SAQs
1. Compare and contrast the pharmacology of ibuprofen and paracetamol
Examiner Comments
2017B 01: 65% of candidates passed this question.
This was a standard compare and contrast question of common analgesic pharmacology and it was generally well answered. The use of a table ensured all areas were covered including class, indications, pharmaceutics, mode of action, pharmacodynamics, pharmacokinetics and adverse effects. The uncertain nature (and possibilities) of the mechanism of action of paracetamol was alluded to in better responses.
Details of the comparative pharmacokinetics were often lacking. Answers should have included a comment on first-pass effect, the significance of the difference in protein binding and the details of metabolism, particularly paracetamol. Metabolism limited to “hepatic metabolism and renal excretion” gained no marks as better responses were more detailed and clearer about the differences between the two drugs. Knowledge of metabolism at therapeutic doses and the effect of overdose were expected. Better answers included potential interactions with other drugs (e.g. warfarin) and contraindications to the use of these drugs.
2. Outline the daily nutritional requirements, including electrolytes, for a normal 70 kg adult
CICMWrecks Answer
MACRONUTRIENTS
CHNOPS (carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulphur)
Provide bulk energy
- Carbohydrates: compounds made up of types of sugar.
- monosaccharides (such as glucose and fructose)
- disaccharides (such as sucrose and lactose)
- oligosaccharides
- polysaccharides (such as starch, glycogen, and cellulose).
- Proteins: are organic compounds that consist of amino acids joined by peptide bonds.
- Essential (cannot be manufactured in the body: phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine) and non-essential
- Fats: glycerin molecule with three fatty acids attached.
- Essential (alpha-linolenic acid (ω-3) and linoleic acid (ω -6)) and non-essential
- Saturated and Unsaturated
| Biomolecule | Kilocalories per 1 gram |
|---|---|
| Protein | 4 |
| Carbohydrate | 4 |
| Fat | 9 |
MICRONUTRIENTS
Support metabolism
- Dietary minerals: exogenous chemical elements indispensable for life
- Vitamins: organic molecules essential for an organism that are not classified as amino acids or fatty acids. They commonly function as enzymatic cofactors, metabolic regulators, or antioxidants.
Suggested daily nutritional requirements in humans
| Requirement | 70 kg Adult | |
|---|---|---|
| ENERGY REQUIREMENTS | ||
| Calories | 25kcal/kg | 1750kcal |
| Energy | 100kJ/kg | 7000kJ |
| MACRONUTRIENTS | ||
| Carbohydrate | 4g/kg | 280g |
| Protein | 1.5g/kg | 105g |
| Fat | 1g/kg | 70g |
| WATER AND ELECTROLYTES | ||
| H2O | 30 mL/kg | 2100ml |
| Na+ | 2mmol/kg | 140mmol |
| K+ | 1mmol/kg | 70mmol |
| Ca2+ | 0.1mmol/kg | 7mmol |
| Mg2+ | 0.1mmol/kg | 7mmol |
| PO4 | 0.1mmol/kg | 7mmol |
| VITAMINS | |
| Water sol B complex folate vitamin C vitamin B12 | |
| Fat soluble ADEK | |
| TRACE ELEMENTS | |
| Fe | 10mg |
| Zn | 15mg |
| Cu | 3mg |
| iodine | 150μg |
| manganese | 5mg |
| chromium | 200μg |
| selenium | 200μg |
Please include common sources of specific nutrients – This is best done based on individual candidate’s method of presentation
JC 2020
Examiner Comments
2017B 02: 21% of candidates passed this question.
The provision of nutrition is a core skill in ICU. An understanding of its key elements enables prescription and modification. However, most answers lacked detailed information which is available in the standard texts. Better responses outlined the caloric requirements including each major element (water, carbohydrate, fat and protein) along with the caloric values and potential sources. Essential amino acids, fatty acids, fat and water-soluble vitamins were expected. A list of the requirements for major electrolytes and some of the trace elements were expected. Some candidates seemed to confuse calories, kilocalories and kilojoules. Some answers did not provide the nutritional requirements, as asked, but instead discussed the fate of the nutrients; hence did not score marks. Candidates are reminded to read the question carefully.
3. Describe the factors that determine the filtered load of a substance at the renal glomerulus
CICMWrecks Answer
Filtered Load
- It is the amount of substance that is filtered per unit time.
- For freely filtered substances, The filtered load is equal to:
- E.g: Na: Filtered load = GFR x Px = 125ml/min x 0.14mEq/ml(or 140mEq/L) = 17.5 mEq/min.
- The filtered load is what is presented to the rest of the nephron to handle.
- For substances that are freely filtered:
- The filtered load varies only with plasma concentration and GFR.
- A rise in GFR, at constant plasma concentration increases the filtered load, as does a rise in plasma concentration at constant GFR.
- For substances which are not freely filtered, filtration also depends on
- protein binding
- molecular size
- charge
GFR
Direct Determinants
The GFR (Net flux across the membrane) is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation.
where
Kf = Filtration coefficient
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
| Kf | Filtration coefficient | = LpS = Hydraulic conductivity x Surface Area Glomerular surface area = 0.8m2 • Altered by Mesangial cell contraction (see circulating factors e.g. Angiotensin II → ↓SA → ↓GFR) Patency of the normal capillary wall structure (ie in tubular dysfunction Kf ↑’s ↑GFR) |
| PGC | hydrostatic pressure in capillary | relates to • RBF which is autoregulated for MAP 70-170mmHg • relative afferent/efferent arteriolar tone Affected by • Catecholamines • Local autoregulation -> Myogenic -> Tubuloglomerular feedback -> Hormones (see below) |
| PT | hydrostatic pressure in tubule | relates to • obstruction to urinary flow (usually pathological) • ↑PT → ↓ GFR (ie post renal obstructiion causing renal failure) |
| πGC | oncotic pressure in capillary | relates to • plasma protein concentration (incr in dehydration, decreased in heart failure) • ↑Systemic plasma oncotic pressure → ↑πGC → ↓GFR • ↓Renal plasma flow → ↑πGC → ↓GFR |
| πT | oncotic pressure in tubule | • usually zero, but can increase in renal failure/proteinuria • ↑’d πT → ↑GFR |
| σ | Staverman’s reflection coefficient | • Permeability of membrane to protein usually 1 (no protein leak) • can decrease with nephritis/proteinuria |
Circulating Factors
- Prostaglandins
- PGI2 and PGE2 → vasodilates → ↑ renal blood flow → ↑ GFR
- ↓ PGE2, PGI2 and NO levels → inhibits afferent arteriolar vasodilation → ↓ GFR
- Long term → ↓ renin release → ↓ ATII-induced efferent arteriolar vasoconstriction → ↓ GFR
- Noradrenaline / Adrenaline
- constrict renal afferent and efferent arterioles → ↓ renal blood flow and reducing filtration → ↓GFR
- Constrict mesangial cells to → ↓ GFR.
- Mesangial cell tone:
- Angiotensin 2, Endothelin, vasopressin → ↓ glomerular surface area and GFR
- ANP, PGE2, Dopamine and cAMP → all increase GFR
Disease Factors
- Filtration decreases
- Shock → decreased glomerular pressure
- Obstruction → increased bowman’s capsule hydrostatic pressure
- Hypoproteinaemia → hepatic failure, nephrotic syndrome
Plasma Concentration
Plasma concentration is a complex interplay of factors:
- Extrinsic substances like drugs: dosage, time and route of administration, the bioavailability of the drug
- Absorption / Production of substance
- Distribution
- Metabolism
- Excretion
- Inter-relationships with other substances or drugs
- Concurrent drug administration may profoundly affect plasma drug concentrations
Additional Factors for Substances not Freely filtered
- Molecular Size
- < 7 kDa (Eg. glucose, ions, urea, H2O) filtered freely,
- > 70 kDa (Eg. albumin) are not filtered;
- neutral particles < 4 mm diameter filtered freely, > 8 mm are excluded
- Molecular Charge
- Negatively charge (Anionic) particles repulsed
- Cationic substances filtered more readily
- Protein binding
- Albumin excluded
JC / Gladwin / Sakurai / Kerr 2020
Examiner Comments
2017B 03: 67% of candidates passed this question.
A good place to start was with the correct equation for a filtered load and a description of the components. Better answers described the components and how they differ and change over the glomerulus. Many candidates usefully based answers around the Starling forces.
A summary of factors including the role of plasma concentration, protein binding, molecular size and charge was required to pass. Many answers gave examples for the effects of size and charge and relate endocrine responses to specific alterations in arteriolar tone and how this affected filtration. A detailed discussion of cardiovascular and endocrine responses to hypovolaemia was not required.
Some candidates confused clearance with filtered load. Candidates are reminded to write legibly – especially where subscripts and Greek letters are used. Directional arrows (if used) should correlate with text.
4. Describe how interstitial fluid recirculates to the vascular system
CICMWrecks Answer
Interstitial fluid:
- Interstitial fluid provides the immediate microenvironment that allows for movement of ions, proteins and nutrients across the cell barrier.
- In the average male (70 kg) human body, the interstitial space has approximately 10.5 litres of fluid.
- This fluid is not static, but is continually being refreshed by the blood capillaries and recollected by lymphatic capillaries.
- Summary of drainage:
- The blood and lymphatic vasculatures constitute two parallel circulatory organs, connected by the emptying of lymph into the venous system.
- Blind-ending lymphatic capillaries collect interstitial fluid by pumping the liquid, which will pass lymphatic valves that close to prevent ‘back-flow’.
- Tissue oedema facilitates the draining of the interstitial fluid through the initial lymphatic vessels by pulling on the vessels through their tissue-anchored filaments
- Approx. 24L fluid filtered / day
- 85% reabsorbed into capillaries
- Rest reabsorbed via lymphatics (~3.5L/day) = Net fluid loss from filtration
Anatomy of Veins:
- Veins are thin-walled, being thinner and larger than the arteries.
- Veins have valves which maintain the unidirectional flow of blood, even against gravity.
- The muscular and elastic tissue content of the venous walls is much less than that of the arteries. This is directly related to the low venous pressure.
- Since the venous pressure is low (7 mm Hg) the valves are of utmost value in the venous return.
- However, the valves are absent in:
- The veins of less than 2 mm diameter.
- The venae cavae.
- The hepatic, renal, uterine, ovarian (not testicular), cerebral, spinal, pulmonary, and umbilical veins.
- Large veins have dead space around them for their dilatation during increased venous return. The dead space commonly contains regional lymph nodes.
Vascular permeability:
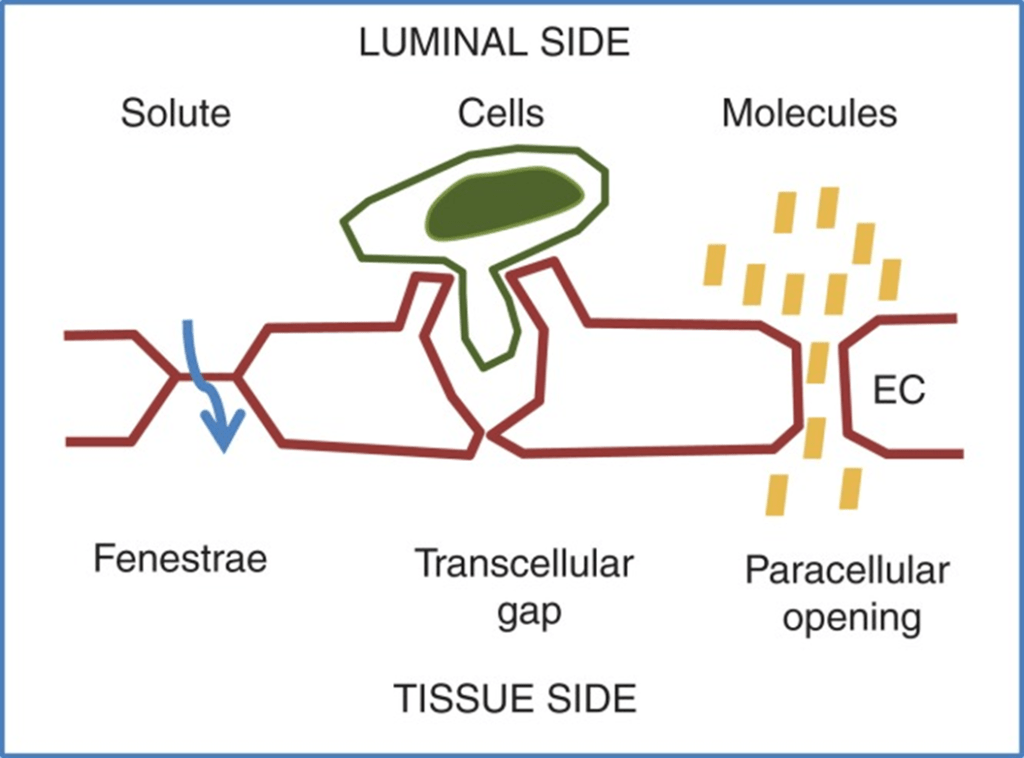
- Vascular permeability, often in the form of capillary permeability or microvascular permeability, characterizes the capacity of a blood vessel wall to allow for the flow of small molecules (drugs, nutrients, water, ions) or even whole cells (lymphocytes on their way to the site of inflammation) in and out of the vessel.
- Blood vessel walls are lined by a single layer of endothelial cells. The gaps between endothelial cells (cell junctions) are strictly regulated depending on the type and physiological state of the tissue.
- Vascular permeability is Regulated by Angiogenic growth factors (VEGF) and Inflammatory cytokines (histamine, bradykinin).
- Vascular permeability is affected in disease states like cancer, MI, Sepsis.
Anatomy of Lymph:
- Lymph is the name given to interstitial fluid which enters the lymphatic vessels.
- Lymphatic capillaries are present in nearly all tissues.
- Significant exceptions are the central nervous system and bone.
- Small interstitial channels are present in the brain and the fluid flows into the CSF and then passes back into the circulation via the arachnoid villi.
- The lymph capillaries are blind-ending and possess flap valves between adjacent lymphatic endothelial cells.
- These functional valves permit entry of ISF but prevent its return to the interstitium.
- The pressure inside the lymph capillary is about 1 mmHg at rest and the flap valves are closed.
- The lymph capillaries interconnect and join together to form lymph venules, and then large lymph veins which drain via lymph nodes into the thoracic duct (on the left) and the right lymphatic duct.
- By these two final pathways, lymph returns into the circulation.
Role of Forces:
Starling Forces (Osmotic and Hydrostatic)
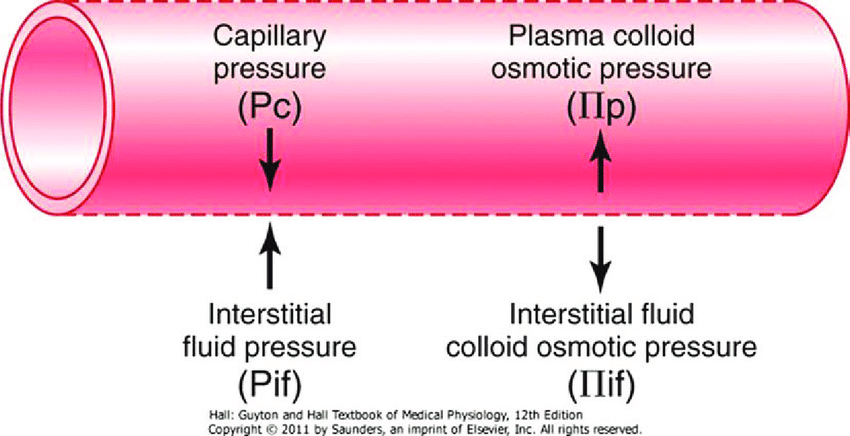
- The NET flux across the membrane is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation:
where
Jv is the trans endothelial solvent filtration volume per second
( [ Pc – Pi ] – σ [ πp – πi ] ) is the net driving force
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
κ = filtration constant = LpS = Hydraulic conductivity x Surface Area
- Typically quoted values for the variables in the classic Starling equation:
| Hydrostatic pressure | Oncotic pressure |
|---|---|
| Pressure moving fluid | pressure exerted by proteins which draw water into and keep it within a compartment |
| Pc ~35 → 15mmHg (Arterial → venous) Capillary hydrostatic pressure Pressure moving fluid out of capillary | πp ~ 20mmHg Plasma oncotic pressure Pressure keeping fluid within capillary |
| Pif = 5mmHg Interstitial hydrostatic pressure Pressure moving fluid into capillary | πif ~ 0mmHg Interstitial fluid oncotic pressure Pressure keeping fluid out of capillary |
- In general,
- at the arterial end of capillary NFP is positive (filtration) +10mmHg
- At the venous end NFP is negative (absorption) -10mmHg
- Approx. 24L fluid filtered / day
- 85% reabsorbed into capillaries
- Rest reabsorbed via lymphatics (~3.5L/day) = Net fluid loss from filtration
Electric forces:
- The ionic composition of the interstitial fluid and blood plasma vary due to the Gibbs–Donnan effect [“Opposing osmotic and electro-chemical gradients in the presence of a non–diffusable ion resulting in unequal distribution of the diffusable ions”]
- This causes a slight difference in the concentration of cations and anions between the two fluid compartments.
JC 2019
Examiner Comments
2017B 04: 10% of candidates passed this question.
Candidates had a limited understanding of this area of the syllabus. It was expected that answers would describe important concepts including the anatomy of venous structures, valves and lymphatics, permeability and factors which influence permeability. A description of hydrostatic forces, other pressures involved, and the role of osmotic and electric forces were required.
5. Compare and contrast unfractionated heparin with low molecular weight heparin
Examiner Comments
2017B 05: 68% of candidates passed this question.
This question was generally well answered and lent itself well to a tabular format. Expected information included an approximation of the molecular weights / significance of the differences in size and therefore its mechanism of action.
Other pertinent areas to mention included pharmacokinetic differences and its use in renal failure, side effect profiles, monitoring, predictability of response and reversibility for the two agents.
6. Describe the effects of Ventilation/Perfusion (V/Q) inequality on the partial pressure of oxygen (PaO2) in arterial blood
CICMWrecks Answer
Normal V/Q variation
- Both V and Q ↓ as you move from base to apex.
- ↓ total number of alveoli → ↓ diffusive area → ↓ V
- ↑ compression of intra-alveolar vessels → ↑ west zone 1 → ↓ Q
- V ↓’s slower than Q
- The intersection of V/Q is at rib 3, here V/Q = 1
- V/Q = 3 at the apex
- V/Q = 0.67 at bases
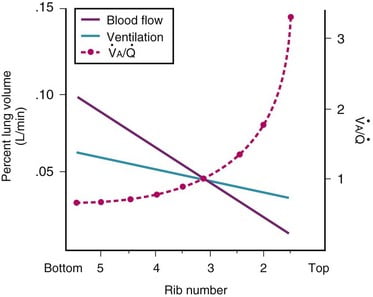
Normal Variation in V/Q causes:
- Shunt
- PACO2 and PAO2 = mixed venous blood
- PACO2 = 46mmHg
- PAO2 = 40mmHg
- Matched ventilation and perfusion
- PACO2 and PAO2 = normal blood gas for healthy patient
- PACO2 = 40mmHg
- PAO2 = 100mmHg
- Dead space
- Alveolar pressures equal that of inspired air
- PACO2 = 0mmHg
- PAO2 = 150mmHg
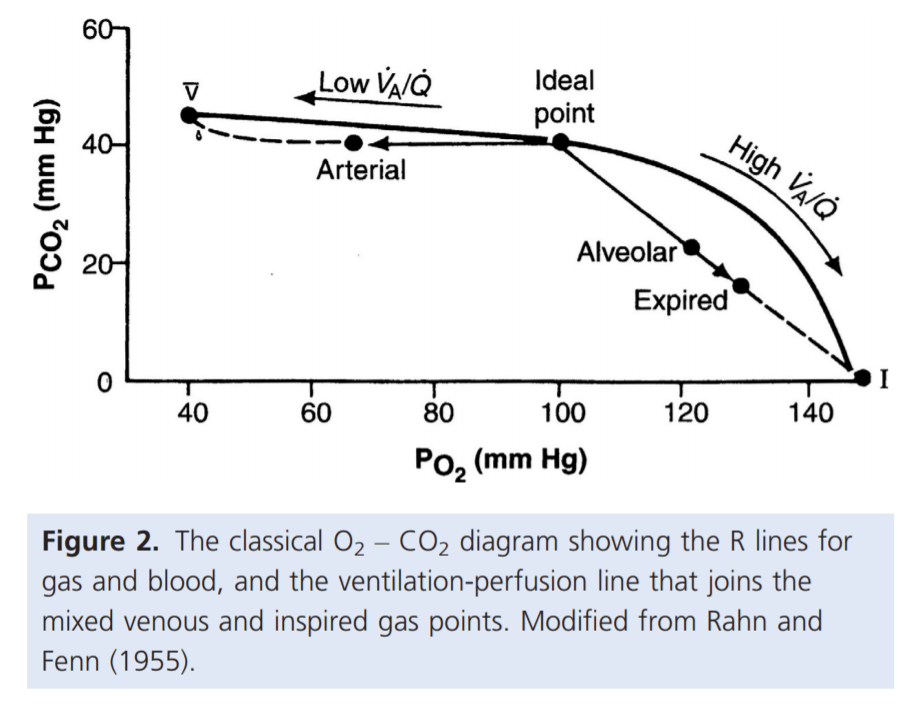
For PaO₂:
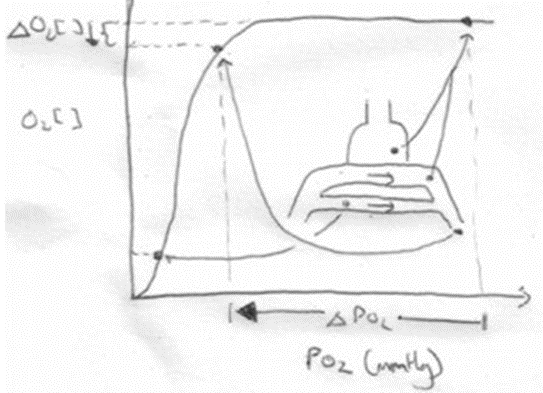
- Increased V/Q inequality causes:
- Mild decrease in PaO2
- At Apex
- decrease in dissolved O2
- Minimal decrease in Hb saturation due to flat part of OHDC
- Thus overall decrease in CaO2
- Base
- PAO2 = PvO2 = 40
- Addition of a small quantity of poorly oxygenated shunted blood flow (PO2 < PcO2) added to a large volume well-oxygenated PECB → small fall in CaO2 BUT a LARGER fall in PaO2
- Because carrying capacity of blood to apex is decreased there is a decrease in PaO2
For PaCO₂:
Remembering that the relationship for the Alveolar gasses:
- Increased V/Q inequality causes:
- Mild increase in PaCO2 which is rapidly compensated for due to Chemoreceptor reflexes
- Apex to base
- ↓PACO2 towards the apices of the lungs
- ↓gas transfer → ↑PaCO2
- Sensed by chemoreceptors (sensitive to ±3mmHg)
- ↑RR and Tv as per the CO2 dissociation curve
- Normal or slightly lower (after compensation) CO2
Gladwin 2016
Examiner Comments
2017B 06: 48% of candidates passed this question.
