1. Explain the control of breathing
CICMWrecks Answer
Respiration
- Normal RR ~ 10 breaths per minute
- Normal tidal volume ~ 500ml/kg (in 70kg male)
- Therefore normal minute ventilation approx 5l/min
Afferents / Sensors
Chemoreceptors
- Central
Located in retrotrapezoidal nucleus- Sensitive to changes in CSF [H+]
- CO2 readily diffuses across BBB and converted to H+ and HCO3– via carbonic anhydrase
- Increased [H+] (and therefore CO2) stimulated respiration
- Sensitive to changes in CSF [H+]
- Peripheral
Located in aortic bodies (innervated by vagus nerve) and carotid bodies (innervated by glossopharyngeal nerve)- Sensitive to PO2, [H+], PCO2 and blood flow
- O2 dependent K+ channels
- Respiration increases as O2 drops below 50mmHg
- O2 dependent K+ channels
- CO2
- Linear increase in respiration as CO2 increases
- Sensitive to PO2, [H+], PCO2 and blood flow
Baroreceptors:
- Located in aortic arch and carotid sinuses
- Responds to stretch
- As MAP drops → less stretch on vessel walls → increased sympathetic outflow from medullary vasomotor centre → triggers increase in respiration
- Responds to stretch
Pulmonary receptors
- Stretch receptors
- Increased stretch of pulmonary parenchyma triggers inflation reflex → inhibits inspiration to prevent overdistention
- Collapse of pulmonary parenchyma triggers deflation reflex → inhibits expiration to prevent atelectasis and loss of FRC
- J fibres
- Nociceptive mechano-chemoreceptors
- On stimulation → Bronchospasm, apnoea, bradycardia and hypotension
- Nociceptive mechano-chemoreceptors
Others
- Joint and muscle receptors stimulate ventilation
- Pain and temperature sensation can alter ventilation via the cortex and limbic system
Central control of breathing
Central input
- There is input from the hypothalamus and cortex, with the ability of the cortex to override the medulla and bring ventilation under voluntary control
Controller
- Medullary Respiratory Centre
- Dorsal respiratory group (DRG)
- Located in and adjacent to Nucleus Tractus Solitarus
- Associated with inspiration and timing
- Works as an ‘integrating centre’ with VRG
- Ventral respiratory group (VRG)
- Including Pre-Botzinger and Botzinger complex → Central Pattern Generator
- Associated with control of expiration, airway dilation and central pattern generation
- sends inhibitory impulses to Apneustic centre
- Dorsal respiratory group (DRG)
- Pontine respiratory group (PRG)
- Pneumotaxic centre
- controls both the rate and the pattern of breathing
- Sends inhibitory impulses to the inspiratory area
- Antagonist to apneustic center
- decreases tidal volume
- Apneustic centre:
- sends signals for inspiration for long and deep breaths
- controls the intensity of breathing and is inhibited by the stretch receptors of the pulmonary muscles at maximum depth of inspiration, or by signals from the pnuemotaxic center
- increases tidal volume.
- Pneumotaxic centre
- Inspiratory phase:
- Gradual ramping up of nerve activity – ↑muscle contraction
- Expiratory phase I:
- Gradual reduction of nerve activity – ↓muscle contraction
- Expiratory phase II:
- Inspiratory muscles inactive
- If increased respiratory drive, expiratory muscles are activated
Efferents
- Phrenic nerve (C3, 4, 5) → Innervates diaphragm – Main inspiratory muscle
- Spinal nerves to intercostal muscles (external intercostal → inspiration, internal intercostal → expiration)
- Brachial plexus → Pectoralis major (forced breathing inspiration)
- Accessory muscle → Sternocleidomastoid (forced breathing inspiration)
- Spinal nerves to abdominal muscles (forced expiration)
Mooney / Sakurai / JC 2020
Examiner Comments
2015A 01: 71 % of candidates passed this question.
This question was generally well done. It was expected answers would include discussion of the three core elements of sensors, a central controller and effectors. Central control involves three main groups of neurones in the brainstem with some cortical voluntary control also possible. More in depth answers included graphs of the ventilatory response to oxygen and carbon dioxide tensions.
2. Describe the Foetal Circulation and the changes that occur at birth
CICMWrecks Answer
Foetal Circulation
- Placenta
- Supplied by paired umbilical artery and drains to single umbilical vein
- Capillary networks in parallel à high flow, low resistance system, decreases overall systemic vascular resistance.
- Oxygenated blood (SpO2 80% PO2 35mmHg) from placenta returns to foetus via umbilical vein
- 60% of blood from umbilical vein bypasses liver via ductus venosus into IVC
- Blood in IVC from systemic circulation is hypoxaemic (SpO2 30%)
- Heart
- Blood from IVC enters right atrium and oxygenated blood, directed via the Eustachian valve, preferentially flows across foramen ovale to left ventricle à supplies ascending aorta (SpO2 65%, PaO2 25mmHg)
- This allows most oxygenated blood to supply the brain and coronary arteries
- Blood from SVC enters right atrium and continues to right ventricle and pulmonary arteries → flows into aorta via ductus arteriosus and predominantly supplies descending aorta (SpO2 60%, PaO2 20mmHg) and organs
- Blood from IVC enters right atrium and oxygenated blood, directed via the Eustachian valve, preferentially flows across foramen ovale to left ventricle à supplies ascending aorta (SpO2 65%, PaO2 25mmHg)
- Lungs
- High pulmonary vascular resistance due to;
- Unexpanded lungs
- Hypoxic pulmonary vasoconstriction
- Therefore blood in pulmonary artery preferentially perfuses systemic circulation via ductus arteriosus
- Only approx 12% of pulmonary arterial blood enters the pulmonary circulation
- High pulmonary vascular resistance due to;
Changes at birth
- Placenta
- Vasoconstriction occurs due to longitudinal stretch of umbelical vessels and increase in PaO2
- Augemented by clamping of vessels
- Loss of placenta increases systemic vascular resistance
- Increased left heart pressures
- Lungs
- After first breath…
- Hypoxic pulmonary vasoconstriction is relieved,
- Lung expands
- Recruitment of collapsed parallel capillary networks
- Dilation of existing vasculature
- Pulmonary blood flow increases 10-fold and pulmonary vascular resistance decreases
- Combined with decreased venous return (from placenta), decreases right heart pressures
- After first breath…
- Heart
- Foramen ovale
- Equilibration of right and left heart pressures closes flap of foramen ovale (occurs within minutes to hours)
- Pulmonary arterial blood flow further increases
- Ductus arteriosus
- The ductus arteriosus shunt become bi-directional
- Closure
- Due vasospasm caused by increased PO2
- Also, loss of PGE2 produced by placenta causes loss of vasodilation
- Occurs within 96 hours
- Foramen ovale
Sakurai 2016
Examiner Comments
2015A 02: 55 % of candidates passed this question.
This topic is well covered in standard texts and has been asked previously. Better answers displayed knowledge of the key concepts, such as the parallel circulations in the foetus and preferential flow of better oxygenated blood to the brain and upper limbs. Some candidates spent time on the maternal and placental circulations which were not required to answer the question asked.
3. Describe the structure and function of the blood brain barrier
CICMWrecks Answer
Blood brain barrier
- Anatomical and chemical partition separating the intravascular space from CNS interstitial space
Structure
- Mechanical barrier
- Endothelial cells
- Tight junctions between cells formed by membrain proteins (e.g. occludin) prevents paracellular flow
- Lack fenestrations
- Lack transcellular pathways such as vescicles
- Selective transport proteins (e.g. GLUT, various amino acid transporters)
- Pericytes embedded in basement membrane
- Astrocyte end feet
- Supportive role for endothelium
- Aquaporin regulation
- Endothelial cells
- Physiological barrier
- Enzymatic inactivation
- Enzymtes within endothelial cells metabolise substances absorbed from capillary lumen
- Monoamine oxidase
- Cholinesterase
- Aminopeptidase and endopeptidase
- Enzymtes within endothelial cells metabolise substances absorbed from capillary lumen
- Efflux pumps
- Substances that are absorbed across the luminal capillary membrane may be pumped back into capillary lumen by efflux pumps
- P-Glycoprotein
- ABC-Transporter
- Substances that are absorbed across the luminal capillary membrane may be pumped back into capillary lumen by efflux pumps
- Enzymatic inactivation
- Areas of brain outside BBB
- Subfornical organ
- Organum Vasculosum Lamina Terminalis
- Pituitary
- Area postrema
Function
- Regulate uptake of nutrients and electrolytes into brain
- Regulate migration of leukocytes and inflammatory responses in the brain
- Buffer brain parenchyma and interstitium from fluctuations in blood
- Prevent toxins and pathogens entering brain
- Some substances (such as water) pass BBB readily. These are characterised by:
- Small molecular weight
- Lipophilic (thiopentone)
- Uncharged (e.g. atropine vs. glycopyrollate)
- Poorly protein bound
Sakurai 2016
Examiner Comments
2015A 03: 33 % of candidates passed this question.
There was general lack of understanding of the conceptual framework of the blood brain barrier (BBB) and its function. To attain a pass, candidates were required to describe the concept of BBB as a physical and a transport barrier, describe the role of tight junctions and glial cells and identify important barrier functions with some examples of things commonly transported across or excluded.
4. Describe the mechanisms by which water and electrolytes are reabsorbed across the renal tubules.
CICMWrecks Answer
This is a list of all the mechanisms by which the renal tubules reabsorb water and electrolytes.
Most substances involve multiple mechanisms, the answer is structured to describe the different transport “mechanisms” and hence the examples are not extensive.
| Mechanism | Energy Expenditure | Electrochemical gradient | Example | ||||
|---|---|---|---|---|---|---|---|
| Diffusion | Passive diffusion | Molecule crosses a membrane to which it is permeable by diffusion | No | With | Most electrolytes and molecules at different levels of kidney (along with other mechanisms) - H+, NH4+, HCO3-, Urea | ||
| Facilitated diffusion | Molecule crosses a membrane via a channel, without energy expenditure | No | With | Amino acid movement from tubules to blood | |||
| Active transport | Primary | Primary active transport | Molecule crosses a membrane via a channel, with energy expenditure (ATP) | Yes | Against | 3Na+ /2K+ ATPase pump - Na from cell into interstitium, in exhange for K | |
| Secondary | Symport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Co-transported) | Not directly | May be Passive or Active based on gradient of 2nd | sodium and an amino acid | ||
| Antiport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Anti-transported) | Not directly | May be Passive or Active based on gradient of 2nd | Na+ / H+ antiporter in proximal convoluted tubule | |||
| Ligand-gated ion channel | Binding of a ligand causes conformational change in membrane channel, allowing movement of ion across membrane | No | With | ENaC channel - Control resorption of Na Located in collecting duct | |||
| Exocytosis | Substance packed in vesicle, moves to cell membrane, two membranes merge -> substance exits cell | Yes | Usually against | Exocytosis of Water by Aquaporin2 channel (regulated by vasopressin) | |||
| Endocytosis | Cell membrane extends to engulf a substance or object, which is then contained in the cell within a vesicle | Yes | Can be with, against or refer to a macroscopic object | Reabsorption of filtered proteins by receptor-mediated endocytosis in the proximal tubule | |||
| Reabsorption | Multi-mechanism: Starling forces, diffusion, and active transport. | Depending on mechanism | Depending on mechanism | Resorption of glucose, water | |||
| Osmosis | Spontaneous net movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides (Diffusion of water across semi-permeable membrane) | No | Yes | Water | |||
| Solvent Drag | solutes in the ultrafiltrate that are transported back from the renal tubule by the flow of water rather than specifically by ion pumps or other membrane transport proteins. Occurs in paracellular pathways between tubular cells | Usually No | NA | Renal Na and Cl resorption, Renal urea handling | |||
JC 2019
Examiner Comments
2015A 04: 33 % of candidates passed this question.
The intent of the question was to have the candidate describe in the context of a classification the mechanisms by which water and electrolytes may cross a cell membrane and use the renal tubule to provide an example of each mechanism.
It was expected the answer would talk about transport “mechanisms” across membranes. These would include processes such as reabsorption, diffusion, facilitated diffusion, primary and secondary active transport, endocytosis, osmosis and solvent drag. Many candidates used colloquial and vague language to describe precise concepts.
Some candidates structured their answer as an outline of the principal mechanisms at each segment of the tubule. Thus there was repetitive reference to mechanisms without a description as requested in the question. This approach also resulted in some candidates omitting some mechanisms altogether.
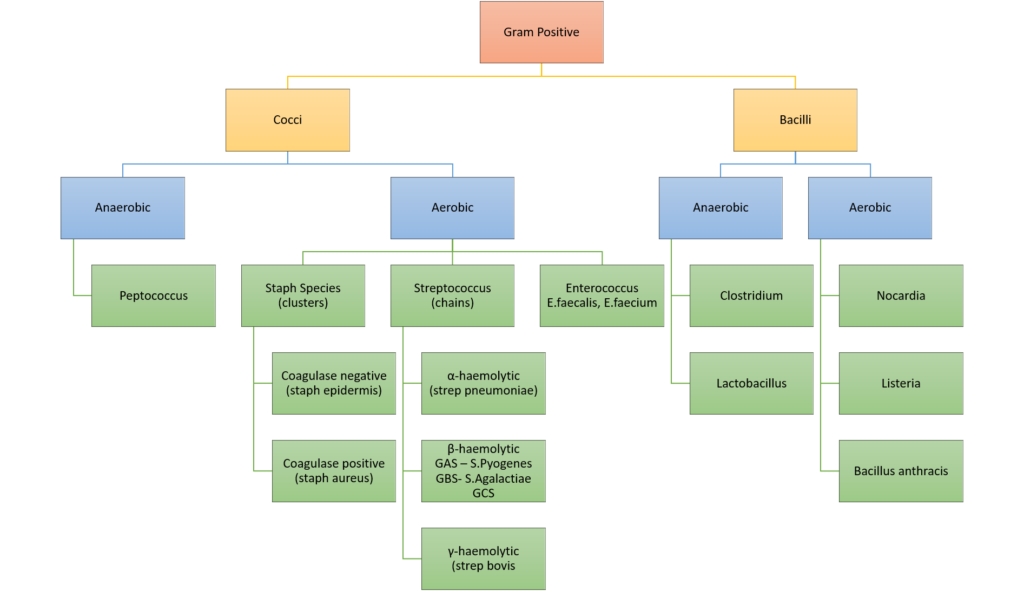
5. Classify gram positive bacteria with examples (20% of marks). Outline the pharmacology of vancomycin (80% of marks).
CICMWrecks Answer: Gram Positive Bacteria
GRAM POSITIVE BACTERIA
Gram Stain
Method of differentiated bacteria according to their cell wall characteristics, using
staining with crystal violet dye
- Gram positive bacteria have a thick outer cell wall composed of peptidoglycan,
which stains positive with crystal violet dye - Gram negative bacteria have an outer cell membrane enclosing a thinner
peptidoglycan cell wall, which has decreased affinity to the crystal violet dye
PHARMACOLOGY
CICMWrecks Table – Vancomycin | Flucloxacillin (Click to Open)
CICMWrecks Table – Vancomycin (Click to Open)
Gladwin / Sakurai / JC 2020
CICMWrecks Answer: Pharmacology: Vancomycin
PHARMACOLOGY OF VANCOMYCIN
Examiner Comments
2015A 05: 75 % of candidates passed this question.
The classification should have demonstrated a framework that covered relevant gram positive pathogens. Examples should have included both genus and species. More detail than “strep” or “Staph” was expected.
Knowledge of vancomycin was expected to include an outline of pharmaceutics, pharmacodynamics, pharmacokinetics, dosing and adverse effects. In particular, pharmacokinetics should be well understood as there are significant implications for dosing.
Common errors included incorrect examples such as “clostridium” or classifications lacking detail with respect to examples.
6. Outline the physiology of pancreatic secretion (80% of marks) and outline the pharmacology of octreotide (20% of marks).
CICMWrecks Answer: Pancreas Secretions
Pancreatic Secretion
| EXOCRINE SECRETIONS | HCO3– | |
| Water | ||
| Enzymes | Proteases | |
| Amylase | ||
| Lipase | ||
| Others | ||
| ENDOCRINE SECRETIONS | Insulin | |
| Glucagon | ||
| Somatostatin | ||
Exocrine Secretions
- produced by acinar and ductal cells
- ~1.5L per day
- HCO3–
- alkalinize gastric contents
- enzyme carbonic anhydrase
- Water
- Enzymes
- Proteases: Trypsinogen → Trypsin (by enterokinase in gut), Chymotrypsinogen → Chymotrypsin. Proteolysis.
- Amylase: Hydrolysis of glycogen, starch, and complex carbohydrate.
- Lipase: Hydrolysis of dietary triglycerides.
- Others: ribonuclease, deoxyribonuclease, gelatinase and elastase
Endocrine Secretions
- Insulin
- polypeptide hormone
- synthesized from proinsulin in RER of B cells in Islets of Langerhans
- Excreted via exocytosis in response to ↑intracellulae Ca2+
- Minimal protein binding, tiny Vd 0.075 L/kg
- Metabolized in liver, muscle, kidney byt Glutathione insulin transhydrogenase
- Renal elimination of inactive metabolites
- Circulatory hald life ~5mins
- Increased anabolism, decreased catabolism
- maintains normal blood glucose levels by facilitating cellular glucose uptake, regulating carbohydrate, lipid and protein metabolism
- promotes cell division and growth through its mitogenic effects
- Glucagon
- polypeptide hormone
- synthesized in pancreatic A cells
- secreted into portal vein
- Circulating half life ~5mins
- Metabolized in liver (1st pass) – low circulating level
- Glycogenolysis, gluconeogenesis, glucose release, ketone formation
- Lipolysis
- Inotopy, increases metabolic rate
- Stimulates somatostatin, insulin release
- Somatostatin
- polypeptide hormone
- Inhibits Glucagon, Insulin, other pancreatic peptides
- may function as CNS neurotransmitter
CICMWrecks Answer: Pharmacology of Octreotide
Pharmacology of Octreotide
Examiner Comments
2015A 06: 38 % of candidates passed this question.
An outline of exocrine function should have included the sources of secretions, secretions involved in the digestion of proteins, carbohydrates and fats, the roles of trypsin inhibitors and bicarbonate secretion and the regulation of enzyme and bicarbonate secretion.” Knowledge of endocrine physiology was good whereas the depth of knowledge regarding exocrine function was generally shallow with many errors.
Only some general facts around the pharmacology of octreotide were required to pass this section of the question. Responses revealed limited knowledge and contained many errors.
7. Outline the physiological responses to the rapid intravenous administration of 1 litre of 0.9 % saline to a 70 kg euvolaemic person.
CICMWrecks Answer
Assumptions
- TBW is one-third ECF & two-thirds ICF
- ECF is one-quarter plasma & three-quarters ISF
- The threshold of the volume receptors is 7-10% change in blood volume
- The osmoreceptors are sensitive to a 1-2% change in osmolality.
- Plasma osmolality is normal prior to the transfusion (ie 287-290 mOsm/kg)
Key
- TBW = Total Body Water
- ECF = Extracellular Fluid
- ICF = Intracellular Fluid
- ISF = Interstitial Fluid
1L NS has 9gm NaCl – It is an ECF replacement fluid
| Volume Litre | [Na+] mmol/L | [Cl–] mmol/L | Osmolarity mOsml/L | |
|---|---|---|---|---|
| NS | 1 | 154 | 154 | 308 |
| Plasma | 5 | 140 | 100 | 290 |
Response to 1L Normal Saline Bolus
| INITIAL PHASE | ||
| Brief Hypervolemia | → ↑ arterial BP | → Reflex response (baroceptors, atrial stretch receptors etc) |
| ↑ Renal perfusion → Stimulation of intra renal apparatus (juxtaglomerular apparatus) | ||
| [Na+] | 154/L into 140/L | very negligible rise |
| [Cl–] | 154/L into 100/L | rises 1L NS unlikely to cause hyperchloraemic metabolic acidosis |
| REDISTRIBUTION PHASE (~15 minutes) | ||
| [Na+] similar to ECF → Distributes | ISF (3) | ↑750ml |
| Plasma (1) | ↑250ml | |
| EXCRETION PHASE (~6 hours) | ||
| Plasma osmolality and tonicity mostly unchanged | no osmoreceptor stimulation | |
| Plasma volume ↑ to 5250mls (5% increase) | below sensitivity of volume receptor → no volume receptor stimulation | |
| No protein → oncotic pressure slightly lowered | Fluid moves into ISF (Starling’s hypothesis) | |
| Glomerulo-tubular Imbalance → ↑GFR → ↓ reabsorption of water in proximal tubule → ↑ Urine flow | ||
| Fluid moves back into intravascular compartment till all infused fluid is excreted (~6hrs) | ||
Examiner Comments
2015A 07: 25 % of candidates passed this question.
Answering this question required the integration of information from areas of cardiovascular, body fluid and renal physiology which proved difficult for most candidates. Both breadth and depth was expected so as to score well.
This question is best answered using a time-based approach. For example, upon the rapid infusion of a litre of normal saline there will be a brief period of hypervolemia, increase in arterial blood pressure and an associated physiological reflex response to these changes (e.g. baroreceptors, atrial stretch receptors, etc.). There will also be an associated increase in renal perfusion and stimulation of intrarenal receptors (e.g. juxtaglomerular apparatus).
Candidates were expected to outline these changes, their effector responses (e.g. autonomic nervous system reflexes and humoral changes) and their physiological consequences.
A more prolonged redistribution phase of the administered saline then occurs. This saline redistributes throughout the extracellular fluid space. Candidates were expected to briefly describe this effect as well as the subsequent management of the sodium and water load by the kidney.
Most candidates spoke about the pressure effects, and only some compared these with the volume effects. The effect of redistribution and other effects were not considered by the majority of the candidates.
8. How does warfarin exert its anti-coagulant effect (50% of marks)? Outline the pharmacology of the agents that can be used to reverse the effects of warfarin, giving examples (50% of marks).
CICMWrecks Answer: Pharmacology of Warfarin
Warfarin Pharmacology
CICMWrecks Answer: Warfarin Reversal
Warfarin Reversal
The following reversal strategies may be either used alone or in combination:
- Stopping warfarin
- S-warfarin is eliminated via oxidation predominantly by CYP2C9 (and to a lesser extent by CYP3A4 and 1A2) → inactive metabolites
- Elimination half-life of S-warfarin is 29 hours
- ∴ in patients with normal hepatic metabolic and synthetic functions take 4 ~ 5
- days for INR to normalise
- Vitamin K
- Can be given either enterally or parenterally (IV, IM) (bioavailability ≈ 100%)
- Requires few hours to work
- Enteral administration Requires presence of bile salts to be absorbed by gut
- Parenteral administration has rare but life threatening complication of hypersensitivity probably related to its preservative, benzyl alcohol
- Low dose vitamin K (1 ~ 2.5 mg) will slowly reduce INR over 24 hours
- but often will not result in complete reversal and would not significantly affect re-establishment of anticoagulation with warfarin
- High dose vitamin K (10 mg) will slowly reduce INR over 12 hours
- ∴ often given together with clotting factors (e.g. FFP)
- Can be given either enterally or parenterally (IV, IM) (bioavailability ≈ 100%)
- Fresh frozen plasma
- Plasma from donated blood, which contains all clotting factors
- ∴ immediately reverses coagulopathy
- However, short duration of action (24 ~ 48 hours) ∴ reserved for active bleeding
- Used when PCC not available
- Dose 2 ~ 4 units (10-15ml/kg) depending on INR and bleeding risk
- Disadvantages = relatively large fluid volume, possible transfusion reactions (e.g. immune reaction, TRALI, infection, etc)
- PCC – Prothrombin complex concentrates (Prothrombinex)
- Human plasma derivative containing concentrates of factors II, IX and X
- 500IU PCC has 500IU of II, IX, X each in a vial
- ∴ immediately reverses coagulopathy
- Dose 25 ~ 50 IU/kg
- Advantages = reliable reversal, smaller fluid volume, avoids transfusion
- complications associated with FFP
- Disadvantages = expensive
- Recombinant activated factor VII (NovoSeven)
- Controversial treatment, present in some guidelines
- Indication = life threatening bleed with significantly elevated INR
- Given together with vitamin K and FFP
- Disadvantage = thromboembolic complications
Examiner Comments
2015A 08: 38 % of candidates passed this question.
Warfarin is a competitive inhibitor of the enzyme vitamin K epoxide reductase which converts oxidised or inactive vitamin K to reduced or active vitamin K Reduced vitamin K is required for the gamma carboxylation of the glutamate residues in the vitamin K dependant factors (II, VII, IX and X) and proteins C and S. This gamma carboxylation converts these clotting factors from their inactive to their active form resulting in coagulation. The presence of warfarin inhibits this conversion process resulting in anticoagulation. The presence of inactive protein C and S explains the initial hypercoaguable effect of warfarin.
The three main agents used to reverse the effects of warfarin are vitamin K, prothrombinex and fresh frozen plasma (FFP). It was expected answers would provide a brief overview of all three agents. Most candidates did not highlight the fact that parenteral vitamin K requires a few hours to work whereas prothombinex and FFP work immediately.
Better answers noted additional facts such as oral vitamin K because it is fat soluble requires the presence of bile salts to be absorbed from the gut or the rare but life threatening hypersensitivity reaction caused by intravenous vitamin K possibly related to its preservative benzyl alcohol.
A common omission was the amount of coagulation factors in international units (IU) in an ampoule of prothormbinex or the dose required to reverse warfarin anticoagulation. A description of the clinical pros-cons of the various agents was not required to answer the question.
9. Classify and describe mechanisms of drug interactions with examples
CICMWrecks Answer
Pharmaceutical interactions:
- Physicochemical incompatibility b/t drugs
- causes precipitation of drug (Eg. STP (alkaline) + SCh (acidic))
- Absorption or binding to containers (Eg. GTN + PVC lines)
- Degradation of drug (Eg. insulin denatures in solution of dextrose)
Pharmacodynamic interactions:
- One drug alters the body’s response to another at a given plasma [drug] and can be either
- antagonistic
- Direct (morphine and naloxone) or indirect
- additive
- synergistic
- Inhibition of enzymatic inactivation e.g. clavulanic acid and amoxicillin
- Enhanced agent uptake e.g. gentamicin and penicillin
- antagonistic
- Direct interaction
- Drugs act at same receptor site for effect
- naloxone + opioids → direct antagonism
- N2O + volatiles → direct additivity
- Drugs act at same receptor site for effect
- Indirect interaction
- Drugs act at different receptor sites for same effect
- Opioids + volatiles → indirect synergism
- atropine + neostigmine → indirect antagonism
- Drugs act at different receptor sites for same effect
Pharmacokinetic interactions:
- Absorption
- Mainly due to altered oral absorption a/w:
- Complex formation: tetracycline + Ca in milk/antacids
- Altered gastric emptying/intestinal motility
- opiates ↓ intestinal motility → ↓ absorption of drugs absorbed in small intestine (paracetamol)
- metoclopramide ↑ intestinal motility → ↓ absorption of drugs absorbed in stomach (Eg. cimetidine)
- Altered gastric and intestinal pH
- ↑ gastric pH by antacids impairs absorption of weakly acidic drugs
- Complex formation: tetracycline + Ca in milk/antacids
- Altered parental absorption a/w localised vasoconstriction (Eg. adrenaline + LA)
- Mainly due to altered oral absorption a/w:
- Distribution
- Competition by drugs for plasma protein binding site → affects drugs that:
- Are highly protein-bound drugs (Eg. warfarin, diazepam, phenytoin)
- → ↑ unbound % in plasma
- Have enzyme system close to saturation or zero-order kinetics (Eg. phenytoin)
- →↑ displacement of drug and ↑ unbound % cannot be cleared effectively
- → large ↑ unbound % in plasma
- Are highly protein-bound drugs (Eg. warfarin, diazepam, phenytoin)
- Drugs that alter C.O. impact on distribution of drugs to target, peripheral tissues
- β-blockers ↓ C.O. → slow onset/offset times of drugs reliant on distribution
- Competition by drugs for plasma protein binding site → affects drugs that:
- Metabolism
- Inhibition/induction of microsomal enzymes (Eg. CYP450)
- Enzyme induction → resulting in ↓ plasma [drug]
- Enzyme inhibition → resulting in ↑ plasma [drug]
- Inhibitors of non-microsomal enzymes (Eg. MAOi, COMTi)
- Inhibition/induction of microsomal enzymes (Eg. CYP450)
- Elimination
- ↓ urinary excretion
- Competition for tubular transport system occurs with weak organic acids (Eg. probenecid + penicillin)
- Changes in urine pH
- Alkalinising agents (Eg. NaHCO3/acetazolamide) → ↑ excretion of weak acids
- Changes in urine volume
- Changes in biliary excretion
- Phenobarbital ↑ bile flow and biliary conjugation of drugs
- ↓ urinary excretion
Gladwin 2016
Examiner Comments
2015A 09: 33 % of candidates passed this question.
This question was best approached by classifying drug interactions as physicochemical or pharmaceutical, then pharmacokinetic and finally pharmacodynamic. Pharmacokinetic drug interactions could then be further sub classified into those affecting the rate and extent of absorption of other drugs by mechanisms such as surface adsorption, chelation, altering gastric pH and altering gastrointestinal motility. Drug interactions affecting the distribution of drugs mainly involve competition for protein binding and the displacement of highly protein bound drugs. Drug metabolism interactions usually involve drug induction or inhibition of hepatic microsomal enzymes either increasing or decreasing the metabolism of other drugs.
Examples of drug interactions affecting drug excretion include drugs altering urinary pH or drugs altering the tubular rate of secretion of other drugs. Pharmacodynamic drug interactions include potentiation of one drug by another, antagonism and combined toxicity at the tissue level. Combined toxicity can be due to the potentiation of adverse effects of two drugs.This is a broad question with plenty of opportunity to score marks. A structured approach such as that described above and providing an example for each mechanism was important.
10. Compare and contrast the pharmacology of mannitol and hypertonic saline
Examiner Comments
2015A 10: 8 % of candidates passed this question.
A structured approach is important and a table worked best for most candidates, although a few attempted this in free text. Despite attempting a structured answer very few candidates provided information in regards to preparation, dose, monitoring of osmolarity, adverse effects or contraindications. Understanding of the action of these drugs was expected and factual inaccuracies were common with many candidates suggesting hypertonic saline acts as an osmotic diuretic. Better answers mentioned other potential mechanisms of action of mannitol. Many candidates failed to appreciate the impact on raised intracranial pressure.
11. Outline the pharmacology of sodium nitroprusside (50% of marks). Discuss the mechanisms of toxicity and their management (50% of marks)
Examiner Comments
2015A 11: 38 % of candidates passed this question.
Most candidates presented a structured answer and exhibited a good understanding of the pharmacology of sodium nitroprusside. Few candidates demonstrated an understanding of the mechanisms of SNP toxicity and details on management of cyanide toxicity were lacking.
Cobalt EDTA is no-longer recommended as initial therapy in the management for cyanide toxicity.
More specific detail was expected beyond a generic comment on “mechanisims of toxicity” such as potentially causes of respiratory, renal, hepatic or CNS failure.
Few candidates mentioned adverse effects other than that of cyanide toxicity. Many
candidates also failed to outline the management of sodium nitroprusside toxicity.
12. Describe the effects of resonance and damping on an invasive arterial blood pressure tracing.
CICMWrecks Answer
Definitions
Natural frequency: the frequency at which a system oscillates when not subjected to a continuous or repeated external force.
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of a periodically applied force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts.
Resonant frequency: The frequency of a dynamic system at which the amplitude of response to an oscillating force is a relative maximum is known as a resonant frequency (OR) A natural frequency of vibration determined by the physical parameters of the vibrating object.
Damping: the property of a system that diminishes resonance.
Critical Damping: Occurs when the damping coefficient (DC or γ gamma) is equal to the undamped resonant frequency of the oscillator.
Optimum Damping: Level of damping which provides a compromise between the speed of the system with its accuracy
Undamped simple harmonic oscillator
- obeys hooke’s law
- motion is sinusoidal in time
- demonstrates a single resonant frequency.
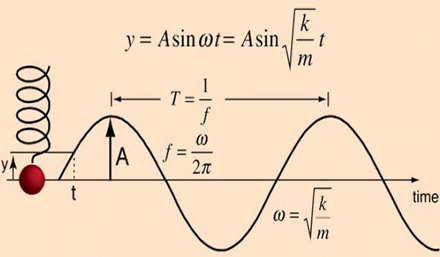
Resonance
Invasive arterial blood pressure is a driven harmonic oscillator
- An external force (pulsations of the heart) adds to the oscillations of the system (the tubing/transducer etc).
- If the frequency of the driving force is similar to, or a harmonic of, the natural frequency of the system then the result is amplification
Arterial waveform is a complex summation of multiple simple sinusoidal waveforms (a fourier series)
- The frequency of the arterial wave (i.e., the pulse rate) is known as the natural or fundamental frequency
- The sine waves used to reproduce it must have a frequency that is a multiple (or harmonic) of the fundamental frequency
- Increasing the number of harmonics allows better reproduction of high-frequency components, such as a steep systolic upstroke
In most clinical systems
- Natural resonant frequency is 10 to 15 Hz
- Primary frequency of the arterial waveform (the heart rate is 60 to 120 bpm or 1 to 2 Hz)
- However the higher frequency components of the more complex arterial waveform are closer to the natural resonant frequency and are amplified to a larger degree.
To minimize the potential of amplification and improve the dynamic accuracy of the real arterial pressure, system should have resonant frequency >> than 6-8 times frequency of the system being measured:
- Should have noncompliant (i.e. stiff) tubing
- Total mass of liquid in the system should also be minimized (shorter/thinner tubes)
- air bubbles or blood clots or occlusion should be eliminated
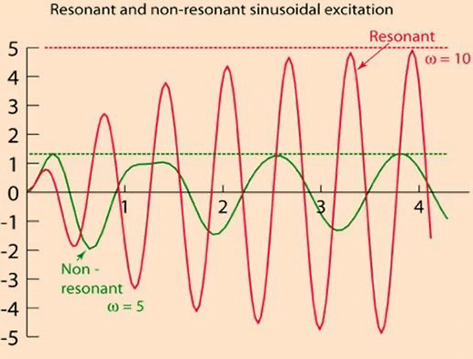
Damping
the property of a system that diminishes resonance.
- Damping:
- decreases the magnitude of the oscillations
- allows the system to come to rest at a new value.
- Degree of damping determines the rate at which the system achieves that new value.
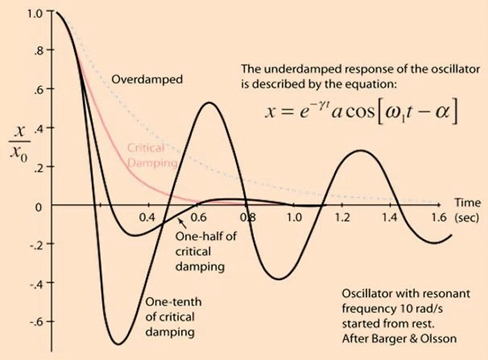
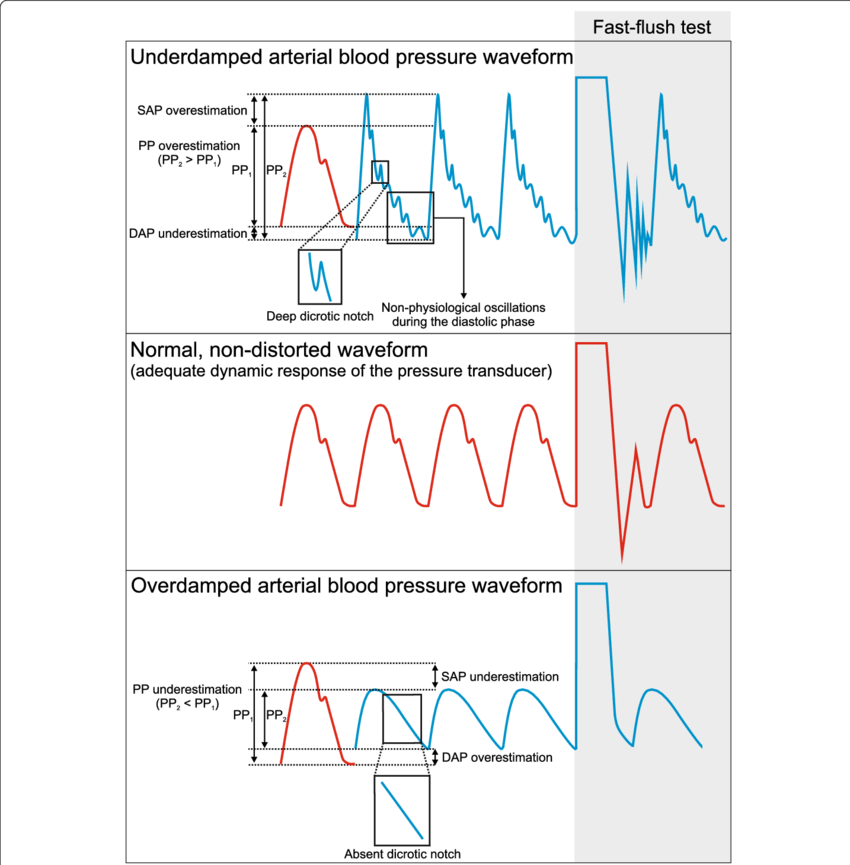
System can be:
- Underdamped (DC < 0.7):
- Reaches the zero position quickly
- Oscillates around it
- Causes falsely ↑ SBP and falsely ↓ DBP
- Critically damped (DC = 1):
- Quickest approach to zero amplitude for a damped oscillator without overshoot.
- Occurs when the damping coefficient (DC or γ gamma) is equal to the undamped resonant frequency of the oscillator.
- Optimal damping (DC = 0.64):
- Provides a compromise between the speed of the system with its accuracy
- Minimises overshoot of oscillations, phase and amplitude distortion, and provides maximal frequency response
- Overdamped (DC > 1):
- The approach to zero is slower
- Very slow to respond
- Causes falsely ↓ SBP, falsely ↑ DBP and loss of fine details of waveform (Eg. dichrotic notch)
Bedside Flush Test:
- The degree of damping in an invasive arterial line system can be determined by the bedside flush test
- Briefly open the continuous flush device to produce a square wave
Gladwin / Sakurai / JC 2020
Examiner Comments
2015A 12: 33 % of candidates passed this question.
Many candidates seemed to get some of the basic concepts but few were able to expand on simple concepts.
It was expected that candidates could describe that the arterial pressure waveform is made up of many different sine waves (as determined by Fourier Analysis) with each sine wave having a specific frequency. Every system has its own natural oscillatory frequency, or resonant frequency. If this is less than 40 Hz, it falls within the range of frequencies present in the blood pressure waveform and oscillations may produce a sine wave which is superimposed on the blood pressure wave form.
Some damping is inherent in any system and acts to slow down the rate of change of signal between the patient and pressure transducer. It may be caused by air bubbles or blood clots or occlusion. This reduces the deflection of the transducer diaphragm and hence the size of the waveform. The effect of damping on temporal response was rarely mentioned. Accurate graphical representations of invasive pressure traces are important. Many candidates provided poor drawings without axis, labels, reference to normal or discussion in text.
13. Describe how carbon dioxide (CO2) is carried in the blood?
CICMWrecks Answer
CO2
- End-product of aerobic metabolism
- Produced almost entirely in mitochondria
- Normal production approx. 200ml/min
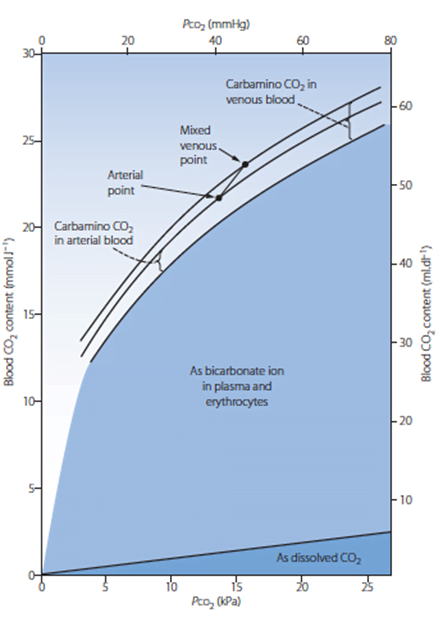
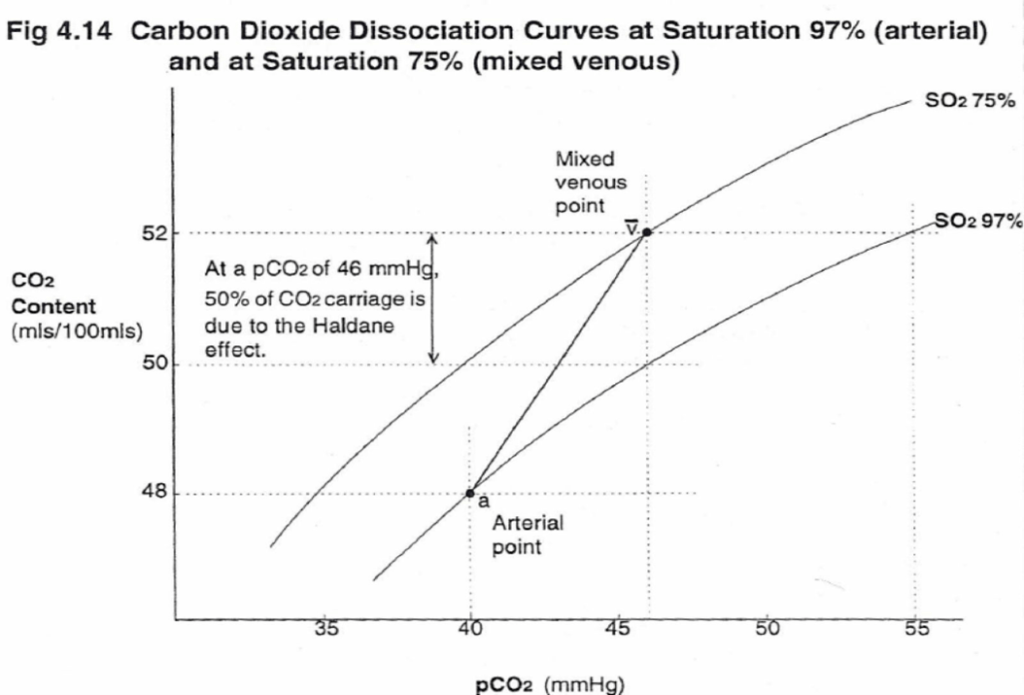
- Total CO2 in blood
- Venous 52mL/dL
- Arterial 48mL/dL
CO2 Carriage in 4 forms:
| Arterial % | % of A-V diff | Details | |
|---|---|---|---|
| 1. Bicarbonate | 90 | 60 | Main form of CO2 delivery in blood Via Hendersen-hasselbach: CO2 + H2O ⇔ H2CO3 ⇔ H+ + HCO3– Process 1. CO2 diffuses into RBCs 2. Carbonic anhydrase converts it to Bicarb 3. Hydrogen ions are buffered by binding to Hb molecules inside RBCs (30% Haldane.) Higher percentage in venous blood as: • Deoxygenated pKa 8.2 • Oxygenated pKa 6.6 • decreased pH = greater increase in [A-]/[HA] of DeoxyHb rel to OxyHb thus greater ability to accept protons 4. Bicarb is transferred out of cell in exchange for Cl ion by an exchange transporter called Band 3. (Hamburger Shift) |
| 2. Carbamino compounds | 5 | 30 | CO2 able to bind amino end of proteins Hb largely responsible as in much greater concentrations than any other protein (15 g/dL vs 7 g/dL) in serum CO2 combines with terminal amine to form carbamic acid dissosciates → at physiological pH to carbamate Affinity of Hb for CO2 increases in venous blood via Conformational change (70% Haldane): • Unbinding of O2 causes a conformational change in the beta chain • CO2 binding site at the N-terminus moves 1nm • Affinity for CO2 is increased. • Deoxy-Hb is 2-3x better at forming carbamino compounds than oxy-Hb |
| 3. Dissolved | 5 | 10 | CO2 ~20 x more soluble than O2 thus via Henry’s Law more CO2 is dissolved PvCO2 ~46mmHg (PaCO2 40mmHg) |
| 4. Carbonic Acid | <1% | <1% | Carried as dissolved H2CO3 Conversion to CO2 via Hendersen-Hasselbach equation above has pKa 6.1 At physiological pH Carbonic acid is ~96% dissociated Reaction is catalysed by Carbonic Anhydrase Not present in plasma, present in high concentrations in erythrocytes and pulmonary capilaries |
Gladwin / Sakurai 2016
Examiner Comments
2015A 13: 79 % of candidates passed this question.
It was expected answers would describe each of the main categories of how CO2 is carried: Dissolved (10%), Plasma Bicarbonate (70%) and conjunction with plasma proteins and Hb as Carbamino Hb (20%). An opening statement quantifying the amount of CO2 dissolved in arterial (48mL/100mL) and venous blood (52mL/100mL) (4mL/100mL) and how this compares with Oxygen was expected (20 times more soluble).
For dissolved CO2, the application and description of Henry’s Law was awarded marks. A description of the consequences of the Haldane effect: difference in CO2 carriage of oxygenated and deoxygenated blood was expected. A diagram of pCO2 v CO2 content was helpful
14. How is blood typed and cross-matched?
CICMWrecks Answer
Blood Typing
- The purpose of blood typing is the streamlining of blood products on the basis of the presence of antiogenetic surface membrane proteins in order to minimise patient harm.
- Main Types of Antigens:
- ABO
- Rhesus
- Others:
- Kell, P, MN, Lewis
- less antigenic
ABO system:
Red blood cells possess genetically determined antigens on their cell membrane, termed
“agglutinogens”
Agglutinogens: glycoproteins, with differing terminal oligosaccharides
- H gene:
- Forms “H-antigen” by addition of fructose to membrane glycoprotein
- A gene:
- Forms “A-antigen” by extension of H-antigen with “N-acetyl-D-galactosamine”
- B gene:
- Forms “B-antigen” by extension of H-antigen with “D-galactose”
Blood Groups:
| Group | Antigen | Possess Antibody | Incidence |
|---|---|---|---|
| A | A-antigen only | Anti-B antibody | 45% |
| B | B-antigen only | Anti-A antibody | 9% |
| A/B | A and B-antigen | Lack both Anti-A and Anti-B antibodies | 3% |
| O | H-antigen only | possess anti-A and anti-B Ab’s | 43% |
Blood Group antibodies:
- Naturally-occurring Ab to A- and/or B-antigens (as anti-A and anti-B antibodies)
- IgM only
- Develop naturally after 3 months of age due to presence of Ag in bacteria/food that resemble A/B-Ag’s
Typing / Blood Group determination (Coombs)
- Red cells tested using anti-sera (Ab added to sample)
- IgM anti-A and anti-B Abs added to sample
- +ve agglutination indicates blood type
- “Reverse blood grouping” (Sample added to Ab)
- Test individual’s serum using known group A, B, and O red cells
- +ve agglutination indicates blood type
Antibody screen:
- Used to detect if an individual possesses antibodies other than those against agglutinogens A and B
- Individual’s blood is exposed to a number of red cells with known antigens, and if a reaction, an antibody is present
- A common antibody is the D antigen, or Rhesus factor
- Highly antigenic
- Present on RBC only
- Three subtypes
- Cc; Dd; Ee
- d-Ag does not exist
- Any combination of the three with a D-Ag is highly antigenic.
- Rh +ve (if D-Ag present)
Cross Matching
Major Crossmatch: Test in vitro recipient’s serum and donor’s red cell
Minor Crossmatch: In vitro serological compatibility between donor’s serum and recipient’s red cells
Major Crossmatch:
- Saline agglutination test
- To reconfirm ABO grouping
- It tests for presence of IgM (Eg. anti-A and anti-B Ab’s) in recipient serum against donor RBC
- Donor’s RBCs + saline + recipient serum
- +ve agglutination means incompatible ABO
- Indirect Coomb’s test
- To Reconfirm presence of “minor” Ab’s in recipient serum
- Two stages:
- Donor RBCs + recipient serum
- RBC will be coated if Abs present
- AHG test
- RBC washed
- “anti-human globulin” (AHG) added
- agglutination
- → recipient serum contains an Ab against donor RBC
- Donor RBCs + recipient serum
Serological testing:
All donor blood is tested for:
HIV 1 + 2
Hepatitis B
Hepatitis C
HTLV-1
Mooney / Gladwin 2016
Examiner Comments
2015A 14: 38 % of candidates passed this question.
An opening statement of the importance of compatibility testing helped explain the relevance of the process. A brief description/table of agglutinogens (membrane antigens) along with Agglutinins (IgM Antibodies) was helpful.
Typing is the testing of individual red blood cells (donor and recipient) with anti-sera containing anti-A, B and AB antibodies. A positive test results in agglutination. Red cells with known antigens (A, B and O) are then tested with sera (reverse grouping). When discussion antibody screening, a mention of Rhesus antibodies along with testing for minor antibodies (Kell, Duff etc.) was expected.
Cross matching consists of the saline agglutination test andiIndirect Coombs testing. (This involves incubation, washing and testing with antiglobulin serum).
Many answers confused the processes of typing, antibody screening and cross matching.
15. Describe the different types of hypersensitivity reactions including an example of each.
CICMWrecks Answer
HYPERSENSITIVITY REACTIONS
| Type | Pathophysiology | Disease types |
|---|---|---|
| Type I Immediate Hypersensitivity IgE mediated | Sensitisation Plasma B cell production of IgE Mast cell proliferation → IgE binds to mast cells On re-exposure Allergenic antigen binds to mast cell (expressing IgE) → Mast cell degranulates Mast cell degranulation leads to Primary mediators: – Serotonin – Histamine Secondary mediators – Leukotrienes (SRSA) – Prostaglandins – ECFA, NCF | Anaphylaxis Atopy |
| Type II Cell Cytotoxicity IgG, IgM Mediated | Antibody attaches to antigen on target cell Complement release C5-9 “membrane attack complex” → Causes cell lysis | Blood Transfusions Goodpasteur’s syndrome Autoimmune cytopaenias |
| Type III Immune Complex IgG, IgM, IgA mediated | Circulating antibody-antigen complexes are deposited in basement membranes e.g. peritoneum, blood vessels, joints, glumeruli Complement cascade activation Attract granulocytes → inflammation | SLE Serum Sickness Necrotising vasculitis |
| Type IV Delayed hypersensitivity T-cell mediated | T cell mediated Cell mediated, as opposed to previous humoral Peak reaction 2-3 days CD4 helper T cells cause recruitment of Neutrophils Macrophages CD8 cytotoxic T cells | TB, Sarcoid Granulomatosis with polyangiitis (Wegener’s) Granulomatous vasculitis |
SRSA: “slow reacting substance” A – Mixture of Leukotrienes (LTC4, LTD4, LTE4)
ECFA: Eosinophil chemotactic factor of anaphylaxis
NCF: Neutrophil chemotactic factor of anaphylaxis
Key Points:
- “Anaphylaxis” may be:
- True anaphylaxis: a symptoms complex following exposure of a sensitised individual to an antigen, produced by a type I hypersensitivity reaction, associated with IgE mediated mast cell degranulation
- Anaphylactoid reactions: Indistinguishable from true anaphylaxis, however the immune nature of the reaction is either unknown, or not due to a type I hypersensitivity reaction, immediate generalised reaction is a better term.
Gladwin / Mooney 2016
Examiner Comments
2015A 15: 79 % of candidates passed this question.
This question can be answered in tabular form and details have been described in a previous exam report (2007). Details of each of the four main types were expected. A description of the timing of reactions was also expected.
16. Describe the physiology of cerebrospinal fluid.
CICMWrecks Answer
Formation / Production of CSF
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
Distribution / Circulation of CSF
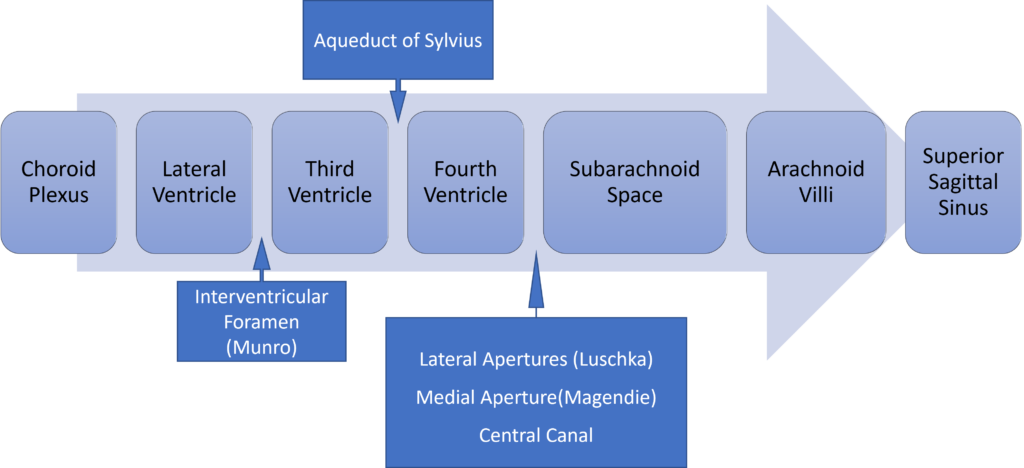
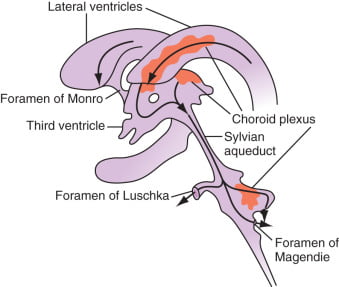
Absorption of CSF
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
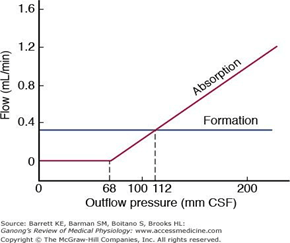
Composition of CSF
IDENTICAL to brain ECF, but differs in several manners from plasma
Compared with plasma:
- ↑ pCO2 (50 mmHg)
- ↓ pH (7.33)
- ↓ protein content
- 0.5% of plasma; 20 mg/dL
- poor acid-base buffering capacity
- ↓ content of glucose (by 60%) and cholesterol
- ↑ [Cl-] (by 7-14%) and ↑ [Mg2+] (by 40%)
- ↓ [K+] (by 40%), ↓ [Ca2+] (by 50%) and ↓ [Pi] (by 20-30%)
- ↑ creatinine (by 25%) but ↓ urea
- IDENTICAL osmolality (295), [Na+] (145), and [HCO3] (25)
Role / Functions of CSF
- Protective role (main function)
- Water bath effect
- Attributed to the low specific gravity of CSF (1.007)
- Causes brain to be buoyant
- ↓ its effective net weight from 1400 g to 50 g
- Mechanical cushion against acceleration/deceleration forces
- Buffer ↑ ICP by CSF translocation to extracranial subarachnoid space
- Abrupt ↑ ICP buffered by translocation of CSF within the vault to extracranial compartments
- Water bath effect
- Maintains constant ionic environment conducive to neuronal electrical activity CSF
- Supply role of nutrients (Eg simple sugars, amino acids) and O2 to brain
- Excretion of toxic substances, metabolic by-products, and CO2 from brain
- “Lymph-type” function → interstitial proteins in brain ECF return to circulation by
- CSF absorption across arachnoid villi
- Acid-base regulation → due to content, CSF allows for tight respiratory control
- Endocrine transport function → transports hormones to other brain regions
Gladwin / JC 2020
Examiner Comments
2015A 16: 83 % of candidates passed this question.
Most candidates answered the question well. The most common mistake was incorrect CSF composition. Better answers also discussed raised ICP and CSF’s role in the compensation for raised ICP.
17. Describe the oxygen cascade in a person breathing room air at sea level
CICMWrecks Answer
- Fraction of O2 in atmosphere = 0.21
- Oxygen cascade:
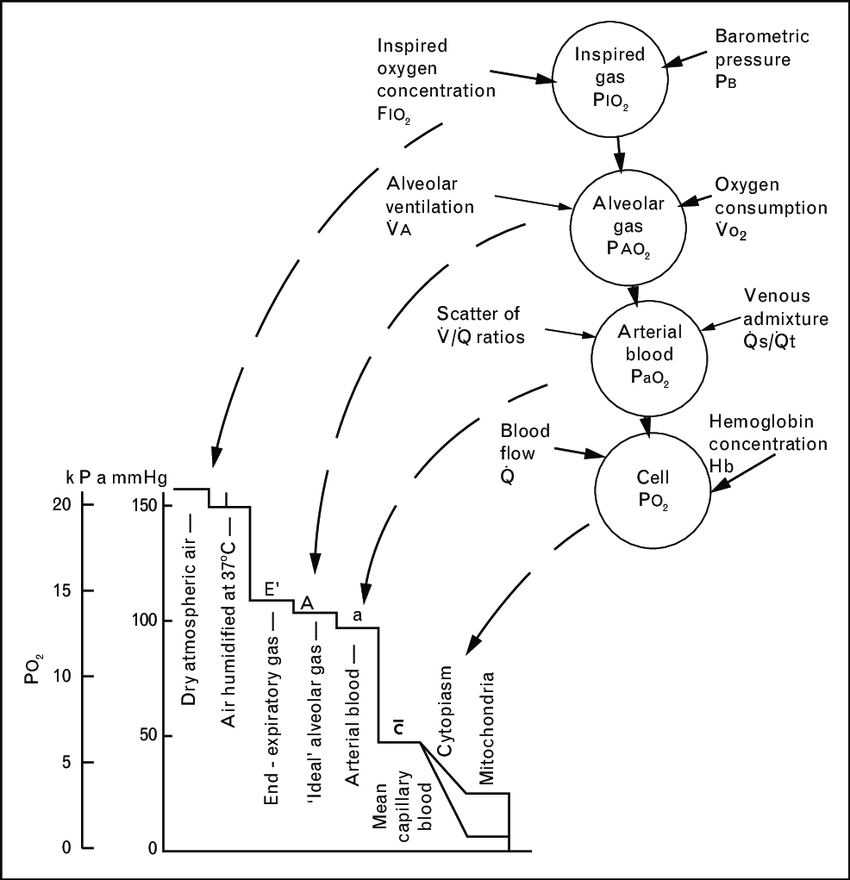
1. Oxygen in the atmosphere
- Oxygen in the atmosphere at sea-level
- Atmospheric pressure = 760mmHg
- pO2 = 760mmHg x 0.21 = 160mmHg
2. Oxygen in the bronchi
- Inspired air at sea level is humidified and saturated by H2O vapour at the isothermic saturation boundary 3cm distal to the carina
- pO2 = 0.21 (760mmHg – 47mmHg) = 150mmHg
3. Oxygen in the alveoli
- In the alveoli, inspired air is diluted with CO2 diffusing into the alveoli from the alveolar capillaries.
- pAO2 = 0.21(760mmHg – 47mmHg) – paCO2/R + f
- where, R = respiratory quotient, approx. 0.8
- f = correctional factor
- paCO2 = 40mmHg
- pAO2 = 100mmHg
- pAO2 = 0.21(760mmHg – 47mmHg) – paCO2/R + f
4. Oxygen in the arterial blood
- Oxygen diffuses from the alveoli to pulmonary capillaries (solubility coefficient 0.024) however paO2 is lower than pAO2 due to venous admixture
- Factors contributing to venous admixture
- V/Q mismatch
- Shunt
- The degree of venous admixture can be calculated by
- Oxygen in carried in blood as a predominant Hb bound portion and a minor diffused portion, governed by the oxyhaemoglobin dissociation curve
- In healthy adult, A-a gradient is no more than 15mmHg
5. Oxygen in the cytosol and mitochondria
- Oxygen diffuses from capillary blood across the plasma membrane (according to Fick’s law of diffusion) into the cell cytosol and then across the mitochondrial membranes into the mitochondria
- There is paucity of evidence to determine mitochondrial pO2 but estimated to be between 5mmHg and up to 40 mmHg in recent studies
Sakurai / Gladwin 2016
Examiner Comments
2015A 17: 63 % of candidates passed this question.
This question could be answered with a description or a diagram but required an ordered journey from the atmosphere to the mitochondria. This is commonly available in many texts and most candidates answered the question well. Most candidates said the alveolar PO2 fell solely because CO2 came out of the pulmonary capillary. Very few talked about oxygen uptake into the capillary. Another common omission was failure to state normal values for the A-a gradient.
18. Outline the role of the liver in the metabolism of fat (1/3 of marks), carbohydrate (1/3 of marks) and proteins (1/3 of marks)
CICMWrecks Answer
Fat
Anabolic Role
Synthesis or lipoproteins for transport of lipids from dietary FFA’s
- Chylomicrons
- LDL/HDLs
Lipogenesis via citrate in the TCA:
- Citrate leaves mitochondria and is converted back to Acetyl-CoA
- Acetyl-CoA goes to Malonyl-CoA (conversion ↑d by insulin)
- Fatty acids: via fatty acyl-CoA
- Malonyl-CoA → Fatty-Acyl CoA in the reverse of B-oxidation and creates Triglycerides
- Cholesterol: via HMG-CoA
- blocked by statins
Catabolic Role
Β-oxidation:
- FACoA synthase in cytosol makes FACoA
- FACoA → Acyl carnitine by CPT1 and transported into cell
- Acyl carnitine → FACoA by CPT2
- Undergoes B oxidation
- 1 Acetyl CoA (by cleaving the 2 carbon-CoA from the whole molecule) → Used in TCA cycle
- 1 FADH2 + 1 NADH to be used in the Electron Transport Chain.
- → up to 17 molecules of ATP
Carbohydrate
Anabolic Role
Glucostat Function
- Gluconeogenesis from pyruvate derived from
- Complex polysaccharides (fructose and galactose)
- Glycogen
- Gluconeogenesis
- lactate, pyruvate, glycerol (from TAGs), glucogenic a.a’s (esp Ala and Glu)
- Pentose phosphate shunt
- Glycerol (but not FFAs)
- Glycogen synthesis
Catabolic Role
Glycolysis:
- Primary function
- For generation of pyruvate + 2 ATP
- Pyruvate then utilised to form
- fat, AA’s and ketones
- Lactate
Glycogenolysis:
- From G6P.
Protein
Anabolic Role
Generation of functional proteins in serum:
- Albumin
- Fibrinogen
- Coagulation proteins
- Regulatory proteins
- Coagulation factors
- Complement proteins
- Globulins
- α1 (α1 anti-trypsin, α1-fetoprotein)
- α2 (haptoglobulin),
- β (transferrin)
Amino acids and nucleosides from α-ketogluterate in the TCA
Haeme from Succinyl-CoA in the TCA
Catabolic Role
- Breakdown of proteins to form
- ketones as energy source (liver, heart and brain)
- free amino acids for addition to the AA pool.
- Amino acids utilisation for energy or protein synthesis
- Ammonia metabolism and recycling
- Deamination of fatty acids
- Urea formation for ammonia removal Part of the urea cycle takes place in liver
Gladwin 2016
Examiner Comments
2015A 18: 50 % of candidates passed this question.
Most candidates seemed not to have thought about this before and so collated information from answers about insulin and glucagon, and starvation. Many added information about absorption and digestion which was not required.
Metabolic functions of the liver form part of the “standard list” of functions of the liver yet few details could be provided beyond that. It was expected answers would detail the central role of the liver as a “glucostat” and its role in glucose utilization. It has two main roles in lipid metabolism, the synthesis of fatty acids and the partial oxidation of fatty acids to ketone bodies. The liver also plays a central role in protein catabolism and anabolism. It plays a major role in the breakdown of amino acids gluconeogenesis and protein synthesis. The liver also releases amino acids into the blood for utilization by peripheral tissues.
19. Describe the effects of ageing on the cardiovascular system.
CICMWrecks Answer
Ageing = physiological time-dependent process which results in ↓ cellular function + ↓ reserve
- Heart
- ↑ fibrous infiltration of myocardium → ↓ ventricular compliance (esp LV > RV)
- ↓ # myocytes but compensated by hypertrophy → ↑ myocardial wall thickness (concentric) → ↓ ventricular compliance (esp LV > RV)
- ↑ fibrous infiltration of endocardium → ↓ ventricular compliaince (esp LV > RV)
- Calcification of heart valves → valvular incompetence (stenosis/regurgitation)
- Conducting system (Ie. SAN, AVN, bundle of His, Purkinje):
- ↑ fibrous/fatty infiltration
- ↑ loss of pacemaker cells
- ↓ # and sensitivity of β receptors within pacemaker cells
- Peripheral vasculature:
- ↓ elasticity of large arteries due to thickening and calcification of vessel wall
| Change with ageing | Reason | |
|---|---|---|
| HR | Resting HR → unchanged ↓ maximum HR (Max. HR = 220 – age) ↑ arrhythmias (AF, AV heart block, VEBs, BBB) | Conduction system (SAN, AVN, bundle of His, Purkinje) → fibrous/fatty infiltration and loss of pacemaker cells ↓ # and sensitivity of β receptor of pacemaker cells |
| SV | ↓ resting SV ↓ maximum SV | ↓ ventricular compliance (2° to fibrous infiltration of myocardium/endocardium, and ventricular hypertrophy) Valvular disease |
| CO | ↓ due to preconditioning (Ie. sedentary lifestyle) or age-related disease Unchanged in “healthy” subjects Nb. ↑ C.O. occurs mainly by ↑ SV (preload) via Frank-Starling mechanism (rather than ↑ HR) | ↓ HR – see above ↓ preload (↓ SV) – see above ↑ afterload (↑ SVR) – see below ↑ reliance on “atrial kick” (30% of C.O. vs 5% in adults) → AF/↑HR not well tolerated |
| BP | ↑ MAP and SBP DBP ↑ slightly (until age 60), then ↓ ↑ pulse pressure (due to ↑ SBP > DBP) | ↓ elasticity of large arteries due to thickening and calcification of vessel wall |
| Pulmonary circulation | ↑ PAP a/w ↑ PVR | |
| Control of CVS | ↓ maximum exercise tolerance (due to age-related ↓ maximum HR, SV, C.O.) Impaired BRR → postural hypotension ↓ β receptor response of CVS to catecholamines (↓ # or affinity of receptor, ↓post-receptor signalling, ↓ myocardial contractile response with stimulation) ↑ vagal tone |
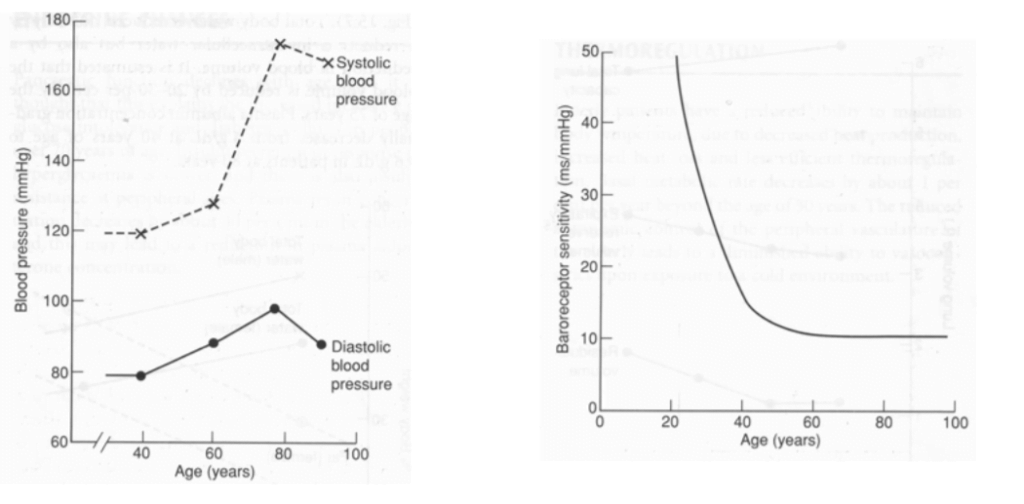
Bianca 2016
Examiner Comments
2015A 19: 29 % of candidates passed this question.
Many candidates described the pathological processes which might affect the aging heart rather than the physiological ones.
Recognition that aging reduces cardiovascular reserve followed up with an outline of the effects of aging on the heart, the vasculature, endothelial function and the conducting system would be rewarded with a good mark.
Few answers quantified the decrease of cardiac output with age and only even fewer ventured into the contribution of ventricular filling by atrial systole. No answer discussed
endothelial changes with aging.
Some answers were repetitious. Some answers included a significant discussion of information that was not asked for (Laplace law/Poiseuille’s law).
20. Describe the anatomy of the sympathetic nervous system
CICMWrecks Answer
Anatomy of SNS
- Posterior hypothalamus is the main site of sympathetic nervous outflow
- Receives input from cardiovascular centres of medulla and pons
- Sympathetic innervation from Sympathetic trunks
- Paired bundle of sympathetic neurons run lateral from vertebral bodies from T1 to L2
- Consists of two neurons in series
- Short Pre-ganglionic neuron → Sympathetic ganglion → Long Post-ganglionic neuron
Origin
- Origin: Preganglionic fibres originate in the grey matter of the spinal cord lateral horn
- Nerve Fibres and Pathway: Leave the spinal cord through ventral roots (with spinal nerves)
- Leave spinal nerves, travel as white rami communicantes (short myelinated B fibres)
- Meet with the ganglia of the sympathetic trunk
- Synapse with post-ganglionic neurons
- Neurotransmitter: Preganglionic neurotransmitter is acetylcholine
- Receptor: nicotinic receptors
Sympathetic trunk:
4 parts
- Cervical part
- Head
- Neck
- Thorax
- Thoracic part (T1-T5)
- Aortic plexus
- Pulmonary plexus
- Cardiac plexus
- Thoracic splanchnic nerves
- Lumbar sympathetic ganglia
- Coeliac plexus
- Pelvic part
- Hypogastric plexus
- Pelvic plexus
Post-ganglionic
- Nerve Fibres and Pathway: Post-ganglionic neurons leave the ganglia as grey rami communicantes
- Long unmyelinated C fibres
- Rejoin the spinal nerves to travel to target organs
- Neurotransmitter: Postganglionic neurotransmitter is noradrenaline
- (acetylcholine in muscles, sweat glands and hair follicles)
- Receptor: Adrenergic alpha and beta receptors on target organs/vessels
Exception is adrenal medullary outflow:
- Modified post-ganglionic cells release adrenaline into circulation
- Innervated directly by pre-ganglion sympathetic fibres
Effects of SNS
| Function | Control the body’s response during perceived threat. Diffuse physiological accelerator |
| Activates response of | Fight-or-flight |
| General Body Response | Body speeds up, tenses up, becomes more alert. Functions not critical to survival shut down. |
| Cardiovascular System | ↑↑↑ Chronotropy, ↑↑↑ inotropy, ↑↑↑ lusiotropy, ↑↑ dromotropy |
| Vasculature | Constriction |
| Pulmonary System | Bronchial tubes dilate |
| Musculoskeletal System | Sweating, contraction, lipolysis |
| Pupils | Dilate |
| Gastrointestinal System | Decreased salivation and GIT motility, increased sphincter tone, gluconeogenesis |
| Salivary Glands | Saliva production decreases |
| Endocrine | Adrenaline and noradrenaline release |
| GU | Detrusor relaxation, sphincter contraction, ↑ uterine tone |
Mooney / JC 2020
Examiner Comments
2015A 20: 25 % of candidates passed this question.
A definition of the sympathetic system, followed by a systematic description of the central sympathetic centres; what happens at the spinal cord; the anatomy of the pre and post ganglionic fibres would have been awarded with a pass mark. Additional information about the sympathetic ganglia and the neurotransmitters involved would have rounded off a good answer.
Many answers lacked anatomical detail and described the actions (function) of the sympathetic system which was not asked for.
Most answers lacked any structure. The most common reason for not passing this question was that significant sections of the anatomy from central to peripheral were not mentioned. Most had a simple sketch understanding of the question asked but could not add enough of the next layer to be awarded a pass mark.
21. Describe the pharmacodynamic effects and indications for the use of anticholinesterase drugs.
CICMWrecks Answer
Background
- Acetylcholinesterase (AChE) is an enzyme that hydrolyse acetylcholine (ACh) into choline & acetate
- AChE is found in synaptic clefts and is responsible for the termination of synaptic transmission
- Common action of anti-cholinesterases = allow build up of Ach and prevent it from being destroyed.
Naturally Occuring Cholinesterases
Two types of naturally occurring cholinesterases:
- Achesterase – nerve endings & in RBCs
- Non-specific or pseudocholinesterases – destroy other esters – tissues & plasma
Anticholinesterase drugs
AntiAchE drugs are administered in anaesthesia when spontaneous recovery from NDNMB is occurring to accelerate it.
Classification:
Drugs are classified by the way in which they inhibit the activity of AchE.
3 main types of anticholinesterase:
- Reversible antagonist via electrostatic binding
- ie. edrophonium
- causes electrostatic attachment to the anionic site of the enzyme -> stabilising the H+ bond at the esteratic site -> edrophonium-AchE complex prevents Ach from binding
- Reversible antagonist via covalent bonding [Formation of carbamyl esters (carbamates)]
- ie. neostigmine, physostigmine & pyridostigmine
- antagonise AchE enzyme by being competitive substrate for Ach -> forms a carbamyl-ester complex at the esteratic site of enzyme.
- longer lasting bond (15-30min)
- Irreversible antagonist via covalent bonding
- ie. organophosphate anticholinesterase drugs (echothiopate), insecticides & nerve gases
- combine with Ach at the esteraic site to form a stable covalent bond -> does not undergo hydrolysis.
- synthesis of a new AchE is required.
Pharmacodynamic effects
Anticholinesterase drugs inhibit AChE, thereby increase the concentration of ACh at both nicotinic and muscarinic ACh receptors
Muscarinic effects occur at lower doses than nicotinic effects
Muscarinic effects
- CVS – bradycardia ± hypotension
- RESP – bronchoconstriction ± bronchospasm
- CNS – miosis, cholinergic syndrome – confusion, agitation, nausea/vomiting
- GIT – hypersalivation, ↑GIT motility, nausea/vomiting, diarrhoea
- GUT – urination, incontinence
- OTHER – lacrimation, diaphoresis
Mnemonic: SLUDGE-BM: Salivation/Sweating, Lacrimation, Urination, Diaphoresis/Diarrhoea, GI upset, Emesis, Bradycardia/bronchospasm, Miosis
Nicotinic effects
- Reversal of non-depolarising neuromuscular blockers
- Prolongs effect of suxamethonium (depolarising NMB)
- Anticholinesterase overdose → excess synaptic ACh → depolarisation block ± fasciculation
Clinical uses
- Reversal of non-depolarising neuromuscular blocker
- Mechanism – anticholinesterase drugs ↑ synaptic ACh → competes with ND-NMB in synapse for nAChR → reversal of neuromuscular block
- Drugs – usu. neostigmine, administered together with glycopyrrolate or atropine
- Diagnosis and treatment of myasthenia gravis
- Mechanism – anticholinesterase drugs ↑ synaptic ACh → competes with myasthenia auto-antibodies for post-synaptic nAChR → ↑muscle strength
- Drugs – e.g. edrophonium for diagnosis, pyridostigmine for maintenance
- Treatment of cognitive impairment in neurodegenerative diseases (e.g. Alzheimer’s disease, Lewy body dementia, etc)
- Mechanism – ↑ synaptic ACh in CNS → ↑ cholinergic transmission
- Drugs – e.g. rivastigmine, galantamine, donepezil
- Treatment of glaucoma
- Mechanism – constriction of sphincter pupillae and ciliary muscles → miosis → facilitate outflow of aqueous humor → IOP decreases
- Drugs – e.g. echothiophate eye drops, physostigmine
- Treatment of anticholinergic syndrome
- Anticholinergic syndrome caused by: anti-histamines, anti-parkinsonians, atropine, anti-spasmodics, mydriatics, skeletal muscle relaxants, plants
- CLINICAL FEATURES: delirium, tachycardia, dry and flushed skin, dilated pupils, myoclonus, hyperthermia, urinary retention, bowel sounds, seizures, dysrhythmias(tachy)
- Mechanism – increase synaptic ACh
- Drugs – e.g. physostigmine (tertiary amine + lipophilic → readily crosses BBB)
JC 2019
Examiner Comments
2015A 21: 25 % of candidates passed this question.
It was expected the answer would provide a structured approach to describing the pharmacodynamics (what the drug does to the body) of this discreet class of drugs. A brief acknowledgement of the drugs in this class followed by a catalogue of the various clinical uses of this class of drugs would be a good start. If this was followed up with a description of the effects of these drugs on the CVS, GIT, Salivary glands, eye, NMJ and the lungs a good mark would have been awarded.
A number of candidates described the actions at the receptors in detail which did not attract marks. The extensive range of clinical uses for this class of drugs was poorly appreciated. Few answers demonstrated any understanding of the PD effects of the drug class. There was generally a good knowledge of representative drugs within this class. Failing to achieve a pass mark reflected scant/brief answers that just did not cover enough of the expected material.
22. Compare and contrast dexmedetomidine and ketamine
Examiner Comments
2015A 22: 50 % of candidates passed this question.
The majority of candidates were able to describe the mechanism of action, uses, dose and some side effects of each drug. The better answers were in a table format. It is of course possible to include much of the relevant information without using a table; however without the visual prompt of a table it makes it likely sections will be omitted.
When comparing two drugs it would be useful to note that though they both provide sedation with analgesia they are used in different circumstances. In ICU, dexmedetomidine is mainly used for sedation peri-extubation and may be continued post-extubation but this was not often mentioned.
The pharmacodynamic effects often omitted the cardiovascular and respiratory effects of ketamine (particularly bronchodilation).
The pharmacokinetic information required was not detailed but only minimal marks can be awarded for ‘administered IV with 100% bioavailability, liver metabolism and renal excretion’ which was a common answer. Noting dexmedetomidine is metabolised to inactive metabolites and ketamine is metabolised to norketamine gained marks, specific pathways were not required. Both drugs are licenced for administration intravenously (and ketamine may be administered IM); however other routes of administration are emerging in clinical practice for both drugs.
23. Statistics (not in current primary syllabus)
24. Outline the anatomy of the larynx
CICMWrecks Answer
Cartilages
- Unpaired
- Thyroid cartilage – Level of C4~5
- Cricoid cartilage
- Epiglottis
- Paired
- Arytenoid
- Cuneiform
- Corniculate
Muscles
- Intrinsic
- Cricothyroid
- Originates in cricoid cartilage and inserts into thyroid
- Tenses vocal cords and elevates voice
- Thyroarytenoid
- Originates in thyroid cartilage and inserts into arytenoid cartilage
- Relaxes vocal cords and depresses voice
- Posterior cricoarytenoid
- Abducts the vocal cords
- Lateral cricoarytenoid
- Adducts the vocal cords
- Oblique and transverse arytenoids
- Adducts the vocal cords
- Cricothyroid
- Extrinsic
- Strap muscles
Innervation
Sensory
- Internal branch of superior laryngeal nerve
Motor
- Cricothyroid – External branch of the superior laryngeal nerve
- All other intrinsic muscles – Recurrent laryngeal nerve
Blood supply
- Superior laryngean artery (branch of external carotid)
- Inferior laryngeal artery (branch of thyrocervical trunk from subclavian artery)
Associations
- The thyroid glands lie inferolateral to the larynx (lateral to the cricoid, the isthmus is inferior to the cricoid)
- The oesophagus, anterior longitudinal ligament, cervical vertebrae lies posterior to the larynx
- The brachiocephalic trunk may arch superiorly close to the cricothyroid membrane in an anatomical variant
Sakurai 2016
Examiner Comments
2015A 24: 13 % of candidates passed this question.
It was expected that an answer would include the names of the three single and three paired laryngeal cartilages, intrinsic and extrinsic muscles (names were not required), nerve supply (motor and sensory) and blood supply. Many candidates had good illustrations though a drawing was not essential.
The majority of candidates failed to name the laryngeal cartilages. There was much confusion about whether certain structures were bones or cartilage or even muscle. The relation of the larynx to the thyroid gland was frequently misunderstood.
Many answers focussed on the relations of the larynx but omitted basic information about the larynx itself. No marks were awarded for the contents of the carotid sheath or the course of the recurrent laryngeal nerve both of which were frequently included in answers.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | respiratory physiology, capnography |
| G. CVS | cardiovascular physiology, ECG and Antiarrhythmic cardiovascular pharmacology and pharmacodynamics obesity and focused on the physiological cardiovascular changes, consequences for various other organ systems and drug pharmacology. |
| H. Renal | renal physiology and related pharmacology |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | |
| L. Musculoskeletal | neuromuscular physiology and pharmacology |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | nutrition and metabolism |
| Q. Haematology | physiology and pharmacology of iron and haemoglobin |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments