑ ICP by CSF translocation to extracranial subarachnoid space
- Abrupt ↑ ICP buffered by translocation of CSF within the vault to extracranial compartments
- Water bath effect
- Maintains constant ionic environment conducive to neuronal electrical activity CSF
- Supply role of nutrients (Eg simple sugars, amino acids) and O2 to brain
- Excretion of toxic substances, metabolic by-products, and CO2 from brain
- “Lymph-type” function → interstitial proteins in brain ECF return to circulation by
- CSF absorption across arachnoid villi
- Acid-base regulation → due to content, CSF allows for tight respiratory control
- Endocrine transport function → transports hormones to other brain regions
Gladwin / JC 2020
CICMWrecks Answer: Anatomy of LP
Anatomy:
- Position in sitting or lateral decubitus position
- Level:
- Any of the interspaces between between L2-L5
- L4/5 interspace: Tuffier’s Line: line between iliac crests
- (or) L3/4 interspace: Line joining PSISs (Posterior superior iliac spines)
- Discrepancy between identified and actual intervertebral space in 50% of cases
- Conus medullaris ends at L1 in about 94% of patients
Tissues and target for LP:
- In the subarachnoid space between the arachnoid mater and the pia mater.
- The tissues pierced are (in order):
- skin
- subcutaneous tissue
- supraspinal ligament,
- interspinal ligament,
- ligamentum flavum,
- dura mater,
- the arachnoid mater into the subarachnoid space.
- Lateral/paraspinal approach: Skin – subcutaneous tissue – erector spinae muscles – ligamentum flavum – dura – arachnoid – subarachnoid space
Epidural Space
- Posterolateral Epidural Space
- Posterolateral epidural space extends vertically down the spinal canal and contains arteries, venous plexus, and fat
- Posterolateral epidural space is larger than the anterior epidural space
- Posterolateral epidural space is larger in the sacral region than it is in the cervical region
- Anterior Epidural Space
- Anterior epidural space is a virtual space under normal circumstances (due to adherence of dura to bone of vertebral bodies from the foramen magnum down to L1)
Gladwin / JC 2020
Examiner Comments
2019A 11: 86% of candidates passed this question.
Better answers had a structure with headings such as function, formation, circulation, absorption and composition with dot point facts under each heading. The second part of the question lent itself to a diagram with labelling which scored well. Precise surface anatomy and mentioning all layers from the skin to the sub-arachnoid space scored well.
12. Compare and contrast the pharmacology of salbutamol and ipratropium bromide.
Examiner Comments
2019A 12: 46% of candidates passed this question.
Overall candidates had a superficial knowledge of these level 1 drugs. To pass candidates
needed to identify points of difference and overlap in various areas such as structure,
pharmaceutics, pharmacokinetics, pharmacodynamics, mechanism of action, adverse effects
and contraindications.
13. Classify circulatory shock and provide examples (40% of marks).
Outline the cardiovascular responses (60% of marks).
CICMWrecks Answer
Shock
Shock is defined as a state of cellular and tissue hypoxia due to reduced oxygen delivery and/or increased oxygen consumption or inadequate oxygen utilization. This most commonly occurs when there is circulatory failure manifested as hypotension (ie, reduced tissue perfusion).
Distributive:
Distributive shock is characterized by severe peripheral vasodilatation (vasodilatory shock)
e.g: Sepsis with shock
Cardiogenic:
Cardiogenic shock is due to intracardiac causes of cardiac pump failure that result in reduced cardiac output
e.g: Cardiomyopathy from MI
Hypovolemic:
Hypovolemic shock is due to reduced intravascular volume (ie, reduced preload), which, in turn, reduces CO.
e.g: Haemorrhage
Obstructive:
Obstructive shock is mostly due to extracardiac causes of cardiac pump failure and often associated with poor right ventricle output.
e.g: Pulmonary Embolism
Mixed / Unknown:
Unknown or more than one cause of shock contributing.