Overall answers lacked sufficient detail on a core area of respiratory physiology. Answers expected included a description of V/Q ratios throughout the lungs and an explanation of how V/Q inequality lowers PaO2
7. Compare and contrast the sympathetic and parasympathetic nervous systems
CICMWrecks Answer
| Sympathetic nervous system | Parasympathetic nervous system | |
|---|---|---|
| Introduction | The sympathetic nervous system (SNS) is one of two main divisions of the autonomic nervous system (ANS). Its general action is to mobilize the body’s fight-or-flight response. | The parasympathetic nervous system is one of the two main divisions of the autonomic nervous system (ANS). Its general function is to control homeostasis and the body’s rest-and-digest response. |
| Function | Control the body’s response during perceived threat. Diffuse physiological accelerator | Control the body’s response while at rest. Acts as local brake |
| Activates response of | Fight-or-flight | Rest and digest |
| Neuron Pathways | Very short neurons, faster system | Longer pathways, slower system |
| Supplies | Viscera and skin | Only viscera |
| Sympathetic nervous system | Parasympathetic nervous system | |
|---|---|---|
| Originates in | Thoracic and lumbar regions of spinal cord | Sacral region of spinal cord, medulla, cranial nerves 3, 7, 9, and 10 |
| Pre-Ganglionic fibres | Cholinergic | Cholinergic |
| Ganglion | Sympathetic ganglion – Synapse between Short pre-ganglionic fibre Long post-ganglionic fibre | PNS Ganglion sit close to target organ. Long pre-ganglionic fibre Short post ganglionic fibre |
| Post-ganglionic | Release Norad Ach at Adrenal medulla & Sweat glands | All release Ach |
| Target organ receptors | Adrenoreceptors (Alpha 1&2, Beta 1&2) G protein coupled | Ach receptors: Muscarinic M1-5: G protein couples Nicotinic: Ion channel |
| Sympathetic nervous system | Parasympathetic nervous system | |
|---|---|---|
| General Body Response | Body speeds up, tenses up, becomes more alert. Functions not critical to survival shut down. | Counterbalance; restores body to state of calm. |
| Cardiovascular System (heart rate) | ↑↑↑ inotropy ↑↑↑ Chronotropy ↑↑↑ lusitropy ↑↑ dromotropy | ↓ inotropy (Atria > Vent) ↓↓↓ Chronotropy ↓ lusitropy (Atria > Vent) ↓↓↓ dromotropy |
| Vasculature | Constriction | |
| Pulmonary System (lungs) | Bronchial tubes dilate | Bronchoconstriction increased mucous production |
| Musculoskeletal System | Sweating, contraction, lipolysis | Muscles relax |
| Pupils | Dilate | Constrict, lacrimation |
| Gastrointestinal System | Decreased salivation and GIT motility, increased sphincter tone, gluconeogenesis | Increases stomach movement and secretions, decreased sphincter tone |
| Salivary Glands | Saliva production decreases | Saliva production increases |
| Endocrine | Adrenaline and noradrenaline release | No involvement |
| GU | Detrusor relaxation, sphincter contraction, ↑ uterine tone | Detrusor contraction, erection |
JC 2019
Examiner Comments
2017B 07: 75% of candidates passed this question.
This question was generally well answered A table or diagram lent structure to the answer. More complete answers included details on the function, anatomy, a description of the pre- and post-ganglionic fibres, ganglia, receptors and neurotransmitters involved. Whilst most commented on ‘fight or flight’ for the SNS and ‘rest and digest’ for the PNS, no candidate observed that the SNS is a diffuse physiological accelerator and that the PNS acts as a local brake. No candidate included the fact that the SNS supplies viscera and skin whilst the PNS only supplies the viscera. Many candidates failed to make reference to the fact that the postganglionic SNS receptor is G protein coupled and the PNS postganglionic receptor is Gcoupled on muscarinic receptors but operates an ion channel when nicotinic. Candidates may have scored higher if they had provided a little more detail in their answers.
8. Classify calcium channel antagonists and give one example of each class (30% of marks). Describe the pharmacology of Nimodipine including important drug interactions (70% of marks).
CICMWrecks Answer
Calcium Channel Blocker Classification
- Class I – Phenylalkylamines:
- Verapamil
- Reduces HR, contractility and causes vasodilatation, decrease in cardiac contractility, heart rate, and both coronary and peripheral vascular tone.
- Class II – Dihydropyridines:
- main effects are on vasodilatation
- 1st gen = nifedipine
- 2nd gen = felodipine, nimodipine
- 3rd gen = amlodipine
- Class III – Benzothiazepines:
- diltiazem
- some reduction in HR and contractility, also causes vasodilatation, mild to moderate decrease in myocardial contraction, and decreases heart rate by decreasing both the automaticity of the SA node and the rate of conduction in the AV node.
Calcium Channel Blocker Pharmacology
(Include as per the question)
Click here to Open CICMWrecks Table: Calcium Channel Blockers
Class Effects:
- Heart
- V-W Class 4 antiarrhythmic
- Decr SA node automaticity
- Decr AV node conductivity
- Vasodilation
- Negative inotropy
- reflex tachycardia
- 2) Vasculature
- Decr SVR and BP
- Decr Hypoxic pulmonary vasoconstriction
- Incr coronary and cerebral blood flow
- 3) Other
- Tocolytic
- NDMR potentiation.
Gladwin / JC 2021
Examiner Comments
2017B 08: 19% of candidates passed this question.
The classification was done well. Most candidates demonstrated that they had a structure for a “drug” question, but were often challenged to fill in the detail of that structure and failed to deliver enough content to secure a pass. Many candidates wrote a generic answer for calcium channel blockers instead of the specifics of nimodipine.
Frequently the pharmacokinetic data recounted was incorrect. Candidates failed to distinguish between absorption and bioavailability. The difference between oral and intravenous dosing was often omitted. Few answered the section on important drug interactions.
9. Briefly outline the formation, absorption, distribution, role and composition of cerebrospinal fluid
CICMWrecks Answer
Formation / Production of CSF
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
Distribution / Circulation of CSF
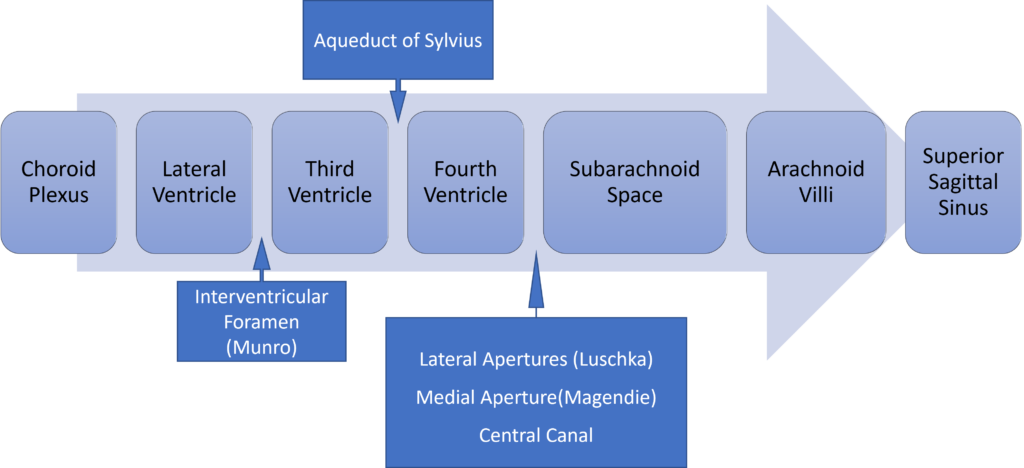
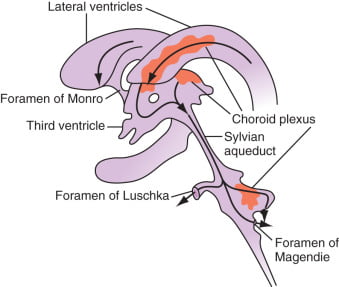
Absorption of CSF
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
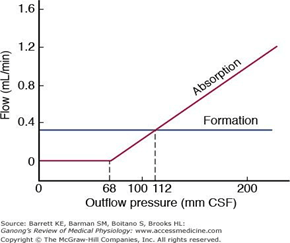
Composition of CSF
IDENTICAL to brain ECF, but differs in several manners from plasma
Compared with plasma:
- ↑ pCO2 (50 mmHg)
- ↓ pH (7.33)
- ↓ protein content
- 0.5% of plasma; 20 mg/dL
- poor acid-base buffering capacity
- ↓ content of glucose (by 60%) and cholesterol
- ↑ [Cl-] (by 7-14%) and ↑ [Mg2+] (by 40%)
- ↓ [K+] (by 40%), ↓ [Ca2+] (by 50%) and ↓ [Pi] (by 20-30%)
- ↑ creatinine (by 25%) but ↓ urea
- IDENTICAL osmolality (295), [Na+] (145), and [HCO3] (25)
Role / Functions of CSF
- Protective role (main function)
- Water bath effect
- Attributed to the low specific gravity of CSF (1.007)
- Causes brain to be buoyant
- ↓ its effective net weight from 1400 g to 50 g
- Mechanical cushion against acceleration/deceleration forces
- Buffer ↑ ICP by CSF translocation to extracranial subarachnoid space
- Abrupt ↑ ICP buffered by translocation of CSF within the vault to extracranial compartments
- Water bath effect
- Maintains constant ionic environment conducive to neuronal electrical activity CSF
- Supply role of nutrients (Eg simple sugars, amino acids) and O2 to brain
- Excretion of toxic substances, metabolic by-products, and CO2 from brain
- “Lymph-type” function → interstitial proteins in brain ECF return to circulation by
- CSF absorption across arachnoid villi
- Acid-base regulation → due to content, CSF allows for tight respiratory control
- Endocrine transport function → transports hormones to other brain regions
Gladwin / JC 2020
Examiner Comments
2017B 09: 44% of candidates passed this question.
The question spelt out very specific areas of CSF physiology to outline and the marks were evenly distributed among these areas. The candidates who did not pass this question usually did not provide enough detailed information.
Details of the production and absorption of CSF were commonly lacking. The majority of candidates correctly described the composition of CSF; indicating whether a particular variable was higher or lower than in plasma, scored less marks than more specific information.
10. Compare and contrast two methods of measuring cardiac output
CICMWrecks Answer
Cardiac Output
Cardiac output = Volume of blood ejected by the heart per unit time (minute)
HR determined by autonomic input
- Resting HR approx 60
- Sympathetic blockade causes HR to slow to 50
- Sympathetic output to heart governed by the medullary vasomotor area
- Parasympathetic blockade causes HR to hasten to 110
- Both sympathetic and parasympathetic blockade causes HR to hasten to 100
- Electrolytes
- Drugs
- Sympathetic blockade causes HR to slow to 50
SV = Amount of blood ejected by the heart per contraction
Governed by:
- Preload
- Tension applied to myocyte immediately prior to contraction
- Starling relationship
- Optimal sarcomere length ~2.2um
- Over-stretch inhibited by fibrous pericardium
- Preload governed by
- Blood volume and MSFP (increases preload)
- RAP (decreases preload)
- Resistance to venous return (decreases preload)
- Negative intrathoracic pressure (aids in venous return)
- Musculovenous pumps (aids in venous return)
- One-way venous valves (aids in venous return)
- Starling relationship
- Tension applied to myocyte immediately prior to contraction
- Afterload
- Load against which the ventricle must exert its contractile force
- According to Laplace law
- Afterload ∝ (Aortic pressure x ventricular radius) / thickness of ventricular wall
- Other factors affecting afterload
- Resistance
- Viscosity inversely proportional to flow
- As viscosity decreases (e.g. Hb decrease), output increases
- Flow is proportional to vessel radius to the 4th power
- Viscosity inversely proportional to flow
- Resistance
- Contractility
- Intrinsic ability of myocardium to contract at given preload and afterload
- Governed
- Sympathetic increases contractility
- Parasympathetic decreases contractility
- Other hormones
- Electrolytes
Measurement
Cardiac output measurement can be performed:
- Invasively
- Pulmonary Artery Catheter
- Thermodilution
- Fick Principle
- Indicator Dilution Technique
- TOE
- Arterial waveform analysis
- PiCCO
- FloTrak/Vigileo
- Acumen/Hemosphere
- Pulmonary Artery Catheter
- Non-invasively
- TTE
- MRI
- Thoracic impedance
Thermodilution
Indicator Dilution (Stewart-Hamilton)
Introduction
Invasive
Thermodilution remains the gold standard of cardiac output measurement.
Invasive
Equipment
Pulmonary Artery Catheter
- A proximal port at the RA/SVC
- A temperature probe at the tip: Typically a silicon oxide thermistor.
- A balloon at the tip: To float it into position.
- A distal (PA) port is required for measuring PAP and the PCWP, but is not required for CO calculation
Can also be done using other devices like PiCCO
- dyes: indocyanine green, isobestic or radioactive isotope.
- Catheter for injection
- Arterial Sampling catheter
- Measurement device
Method / Theoretical Basis
- Catheter passed into right atrium
- Known quantity of fluid of known temperature injected into right atrium
- Second catheter in pulmonary artery
- Measured change in temperature
- Change in temperature over time graphed
- Return of temperature to baseline extrapolated to zero to account for
recirculation - Average change in temperature for duration of curve calculated
- CO = temperature of indicator / (average change in temp x duration of curve)
Method:
- rapidly inject 10mL of dye into venous circullation in RA.
- indicator mixes quickly with cardiac contents.
- during next few beats, blood-indicator mixture is measured by continual sampling from proximal artery in arm for one complete circulation (30 seconds).
- mean concentration of mixture for one circulation determined.
Q = amount of indicator injected / indicator concentration over time.
Assumptions:
- retention of indicator
- complete mixing
- constant flow rate
Advantages / Disadvantages / Errors
Advantages:
- Simple
- No toxicity
- Repeated measurements possible
Disadvantages / Errors:
- Natural variability (reduced by using mean of 3-5 measurements)
- Issues with incorrect volume and temperature of injectate
- Improper positioning of PAC and complications associated with insertion and use
- Tricuspid regurgitation
Results in retrograde ejection of injectate back past the valve. - Arrhythmia
Advantages:
- rapid Q determination
Disadvantages:
- need to continuously withdraw arterial blood to plot the concentration curve.
- dye can build up.
- intracardiac shunt will effect recirculation.
Sakurai / JC 2019
Examiner Comments
2017B 10: 35% of candidates passed this question.
Good answers began with a definition of cardiac output. For each method, it was expected that candidates discuss the theoretical basis, equipment, advantages and disadvantages / sources of error and limitations. Additional marks were awarded when an attempt was made to compare and contrast the two methods (often helped by the use of a table).
11. Describe the pharmacology of propofol
Examiner Comments
2017B 11: 76% of candidates passed this question.
A structured approach proved a good basis to answer this question. It was expected candidates would outline the uses such as anaesthesia, more prolonged sedation or possible additional roles in patients with seizures or head injuries. Discussion of the presentation and pharmaceutics, including a comment on antibacterial preservatives or lack thereof was expected. The mechanism of action should have been described. It was expected candidates could provide an indication of the usual dose (and how it differs in the more unwell / elderly patient population). A maximal rate and possible toxicity was expected.
A discussion on the pharmacodynamics by major organ systems was expected and credit was given for additional comments about hyperlipidaemia, urine colour changes or metabolic alterations. It was expected that candidates would mention propofol infusion syndrome at some point in their answer with some mention of clinical features or pathophysiology.
The important aspects of its pharmacokinetics should have been mentioned (high protein binding, large volume of distribution, termination of effect by redistribution, hepatic metabolism, context sensitive half life). A mention of adverse effects would complete the answer
12. Compare and contrast aspirin and clopidogrel
Examiner Comments
2017B 12: 68% of candidates passed this question.
Both of these commonly used agents are level A in the syllabus and thus a high level of detail was expected. Marks were awarded in the following areas – pharmaceutics, mechanism of action, pharmacokinetics (PK) and side effects. For the PK parameters a general description rather than exact values was sufficient (i.e. ‘high protein binding’ rather than ‘98% protein bound’). It was expected that candidates would mention the fact that clopidogrel is a pro-drug and the factors which influence its conversion to the active form. Additional marks were awarded for well-structured answers which attempted a comparison between the two drugs (helped by the use of a table).
13. Compare and contrast the pharmacology of intravenous fentanyl and morphine
Examiner Comments
2017B 13: 68% of candidates passed this question.
Good candidates produced a well-structured answer and highlighted the differences between the two drugs. It was important to include the dose, potency, time course of effect of both agents, and differences in pharmacokinetic and pharmacodynamic effects. Candidates should have specific knowledge of these important drugs. Many candidates failed to focus the question on intravenous fentanyl and intravenous morphine as asked. No marks were given for information about other routes of administration.
14. Explain the mechanisms responsible for the cell resting membrane potential (60% of marks) and describe the Gibbs Donnan effect (40% of marks)
CICMWrecks Answer
Equilibrium Potential
Nernst Equation
The potential difference generated by a permeable ion in electrochemical equilibrium when there are different concentrations on either side of the cell can be calculated via the Nernst Equation:
where
E is the equilibrium potential for the ion
R is the gas constant (8.314 J.K-1.mol-1 )
T is the temperature in Kelvin
F is Faraday’s Constant
z is the ionic valency (e.g. +2 for Mg2+, -1 for Cl–)
EK = -90 mV
ENa = +55mV
ECl = -65mV
Goldman-Hodgkin-Katz Equation
The Nernst equation describes the equilibrium potential for a single ion, and assumes that the membrane is completely permeable to that ion.
However, calculation of membrane potential requires examining the effects of many different ions with different permeability. This can be performed with the Goldman-Hodgkin-Katz equation:
where,
Px is the permeability constant for the ion, x
If the membrane is impermeable to x, then Px = 0
Note that:
This model does not consider valency
The concentrations of negative ions are reversed relative to positive ions
Resting Membrane Potential
- Definition: The potential difference (units volts) that exists across the cell membrane (i.e. between the intracellular and extracellular environment) when the cell is in a unexcited state, where the reference voltage is the extracellular environment.
- Resting Membrane Potential (RMP) is the result of two key properties of cells:
- the cell membrane is semi-permeable, that is it has variable permeability to different species in solution.
- differing concentration gradients exist across the semi-permeable cell membrane for different ions in solution, the most important determinant of these gradients being the Na+-K+-ATPase.
- At rest, membranes are:
- Permeable to potassium
- Impermeable to other ions
- Generation of membrane potential:
- Intracellular potassium concentration is much higher than extracellular potassium concentration – Due to the action of the Na+-K+pump.
- As the membrane is permeable to potassium, potassium will attempt to diffuse down this gradient, generating a negative intracellular charge which opposes further diffusion
- At some point, an electrochemical equilibrium is reached between:
- The concentration gradient dragging potassium out of the cell
- Negative electrical charge pulling it in
Determinants of RMP
- K+ Diffusion Potential
- From the Nernst equation above we can see that the normal RMP of most tissue is relatively close to the K+ equilibrium potential (-94mV). From this (and the Goldman equation) we can infer that the membrane is likely to be most permeable to K+ at rest. Indeed the primary determinant of the RMP is K+. Relative permeability of the membrane to K+ vs Na+ is 100:1.
- This has the corollary that changes in K+ concentration will have the most major effect on RMP. This is particularly the case with [K+]o due to its low value – small absolute changes in [K+]o are a relatively large proportion of [K+]o.
- Note that while the RMP is as close (or closer) to the equilibrium potential for Cl–, Cl– is not the primary determinant of the RMP as the concentration gradient for Cl– is largely the passive result of the electrochemical gradient created by the Na+-K+-ATPase.
- Na+ Influx
- While the resting membrane is very impermeable to Na+ the electrochemical gradient for its movement into the cell is so large that there is a small ‘leak current’ of Na+ into the cell.
- This Na+ leak is the single factor responsible for most of the deviation of the RMP from the equilibrium potential for K+ (contributes roughly +8mV to the RMP).
- Na+-K+-ATPase
- As noted there is a constant slow leak of Na+ into the cell. Because of the deviation away from the K+ equilibrium potential that this causes, there is also an electrochemical gradient to cause a slow leak of K+ out of the cell. As such the Na+-K+-ATPase is essential to maintain the relative concentration gradients of these ions and thus the RMP.
- In addition the Na+-K+-ATPase itself is electrogenic, transferring as it does 3 Na+ out for every 2 K+ pumped in, leaving a net negative charge balance on the inside of the cell membrane. This contributes roughly -4mV to the RMP.
- Gibbs-Donnan Effect
- The Gibbs-Donnan effect accounts for the effect of non-diffusible ions on the RMP. In vivo the high concentration of negatively charged intracellular proteins has a small but significant effect on RMP. The presence of this net fixed negative charge on the inside of the cell effects the distribution of permeable ions across the membrane.
Resting Membrane Potential in Different Tissues
Typical values of RMPs:
- Ventricular Myocyte: -90mV
- Cardiac Pacemaker cell: -60mV
- Skeletal Muscle cell: -80mV
- Myelinated Axon: -70mV
Gibbs – Donnan Effect
- Describes the tendency of diffusable ions to distribute themselves such that the ratios of the concentrations are equal when they are in the presence of non-diffusable ions.
- Occurs when:
- A semi-permeable membrane separates two solutions
- At least one of those solutions contains a non-diffusable ion
- The distribution of permeable charged ions will be influenced by both their valence and the distribution of uncharged ions, such that at equilibrium the products of the concentrations of paired ions on each side of the membrane will be equal:
- The two main contributors to the Gibbs-Donnan effect in the body are sodium and protein. This occurs because cell membranes:
- Are impermeable to protein
Intracellular protein concentration is high. - Effectively impermeable to sodium
Due to the Na+-K+ ATP-ase pump.
- Are impermeable to protein
- Changing Gibbs-Donnan equilibriums also change the tonicity on each side of the cell membrane, causing movement of water which then upsets the Gibbs-Donnan effect – therefore there is no stable state.
- The Gibbs-Donnan Effect is important for:
- Maintenance of cell volume
Na+ acts as an effective osmole, reducing cellular swelling. - Plasma oncotic pressure
Increased plasma ion concentration increases oncotic pressure. - Resting Membrane Potential
- Maintenance of cell volume
JC 2019
Examiner Comments
2017B 14: 35% of candidates passed this question.
A good answer included a definition of the resting membrane potential and a clear description of the factors that determine it. Explanation of these factors should have included a detailed description of the selective permeability of the membrane, electrochemical gradients and active transport mechanisms. Answers should demonstrate awareness of the Nernst equation and the Goldman-Hodgkin-Katz equation. These were often confused, sometimes with the GibbsDonnan effect. Descriptions of the Gibbs-Donnan effect generally lacked detail and understanding. The better answers included a definition and discussed in detail the influence of non-diffusible ions (intracellular proteins) on the distribution of diffusible ions.
15. List the properties of an ideal inotrope (50% of marks). How does adrenaline compare with respect to these ideal properties? (50% of marks).
CICMWrecks Answer
An Ideal Inotrope is pharmaceutically suitable, has beneficial pharmacodynamic properties, has an excellent pharmacokientic profile.
PHARMACEUTIC
| IDEAL INOTROPE | ADRENALINE |
|---|---|
| Non-toxic | Y |
| Cost effective | Y |
| Stable preparation | Y |
| Compatible with other drugs | Y |
| Peripherally deliverable | Y |
| Multiple preparations | Y |
| Stable in all solutions | N (Oxidises to adenochrome in alkaline solutions, turns pink) |
PHARMACODYNAMIC
| IDEAL INOTROPE | ADRENALINE |
|---|---|
| Increases contractility | Y |
| Increases mean arterial pressure, Maintenance of diastolic blood pressure | Y |
| Increases cardiac output | Y |
| Improves regional perfusion, without pharmacodynamic costs | N |
| No increase in myocardial oxygen consumption | N (increases myocardial oxygen consumption) |
| Avoidance of tachycardia, Non-arrhythmogenic | N (causes tachyarrhythmias) |
| Suitable in pregnancy and paediatric populations | Y |
| No Adverse effects | N (hyperglycaemia, lactic acid production, worsens pulm HTN, Peripheral necrosis) |
PHARMACOKINETIC
| IDEAL INOTROPE | ADRENALINE |
|---|---|
| Does not develop tolerance | N |
| Titratable | Y |
| Rapid onset, Rapid termination of action | Y |
| Metabolised independent of liver and kidney function | N (some hepatic metabolism) |
| Doesn’t require concentration monitoring | Y |
JC 2019
Examiner Comments
2017B 15: 98% of candidates passed this question.
Many candidates scored very highly on this core topic. It was expected information be included on pharmaceutics, cost, availability and compatibilities. Relevant pharmacokinetics (onset/offset, titratability) and pharmacodynamics (including relevant receptors, nuances of haemodynamic effects e.g. effect on diastolic pressure and regional perfusion) should have been detailed. Adverse effects and safety profile (e.g. use in pregnancy, therapeutic index) should also have been included.
Good answers were structured and highlighted differences with specific facts and data
16. Classify and describe adverse drug reactions with examples of each
CICMWrecks Answer
Adverse Drug Reaction: An unwanted, unintended or harmful reaction as a consequence of drug administration
| Explanation | Example | |
|---|---|---|
| A. Augmented | • Predictable • Related to pharmacological action of drug • Common | Hypoglycaemia from insulin |
| B. Bizarre | • Unpredicatable • Not related to pharmacological action of drug • Uncommon | Anaphylaxis to penicillin |
| C. Chronic | Related to cumulative dose | Suppression of hypothalamic-pituitary-adrenal axis by chronic corticosteroid use |
| D. Delayed | • Becomes apparent some time after use of drug • Uncommon | Carcinogenesis Teratogenesis |
| E. Withdrawal | Occurs on withdrawal of drug treatment | • Opioid withdrawal • Angina from β blocker withdrawal |
| F. Failure | Unexpected failure of drug therapy | Clopidogrel in non-metabolizers |
Sakurai 2016
Examiner Comments
2017B 16: 44% of candidates passed this question.
Candidates should have provided a definition of adverse drug reactions and then a classification. There are at least two widely accepted systems for classification, either was acceptable; though candidates often switched between both which led to a less structured answer. The WHO classification is comprehensive and logical, though both Rang and Dale and Goodman and Gilman also have sections on this topic.
Common errors were the citing of examples with the incorrect mechanism, describing only drug interactions rather than all adverse reactions and focussing the answer on the 4 hypersensitivity reactions which could only score a low mark. Some candidates confused drug errors with adverse reactions.
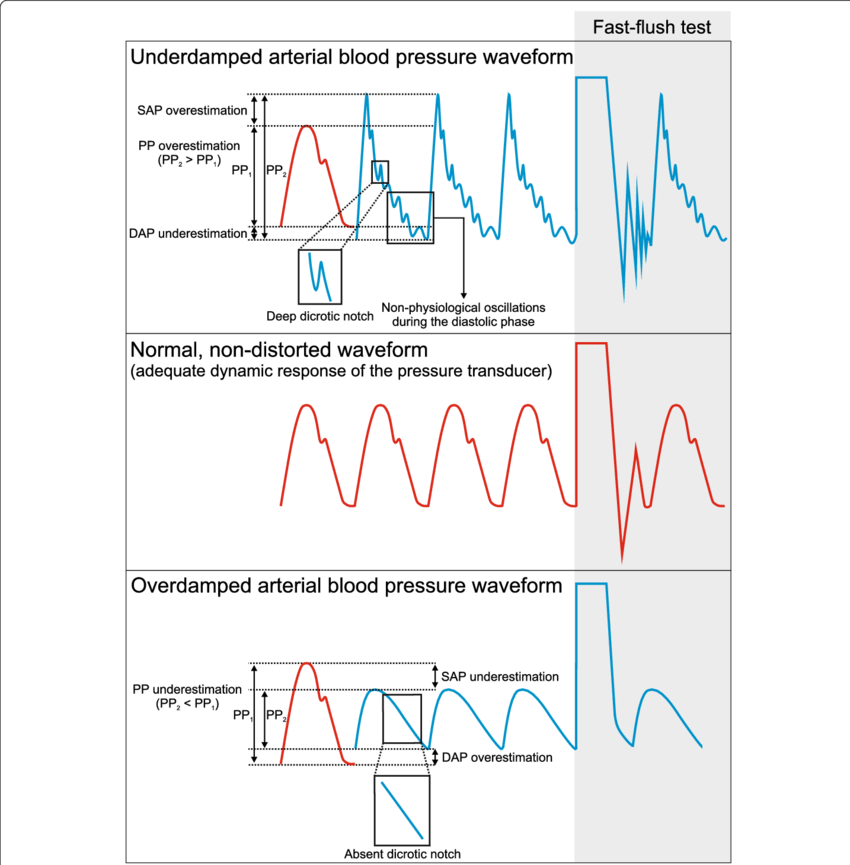
17. Define and explain damping, resonance, critical damping and optimum damping
CICMWrecks Answer
Definitions
Natural frequency: the frequency at which a system oscillates when not subjected to a continuous or repeated external force.
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of a periodically applied force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts.
Resonant frequency: The frequency of a dynamic system at which the amplitude of response to an oscillating force is a relative maximum is known as a resonant frequency (OR) A natural frequency of vibration determined by the physical parameters of the vibrating object.
Damping: the property of a system that diminishes resonance.
Critical Damping: Occurs when the damping coefficient (DC or γ gamma) is equal to the undamped resonant frequency of the oscillator.
Optimum Damping: Level of damping which provides a compromise between the speed of the system with its accuracy
Undamped simple harmonic oscillator
- obeys hooke’s law
- motion is sinusoidal in time
- demonstrates a single resonant frequency.
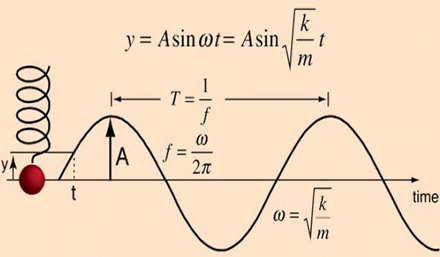
Resonance
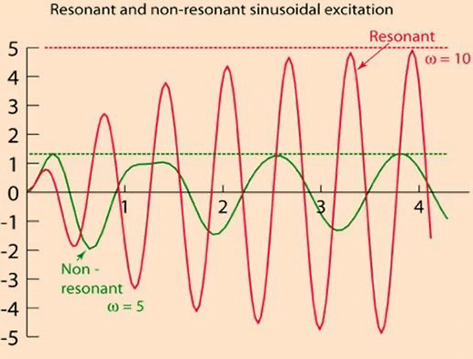
Invasive arterial blood pressure is a driven harmonic oscillator
- An external force (pulsations of the heart) adds to the oscillations of the system (the tubing/transducer etc).
- If the frequency of the driving force is similar to, or a harmonic of, the natural frequency of the system then the result is amplification
Arterial waveform is a complex summation of multiple simple sinusoidal waveforms (a fourier series)
- The frequency of the arterial wave (i.e., the pulse rate) is known as the natural or fundamental frequency
- The sine waves used to reproduce it must have a frequency that is a multiple (or harmonic) of the fundamental frequency
- Increasing the number of harmonics allows better reproduction of high-frequency components, such as a steep systolic upstroke
In most clinical systems
- Natural resonant frequency is 10 to 15 Hz
- Primary frequency of the arterial waveform (the heart rate is 60 to 120 bpm or 1 to 2 Hz)
- However the higher frequency components of the more complex arterial waveform are closer to the natural resonant frequency and are amplified to a larger degree.
To minimize the potential of amplification and improve the dynamic accuracy of the real arterial pressure, system should have resonant frequency >> than 6-8 times frequency of the system being measured:
- Should have noncompliant (i.e. stiff) tubing
- Total mass of liquid in the system should also be minimized (shorter/thinner tubes)
- air bubbles or blood clots or occlusion should be eliminated
Damping
the property of a system that diminishes resonance.
- Damping:
- decreases the magnitude of the oscillations
- allows the system to come to rest at a new value.
- Degree of damping determines the rate at which the system achieves that new value.
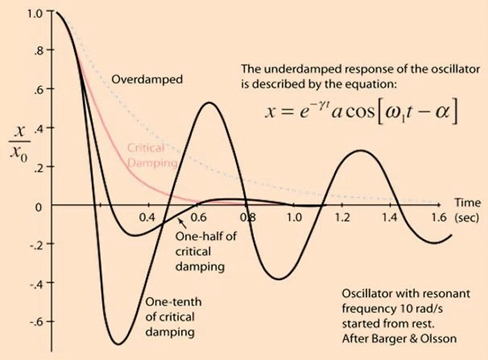
System can be:
- Underdamped (DC < 0.7):
- Reaches the zero position quickly
- Oscillates around it
- Causes falsely ↑ SBP and falsely ↓ DBP
- Critically damped (DC = 1):
- Quickest approach to zero amplitude for a damped oscillator without overshoot.
- Occurs when the damping coefficient (DC or γ gamma) is equal to the undamped resonant frequency of the oscillator.
- Optimal damping (DC = 0.64):
- Provides a compromise between the speed of the system with its accuracy
- Minimises overshoot of oscillations, phase and amplitude distortion, and provides maximal frequency response
- Overdamped (DC > 1):
- The approach to zero is slower
- Very slow to respond
- Causes falsely ↓ SBP, falsely ↑ DBP and loss of fine details of waveform (Eg. dichrotic notch)
Bedside Flush Test:
- The degree of damping in an invasive arterial line system can be determined by the bedside flush test
- Briefly open the continuous flush device to produce a square wave
Gladwin / Sakurai / JC 2020
Examiner Comments
2017B 17: 25% of candidates passed this question.
Concise definitions were required with a clear explanation of the underlying physical principles. The response time of the system, degree of overshoot, effect on amplitude, noise and ability to faithfully reproduce frequencies relative to the natural resonant frequency were important considerations. Many candidates interpreted the question as relating to arterial lines and a detailed discussion of the components and characteristics of an intra-arterial catheter and transducer system did not attract marks
18. Draw and numerically label, on a spirogram, the lung volumes and capacities of a 30 kg child
CICMWrecks Answer
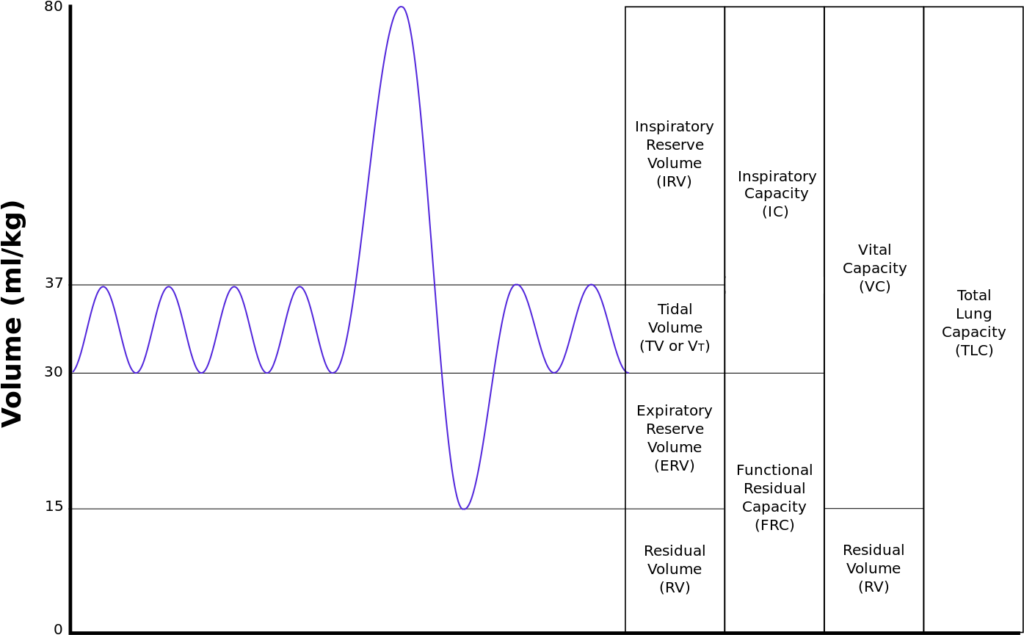
| Volumes | per kg | 30kg Child |
|---|---|---|
| Inspiratory reserve volume | 45ml/kg | 1.35 L |
| Tidal volume | 7ml/kg | 210 ml |
| Expiratory reserve volume | 15ml/kg | 450 ml |
| Residual volume | 15ml/kg | 450 ml |
| Total Lung Capacity | 82ml/kg | 2.46 L |
| Vital Capacity | 67ml/kg | 2.01 L |
| Functional Residual Capacity | 30ml/kg | 900 ml |
JC 2020
Examiner Comments
2017B 18: 87% of candidates passed this question.
This core respiratory physiology topic was well answered by most candidates. Candidates
generally were able to draw a spirogram. A common omission was inspiratory capacity.
19. Describe the physiology of a vasovagal syncope
CICMWrecks Answer
Vasovagal Syncope
- Loss of consciousness from excessive autonomic reflex activity
- typically triggered by seeing blood, pain, emotional stress, or prolonged standing, Time varying magnetic field (i.e. transcranial magnetic stimulation)
- Underlying mechanism involves the nervous system slowing the heart rate and dilating blood vessels resulting in low blood pressure and therefore not enough blood flow to the brain
- Recovery happens without specific treatment. Prevention involves avoiding the triggers.
- Drinking sufficient fluids, salt, and exercise may also be useful.
Mechanism
Regardless of the trigger, the mechanism of syncope is similar
- The nucleus tractus solitarii of the brainstem is activated directly or indirectly by the triggering stimulus
- Results in simultaneous enhancement of parasympathetic nervous system (vagal) tone and withdrawal of sympathetic nervous system tone.
This results in a spectrum of hemodynamic responses:
- On one end of the spectrum is the cardioinhibitory response
- ↓ HR, ↓ contractility → ↓ CO → LOC
- This response results primarily from enhancement in parasympathetic tone.
- On the other end of the spectrum is the vasodepressor response
- ↓ BP without change in HR.
- This occurs due to dilation of the blood vessels, probably as a result of withdrawal of sympathetic nervous system tone.
The majority of people with vasovagal syncope have a mixed response somewhere between these two ends of the spectrum.
One account for these physiological responses is the Bezold-Jarisch reflex.
CVS Challenges
- ↓ in MAP: = due to ↓ CO
- Hydrostatic effects on CPP:
- ↓MAP at level of brain
- effect = immediate.
- NB : ↓MAP at brain level is offset by a similar:
- ↑ CVP venous side (brain circulation is like an inverted U-tube) as well as on the ↓CSF pressure.
- CPP is further augmented by an increase in VR from the brain to the heart in the erect position
- Summary: the main challenge to the CVS (and the brain circulation) is ↓MAP
The CVS response
- baroreceptor reflex mechanism:
- ↓ MAP ⇒ sensed by carotid (mainly) and aortic baroreceptors ⇒ ↓ traffic up to NTS ⇒ via medullary control centre ⇒ ↑ SNS outflow and ↓ PNS outflow.
- The ↑ SNS outflow causes: [ remember: MAP (minus RAP) = CO x SVR ]
- [↑preload] peripheral venoC ⇒ ↑ VR ⇒ ↑ CO ⇒ ↑ MAP
- [↑afterload] peripheral vasoC ⇒ ↑ SVR ⇒ ↑ MAP (slight ↓ in SV due to afterload increase, but net effect = ⇑ MAP)
- ↑ cardiac contractility ⇒ ↑ CO ⇒ ↑ MAP
- ↑ Heart rate ⇒ ↑ CO ⇒ ↑ MAP
NB: Baroreflex ⇒ vasoconstriction = more effective than venoconstriction to restore MAP
! (not to be confused with the vascular function curves where venoconstriction shifts the curve more up than what vasoconstriction rotates it downwards)
- Cerebral pressure autoregulation: a.k.a. the myogenic mechanism:
- effective at maintaining constant cerebral blood flow in a MAP range of 50–150 mmHg
- It effects this by changing the CVR.
- Onset is not immediate though.
- Activity: Mm pump further augments VR
- in conjunction with the one-way valves in the veins to prevents further venous pooling
JC 2019
Examiner Comments
2017B 19: 41% of candidates passed this question.
Generally, there was a lack of knowledge about this topic with many candidates confusing vasovagal syncope with a Valsalva or orthostatic hypotension. A “vasovagal” is from excessive autonomic reflex activity in contrast to orthostatic hypotension which is a failure of the autonomic reflex response.
A good place to start was with a description of vasovagal syncope, also known as neurocardiogenic syncope. It is benign, self-limiting and caused by an abnormal or exaggerated autonomic response to various stimuli (which should have been listed). The mechanism should have been described including the various receptors involved.
20. Outline the functions of the liver
CICMWrecks Answer: Liver Functions
Liver:
- Largest abdominal solid organ
- 25 % CO at rest. approx 1,500 mls/min
- 15% of total blood volume at rest
Functions of Liver
| Filtration | |
| Immune defence | (via Kuppfer cells) against agents entering the portal circulation |
| 80% of circulating cholesterol | → bile salt |
| Biliary excretion of drugs/hormones | penicillins, amp, erythro |
| thyroxine, cortisol, estrogen | |
| calcium | |
| Immune | |
| Filtration of portal circulation | |
| Kuppfer cells | bacteria/ virus/ endotoxins/ immune complexes/ thrombin/ tumour |
| Phagocytosed, fused with lysozomes and degraded by lysosomal enzymes | |
| Antigen presentation | |
| Endotoxin neutralisation = pinocytosed | |
| Complement/CRP production | |
| Storage of metabolic substrate/fluids | |
| glycogen ~ 400g | |
| fat | |
| Fe++, B12, folate, Cu | |
| Vitamin A | |
| Blood Reservoir | |
| Metabolic | |
| CHO and intermediary metabolism | Hepatic Glucostat |
| gluconeogenesis, glycogen storage and utilisation, galactose/fructose to glucose | |
| Conversion to fat, AA’s and ketones | |
| Protein metabolism | amino acids utilisation |
| protein synthesis | |
| production of ketones | |
| deamination of fatty acids | |
| urea formation for ammonia removal | |
| plasma protein formation | |
| Fat homeostasis | metabolism (beta oxidation (rapid in hepatic cells)), synthesis and transport as lipoprotein |
| cholesterol homeostasis | |
| Endocrine | hormone synthesis & metabolism |
| Synthesis of 25 OH cholecalciferol, Metabolism of steroid hormones, Synthesis of somatomedins, Erythropoietin | |
| biotransformation | ammonia & urea cycle |
| drugs & toxins | |
| Acid-Base | Lactate metabolism |
| Synthetic functions | |
| Bile production | bile salts (incr fat absorption) |
| bilirubin (incr haem excretion) | |
| Protein synthesis | albumin 120-300mg/kg/d |
| alpha1/2 & beta globulins (transport) | |
| coagulation & fibrinolytic factors (fibrinogen, prothrombin, II, V, VII,VII, IX, X, XI, XII, XIII, antithrombin) | |
| Lymph synthesis | Up to 50% |
| Erythropoietin (10%) | |
Gladwin / JC / Bianca 2019
CICMWrecks Answer: Paracetamol Toxicity
Paracetamol Overdose
Hepatotoxicity
Toxic daily dose > 4-6 g (while lethal dose occurs at > 10-15 g (or > 300 mg/kg LBM)) → but the toxic and lethal doses are lower in “at-risk” groups:
- EtOH abuse (due to CYP450 induction (↑ toxic metabolite produced) and ↓ glutathione stores)
- Malnutrition (due to ↓ glutathione stores)
- Elderly (due to ↓ glutathione stores)
- Preexisting liver dysfunction
Mechanism:
- At therapeutic doses – “N-acetyl-p-amino-benzoquinoneimine” (highly toxic metabolite) is produced in small amounts, but is rapidly conjugated with hepatic glutathione (anti-oxidant) into a harmless metabolite
- BUT with toxic doses – Hepatic conjugation pathway is saturated and ↑↑↑ N-acetyl-p-amino-benzoquinoneimine is produced → this depletes hepatic glutathione stores, causing remaining N-acetyl-p-aminobenzoquinoneimine to then forms covalent bonds with sulphydryl groups on hepatocytes → results in centrilobular hepatic necrosis
Clinical features:
- Generally conscious and c/o N/V, epigastric pain, erythema, sweating → later developing:
- Acute haemolytic anaemia
- Develop hepatic failure (Ie. jaundice and cholestasis) after 48 hrs
- LFT/INR derangements at 3-5 days
- Fulminant hepatic failure at 3-7 days
- With severe OD, can present with hypotension/shock
Diagnosis :
Serum paracetamol levels → correlate with “nomogram” to predict likelihood of liver damage → used as a guide to dictate therapy
Treatment:
- Activated charcoal/gastric lavage→ limit paracetamol absorption
- Replace hepatic glutathione store within 12 hrs of OD → permits glucuronidation of toxic metabolite
- Oral methionine → ↑ glutathione synthesis
- IV N-acetylcysteine → hydrolysed to cysteine (which is a glutathione precursor)
- Nb. IV NAC is preferred b/c of N/V a/w toxicity (Ie. ↓ oral methionine absorption)
- IV glucose → due to risk of ↓ BGL with liver dysfunction
- Serial monitoring of LFTs and coagulation studies
- Referral to a specialist centre
Gladwin / JC / Bianca 2019
Examiner Comments
2017B 20: 86% of candidates passed this question.
This is a very straightforward question testing breadth of knowledge rather than depth. It was well answered by the majority of candidates.
21. Describe and compare the action potentials from cardiac ventricular muscle and the sinoatrial node
CICMWrecks Answer
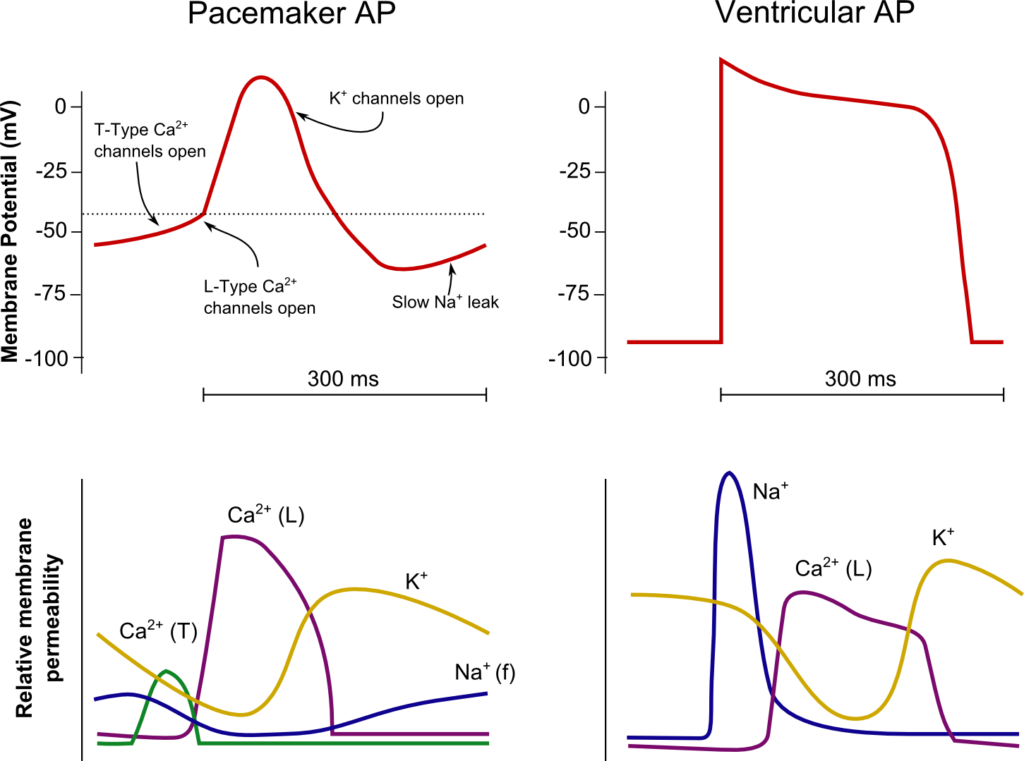
SA Node
Ventricle
Resting membrane potential
- No set RMP, however approx. -60mV
- βadrenergic stimulation causes a less negative RMP and increased slope of the initial upstroke
- muscarinic stimulation causes a more negative RMP and a decreased slope of the initial upstroke
Resting membrane potential
- Approx -90mV
Threshold potential
- Approx 40mV
Threshold potential
- Approx. -50mV
Phase 0
- Funny Na+ current allow leakage of Na+ into the the cell slowly increasing membrane potential to the threshold potential
T and L-type Ca2+ channels (voltage gated) open when threshold potential reached
- Ca2+ influx
- Depolarization
- Shallow upstroke compared to ventricular myocyte
Phase 0
- Rapid depolarization
- Opening of fast Na+ channels
- Influx of Na+
- Overshoot to +20mV
Phase 1
- Early repolarization
- Closure of fast Na+ channels and opening of K+ channels (transient outward, inward rectifier)
- Efflux of K+
Phase 2
- Plateau
- Opening of intially T-type Ca2+ channels and subsequently L-type Ca2+ channels
- Ca2+ influx balances K+ efflux
- Na+ channels in closed state à absolute refractory period
- No action potential can be generated in this state
Phase 3
K+ channels open and Ca2+ channels slowly close
- K+ efflux
- Repolarization
- No plateau phase
Phase 3
- Repolarization
- Closure of Ca2+ channels while K+ channels remain open (delayed rectifier)
- K+ efflux
- Returns potential towards RMP
- Na+ channels transition to inactive state à relative refractory period
- Another action potential can be generated with greater adequate stimulus
Phase 4
- Na+/K+ ATPase and Na+/Ca2+ ATPase maintain ionic gradients
Phase 4
- RMP
- Na+/K+ ATPase and Na+/Ca2+ ATPase maintain ionic gradients
Sakurai 2016
Examiner Comments
2017B 21: 95% of candidates passed this question.
This topic was well understood and answered by most candidates. Some candidates had a good knowledge base but missed out on potential marks by failing to compare and contrast. A diagram outlining the various phases was a useful way to approach the question.
22. Define bioavailability. Outline the factors which affect it.
CICMWrecks Answer
DEFINITION
The fraction of drug that reaches the circulation compared with the same dose given intravenously. (%)
(or)
The ratio of the area under the stated concentration–time curve (AUC) divided by the area under the i.v. concentration–time curve. (%)
FACTORS AFFECTING BIOAVAILABILITY
- Pharmaceutical factors
- Preparation: Decreasing order: Solutions > Suspensions > Capsule > Tablet > Coated tablet
- Particle Size
- Salt form
- Crystal forms have better availability compared to amorphous forms
- Degree of ionization: – Non-ionised, lipid soluble drugs – higher bioavailability
- Pharmacological factors
- Gastric Emptying and GI Motility
- GI Diseases: Coeliac, Crohn’s
- Timing in relation to food intake
- First pass metabolism: The degree of metabolic breakdown of an orally administered drug that occurs in the intestine or liver before it reaches the systemic circulation.
- Drug-Drug interactions
- Pharmacogenetic factors
- Other factors:
- Area of absorptive surface
- State of circulation (shock, tissue perfusion)
- Hepatic Insufficiency, Poor renal function
- Route of administration
Sources: Cross and Plunkett. Physics, Pharmacology, Physiology for Anaesthetists. Goodman & Gilman’s Pharmacological Basis of Therapeutics. Katzung Clinical Pharmacology
JC 2020
Examiner Comments
33% of candidates passed this question.
Many candidates did not specify that bioavailability describes the proportion/fraction of drug reaching the systemic circulation (to differentiate from the portal circulation). Some candidates considered only factors impacting absorption from the GI tract or stated that bioavailability related only to orally administered drugs. Candidates failed to provide an equation, or got equations or graphs wrong. Nearly all candidates failed to provide a comprehensive list of factors affecting bioavailability.
23. Outline the anatomy of the internal jugular vein relevant to central venous line cannulation (80% of marks)
CICMWrecks Answer: IJV (Internal Jugular Vein) Anatomy
Origin / Course / Termination
- Originates at the jugular foramen where it continues the sigmoid sinus
- Exits the foramen accompanies by the glossopharyngeal (IX), vagus (X) and accessory (XI) nerves
- runs caudally down the neck – The vein lies most superficially in the upper part of the neck
- The vein lies lateral, first, to the internal carotid artery, separated by the hypoglossal (XII) nerve, and then to the common carotid artery, with the vagus nerve between and posterior to the artery and vein.
- These three structures lie in a common fascial compartment (the carotid sheath) with the cervical sympathetic chain lying posterior to the sheath.
- These four structures, two vessels and two nerves, form a quartet throughout the neck.
- The deep cervical chain of lymph nodes lies close against the carotid sheath
- The vein is initially posterior to, then lateral and then anterolateral to the carotid artery during its descent in the neck
- Terminates behind the sternoclavicular joint, where it joins the subclavian vein to form the brachiocephalic vein
Relations of the IJV
- Anterior –
- Internal carotid artery and vagus nerve
- Superficially, the internal jugular vein is overlapped above and then lower down covered by the sternocleidomastoid muscle and is crossed above by the posterior belly of the digastric and lower down by the inferior belly of the omohyoid
- Posterior –
- C1, sympathetic chain, dome of the pleura. On the left side, the IJV lies anterior to the thoracic duct
- Posteriorly, the vein rests on (from above downwards) the rectus capitis lateralis, the transverse process of the atlas, the scalenus medius, and the scalenus anterior, with the phrenic nerve crossing it; and all are covered by the precervical fascia
- Medial – Carotid arteries, cranial nerves IX-XII
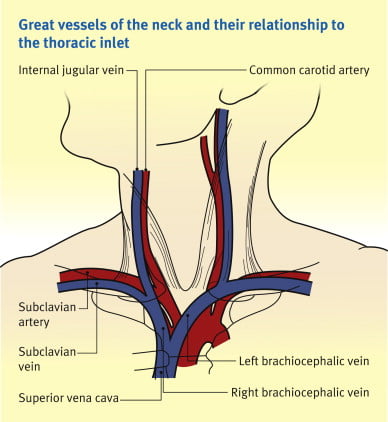
Anaesthesia & Intensive Care Medicine,
https://doi.org/10.1016/j.mpaic.2009.10.005.
Surface Anatomy for Venepuncture
- Lies lateral to the carotid artery within triangle formed by: Two heads of Sternocleidomastoid muscle and clavicle
- On Ultrasound: Non-pulsatile, thin walled, compressible vessel lateral to the carotid artery
Anatomical Variations
- The right IJV (80.5%) was more often larger than the left IJV.
- With reference to the CCA, 85.2% of the IJV were found in the lateral position, 12.5% anteriorly, 1.1% medially and 1.1% posteriorly.
- IJV were sometimes found to be hypoplastic, and in rare cases this was seen bilaterally in both the right and left IJV.
- 5.5% have an IJV that does not correspond to the site predicted by external markings
- Other variations:
- IJV bifurcation
- IJV duplication
- IJV fenestration
- IJV trifurcation
- Posterior tributary
Sources:
Ellis H. The clinical anatomy of the great veins of the neck. Br J Hosp Med (Lond). 2010 Feb;71(2):M20-1. doi: 10.12968/hmed.2010.71.Sup2.46501. PMID: 20220708.
Asari AIA, Barros RAV, Borges MAP. Anatomic variant of the internal jugular vein and its importance in vascular access for hemodialysis. J Vasc Bras. 2019 Oct 23;18:e20190014. doi: 10.1590/1677-5449.190014. PMID: 31692937; PMCID: PMC6822959.
Gladwin / JC 2020
Examiner Comments
2017B 23: 14% of candidates passed this question.
Good answers were structured including origin, termination, tributaries, relationships, surface anatomy and common variations.
Factual inaccuracies were common and there was confusion about the relations of the internal jugular vein. Many candidates did not mention the changing relationship between the internal jugular and the carotid artery as they travel through the neck or the changes that result from repositioning for insertion. Many candidates also forgot to mention surface anatomy and a number talked about ultrasound and views used for insertion of central lines. Common omissions included the origin, tributaries, relationship with the correct cranial nerves and the fact that it is usually larger on the right. Almost nobody mentioned the relationship to the pleura
24. What is functional residual capacity (30% of marks)? Describe two methods of measuring functional residual capacity (70% of marks)
CICMWrecks Answer
Functional residual capacity
- Volume of air in lungs at the end of normal tidal expiration
- Occurs at equilibrium point between the recoil of chest/diaphragm and lungs to collapse inwards
- Sum of residual volume and expiratory reserve volume
- Normal FRC 30ml/kg (approx 2.2l in 70kg male)
- Functions:
- Oxygen reservoir
- PaO2 buffer
- Prevents atelectasis
- Minimizes work of breathing
- Lung kept at most compliant segment of compliance curve
- Decreases pulmonary vascular resistance

Measurement of FRC
- Cannot be directly measured by spirometry
- Body plethysmography
- Subject contained within glass tight box
- Breath against occluded airway
- Changes in alveolar pressure measured at mouth
- Boyle’s law used to calculate FRC
- Nitrogen wash-out technique
C1V1=C2V2- Initial alveolar concentration of nitrogen = 79% (if breathing room air)
- Subject breathes 100% O2
- Volume of total nitrogen exhaled measured (e.g 4L)
- FRC = 4L/79% = approx 5L
- Helium wash-in technique
C1V1=C2V2- Known volume and concentration of helium inhaled and allowed to
equlibrate but not diffuse into blood - Concentration and volume of exhaled helium measured and used to
calculate FRC, where
- Known volume and concentration of helium inhaled and allowed to
Sakurai / Gladwin / JC 2020
Examiner Comments
2017B 24: 59% of candidates passed this question.
Most candidates could state 2 methods of measuring FRC. Some candidates (especially for nitrogen wash out) failed to provide enough information e.g. statements such as “if the amount of nitrogen is measured then FRC can be derived” were insufficient to score many marks.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | CO2 physiology – relationship of PCO2 and CO2 conc respiratory physiology, oxygen cascade, and the renal handling of sodium physiology and pharmacology of work of breathing, graph structure and function of the respiratory system and inhaled drug delivery role of oxygen in the body |
| G. CVS | LV PV relationship. Cardiac cycle with image cardiovascular physiology, afterload and components, and calcium physiology and pharmacology as it relates to blood volume and tonicity. Outline the response to loss of 1 L of circulating blood in an adult. cardiac electrical physiology and anti-arrhythmic drugs. Normal lead II ECG trace. Right heart physiology. Draw the right atrial pressure trace. |
| H. Renal | renal physiology and potassium |
| I. Body Fluids and Electrolytes | intravenous fluids and buffers. Diagram – dluid distribution healthy 70kg male |
| J. Acid Base | Arterial blood gases and oxygen measurement. Hypoxia vs hypoxaemia |
| K. Neuro | Neurophysiology and neuropharmacology. PNS, LA pharm, Femoral nerve in triangle diagram pharmacokinetics and opioids. Draw the concentration-time curve following an intravenous bolus of 100 ug fentanyl Neurophysiology and pharmacology. Response of the nervous system to standing on a nail. |
| L. Musculoskeletal | NM physio, NM blockers |
| M. ANS | |
| N. Liver | liver physiology and diuretics |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | Coagulation and lab assessment. Injury to vessel wall that lead to formation of a haemostatic plug ABO, blood transfusion, CNS physiology and pharmacology |
| R. Thermoregulation | |
| S. Immunology | immunology and associated pharmacology. Innate immunity |
| T. Microbiology | classification of micro-organisms and antimicrobial pharmacology. What microorganisms are demonstrated on Gram stain |
| U. Endocrine | Endocrine functions of the kidney and renal pharmacology. RAS. |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments