1. Outline the determinants of oxygen delivery to the tissues.
CICMWrecks Answer
O2
- Total body O2 content ~ 1.55L
- ~850mls – blood (20.4ml/100ml blood) (O2 Carriage)
- 20.1ml/100ml – bound to Hb
- 0.3ml/100ml – dissolved
- ~200ml – bound to myoglobin
- ~450ml in FRC
- ~50ml dissolved in tissue
- ~850mls – blood (20.4ml/100ml blood) (O2 Carriage)
- O2 Content
- O2 Delivery
- Determinants
- O2 Extraction / Extraction Ratio
Carriage of O2
- Normal arterial blood has a PO2 of 100mmHg and is 97.5% saturated
- Venous blood has a PO2 of 40mmHg and is 75% saturated
- O2 is carried either bound to Hb or dissolved in solution
- Dissolved O2 (2%)
- obeys Henry’s Law: amount dissolved ∝ the partial pressure.
- For each mmHg of PO2, there is 0.003ml O2/100ml blood
- therefore normal arterial blood with PO2 of 100mmHg contains 0.3ml O2/100ml
- Bound to Hb (98%)
- each gram of fully saturated Hb can bind 1.34ml of O2 (= Hufners constant)
- 98% O2 in blood = bound to Hb, a protein tetramer with an iron-porphyrin ring attached to each chain
- O2 coordinates with each Fe atom → inducing a conformational change → promotes binding of O2 to the other Fe atoms.
- total O2 binding capacity of Hb in blood (at normal p, temp, and PCO2) = 1.34ml/g → giving a total O2 carrying capacity of blood which an Hb of 150g/l of 20.1ml/100ml
O2 content
- 1.34 = Hufners constant at 37 degrees
- 0.03 = solubility coefficient for O2 in water
- O2 content per 100mL:
| CaO2 (Arterial O2 content) | CvO2 (Venous O2 content) |
|---|---|
| = (15 x 1 x 1.34) + (0.003 x 100) | = (15 x 0.75 x 1.34) + (0.003 x 40) |
| = 20.1 + 0.3 | = 15.08 + 0.12 |
| = 20.4 ml | = 15.2 ml |
Relevant Anatomy (for O2 Carriage)
- RBCs
- small, flexible, biconcave discs
- cell membrane contains carbohydrate based antigens (ABO) and transmembrane proteins (Rhesus)
- No nucleus; no mitochondria (therefore aerobic metabolism not possible – entirely dependent on glucose and glycolytic pathway)
- Hb: large, ion containing protein contained within RBCs
- HbA
- Most common form of adult Hb (95%)
- Quaternary structure comprising 4 polypeptide globin subunits (2 alpha and 2 beta chains) in tetrahedral arrangement
- 4 globin chains held together with weak electrostatic forces
- each globin chain has its own haem group – an ion containing porphyrin ring with iron in the ferrous state (Fe2+)
- O2 molecules are reversibly bound to each haem group through a weak coordinate bond to the Fe ion
- In total: 4 O2 molecules can be bound to each Hb molecule – one for each heme group
- Different Hb types have different structures of globin chains
- HbA: 2 alpha, 2 beta (Adult)
- HbF: 2 alpha, 2 gamma (Foetal – replaced by HbA by 6/12 age)
- HbA2: 2 alpha, 2 delta (2-3% adults)
- Different O2 carriage and dissociation curves
- Disorders of Hb synthesis:
- Decreased production of normal globin change: Thalassaemia
- Abnormal globin chains: Sickle cell anaemia
- Affects O2 Carriage
O2 Delivery, Uptake
O2 Delivery and Uptake
- Normal O2 Delivery = 1000ml/min
- Determinents of tissue O2 delivery
- Diffusion from alveolus to blood: Fick’s law of diffusion and factors affecting rate
- Diffusion from capillaries into tissues and cells: partial pressure difference
- O2ER: O2 Extraction ratio = % of oxygen removed = O2 extraction / CaO2
| Oxygen Delivery (DO2) | Oxygen Return | Oxygen uptake (VO2) |
|---|---|---|
| = CO x Art O2 content | = CO x Ven O2 content | = O2 delivery – O2 return |
| = 5000 x 20 x 0.1 | = 5000 x 15.2 x 0.1 | = 1000 – 750 |
| = 1000ml/min | = 750ml/min | = 250ml/min |
Determinants of O2 Delivery
- Cardiac output = HR x SV
- Pathological states like low output or hyperdynamic states can alter CO
- Oxygen content dependent on Hb, Sats, PaO2
- O2 delivery decreased in:
- decreased cardiac output (decreased preload, contractility, HR or increased afterload)
- decreased saturations (V/Q mismatch, decreased V, decreased PiO2, Shunt, increased CO2)
- decreased Hb (blood loss, iron deficient anaemia, anaemia of chronic disease, etc)
- O2 Uptake increased in:
- increased metabolic activity (sepsis, exercise, malignancy, pregnancy)
- right shift of HbO2 curve (CO2, ¯pH, 2,3 DPG)
HR
affected by automatic rhythmicity of cell and balance between sympathetic & parasympathetic systems
Stroke Volume
- = Amount of blood pumped into the circulation per contraction
- dependent on preload, contractility, afterload
- Preload
- ~ Venous return
- (MSFP-RAP)/RVR
- MSFP – Mean Systemic Filling Pressure
Equilibration of pressures in the systemic circulation if cardiac output was abolished
Describes the filling state of the circulation and tone of capacitance vessels - RAP – Right atrial pressure
- Resistance to venous return
- Calibre of transmitting venous system according to Poisuille-Hagen equation (where resistance ∝ 1/r4 )
- Physical obstructions to venous return: Mechanical obstruction, Valvular stenosis
- MSFP – Mean Systemic Filling Pressure
- Thoracic pump (negative intrathoracic pressure created during inspiration)
- Skeletal muscle pump
- One way valves
- Pump function of the ventricle
- Venous tone
- Skeletal muscle pump
- One way valves
- Pump function of the ventricle
- Venous tone
- Afterload
- Proportional to aortic pressure and ventricular size
- Inversely proportional to ventricular wall thickness
- Affected by obstruction to the LVOT
- Contractility
- Affected by adrenergic or muscarinic stimulation
- Availability of Ca
O2 diffusion into blood:
- Fick equation:
- Describes Diffusion through tissues
- Rate of movement of solute across semi-permiable membrane J is
where
C = concentration (or partial pressure for gasses)
A = cross-sectional area
T = thickness of the membrane or distance over which diffusion takes place.
- Thickness of membrane (normal: 0.3μm)
- Surface area of membrane (normal: 50-100m2)
- Age, posture, degree of inflation (1 mark for any)
- Partial pressure gradient across membrane
- Rate of blood flow through lungs
- (no points for mentioning diffusion constant of gas – question relates to only oxygen)
- Rate of oxygenation of reduced Hb
- Shift of oxygen dissociation curve (pH, temperature, PCO2, 2,3-DPG)
- Haematocrit
- Abnormalities of haemoglobin
Kerr / JC 2020
Examiner Comments
2016A 01: 61% of candidates passed this question.
An opening statement such as oxygen delivery = cardiac output x oxygen content then allowed a more detailed description of the determinants of both oxygen content and cardiac output. It was expected candidates could detail the formula for Oxygen Content = Sat O2 % x 1.34 x Hb x 10 (depending on units) + PaO2 x .003, or x .03 (units) and accurately describe each element. Determinant of cardiac output completed the answer, often starting with CO = HR x SV while discussing the importance of preload, afterload and contractility. Many candidates spent little time on cardiac output which cost valuable marks. Some candidates provided a lot of detail on how oxygen is managed within the lung, the majority of which was not required as part of the answer. Some candidates answered the question describing the oxygen cascade only which was not sufficient to score well.
2. Describe the Respiratory and cardiovascular effects of applying 10 cm of PEEP (positive end-expiratory pressure) to a healthy mechanically ventilated adult.
CICMWrecks Answer
Positive Pressure Ventilation
- PEEP = Positive End Expiratory Pressure.
- Equivalent to a constant pressure applied throughout the respiratory cycle.
- Intrinsic PEEP = unintentional or un-measured end-expiratory hyperinflation
- Physiological effects of Positive Pressure Ventilation mostly related to increased mean airway pressure
Cardiovascular Effects
- Causes constant ↑ intrathoracic pressure (ITP) throughout respiratory cycle
- Left Heart
- On Preload
- Initially: → ↑ LV PL → ↑ CO
- Secondarily: → ↓RV output → ↓ LV PL → ↓ CO
- On Afterload
- → ↓myocardial transmural pressure → ↓LV AL → ↑CO
- PEEP > Ao diastolic pressure
- → Collapse of intrathoracic aorta → Starling resistor mechanism → ↑LV AL → ↓ CO
- PEEP < Ao diastolic pressure
- ↑ pressure gradient for flow to systemic circulation
- ↓ AL → ↓ myocardial work
- On Compliance
- diastolic buldging of septum → ↓ LV compliance → ↓LV CO
- On Preload
- Right Heart
- → ↑ Pulmonary vascular resistance → ↑ RV AL → ↓ RV output
- → ↓ Venous return → ↓ RV PL → ↓ RV output
- In the failing LV → ↑CO
- ↓ LV afterload
- ↑ pressure gradient thorax to abdomen
- ↓ transmural pressure → ↓ LV wall tension = ↓afterload
- ↓ LV preload (ie more favourable position on compliance curve)
- ↓ LV afterload
Respiratory Effects
Beneficial Effects:
- ↓ atelectasis and gas trapping
- ↑’s FRC > “closing capacity”
- Shifts position on P-V curve right, above the “closing point”
- ↑ lung compliance → shifts back to steep/compliant part of P-V curve
- ↓ intrapulmonary shunt thus improved V/Q match and ↑ PaO2
- ↓ atelectasis and gas trapping
- ↓ extravascular lung water → ↓interstitial/alveolar oedema
- ↓ AWR
- ↑ lung volume → ↑ radial traction by parenchyma → ↑ airway calibre
- ↓ work of breathing
- ↑ lung compliance = ↓ elastic work
- ↓ AWR = ↓resistance work
Negative Effects:
- ↑ V/Q mismatch
- ↑ west zone 1
- ↓ lung compliance
- shift to flat part of P-V curve
- ↑ Work of breathing (due to compliance change)
- ↑ PVR and RV afterload
- extrinsic compression of pulmonary vessels
- Barotrauma
End-Organ Effects
- Renal:
- ↓CO & ↑renal venous pressure
- → Reduced renal blood flow → Reduced GFR → Reduced urine output
- → Reduced atrial stretch and ANP release → Increased ADH → Fluid retention → Oedema
- ↓CO & ↑renal venous pressure
- Hepatic:
- Reduced hepatic blood flow due to:
- Increased CVP and decreased CO lowering the pressure gradient for hepatic flow
- May result in circulation only intermittently throughout the cardiac cycle
- Hepatocyte dysfunction
- Haematological:
- Neutrophil sequestration in the compressed pulmonary vasculature
- CNS:
- ↓VR ⇒ ↑CVP ⇒ ↑ICP
Gladwin / Mooney / JC 2020
Examiner Comments
2016A 02: 29% of candidates passed this question.
This topic has been asked previously. It was expected candidates could detail the impact of PEEP on a variety of respiratory parameters such as lung volume, dead space, arterial pO2 and intrapleural pressure. The cardiovascular consequences are well described including the effect on cardiac output, blood pressure and oxygen delivery. The physiological impact of lower levels PEEP in a young healthy person is different to that often seen in the critically ill and this was not appreciated by most candidates.
3. Compare the physiology of the apex of the lung with the base of the lung in the upright position.
CICMWrecks Answer
Vertical gradient of pleural pressure and alveoli
Inspiration
- Inspiratory muscle contraction → Overcomes resistance to insp flow → negative alveolae pressure -1cm H2O → VT 500ml
- Weight of lung on alveoli and pleura above:
- causes gradient of pleural pressure: Apex (-10cmH2O) Base (-2.5cm H2O)
- Apical alveoli larger
- But compliance lesser (due to baseline distension)
- Lesser ventilation for same pressure
- Apex and base on different part of compliance curve
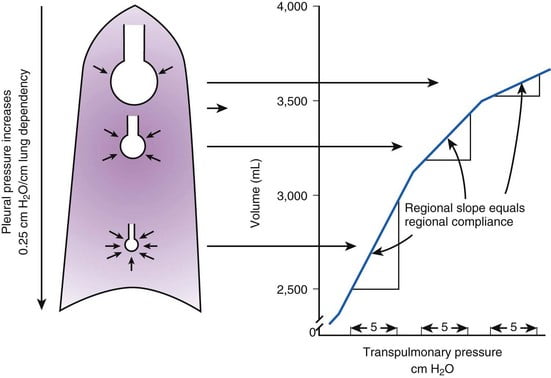
Expiration:
- Gradient of pleural pressure smaller
- Apex: -4cm H2O → on steeper part of compliance curve → ventilation better
- Base +3.5cm H2O →airway closure → gas trapping & shunt
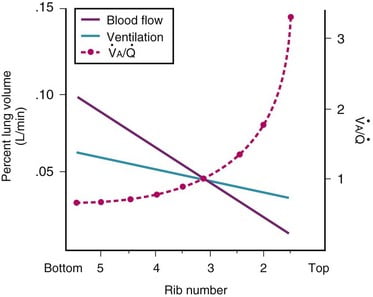
Ventilation, perfusion, V/Q matching
Vertical segments based on V and Q
- Both V and Q ↓ as you move from base to apex.
- ↓ total number of alveoli → ↓ diffusive area → ↓ V
- ↑ compression of intra-alveolar vessels → ↑ west zone 1 → ↓ Q
- V ↓’s slower than Q
| Level | V/Q | Ventilation | Perfusion | PO2 | PCO2 |
|---|---|---|---|---|---|
| Apex | ~3 High | Lower Alveoli largest (no wt of lung) | Very Low Blood hydrostatic pressure low (high gravity) | High 132 | Low 28 |
| At level of heart | ~1 | Slightly more ventilated (Optimized) | Optimized (less effect of gravity) | 108 | 39 |
| Base | ~0.67 towards mixed venous point | Well ventilated (in normal lung): small alveoli, more compliance (effect of wt of lung) | Highly perfused Maximal Hydrostatic pressure | relativly hypoxic 89 | relatively hypercapnoeic 42 |
West Zones:
- Divided into zones based on relationship between Alveolar pressure (PA) Arterial pressure (Pa) Venous pressure (Pv) Interstitial pressure (Pi)
- West Zones 1,2,3: Due to changes in hydrostatic pressure when pumping to top of lung vs. bottom of lung
| West Zone | Pressure Relationships | Physiology | Location |
|---|---|---|---|
| Zone 1 | PA > Pa > Pv | No flow of blood, as arterial pressure completely opposed by alveolar pressure | not seen in normal lung |
| Zone 2 | Pa > PA > Pv | Resistance to flow is determined by alveolar pressure (Starling resistor effect) | about 3cm above the heart |
| Zone 3 | Pa > Pv > PA | Resistance to flow is determined by venous pressure Venous pooling causes increased distension of pulmonary capillaries | majority of healthy lung |
| Zone 4 | Pa > Pi > Pv > PA | Low lung volume causes narrowing of extra-alveolar vessels | Lung bases at low lung volume or in pulmonary edema |
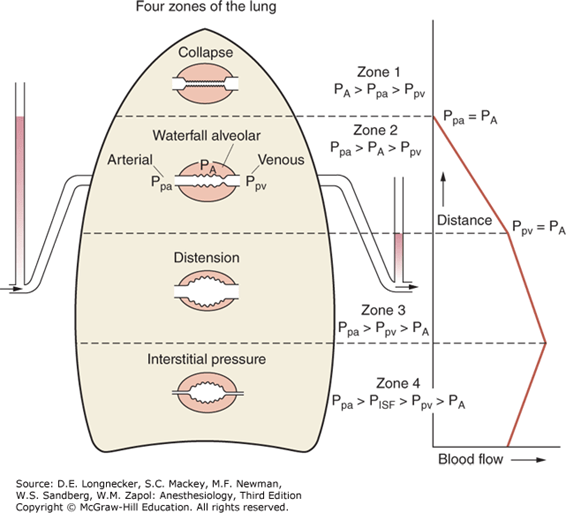
JC 2019
Examiner Comments
2016A 03: 33% of candidates passed this question.
The majority of candidates gave extensive detail on West’s zones of the lungs and did not describe other parameters that vary from base to apex. Ventilation, resistance, compliance, alveolar and lung size all vary. Some candidates mixed up the changes at the apex versus the base.
4. Discuss the factors that influence filtration across the glomerular basement membrane.
CICMWrecks Answer
GFR
GFR:
- “The amount of glomerular ultrafiltrate formed divided by the time of filtration”
- Normal value is approx 125 mL/min or 180 L/day
- Renal blood flow 1.25l/min in 70kg male → Filtration fraction 0.2
Functional anatomy:
3 Distinct Layers:
- glomerular capillary endothelium
- highly specialised endothelium with fenestrations to ↓ filter thickness
- prevents cellular components of blood from coming into contact with BM
- glomerular BM
- made of CT; -vely charged
- acts as filter
- bowmans epithelial cells (podocytes)
- epithelial cells with foot processes → large SA
- negatively charged
- maintain BM + phagocytic functions
Direct GFR Determinants
The GFR (Net flux across the membrane) is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation.
where
Kf = Filtration coefficient
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
- Normally NFP = 17mmHg
- Tubular oncotic pressure = zero throughout
- GC oncotic pressure varies from 21 to 33 mmHg as filtrate is removed.
- Thus less GFR produced at distal end of tubule.
| Afferent end of Glomerular Capillary (mmHg) | Efferent end of Glomerular Capillary (mmHg) | |
|---|---|---|
| PGC | 60 | 58 |
| PT | 15 | 15 |
| πGC | 21 | 33 |
| NFP | 24 | 10 |
| Kf | Filtration coefficient | = LpS = Hydraulic conductivity x Surface Area Glomerular surface area = 0.8m2 • Altered by Mesangial cell contraction (see circulating factors e.g. Angiotensin II → ↓SA → ↓GFR) Patency of the normal capillary wall structure (ie in tubular dysfunction Kf ↑’s ↑GFR) |
| PGC | hydrostatic pressure in capillary | relates to • RBF which is autoregulated for MAP 70-170mmHg • relative afferent/efferent arteriolar tone Affected by • Catecholamines • Local autoregulation -> Myogenic -> Tubuloglomerular feedback -> Hormones (see below) |
| PT | hydrostatic pressure in tubule | relates to • obstruction to urinary flow (usually pathological) • ↑PT → ↓ GFR (ie post renal obstructiion causing renal failure) |
| πGC | oncotic pressure in capillary | relates to • plasma protein concentration (incr in dehydration, decreased in heart failure) • ↑Systemic plasma oncotic pressure → ↑πGC → ↓GFR • ↓Renal plasma flow → ↑πGC → ↓GFR |
| πT | oncotic pressure in tubule | • usually zero, but can increase in renal failure/proteinuria • ↑’d πT → ↑GFR |
| σ | Staverman’s reflection coefficient | • Permeability of membrane to protein usually 1 (no protein leak) • can decrease with nephritis/proteinuria |
Factors influencing GFR
Circulating Factors
- Prostaglandins
- PGI2 and PGE2 → vasodilates → ↑ renal blood flow → ↑ GFR
- ↓ PGE2, PGI2 and NO levels → inhibits afferent arteriolar vasodilation → ↓ GFR
- Long term → ↓ renin release → ↓ ATII-induced efferent arteriolar vasoconstriction → ↓ GFR
- Noradrenaline / Adrenaline
- constrict renal afferent and efferent arterioles → ↓ renal blood flow and reducing filtration → ↓GFR
- Constrict mesangial cells to → ↓ GFR.
- Mesangial cell tone:
- Angiotensin 2, Endothelin, vasopressin → ↓ glomerular surface area and GFR
- ANP, PGE2, Dopamine and cAMP → all increase GFR
Solute factors
- Size
- < 7 kDa (Eg. glucose, ions, urea, H2O) filtered freely,
- > 70 kDa (Eg. albumin) are not filtered;
- neutral particles < 4 mm diameter filtered freely, > 8 mm are excluded
- Charge
- Negatively charge (Anionic) particles repulsed
- Cationic substances filtered more readily
- Protein binding
- Albumin excluded
Disease Factors
- Filtration decreases
- Shock → decreased glomerular pressure
- Obstruction → increased bowman’s capsule hydrostatic pressure
- Hypoproteinaemia → hepatic failure, nephrotic syndrome
JC / Gladwin / Sakurai / Kerr 2020
Examiner Comments
2016A 04: 38% of candidates passed this question.
It was expected this answer would involve discussion about membrane structure, the unique blood vessel structure (afferent and efferent arterioles allowing a high net pressure to be maintained) and Starlings forces all influencing ultrafiltation. Better answers included comment on mesangial cells contraction to decrease surface area ( caused by angiotensin 2). Details regarding molecular weight cut offs (> 7000 Da are not filtered freely) gained additional credit
5. Describe the composition, formation and functions of bile.
CICMWrecks Answer
Bile
- dark green solution produced by the liver to facilitate absorption of fat and fat-soluble vitamins (ADEK) through emulsification.
- Liver produces 1 L bile/day → passes into gallbladder for concentration to 20% volume
- Produced by hepatocytes
- Excreted via canaliculus and biliary tree
- Stored and concentrated in the gallbladder
- Bile contains
- Bile salts (sodium and potassium salts of bile acids)
- Bilirubin
- Water and electrolytes: Na, Cl, K, Ca, bicarb
- Lipids: Cholesterol, Fatty acids, lecithin
- Proteins
Bile Acids:
- Are produced from cholesterol (from diet or from fat metabolism)
- Cholesterol is first converted to cholic acid or chenodeoycholic acid
- These acids combine with glycine and taurine to form glycol- and tauro- conjugated bile acids.
- The salts of these acids (mainly sodium, and potassium) are then secreted into bile.
Bile salts:
- Are amphipathic, and act as emulsifiers of lipid
- Break up large fat globules into smaller micelles, which can then be absorbed.
- Form micelles
- help in the absorption of (1) fatty acids, (2) monoglycerides, (3) cholesterol, and (4) other lipids from the intestinal tract.
- Are absorbed in the terminal ileum, and recycled by the portal circulation (Enterohepatic circulation) along with secondary bile salts produced by gut bacteria such as deoxycholate and lithocholic acid
Bilirubin:
- Catabolic product of heme metabolism
- hemoglobin, myoglobin, cytochromes, catalase, peroxidase, and tryptophan pyrrolase
- 80% from Hb (250 to 400 mg daily)
- 20% other heme proteins and a rapidly turning-over small pool of free heme
- Pathway:
- Haeme + Haem oxygenase → biliverdin
- Biliverdin + biliverdin reductase → bilirubin
- Free bilirubin + albumin → transported to hepatocytes
- Conjugated with glucuronide to ↑ water solubility
- Excreted to bile canniliculus
- Secreted to gut at D2 (duodenum)
- Intestinal flora hydrolyse and reduce → urobilinogen
- 3 fates:
- Gut bacteria form the dark pigment stercobilin, which is egested in the faeces
- Urobilinogen reabsorbed unchanged by the portal system and recycled by the liver
- Remainder is reabsorbed by the portal system and then excreted in the urine
Formation and Handling
Formation and handling of bile salts:
- 1° bile acids (cholic acid and chenodeoxycholic acid) are produced in hepatocytes from cholesterol → then conjugated with glycine (75%) and taurine (25%) to ↑ their H2O solubility → then actively excreted in bile
- They are metabolised by intestinal bacteria (esp large intestines) to form 2° bile acids (deoxycholic acid and lithocholic acid)
- 1° and 2° bile acids become “Bile salts” when they combine with Na+ or K+
- 95% of bile salts are reabsorbed in terminal ileum → recirculated to liver via the portal circulation (“enterohepatic circulation”) → so both 1° and 2° bile salts can be found in bile!
- Only 5% of bile salts are lost in faeces
Turnover of bile salts:
- Rate of hepatic production of bile acid (~0.2-0.5 g/day) EQUALS rate of bile acid loss in the intestines → this low rate of production/loss is due to bile acid recycling via “enterohepatic circulation”
- The body has a “total” storage pool of bile acids of 3 g → during a meal, 9 g of bile salts are needed (Ie. this pool is recycled 3X). Thus, with 3x meals/day the “effective” bile acid pool can ↑ up to 27 g (Ie. this 3g pool is recycled up to 9X)
Release: (relaxation of sphincter of Oddi) takes place via
- negative feed back from bile salt reabsorption in terminal ileum
- acid chyme in duodenum releasing secretin -> increases HCO3- rich secretions from duct cells
- CCK
- gastrin
- nervous control
Functions
- Facilitate intestinal digestion of lipids → aids pancreatic lipase activity
- Enhances intestinal absorption of lipids (fat, cholesterol, phospholipids, and fat-soluble vitamins)
- by emulsifying lipids (by ↓ surface tension → breaks down fat), and
- forming “micelles” (as it is amphipathic)
- Keeps cholesterol solubilised within gallbladder (Ie. prevents gallstones!)
- Induces intestinal motility (Ie. endogenous laxative)
- Choleretic action → bile salts stimulate the hepatocytes to produce MORE bile (via Bile-dependent biliary secretion)
Bianca / JC 2020
Examiner Comments
2016A 05: 31% of candidates passed this question.
Bile is produced by hepatocytes, excretion via cannaliculus and biliary tree. It is stored and concentrated in the gallbladder. Bile contains water, bile acids and bile salts, bile pigments and electrolytes. Some detail was expected regarding each of these and comment on “Primary bile acids”, conjugation with taurine and glycine and production of “bile salts”. It was often not appreciated bacteria in gut produce “secondary” bile acids such as deoxycholate and lithocholic acid. Additional credit was given for discussing “Unconjugated bilirubin” being derived from “heme” component of haemoglobin (85%) is carried via albumin to liver for conjugation via UDP-gluconuryl transferase to “conjugated bilirubin” and that gut bacteria generate water soluble urobilinogen, enterohepatic recycling and excretion in urine. The major role of bile is in lipid, cholesterol and lipid soluble vitamin absorption with a minor role in excretion of bile pigments. It was expected candidates would describe the emulsification of fat via bile salts and lipid micelle formation to facilitate absorption.
6. Outline the formation, structure and function of the platelet.
CICMWrecks Answer: Platelet
PLATELET
Formation
- produced during hematopoiesis in a sub-process called thromopoiesis, or production of thrombocytes.
- Bone marrow: Common myeloid progenitor cells → promegakaryocytes → megakaryocytes
- Megakaryocytes produce protoplatelets within their cytoplasm → released in cytoplasmic extensions upon cytokine stimulus
- Protoplatelets break up into hundreds of platelets that circulate throughout the bloodstream
- The remaining nucleus of the ruptured megakaryocyte is consumed by macrophages.
- Megakaryocyte and platelet production is regulated by thrombopoietin (hormone produced by the liver and kidneys)
- Thrombopoietin stimulates differentiation of myeloid progenitor cells into megakaryocytes and causes the release of platelets.
- Thrombopoietin is regulated by a negative feedback mechanism based on platelet levels
- Each megakaryocyte produces between 5,000 and 10,000 platelets
- Altogether, around 1011 platelets are produced each day in a healthy adult
Fate
- Average lifespan of a platelet is 5 to 10 days
- Old platelets are destroyed by macrophage phagocytosis in the spleen and by Kupffer cells in the liver
- Up to 40% of platelets are stored in the spleen as a reserve, released when needed by sympathetically-induced splenic muscle contractions during severe injury.
Structure
- Small anucleated cells derived from megakaryocytes which originate from haematopoietic stem cell
- Normally 150~300 x 103 /μl
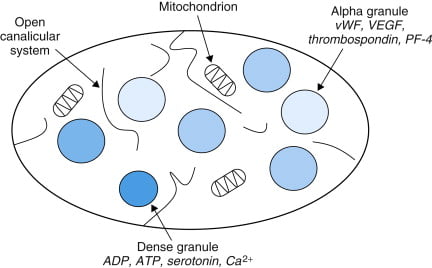
- Actin and myosin
- Remnants of the ER, SR storing calcium
- Mitochondria
- Enzyme systems → production of prostaglandins
- α granules
- Thrombin
- PDGF
- P-selectin
- Fibronectin
- vWF
- Dense granules
- ADP
- ATP
- Ca2+
- Serotonin
- Histamine
Function
- Haemostasis
- Formation of platelet plug
- Interact with collagen exposed at damaged endothelium by GPIa
- Activated by PAF, Thrombin
- Morphological change → irradiating pseudopods
- Degranulation
- Aggregation
- Expression of GPIIb/IIIa → binds fibrin and vWF
- Formation of platelet plug
- Immunomodulation
- Deployed to sites of inflammation and infection and secrete cytokines and chemokines
Sakurai / JC 2019
Examiner Comments
2016A 06: 50% of candidates passed this question.
The structure of the question outlined exactly what was expected. Platelets are formed in the bone marrow from budding of megakaryocytes. Granulocyte colony stimulating factor and thrombopoeiten play a role in the process and they have a life span of about 10 days. It was expected candidates could describe or draw the structure detailing they have no nucleus, the presence of mitochondria and granules and provide some detail of the important external surface proteins (glycoproteins, ABO, human platelet antigens). Better answers also described the internal microtubule structure and related this to function (allows contraction and shape change). The description of function required detail around the importance of platelet plug formation and the role of adhesion, aggregation and activation in this process.
7. Describe the cardiovascular changes of pregnancy including parturition.
CICMWrecks Answer
- Pregnancy is a time of increased metabolic demand, which cardiovascular changes reflect.
- Changes begin from week 8 and ↑ to plateau at 32 weeks → return to normal 2-8 weeks post delivery
- Changes depend on stage of pregnancy
- Hormonal changes: ↑ circulating concentrations of oestrogen, progesterone, hCG
- ↑ metabolic demand esp. during labour: ~↑60% O2 consumption/ CO2 production during labour
- Mechanical effects from gravid uterus
Characteristics
- Mechanical Effects:
- Thoracic changes:
- Anatomical compression of chest
- Diaphragm pushed upwards by 4cm
- ↑ AP + transverse diameter of chest wall (2-3cm)
- placental circulation: ↓pressure, ↓resistance AV shunt
- Aortocaval compression
- Collateral blood flow via collateral paravertebral epidural veins
- Thoracic changes:
- Hormonal Changes:
- ↑ circulating concentrations of oestrogen, progesterone, hCG
- Oestrogen stimulation of RAAS
- Increased plasma volume (40% or 1~1.5L positive)
- Erythropoietin secretion
- Increased erythropoiesis and red blood cell volume (20%)
Changes in CVS
- Anaemia of pregnancy
- Disproportionate plasma volume expansion relative to erythropoiesis
- Increased cardiac output (40%)
- Increased uterine blood flow (750ml/min)
- Increased renal blood flow
- Increased HR (25% by second trimester)
- Increased SV (25% in first trimester)
- Decreased peripheral vascular resistance (30%)
- Progesterone
- Prostaglandins
- Down-regulation of α receptors
- Decreased plasma oncotic pressure (15%) → peripheral oedema
During labour
- Contraction → 300ml return to central maternal circulation
- CO increases 15%, 30% and 45% in latent, active and expulsive phases of labour respectively
- Immediately after labour CO 80% pre-labour levels due to autotransfusion due to uterine involution
- Return to non-pregnant levels 2 weeks after delivery
Sakurai / Kerr / JC 2020
Examiner Comments
2016A 07: 62% of candidates passed this question.
Significant CVS changes can occur by eight weeks and then progressively over the term of the pregnancy. Structured answers helped candidates avoid missing important areas of the answer. It was expected candidates could detail the major changes such as a 40 – 50% increase in blood volume, a 30 – 50% increase in cardiac output, a slight decrease in blood pressure, the heart size and position changes, the impact of aortocaval compression and alterations in colloid osmotic pressure. Some mention of the changes during labour and delivery was expected noting uterine contraction squeezes blood to maternal circulation (auto transfusion), cardiac output increases (immediately after delivery up by about 60 – 80%) and blood pressure increases (both systolic and diastolic) during labour.
Hormones, particularly the effects of foetoplacental production or transformation of hormones, and their cardiovascular effects, especially on total body composition / filling pressures were under explained. The cardiovascular changes at parturition were not well explained.
8. Describe the characteristics of a drug that influence its excretion by the kidneys.
CICMWrecks Answer
Excretion by kidneys can be influenced by drug characteristics
1) Which affect filtration at glomerulus
2) Which affect secretion into tubules
3) Which affect reabsorption in the tubules
1) Which affect filtration at glomerulus
Filtration at the glomerulus – Dependent on Filtration fraction [GFR / RBF] and net starling force.
Normal filtration is 180 L/day (20% of renal plasma flow)
- Protein binding:
- Only free drug present in filtered plasma will be excreted.
- Concentration of filtered drug will be the same as in unfiltered plasma
- Highly protein bound drugs are poorly filtered
There is only a weak concentration gradient favouring dissociation from plasma proteins.
- Molecule size:
- Size:
- Molecules larger than 30 Angstrom is not filtered
- Weight:
- Substances less than 7,000 Da are freely filtered
- Substances greater than 70,000 Da are essentially impermeable
- Size:
- Hydrophilic/lipophobic
Lipophilic drugs may be filtered at the glomerulus but will be freely reabsorbed during their passage down the tubule, such that only trivial amounts are eliminated in urine. - Charge:
- Positive ions are filtered (Glomerulus is slightly negative charged)
2) Which affect secretion into tubules
Active process, allows secretion against concentration gradients
- Acid/Base:
- Saturable process – Saturation may occur of a basic transporter whilst still allowing excretion of acidic drugs, and vice versa.
- Size:
- Molecules which are too large to be filtered in the glomerulus may still be cleared renally by these mechanisms
- Protein binding:
- Protein-bound drugs are not cleared this way; only the unbound fraction is available for active secretion.
3) Which affect reabsorption in the tubules
Passive diffusion down a concentration gradient.
- Hydrophilic molecules can only be reabsorbed by a specialised transport mechanism
- Drug ionization:
- Only non-ionised drug can be reabsorbed passively
- Acidic drugs will become ionised in an alkaline urine (and vice versa), reducing their solubility
This is the physiological justification for urinary alkalinisation.
JC 2019
Examiner Comments
2016A 08: 29% of candidates passed this question.
Drug characteristics that might influence the renal excretion processes include charge, size, solubility, and binding to specific structures or protein. Whether the drug is unchanged versus metabolised can influence these factors.
This question tests core knowledge of pharmacology principles and should be answered with equations, graphs or simple clear descriptions of physical and chemical principles. Extended examples and hedged statements about “influencing” without the direction, magnitude and necessary conditions for the influence did not score marks
9. Describe the essential components of an ECG monitor (80% of marks). Outline the methods employed to reduce artefact (20% of marks).
CICMWrecks Answer
ECG
- 12 electrodes on the body surface to detect small potentials (0.5-2 mV) generated by the heart.
- Electrodes made of Ag/AgCl
- ECG signals detected by these electrodes
- Sent to electronic device that filters out noise and boosts the signal
- Displayed on oscilloscope
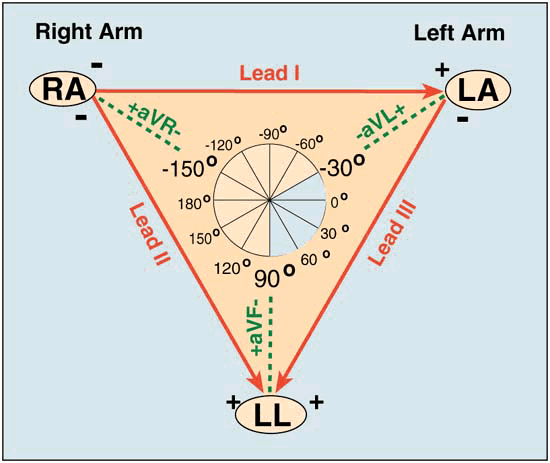
Signal Interpretation from oscilloscope readings via Enthoven’s triangle
- For orientation of the Bipolar and unipolar limb leads synthesised from the 12-lead ECG.
- Bipolar limb leads
- Lead I: RA (-) to LA (+) at 0°
- Lead II: RA (-) to LL (+) at 60°
- Lead III: LA (-) to LL (+) at 120°
- Unipolar limb leads
- aVR: RA (+) at -150°
- aVL: LA (+) at -30°
- aVF: LL (+) at 90°
- Positive deflection in a lead indicates depolarisation wave moving towards lead’s positive terminal
Calibration
- Vertical calibration
- 1 mV signal → vertical deflection of 10 mm (2x large square or 10x small squares)
- 0.1 mV per mm (or small square)
- Horizontal calibration
- paper speed is 25 mm/s → 0.04 s/mm (or small square)
Errors:
Noise and interference caused by:
- Electrical interference by any device using AC current (esp high-frequency diathermy)
- → Minimise by ECG filters, shielding of leads/cables, differential amplifers, Etc.
- Movement or shivering
- → minimised by placement over bony prominences and use of ECG filters
- High skin impedance
- → minimised by degreasing skin with EtOH and use of conducting gel with electrodes
- Incorrect electrode placement relative to heart
It looks like this Examiner reads Hemmings.
Gladwin 2016
Examiner Comments
2016A 09: 50% of candidates passed this question.
The ECG device detects and amplifies the small electrical changes on the skin that are caused when the heart muscle depolarizes (0.5 – 2 mV). This is reflected as rises and falls in the voltage between two electrodes placed either side of the heart which is displayed either on a screen or on paper. Usually more than 2 electrodes are used and they can be combined into a number of pairs (For example: Left arm (LA), right arm (RA) and left leg (LL) electrodes form the three pairs LA+RA, LA+LL, and RA+LL). The output from each pair is known as a lead. Each lead is said to look at the heart from a different angle.
Electrodes are commonly made of silver or silver chloride components that are attached to the main unit of the machine. Most ECG machines use 12 electrodes. Better answers made mention of the two lead types: unipolar and bipolar.
Methods to reduce artefact include improving signal detection (conductive paste, skin preparation (dry, no hair, etc.)) and minimizing external electrostatic forces (common earthed environment, diathermy, etc.,) or patient environment (avoid shivering).
The amplifier has three essential functions: High input impedance so as to minimize signal loss and reject interference (50 – 60 Hz), differential amplification, (to amplify the potential difference detected by the skin electrodes), and high common mode rejection (e.g. > 50Hz) to aid eliminating muscle artefact or electrical interference from the power grid.).
Vector analysis is not a component of the ECG machine and so was not required to answer the question.
10. Outline the pharmacology of warfarin.
Examiner Comments
2016A 10: 61% of candidates passed this question.
The “traditional” pharmacology answer structure was useful to avoid omitting key details.
Warfarin is a synthetic coumarin derivative presented in tablet form for oral use . It is a racemic mixture. S-enantiomer is 2-5 times more potent than the R-enantiomer. It is used for anticoagulation and the usual dosing involves a loading dose ( 3 to 5 mg for 1 to 3 days) then maintenance dose titrated to INR. It was expected answers would then detail mechanism of action, absorption (commenting on bioavailability), distribution, elimination, excretion and adverse effects. Warfarin has contraindications in pregnancy being teratogenic in first trimester and increasing the risk of fetal haemorrhage in third trimester.
Better answers provided increased detail on mechanism of action including the initial procoagulant effect due to protein C and S inhibition and some details about monitoring effect with INR / PT.
Warfarin has several important drug interactions and detailing these gained additional marks.
Additional credit was given for discussion of reversal options, which includes 1) Stop administration – days 2) Prothrombinex – hours 3) FFP – hours 4) Vitamin K depends on dose given.
11. Provide a detailed account of the side effects of Amiodarone.
CICMWrecks Answer
Side Effects of Amiodarone:
Side effects reflect long elimination half-life and significant accumulation in tissues. Side affects increase when maintenance doses are above 400mg daily.
- Respiratory
- Most serious is pneumonitis. Risk is 5-10% at 3 years with a mortality of 10% long term.
- May be due to increased oxygen free radicals and risk increased by high FiO2
- 2 phenotypes- slow insidious and acute onset
- May be reversible if treatment stopped early
- Cardiac
- Prolonged QTc with Torsade’s
- Proarrhythmic (ventricular tachyarrhythmia)
- Bradycardias resistant to atropine
- Peripheral vasodilation leading to hypotension with decreased responsiveness to adrenergic stimuli
- Neurological
- Peripheral neuropathy, tremor, sleep disturbance
- Myopathy
- Endocrine
- Both hyperthyroidism and hypothyroidism may occur (usually reversible)- 2-4% of patients
- Prevents peripheral conversion of T4 to T3 (detected by increased plasma [TSH])
- 37% of weight of Amiodarone is iodine, which may precipitate hyperthyroidism
- Pre-existing thyroid disease increases risk
- GIT
- Hepatitic dysfunction, LFT derangement and cirrhosis has been observed
- Metallic taste during loading
- Ophthalmic
- Corneal microdeposits but visual impairment less likely
- Optic neuropathy (insidious onset, bilateral). Often reversible if discontinued
- Dermatological
- Photosensitivity
- Slate gray pallor of the face, continues after drug discontinuation
- Pregnancy and lactation
- Avoid in from 3 months pre-pregnancy to after breast feeding has finished
- May cause hypothyroidism and bradycardia in the fetus. Foetal hypothyroidism may lead to impairment of myelination
- Contraindicated in porphyria
- Miscellaneous
- IV preparation is irritant and should be administered through a central vessel
Examiner Comments
2016A 11: 26% of candidates passed this question.
The question asked for a detailed account and the expected marks were spread across a range of systemic side effects, not just the cardiovascular and pulmonary side effects. Many candidates provided irrelevant and lengthy descriptions of the mechanisms of action of amiodarone which was not asked for in the question and gained no additional marks. Most successful answers used an organ systems approach to include the many side effects of amiodarone.
Many candidates failed to mention skin side effects, neurological side effects, GI/hepatic side effects, pregnancy and breast feeding considerations, and interactions with other highly protein bound drugs. The predominant mechanism for hypotension with rapid IV administration of amiodarone was incorrectly given in a number of answers.
12. In relation to neuromuscular blocking drugs – Discuss the factors that influence the speed of ONSET of neuromuscular block.
CICMWrecks Answer
Drug Factors
- Dose: Higher dose (eg. multiples of ED95) = faster onset.
- Plasma clearance: Postulated that drugs with faster clearance (eg. sux) have faster onset.
- Potency:
- Inverse log relationship between potency and speed of onset (non-depolarisers).
- Low potency = bigger dose needed (more molecules), so higher conc gradient between plasma and site of action = more rapid onset.
- Depolarisers vs non-depolarisers.
Patient Factors
- Rate of injection: faster rate = faster onset.
- Site of injection: Central vein (more rapid distribution)> peripheral vein > IM
- Cardiac output and muscle blood flow: Higher CO and muscle flow = faster delivery to site of action.
- Priming: small dose of non-depolariser as a premed can partially block NMJ, followed by intubating dose. This decreases time from intubating dose to intubation conditions.
- Disease states: eg. myasthenia gravis (fewer ACh receptors, so needs more sux, but about 10% dose of non-depolariser).
Other factors
- Muscle group: Fastest affected = small, rapidly moving muscle groups (eye, digits), with trunk/abdo muscles last affected.
- Recovery is in reverse order: diaphragm/intercostals recover first.
- Onset more rapid in muscle groups relevant to intubation: laryngeal, masseters. Slower in monitored muscles (eg.adductor pollicus).
- Probably due to muscle blood flow.
- Smaller volume of distribution due to blood loss can augment potency ie: speed of onset(Stoelting)
Drugs:
eg. ephedrine can increase speed of rocuronium onset. Inhalationals/aminoglycosides.
JC 2019
Examiner Comments
2016A 12: 38% of candidates passed this question.
The question specifically asked for factors that influenced the speed of onset of neuromuscular block. This information is different to the factors that influence neuromuscular blockade in general which was what many candidates focussed on. It was expected candidates would address the main factors known to influence the speed of onset of neuromuscular block: – potency of the agent used (inverse relationship to speed of onset); rate of delivery of the agent to the NMJ (blood flow / muscle group); and mechanism of the neuromuscular block (nondepolarising vs depolarising).
13. Describe the cardiovascular effects of a sudden increase in afterload
CICMWrecks Answer
Afterload
- Load against which the muscle exerts its contractile force (Guyton)
- It is represented by the gradient of the line connecting the end-diastolic volume, to the end-systolic point
- pressure which the ventricle has to contract against (Power & Kam)
Determinants of Afterload
Modified Laplace Equation
- where
- T represents afterload
- P represents aortic pressure
- Therefore, afterload increases as aortic (or pulmonary arterial) pressure increases
- R represents ventricular radius
- Afterload increases as ventricular radius increases
- H represents ventricular thickness
- Afterload decreases as the thickness of the ventricular wall increases (hypertrophy secondary to chronic hypertension and cardiac remodelling)
Modified Hagen-Poiseuille Equation
- Afterload is affected by resistance to cardiac output
- Afterload increased by reduced radius of systemic vasculature
- Afterload increased by increasing viscosity of blood
Resistance in parallel
- Afterload is affected by addition, or loss of large capillary networks in parallel
- Systemic vascular resistance is increased significantly by loss of placenta, with parallel vascular networks
- Pulmonary vascular resistance is decreased significantly by inflation of lung, causing creation of vast capillary network in parallel
Cardiovascular Effects of a sudden increase in afterload
Effects of sudden increase in afterload can be demonstrated using a Left ventricular PV loop:
- End-Systolic Volume is increased, causing a reduction in stroke volume
- Initially, Left atrial pressure decreases
- Increased End systolic volume leads to secondary increase in end diastolic volume, hence increasing ventricular filling
- This secondary increase in preload enables the ventricle to contract with greater force (Frank-Starling mechanism) which partially offsets the reduction in stroke volume
- In patients with impaired left ventricular function, the decreased in stroke volume cannot be compensated
- ventricular end-systolic pressure: increases
- ventricular end-diastolic pressure: increases
- cardiac output (=HRxSV) – remains the same
- Initial drop in SV is compensated by increased pre-load
- In a failing heart, the drop in SV causes subsequent stimulation of baroceptors, which in turn causes increased heart rate and can potentially return cardiac output to normal
- Increase in afterload → Anrep → Small ↑Contractility to compensate
- Mechanism: ↑AL → sustained ↑stretch (prolonged isovolumetric contration) → ↑ Ca induced Ca release → ↑ contractility
- Purpose: ↑AL → ↓SV and ↑ ESV
- myocardial oxygen demand and myocardial work: Increased Afterload will increase the pressure during contraction, hence increasing MVO2 for internal work. This might be partially offset by the reduction in external work (due to decreased stroke volume) depending on the cause of the increased afterload
- coronary blood flow is autoregulated to remain normal
JC 2019
Examiner Comments
2016A 13: 21% of candidates passed this question.
It was expected the answer would start with a definition of afterload and then proceeded to indicate what effects this increase in afterload would have on ventricular end-systolic pressure, ventricular end-diastolic pressure, left atrial pressure, cardiac output, myocardial oxygen demand and myocardial work, coronary blood flow and systemic blood pressure.
Most candidates who failed to pass this question submitted answers that were just too brief, only including a small subset of the material required. Very few candidates included any mention of myocardial oxygen demand or myocardial work or the impact upon the cardiac output. A number of candidates included a detailed description of the Sympathetic Nervous System and the Renin-Angiotensin system, material which was not asked for. There were quite a number of incorrect perceptions about what effect a sudden increase in afterload would have on the systemic blood pressure. Candidates who mentioned the baroreceptor response and the stretch receptor response where rewarded with additional credit.
14. Describe the factors that influence intracranial pressure.
CICMWrecks Answer: ICP
Definition:
- ICP: hydrostatic pressure within the cranial vault
- Normal value is 5-15 mmHg
- focal ischaemia when ICP > 20 mmHg
- global ischaemia when ICP > 50mmHg
Munro-Kellie Doctrine
- The rigid and closed cranial vault forms a fixed brain volume containing
- Brain parenchyma (80%, 1400 g)
- CSF (10%; 75 mL)
- Cerebral blood and vessels (10%; 75 mL)
- Δ’s in volume of any components → Δ’s in others or and increase in ICP
CSF Production / Absorption
CSF Production:
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
CSF Absorption:
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
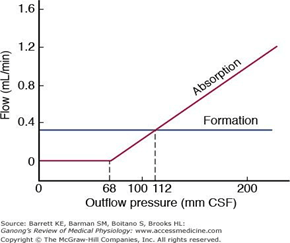
Control of ICP
- ICP is regulated via volume buffering
- i.e. increase in volume of one intracranial component leads to compensatory decrease in volume of other intracranial components
- When volume buffering mechanism is exhausted → rapid increase in ICP (decompensation)
- Movement of cerebral venous blood = rapid compensation, lower capacity
- Movement of CSF = gradual compensation, larger capacity
Determinants of ICP:
- Brain
- Age / Mass
- Space occupying lesions
- Cerebral Oedema
- CSF
- CSF production
- CSF Absorption
- Cerebral Blood Volume
- Cerebral autoregulation: Flow-metabolic coupling
- Cerebral metabolic rate
- Increase in systemic blood pressure / flow
- Venous Outflow obstruction
- Vasoactive agents
- Monro-Kellie Doctrine
- Loss of above – e.g. Fractures, surgery
Compensation for Elevated ICP (Intracranial Pressure)
Early compensation
- Δ CSF distribution and flow
- CSF is displaced to spinal subarachnoid space
- ↑’d resorption rate
Late compensation
- ↑ ICP → ↓ CBF → ↓ in cerebral blood volume → cerebral ischaemia
Decompensation
- ↑ICP → ↓ in cerebral tissue volume → brain herniation
- Cushing Reflex
- Hypertension, bradycardia and abnormal breathing associated with raised ICP
- Mechanism:
- Stage 1:
- ↑ ICP → ↓ blood supply to vasomotor area → Local hypoxia/hypercarbia → ↑ SNS >> ↑ PSPS vasomotor stimulation
- ↑ TPR → ↑ MAP
- ↑ HR → ↑ CO
- → compensatory ↑CBF
- Stage 2:
- ↑ CO → Baroreceptor stimulation → ↑ Vagus nerve stimulation → Bradycardia and ↓ contractility.
- Stage 1:
Gladwin / JC 2020
Examiner Comments
2016A 14: 69% of candidates passed this question.
A structure approached works well for “describe the factors …” questions. Better answers provided a definition of ICP, explained the Monro-Kellie doctrine and then detailed the factors which affect the volume of each of the components – cerebro spinal fluid (CSF), cerebral blood flow and brain parenchyma. Some candidates focused only on factors which cause intracranial hypertension and were thus unable to score full marks. Many candidates stated that CSF production was ICP dependant which is incorrect.
15. Describe the structure and function of the alveolus.
CICMWrecks Answer
Alveolus
- Hollow cavity found in the lung parenchyma, Ends of the respiratory tree.
- There are ~300 million alveoli that are engulfed by a rich capillary network
- cumulative Surface Area of all alveoli is ~85 m2 with a volume of 4000 mL
- Polyhedral-shaped → 0.33 mm in diameter
- Alveolar walls contain dense mesh of capillaries large enough for a red cell to pass through
- Each alveolar wall facing air is lined by Type I cells (one-cell-thick) → parts of alveolar
wall consists of interstitial space that contain pulmonary capillaries - Diffusion barrier that alveolar gas has to travel to pulmonary blood is short (0.3 um only!)
→ consists of (i) Type I cell (epithelium), (ii) Basement membrane of type I cell, (iii)
Basement membrane of pulmonary capillary endothelium, and (iv) Pulmonary capillary
endothelium - In some alveolar walls, there are pores between alveoli called Pores of Kohn
- Alveoli are inherently unstable (Ie. collapse) given their small size and surface tension of
their liquid-lining → this is offset by surfactant produced by Type II cells (round cells
with large nuclei and cytoplasmic granules containing surfactant)
Alveolar cells
Type 1 Pneumocytes
- 90% of alveolar surface area
- Thin walled, Involved in gas exchange
- Unable to replicate, Susceptible to toxic insults,
Type II pneumocytes
- 10% of alveolar surface area
- Secrete surfactant
- Proliferate and differentiate into type I and type II cells
Alveolar Macrophages
- Present in alveolar septae, lung interstitium
- Phagocytosis of foreign particles
JC / Bianca 2019
Examiner Comments
2016A 15: 52% of candidates passed this question.
Better answers related structure to function. Many answers lacked key anatomical features (for example pores of Kohn, basement membrane, interconnecting walls / alveolar interdependence etc.). There was little understanding of the role and origin of the basement membrane of the alveolus. Some candidates went into detailed discussions of Work of Breathing, respiratory mechanics and the renin-angiotensin system which were not asked for.
Answers not reaching a pass mark generally suffered from lacking detail and suggested only a
superficial understanding of the area.
16. Outline the anatomy of the subclavian vein relevant to central venous line insertion.
CICMWrecks Answer:
Origin
- Continuation of axillary vein
- Lateral border of 1st rib
Course
- Follows subclavian artery
- Deep to clavicle
- Superior to 1st rib
Termination
- Deep to sternoclavicular joint at medial border of scalenus anterior
- Joints internal jugular vein to form bilateral brachiocephalic veins vein on left
Relations
- Anterior
- Clavicle, subclavius
- Posterior
- Subclavian artery runs deep/posterior (separated by scalenus anterior)
- Internal mammary artery is posterior medially
- Phrenic nerve is posterior
- Superior
- Skin, superficial aponeurosis
- Inferior
- Apex of lung and 1st rib
- Medial
- Brachiocephalic trunk, thoracic duct and trachea and vagal trunks
- Lateral
- Inferior trunk of brachial plexus
Surface Anatomy
- Clavicle
- Deltopectoral groove
- 2 heads of sternocleidomastoid
- Suprasternal notch
Sakurai 2016
Examiner Comments
2016A 16: 38% of candidates passed this question.
Answers to anatomy questions can be generally structured by considered the origin and ending of the structure, the surface landmarks and the relations (medial / lateral / anterior / posterior) and this would have worked well in this question. It was expected candidates could detail course (from origin to end) and relations of subclavian vein. This could then be used to highlight how these features may be relevant to central venous line insertion (proximity of subclavian artery or pleura creating the possibility of inadverant arterial puncture or pneumothorax. Many candidates failed to mention drainage of external jugular vein and thoracic duct and right lymphatic ducts into the subclavian veins. Candidates should ensure diagrams are accurate and well labelled and they use appropriate anatomical terminology rather than vague terms such as “in front”. Care should be taken ensuring accuracy (e.g. some mentioned dome of diaphragm instead of pleura or IVC instead of SVC)
17. Describe the physiology of the thyroid hormones.
CICMWrecks Answer
Thyroid Hormones
- 3 main thyroid hormones
- thyroxine (T4): 95%; ½ life 7 days; less active
- tri-iodothyronine (T3): 7%; ½ life 24hrs; 3-5x activity of T4
- reverse T3 (rT3): inactive
- T3 + T4 formed from iodination of aa tyrosine
- iodine obtained from diet in form of iodide + actively taken up into thyroid follicular cells (req 120-150ug/day)
- Release
- TRH from hypothalamus → stimulates TSH release → binds to R on cell membrane of follicular cells, GPCR → ↑cAMP → ↑AC →
- ↑iodine uptake into follicular cells
- ↑synthesis of T3 + T4 via ↑iodination + ↑rate coupling reactions
- ↑proteolysis of thyroglobulin within follicular cells → liberate T3 + T4
- MoA
- T3 + T4 highly protein bound (>99%) predominantly to thyroxine binding globulin, albumin, thyroxin binding pre-albumin
- Thyroid hormones enter cell → T3 binds to intracellular thyroid receptors (TR) → hormone receptor complex = transcription factors (bind to DNA via zinc fingers) → alter gene transcription → clinical effects
- T4 de-iodinated to T3
Production and Regulation
Synthesis
- Steps:
- dietary iodine converted to iodide for absorption
- iodide actively transported into follicular cells in thyroid (trapping) → oxidised to iodine (via thyroid peroxidase)
- iodine binds tyrosine in thyroglobulin molecule (iodinase) → forms mono-iodotyrosine then di-iodotyrosine (peroxidase)
- di-iodotyrosine + di-iodotyrosine = thyroxine (T4) (perixodase) → binds thyroxine binding globulin + thyroxine binding pre-albumin
- ↑activity with TSH of:
- iodide pump activity
- thyroid peroxidase
- iodinase
- peroxidase
- vesicular lysosomal activity
- thyroid hormones formed within thyroglobulin; synthesised in golgi apparatus
- thyroglobulin stored in follicular colloid → vesicular lysosomal activity breaks down thyroglobulin to release T3 + T4 which diffuse out of follicular cells and into circulation
Metabolism of thyroid homrones
- T4 deiodinated to T3/ rT3 (inactive compound 1:1) → deiodinated in liver, kidney, skeletal muscle to inactive compounds
Negative feedback
Physiological Action
| System | Action | Physiological + pathological effects |
|---|---|---|
| CNS | Development | – normal CNS development – ↓T → ↓CNS development → retardation, rigidity, deaf-mutism – sexual function |
| CVS | Chronotrope Inotrope Vasodilation | – ↑number β-adrenoceptors → ↑HR – ↑circulating catecholamines → ↑contractility + ↑CO – ↑T → ↑body temp → vasodilation → ↓SVR |
| Resp | Metabolic | – ↑T → ↑metabolic rate → ↑MV |
| Bone | Anabolic | – essential for normal bone growth |
| ANS | Stimulatory | – ↑T → synergy with circulating catecholamines → ↑SNS |
| Metabolic / endocrine | Cellular effect Feedback Anabolic/ catabolic | – ↑Na/K ATPase activity → ↑MR of cells + calorigenic – feedback inhibition → ↓TRH + ↓TSH ; ↑T → ↑GH release – CHO: ↑CHO absorption – Fat: ↑lipolysis; ↑LDL Rs → ↑ liver uptake circulating cholesterol – Protein: physiological amounts: ↑protein synthesis; excess amounts: protein breakdown (thyrotoxic myopathy) |
Kerr 2016
Examiner Comments
2016A 17: 40% of candidates passed this question.
Thyroid hormones consist of thyroxine (T4), tri-iodothyronine (T3) and reverse T3 (rT3). It was expected candidates would briefly describe each of these. T4 is a pro-hormone synthesized from tyrosine in follicular cells of the thyroid gland and represents 80% of body’s thyroid hormone production. It exists in free form, plasma protein bound (albumin and pre-albumin (TBPA) and tissue protein bound thyroid-binding globulin (TBG) and has a half-life around 7 days. Tri-iodothyronine (T3) is the most biologically active thyroid hormone (5 times T4), is produced directly from tyrosine (20%) or in the periphery by conversion of T4 (80%) with a
half-life 1.5 days. Reverse T3 (rT3) is formed via peripheral conversion of T4 by de-iodination.
A classic negative feedback loop exists to control thyroid hormone secretion. Thyroid Stimulating Hormone (TSH) from the anterior pituitary is controlled by Thyrotropin Releasing Hormone (TRH) from the Hypothalamus via hypothalamic-hypophyseal portal system. Both of these factors are inhibited by elevated levels of T4 and T3.
The mechanism of action is by binding to nuclear receptors to effect protein synthesis. Thyroid hormone has a wide variety of physiological effects across many systems including respiratory, cardiovascular, metabolic and growth and sexual function.
The answer required candidates to detail both the synthesis and control of thyroid hormones as well discussing the action of thyroid hormones. Few candidates could differentiate the roles and actions of T3 and T4.
18. Describe the structure and function of the mitochondrion.
CICMWrecks Answer
Structure of the Mitochondrion
Mitochondria have their own genome & ability to manufacture own RNA and proteins
Their ribosomes = 70S type (30S & 50S) i.e same as bacteria (rest of cell has 80S ribosomes)
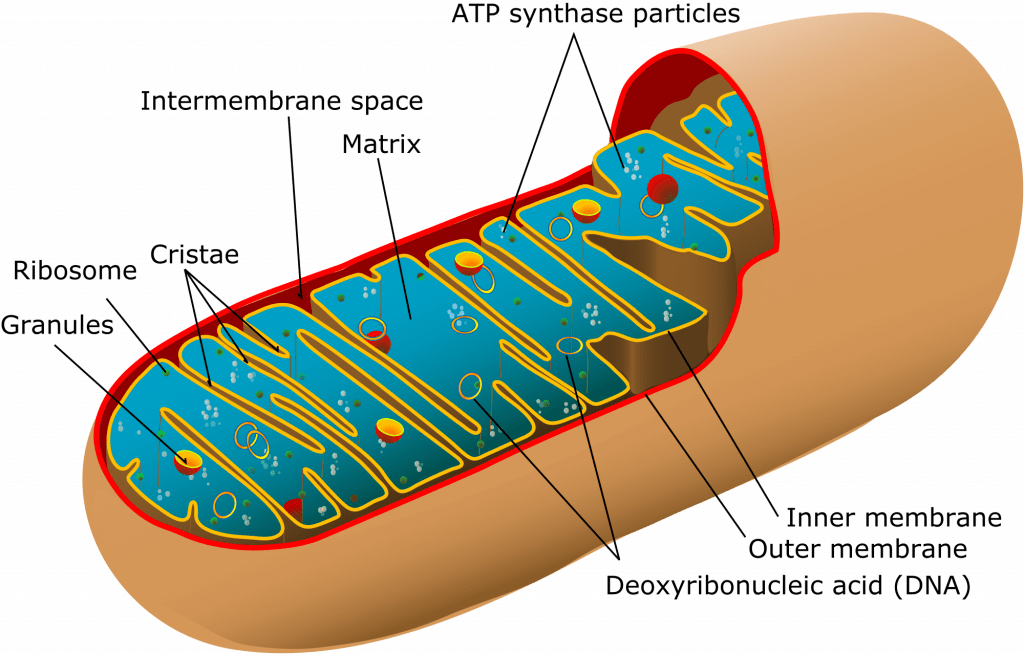
- 1-10µ
- Consist of two membranes (outer and inner)
- Outer membrane
- encloses whole organelle
- contains several integral proteins = porins
- porins form large aqueous channels which allow passage of movement of molecules up to 5000D (pyruvate, amino acids, fatty acids)
- Intermembrane space
- between outer & inner membrane
- chemically equivalent to cells cytosol
- contains cytochrome-c
- Inner membrane:
- No porins
- controlled permeability via transporter proteins
- Different functions
- proteins carrying out oxidative reactions of resp chain
- ATP synthase – makes ATP in matrix
- Transport proteins
- Protein import machinery
- Cristae:
- Formed by folded inner membrane
- Vastly ↑s surface area for ATP production
- Cells which more active e.g myocardium have more cristae
- Inner mitochondrial matrix
- Space enclosed by inner membrane
- Impt in ATP production
- Contains highly conc mixture of
- hundreds of enzymes
- mitochon ribosomes (70S)
- tRNA
- several copies of DNA genome
- Contents important in many metabolic processes:
- Citric acid cycle
- Pyruvate metabolsim
- Fatty acid metabolism
- Urea cycle
- Haeme synthesis
- Outer membrane
- There are several mitochondria are found within a cell – They replicate independently of the cell’s state of division (as they possess their own DNA), and they replicate in response to the metabolic demands of the cell (Ie. number of mitochondria reflects metabolic activity of the cell)
- Mitochondria DNA is unique from nuclear DNA in that it is:
- Contains both double-stranded circular DNA and plasmid DNA, which are both maternally-inherited
- only 1% of mitochondrial proteins (esp enzymes for oxidative phosphorylation) – Remaining 99% of proteins are encoded by nuclear DNA
Function of the Mitochondrion
- Form ATP via oxidative phosphorylation (Major), via Kreb’s cycle and Electron Transport Chain
- Regulation of Cellular proliferation regulation including cell division and differentiation (contributes ATP)
- Regulation of cellular metabolism
- Regulate apoptosis
- Xenobiotic metabolism (esp role of MAO)
- Heat production (esp in brown fat)
- By proton leak or mitochondrial uncoupling
- proton re-enters mitochondrial matrix without contributing to ATP synthesis → heat released
- mediated by therminogenin (proton channel)
- By proton leak or mitochondrial uncoupling
- Sequestration of Ca2+ ions (with swelling/damage post-ischaemia)
- acts as cytosolic buffers for calcium
- Significant interplay with Endoplasmic reticulum
- primary driven by mitochondrial membrane potential
- released back into cell’s interior via Na+-Ca2+ exchange protein or Calcium-induced-Calcium-release pathways
- Calcium also necessary to activate isocitrate dehydrogenace (Kreb’s cycle)
- Signaling through mitochondrial reactive oxygen species
- Cholesterol and steroid synthesis
- Certain haeme synthesis reactions
- Organ specific functions:
- Neuronal: contribute to cellular quality control by reporting neuronal status towards microglia through specialised somatic-junction
- Liver: Detoxify ammonia
Oxidative Phosphorylation – Mitcochondria Energy Production
- mitochon found in high conc in cells with high metabolic demands eg myocardium (23% of cell), brown fat (neonate)
- exercise ↑s numbers
- OP = production of ATP associated with oxidation by the flavoprotein cytochrome system in mitochondria
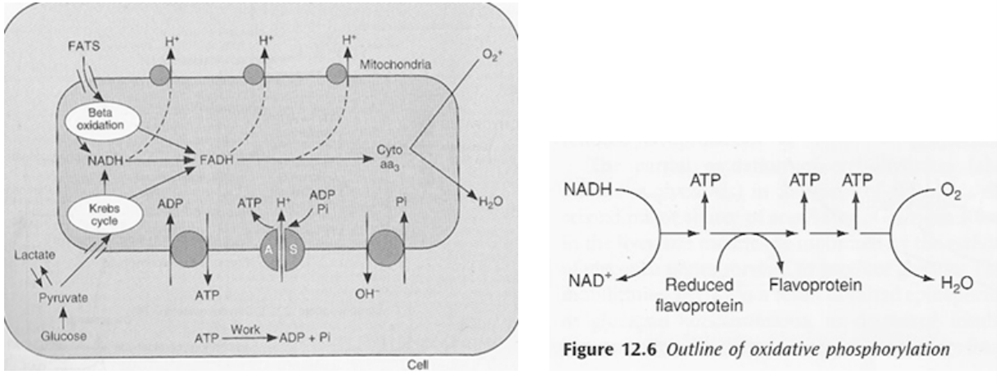
- ATP formed in electron transfer chain:
- Substrate diffuses into mitochon cytoplasm
- Hydrogen removed by a dehydrogenase
- NAD carries hydrogen to respiratory chain
- Hydrogen ionises and protons pass along series of carrier molecules across insulating membrane (inner membrane of mitochondria – forms cristae)
- Movement of protons creates an electrochemical gradient for transport of protons from intermediate space back into matrix ⇒ this drives a reversible ATPase in inner membrane (ATP synthase)
- ATP synthase: ADP + Pi ⇒ ATP
- @end:
- ATP produced
- Reduction of O2 to water – catalysed by cytochrome oxidase
- cyanide inhibits this oxidase ∴ inhibits OP in mitochon
- O2 required to oxidise NADH
- Eg’s of carrier molecules in electron transfer chain
- Flavoprotein
- Cytochromes A, A3, B, C, C1
- Ubiquinone
- Several iron sulphide proteins
- OP depends on:
- Adequate supply of ADP: +ve feedback loop e.g. ↑ATP utilisation ⇒ ↑ADP ⇒ ↑OP
- Rate of delivery of fats, lactate, glucose to interior of mitochon
- Availability of O2: Pasteur point = 1-2mmHg i.e. point below which OP cannot occur
- ∴ cardioresp works in harmony to ensure o2 reaches cells
- defined by oxygen flux equation:
- lack of oxygen causes:
- nothing to scavenge H+ at end of transfer chain
- transfer chain ceases
- build up of reduced compounds ⇒ inhibits TCA cycle ⇒ inhibition of glycolysis
- but glycolysis continues as lactate dehydrogenase removes reduced compounds
Side note: breakdown of 1 glucose molecule:
| Stage | Direct products (net) | Ultimate ATP yield (net) |
| Glycolysis | 2 ATP | 2 ATP |
| 2 NADH | 4 ATP | |
| Pyruvate oxidation | 2 NADH | 6 ATP |
| Citric acid cycle | 2 ATP/GTP | 2 ATP |
| 6 NADH | 18 ATP | |
| 2 FADH2 | 4 ATP | |
| Total | 36 ATP |
JC 2019
Examiner Comments
2016A 18: 19% of candidates passed this question.
Most candidates had at least a basic understanding of mitochondral function although some detail was required for a pass and many did not provide this. A well labelled diagram was used by many candidates and scored marks. Repetition of the same information illustrated on a labelled diagram in subsequent text was not required and did not score additional marks.
It was expected answers would cover basic structure (double membrane structure with cristae and enzymes lining the membrane and within the matrix), details of the electron transport chain, the citric acid cycle and beta-oxidation of long chain fatty acids and mention the maternal origin of DNA. Better Answers provided some information on other functions such as production of reactive oxygen species, role in calcium homeostasis and apoptosis, urea cycle, haem synthesis and heat production
19. Describe the methods of temperature measurement.
CICMWrecks Answer
Temperature is the tendency of a body to transfer heat energy to another body, and is measured in degrees.
It is distinct from heat, which is the kinetic energy content of a body, and is measured in Joules. The two are related by the specific heat capacity, which describes how much energy (J) must be applied to a body to raise its temperature from 14°C to 15°C, without a change in state.
Measurement of Temperature
Temperature is measured by a number of methods:
- Liquid Expansion Thermometry
- Electrical methods:
- Resistance thermometry
- Thermistor
- Thermocouple
1. Liquid Expansion Thermometry
This is used in mercury thermometers. These consist of:
- A graduated evacuated capillary of negligible volume, attached to
- A mercury reservoir, of much greater volume, separated by
- A constriction ring
Prevents travel of mercury up the capillary by gravity.
Mechanism:
- When heated, the kinetic energy of the mercury increases and it expands, forcing it up the capillary.
- As the thermal expansion coefficient for all liquids is very small, the capillary must be of a very small volume to create a useable device.
- The speed that this occurs is related to the time-constant of the system
This is typically 30 seconds. Measurement therefore takes ~4 time-constants, or 2 minutes.
Pros
- Easy to use
- Accurate
- Reusable
- Sterilisable
- Cheap
Cons
- Slow response: Only accurate once it has reached thermal equilibrium.
- Glass can break: May cause release of mercury or alcohol.
- Inaccurate at:
- Low temperatures with mercury
Freezes at -38.8°C. - High temperatures with alcohol
Boils at 78.5°C.
- Low temperatures with mercury
2. Electrical
Electrical methods include:
- Resistance thermometer
Platinum wire increases electrical resistance with increasing temperature.- Therefore the voltage drop across the wire will correspond to the temperature of the wire
- Change in resistance is linear across the temperature range
- However, these are expensive.
- Thermistor
Metal (e.g. SiO2) semiconductor which changes its resistance in a predictably non-linear fashion (run-away exponent) with temperature.- Can be manufactured so that change is linear over the clinical range
- Much cheaper than wire resistance methods
- The degree of voltage drop is usually very small, however this can be amplified using a wheatstone bridge
- Thermocouple
At the junction of two dissimilar metals, a potential difference will be produced proportional to their temperature. This is known as the Seebeck effect.- Non-linear (wash in exponent)
- Degrade over time
JC 2019
Examiner Comments
2016A 19: 36% of candidates passed this question.
A good answer included a definition of temperature and a classification of the methods of measuring temperature such as electrical, non-electrical and infrared. There followed a brief description of the physical principles of thermistors, thermocouples and resistance thermometers; mercury and alcohol thermometers, bimetallic strips; and of infrared methods. Candidates who did well reproduced the content of the chapter on temperature measurement in the recommended text book. Candidates who were not familiar with this material attempted to answer the question by falling back on clinical experience of measuring temperature in different sites or occasionally referring to concepts of thermoregulation. Neither approach gained credit.
Some candidates interpreted “methods” incorrectly as “site of measurement” so scored poorly.
20. Outline the role of the liver in drug pharmacokinetics.
CICMWrecks Answer
This answer could be structured by splitting the content into major categories. These could be:
- the clearance functions of the liver
- first pass metabolism
- mechanisms of hepatic metabolism
- the effects of liver disease.
The role of the liver in drug clearance
- The role of the liver in pharmacokinetics is as an organ of clearance.
- The two major determinants of hepatic clearance are the efficiency of drug removal from the blood and the efficiency of blood delivery to the liver.
- Efficiency of drug removal by the liver is described by the hepatic extraction ratio, which is the fraction of the drug entering the liver in the blood which is irreversibly removed (extracted) during one pass of the blood through the liver.
- The hepatic extraction ratio is determined largely by the free (unbound) fraction of the drug and by the intrinsic clearance rate, which is the intrinsic ability of the liver to remove (metabolise) the drug in absence of restrictions imposed on drug delivery to the liver cell by blood flow or protein binding.
- The effect of liver blood flow on hepatic clearance depends on the hepatic extraction ratio of the drug.
- With increasing hepatic blood flow, hepatic extraction ratio will decrease for all drugs.
- For drugs with low intrinsic clearance:
- Hepatic extraction ratio will drop more rapidly with increasing hepatic blood flow
- Hepatic clearance will not increase significantly with increasing blood flow
- For drugs with high intrinsic clearance:
- Hepatic clearance will increase in a fairly linear fashion, in proportion to hepatic blood flow
- Increasing the intrinsic clearance will have diminishing effect on total hepatic clearance
The effect of the liver on first pass metabolism
- First pass clearance is not just hepatic but is a combination of metabolism by gut bacteria, metabolism by intestinal brush border enzymes, metabolism in the portal blood and metabolism by liver enzymes.
- For drugs with low hepatic extraction ratio:
- First pass clearance will be low
- Changes in hepatic enzyme function will have little effect on first pass clearance
- For drugs with high hepatic extraction ratio:
- First pass clearance will be high
- Changes in hepatic enzyme function will have a significant effect on first pass clearance
Biotransformation in the liver
By convention, the metabolic functions of the liver are divided into Phase I and Phase II reactions
- Phase I reactions:
- Examples of Phase I reactions:
- Hydrolysis
- Reduction
- Oxidation.
- Characteristics of Phase I reactions:
- these reactions expose or introduce a functional group (–OH, –NH2, – SH or –COOH)
- They usually result in a small increase in hydrophilicity.
- Examples of Phase I reactions:
- Phase II reactions:
- Examples of Phase II reactions:
- Glucuronidation
- Sulfation
- Acetylation
- Methylation
- Conjugation with glutathione
- Conjugation with amino acids eg. taurine, glutamine, glycine
- Characteristics of Phase II reactions:
- The products are supposed to be significantly more hydrophilic than the original substrate
- Examples of Phase II reactions:
Effects of changes in liver function
- The effects of changes in synthetic function
- The liver synthesises plasma proteins; plasma protein binding influences the volume of distribution
- Low plasma protein levels lead to raised free drug levels (the free fraction increases)
- This process is therefore synergistic with the concurrent decrease in liver blood flow and hepatic extraction ratio
- The liver synthesises plasma esterases and peptidases; these metabolise certain drugs
- Significant liver disease can result in prolonged clearance of drugs which are susceptible to these enzymes (eg. suxamethonium)
- The effect of changes in secretory function
- Drugs and metabolites which rely on biliary excretion will be retained, and may require dose adjustment
- Drugs which enjoy enterohepatic recirculation may have decreased halflives due to failure of recirculation
- High bilirubin levels may result in the displacement of drugs from albumin as it competes for binding sites
- Decreased secretion of bile may result in malabsorption
- The effects of portal hypertension on pharmacokinetics
- Portal venous hypertension leads to shunting of portal venous blood into the systemic circulation
- This has the effect of decreasing first pass metabolism
JC 2019
Examiner Comments
2016A 20: 62% of candidates passed this question.
Most candidates structured their answer to this question well – they were aware of first pass metabolism and the effect of protein synthesis upon volume of distribution of drugs. Knowledge concerning Phase I and Phase II reactions was frequently inadequate. Many candidates were aware that these processes as well as inactivating or activating drugs resulted in increased water solubility to aid excretion via bile or urine. Few candidates discussed the significance of the large blood flow to the liver or the implications of high and low extraction ratios especially in relation to liver blood flow.
21. Statistics (not in current primary syllabus)
22. Compare and contrast the mechanism of action (25% of marks), antimicrobial profile (25% of marks), pharmacokinetics (25% of marks) and adverse effects (25% of marks) of Flucloxacillin and Vancomycin.
Examiner Comments
2016A 22: 83% of candidates passed this question.
The structure required to score well was provide by the questions asked. Marks were lost by not mentioning that flucloxacillin is a beta lactam that it does not cover MRSA and that vancomycin covers enterococcus. Better answers could identify that vancomycin is slower at killing sensitive staph than flucloxacillin. Adverse effects were specifically asked for in the question so omitting facts such as associated nausea/ vomiting/ diarrhoea/ anaphylaxis etc. cost some candidates marks. If 25% of marks are allocated to side effects then it is expected more than one adverse effect would be mentioned. Some candidates had incorrect facts – Enterococcus is not a gram negative organism.
23. Classify anti-emetic agents by describing their mechanism of action and provide examples.
CICMWrecks Answer
Anti-emetic Agents
| CLASS | EXAMPLES | MECHANISM OF ACTION |
|---|---|---|
| Anticholinergics | Hyoscine Atropine | – M1-Ach-R antagonism (NTS, CTZ, VC) – Small antihistamine and D2 antagonist effects |
| Antihistamines (H1) | Cyclizine Promethazine | – H1 antagonism- VC, vestibular nucleus and CTZ – Anti-muscarinic effects- NTS, CTZ, vomit center – D2 antagonism (GIT, CTZ) |
| 5-HT3 Antagonists | Ondansetron | – Peripheral in GIT – Central at VC and CTZ |
| Dopamine Antagonists | 1. Phenothiazines – Prochlorperazine (stemetil) 2. Butyrophenones – Droperidol, domperidone 3. Benzamides – Metoclopramide | – Decreased sensitivity of visceral afferents to vomit center – Central D2 blockade increased threshold at CTZ Other effects: – Inhibition of 5-HT3 – Anti-H1 effects |
| Steroids | Dexamethasone | – Proposed to act centrally to inhibit prostaglandin synthesis and inhibit endorphin receptors |
| Miscellaneous | 1. Propofol 2. Benzos 3. Cannabinoids 4. NK1- Receptor antagonists (aprepitant) | – Propofol and BZD- GABAergic inhibition of VC – Cannabinoids- Direct CTZ and VC inhibition – NK1 antagonists inhibit VC |
Examiner Comments
2016A 23: 52% of candidates passed this question.
Successful candidates were those who discussed the multiple locations in the vomiting pathway where a drug acts, the receptors involved and discussed 5 or more classes. Several approaches can be taken to this topic. Classification by drug group works well and then allows more detail to be provided about possible receptor activity.
For example:
Anithistamines: Promethazine H1 ++++ M ++ D2 ++ 5-HT3
Anitcholinergics: Scopalamine H1 + M ++++ D2 + 5-HT3
Benzamines: Metoclopromide H1 + M — D2 +++ 5-HT3 ++,
Neuroleptics: Droperidol H1 + M – D2 +++ 5-HT3 +,
5-HT3 Antagonists: Ondansetron H1 – M – D2 – 5-HT3 ++++, Granisetron H1 ++++ M ++
D2 ++ 5-HT3 ; Glucocorticoids: Dexamethasone H1 – M – D2 – 5-HT3 -,
Propofol: GABBA
Cannabinoids: direct on vomiting center.
An alternative approach involves a discussion of the distribution of receptor sites: nucleus vestibularis H1 M, area postrema (chemoreceptor trigger zone) 5-HT3 D2 M1 H1, nucleus tractus solitarius 5-HT3 D2 M H2 and then discuss which drugs act where. This material has also been previously covered in the viva examination and knowledge of both drug properties and receptor distribution is often required.
Few answers detailed relative activity at various receptors types – “+” scale illustrated above would be sufficient to convey an understanding to the examiners.
24. Describe the ideal sedative agent for an Intensive Care patient (50% of marks). How does midazolam compare to this (50% of marks)?
CICMWrecks Answer
(Please note Propofol was not asked in this paper)
PHARMACEUTIC
| IDEAL SEDATIVE | MIDAZOLAM Y/N | PROPOFOL Y/N |
|---|---|---|
| long shelf life | Y | Y |
| stable when drawn up and on exposure to light | Y | Y |
| water soluble | Y | N |
| no refridgeration | Y | Y |
| cheap | Y | Y |
| mixes well with other agents in the central line lumen | Y | Y |
| Bacteriostatic | Y | N |
PHARMACODYNAMIC
| IDEAL SEDATIVE | MIDAZOLAM Y/N | PROPOFOL Y/N |
|---|---|---|
| Known MOA | Y | N |
| Affects only CNS | N | N |
| No excitatory or emergence phenomenon | Y | N |
| No pain on injection | rare | N |
| Safe on Intra-arterial injection | N | N |
| No trigger for MH or porphyria | Y | N |
| Safe in pregnancy | N | Y |
| Reliable dose – effect curve with little inter- individual variation in effect | N | Y |
| Anxiolysis | Y | Y |
| Analgesic properties | N | N |
| Amnesia | Y | Y |
| No effect on cardiovascular performance | N ↓MAP, ↓SVR, ↓HR | N ↓MAP, ↓SVR, ↓HR |
| Does not depress respiratory drive | N Significant, Dose-dependent | N Mild, dose-dependent |
| Rousable sedation | Y | Y |
| Minimal side effects | N | N |
| No incidences of allergy / anaphylaxis | Y rare | Y rare |
| No idiosyncratic reactions | N | N |
| No tachyphylaxis | N | Y |
PHARMACOKINETIC
| IDEAL SEDATIVE | MIDAZOLAM Y/N | PROPOFOL Y/N |
|---|---|---|
| Low volume of distribution | Y 0.8-1.5L/kg | N 2-10 L/kg |
| rapid onset | Y 2 mins | Y 30sec |
| low protein binding | N 96% PB | N 98% PB |
| Largely unionized (high pKa) | Y pKa=6.5 (85% ionized at 7.4) | Y pKa=11 (99% unionized at 7.4) |
| Lipophilic | Y Highly lipophilic with closed ring | Y |
| inactive metabolites | N | Y |
| non-toxic metabolites | Y | Y |
| rapid clearance (context-sensitive half-life) | Y/N (CSHT 40mins at 2hrs) | Y (CSHT 20mins at 2hrs) |
| clearance not affected by either renal or hepatic dysfunction | Y | N |
| Little inter-individual variation in pharmacokinetics | N | Y |
| Availability of an antagonist | Y Flumazenil | N |
Propofol infusion syndrome:
When used at >4mg/Kg/Hr for more than 24 hours
- Metabolic acidosis,
- Lipaemia,
- rhabdomyolysis
- Cardiac failure
- arrhythmias and death
Gladwin / JC 2020
Examiner Comments
2016A 24: 60% of candidates passed this question.
Candidates who had a structured approach (i.e. pharmaceutical, pharmacokinetic, pharmacodynamic) provided more content and scored higher. Candidates who also approached pharmacodynamic effects in an organ system based approach scored higher.
Relating a pharmacokinetic property of midazolam (e.g. volume of distribution or half-life) to a un/desirable attribute e.g. offset of action and accumulation displayed a greater understanding of the question. For many candidates, the description of an ideal drug contained more detail and candidates were not able to adequately state how midazolam compares.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Resp physio, Abdominal pressure, splanchnic blood flow, terlipressin |
| G. CVS | Cardiac output, SV, myocardial contractility, calcium, catecholamines, digoxin Aging physio + pharm, oPioid pharmacology |
| H. Renal | |
| I. Body Fluids and Electrolytes | fluid flow and related physical principles around turbulent and laminar flow, invasive BP monitoring |
| J. Acid Base | buffer systems in the body and the renal handling of acid. Pharmacology, LA. |
| K. Neuro | Cerebral physio + pharm, propofol, ketamine Aging physio + pharm, oPioid pharmacology |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | Antibacterial agents, resistance, dosing rationale for aminoglycosides |
| U. Endocrine | blood glucose homeostasis and the pharmacology of insulin |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments