SAQs
1. Outline the distribution of calcium in normal plasma (20% of marks). Describe the hormonal control of the calcium concentration in the plasma (80% of marks)
CICMWrecks Answer
Normal Calcium: 25,000 mmol (400 mmol/kg)
Distribution:
- Readily exchangeable pool (1%) (ECF esp plasma)
- Total plasma [Ca2+] = 2.12-2.65 mmol/L
- Ionised [Ca2+] = 1.2 mmol/L
- Only the plasma free Ca is physiologically active and regulated by homeostatic mechanisms
- Two pools
- Diffusible (55%)
- 45% free/ionised
- Active form (above)
- 10% complexed
- 45% free/ionised
- Non-diffusible (45%)
- protein bound (esp to albumin)
- pH-dependent (↑ binding with ↑ pH)
- Diffusible (55%)
- Poorly exchangeable pool (99%)
- Bone/teeth (as hydroxyapatite, phosphates, carbonates)
Calcium balance
ECF and hence plasma Ca is the result of a balance between dietary intake, gastrointestinal absorption and excretion, renal excretion and exchange with bone Ca.
Normal Losses:
- Kidneys (40%) → 2.5-7.5 mmol/day
- Filtration of 250 mmol/day
- 95% reabsorbed by tubules
- PCT 65% with Na
- TAL of LoH 20%
- distal nephron 10%
- 5% excreted
- ↑ reabsorption at LoH/distal nephron
- PTH
- 1,25-dihydroxy-vitamin D
- GIT in faeces (60%) → 6-14 mmol/day
Calcium Regulation
- Calcitonin
- 32 AA peptide with 1 disulfide bond it is the hormone of Procalcitonin
- Released from Parafollicular cells (C-cells) of thyroid
- Release Stimuli: Hypercalcaemia, gastrin, beta-agonists, dopamine, oestrogen, CCK, glucagon and secretin
- Effect:
- Increases osteoblast function/Inhibits osteoclast function
- ↓intestinal calcium reabsorption
- inhibition of renal Ca and PO4 reabsorption
- Vitamin D
- Fat soluble sercosteroid from either
- diet or
- synthesis in skin of cholecalciferol from cholesterol then processed in liver and activated in PCT of the kidney
- Release stimuli
- ↑ concentration of PTH causes ↑ 1-alpha-hydroxylase activity in kidney or ↓ Ca or PO4
- Effect:
- ↑ bone release of Ca/PO4
- ↑ intestinal and renal reabsoption of Ca and PO4
- negative feedback on PTH release
- Fat soluble sercosteroid from either
- Parathyroid hormone
- Polypeptide hormone secreted from the parathyroid gland
- Release stimuli:
- ↓ Ca (primary or due to ↑ PO4) sensed by CaSR (Ca sensing receptor) in PT chief cell
- ↓ vitamin D levels
- Effect:
- ↑ Ca/PO4 reabsorption due to ↑ osteoclast activity
- ↑ Vit-D production by kidney (effect on 25 alpha hydroxylase)
- ↑ illeal Ca reabsorption
- ↑ renal reabsoption of Ca/Mg from DCT and TAL loop of henle
- ↓ PCT phosphate reabsorption
Gladwin 2016
Examiner Comments
2016B 01: 53 % of candidates passed this question.
As stated in the question, the distribution in plasma (not body) was expected. Candidates are reminded to include units [mmol/l].
Many candidates spent considerable effort describing the roles of calcium and particularly its role in excitation contraction coupling – which was not asked and hence scored no marks. It was expected that candidates were able to identify the roles of parathormone (PTH), 1,25 OH vitamin D and calcitonin as the major hormonal regulators. Better answers were able to describe the physiology and integration of these hormones at the gut, kidney and bone. Calcitonin and its transient and (probable) minor opposite role were also identified in these answers.
Better answers were also able to identify the permissive roles of growth hormone, cortisol and thyroxine.
2. Compare and contrast the mechanisms of action and toxicity of sodium nitroprusside and glyceryl trinitrate (GTN).
Examiner Comments
2016B 02: 55% of candidates passed this question.
Some excellent responses to this question showed a clear understanding of the pharmacology of these agents – the differing mechanisms of action involving both involving nitric oxide. Better answers were able to use this to explain the altered vascular specificity.
Toxicity was similarly well prepared for with a good understanding of the role of cyanide in SNP and the low rates of toxicity with GTN. This question was best handled in a tabular format which minimised omissions.
Some candidates focused on pharmaceutics, indications and side effects which were not allocated any marks.
‘Compare & contrast’ means the similarities; differences & unique features need to be related to each other. Several candidates confused ‘nitrous oxide’ with nitric oxide.
3. Describe the factors that determine glomerular filtration rate (GFR) in the kidney (70% of marks). Outline methods by which GFR can be measured (30% of marks).
CICMWrecks Answer – GFR, Determinants, Factors
GFR
GFR:
- “The amount of glomerular ultrafiltrate formed divided by the time of filtration”
- Normal value is approx 125 mL/min or 180 L/day
- Renal blood flow 1.25l/min in 70kg male → Filtration fraction 0.2
Functional anatomy:
3 Distinct Layers:
- glomerular capillary endothelium
- highly specialised endothelium with fenestrations to ↓ filter thickness
- prevents cellular components of blood from coming into contact with BM
- glomerular BM
- made of CT; -vely charged
- acts as filter
- bowmans epithelial cells (podocytes)
- epithelial cells with foot processes → large SA
- negatively charged
- maintain BM + phagocytic functions
Direct GFR Determinants
The GFR (Net flux across the membrane) is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation.
where
Kf = Filtration coefficient
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
- Normally NFP = 17mmHg
- Tubular oncotic pressure = zero throughout
- GC oncotic pressure varies from 21 to 33 mmHg as filtrate is removed.
- Thus less GFR produced at distal end of tubule.
| Afferent end of Glomerular Capillary (mmHg) | Efferent end of Glomerular Capillary (mmHg) | |
|---|---|---|
| PGC | 60 | 58 |
| PT | 15 | 15 |
| πGC | 21 | 33 |
| NFP | 24 | 10 |
| Kf | Filtration coefficient | = LpS = Hydraulic conductivity x Surface Area Glomerular surface area = 0.8m2 • Altered by Mesangial cell contraction (see circulating factors e.g. Angiotensin II → ↓SA → ↓GFR) Patency of the normal capillary wall structure (ie in tubular dysfunction Kf ↑’s ↑GFR) |
| PGC | hydrostatic pressure in capillary | relates to • RBF which is autoregulated for MAP 70-170mmHg • relative afferent/efferent arteriolar tone Affected by • Catecholamines • Local autoregulation -> Myogenic -> Tubuloglomerular feedback -> Hormones (see below) |
| PT | hydrostatic pressure in tubule | relates to • obstruction to urinary flow (usually pathological) • ↑PT → ↓ GFR (ie post renal obstructiion causing renal failure) |
| πGC | oncotic pressure in capillary | relates to • plasma protein concentration (incr in dehydration, decreased in heart failure) • ↑Systemic plasma oncotic pressure → ↑πGC → ↓GFR • ↓Renal plasma flow → ↑πGC → ↓GFR |
| πT | oncotic pressure in tubule | • usually zero, but can increase in renal failure/proteinuria • ↑’d πT → ↑GFR |
| σ | Staverman’s reflection coefficient | • Permeability of membrane to protein usually 1 (no protein leak) • can decrease with nephritis/proteinuria |
Factors influencing GFR
Circulating Factors
- Prostaglandins
- PGI2 and PGE2 → vasodilates → ↑ renal blood flow → ↑ GFR
- ↓ PGE2, PGI2 and NO levels → inhibits afferent arteriolar vasodilation → ↓ GFR
- Long term → ↓ renin release → ↓ ATII-induced efferent arteriolar vasoconstriction → ↓ GFR
- Noradrenaline / Adrenaline
- constrict renal afferent and efferent arterioles → ↓ renal blood flow and reducing filtration → ↓GFR
- Constrict mesangial cells to → ↓ GFR.
- Mesangial cell tone:
- Angiotensin 2, Endothelin, vasopressin → ↓ glomerular surface area and GFR
- ANP, PGE2, Dopamine and cAMP → all increase GFR
Solute factors
- Size
- < 7 kDa (Eg. glucose, ions, urea, H2O) filtered freely,
- > 70 kDa (Eg. albumin) are not filtered;
- neutral particles < 4 mm diameter filtered freely, > 8 mm are excluded
- Charge
- Negatively charge (Anionic) particles repulsed
- Cationic substances filtered more readily
- Protein binding
- Albumin excluded
Disease Factors
- Filtration decreases
- Shock → decreased glomerular pressure
- Obstruction → increased bowman’s capsule hydrostatic pressure
- Hypoproteinaemia → hepatic failure, nephrotic syndrome
JC / Gladwin / Sakurai / Kerr 2020
CICMWrecks Answer – GFR Measurement
GFR Measurement
Clearance:
- Renal clearance = vol of plasma completely cleared of a given substance by the kidneys per unit time (ml/min)
- Involves: glomerular filtration, secretion, reabsorption, and rarely tubular metabolism
- Renal clearance = V x [U]/[P]
- V = volume of urine or urine flow rate in ml/min
- [U] = urinary concentration of substance in mg/ml
- [P] = plasma concentration of substance in mg/ml
Estimating GFR:
- GFR = renal clearance of a substance if it is:
- Freely filtered at glomerulus
- Not secreted
- Not reabsorbed
- Not synthesized
- Not metabolised
- The amount excreted in the urine = amount filtered
- i.e. [plasma] x GFR = [urine] x urine vol
- Rearrange: GFR = urine vol x [urine] / [plasma]
Substances used to estimate GFR:
- Criteria
- easily measured in urine + plasma
- non-toxic
- easily administered
- Inulin
- Protein; polymer of fructose
- MW 5800 Da
- freely filtered
- Not reabsorbed, secreted, metabolised, or synthesized by the kidney
- Therefore clearance of inulin = GFR
- Requires continuous infusion for several hours to achieve steady state
- Creatinine
- used to approximate GFR as is more practical
- Released at a steady state from skeletal muscle cells (phosphocreatine)
- Freely filtered + not reabsorbed
- *Small amount secreted → overestimates GFR by small amount
- Cockcroft Gault formula used
JC / Gladwin / Sakurai / Kerr 2020
Examiner Comments
2016B 03: 57% of candidates passed this question.
Good answers included a description of Starling forces acting at the glomerular basement membrane. A description of the local and systemic factors influencing each component was expected.
It was expected candidates would discuss autoregulation of GFR & RBF, tubuloglomerular feedback, and integrated responses the body uses to keep GFR steady. Confusion about the nature of induced effects on afferent or efferent arteriolar dilation and constriction limited marks for some candidates. Many failed to mention the effects of mesangial surface area, Bowmans space pressure or serum protein content.
Candidates were expected to outline the methods of GFR estimation. Better responses described the rationale behind the use and limitations. Creatinine clearance, inulin and nuclear medicine techniques all scored marks. Some candidates made no attempt at this section and missed the opportunity to score marks. Estimates of CrCl/GFR [eGFR by formulae such as Cockcroft Gault, and serum Cr] are not measurement of GFR.
