SAQs
1. Describe the carriage of oxygen in the blood, including total oxygen delivery per minute.
CICMWrecks Answer
O2
- Total body O2 content ~ 1.55L
- ~850mls – blood (20.4ml/100ml blood) (O2 Carriage)
- 20.1ml/100ml – bound to Hb
- 0.3ml/100ml – dissolved
- ~200ml – bound to myoglobin
- ~450ml in FRC
- ~50ml dissolved in tissue
- ~850mls – blood (20.4ml/100ml blood) (O2 Carriage)
- O2 Content
- O2 Delivery
- Determinants
- O2 Extraction / Extraction Ratio
Carriage of O2
- Normal arterial blood has a PO2 of 100mmHg and is 97.5% saturated
- Venous blood has a PO2 of 40mmHg and is 75% saturated
- O2 is carried either bound to Hb or dissolved in solution
- Dissolved O2 (2%)
- obeys Henry’s Law: amount dissolved ∝ the partial pressure.
- For each mmHg of PO2, there is 0.003ml O2/100ml blood
- therefore normal arterial blood with PO2 of 100mmHg contains 0.3ml O2/100ml
- Bound to Hb (98%)
- each gram of fully saturated Hb can bind 1.34ml of O2 (= Hufners constant)
- 98% O2 in blood = bound to Hb, a protein tetramer with an iron-porphyrin ring attached to each chain
- O2 coordinates with each Fe atom → inducing a conformational change → promotes binding of O2 to the other Fe atoms.
- total O2 binding capacity of Hb in blood (at normal p, temp, and PCO2) = 1.34ml/g → giving a total O2 carrying capacity of blood which an Hb of 150g/l of 20.1ml/100ml
O2 content
- 1.34 = Hufners constant at 37 degrees
- 0.03 = solubility coefficient for O2 in water
- O2 content per 100mL:
| CaO2 (Arterial O2 content) | CvO2 (Venous O2 content) |
|---|---|
| = (15 x 1 x 1.34) + (0.003 x 100) | = (15 x 0.75 x 1.34) + (0.003 x 40) |
| = 20.1 + 0.3 | = 15.08 + 0.12 |
| = 20.4 ml | = 15.2 ml |
Relevant Anatomy (for O2 Carriage)
- RBCs
- small, flexible, biconcave discs
- cell membrane contains carbohydrate based antigens (ABO) and transmembrane proteins (Rhesus)
- No nucleus; no mitochondria (therefore aerobic metabolism not possible – entirely dependent on glucose and glycolytic pathway)
- Hb: large, ion containing protein contained within RBCs
- HbA
- Most common form of adult Hb (95%)
- Quaternary structure comprising 4 polypeptide globin subunits (2 alpha and 2 beta chains) in tetrahedral arrangement
- 4 globin chains held together with weak electrostatic forces
- each globin chain has its own haem group – an ion containing porphyrin ring with iron in the ferrous state (Fe2+)
- O2 molecules are reversibly bound to each haem group through a weak coordinate bond to the Fe ion
- In total: 4 O2 molecules can be bound to each Hb molecule – one for each heme group
- Different Hb types have different structures of globin chains
- HbA: 2 alpha, 2 beta (Adult)
- HbF: 2 alpha, 2 gamma (Foetal – replaced by HbA by 6/12 age)
- HbA2: 2 alpha, 2 delta (2-3% adults)
- Different O2 carriage and dissociation curves
- Disorders of Hb synthesis:
- Decreased production of normal globin change: Thalassaemia
- Abnormal globin chains: Sickle cell anaemia
- Affects O2 Carriage
O2 Delivery, Uptake
O2 Delivery and Uptake
- Normal O2 Delivery = 1000ml/min
- Determinents of tissue O2 delivery
- Diffusion from alveolus to blood: Fick’s law of diffusion and factors affecting rate
- Diffusion from capillaries into tissues and cells: partial pressure difference
- O2ER: O2 Extraction ratio = % of oxygen removed = O2 extraction / CaO2
| Oxygen Delivery (DO2) | Oxygen Return | Oxygen uptake (VO2) |
|---|---|---|
| = CO x Art O2 content | = CO x Ven O2 content | = O2 delivery – O2 return |
| = 5000 x 20 x 0.1 | = 5000 x 15.2 x 0.1 | = 1000 – 750 |
| = 1000ml/min | = 750ml/min | = 250ml/min |
Determinants of O2 Delivery
- Cardiac output = HR x SV
- Pathological states like low output or hyperdynamic states can alter CO
- Oxygen content dependent on Hb, Sats, PaO2
- O2 delivery decreased in:
- decreased cardiac output (decreased preload, contractility, HR or increased afterload)
- decreased saturations (V/Q mismatch, decreased V, decreased PiO2, Shunt, increased CO2)
- decreased Hb (blood loss, iron deficient anaemia, anaemia of chronic disease, etc)
- O2 Uptake increased in:
- increased metabolic activity (sepsis, exercise, malignancy, pregnancy)
- right shift of HbO2 curve (CO2, ¯pH, 2,3 DPG)
HR
affected by automatic rhythmicity of cell and balance between sympathetic & parasympathetic systems
Stroke Volume
- = Amount of blood pumped into the circulation per contraction
- dependent on preload, contractility, afterload
- Preload
- ~ Venous return
- (MSFP-RAP)/RVR
- MSFP – Mean Systemic Filling Pressure
Equilibration of pressures in the systemic circulation if cardiac output was abolished
Describes the filling state of the circulation and tone of capacitance vessels - RAP – Right atrial pressure
- Resistance to venous return
- Calibre of transmitting venous system according to Poisuille-Hagen equation (where resistance ∝ 1/r4 )
- Physical obstructions to venous return: Mechanical obstruction, Valvular stenosis
- MSFP – Mean Systemic Filling Pressure
- Thoracic pump (negative intrathoracic pressure created during inspiration)
- Skeletal muscle pump
- One way valves
- Pump function of the ventricle
- Venous tone
- Skeletal muscle pump
- One way valves
- Pump function of the ventricle
- Venous tone
- Afterload
- Proportional to aortic pressure and ventricular size
- Inversely proportional to ventricular wall thickness
- Affected by obstruction to the LVOT
- Contractility
- Affected by adrenergic or muscarinic stimulation
- Availability of Ca
O2 diffusion into blood:
- Fick equation:
- Describes Diffusion through tissues
- Rate of movement of solute across semi-permiable membrane J is
where
C = concentration (or partial pressure for gasses)
A = cross-sectional area
T = thickness of the membrane or distance over which diffusion takes place.
- Thickness of membrane (normal: 0.3μm)
- Surface area of membrane (normal: 50-100m2)
- Age, posture, degree of inflation (1 mark for any)
- Partial pressure gradient across membrane
- Rate of blood flow through lungs
- (no points for mentioning diffusion constant of gas – question relates to only oxygen)
- Rate of oxygenation of reduced Hb
- Shift of oxygen dissociation curve (pH, temperature, PCO2, 2,3-DPG)
- Haematocrit
- Abnormalities of haemoglobin
Kerr / JC 2020
Examiner Comments
2018A 01: 32% of candidates passed this question.
Better answers divided oxygen carriage into that bound to haemoglobin and that carried in the dissolved form. A reasonable amount of detail on the haemoglobin structure and its binding of oxygen was expected, including an explanation of co-operative binding and the oxygen carrying capacity of haemoglobin. Better answers mentioned Henry’s law in the description of dissolved oxygen, along with an estimation of the amount of oxygen that is normally in the dissolved form.
It was expected that answers include the equation for oxygen delivery, a brief description of the components of that equation and the normal value, which a large number of candidates omitted.
2. Compare and contrast the pharmacology of adrenaline and milrinone.
Examiner Comments
2018A 02: 45% of candidates passed this question.
This question was best answered using a table. Better answers included: the mechanisms of action, the pharmacokinetics and pharmacodynamics, indications for use and adverse effects. To complete the answer, the two drugs should have been compared and contrasted. There are many areas which could be contrasted e.g. different indications, different mechanisms of action, different half-lives and duration of action, different metabolism and different pharmacodynamic effects, in particular the effects on the cardiovascular system and the pulmonary circulation. Similarities should also have been highlighted.
3. Define dead space and its components (30% of marks). Explain how these may be measured (35% of marks) and describe the physiological impact of increased dead space (35% of marks).
CICMWrecks Answer
Dead space
- Ventilated lung volume in which no gas exchange occurs
- Anatomical dead space
- Dead space volume of conducting airways
- Oropharynx, nasopharynx and first 16 divisions of the tracheobronchial tree
- Approx 2ml/kg
- Measured by fowlers method (N2 washout)
- Dead space volume of conducting airways
- Alveolar dead space
- Dead space volume of parts of the respiratory airways that are ventilated but not perfused
- West Zone 1 (PA > Pa)
- Low cardiac output
- Positive pressure ventilation
- Posture
- Disease states
- Pulmonary Embolism
- West Zone 1 (PA > Pa)
- Measured by subtracting anatomical deadspace from physiological dead space
- Dead space volume of parts of the respiratory airways that are ventilated but not perfused
- Physiological dead space
- Alveolar + anatomical dead space
- Calculated from Bohr equation
- Normal arterial to end-tidal CO2 gradient is approx 5mmHg
- Increases if increased dead space
- Apparatus dead space
- Dead space contributed by artificial airway devices (endotracheal tubes, LMA, HME filters)
- Usually reduces anatomical deadspace (due to bypass of oropharynx)
Calculation / Measurement of Dead Space
Bohr’s Method
- Bohr’s Equation:
- Based on the principle that all CO2 exhaled must come from ventilated alveoli
- PĒCO2 is mixed-expired CO2 in an expired tidal breath
- Alveolar PCO2 is difficult to measure, so the Enghoff modification is used
- which assumes PACO2 = PaCO2
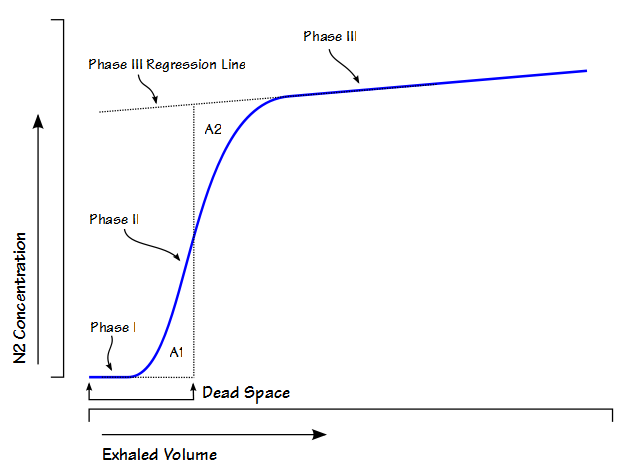
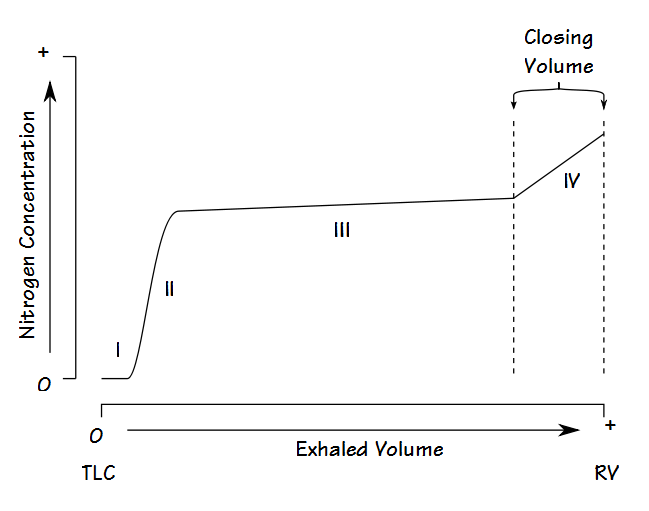
Fowler’s Method
Single-breath nitrogen washout test
- Single Vital Capacity Breath – 100% O2 → Exhales to Residual volume
- Expired Nitrogen concentration and volume is measured
- Plot of concentration by volume generated
- Phase I: Pure dead space. No Nitrogen present.
- Phase II: Midpoint is volume of Anatomical dead space.
- Phase III: Expired N2 plateaus
- Phase IV: Closing capacity. Sudden Increase in Nitrogen.
Consequences of increasing dead space
- Alveolar ventilation
- RR x (tidal volume – dead space)
- Therefore, as dead space approaches tidal volume, alveolar ventilation approaches zero, and cannot be compensated by increasing respiratory rate
- PaCO2 is inversely proportional to alveolar ventilation → PaCO2 increases as alveolar ventilation decreases
- Decreased pH
- Increased central and peripheral stimulation of ventilation → Reduction in PaCO2
- Central chemoreceptors activate medullary vasomotor centre → increased sympathetic discharge
- If PaCO2 increases, alveolar CO2 in non-dead space alveoli will increase
- PAO2 will decrease according to alveolar gas equation
Sakurai / JC 2016
Examiner Comments
2018A 03: 59% of candidates passed this question.
Some candidates failed to provide a correct definition of dead space. An outline of anatomical, alveolar and physiological dead space was expected. The Bohr equation was commonly incorrect, and many did not comment on how to measure the components of the Bohr equation. Fowler’s method was generally well described though some plotted the axes incorrectly. The impact of increased dead space was not often well explained. Very few people stated the major impact of increased dead space is reduced minute ventilation and how this would affect CO2.
4. Describe the renal handling of sodium.
CICMWrecks Answer
NORMAL SODIUM
- Total Sodium (Na) = 4000 mmol (60 mmol/kg)
- Distributed:
- Bone (45%)
- ECF (50%)
- 1°ly found in ECF (major EC cation)
- ICF (5%)
- 10-15 mmol/L maintained by
- Na+/K+ ATPase
- low gNa → prevents influx of Na
- Role of Na
- Main determinant of ECF osmolality and tonicityNa/Cl ~ 90% ECF osmotic solute load
- Main determinant of ECFV
- Depolarisation in action potential 2° to ↑Na conductance
- Co-transport of substances across membranes (Eg. Glucose)
- Involved in Na+/K+ ATPase in cell membranes
- Total output =Total input
- 1-1.4 mmol/kg/day
- ~100-300 mmol/day in 70 kg adult
- 10.5 g/day
- Sodium Control – Lost via:
- Kidneys (main) ~ lose 150 mmol/day
- Filters 25000 mmol Na+/day
- 99.5% reabsorbed
- 65% PCT, 25% TAL of LoH, 5% EDCT, 4-5% LDCT and CD
- Sweat and GIT loss in faeces ~ lose 10 mmol/day (0.25 g/day each)
- Kidneys (main) ~ lose 150 mmol/day
Sodium Reabsorption in Kidney
| Location | Contribution | Mechanism |
|---|---|---|
| PCT | 65% | Secondary active transport: – Luminal Na/organic cotransporters with glucose and AAs – Luminal Na/K (NHE-3) exchanger with H from Henderson-Hasselbach intracellularly Passive transcellular – Via solvent drag passively – Down electrical gradient from positive lumenal charge |
| TAL of LoH | 25% | Secondary Active transport: – NKCCT on luminal surface – Dominant mechanism Small amount continues via Secondary active transport as per PCT Paracellular movement driven by net positive charge in lumen |
| Early DCT | 6-10% | 2° active means – Apical Na/K ATPase generates Na gradient – Basal Na/Cl symporter – No alteration in the luminal charge as electrically neutral |
| Late DCT and CD | 5-10% | – Facilitated diffusion across principle cells – Basolateral Na/K ATPase → intracellular Na deficit – Na reabsorbed from lumen via ENaC channels in principle cells up regulated by Aldosterone |
Overview of renal Na+ regulation:
- Thus, renal Na+ regulation depends on:
- Degree of glomerular filtration of Na+ → GFR (minor)
- Changes in GFR due to hyper or hypovolaemia will (indirectly) adjust sodium elimination. Increased plasma volume increases GFR, and vice versa.
- Degree of tubular reabsorption of Na+ (major)
This is the main mechanism for controlling sodium in euvolaemia.
In terms of long term Na excretion; Na reabsorbed is more impt than GFR because:- (i) GFR is heavily autoregulated
- (ii) Glomerulotubular balance blunts any major changes in Na+ excretion that would have resulted from minor changes in GFR changes that actually occurs
- Na reabsorption through GIT/sweat/salivary glands:
- Varies with diet and exercise
- Mainly action of Aldosterone via Na/K ATPase
- Degree of glomerular filtration of Na+ → GFR (minor)
- Normal values
- Na+ filtration: 140mmol/L x 180L/day = 25000mmol Na/day
- Na+ excretion: 140mmol excreted
- rest reabsorbed
- Fractional excretion = 0.5%
Na+ regulation: Control of GFR
- Intrinsic autoregulatory factors (tubuloglomerular feedback and myogenic mechanism)
- MAP has minor effect on GFR over MAP range 70-175 mmHg → BUT changes
in BP that invoke baroreceptor reflexes (BRR) can override these autoregulatory
mechanisms → alter GFR and amount of Na+ filtered
- MAP has minor effect on GFR over MAP range 70-175 mmHg → BUT changes
- Extrinsic factors: Body Na+ content (via ECFV)
- Direct renal effects – ↓ [Na+] (or ↓ ECFV) → results in ↓ GFR due to a ↓ glomerular capillary P(HYDROSTATIC) and ↑ glomerular capillary P(ONCOTIC) → ↓ GFR and Na+ filtered
- Indirect renal effects – ↓ [Na+] (or ↓ ECFV) → stimulates arterial, venous and cardiac BRR → neurohormonal response → to ↓ GFR and Na+ filtered via:
- (i) ↑ SNS and RAAS activity → cause afferent and efferent arteriolar constriction and mesangial cell contraction
- (ii) ↑ ADH → cause afferent arteriolar constriction and mesangial cell contraction
- (iii) ↓ ANP → inhibit afferent arteriolar dilation and mesangial cell relaxation
Na+ Regulation: Control of Reabsorption
- Glomerulotubular balance:
- Intrinsic autoregulatory mechanism that minimises the effect of changes in GFR on Na+ and H2O excretion
- It functions on the basis that the PCT reabsorbs a constant proportion of glomerular filtrate (65% of filtered Na+ /H2O), rather than a constant amount
- In effect – ↑ GFR = ↑ filtration of Na+/H2O = ↑ Na+/H2O reabsorption
- Mechanism:
- With ↑ GFR → large amount of plasma is filtered at the glomerulus → leads to ↑ π(ONCOTIC) of plasma in peritubular capillaries
- This results in an ↑ gradient that –
- (i) Favours tubular reabsorption, and
- (ii) Counteracts the effect of ↑ GFR on fluid leaving the PCT
- Renal interstitial hydrostatic pressure (Intrarenal physical factors)
- ↓ ECFV (and ↓ Na+) results in ↓ MAP → leads to (i) ↓ PHYDROSTATIC and (ii) ↑ πONCOTIC of peritubular capillaries → thus, ↑ Na+ (and ↑ H2O) reabsorption from tubular interstitium into peritubular capillaries
- Hormonal Influences:
- Renin
- Released by ↓ Na delevery to macula densa or β1 stimulation secondary to volume underload
- Tubular effects:
- increased PCT Na/Cl reabsorption, increased tubular K secretion
- Direct PCT effect
- Aldosterone release
- Aldosterone
- Most important regulator of Na+ reabsorption
- Alters protein translation (inducing production of tubular basolateral Na+/K+ATPase and luminal ENaC and K+channels) → causes ↑ Na+ reabsorption by DCT and Principal cells of CCD
- Increased Na reabsorption throughout the GIT/sweat and salivary glands via Na/K ATPase
- Increased H2O reabsorption and increased Na via solvent drag
- Angiotensin II
- Negative feedback on renin release
- Increased aldosterone release
- Decreased RBF and GFR
- Direct renal arteriole constriction (efferent = afferent)
- Mesangial cell contraction thus decreased Kf and GFR
- Direct stimulation of Na+ reabsorption at PCT, and
- Indirect stimulation of Na+ reabsorption via SNS, AII, and aldosterone
- SNS
- Direct stimulation of Na+ reabsorption at the PCT (α1 and β1 receptors), and
- Indirect stimulation of Na+ reabsorption via RAAS
- ADH
- → ↑ Na+ reabsorption at the CCD (principal cells) → acts synergistically with aldosterone here
- ANP
- Inhibition of Na+ reabsorption (blockage of ENaC) in the CDs
- ↓ RAAS and ↓ ADH activity
- Renin
- Other causes ↑Na reabsorb:
- Cortisol
- Oestrogen
- GH
- Thyroid hormone
- Insulin
- Dopamine
- Other cause ↓Na reabsorb:
- PGE2 inhibits NaK ATPase to reduce Na reabsorption
- Glucagon
- Progesterone
- PTH
- Renal vasoDilators:
- PGs
- Kinins
- Pressure natriuresis & diuretics
- renal compensatory mechanism that maintains long-term regulation of arterial BP by controlling the kidney’s excretory ability of Na+and H2O
- Pharmacological agents:
- Ouabain (a cardiac glycoside) inhibits NaK ATPase decreasing excretion
- Loop Diuretics → ↑ Na loss
Gladwin / Bianca / JC 2019
Examiner Comments
2018A 04: 6% of candidates passed this question.
A description of filtration and reabsorption, including amounts was required. Better answers described sodium handling in a logical sequence as it progressed through the nephron including the percentages reabsorbed in each segment. In addition to the amounts reabsorbed, the mechanisms of transport across the tubular luminal and basolateral membranes into interstitial space should have been described.
- aldosterone = stimulates Na+ reabsorption at the collecting tubules
- intra renal factors such as interstitial pressure which is lowered during hypovolaemia and reduced renal perfusion, thus promoting Na+ reabsorption gradient
- sympathetic nervous system – influences interstitial pressure and increases renin production
- angiotensin II – stimulates reabsorption at proximal tubule
- Atrial Naturetic Peptide/Factor – inhibits Na+ reabsorption
- Others – dopamine, cortisol, insulin => increase Na+ reabsorption, but minor factors
Syllabus – D1, 2f
Reference – Textbook of medical Physiology, Guyton, Chp 28
5. Compare and contrast the pharmacokinetics and pharmacodynamics of IV fentanyl and IV remifentanil (60% of marks). Discuss the concept of context sensitive half-time using these drugs as examples (40% of marks).
CICMWrecks Answer
PK and PD of Fentanyl and Remifentanil
Context sensitive half-time
- Defined as the time for plasma concentration to fall to half of its value at the time of stopping an infusion
- A method to describe the variability in plasma concentrations after ceasing an infusion
The “context” is the duration of infusion. - Used because terminal elimination half-life has little clinical utility for predicting drug offset
Half-lives are often misleading when discussing drug infusions. - Dependent on:
- Duration of infusion
During an infusion, drugs distribute out of plasma into tissues. When the infusion ceases, drug is cleared from plasma and tissue drug redistributes back into plasma.- The longer an infusion, the more drug has distributed out of tissues, and the longer the redistribution phase
- The longest context-sensitive half time occurs when an infusion is at steady-state
- Redistribution
The maximal CSHT reached depends on the:- VDss
Drugs with a larger VDss have a longer CSHT, as only a small proportion of the drug in the body will be in plasma and able to be cleared. - Rate constant for elimination
Drugs with a smaller rate constant for elimination have a longer CSHT.
- VDss
- Duration of infusion
Drugs with longer context-sensitive half-times will wear off less predictably.
Context sensitive half time – Fentanyl vs Remifentanil:
- Fentanyl shows variable CSHT – Has high redistribution, with VdSS 3-5L/kg, and a terminal half life of 3 hours. Hence, prolonged infusion may lead to increased duration of action and SE
- Remifentanil has little redistribution and a small Vd (0.2-0.3L/kg) in the peripheral compartments, and a very short elimination half life of 3 minutes. Hence, it has a very short, context-insensitive half time
It wears off reliably and quickly following cessation of infusion.

Courtesy: icuprimaryprep.com
Examiner Comments
2018A 05: 66% of candidates passed this question.
Well-constructed answers were presented in a table to compare pharmacokinetics and pharmacodynamics with a separate paragraph to discuss the concept of context sensitive halftime. Important pharmacokinetic points included: the differences in lipid solubility, ionised fractions and onset, and differences in metabolism. Marks were awarded for a definition of context-sensitive half-time. A discussion of these two drugs’ context-sensitive half-times should have included the differences in re-distribution into other compartments and rates of elimination.
6. Define a buffer (25% of marks). Describe how acid and base shifts in the blood are buffered (75% of marks).
CICMWrecks Answer – Buffer Systems
Buffer
- Weakly ionised acid or base in equilibrium with its full ionised salt
- A buffer can “resist” change in pH by absorbing or releasing H+ ions
- Works best when pKa is closest to the target pH (7.4)
- Isohydric Principle
- All buffer systems which participate in defence of acid-base changes are in equilibrium with each other. There is after all only one value for [H+] at any moment. This is known as the Isohydric Principle.
Effectiveness:
- buffer pKa (most effective if pKa = pH of carrying solution)
- pH of carrying solution
- amount of buffer present
- open (physiological) vs closed (chemical) system
Buffer Systems by site
| Site | Buffer System | Comment |
|---|---|---|
| ISF | Bicarbonate | For metabolic acids |
| Phosphate | Not important because concentration too low | |
| Protein | Not important because concentration too low | |
| Blood | Bicarbonate | Important for metabolic acids |
| Haemoglobin | Important for carbon dioxide | |
| Plasma protein | Minor buffer | |
| Phosphate | Concentration too low | |
| ICF | Proteins | Important buffer |
| Phosphates | Important buffer | |
| Urine | Phosphate | Responsible for most of ‘Titratable Acidity’ |
| Ammonia | Important – formation of NH4+ | |
| Bone | Ca carbonate | Important in prolonged metabolic acidosis |
| CSF | Bicarbonate | Important – (as very low proteins for buffering) free Bicarbonate flow in CSF |
| Phosphate | negligible |
Buffer Systems
| Name | Location |
|---|---|
| Protein | Intra cellular |
| Haemo globin | Blood |
| Bicarb | Plasma, Interstitium, Urine (minimal) |
| Ammonia | Urine |
| Phosphate | Urine, Bone |
| CaCO3 | Bone |
Protein (Intracellular)
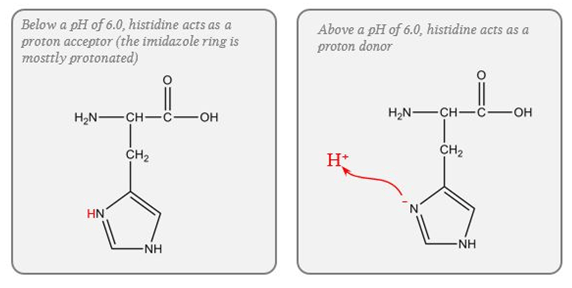
- pKa of imidazole group is 6.0
- pKa of the histadine residues themselves = 6.8
- Protein buffer ability is proportional to Histadine residue content.
Haemoglobin (Blood)
- Protein buffering system
- Important intracellular buffer in RBC
- Exists as weak acid – HHb and potassium salt KHb.
- Hb a pKa of 8.2 in deoxy form and 6.6 in oxyHb
- Buffering capacity due to imidazole residues on 38 histidine residues on Hb molecule
- pKa of residues 6.8 à close to physiological pH
- Also important in extracellular buffering (following bicarbonate buffer system)
- Due to fast equilibration of HCO3– (Hamburger Shift)
- Band3 Transporter (HCO3–/Cl– antiporter)
- Allows carbonic anhydrase reaction to take place by limiting build-up of HCO3– (la chatelier principle)
- Formed in erythrocytes as a tetramer of 4 subunits
- Hb ~ 6 times the number of histadine (38) residues compared with albumin. (3-6x buffering capacity)
- Hb is in much greater concentrations than any other protein (15 g/dL vs 7 g/dL)
- For each mmol OxyHb, 0.7mmol H+ is buffered and 0.7mmol of CO2 can enter circulation without a change in pH
- Available at high concentrations in RBC
- Isohydric exchange
- the buffer system (HHbO2-HbO2-) is converted to another more effective buffer (HHb-Hb-) exactly at the site where an increased buffering capacity is required
- Deoxyhaemoblobin is a much more effective buffer
- oxygen unloading increases the amount of deoxyhaemoglobin and this better buffer is produced at exactly the place where additional H+ are being produced because of bicarbonate production for CO2 transport in the red cells.
Bicarb (Plasma, Interstitium, Urine (minimal))
- Catalysed by carbonic anhydrase (slow where CA is absent (plasma))
- Open at both ends
- Bicarb regulation at kidneys
- CO₂ regulation at lungs
Ammonia (Urine)
Equlibrium between ammonia and ammonium
Steps:
- Glutamine enters PCT cells
- 20% from filtrate
- 80% from peritubular capillaries
- Ammonia (NH3) produced from glutamine in PCT by glutaminase secreted into lumen
- Reabsorbed in TAL of LoH
- Diffuses into peritubular cells of CD down conc Grad
- H+ secreted into lumen of CD
- Combines with ammonia to form ammonium
- Ammonium positive charge prevents reabsorption
- Thus “excess hydrogen can be secreted with ammonium”
Phosphate (Urine, Bone)
Effect in urine is limited due to:
- Minimal urinary pH is 4.5 → equation B is >99% ionised → of little use.
- Requires filtered PO4 → Normally low levels.
- Depends on Diet and PTH.
- Where H is secreted in distal tubules, there is minimal PO4 excretion
CaCO3 (Bone)
- Important in chronic metabolic acidosis
- Release of calcium carbonate from bone is the most important buffering mechanism involved in chronic metabolic acidosis.
Please Note: This answer is viewed from the Traditional approach to Acid-Base theory. Alternative (physicochemical approaches) deemphasise bicarb due to its equilibrium with water and relatively low concentration compared with the strong ions.
Gladwin / Sakurai / JC 2020
Examiner Comments
2018A 16: 45% of candidates passed this question.
Few candidates defined a buffer making it difficult to award 25% of the marks for this question.
The three main buffers in blood should have been described: bicarbonate system, haemoglobin and proteins. The pKa, the buffering mechanism and the capacity of the system should have been described. The Henderson Hasselbach equation was sometimes incorrect. Marks were only awarded for buffers in blood and unfortunately some candidates described non-blood buffers.
7. Outline the blood supply to the gastrointestinal system (arteries and veins).
CICMWrecks Answer
Blood supply to the Gastrointestinal system
- Portal circulatory system + arterial blood flow into liver
- 1100ml of portal blood + 400ml from hepatic artery = 1500ml (30% CO)
- Oxygen consumption – 20-35% of total body needs
Arterial Supply
| Aorta | Division / Branches | Organ supplied | |||
|---|---|---|---|---|---|
| Thoracic A. | Subclavian A. | Thyrocervical trunk | Inf. Thyroid A. | Cervical Oesophagus | |
| Bronchial A. | branches | Thoracic Oesophagus | |||
| direct branches | |||||
| Abdominal Aorta / Coeliac | Inf. Phrenic A. | Abdominal Oesophagus | |||
| Anterior branches of Abdominal Aorta | COELIAC TRUNK | Lt. GASTRIC A. | |||
| Foregut (Stomach → major duodenal papilla | |||||
| SPLENIC A. | Short Gastric A.s | ||||
| Lt. Gastroepiploic A. | |||||
| COMMON HEPATIC A. | Hepatic A. Proper | Lt. Hepatic A. | |||
| Rt. Hepatic A. | |||||
| Gastroduodenal A. | Rt. Gastroepiploic A. (gastro-omental) | ||||
| Sup. Pancreatoduodenal A. | |||||
| Supraduodenal A. | |||||
| SUPERIOR MESENTERIC ARTERY | Inf. Pancreaticoduodenal A. | Midgut (Major duodenal papilla → 2/3 of transverse colon) | |||
| Jejunal and Ileal A.s | |||||
| Middle colic A. | |||||
| Rt colic A. | |||||
| Ileocolic A. | |||||
| INFERIOR MESENTERIC ARTERY | Lt colic A. | (2/3 transverse colon → rectum) | |||
| several sigmoid A.s | |||||
| Superior rectal A. | |||||
| Distal Abdominal Aorta | Internal Iliac A. | Middle Rectal A. | Rectum | ||
| Internal Pudendal A. | Inferior Rectal A. | ||||
Venous Drainage
| Organs drained | Branches / Tributaries | IVC | |||
|---|---|---|---|---|---|
| Liver | Hepatic V.s | IVC | |||
| Fundus + L greater curvature Stomach | Short gastric V.s | Splenic V | Portal Vein | Liver parenchyma → Hepatic Veins → IVC | |
| Greater curvature Stomach | Lt. gastro-omental V. | ||||
| Pancreas | Pancreatic V. | ||||
| colon | Lt. colic V. | IMV | |||
| sigmoid | Sigmoid V. | ||||
| rectum | Superior rectal V. | ||||
| Rectum, Colon | direct | ||||
| Rt greater curvature Stomach | Rt gastro-omental V. | SMV | |||
| Pancreas, duodenum | Ant sup pancreatocoduodenal V. | ||||
| Ant and Post inferior pancreaticoduodenal V.s | |||||
| Jejunum | Jejunal V. | ||||
| Ileum | Ileal V. | ||||
| Ileum, colon, cecum | Ileocolic V. | ||||
| ascending colon | Rt. colic V. | ||||
| transverse colon | Middle colic V. | ||||
| pancreas, duodenum | Post Sup pancreaticoduodenal V. | ||||
| stomach | Rt. gastric V. | ||||
| stomach | Lt. gastric V. | ||||
| gall bladder | cystic veins | ||||
| Skin of umbilical region | para-umbilical vein | ||||
Portosystemic Anastomosis
- Areas:
- Lower end of oesophagus
- Upper part of anal canal
- Umbilicus
- Retroperitoneal Bare area of liver
- The gastroesophageal junction around the cardia of the stomach-where the left gastric vein and its tributaries form a portosystemic anastomosis with tributaries to the azygos system of veins of the caval system.
- The anus-the superior rectal vein of the portal system anastomoses with the middle and inferior rectal veins of the systemic venous system.
- The anterior abdominal wall around the umbilicus-the para-umbilical veins anastomose with veins on the anterior abdominal wall.
Examiner Comments
2018A 07: 7% of candidates passed this question.
An outline of the blood supply from the oesophagus down to the anus was expected. Very few candidates knew the branches of the main 3 arteries and which portion of the gastrointestinal system they supplied. Concepts related to control of blood flow and autoregulation of blood flow were not asked and therefore marks were not awarded for this information.
8. Outline the principle of co-oximetry (40% of marks), describe what a co-oximeter is able to measure (30% of marks), and compare its limitations to those of a pulse oximeter (30% of marks).
CICMWrecks Answer
Pulse oximeters assumes that only oxy-Hb and deoxy-Hb are present in the blood. It cannot differentiate other species of haemoglobin such as carboxyhaemoglobin (carboxy-Hb) or methaemoglobin (met-Hb) because it only uses two wavelengths of light (660 nm and 940 nm).
Co-oximeter:
- A co-oximeter is a blood gas analyzer that, in addition to the status of gas tensions provided by traditional blood gas measurements, measures concentrations of oxygenated hemoglobin (oxyHb), deoxygenated hemoglobin (deoxyHb or reduced Hb), carboxyhemoglobin (COHb), and methemoglobin (MetHb) as a percentage of the total hemoglobin concentration in the blood sample
- Use of co-oximetry is indicated:
- when a history is consistent with toxin exposure
- hypoxia fails to improve with the administration of oxygen
- there is a discrepancy between the Pao2 on a blood gas determination and the oxygen saturation on pulse oximetry (Spo2)
- clinician suspects other dyshemoglobinemias such as methemoglobinemia or carboxyhemoglobinemia.
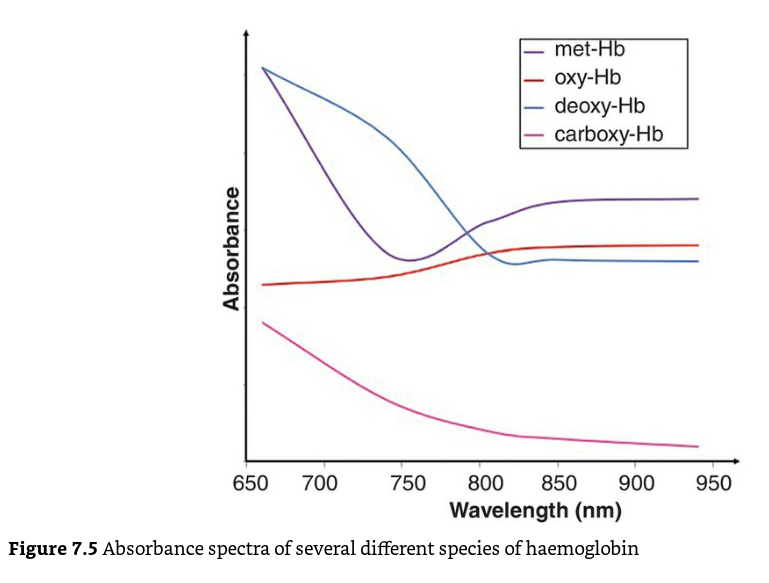
- Different haemoglobin species have different absorption spectra.
Mechanism / Measurement
- Uses Beer-Lambert law to detect different Hb species.
- “Incidence of light is inversely proportional to the path distance and concentration of light absorbing particles within the path”
- multi-wavelength spectrophotometry (measures the absorption of light passing through blood from several dozens of wavelengths)
- Complex, but straightforward internal computations
- enables the instrument to distinguish between oxy-Hb, deoxy-Hb, and carboxyhemoglobin,-COHb, methemoglobin -metHb, other hemoglobin moieties and ‘background’ light-absorbing species
- This is reported as the fractional oxyhaemoglobin content (FO2Hb), which is defined as:
- The FO2Hb measure provides a more accurate picture of the availability of oxygen to the tissues in the presence of haemoglobin variants.
- If the original blood sample contained no carboxy-Hb or met-Hb, then the values for FO2Hb and SaO2 would be identical.
Advantages:
- Accurate measure of oxygen saturation: low readings indicate true hypoxia, and high readings always represent true hypoxia
- Able to detect different Hb species, like deoxy-Hb, carboxy-Hb, met-Hb
- Not confused by ambient light, absence of pulsatile flow, tricuspid regurgitation, methylene blue dye.
Limitations of co-oximetry:
- Expensive (depending on device
- Invasive (Blood sample needed)
- Variations with different devices, especially due to Limitations on Quality control
- Possible false values with severe hypoxemia, and extremes of Hb concentrations (better with newer generation devices)
- Not available in all blood gas analyzers
- Continuous monitoring not commercially availabe yet
Limitations of pulse oximetry:
| PATIENT FACTORS | ||
| Low or High SpO2 | Low SpO2 | Normal or High SpO2 |
| Met-Hb Sulph-Hb | Poor perfusion of finger Movement artifact Venous pulsations Fingernail polish Intravenous pigmented dyes Haemoglobinopathy Anaemia with co-existing hypoxia | Carbon Monoxide poisoning |
| EQUIPMENT FACTORS | ||
| – Ambient light interference – Poorly fitting probe – Assay calibrated using healthy volunteers only down to SpO2 80%. Unknown significance if tested SpO2 less than 80% | ||
| PHYSIOLOGICAL FACTORS | ||
| – Due to O2 dissociation curve, insensitive to changes above PaO2 80mmHg – Does not measure tissue oxygenation | ||
JC 2020
Examiner Comments
2018A 08: 32% of candidates passed this question.
Most candidates confused co-oximetry with other methods of measuring oxygenation of blood. Whilst there were a number of excellent descriptions of pulse oximetry, these attracted no marks for the first two sections. Structuring the answer based on the three parts asked, would have improved answers ensuring all aspects of the question were addressed.
9. Describe the functions of the placenta (80% of marks). Outline the determinants of placental blood flow (20% of marks).
CICMWrecks Answer
Functions of the Placenta
- Organ of foetal + maternal origin; supports developing foetus
- Low pressure, low resistance AV shunt that provides metabolic nutrients necessary for growing foetus
- Functions
- Transfer: gas exchange; nutrient + waste exchange; drugs; heat
- Immunological barrier
- Metabolic
- Endocrine
1. Transfer
a. Gas Exchange
- Diffusion dependent on Fick’s Principle
- Maternal placental flow ~600ml/min at term (2x foetal flow) → ↑diffusion by ↑concentration gradient for solutes
- Molecules <600Da more readily diffuse down concentration gradient
- O2 diffusion
- PO2 entering placenta via uterine artery = 18mmHg (SpO2 45%)
- PO2 leaving placenta via uterine vein 28mmHg (SpO2 70%)
- Foetus able to absorb large enough vol O2 despite low PO2 because:
- Foetal Hb
- 2 y subunits cf β → prevent binding of 2,3-DPG → L shifted OHDC → favours O2 loading at ↓PaO2
- [FHb] 50% > maternal [Hb]
- Double Bohr effect
- Describes Bohr effects happening on either side of placental gas exchange in mother + foetal circulations
- Accounts for 2-8% of O2 transfer
- Bohr effect 1: placenta: foetus unloads CO2 in placenta → ↑placental CO2 → RIGHT shift HbA OHDC → ↓HbA affinity for O2 → ↑placental O2 unloading
- Bohr effect 2: foetus: foetus unloads CO2 → ↓foetal CO2 → LEFT shift HbF OHDC → ↑HbF affinity for O2 → ↑foetal O2 binding/ uptake
- Foetal Hb
- CO2 diffusion
- Foetal PaCO2 50mmHg; intervillous PCO2 37mmHg
- CO2 offloading favoured in foetus by:
- High foetal [Hb] ↑s amount of CO2 that can be carried as carbaminoHb
- Double Haldane effect
- Haldane effect: describes how ∆O2 sat of Hb affect CO2 transport: deoxyHb has ↑affinity for CO2 than oxyHb
- Double Haldane: describes Haldane effects happening across the placenta
- Accounts for 45% of CO2 transfer between maternal and foetal circulation
- Haldane 1: placenta: placenta unloads O2 → ↓placental O2 → deoxyHbA has ↑affinity of CO2 → ↑placental CO2 uptake from foetus
- Haldane 2: foetus: foetus takes up O2 → ↑HbF O2 → ↓HbF affinity for CO2 → ↑HbF CO2 release to placenta
b. Nutrient delivery
- Nutrient diffusion
- High foetal caloric requirements in late pregnancy
- Facilitated diffusion of glucose via carrier molecules in trophoblasts
- Active transport for amino acids, Ca2+, Fe, folate, vit A and C
c. Waste removal – Urea, Uric acid, Creatinine, Br
d. Heat transfer
2. Immunological function
- permeable to IgG via pinocytosis → allows maternal abs to provide passive immunity to foetus
- Trophoblast cells lose many cell surface MHC molecules → making them less immunogenic; also cells cover themselves in mucoprotein which
disguises them from maternal immunie system - Chorionic cells act as immunological barrier – preventing maternal T cells and abs from reaching foetal circulation
- Some bacteria (listeria) and viruses (rubella, parvovirus B19, HIV) can cross into foetal circulation
- Progesterone + alpha-foetoprotein produced by yolk sac act as maternal immunosuppressive agents
3. Metabolic
- synthesis of glycogen, cholesterol, FA, enzymes
4. Endocrine function
- synthesis of 4 main hormones:
- bHCG
- hPL: human placental lactogen (human chorionic somatomammotrophin)
- Oestriol
- Progesterone
- Synthesis of other hormones and growth factors
- Placental corticotrophin
- Human chorionic somatostatin
- Human chorionic thyrotropin
- Epidermal growth factor
- Somatomedin
Placenta
- Placenta:
- temporary fetal organ that begins developing from the blastocyst shortly after implantation
- ~22cm length, 2.5cm thickness. ~500gm
- Connects to fetus by umbillical cord
- “Chorionic villus” is the basic structural unit of the placenta → it is a vascular projection of foetal tissue that is bathed by maternal blood within the “Intervillous space”. It consists of:
- Foetal connective tissue containing foetal capillaries
- Chorion → outermost layer of foetal tissue that is made of 2 layers →
- (a) Syncytiotrophoblast (directly contacts maternal blood in intervillous space) and
- (b) Cytotrophoblast (b/t syncytiotrophoblasts and foetal CT)
Placental Circulation
- Placental circulation = Two circulation systems in parallel – maternal and the fetal
- Maternal circulation system
- Uterine Arteries (600ml/min, 100mm Hg) → arcuate arteries → radial arteries → spiral arteries (70 mm Hg) & basal arteries (myometrium and deciduas)
- Spiral arteries → intervillous spaces (10mm Hg) → uterine veins that are arranged in the periphery of the intervillous space.
- Blood in the intervillous space is exchanged 2-3 times per minute.
- Fetal circulation system:
- Two villus Umbilical Arteries (50mmHg) → finer vessels that cross through the chorionic plate → villus capillaries (30mmHg) → leaves placenta through umbillical vein (20mmHg)
- Their supply amounts to approximately 40% of the fetal heart blood volume per minute.
- The pressure in the fetal vessels and their villus branches always lies over that of the intervillous space. This protects the fetal vessels from collapse.
- Substances traverse between foetal and maternal blood via the following layers:
- Maternal blood (intervillous space) ↔ Chorion (2x layers of trophoblasts) ↔ Foetal connective tissue ↔ Endothelium of foetal capillaries ↔ Foetal blood
Determinants of Placental Blood Flow
Uteroplacental blood flow
- At term → uteroplacental BF is 500-700 mL/min (10% maternal C.O.) of which:
- 70-90% of this BF enters the intervillous space (via the spiral arteries) → NOT autoregulated as blood flow is “pressure-dependent” (see factors below)
- 10-30% of this BF supplies the myometrium/deciduas (via the basal arteries) → autoregulated blood flow
- Blood flow to the intervillous space (which participates in substance exchange with foetal blood) is affected by the following factors:
- Uterine arterial pressure (UAP):
- Maternal arterial BP → ↓ MABP (Ie. due to SNS block 2° neuraxial block,
hypovolaemia, supine hypotension syndrome, Etc.) causes ↓ UAP → ↓ UBF
- Maternal arterial BP → ↓ MABP (Ie. due to SNS block 2° neuraxial block,
- Uterine venous pressure (UVP):
- Uterine tone and contractions → ↑ tone/contractions (Ie. due to contractions,
oxytoxics, ketamine, Etc.) causes ↑ UVP → ↓UBF
- Uterine tone and contractions → ↑ tone/contractions (Ie. due to contractions,
- Uterine vascular resistance (UVR):
- Uterine arteriolar tone → ↑ vasoconstriction (Ie. a/w essential HT and PET, α-adrenoceptor stimulation (by endogenous SNS innervation, catecholamines or sympathomimetics), and vasopressin) causes ↑ UVR → ↓ UBF
Umbilical blood flow
- At term → umbilical BF is 360 mL/min (25-50% of foetal C.O. (≈ 1000 mL/min))
- It is “autoregulated” (cf. uterine BF) → involves vasodilators (PCI-2/NO) derived from vascular endothelium
- BF is ↓ with severe hypoxia, ↑ BGL, catecholamine and cord compression
Kerr / Bianca 2020
Examiner Comments
2018A 09: 32% of candidates passed this question.
Many candidates provided a broad overview of functions of the placenta but lacked detail.
Placental blood flow has maternal and foetal components, though most only considered the maternal circulation to the placenta and didn’t mention the foetal vessels. Many were not specific as to what blood vessels were described.
Many stated that uterine blood flow is not autoregulated, however went on to describe myogenic and neuro-humoral mechanisms of autoregulation
10. Outline the advantages (15% of marks) and disadvantages (85% of marks) of the clinical use of suxamethonium.
CICMWrecks Answer
- Suxamethonium is the dicholine ester of succinic acid which acts as an ultrashort acting depolarising muscle relaxant.
- It is used in rapid sequence induction and to modify seizures caused by ECT.
- Mechanism of Action:
- mimics the actions of Ach by binding to the nicotinic Ach receptor and causing membrane depolarization.
- However, because its hydrolyzing enzyme is not present at the NMJ, the effect lasts longer than for Ach.
- The persistent depolarization renders the voltage sensitive Na channels inactive within 1-2 mins.
- This prevents the transmission of further APs. Initially causes fasciculations, then muscle relaxation.
- Partial agonists are drugs that bind to and activate a given receptor, but have only partial efficacy at the receptor relative to a full agonist
- A partial agonist is unable to achieve a maximal biological response following active site binding, despite increasing doses
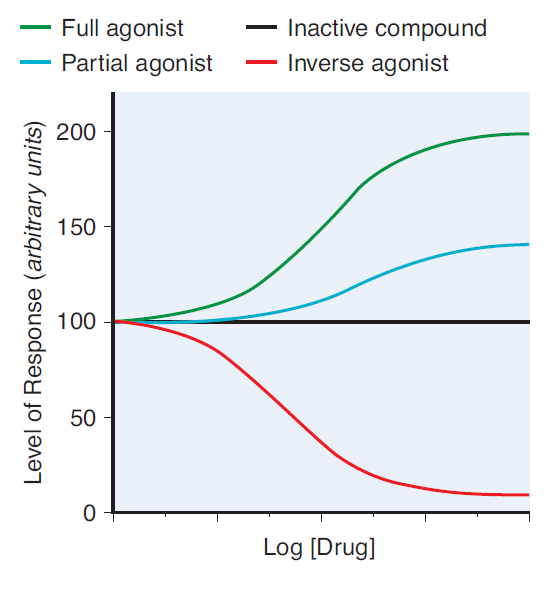
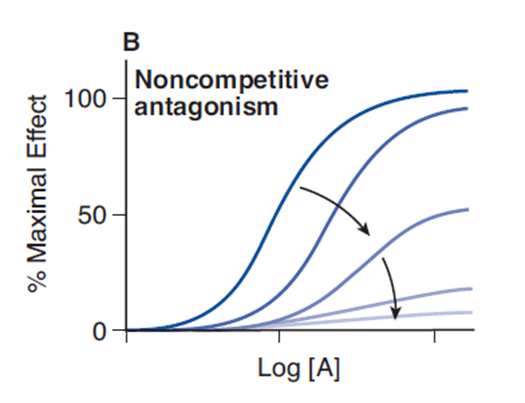
- A non-competitive antagonist binds to an allosteric (non-agonist) site on the receptor to prevent activation of the receptor
- Since it induces a lower biological response on active site binding than acetylcholine, it is a non-competitive antagonist of acetylcholine at the nicotinic receptor
- No matter how high levels of acetylcholine are at the neuromuscular junction, maximal response will not be reached
Advantages and Disadvantages of Sux in ICU
| Effect | Advantage | Disadvantage |
|---|---|---|
| Pharmaceutics | Inexpensive | Needs to be stored at 4 degrees -Shelf life only 7 days when kept in resuscitation trolley |
| Can be given peripherally | ||
| Excretion of 10% of drug unchanged in urine means active metabolites may accumulate and prolong the effects. | ||
| 0.03% of population are abnormal metabolisers resulting in prolonged apnoea | ||
| Causes fasiculations | Offset of fasiculations indicates paralysis | Muscle pains |
| Causes malignant hyperthermia | ||
| Prolonged suxemethonium apnoea | If intubation fails, prolonged apnoea | |
| Some agonism at muscarinic receptor | Second dose of suxemethonium commonly causes bradycardia | |
| Widespread neural depolarisation | Hyperkalaemia Raised intraocular pressure Raised GIT sphincter pressures | |
| Rapid onset (30-60 seconds) | Short period of time before intubating conditions achieved – Less risk of desaturation / apnoea when intubating | |
| Rapid offset (5-10 minuts) | If intubation fails, apnoea will not be prolonged | Muscle paralysis may disappear before airway secured or patient made safe |
| Metabolised by plasma cholinesterase | Metabolism not dependant on organ function | Plasma cholinesterase may be lower in some patients – myriad reasons |
Mooney / JC 2020
Examiner Comments
2018A 10: 46% of candidates passed this question.
This commonly used drug should be very well-known. The question asked for an outline, hence long explanations of various aspects of pharmacology (e.g. pseudocholinesterase deficiency) were unnecessary.
Headings should have included: advantages (e.g. rapid onset, rapid offset, short acting, IV or IM administration, not end organ dependent for metabolism, premixed, safe in pregnancy and neonates). The disadvantages section should have included the following headings: pharmaceutical, adverse drug reactions (including several potentially fatal ones), numerous contraindications, unpleasant side-effects and potential problems with repeat dosing.
2012B 02: 13 (59.1%) of candidates passed.
For a good answer candidates were expected to mention that an agonist is a drug that elicits a maximal response on binding to a receptor. A partial agonist has intrinsic affinity with only partial efficacy and hence is unable to elicit a maximal response. A competitive drug acts at the same binding site of a receptor as an endogenous ligand (e.g. acetylcholine at the neuromuscular junction) and its action therefore is surmountable with increasing concentrations of drug and how this concept relates to suxemethonium. For the remainder of the question, Candidates were expected to mention the advantages and disadvantages of suxamethonium within Intensive Care practice. Good answers included a systematic approach and use of tables and/or well organised lists.
11. Describe the regulation of the coronary circulation.
CICMWrecks Answer
Arteries
- LCA to
- LCx to
- OM1
- OM2
- LAD has branches
- Diagonal 1 or 2
- LCx to
- RCA
- Right Ventricular
- Acute Marginal
- Posterior descending
Veins
- Great, small, middle
- Drain into the thebesian veins → ventricles directly
- Empty into the coronary sinus on the posterior wall or the RV
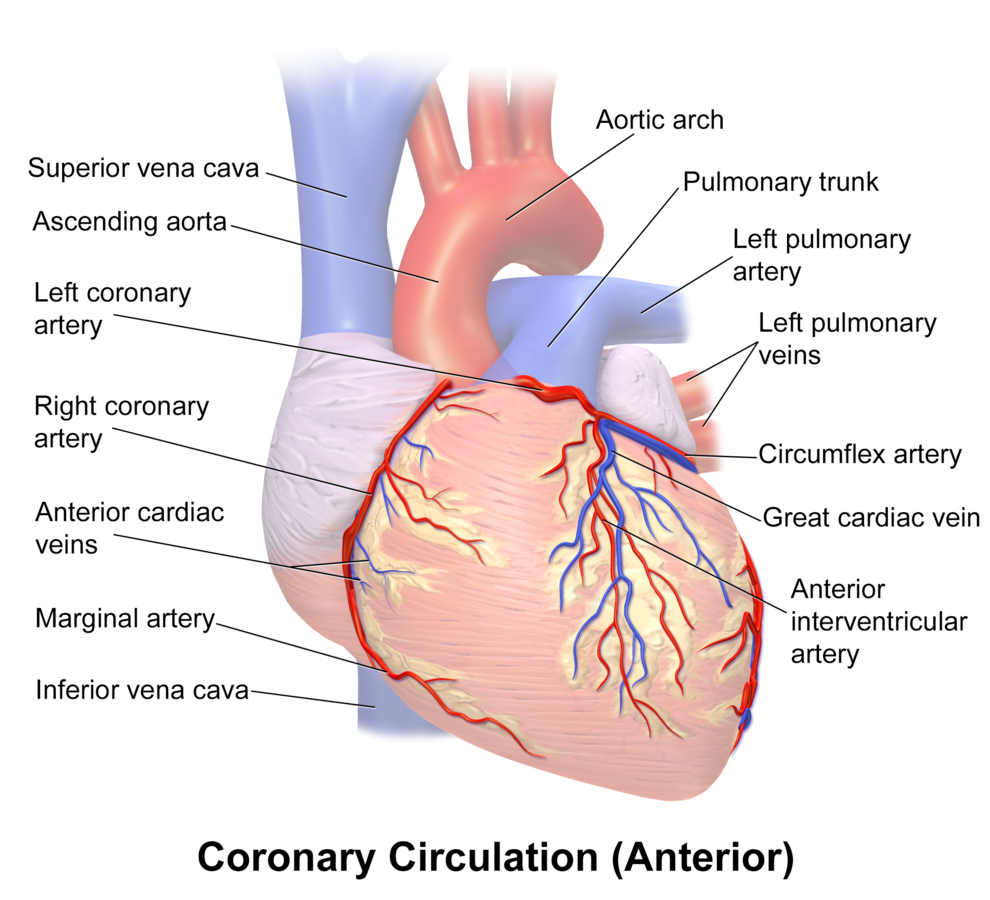
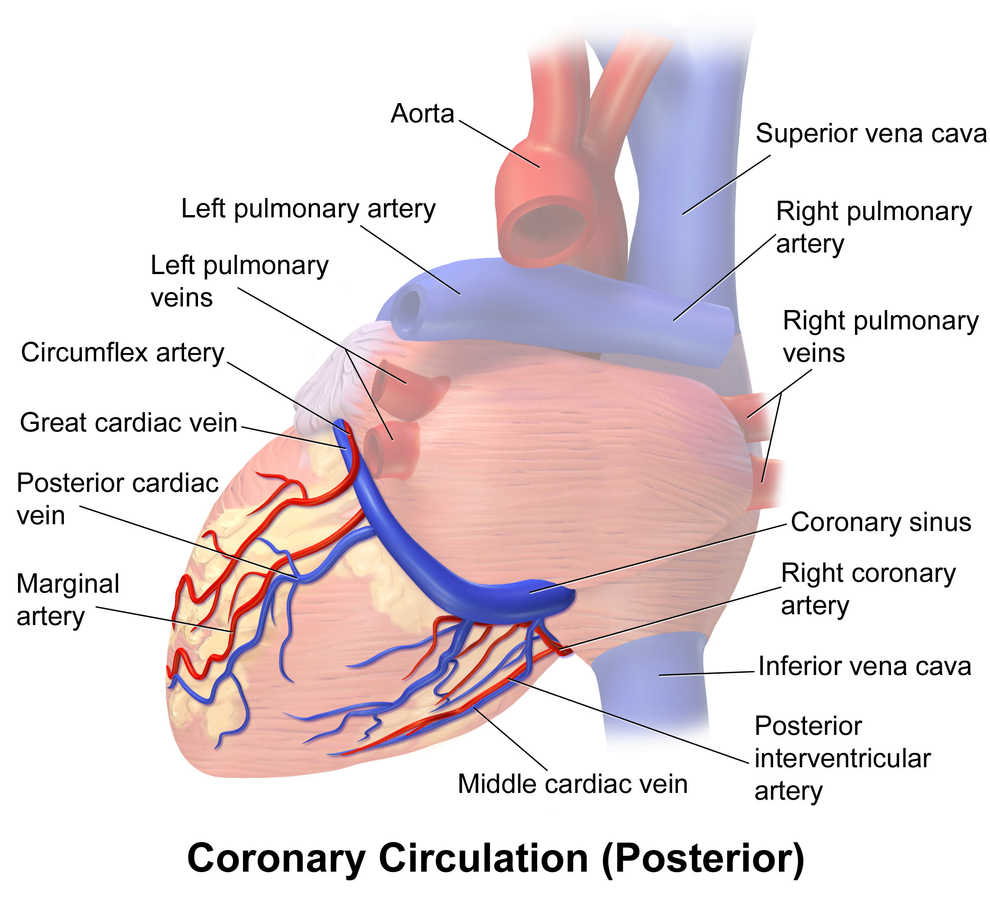
Regional Supply
| Artery | Anatomical region | ECG abnormality on occlusion |
|---|---|---|
| RCA | Down Anterior aspect of the heart, right of the pulmonary trunk Supplies RA SAN and AVN Posterior IVS | RCA occlusion causes → inferior: 2,3 aVF → true posterior: tall R in V1 |
| LCA | Left coronary artery ostia adjacent to left leaflet → Left Main → LCx and LAD Supplies LA, LV IVS AV Bundles | LCA occlusion → Lateral: 1, aVL, V1-V6 |
| LCx | LA and superior LV | anterolateral leads (1, aVL, V5 and V6) |
| LAD | RV, LV Anterior 2/3rds Interventricular Septum | Anteroseptal (V1 and V2) Anteroapical (V3 and V4) with some RCA supply. |
| Left Marginal | The continuation of the LCA after LAD branch Supplies LV laterally | Isolated Abnormalities rare V5, V6 |
| Right Marginal | The anterior branch of the RCA along the inferior border Supplies right ventricle and apex | Isolated Abnormalities rare RCA occlusion causes 2,3 aVF |
Coronary Blood Flow
- 80 mL/min/100 g
- or 200-250 mL/min
- 5% of CO at rest
- Can increase by 3-4 times (up to 400mL/min/100g)
High Extraction Ratio
High at rest (55-65%) body average of 25%
- Extraction ratio can only rise by factor of < 2 to 90%
- AV Δ O₂ = 11 mL/dL
- Coronary venous O₂ content = 5 mL/dL
- Coronary sinus SpO₂= 20mmHg
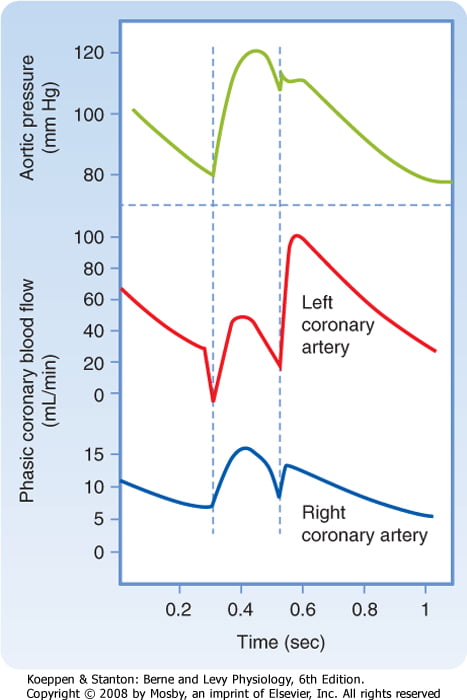
Coronary perfusion pressure (CPP)
- ADP = Ao Diastolic Prssure
- It is different throughout the cycle and b/w ventricles
Phasic Supply as per diagram.
Determinants of Coro BF
- Physical Factors
- Extravascular compression (CPP factors)
- Neural and Neurohumoral Factors
- ↑ SNS tone →
- α receptor mediated vasoconstriction
- β receptor mediated vasodialtion
- ↑ force and rate of contractions → ↑ vasodialtor metabolite release
- Overall effect is dilation
- ↑ PSNS tone → KACh stimulation → mild ↓ Coronary vascular resistance
- ↑ SNS tone →
- Metabolic Factors (main)
- Vasodilatory
- ↑ Adenosine, H, K, CO2, Lactate
- NO → GTP
- ↑ O2 demand → ↓ ATP → ↑KATP channel activation → hyperpolarisation → vasodilation
- Vasodilatory
- Myogenic autoregulation (keep CPP 60-180 mmHg)
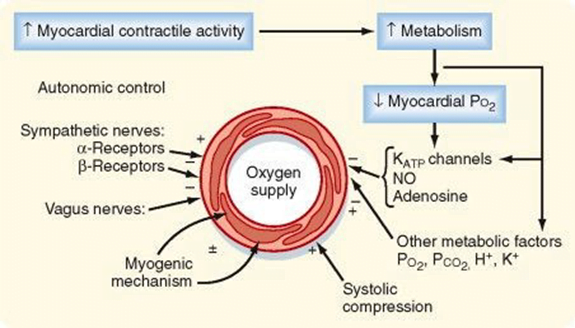
Gladwin 2016
Examiner Comments
2018A 11: 46% of candidates passed this question.
Some answers suffered from listing things rather than describing things as the question required.
Better answers included a description of metabolic, physical and neuro-humoral factors and the relative importance of each.
Many described detailed anatomy which was not necessary.
12. Briefly describe the cardiac events that occur during ventricular diastole.
CICMWrecks Answer
Diastole in the cardiac cycle corresponds with cardiac myocyte relaxation, and includes periods of isovolumetric relaxation and ventricular filling. It lasts approximately 430ms.
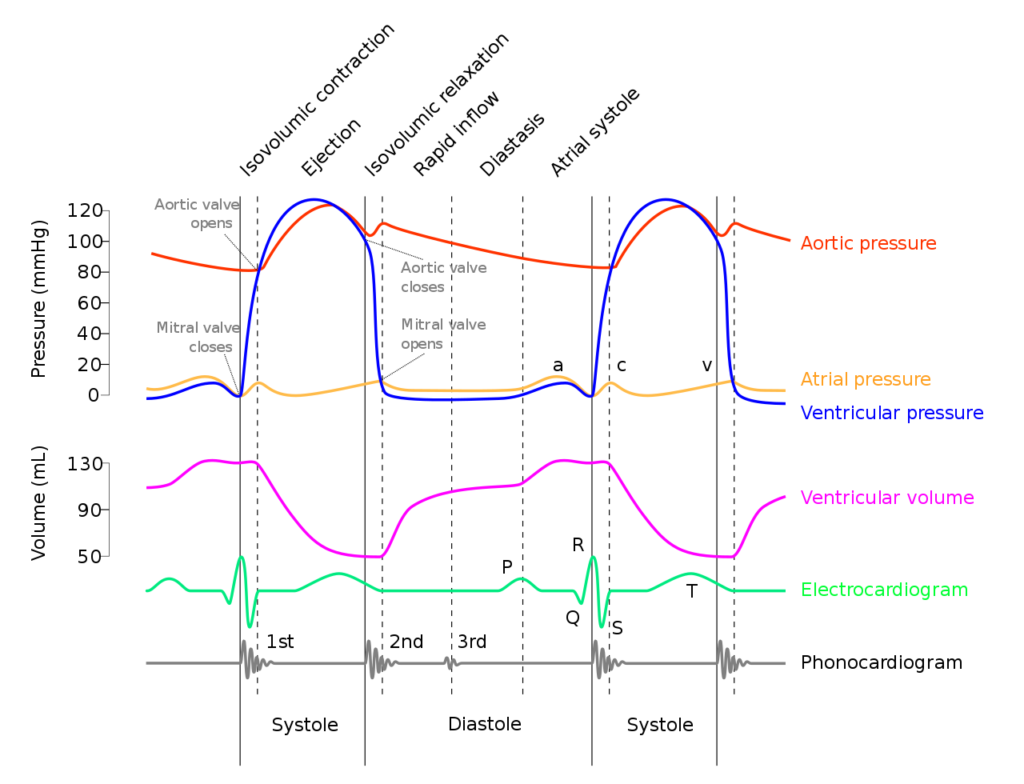
Isovolumetric relaxation
- As aortic pressure overcomes ventricular pressure, and the inertia of forward flow of blood out of the LVOT, the aortic valve closes
- The ventricular myocytes relax, while both the mitral (or tricuspid) and aortic (or pulmonary valves) remain closed
- The volume of blood within the ventricle remains constant (LV End-Systolic Volume ~50mls) , however ventricular pressure decreases from 120mmHg to close to 0mmHg (or 20 to 0mmHg in R ventricle
Ventricular filling
- Passive phase
- As ventricular pressure decreases below the atrial pressure, the mitral (or tricuspid) valve opens allowing forward flow of blood from the atria to the ventricles leading to a progressive increase in ventricular volume and pressure.
- The first two-thirds of the ventricular filling is due to passive filling
- Active phase
- 20% of ventricular filling is provided by the atrial kick during the final quarter of ventricular filling resulting in a slight increase in ventricular pressure
- Ventricular pressure overcomes atrial pressure and the mitral (or tricuspid) valves close
Electrochemical events
- The cardiac action potential is tied to contraction via excitation-contraction mechanism.
- Diastole corresponds to phase IV where voltage gated L and T-type Ca channels close, ceasing Ca influx, and ongoing K efflux repolarizes the myocyte.
- ECG
- The T wave corresponds with ventricular repolarization and occurs immediately prior to ventricular diastole.
- The QRS complex corresponds with ventricular depolarization and occurs immediately prior to ventricular systole
- Diastole corresponds with the terminal part of the T wave to immediately after the QRS complex
- Electrical current through cardiac cycles
- During depolarization, current runs from the base of the heart to the apex (corresponding to flow down bundle of His) the just before the end of depolarization, the direction of flow reverses (corresponding to flow down Purkinje fibres)
- During repolarization, the order reverses, from Purkinje system → Bundle of His
Coronary artery perfusion
- Due to the high ventricular pressures impeding blood flow during systole in a starling-resistor model, left ventricular perfusion (or right ventricular perfusion during pressure-overloaded states) occurs predominantly during diastole.
- As right ventricular pressures are significantly lower than aortic pressures even during systole, perfusion continues (in a normal right ventricle)

Sakurai 2016
Examiner Comments
2018A 12: 29% of candidates passed this question.
Many answers lacked structure and contained insufficient information. Better answers defined diastole and described the mechanical events in the 4 phases of diastole. A common error was the ECG events in diastole. The electrical events and coronary blood flow should have been mentioned.
13. Explain the difference between viscosity and density (10% of marks). Describe the effects of changes in viscosity and density on the flow of gases and liquids (90% of marks).
CICMWrecks Answer
DEFINITIONS
Viscosity (η)
Used to indicate a fluid’s internal resistance to flow. Also thought of as a measure of the friction of a fluid.
e.g. honey has a high viscosity than water and as such moves much slower if poured
Density (ρ)
relates the mass of a substance to its volume such that ρ = kg/m3
Although some gases may have similar viscosity, their densities may be different (eg. O2 and He)
Flow
measure of volume of fluid / gas moved per unit of time (e.g. ml/min).
FLOW
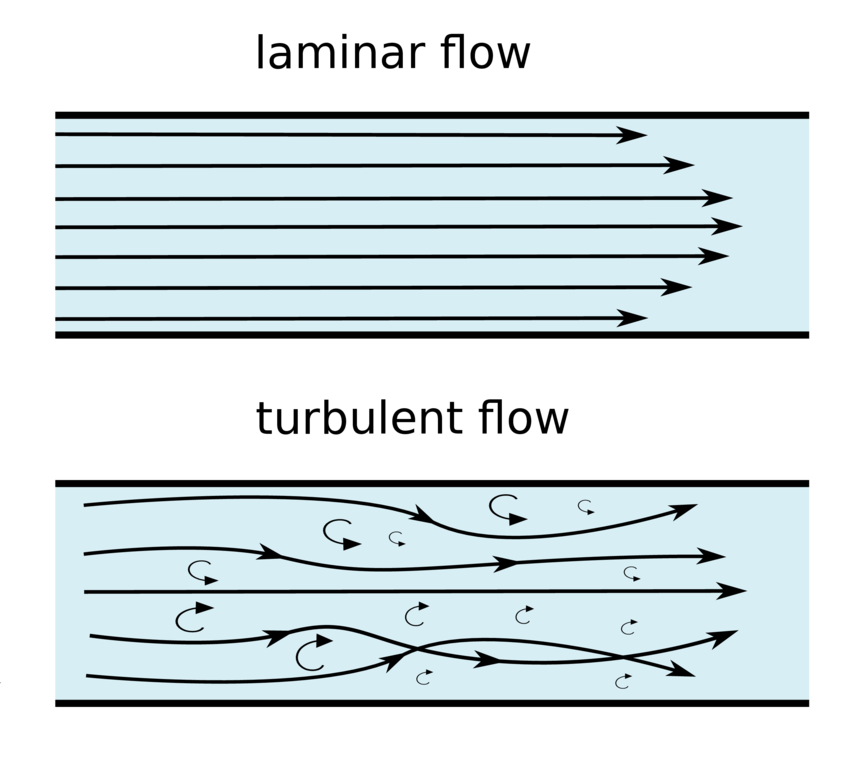
TYPES OF FLOW
Laminar
Turbulent
Transitional
Reynold’s Number
- The Reynolds Number is defined as the ratio of inertial forces to viscous forces in a flowing fluid.
- It is used in many fluid flow correlations and is used to describe the boundaries of fluid flow regimes (laminar, transitional and turbulent).
- Reynold’s number can predict the likelihood of turbulent flow occurring.
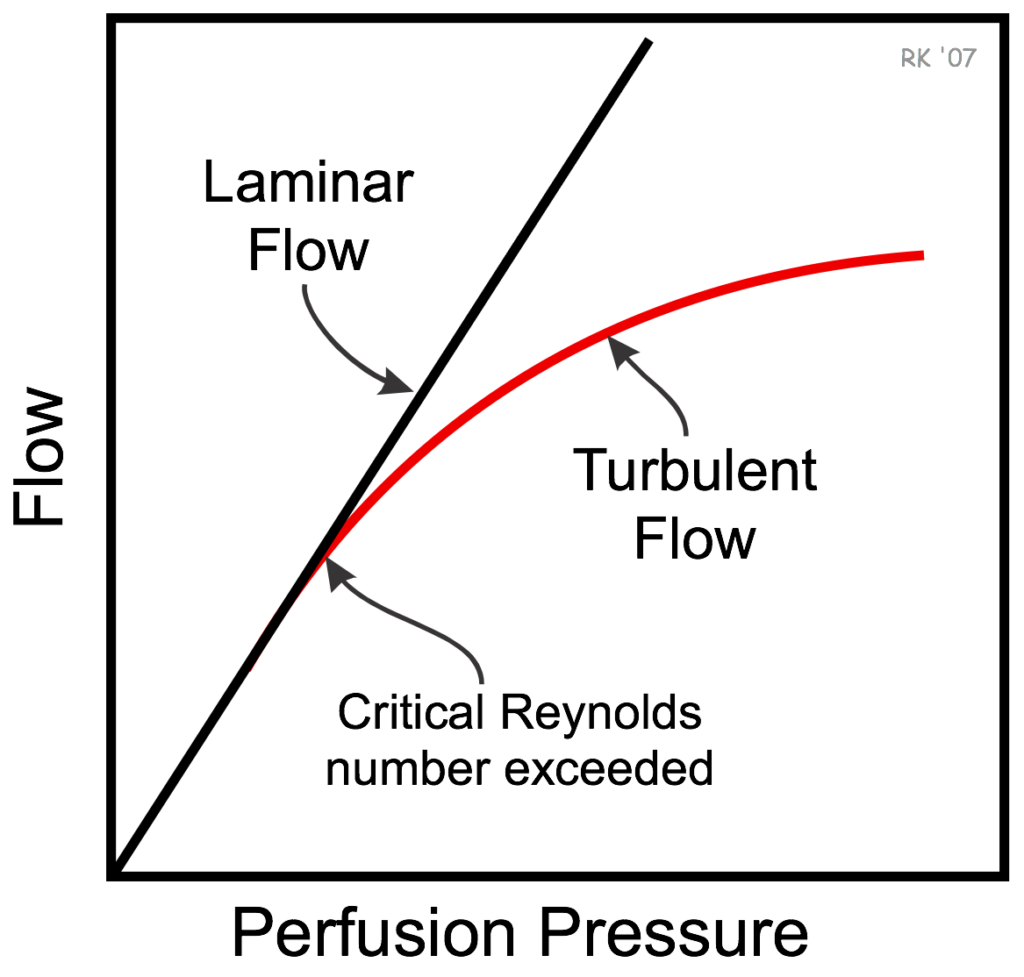
| Flow | Reynold’s Number |
|---|---|
| Laminar | <2300 |
| Transitional | 2300-4000 |
| Turbulent | >4000 |
where
Re is Reynold’s number
r is radius
ρ is density
v is velocity
η is viscosity
- Density (ρ) is the major determinant of Re: ↑ρ (N2 v He) → ↑prob of turbulent flow
- Viscosity (η) has a much smaller contribution to the determination of Re: ↓η → ↑probability of turbulent flow
Laminar Flow
- Laminar flow is the flow that corresponds with low velocities and Reynolds numbers less than 2300.
- In this type of flow, the fluid flows in parallel layers, with no disruption between the layers.
- At low enough velocities, the fluid will tend to flow without lateral mixing, while adjacent layers simply slide past one another
- The movement of substance in the centre is twice the velocity of the movement of substance at the walls (there is no flow at the walls)
- For flow to be laminar, the tube in which it is travelling must have smooth, parallel sides with no branches in the system
Resistance
(Hagan – Poisuelli Equation)
where
R is vessel resistance
η is viscosity
L is length of vessel
r is radius of vessel
Laminar Flow
where
Q is Laminar Flow
r is radius of vessel
ΔP is pressure gradient
η is viscosity
L is length of vessel
- There is a linear relationship between pressure and flow
- Viscosity of the gas / fluid is directly proportionate to resistance:
- ↑η → ↑R → ↓flow
- Density has no effect on the flow of gas during laminar flow
- Altering the other variables will change R as seen
Turbulent Flow
- Turbulent flow is the most common form of flow in nature, and corresponds to the Reynolds numbers higher than a value of 4000
- chaotic and unpredictable, and is often seen with fluids at high velocities
- undergoes irregular fluctuations, or mixing, and continuously changes magnitude and direction
- Flow is disorganised with small eddies forming → creates a square wavefront
Resistance
where
R is Resistance to flow
ρ is density
l is length of vessel
r is radius of vessel
Turbulent Flow
where
Q is flow
ΔP is pressure gradient
ρ is density
l is length
- Flow is inversely proportional to √ρ
- Pressure is proportionate to flow2
- Resistance proportional to density and not viscosity
Transitional Flow
- Transitional or Transient flow is the phase of flow that occurs between laminar and turbulent flow, and corresponds to Reynolds numbers that land between 2300 and 4000.
- In this type of flow, there is a mixture of laminar and turbulent flows present.
- As Reynolds number increase from 2300 to ~4000, there are an increasing amount of disturbances appearing within the flow.
- Transition can occur in either direction, that is laminar–turbulent transitional or turbulent–laminar transitional flow.
Sources: cfdsupport.com, cvphysiology.com
JC 2019
Examiner Comments
2018A 13: 46% of candidates passed this question.
Whilst most candidates defined density correctly, there was a lot of uncertainty regarding viscosity. Most candidates recognised that flow may be laminar, turbulent or transitional. Most accurately recounted Reynolds number and applied this correctly. Additionally, the Poiseuille equation was correctly stated by most candidates and correctly related to laminar flow. Few candidates recalled the equation describing turbulent flow
14. Classify anticholinesterase drugs according to chemical interaction with an example of each (30% of marks). Outline the pharmacodynamic effects of anticholinesterase drugs and their clinical indications (70% of marks).
CICMWrecks Answer
Background
- Acetylcholinesterase (AChE) is an enzyme that hydrolyse acetylcholine (ACh) into choline & acetate
- AChE is found in synaptic clefts and is responsible for the termination of synaptic transmission
- Common action of anti-cholinesterases = allow build up of Ach and prevent it from being destroyed.
Naturally Occuring Cholinesterases
Two types of naturally occurring cholinesterases:
- Achesterase – nerve endings & in RBCs
- Non-specific or pseudocholinesterases – destroy other esters – tissues & plasma
Anticholinesterase drugs
AntiAchE drugs are administered in anaesthesia when spontaneous recovery from NDNMB is occurring to accelerate it.
Classification:
Drugs are classified by the way in which they inhibit the activity of AchE.
3 main types of anticholinesterase:
- Reversible antagonist via electrostatic binding
- ie. edrophonium
- causes electrostatic attachment to the anionic site of the enzyme -> stabilising the H+ bond at the esteratic site -> edrophonium-AchE complex prevents Ach from binding
- Reversible antagonist via covalent bonding [Formation of carbamyl esters (carbamates)]
- ie. neostigmine, physostigmine & pyridostigmine
- antagonise AchE enzyme by being competitive substrate for Ach -> forms a carbamyl-ester complex at the esteratic site of enzyme.
- longer lasting bond (15-30min)
- Irreversible antagonist via covalent bonding
- ie. organophosphate anticholinesterase drugs (echothiopate), insecticides & nerve gases
- combine with Ach at the esteraic site to form a stable covalent bond -> does not undergo hydrolysis.
- synthesis of a new AchE is required.
Pharmacodynamic effects
Anticholinesterase drugs inhibit AChE, thereby increase the concentration of ACh at both nicotinic and muscarinic ACh receptors
Muscarinic effects occur at lower doses than nicotinic effects
Muscarinic effects
- CVS – bradycardia ± hypotension
- RESP – bronchoconstriction ± bronchospasm
- CNS – miosis, cholinergic syndrome – confusion, agitation, nausea/vomiting
- GIT – hypersalivation, ↑GIT motility, nausea/vomiting, diarrhoea
- GUT – urination, incontinence
- OTHER – lacrimation, diaphoresis
Mnemonic: SLUDGE-BM: Salivation/Sweating, Lacrimation, Urination, Diaphoresis/Diarrhoea, GI upset, Emesis, Bradycardia/bronchospasm, Miosis
Nicotinic effects
- Reversal of non-depolarising neuromuscular blockers
- Prolongs effect of suxamethonium (depolarising NMB)
- Anticholinesterase overdose → excess synaptic ACh → depolarisation block ± fasciculation
Clinical uses
- Reversal of non-depolarising neuromuscular blocker
- Mechanism – anticholinesterase drugs ↑ synaptic ACh → competes with ND-NMB in synapse for nAChR → reversal of neuromuscular block
- Drugs – usu. neostigmine, administered together with glycopyrrolate or atropine
- Diagnosis and treatment of myasthenia gravis
- Mechanism – anticholinesterase drugs ↑ synaptic ACh → competes with myasthenia auto-antibodies for post-synaptic nAChR → ↑muscle strength
- Drugs – e.g. edrophonium for diagnosis, pyridostigmine for maintenance
- Treatment of cognitive impairment in neurodegenerative diseases (e.g. Alzheimer’s disease, Lewy body dementia, etc)
- Mechanism – ↑ synaptic ACh in CNS → ↑ cholinergic transmission
- Drugs – e.g. rivastigmine, galantamine, donepezil
- Treatment of glaucoma
- Mechanism – constriction of sphincter pupillae and ciliary muscles → miosis → facilitate outflow of aqueous humor → IOP decreases
- Drugs – e.g. echothiophate eye drops, physostigmine
- Treatment of anticholinergic syndrome
- Anticholinergic syndrome caused by: anti-histamines, anti-parkinsonians, atropine, anti-spasmodics, mydriatics, skeletal muscle relaxants, plants
- CLINICAL FEATURES: delirium, tachycardia, dry and flushed skin, dilated pupils, myoclonus, hyperthermia, urinary retention, bowel sounds, seizures, dysrhythmias(tachy)
- Mechanism – increase synaptic ACh
- Drugs – e.g. physostigmine (tertiary amine + lipophilic → readily crosses BBB)
JC 2019
Examiner Comments
2018A 14: 32% of candidates passed this question.
Many candidates who scored poorly confused anticholinesterase drugs with anticholinergic drugs. Some described pharmacokinetics when it was not asked. Similarly, treatment of organophosphate poisoning and/or cholinergic crisis was not asked for in the question.
15. Describe the physiological regulation of intracranial pressure.
CICMWrecks Answer: ICP
Definition:
- ICP: hydrostatic pressure within the cranial vault
- Normal value is 5-15 mmHg
- focal ischaemia when ICP > 20 mmHg
- global ischaemia when ICP > 50mmHg
Munro-Kellie Doctrine
- The rigid and closed cranial vault forms a fixed brain volume containing
- Brain parenchyma (80%, 1400 g)
- CSF (10%; 75 mL)
- Cerebral blood and vessels (10%; 75 mL)
- Δ’s in volume of any components → Δ’s in others or and increase in ICP
CSF Production / Absorption
CSF Production:
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
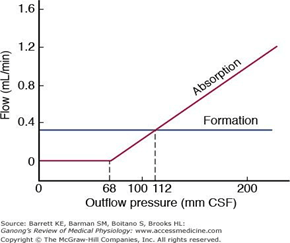
CSF Absorption:
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
Control of ICP
- ICP is regulated via volume buffering
- i.e. increase in volume of one intracranial component leads to compensatory decrease in volume of other intracranial components
- When volume buffering mechanism is exhausted → rapid increase in ICP (decompensation)
- Movement of cerebral venous blood = rapid compensation, lower capacity
- Movement of CSF = gradual compensation, larger capacity
Determinants of ICP:
- Brain
- Age / Mass
- Space occupying lesions
- Cerebral Oedema
- CSF
- CSF production
- CSF Absorption
- Cerebral Blood Volume
- Cerebral autoregulation: Flow-metabolic coupling
- Cerebral metabolic rate
- Increase in systemic blood pressure / flow
- Venous Outflow obstruction
- Vasoactive agents
- Monro-Kellie Doctrine
- Loss of above – e.g. Fractures, surgery
Compensation for Elevated ICP (Intracranial Pressure)
Early compensation
- Δ CSF distribution and flow
- CSF is displaced to spinal subarachnoid space
- ↑’d resorption rate
Late compensation
- ↑ ICP → ↓ CBF → ↓ in cerebral blood volume → cerebral ischaemia
Decompensation
- ↑ICP → ↓ in cerebral tissue volume → brain herniation
- Cushing Reflex
- Hypertension, bradycardia and abnormal breathing associated with raised ICP
- Mechanism:
- Stage 1:
- ↑ ICP → ↓ blood supply to vasomotor area → Local hypoxia/hypercarbia → ↑ SNS >> ↑ PSPS vasomotor stimulation
- ↑ TPR → ↑ MAP
- ↑ HR → ↑ CO
- → compensatory ↑CBF
- Stage 2:
- ↑ CO → Baroreceptor stimulation → ↑ Vagus nerve stimulation → Bradycardia and ↓ contractility.
- Stage 1:
Gladwin / JC 2020
Examiner Comments
2018A 15: 45% of candidates passed this question.
A definition and a normal value were expected. A description of the Monro-Kellie doctrine was expected. Better answers divided into the various components of the cranium with the answer focussing on cerebral blood volume and CSF volume as the brain tissue as no capacity to change its volume.
16. Compare and contrast the pharmacology of furosemide (frusemide) and acetazolamide.
Examiner Comments
2018A 16: 30% of candidates passed this question.
The use of a table assisted with both clarity and the ability to compare the two drugs. Writing separate essays about each makes it difficult to score well. It was expected that candidates would follow a standard pharmacology format and discuss pharmaceutics, pharmacokinetics, pharmacodynamics and adverse drug reactions. Both of these drugs are ‘Level A’ in the syllabus and a suitable level of detail was expected.
It was expected candidates would discuss in detail the mechanism of action, electrolyte and acid-base effects. Pharmacokinetic values were poorly answered. Qualitative terms such as ‘moderate, good and some’ are vague and should be avoided. Only correct numerical values (or ranges) attracted full marks.
17. Define the osmolality and tonicity of an intravenous fluid (20% of marks). Compare and contrast the pharmacology of intravenous Normal Saline 0.9% and 5% Dextrose (80% of marks).
CICMWrecks Answer
Definitions
Osmolality is a measure of solute concentration as defined as the number of osmotically active particles per kilogram water.
Tonicity is the effective osmolality and is equal to the sum of the concentrations of the solutes which have the capacity to exert an osmotic force across the membrane.
Pharmacology
Examiner Comments
2018A 17: 29% of candidates passed this question.
Most candidates gave an adequate definition of osmolality and tonicity. A single concise sentence for each attracted full marks. Some candidates drew diagrams & equations, which added few marks. Some candidates confused osmolarity (mOsm/L) and osmolality (mOsm/kg).
Tonicity was best defined as the number of ‘effective’ osmols (those that cannot cross the cell membrane) in a solution relative to plasma. The use of a table greatly facilitated the comparison of 0.9% saline and 5% dextrose solutions. Values for composition, osmolarity and osmolality were poorly done. Some manufacturers state calculated values and some approximate values on the bags – both were accepted.
No candidate correctly pointed out the fluids respectively have 9g NaCl and 50g dextrose per litre.
18. Compare and contrast non-invasive oscillometric and invasive arterial blood pressure monitoring.
CICMWrecks Answer
NIBP
Invasive BP
Overview
Cuff applied on arm, connected to an inflating device, and Koratkoff sounds are heard to determine BP
arterial catheter connected to a pressure transducer
Uses
BP measurement (SBP,DBP)
- blood pressure (systolic, diastolic, mean and pulse pressure)
- arterial blood sampling
Specific Indications
BP monitoring
- Labile blood pressure
- Anticipation of haemodynamic instability
- Titration of vasoactive drugs
- Frequent blood sampling
- Morbid obesity (unable to fit an appropriately sized NIBP cuff)
Description / Components
- Two cuffs
- Occlusive cuff
- Measurement cuff
- Tubing
- Device for inflating the occlusive cuff and gradually deflating it
- Aneroid barometer for transducing pressure
- Display
- arterial line
- 48 inches of non-compressible rigid-walled, fluid filled tubing
- pressure transducer and automatic flushing system
- pressure bag and automated slow infusion (1-3mL/h) of pressurised saline
- electronic transducer amplifier display
Method of Insertion / Use
- Cuff is inflated until the radial pressure is no longer palpable
This is approximates SBP. - Cuff is deflated, and reinflated to 20mmHg above the estimated SBP
- Cuff is deflated at a rate of 2-3mmHg.s-1 whilst auscultating the brachial artery
When cuff pressure equals:- SBP
Turbulent flow occurs past the cuff, turbulent flow causes the first of the Korotkoff sounds (clear tapping pulsations) to be heard. - DBP
The cuff no longer compresses the vessel at all, so no turbulent flow occurs and nothing is auscultated.
- SBP
- fluctuations of vascular pressure cause a pulsation of the saline column
- displaces electromanometer’s diaphragm which has a built in strain gauge (Wheatstone bridge principle)
- deformation leads to a change in resistance of the strain gauge which is sensed electronically
- wave form built up by Fourier analysis from sinusoids or simple wave forms
- wave forms differ depending on where the cannula is inserted
Needs Calibrating (‘zeroing’) and Square wave test (aka fast flush test) to check for damping of system
Accuracy and Errors
- Requires an appropriately sized cuff
Cuff should be ~20% greater than arm diameter.- Cuffs that are too small will over-read
- Cuffs that are too wide will under-read
- Requires a regular rhythm
- Inaccurate at extremes of blood pressure
- Inaccurate when used more frequently than once per minute
- Inaccurate when the vessel is incompressible
- Heavily calcified vessels
- When applied to forearm/foreleg
- Time consuming, cannot provide continuous monitoring
- Does not require calibration
Common sources of error
- Cannula insertion/position error
- bubbles in catheter-transducer system → decreased resonant frequency
- clotting in arterial catheter
- elastic walls causes increased damping
- Transducer not levelled (Not calibrated properly)
- cannula won’t flush – kinked, clotted, tissued
Complications
- Pain
- Local pressure injury
- May cause neuropraxia
- Pain
- thrombosis and distal ischaemia
- infection
- increased diagnostic blood loss and anemia
- retrograde air embolism
- inadvertent drug/air injection
- haematoma (+/- nerve compression)
- retroperitoneal haematoma (femoral)
- bowel perforation (femoral)
- vessel damage may lead to stricture and prevent future AV fistula formation for haemodialysis
- pseudo-aneurysm
- arterial dissection
- arteriovenous fistula
Other Information
- MAP can be calculated
- Variation seen at different limbs
Information other than blood pressure can be obtained:
- pulse rate and rhythm
- effects of dysrhythmia on perfusion
- ECG lead disconnection
- continuous cardiac output using pulse contour analysis
- specific wave form morphologies might be diagnostic
— e.g. slow rising = AS, pulsus alternans = tamponade - pulse pressure variation (suggests fluid responsiveness)
- steeper upstroke of pulse pressure = increased contractility
- area under upstroke = SV
- steep downstroke = low SVR
Variation in arterial waveform at different sites
JC 2019
Examiner Comments
2018A 18: 52% of candidates passed this question.
There were some good answers, though invasive BP measurement was better answered than oscillometry. Many candidates provided extensive detail in one area i.e. the workings of a Wheatstone bridge, to the detriment of a balanced answer. Few seemed to have a structure consisting of “equipment, method, sources of error, advantages, disadvantages” or similar and missed providing important information as a result. Several described auscultatory non-invasive blood pressure measurement, rather than oscillometry, which although related in principle is a different process.
19. Explain the mechanisms by which normal body temperature is maintained and regulated.
CICMWrecks Answer: Temperature Regulation
Definitions
- Heat – A form of kinetic energy → being a state of “thermal agitation” of molecules in a substance
- Temperature – A measure of a physical property of a substance that determines the tendency for heat to flow from one object to another → heat energy is transferred from a region of higher temperature to a region of lower temperature
- Temperature setpoint is the level at which the body attempts to maintain its temperature.
- Core body temperature:
- Refers to deep body temperature of main internal organs (in head, trunk, abdomen) →
sites where metabolic activity occur (Ie. heat production) - Kept constant at 37 +/- 0.4 °C (“Normothermia”) → displays normal variations:
- Diurnal variation – ↑ in evening (37.3 °C) and ↓ in early morning (35.8 °C)
- Menstrual variation – ↑ 0.5 °C in latter half of cycle
- Refers to deep body temperature of main internal organs (in head, trunk, abdomen) →
- Variations in core body temperature:
- Normothermia → core body temperature 37 +/- 0.4 °C
- Hypothermia → core body temperature < 36 °C
- Hyperthermia → core body temperature > 37.5 °C
- Peripheral body temperature:
- Refers to body temperature peripherally (Ie. skin, arms, legs, superficial tissues of core
sites) → sites where heat loss occur - Temperature varies widely → always LESS than core body temperature
- Refers to body temperature peripherally (Ie. skin, arms, legs, superficial tissues of core
- Regulation of body temperature is done by balancing heat loss and heat production, predominantly through behavioural mechanisms and skin
Thermoneutral Zone
- The range of environmental temperatures in which the metabolic heat production (and oxygen consumption) is minimal and steady and where core temperature is maintained by vasomotor activity alone.
- 25-30 °C for a naked, upright man in still air
Interthreshold Range
- The range of core temprature over which no autonomic thermoregulatory responses occur
- Normally 0.2 -0.4 °C in a non-anaesthetized state
Regulation of Body Temperature
Temperature sensors are central and peripheral, whilst regulation occurs centrally, and has multiple
effectors
| Cold | Warm | |
|---|---|---|
| Receptor | Bulbs of Krause | Bulbs of Ruffini |
| Afferent nerve type | via Aδ and unmyelinated C sensory fibres | Unmyelinated C fibres |
| Spinal synapse location | Rexed lamina 1,5 | Rexed lamina 1, 2 |
| Ascending tract | Lateral spinothalamic tract in anterior spinal cord | Lateral spinothalamic tract in anterior spinal cord |
| Brainstem synapse | Reticular system of medulla | Reticular system of medulla |
| Central Processor | Hypothalamus (posterior) | Hypothalamus (anterior) |
| Thresholds | No activity at 40 degrees ↑ing activity between 10-27 degrees (remember thermoneutral zone) | Activity with increasing between 30-45 °C Upper threshold limit = 46 °C |
Sensors:
- Central
- Deep tissues/viscera (Eg. in intestinal wall)
- Brain (anterior hypothalamus and extra-hypothalamic areas)
- Spinal cord
- Peripheral
- Dermis (main)
- Corneas
Central Controller:
Hypothalamus is the main body temperature regulatory centre
- Posterior hypothalamus responds to cold
- inputs from peripheral afferents
- responsible for the temperature set point
- ACh is the major neurotransmitter here.
- Anterior hypothalamus responds to heat
- both peripheral input and change in blood temperature
- Major neurotransmitters are Nad, 5HT, dopamine and prostaglandins.
Effector Mechanisms:
- Skin
- Sweating (evaporation)
- Secrete plasma-like fluid Na142, Cl- 104 (urea/K, etc v low amounts) osmolality controlled by rate of secretion and aldosterone
- loss via evaporation (latent heat of vapourisation of water)
- 1-2L in extreme exercise up to 10 L max per 25 hours.
- 0.54 kcal/gram of H2O evaporated
- Degree of evaporation determined by ambient temperature and relative humidity
- Blood Flow (radiation)
- 8 (300 mL/min) – 30% CO skin
- Vasodilation up to 30 fold increase
- Vasoconstriction up to 10 fold decrease
- vasoconstriction/dilation controlled by SNS (α1-mediated) vascular SM contraction and hypothalamic feedback
- Fat/Clothing
- Fat conducts heat 1/3 as readily as other tissues (decreased radiation)
- Clothes reduce conductive heat loss via private zone of air adjacent to skin and decreased convection air currents -50% and more if specialised
- Sweating (evaporation)
- Non-Skin
- Non-shivering thermogenesis
- Predominantly in brown fat
- Uncoupling of oxadative phosphorylation
- Increased heat gain without oxygen consumption/ATP production
- Shivering
- Increased heat gain by metabolism
- Behaviour
- For gain (exercise) or loss (submerging in water)
- Voluntary muscle contraction (Ie. ↑ activity with cold stress; ↓ with hot stress) – Affects heat production
- Body posturing (Ie. ↓ BSA with cold stress;↑ with hot stress) – Affects heat loss
- Clothing (Ie. ↑ clothing with cold stress; ↓ with hot stress) – Affects heat loss
- Appetite
- Cold stress stimulates food-induced thermogenesis → ↑ metabolic rate and heat production
- Thyroid hormone secretion
- Cold stress stimulates thyroid hormone secretion → long-term ↑ metabolic rate and heat production
- Non-shivering thermogenesis
Gladwin / JC 2019
Examiner Comments
2018A 19: 52% of candidates passed this question.
The best answers were systematic, using a sensor, integrator, effector approach, while also mentioning physiological variations i.e. diurnal, with ovulatory cycle etc.
Few candidates raised the concept of central and peripheral compartments. The differentiation of the concepts of set point, interthreshold range and thermoneutral zone was often confused.
20. Outline the structure (20% of marks) and function (80% of marks) of the hypothalamus.
CICMWrecks Answer
Hypothalamus
- Hypothalamus
- organ that regulates large number of autonomic/ endocrine processes
- acts as control centre
- Cells
- magnocellular neurosecretory cells in the paraventricular nucleus and the supraoptic nucleus of the hypothalamus produce neurohypophysial hormones, oxytocin and vasopressin. These hormones are released into the blood in the posterior pituitary.
- Much smaller parvocellular neurosecretory cells, neurons of the paraventricular nucleus, release corticotropin-releasing hormone and other hormones into the hypophyseal portal system, where these hormones diffuse to the anterior pituitary.
- Location
- Part of the fore brain. Considered part of the diencephalon
- Located below the thalamus and forms the floor and lower part of the lateral walls of the third ventricle
- Anteriorly extends up to the optic chiasma
- Posteriorly continuous with the tegmentum of midbrain
- Structure
- formed by gray matter conglomeration of neurons that organize in nuclei and also by white-matter substance formed by myelinated nervous fibers.
- Nuclei send and receive fibers to other parts of brain
- divided by the anterior horns of the fornix in a lateral, medial, and periventricular (median) region and by a coronal plane passing through the infundibulum in an anterior and posterior region.
- Regions
- Anterior / Prechiasmatic / Supraoptic
- located above the optic chiasm
- contains: supraoptic, preoptic and medial preoptic, the suprachiasmatic and the anterior hypothalamic nucleus, alongside with the paraventricular nucleus
- Infundibular / Tuberal
- located above tuber cinereum (between anterior and posterior)
- composed of two parts, anterior and lateral
- contains: dorsomedial, ventromedial, paraventricular, supraoptic, and arcuate nucleui
- Posterior / Mamillary
- formed by medial and lateral area
- medial area contains mamillary nuclei and posterior nucleus
- lateral area contains lateral nucleus and tuberomammillary nucleus
- Anterior / Prechiasmatic / Supraoptic
- Connections: Nervous, Endocrine, CSF, Direct to pituitary
- Blood supply
- mainly hypophyseal artery (branch of anterior cerebral artery)
- Detailed arterial supply:
- Anterior: branches of ACA and ACOM
- Tuberal: branches of PCOM and superior hypophyseal artery
- Posterior: branches of PCA
- Infundibulum: superior hypophyseal arteries from the ophthalmic branch of ICA (C6)
- drains into hypothalamohypophyseal system of veins to anterior pituitary → hypophyseal vein
Function of Hypothalamus
Autonomic nervous system activity
- CVS:
- Ant hypothalamic stimulation → ↓BP + ↓HR
- Post hypothalamic stimulation → ↑BP + ↑HR
- Thermoregulatory: integrates thermoreceptor input + controls activity of heat loss + heat gain mechanisms
- Satiety: hunger modulated by glucose, CCK, glucagon, and leptin
- Water balance:
- Osmoreceptors: control ADH release from posterior pituitary
- ATII: stimulates thirst + ADH release via subfornical organ + organum vasculosum
- Circadian rhythm
- Behaviour
- Sexual function
Endocrine/ hormonal activity
- Direct neural control of posterior pituitary gland
- Pituitary neurosecretory neurons
- Magnocellular neurons:
- consists of SON + PVN
- synthesise and secrete ADH and oxytocin
- Parvocellular neurons:
- form tuberoinfundibular tract → secrete hypophysiotropic hormones
- release controlled by Nad, dopamine, 5-HT
- Magnocellular neurons:
- Hypothalamic hormones → action in pituitary
- Anterior pituitary by hormone secretion into long portal vein
- GnRH → stimulates FSH + LH release
- CRH → stimulates ACTH release
- GHRH → stimulates GH release
- TRH → stimulates TSH release
- Somatostatin (GH inhibiting hormone) → inhibits GH, TSH, ACTH, and PRL release
- PRL releasing hormone (PRH) → stimulates PRL release
- Dopamine → inhibits PRL release
- Posterior pituitary by neuronal innervation
- ACh → stimulates release of ADH + oxytoxin
- NAd → inhibits ADH + oxytocin secretion
- Anterior pituitary by hormone secretion into long portal vein
(See next tab for detailed table of nuclei with functions, and communications of hypothalamus)
Sources: https://www.intechopen.com/chapters/63258
https://human-memory.net/hypothalamus/#Structure
JC / Kerr 2022
Additional: Nuclei and functions Table, Communications
Nuclei and functions
| Region | Area | Nucleus | Function |
|---|---|---|---|
| Anterior (Supraoptic / prechiasmatic) | Preoptic | Preoptic nucleus | – Thermoregulation |
| Medial | Medial preoptic nucleus | – Regulates the release of gonadotropic hormones from the adenohypophysis – Contains the sexually dimorphic nucleus, which releases GnRH, differential development between sexes is based upon in utero testosterone levels – Thermoregulation | |
| Supraoptioc nucleus | – Vasopressin release – Oxytocin release | ||
| Paraventricular nucleus | – thyrotropin-releasing hormone release – corticotropin-releasing hormone release – oxytocin release – vasopressin release – somatostatin round | ||
| Anterior hypothalamic nucleus | – thermoregulation – panting – sweating – thyrotropin inhibition | ||
| Suprachiasmatic nucleus | – Circadian rhythms | ||
| Lateral | Lateral nucleus | primary source of orexin neurons that project throughout the brain and spinal cord – Mainly promotion of feeding behaviour and arousal | |
| Infundibular (Middle / tuberal) | Medial | Dorsomedial hypothalamic nuclei | – blood pressure – heart rate – GI stimulation |
| Ventromedial nucleus | – satiety – neuroendocrine control | ||
| Arcuate nucleus | – Growth hormone-releasing hormone (GHRH) – feeding – Dopamine-mediated prolactin inhibition | ||
| Lateral | Lateral nucleus | primary source of orexin neurons that project throughout the brain and spinal cord – Mainly promotion of feeding behaviour and arousal | |
| Lateral tuberal nuclei | – regulation of feeding. Its absence or destruction has been implicated in extremes of starvation such as anorexia nervosa. | ||
| Posterior (mamillary) | Medial | Mamillary nuclei (part of mamillary bodies) | – memory |
| Posterior nucleus | – Increase blood pressure – pupillary dilation – shivering – vasopressin release | ||
| Lateral | Lateral nucleus | primary source of orexin neurons that project throughout the brain and spinal cord – Mainly promotion of feeding behaviour and arousal | |
| Tuberomammillary nucleus | – arousal (wakefulness and attention) – feeding and energy balance – learning – memory – sleep |
Communications of Hypothalamus
- Nervous connections: divided into afferent and efferent fibers
- Afferent fibres: carry somatic and visceral sensations as well as from special senses
- Somatic and visceral afferents via lemniscal afferent fibers and nucleus of tractus solitarius, that reach the hypothalamus via reticular formation
- Visual afferents from the optic chiasma reach the suprachiasmatic nucleus
- Olfactory afferents are received through medial forebrain bundle
- Auditory afferents though not identified completely but are influenced by the hypothalamus
- Hippocampo-hypothalamic afferents reach via fornix to mamillary bodies
- Tegmental fibers from midbrain
- Thalamo-hypothalamic fibers from the midline and dorsomedial nuclei of the thalamus
- Amygdalo-hypothalamic fibers from the amygdaloid complex reach the hypothalamus via stria terminalis
- Efferent fibers: complex and numerous
- To brain stem and spinal cord: The hypothalamic nuclei send efferent fibers to nuclei present in the brainstem and spinal cord. In this way, they control the autonomic nervous system.
- Mammillothalamic Tract: This tract consists of fibers arising in the mamillary body and terminating in the anterior nucleus of thalamus.
- Mammillotegmental Tract: These fibers terminate in the reticular formation, present in the tegmentum of the midbrain.
- Limbic System: The nuclei in the hypothalamus also send efferent fibers to the various nuclei of the limbic system.
- Afferent fibres: carry somatic and visceral sensations as well as from special senses
- Endocrine:
- regulating factors or hormones released by hypothalamus → reach pituitary via the hypophyseal portal system of veins → cells of pituitary release hormones
- Direct:
- Cell bodies present in hypothalamus → axonal terminals in posterior pituitary gland
- Bodies synthesise oxytocin and vasopressin, stored in axonal terminals in posterior pituitary and released on demand
- CSF:
- clear metabolic waste
- distribute glucose, amino acids, lipids, and neurotransmitters
Examiner Comments
2018A 20: 21% of candidates passed this question.
Most candidates understood the endocrine functions of the hypothalamus, and to some degree its interactions with the pituitary. Fewer candidates mentioned the importance of the hypothalamus as an integrator for the autonomic nervous system, or its roles in arousal/emotions.
Many candidates had only a vague idea of the structure of the hypothalamus, while the best candidates were able to relate function to structure quite accurately
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | respiratory changes in pregnancy. Explain why pregnant patients desaturate so quickly. “High Flow” Oxygen Therapy – diagram In relation to respiration, describe the central chemoreceptors, and explain their role in the control of ventilation. lung volumes and capacities – Why does the lung not “collapse” at the end of expiration? |
| G. CVS | This viva will examine pacemaker cells and antiarrhythmic drugs – ionic currents responsible for spontaneous electrical activity of sinus nodepacemaker cells. antibiotics and antiarrhythmics – Which classes of antibiotics are effective against Gram positive organisms? cardiovascular physiology and blood – Describe the important features of a left ventricular pressure-volume loop. adreno-receptors, second messengers and calcium |
| H. Renal | Describe the physiological processes which cause oliguria in response to acute hypovolaemia? Renal clearance and aspects of the ECG. |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | Define pain and describe how pain is detected in response to a peripheral noxious stimulus. nerve action potentials and neuromuscular blockade. |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | |
| O. GIT | Define vomiting. Describe the inputs to the vomiting centre. |
| P. Nutrition and Metabolism | |
| Q. Haematology | Outline the different blood groups and explain why they are different. The pharmacology of common anticoagulants (UFH) and the physics of arterial lines. |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | insulin and glomerular filtration. |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |


Recent Comments