1. Compare and contrast 0.9% saline and 4% albumin
Examiner Comments
2015B 01: 21% of candidates passed this question.
It was expected answers would include a comparison of the composition, physicochemical features and relevant physiology. Many candidates failed to adequately describe the differences in distribution across the body compartments or differences in physical properties of both fluids. In particular, how albumin is manufactured did not appear to be well understood by candidates.
2. Describe the consequence of mild hypothermia in the early post-operative setting
CICMWrecks Answer
- Temperature
- average kinetic energy of the atoms and molecules that make up a substance
- Mild hypothermia
- Temp <35 degrees
- Interthreshold range
- the range of core temperature over which no autonomic thermoregulatory responses occur.
- Under normal conditions: 36.8 ~37.2 degrees
- Under anaesthesia
- Lower threshold decreases 2.5 degrees and upper threshold increases 1.3 degrees
- Therefore decreased ability to maintain core normothermia
Physiology
Cardiovascular system
- Decreased cardiac output
- Peripheral vasoconstriction
- Increased afterload
- Decreased diastolic relaxation
- Decreased preload
- Decreased contractility
- Increased blood viscosity
- Haemoconcentration
- Cold diuresis: Due to decreased responsiveness to ADH and shunting of peripheral blood to central circulation
- Extravasation of intravascular H2O
- Haemoconcentration
- Increased risk of myocardial ischaemia
- Peripheral vasoconstriction
- Coagulopathy
- PT and aPTT increase 9% at 34c compared to 37c
- Increase incidence of atrial arrythmias → ventricular fibrillation at severe hypothermia
- Every degree decrease in temp increases pH 0.015 and decreases pCO2 4.4%
Respiratory
- Initially tachypnoea due to sympathetic stimulation
- Decreased responsiveness to CO2
- Oxyhaemoglobin dissociation curve shifts to left → decreased tissue O2 delivery
CNS
- CMRO2 decreases 7% per 1 degree decrease in core body temperature
GI
- Decreased GI motility
Other
- Post-operative shivering increases total body O2 consumption
- Decreased enzyme activity
- Decreased temperature decreases rate constant in Arrhenius equation
- Decreasesd neutrophil degranulation
- Impaired macrophage phagocytosis
Pharmacology
Pharmacodynamics
- Arrhenius equation for rate constant K
- Where T = temperature
- Therefore as T decreases, the rate constant decreases
- Drug-Receptor interactions are slowed as temperature decreases
- Where T = temperature
Pharmacokinetics
- Decreased absorption of drugs due to delayed gut transit times
- Metabolism of drugs reduced → slow offset of sedative agents given in anaesthesia
- Decreased cardiac output → decreased renal filtration à decreased drug elimination of unchanged drugs → slow offset
- Specific drugs
- Prolonged muscle relaxation from non-depolarizing
- Opioids blunt shivering thermogenesis mechanism → decreased ability to thermoregulate
Sakurai 2016
Examiner Comments
2015B 02: 35% of candidates passed this question.
A well organised answer would provide details on domains of physiology, pharmacology and “other”, such as patient centred effects. Few answers mentioned pharmacology. Providing a definition of “mild” was often overlooked but doing so assisted a focused answer. Many answers spread beyond “mild” hypothermia or address the “causes” rather than the consequence – neither of which gained marks as they were not answering the question asked.
3. Compare and contrast renal and hepatic blood flow, and their regulation
CICMWrecks Answer
Blood flow
| Renal | Hepatic | |
|---|---|---|
| Blood Flow | 1.25l/min Highest blood flow per tissue mass | 1.5l/min Highest blood flow per organ |
| Afferent | Renal artery (from aorta) | – Hepatic artery (30%) from coeliac trunk – Portal vein (70%) from SMV, IMV and splenic veins – Hepatic artery supplies 50% of O¬2 |
| Course | Portal system → Afferent arteriole → glomerulus → efferent arteriole → peritubular capillary (inc. vasa recta) → renal vein | Via portal triad → hepatic sinusoids → central vein → hepatic vein |
| Efferent | Renal vein to IVC | Hepatic vein to IVC |
| Liver has blood reservoir function – 400ml |
Regulation
| Renal | Hepatic | |
|---|---|---|
| Hepatic arterial tone above sBP >80mmHg | ||
| Local | Myogenic mechanism – Stretch of afferent arterioles causes contraction decreasing blood flow Tubuloglomerular feedback – Na and Cl are sensed by the macula densa causing adenosine release – Adenosine constricts the afferent arteriole reducing renal blood flow | Myogenic autoregulation – Stretch of arterioles causes contraction decreasing blood flow Semi-reciprocal relationship between portal venous and hepatic arterial blood flow – Decreased blood flow via portal vein increases hepatic arterial blood flow |
| External – Hormonal | RAAS – Juxtaglomerular apparatus secretes renin in response to decreased tubular [Na] and [Cl], as well as to β stimulation – Angiotensin II constricts both afferent and efferent arterioles reducing renal blood flow | Adrenaline – Constricts portal vein and dilates hepatic artery – increasing hepatic artery contribution to blood flow Angiotensin II – Vasoconstricts both portal vein and hepatic artery decreasing hepatic artery blood flow Vasoactive intestinal peptide and secretin – Increase hepatic artery blood flow Glucagon – Increased hepatic blood flow |
| External -Neural | – Sympathetic stimulation constricts the renal afferent and efferent arterioles reducing renal blood flow | – Sympathetic (α adrenergic) stimulation by noradrenaline causes portal venous constriction and decreased hepatic blood flow |
| External -Starling resistors | – Increased intra-abdominal pressure will decrease blood flow in starling resistor model – Increased intra-capsular pressure will decrease renal blood flow | Respiration – Inspiration decreases hepatic venous blood flow |
| External – dietary | High blood amino acid/ glucose level – vasodilation and increased renal blood flow | Post-prandial – Increased splanchnic blood flow – increased portal venous blood flow |
Examiner Comments
2015B 03: 54% of candidates passed this question.
It was expected candidates would describe the salient features of the anatomy, distribution and content of blood flow and influences on each circulation. Answers with a clear organisation and context for the normal influences of blood flow on the functioning of each organ system scored highly. Anatomy was often sufficiently covered, but candidates often did not take advantage of that by linking the anatomical features to the functional concepts. Figures should be clearly and accurately labelled to score well. Many answers failed to demonstrate a depth of understanding of key concepts. For example tubuloglomerular feedback, relationship between hepatic arterial and portal venous flows and autoregulation within both those systems was often poorly described.
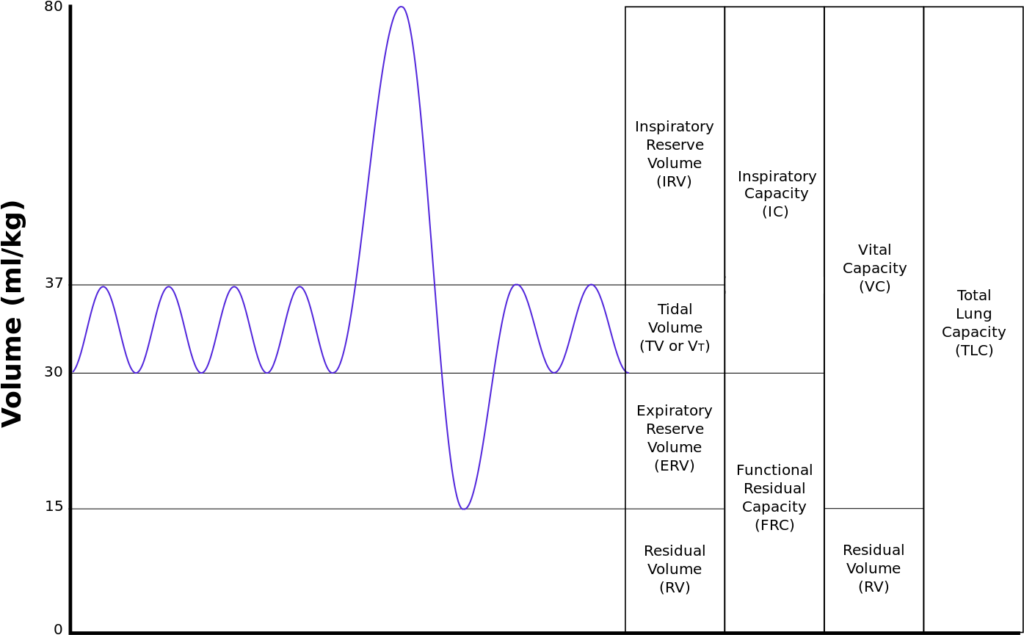
4. What is functional residual capacity and describe how it is measured
CICMWrecks Answer
Functional residual capacity
- Volume of air in lungs at the end of normal tidal expiration
- Occurs at equilibrium point between the recoil of chest/diaphragm and lungs to collapse inwards
- Sum of residual volume and expiratory reserve volume
- Normal FRC 30ml/kg (approx 2.2l in 70kg male)
- Functions:
- Oxygen reservoir
- PaO2 buffer
- Prevents atelectasis
- Minimizes work of breathing
- Lung kept at most compliant segment of compliance curve
- Decreases pulmonary vascular resistance
Measurement of FRC
- Cannot be directly measured by spirometry
- Body plethysmography
- Subject contained within glass tight box
- Breath against occluded airway
- Changes in alveolar pressure measured at mouth
- Boyle’s law used to calculate FRC
- Nitrogen wash-out technique
C1V1=C2V2- Initial alveolar concentration of nitrogen = 79% (if breathing room air)
- Subject breathes 100% O2
- Volume of total nitrogen exhaled measured (e.g 4L)
- FRC = 4L/79% = approx 5L
- Helium wash-in technique
C1V1=C2V2- Known volume and concentration of helium inhaled and allowed to
equlibrate but not diffuse into blood - Concentration and volume of exhaled helium measured and used to
calculate FRC, where
- Known volume and concentration of helium inhaled and allowed to
Sakurai / Gladwin / JC 2020
Examiner Comments
2015B 04: 25% of candidates passed this question.
This question requested a definition AND a description of measurement (one method if correctly discussed could and did generate a pass mark) although additional marks were awarded if multiple measurement methods were mentioned or described. Detailed descriptions of the factors effecting FRC and its functions were NOT requested and scored no marks. “Fowlers method” uses 100% oxygen and nitrogen analysis to calculate anatomical dead space – NOT FRC – so scored no marks. Both Helium dilution and nitrogen washout (with 100%oxygen) enable calculation of FRC using C1V1=C2V2 where V2 = V1+FRC. Body plethysmography requires more complex calculations of P1V1= P2V2 (Boyles Law) applied twice = for the box and then the lung. Few candidates had a clear understanding of this method. Most answers did not demonstrate the depth of understanding of the measurement techniques that was required to score highly
5. Explain the oxyhaemoglobin dissociation curve and the factors that may alter it
CICMWrecks Answer
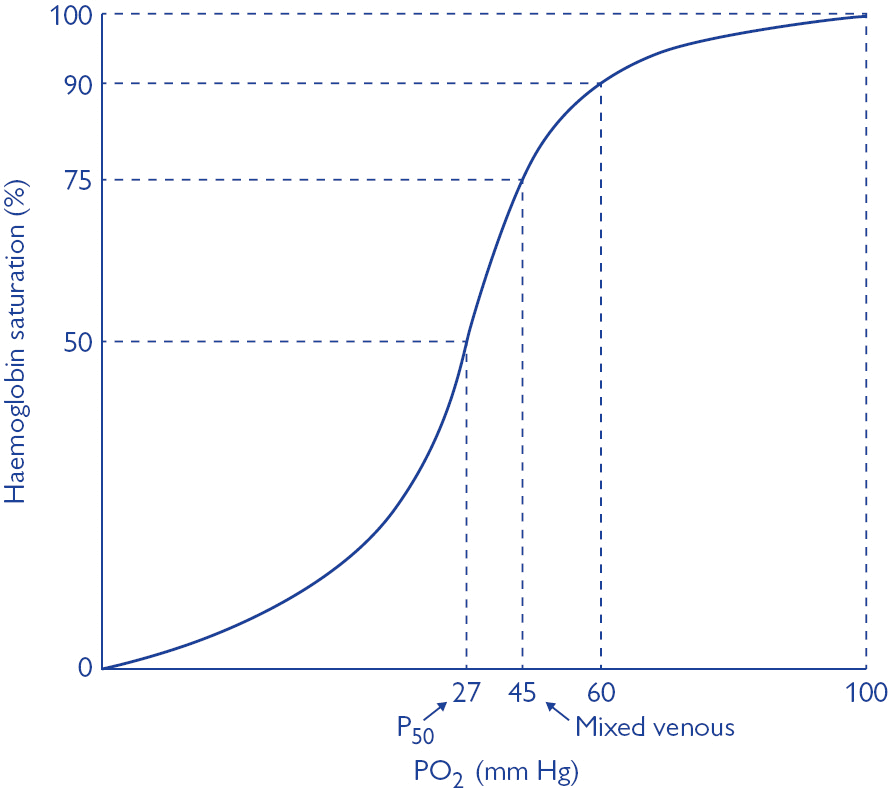
Oxyhaemoglobin dissociation curve
- OHDC describes the relationship between SaO2 and PaO2 at 37 degrees
- Demonstrates cooperative binding of Hb:
- ↑ in O2 affinity of Hb with each successive O2 binding
- 1st O2 molecule = difficult to bind – 2° strong electrostatic charges to be overcome to achieve conformational changes in Hb molecule: tense conformation → β chains far apart
- once 1st O2 has bound confirmation of Hb changes → β chains closer together → 2nd O2 has ↑ binding affinity → ↓ energy to bind
- 4th molecule binds 300 times more easily than 1st
- once 4th O2 bound → Hb in relaxed state
- The max amount of O2 that can be combined with Hb is the O2 capacity → all available binding sites are occupied by O2
- ↑ in O2 affinity of Hb with each successive O2 binding
- Importance of sigmoidal shape
- Upper portion is flat: if PO2 in alveolar gas ↓s slightly, loading of O2 will be little affected. I.e. a small ↓ in PO2 at normal O2 levels → causes only a slight ↓ in arterial saturation
- However, where O2 levels are already low, and on the steep part of the curve, the same small ↓ in PO2 will cause a sharp ↓ in SaO2
- Oxygen saturation of Hb = the % of the available binding sites that have O2 attached:
- (O2 combined wit Hb / O2 capacity) x 100
- In arterial blood, O2 sats are usually >97%. This corresponds to a PO2 of 100mmHg and is on the flat part of the curve
- In venous blood, the saturations are ~75%. This corresponds to the start of the steep part of the curve and a PO2 of ~40mmHg
Factors that may alter the OHDC
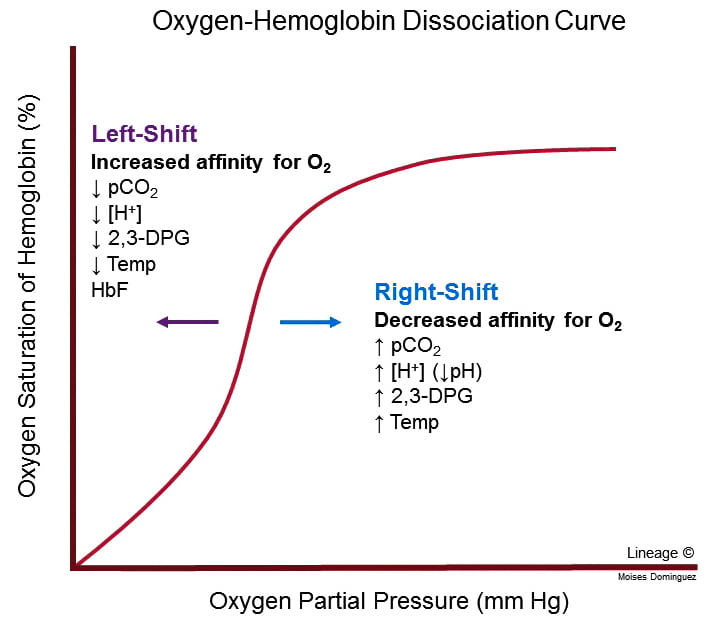
- Position of OHDC is described by P50 value = the PO2 at which 50% of Hb is bound to O2; corresponds to PaO2 of 26mmHg
- Right shift
- ↓ affinity of O2 for Hb → O2 ↑ easily offloaded (i.e. for a given PO2, SaO2 is lower)
- ensures ↑ tissue oxygenation in states of ↓ perfusion
- Causes: ↑ PCO2; acidosis; ↑ 2, 3, DPG; exercise; ↑ temp; HbS
- Bohr effect: ∆ PCO2 and pH affect O2 transport → ↑ PaCO2 and ↓ pH sabilises deoxyHb → facilitates release of O2 → right shift
- Left shift
- Left shift → ↑ O2 binding affinity
- Causes; ↓PaCO2; alkalosis; ↓2, 3, DPG; hypothermia; methaemoglobin; HbF
2,3 DPG
- Formed by RBC during glycolysis
- Binds to beta chains of deoxy-Hb
- DPG binds strongly with beta chains → changing protein conformation → ↓O2 affinity
- ↑2,3DPG → ↑unloading of O2 from Hb → ↑ tissue supply
- Factors ↑ DPG:
- High altitude: aclimatisation response
- Anaemia
- Alkalosis
- Chronic hypoxaemia
- Exercise
- Pregnancy
- Hyperthyroidism
- Factors ↓DPG
- Stored blood: DPG ↓ in stored blood: storage ↓ glycolysis (only issue with MTP); levels return to normal after 24-48hr
Kerr
Examiner Comments
2015B 05: 77% of candidates passed this question.
Marks were awarded for an appropriate curve with values, an explanation of the nature of positive cooperatively and notes on those factors causing changes in the p50 or “shifts” in the curve.
Most candidates were able to provide the required sigmoid shaped curve with appropriate key value points (p50, venous and arterial points). Better candidates were able to identify p50 as a measure of avidity or affinity for oxygen and commented on T- Tense and R- Relaxed states, the role and production of 2,3 DPG , binding to the beta chains (nature of the lack effect on foetal haemoglobin). Describing the mechanisms associated with factors shifting the curve and commenting on changes in oxygen content over the steep and flatter parts of the curves gained additional marks. Candidates are reminded to answer the question asked – no marks were awarded for a description of dissolved oxygen delivery. Some answers confused the Bohr and Haldane effects.
6. Describe the formation and the metabolic fate of lactate. Outline its role in energy production
CICMWrecks Answer
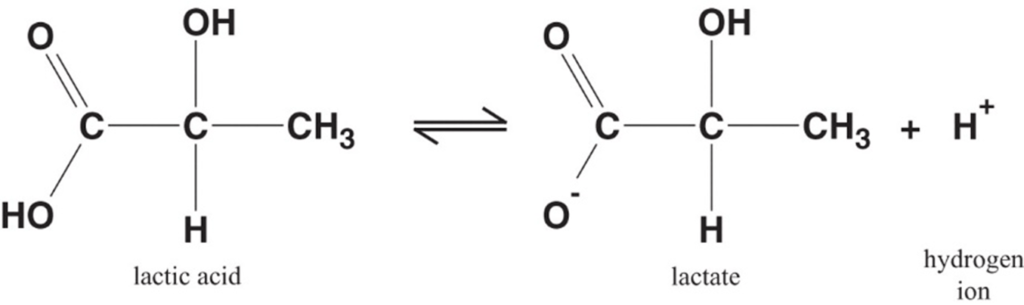
Lactate is the conjugate base of lactic acid (an organic 3-C acid)
Production:
- It is produced by anaerobic metabolism of pyruvate either:
- Physiologically → in RBC (no mitochondria), renal medulla (↓ PO2), cornea/ lens (↓ PO2) → hence, normal plasma [lactate] is 0.5-2 mmol/L (and NOT zero!)
- Pathologically → reduced tissue perfusion and/or O2 delivery (Eg. shock, hypoxaemia) → thus, plasma [lactate] ↑↑↑ (> 2 mmol/L)
- Plasma [lactate] is 0.5 – 2 mM (and NOT zero) due to physiological production → it can be measured clinically as an indicator of anaerobic metabolism (Ie. ↑ anaerobic metabolism due to pathological situations lead to ↑ [lactate])
Fate of lactate:
- Persistent anaerobic metabolism (Ie. ongoing hypoxia) causes an accumulation of cellular lactate → this diffuses out of the cell into plasma along its [ ] gradient → lactate can then be:
- Used as a fuel source by the heart and brain
- Transported to the liver where it is:
- Converted back to glucose via gluconeogenesis (requires 6x ATP), which is then transported back peripherally for use → “Cori cycle”
- Converted to pyruvate intermediate → utilised locally in TCA cycle for ATP production via oxidative phosphorylation
- Resolution of hypoxia (Ie. tissue O2 tension restored) → intracellular lactate can be oxidised back to pyruvate for use in local tissue aerobic metabolism (Ie. fed into TCA cycle)
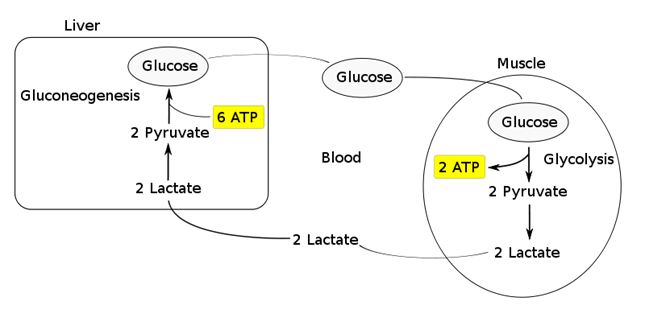
Functions of Lactate:
Lactate Sink:
- Lactate acts as a sink in heart, liver, muscle etc, allowing a period of ongoing ATP production from glycolysis when:
- cells become oxygen deplete
- Kreb’s cycle is inhibited
- Other causes of pyruvate accumulation: circulating catecholamines, exercise, sepsis or lack of mitochondria (RBCs)
Lactate Shuttle:
- Intracellular shuttle:
- Lactate may be shuttle out of:
- mitochoncrial membrane
- peroxisomes
- into cytoplasm of myocytes, neurons, astrocytes
- Ixydised by cytoplasmic LDH to pyruvate, generating NADH for energy use
- Lactate may be shuttle out of:
- Intercellular shuttle:
- Excess lactate, formed within fast-twitch fibres, is transported to other cells within the body with the oxidative capability to metabolize lactate, such as type I (slow-twitch) muscle cells, enhancing their excitability and limiting fatigue
- Furthermore, once in circulation, lactate attaches to red blood cells (RBCs) and is disassociated in the liver where inter-conversion via gluconeogenesis facilitates glucose formation, providing an alternative aerobic energy source.
Lactate as a signaling molecule:
- Redox signaling by intracellular shuttles
- Gene expression
- Increased intracellular levels of lactate can act as a signalling hormone, inducing changes in gene expression that will upregulate genes involved in lactate removal, and stimulates mitochondrial biogenesis.
- Control of lipolysis
- the shuttle regulates FFA mobilization by controlling plasma lactate levels.
- lactate functions to inhibit lipolysis in fat cells through activation of an orphan G-protein couple receptor (GPR81) that acts as a lactate sensor, inhibiting lipolysis in response to lactate
Bianca / JC 2021
Examiner Comments
2015B 06: 21% of candidates passed this question.
It was expected that the answer would include comments on lactate generation from glucose via pyruvate and the metabolic linkage of nicotinamide adenine dinucleotide (NAD). Lactate regenerates NAD+ (pyruvate is reduced to lactate while NADH is oxidized to NAD+ ). The citric acid cycle and the electron transport chain occur in the mitochondria of cells, and will only proceed in the presence of oxygen.
One molecule of glucose produces 2 ATP anaerobically (pyruvate to lactate) vs 26 aerobically (pyruvate enters TCA cycle) .Total production is about 1500 mmols/day with blood levels resting value of 1–1.5 mmol/L to a peak of 10–15 mmol/L.
Lactate can be used in 3 ways:
1. Conversion to glucose via gluconeogenesis in the liver and release back into circulation (Cori cycle). This is the fate of 80 % circulating lactate from tissues low in oxygen (e.g. exercising muscle with low pO2) or red blood cells (no mitochondria). The production from glucose in RBC’s is the Embden-Meyerhoff pathway.
2. Consumed as a fuel e.g. heart (20% of circulating lactate)
3. Mitochondria and oxygen Oxidation back to pyruvate by well-oxygenated muscle cells, heart cells, and brain cells pyruvate is then directly used to fuel the Krebs cycle (generating 28 mmols ATP)
Lactate generation from muscle is increased with B1 mediated stimulation e.g. from adrenalin. This topic is well covered in Power and Kam Principles of Physiology for the Anaesthetist, 3rd Edition, although some of the details are in several different sections.
Most candidates showed some understanding of the role of glucose in the production of pyruvate to lactate. However, the differential ATP production, the role of NADH availability and how oxygen and the role of mitochondria were involved was less well handled. Better answers described the normal generation of lactate in some tissues (e.g. RBC) and role of muscle and liver in metabolism back to glucose (Cori cycle) and the role of lactate as a metabolic substrate in some organs. Marks were awarded for normal production values and blood levels.
7. Draw and label a cross section of the lumbar epidural space (50% of marks). Describe the pharmacology of bupivacaine (50% of marks).
CICMWrecks Answer: Anatomy of LP
Anatomy:
- Position in sitting or lateral decubitus position
- Level:
- Any of the interspaces between between L2-L5
- L4/5 interspace: Tuffier’s Line: line between iliac crests
- (or) L3/4 interspace: Line joining PSISs (Posterior superior iliac spines)
- Discrepancy between identified and actual intervertebral space in 50% of cases
- Conus medullaris ends at L1 in about 94% of patients
Tissues and target for LP:
- In the subarachnoid space between the arachnoid mater and the pia mater.
- The tissues pierced are (in order):
- skin
- subcutaneous tissue
- supraspinal ligament,
- interspinal ligament,
- ligamentum flavum,
- dura mater,
- the arachnoid mater into the subarachnoid space.
- Lateral/paraspinal approach: Skin – subcutaneous tissue – erector spinae muscles – ligamentum flavum – dura – arachnoid – subarachnoid space
Epidural Space
- Posterolateral Epidural Space
- Posterolateral epidural space extends vertically down the spinal canal and contains arteries, venous plexus, and fat
- Posterolateral epidural space is larger than the anterior epidural space
- Posterolateral epidural space is larger in the sacral region than it is in the cervical region
- Anterior Epidural Space
- Anterior epidural space is a virtual space under normal circumstances (due to adherence of dura to bone of vertebral bodies from the foramen magnum down to L1)
Gladwin / JC 2020
CICMWrecks Answer: Pharmacology of Bupivacaine
Examiner Comments
2015B 07: 35% of candidates passed this question.
It was expected answers would include a diagram of a cross section and label the lumbar epidural space and the key landmarks namely dura, subarachnoid space, epidural space. Most candidates were able to give a schematic representation even if not being able to draw. Some candidates confused the subdural space with the epidural space. Pharmacology of bupivacaine needed to cover both pharmacokinetics and pharmacodynamics. Several candidates addressed only one of these components and so missed the opportunity to score marks.
8. Compare and contrast the physiological changes in the cardiovascular system in pregnancy at term and morbid obesity (BMI > 30).
CICMWrecks Answer
Pregnancy at Term
Morbid Obesity
- Pregnancy is a time of increased metabolic demand, which cardiovascular changes reflect.
- Changes begin from week 8 and ↑ to plateau at 32 weeks → return to normal 2-8 weeks post delivery
- excessive fat accumulation in adipose tissue
- WHO/NIH classification based on BMI:
- Overweight: BMI 25 – 30
- Obesity: BMI ≥ 30
- Obesity Class 1: 30 to 34.9 kg/m2
- Obesity Class 2: 35 to 39.9 kg/m2
- Obesity Class 3: BMI ≥40 kg/m2
- Alternate classifications:
- Severe Obesity: ≥ 35 or 40 kg/m2
- Morbidly Obese: BMI ≥ 40 (or) Obesity related disease and a BMI ≥ 35
- Super Obesity: ≥ 45 or 50 kg/m2
- Changes depend on stage of pregnancy
- Hormonal changes: ↑ circulating concentrations of oestrogen, progesterone, hCG
- ↑ metabolic demand esp. during labour: ~↑60% O2 consumption/ CO2 production during labour
- Mechanical effects from gravid uterus
- Changes depend on extent + duration of obesity
- Complex genetic and environmental causes
- Increased caloric intake
- Increased metabolic rate (normal for BSA)
- associated with HTN, HF, IHD, cardiomyopathy, sudden cardiac death, arrhythmias, PVD, DVT, CVD
- Thoracic changes:
- Anatomical compression of chest
- Diaphragm pushed upwards by 4cm
- ↑ AP + transverse diameter of chest wall (2-3cm)
- placental circulation: ↓pressure, ↓resistance AV shunt
- Aortocaval compression
- Collateral blood flow via collateral paravertebral epidural veins
- Compression of abdominal + leg vessels
- ↓ VR → supine hypotension + ↑ risk DVTs
- ↑ circulating concentrations of oestrogen, progesterone, hCG
- Oestrogen stimulation of RAAS
- Increased plasma volume (40% or 1~1.5L positive)
- Erythropoietin secretion
- Increased erythropoiesis and red blood cell volume (20%)
- ↑ SNS
- ↑ HR + ↑ SV
- ↑ RAAS → Na+ retention → ↑ blood vol → ↑ MAP (Systemic HTN)
- ↑ MAP → LVH → LV dilation → LV failure
- LV diastolic failure + ↑ PVR → RV hypertrophy
- ↑ Leptin → Cardiac remodelling + LVH
- Plasminogen Activator Inhibitor-1 → ↓ fibrinolysis → predisposes to VTE
- Inflammatory Adipokines → Impairs endothelial function → ↑ SVR
- insulin resistance + hyperlipidaemia → inflammatory mediator upregulation → disrupt endothelial function → IHD + cerebrovascular disease + PVD
- Anaemia of pregnancy
- Disproportionate plasma volume expansion relative to erythropoiesis
- Increased cardiac output (40%)
- Increased uterine blood flow (750ml/min)
- Increased renal blood flow
- Increased HR (25% by second trimester)
- Increased SV (25% in first trimester)
- Increased VO2 : Due to increased LBM and fat mass.
- Increased Cardiac Output: To maintain DO2.Initially with preserved ejection fraction
- Increased Stroke Volume: Due to:
- Increased preload (major factor)
- Increased contractility (minor factor) due to increased circulating adrenal hormones.
- Decreased peripheral vascular resistance (30%)
- Progesterone
- Prostaglandins
- Down-regulation of α receptors
- Decreased plasma oncotic pressure (15%) → peripheral oedema
- ↑ Peripheral vascular resistance
- Inflammatory Adipokines → Impairs endothelial function
- ↑ SNS
- Diastolic dysfunction: Due to myocardial fibrosis impairing relaxation.
- direct deposition of fat in myocardium → conduction disease (& predisposition to arrhythmias) + cardiomyopathy
- OSA
- Pulmonary HTN → cor pulmonale
- Polycythaemia → ↑ viscosity
Sakurai / Kerr / JC 2020
Examiner Comments
2015B 08: 2% of candidates passed this question.
The question was very specific for the cardiovascular system and therefore answers that
described respiratory changes and airway modulation failed to score marks. This answer leant itself to a tabular format. Candidates are reminded to ensure they document the facts in the correct column i.e. obesity facts in the obesity column. The cardiovascular changes associated with term pregnancy are well described in various texts. Those associated with morbid obesity required some integration from various sources and would include a structured series of comments such as heart rate (unchanged), blood pressure (tendency for hypertension), stroke volume (increased), cardiac output (increased), blood volume (increased – although perhaps decreased on a ml/kg basis), systolic function (preserved or increased), LV wall thickness increased. The pathological changes seen with the diseases associated with obesity are difficult to tease out and better answers identified this. Morbid obesity has a specific definition and
stating this aided focus of the answers.
9. Describe the principles of measurement of end-tidal CO2, including the sources of error.
CICMWrecks Answer
Principles
- Beer Lambert law: At a given wavelength, the amount of infrared radiation absorbed by gas is proportional to the concentration of gas present
- CO2 is a heteronucleic molecule, and so absorbs infra-red light
- Infrared light shone across a sample of gas
- Narrow band light emitted from infrared source
- Band frequency chosen which fits the peak absorption frequency of CO2
- 4.23micrometres
- Shone across gas, and absorbed at a detector
- Detector emits signal to analyser -> screen
- CO2 reading outputted is inversely proportional to CO2 present in sample, as per Beer-Lambert Law
- Narrow band light emitted from infrared source
- Gas can be sampled at the patient within the ventilation circuit (in-line), or in a sample of gas diverted away to a separate analysing chamber (sidestream)
Sources of error
- Sampling
- Entrainment of atmospheric gas if leak in sidestream line
- Occlusion of sidestream line causes loss of gas sampling
- Water condensation absorbs IR light -> erroneously high ETCO2
- Modern capnography includes a water trap and heater (to reduce condensation)
- Calibration
- Incorrect calibration of analyser
- Interference
- Other gases (notably N2O) have a similar absorption spectrum
- Presence may falsely elevate measured ETCO2 (esp. if infrared frequency band too broad)
- Presence of other gases causes ‘collision broadening’
- Absorption spectrum of CO2 is broadened
- Pressure
- Partial pressure, rather than percentage composition, is measured
- If pressure ↓ (e.g. by suction drawing gas into sampling chamber), erroneously low measured ETCO2
- Sampling chamber
- If too large, mixing of gas between respiratory cycles -> compression of waveform and erroneously low measured ETCO2
Mooney 2016
Examiner Comments
2015B 09: 19% of candidates passed this question.
Candidates were expected to be detail the principles required to measure carbon dioxide in expired gas. This would involve some comment on ways to sample end tidal gas (in line vs side stream) and also ways to measure carbon dioxide. It was expected candidates could provide a detailed description of infrared analysis including the apparatus design and principles such as the asymmetric nature of CO2 as a polyatomic gas allowing absorption if infrared radiation with some discussion of response times and equipment design. It was expected these principles would be related to the potential errors (e.g. other gases, collision broadening)
10. Describe the pharmacology of magnesium sulphate.
Examiner Comments
2015B 10: 27% of candidates passed this question.
The standard “pharmacology template” approach would have served well to cover this question. Answers were generally lacking in detail and focussed on extraneous physiology rather than pharmacology. Toxicity and side-effects were important to emphasise, especially in the context of infusions for treatment of asthma and/or pre-eclampsia
11. Compare and contrast the anatomy and physiology of skeletal and smooth muscle.
CICMWrecks Answer
Anatomy
| Skeletal Muscle | Smooth Muscle |
|---|---|
| Long, cylindrical, multi-nucleated cells | Spindle shaped single-nucleated cells |
| 10 μm diameter 100 μm long | 2 μm diameter 400 μm long |
| Striated | Not striated |
| Actin and Myosin arranged in sarcomeres | More actin than myosin Actin inserts into dense bodies and cell membrane |
| Well developed sarcoplasmic reticulum and transverse tubules | Poorly developed sarcoplasmic reticulum No Transverse tubules |
| Containes troponin in the thin filaments | Contains calmodulin → binds to Ca2+ → activates myosin light-chain kinase |
| Ca2+ released from cytoplasm from sarcoplasmic reticulum | Ca2+ enters cytoplasm from extracellulr fluid, sarcoplasmic reticulum and mitochondria |
| Cannot contract without nerve stimulation | Maintains tone in absence of nerve stimulation Visceral smooth muscle produces pacemaker potentials |
| Denervation results in muscle atrophy | denervation results in hypersensitivity to stimulation |
| Muscle fibers stimulated independently No gap junctions | Gap junctions usually present |
| Two Types 1. Extrafusal (α-motor neuron supply) a) Slow-type (tonic) b) Fast-type (twitch) – Slow fatigue (type 1/red – slow oxidative) – Fast fatigue-resistant (type 2B/red – fast oxidative) – Fast fatiguable (type 2B/white) 2. Intrafusal (γ-motor neuron supply) – Non-contractile sensory fibers associated with muscle spindles | Two types 1. Visceral – located in hollow viscera – linked in sheets by gap junctions – contractile elements extend across multiple cells – Spontaneous depolarisation 2. Multiunit – located in eye (iris and ciliary body), large blood vessels, small airways, hair follicles – No gap junctions – Under ANS control without spontanaetiy |
Physiology
| Skeletal Muscle | Smooth Muscle |
|---|---|
| Excitation | |
| – Motor neuron depolarisation – ACh vesicular release – Multiple MEPs → End plate potential – Muscle AP propagates via T-tubles – Opens L-type Ca channels – Calcium induced calcium release from SR – Ca-troponin C interaction – Alteration and movement of Trop-tropomyocin complex – Exposure of Actin – Removal of Troponin I inhibition of myosin ATPase – Cross bridge cycling | – Motor neuron depolarisation – ACh vesicular release – Multiple MEPs → End plate potential – Muscle AP propagates – Opens L-type Ca channels |
| Contraction | |
| Sliding filament cross bridge cycling – “Flexed” Myosin without ATP binds to Actin. – Binding of ATP to Myosin causes release of Actin/Myosin complex and extension of the head – In the presence of Ca, Actin binding sites are open and binding of myosin/Actin/ATP complex is formed – Hydrolysis of ATP to ADP + P in myosin head causes a conformational change “initial flexing” the myosin head and releasing phosphate – Release of ADP further flexes Myosin head – In presence of Ca return to step 2. | – Influx of EC Ca → ↑ IC [Ca] – Ca binds Calmodulin → Ca-Calmodulin complex – Ca-Calmod → ↑ MLCK activity → – Phosphorylation of Myosin Light chain (MLC) → Activation of Myosin ATPase – Normal Cross-bridge cycling as per Skeletal muscle cross bridge cycling – Latch-bridging (Sustained SM contraction with minimal O₂ or ATP use) ◦ ↓ IC [Ca] + dephosphorylation of MLC does not cause unbinding of actin/myosin ◦ Unbinding occurs slowly |
| Relaxation | |
| – AChE metabolises ACh in the NMJ ◦ ↓ nAChR activation at MEP → ↓ EPP and muscle AP generation – SR actively sequesters Ca (via Ca-Mg ATPase) ◦ ↓ IC [Ca] → Ca removed from troponin C ◦ tropomyosin mediated inhibition of actin-myosin – Titin ◦ Elastic component of sarcomere → ↓ to normal length | – Ca removed from cell ◦ Ca ATPase ◦ Ca2+/Na+ antiport – Myosin Light Chain Phosphatase ◦ dephosphorylates MLC → inhibition of myosin ATPase |
Gladwin / JC 2020
Examiner Comments
2015B 11: 23% of candidates passed this question.
It was expected answers would describe in detail the role of troponin, tropomyosin and calmodulin in mediating muscle contraction. Detail on the structure (histology) of the skeletal and smooth muscle cells was often lacking. Many answers omitted the mechanism of muscle relaxation.
12. Define “volume of distribution” and describe the factors that influence it.
CICMWrecks Answer
Definition
Volume of Distribution (Vd) = The theoretical volume of bodily fluid in which a drug would need to be distributed following its administration to produce the observed / desired plasma concentration
Mathematically, at time t=0:
Factors Influencing Volume of Distribution
Drug factors
- ↑ Plasma protein binding
- ↓’s Vd
- ↑ Tissue binding
- Binds to tissues → ↓ circulating drug → apparent ↑ Vd
- Physicochemical properties of drug
- ↑ Lipophilicity → ↑ Vd
- ↑ Hydrophilicity → ↓Vd
- ↓ Size → ↑ Vd
- ↓ Charge → ↑ Vd
- pKa
- Basic drug → lower pKa = ↑ unionised portion → ↑ Vd
- Acidic drug → higher pKa = ↑ unionised portion → ↑ Vd
Patient factors
- ↓ pH → ↑’d ionisation of basic drugs → ↓ diffusion → ↓ Vd
- Blood flow
- ↑ blood flow → ↓d concentration → ↑ Vd
- Age
- ↑ Vd in neonates
- Total body water ↑ with
- ↓ age (neonates highest)
- Male gender
- ↑ volume of distribution for water soluble drugs.
- total body fat
- ↓’d in children and men → ↓ Vd of lipophillic drugs
- skeletal muscle bulk
- ↑’d in men → ↑ Vd
- disease
- Sepsis: alterations in pH alter ionised and bound fractions of drugs, increased permeability, organ failure and acute phase reactants all alter protein binding and Vd.
Measurement of Volume of Distribution
It is calculated based on the formula:
Since it is impossible to measure plasma concentration at time zero, an extrapolated plasma concentration is used.
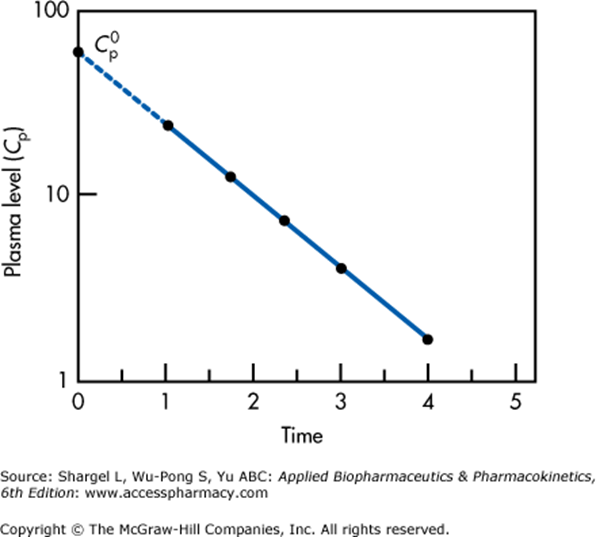
Semilogarithmic plot above illustrates extrapolation of plasma level to time 0 required to determine the volume of distribution
This graph applied for a single compartment model only.
For multiple compartments which will appear as a non-linear relationship, extrapolation back to t = 0 must be performed for each compartment separately.
Gladwin / JC 2019
Examiner Comments
2015B 12: 35% of candidates passed this question.
A definition was required that included reference to plasma concentration, total body content / dose, and the theoretical nature of the volume which can exceed the physical volume of the body.
A broad answer listing patient and drug-related factors was required. Patient factors could include age, gender, muscle mass, fat mass and abnormal fluid distribution (oedema, ascites, pleural effusion). The drug factors would include tissue binding, plasma protein binding and physicochemical properties of drug (size, charge, pKa, lipid solubility, water solubility).
13. Describe the control of alveolar ventilation.
CICMWrecks Answer
Respiration
- Normal RR ~ 10 breaths per minute
- Normal tidal volume ~ 500ml/kg (in 70kg male)
- Therefore normal minute ventilation approx 5l/min
Afferents / Sensors
Chemoreceptors
- Central
Located in retrotrapezoidal nucleus- Sensitive to changes in CSF [H+]
- CO2 readily diffuses across BBB and converted to H+ and HCO3– via carbonic anhydrase
- Increased [H+] (and therefore CO2) stimulated respiration
- Sensitive to changes in CSF [H+]
- Peripheral
Located in aortic bodies (innervated by vagus nerve) and carotid bodies (innervated by glossopharyngeal nerve)- Sensitive to PO2, [H+], PCO2 and blood flow
- O2 dependent K+ channels
- Respiration increases as O2 drops below 50mmHg
- O2 dependent K+ channels
- CO2
- Linear increase in respiration as CO2 increases
- Sensitive to PO2, [H+], PCO2 and blood flow
Baroreceptors:
- Located in aortic arch and carotid sinuses
- Responds to stretch
- As MAP drops → less stretch on vessel walls → increased sympathetic outflow from medullary vasomotor centre → triggers increase in respiration
- Responds to stretch
Pulmonary receptors
- Stretch receptors
- Increased stretch of pulmonary parenchyma triggers inflation reflex → inhibits inspiration to prevent overdistention
- Collapse of pulmonary parenchyma triggers deflation reflex → inhibits expiration to prevent atelectasis and loss of FRC
- J fibres
- Nociceptive mechano-chemoreceptors
- On stimulation → Bronchospasm, apnoea, bradycardia and hypotension
- Nociceptive mechano-chemoreceptors
Others
- Joint and muscle receptors stimulate ventilation
- Pain and temperature sensation can alter ventilation via the cortex and limbic system
Central control of breathing
Central input
- There is input from the hypothalamus and cortex, with the ability of the cortex to override the medulla and bring ventilation under voluntary control
Controller
- Medullary Respiratory Centre
- Dorsal respiratory group (DRG)
- Located in and adjacent to Nucleus Tractus Solitarus
- Associated with inspiration and timing
- Works as an ‘integrating centre’ with VRG
- Ventral respiratory group (VRG)
- Including Pre-Botzinger and Botzinger complex → Central Pattern Generator
- Associated with control of expiration, airway dilation and central pattern generation
- sends inhibitory impulses to Apneustic centre
- Dorsal respiratory group (DRG)
- Pontine respiratory group (PRG)
- Pneumotaxic centre
- controls both the rate and the pattern of breathing
- Sends inhibitory impulses to the inspiratory area
- Antagonist to apneustic center
- decreases tidal volume
- Apneustic centre:
- sends signals for inspiration for long and deep breaths
- controls the intensity of breathing and is inhibited by the stretch receptors of the pulmonary muscles at maximum depth of inspiration, or by signals from the pnuemotaxic center
- increases tidal volume.
- Pneumotaxic centre
- Inspiratory phase:
- Gradual ramping up of nerve activity – ↑muscle contraction
- Expiratory phase I:
- Gradual reduction of nerve activity – ↓muscle contraction
- Expiratory phase II:
- Inspiratory muscles inactive
- If increased respiratory drive, expiratory muscles are activated
Efferents
- Phrenic nerve (C3, 4, 5) → Innervates diaphragm – Main inspiratory muscle
- Spinal nerves to intercostal muscles (external intercostal → inspiration, internal intercostal → expiration)
- Brachial plexus → Pectoralis major (forced breathing inspiration)
- Accessory muscle → Sternocleidomastoid (forced breathing inspiration)
- Spinal nerves to abdominal muscles (forced expiration)
Mooney / Sakurai / JC 2020
Examiner Comments
2015B 13: 42% of candidates passed this question.
The most comprehensive answers were those structured as sensor-controller-effector with an explanation of each part and how homeostasis was maintained. Insufficient detail was generally provided as to how central and peripheral chemoreceptors were stimulated. A description of central control was required, rather than listing nuclei or areas. Many failed to address all three components of a control question and focused primarily on the sensors. Many answers were just too brief and did not present enough information to demonstrate understanding.
14. Describe the pharmacology of propofol
Examiner Comments
2015B 14: 60% of candidates passed this question.
Those candidates who did poorly lacked any structure for answering a pharmacology question.
Pharmacokinetics was generally poorly handled and many answers revealed a lack of knowledge about this drug. Adverse effects and mechanism of action were generally well known. Doses of the drug were often incorrectly stated.
Important aspects such as dose or pharmacodynamics were often omitted and a structured approach helps avoid this
15. Define cardiac preload and describe its determinants
CICMWrecks Answer
Definitions of Preload:
- The mean tension in the ventricular fibres or the initial myocardial fibre length prior to contraction.
- Mathematically:
- where:
- ITP = intrathoracic pressure; LVEDP = left ventricular end-diastolic pressure; LVEDR = left ventricular end-diastolic radius (at midpoint of ventricle); h = thickness of ventricle
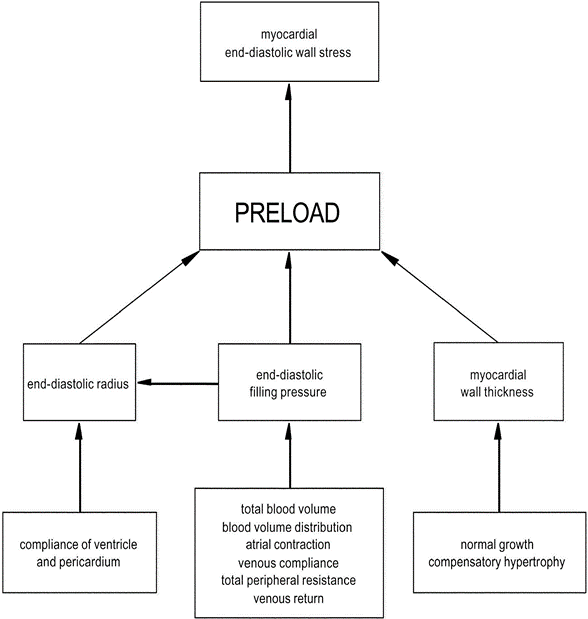
Factors determining Preload
1. LaPlace Factors
- Extra-cardiac: End-diastolic filling pressure
- CVP
- Venous compliance
- Thoracic Blood Volume
- Total Blood Volume
- ↑ TBV → ↑MSFP → ↑ CVP → ↑ preload.
- Blood Volume distribution
- Total Blood Volume
- Venous return (VR)
- ↑VR → ↑ CVP → ↑ preload
- Afterload factors
- ↑Total peripheral resistance → ↓ VR → ↓ Preload
- Compression
- Pericardial effusion/tamponade → ↓ ventricular filling
- PEEP → ↑ ITP → ↓ VR
- Aortic pressures
- ↑ afterload (or raised aortic pressure) → ↓ SV → ↑ ESV → ↑ EDV (or ventricular preload).
- CVP
- Intra-cardiac:
- End-diastolic radius
- Ventricular and Pericardial compliance
- ↑ compliance → ↑ filling for constant pressure → ↑preload
- Atrial contraction/coordination
- ↑ atrial contraction/contractility → ↑ preload
- eg ↑ SNS stimulation / fluid bolus
- ↓ atrial contractile force/coordination in AF
- ↑ atrial contraction/contractility → ↑ preload
- HR: ↓ HR → ↑ filling time → ↑ preload
- Myocardial Wall thickness
- ↑Normal Growth → ↓ compliance and ↑ thickness (h) → ↓ Preload
- ↑Compensatory hypertrophy
- End-diastolic radius
2. Venous Return Factors (↑/↓ VR → ↑/↓ Preload)
- Venous Factors
- ↓Venomotor tone → ↓ VR
- Venous Values prevent retrograde flow → failure → ↓VR
- Pump Factors
- Skeletal Muscle pump/Respiratory pump both ↑ VR
- Inspiration
- → ↓ ITP → ↓ RAP → ↑VR
- → Diaphragmatic contraction → ↑IAP → ↑VR
- Inspiration
- Effect of ventricular contraction and relaxation
- → Systolic Rapid ejection phase → ↓RAP → ↑VR
- → Early diastole → Rapid vetricular filling (open AV valve) → ↑VR
- Skeletal Muscle pump/Respiratory pump both ↑ VR
- Posture
- Intrapericardial pressure (tamponade)
- Afterload
- ↑ Arteriolar tone → ↑ Resistance to VR → ↓ VR
3. Pathological Factors
- ↑ preload
- Ventricular systolic failure
- Outflow valve stenosis or regurgitation (AS or AR),
- Inflow valve regurgitation (MR/TR)
- ↓ preload
- Ventricular diastolic failure,
- Inflow valve stenosis (MS/TS)
Gladwin 2015
Examiner Comments
2015B 15: 29% of candidates passed this question.
This question required synthesis and application of knowledge derived from multiple sources rather than regurgitation of a published list in a text. Many candidates failed to recognise that venous return is not the only determinant of preload. Most candidates failed to discuss determinants of venous return. Factors such as contractility, afterload or chamber filling and emptying can all impact preload. In addition to listing determinants the question required an explanation of their relationship with preload (e.g. the direction of change).
A discussion about the determinants of cardiac output was not asked for as did not score marks.
16. Describe ammonia metabolism and excretion (70% of marks). Outline the pharmacology of lactulose (30% of marks).
CICMWrecks Answer
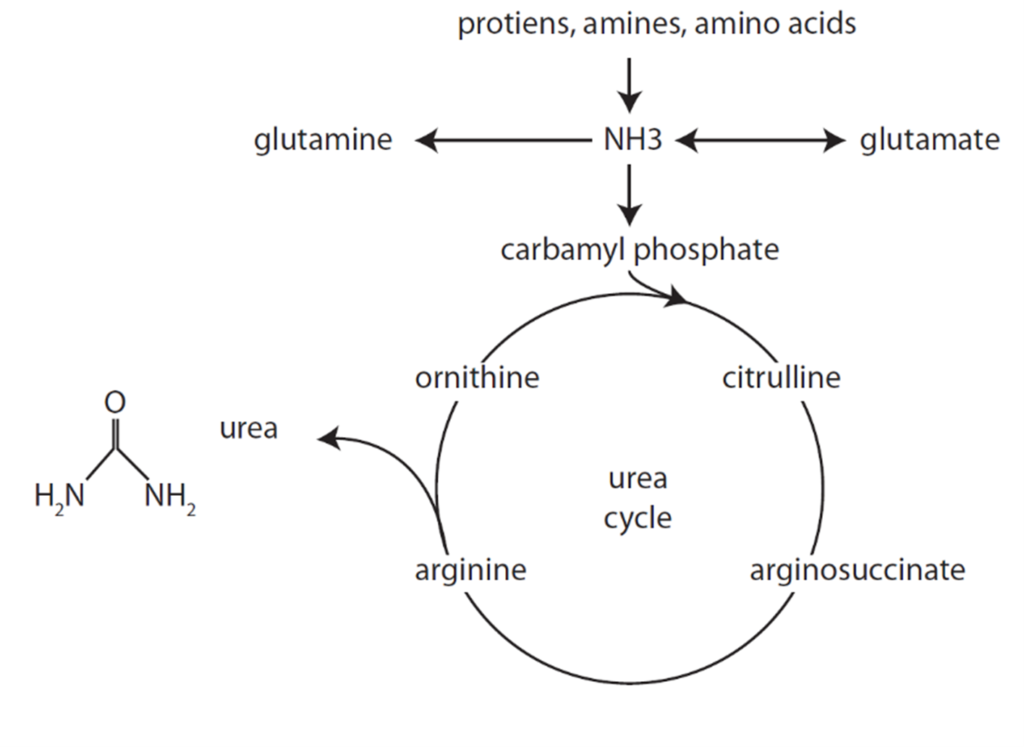
Formation of Urea from Ammonia
- Liberation of ammonia from AAs
- Formation of carbamoyl phosphate from NH3
- Reactions of the urea cycle
Urea Cycle
- Ammonia carried in blood from tissues to liver mitochondria by glutamate as amino groups.
- Hepatocyte Mitochondria
- 2ATP+CO2+NH3 + Carbamoyl Phosphate → citrulline using enzyme Carbamoyl phosphate synthetase 1
- Hepatocyte Cytosol
- Citrulline + ATP + H2O → ornathine + urea
- Urea Fate:
- Enterohepatic circulation (25%)
- Excreted by kidneys (75%).
- Ornathine reenters the mitochondria and is combined with new carbamoyl phosphate to form citrulline
Excretion of ammonia by Kidney
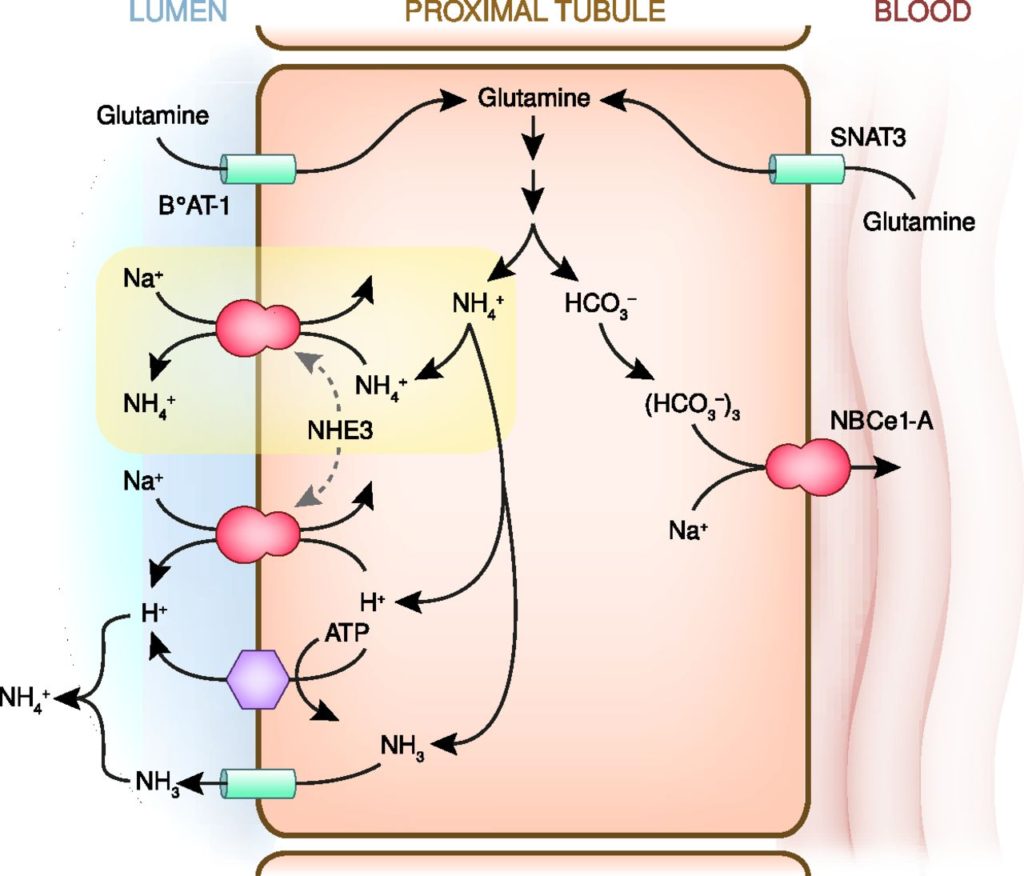
- Ammoniagenesis:
- Glutamine enters the peritubular cells of the PCT
- 80% from the peritubular capillaries
- 20% from the filtrate, reabsorbed by PCT
- Deaminated via Glutaminase (increased activity in acidosis)
- HCO3 reabsorbed into peritubular capillary blood
- NH4 excreted via Na/NH4 antiporter
- Glutamine enters the peritubular cells of the PCT
- Ammonium cycling:
- 80% reabsorbed TAL of LoH via replacing K on Na/K/2Cl cotransporter
- Increased conc in medullary intersitium
- Diffuses down conc grad into CD
- Buffer ability can be utilised to excrete H.
Pharmacology of Lactulose:
| PC | Non-absorbable synthetic, non-digestible disaccharide. osmotic laxative |
| PD | MOA: ↑ intraluminal osmotic pressure → retention of water in stool Abdminal cramping and flatulance |
| PK | A: Not absorbed (PO BA 0%) D: Not applicable M: fermented by gut flora producing metabolites (such as acetate) which have osmotic and peristalsis-stimulating effects E: Faecal |
Gladwin 2016
Examiner Comments
2015B 16: 37.5% of candidates passed this question.
It was expected candidates would identify sources of ammonia (colon from metabolism of proteins, kidney, small amounts from breakdown of red blood cells and metabolism in muscles). The liver converts all circulating ammonia to urea (the urea cycle) (2NH3+CO2 = urea +H2O). Urea then undergoes enterohepatic circulation (25%) or is excreted by kidneys (75%). Ammonia (NH3) is lipid soluble and diffuses into the interstitial cell and tubular fluid by non-ionic diffusion where is buffers H+ to become non diffusible NH4+. No candidate mentioned enterohepatic circulation and most answers had very little detail on the metabolism and excretion and lacked depth.
Lactulose is a non absorbable synthetic, non-digestible disaccharide. It is an osmotic laxative fermented by gut flora producing metabolites (such as acetate) which have osmotic and peristalsis-stimulating effects, and methane causing in flatulence. Few could describe how lactulose decrease absorption of ammonia and a surprising number of people did not even state that lactulose was an osmotic laxative.
17. Statistics (not in current primary syllabus)
18. Describe the stages of sleep (50%) Describe the respiratory physiological changes that occur in sleep (50%)
CICMWrecks Answer
Stages of sleep
- Slow-wave
- Deep, restful sleep occuring during first hour
- Stage I
- As person drifts to sleep
- α waves replaced by theta waves of low frequency (4~8Hz)
- Stage II
- Occasional bursts of high frequency waves (sleep spindles)
- Stage III
- High voltage, low frequency (1~2Hz) δwaves with bursts of high frequency K complexes
- Stage IV
- Deepest sleep
- High voltage δ waves
- REM
- Rapid Eye Movements
- Occurs every 90 minutes and lasts 30 minutes
- β brain waves resembling alert wakefulness
- Rapid Eye Movements
Respiratory changes during sleep
- Tidal volume
- Declines progressively as slow wave sleep progresses → minimal in REM sleep
- Vt 25% of awake volume during REM sleep
- Respiratory Rate
- Remains relatively unchanged
- Irregular pattern during REM sleep
- Response to pO2 and pCO2 dampened
- PaCO2 increases 3mmHg
- PaO2 decreases 3mmHg
- Upper airway resistance
- Increased
- Especially in REM sleep
- At hypopharynx and soft palate
- Loss of tonic activity of tensor palati
- Increased
Sakurai 2016
Examiner Comments
2015B 18: 29% of candidates passed this question.
Few candidates demonstrated a good knowledge of this topic. Few answers described the EEG changes associated with the stages of sleep. Respiratory changes in sleep were more commonly known though many candidates made no reference to the change in resistance associated with reduction in upper airway tone.
Confusion existed about the tidal volume changes in sleep. The question asked specifically for respiratory changes and marks were not awarded for discussion about cardiovascular or metabolic responses.
19. Describe the fibrinolytic pathway and identify areas of interaction with the coagulation pathway (80% of marks). List two anti-fibrinolytic agents and state their specific mechanism of action (20% of marks).
CICMWrecks Answer: Fibrinolysis
Fibrinolysis
- Process by which fibrin clot is degraded to prevent excessive clot formation
- Plasminogen incorporated into clot
- Injured tissues and vascular endothelium gradually produce Tissue Plasminogen Activator (t-PA) in a delayed manner.
- Urokinase is produced by monocytes, macrophages and urinary epithelium
- Factor XII (Hageman factor) acts as a weak activator of plasminogen
- Plasminogen activated by t-PA, u-PA and factor XII to plasmin, a serine protease
- Plasmin cleaves fibrin into fibrin degradation products → reducing clot stability → Clot breakdown
Regulation of fibrinolysis
- Plasmin Activator Inhibitor (PAI) 1 & 2 – produced by vascular endothelium and inhibits tPA and uPA
- Protein C inactivates PAI, TAFI
- Thrombomodulin – expressed on in tact endothelium – binds thrombin and subsequently activates protein C
- Thrombin Activatable Fibrinolysis Inhibitor – produced by liver and cleaves plasmin binding sites on fibrin → decreased degradation
- α2 anti-plasmin – produced by liver, inhibits circulating plasmin, not fibrin bound plasmin
Sakurai 2016
CICMWrecks Answer: Anti-fibrinolytic agents
Anti-Fibrinolytic Agents
- Aprotinin
- Proteolytic enzyme inhibitor that acts on trypsin, plasmin and tissue kallikrein → forms reversible enzyme-inhibitor complex that inactivates free plasmin → ↓ clot lysis
- Tranexamic acid:
- Competitive inhibition of plasminogen conversion to active plasmin → ↓ fibrin (clot) lysis
- Aminocaproic acid
- Forms reversible complex with plasminogen → ↓ conversion to active plasmin → ↓ fibrin (clot) lysis
Sakurai 2016
Examiner Comments
2015B 19: 8% of candidates passed this question.
The fibrinolytic pathway is a cascade largely made up of proteolytic enzmes and other factors synthesized in the liver that circulate in inactive precursor forms. Marks were awarded for description of the principal members of the cascade and the pathway relations between them. Endothelium is also important in the fibrinolytic pathway.
Regulation of the pathway to localise the site and size of clot as well as delayed onset of action of fibrinolysis is central to any description. Regulation of fibrinolysis by the coagulation cascade and a description of this area of interaction were expected.
Many candidates provided a reasonable description of the fibrinolytic cascade. Marks were not awarded for description of the coagulation cascade that did not have relevance to fibrinolysis. Understanding of regulation of fibrinolysis and it’s interaction with coagulation was poorly answered.
Most candidates were able to name two antifibrinolytic agents. Few were able to describe mechanism of action.
20. Compare and contrast the pharmacology of aspirin and clopidogrel
Examiner Comments
2015B 20: 46% of candidates passed this question.
Both agents are principally used as anti-platelet agents. Aspirin however has wider clinical applications. Mechanism of action of both agents involves irreversible inhibitions of enzymes and/or receptors. The inability of platelets to regenerate these means that physiological effects can not be fully explained by pharmacodynamics or pharmacokinetics alone.
Candidates who followed a traditional template for pharmacology answers scored better, providing answers that covered the breath of the topic.
21. Outline the anatomy of the diaphragm (70%) Describe the function of the diaphragm in respiration (30%)
CICMWrecks Answer
Anatomy of the Diaphragm
- Insertion
- Anterior
- Xiphoid process
- Costal cartilage of ribs 6~12
- Posterior
- Lateral arcuate ligament (overlies quadratus lumborum)
- Medial arcuate ligament (overlies psoas major)
- Right and left crux (blends with anterior longitudinal ligament from vertebral bodies L1~3 on right, L1~2 on left)
- Median arcuate ligament (between right and left crux, overlying aorta)
- Central tendon – blends with pericardium superiorly and fibrous capsule of liver inferiorly
- Anterior
- Openings
- Caval orifice (T8) – Within central tendon – Passage of the IVC
- Oesophageal hiatus (T10) – Within sling of right crura – Passage of oesophagus and vagus nerve
- Aortic hiatus (T12) – Posterior to median arcuate ligament between right and left crura – Passage of aorta, azygous vein and thoracic duct
- Innervation
- Motor – Phrenic nerve (C3~5)
- Sensory – Phrenic and inferior intercostal nerves
- Blood supply
- Branches of the lower intercostal arteries, superior phrenic arteries
- Inferior phrenic artery (branch of aorta) from inferior surface
Function of the Diaphragm
- On inspiration
- Diaphragm contracts
- Dome of diaphragm flattens and descends
- Pushes lower thoracic ribs outwards
- Increases AP diameter and superior-inferior distance of thorax → increased thoracic volume → Negative intrathoracic pressure → inspiration of air
- Caval orifice dilates → increases venous return (Liver compressed squeezing hepatic blood reservoir, increased intraabdominal pressure due to descending diaphragm)
- Aortic hiatus constricts → prevents arterial blood regurgitation
- Oesophageal hiatus constricts → aids lower oesophageal sphincter and prevents regurgitation of gastric contents
- Diaphragm contracts
- On expiration
- Diaphragm relaxes
- Elastic recoil of lung causes deflation and expiration
- Diaphragm relaxes
Sakurai 2016
Examiner Comments
2015B 21: 27% of candidates passed this question.
The diaphragm is the principal muscle of respiration. Important and unique features of its anatomy include a central tendon that blends with the pericardium above and the fibrous capsule of the liver below, arcuate ligaments and crura that are important points of muscle insertion. There are also three major and three minor openings that allow passage of structures between the thoracic and abdominal cavities. Candidates who had studied anatomy of the diaphragm were clearly distinguishable from those who had not.
Candidates who followed a traditional template for anatomy answers scored better, providing answers that covered the breath of the topic.
22. Describe the counter-current mechanisms in the kidney (60% of marks) and in the skin (40% of marks).
CICMWrecks Answer – Kidney counter-current
Medullary concentrating gradient
- physiological process which sets up a concentration gradient from cortex through to medulla
- allows formation of concentrated urine
- Mechanisms:
- Counter current Multiplier: creates concentrated medullary interstitium
- Counter current Exchange (vasa recta): maintains intersisital osmotic gradient
- Recycling of urea: contributes to high osmolarity of medullary interstitium
- Normal cortico-medullary gradient 300-1400mOsmol.
Counter-current Mechanism in Kidney
Counter Current Multiplier:
Set-up
- Descending limb LoH Permeable to water only
- Ascending limb LoH Permeable to NaCl only
- Thin passive NaCl reabs
- Thick NaCl via active NKCCT cotransport and paracellular
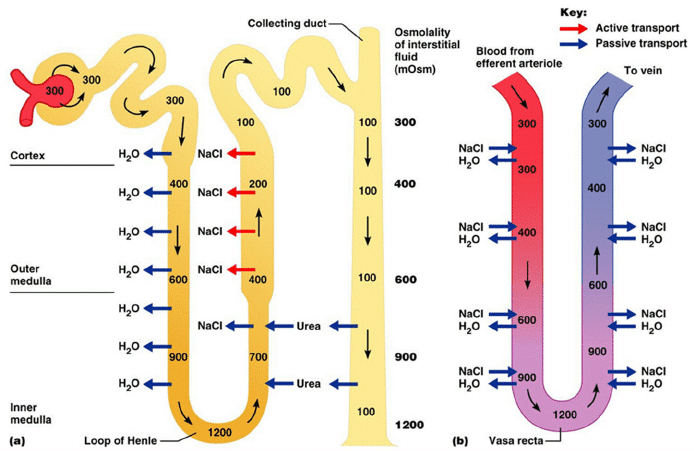
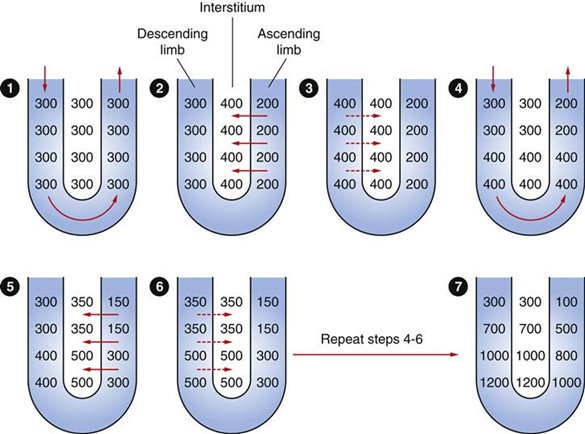
Generation
- Enters LoH osmolarity throughout = 300mOsm
- Max effort of NKCCT = 100mOsm/kg
- Tubular OSM = 200/kg
- Interstitial Osm = 400/kg
- Descending limb diffuses water down gradient
- Descend limb OSM now 400
- Process repeats (flow, pump salt, equilibrate water)
Counter Current Exchange:
- Hairpin loop arrangement of the vasa recta in the juxta-medullary nephrons.
- Provides blood flow to medullary tissue without impact of the cortico-medullary gradient.
- Relies on slow flow of blood.
- Mechanism
- On descent, water is lost from capillaries and NaCl is absorbed into capillaries increasing osmolarity
- On ascent water is reabsorbed and solute is lost
Role of Urea in Medullary Concentration Gradient
- 900mmol/day filtered
- 350-550mmol/day reabsorbed.
- Comprises 50% of osmolality (300-650 mOsm/kg)
- Freely filtered at the glomerulus
- Secreted by thin limbs of LoH down concentration gradient from medullary interstitium to tubular fluid.
- As water and Na are reabsorbed, tubular [Urea] increases
- [Urea] = >500mmol/L at collecting ducts
- Equilibrates with interstium due to slow flow rates.
- ↑ADH
- urea transporters UT1 and UT3 insertion → ↑ CD permeability to urea
- → ↑ medullary gradient (further 10% reabsorption if required)
Gladwin / JC 2020
CICMWrecks Answer – Skin counter-current
Counter-current Mechanism in Skin
- Counter current mechanism in skin
- Unknown significance in man
- In arctic fox
- Deep veins run parralel to and adjacent to arteries
- Heat from arterial blood is reabsorbed by venous blood returning to the body
- minimizes heat transfer to periphery and subsequent heat transfer to the
environment
- Counter-current system within the limbs
- one of the mechanisms to keep core temperature fairly constant
- the warm outbound arterial blood transfers heat to the cold inbound venous blood
- Three types:
- One artery and one vein in parallel
- as exist down the lengths of our arms and legs,
- One artery surrounded by many veins
- as in human fingers
- A net where 20–40, or sometimes several hundred, small arteries and veins run parallel and are intermingled
- Extremely rare in humans – usually pathological
- Extremely rare in humans – usually pathological
- One artery and one vein in parallel
Gladwin / JC 2020
Examiner Comments
2015B 22: 48% of candidates passed this question.
Most answers gave a good account of counter currents in the kidney but few described the generation of the counter current multiplier arrangement. Many candidates did not discuss counter current mechanisms in the skin but instead, focussed on thermal control by the skin.
23. Describe the factors that affect partial pressure of CO2 in mixed venous blood
Link to F12. Respiratory Measurement
CICMWrecks Answer
Mixed venous blood
- Mixture of all venous blood from all tissue capillary beds.
- Sampled from pulmonary artery
- In reality, omits venous blood from physiological shunt
PCO2
- Small portion of CO2 carriage in blood – approx. 5%
- The rest being HCO3 (approx. 90%), Carbamino compounds (approx. 5%), and carbonic acid (negligable)
- Constitutes 10% of A-V difference in CO2 carriage
- The rest being HCO3 (approx. 60%) and Carbamino compounds (approx. 30%)
- Obeys Henry’s Law
- Mass of dissolved gas is proportional to its partial pressure
- Solubility coefficient of CO2 ~0.54
- Obeys Dalton’s Law
- Sum of all partial pressures equals the environmental atmospheric pressure
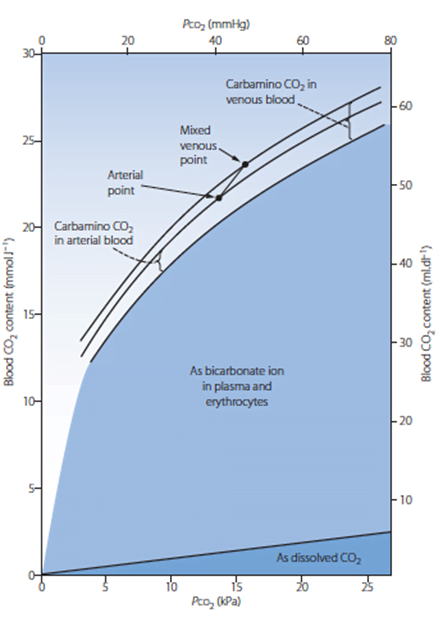
PMVCO2
- Sum of PaCO2 and dissolved portion of CO2 produced
- PaCO2
- In ideal system equal to PACO2 – 10% shunt only increases CO2 by 0.7mmHg
- PACO2 = (CO2 delivery to lung)/(Alveolar ventilation)
- Alveolar ventilation = (Vt – Vd) x RR
- Vt = Tidal volume – Approx. 7ml/kg
- Vd = Dead space volume
- Anatomical deadspace = first 16 divisions of airway – approx 2ml/kg
- Alveolar deadspace – Volume of non-perfused lung
- Increases with
- West zone 1
- PE
- Decreased lung perfusion
- Decreased cardiac output
- Positive pressure ventilation
- Upright posture
- Increases with
- RR – Regulated by medullary respiratory centre
- Chemoreceptor
- Peripheral
- Sensitive to decreased pO2, decreased pH, decreased blood flow and increased pCO2
- Increases respiratory rate
- Central
- Sensitive to CO2 via conversion to [H+]
- Increases respiratory rate
- Peripheral
- Baroreceptor
- Decreased stretch of aortic and carotid baroreceptors increases respiratory rate
- Pulmonary receptors
- J fibres
- Stimulation causes apnoea, bronchoconstriction, bradycardia and hypotention
- Stretch receptors
- Inflation reflex – Inhibits inspiration on lung inflation
- Deflation reflex – Inhibits expiration on lung deflation
- J fibres
- Chemoreceptor
- CO2 production
- Occurs predominantly in mitochondria
- From metabolism of glucose
- Glucose + 6O2 → 6CO2 + 6H2O
- From
- BMR – 40kcal/hr/m2
- Increased muscle activity
- Post-prandial metabolism
- Thyroid hormones increase metabolism
- Catecholamines increase metabolism
- Disease states
- Fever
- Malignant hyperthermia
- Tourniquet
- Relative perfusion of tissues
- Blood from highly metabolic tissues contributes relatively greater amounts of CO2 per weight
- Blood from less metabolic tissues contributes relatively less CO2 per weight
- From metabolism of glucose
- Occurs predominantly in mitochondria
Sakurai 2016
Examiner Comments
2015B 23: 15 % of candidates passed this question.
It was expected candidates would define key concepts, particularly ‘mixed venous’. Many candidates knew some of the elements that contributed to mixed venous PCO2 but few described all of the main factors. There was little mention of tissue capillary flow as a factor affecting mixed venous CO2.
24. Compare and contrast the pharmacology of valproic acid and carbamazepine.
Examiner Comments
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Resp physio, Oxygen cascade, humidity |
| G. CVS | Response to 10% blood loss, volureceptors, baroreceptors, RAS, mechanism and limitations of automated NIBP (DINAMAP), Coronary artery blood flow |
| H. Renal | |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | knowledge of blood gas interpretation |
| K. Neuro | Cerebral physio+pharm, mannitol, ketamine opioid pharmacology including classification and mechanism |
| L. Musculoskeletal | Calcium, cardiac myocyte, pharm, regulation NMJ, NM blockers |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | Immunology related to anaphylaxis and immunoglobulins |
| T. Microbiology | Classification and mechanisms of Abx resistance. |
| U. Endocrine | insulin physiology |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |




Recent Comments