4. Describe the mechanisms of action of drugs used to treat acute severe asthma and give examples.
CICMWrecks Answer
Asthma:
- Airway obstruction that is reversible (completely or partially) either spontaneously or with treatment,
- Airway inflammation (oedema and hypersecretion)
- Increased airway responsiveness to a variety of stimuli
General Measures:
- Oxygen.
- Repeated assessment
- ABGs
Specific Pharmacology:
| Class | Example | MoA |
|---|---|---|
| Adrenergic Agonists | Salbutamol Adrenaline | Predominantly acting at the β2-adrenergic receptor Gs-PRC → ↑AC → ↑cAMP → ↑PLC activity – ↓ [Ca] via ↑ uptake and removal from cytoplasm – Active uncoupling of actin-myosin via i) Phosphorylation of MLCK ii) Phosphorylation of MLCK Phosphatase – ↑ K channel activation → hyperpolarisation → SMC relaxation Mast cell stabilisation (adrenaline) Improved mucocillary function. |
| Methyxanthines (PDE3 inhibitors) | Theophylline Aminophylline | Multiple Actions ↓ PDE acitons → ↑ cAMP in bronchial SM → sim to β2 effect Adenosine receptor antagonism → ↓ adensosine related bronchoconstriction |
| Antimuscarinics | Tiotropium, Ipratropium | M3 receptor antagonists Normally ACh stimulates M3 → Gq-PRC → ↑ DAG/IP3 → ↑ bronchiolar tone Antagonism → ↓ Vagally mediated Decreased Gq mediated effects |
| Steroids | Prednisone, Hydrocortisone | Reverse the activating effect of pro-inflammatory transcription factors → decrease inflammation – Inhibit the formation of cytokines secreted in asthma by T-lymphocytes, macrophages, and mast cells – Decreased vascular permeability – Inhibitory effect on mucus glycoprotein secretion – ? enhance B2 effects. |
| Other | Mag Sulphate | Acts as a bronchodilator by decreasing cytosolic Ca2+ concentrations. |
| Heliox | Decreased density – thus increased air flow via Hagan Pouiselle | |
| Ketamine | Bronchial smooth muscle relaxant by inhibiting Ach mediated SM constriction | |
| Sevoflurane | Direct beta agonism and inhibition of histamine release from mast cells. |
Gladwin 2016
Pharmacopeia Table
Examiner Comments
2016B 04: 71% of candidates passed this question.
Asthma drugs are typically categorised according to mechanism of action. A reasonable alternative is to categorise by clinical use, e.g. short acting, long acting, preventer, rescue etc. A lot of emphasis in marking was placed on an understanding of the beta-adrenergic pathway, its secondary messenger system and how this medicates smooth muscle relaxation. Candidates whose answers had structure as well those who described the wide range of drugs used to treat asthma scored well.
5. Describe the physiological factors that affect pulmonary arterial pressure (65% of marks). Write short notes on the use of inhaled nitric oxide as a pulmonary vasodilator (35% of marks).
CICMWrecks Answer
Factors affecting Pulmonary Arterial Pressure
Pressure in a system is generated by the interaction between flow and resistance
Factors affecting Pulmonary artery pressure are: PBF x PVR
Factors affecting Pulmonary Vascular Resistance
- Recruitment and Distension
- At higher pressures, decrease in resistance due to distension of partially collapsed capillaries and recruitment of completely collapsed capillaries
- Gravity – West Zones
- Zone 1 – PA > Pa > Pv – no flow of blood
- Zone 2 – Pa > PA > Pv – Resistance determined by alveolar pressure (starling resistor effect)
- Zone 3 – Pa > Pv > PA – Resistance determined by venous pressure
- Zone 4 – Pa > Pi > Pv > PA – Low lung volumes causes narrowing of extra-alveolar pressures
- Autonomic Innervation
- Sympathetic innervation from sympathetic trunkNoradrenaline
- α1 (vasoconstriction) and β (vasodilation) receptors
- α effects dominate (vasoconstricton)
- Parasympathetic innervation from vagus nerve
- Acetylcholine (M3 receptors)
- Cause vasodilation
- Sympathetic innervation from sympathetic trunkNoradrenaline
- Volume
- Balance of extra vascular lateral stretch (↓resistance) by increasing volumes and capillary longitudinal stretch (↑ resistance)
- Hypoxia, Hypercapnoea, Acidosis
- Alveolar hypoxia causes local vasoconstriction
- Similar but less potent effect of hypercapnoea and acidosis
- Local Mediators
- Dilators: Nitric oxide, prostacycline, Isoprenaline
- Constrictors: Histamine, Serotonin, Endothelins
Factors affecting Pulmonary Blood Flow
- Venous return
- MSFP – RAP
- Resistance to venous return
- LV output, RV output
- Gravity dependant
- Recruitment and distension of vasculature
Inhaled Nitric Oxide
Click to open CICMWrecks Table: Nitric Oxide
JC 2019
Examiner Comments
2016B 05: 51% of candidates passed this question.
Pressure in a system is generated by the interaction between flow and resistance. A structured approach to defining and describing the many factors that influence fluid flow and resistance was required to score well. Poiseuille’s law describes the determinants of resistance to laminar fluid flow and provides a useful answer structure. It is also necessary to describe factors that determine flow. This includes factors that determine venous return, as well right and left heart output.
A standard structured answer to the pharmacology of nitric oxide enabled concise and high scoring answering of this question.
6. Describe the factors that affect airways resistance.
CICMWrecks Answer
Airway Resistance
- Resistance is the impedence to flow.
- Normal Airways Resistance (AWR):
- Adult: ~2 cmH2O/L/s
- Newborn: ~20 cmH2O/L/s – declines markedly
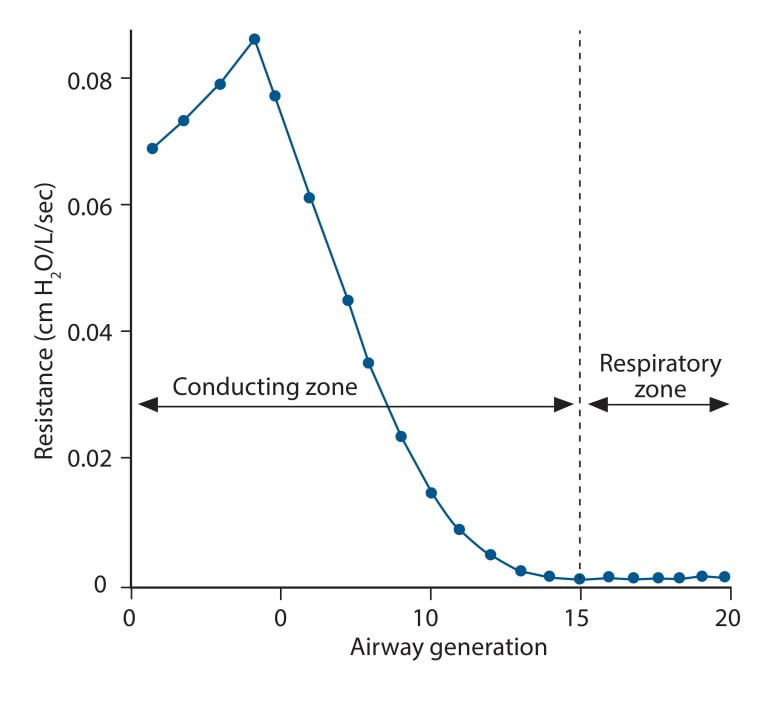
- Main Site of AWR:
- Mid-sized bronchi 7th/8th generation
- comparatively smaller cross-sectional area
- (note that in neonates, a greater proportion of Raw comes from the smaller peripheral airways)
- In the airway flow can be laminar or turbulent
- Flow depends on Depends on Reynolds number
where
Re is Reynold’s number
r is radius
ρ is density
v is velocity
η is viscosity
Laminar Flow
- Ordered flow occuring in concentric layers within a tube
- Flow in the center is fastest and flow in the most peripheral layer is the slowest
- Resistance to laminar flow obeys the Poisuille-Hagen Equation
where
R is vessel resistance
η is viscosity
L is length of vessel
r is radius of vessel
- Increases in viscosity of gas, or length of the tube increase resistance
- Increases in radius of the tube, decreases resistance by a power of 4
Turbulent Flow
- In turbulent flow, due to the disorganized flow and increased likelihood of friction
with the static airway wall, resistance is markedly increased. - Resistance to turbulent flow obeys the following equation
where
R is Resistance to flow
ρ is density
l is length of vessel
r is radius of vessel
- Increases in density of gas, or length of the tube increase resistance
- Increases in radius of the tube, decreases resistance by a power of 5
Factors affecting Airway Resistance
- Physics factors (see above):
- Location in the airway (see above): Mid-sized bronchi are the location of greatest airway resistance, resistance progressively declines with successive airway generations
- Flow:
- Laminar Flow vs turbulent flow
- Depends on Reynolds number factors
- density more important than dynamic viscosity, velocity (flow rate) as Main Site is Turbulent.
- Flow rate:
- Flow related airway collapse
- Airways beyond generation 11 have no structural rigidity
- High flows can reverse transmural pressure gradient and cause airway collapse
- Flow related airway collapse
- Newborn: ~20 cmH2O/L/s – declines markedly with age to ~2 cmH2O/L/s
- Radius changes
- Muscular control of airway diameter
- Neural:
- Parasympathetics important in bronchomotor tone → airway constriction via ACh and muscarinic receptors
- Sympathetic system virtually absent in lung
- Hormonal:
- Although no symppathetic innervation, abundance of β2 adrenoceptors → airway dilation
- Neural:
- Smooth mm tone:
- (↓r) bronchospasm, Musc antag (PSNS), LTs, PGF2-alpha
- (↑r) β2-agonists, adrenaline neb, SNS
- ↓intramural radius:
- oedema, ↑mucous, wall hypertrophy
- External compression:
- tumour, haemorrhage, PTX
- dynamic airways compression with forced expiration
- Muscular control of airway diameter
- Lung Volume
- ↑ lung vol →
- ↑ radial traction → ↓ AWR
- ↑ -ve intrapleural → ↑ patency of small airways
- ↓ lung vol → ↑ AWR
- CPAP or PEEP → ↑ FRC → reduces flow-related airway collapse at low lung volumes
- ↑ lung vol →
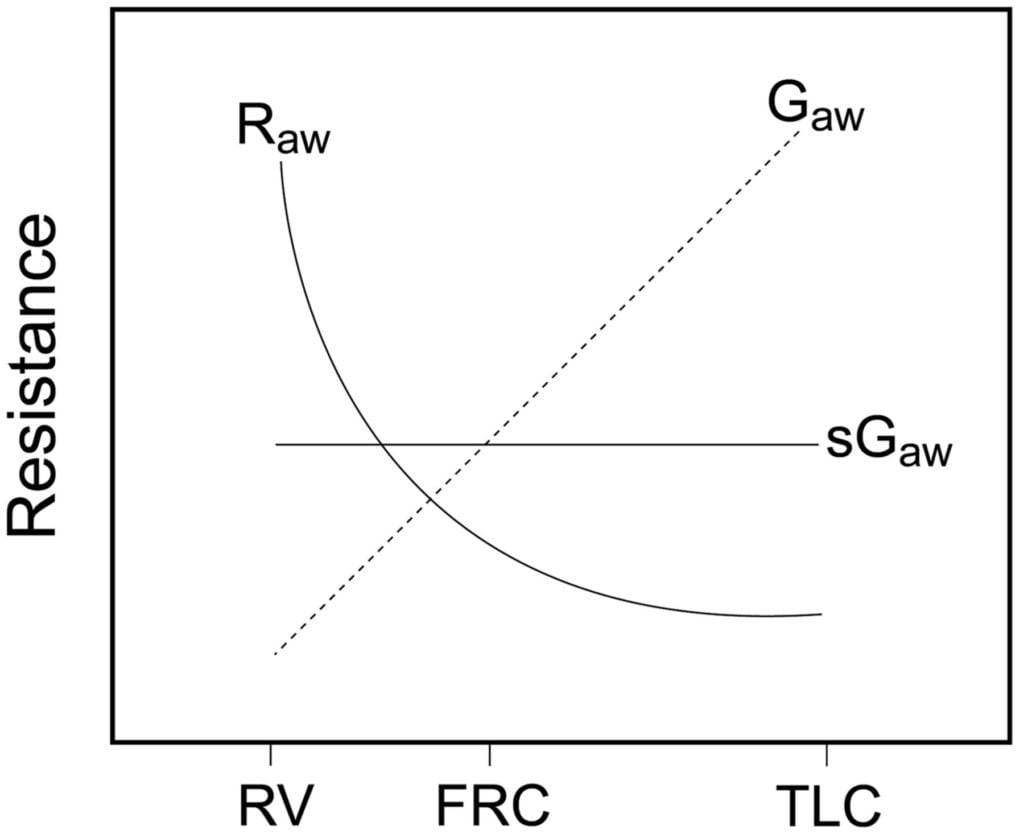
Relationship between Lung volume, Airway Resistance (R) , reciprocal of resistance (Conductance G, specific conductance sG)
Notice that only sGaw is independent of lung volume
Measurement of Airway Resistance
Body Plethysmography:
- Q measured with flow meter
- Lung Volume is measured with Plethysmography
- ∆P via Plethysmography and Boyle’s Law
- (A) box pressure is atmospheric
- (B) inspiration
- ∆V allows for ∆P measurement for given Q since
- PV = constant
- Then AWR can be calculated from 1
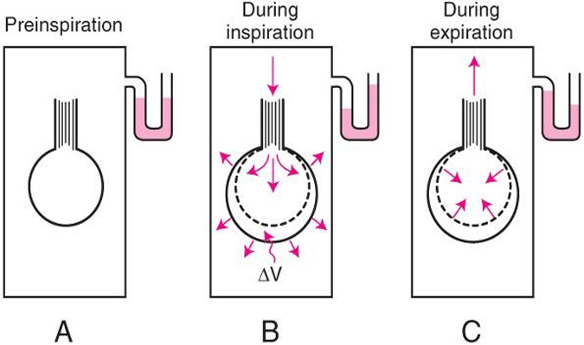
Interrupter Technique (direct from AWR eqn)
- Method
- Manometer distal to shutter
- Used to measure mouth and alveolar pressure
- Flow during inspiration or expiration in interrupted for 50–100 ms repeatedly throughout the respiratory cycle.
- Measure
- Q – flow rate (measured before interruption)
- P2 – mouth pressure (measured before interruption)
- P1 – pressure in alveoli (measured at the end of the interruption at te level of the mouth after equilibration)
- Apply Ohm’s Law (AWR equation above).
- Adequate for normal lungs not diseased.
Gladwin / Sakurai 2016
Examiner Comments
2016B 06: 47% of candidates passed this question.
Candidates who used a structured approach of using formulae that describe resistance fluid flow scored well. Poiseuille’s law describes the determinates of resistance to laminar fluid flow and provides a useful answer structure. The most common mistakes were confusion between resistance and compliance as well as failure to describe turbulent as well as laminar flow.
7. Compare and contrast the supply and demand of oxygen for the right and left ventricle.
CICMWrecks Answer
Myocardial O2 Supply
RIGHT VENTRICLE
LEFT VENTRICLE
- Supply:
- Right coronary artery and its branches
- conus artery supplies the infundibulum
- acute marginal arteries supply the anterior free wall
- posterior descending artery via septal branches supply the posterior 1/3 IV septum (PDA arises from RCA in Right dominant circulation and LCx in Left dominant circulation
- the other 2/3 of the IV septum is supplied by septal branches of the left anterior descending artery
- Supply: Left Main Coronary
- LAD (supplies the free wall and most of the papillary muscles)
- Diagonals
- Septal perforators
- LCx: inferolateral LV wall
- Obtuse Marginal branches
- LAD (supplies the free wall and most of the papillary muscles)
- RV Coronary blood flow
- RV pressures are low during both systole and diastole.
- flow during both systole and diastole
- LV Coronary blood flow
- The pressure inside the left ventricle is slightly higher than in the aorta during systole.
- Flow predominantly during diastole.
- Subendocardial flow ceases during systole.
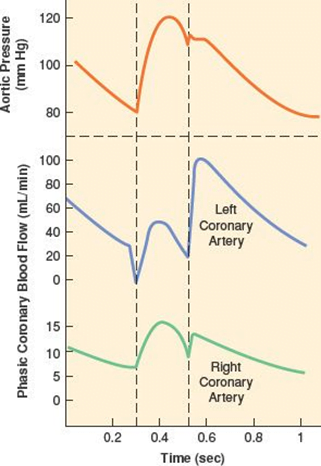
Myocardial O2 Demand
Determinants of myocardial O₂ demand:
- MVO₂ (ie extraction)
- High at rest (55-65%) cf. body average of 25%
- Extraction ratio can only rise by factor of < 2 to 90%
- AV Δ O₂ = 11 mL/dL
- Coronary venous O₂ content = 5 mL/dL
- Normally consumption 21 to 27 mls of O2/min.
- Determined by
- Wall tension.
- preload (EDV/EDP) and afterload (SVR)
- Contractility.
- Heart rate.
- ↑HR → ↓ supply
- ↑HR → ↑ demand (as ↑ MRO2)
- Wall tension.
RIGHT VENTRICLE
LEFT VENTRICLE
| Pumps against less resistance (PVR) → less systolic pressure → less O2 demand may be compromised during acute or chronic increases in right ventricle afterload resulting from pulmonary arterial hypertension | Pumps against higher afterload, thus more demand |
| ability to downregulate its metabolic demand during coronary hypoperfusion and thereby maintain contractile function and energy stores | |
| Activation of SNS causes relatively small changes in O2 demand | Activation of SNS causes more pronounced increase in O2 demand |
Examiner Comments
2016B 07: 29% of candidates passed this question.
An integrated answer to supply and demand of oxygen was expected, as a comparison between the right and left ventricles. Many candidates concentrated on differences not similarities. Myocardial oxygen demand was in general poorly described.
About 85 – 90% of oxygen demand is for internal work (major determinants wall tension 30 – 40%, heart rate 15 – 25%, myocardial contractility 10 – 15%, basal metabolism 25%). 10 – 15% of oxygen demand for external work or pressure volume work, determined by MPAP x CO.
It was expected answers would comment on the phasic nature of coronary blood flow which differs between left and right and the consequence of this to subendocardial oxygen supply during systole usually. Coronary blood flow is affected by coronary perfusion pressure (determined by aortic pressure and RV pressure) & coronary vascular resistance (determined by autoregulation, metabolic factors, humoral factors, nervous control interacting with local endothelial factors)
Generally, coronary blood flow is tightly coupled to oxygen demand/consumption due to high basal oxygen consumption (8 – 10 ml/ min/100g) and high oxygen extraction ratio (75%). Better answers noted that oxygen supply can only be increased to cope with increased demand only by increased coronary blood flow.
8. Compare and contrast the pharmacology of ranitidine and omeprazole.
Examiner Comments
2016B 08: 14% of candidates passed this question.
These agents are both commonly used in the ICU. The expectation was weighted towards the interesting and important aspects of pharmacology as outlined for category B drugs.
It was expected candidates could detail that both drugs are used to suppress acid secretion in the stomach. Ranitidine is a competitive, reversible inhibitor of the action of histamine at the histamine H2 receptors found in gastric parietal cells. This results in decreased gastric acid secretion and gastric volume, and reduced hydrogen ion concentration.
In contrast to Omeprazole which is a proton pump inhibitor that irreversibly blocks
the hydrogen potassium ATPase the gastric parietal cells.
Some general description of dosing, route of administration, pharmacokinetics and possible adverse effects was expected.
9. Describe the immunology and drug treatment of anaphylaxis.
CICMWrecks Answer
Anaphylaxis
- acute severe type I hypersensitivity reaction affecting multiple organ systems
- most common triggers:
- Food, Environmental
- Drugs: antibiotics, muscle relaxants
- Iodine contrast
- Chlorhexidine
- Latex
- Clinical effects
- Circulatory collapse: profound vasoplegia distributive shock
- Airway obstruction: angioedema, bronchospasm,
Pathophysiology
- Multiple exposures to target allergen
- 1st exposure: allergen presented by T helper cells to B cells → B cells produce specific IgE to target allergen
- Circulating IgE attach to mast cells → sensitization
- Subsequent exposure to allergen → allergen binds specific IgE on mast cells → mast cell degranulation → release of allergenic mediators
- Mediators trigger disseminated vasodilation + ↑ vascular permeability +/- bronchospasm → anaphylaxis
- Histamine: Localised release → urticaria; Systemic release → H1 + H2 → vasodilation, ↑vascular permeability → ↓MAP
- Bradykinin: ↑production PGI2, NO → vasodilation, ↑ vascular permeability → ↓MAP
- Prostaglandins: PGID2 → vasodilation, ↑ vascular permeability, bronchoconstriction, chemotactic for neutrophils + activates eosinophils
- Leukotrienes: LTE B4: chemotactic agent; LTE D4 + E4: angioedema, clotting/ thrombolysis/ DIC
- Tryptase: activates complement, coagulation, kallikrein-kinin pathways → ↓ BP, angioedema, clotting/ thrombolysis/ DIC
- TNF-a: cytokine → propagates anaphylactic reaction
- NO: vasodilates
- Serotonin: vasodilation, bronchoconstriction, aplatelet activation
- Platelet activating factor: acts at PAF Rs
Management of Anaphylaxis
- Oxygen
- IV Fluids
- Adrenaline is the drug of choice, as it treats cardiovascular collapse, bronchospasm, and decreases oedema formation.
- Stabilises mast cells to prevent degranulation
- Low doses: Beta agonism at (inotropy and chronotropy) and causing smooth muscle relaxation (including bronchorelaxation).
- Higher doses: alpha effects and causes vasoconstriction.
- In adults, 0.3-0.5mg IM Q5-15min
- In children, 0.01mg/kg IM Q5-15min
- Glucagon may be used in β-blocked patients resistant to adrenaline.
- In adults, 1-5mg IV over 5 minutes, followed by infusion at 5-15microg/min
- In children, 20-30mcg/kg up to 1mg over 5 minutes
Adjunctive agents include antihistamines and steroids. They are second line as they do not attenuate cardiovascular collapse, resolve airway obstruction, or have strong evidence behind their use. They include:
- Steroids:
- Work by binding to the cell nucleus and switching off the transcription of various genes which encode for inflammatory mediators such as cytokines, chemokines, adhesion molecules and arachidonic acid.
- They also activate anti-inflammatory genes such as MAP (mitogen activated protein) and increase the expression of beta2 receptors.
- Methylprednisolone, Hydrocortisone or Prednisolone
- Antihistamines:
- Binds to H1 receptors to block the effects of the histamine released from mast cells-
- inhibition of vasodilatation
- increased vascular permeability
- contraction of non-vascular smooth muscle
- Diphenhydramine 25-50mg IV (Children: 1mg/kg up to 40mg) up to 200mg in 24/24
- Binds to H1 receptors to block the effects of the histamine released from mast cells-
- Salbutamol, for bronchodilation
- Other Non-pharmacological management includes early intubation to protect against airway obstruction due to angioedema
Kerr / JC 2019
Examiner Comments
2016B 09: 32% of candidates passed this question.
It was expected candidates would detail the process of IgE mediated type I hypersensitivity reaction with some discussion of the mediators (Histamine / tryptase and others) and their consequences. Some detail describing time frame of response and the pre-exposure to Antigen (or a similar Antigen) was expected. Drug treatments would include oxygen and fluids as well as more specific agents such as adrenaline and steroids. Adrenaline is the mainstay of therapy and some comment on its haemodynamic role and prevention of ongoing mast cell degranulation was required. Better answers noted steroids take time to work and some also discussed the role of histamine blocking agents.
10. Statistics (not in current primary syllabus)
11. Compare the action potentials of a sino-atrial node cell and a myocardial cell.
CICMWrecks Answer
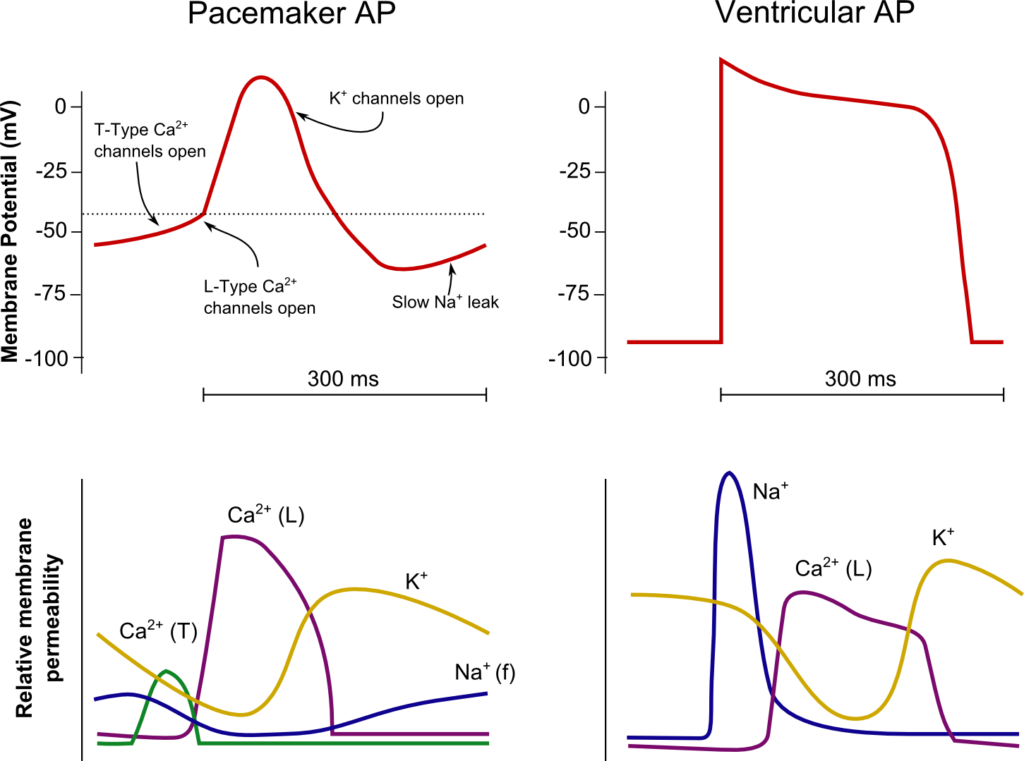
SA Node
Ventricle
Resting membrane potential
- No set RMP, however approx. -60mV
- βadrenergic stimulation causes a less negative RMP and increased slope of the initial upstroke
- muscarinic stimulation causes a more negative RMP and a decreased slope of the initial upstroke
Resting membrane potential
- Approx -90mV
Threshold potential
- Approx 40mV
Threshold potential
- Approx. -50mV
Phase 0
- Funny Na+ current allow leakage of Na+ into the the cell slowly increasing membrane potential to the threshold potential
T and L-type Ca2+ channels (voltage gated) open when threshold potential reached
- Ca2+ influx
- Depolarization
- Shallow upstroke compared to ventricular myocyte
Phase 0
- Rapid depolarization
- Opening of fast Na+ channels
- Influx of Na+
- Overshoot to +20mV
Phase 1
- Early repolarization
- Closure of fast Na+ channels and opening of K+ channels (transient outward, inward rectifier)
- Efflux of K+
Phase 2
- Plateau
- Opening of intially T-type Ca2+ channels and subsequently L-type Ca2+ channels
- Ca2+ influx balances K+ efflux
- Na+ channels in closed state à absolute refractory period
- No action potential can be generated in this state
Phase 3
K+ channels open and Ca2+ channels slowly close
- K+ efflux
- Repolarization
- No plateau phase
Phase 3
- Repolarization
- Closure of Ca2+ channels while K+ channels remain open (delayed rectifier)
- K+ efflux
- Returns potential towards RMP
- Na+ channels transition to inactive state à relative refractory period
- Another action potential can be generated with greater adequate stimulus
Phase 4
- Na+/K+ ATPase and Na+/Ca2+ ATPase maintain ionic gradients
Phase 4
- RMP
- Na+/K+ ATPase and Na+/Ca2+ ATPase maintain ionic gradients
Sakurai 2016
Examiner Comments
2016B 11: 75% of candidates passed this question.
A good answer included a well labelled sketch with a description of ion channels and relative directional flow. When using sketches in an answer they should be correctly labelled, and when used as a comparison with another sketch, the differences should be clear e.g. shape, duration and voltage difference.
12. Compare and contrast the pharmacokinetics and adverse effects of morphine and fentanyl.
Examiner Comments
2016B 12: 20% of candidates passed this question.
This question is best answered in a tabular form, comparing absorption, distribution, metabolism, excretion and the adverse effects.
Common omissions were lack of details on distribution, and not relating lipid solubility to effect. A description of the relative adverse effects was expected, e.g., more histamine release, less bradycardia, rather than listing similar adverse effects, e.g. respiratory depression.
Comparisons of pharmaceutics and pharmacodynamics did not attract any additional marks.
13. Outline the anatomy and physiology of liver blood flow (60% of marks). Explain the changes to drug metabolism when liver blood flow decreases (40% of marks).
CICMWrecks Answer
Hepatic blood flow
- 1.5l/min
- Anatomy
- Afferent via Hepatic Artery (branch of ceoliac trunk) contributing 30% and Portal Vein (confluence of inferior mesenteric, superior mesenteric and splenic veins) contributing 70% of hepatic blood flow
- Hepatic artery supplies 50% of O2 delivery
- Portal venous blood has PO2 of 80% due to mesenteric AV anastamosis
- Blood flows through low pressure hepatic sinusoidal system to ventral vein
- Efferent via Hepatic Vein → IVF
- Afferent via Hepatic Artery (branch of ceoliac trunk) contributing 30% and Portal Vein (confluence of inferior mesenteric, superior mesenteric and splenic veins) contributing 70% of hepatic blood flow
Regulation
Intrinsic
- Hepatic arterial and Portal venous pressure – Hepatic venous pressure / Hepatic vascular resistance (low)
- Autoregulation to systolic blood pressure 80mmHg
- Metabolic autoregulation
- Vasodilation in response to H+, CO2, lactate
- Semi-reciprocal relationship
- Hepatic arterial blood flow increases when protal venous flow decreases
Extrinsic
- Respiration
- Diaphragmic contraction → Decreased intrathoracic (and IVC) pressure and increased abdominal pressure → Favours hepatic blood flow
- Post-prandial
- Increased splanchnic blood flow post-prandially → Increased hepatic blood flow
- Neural
- Sympathetic
- α adrenoceptors present in portal vein AND hepatic artery
- β adrenoceptors only present in hepatic artery
- Therefore sympathetic input tends to venoconstrict the portal vein and dilate the hepatic artery
- Sympathetic
- Hormonal
- Adrenaline
- α constricts protal veins and decreases HBF
- β dilates hepatic arteries and increases HBF
- Glucagon increases hepatic blood flow
- Vasopressin decreases hepatic blood flow
- Angiotensin II decreases hepatic blood flow
- Vasoactive Intestinal Peptide (VIP) and secretin increase hepatic artery flow
- Adrenaline
Changes to drug metabolism when liver blood flow decreases:
- The two major determinants of hepatic clearance are the efficiency of drug removal from the blood and the efficiency of blood delivery to the liver.
- Efficiency of drug removal by the liver = hepatic extraction ratio
- fraction of the drug entering the liver in the blood which is irreversibly removed (extracted) during one pass of the blood through the liver.
- determined largely by:
- the free (unbound) fraction of the drug and
- the intrinsic clearance rate (the intrinsic ability of the liver to remove (metabolise) the drug in absence of restrictions imposed on drug delivery to the liver cell by blood flow or protein binding.)
- The effect of liver blood flow on hepatic clearance depends on the hepatic extraction ratio of the drug.
With decreasing hepatic blood flow, hepatic extraction ratio will increase for all drugs.
- For drugs with low intrinsic clearance:
- Hepatic extraction ratio will increase with decreasing hepatic blood flow
- Hepatic clearance will not decrease significantly with decreasing blood flow
- For drugs with high intrinsic clearance:
- Hepatic extraction ratio will increase slowly with decreasing blood flow
- Hepatic clearance will decrease in a fairly linear fashion, in proportion to hepatic blood flow
Also, Drugs which enjoy enterohepatic recirculation may have decreased half-lives due to failure of recirculation
Sakurai / JC 2019
Examiner Comments
2016B 13: 55% of candidates passed this question.
A statement regarding the quantum of hepatic blood flow with recognition of the contributions made by the Hepatic Artery and Portal Vein, drainage into the sinusoids before entering the hepatic vein which drains into the IVC would have been a good start. Discussion was then expected to revolve around how the liver blood flow is controlled. Answering this with respect to intrinsic and extrinsic factors along with an understanding of the semi-reciprocal relationship between hepatic arterial and portal venous blood flow would have rounded out a good answer to the first part of the question.
The second part of the question required recognition that hepatic clearance is the product of hepatic blood flow and the hepatic extraction ratio and considering the impact on drugs with a high or a low extraction ratio. Many answers failed to adequately mention how hepatic blood flow was controlled/regulated thus limiting the marks available. Similarly, in the second part of the question, many candidates spent considerable time mentioning the principals of drug metabolism rather than focusing on the question asked.
The concept of hepatic drug clearance as the product of blood flow and its extraction ratio was poorly appreciated.
14. Describe the features of a red blood cell that facilitate oxygen transport.
CICMWrecks Answer
RED BLOOD CELL
Formation
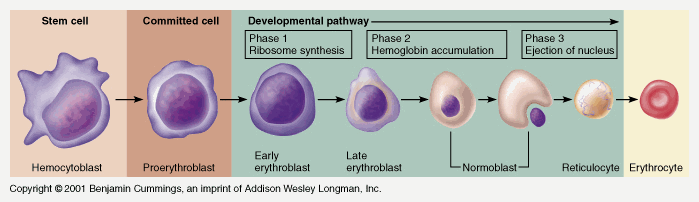
Pluripotent haemopoetic stem cell → Colony Forming unit → Proerythroblast → Reticulocyte → Erythrocyte
Simplified – main steps.
Takes 7 days
- Proerythroblast propagates
- From proerythroblast to reticulocyte
- Concentration of haemoglobin increases
- Nucleus disappears
- Endoplasmic reticulum disappears
- Reticulocyte
- Ribosomes
- Mitochondria
- Reticulocyte released into circulation
- Matures to RBC in 1-2 days
EPO increases rate of differentiation
Produced in the kidney (in response to low PO2)
Structure
- Are 7.5μm in diameter
- Are 2um thick
- biconcave disc, contains haemoglobin A (Hb F in-utero), has a central Fe moiety and demonstrates positive cooperativity in binding oxygen
- Have a lifespan of 120 days
- make up 40-50% of the blood volume, usual value 4-5 x 1012/L
- Carry ~29pg of haemoglobin
| Structure | Function |
|---|---|
Biconcave disc shape | Maximises surface area (optimising gas transfer) |
| Flexible membrane with shape maintained by structural proteins | makes the cells flexible enough to pass through capillary beds (which are narrower than the cell). |
| No nucleus & other organelles | Maximises cell volume available for Hb. |
| No mitochondria (No oxygen utilization) | Cannot perform aerobic metabolism – all ATP is generated via glycolysis. 3 shunts that come off the anaerobic glycolytic pathway (RBC’s only means of ATP generation): i) the production of 2-3 DPG via the Rapoport-Luebering shunt ii) generation of NADPH by the hexose monophosphate shunt (protects RBC from oxidative damage) iii) reduction of metHb back to Hb by way of the NADH. Optimizes Oxygen carriage |
| No ribosomes | Incapable of producing protein |
| Haemoglobin Four globulin chains (2α + 2β) links to heme Heme: Porphyrin ring chelated to iron Formed in the mitochondria | High affinity for oxygen O2 in haemoglobin is Hb x SatO2 x 1.34, compared to 0.003 x PO2 dissolved in blood Haemoglobin is a good buffer |
| Contains carbonic anhydrase | CO2 + H2O → H2CO3 Enables transport of CO2 as bicarbonate |
JC / Mooney 2019
Examiner Comments
2016B 14: 33% of candidates passed this question.
This question was best answered by considering form and then function. Detailing red cell size, that it is a biconcave disc, contains haemoglobin A (Hb F in-utero), has a central Fe moiety and demonstrates positive cooperativity in binding oxygen would be a good start. Additionally, noting that the RBC has a flexible membrane with shape maintained by structural proteins and that it lacks a nucleus, organelles and mitochondria, but contains carbonic anhydrase would pass this question.
A complete answer would mention the3 shunts that come off the anaerobic glycolytic pathway (RBC’s only means of ATP generation), namely the production of 2-3 DPG via the RapoportLuebering shunt, generation of NADPH by the hexose monophosphate shunt (protects RBC from oxidative damage) and the reduction of metHb back to Hb by way of the NADH.
Many answers lacked sufficient information to pass this question. Many answers included lengthy discussions about the production of RBC’s, the Oxyhaemoglobin dissociation curve or calculated the oxygen content of blood. RBC metabolic adaptations (e.g. 2, 3-DPG, NADPH production by the HMP shunt/ G6phosphatase to regenerate glutathione and metHb reductase) were rarely mentioned, as were vasodilatory mediators released by RBCs.
15. Compare and contrast the pharmacology of noradrenaline and dobutamine.
Examiner Comments
2016B 15: 84% of candidates passed this question.
The best answers used tables and key pharmacological headings for comparisons, and avoided long sentences/ paragraphs.
An answer that correctly considered the following sections would be awarded a very good pass: Presentation, pharmacodynamics, mechanism of action, organ effects, side effects and pharmacokinetics.
Many candidates failed to identify agents as natural / synthetic catecholamines. Few answers correctly mentioned the available preparations of these drugs or considered the structure activity relationships. Only 3 candidates commented that dobutamine is a racemic mixture.
Intracellular second messenger pathways were often incorrectly recounted or not mentioned at all. Pharmacodynamic effects on all organ systems, and all CVS parameters (HR, inotropy, PVR, SVR, SBP/DBP/MAP, regional circulations) should be considered. Metabolic fate and clinical dosage ranges were frequently incorrectly quoted.
16. Outline the influence of pregnancy on pharmacokinetics.
CICMWrecks Answer
Pharmacokinetics:
Absorption:
- ↓ Oral absorption
- ↑ N/V
- ↓ gastric emptying during labour
- ↓ gastric motility 2° to intestinal compression
- ↑ gastric absorption
- ↓intestinal absorption due to ↓ intestinal blood flow
- ↑ IM/SC/transdermal absorption
- ↑ skin blood flow
- ↑ C.O. (by 30-40%)
- ↓ SVR
- ↑ skin blood flow
- IV (↑ onset)
- Neuraxial
- ↓ epidural space 2° to EDVs
- ↓ spinal and epidural doses
- Inhalational
- Progesterone-mediated ↑ MV (by 50-70%)
- ↑ FA/FI ratio (= uptake
Distribution:
- ↑ VD (↑ TBW/ECF and fat)
- ↑ TBW/ECF (by 50%) (important for polar/ionized drugs)
- ↑ body fat % (important for lipid soluble drugs)
- ↓ plasma protein 2° to dilutional effect
- ↓ albumin →
- ↑ free % of acidic drugs (Eg. STP, propofol)
- ↓ dose required
- ↑ transplacental transfer of drug.
- ↓ A1AGP (by 30%) →
- ↑ free % of basic drugs (Eg. LA, β blockers)
- ↓ dose required
- ↑ transplacental transfer of drug
- ↓ albumin →
- Ionisation (mild ↑pH alters ionisation based on pKa)
- ↑ MV = mild respiratory alkalosis
- ↑ transplacental transfer of basic drugs as they will have ↑ % in unionized form
- (Base in base is less ionised)
- ↑ transplacental transfer of basic drugs as they will have ↑ % in unionized form
- ↑ ion trapping in more acidotic foetal circulation
- ↑ MV = mild respiratory alkalosis
Metabolism:
- Progesterone:oestrogen ratio
- Progesterone → induces hepatic enzymes
- Oestrogen → inhibits hepatic enzymes
- ↓ plasma cholinesterase (30%)
- Placenta metabolises some drugs
- Foetal liver has functioning CYP450
- Can metabolise drugs
- But requires transfer back to maternal circ for conjugation
Excretion:
- ↑ RBF/GFR (50%)
- ↑ clearance/↓ elimination t1⁄2 of water-soluble drugs
- ↑ MV/↓FRC
- ↑ washout of volatile agents
Pharmacodynamics:
- Decreased MAC – Increased sensitivity to volatile anaesthetics
- Increased LA sensitivity due to decreased α1-glycoprotein
- Increased sensitivity to IV anaesthetics
Gladwin / JC 2019
Examiner Comments
2016B 16: 84% of candidates passed this question.
The best answers used tables and key pharmacological headings for comparisons, and avoided long sentences/ paragraphs.
An answer that correctly considered the following sections would be awarded a very good pass: Presentation, pharmacodynamics, mechanism of action, organ effects, side effects and pharmacokinetics.
Many candidates failed to identify agents as natural / synthetic catecholamines.
Few answers correctly mentioned the available preparations of these drugs or considered the structure activity relationships. Only 3 candidates commented that dobutamine is a racemic mixture.
Intracellular second messenger pathways were often incorrectly recounted or not mentioned at all. Pharmacodynamic effects on all organ systems, and all CVS parameters (HR, inotropy, PVR, SVR, SBP/DBP/MAP, regional circulations) should be considered. Metabolic fate and clinical dosage ranges were frequently incorrectly quoted.
17. Draw a labelled diagram of both the aortic root and radial artery pressure waveforms in a young adult using the same axis (60% of marks). Explain the factors that account for the differences between these two waveforms (40% of marks).
CICMWrecks Answer
The arterial pulse waveform:
- The arterial pulse waveform can be separated into three distinct components
- The systolic phase, characterised by a rapid increase in pressure to a peak, followed by a rapid decline. This phase begins with the opening of the aortic valve and corresponds to the left ventricular ejection
- The dicrotic notch, which represents the closure of the aortic valve
- The diastolic phase, which represents the run-off of blood into the peripheral circulation.
Windkessel effect
is a term used in medicine to account for the shape of the arterial blood pressure waveform in terms of the interaction between the stroke volume and the compliance of the aorta and large elastic arteries (Windkessel vessels) and the resistance of the smaller arteries and arterioles.
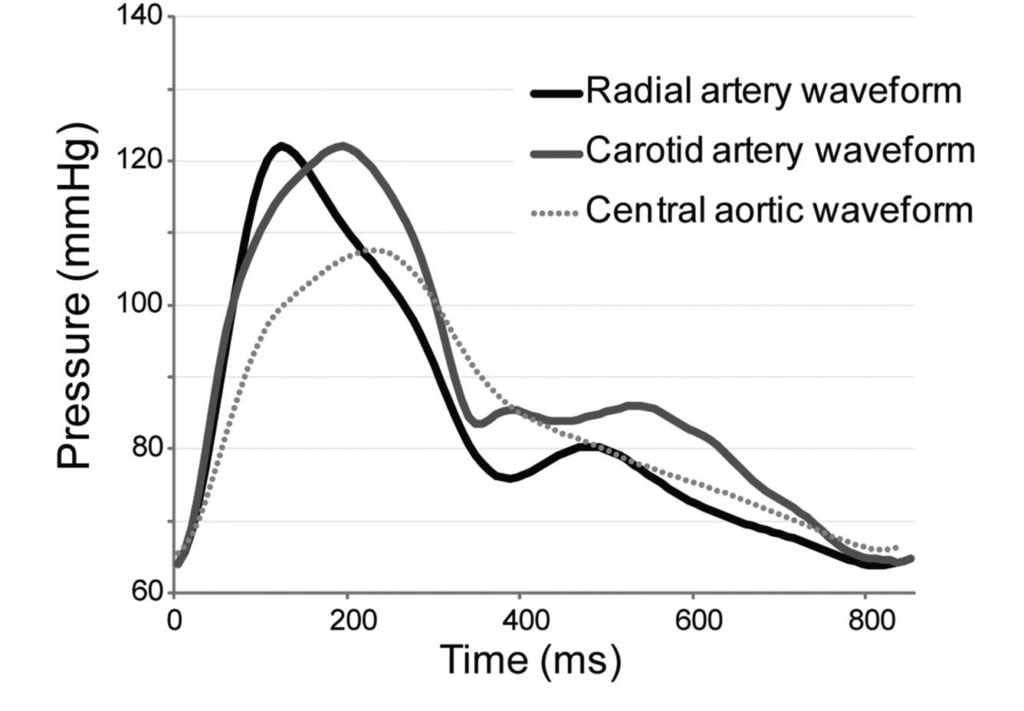
The further you get from the aorta
- The taller the systolic peak (i.e. a higher systolic pressure)
- The further the dicrotic notch
- The lower the end-diastolic pressure (i.e. the wider the pulse pressure)
- The later the arrival of the pulse (its 60msec delayed in the radial artery)
Waveform Difference: Radial vs Aortic
| Radial waveform (vs Aortic) | Reason |
|---|---|
| Delayed start 60msec | · Time taken for wave front to travel (note much faster than blood flow) |
| Narrower and higher systolic component Steeper upstroke = ↑dp/dt max | · Less elastic tissue · Relatively more muscular tissue · ↓ compliance (δv/δp) |
| Lower diastolic component | · Less elastic tissue · Less Windkessel effect (storage and release of energy) |
| Slightly lower mean | · Above factors |
| Widened pulse pressure | · Above factors |
| Disappearance of high freq components | · Damping affects high > low freq · Damping ∝ frictional tissue interactions per length or time |
| Appearance of dicrotic notch | · Reflection and resonance. (peripheral dicrotic notch owes more of its shape to the vascular resistance of peripheral vessels than to the closing of the aortic valve) |
Source: Deranged Physiology, Ketamine nightmares
Examiner Comments
2016B 17: 41% of candidates passed this question.
A well labelled diagram drawn clearly to demonstrate the salient features of the aortic and radial arterial pulses was expected. This would include the different systolic pressure, the absence of a dicrotic notch in the radial pulse (instead a diastolic hump), the narrowness and the delay of the radial pulse, garnered many marks.
Marks were lost for insufficient explanation such as the distance needed to travel for the pressure wave accounting for the delay, the sharper rise and decline of the radial pulse due to loss of the WIndkessel effect and the different compliance and the loss of the dicrotic notch due to summation and damping out of high frequency components of the pressure wave.
18. Discuss the determinants of intracranial pressure (80% of marks). Outline how it can be measured (20% of marks).
CICMWrecks Answer: ICP
Definition:
- ICP: hydrostatic pressure within the cranial vault
- Normal value is 5-15 mmHg
- focal ischaemia when ICP > 20 mmHg
- global ischaemia when ICP > 50mmHg
Munro-Kellie Doctrine
- The rigid and closed cranial vault forms a fixed brain volume containing
- Brain parenchyma (80%, 1400 g)
- CSF (10%; 75 mL)
- Cerebral blood and vessels (10%; 75 mL)
- Δ’s in volume of any components → Δ’s in others or and increase in ICP
CSF Production / Absorption
CSF Production:
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
CSF Absorption:
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
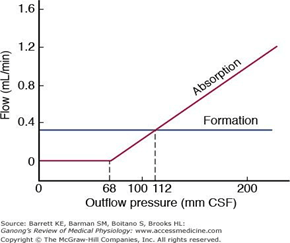
Control of ICP
- ICP is regulated via volume buffering
- i.e. increase in volume of one intracranial component leads to compensatory decrease in volume of other intracranial components
- When volume buffering mechanism is exhausted → rapid increase in ICP (decompensation)
- Movement of cerebral venous blood = rapid compensation, lower capacity
- Movement of CSF = gradual compensation, larger capacity
Determinants of ICP:
- Brain
- Age / Mass
- Space occupying lesions
- Cerebral Oedema
- CSF
- CSF production
- CSF Absorption
- Cerebral Blood Volume
- Cerebral autoregulation: Flow-metabolic coupling
- Cerebral metabolic rate
- Increase in systemic blood pressure / flow
- Venous Outflow obstruction
- Vasoactive agents
- Monro-Kellie Doctrine
- Loss of above – e.g. Fractures, surgery
Compensation for Elevated ICP (Intracranial Pressure)
Early compensation
- Δ CSF distribution and flow
- CSF is displaced to spinal subarachnoid space
- ↑’d resorption rate
Late compensation
- ↑ ICP → ↓ CBF → ↓ in cerebral blood volume → cerebral ischaemia
Decompensation
- ↑ICP → ↓ in cerebral tissue volume → brain herniation
- Cushing Reflex
- Hypertension, bradycardia and abnormal breathing associated with raised ICP
- Mechanism:
- Stage 1:
- ↑ ICP → ↓ blood supply to vasomotor area → Local hypoxia/hypercarbia → ↑ SNS >> ↑ PSPS vasomotor stimulation
- ↑ TPR → ↑ MAP
- ↑ HR → ↑ CO
- → compensatory ↑CBF
- Stage 2:
- ↑ CO → Baroreceptor stimulation → ↑ Vagus nerve stimulation → Bradycardia and ↓ contractility.
- Stage 1:
Gladwin / JC 2020
CICMWrecks Answer: Measurement of ICP
Methods of Measurement of ICP (Outline)
Invasive
| Method | Advantages | Disadvantages |
|---|---|---|
| Intraventricular catheter (EVD) | Provides ‘ture’ global ICP Allows for CSF drainage and administration of drugs In-vivo calibration possible via external pressure transducer | Infection Difficult iinsertion |
| Epidural catheter | Ease of insertion Minimal risk of infection (no penetration of dura) | Low accuracy |
| Lumbar CSF puncture | Extracranial procedure Can be performed ambulatory | May not reflect ICP Dangerous if ICP high |
| Catheter-tip micro transducers (subdural or intra-parenchymal) | Rare complications during procedure Low risk of infections Can be made permanent implants | Drift of transducer output over time In-vivo calibration not possible Inaccurate if intraparenchymal gradient exists |
Non-Invasive
Non-invasive methods like pupillometry, CT, MRI, TCD provide an adjunct to clinical examination of high ICP, but are not a surrogate for invasive ICP measurement.
Gladwin / JC 2020
Examiner Comments
2016B 18: 55% of candidates passed this question.
It was expected answers would include an explanation of the Monro-Kellie Doctrine. Many candidates gave insufficient details of compensatory mechanisms especially regarding decreased total cerebral blood volume (primarily venous) in response to increased intracranial pressure.
Most candidates had all the information but had difficulty synthesising the information to write a cohesive answer. Factors affecting ICP could be divided into factors affecting CBV, factors affecting CSF and factors affecting brain tissue. Under factors affecting CBV the effect of blood gases, autoregulation, temperature, metabolism, drugs and venous obstruction could have been detailed.
19. Describe how Starling forces determine fluid flux within the pulmonary capillary bed.
CICMWrecks Answer
Introduction
- Capillaries contain semipermeable membranes to allow the movement of fluid and solutes.
- it is normally impermeable to large protein
- Plasma ultrafiltrate is filtered by bulk flow through the capillary wall by the action of opposing hydrostatic and oncotic pressures
- The key features of the pulmonary microcirculation are:
- The pulmonary capillaries (and the alveoli) have very thin walls which minimises the barrier to diffusion.
- In the alveolar walls, the capillaries form a dense network which has been considered to be almost a continuous thin film of blood. This provides a large capillary surface area.
- The pressures in the pulmonary circuit are much lower than in the systemic circulation and the pulmonary vascular resistance is very low. The pressure is just sufficient to perfuse the apical areas of the lungs in the erect healthy adult.
Starling Forces
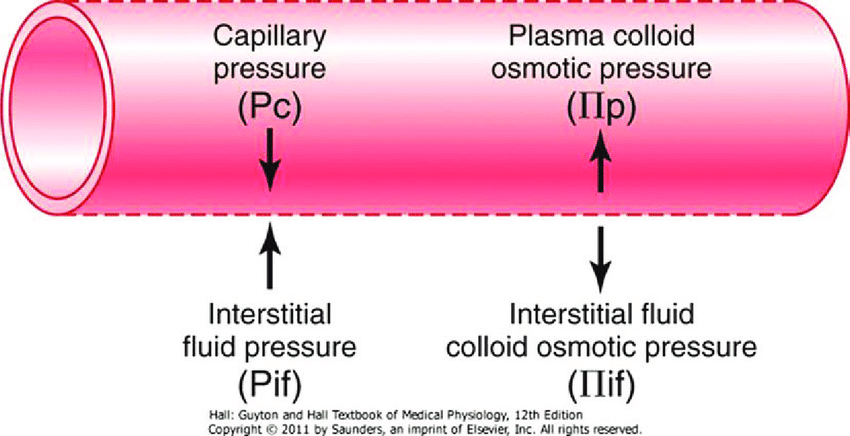
- The NET flux across the membrane is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation:
where
Jv is the trans endothelial solvent filtration volume per second
( [ Pc – Pi ] – σ [ πp – πi ] ) is the net driving force
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein (0.5 for lung)
κ = filtration constant = LpS = Hydraulic conductivity x Surface Area
| Pulmonary | Systemic | ||
|---|---|---|---|
| Pc Capillary hydrostatic pressure | Pressure moving fluid out of capillary | 13→6 mmHg Arterial → venous Variable due to hydrostatic effects of gravity in different parts of lung | ~35→15 mmHg Arterial → venous |
| Pi Interstitial hydrostatic pressure | Pressure moving fluid into capillary | Variable, but 0 to slightly negative | 5 mmHg |
| πp Plasma oncotic pressure | Pressure keeping fluid within capillary | 25 mmHg | ~20 mmHg |
| πi Interstitial fluid oncotic pressure | Pressure keeping fluid out of capillary | 17 mmHg | ~0 mmHg |
Oncotic pressure gradient
- The interstitial oncotic pressure is high indicating significant leak of protein (mostly albumin) across the thin capillary walls under normal circumstances. The reflection coefficient has been estimated at about 0.5
- Considering the typical values and allowing for the reflection coefficient, it can be estimated that the net oncotic gradient is small but favours reabsorption.
Hydrostatic pressure gradient
- The capillaries are called intra-alveolar vessels and the presssure they are exposed to is close to alveolar pressure (zero to slightly negative, due to surfactant) and become more negative closer to the hilum
- This favours flow of fluid from the alveolar intersitium into the pulmonary lymphatics.
- The capillary hydrostatic pressure is variable because of the effects of gravity.
- The pulmonary circuit has a low resistance and about half of this resistance is due to the pulmonary capillaries which have no muscle in their walls. The capillary hydrostatic pressure is quickly affected by changes in pulmonary artery pressure and left atrial pressure without much protective buffering.
Overall Effect
The balance of Starling forces in the lung is generally stated as favouring reabsorption because of the clinical fact that the lungs are generally dry and clearly need to be to facilitate gas exchange
Safety Factors Preventing Pulmonary Oedema
- Increased lymph flow
- Decrease in interstitial oncotic pressure (oncotic buffering mechanism)
- High interstitial compliance
JC 2019
Examiner Comments
2016B 19: 25% of candidates passed this question.
The equations for nett fluid flux and for nett filtration pressure were incorrect in many answers. Better answers presented the equations and discussed each of the elements as relevant to the pulmonary capillary bed, including difference from systemic capillary beds.
Mention of the role of lymphatics and of the effect of surfactant, left atrial pressure, gravity and posture gained marks, also.
20. Describe the mechanisms by which heat is lost from the body (40% of marks). Discuss the importance of each of these in a sedated and intubated adult patient (60% of marks).
CICMWrecks Answer
Definitions
- Thermoneutral zone:
- The range of environmental temperatures in which the metabolic heat production (and oxygen consumption) is minimal and steady and where core temperature is maintained by vasomotor activity alone.
- 25-30 C for a naked, upright man in still air
- Inter-threshold range:
- The range of core temprature over which no autonomic thermoregulatory responses occur
- Normally 0.2 -0.4 °C in a non-anaesthetized state
Mechanisms of Heat production
Total body heat production = Metabolic rate – Total external work
- Metabolism of food to form ATP
- <100% efficient
- Transfer of ATP to functional systems of cells
- <100% efficient
- Basal metabolism of cells
- Muscle contraction
- Overcoming resistance in muscles and other tissues -> friction
- Useful in shivering
- Overcoming resistance in muscles and other tissues -> friction
- Pumping blood
- Overcoming shear forces -> friction
- Breaking chemical bonds
- Flow of ions across membranes
Mechanisms of Heat loss
- Heat is lost by conduction, convection and radiation and evaporation
- The movement of warm blood from the body to skin allows heat loss to the environment
- Conduction:
- Heat transfer occurs due to direct contact to another solid body
- Comprises 3% of total heat loss
- Convection:
- Heat is lost to air via conduction
- This air then moves away, restoring the temperature gradient
- Comprises 15% of total heat loss
- Radiation:
- Heat is emitted from the skin as infrared radiation
- Comprises 60% of total heat loss
- Evaporation:
- There is a distribution of kinetic energies in liquids
- Warmer particles transition to the gas phase, lowering the average temperature of the skin
- Sweat, comprises 22% of total heat loss
Importance in sedated and intubated patient
Radiation:
- Heat loss increased
- if the patient is uncovered and surrounded by cold objects.
- by vasodilatation due to sedative drugs or cholinergics.
- by cool ambient temperature
- in Extracorporeal circuits
- Heat loss Decreased
- by vascoconstriction due to adrenergics
Conduction:
- usually a less important route
- Heat loss increased by use of cold irrigating solutions, and in extracorporeal circuits
Convection:
- Heat loss increased
- if the patient is uncovered
- by cool ambient temperature
- in Extracorporeal circuits
- Heat loss decreased
- Use of Neuromuscular blocking drugs (NMBDs can inhibit shivering, hence decreasing possible convective heat loss via bodily movement)
Evaporation:
- increased if a body cavity is opened, especially if ambient humidity is low.
- Heat loss via evaporation in the trachea and airways may be considerable if inspired gases are not humidified.
- Drugs affecting vascular tone can also alter heat loss by evaporation
Other:
- impaired temperature regulation
- peripheral (e.g. vasodilatation, shivering and impaired piloerection)
- central (central effects of drugs)
- Impairment of neurological or cardiovascular function, or deficiencies of substrate for thermogenesis → alter body’s ability to maintain thermal homeostasis
Mooney / JC 2019
Examiner Comments
2016B 20: 59% of candidates passed this question.
A satisfactory answer required use of terms radiation, convection, conduction etc. in the manner defined in the texts, rather than the layman’s use of the terms. Better answers displayed understanding of the meaning, relative importance and mechanism of methods of heat loss.
The second part of the question required application of these concepts in patients with artificial airway and sedatives, particularly change in control of vascular tone and voluntary behavioural control.
21. Classify anti-arrhythmic drugs by mechanism of action, giving examples of each (75% of marks). Describe the electrophysiological and ECG effects of sotalol (25% of marks).
CICMWrecks Answer
| CLASS | ACTION | ELECTRO- PHYSIOLOGY | DRUGS | THERAPEUTIC INDICATIONS |
|---|---|---|---|---|
| I. Na – Channel Blockers | ||||
| Ia | Intermediate dissociation | ↓ phase 0 ↓↓ conduction v ↑ repolarisation ↑ APD | Quinidine Disopyramide Procainamide | Atrial and ventricular arrhythmias esp. post MI |
| Ib | Fast dissociation | ↔,↓ phase 0 ↓ conduction v ↓ repolarisation ↓ APD | lignocaine phenytoin tocainide, mexilitine | Ventricular arrhythmias post MI, digoxin induced arrhythmias |
| Ic | Slow dissociation | ↓↓↓↓ phase 0 ↓↓↓↓ conduction v ± repolarisation ↔ APD | flecainide ecainide, lorcainide | Refractory arrhythmias |
| II. β – Blockers | ||||
| ↓ SA firing – ↓ rate, conduction | propranalol* atenolol, esmolol | Rate control in AF, AT, Flutter and VT | ||
| III. K – Channel Blockers | ||||
| Delay phase 3 (repol) ↑ APD ↑ ERP | amiodarone bretylium, sotalol^ | AF / Flutter termination | ||
| IV. Ca – Channel Blockers | ||||
| ↓ AV conduction ↑ PR interval ↓ rate/conduction | verapamil diltiazem | SVT and AF or Flutter | ||
| OTHERS (Some call this Class V) | ||||
| Blocks Na+/K+ ATPase → ↑Ca2+, ↓K+, ↑Ach | ↑ contractility ↓ AV conduction | Digoxin | AF rate control Heart Failure | |
| Opens K+ channels via adenosine receptors | Hyperpolarizes myocardium ↓ AV conduction ↓ SA firing | Adenosine# Ibutilide | Terminate SVT or reveal underlying rhythm in tachycardias | |
| Stimulates Na+/K+ATPase | Membrane stabilization | Magnesium | VF / Torsades de pointes | |
| Notes | * Propranalol also has Na blocking activity ^ l-sotalol has beta blocking and class III activities; d-sotalol is a pure class III agent. Commercially available sotalol is a racemic (equal part) mixture. # Amiodarone – blocks Na, Ca, K channels, and exhibits beta blockade. | |||
The effect on the myocardial action potential of each class is:
| Class Ia | Class Ib | Class Ic | Class II | Class III | Class IV | |
|---|---|---|---|---|---|---|
| Depolarisation rate (phase 0) | ↓ | ↔ or ↓ | ↓↓↓↓ | ↔ | ↔ | ↓↓↑↔± ↔ |
| Conduction velocity | ↓↓ | ↓ | ↓↓↓↓ | ↓ | ↓ | ↔ |
| Effective Refractory Period | ↑↑↑↑ | ↓ | ↑ | ↓ | ↑↑↑↑ | ↔ |
| Action potential duration | ↑ | ↓ | ↔ or ↑ | ↑ | ↑↑↑↑ | ↓ |
| Automaticity | ↓ | ↓ | ↓ | ↓ | ↓ | ↔ |
| P-R duration | ↔ | ↔ | ↑ | ↔ or ↑ | ↑ | ↔ or ↑ |
| QRS duration | ↑ | ↔ | ↑↑↑↑ | ↔ | ↑ | ↔ |
| QTc duration | ↑ | ↔ or ↓ | ↑ | ↓ | ↑↑↑↑ | ↔ |
Sotalol Effects
- l-sotalol has beta blocking and class III activities
- d-sotalol is a pure class III agent
- Commercially available sotalol is a racemic (equal part) mixture.
- Class III Effects (K – channel blocking)
- Blocking of outward K+ channels slows cardiac repolarisation, which increases the cardiac refractory period.
- Delay phase 3 (repol), ↓ AV conduction,
- ↑↑↑↑ APD, ↑↑↑↑ ERP
- ↑↑↑↑ QTc
- ↓ automaticity
- ↓ ectopy
- ↓ defibrillation energy requirement
- May ↑ inotropy
- Class II Effects (β – Blocker)
- Antagonizes β1 & β2 receptors
- ↓ SA firing – ↓ heart rate, conduction
- can prolong the QT interval.
- can cause polymorphic ventricular tachycardia (Torsades de Pointes)
- recommended to be initiated in the inpatient setting to monitor the QT interval after each dose
- can cause severe bradycardia necessitating drug discontinuation
Gladwin / Sakurai / JC 2020
Examiner Comments
2016B 21: 88% of candidates passed this question.
Most answers displayed a good knowledge of the Vaughan Williams classification, classes I to IV and the relevant electrophysiological characteristics of the classes.
Answers should also have included mention of other antiarrhythmics, such as digoxin, magnesium and adenosine. The second part of the question required comment about K ion blockade and its effects. It was helpful to mention prolongation of QT and risk of torsade. Most answers omitted reference to its being a racemic mixture, with different actions of the isomers.
22. Outline the physiological factors that affect the diffusion of oxygen and carbon dioxide within the lung.
CICMWrecks Answer
Fick’s law of diffusion
- Describes Diffusion through tissues
- Rate of movement of solute across semi-permiable membrane J is
where
C = concentration (or partial pressure for gasses)
A = cross-sectional area
T = thickness of the membrane or distance over which diffusion takes place.
The rate of diffusion of a gas through a tissue is:
Directly ∝:
- Surface area of the barrier
Affected by:- Parenchyma volume: Changes with Body size and in disease states
- V/Q mismatch: reduced in shunt and dead space
- Pulmonary Blood Volume: changed with vascular distension and recruitment
- Cardiac output: increased recruitment in high output states, decreased recruitment and increased V/Q mismatch in shock states
- Posture: Increased surface area while supine (compared to sitting or standing)
- Solubility of the gas
- CO2 is ~20 times as soluble as O2 in blood
- Concentration gradient (partial pressure difference)
- Rate of blood flow through lungs
- Transit time of blood across capillary (not part of Fick’s Equation) – based on HR
- alters total gas exchange across alveolus
- Can potentially change perfusion limited gas exchange to diffusion limited
- Alveolar partial pressure affected by:
- Atmospheric pressure
- Ventilation: Alveolar hypoventilation → ↑ PACO2 , ↓ PAO2
- Partial pressure in blood affected by:
- Binding of gas to protein:
- Rate of oxygenation of reduced Hb:
- Shift of oxygen dissociation curve (pH, temperature, PCO2, 2,3-DPG)
- Haematocrit
- Abnormalities of haemoglobin
- Formation of carbamino compounds
- Anaesthetic agents to plasma contents, e.g Albumin, cholesterol
- Enzymatic Action
- carbonic anhydrase (conversion of HCO3 to CO2)
- Rate of blood flow through lungs
Inversely ∝:
- Membrane thickness
- Increased thickness impedes gas exchange – Pathological states like pulmonary edema and cardiac failure
- Square root of Molecular Weight
- smaller substances diffuse more quickly
Diffusion vs. perfusion limited
Transfer of gases can be diffusion or perfusion limited dependent on the rate limiting step
Diffusion Limited
- Occurs in gases which do not reach equilibration of Pa and PA
- The rate of gas diffusion across the alveolar membrane limits its transport away from the lung
- Rate limiting step = rate of diffusion
- E.g. CO
- CO binds so avidly to Hb (250x that of O2) → virtually no CO dissolved in plasma → PaCO rises only slightly
- Even when RBC traversed entire length of pulmonary capillary, there is still substantial partial pressure difference across alveolar-capillary barrier → equilibrium of PaCO and PACO never reached
Perfusion Limited
- Characterized by complete equilibration i.e. Pa = PA
- amount of gas transferred between alveolus and capillary = dependent on amount of blood passing through the capillary
- rate limiting step = rate of blood flow
- E.g. N2O
- N2O rapidly diffuses across alveolar-capillary barrier
- Insoluble; does not bind to Hb; only carried in plasma in dissolved form
- PaN2O = PAN2O (<0.07sec); well before RBC has traversed pulmonary capillary
- ↑ diffusion rate will not ↑ blood transport away from the lungs → limiting factor = rate of blood flow / perfusion
- e.g. CO2 (ventilation limited i.e. perfusion limited in reverse)
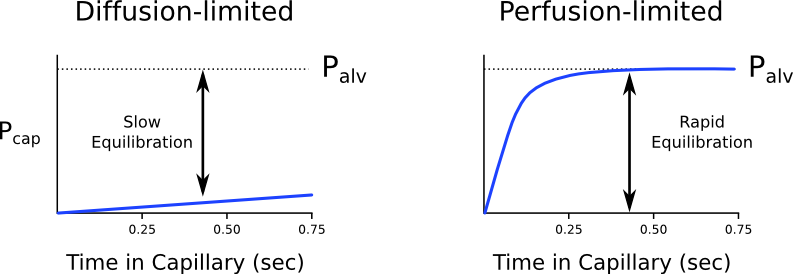
Is the transfer of O2 perfusion or diffusion limited?
- Can behave as both perfusion and diffusion limited
- O2 diffusion takes 0.25s; pulmonary capillary transit time is 0.75s
- Normal conditions
- Transfer of O2 across the alveolar capillary barrier is perfusion limited
- Equilibrium is reached between alveolar and capillary PO2 before the RBC has traversed the pulmonary capillary
- Conditions where transfer of O2 may become diffusion limited
- Disease of the alveolar capillary barrier
- Pulmonary fibrosis: thickening of alveolar-capillary barrier → ↓ rate of diffusion
- Exercise: ↑ CO → ↓ RBC transit time
- Altitude: ↓ PaO2
- Disease of the alveolar capillary barrier
Diffusion of O2 and CO2
Oxygen
- Oxygen diffusion takes ~0.25s
- Pulmonary capillary transit time is 0.75s
- Therefore, under normal conditions oxygen is a perfusion limited gas
- However, oxygen may become diffusion limited in certain circumstances:
- Alveolar-capillary barrier disease
Decreases the rate of diffusion.- Decreased surface area
- Increased thickness
- High cardiac output
Decreases pulmonary transit time. - Altitude
Decreases PAO2
- Alveolar-capillary barrier disease
Carbon Dioxide
- Carbon dioxide is ventilation limited, rather than diffusion or perfusion limited
- This is because it is:
- 20x more soluble in blood than oxygen
- Rapidly produced from bicarbonate and carbamino compounds
- Present in far greater amounts than oxygen
1.8L.kg-1 exist in the body (though 1.6L-1 of this are in bone and other relatively inaccessible compartments).
- Impairment of diffusion capacity causes type 1 respiratory failure as oxygen is affected to a much greater extent than carbon dioxide
Kerr / JC 2020
Examiner Comments
2016B 22: 43% of candidates passed this question.
Good answers to this question were those that included a definition of diffusion; an outline of Fick’s Law of Diffusion and then a further description how each of the variables in the this Law affect the diffusion of oxygen and carbon dioxide in the lung; and an outline of the other factors that affect diffusion not covered by the above. Most candidates included Fick’s Law in their answers and at least briefly expanded on the associated variables.
Few candidates defined the process of diffusion. The other common omissions were the factors that affect diffusion that aren’t directly encompassed in Fick’s Law, such as cardiac output, capillary transit time, carbonic anhydrase (conversion of HCO3 to CO2) and combination of oxygen with haemoglobin.
23. Compare and contrast the mechanism of action, spectrum of activity and adverse effects of benzyl penicillin and fluconazole.
Examiner Comments
2016B 23: 8% of candidates passed this question.
To pass this question each of the three components needed to be compared and contrasted for both agents. A tabulated answer helped in this regard but was not essential.
Some answers included information that could not gain marks, as it was not directly relevant to the question asked (e.g. presentation and dose).
In spectrum of activity, as well as what important organisms the agents were effective against, marks were also given for the important organisms that they were not effective against (e.g. MRSA and beta-lactamase producing organisms for penicillin G; and aspergillus for fluconazole).
In general, of the two agents, fluconazole was the least well answered. For example, a common omission either in mechanism of action or in adverse effects was that fluconazole inhibits microsomal P450 enzymes.
Some candidates confused fluoroquinolone with fluconazole.
24. Outline the tracheal (60% of marks) and left and right main bronchial anatomy (40% of marks) in an adult.
CICMWrecks Answer
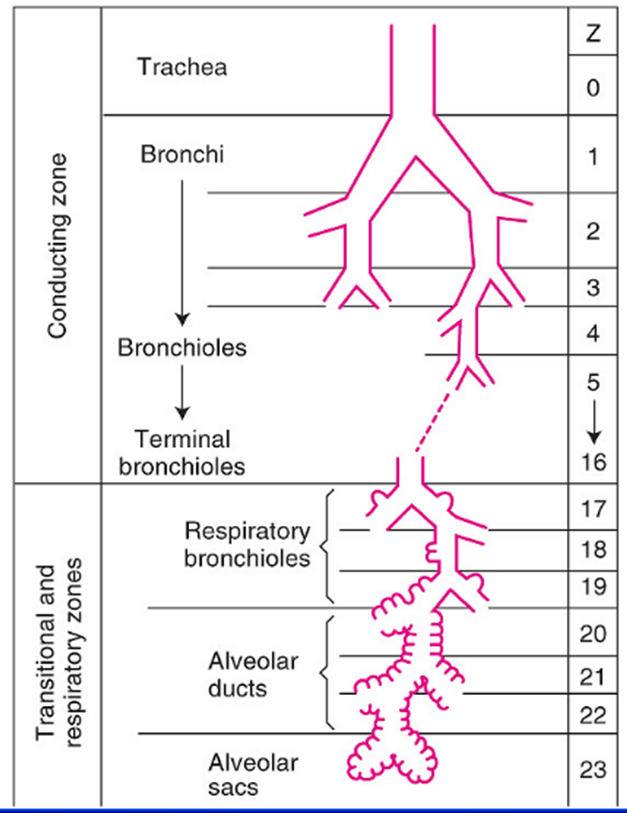
Tracheobronchial Tree
Trachea →
Primary main bronchi →
Lobar bronchi →
segmental bronchi (each supplies one bronchopulmonary segment) →
divide dichotomously→→→→
terminal bronchioles →
respiratory bronchioles →
2-11 alveolar ducts →
alveoli
Trachea
- Midline. 10-12 cm long 15-20mm diameter.
- From Cricoid (C6 level) to bifurcation (T4-T6 level)
- 16-20 C-shaped cartilage rings
- Vertical fibroelastic tissue, and posterior trachealis muscle
- Arterial supply: Upper 2/3 – Inferior thyroid artery, Lower 1/3 Bronchial artery
- Venous drainage: Inferior thyroid vein
- Lymphatics: Drain into deep cervical, pre-tracheal, paratracheal lymph nodes
- Nerve supply: Recurrent laryngeal nerve, Sympathetic fibres from middle cervical ganglion
Bronchi
- Supplied by bronchial arteries which run along the bronchi (with vessels from pulmonary circulation)
- Drains into bronchial veins
- Similar innervation as trachea
- A bronchopulmonary segment is a portion of lung supplied by a specific segmental bronchus and arteries.
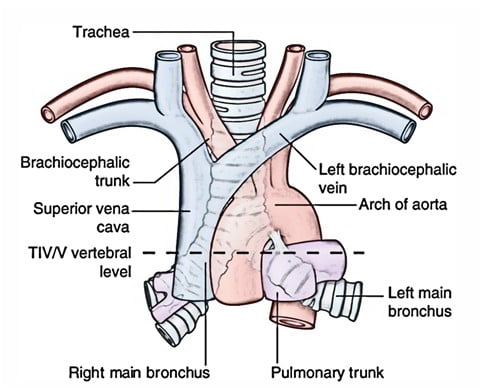
Right main bronchus
2cm long, I.D. 10-16mm
Shorter, wider, more nearly vertical than left
Branches:
- Right upper lobe
- Apical
- Anterior
- Posterior
- Bronchus intermedius
- Middle lobe
- Medial
- Lateral
- Lower lobe
- Superior
- Medial basal
- Anterior basal
- Lateral basal
- Posterior basal
- Middle lobe
Left main bronchus
4-5cm long, I.D. 8-14mm
Crosses anterior to the esophagus, which it indents.
Branches:
- Left upper lobe
- Apicoposterior
- Anterior
- Lingular
- Superior
- Inferior
- Left lower lobe
- Superior
- Anterior basal
- Lateral basal
- Posterior basal
Lining Epithelium
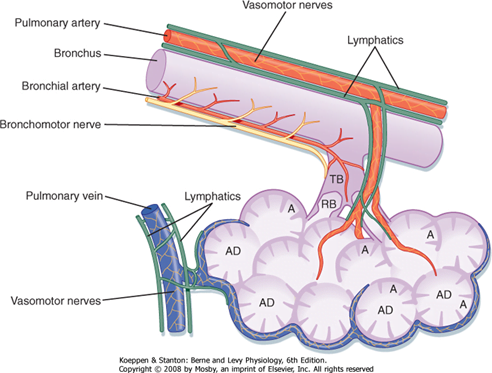
Trachea → Terminal bronchioles: ciliated pseudostratified columnar epithelium
Respiratory bronchioles → alveolar ducts → alveoli: Non ciliated cuboidal epithelium
Sources: Nunn’s Applied Respiratory Physiology, West’s Respiratory Physiology, Berne and Levy Physiology
JC 2019
Examiner Comments
2016B 24: 24% of candidates passed this question.
Better answers included details of the significant structures related to the cervical and mediastinal trachea and bronchi. The lobar branches and bronchopulmonary segments requiring naming to attract full marks. Many answers lacked sufficient detail or contained inaccuracies regarding vertebral levels and key structural relations. Some candidates discussed the general anatomy of the airway, including the larynx, structure of the airways, blood supply and innervation. This did not attract marks.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Respiratory physio, obesity, LES, gastric emptying, Metoclopramide |
| G. CVS | Cardiovascular physio – CO, Valsalva, aleterations with acute blood loss Measurement of Arterial BP, Beta blockers |
| H. Renal | Renal clearance, Diuretics |
| I. Body Fluids and Electrolytes | Physical principles around flow and viscosity, Venous anatomy of UL+chest, in vivo blood clotting |
| J. Acid Base | |
| K. Neuro | Cerebral physio + pharm, propofol, ketamine |
| L. Musculoskeletal | NM blockers, physio of Deep tendon reflex |
| M. ANS | Cholinergic receptors, Atropine, Propofol |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |


Recent Comments