1. Outline the motor and sensory pathways involved in withdrawing the lower limb from a painful stimulus.
CICMWrecks Answer
Withdrawal reflex is a spinal reflex intended to protect the body from damaging stimuli.
The upper centres are not involved.
Afferent
- Nociceptors detect the painful stimulus
- Conveyed to the dorsal horns of spinal cord by Aδ (fast) fibres and C (slow) fibres
- Through the cauda equina
- Synapse at the same spinal level, or 1-2 levels above via Lissauer’s tract
Reflex arc
- (fast response to painful stimulus)
- (outside of conscious control, but can be modulated by the cerebral cortex)
- Interneurons:
- Travel to the ipsilateral anterior horn, synapse with motor neurons
- Cross the midline to the contralateral anterior horn
- Efferent
- Motor neurons in the ipsilateral limb cause contraction of flexors, and relaxation of extensors
- Contralateral limb motor neurons cause relaxation of flexors, and contraction of extensors
- Causes withdrawal of painful limb, and shift In centre of gravity to the opposite limb
Conscious pathways
(slow response to painful stimulus)
Interneurons:
- Cross the midline via the anterior white commissure
- Ascend in the spinothalamic tract to the thalamus
- Synapse in the thalamus
- Travel to the post-central gyrus of the cerebral cortex
Efferent:
- Originate in the motor cortex (pre-central gyrus)
- Project to medulla
- 90% decussate at pyramids to innervate limbs
- Travel down the lateral corticospinal tract
- 10% continue down ipsilateral anterior corticospinal tract to innervate axial muscles
- Decussate at the target spinal level
- Synapse at the anterior horn to lower motor neurons
Mooney / Sakurai 2016
Examiner Comments
2014A 01: 26% of candidates passed this question.
It was expected that candidates would outline both motor and sensory pathways and mention
a reflex arc and conscious pathways.
2. Classify calcium channel blockers, and give an example for each classification. (30% of marks) Describe the pharmacology of verapamil. (70% of marks)
CICMWrecks Answer
Calcium Channel Blocker Classification
- Class I – Phenylalkylamines:
- Verapamil
- Reduces HR, contractility and causes vasodilatation, decrease in cardiac contractility, heart rate, and both coronary and peripheral vascular tone.
- Class II – Dihydropyridines:
- main effects are on vasodilatation
- 1st gen = nifedipine
- 2nd gen = felodipine, nimodipine
- 3rd gen = amlodipine
- Class III – Benzothiazepines:
- diltiazem
- some reduction in HR and contractility, also causes vasodilatation, mild to moderate decrease in myocardial contraction, and decreases heart rate by decreasing both the automaticity of the SA node and the rate of conduction in the AV node.
Calcium Channel Blocker Pharmacology
(Include as per the question)
Click here to Open CICMWrecks Table: Calcium Channel Blockers
Class Effects:
- Heart
- V-W Class 4 antiarrhythmic
- Decr SA node automaticity
- Decr AV node conductivity
- Vasodilation
- Negative inotropy
- reflex tachycardia
- 2) Vasculature
- Decr SVR and BP
- Decr Hypoxic pulmonary vasoconstriction
- Incr coronary and cerebral blood flow
- 3) Other
- Tocolytic
- NDMR potentiation.
Gladwin / JC 2021
Examiner Comments
2014A 02: 61% of candidates passed this question.
Most candidates managed to provide a classification of calcium anatagonists. The pharmacology of verapamil was less well understood. The structure for answering a pharmacology question was often poor and there was commonly a lack of precision in pharmacokinetics. We suggest that candidates look at a general pharmacokinetic structure when answering these questions. One approach would be a structure that covers drug name and description, pharmaceutics (chemistry/ ampoule contents), Pharmacokinetics, Pharmacodynamics (Think CNS/CVS/Resp/GIT etc. if relevant), Dose and Side effects then Indications and Contraindications can help organize the information.
3. Describe the neural integration of vomiting. (60% of marks) Describe the pharmacology of ondansetron. (40% of marks)
CICMWrecks Answer: Physiology of Vomiting
Physiology of Vomiting
Vomiting
- Involuntary, forceful and rapid expulsion of gastric contents through the mouth
Triggers
- Excessive GI tract distension
- Stimulation of CTZ
- Directly by certain drugs eg apomorphine, morphin
- Rhythmic motion of the body stimulating the vestibular labyrinth of the inner ear
- Cerebral excitation secondary to odours, pain, stress
Central control of vomiting
- Vomiting center (5HT3, NK1, Muscarinic and Histamine), near NTS
- Inputs
- Chemoreceptor trigger zone (NK1, 5HT3, Dopamine), area postrema outside BBB
- Vestibular system (Histamine and Muscarinic) via CNVIII
- GI Tract (5HT3, stretch and chemoreceptors) via vagal
- Higher centres
- Efferent arc
- via CN V,VII,IX,X,XII and spinal nerves to abdominal wall musles and diaphragm
- Inputs
| Afferent signal | Sensor | Efferent signal | Effect |
|---|---|---|---|
| Chemo/ baroreceptor input Drugs Stim from NTS Stim from GIT (5HT3) | CTZ (area postrema) | 5HT3, D2 and Opioid neurons | Direct stimulation of vomit centre in lateral reticular formation |
| Surgery/ Rhythmic motions | Labyrinths | ACh and H1 neurons | |
| Memory/ Emotions Sensory (sight, smell, taste) | Cortex | ||
| Severe painful stimuli | Pain | H1 neurones | |
| Irritation Manipulation during surgery Distension | GIT | NK1, NAdr, ACh neurons | |
| Via CNX, to NTS | Stimulation of CTZ then Vomit centre | ||
| Via 5HT3 to CTZ | CTZ to vomit centre | ||
| Manipulation of pharynx via CN9 to.. | NTS | Direct to CTZ | To vomit centre |
Vomiting act
- Antiperistalsis as the prelude to vomiting
- At the onset of vomiting, strong intrinsic contractions occur in both the duodenum and the stomach
- Partial relaxation of the lower oesophageal sphincter (LOS)
- Deep inspiration
- Raising of the hyoid bone and larynx to open the upper oesophageal sphincter
- Glottic closure
- Lifting of the soft palate to close the posterior nares
- Strong down ward contraction of diaphragm and simultaneous contraction of all the abdominal wall muscles
- Complete relaxation of the LOS
Gladwin / Sakurai 2016
CICMWrecks Answer: Pharmacology of Ondansetron
Pharmacology of Ondansetron
Examiner Comments
2014A 03: 58% of candidates passed this question.
Candidates who failed the question did not answer the actual question. They did not discuss the neural integration but instead listed various inputs into the CTZ and vomiting centre. It was expected candidates could detail the pathways involved (afferent and efferent limbs) and describe the relationship with the coordinating centres. For example, afferent pathways to the vomiting centre include stretch and chemoreceptors located throughout the GIT via vagal and sympathetic nerves, pharyngeal touch receptors via glossopharyngeal nerves etc. Again structuring pharmacology answers was often poor.
4. Describe the respiratory changes during pregnancy
CICMWrecks Answer
Respiratory Changes In Pregnancy
Anatomical
- Diaphragm ascends ~4cm due to foetus
- AP and lateral diameters of thorax increase 2~3cm due to relaxin effect on chest
wall - Thoracic circumference increases 5~7cm (AP and lateral diameters increased 2~3cm)
- Airway dilatation → decreased resistance (35%) and increased deadspace (45%)
- Increased cardiac output, intravascular volume, and decreased oncotic pressure
- Increased plasma volume → increased pulmonary blood volume → decreased compliance
- Venous engorgement → Upper airway oedema
Mechanical
- Lung volume changes occur from 20 weeks
- TLV decreases 5% from baseline
- FRC decreased 20% due to both ERV and RV
- Significant postural change in FRC of 70%
- Progesterone → increased sensitivity to CO2 → Minute ventilation increases 50%
- TV increases (25%)
- RR increases
- Results in hypocarbia and compensated respiratory alkalosis
- pCO2 ~ 26-32mmHg
- HCO3 ~ 18-20mmol/l
- Further increase in MV during contractions
- Inspiratory capacity increased by 10%
- Closing capacity unchanged
- Increased O2 consumption during labour (60%)
After birth
- FRC and RV return to normal within 48 hours
- TV returns to normal within 5 days
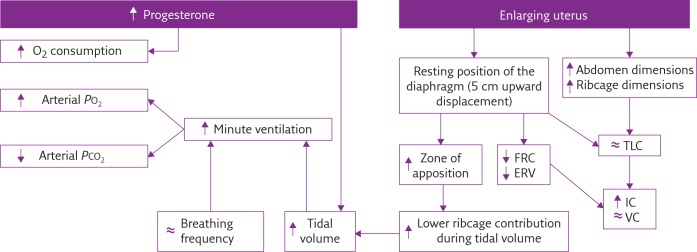
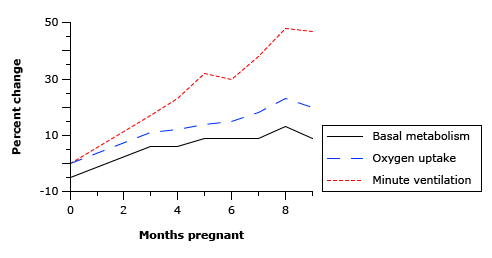
Sakurai 2016
Examiner Comments
2014A 04: 26% of candidates passed this question.
This question was variably answered, with a wide range of marks. Candidates often mentioned central regulation, anatomy, respiratory mechanics, static lung volumes or gas transfer, but did not provide comprehensive cover of all areas. Very few clearly described the extent of any change (either as a percentage or actual amount relative to the non-pregnant state). A number of candidates presented information well in diagrammatic form (e.g. static lung volumes) however then repeated this same information in text, which was unnecessary.
5. Describe the pharmacological effects of paracetamol. (40% of marks) Outline its toxic effects and their management. (60% of marks)
CICMWrecks Answer
Pharmacological effects of Paracetamol
- Arachidonic acid ——(COX)—–>
- Prostaglandins → inflammation, sensitise spinal nerves to pain
- Prostacycline → vasodilation
- Thromboxane → platelet aggregation and activation
- Paracetamol: Weak COX-1 and COX-2 inhibitor in peripheral tissues
- Relatively selective for COX-2 → less inhibition of thromboxane production
- Possible cause for antipyretic effect:
- Inhibition of COX-3 (variant of COX-1), leading to central effects
Toxic Effects and Management
Toxicity
- Occurs in doses >4g/day
- Overdose dose lower in chronic alcoholics, elderly and children
- Paracetamol
- → glucuronidation
- → sulphation
- → N-hydroxylation to NAPQI
- In overdose, glucuronidation and sulphation pathways saturated → increased production of toxic NAPQI
- NAPQI usually conjugated with hepatic glutathione
- In overdose, glutathione exhausted → accumulation of NAPQI
- NAPQI bonds with hepatocyte sulfhydryl groups → cell death and necrosis
Therapy
- Decision to treat based on paracetamol overdose nomogram
- Serum paracetamol level, time since dose
- If within 4 hours → activated charcoal
- Hepatic failure, GI upset, renal failure (less common)
- N-acetyl-cysteine should be given
- Replenishes glutathione stores
- Directly conjugates NAPQI
- Monitoring and supportive care:
- Bilirubin
- Transaminases
- Creatinine (dialysis)
- Liver synthetic function (albumin, INR) (vitamin K, maybe FFP, but discuss with liver unit first)
- Blood sugar (glucose infusion)
- Conscious level (hepatic encephalopathy) (lactulose, intubation)
Mooney 2016
Examiner Comments
2014A 05: 63% of candidates passed this question.
This question was generally well answered with narrow variance; very few candidates discussed factors predisposing to hepato-toxicity or renal toxicity. Discussion of pharmacokinetics only gained marks when relevant to toxicity.
6. Statistics (not in current primary syllabus)
7. Outline the principles underlying pulse oximetry. ( 80% of marks)
Briefly describe the effect of an elevated level of the following upon pulse oximetry values. (20% of marks) a. Carboxyhaemoglobin b. Methaemoglobin
CICMWrecks Answer
Principles
Beer-Lambert Law
- Incidence of light is inversely proportional to the path distance and concentration of light absorbing particles within the path.
Hb absorbance at 660nm and 940nm utilized
- 660nm maximally absorbed by deoxyhaemoglobin
- Ratio of absorbance at 660nm and 940nm utilized to calculate SpO2
using healthy volunteers derived R values
Sats 100% R = 0.4
Sats 85% R = 1.0
Sats 50% R = 2
Sats 0% R = 3.4
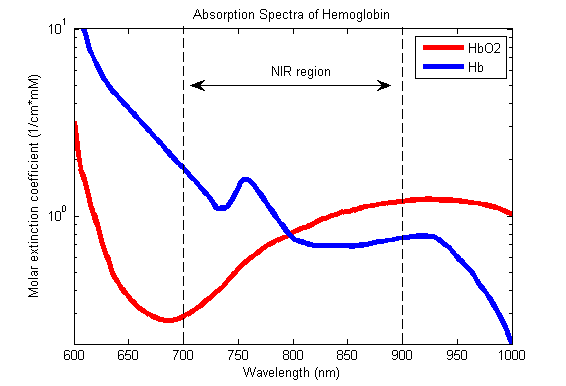
Pulse oximeter
- Light emitter
- Produces infra-red light at 660nm and 950nm
- Light detector
- Detects light at 660nm and 950nm
- Processor
- Calculates SpO2 using the ratio of absorbance at 660nm and 950nm
- Light detected (incidence) represents light passing through both pulsatile (arterial) blood and non-pulsatile elements (venous blood and other tissues)
- Processor differentiates light incidence during maxima and minima of pulse wave and calculates SpO2 from pulsatile blood only giving SaO2
Limitations
| PATIENT FACTORS | ||
| Low or High SpO2 | Low SpO2 | Normal or High SpO2 |
| Met-Hb Sulph-Hb | Poor perfusion of finger Movement artifact Venous pulsations Fingernail polish Intravenous pigmented dyes Haemoglobinopathy Anaemia with co-existing hypoxia | Carbon Monoxide poisoning |
| EQUIPMENT FACTORS | ||
| – Ambient light interference – Poorly fitting probe – Assay calibrated using healthy volunteers only down to SpO2 80%. Unknown significance if tested SpO2 less than 80% | ||
| PHYSIOLOGICAL FACTORS | ||
| – Due to O2 dissociation curve, insensitive to changes above PaO2 80mmHg – Does not measure tissue oxygenation | ||
Absorption spectra confounded by:
- Carboxyhaemoglobin
- Absorbs 660nm, not at 940nm
- R closer to 0.4
- causes the pulse oximeter to read artificially high
- Methemoglobin
- though it absorbs 660nm light, it also absorbs 940nm light to a greater degree
- R closer to 1
- causes the SpO2 to trend towards 85%
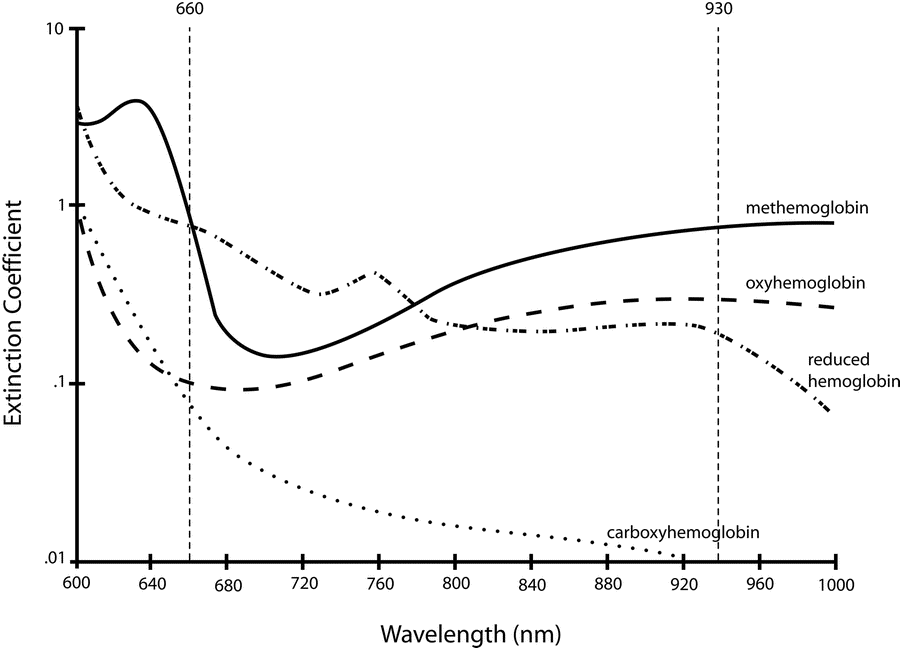
Sakurai 2016
Examiner Comments
2014A 07: 34% of candidates passed this question.
Explanation of several crucial principles was expected for a good answer. These would include that Haemoglobin can be measured and quantified with a light absorbance technique; based on the Beer Lambert law (a description of this was required). In addition, oxygenated haemoglobin must be distinguished from reduced haemoglobin (the 2 dominant species of Hb) and that the oximeter determines pulsatile from non pulsatile blood. The oximeter accounts for ambient light and that “R”, a ratio of absorbances during pulsatile and non pulsatile flow is calculated and compared within a computer algorithm to standardised values of SaO2 to deliver a final value.
Mention of limitations was not required except to answer the second part of question. Common omissions included failure to describe accurately the Beer Lambert Law, and no explanation of how pulsatile component was detected, or ambient light accounted for. Many candidates understood the clinical inaccuracy associated with CO and Met HB, but failed to identify the spectrophotometric reason and application of the R value for this discrepancy.
8. Describe the physiology of skeletal muscle cell contraction.
CICMWrecks Answer
Sarcomere
“Sarcomere” → basic unit of muscle contraction within myofibril
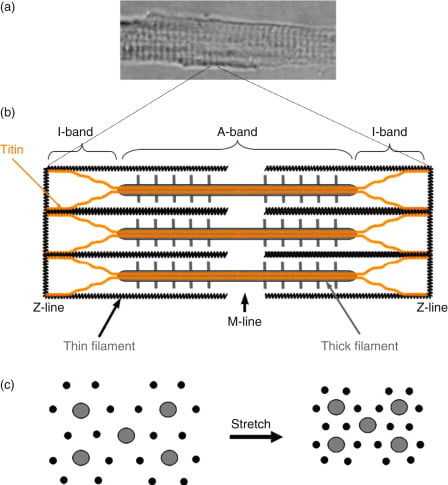
I band – Has actin filaments only
A band – Contains myosin and some actin
H band – Band of myosin only
M line – Joins myosin filaments together
Components of the sarcomere:
- Myosin
- Large protein that consists of:
- 1x long tail – 2 interwound α-helices with a flexible hinge (S2 segment)
- 2x globular heads (S1 segment) – Connected to long tail via S2 segment. Each head has 1x heavy chain that binds 1x G-actin, and 2x short chains that bind ATP
- 2x myosins fuse together at M-line via their long tails → forms “thick filament”
- Large protein that consists of:
- Actin (thin filament)
- Consists of 2x chains of F-actin (≈ double-strand cord) formed from the polymerisation of G-actin
- Tropomyosin
- 2x α-helical chains that lie between 2x chains of actin polymers → under the influence of Troponin, it can acts to inhibit or permit myosin-actin interaction
- Troponin
- Present with tropomyosin on thin filament at regular intervals (every 7 actin monomers)
- Consists of 3 subunits – (a) T (binds tropomyosin), (b) I (inhibits myosin ATPase) and (c) C (binds Ca2+)
- Binding to Ca2+ causes a conformation change in troponin-tropomyosin complex → allows actin to interact with myosin
- Titin
- Large elastic molecule that attaches myosin to Z-line → acts to return muscle to
resting length
- Large elastic molecule that attaches myosin to Z-line → acts to return muscle to
Process of Muscle Contraction and Relaxation:
(1) Excitation-contraction coupling:
- Motor neuron depolarisation
- ACh vesicular release
- Multiple MEPs → End plate potential
- Muscle AP propagates via T-tubles
- Opens L-type Ca channels
- Calcium induced calcium release from SR
- Ca-troponin C interaction
- Alteration and movement of Trop-tropomyocin complex
- Exposure of Actin
- Removal of Troponin I inhibition of myosin ATPase
- Cross bridge cycling
2) Contraction (Sliding filament cross bridge cycling):
- “Flexed” Myosin without ATP binds to Actin.
- Binding of ATP to Myosin causes release of Actin/Myosin complex and extension of the head
- In the presence of Ca, Actin binding sites are open and binding of myosin/Actin/ATP complex is formed
- Hydrolysis of ATP to ADP + P in myosin head causes a conformational change “initial flexing” the myosin head and releasing phosphate
- Release of ADP further flexes Myosin head
- In presence of Ca return to step 2
(3) Muscle relaxation:
- AChE metabolises ACh in the NMJ → ↓ nAChR activation at MEP → ↓ EPP and muscle AP generation
- SR actively sequesters Ca2+ (via Ca2+-Mg2+ ATPase) → ↓ IC [Ca2+] → Ca2+ removed from troponin C → tropomyosin mediated inhibition of actin-myosin interaction returns
- Elastic components of the sarcomere causing the muscle to return to its normal length
Energy Source for Muscle Contraction
- Energy (in the form of ATP) is needed for muscle contraction AND relaxation
- The method of ATP generation depends on the level of activity present:
- Creatinine phosphate store
- ATP generated from Creatine phosphate during intense exercise
- ADP + Creatine phosphate → ATP + creatinine (enzyme: Creatine Phosphokinase)
- Replenished at rest by reversing the above reaction by aerobic/anaerobic generation of ATP
- Aerobic generation of ATP:
- Glucose (and Glycogen stores) – ATP is produced during exercise/activity by substrate phosphorylation (via glycolysis) and oxidative phosphorylation (via Kreb’s cycle and the ETC)
- Fat – β-oxidation of fat produces ATP during light exercise and at rest
- Anaerobic generation of ATP:
- When O2 supplies are insufficient, ATP is produced in smaller amounts from the anaerobic glycolysis of glucose → produces lactic acid
- Creatinine phosphate store
JC / Gladwin 2020
Examiner Comments
2014A 08: 34% of candidates passed this question.
This question required a description of excitation- contraction coupling. Marks were gained for a brief outline of the structure of a sarcomere and how it facilitates shortening. An explanation of membrane processes, receptor interactions and the contraction processes was required. Mention of the role of ATP was also required and marks were gained for commenting on the mechanism of return to the relaxed state.
Most candidates wrote extensively on the nerve action potential and neuromuscular junction transmission, with minimal reference to events occurring within the skeletal muscle cell membrane. They could not gain marks for this. Few candidates demonstrated knowledge of the ATP dependent walk along processes of myosin heads during contraction.
9. Discuss the factors that may potentially influence the speed of onset of neuromuscular blockade.
CICMWrecks Answer
Drug Factors
- Dose: Higher dose (eg. multiples of ED95) = faster onset.
- Plasma clearance: Postulated that drugs with faster clearance (eg. sux) have faster onset.
- Potency:
- Inverse log relationship between potency and speed of onset (non-depolarisers).
- Low potency = bigger dose needed (more molecules), so higher conc gradient between plasma and site of action = more rapid onset.
- Depolarisers vs non-depolarisers.
Patient Factors
- Rate of injection: faster rate = faster onset.
- Site of injection: Central vein (more rapid distribution)> peripheral vein > IM
- Cardiac output and muscle blood flow: Higher CO and muscle flow = faster delivery to site of action.
- Priming: small dose of non-depolariser as a premed can partially block NMJ, followed by intubating dose. This decreases time from intubating dose to intubation conditions.
- Disease states: eg. myasthenia gravis (fewer ACh receptors, so needs more sux, but about 10% dose of non-depolariser).
Other factors
- Muscle group: Fastest affected = small, rapidly moving muscle groups (eye, digits), with trunk/abdo muscles last affected.
- Recovery is in reverse order: diaphragm/intercostals recover first.
- Onset more rapid in muscle groups relevant to intubation: laryngeal, masseters. Slower in monitored muscles (eg.adductor pollicus).
- Probably due to muscle blood flow.
- Smaller volume of distribution due to blood loss can augment potency ie: speed of onset(Stoelting)
Drugs:
eg. ephedrine can increase speed of rocuronium onset. Inhalationals/aminoglycosides.
JC 2019
Examiner Comments
2014A 09: 16% of candidates passed this question.
Speed of onset is related to how quickly an effective dose reaches the neuromuscular junction, the type of interaction with the receptor and the margin of safety of the receptors. Examples of parameters that increase speed of onset include a high drug rate of delivery (high CO, high muscle
group blood flow, and fast injection rate), high drug concentration (higher dose, low potency, higher ED 95, lower protein binding) or a depolarising block. A good answer would include a list of these factors, with a brief explanation. Mention of other factors such as electrolyte disturbances gained additional marks. It was expected that the direction of an effect would be clearly indicated (e.g. “potency” would not score a mark unless the candidate wrote – “low potency increases speed of onset”, etc.). These drugs are charged molecules which do not cross cell membranes and have a low volume of distribution. Absorption from GIT, Lipid solubility, pKa, metabolism and clearance have minimal relevance to speed of onset.
10. Classify bacteria according to the Gram stain system and their shape. Give two examples for each classification. (40% of marks) List with examples the mechanisms of bacterial antibiotic resistance. (60% of marks)
CICMWrecks Answer: Classify Bacteria
Gram Stain
Method of differentiated bacteria according to their cell wall characteristics, using
staining with crystal violet dye
- Gram positive bacteria have a thick outer cell wall composed of peptidoglycan,
which stains positive with crystal violet dye - Gram negative bacteria have an outer cell membrane enclosing a thinner
peptidoglycan cell wall, which has decreased affinity to the crystal violet dye
Classification of bacteria
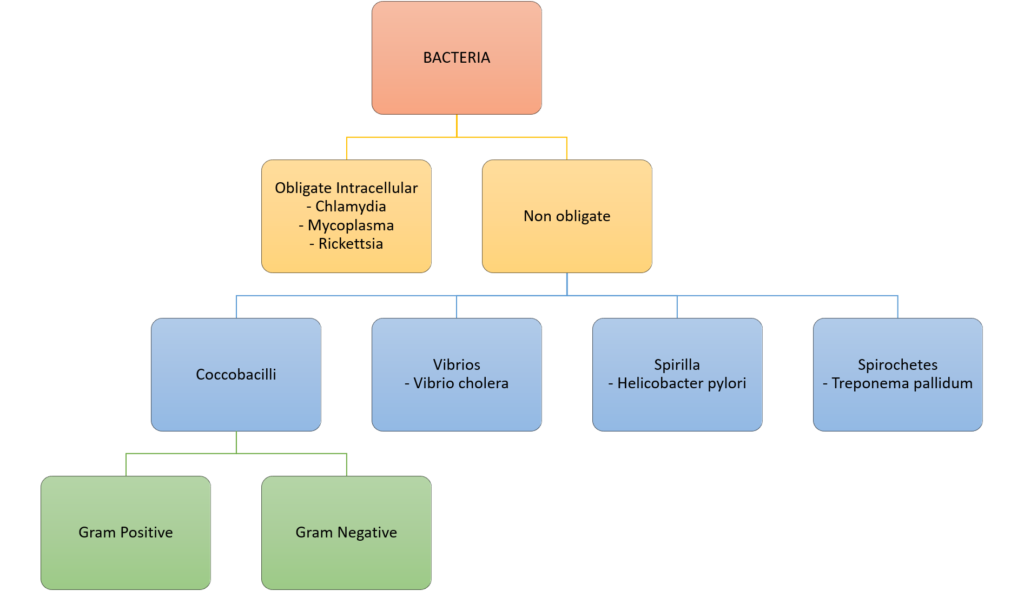
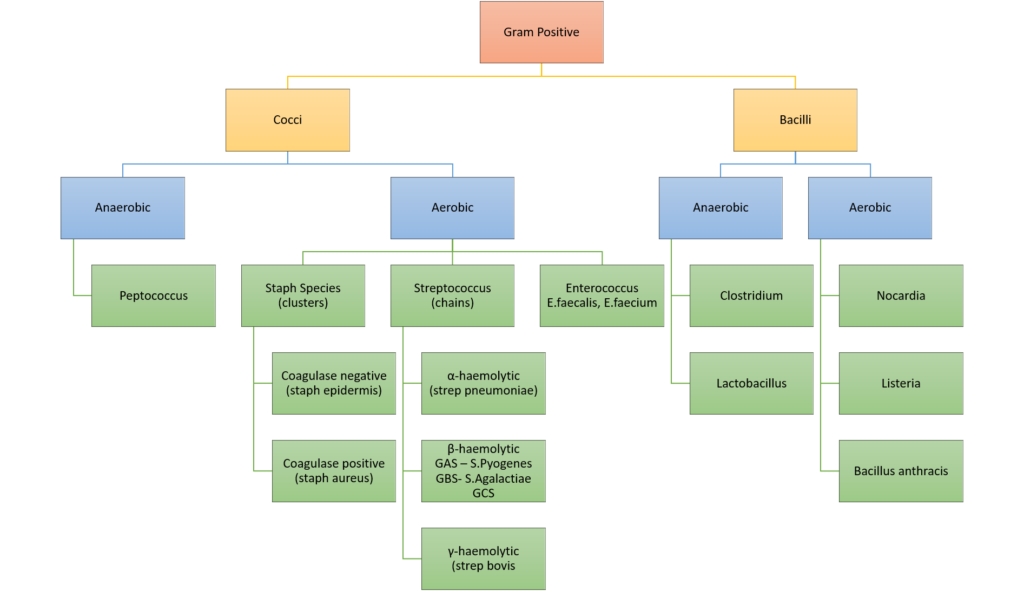
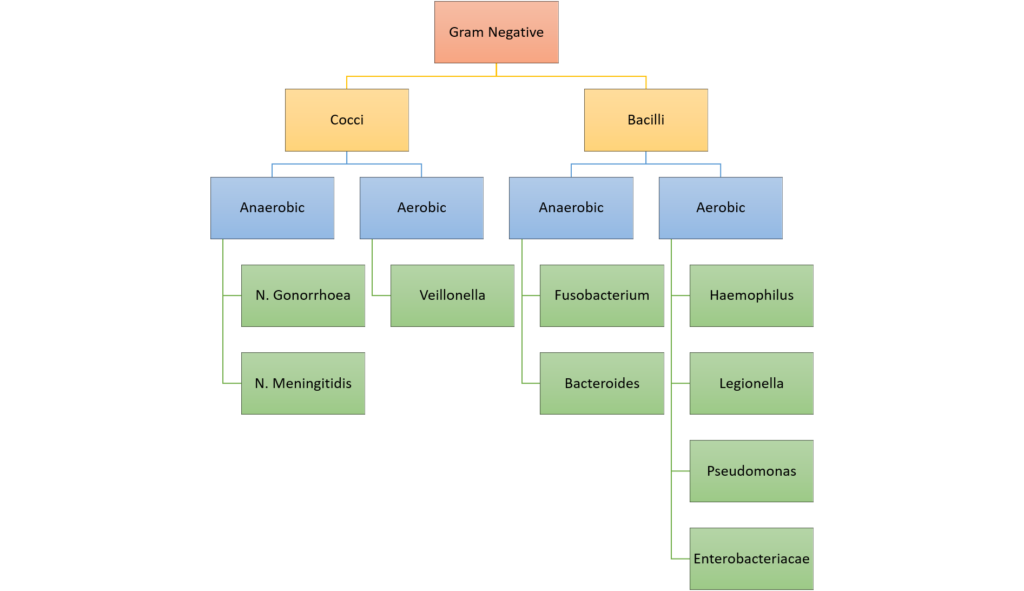
Gladwin / Sakurai / JC 2020
CICMWrecks Answer: Antibiotic resistance
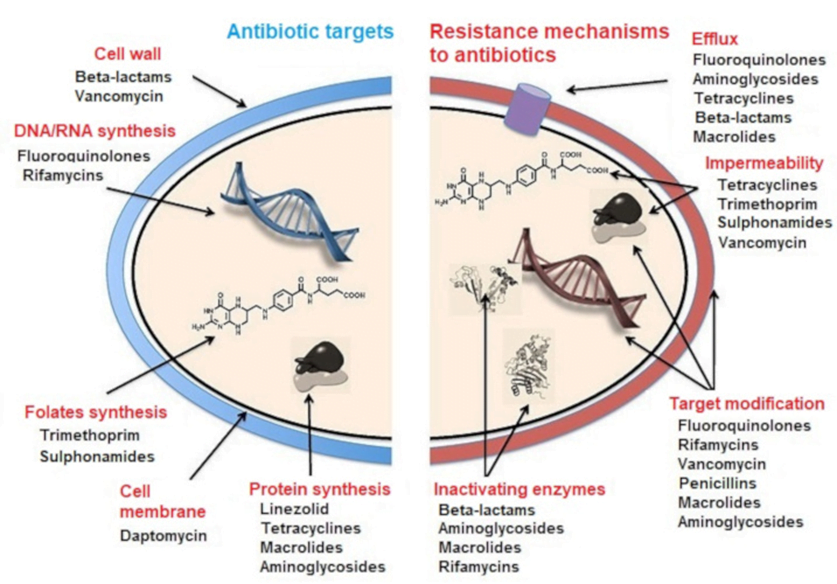
MECHANISMS OF ANTIBIOTIC RESISTANCE
- Mechanisms of Antibiotic Resistance can be classified broadly:
- Efflux Pumps
- Blocked Penetration / Alteration in access to target site
- Target Modification
- Modification of Drug or pathways
- There might be multiple resistance mechanisms at play in the same organism
| Efflux Pumps: Increased efficiency or expression of efflux pumps (inner membrane proteins). | |
| Removed from cell | Active transport of ciprofloxacin out of the bacterial cell |
| E.Coli to tetracycline. | |
| Trapped between cell wall layers | glycopeptide resistance in VRSA. |
| Blocked Penetration / Alteration in access to target site | |
| Narrowing of porin channels | Streptococcal resistance to penicillins typically occurs by reducing access to PBPs. |
| Alteration of porins in gram negative outer membranes | downregulation of Outer Membrane Proteins (eg enterobacteriaceae, pseudomonas vs penems/cephs) |
| increased selectivity of Outer Membrane Proteins (eg klebsiella outbreaks) | |
| Loss of non-essential transporter channels | Anaerobes have no oxygen-transport channel which prevents penetration by aminoglycosides (Gentamicin) |
| Reduced binding of the antibiotic | VanA and VanB vancomycin resistance involves a gene mutation leading to decreased affinity of vancomycin for the binding sites of peptidoglycan precursors |
| Changes to the DNA-binding surface of DNA supergyrase infers resistance against ciprofloxacin | |
| Target Modification | |
| Modifying the enzyme that the antibiotic inhibits | Rifampicin resistance by point mutations, insertions, or deletions in RNA polymerase gene |
| Production of an alternative enzyme for that which the antibiotic inhibits | MRSA develop or acquire the gene mecA which synthesizes an additional penicillin binding protein that enables it to continue cell wall synthesis in the presence of a beta lactam drug |
| Overproduction of the target of the antibiotic | Trimethoprim DHFR enzyme overproduction in Escherichia coli and Haemophilus influenzae. |
| Synthesis of target-protecting proteins | Ribosomal protection proteins against Tetracyclines in multiple Gram positive and gram negative bacteria |
| Modification of Drug or pathways | |
| Development of metabolic pathways to bypass site of action of antibiotic | Resistance to TMP-SMX by allowing bacteria to synthesize or absorb folic acid. |
| Enzymes produced to Metabolize the drug | β-lactamases and cephalosporinases hydrolyse β-lactam rings |
| Enzymes produced that add a chemical group to antibiotic to inhibit its activity | Aminoglycoside resistance by Staphylococcus aureus or Pseudomonas (Multiple enzymes acetyltransferase, adenyltransferase, and phosphotransferase) |
Spread of Bacterial Resistance
- Selective pressure selects for favourable mutations of resistance
- mosaic genes (from other bacteria eg strep pneumo from strep mitus) or uptake of DNA from environment
- transfer of resistant bacteria from person to person
- horizontal gene transfer;
- transduction – acquisition of bacterial DNA from a phage (a virus that propagates in bacteria);
- transformation – uptake and incorporation of free DNA released into the environment by other bacterial cells;
- conjugation – gene transfer (usually on plasmids), by direct cell-to-cell contact through a bridge.
Sources:
Microbiology Lippincott Williams & Wilkins
https://courses.lumenlearning.com/microbiology/chapter/drug-resistance/
https://www.encyclopedie-environnement.org/en/health/antibiotics-antibiotic-resistance-and-environment/
Gladwin / Sakurai / JC 2019
Examiner Comments
2014A 10: 63% of candidates passed this question.
Generally candidates provided an accurate classification of bacteria based upon Gram staining and shape. None or poorly staining organisms were often overlooked. Mechanism of resistance was also well covered, with examples. Areas of weakness were a lack of descriptive detail and/or omission of an example for each mechanism. Mechanisms such as metabolic blockade of essential pathways for antibacterial action and active removal of intracellular antibiotic were more commonly omitted.
11. Outline the formation, structure and function of the adult red blood cell.
CICMWrecks Answer
RED BLOOD CELL
Formation
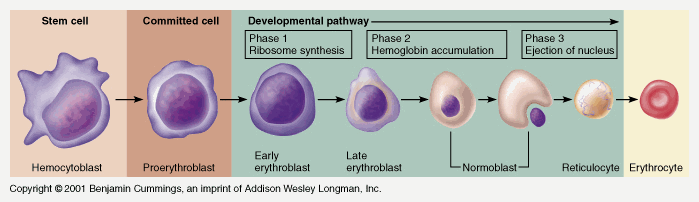
Pluripotent haemopoetic stem cell → Colony Forming unit → Proerythroblast → Reticulocyte → Erythrocyte
Simplified – main steps.
Takes 7 days
- Proerythroblast propagates
- From proerythroblast to reticulocyte
- Concentration of haemoglobin increases
- Nucleus disappears
- Endoplasmic reticulum disappears
- Reticulocyte
- Ribosomes
- Mitochondria
- Reticulocyte released into circulation
- Matures to RBC in 1-2 days
EPO increases rate of differentiation
Produced in the kidney (in response to low PO2)
Structure
- Are 7.5μm in diameter
- Are 2um thick
- biconcave disc, contains haemoglobin A (Hb F in-utero), has a central Fe moiety and demonstrates positive cooperativity in binding oxygen
- Have a lifespan of 120 days
- make up 40-50% of the blood volume, usual value 4-5 x 1012/L
- Carry ~29pg of haemoglobin
| Structure | Function |
|---|---|
Biconcave disc shape | Maximises surface area (optimising gas transfer) |
| Flexible membrane with shape maintained by structural proteins | makes the cells flexible enough to pass through capillary beds (which are narrower than the cell). |
| No nucleus & other organelles | Maximises cell volume available for Hb. |
| No mitochondria (No oxygen utilization) | Cannot perform aerobic metabolism – all ATP is generated via glycolysis. 3 shunts that come off the anaerobic glycolytic pathway (RBC’s only means of ATP generation): i) the production of 2-3 DPG via the Rapoport-Luebering shunt ii) generation of NADPH by the hexose monophosphate shunt (protects RBC from oxidative damage) iii) reduction of metHb back to Hb by way of the NADH. Optimizes Oxygen carriage |
| No ribosomes | Incapable of producing protein |
| Haemoglobin Four globulin chains (2α + 2β) links to heme Heme: Porphyrin ring chelated to iron Formed in the mitochondria | High affinity for oxygen O2 in haemoglobin is Hb x SatO2 x 1.34, compared to 0.003 x PO2 dissolved in blood Haemoglobin is a good buffer |
| Contains carbonic anhydrase | CO2 + H2O → H2CO3 Enables transport of CO2 as bicarbonate |
JC / Mooney 2019
Examiner Comments
2014A 11: 19% of candidates passed this question.
Candidates generally provided detailed description of the cell lineage that led up to the production of the mature red blood cell (RBC), but often omitted to mention those aspects unique to the RBC that were essential to its functions (e.g. biconcave shape gives the RBC a greater surface area and shorter distance to central regions, thus optimising diffusion of gases; the RBC enhanced ability to change shape and travel through narrow capillaries; lack of organelles maximises space for Hb, etc.). Mention of RBC function often lacked detail (e.g. restricted to just mentioning “O2 carriage”) or failed to mention, and describe, the RBC’s role in acid base buffering and HCO3- production.
12. Describe autoregulation within peripheral circulations.
CICMWrecks Answer
- Autoregulation: “intrinsic ability of an organ to maintain a constant blood flow despite changes in perfusion pressure“
- if perfusion pressure is decreased to an organ, blood flow initially falls, then returns toward normal levels over the next few minutes
- This autoregulatory response occurs in the absence of neural and hormonal influences and therefore is intrinsic to the organ, although these influences can modify the response
Mechanisms of Autoregulation
Local
- Myogenic
- Stretch of vessel walls causes constriction due to stretch mediated Ca2+ release
- Prominent in small arterioles throughout body
- Prevents excessive increases in blood flow in response to pressure
- Sheer stress on vessels due to increased flow causes release of Endothelial Derived Relaxing Factors such as Nitric Oxide which dilates the vessel, increasing vessel diameter and reducing sheer stress
- Stretch of vessel walls causes constriction due to stretch mediated Ca2+ release
- Metabolic
- Increased concentration of metabolic byproducts such as CO2, lactate, adenosine and H+, K+, cause upstream vasodilation
- This diffuse through the interstitium to upstream pre-capillary sphincters, arterioles and meta-arterioles
- Due to alteration of vessel radius, flow increases, according to the Poisuille-Hagen Equation
- Conversely, presence of excess nutrients (and O2) leads to vasoconstriction and decreased flow
- Increased concentration of metabolic byproducts such as CO2, lactate, adenosine and H+, K+, cause upstream vasodilation
- Reactive hyperaemia
- During occlusion to blood flow to a tissue, metabolic products build-up
- On restoration of flow to the tissue, flow can increase 4~7 fold due to buildup of metabolites and autoregulatory mechanisms
- During occlusion to blood flow to a tissue, metabolic products build-up
- Active hyperaemia
- During periods of increased metabolic work, due to the consumption of nutrients and production of metabolites, blood flow increases due to autoregulation
Regional Circulations
| System | Blood Flow | Circulation & Autoregulation |
|---|---|---|
| Heart | 250ml/min | 80% of perfusion occurs during diastole – Maintenance of diastolic pressure is imperative – Tachycardia reduces perfusion Coronary blood flow increases in response to myocardial O2 consumption approx. 5 fold Autoregulated between 60~180mmHg |
| Skin | 500ml/min | Can increase 30x or decrease 10x Glomus bodies (A-V anastamosis allowing shunting away from skin) |
| Brain | 750ml/min | Autoregulated between 50~150mmHg Munroe-Kellie Doctrine CO2, O2 play role in regulation |
| Skeletal muscle | 1000ml/min | Can increase 20 fold during exercise and decreases to 20% during severe hypovolaemia β2 innervation causing vasodilation at low concentrations of adrenalin (α predominates at higher) Skeletal muscle pump |
| Kidneys | 1250ml/min | Autoregulated between 70~170mmHg Tubuloglomerular feedback – Adenosine produced in response to increased perfusion pressure causing vasoconstriction and reduction in flow Sympathetic constriction Angiotensin II constriction Prostaglandins |
| Liver | 1500ml/min | In GI tract, counter-current conformation of the A-V system allows nutrient uptake however predisposes to necrosis with hypoxia Gastrin and CCK increase blood flow |
| Uterus | 100ml/min → 700ml/min at term | NOT AUTOREGULATED |
Other Influences which modify Autoregulation
Neural factors
- Sympathetic
- Sympathetic α adrenergic stimulation causes vasoconstriction (integral in baroreceptor response)
- Resting sympathetic tone contributes significantly to peripheral vascular resistance
- Most tissues except coronary and cerebral circulations
- Sympathetic cholinergic vasodilator supply under cortical control to skeletal muscle
- Sympathetic α adrenergic stimulation causes vasoconstriction (integral in baroreceptor response)
Hormonal factors
- β2 mediated vasodilation in response to adrenaline in skeletal muscle
- α1 mediated vasocontriction in response to circulating adrenaline
Long term
- Arterioles and vessels increase in size and number due to increased metabolic requirements of a tissue and chronic hypoxia
- Important factors
- VEGF
- Angiogenin
- Important factors
Sakurai 2016
Examiner Comments
2014A 12: 8% of candidates passed this question.
Most candidates failed to fully comprehend the question. Candidates displayed some difficulty in differentiating regulation at a local level (which is what the question asked for) from that of central regulation (e.g. sympathetic nervous system activity, cardiac output, etc.), which was not what the question asked for. Other omissions were a failure to define and explain autoregulation. Most candidates mentioned the myogenic and the metabolic theories, but failed to provide sufficient details as to their mechanisms. It was expected candidates would provide some detail as to locally acting factors. Adenosine and nitric oxide were mentioned on occasions but others such as endothelin and prostacyclin were often omitted.
13. Describe the cough reflex.
CICMWrecks Answer
Afferent
- In response to chemical, mechanical or noxious stimuli in larynx, trachea, carina or bronchi
- Signal transduced via internal laryngeal nerve (vagus nerve)
Central processor
- Medullary respiratory centre
- No single “cough centre” isolated
Efferent
Co-ordinated action of inspiratory muscles, pharyngeal muscles, and expiratory muscles
- Inspiratory phase
- Inhalation of a volume of air sufficient for cough generation (phrenic nerve and intercostal nerves)
- Compressive phase
- Closure of the glottis (recurrent laryngeal nerve)
- Activation of expiratory muscle against closed glottis
- Generation of transient increase in pressure of 300mmHg in thorax arterial blood and CSF
- Expulsive phase
- Opening of glottis due to posterio-cricoarytenoid muscle activation
- High velocity flow generated in bronchi, trachea and larynx
- “Choke point” – high velocity flow causes turbulant flow → increased shear stress between gas and airway lining → drag and expulsion of irritant
Intrinsic muscles of the larynx
- Cricothyroid
- Upper lip of cricoid cartilage → Inferior aspect of thyroid cartilage
- Innervated by superior laryngeal nerve
- Tenses vocal cords – elevates vocal tone
- Thyroarytenoid
- Anterior inner surface of thyroid à arytenoid cartilage
- Innervated by inferior laryngeal nerve
- Relaxes vocal cords – depresses vocal tone
- Posterior cricoarytenoid
- Posterior aspect of cricoid cartilage → arytenoid cartilage
- Innervated by inferior laryngeal nerve
- Abducts vocal cords
- Lateral cricoarytenoid
- Lateral aspect of cricoid cartilage → arytenoid cartilage
- Innervated by inferior laryngeal nerve
- Adducts vocal cords
- Transverse and Oblique arytenoids
- Spans the arytenoid cartilages
- Innervated by the inferior laryngeal nerve
- Adducts vocal cords
Sakurai 2016
Examiner Comments
2014A 13: 26% of candidates passed this question.
Many candidates struggled with this question. A good answer included a brief description of the role of the cough reflex, an outline of the sensory pathways, central integration and motor pathways of the reflex, and a description of the components of the cough reflex. Most candidates failed to accurately describe the sequence of events involved in the cough reflex. Very few gave sufficient detail on the nerve supply of the larynx
14. Using a diagram, explain the effect of PaO2, PaCO2 and MAP (Mean Arterial Pressure) on cerebral blood flow (CBF). (60 % of marks) Outline the effects of propofol and ketamine on CBF and cerebral metabolic requirement for oxygen (CMRO2). (40% of marks)
CICMWrecks Answer: Cerebral Blood Flow
Cerebral Blood Flow
- CBF = CPP / CVR (Cerebral Perfusion Pressure / Cerebral Vascular Resistance)
- CBF 15% resting CO → ~750ml/min or 50ml/100g brain tissue/min
- Gray Matter: 75–80 mL/100 g/min – Significantly higher due to the high metabolic activity and dense synaptic connections
- White Matter: 20–30 mL/100 g/min
- Abnormal CBF
- CBF<50ml/100g/min → cellular acidosis
- CBF<40ml/100g/min → impaired protein synthesis
- CBF <30ml/100g/min → cellular oedema
- CBF <20ml/100g/min → failure of cell membrane ion pumps, loss of transmembrane electrochemical gradients
- CBF <10ml/100g/min → cell death
Determinants of CBF
- CPP
- Net pressure gradient driving blood flow through the cerebral circulation
- CPP = MAP – ICP
- MAP = CO x SVR; CO = HR x SV; SVR = MAP / CO
- ICP dependent on: brain, blood, CSF
- CVR
Regulated by 4 primary factors:
- Cerebral metabolism
- flow-metabolism coupling:
↑ metabolic demand → ↑ CBF + substrate delivery - Controlled by vasoactive metabolic mediators:
H+ ions, K, CO2, adenosine, glycolytic intermediates, NO
- flow-metabolism coupling:
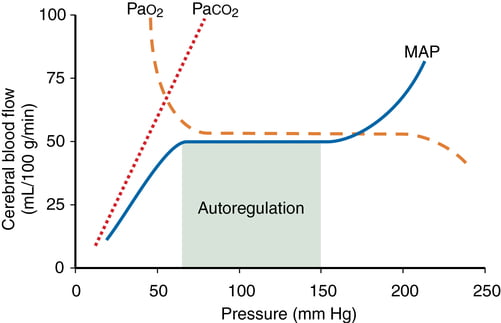
- CO2 and O2
- CO2
- At normotension: relationship between PaCO2 and CBF = almost linear
- ↑ PaCO2 → cerebral arteriolar vasodilation → ↓ CVR + ↑ CBF
- 2~4% increase for every 1mmHg increase in CO2
- ↓ PaCO2 → cerebral arteriolar vasoconstriction → ↑ CVR + ↓ CBF
- Initial stimulus = ↓ brain ECF pH
- Effects regulated by: NO, prostanoids, K channels, intracellular [Ca2+]
- PaO2
- little effect at normal PaO2
- PaO2 <60mmHg → cerebral arteriolar vasodilation → ↑ CBF
- Mechanism: hypoxia acts on →
- cerebral tissue to promote release of adenosine → cerebal vasodilation
- cerebrovascular smooth muscle → hyperpolarisation → ↓ Ca2+ uptake → vasodilation
- CO2
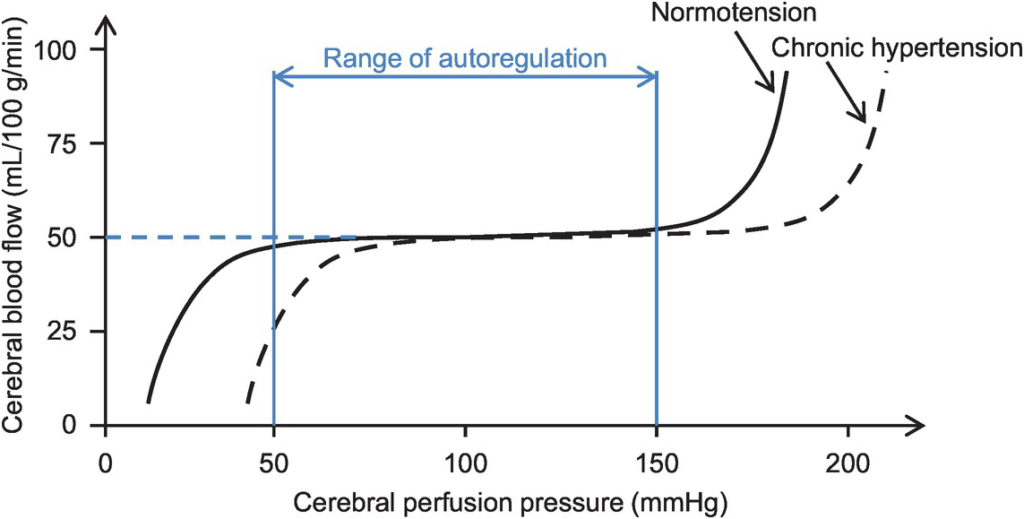
- Autoregulation
- Constant across CPP 50-150mmHg
- CPP >150mmHg: CBF µ CPP
- CPP <50mmHg: CBF <50ml/100g brain tissue/min → ischaemia
- Stimulus to autoregulation = CPP (not MAP)
- autoregulation curve R shifted in HTN; L in neonates
- Mechanism:
- Myogenic mechanism: arterioles vasoconstrict in response to ↑ wall tension + vasodilate in response to ↓ wall tension → ↓ or ↑ CVR
- May involve adenosine
- Can be impaired in SAH, tumour, stroke, head injury
- Constant across CPP 50-150mmHg
- Neurohumeral factors
- Relative lack of humoral + autonomic control on normal cerebrovascular tone
- Main action of SY nerves = vasoconstriction
- Other factors
- Blood viscosity: directly related to HCt; ↓ viscosity → ↑ CBF as per Hagen-Poiseuille law
- Temperature: ↓ CMRO2 by 7% for each ↓ 1°C in temp
- Drugs
- E.g. barbiturates ↓ cerebral metabolism
- Volatile agents → ↓ tension cerebral vascular smooth muscle → vasodilation + CBF
Kerr 2018
CICMWrecks Answer: Effect of Propofol and Ketamine
Effect of Propofol and Ketamine
| Propofol | Ketamine | |
|---|---|---|
| Action | Sedative agent | Dissociative sedative and analgesic agent |
| MoA | Effects on GABAA potentiation and Na channel blockade | Effects on NMDA receptor antagonism |
| CBF | ↓ (dose-dependant) | ↑ |
| CMRO2 | ↓ | ↑ |
| ↓ CBF ∝ ↓ CMRO2 | ↑ CBF > ↑ CMRO2 | |
| SjvO2 | minimal change | ↓ |
| doesn’t affect the autoregulatory curve of CBF and the PaCO2 response |
Kerr 2018
Examiner Comments
2014A 14: 74% of candidates passed this question.
This question was well answered. It is a structured question that guides candidates through exactly what is required. Well drawn graphs were a particularly effective means of scoring marks.
15. What is lung compliance and how is it measured?
CICMWrecks Answer

Lung compliance
- Change in lung volume per unit change in transmural pressure
- Normal lung compliance ~200ml/cmH2O
- Static compliance (Cs)
- ” Compliance of lung measured when lung held at constant lung volume”
- no pressure componet due to resistance
- is a function of: Elastic recoil of the lung, Surface tension of alveoli
- Dynamic compliance (Cd)
- ” Compliance of lung measured during cycles of inspiration and expiration”
- includes the pressure required to generate flow by overcoming resistance forces
- is a function of: respiratory rate
- Dynamic Compliance usually < Static Compliance due to the degree of time dependence in the elastic behaviour of a particular lung
- Specific compliance
- Compliance per unit volume lung
- Used to compare different lungs
- Hysteresis
- any process where the future state of a system is dependent on its current and previous state
- Compliance of the lung is different in inspiration and expiration
- In dynamic compliance curves:
- Airways resistance is a function of flow rate. Flow rate (therefore resistance) is maximal at the beginning of inspiration and end-expiration.
- In static compliance curves:
- There is no resistive component. Hysteresis is due to viscous resistance of surfactant and the lung.
