1. Describe the pharmacology of suxamethonium.
Examiner Comments
2013B 01: 16 candidates passed (59.3 %).
This question was generally well answered. A structured approach that included headings such as pharmaceutics, mechanism of action, pharmacodynamics, kinetics, dose and side effects was associated with a good answer.
2. Describe the various rapidly acting cardiac reflexes that influence cardiac function and the mechanisms by which they act.
CICMWrecks Answer
Cardiac reflex: “a fast-acting reflex loop between the heart and central nervous system that contributes to regulation of cardiac function and maintenance of physiologic homeostasis”
1. Chemo receptor reflex
- Sensor
- Carotid body, central chemoreceptors
- Stimulus
- ↓in PaO2, ↑ in PaCO2
- Response
- Increased SNS stimulation
- Increased HR, SV (via increased contractility), PVR
2. Baroreceptor response (high pressure)
- Sensor
- Arterial baroreceptors
- Aortic arch (CN X)
- Carotid sinus (CN IX)
- Arterial baroreceptors
- Stimulus
- Increased MAP
- Continuous resting tone when MAP >60mmHg
- Increased MAP
- Response
- Increase SNS stimulation
- Increased HR, SV (via increased contractility), PVR
3. Baroreceptor response (low pressure)
- Sensor
- Atrial pressure sensors
- Stimulus
- Atrial filling
- Atrial contraction
- Response
- Peripheral vasodilation
- Increased HR
- Also renal and hormonal effects to decrease circulating volume
4. Bainbridge reflex
- Sensor
- Atrial stretch receptors – right atrial wall, cavoatrial jucntion
- Stimulus
- Increased atrial blood volume
- Response
- Increased HR
5. Cushing reflex
- Sensor
- Medullary sympathetic cell bodies
- Stimulus
- Compression of cell bodies with increased intracranial pressure
- Response
- Increased SNS stimulation -> increased SVR
- Reflex bradycardia
- Via reflex #3
6. Oculocardiac
- Sensor
- Via CN III
- Stimulus
- Ocular compression
- Response
- Vagal stimulation -> decreased HR
7. Bezold-Jarisch reflex
- Sensor
- Chemoreceptors and mechanoreceptors in LV wall
- Stimulus
- Noxious chemical stimuli
- Response
- Increase in parasympathetic outflow
- Bradycardia
- Peripheral vasodilation
Mooney 2016
Examiner Comments
2013B 02: 6 candidates passed (22.2%).
Cardiac reflexes are fast-acting reflex loops between the heart and central nervous system that contribute to regulation of cardiac function and maintenance of physiologic homeostasis. It was expected candidates would include within their answer a mention of the stimulus and how it is sensed, the reflex arc and the resultant effect. Thus candidates could have mentioned the Baroreceptor Reflex/Carotid Sinus Reflex, Chemoreceptor, Bainbridge, Cushing, Oculocardiac and Bezold-Jarisch (involves response to ventricular stimuli, sensed by receptors within the LV wall that trigger vagal afferent type C fibers and the resultant triad of hypotension, bradycardia, and coronary artery dilatation) reflexes.
3. Outline the mechanisms of action of anti-platelet drugs. (50% of marks) Briefly describe the mechanism of action, and pharmacokinetics of aspirin, in relation to its use as an anti-platelet drug. (50% of marks).
CICMWrecks Answer: Anti-platelet drugs
Anti-platelet drugs
| COX Inhibitors | Aspirin | MoA | Cyclooxygenase-1 (COX-1) produces precursor to thromboxane A2 (TxA2) Aspirin irreversibly binds and inactivates |
| A/E | GI bleeding and ulceration Kidney injury Reye’s syndrome Bronchospasm In overdose – metabolic acidosis | ||
| Elimination | CYP2C19, metabolites in urine | ||
| Duration | Irreversible COX-1 inhibition → last until platelet turnover (7-10 days) | ||
| P2Y12 receptor antagonists | Clopidogrel Ticagrelor | MoA | P2Y12 is an ADP receptor, which causes platelet recruitment and activation (by inducing activation of GPIIb/IIIa) |
| A/E | Bleeding, itch | ||
| Elimination | dogrel is a prodrug, requiring activation by CYP450 Prasugrel is inactivated by CYP450 | ||
| Duration | Irreversible receptor inactivation → 7-10 days | ||
| Glycoprotein IIb/IIIa inhibitors | Abciximab Tirofiban | MoA | glycoprotein IIb/IIIa binds von Willebrand factor on damaged vascular wall, and fibrinogen to create a pro-clot with other platelets |
| AE | Higher rate of bleeding than aspirin and ADP receptor antagonists | ||
| Elimination | Variable | ||
| Duration | 6-12 hours after cessation of infusion | ||
| Phosphodiesterase inhibitors | Dipyridamole | MoA | Inhibits phosphodiesterase → increased platelet cAMP Also causes coronary vasodilation → useful in angiograms |
| AE | Bleeding, hypotension | ||
| Elimination | Mainly glucuronides in bile 5% in urine | ||
| Duration | 3 hours |
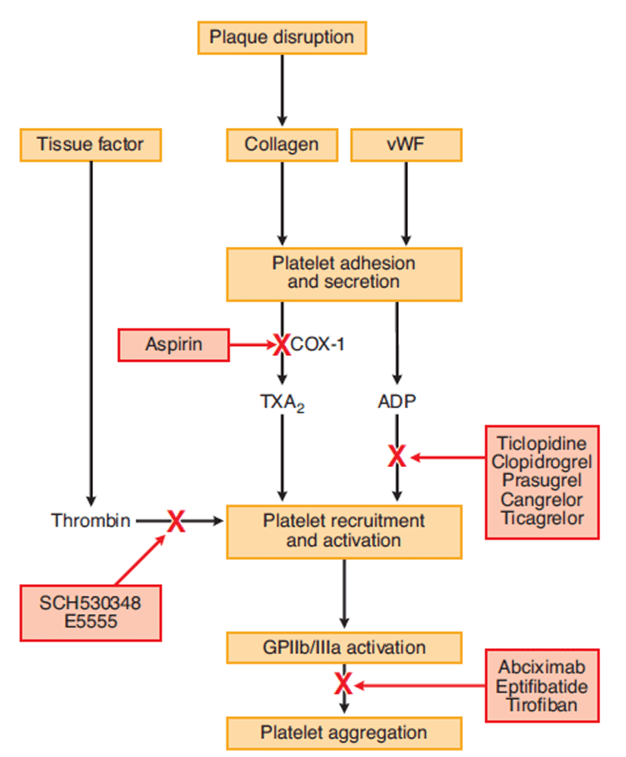
CICMWrecks Answer: Aspirin
Examiner Comments
2013B 03: 3 candidates passed (11.1%).
Candidates should take note of how marks are apportioned to multi part questions and to avoid rewriting the same point more than twice. Generally there was a lack of sufficient breadth in knowledge in responses given for mechanism of action of anti-platelet drugs and sufficient depth of knowledge in relation to aspirin, in particular aspirin pharmacokinetics.
4. Briefly outline the functions of the liver.
CICMWrecks Answer: Liver Functions
Liver:
- Largest abdominal solid organ
- 25 % CO at rest. approx 1,500 mls/min
- 15% of total blood volume at rest
Functions of Liver
| Filtration | |
| Immune defence | (via Kuppfer cells) against agents entering the portal circulation |
| 80% of circulating cholesterol | → bile salt |
| Biliary excretion of drugs/hormones | penicillins, amp, erythro |
| thyroxine, cortisol, estrogen | |
| calcium | |
| Immune | |
| Filtration of portal circulation | |
| Kuppfer cells | bacteria/ virus/ endotoxins/ immune complexes/ thrombin/ tumour |
| Phagocytosed, fused with lysozomes and degraded by lysosomal enzymes | |
| Antigen presentation | |
| Endotoxin neutralisation = pinocytosed | |
| Complement/CRP production | |
| Storage of metabolic substrate/fluids | |
| glycogen ~ 400g | |
| fat | |
| Fe++, B12, folate, Cu | |
| Vitamin A | |
| Blood Reservoir | |
| Metabolic | |
| CHO and intermediary metabolism | Hepatic Glucostat |
| gluconeogenesis, glycogen storage and utilisation, galactose/fructose to glucose | |
| Conversion to fat, AA’s and ketones | |
| Protein metabolism | amino acids utilisation |
| protein synthesis | |
| production of ketones | |
| deamination of fatty acids | |
| urea formation for ammonia removal | |
| plasma protein formation | |
| Fat homeostasis | metabolism (beta oxidation (rapid in hepatic cells)), synthesis and transport as lipoprotein |
| cholesterol homeostasis | |
| Endocrine | hormone synthesis & metabolism |
| Synthesis of 25 OH cholecalciferol, Metabolism of steroid hormones, Synthesis of somatomedins, Erythropoietin | |
| biotransformation | ammonia & urea cycle |
| drugs & toxins | |
| Acid-Base | Lactate metabolism |
| Synthetic functions | |
| Bile production | bile salts (incr fat absorption) |
| bilirubin (incr haem excretion) | |
| Protein synthesis | albumin 120-300mg/kg/d |
| alpha1/2 & beta globulins (transport) | |
| coagulation & fibrinolytic factors (fibrinogen, prothrombin, II, V, VII,VII, IX, X, XI, XII, XIII, antithrombin) | |
| Lymph synthesis | Up to 50% |
| Erythropoietin (10%) | |
Gladwin / JC / Bianca 2019
Examiner Comments
2013B 04: 24 candidates passed (88.9%).
This question was generally well answered with a good response being in some structured format, e.g. a mention, followed by a description for each function of the liver. For questions asking to outline a particular topic, a general overview of the topic is expected and not merely a “dot-point” list of the functions of the liver without actually delving into the way the liver does those functions. In general candidates should avoid making broad-brush statements, which do not get them any marks like “the Liver is the major organ in the body”. Candidates were expected to list, and provide an overview for each, function of the liver.
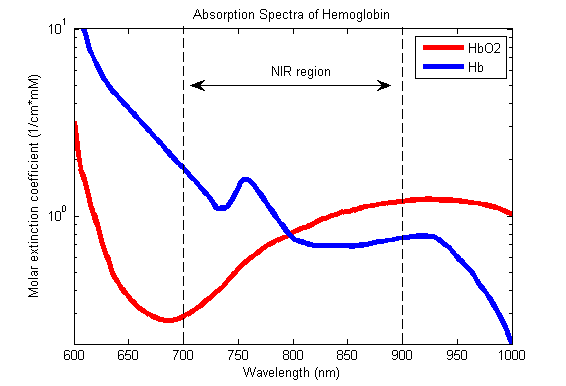
5. Describe the principles of measurement of arterial haemoglobin O2 saturation using a pulse oximeter. (60% of marks) Outline the limitations of this technique. (40% of marks)
CICMWrecks Answer
Principles
Beer-Lambert Law
- Incidence of light is inversely proportional to the path distance and concentration of light absorbing particles within the path.
Hb absorbance at 660nm and 940nm utilized
- 660nm maximally absorbed by deoxyhaemoglobin
- Ratio of absorbance at 660nm and 940nm utilized to calculate SpO2
using healthy volunteers derived R values
Sats 100% R = 0.4
Sats 85% R = 1.0
Sats 50% R = 2
Sats 0% R = 3.4
Pulse oximeter
- Light emitter
- Produces infra-red light at 660nm and 950nm
- Light detector
- Detects light at 660nm and 950nm
- Processor
- Calculates SpO2 using the ratio of absorbance at 660nm and 950nm
- Light detected (incidence) represents light passing through both pulsatile (arterial) blood and non-pulsatile elements (venous blood and other tissues)
- Processor differentiates light incidence during maxima and minima of pulse wave and calculates SpO2 from pulsatile blood only giving SaO2
Limitations
| PATIENT FACTORS | ||
| Low or High SpO2 | Low SpO2 | Normal or High SpO2 |
| Met-Hb Sulph-Hb | Poor perfusion of finger Movement artifact Venous pulsations Fingernail polish Intravenous pigmented dyes Haemoglobinopathy Anaemia with co-existing hypoxia | Carbon Monoxide poisoning |
| EQUIPMENT FACTORS | ||
| – Ambient light interference – Poorly fitting probe – Assay calibrated using healthy volunteers only down to SpO2 80%. Unknown significance if tested SpO2 less than 80% | ||
| PHYSIOLOGICAL FACTORS | ||
| – Due to O2 dissociation curve, insensitive to changes above PaO2 80mmHg – Does not measure tissue oxygenation | ||
Absorption spectra confounded by:
- Carboxyhaemoglobin
- Absorbs 660nm, not at 940nm
- R closer to 0.4
- causes the pulse oximeter to read artificially high
- Methemoglobin
- though it absorbs 660nm light, it also absorbs 940nm light to a greater degree
- R closer to 1
- causes the SpO2 to trend towards 85%
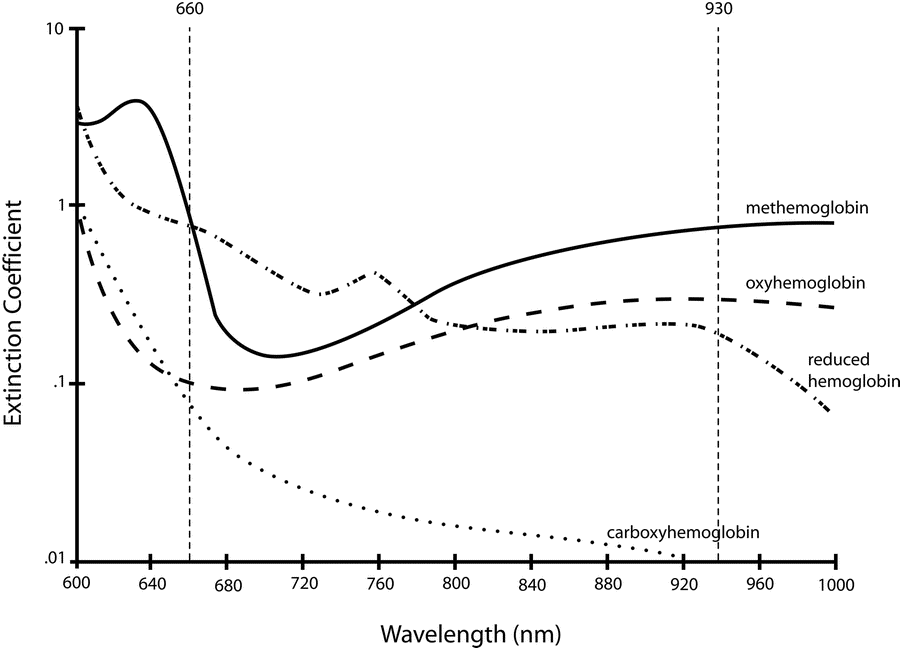
Sakurai 2016
Examiner Comments
2013B 05: 18 candidates passed (66.7%).
A lack of understanding of the physics behind pulse oximetry was a common area of weakness amongst most candidates. Candidates were expected to mention the underlying principle of the “Beer Lambert” Law, absorption spectra and that the differential absorption of light at different wavelengths by different haemoglobin species is used to determine the fractions of haemoglobin types. Limitations should include mention of errors due to calibration as well as sources of false positive and false negative
readings.
6. Describe the pharmacology of short acting insulin (actrapid).
Examiner Comments
2013B 06: 13 candidates passed (48.1%).
In general candidates lacked a sufficient depth of knowledge for this commonly used drug. Some candidates confused actrapid with novo rapid. A structured approach (e.g. pharmaceutics, mode of action, pharmacokinetics, etc.) was expected.
7. Outline the non-respiratory functions of the lung.
CICMWrecks Answer
Filter
- Narrow capillary bed
- Large particles prevented from returning to systemic circulation
- Blood clots → stroke
- Bacteria → killed by macrophages
Immunological
- Numerous macrophages present in lung
- Secretes IgA
Reservoir
9% of blood normally contained within pulmonary vasculature
- Sympathetic stimulation or straining (increase intrathoracic pressure
- Blood mobilised to systemic circulation
- Able to absorb blood volue
- Increase pulmonary artery pressure
- → Recruitment of pulmonary alveoli
- → Increased lung blood volume
- → Recruitment of pulmonary alveoli
- Increase pulmonary artery pressure
Metabolic
- α1-antitrypsin
- Removal of proteases
Endocrine
- Renin/angiotensin/aldosterone axis
- Lung is main source of ACE
- Angiotensin I —ACE—> angiotensin 2
Thermoregulation
- Humidification and warming on inspiration
- Cooling and condensation on expiration
- Prevents excessive loss of heat
Inhalational agents
- Route of administration for drugs e.g. bronchodilators
Sequestration of drugs (“taking up drugs”)
- Fentanyl has affinity for lungs
- Contributes to large VoD
Surfactant production
- Reduces alveolar surface tension
- Prevents alveolar collapse and overdistension
- From type 2 pneumocytes
Mnemonic: FIRM TITS
Mooney 2016
Examiner Comments
2013B 07: 5 candidates passed (18.5%).
In general, this question was not well answered. In particular candidates did not demonstrate sufficient depth of information. Nunn’s Applied Respiratory Physiology devotes an entire chapter to this topic (Ch. 12, in the 6th edition).
8. Statistics (not in current primary syllabus)
9. Define basal metabolic rate and list the factors that affect it. (60% of marks) Describe the ways it may be measured. (40% of marks)
CICMWrecks Answer
Basal Metabolic Rate
- Energy expenditure required to maintain the body’s basic homeostatic mechanisms
- Normal BMR = 40kcal/m2/hr or approx. 70kcal/hr for 70kg male
- Relative organ contribution to BMR
- Liver 30%
- Brain 20%
- Muscle 20%
- Kidney 10%
- Heart 10%
- Other 10%
Factors that affect BMR
- Prandial effects
- Metabolic rate increases post-prandially for the digestion and absorption of nutrients
- After prolonged fasting metabolic rate decreases
- Thermoregulatory effects
- Temperatures above and below the thermoneutral zone cause metabolic rate to increase to support extra thermoregulatory mechanisms
- Response to heat
- Sweating
- Vasodilatation
- Response to cold
- Shivering
- Non-shivering thermogenesis
- Vasoconstriction
- Response to heat
- Temperatures above and below the thermoneutral zone cause metabolic rate to increase to support extra thermoregulatory mechanisms
- Physical activity
- Increased muscle activity
- Exercise increases metabolic rate in order to supply ATP to sarcomeres for contraction
- Increased muscle activity
- Inflammation
- Metabolic rate increases
- Chemotaxis
- Increased oxidative bursts
- Cell proliferation and clonal expansion
- Metabolic rate increases
- Psychological stress
- Hormonal factors
- Thyroid hormones → increase metabolic rate
- Maximal thyroid hormone activity increases MR 100%
- Thyroidectomy decreases MR 50%
- Catecholamines → increase metabolic rate
- Growth hormones → increase metabolic rate ~15~20%
- Testosterone → increase metabolic rate ~10~15%
- Thyroid hormones → increase metabolic rate
- Age
- Decreases with age >20
- Sex
- Male BMR > Female BMR possibly due to testosterone effect
- Body mass
- Muscle mass accounts for significant proportion of BMR, therefore increases BMR
- Sleep
- Decreases BMR 10~20%
Measurement of BMR
- Direct Calorimetry
- Atwater Chamber
- Subject placed in insulated chamber surrounded by H2O
- Change in temperature of water proprotional to metabolic rate
- Atwater Chamber
- Indirect calorimetry
- Metabolic carts
- Measurement of O2 consumption (and CO2, Urea production) to determine Metabolic rate
- Respiratory quotient
- Carbohydrates = 1
- Fats = 0.7
- Proteins = 0.8
- Metabolic carts
Conditions required to measure BMR
- Thermoneutral zone
- Free from psychological or physical stimulus
- After a night of restful sleep
- 12 hours after last meal
- 2 hours after last exercise
Sakurai 2016
Examiner Comments
2013B 09: 12 candidates passed (44.4%).
Basal metabolic rate is the amount of energy liberated by catabolism of food per unit time, under standardised conditions. Knowledge of those conditions (e.g. after a night’s sleep, at rest and with no strenuous activity for at least 1 hour, a relaxed subject at a comfortable ambient temperature, at least 12 hours after a meal) was weak. Factors affecting (e.g. body temperature, various hormones, malnutrition, pregnancy, drugs, disease, etc.) it was often incomplete.
Knowledge of measurement was very poor. Candidates were expected to describe the direct (e.g. by using a whole body calorimeter) and indirect (e.g. measuring oxygen consumption) methods.
10. Compare and contrast the mechanism of action, pharmacokinetics, pharmacodynamics, and adverse effects of digoxin and levosimendan.
Examiner Comments
2013B 10: 11 candidates passed (40.8%).
This question provided candidates with a clear structure and headings that were often ignored. Candidates wasted time on pharmaceutics, derivation (“foxglove” mentioned often) and dosing – these were not requested and scored no marks. Superficial answers such as “cardiac glycoside” or “calcium sensitiser” were not adequate. Responses such as “modest” for Vd are inadequate – marks could be gained for identifying at least the direction of the difference between the two agents. Likewise “hepatic metabolism and renal excretion” is inadequate. Both agents had quantitative and qualitative differences in outcome of metabolic products and the renal elimination of active drug. Confusing diagrams with inadequate labelling, arrows with two heads and the use of uncommon abbreviations without definition all served to confuse the examiners rather than help the candidate. Candidates should read the questions carefully.
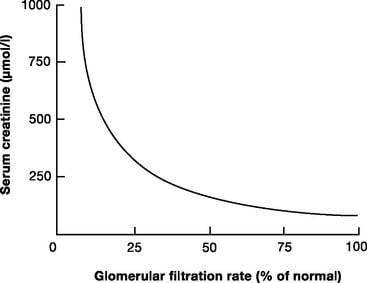
11. Describe the relationship between creatinine clearance and serum creatinine. (60% of marks) What are the limitations in using serum creatinine to assess renal function in the critically ill? (40% of marks)
CICMWrecks Answer
Clearance:
- Renal clearance = vol of plasma completely cleared of a given substance by the kidneys per unit time (ml/min)
- Involves: glomerular filtration, secretion, reabsorption, and rarely tubular metabolism
- Renal clearance = V x [U]/[P]
- V = volume of urine or urine flow rate in ml/min
- [U] = urinary concentration of substance in mg/ml
- [P] = plasma concentration of substance in mg/ml
Estimating GFR:
- GFR = renal clearance of a substance if it is:
- Freely filtered at glomerulus
- Not secreted
- Not reabsorbed
- Not synthesized
- Not metabolised
- The amount excreted in the urine = amount filtered
- i.e. [plasma] x GFR = [urine] x urine vol
- Rearrange: GFR = urine vol x [urine] / [plasma]
Creatinine
- used to approximate GFR as is more practical
- Released at a steady state from skeletal muscle cells (phosphocreatine)
- Freely filtered + not reabsorbed.
- *Small amount secreted → overestimates GFR by small amount
- Serum creatinine levels can be used as a surrogate marker of GFR
- Creatinine clearance calculation better than creatinine levels
- most accurate to collect urine and use the formula to assess creatinine clearance
- because serum creatinine it is at steady state, eGFR can be calculated by Cockroft-Gault, MDRD or CKD-Epi
- During acute changes in GFR, serum creatinine will underestimate GFR until a new steady state is reached.
Creatinine Clearance Estimation from Serum Creatinine
- CG (Cockcroft-Gault Equation): common method which has a correlation of ~0.83 with CrCl:
- CrCl = [(140−A) × W x S)] / (72 × Cr) , where:
- Cl = Clearance (mL/min), A = Age, W = Lean body Wt (kg)
- S = Sex coefficient (Male = 1, Female = 0.85), Cr = Creatinine in µmol.L-1
- CrCl = [(140−A) × W x S)] / (72 × Cr) , where:
- Alternative formulas are MDRD and CKD-EPI. These equations have two advantages over Cockcroft-Gault:
- They are better predictors of GFR
- They do not require weight, and so can be calculated by the laboratory automatically, Other required data (gender, race, age, creatinine) can be taken from hospital records.
- MDRD (Modification of Diet in Renal Disease Study): useful in estimating glomerular filtration rate (GFR) in stable chronic kidney disease
- CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): more precise formula to estimate glomerular filtrate rate (GFR) from serum creatinine and other readily available clinical parameters, especially at when actual GFR is >60 mL/min per 1.73m2
Limitations
- General limitations
- Dependent on serum creatinine, which can be highly variable. Formulas are derived from average values of dependent variables, and so will be unreliable at extremes of:
- Age
- Muscle mass
- Critically ill
- Malignancy
- Diet (High production with red meat)
- the relationship between creatinine clearance and serum creatinine is non-linear
- filtration is only one component of a complex kidney, although GFR is used as a surrogate of function
- Small amount of creatinine is secreted by proximal tubule – so Creatinine clearance is ~10-20% higher than GFR
- More severely overestimates GFR in patients with very low GFR
- Dependent on serum creatinine, which can be highly variable. Formulas are derived from average values of dependent variables, and so will be unreliable at extremes of:
- Critically ill patients
- the amount of creatinine produced varies with muscle mass, nutrition, steroid use, muscle injury
- there can be a decline of almost 50% of function before serum creatinine levels rise
- they do not indicate dynamic changes in renal function
- are modified by aggressive fluid resuscitation
JC 2019
Examiner Comments
2013B 11: 4 candidates passed (14.8%).
This was a straightforward question of core CICM material. Most candidates were able to describe the Fick equation as it related to clearance, and then relate it to Glomerular Filtration Rate (GFR). Formula relating GFR to serum creatinine were often quoted incorrectly, graphs poorly constructed and/or labelled and many answers were very superficially answered.
Better candidates were able to relate the graph to functional nephron loss and hence serum creatinine. The non-linear relationship between nephron mass (and function) and the serum creatinine was poorly appreciated by many. The nature of the variability of creatinine production with age, sex, ethnicity etc. was often omitted, as were the factors involved in the variability in an ICU patient.
12. Explain the following laws:
a. Dalton’s
b. Boyle’s
c. Henry’s
d. Graham’s
e. Fick’s Law of Diffusion
CICMWrecks Answer
Dalton’s Law (of partial pressures)
The total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the individual gases.
Boyle’s Law
The pressure exerted by a gas is inversly proportional to the volume it occupies, assuming the amount of gas and the temperature is constant
Henry’s Law
where c=solubility of a gas at a fixed temp, k=Henry’s Law (equilibrium) constant p = partial pressure of the gas
Given a constant temperature, the amount of gas dissolved in a liquid, is proportional to the partial pressure of that gas
Graham’s Law
The rate of diffusion of a molecule is inversely proportional to the square root of its molecular weight
Fick’s Law of Diffusion
Diffusive flux goes from a high-concentration area to a low-concentration area proportional to both the concentration gradient and surface area and is inversely proportional to the thickness of the membrane.
JC / Sakurai 2016
Examiner Comments
2013B 12: 7 candidates passed (25.9%).
The universal gas laws form the basis of oxygen therapy and delivery, pressure and volumetric monitoring as well as a key to understanding the solubility of gases in blood. All the equations and relationship are straightforward so this question provided a good opportunity to score marks. Unfortunately many candidates were aware of the properties of the ideal gases but not the named laws. This led many candidates to omit major sections of the answer and thus scored no marks. Several candidates wasted time with complicated diagrams as well as equations and descriptions (scoring no additional marks). Many candidates were unable to identify Grahams Law (rate of diffusion inversely proportional to the square root of the molecular weight) but included it in an expanded Fick’s Equation.
13. Outline the adverse consequences of a blood transfusion. (75% of marks) Define massive blood transfusion and list the adverse consequences associated with a massive blood transfusion. (25% of marks)
CICMWrecks Answer
Adverse Consequences of Blood Transfusion
1. Acute (<24 hours)
1a) Immune-mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Allergic reaction to plasma proteins | mild (urticarial) or severe (anaphylaxis) | Pre-treatment in high risk patients |
| Acute Haemolytic transfusion reaction | incompatibility of donor and recipient blood leads to widespread haemolysis and circulatory collapse | Group and screening |
| Febrile non-haemolytic transfusion reaction (FNHTR) | due to stored cytokines and/or the presence of recipient alloantibodies | Careful monitoring and early cessation of transfusion |
| Transfusion related acute lung injury (TRALI) | noncardiogenic pulmonary oedema caused by HLA antibodies in donor plasma directed against recipient leukocytes or bioactive lipids which accumulate during storage | Careful monitoring and early cessation of transfusion |
1b) Non-immune mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Sepsis | bacterial infections are most common with platelets as they are stored at room temperature | Safe and sterile methods of storage |
| Transfusion Related Circulatory overload (TACO) | fluid overload usually due to rapid or massive transfusion | Careful monitoring and early cessation of transfusion |
| Non-immune mediated haemolysis | Careful monitoring and early cessation of transfusion | |
| Hypothermia | Careful monitoring and early cessation of transfusion | |
| Dilutional coagulopathy | Careful monitoring and early cessation of transfusion |
2. Delayed (>24 hours)
2a) Immune-mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Delayed haemolytic transfusion reaction | Production of anti-donor antibodies post-transfusion May be associated with transfused malaria | Careful monitoring and early cessation of transfusion |
| Transfusion-related immunomodulation (TRIM) | transient immunosuppression in blood recipients which may be due to release of cytokines from donor lymphocytes | Careful monitoring and early cessation of transfusion |
| Alloimmunisation | development of antibodies during exposure to blood products, resulting in an amplified reaction on subsequent exposure Can cause post transfusion thrombocytopenia and purpura | Re-screening every 48-72 hours |
| Transfusion Associated Graft vs. Host Disease | Profound bone marrow aplasia >90% mortality Viable donor T cells implant and attack recipient tissues | Careful monitoring. Re-screening |
2b) Non-immune mediated
| Problem | Methods to Minimize | |
|---|---|---|
| Iron overload | most common in chronically transfused patients | Monitoring of Iron levels |
| Transfusion-related infection | Viral – the risk of HIV, HTLV 1&2 and HCV is <1/1 million. The risk of contracting HBV is slightly higher at 1/500,000. Other – malaria, vCJD, Dengue Fever, West Nile Virus | Pre-screening of donors, screening of collected blood as per local guidelines (different for different areas) |
3. Storage Lesions
A storage lesion refers to the changes that occur to a sample of blood during storage. (Note: Australian Red cross anti-coagulates Whole blood with CPD, but washes and stores Red Cells in SAGM).
| Problem | Methods to Minimize | |
|---|---|---|
| Physical changes | Reduction in the viability of RBCs due to shape changes and reduced deformability Formation of microaggregates | Careful monitoring and usage of products within a specified period |
| Hyperkalaemia | plasma K can be >20 at 28 days in stored blood due to inactivation of the red cell Na/K ATPase pump. | Careful monitoring and usage of products within a specified period |
| High citrate load | can lead to hypocalcaemia and alkalosis (less or nil in SAGM) | Use of SAGM for storage |
| Reduction in 2,3-BPG | causes left shift of the oxygen/haemoglobin dissociation curve (less in CPDA1) | Use of CPDA1 |
| Renal impairment | Due to Increase in free haemoglobin from cell lysis | Routine monitoring of renal function and careful monitoring during transfusion |
Massive Transfusion
- Replacement of >1 blood volume in 24 hours
- 50% of blood volume in 4 hours
Adverse consequences of massive transfusion
| Problem | Methods to Minimize | |
|---|---|---|
| Hypothermia | Cooled products | Appropriately re-warm products. Use of blood warmers |
| Poor O2 delivery | Depletion of 2,3 DPG in PRBC | |
Haemostatic abnormalities | Dilutional coagulopathy | Monitor coagulation profile at appropriate intervals during massive transfusions. Use of appropriate factors like FFPs, Cryoprecipitate during massive transfusions |
| Hypocalcaemia | Consumption with coagulopathy and bound to citrate added to transfused units | Monitor and replace calcium as necessary |
| Hypomagnesaemia | Bound to citrate in transfused units | Monitor and replace magnesium as necessary |
| Citrate toxicity | Citrate is added to stored units as an anticoagulant | Monitor for acidosis during massive transfusions |
| Lactic acidosis | Hyperlactataemia due to anaerobic metabolism in stored units | Monitor for acidosis during massive transfusions |
| Hyperkalaemia | Potassium migrates from stored erythrocytes into plasma whilst in storage | Careful monitoring and usage of products within a specified period |
| Air embolism | Inadvertent infusion | Careful monitoring, use of transfusion sets with air vents, filters |
JC / Sakurai 2019
Examiner Comments
2013B 13: 17 candidates passed (63.3%).
In general this question was well answered; however candidates often had difficulty differentiating specifically for a massive blood transfusion (defined as replacement of circulating volume in 24 hours, or greater than 4 units blood transfused in 1 hour of continuing blood loss, or loss of 50% circulating volume in 3 hours). Responses to the first part of the question generally lacked depth. It is suggested that candidates take a systematic approach (e.g. use of categories such as immune, infectious, storage, etc.) when answering these type of questions. Candidates are reminded that when asked to “outline”, that the expectation for them to include a description that reflects understanding, and not just “dotpoints”.
14. Compare and contrast the mechanism of action, pharmacokinetics and central nervous system effects of morphine and tramadol.
Examiner Comments
2013B 14: 15 candidates passed (55.6%).
In general candidates either lacked a depth of knowledge, or a deep enough understanding of the drugs so as to apply their knowledge specifically to the central nervous system (CNS). Mechanism of action and pharmacokinetics for morphine was better understood, in comparison to tramadol. Mention of the non-CNS effects of morphine and tramadol, was not expected, did not score marks, and would have wasted valuable exam time.
15. Explain the role of the skin.
CICMWrecks Answer
Skin
- Largest organ in body ~15% body weight
- Resting blood flow ~500ml/min in 70kg male
- Can increase 30-fold or decrease 10-fold
- Largest barrier between human body and external environment
- Composed of epidermis, dermis and subcutaneous tissue
Role of Skin
- Homeostasis
- Relatively impermeable
- Prevents fluid loss
- Only ~300ml/day lost through skin
- Immunological
- Physical barrier
- Low pH and commensal organisms
- Abundant Antigen Presenting Cells (APC) such as dendritic cells
- Sensory function
- Embedded with nerve endings
- Sensory signals to CNS and spinal reflex arcs
- Touch
- Pain
- Temperature
- Sensory signals to CNS and spinal reflex arcs
- Embedded with nerve endings
- Thermoregulation (SEE BELOW FOR DETAILS)
- Sensory
- C fibres – heat
- A δ fibres – cold
- Effector
- Heat loss
- Occurs via
- Radiation
- Conduction
- Convection
- Evaporation
- Requires transfer of core body heat to skin
- Regulated by AV shunts (glomus body)
- Occurs via
- Heat gain
- Opening of AV shunts prevents loss of core body heat to skin
- Heat loss
- Sensory
- Synthesis of cholecalciferol
- Protection against UV
Role of Skin in Thermoregulation
Sensors:
- Thermoreceptors, on free endings of sensory nerves
- Aδ fibres (myelinated, cold, sense below 38°c)
- C fibres (unmyelinated, warm, sense above 30°c)
- Anterior hypothalamus is sensitive to changes in blood temperature
Controller:
- Hypothalamus
- Maintains temperature around a set temperature
- Influenced by many factors including circadian rhythms, thyroid function and ambient temperature
Effector:
- Heat exchange is due to convection, conduction, radiation and evaporation
- Depends on the direction and size of the gradient between skin and the environment
Skin blood flow:
- Heat loss through radiation and convection
- Increased blood flow to skin increases convection and conduction (blood is warm, if temperature gradient present will lose heat to air at increased rate when more flow)
- Assuming skin temperature is below ambient temperature
- When too cold: Sympathetic outflow from hypothalamus
- Noradrenaline acts on α adrenoreceptors (abundant in skins)
- Constriction of cutaneous precapillary sphincters
- Shunting of blood through arteriovenous shunts, bypassing skin
- Constriction of cutaneous precapillary sphincters
- Noradrenaline acts on α adrenoreceptors (abundant in skins)
- When too warm: Decreased sympathetic outflow
- Cutaneous vasodilation
Sweating:
- Heat lost through evaporation and convection
- Energy of molecules in a liquid is unevenly distributed
- High energy molecules will enter gas phase, be liberated from the liquid
- Lowering the average temperature of the liquid
- Sweat glands in dermis
- Secretion of precursor fluid from the gland
- Ducts ascend through the dermis to exit from the dermis
- Pre-ganglionic sympathetic (cholinergic) innervation
- When too cold, Decreased stimulation
- Fluid moves slowly through duct
- Fluid mostly absorbed, minimal sweat
- When too hot, increased sympathetic stimulation
- Fluid moves quickly through duct
- Fluid not resorbed, lots of sweat
Galwin 2016
Examiner Comments
2013B 15: 13 candidates passed (48.1%).
The skin is the largest organ of the body, accounting for about 15% of the total adult body weight, with a rich, but tightly regulated blood flow. It performs many vital functions, including the protection against external physical, chemical, and biologic threats, the prevention of excess water loss from the body and thermoregulation. It is composed of three principle layers, the epidermis, dermis and subcutaneous tissue, each with their own purpose. In addition candidates were expected to describe those aspects of the skin that play a role in physical protection, immune, sensory, thermoregulatory and water regulation. Candidates lacked a sufficient breath and depth of knowledge in this area and often digressed beyond areas specific to the skin (e.g. thermoregulation not specific to the skin).
16. Describe the pharmacology of vancomycin.
Examiner Comments
2013B 16: 16 candidates passed (59.3%).
A commonly used drug in intensive care practice, for which a high level of understanding is required (Level A). In general answers were sufficient for a pass, but there was still a lack of sufficient breadth of knowledge, in particular to pharmacokinetics and detailed mechanism of action.
17. Describe the anatomy of the sympathetic nervous system.
CICMWrecks Answer
Anatomy of SNS
- Posterior hypothalamus is the main site of sympathetic nervous outflow
- Receives input from cardiovascular centres of medulla and pons
- Sympathetic innervation from Sympathetic trunks
- Paired bundle of sympathetic neurons run lateral from vertebral bodies from T1 to L2
- Consists of two neurons in series
- Short Pre-ganglionic neuron → Sympathetic ganglion → Long Post-ganglionic neuron
Origin
- Origin: Preganglionic fibres originate in the grey matter of the spinal cord lateral horn
- Nerve Fibres and Pathway: Leave the spinal cord through ventral roots (with spinal nerves)
- Leave spinal nerves, travel as white rami communicantes (short myelinated B fibres)
- Meet with the ganglia of the sympathetic trunk
- Synapse with post-ganglionic neurons
- Neurotransmitter: Preganglionic neurotransmitter is acetylcholine
- Receptor: nicotinic receptors
Sympathetic trunk:
4 parts
- Cervical part
- Head
- Neck
- Thorax
- Thoracic part (T1-T5)
- Aortic plexus
- Pulmonary plexus
- Cardiac plexus
- Thoracic splanchnic nerves
- Lumbar sympathetic ganglia
- Coeliac plexus
- Pelvic part
- Hypogastric plexus
- Pelvic plexus
Post-ganglionic
- Nerve Fibres and Pathway: Post-ganglionic neurons leave the ganglia as grey rami communicantes
- Long unmyelinated C fibres
- Rejoin the spinal nerves to travel to target organs
- Neurotransmitter: Postganglionic neurotransmitter is noradrenaline
- (acetylcholine in muscles, sweat glands and hair follicles)
- Receptor: Adrenergic alpha and beta receptors on target organs/vessels
Exception is adrenal medullary outflow:
- Modified post-ganglionic cells release adrenaline into circulation
- Innervated directly by pre-ganglion sympathetic fibres
Effects of SNS
| Function | Control the body’s response during perceived threat. Diffuse physiological accelerator |
| Activates response of | Fight-or-flight |
| General Body Response | Body speeds up, tenses up, becomes more alert. Functions not critical to survival shut down. |
| Cardiovascular System | ↑↑↑ Chronotropy, ↑↑↑ inotropy, ↑↑↑ lusiotropy, ↑↑ dromotropy |
| Vasculature | Constriction |
| Pulmonary System | Bronchial tubes dilate |
| Musculoskeletal System | Sweating, contraction, lipolysis |
| Pupils | Dilate |
| Gastrointestinal System | Decreased salivation and GIT motility, increased sphincter tone, gluconeogenesis |
| Salivary Glands | Saliva production decreases |
| Endocrine | Adrenaline and noradrenaline release |
| GU | Detrusor relaxation, sphincter contraction, ↑ uterine tone |
Mooney / JC 2020
Examiner Comments
2013B 17: 3 candidates passed (11.1%).
Knowledge of the anatomy of the sympathetic nervous system is important in helping to understand its physiology, and the pharmacology of drugs that affect it. Such information is widely available within most physiology, and even pharmacology texts when mentioning the sympathetic nervous system. In general candidates lacked depth and often were inaccurate in their description. A systematic approach (e.g. spinal levels, pre-ganglionic, post-ganglionic, etc.) was often lacking.
18. Describe the anatomy relevant to the cannulation of the left subclavian vein.
CICMWrecks Answer:
Origin
- Continuation of axillary vein
- Lateral border of 1st rib
Course
- Follows subclavian artery
- Deep to clavicle
- Superior to 1st rib
Termination
- Deep to sternoclavicular joint at medial border of scalenus anterior
- Joints internal jugular vein to form bilateral brachiocephalic veins vein on left
Relations
- Anterior
- Clavicle, subclavius
- Posterior
- Subclavian artery runs deep/posterior (separated by scalenus anterior)
- Internal mammary artery is posterior medially
- Phrenic nerve is posterior
- Superior
- Skin, superficial aponeurosis
- Inferior
- Apex of lung and 1st rib
- Medial
- Brachiocephalic trunk, thoracic duct and trachea and vagal trunks
- Lateral
- Inferior trunk of brachial plexus
Surface Anatomy
- Clavicle
- Deltopectoral groove
- 2 heads of sternocleidomastoid
- Suprasternal notch
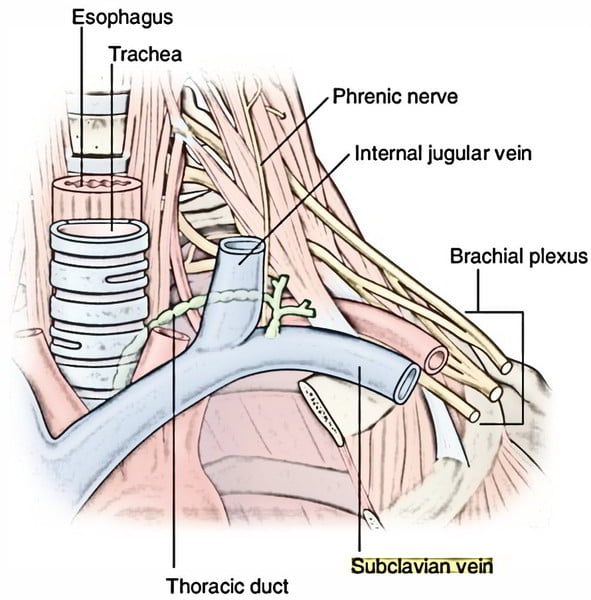
Sakurai 2016
Examiner Comments
2013B 18: 9 candidates passed (33.3%).
Understanding the anatomy of the subclavian is essential knowledge in intensive care practice. It was expected that candidates mention where it starts, where it ends, relationship to surrounding structures (in all directions, i.e. medial, lateral, anterior, posterior, etc.) and surface anatomy. In general, relationships and/or surface anatomy was either poorly understood or not mentioned.
19. Describe the cardiovascular changes during pregnancy.
CICMWrecks Answer
- Pregnancy is a time of increased metabolic demand, which cardiovascular changes reflect.
- Changes begin from week 8 and ↑ to plateau at 32 weeks → return to normal 2-8 weeks post delivery
- Changes depend on stage of pregnancy
- Hormonal changes: ↑ circulating concentrations of oestrogen, progesterone, hCG
- ↑ metabolic demand esp. during labour: ~↑60% O2 consumption/ CO2 production during labour
- Mechanical effects from gravid uterus
Characteristics
- Mechanical Effects:
- Thoracic changes:
- Anatomical compression of chest
- Diaphragm pushed upwards by 4cm
- ↑ AP + transverse diameter of chest wall (2-3cm)
- placental circulation: ↓pressure, ↓resistance AV shunt
- Aortocaval compression
- Collateral blood flow via collateral paravertebral epidural veins
- Thoracic changes:
- Hormonal Changes:
- ↑ circulating concentrations of oestrogen, progesterone, hCG
- Oestrogen stimulation of RAAS
- Increased plasma volume (40% or 1~1.5L positive)
- Erythropoietin secretion
- Increased erythropoiesis and red blood cell volume (20%)
Changes in CVS
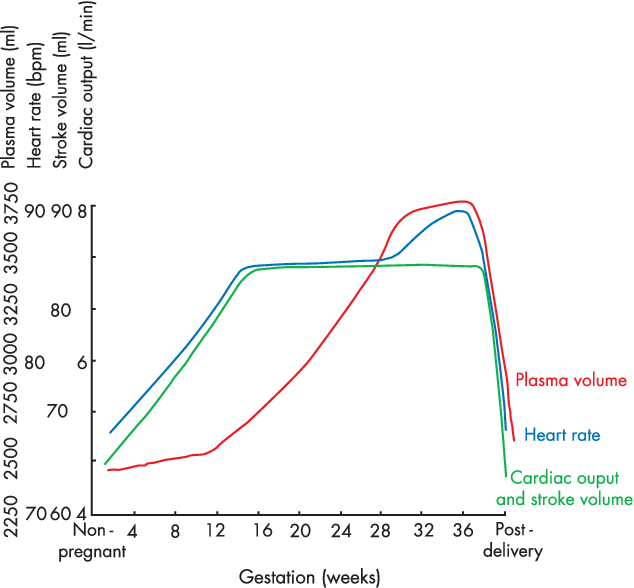
- Anaemia of pregnancy
- Disproportionate plasma volume expansion relative to erythropoiesis
- Increased cardiac output (40%)
- Increased uterine blood flow (750ml/min)
- Increased renal blood flow
- Increased HR (25% by second trimester)
- Increased SV (25% in first trimester)
- Decreased peripheral vascular resistance (30%)
- Progesterone
- Prostaglandins
- Down-regulation of α receptors
- Decreased plasma oncotic pressure (15%) → peripheral oedema
During labour
- Contraction → 300ml return to central maternal circulation
- CO increases 15%, 30% and 45% in latent, active and expulsive phases of labour respectively
- Immediately after labour CO 80% pre-labour levels due to autotransfusion due to uterine involution
- Return to non-pregnant levels 2 weeks after delivery
Sakurai / Kerr / JC 2020
Examiner Comments
2013B 19: 4 candidates passed (14.8%).
Many candidates’ answers included respiratory and other systems (e.g. endocrine) when only cardiovascular changes were asked for. Candidates are reminded to read the questions carefully. Poor candidates lacked detail of the progressive changes through the trimesters. Changes during (and immediately post) labour and delivery were often overlooked. This is listed as a core topic within the syllabus, that is readily covered by most physiology texts and candidates are expected to have a very sound knowledge of it.
20. Describe the electrocardiographic (ECG) changes seen with hyperkalaemia. (30% of marks) Outline the pharmacologic principles of drugs used in the management of severe hyperkalemia. (70% of marks)
CICMWrecks Answer
Serum Potassium
- Normally 3.5~5mmol/L
- Hyperkalaemia [K+] >5mmol/L
- Severe hyperkalaemia [K+] >7mmol/L
ECG Changes
- Peaked T waves at [K+] > 5.5mmol/L
- Loss of P wave at [K+] > 6.5 mmol/L
- QRS widening at [K+] > 7.5mmol/L
- VF or asystole
Consequences of Acute hyperkalaemia
- CVS
- ECG changes
- Tenting of T wave
- Flattening of P wave and increase PR interval
- Widening of QRS
- VF
- Arrythmia
- VF
- ECG changes
- CNS
- Lethargy
- Other
- Flaccid paraylis
- Muscle weakness
- Respiratory failure
Management
Drugs that eliminate body K+
- Loop diuretics e.g. frusemide
- 40mg IV
- Inhibition of the Na+/K+/2Cl- cotransporter on luminal membrane of the thick ascending Loop of Henle
- This causes loss of normal positive charge in lumen, leading to the loss of paracellular resorption of K+
- Net result is K+ excretion
- Elimination half-life 45-9 mins
- Cation exchange resins e.g. sodium polystyrene sulfonate
- 15~45gm
- Not absorbed in GI tract
- Prevents potassium reabsorption in the GI tract (predominantly in the colon where K+ secretion occurs)
- Onset of action: 1 hr PR, 4-6hrs PO
- Duration: variable
- No effect acutely
Drugs causing increased intracellular uptake of K+
- Insulin/glucose
- 10 units IV (with 50ml 50% dextrose)
- Insulin receptor agonism → transcellular shift of K from extracellular to intracellular space via upregulation of Na/K ATPase → transient reduction in serum [K+]
- Onset: 20-30 mins
- Duration: 2-6 hours (prolonged if i.v. infusion)
- Sodium bicarbonate
- Alkalosis increases the activity of the Na+/K+ ATPase pump, increasing K+ uptake
- Onset: 30-60 mins
- Duration: 2-3 hours
- β2 agonists e.g. salbutamol
- 5~20mg via nebulizer, repeated (or 6-12 puffs via MDI, repeated)
- β2 agonism → increased intracellular [cAMP] → transcellular shift of K from extracellular to intracellular space → transient reduction in serum [K+]
- Onset: 30 mins
- Duration: 2-3 hours
- I.V. fluid
- Causes haemodilution
- Can increase renal excretion of K+ by increasing renal perfusion and increasing urine output
Other Treatments in Hyperkalaemia:
- Calcium (No effect on K+ level)
- 10ml 10% CaCl (6.8mmol) or 10% CaGluconate (2.2mmol)
- Immediate onset
- Stabilizes cardiac membrane by reducing threshold potential
- Dialysis
- via continous Veno-Venous Dialysis or intermittent haemodialysis
- Dialysis fluid adjusted to create large concentration gradient for potassium
extraction via dialysis of filtration
JC / Sakurai 2019
Examiner Comments
2013A 12:
Potassium is the second most common cation in the body and the main intracellular cation. It is widely distributed and has many important roles. Maintenance of potassium balance depends mainly on secretion by the kidneys in the distal and collecting tubules. Candidates were expected to mention the influence of aldosterone, and other hormones such as glucocorticoids, catecholamines and vasopressin have as well as factors such as acidosis/alkalosis. Candidates who had a systematic and structured approach performed better.
21. Describe the pharmacology of propofol.
Examiner Comments
2013B 21: 19 candidates passed (70.4%).
A high level of knowledge was expected as it is a commonly used drug in intensive care. Overall most candidates performed very well. Areas of weakness were those relating to propofol pharmacokinetics and pharmacodynamics.
22. Define pain. (10% of marks) Describe the anatomical and immediate physiological components of the response to pain arising from the insertion of an arterial line. (90% of marks)
CICMWrecks Answer
Pain is an unpleasant sensory and emotional experience, associated with actual or potential tissue damage, or described in terms of such damage
Anatomical and physiological components of the response to pain
- Primary afferent nociceptors
- Pseudo-Unipolar
- Cell body in dorsal root ganglion
- Free nerve endings or specialized terminal structures
- Pacinian corpuscles
- Location
- Skin
- Connective tissue
- Muscle
- Blood vessels
- Viscera
- Responds to simuli
- Mechanical
- Chemical
- Thermal
- Pseudo-Unipolar
Nociception
- Transient Receptor Potential (TRP) Ion Channels
- Capsaicin
- Heat
- Acid
- Inflammation
- Ischaemia
- Acid-Sensing Ion Channel (ASIC)
- 5-HT3 receptors
- On activation of nociceptors
- Na channels open
- Depolarization from < -60mV to -40mV
- Rapid membrane depolarization
- Voltage Dependent Calcium Channels (VDCC) open and increase intracellular Ca
- Substance P and Calcitonin Gene-Related Peptide (CGRP), Neurokinin A released in response to Ca
- Mediate inflammation → macrophages, neutrophils, mast cells activated
- Increase excitability of sensory and sympathetic fibres
- Vasodilation, extravasation of plasma proteins
- Inflammatory cells release substances
- Histamine
- Bradykinin
- Serotonin
- Nitric Oxide
- Peripheral sensitization → Primary hyperalgesia
- to dampen pain response
- Silent nociceptors – Do not respond to external stimulus, however activated by inflammatory mediators
- Voltage-gated Potassium Channels stabilize the membrane potential
Signal Pathway
- Signal transduced via C fibres (0.5~2m/sec) or Aδ fibres (6~30m/sec) to dorsal horn
- Aδ activation → short, pricking pain
- C activation → burning, aching pain
- Primary afferent enters spinal cord at the spinal level, or above or below the spinal level via lissauer tracts and synapse with interneurons
- Via C fibres or Aδ fibres
- Signal relay via spinal interneurons
- Signalling via Glutamate and NMDA or AMPA receptors
- Nociceptor-specific
- Respond only to pain
- Wide Dynamic Range (WDR)
- Respond to nociceptors and other stimulus
- Central sensitization → allodynia
- Inhibitory interneurons and descending pathways modulate pain
- “Gate control theory of pain” → Aβ fibres carrying signals from benign stimuli inhibit noxious interneuron transmission
- Signal transduction to thalamus via anterolateral spinothalamic tract
- Small but significant proportion of signal transduction also occurs ipsilaterally
- Signal distributed to cerebral cortex via thalamus
- Putamen
- Hypothalamus
- Amygdala
- Periaquaductal grey matter
- Hippocampus
- Cerebellum
- Somatosensory cortex
Descending inhibitory pathways
- Noradrenaline
- Serotonin
- Substance P
- CCK
- GABA
Sakurai 2016
Examiner Comments
2013B 22: 11 candidates passed (40.8%).
The pain pathways following arterial line insertion involve sensing the stimulus and transmitting the sensation to the central nervous system. It was expected candidates could provide some detail about the major features along this pathway including a description of sensors, nerve types, spinal cord input and decussation with subsequent projection to higher centres. Better answers provided additional details about modulation and descending pathways. The question also required a description of the physiology with some discussion of the mediators involved and explanation of how a stimulus or tissue may result in the perception of pain. Common omissions included insufficient detail of the pain pathway and limited or no discussion of the physiological components.
23. What factors affect airway resistance? (80% of marks) Briefly outline how it may be measured and/or changes in flow are detected. (20% of marks)
CICMWrecks Answer
Airway Resistance
- Resistance is the impedence to flow.
- Normal Airways Resistance (AWR):
- Adult: ~2 cmH2O/L/s
- Newborn: ~20 cmH2O/L/s – declines markedly
- Main Site of AWR:
- Mid-sized bronchi 7th/8th generation
- comparatively smaller cross-sectional area
- (note that in neonates, a greater proportion of Raw comes from the smaller peripheral airways)
- In the airway flow can be laminar or turbulent
- Flow depends on Depends on Reynolds number
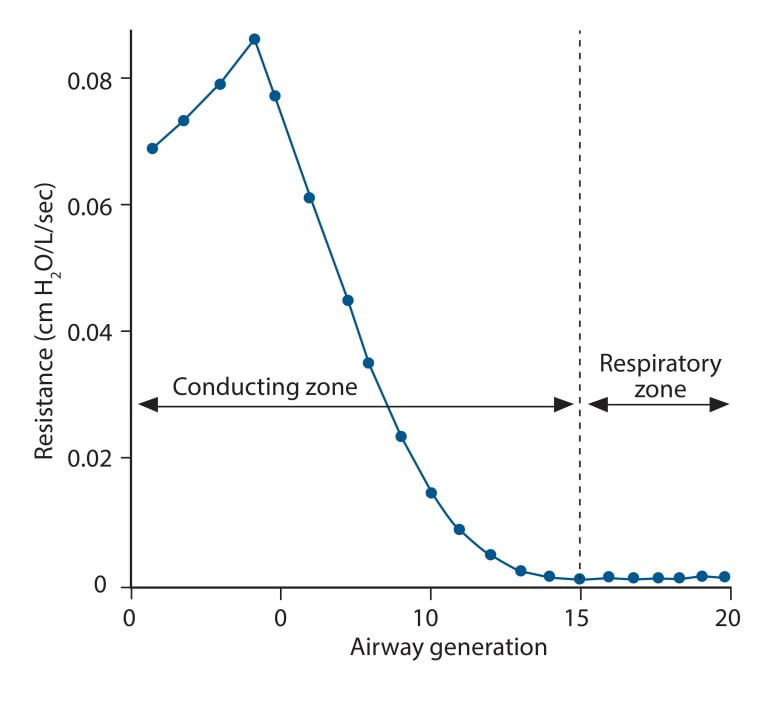
where
Re is Reynold’s number
r is radius
ρ is density
v is velocity
η is viscosity
Laminar Flow
- Ordered flow occuring in concentric layers within a tube
- Flow in the center is fastest and flow in the most peripheral layer is the slowest
- Resistance to laminar flow obeys the Poisuille-Hagen Equation
where
R is vessel resistance
η is viscosity
L is length of vessel
r is radius of vessel
- Increases in viscosity of gas, or length of the tube increase resistance
- Increases in radius of the tube, decreases resistance by a power of 4
Turbulent Flow
- In turbulent flow, due to the disorganized flow and increased likelihood of friction
with the static airway wall, resistance is markedly increased. - Resistance to turbulent flow obeys the following equation
where
R is Resistance to flow
ρ is density
l is length of vessel
r is radius of vessel
- Increases in density of gas, or length of the tube increase resistance
- Increases in radius of the tube, decreases resistance by a power of 5
Factors affecting Airway Resistance
- Physics factors (see above):
- Location in the airway (see above): Mid-sized bronchi are the location of greatest airway resistance, resistance progressively declines with successive airway generations
- Flow:
- Laminar Flow vs turbulent flow
- Depends on Reynolds number factors
- density more important than dynamic viscosity, velocity (flow rate) as Main Site is Turbulent.
- Flow rate:
- Flow related airway collapse
- Airways beyond generation 11 have no structural rigidity
- High flows can reverse transmural pressure gradient and cause airway collapse
- Flow related airway collapse
- Newborn: ~20 cmH2O/L/s – declines markedly with age to ~2 cmH2O/L/s
- Radius changes
- Muscular control of airway diameter
- Neural:
- Parasympathetics important in bronchomotor tone → airway constriction via ACh and muscarinic receptors
- Sympathetic system virtually absent in lung
- Hormonal:
- Although no symppathetic innervation, abundance of β2 adrenoceptors → airway dilation
- Neural:
- Smooth mm tone:
- (↓r) bronchospasm, Musc antag (PSNS), LTs, PGF2-alpha
- (↑r) β2-agonists, adrenaline neb, SNS
- ↓intramural radius:
- oedema, ↑mucous, wall hypertrophy
- External compression:
- tumour, haemorrhage, PTX
- dynamic airways compression with forced expiration
- Muscular control of airway diameter
- Lung Volume
- ↑ lung vol →
- ↑ radial traction → ↓ AWR
- ↑ -ve intrapleural → ↑ patency of small airways
- ↓ lung vol → ↑ AWR
- CPAP or PEEP → ↑ FRC → reduces flow-related airway collapse at low lung volumes
- ↑ lung vol →
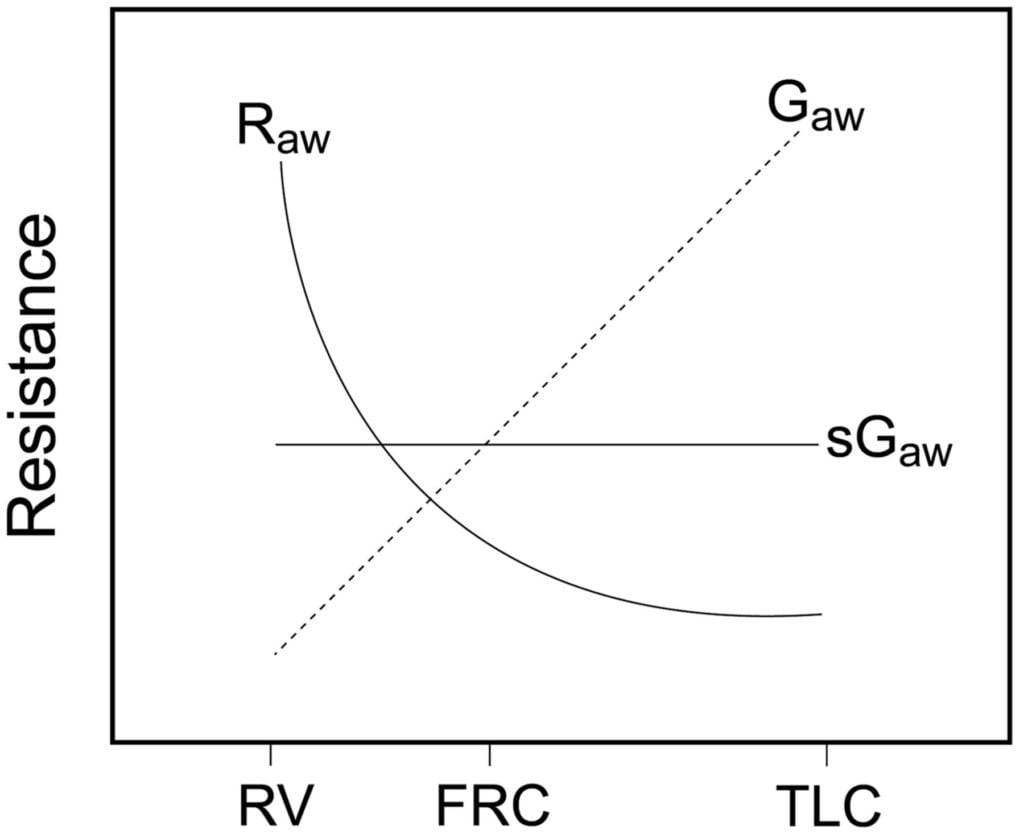
Relationship between Lung volume, Airway Resistance (R) , reciprocal of resistance (Conductance G, specific conductance sG)
Notice that only sGaw is independent of lung volume
Measurement of Airway Resistance
Body Plethysmography:
- Q measured with flow meter
- Lung Volume is measured with Plethysmography
- ∆P via Plethysmography and Boyle’s Law
- (A) box pressure is atmospheric
- (B) inspiration
- ∆V allows for ∆P measurement for given Q since
- PV = constant
- Then AWR can be calculated from 1
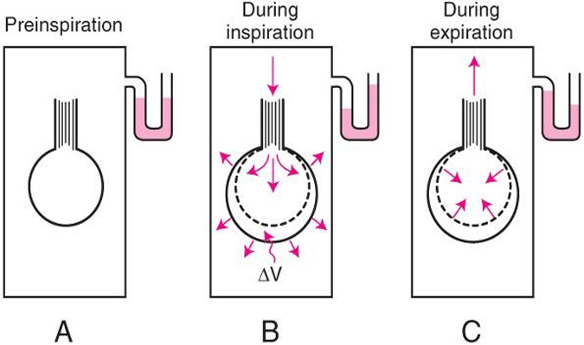
Interrupter Technique (direct from AWR eqn)
- Method
- Manometer distal to shutter
- Used to measure mouth and alveolar pressure
- Flow during inspiration or expiration in interrupted for 50–100 ms repeatedly throughout the respiratory cycle.
- Measure
- Q – flow rate (measured before interruption)
- P2 – mouth pressure (measured before interruption)
- P1 – pressure in alveoli (measured at the end of the interruption at te level of the mouth after equilibration)
- Apply Ohm’s Law (AWR equation above).
- Adequate for normal lungs not diseased.
Gladwin / Sakurai 2016
Examiner Comments
2013B 23: 5 candidates passed (18.5%).
This topic required a definition and understanding of airways resistance. It was expected candidates could identify that issues around the nature of flow (turbulent vs. laminar) and airway diameter were central determinants. It was expected candidates would describe the determinants of turbulent flow.
The provision of formula and comments about Reynolds number helped demonstrate an understanding of this. Better answers discussed the transitional point in the airway and the paradox about size vs. total cross sectional area and its influence on total resistance. Several candidates confused pulmonary vascular resistance with airways resistance. Using graphs to help illustrate certain concepts would have been helpful. Measurement of resistance (indirectly via measurement of flow and pressure difference by a body plethysmography, spirometry) and detection of flow (spirometry, capnography) was in general poorly understood.
24. Outline the physiological responses to anaemia. (The specific physiological responses to hypovolaemia are NOT required.)
CICMWrecks Answer
Anaemia
- condition (acute or chronic) a/w deficiency of RBC or Hb in blood
- Low haemoglobin
- Males <140g/L
- Females <120g/L
- Eg. due to haemolysis, chronic disease, Fe-deficiency, Vitamin B12/folate deficiency, haemorrhage, etc.
Normal O2 supply to tissues
Approx 1l/min
Consequence of anaemia
- ↓ O2 content of blood (CaO2), and thus ↓ O2 delivery to tissues (DO2)
- Risk of tissue hypoxia – Increase products of anaerobic metabolism
- CO2, Lactate
- Decrease in pH as a consequence
Physiological Responses /
Compensatory Mechanisms that maintain Tissue Oxygenation
Effects on oxyhaemoglobin dissociation
- Increased PaCO2, Decreased pH and Decreased O2 cause right shift in Hb O2 dissociation curve → ↑ Tissue O2 extraction
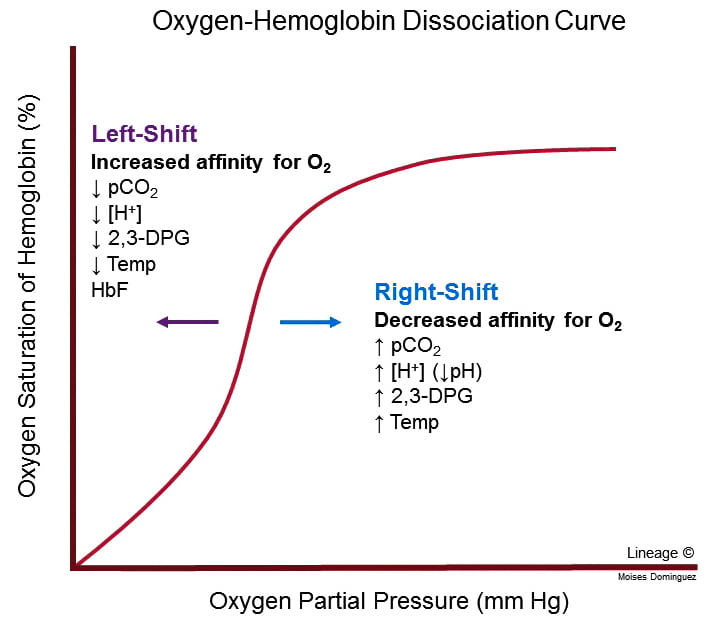
- For given PO2, Hb O2 affinity is reduced → increased offloading of O2 to tissues
- Decreased pH → Production of 2,3-DPG via Leubering-Rapaport Pathway and the enzyme BPG mutase
- Net loss of 1 ATP per 2,3-DPG generated
- Further right shift in the Oxyhaemoglobin dissociation curve
Cardiac Output Increases
- Blood flow and resistance governed by Poisuille-Hagen Equation
- Hb significant factor contributing to blood viscosity
- Anaemia → Decreased viscosity → Increased cardiac output
- Decreased viscosity → decreased resistance to venous return → increased preload → increased cardiac output
- Hb significant factor contributing to blood viscosity
- Stimulation of aortic chemoreceptors by decreased pH and increased CO2
- Increased signalling via vagus nerve NTS vasomotor sensory area → increase sympathetic output by anterolateral upper medulla → increased HR and contractility → Increased CO
Redistribution of blood flow
- To tissues where adequate O2 delivery is critical
- esp heart and brain
Respiratory effects
- Increased CO2 and decreased pH stimulates central and peripheral chemoceptors
- Medullary respiratory centre increases minute ventilation
- Increased respiratory clearance of CO2
- Alveolar CO2 ∝ arterial CO2 → increased PAO2 and hence increased PaO2
Renal compensation
- Decreased O2 delivery to renal interstitium → Hypoxia Inducible Factor (HIF) → Erythropoietin → Stimulates erythropoiesis via EPO-R JAK/STAT signalling.
#Extra:
Techniques to maintain tissue O2 balance in a patient with anaemia:
- ↑ tissue O2 supply (or DO2) by:
- ↑ C.O. → volume load to significantly ↑ C.O. as per Frank-Starling mechanism (preferably with PRBC to replace Hb also, but OK with colloids/crystalloids)
- ↑ [Hb] → replace Hb with PRBC transfusion, and support ongoing haemopoiesis with haemotinic factors (Eg. Fe, vitamin B12, folate)
- ↑ SpO2 and PaO2 → supplemental O2 (FiO2 100%)
- ↓ tissue O2 demand by:
- Sedation, paralysis and artificial ventilation → ↓ muscle MRO2 a/w activity and respiration
- Maintain normal core body temperature → avoid hypothermia and shivering 2° to hypothermia, which ↑ MRO2
- Minimal use of inotropes to maintain C.O. → avoid ↑ cardiac MRO2
CICMWrecks 2016
Examiner Comments
2013B 24: 10 candidates passed (37.0%).
It was expected candidates would expand on the central role haemoglobin has in oxygen delivery and that in the presence of reduced haemoglobin there are various efforts aimed at maintaining oxygen delivery. Cardiac output is increased, systemic vascular resistance is reduced, modifications are seen in regional circulations and as tissue oxygenation begins to falter then the end products of anaerobic metabolism provide a further stimulus to enhance cardiac out and tissue oxygen delivery.
Better answers also included a mention of additional factors that enhance tolerance of chronic anaemia (e.g. angiogenesis).
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Respiratory physiology, measurement, oxygen delivery, shunt, deadspace, O2 measurement |
| G. CVS | ECG – principles, error, statistics Control of HR, regulation, catecholamines, dobutamine pharmacokinetics Calcium Caclcium channel antagonists |
| H. Renal | Renal physiology and pharm, GFR and determinants, values, hypovolaemia scenario, cointer current mechanism and multiplier effect, diuretics |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | |
| O. GIT | Physiology of swallowing, food passage, stages, cough reflex an dinnervation of larynx, LES, Metochlopramide |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | Hypersensitivity reactions, histamine, H receptors, adrenaline |
| T. Microbiology | |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures | Anatomy of neck, US, CVC |

Recent Comments