e.g: Pancreatitis with Septic (Distributive) and Hypovolemic shock
Cardiovascular Responses
- Arterial Baroreceptor Reflex:
- Short term regulation of arterial blood pressure
- Sensor: Stretch sensitive baroreceptor nerve endings in walls of arteries, nonarterial baroceptor units
- Senses: pressure (stretch)
- Integrator: Medullary cardiovascular centres
- Efferent pathways: Cardiovascular sympathetic and cardiac parasympathetic
- Effector organs: Heart, peripheral blood vessels
- Effect: Operates such that an increase in arterial pressure leads to an essentially immediate decrease in sympathetic nerve activity and a simultaneous increase in parasympathetic nerve activity (and vice versa).
- Reflexes from Receptors in Heart and Lungs:
- Mechanoreceptors and chemo receptors in atria, ventricles, coronaries, lungs
- Neurohumoral control of CVS
- Regulate blood volume and body fluid balance
- Chemoreceptor reflexes:
- Hypoxia (low Po2), hypercapnia (high Pco2), and/or acidosis (low pH) levels in the arterial blood cause reflex increases in respiratory rate and mean arterial pressure
- Arterial Chemoreceptors in carotid bodies at bifurcation of CCAs, and aortic bodies in arch of aorta
- Central Chemoreceptors in CNS respond to hypercapnia and acidification of CSF to regulate resp patterns and influence autonomic output
- Exercise responses:
- Reflexes from Receptors in Exercising Skeletal Muscle
- Chemoreceptors respond to muscle ischaemia
- Increased blood pressure
- Central command
- Input from cerebral cortex to lower brain centres during voluntary exercise simultaneous to cortical drives initiating exercise
- Cause reflex tachycardia and increased arterial pressure
- Reflexes from Receptors in Exercising Skeletal Muscle
- The Dive Reflex
- Bradycardia produced by enhanced cardiac parasympathetic activity and peripheral vasoconstriction caused by sympathetic activity
- Cardiovascular Responses associated with Emotion and Stress
- Originate in cerebral cortex – corticohypothalamic pathways – medullary cardiovascular centers
- Response is complex and depends on multiple factors
- E.g blushing, excitement, vasovagal syncope
- Reflex responses to Pain
- Superficial pain: rise in BP
- Deep pain: response similar to vasovagal syncope with dec sympathetic tone, inc parasympathetic tone, dec BP/shock
- Temperature Regulation Reflexes
- Controlled by hypothalamus, operate through cardiovascular centers, discretely control sympathetic activity
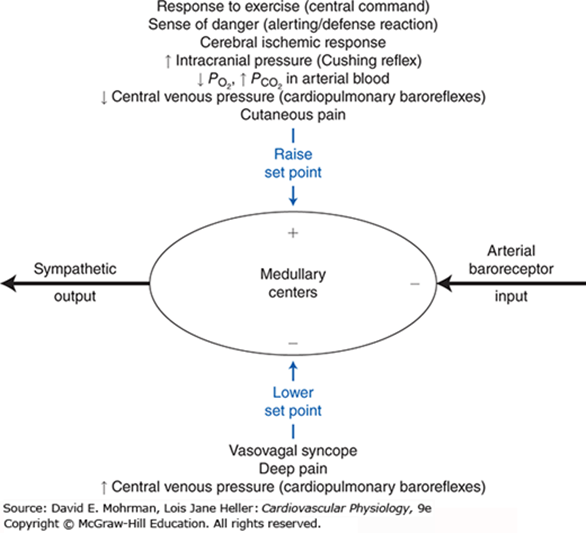
JC 2019
Examiner Comments
2019A 13: 83% of candidates passed this question.
Answers should have included the various types of shock and provided clear examples.
Cardiovascular responses including sensor, integrator, effector mechanisms were necessary to pass.
14. Compare and contrast the mechanism of action, pharmacokinetics and adverse effects of digoxin and sotalol.
Examiner Comments
2019A 14: 19% of candidates passed this question.
Good answers listed class and the multiple mechanisms of action for both these antiarrhythmics, briefly outlining relevant downstream physiological effects and contrasting effects on inotropy. Knowledge of specific pharmacokinetic parameters of these agents was generally lacking. Clinically relevant adverse effects were frequently omitted (e.g. prolonged QT/Torsades for sotalol, hypokalaemia potentiating toxicity of digoxin).
15. Describe the physiology of the NMDA (N-Methyl D-aspartate) receptor (40% of marks).
Outline the pharmacology of ketamine (60% of marks).
CICMWrecks Answer: NMDA Receptor
N-Methyl D-Aspartate (NMDA) Receptor
- ligand-gated voltage dependent ion channel
- Natural ligand = glutamate
- Associated with the family of glutamate receptors (AMPA, kainite and neurokinin)
Structure:
- transmembrane receptor
- 5 subunits forming central cation ionophore
- at baseline Mg2+ plugs and inhibits central cation pore
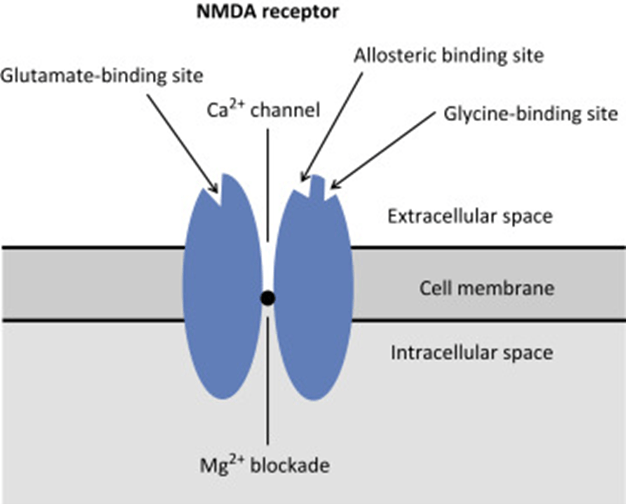
- Location: abundant throughout brain (esp. hippocampus) and spinal cord (esp. dorsal horn) on post-synaptic membranes
- Activation: NMDA receptor contains central Mg2+ plug, which block channel at rest
- Glycine binding + voltage stimulus are required (via activation of adjacent AMPA and neurokinin receptors) → remove Mg plug → primes NMDA receptor for activation by glutamate
- When channel activated→ opens cation channel → ↑cation conductance (Ca2+ and Na+ in; K+ out) → excitatory postsynaptic potential
Physiological Roles of NMDA Receptors in the CNS
- Wind up phenomenon
- Wind up = repeated stimuli of same strength cause increase in pain response
- Proposed mechanism: C fibres synapse in lamina II of dorsal horn → repeated stimulation → glutamate release → NMDA activation → ↑response of dorsal horn neurons to excitatory neurotransmitter input àhyperalgesia and allodynia
- Long term potentiation
- LTP = strengthening of synaptic transmission that occurs following ↑activity across that synapse
- i.e. recurrent painful stimuli → neuroplasticity → chronic pain
- NMDA receptor stimulation leads to various intracellular changes:
- production of NO
- activate second messengers (IP3, DAG, cGMP, PKC)
- PKC → increases NMDA activity → vicious cycle
- second messengers induce oncogenes (e.g. c-fos)
- Memory
- NMDA receptors are highly expressed in hippocampus and throughout cortex Important roles in neuroplasticity and memory formation
- Apoptosis
- Post cerebrovascular accident → significant NMDA activation → ↑↑Ca influx → excitotoxicity → triggers neuronal apoptosis
CICMWrecks Answer: Ketamine Pharmacology
Pharmacology of Ketamine
Examiner Comments
2019A 15: 49% of candidates passed this question.
The NMDA receptor is a ligand gated voltage dependent ion channel located on post synaptic membranes throughout the CNS, with glutamate, an excitatory neurotransmitter, its natural ligand. A brief description of its structure, roles of glycine and magnesium, ions conducted, result of activation, role in memory and learning and agonists/antagonists was expected. Detail on structure and functions of the receptor were a common omission.
Ketamine, a phencyclidine derivative, is a non-competitive antagonist at the NMDA receptor. It is presented as a racemic mixture or as the single S(+) enantiomer (2-3 X potency).
Administration routes and doses scored marks. Pharmacodynamics were generally well covered including CVS (direct and indirect effects), CNS (anaesthesia, analgesia, amnesia, delirium, effects on CBF and ICP) respiratory (bronchodilator with preservation of airway reflexes) GIT effects (salivation, N and V). Knowledge of specific pharmacokinetic parameters was less well covered, including low oral bioavailability and protein binding and active metabolite (norketamine).
16. Describe the role of carbon dioxide in the control of alveolar ventilation.
CICMWrecks Answer
Role of CO2 in control of alveolar ventilation
Sensors
Central chemoreceptors
Located in the medulla, just below the central surface
Stimulated by increased pH of CSF. Not stimulated by low PO2
CO2 crosses the blood brain barrier easily
-> combines with water to make carbonic acid
-> dissociates to produce hydrogen ions lowering pH
Peripheral chemoreceptors
Carotid bodies at bifurcation of common carotid artery (supplied by glossopharyngeal nerve)
Aortic bodies (supplied by the vagus nerve)
Sense changes in PO2, PCO2 and pH (not in aortic bodies)
Globus cells release neurotransmitters (dopamine, noradrenaline, acetylcholine)
-> stimulates afferent nerves -> medulla -> increased ventilation
Lung receptors, Baroreceptors
No role of CO2
Central input
There is input from the hypothalamus and cortex, with the ability of the cortex to override the medulla and bring ventilation under voluntary control
Controller
- Medullary respiratory centre
- Dorsal group
- Part of nucleus tractus solitarius
- Timing of ventilation
- Ventral group
- In nucleus ambiguous and nucleus retroambigualis
- Controls inspiration
- Botzinger complex
- Rostral to nucleus ambuguus
- Controls expiration (inactive at quiet breathing)
- Dorsal group
- Inspiratory phase:
- Gradual ramping up of nerve activity – ↑muscle contraction
- Expiratory phase I:
- Gradual reduction of nerve activity – ↓muscle contraction
- Expiratory phase II:
- Inspiratory muscles inactive
- If increased respiratory drive, expiratory muscles are activated
Effectors
Mainly diaphragm (via phrenic nerves)
Also intercostal muscles and abdominal wall (forced expiration)
Effect of CO2 on Alveolar Ventilation
- ↑ CO2
- ↑ RR + ↑ Depth of breathing → Steady state hyperventilation in few mins
- Linear response in usual range ( MV ↑ 2L/min for 1mmHg rise on PaCO2)
- Max Ventilatory stimulation at ~100mmHg → Respiratory fatigue, CO2 narcosis
- ↓ CO2
- ↓ Alveolar ventilation
- Once PaCO2 <30mmHg
- Some reduce ventilation to point of apnoea
- Some continue to breath (Due to cortical control of respiration especially when awake)
- Responses may be blunted by partial neuromuscular blockade, Restrictive or obstructive lung disease, airway obstruction
- Prolonged periods of CO2 retention:
- → Active secretion of bicarb into CSF → CSF pH normalized → central chemoreceptor stimulation ceases
- → renal reabsorption of bicarb → normalizes arterial pH normalized → ↓ peripheral chemoreceptor stimulation
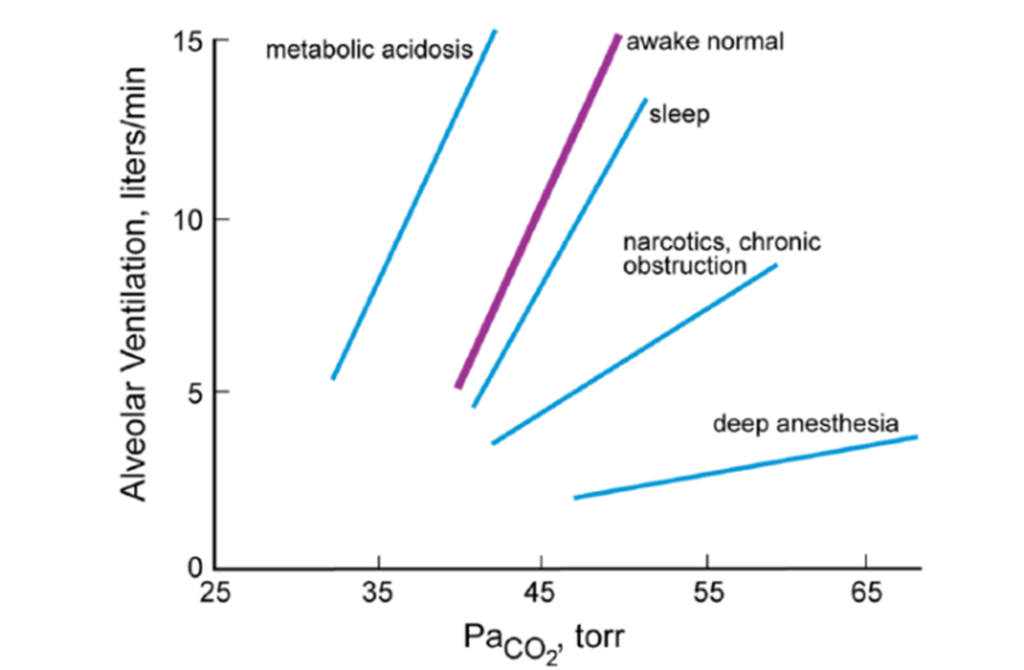
Source: Pulmonary Physiology, 9e. Michael G. Levitzky
References: Nunn’s Applied Respiratory Physiology, Pulmonary Physiology.
Mooney / JC 2019
Examiner Comments
2019A 16: 57% of candidates passed this question.
Better answers considered the role of CO2 in the control of alveolar ventilation in terms of sensors, central processing and effectors – with an emphasis on sensors. Features of central and peripheral chemoreceptors should have been described in detail. The PCO2/ventilation response curve is best described using a graph, with key features of the curve identified (including gradient and axes). Various factors affecting the gradient of this curve and how CO2 affects the response to hypoxic drive should be described.
17. Explain the physiology of neuromuscular transmission.
CICMWrecks Answer
Neuromuscular Transmission
- In nerve cells, a reduction in cell membrane potential above RMP (ie. – 70mV) results in a voltage dependent increase in Na+ permeability.
- the wave of excitation moves along the axon unidirectionally at a constant amplitude & velocity.
- At the neuromuscular junction presynaptic motor axons terminate 30 nanometers from the cell membrane or sarcolemma of a muscle fiber.
- The sarcolemma at the junction has invaginations called postjunctional folds, which increase its surface area facing the synaptic cleft.
- These postjunctional folds form the motor endplate, which is studded with nicotinic acetylcholine receptors (nAChRs)
Neuromuscular Junction
Acetyl Choline:
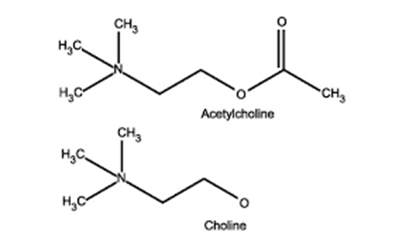
- stored in vesicles within the terminal button
- approx. 10,000 molecules in each vesicle
- 3 pools (1% immediately releasable, 80% readily releasable, rest stationary store)
- Ach inhibits Choline Acetyltransferase
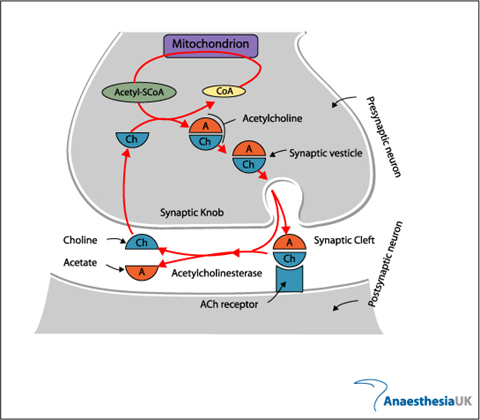
- Vesicles exocytose in response to depolarization of presynaptic terminal and influx of calcium
- Acetylcholine released into the synaptic cleft
- Pyruvate in Mitochondria → Acetyl-CoA
- Choline – 50% from extracellular (diet) + 50% recycled from Ach
ACh Receptor
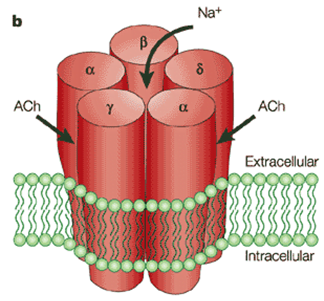
- Nicotinic subtype
- 5 polypeptide subunits – ring structure with central pore
- Two α (Bind ACh), One β, One g, One δ
- Locations:
- Post junctional membrane receptors on motor end plate
- Extra-junctional
- Pre-junctional
Activation:
- Ach bind to alpha subunits → opens the ion gated channed
- Movement of all cations, but mainly sodium → depolarizes membrane
- Once reaches threshold of -50mV (from resting of -80) → voltage gated sodium channels open → rapid depolarization → end plate potential (EPP) of 50-100mV
- This triggers muscle action potential → muscle contraction
- Muscle AP propogates along muscle membrane to cause contraction
- Pre-junctional receptors → positive feedback → increased Ach by second messenges system
- Play role in “fade” seen in non-depolarizing muscle blockers
Fate:
- Acetyl choline hydrolysed to choline and acetate by AChE (Acetyl Choline Esterase)
- Breakdown is very rapid, so the inward current through receptor is transient, followed by rapid repolarisation to resting state
Sources:
AnaesthesiaUK
Arthur Karlin, Nicotinic Acetylcholine receptors, Nature reviews Neuroscience
JC 2019
Examiner Comments
2019A 17: 60% of candidates passed this question.
Description of sequential events from axon conduction to detail at the neuromuscular junction was required. Well-constructed answers defined neuromuscular transmission, elucidated the structure of the neuromuscular junction (best done with a detailed diagram), described the central importance of acetylcholine, including synthesis, storage, receptors, and degradation. An ideal answer also described both pre-synaptic (e.g. voltage-gated calcium channels, exocytosis of vesicles) and post-synaptic events (acetylcholine receptors, end plate potentials, and the events that lead to excitation-contraction coupling in skeletal muscle).
18. Describe the pharmacology of frusemide.
Examiner Comments
2019A 18: 13% of candidates passed this question.
The majority of answers were well structured, some using tables and others using key headings. In general, for a commonly used drug that is listed in the syllabus as Level 1 of understanding, detailed information was lacking. In particular, mechanism of action, dose threshold and ceiling effect and pharmacokinetics lacked detail and/or accuracy.
19. Describe the effects of ageing on the respiratory system.
CICMWrecks Answer
Basic Changes
- Limited reserve
- ↓ Airway protective reflexes
- ↓ Respiratory Muscle Strength
- ↓ Maximal inspiratory pressure, Transdiaphragmatic pressure
- ↓ Maximum voluntary ventilation
- ↓ Elastic recoil of lung tissue (changes in the spatial arrangement and/or crosslinking of the elastic fibre network)
- Stiffening of chest wall and calcification of costal cartilages, ↓ in size of intervertebral spaces
- Loss of alveolar surface area and pulmonary capillary blood volume
- Compliance
- Chest wall compliance decreased (due to stiffening and structural changes)
- Lung compliance – normal or increased
- Total respiratory compliance normal or decreased
Static Respiratory Compliance (= Slope)
W = Chest wall
L = Lung
RS = Respiratory system
A – 20yr old man
B – 60 year old man
In elderly:
Compliance ↓ , RV & FRC ↑ , Static recoil pressure ↓
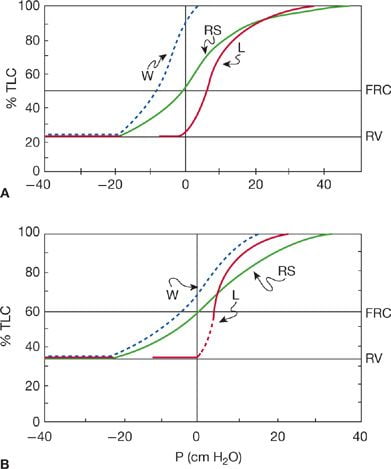
Static pressure–volume curves of the lungs.
Static recoil pressure (~transpulmonary pressure) plotted against lung volume
Lung Function
PFT:
- ↓ FEV1 (non-linear, accelerating in decline after 70 yrs)
- ↓ FVC
- TLC unchanged
- ↓ VC
- ↑ FRC (due to structural chest wall changes – kyphosis, increasing AP diameter)
- ↑ RV (Increased air trapping)
- ↑ CV/CC → airways close during normal TV (due to reduction in supporting tissues)
Gas Exchange:
- Anatomical dead space increases
- Ventilation-perfusion matching is increasingly mismatched
- Increase in units with low V/Q (dependent parts)
- pO2 falls ~ 0.3mmHg/yr
- Decreased Diffusion capacity
Others
Exercise capacity
- ↓ VO2 max
- ↑ Dead space ventilation
Immunology
- Bronchial fluid
- ↑ Neutrophils %
- ↑ Ratio of CD4+/CD8+ cells
- ↓ Epithelial lining fluid antioxidants
Regulation of Breathing
- Diminished Ventilatory response to hypoxia and hypercapnoea
- Increased oxygen cost of breathing for a given Minute ventilation
- Obstructive pattern develops even in lifetime non-smokers
Source: https://thoracickey.com/aging-of-the-respiratory-system/
JC 2019
Examiner Comments
2019A 19: 5% of candidates passed this question.
Answers should have included the effects of ageing on the efficiency of gas exchange, how the expected PaO2 changes with age, and its causation. Anatomical changes should have been included as should changes in lung volumes, particularly the significance of an increased closing volume. Marks were not awarded for the effects of disease states.
20. Describe the cardiovascular effects of positive pressure ventilation on a patient who has received a long acting muscle relaxant.
CICMWrecks Answer
Positive Pressure Ventilation
- PEEP = Positive End Expiratory Pressure.
- Equivalent to a constant pressure applied throughout the respiratory cycle.
- Intrinsic PEEP = unintentional or un-measured end-expiratory hyperinflation
- Physiological effects of Positive Pressure Ventilation mostly related to increased mean airway pressure
Cardiovascular Effects
- Causes constant ↑ intrathoracic pressure (ITP) throughout respiratory cycle
- Left Heart
- On Preload
- Initially: → ↑ LV PL → ↑ CO
- Secondarily: → ↓RV output → ↓ LV PL → ↓ CO
- On Afterload
- → ↓myocardial transmural pressure → ↓LV AL → ↑CO
- PEEP > Ao diastolic pressure
- → Collapse of intrathoracic aorta → Starling resistor mechanism → ↑LV AL → ↓ CO
- PEEP < Ao diastolic pressure
- ↑ pressure gradient for flow to systemic circulation
- ↓ AL → ↓ myocardial work
- On Compliance
- diastolic buldging of septum → ↓ LV compliance → ↓LV CO
- On Preload
- Right Heart
- → ↑ Pulmonary vascular resistance → ↑ RV AL → ↓ RV output
- → ↓ Venous return → ↓ RV PL → ↓ RV output
- In the failing LV → ↑CO
- ↓ LV afterload
- ↑ pressure gradient thorax to abdomen
- ↓ transmural pressure → ↓ LV wall tension = ↓afterload
- ↓ LV preload (ie more favourable position on compliance curve)
- ↓ LV afterload
Respiratory Effects
Beneficial Effects:
- ↓ atelectasis and gas trapping
- ↑’s FRC > “closing capacity”
- Shifts position on P-V curve right, above the “closing point”
- ↑ lung compliance → shifts back to steep/compliant part of P-V curve
- ↓ intrapulmonary shunt thus improved V/Q match and ↑ PaO2
- ↓ atelectasis and gas trapping
- ↓ extravascular lung water → ↓interstitial/alveolar oedema
- ↓ AWR
- ↑ lung volume → ↑ radial traction by parenchyma → ↑ airway calibre
- ↓ work of breathing
- ↑ lung compliance = ↓ elastic work
- ↓ AWR = ↓resistance work
Negative Effects:
- ↑ V/Q mismatch
- ↑ west zone 1
- ↓ lung compliance
- shift to flat part of P-V curve
- ↑ Work of breathing (due to compliance change)
- ↑ PVR and RV afterload
- extrinsic compression of pulmonary vessels
- Barotrauma
End-Organ Effects
- Renal:
- ↓CO & ↑renal venous pressure
- → Reduced renal blood flow → Reduced GFR → Reduced urine output
- → Reduced atrial stretch and ANP release → Increased ADH → Fluid retention → Oedema
- ↓CO & ↑renal venous pressure
- Hepatic:
- Reduced hepatic blood flow due to:
- Increased CVP and decreased CO lowering the pressure gradient for hepatic flow
- May result in circulation only intermittently throughout the cardiac cycle
- Hepatocyte dysfunction
- Haematological:
- Neutrophil sequestration in the compressed pulmonary vasculature
- CNS:
- ↓VR ⇒ ↑CVP ⇒ ↑ICP
Gladwin / Mooney / JC 2020
Examiner Comments
2019A 20: 33% of candidates passed this question.
Structured answers separating effects of positive pressure on right and left ventricle, on preload and on afterload were expected. Overall there was a lack of depth and many candidates referred to pathological states such as the failing heart. Simply stating that positive pressure ventilation reduced right ventricular venous return and/or left ventricular afterload, without some additional explanation was not sufficient to achieve a pass level.
VIVAs
| A. Pharmaceutics | Drugs: Role of excipients (additives) |
| B. Pharmacokinetics | Pharmacokinetics, Plasma concentration-time curve |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Estimate ABG values for sats 85% on fiO2 0.5 Spirometry View of larynx during intubation |
| G. CVS | ECG, electrical currents in heart, phases of Ventricular myocyte Action potential Components of PA catheter trace |
| H. Renal | Renal clearance, GFR measurement |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | Acids and bases |
| K. Neuro | Paracetamol and Toxicity Opioid Pharmacology, MoA Morphine P-V relationship for ICP |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | Functional Anatomy of Liver |
| O. GIT | |
| P. Nutrition and Metabolism | Caloric requirement for 65 yo female |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | Thyroid hormone synthesis, pharmacology |
| V. Obstetrics | Functions of placenta |
| W. Measurement and Monitoring | |
| X. Procedures |






Recent Comments