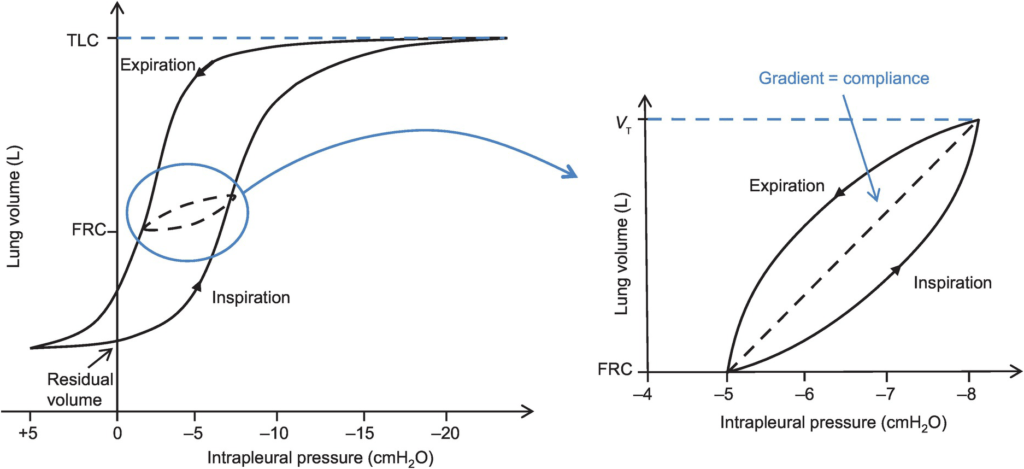
Factors Affecting Compliance
Lung Compliance
- Surfactant
- increases lung compliance
- decreases surface tension at alveolar air – water interface
- prevents small alveoli from collapsing
- accounts for most of hysteresis in intact lungs
- Lung volume
- Lung Compliance decreases at higher lung volumes
- Specific compliance (Compliance/FRC) remains constant
- Elephants have greater lung compliance than mice!
- Pulmonary blood volume
- Increased PBV decreases lung compliance
- Pulmonary venous congestion from L heart failure or mitral regurgitation
decreases lung compliance
- Bronchial smooth muscle tone
- Increased bronchial smooth muscle tone decreases compliance
- Decreased dynamic lung compliance by 50% in animal models of methacoline
challenge
- Disease
- ARDS, pneumonia decreases lung compliance
- pulmonary fibrosis → Impraired elasticity → decreases lung compliance
- Asthma, Emphysema increases lung compliance
Chest Wall Compliance
- Chest wall restriction – reduced chest wall compliance
- Obesity
- Spastic paralysis of chest wall musculature
- Ossification of costal cartilages
- Kyphosis/scoliosis
- Scarring/constriction (e.g. circumferential burns)
- Position: Prone (60% reduced compliance)
- Collagen Disorders: Increased Chest Wall Compliance
Measurement of Compliance
Dynamic Compliance
- ” Compliance of lung measured during cycles of inspiration and expiration”
- Measure:
- Measure with spirometer
- Using Volume/Pressure curve during normal rhythmic breathing at points of no flow
- Equation above then gives dynamic compliance
Static Compliance
- ” Compliance of lung measured when lung held at constant lung volume”
- Volume vs Pressure loops during inspiration and expiration demonstrate Hysteresis
- Measure:
- @ no flow in or out of the lung, AND time for pressure to equlibrate across the lung (>10sec).
- Patient exhales in steps holding the volume with open glottis
- Intrapleural pressure measured as oesophageal pressure
- Measured compliance secondary to viscoelastic properties and accounts for both short and long time-constant alveoli.
Specific Compliance
- Measure of the average compliance across all lung units
- Children = Adults ~ 0.05 cmH2O-1
Gladwin / Sakurai / JC 2020
Examiner Comments
2014A 15: 34% of candidates passed this question.
There was a good understanding of the definitions of compliance but many candidates failed to clearly demonstrate an understanding of the difference between static and dynamic compliance. Many candidates had little knowledge of how compliance is measured. It was expected that descriptions of methods to measure static and dynamic compliance would be provided. There were frequent errors in descriptions that were provided
16. Describe, with the aid of a diagram, the structure of the cell membrane, (40% marks) and transmembrane transport processes. (60% marks)
CICMWrecks Answer
Structure of Cell Membrane
Cell Membrane
- Made of
- Phospholipids
- Proteins
- Cholesterol – Found in eukarocytes ie cells with nuclei
- Cell membrane = 7.5nm thick semi permeable structure
Lipid Bilayer
- Fluid rather than solid
- Phospholipids have:
- eg phosphatidyl-choline & phosphatidyl-ethanol-amine
- Hydrophilic head
- Water soluble
- Exposed to aqueous exterior & interior
- Glycerol backbone
- Fatty acid tails –
- Hydrophobic
- Meet in middle of cell membrane
- Proteins can be either:
- Integral – ie pass through bilayer eg ion channels
- Peripheral = straddling
- make up 50% of cell membranes mass
Function of CM Proteins
- Structural
- Carriers for facilitated diffusion (i.e. down electrochemical gradient)
- Pumps for ion active transport
- Ion channels (diffusion down electro- or chemical gradient or both; e.g. K-“leak” channels)
- Receptors for chemical messengers (i.e. hormones , neurotransmitters , autacoids…)
- Enzymes
- Glycoproteins involved in AB processing or anticoagulation (e.g. the mucopolysaccharide glycocalyx of the endothelium which repels clotting factors + PLT’s → helps prevent blood from clotting in intact blood vessels)
Transmembrane Transport Processes
| Mechanism | Energy Expenditure | Electrochemical gradient | Example | ||||
|---|---|---|---|---|---|---|---|
| Diffusion | Passive diffusion | Molecule crosses a membrane to which it is permeable by diffusion | No | With | Carbon dioxide across vascular endothelium | ||
| Facilitated diffusion | Molecule crosses a membrane via a channel, without energy expenditure | No | With | Potassium across excitable cell membranes, via rectifier channels | |||
| Active transport | Primary | Primary active transport | Molecule crosses a membrane via a channel, with energy expenditure (ATP) | Yes | Against | 3Na+ /2K+ ATPase | |
| Secondary | Symport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Co-transported) | Not directly | May be Passive or Active based on gradient of 2nd | sodium and an amino acid | ||
| Antiport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Anti-transported) | Not directly | May be Passive or Active based on gradient of 2nd | Na+ / H+ antiporter in proximal convoluted tubule | |||
| Ligand-gated ion channel | Binding of a ligand causes conformational change in membrane channel, allowing movement of ion across membrane | No | With | Nicotinic acetylcholine receptor. ACh as ligand, Na+ and K + as ions | |||
| Exocytosis | Substance packed in vesicle, moves to cell membrane, two membranes merge -> substance exits cell | Yes | Usually against | Exocytosis of ACh by presynaptic neuron | |||
| Endocytosis | Cell membrane extends to engulf a substance or object, which is then contained in the cell within a vesicle | Yes | Can be with, against or refer to a macroscopic object | Phagocytosis of bacteria by macrophage | |||
Mooney / JC 2019
Examiner Comments
2014A 16: 60% of candidates passed this question.
The structure of the cell membrane was generally well covered by most candidates. Many had difficulties structuring an answer for the transmembrane transport processes. Dividing this section into proteins (some receptors, channels etc.) and carbohydrates (some receptors, immune reactions etc) followed by a very brief discussion of each type of process would have aided candidates towards providing a good answer.
17. Classify local anaesthetic agents and give examples. (30% of marks) Describe the pharmacology of lignocaine. (70% of marks)
CICMWrecks Answer: Local Anaesthetic Classification
Local Anaesthetics
- Local anaesthetic agents act via inhibition of voltage gated Na+ channels, by binding to the intracellular portion of the channel
- → Decreased Na influx
- → Inhibition of depolarization
- → Inhibition of action potential formation and propagation
- Local anaesthetics are formed by a hydrophobic and hydrophillic component, and are classified according to the linkage between the two, either amide or ester
- Amide Local Anaesthetics
- e.g. Lignocaine, ropivacaine, bupivacaine
- Metabolized by hepatic dealkylation by P450 enzymes
- Longer duration of action
- Ester Local Anaesthetics
- e.g. Procainamide, cocaine
- Metabolized by plasma esterases
- Shorter duration of action
Mooney / JC 2019
CICMWrecks Answer: Pharmacology of Lidocaine / Lignocaine
Pharmacology of Lignocaine
Examiner Comments
2014A 17: 71% of candidates passed this question.
The first part of this question was answered well by most candidates.
Generally, the second part of the question was poorly organised by many candidates, the consequence being that many opportunities for picking up marks were lost. A brief statement as to what lignocaine is, its presentations and dose, some facts about PD and PK followed by a few lines on toxicity (CC/CNS ratio) was mostly what was required. Only a few candidates mentioned lignocaine toxicity
18. Outline the anatomy of the internal jugular vein. (80% of marks) Describe the Doppler Effect. (20% of marks)
CICMWrecks Answer: IJV (Internal Jugular Vein) Anatomy
Origin / Course / Termination
- Originates at the jugular foramen where it continues the sigmoid sinus
- Exits the foramen accompanies by the glossopharyngeal (IX), vagus (X) and accessory (XI) nerves
- runs caudally down the neck – The vein lies most superficially in the upper part of the neck
- The vein lies lateral, first, to the internal carotid artery, separated by the hypoglossal (XII) nerve, and then to the common carotid artery, with the vagus nerve between and posterior to the artery and vein.
- These three structures lie in a common fascial compartment (the carotid sheath) with the cervical sympathetic chain lying posterior to the sheath.
- These four structures, two vessels and two nerves, form a quartet throughout the neck.
- The deep cervical chain of lymph nodes lies close against the carotid sheath
- The vein is initially posterior to, then lateral and then anterolateral to the carotid artery during its descent in the neck
- Terminates behind the sternoclavicular joint, where it joins the subclavian vein to form the brachiocephalic vein
Relations of the IJV
- Anterior –
- Internal carotid artery and vagus nerve
- Superficially, the internal jugular vein is overlapped above and then lower down covered by the sternocleidomastoid muscle and is crossed above by the posterior belly of the digastric and lower down by the inferior belly of the omohyoid
- Posterior –
- C1, sympathetic chain, dome of the pleura. On the left side, the IJV lies anterior to the thoracic duct
- Posteriorly, the vein rests on (from above downwards) the rectus capitis lateralis, the transverse process of the atlas, the scalenus medius, and the scalenus anterior, with the phrenic nerve crossing it; and all are covered by the precervical fascia
- Medial – Carotid arteries, cranial nerves IX-XII
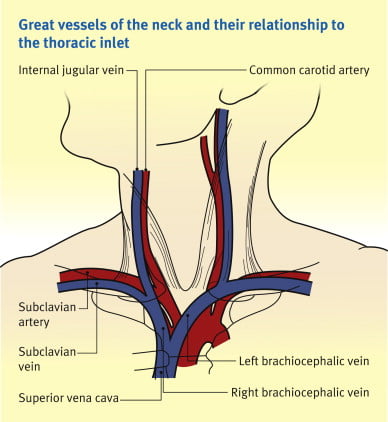
Anaesthesia & Intensive Care Medicine,
https://doi.org/10.1016/j.mpaic.2009.10.005.
Surface Anatomy for Venepuncture
- Lies lateral to the carotid artery within triangle formed by: Two heads of Sternocleidomastoid muscle and clavicle
- On Ultrasound: Non-pulsatile, thin walled, compressible vessel lateral to the carotid artery
Anatomical Variations
- The right IJV (80.5%) was more often larger than the left IJV.
- With reference to the CCA, 85.2% of the IJV were found in the lateral position, 12.5% anteriorly, 1.1% medially and 1.1% posteriorly.
- IJV were sometimes found to be hypoplastic, and in rare cases this was seen bilaterally in both the right and left IJV.
- 5.5% have an IJV that does not correspond to the site predicted by external markings
- Other variations:
- IJV bifurcation
- IJV duplication
- IJV fenestration
- IJV trifurcation
- Posterior tributary
Sources:
Ellis H. The clinical anatomy of the great veins of the neck. Br J Hosp Med (Lond). 2010 Feb;71(2):M20-1. doi: 10.12968/hmed.2010.71.Sup2.46501. PMID: 20220708.
Asari AIA, Barros RAV, Borges MAP. Anatomic variant of the internal jugular vein and its importance in vascular access for hemodialysis. J Vasc Bras. 2019 Oct 23;18:e20190014. doi: 10.1590/1677-5449.190014. PMID: 31692937; PMCID: PMC6822959.
Gladwin / JC 2020
CICMWrecks Answer: Doppler Effect
Doppler effect
- Change in apparent frequency of sound for an observer moving relative to its source
- Use in ultrasound
- Sound waves reflected off objects moving toward or away from transducer
(usually blood)- If object moving toward transducer, frequency appears increased – displayed as red (however this is not standardized across machines)
- If object moving away from transducer, frequency appears decreased –
displayed as blue
- Can be used to measure velocity of flow, as well as direction
- Sound waves reflected off objects moving toward or away from transducer
Sakurai 2016
Examiner Comments
2014A 18: 40% of candidates passed this question.
An overview of the Internal Jugular vein stating where it is formed and terminates would be a good start. The important surface anatomy of the vein (left and right) followed by mention of the important anatomical relationships was then required. Few candidates mentioned anatomical variations.
Many answers had no identifiable structure and went back and forth around the topic as information came to mind. A number of answers contained very rough diagrams that did not contain mark worthy information but took time to draw.
The second part of the question was generally well answered by those attempting it. Many candidates ignored or forgot about this part of the question. Some candidates wrote 2 or more pages of significant detail in stark contrast to what they had written for the first part of the question. The percentage of marks allocated is a good guide as to the level of detail required in the answer.
19. Define cardiac output. (10% of marks) Outline the factors that affect cardiac output. (60% of marks) Briefly describe the thermo dilution method of measuring cardiac output. (30% of marks)
CICMWrecks Answer
Cardiac Output
Cardiac output = Volume of blood ejected by the heart per unit time (minute)
HR determined by autonomic input
- Resting HR approx 60
- Sympathetic blockade causes HR to slow to 50
- Sympathetic output to heart governed by the medullary vasomotor area
- Parasympathetic blockade causes HR to hasten to 110
- Both sympathetic and parasympathetic blockade causes HR to hasten to 100
- Electrolytes
- Drugs
- Sympathetic blockade causes HR to slow to 50
SV = Amount of blood ejected by the heart per contraction
Governed by:
- Preload
- Tension applied to myocyte immediately prior to contraction
- Starling relationship
- Optimal sarcomere length ~2.2um
- Over-stretch inhibited by fibrous pericardium
- Preload governed by
- Blood volume and MSFP (increases preload)
- RAP (decreases preload)
- Resistance to venous return (decreases preload)
- Negative intrathoracic pressure (aids in venous return)
- Musculovenous pumps (aids in venous return)
- One-way venous valves (aids in venous return)
- Starling relationship
- Tension applied to myocyte immediately prior to contraction
- Afterload
- Load against which the ventricle must exert its contractile force
- According to Laplace law
- Afterload ∝ (Aortic pressure x ventricular radius) / thickness of ventricular wall
- Other factors affecting afterload
- Resistance
- Viscosity inversely proportional to flow
- As viscosity decreases (e.g. Hb decrease), output increases
- Flow is proportional to vessel radius to the 4th power
- Viscosity inversely proportional to flow
- Resistance
- Contractility
- Intrinsic ability of myocardium to contract at given preload and afterload
- Governed
- Sympathetic increases contractility
- Parasympathetic decreases contractility
- Other hormones
- Electrolytes
Measurement
Cardiac output measurement can be performed:
- Invasively
- Pulmonary Artery Catheter
- Thermodilution
- Fick Principle
- Indicator Dilution Technique
- TOE
- Arterial waveform analysis
- PiCCO
- FloTrak/Vigileo
- Acumen/Hemosphere
- Pulmonary Artery Catheter
- Non-invasively
- TTE
- MRI
- Thoracic impedance
Thermodilution
Indicator Dilution (Stewart-Hamilton)
Introduction
Invasive
Thermodilution remains the gold standard of cardiac output measurement.
Invasive
Equipment
Pulmonary Artery Catheter
- A proximal port at the RA/SVC
- A temperature probe at the tip: Typically a silicon oxide thermistor.
- A balloon at the tip: To float it into position.
- A distal (PA) port is required for measuring PAP and the PCWP, but is not required for CO calculation
Can also be done using other devices like PiCCO
- dyes: indocyanine green, isobestic or radioactive isotope.
- Catheter for injection
- Arterial Sampling catheter
- Measurement device
Method / Theoretical Basis
- Catheter passed into right atrium
- Known quantity of fluid of known temperature injected into right atrium
- Second catheter in pulmonary artery
- Measured change in temperature
- Change in temperature over time graphed
- Return of temperature to baseline extrapolated to zero to account for
recirculation - Average change in temperature for duration of curve calculated
- CO = temperature of indicator / (average change in temp x duration of curve)
Method:
- rapidly inject 10mL of dye into venous circullation in RA.
- indicator mixes quickly with cardiac contents.
- during next few beats, blood-indicator mixture is measured by continual sampling from proximal artery in arm for one complete circulation (30 seconds).
- mean concentration of mixture for one circulation determined.
Q = amount of indicator injected / indicator concentration over time.
Assumptions:
- retention of indicator
- complete mixing
- constant flow rate
Advantages / Disadvantages / Errors
Advantages:
- Simple
- No toxicity
- Repeated measurements possible
Disadvantages / Errors:
- Natural variability (reduced by using mean of 3-5 measurements)
- Issues with incorrect volume and temperature of injectate
- Improper positioning of PAC and complications associated with insertion and use
- Tricuspid regurgitation
Results in retrograde ejection of injectate back past the valve. - Arrhythmia
Advantages:
- rapid Q determination
Disadvantages:
- need to continuously withdraw arterial blood to plot the concentration curve.
- dye can build up.
- intracardiac shunt will effect recirculation.
Sakurai / JC 2019
Examiner Comments
2014A 19: 58% of candidates passed this question.
This is a core question. It was expected candidates could provide a definition (heart rate x stroke volume) and then move on to outline factors that affect it (afterload, preload, contractility). Additional marks were awarded for descriptions of the relationship to mean systemic filling pressure and other influences beyond this. Most candidates described a thermodilution cardiac output curve but almost all described the technique as based on the “Fick equation or method” (which is used to estimate cardiac output from oxygen consumption). Very few candidates correctly identified the Stewart Hamilton equation as the integration method used to relate cardiac output (flow) to temperature change as an example of indicator dye dilution. Candidates seemed to lack depth and understanding on this topic.
20. Describe the factors affecting drug absorption from the gastrointestinal tract.
CICMWrecks Answer
Drug Factors
- Lipid solubility
◦ ↑ lipid sol → ↑ absorption
◦ Must still have some water soluble component - Molecular size
◦ ↓ size → ↑ rate of absorption
◦ Larger molecules require processes other than simple diffusion - Degree of ionisation
◦ pH-pKa gives the degree of ionisation
◦ ↓’d ionisation favours absorption - Physical forms
◦ Gases faster than liquids faster than solids - Dosage forms
◦ Capsules more rapidly absorbed than tablets - Formulation
◦ The addition of Na to the formulation for stability can reduce the bioabalability via the oral route - Concentration
◦ From Fick
Patient factors
- Area of absorptive surface
◦ intestinal resection or coeliac → ↓ absorptive surface → ↓ absorption from Fic - Vascularity
◦ Shock → ↓ absorption - pH
◦ effect degree of ionisation
▪ ↓ pH favours absorption of acidic drugs
▪ ↑ pH favours absorption of basic drugs - Presence of other substances
◦ Statins should be taken with food
◦ Milk ↓’s and Vit C ↑’s absorption of iron - GI motility
◦ ↑ motility → ↓ absorption - Functional Integrity of the absorptive surface
◦ Coeliac disease → flattened mucosa → ↓ absorption - Coexisting disease state
◦ diarrhoea → ↓ absorption
Gladwin 2016
Examiner Comments
45% of candidates passed this question.
This is a very broad and open question. While a structured approach was useful, a sound knowledge of first principles or even being able to “think on the fly” would have provided candidates with enough opportunities to generate a pass.
21. Compare and contrast the mechanism of action, pharmacokinetics, adverse effects and monitoring of effect of dabigatran and warfarin.
Examiner Comments
2014A 21: 29% of candidates passed this question.
Most candidates were able to provide some details about warfarin however dabigatran was less well known. The syllabus provides a guide to depth of knowledge required for listed drugs and so more detail was expected for Warfarin as a class “A” drug than was expected for dabigatran (a class “C” drug). It was however expected that candidates would provide more than just generalisations regarding “hepatic metabolism and renal excretion” when applied to both agents that actually have different modes of elimination.
22. What is a buffer? (10% of marks) Discuss the body’s buffer systems and how they work. (90% of marks)
CICMWrecks Answer – Buffer Systems
Buffer
- Weakly ionised acid or base in equilibrium with its full ionised salt
- A buffer can “resist” change in pH by absorbing or releasing H+ ions
- Works best when pKa is closest to the target pH (7.4)
- Isohydric Principle
- All buffer systems which participate in defence of acid-base changes are in equilibrium with each other. There is after all only one value for [H+] at any moment. This is known as the Isohydric Principle.
Effectiveness:
- buffer pKa (most effective if pKa = pH of carrying solution)
- pH of carrying solution
- amount of buffer present
- open (physiological) vs closed (chemical) system
Buffer Systems by site
| Site | Buffer System | Comment |
|---|---|---|
| ISF | Bicarbonate | For metabolic acids |
| Phosphate | Not important because concentration too low | |
| Protein | Not important because concentration too low | |
| Blood | Bicarbonate | Important for metabolic acids |
| Haemoglobin | Important for carbon dioxide | |
| Plasma protein | Minor buffer | |
| Phosphate | Concentration too low | |
| ICF | Proteins | Important buffer |
| Phosphates | Important buffer | |
| Urine | Phosphate | Responsible for most of ‘Titratable Acidity’ |
| Ammonia | Important – formation of NH4+ | |
| Bone | Ca carbonate | Important in prolonged metabolic acidosis |
| CSF | Bicarbonate | Important – (as very low proteins for buffering) free Bicarbonate flow in CSF |
| Phosphate | negligible |
Buffer Systems
| Name | Location |
|---|---|
| Protein | Intra cellular |
| Haemo globin | Blood |
| Bicarb | Plasma, Interstitium, Urine (minimal) |
| Ammonia | Urine |
| Phosphate | Urine, Bone |
| CaCO3 | Bone |
Protein (Intracellular)
- pKa of imidazole group is 6.0
- pKa of the histadine residues themselves = 6.8
- Protein buffer ability is proportional to Histadine residue content.
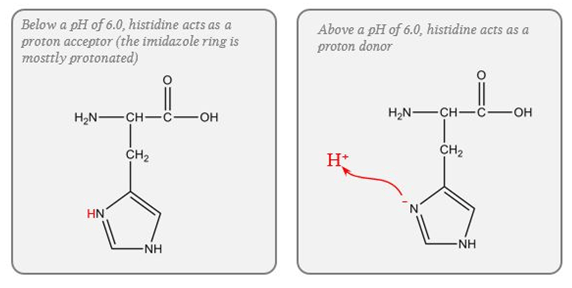
Haemoglobin (Blood)
- Protein buffering system
- Important intracellular buffer in RBC
- Exists as weak acid – HHb and potassium salt KHb.
- Hb a pKa of 8.2 in deoxy form and 6.6 in oxyHb
- Buffering capacity due to imidazole residues on 38 histidine residues on Hb molecule
- pKa of residues 6.8 à close to physiological pH
- Also important in extracellular buffering (following bicarbonate buffer system)
- Due to fast equilibration of HCO3– (Hamburger Shift)
- Band3 Transporter (HCO3–/Cl– antiporter)
- Allows carbonic anhydrase reaction to take place by limiting build-up of HCO3– (la chatelier principle)
- Formed in erythrocytes as a tetramer of 4 subunits
- Hb ~ 6 times the number of histadine (38) residues compared with albumin. (3-6x buffering capacity)
- Hb is in much greater concentrations than any other protein (15 g/dL vs 7 g/dL)
- For each mmol OxyHb, 0.7mmol H+ is buffered and 0.7mmol of CO2 can enter circulation without a change in pH
- Available at high concentrations in RBC
- Isohydric exchange
- the buffer system (HHbO2-HbO2-) is converted to another more effective buffer (HHb-Hb-) exactly at the site where an increased buffering capacity is required
- Deoxyhaemoblobin is a much more effective buffer
- oxygen unloading increases the amount of deoxyhaemoglobin and this better buffer is produced at exactly the place where additional H+ are being produced because of bicarbonate production for CO2 transport in the red cells.
Bicarb (Plasma, Interstitium, Urine (minimal))
- Catalysed by carbonic anhydrase (slow where CA is absent (plasma))
- Open at both ends
- Bicarb regulation at kidneys
- CO₂ regulation at lungs
Ammonia (Urine)
Equlibrium between ammonia and ammonium
Steps:
- Glutamine enters PCT cells
- 20% from filtrate
- 80% from peritubular capillaries
- Ammonia (NH3) produced from glutamine in PCT by glutaminase secreted into lumen
- Reabsorbed in TAL of LoH
- Diffuses into peritubular cells of CD down conc Grad
- H+ secreted into lumen of CD
- Combines with ammonia to form ammonium
- Ammonium positive charge prevents reabsorption
- Thus “excess hydrogen can be secreted with ammonium”
Phosphate (Urine, Bone)
Effect in urine is limited due to:
- Minimal urinary pH is 4.5 → equation B is >99% ionised → of little use.
- Requires filtered PO4 → Normally low levels.
- Depends on Diet and PTH.
- Where H is secreted in distal tubules, there is minimal PO4 excretion
CaCO3 (Bone)
- Important in chronic metabolic acidosis
- Release of calcium carbonate from bone is the most important buffering mechanism involved in chronic metabolic acidosis.
Please Note: This answer is viewed from the Traditional approach to Acid-Base theory. Alternative (physicochemical approaches) deemphasise bicarb due to its equilibrium with water and relatively low concentration compared with the strong ions.
Gladwin / Sakurai / JC 2020
Examiner Comments
2014A: 37% of candidates passed this question.
Candidates who described an overall view of the body’s buffer systems scored well.
Most candidates could define buffer and could discuss the bicarbonate buffer system;
however this was not sufficient to pass the question. To pass there had to also be a discussion of the other buffer systems including phosphate, protein (haemoglobin, plasma proteins and intracellular proteins), ammonia and bone.
Best answers included discussion of open versus closed systems and the relative contribution of the differing buffer systems in blood, intracellular fluid, extracellular fluid and urine.
23. What is the Glomerular Filtration Rate (GFR)? Discuss the physiological factors that can influence it.
CICMWrecks Answer
GFR
GFR:
- “The amount of glomerular ultrafiltrate formed divided by the time of filtration”
- Normal value is approx 125 mL/min or 180 L/day
- Renal blood flow 1.25l/min in 70kg male → Filtration fraction 0.2
Functional anatomy:
3 Distinct Layers:
- glomerular capillary endothelium
- highly specialised endothelium with fenestrations to ↓ filter thickness
- prevents cellular components of blood from coming into contact with BM
- glomerular BM
- made of CT; -vely charged
- acts as filter
- bowmans epithelial cells (podocytes)
- epithelial cells with foot processes → large SA
- negatively charged
- maintain BM + phagocytic functions
Direct GFR Determinants
The GFR (Net flux across the membrane) is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation.
where
Kf = Filtration coefficient
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
- Normally NFP = 17mmHg
- Tubular oncotic pressure = zero throughout
- GC oncotic pressure varies from 21 to 33 mmHg as filtrate is removed.
- Thus less GFR produced at distal end of tubule.
| Afferent end of Glomerular Capillary (mmHg) | Efferent end of Glomerular Capillary (mmHg) | |
|---|---|---|
| PGC | 60 | 58 |
| PT | 15 | 15 |
| πGC | 21 | 33 |
| NFP | 24 | 10 |
| Kf | Filtration coefficient | = LpS = Hydraulic conductivity x Surface Area Glomerular surface area = 0.8m2 • Altered by Mesangial cell contraction (see circulating factors e.g. Angiotensin II → ↓SA → ↓GFR) Patency of the normal capillary wall structure (ie in tubular dysfunction Kf ↑’s ↑GFR) |
| PGC | hydrostatic pressure in capillary | relates to • RBF which is autoregulated for MAP 70-170mmHg • relative afferent/efferent arteriolar tone Affected by • Catecholamines • Local autoregulation -> Myogenic -> Tubuloglomerular feedback -> Hormones (see below) |
| PT | hydrostatic pressure in tubule | relates to • obstruction to urinary flow (usually pathological) • ↑PT → ↓ GFR (ie post renal obstructiion causing renal failure) |
| πGC | oncotic pressure in capillary | relates to • plasma protein concentration (incr in dehydration, decreased in heart failure) • ↑Systemic plasma oncotic pressure → ↑πGC → ↓GFR • ↓Renal plasma flow → ↑πGC → ↓GFR |
| πT | oncotic pressure in tubule | • usually zero, but can increase in renal failure/proteinuria • ↑’d πT → ↑GFR |
| σ | Staverman’s reflection coefficient | • Permeability of membrane to protein usually 1 (no protein leak) • can decrease with nephritis/proteinuria |
Factors influencing GFR
Circulating Factors
- Prostaglandins
- PGI2 and PGE2 → vasodilates → ↑ renal blood flow → ↑ GFR
- ↓ PGE2, PGI2 and NO levels → inhibits afferent arteriolar vasodilation → ↓ GFR
- Long term → ↓ renin release → ↓ ATII-induced efferent arteriolar vasoconstriction → ↓ GFR
- Noradrenaline / Adrenaline
- constrict renal afferent and efferent arterioles → ↓ renal blood flow and reducing filtration → ↓GFR
- Constrict mesangial cells to → ↓ GFR.
- Mesangial cell tone:
- Angiotensin 2, Endothelin, vasopressin → ↓ glomerular surface area and GFR
- ANP, PGE2, Dopamine and cAMP → all increase GFR
Solute factors
- Size
- < 7 kDa (Eg. glucose, ions, urea, H2O) filtered freely,
- > 70 kDa (Eg. albumin) are not filtered;
- neutral particles < 4 mm diameter filtered freely, > 8 mm are excluded
- Charge
- Negatively charge (Anionic) particles repulsed
- Cationic substances filtered more readily
- Protein binding
- Albumin excluded
Disease Factors
- Filtration decreases
- Shock → decreased glomerular pressure
- Obstruction → increased bowman’s capsule hydrostatic pressure
- Hypoproteinaemia → hepatic failure, nephrotic syndrome
JC / Gladwin / Sakurai / Kerr 2020
Examiner Comments
2014A 23: 40% of candidates passed this question.
Generally this question was well answered. Almost all answers gave the correct value for GFR and the correct formula for Starling’s forces across the glomerular membrane. Better answers discussed the physiological factors affecting each force (hydrostatic and osmotic) across the membrane in a stepwise logical manner. Some answers discussed the control of renal blood flow; this was not expected and therefore was not rewarded
24. Describe the mechanism of action of the analgesic effect of opiates. (70% of marks) Explain the mechanism by which morphine causes respiratory depression and constipation. (30% of marks)
CICMWrecks Answer
Mechanism of Action and Effects
- Opiates act on MOP, DOP, KOP and NOP receptors
- MOP are distributed widely:
- Cortex
- Basal ganglia
- Spinal cord – dorsal horn
- Periaqueductal grey – descending inhibition
- DOP, KOP and NOP less affected by commonly used opiates
- Produce analgesia, with different levels of other opiate effects
- Opioid receptors are GiPCR
- Inhibit Ca2+ channels
- Prevent neurotransmitter release at pre-synaptic nerve terminals
- Open K+ channels
- Hyperpolarise post-synaptic nerves, preventing signal transmission
- Inhibit Ca2+ channels
Analgesia
- Analgesia induced by inhibition of ascending pain transmission
- Sites of action
- Primary afferents
- Dorsal horn interneurons
- Descending modulatory neurons
- Inhibition of GABA-releasing inhibitory neurons
- Supraspinally
- Analgesia enhanced by centrally mediated euphoria and sedation
Respiratory depression
- MOP-mediated
- Action on medullary chemoreceptors
- Blunted increase in respiratory drive with increasing CO2
- Effect is antagonised by painful stimulus
Constipation
- Mediated by MOP, KOP and DOP receptors
- Expressed in high density throughout GIT nerve plexus
- Increased GIT tone, with subsequent loss of rhythmic peristaltic movements
Mooney 2016
Examiner Comments
2014A 24: 11% of candidates passed this question.
This question was poorly answered with a low pass rate; which was unexpected for this core topic. In general, completed answers demonstrated good understanding of the analgesic mechanisms of opiates, but only superficial understanding of respiratory depression and constipation. Better answers explained the decreased responsiveness of the respiratory centre to CO2, and the effects of opiates on the enteric nervous system and peristalsis.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | oxygen, measurement of oxygen and principles around humidity Resp physio, effects of obesity |
| G. CVS | Cardiovascular physiology, Venous return, cardiac function curves, ICC anatomy |
| H. Renal | renal physiology and diuretic pharmacology |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | Propofol,pharm, basic kinetics for bolus and infusions, side effects |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | Hepatic physiology, Cerebral physiology |
| O. GIT | glucose and lipid metabolism, daily nutritional requirement, insulin physio |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | measurement of temperature, physical principles of flow, ionic changes across membranes |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments