SAQs
1. Outline the anatomy and physiology of the parasympathetic nervous system.
CICMWrecks Answer
Parasympathetic Nervous System
- The parasympathetic nervous system is one of the two main divisions of the autonomic nervous system (ANS).
- Its general function is to control homeostasis and the body’s rest-and-digest response.
- Control the body’s response while at rest. Acts as local brake
- Activates response of Rest and digest
Parasympathetic (Cranio-sacral) Efferents
- Arises from neurons in cranial (III, VII, IX, X) or sacral segments of spinal cord (S2~S4)
- Pre-ganglionic neuron
- Long fibre
- Ganglia located near or within effector organs
- Post-ganglionic neuron
- Short
- Release Acetylcholine
Cranial:
| Ganglion / Target Organ | Effect | |
|---|---|---|
| CN III | Ciliary ganglion from occulomotor nucleus | • Pupil constriction, lacrimation |
| CN VII | Chorda tympani Submaxillary ganglion | secretion of saliva |
| CN IX | Parotid gland | secretion of saliva |
| CN X | Respiratory: Pulmonary plexus | Bronchoconstriction increased mucous production |
| Cardiovascular: – SA node – AV node | ↓ inotropy (Atria > Vent) ↓↓↓ Chronotropy ↓ lusitropy (Atria > Vent) ↓↓↓ dromotropy | |
| stomach to proximal two-thirds of the transverse colon | Increased gastric motility, secretions Relaxation of pyloric sphincter |
Sacral:
| Ganglion / Target Organ | Effect | |
|---|---|---|
| 2nd, 3rd, 4th sacral segments | Hypogastric plexus – Descending colon – Rectum – Bladder – Uterus | Contracts muscular wall of rectum Relaxes internal sphincter of anus Contracts detrusor muscle |
Effector
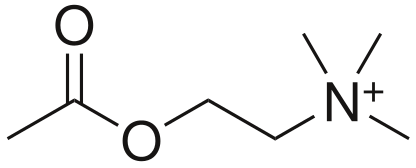
- Acetylcholine (CCOOCCNH3)
- Formed for Choline and Acetyl CoA
- Receptors
- Muscarinic
- G Protein Coupled
- Nicotinic
- Ligand gated ion channels
- Muscarinic
- Metabolism
- Acetylcholinesterase
Mooney / JC 2019
Examiner Comments
2017A 01: 0% of candidates passed this question.
Generally there was a lack of detailed knowledge, incorrect facts and at times confusion between the sympathetic and parasympathetic nervous system functions. A lack of anatomical detail was common (the origin of preganglionic cell bodies was not described clearly, and parasympathetic ganglia were not often named and located). It was expected an answer would mention the central role of Acetylcholine as a neurotransmitter at preganglionic and post ganglionic neurons in the parasympathetic system. Target organs were identified correctly but the exact action was not specified e.g. pupillary constriction vs. dilatation, GI sphincter/bladder – contraction vs. relaxation. Detail concerning receptor physiology was not required. This is a question covering a core topic that no candidate passed. An overview of the arrangement and function of the autonomic nervous system is provided in several core physiology texts, including Ganong and Guyton.
2. Outline the components of dietary fat (20% of marks). Describe their possible metabolic fates (80% of marks)
CICMWrecks Answer
- Fat
- triglycerides -> hydrolysed to FFAs & glycerol
- transported to tissues
- glycerol -> glycerol-3-phosphate & enters glycolytic pathway
- FFA’s transported to mitochondria by carnitine carrier -> degraded to ACoA (beta oxidation) -> enters the citric acid cycle.
- essential fatty acids = linolenic, linoleic & arachidonic acid
- used in the production of leukotrienes, prostaglandins, prostacyclin
- Main Components of Dietary Fat
- Fatty acids: Acylglycerides (esters of glycerol+FA), Phospholipids (2FA+glycerol+Phosphate group)
- Non Glyceride Components: Vitamin E, Carotenoids, Vitamins A & D
- Other components: Sterols, Methylsterols and trierpene alcohols, Squalene, Oryzanols
Lipid Transport
- Digestion: splitting TGs into monoglycerides + FFAs
- Absorption: inside intestinal epithelial cells; resynthesis into TGs + packaged as chylomicrons → circulate in venous system for transport to liver + adipose tissue
- Storage: lipoprotein lipase hydrolyses TGs + phospholipids in chylomicrons → FFAs + glycerol → diffuse into fat (adipose cells) + liver (hepatocytes) → resynthesises as TGs inside cells for storage
- Transport: via synthesised lipoproteins which contain TGs, cholesterol, phospholipids, and protein; formed in liver; transport + deposit lipid components in blood + peripheral tissues. E.g. of lipoproteins – chylomicron, VLDL, LDL, HDL
- Synthesis: liver can synthesise TGs from carbohydrates; cholesterol + phospholipids from FFAs
- Mobilisation: stored fat is hydrolysed by hormone sensitive lipase → released + transported as glycerol + FFAs → ionised and carried on albumin. Activation by starvation
Lipid Metabolism
- Glycerol can be converted to Glucose by the liver
- Free Fatty Acids:
- transported from ECF to ICF by fatty acid transporter
- FACoA synthase in cytosol makes FACoA
- Converted to Acyl carnitine by CPT1 and transported into mitochondrion
- Converted back to FACoA by CPT2
- β-oxidation of FFA (Phase 1 reaction)
- FFA derived from diet and lipolysis of fat stores (via lipoprotein transport) is partially oxidised in mitochondrial matrix by removal of 2-C moieties (as acetyl CoA) at a time
- Lipolysis of fat stores is ↑ by GH, GC and Adr (stimulates TAG lipase)
- TCA cycle (Phase 2 reaction)
- occurs in mitochondrial matrix under aerobic conditions only
- → consumes breakdown products of glucose (as acetyl CoA), FFA (as acetyl CoA) and a.a (as TCA intermediates – α-ketoglutarate, oxaloacetate, fumarate, succinyl CoA) to produce
- → (i) 2x CO2, (ii) 1x ATP, (iii) 3x NADH + H+ / 1x FADH2 – per acetyl-CoA metabolised
- TCA cycle stimulated by ↓ NADH/NAD+ ratio (as NADH inhibits dehydrogenase enzymes of the cycle)
- Electron Transport Chain (Phase 3 reaction)
- Electrons donated to ETC by NADH/FADH2 → passed along series of cytochromes (along inside surface of inner mitochondrial membrane) down its energy gradient until they are accepted by O2 at the end (via cytochrome a)
- transported from ECF to ICF by fatty acid transporter
- Transported as chylomicrons to Adipocytes
- o Degraded by Lipoprotein lipase to FFAs and Glycerol (Heparin is a cofactor)
- o FFAs and Glycerol transported into Adipocyte
- o Converted back to Triglycerides for storage.
- Ketone body metabolism:
- Ketone bodies (acetoacetate, β-OH-butyrate) are formed in liver only when there is extra acetyl CoA formed by β-oxidation of excess FFA (2° to ↑ lipolysis by GH, GC, Adr)
- Ketone bodies are released from liver → utilised peripherally by skeletal muscle, heart, kidney (and brain/nervous tissue during starvation)
Bianca / Kerr 2016
Examiner Comments
2017A 02: 21% of candidates passed this question.
Almost all candidates interpreted “metabolic fate” to mean absorption, digestion and transport of fat. Hence a lot of time was spent on this and little on the fate of fat once it enters the blood stream. The processes of neither beta oxidation, nor lipogenesis were not well understood.
Ketone body production was better understood.
3. Classify and describe the mechanisms of drug interactions with examples.
CICMWrecks Answer
Pharmaceutical interactions:
- Physicochemical incompatibility b/t drugs
- causes precipitation of drug (Eg. STP (alkaline) + SCh (acidic))
- Absorption or binding to containers (Eg. GTN + PVC lines)
- Degradation of drug (Eg. insulin denatures in solution of dextrose)
Pharmacodynamic interactions:
- One drug alters the body’s response to another at a given plasma [drug] and can be either
- antagonistic
- Direct (morphine and naloxone) or indirect
- additive
- synergistic
- Inhibition of enzymatic inactivation e.g. clavulanic acid and amoxicillin
- Enhanced agent uptake e.g. gentamicin and penicillin
- antagonistic
- Direct interaction
- Drugs act at same receptor site for effect
- naloxone + opioids → direct antagonism
- N2O + volatiles → direct additivity
- Drugs act at same receptor site for effect
- Indirect interaction
- Drugs act at different receptor sites for same effect
- Opioids + volatiles → indirect synergism
- atropine + neostigmine → indirect antagonism
- Drugs act at different receptor sites for same effect
Pharmacokinetic interactions:
- Absorption
- Mainly due to altered oral absorption a/w:
- Complex formation: tetracycline + Ca in milk/antacids
- Altered gastric emptying/intestinal motility
- opiates ↓ intestinal motility → ↓ absorption of drugs absorbed in small intestine (paracetamol)
- metoclopramide ↑ intestinal motility → ↓ absorption of drugs absorbed in stomach (Eg. cimetidine)
- Altered gastric and intestinal pH
- ↑ gastric pH by antacids impairs absorption of weakly acidic drugs
- Complex formation: tetracycline + Ca in milk/antacids
- Altered parental absorption a/w localised vasoconstriction (Eg. adrenaline + LA)
- Mainly due to altered oral absorption a/w:
- Distribution
- Competition by drugs for plasma protein binding site → affects drugs that:
- Are highly protein-bound drugs (Eg. warfarin, diazepam, phenytoin)
- → ↑ unbound % in plasma
- Have enzyme system close to saturation or zero-order kinetics (Eg. phenytoin)
- →↑ displacement of drug and ↑ unbound % cannot be cleared effectively
- → large ↑ unbound % in plasma
- Are highly protein-bound drugs (Eg. warfarin, diazepam, phenytoin)
- Drugs that alter C.O. impact on distribution of drugs to target, peripheral tissues
- β-blockers ↓ C.O. → slow onset/offset times of drugs reliant on distribution
- Competition by drugs for plasma protein binding site → affects drugs that:
- Metabolism
- Inhibition/induction of microsomal enzymes (Eg. CYP450)
- Enzyme induction → resulting in ↓ plasma [drug]
- Enzyme inhibition → resulting in ↑ plasma [drug]
- Inhibitors of non-microsomal enzymes (Eg. MAOi, COMTi)
- Inhibition/induction of microsomal enzymes (Eg. CYP450)
- Elimination
- ↓ urinary excretion
- Competition for tubular transport system occurs with weak organic acids (Eg. probenecid + penicillin)
- Changes in urine pH
- Alkalinising agents (Eg. NaHCO3/acetazolamide) → ↑ excretion of weak acids
- Changes in urine volume
- Changes in biliary excretion
- Phenobarbital ↑ bile flow and biliary conjugation of drugs
- ↓ urinary excretion
Gladwin 2016
Examiner Comments
2017A 03: 44% of candidates passed this question.
Candidates with a well organised answer scored highly. A list of drug interactions was not sufficient to pass, as the question asked to ‘describe’ the mechanism of drug interactions. Some candidates described the interaction but did not give examples. Common mistakes included using incorrect examples for a particular mechanism and describing the mechanism of action of drugs instead of drug interactions.
4. Describe the Endocrine functions of the kidney.
CICMWrecks Answer
Non-RAAS Endocrine Functions
Vitamin D
Vitamin D has a complex metabolic pathway which meanders through a number of organ systems:
- Vitamin D2 (ergocalciferol) only in diet
- Vitamin D3 (cholecalciferol) absorbed in diet or produced in skin by the action of UV light
- 7-dehydrocholesterol à 7-dehydroxycholecalciferol à cholecalciferol
- Liver: Cholecalciferol hydrolysed à 25-hydroxycholecalciferol (25-OHD3)
- Enzyme: Vitamin D25-hydroxylase (CYP450 enzyme)
- Released into plasma, binds to Vitamin D-binding protein (a globulin carrier protein)
- Kidney: 25-OHD3 hydrolysed à 1,25-dihydroxycholecalciferol (calcitriol – active form)
- in the proximal tubule, Enzyme: 1alpha hydroxylase
- Released into circulation
- Transported by Vitamin D binding enzyme to intestine, kidney, bone
Erythropoietin
Secretion of EPO to stimulate RBC production.
Erythropoiesis is stimulated by EPO release:
- In adults, EPO is released from the:
- Peritubular capillary fibroblasts (85%)
- Liver (15%)
- EPO is released in response to:
- Hypoxia (near the secretory cells which lie in between the cortex and medulla)
- Hypotension
- Low Hct
- Erythropoiesis is inhibited by:
- High red cell volume
Production of EPO is decreased in renal failure, which is why patients with end-stage renal disease require exogenous EPO.
Prostaglandins:
Production of prostaglandins esp PGE2 and prostacyclin – potent renal vasodilators
Bradykinin:
Production of bradykinin – potent renal vasodilator
RAAS
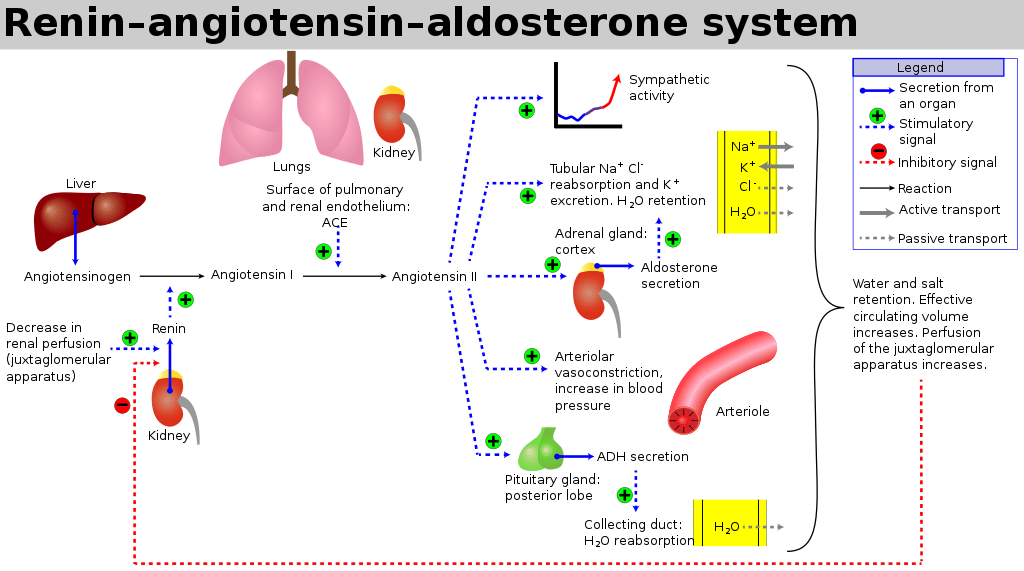
The RAAS is a signaling pathway involved in blood pressure control. It involves a number of hormones:
- Angiotensinogen is produced by the liver
- Renin is a protease enzyme (Half-life 80mins)
- produced by the kidneys
- stored by the granular cells of the juxtaglomerular apparatus, which lies close to the glomerulus and distal tubule
- Secretion is stimulated by:
- Pressure changes in the afferent arteriole
- Stimulation of the macula densa when changes occur in tubular NaCl concentration
- Renal sympathetic nerve activity (a direct β1 effect)
- Baroreceptor reflexes
- Negative feedback from AT II
- Exists to cleave hepatic angiotensinogen to the 10-AA peptide angiotensin I
- Note: Renin is rate-limiting step for activation of RAAS
- Angiotensin I
- has mild vasoconstrictor properties, but not enough to cause significant changes in circulation
- Angiotensin Converting Enzyme (ACE)
- Found mainly in pulmonary vascular endothelial cells – converts angiotensin I to angiotensin II
- Also cleaves bradykinin into inactive metabolites
- Angiotensin II
- Effects include a general reduction in sodium and water excretion, and maintenance of circulating blood volume, GFR and blood pressure.
- Rapidly removed from circulation in 1-2mins, presumably by tissue peptidases (Angiotensinases)
- Aldosterone
- is secreted from the zona glomerulosa of the adrenal cortex
- It acts in the renal CCD to enhance the activity of ENaC and K+ secretion by principal cells.
- increases Na+ (and water) reabsorption in exchange for K+ (kidney, gut, salivary, sweat)
JC 2019
Examiner Comments
2017A 04: 39% of candidates passed this question.
It was expected that candidates would discuss the major hormones produced (or activated) by the kidney. These included erythropoeitin, renin and calcitriol. Good answers included the following: the area where the hormone is produced or modified; stimuli for release; factors which inhibit release; and the subsequent actions / effects. Marks were not awarded for hormones that act on the kidney.
5. Describe the regulation of plasma calcium concentration.
CICMWrecks Answer
Normal Calcium: 25,000 mmol (400 mmol/kg)
Distribution:
- Readily exchangeable pool (1%) (ECF esp plasma)
- Total plasma [Ca2+] = 2.12-2.65 mmol/L
- Ionised [Ca2+] = 1.2 mmol/L
- Only the plasma free Ca is physiologically active and regulated by homeostatic mechanisms
- Two pools
- Diffusible (55%)
- 45% free/ionised
- Active form (above)
- 10% complexed
- 45% free/ionised
- Non-diffusible (45%)
- protein bound (esp to albumin)
- pH-dependent (↑ binding with ↑ pH)
- Diffusible (55%)
- Poorly exchangeable pool (99%)
- Bone/teeth (as hydroxyapatite, phosphates, carbonates)
Calcium balance
ECF and hence plasma Ca is the result of a balance between dietary intake, gastrointestinal absorption and excretion, renal excretion and exchange with bone Ca.
Normal Losses:
- Kidneys (40%) → 2.5-7.5 mmol/day
- Filtration of 250 mmol/day
- 95% reabsorbed by tubules
- PCT 65% with Na
- TAL of LoH 20%
- distal nephron 10%
- 5% excreted
- ↑ reabsorption at LoH/distal nephron
- PTH
- 1,25-dihydroxy-vitamin D
- GIT in faeces (60%) → 6-14 mmol/day
Calcium Regulation
- Calcitonin
- 32 AA peptide with 1 disulfide bond it is the hormone of Procalcitonin
- Released from Parafollicular cells (C-cells) of thyroid
- Release Stimuli: Hypercalcaemia, gastrin, beta-agonists, dopamine, oestrogen, CCK, glucagon and secretin
- Effect:
- Increases osteoblast function/Inhibits osteoclast function
- ↓intestinal calcium reabsorption
- inhibition of renal Ca and PO4 reabsorption
- Vitamin D
- Fat soluble sercosteroid from either
- diet or
- synthesis in skin of cholecalciferol from cholesterol then processed in liver and activated in PCT of the kidney
- Release stimuli
- ↑ concentration of PTH causes ↑ 1-alpha-hydroxylase activity in kidney or ↓ Ca or PO4
- Effect:
- ↑ bone release of Ca/PO4
- ↑ intestinal and renal reabsoption of Ca and PO4
- negative feedback on PTH release
- Fat soluble sercosteroid from either
- Parathyroid hormone
- Polypeptide hormone secreted from the parathyroid gland
- Release stimuli:
- ↓ Ca (primary or due to ↑ PO4) sensed by CaSR (Ca sensing receptor) in PT chief cell
- ↓ vitamin D levels
- Effect:
- ↑ Ca/PO4 reabsorption due to ↑ osteoclast activity
- ↑ Vit-D production by kidney (effect on 25 alpha hydroxylase)
- ↑ illeal Ca reabsorption
- ↑ renal reabsoption of Ca/Mg from DCT and TAL loop of henle
- ↓ PCT phosphate reabsorption
Gladwin 2016
Examiner Comments
2017A 05: 51% of candidates passed this question.
High scoring answers discussed the three major hormones involved in calcium regulation – parathyroid hormone, vitamin D and calcitonin. For each of these it was expected that candidates include: site of production, stimulus for release, inhibitory factors and actions. In the case of renin it was expected that candidates also include the actions of angiotensin and aldosterone. Very few answers discussed inhibitory factors or negative feedback loops.
6. Statistics (not in current primary syllabus)
7. Compare and contrast the systemic circulation with the pulmonary circulation.
CICMWrecks Answer
| Pulmonary circulation | Systemic circulation |
|---|---|
| Movement of blood from the heart to the lungs for oxygenation, then back to the heart again | Movement of blood from the heart through the body to provide oxygen and nutrients to the tissues of the body while bringing deoxygenated blood back to the heart |
| Deoxygenated blood (RA) → RV → Pulmonary Artery →→→ capillary beds → Oxygenated blood →→→ Pulmonary Veins → LA | Oxygenated blood (LA) → LV → Aorta →Major Arteries →→→ Capillaries → Gas and nutrient exchange → Deoxygenated blood →→→ Veins → Vena cavae → RA |
| Accept the entirety of cardiac output, with little capacity to regulate flow (hypoxic vasoconstriction being the exception) | Regulate flow to different organs at different times Contains resistance vessels which allow it to allocate cardiac output accordingly. |
| Low pressure – low resistance | High pressure – high resistance |
| BP – 25/8 (mean 15) mmHg | BP – 120/80 mmHg |
| Pressure differences from inlet to outlet of the pulmonary system is about (15 – 5) = 10 mmHg | Pressure differences from inlet to outlet of the systemic system is about (100 – 2) = 98 mm Hg which is ~ 10 times that in pulmonary system |
| the walls of the pulmonary artery and its branches are remarkably thin and contain relatively little smooth muscle (they are easily mistaken for veins). | the arteries generally have thick walls and the arterioles in particular have abundant smooth muscle. |
| The lung is required to accept the whole of the cardiac output at all times. It is rarely concerned with directing blood from one region to another | The systemic circulation regulates the supply of blood to various organs, including those which may be far above the level of the heart (the upstretched arm, for example). |
| At any given time, contains ~10% of the circulating blood volume, or about 500ml. | At any given time, contains ~90% of the circulating blood volume, or about 5400ml. |
| Blood Flow = Cardiac output (in the abscence of shunts / recirculation) | Blood Flow = Cardiac output (in the abscence of shunts / recirculation) |
| highly compliant – the volume of blood is able to change substantially with minimal change in pressure | Compliance varies depending on distance from heart and as per different regional circulations |
| The distribution of pressures along the pulmonary circulation is far more symmetrical. Much of the pressure drop occurs within the capillary bed itself | Most of the pressure drop is just upstream of the capillaries |
| The pulmonary capillaries are virtually surrounded by gas, and receive little support. Hence are liable to collapse or distend, depending on the pressures within them and alveolar pressure. | All capillaries are surrounded and supported by solid tissue |
| PVR ~ 1/10th of SVR ~ 0.25-1.6 mmHg/L/min 20-120 dynes.Sec.Cm-5 | SVR 9-20 mmHg/L/min 900-1200 dynes.sec.cm-5 |
| Minimal sympathetic effect (as relatively little smooth mm.) | SVR highly regulated by Autonomic tone |
| PVR decreases significantly as pulmonary artery pressure increases because of capillary recruitment (mainly) and distension | This inverse relationship not so significant |
| Both the arteries & veins increase their caliber as the lung expands by radial traction. | No such phenomenon in systemic circulation |
| Vasodilate in response to hypoxia and hypercapnoea | Vasoconstrict in response to hypoxia and hypercapnoea |
| gas exchange function: between blood to air | between blood and tissues |
| metabolic and synthetic functions: Metabolism of: 5HT, PGs ACE: convert AT I → AT II) Synthesize thromboplastin, Heparin | Delivers substance to organ systems for metabolism, synthesis, excretion Synthesizes NO Synthesizes pro- and anti- coagulants |
| role in acid base homeostasis: by CO2 exchange by buffering | by metabolism and excretion via kidneys, liver, skin, GI, etc by buffering |
| Filters large emboli | Filtration in Liver and Kidney |
| Some reservoir function in pulmonary vasculature | Large reservoir in organs (mainly Liver) and venous system |
JC 2019
Examiner Comments
2017A 07: 26% of candidates passed this question.
This question encompasses a wide area of cardiovascular physiology. As a compare and contrast question this question was well answered by candidates who used a table with relevant headings. Comprehensive answers included: anatomy, blood volume, blood flow, blood pressure, circulatory resistance, circulatory regulation, regional distribution of blood flow, response to hypoxia, gas exchange function, metabolic and synthetic functions, role in acid base homeostasis and filter and reservoir functions.
A frequent cause for missing marks was writing about each circulation separately but comparing. For example: many candidates stated ‘hypoxic pulmonary vasoconstriction’, but did not contrast this to ‘hypoxic vasodilation’ for the systemic circulation. Frequently functions of the circulations were limited to gas transport / exchange
8. Describe the physiological consequences of decreasing the functional residual capacity (FRC) in an adult by 1 litre.
CICMWrecks Answer
Functional residual capacity
- Volume of air in lungs at the end of normal tidal expiration
- Occurs at equilibrium point between the recoil of chest/diaphragm and lungs to collapse inwards
- Sum of residual volume and expiratory reserve volume
- Normal FRC 30ml/kg (approx 2.2l in 70kg male)
- Functions:
- Oxygen reservoir
- PaO2 buffer
- Prevents atelectasis
- Minimizes work of breathing
- Lung kept at most compliant segment of compliance curve
- Decreases pulmonary vascular resistance
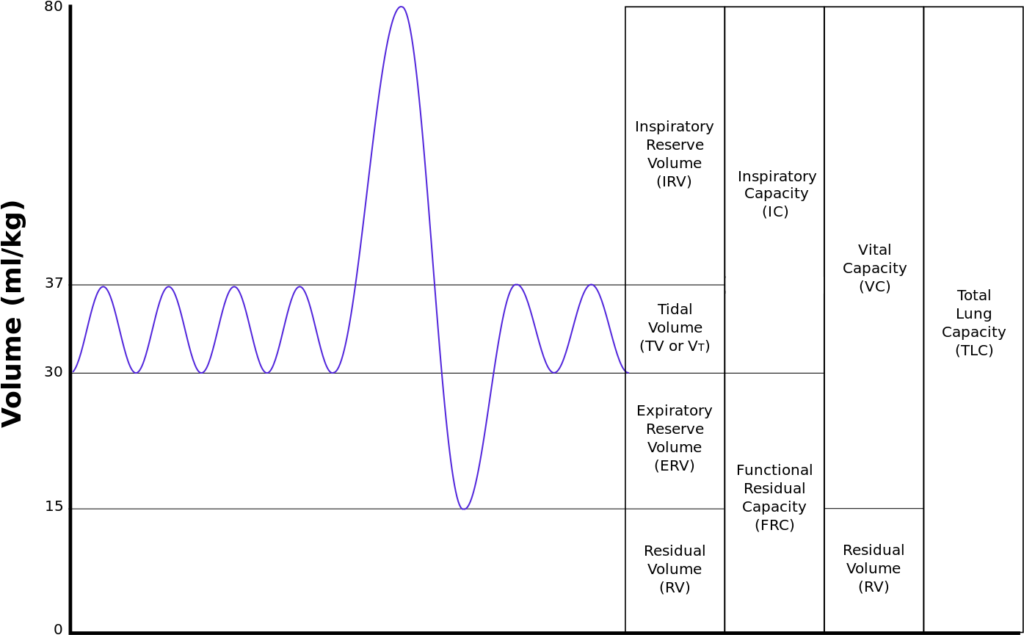
Consequence of 1L loss of FRC
- ↑Work of breathing
- FRC is the volume at the minimum work at rest
- Compliance curve shifted to a flatter portion (greater change in pressure required for change in volume)
- FRC < closing capacity → ↑dynamic air closures/↑air trapping → ↑WOB
- ↑atelectasis (↑with supine position) → ↓ lung compliance → ↑WOB
- ↑airways resistance 2° ↓caliber of airway → ↑WOB
- ↑V/Q mismatch
- 2° to dynamic airways closure, gas trapping, atelectasis
- ↑shunt
- ↓PaO2 due to inability of zone 1 to compensate for the larger zone 3
- ↑Pulmonary Vascular Resistance (PVR)
- PVR minimal at FRC
- ↓FRC → ↓radial traction on extraalveolar vessels → ↓radius of vessels → ↑PVR
- ↑PVR → ↑ RV Afterload
- ↓FRC → ↑West Zone 4 → ↑pressure on extraalveolar vessels by poorly inflated lung → ↓radius → ↓flow
- ↓O Store and Buffer capacity
- Greater variability in saturations thoughout the respiratory cycle
- More rapid desaturation with hypoxia
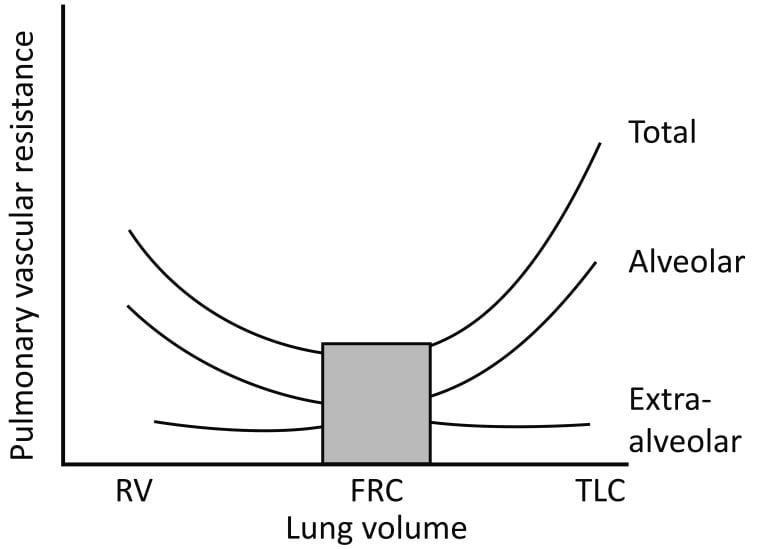
Sakurai / Gladwin / JC 2020
Examiner Comments
2017A 08: 70% of candidates passed this question.
High scoring answers began with a definition and normal values, followed by a detailed list of the consequences of decreasing the FRC.
Some candidates included descriptions of the normal function of FRC, conditions that decrease FRC and ways of improving reduced FRC. These were not required and did not attract marks. Diagrams require correctly labelled axes, values & units.
9. Outline how the following tests assess coagulation: a. Prothombin Time (PT) b. Activated Partial Thromboplastin Time (APTT) c. Activated Clotting Time (ACT) d. Thromboelastography (TEG or ROTEM)
CICMWrecks Answer
Prothrombin Time
Activated partial prothrombin time
What is tested
Extrinsic pathway
Final pathway
Intrinsic pathway
Final pathway
Principles
- Citrate added to chelate calcium
- Sample centrifuged to give plasma
- Calcium returned at time of testing
- Tissue factor added
- Binds to VIIa, activating X
- Time until coagulation measured
- Citrate added to chelate calcium, calcium returned at time of testing
- Sample centrifuged to give plasma
- Calcium returned at time of testing
- Kaolin added
- Activates factor XII
- Cephalin added
- Provides phospholipid surface for binding of tenase and prothrombinase complexes
- Time until coagulation measured
Normal Values
11-13 seconds
30-40 seconds
Causes of Prolonged Time
- Reduction in functional VII
- Warfarin
- Liver disease
- Factor X deficiency
- DIC
Heparin
Haemophilia
DIC
Liver disease
Uses
Warfarin monitoring
Screening test for coagulopathy
Heparin monitoring
Screening test for coagulopathy
Activated clotting time
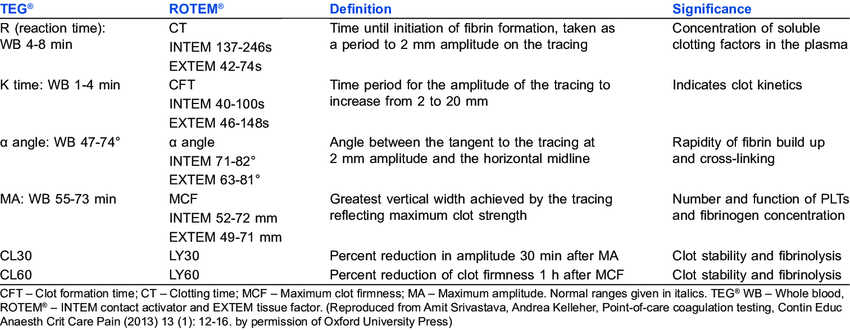
Thromboelastography
What is tested
Entire clotting cascade and platelet function
Entire clotting cascade and platelet function
Principles
- Whole, fresh blood is added to a clotting activator (e.g. kaolin)
- Time until coagulation measured
- Citrate added to chelate calcium, calcium returned at time of testing
- Sample injected into the sampling cup (containing a clotting activator)
- The cup is rotated around a pin, or the pin rotated independently
- As a clot forms, the pin’s movement is restricted by adherence to it
- This restriction is outputted graphically
Normal values
100-110 seconds
Based on test – TEG/ROTEM
Causes of prolonged time
Any coagulopathy, e.g.
• Clotting factor inhibitors e.g. warfarin, heparin
• Thrombocytopaenia or platelet inhibitors
• DIC
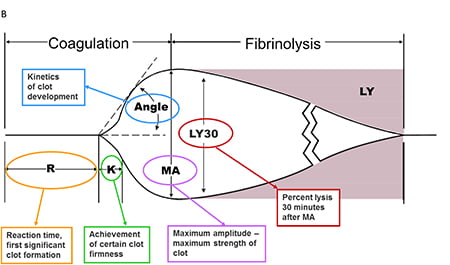
R time – clotting factors
α angle – fibrinogen function and preactivation
MA – platelet number and function
CLT – effectiveness of fibrinolysis
Uses
Monitoring adequacy of coagulation in cardiopulmonary bypass, ECMO, dialysis
Trauma – assess need for blood product replacement
Theatre monitoring
• Cardiac surgery
• Liver transplant
Mooney 2016
Examiner Comments
2017A 09: 61% of candidates passed this question.
Many candidates incorrectly stated that the PT assessed the intrinsic system and that the APTT assessed the extrinsic system. This led to subsequent errors in relating a coagulation test to the appropriate coagulation factors that it assessed. Some candidates produced elaborate diagrams of the coagulation cascade in isolation without relating it to the question.
10. Describe the pharmacology of hydrocortisone.
Examiner Comments
2017A 10: 54% of candidates passed this question.
Hydrocortisone is listed as a Class A drug in the syllabus and as such knowledge of its pharmacokinetics is expected. No marks were awarded for generic pharmacokinetic statements such as: “average bioavailability”, “moderate protein binding”, “bioavailability 100% for IV preparation” etc.
11. Outline the anatomical relations of the trachea relevant to performing a percutaneous tracheostomy.
CICMWrecks Answer
Tracheostomy – insertion of a tube through the anterior portion of the neck into the trachea to facilitate ventilation
Trachea Course:
- Larynx connects to the superior part of the trachea at C6 into the thorax and terminates at the level of the sternal angle, where it divides into the right and left mainstem bronchi.
- Initially anterior, then moves posteriorly as it descends to move behind the sternal notch
Tracheal Structure:
- A fibrocartilaginous tube 10cm long, approx 5cm in neck
- Supported by incomplete cartilaginous tracheal rings, which keep the trachea patent.
- The tracheal rings are joined by fibroelastic tissue.
- They are deficient posteriorly where the trachea lies anterior to the oesophagus; the posterior gap is spanned by the involuntary smooth trachealis muscle
Relationships:
- Lateral – carotid sheaths (common carotid arteries, vagus and internal jugular veins), thyroid lobes, inferior thyroid arteries, recurrent laryngeal nerves
- Inferior to the isthmus of the thyroid gland are the inferior thyroid veins
- Posterior – oesophagus, vertebral column
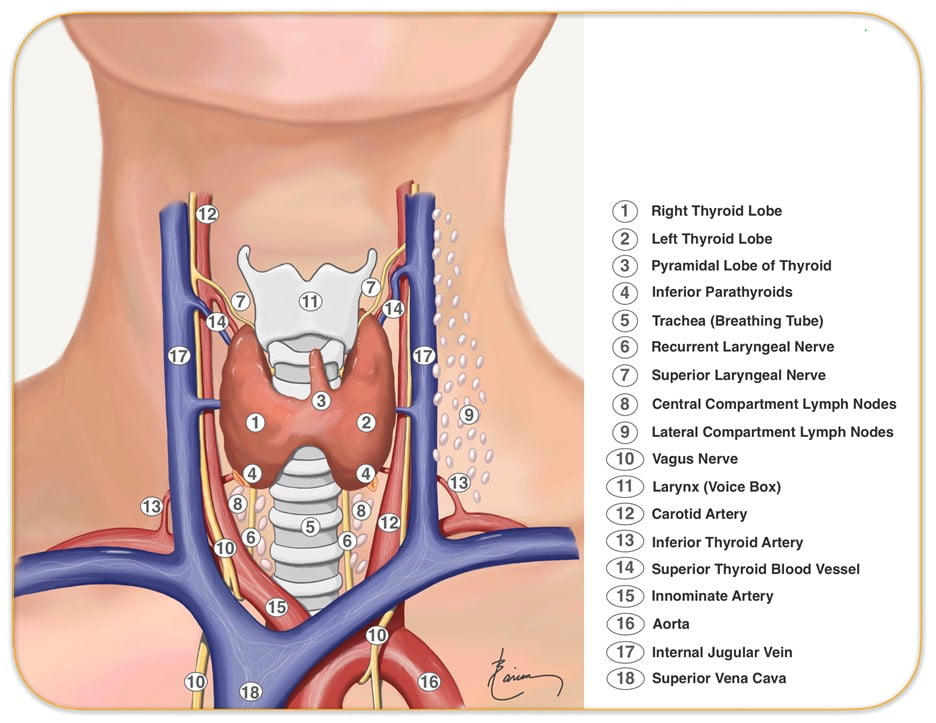
Relevant surface anatomy (in midline of neck):
- Hyoid bone (at level of C3)
- Thyroid cartilage
- Cricothyroid membrane
- Cricoid cartilage (at level of C6)
- Thyroid gland
- Sternohyoid muscle just lateral to midline structures, overlies sternothyroid and thyrohyoid muscles
Layers of dissection in tracheostomy:
- Skin
- Subcutaneous tissue
- Fat
- Pretracheal fascia (superficial and deep)
- Passage through the fibroelastic tissue in between the 1st and 2nd rings (common in perc trache) or 2nd /3rd or 3rd/4th (surgical trache)
- Trachea
Gladwin 2016
Examiner Comments
2017A 11: 44% of candidates passed this question.
Many candidates described how to perform a tracheostomy or the structure of the trachea rather than the relevant anatomical relations. It was expected that answers include anterior, posterior and lateral relations at the correct tracheal level including relevant vascular structures.
12. Describe the pharmacology of oxycodone.
Examiner Comments
2017A 12: 53% of candidates passed this question.
Few candidates covered the pharmacokinetic aspect of the question sufficiently. No marks were awarded for generic comments such as hepatic metabolism and renal excretion.
13. Outline the anatomy relevant to the insertion of a Dorsalis Pedis arterial cannula (50% of marks). Explain the differences between blood pressure measurement at this site compared to measurement at the aortic arch (50% of marks).
CICMWrecks Answer: Dorsalis Pedis Artery
Dorsalis Pedis Artery
- Origin: Continuation of anterior tibial artery at anterior aspect of ankle joint
- Course: Malleoli, over lower end of tibia à Lateral to extensor hallucis longus
- Base of 1st intermetatarsal space
- Termination: Divides into
- First dorsal Metatarsal artery: terminal branch
- Deep Plantar Artery: communicates with plantar blood supply
- Palpation of the dorsalis pedis artery pulse: On dorsal surface of foot
- lateral to extensor hallucis longus tendon (or medially to extensor digitorum longus tendon)
- distal to the dorsal most prominence of the navicular bone which serves as a reliable landmark for palpation
- Acceptable choice for cannulation under most circumstances
- Although it is absent in up to one eighth of the population
- Loop collateral circulation with branches of Posterior tibial artery
- The posterior tibial artery is a similarly useful vessel for arterial pressure monitoring. Catheters placed in this artery are less susceptible to kinking on dorsiflexion of the foot than in the dorsalis pedis artery, and they may be particularly valuable in neonates and infants
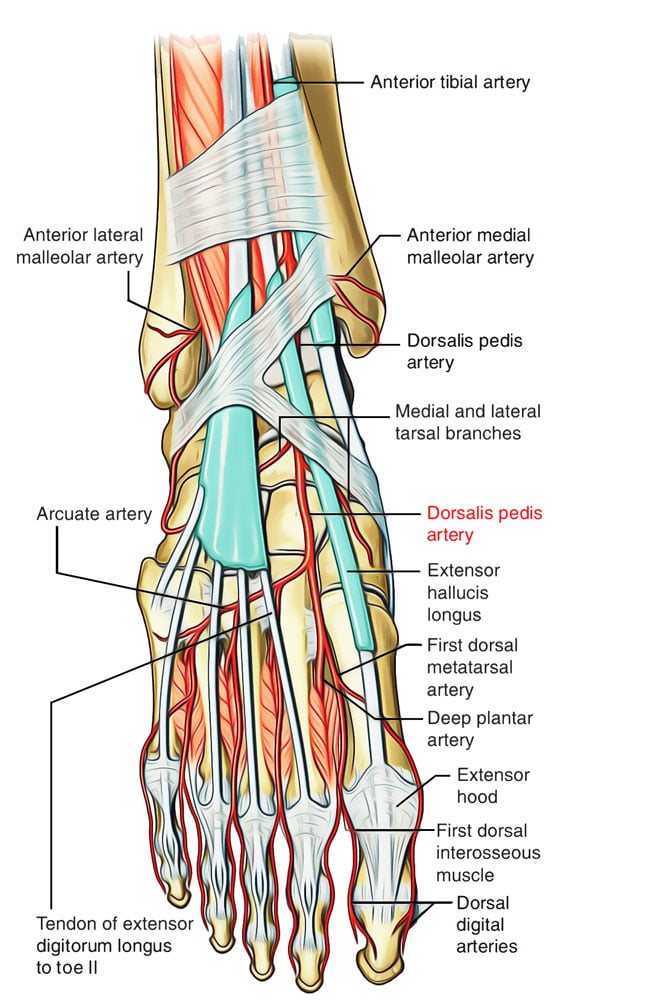
Arterial Line Trace according to site of insertion (Deranged Physiology)
Source: Deranged Physiology – Click to Open
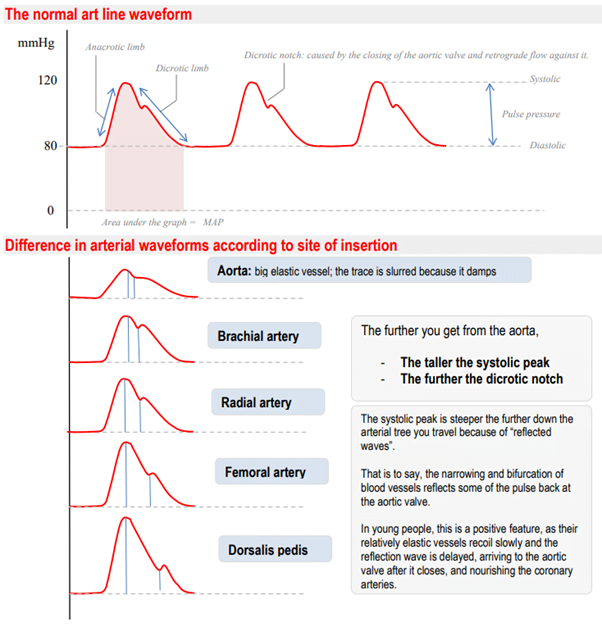
Examiner Comments
2017A 13: 30% of candidates passed this question.
The anatomy component of answers frequently lacked required detail. Many candidates listed the observed differences in the waveforms however an explanation for these differences was required.
14. Define respiratory compliance (20% of marks). Describe the factors that affect it (80% of marks).
CICMWrecks Answer

Lung compliance
- Change in lung volume per unit change in transmural pressure
- Normal lung compliance ~200ml/cmH2O
- Static compliance (Cs)
- ” Compliance of lung measured when lung held at constant lung volume”
- no pressure componet due to resistance
- is a function of: Elastic recoil of the lung, Surface tension of alveoli
- Dynamic compliance (Cd)
- ” Compliance of lung measured during cycles of inspiration and expiration”
- includes the pressure required to generate flow by overcoming resistance forces
- is a function of: respiratory rate
- Dynamic Compliance usually < Static Compliance due to the degree of time dependence in the elastic behaviour of a particular lung
- Specific compliance
- Compliance per unit volume lung
- Used to compare different lungs
- Hysteresis
- any process where the future state of a system is dependent on its current and previous state
- Compliance of the lung is different in inspiration and expiration
- In dynamic compliance curves:
- Airways resistance is a function of flow rate. Flow rate (therefore resistance) is maximal at the beginning of inspiration and end-expiration.
- In static compliance curves:
- There is no resistive component. Hysteresis is due to viscous resistance of surfactant and the lung.
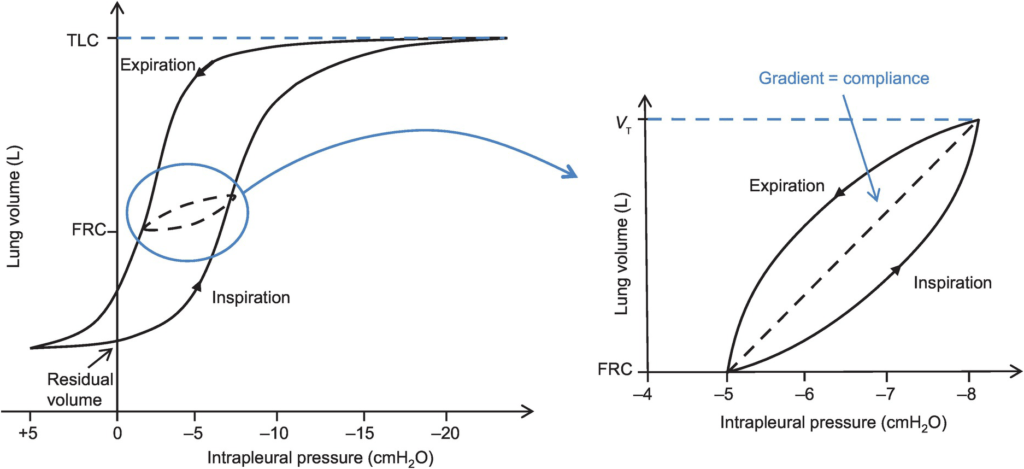
Factors Affecting Compliance
Lung Compliance
- Surfactant
- increases lung compliance
- decreases surface tension at alveolar air – water interface
- prevents small alveoli from collapsing
- accounts for most of hysteresis in intact lungs
- Lung volume
- Lung Compliance decreases at higher lung volumes
- Specific compliance (Compliance/FRC) remains constant
- Elephants have greater lung compliance than mice!
- Pulmonary blood volume
- Increased PBV decreases lung compliance
- Pulmonary venous congestion from L heart failure or mitral regurgitation
decreases lung compliance
- Bronchial smooth muscle tone
- Increased bronchial smooth muscle tone decreases compliance
- Decreased dynamic lung compliance by 50% in animal models of methacoline
challenge
- Disease
- ARDS, pneumonia decreases lung compliance
- pulmonary fibrosis → Impraired elasticity → decreases lung compliance
- Asthma, Emphysema increases lung compliance
Chest Wall Compliance
- Chest wall restriction – reduced chest wall compliance
- Obesity
- Spastic paralysis of chest wall musculature
- Ossification of costal cartilages
- Kyphosis/scoliosis
- Scarring/constriction (e.g. circumferential burns)
- Position: Prone (60% reduced compliance)
- Collagen Disorders: Increased Chest Wall Compliance
Measurement of Compliance
Dynamic Compliance
- ” Compliance of lung measured during cycles of inspiration and expiration”
- Measure:
- Measure with spirometer
- Using Volume/Pressure curve during normal rhythmic breathing at points of no flow
- Equation above then gives dynamic compliance
Static Compliance
- ” Compliance of lung measured when lung held at constant lung volume”
- Volume vs Pressure loops during inspiration and expiration demonstrate Hysteresis
- Measure:
- @ no flow in or out of the lung, AND time for pressure to equlibrate across the lung (>10sec).
- Patient exhales in steps holding the volume with open glottis
- Intrapleural pressure measured as oesophageal pressure
- Measured compliance secondary to viscoelastic properties and accounts for both short and long time-constant alveoli.
Specific Compliance
- Measure of the average compliance across all lung units
- Children = Adults ~ 0.05 cmH2O-1
Gladwin / Sakurai / JC 2020
Examiner Comments
2017A 14: 54% of candidates passed this question.
This question was generally well answered with good structure.
15. Outline the cardiovascular changes associated with morbid obesity.
CICMWrecks Answer
Obesity
- excessive fat accumulation in adipose tissue
- WHO classification based on BMI (weight in kg/ height in m2)
| BMI | |
|---|---|
| Underweight | <18.5 kg/m2 |
| Normal weight | ≥18.5 to 24.9 kg/m2 |
| Overweight | ≥25 to 29.9 kg/m2 |
| Obesity | ≥30 kg/m2 |
| Obesity class 1 | 30 to 34.9 kg/m2 |
| Obesity class 2 | 35 to 39.9 kg/m2 |
| Obesity class 3 | BMI ≥40 kg/m2 |
*Alternate names from different classifications:
| BMI | |
|---|---|
| Severe Obesity | ≥ 35 or 40 kg/m2 |
| Morbid Obesity | ≥ 35 kg/m2 + obesity-related health conditions or ≥ 40 kg/m2 |
| Super Obesity | ≥ 45 or 50 kg/m2 |
Characteristics:
- Changes depend on extent + duration of obesity
- Complex genetic and environmental causes
- Increased caloric intake
- Increased metabolic rate (normal for BSA)
- associated with HTN, HF, IHD, cardiomyopathy, sudden cardiac death, arrhythmias, PVD, DVT, CVD
- Hormonal changes:
- ↑ SNS
- ↑ HR + ↑ SV
- ↑ RAAS → Na+ retention → ↑ blood vol → ↑ MAP (Systemic HTN)
- ↑ MAP → LVH → LV dilation → LV failure
- LV diastolic failure + ↑ PVR → RV hypertrophy
- ↑ Leptin → Cardiac remodelling + LVH
- Plasminogen Activator Inhibitor-1 → ↓ fibrinolysis → predisposes to VTE
- Inflammatory Adipokines → Impairs endothelial function → ↑ SVR
- insulin resistance + hyperlipidaemia → inflammatory mediator upregulation → disrupt endothelial function → IHD + cerebrovascular disease + PVD
- ↑ SNS
- Mechanical Effect: Compression of abdominal + leg vessels
- ↓ VR → supine hypotension + ↑ risk DVTs
Changes in CVS:
- Increased VO2 : Due to increased LBM and fat mass.
- Increased Blood Volume : Due to increased angiotensin II and aldosterone.
- Increased Stroke Volume: Due to:
- Increased preload (major factor)
- Increased contractility (minor factor) due to increased circulating adrenal hormones.
- Increased Cardiac Output: To maintain DO2.
- Initially with preserved ejection fraction
- ↑ Peripheral vascular resistance
- Inflammatory Adipokines → Impairs endothelial function
- ↑ SNS
- Diastolic dysfunction: Due to myocardial fibrosis impairing relaxation.
- direct deposition of fat in myocardium → conduction disease (& predisposition to arrhythmias) + cardiomyopathy
- OSA
- Pulmonary HTN → cor pulmonale
- Polycythaemia → ↑ viscosity
Kerr / JC 2020
Examiner Comments
2017A 15: 42% of candidates passed this question.
Many candidates did not include enough detail in their answers. Higher scoring answers included more depth such as the following: blood volume, left ventricular changes, arterial blood pressure, pulmonary artery pressures, risks of ischaemia, arrhythmias etc.
16. List the potential problems resulting from blood transfusion and methods used to minimise them.
CICMWrecks Answer
Adverse Consequences of Blood Transfusion
1. Acute (<24 hours)
1a) Immune-mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Allergic reaction to plasma proteins | mild (urticarial) or severe (anaphylaxis) | Pre-treatment in high risk patients |
| Acute Haemolytic transfusion reaction | incompatibility of donor and recipient blood leads to widespread haemolysis and circulatory collapse | Group and screening |
| Febrile non-haemolytic transfusion reaction (FNHTR) | due to stored cytokines and/or the presence of recipient alloantibodies | Careful monitoring and early cessation of transfusion |
| Transfusion related acute lung injury (TRALI) | noncardiogenic pulmonary oedema caused by HLA antibodies in donor plasma directed against recipient leukocytes or bioactive lipids which accumulate during storage | Careful monitoring and early cessation of transfusion |
1b) Non-immune mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Sepsis | bacterial infections are most common with platelets as they are stored at room temperature | Safe and sterile methods of storage |
| Transfusion Related Circulatory overload (TACO) | fluid overload usually due to rapid or massive transfusion | Careful monitoring and early cessation of transfusion |
| Non-immune mediated haemolysis | Careful monitoring and early cessation of transfusion | |
| Hypothermia | Careful monitoring and early cessation of transfusion | |
| Dilutional coagulopathy | Careful monitoring and early cessation of transfusion |
2. Delayed (>24 hours)
2a) Immune-mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Delayed haemolytic transfusion reaction | Production of anti-donor antibodies post-transfusion May be associated with transfused malaria | Careful monitoring and early cessation of transfusion |
| Transfusion-related immunomodulation (TRIM) | transient immunosuppression in blood recipients which may be due to release of cytokines from donor lymphocytes | Careful monitoring and early cessation of transfusion |
| Alloimmunisation | development of antibodies during exposure to blood products, resulting in an amplified reaction on subsequent exposure Can cause post transfusion thrombocytopenia and purpura | Re-screening every 48-72 hours |
| Transfusion Associated Graft vs. Host Disease | Profound bone marrow aplasia >90% mortality Viable donor T cells implant and attack recipient tissues | Careful monitoring. Re-screening |
2b) Non-immune mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Iron overload | most common in chronically transfused patients | Monitoring of Iron levels |
| Transfusion-related infection | Viral – the risk of HIV, HTLV 1&2 and HCV is <1/1 million. The risk of contracting HBV is slightly higher at 1/500,000. Other – malaria, vCJD, Dengue Fever, West Nile Virus | Pre-screening of donors, screening of collected blood as per local guidelines (different for different areas) |
3. Storage Lesions
A storage lesion refers to the changes that occur to a sample of blood during storage. (Note: Australian Red cross anti-coagulates Whole blood with CPD, but washes and stores Red Cells in SAGM).
| Problem | Methods to Minimize | |
|---|---|---|
| Physical changes | Reduction in the viability of RBCs due to shape changes and reduced deformability Formation of microaggregates | Careful monitoring and usage of products within a specified period |
| Hyperkalaemia | plasma K can be >20 at 28 days in stored blood due to inactivation of the red cell Na/K ATPase pump. | Careful monitoring and usage of products within a specified period |
| High citrate load | can lead to hypocalcaemia and alkalosis (less or nil in SAGM) | Use of SAGM for storage |
| Reduction in 2,3-BPG | causes left shift of the oxygen/haemoglobin dissociation curve (less in CPDA1) | Use of CPDA1 |
| Renal impairment | Due to Increase in free haemoglobin from cell lysis | Routine monitoring of renal function and careful monitoring during transfusion |
Massive Transfusion
- Replacement of >1 blood volume in 24 hours
- 50% of blood volume in 4 hours
Adverse consequences of massive transfusion
| Problem | Methods to Minimize | |
|---|---|---|
| Hypothermia | Cooled products | Appropriately re-warm products. Use of blood warmers |
| Poor O2 delivery | Depletion of 2,3 DPG in PRBC | |
Haemostatic abnormalities | Dilutional coagulopathy | Monitor coagulation profile at appropriate intervals during massive transfusions. Use of appropriate factors like FFPs, Cryoprecipitate during massive transfusions |
| Hypocalcaemia | Consumption with coagulopathy and bound to citrate added to transfused units | Monitor and replace calcium as necessary |
| Hypomagnesaemia | Bound to citrate in transfused units | Monitor and replace magnesium as necessary |
| Citrate toxicity | Citrate is added to stored units as an anticoagulant | Monitor for acidosis during massive transfusions |
| Lactic acidosis | Hyperlactataemia due to anaerobic metabolism in stored units | Monitor for acidosis during massive transfusions |
| Hyperkalaemia | Potassium migrates from stored erythrocytes into plasma whilst in storage | Careful monitoring and usage of products within a specified period |
| Air embolism | Inadvertent infusion | Careful monitoring, use of transfusion sets with air vents, filters |
JC / Sakurai 2019
Examiner Comments
2017A 16: 53% of candidates passed this question.
This question required a broad answer. It was generally well answered. Those candidates who scored well had a good structure to their answers e.g. grouping potential electrolyte disturbances together, and infectious risks together etc. and including methods used to minimise these risks in appropriate detail.
17. Compare and contrast the pharmacology of phenytoin and levetiracetam.
Examiner Comments
2017A 17: 35% of candidates passed this question.
A table was useful to answer this question. Comparing and contrasting the pharmacology was required to score well rather than listing various aspects of pharmacology. The key properties of the drugs which demonstrate their importance to ICU was required.
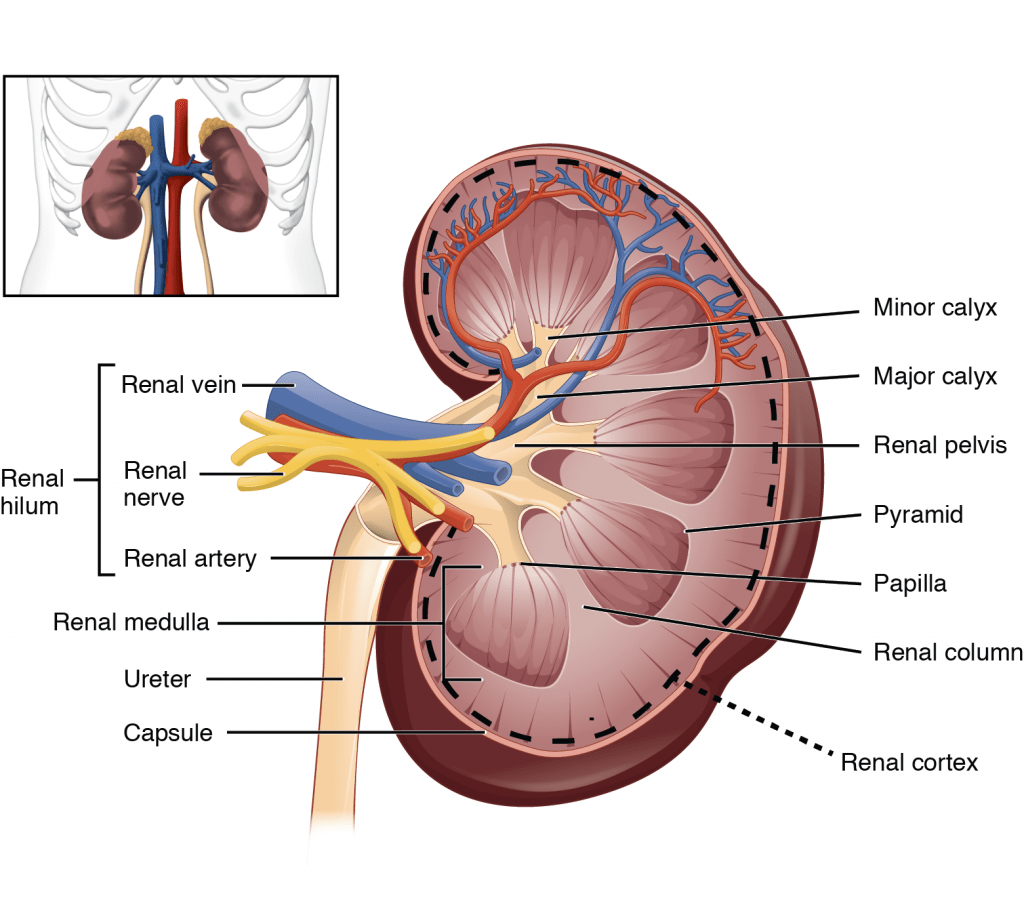
18. Outline the functional anatomy of the kidneys (40% of marks). Outline the regulation of renal blood flow (60% of marks).
CICMWrecks Answer – Anatomy
Kidney
- Lie in the posterior wall of the abdomen – outside peritoneum (retroperitoneal)
- Each kidney approx 150g
- Medial side of each kidney = hilum
- Renal artery and vein
- Lymphatic supply
- Nerves
- Ureter
- Kidney surrounded by tough fibrous capsule
- Kidney organized into outer cortex and inner medulla
- Medulla
- Organised into mutliple pyramids with the base at the corticomedullary junction and apices – the papilla – at the hilum draining into the ureters
- Nephrons = functional unit of the kidney (1 million / kidney)
Nephron
The functional unit of the kidney (1 million / kidney)
Parts:
- Afferent Arteriole: Brings blood to glomerulus. Regulate BP and supply blood to kidneys. Diameter bigger than Efferent Arterioles
- Efferent arteriole: Carries blood away. Regulates GFR
- Glomerulus: Provides driving force for solutes and water
- Bowman’s Capsule: Inner visceral layer, outer parietal layer. Filters bloods
- Renal Tubule: Filled with tubular fluid: aids in excretion and reabsorption
- Proximal convoluted tubule
- Loop of Henle:
- Descending Limb
- Lower thin Ascending limb
- Thick Ascending Limb
- Distal convoluted tubule
- Connecting tubule
Types by length:
- Cortical nephrons
- High in cortex, short loops of Henle.
- Juxtamedullary nephrons
- Low in cortex near medulla, long loops of Henle which penetrate medulla
- Only nephrons with vasa recta
Renal Blood Supply
- Renal blood flow ~1.25L/min (24% of cardiac output or 500ml/100g tissue/min) via renal artery
- 95% blood flow to cortex, ~5% to Medulla
- Renal artery enters through hilum it divides
- → interlobar arteries
- → arcuate arteries
- → interlobular arteries
- → afferent arterioles
- → glomerular capillaries
- → efferent arterioles
- → peritubular capillaries
- Peritubular capillaries supply blood to the renal tubules
- Juxtaglomerular capillaries have specialized vasa recta which supply the LoH and is important in renal concentration of urine
- → venous system → interlobular veins → arcuate veins → interlobar veins → renal vein
Juxtaglomerular Apparatus
Formed where the TAL of LoH and DCT passes between the afferent and efferent
arterioles at the vascular pole of the glomerulus.
Comprises of:
- Macula Densa
- Modified tubular epithelial wall of the DCT (located at the angle between
afferent and efferent arterioles) - Contains specialised cells that sense changes in the filtrate NaCl levels →
involved in intrinsic regulation of GFR and RBF by “Tubuloglomerular
feedback” and control of renin secretion
- Modified tubular epithelial wall of the DCT (located at the angle between
- Juxtaglomerular (Granular) cells
- Specialised smooth muscle cells found in the afferent arteriolar wall (esp
“media”) that possess:- Renin-containing granules → secreted to invoke RAAS
- Intrarenal-baroreceptors → involved in intrinsic autoregulation of RBF/GFR by a myogenic mechanism
- Specialised smooth muscle cells found in the afferent arteriolar wall (esp
- Extraglomerular mesangial (Lacis) cells
- Participate in a signalling pathway of the Tubuloglomerular feedback
- Possess contractile elements (actin and myosin) → contract in response to SNS stimulation → thereby ↓ glomerular surface area and GFR
JC / Sakurai 2019
CICMWrecks Answer – Physiology of Blood flow
Regulation of Renal Blood Flow
- Renal blood flow Autoregulated between 80~170mmHg
- Blood flow maintained by modulating resistance based on pressure
- Renal vascular resistance maintained by interlobular arteries, afferent arterioles and efferent arterioles, amenable to external regulation
- GFR approx 180l/day – Autoregulated by tubuloglomerular feedback – relatively constant in response to fluctuating renal blood flow
INTRINSIC Regulation (Autoregulation) of GFR and RBF:
- Renal blood flow (and consequently GFR) Autoregulated between MAP range of 75~170mmHg
- Blood flow maintained by modulating resistance of AFFERENT based on pressure
- Efferent arteriole is NOT involved in autoregulation!
- Autoregulation of GFR and RBF can be overridden by external influences (Eg. hormones and SNS neurons), even when renal perfusion pressure is between MAP 75-170 mmHg!
Mechanisms of Autoregulation:
- Myogenic autoregulation (Myogenic stretch response):
- In response to vascular wall stretch (due to increased intraluminal pressures), stretch dependent Ca influx occurs causing vasoconstricion of arterioles → increased resistance according to Poisuille-Hagen Equation → Decreased flow
- In response to shear stress (due to increased flow), Endothelial derived relaxation factors released (such as NO) → NO acts on guanylyl cyclase → increased cGMP → smooth muscle relaxation → arteriolar vasodilation
- Tubuloglomerular feedback (TGF)
- Negative feedback – Links the rate of GFR to concentration of salt in tubular fluid at macula densa
- Macula densa in wall of Ascending limb of loop of Henle Detects change in tubular flow (by the changing salt concentrations)
- As a consequence of decreased blood flow → GFR decreases → Tubular flow rate decreases → Increased uptake of [Na] in Ascending LoH → Reduced [Na+] and [Cl-] reaching the DCT and macula densa → Juxtaglomerular apparatus releases prostaglandins (PGE2) → vasodilation of afferent arteriole → increased resistance to glomerular blood flow
- With increased GFR → Increased tubular flow rate → Decreased [Na] uptake by the LoH → Increased [Na+] in macula densa → Juxtaglomerular apparatus secretes adenosine → Vasoconstriction of the afferent arteriole → Decreased blood flow
Note: Flow to Juxtamedullary nephrons is not autoregulated. High blood pressure increases juxtamedullary flow, increasing GFR and impairing renal concentration, resulting in a pressure diuresis.
Extrinsic Control of GFR and RBF:
- Hormonal regulation of blood flow
- Afferent arterioles:
- Dilation → PGE-2, PGI-2, DA, ANP, NO, kinins
- Constriction → High dose AII, NAd, ET-1, Adenosine, ADH, Thirst
- Efferent arterioles:
- Dilation → Inhibition of AT-II
- Constriction → Low dose AT-II
- Mesangial cell → Contracts due to AT-II, ADH and NAd (contraction response inhibited by ANP, DA, PGE2, PGI2)
- Note: Angiotensin II:
- At physiological (low) doses → it maintains GFR by efferent arteriolar vasoconstriction (at expense of RBF)
- With ↑ AT-II levels → causes:
- BOTH afferent and efferent arterioles constriction→ ↓ GFR
and RBF - Mesangial cell contraction in renal corpuscle → ↓ KF → ↓
GFR
- BOTH afferent and efferent arterioles constriction→ ↓ GFR
- Afferent arterioles:
- Neural regulation of renal blood flow (SNS, noradrenergic)
- All renal vescles richly innervated by sympathetic nerves
- 2 Mechanisms:
- Constricts BOTH afferent and efferent arterioles → ↓ RBF >>> GFR
- Stimulates renin secretion (via β1 receptors on JG cells) → ↑ Angiotensin II production → afferent and efferent arteriolar vasoconstriction → ↓ RBF and GFR
- Starling resistors
- Increased intra-abdominal pressure will decrease blood flow in starling resistor model
- Increased intra-capsular pressure will decrease renal blood flow
- High blood amino acid/ glucose level
- High filtered AA/ glucose load → reabsorption in PT with Na → ↓NaCl reaches distal tubules → macula densa → ↓adenosine → vasodilation → ↑RBF
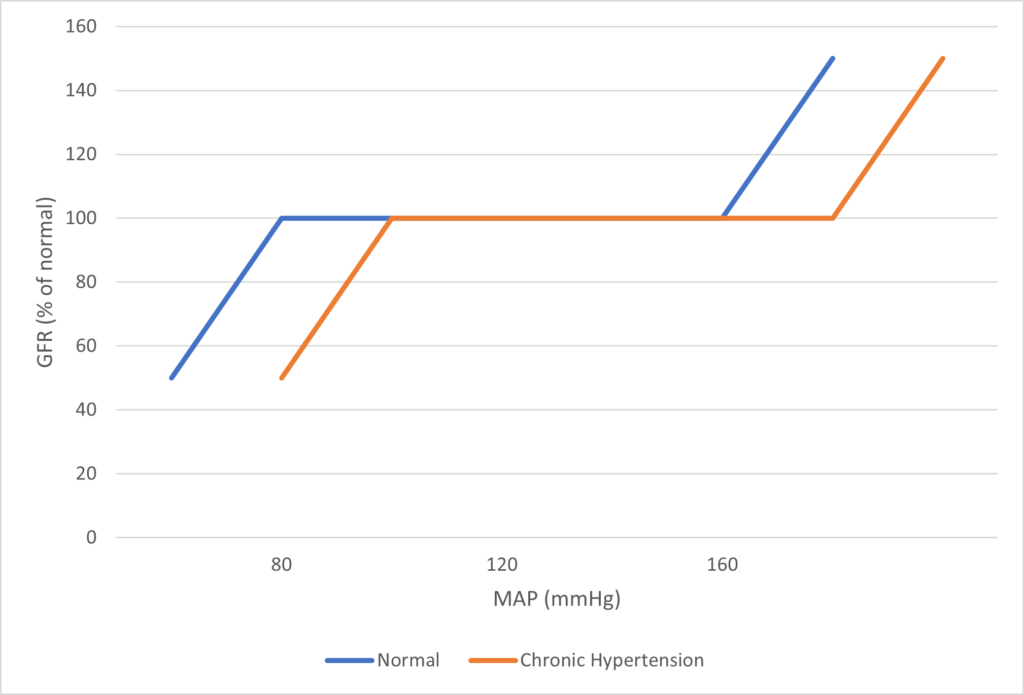
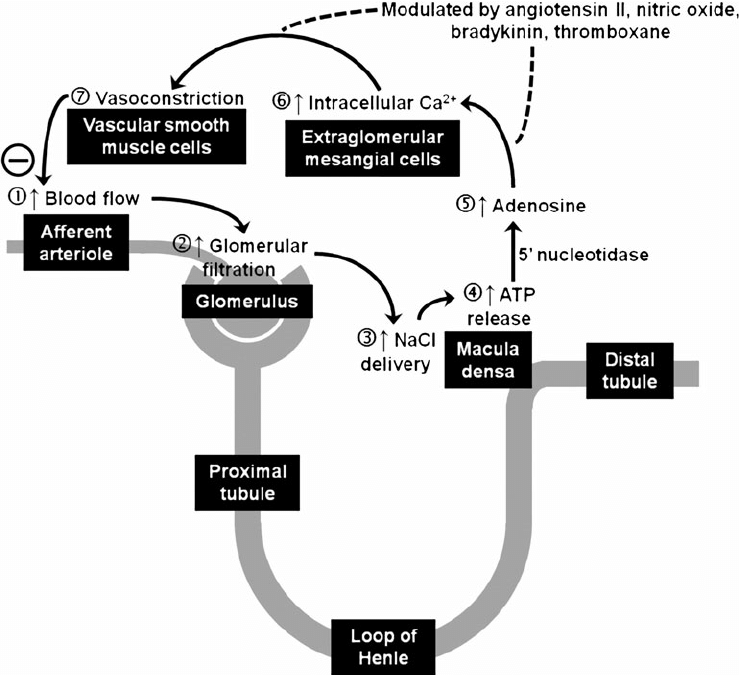
Image Source
JC / Mooney / Sakurai 2019
Examiner Comments
2017A 18: 67% of candidates passed this question.
Candidates who scored well weighted their answers according to the marks allocation outlined in the question and adopted a good structure. A number of candidates confused the roles of tubuloglomerular feedback and the renin angiotensin aldosterone pathway.
19. Define mixed venous PO2 (20% of marks). Outline the factors that affect this value (80% of marks).
CICMWrecks Answer
Mixed Venous Blood
- Mixed Venous Blood = blood taken from the pulmonary artery
- Normal mixed venous partial pressure of oxygen = 40mmHg -> 75% saturation
- Majority of oxygen in blood bound to haemoglobin
- Hb has 4 haem molecules, each can bind one oxygen molecule
- Affinity of Hb for oxygen changes with oxygen saturation, due to conformational change in the haem group on oxygen binding
- Affinity for oxygen decreased by: low pH, high CO2, high temperature, 1-3-DPG
Factors
- Complex interplay of factors which affect Venous Oxygen Partial pressure of oxygen and hence content
- Modified Fick principle: Mixed venous oxygen content = oxygen delivery – oxygen extraction
Oxygen Content
- = 20ml/100ml in arterial blood, 15ml/100ml in venous blood
- Vast majority of oxygen carried by haemoglobin, tiny dissolved portion
- Non-linear relationship between dissolved O2 and O2 content
- PO2 is the fraction of dissolved O2
- PO2 determines Sats based on Oxygen-Haemoglobin Dissociation Curve
Oxygen Delivery
= Cardiac output x Arterial oxygen content
Oxygen Demand
= Cardiac output x Arteriovenous O2 content difference
Oxygen flux
Hence SvO2 and pvO2 are surrogate markers for Oxygen flux
- O2 Delivery
- 1000ml/min in health
- Decrease in cardiac output: ↓HR, ↓SV (↓preload, ↓contractility, ↑afterload)
- Decrease in functional Hb: anaemia, carbon monoxide, congenital abnormalities of haemoglobin
- Decrease in oxygen saturation: V/Q mismatch, anatomical shunt, hypoventilation, low FiO2 (e.g. at altitude), right-shift of oxygen dissociation curve (low pH etc. – see above)
- Oxygen extraction:
- 250ml/min
- Increased by:
- increased tissue metabolism (e.g. sepsis, pregnancy, malignancy, post-operative catabolic state etc.)
- right shift of oxygen dissociation curve (see above)
Mooney / JC 2020
Examiner Comments
2017A 19: 37% of candidates passed this question.
This question was in two parts – the first part was worth 20% and candidates were expected to provide a definition of mixed venous blood as well as the partial pressure of oxygen in mixed venous blood (including normal range). Good answers also provided the varying PO2 from different tissue beds that make up mixed venous blood, where the ‘mixing’ occurs (the right ventricle) and where it is sampled (pulmonary artery).
For the second part of the question, worth 80% of the marks, good answers included the relationship between mixed venous PO2 and mixed venous O2 content (including the shape and position of the HbO2 dissociation curve); the variables encompassed in the modified Fick equation; arterial oxygen content and its determinants; oxygen consumption (VO2); and cardiac output (CO). Including an outline of how each affects the value of mixed venous PO2. A number of candidates wrote about mixed venous oxygen saturation. Other common errors were: missing a number of key factors that affect PO2; and using an incorrect form and/or content of the modified Fick equation.
20. Describe the pharmacology of vasopressin (70% of marks) and its analogues (30% of marks).
Examiner Comments
2017A 20: 28% of candidates passed this question.
A pharmacology answer template outlining pharmacokinetics and dynamics was required. Candidates failed to score marks for describing the physiology of vasopressin secretion. A number of answers demonstrated limited knowledge about its indications for use and its potential adverse effects.
21. Explain the potential causes of a difference between the measured end tidal CO2 and the arterial partial pressure of CO2.
CICMWrecks Answer
PaCO2
- Factors affecting PaCO2
- Rate of metabolism and CO2 production
- FiCO2 (usually negligable)
- Alveolar ventilation
- Increased alveolar ventilation decreased PaCO2
End-Tidal CO2
- CO2 contained in gas at the end of tidal expiration
- Contains:
- Alveolar CO2
- High CO2 diffusion – PACO2 = PaCO2
- Only 0.7mmHg Alveolar-arterial CO2 gradient for 10% shunt
- Alveolar Dead Space CO2
- Anatomical – conducting airways
- Physiological – West zone 1 / Apex
- Varies with posture and pathology
- Alveolar CO2
- Measured by capnometer
- In-line or side-stream
- Used IR absorbance at 4.23 μm
- Side-stream: lag in CO2 detection due to increased dead space between respiratory tract and analyzer
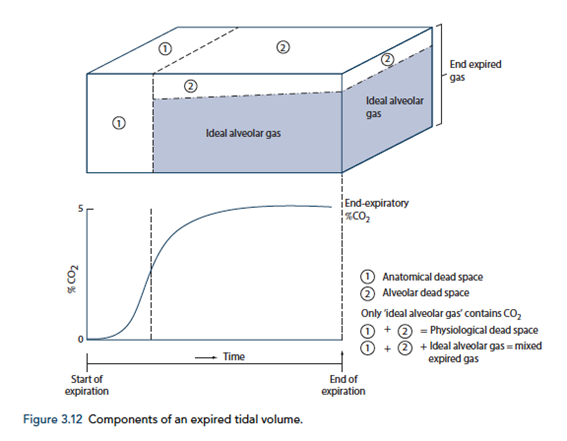
End-tidal to Arterial PaCO2 difference
- Normal ETCO2 – PaCO2 ~5mmHg
- Can be due to Artificially low ETCO2 or Artificially High PaCO2
Artificially low ETCO2
- Dead space (VD)
- Assumed PCO2 however usually low pCO2 but non 0 pCO2
- Contributes to ETCO2-PaCO2 ~5mmHg
- Pathological ↑VD
- ↑alveolar dead space (normal individuals = 0)
- Mixing of gas between perfused and non-perfused alveoli → ETCO2 < PaCO2
- Equivalent to ↑ West Zone 1
- → PE, hypotension, excess PEEP/IPPV
- COAD – poor perfusion of hyper-expanded alveoli
- ↑alveolar dead space (normal individuals = 0)
- Closing capacity (CC):
- Usually 40 CC may exceed FRC
- ↑ Airways collapse/gas trapping → airways closure → ↑alveolar pCO2 within alveoli not exhaling
- Exacerbated by
- low volume ventilation close to FRC
- High FiO2 for extended periods → denitrogenation → loss of airways splinting = CC closer to FRC
- Usually 40 CC may exceed FRC
- Sampling line problems:
- Too far from trachea (ie ↑ mechanical dead space)
- Too long – unable to adequately sample as gas is trapped in line
- Air leakage/line blockage leading to measurement error with entraining of room air, loss of expired air
- Machine:
- Loss of calibration of sampling unit
- Excess H2O in water trap interfering with measurement
- Interference from other gases (N2O) artificially raises ETCO2 due to interference with IR absorber “Collision broadening”
- Incorrect timing of measurement
- Inadequate expiration time → failing to reach plateau = incomplete alveolar expiration = falsely low ETCO2
Artificially high PaCO2
- Should be measured from arterial sample
- Venous sampling will artificially ↑pCO2
- Loss of calibration of machine
Gladwin / Sakurai 2016
Examiner Comments
2017A 21: 30% of candidates passed this question.
Many candidates didn’t distinguish between the different types of dead space. In general this topic was not well understood.
22. Outline the functions of the liver
CICMWrecks Answer: Liver Functions
Liver:
- Largest abdominal solid organ
- 25 % CO at rest. approx 1,500 mls/min
- 15% of total blood volume at rest
Functions of Liver
| Filtration | |
| Immune defence | (via Kuppfer cells) against agents entering the portal circulation |
| 80% of circulating cholesterol | → bile salt |
| Biliary excretion of drugs/hormones | penicillins, amp, erythro |
| thyroxine, cortisol, estrogen | |
| calcium | |
| Immune | |
| Filtration of portal circulation | |
| Kuppfer cells | bacteria/ virus/ endotoxins/ immune complexes/ thrombin/ tumour |
| Phagocytosed, fused with lysozomes and degraded by lysosomal enzymes | |
| Antigen presentation | |
| Endotoxin neutralisation = pinocytosed | |
| Complement/CRP production | |
| Storage of metabolic substrate/fluids | |
| glycogen ~ 400g | |
| fat | |
| Fe++, B12, folate, Cu | |
| Vitamin A | |
| Blood Reservoir | |
| Metabolic | |
| CHO and intermediary metabolism | Hepatic Glucostat |
| gluconeogenesis, glycogen storage and utilisation, galactose/fructose to glucose | |
| Conversion to fat, AA’s and ketones | |
| Protein metabolism | amino acids utilisation |
| protein synthesis | |
| production of ketones | |
| deamination of fatty acids | |
| urea formation for ammonia removal | |
| plasma protein formation | |
| Fat homeostasis | metabolism (beta oxidation (rapid in hepatic cells)), synthesis and transport as lipoprotein |
| cholesterol homeostasis | |
| Endocrine | hormone synthesis & metabolism |
| Synthesis of 25 OH cholecalciferol, Metabolism of steroid hormones, Synthesis of somatomedins, Erythropoietin | |
| biotransformation | ammonia & urea cycle |
| drugs & toxins | |
| Acid-Base | Lactate metabolism |
| Synthetic functions | |
| Bile production | bile salts (incr fat absorption) |
| bilirubin (incr haem excretion) | |
| Protein synthesis | albumin 120-300mg/kg/d |
| alpha1/2 & beta globulins (transport) | |
| coagulation & fibrinolytic factors (fibrinogen, prothrombin, II, V, VII,VII, IX, X, XI, XII, XIII, antithrombin) | |
| Lymph synthesis | Up to 50% |
| Erythropoietin (10%) | |
Gladwin / JC / Bianca 2019
CICMWrecks Answer: Paracetamol Toxicity
Paracetamol Overdose
Hepatotoxicity
Toxic daily dose > 4-6 g (while lethal dose occurs at > 10-15 g (or > 300 mg/kg LBM)) → but the toxic and lethal doses are lower in “at-risk” groups:
- EtOH abuse (due to CYP450 induction (↑ toxic metabolite produced) and ↓ glutathione stores)
- Malnutrition (due to ↓ glutathione stores)
- Elderly (due to ↓ glutathione stores)
- Preexisting liver dysfunction
Mechanism:
- At therapeutic doses – “N-acetyl-p-amino-benzoquinoneimine” (highly toxic metabolite) is produced in small amounts, but is rapidly conjugated with hepatic glutathione (anti-oxidant) into a harmless metabolite
- BUT with toxic doses – Hepatic conjugation pathway is saturated and ↑↑↑ N-acetyl-p-amino-benzoquinoneimine is produced → this depletes hepatic glutathione stores, causing remaining N-acetyl-p-aminobenzoquinoneimine to then forms covalent bonds with sulphydryl groups on hepatocytes → results in centrilobular hepatic necrosis
Clinical features:
- Generally conscious and c/o N/V, epigastric pain, erythema, sweating → later developing:
- Acute haemolytic anaemia
- Develop hepatic failure (Ie. jaundice and cholestasis) after 48 hrs
- LFT/INR derangements at 3-5 days
- Fulminant hepatic failure at 3-7 days
- With severe OD, can present with hypotension/shock
Diagnosis :
Serum paracetamol levels → correlate with “nomogram” to predict likelihood of liver damage → used as a guide to dictate therapy
Treatment:
- Activated charcoal/gastric lavage→ limit paracetamol absorption
- Replace hepatic glutathione store within 12 hrs of OD → permits glucuronidation of toxic metabolite
- Oral methionine → ↑ glutathione synthesis
- IV N-acetylcysteine → hydrolysed to cysteine (which is a glutathione precursor)
- Nb. IV NAC is preferred b/c of N/V a/w toxicity (Ie. ↓ oral methionine absorption)
- IV glucose → due to risk of ↓ BGL with liver dysfunction
- Serial monitoring of LFTs and coagulation studies
- Referral to a specialist centre
Gladwin / JC / Bianca 2019
Examiner Comments
2017A 22: 56% of candidates passed this question.
Most candidates attempted a structure however did not expand the answers within the
categories: e.g. a passing mention of glucose homeostasis is insufficient to score full marks for the carbohydrate metabolism category.
23. Draw and label a left ventricular pressure volume loop in a normal adult (40% of marks). List the information that can be obtained from this loop (60% of marks).
CICMWrecks Answer
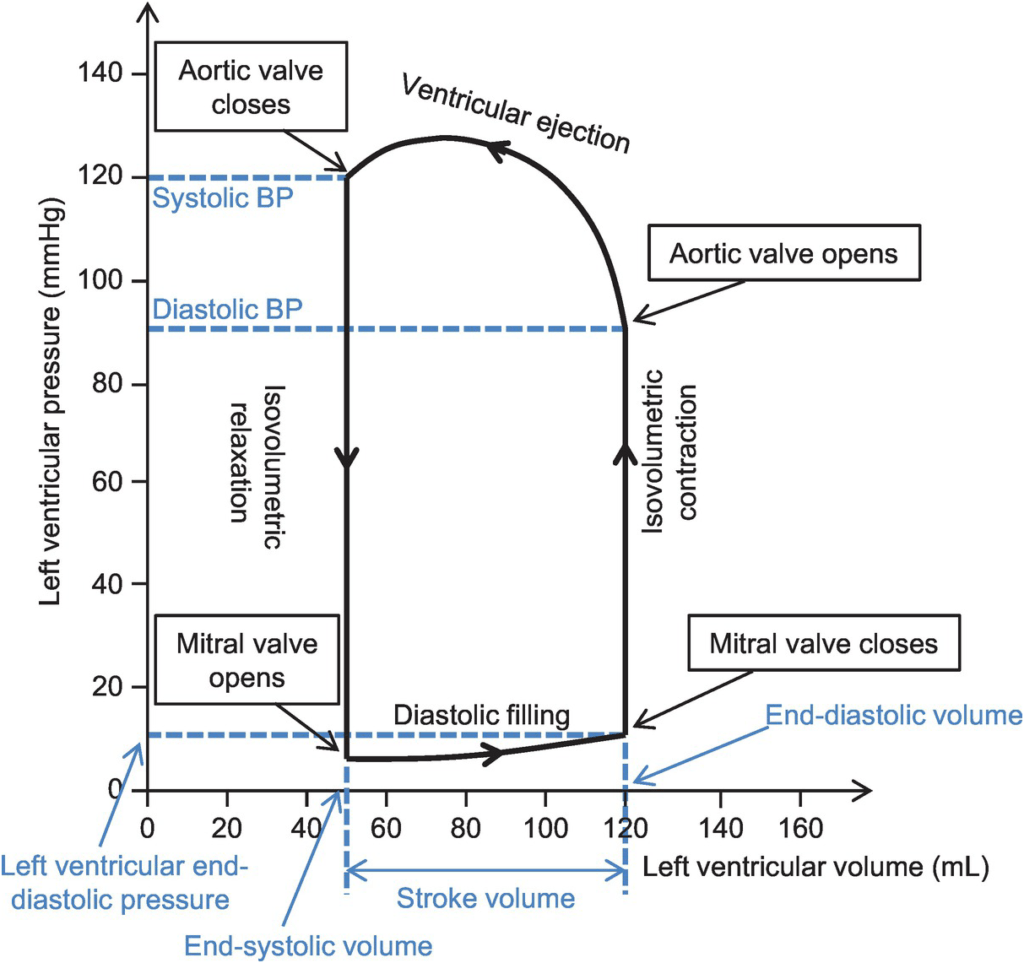
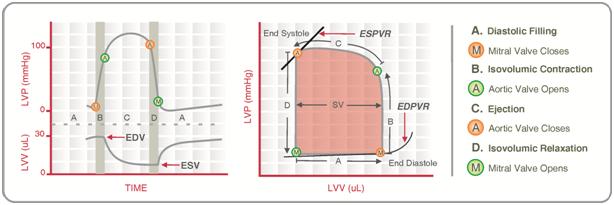
Normal P-V loop:
- Diastole
- (D) Beginning of diastole (isovolumetric ventricular relaxation)
- (A first 60% of vol) Early diastole (rapid ventricular filling – highly compliant Ventrical still relaxing)
- (A 60-90% of vol) Mid-diastole (slow ventricular filling – nearly full of blood → ↓ vent compliance)
- (A last 10% of vol) Late diastole (atrial contraction)
- Systole
- (B) Early systole (isovolumetric contraction)
- (C) Ventricular Ejection
- Early short rapid ejection phase (1st third of time)
- Prolonged reduced ejection phase (last 2/3 of time)
Derived Values:
- Stroke volume: SV = LVEDV-LVESV
- Ejection fraction as per eqn: EF = SV / EDV
- Measure of preload: LVEDV
- Measure of afterload
- Line b/w end-systolic point and locus at (LVEDV, 0)
- ↑ slope → ↑ Afterload
- Measure of contractility (ESPVR)
- End-systolic pressure volume relationship
- Slope is surrogate for contractility
- Measure of elastance (or stiffness) and compliance (or distensibility) of the LV – (EDPVR)
- End-diastolic pressure volume relationship
- Slope of the EDPVR = elastance = 1/compliance
- Peak LV pressure
- Measure of cardiac workload
- Total mechanical energy (or stroke work) (area of P-V loop)
- Heat generated by the heart during contraction
- “Diastolic work”
Gladwin 2016
Examiner Comments
2017A 23: 65% of candidates passed this question.
Many candidates lost marks for poor quality diagrams with inaccurate labelling. An accurate
diagram was required. Many answers lacked sufficient detail regarding contractility and
afterload.
24. Outline the physiology of cerebral spinal fluid (CSF)
CICMWrecks Answer
Formation / Production of CSF
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
Distribution / Circulation of CSF
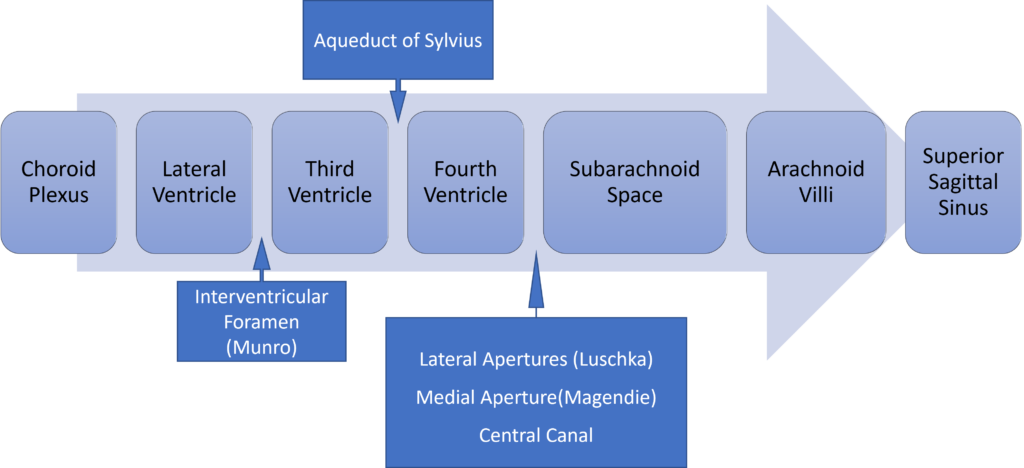
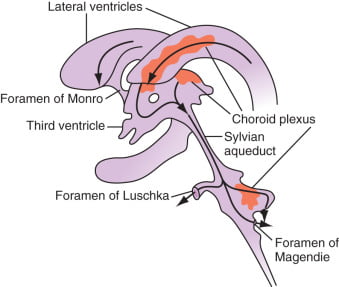
Absorption of CSF
- Absorbed through the arachnoid villi into the cerebral venous sinuses
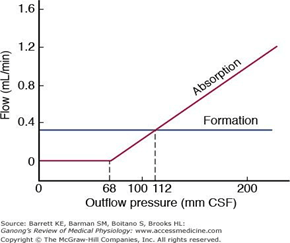
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
Composition of CSF
IDENTICAL to brain ECF, but differs in several manners from plasma
Compared with plasma:
- ↑ pCO2 (50 mmHg)
- ↓ pH (7.33)
- ↓ protein content
- 0.5% of plasma; 20 mg/dL
- poor acid-base buffering capacity
- ↓ content of glucose (by 60%) and cholesterol
- ↑ [Cl-] (by 7-14%) and ↑ [Mg2+] (by 40%)
- ↓ [K+] (by 40%), ↓ [Ca2+] (by 50%) and ↓ [Pi] (by 20-30%)
- ↑ creatinine (by 25%) but ↓ urea
- IDENTICAL osmolality (295), [Na+] (145), and [HCO3] (25)
Role / Functions of CSF
- Protective role (main function)
- Water bath effect
- Attributed to the low specific gravity of CSF (1.007)
- Causes brain to be buoyant
- ↓ its effective net weight from 1400 g to 50 g
- Mechanical cushion against acceleration/deceleration forces
- Buffer ↑ ICP by CSF translocation to extracranial subarachnoid space
- Abrupt ↑ ICP buffered by translocation of CSF within the vault to extracranial compartments
- Water bath effect
- Maintains constant ionic environment conducive to neuronal electrical activity CSF
- Supply role of nutrients (Eg simple sugars, amino acids) and O2 to brain
- Excretion of toxic substances, metabolic by-products, and CO2 from brain
- “Lymph-type” function → interstitial proteins in brain ECF return to circulation by
- CSF absorption across arachnoid villi
- Acid-base regulation → due to content, CSF allows for tight respiratory control
- Endocrine transport function → transports hormones to other brain regions
Gladwin / JC 2020
Examiner Comments
2017A 24: 67% of candidates passed this question.
Better answers included details on CSF production (amount, site), reabsorption and factors which influences CSF and its circulation.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | physiological reasons for an increased respiratory rate and the mechanism understanding of the gas laws understanding of lung volumes CO2, Capnograph Oxygen cascade |
| G. CVS | myocardial performance, lusitropy and blood pressure regulation. cardiac physiology and measurement, cardiac conduction pathway diagram cardiovascular response to a rapid and sudden loss of 1000 mls of blood? CVP anatomy and physio. |
| H. Renal | physiological causes of polyuria |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | acid/base physiology, H-h equation |
| K. Neuro | cerebral perfusion, its control and measurement pharmacology of propofol and ketamine |
| L. Musculoskeletal | NMJ, NM blockers |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | knowledge of blood groups and coagulation |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | classification of bacteria. |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |




Recent Comments