1. List the different mechanisms of drug actions with examples.
CICMWrecks Answer
7 methods by which drugs cause their action include: PEFsPdCpRsig (almost PEEPd CpR sig)
- PHYSICOCHEMICAL ACTIONS
- Antacids exert their effects by neutralizing gastric acid
- Chelating agents reduce the concentration of certain metallic ions within the body (eg, desferoxamine and iron, or activated charcoal)
- CaCl3, KCl, blood, Mg, replase alter or supplement endogenous primary substrates
- ACTIONS ON ENZYMES
- Enzymes are biological catalysts, and most drugs that interact with enzymes are inhibitors
- Increased concentration of the substrate and decreased concentration of the product eg. ACE inhibitors (captopril, enalapril) prevent the conversion of ACEI to ACEII and bradykinin to various fragments
- Neostigmine inhibits acetylcholinesterase reversibly
- DRUGS WHICH ACT AS FALSE SUBSTRATES FOR ENZYMES
- Fluorouracil act as “false substrate”, replaces uracil as an intermediate in purine biosynthesis but cannot be converted into thymidylate
- PRODRUGS
- Require conversion to activated form by metabolic pathway
- Levodopa → Dopamine
- Parecoxib → Valdecoxib
- ALTERATION OF CARRIER PROTEIN PROPERTIES
- Cardiac glycosides (such as digoxin) inhibits Na-K pump
- Loop diuretics inhibits Na/K/Cl co-transporter in loop of Henle
- Cocaine inhibits noradrenaline re-uptake
- VOLTAGE GATED ION CHANNELS
- Involved in the conduction of action potentials in excitable tissues
- Several groups of drugs have specific blocking actions at these ion channels
- local anaesthetics (eg, lignocaine) block Na channels,
- calcium channel blockers (eg, diltiazem) acts on vascular smooth muscle ion channels
- RECEPTORS
- Definition:
- A protein, often integral to a membrane, containing a region to which a ligand binds specifically to elicit a response.
- Binding may be allosteric (at a site distant to the receptor).
- Grouped into three classes based on mechanism of action:
- Altered ion permeability (ion channels / ionotropic)
- Membrane spanning complexes with the potential to form a channel through the membrane
- Three families:
- Pentameric
- nicotinic Ach receptor at the NMJ → allows an Na channel to form
- GABA A receptor which allows a Cl channel to form,
- 5HT3 receptor
- Ionotropic glutamate – NMDA ligand gated ion channels.
- They form Na, K and (NMDA only) Ca channels when glutamate binds
- Purinergic receptors activated by ATP, permeable to Na, K and Ca, and are associated with mechanosensation and pain.
- Pentameric
- Production of intermediate messengers
- Membrane bound systems that transduce a ligand gated signal presented on one side of the cell membrane into an intracellular signal transmitted by intermediate messengers. These messengers:
- G proteins (most common) – eg, Nad and Adr
- Tyrosine kinase – eg, insulin
- Guanylyl cyclase – eg, NO, atrial natriuretic peptide
- Membrane bound systems that transduce a ligand gated signal presented on one side of the cell membrane into an intracellular signal transmitted by intermediate messengers. These messengers:
- Regulation of gene transcription
- Steroids and thyroid hormones act through intracellular receptors to alter the expression of DNA and RNA, and indirectly alter the production of intracellular proteins.
- Altered ion permeability (ion channels / ionotropic)
- Definition:
Gladwin 2016
Examiner Comments
2013A 01: 23% pass.
A good answer to this question required candidates to think broadly about how drugs act and have a system for classifying their actions. One possible classification is action via receptors or non-receptor actions. Many candidates used categories such as physiochemical, receptor and enzymes. Common problems were failure to mention a whole class of drug actions e.g. drugs acting via voltage-gated ion channels or gene transcription regulation. Candidates also gave far too much detail in some sections e.g. a description of zero order and first order kinetics is not required. Candidates often did not give examples of the drug action they described.
2. Describe the physiology of cerebrospinal fluid (CSF). (70% of marks) Describe the anatomy relevant to the performance of a lumbar puncture. (30% of marks)
CICMWrecks Answer: Physiology of CSF
Formation / Production of CSF
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
Distribution / Circulation of CSF
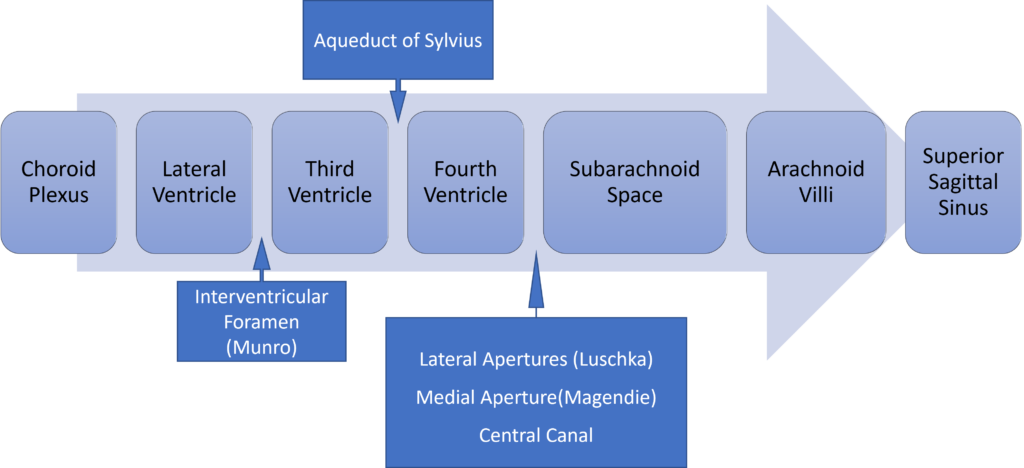
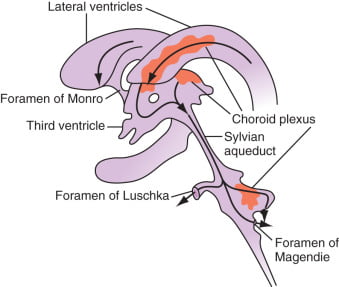
Absorption of CSF
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
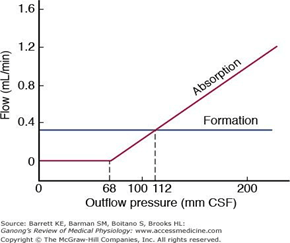
Composition of CSF
IDENTICAL to brain ECF, but differs in several manners from plasma
Compared with plasma:
- ↑ pCO2 (50 mmHg)
- ↓ pH (7.33)
- ↓ protein content
- 0.5% of plasma; 20 mg/dL
- poor acid-base buffering capacity
- ↓ content of glucose (by 60%) and cholesterol
- ↑ [Cl-] (by 7-14%) and ↑ [Mg2+] (by 40%)
- ↓ [K+] (by 40%), ↓ [Ca2+] (by 50%) and ↓ [Pi] (by 20-30%)
- ↑ creatinine (by 25%) but ↓ urea
- IDENTICAL osmolality (295), [Na+] (145), and [HCO3] (25)
Role / Functions of CSF
- Protective role (main function)
- Water bath effect
- Attributed to the low specific gravity of CSF (1.007)
- Causes brain to be buoyant
- ↓ its effective net weight from 1400 g to 50 g
- Mechanical cushion against acceleration/deceleration forces
- Buffer ↑ ICP by CSF translocation to extracranial subarachnoid space
- Abrupt ↑ ICP buffered by translocation of CSF within the vault to extracranial compartments
- Water bath effect
- Maintains constant ionic environment conducive to neuronal electrical activity CSF
- Supply role of nutrients (Eg simple sugars, amino acids) and O2 to brain
- Excretion of toxic substances, metabolic by-products, and CO2 from brain
- “Lymph-type” function → interstitial proteins in brain ECF return to circulation by
- CSF absorption across arachnoid villi
- Acid-base regulation → due to content, CSF allows for tight respiratory control
- Endocrine transport function → transports hormones to other brain regions
Gladwin / JC 2020
CICMWrecks Answer: Anatomy of LP
Anatomy:
- Position in sitting or lateral decubitus position
- Level:
- Any of the interspaces between between L2-L5
- L4/5 interspace: Tuffier’s Line: line between iliac crests
- (or) L3/4 interspace: Line joining PSISs (Posterior superior iliac spines)
- Discrepancy between identified and actual intervertebral space in 50% of cases
- Conus medullaris ends at L1 in about 94% of patients
Tissues and target for LP:
- In the subarachnoid space between the arachnoid mater and the pia mater.
- The tissues pierced are (in order):
- skin
- subcutaneous tissue
- supraspinal ligament,
- interspinal ligament,
- ligamentum flavum,
- dura mater,
- the arachnoid mater into the subarachnoid space.
- Lateral/paraspinal approach: Skin – subcutaneous tissue – erector spinae muscles – ligamentum flavum – dura – arachnoid – subarachnoid space
Epidural Space
- Posterolateral Epidural Space
- Posterolateral epidural space extends vertically down the spinal canal and contains arteries, venous plexus, and fat
- Posterolateral epidural space is larger than the anterior epidural space
- Posterolateral epidural space is larger in the sacral region than it is in the cervical region
- Anterior Epidural Space
- Anterior epidural space is a virtual space under normal circumstances (due to adherence of dura to bone of vertebral bodies from the foramen magnum down to L1)
Gladwin / JC 2020
Examiner Comments
2013A 02:
Most candidates performed well in this question. The physiology of cerebrospinal fluid (CSF) required candidates to write about CSF formation, circulation and absorption, compare the composition of CSF to plasma and describe normal volumes and pressures. The functions of CSF also need to be listed. Some candidates described the displacement of CSF when intracranial pressure rises as a function of CSF. No marks were given for this.
The best approach to the anatomy of a lumbar puncture was to describe the lumbar intervertebral space at which the lumbar puncture is done and then describe the anatomical structures that the needle would traverse from the skin to the subarachnoid space. Mentioning the indications for a lumbar puncture was not required.
3. What is the Valsalva manoeuvre? Explain the cardiovascular response and include graphs in your answer.
CICMWrecks Answer
Valsalva manoeuvre
- Forced expiration against closed glottis
- Requires airway pressures of 40mmHg for 15 seconds
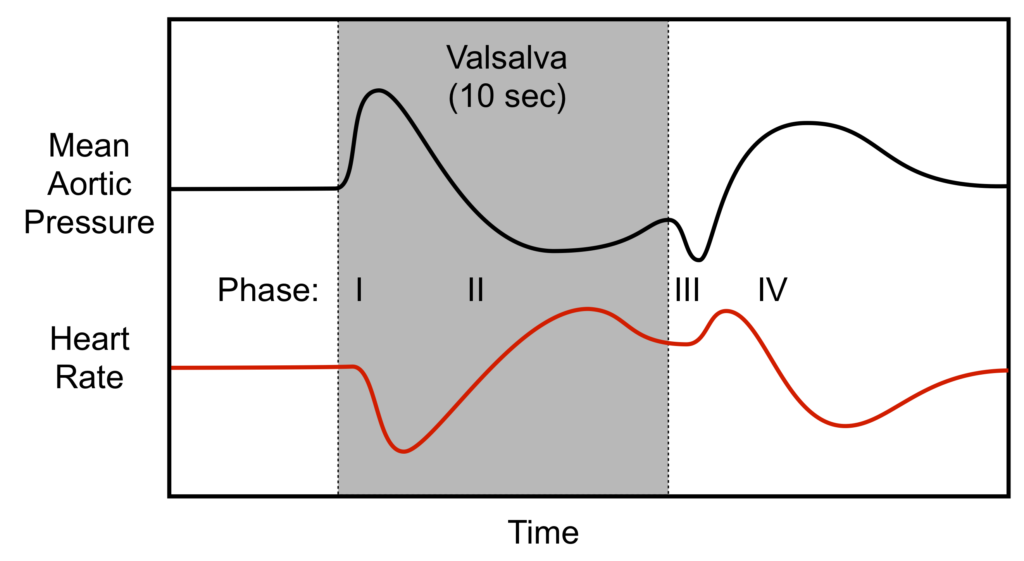
Phase I
- Onset of manoeuvre
- Transient increase in blood pressure with a baroreflex bradycardia
- Intrathoracic pressure transmitted to LV and aorta → Increased ventricular transmural pressure and decreased afterload → Increased cardiac output
- Increased CO causing hypertension and stretch of aortic and carotid baroreceptors → inhibits sympathetic output and promotes vagal output from the medullary vasomotor centre → Bradycardia
- Transient increase in blood pressure with a baroreflex bradycardia
Phase II
- Decreased venous return
- Decreased blood pressure and baroreflex tachycardia
- Continual increase in intrathoracic pressure decreases venous return to RV
- Decreased Venous return → Decreased preload
- Decreased RV CO secondary to Frank-Starling Mechanism
- Decreased RV CO → Decreased LV Preload → Decreased LV Cardiac Output
- Decreased output causes decreased blood pressure → carotid and aortic baroreceptor not stretched → release of sympathetic inhibition and decreased vagal output from medullary vasomotor centre → Tachycardia
- Reflex tachycardia and sympathetic stimulation tends to restore BP
- Decreased blood pressure and baroreflex tachycardia
Phase III
- Release of intrathoracic pressure
- Transient further decrease in blood pressure and further tachycardia
- Decreased transmural pressure on LV and aorta leading to increased afterload → Decreased cardiac output → Decreased stretch of baroreceptors → Tachycardia
- Transient further decrease in blood pressure and further tachycardia
Phase IV
- Normalization of venous return
- Venous return to RV promoted by negative intrathoracic pressure on normal respiration → Increased VR → increased RV CO → increased LV VR → Increased LV Cardiac output
- Increased LV CO → stretch of baroreceptors → Reflex bradycardia
Sakurai 2016
Examiner Comments
2013A 03:
A good answer to this question required attention to detain and an ability to describe changes in many variables at each stage e.g. intrathoracic pressure, blood volumes, baroreceptor firing and the subsequent cardiovascular response (e.g. heart rate and blood pressure). Using graph(s) is a useful way to assist in the explanation and was requires as part of the answer. Dividing the response into four stages makes answering the question much easier. Overall there was a deficiency in a deep understanding of the integrated physiology associated with the Valsalva manouvre. The most common mistakes were describing a change but not saying why it happened, not considering each element at each stage and confusing terms e.g. saying increased cardiac output when the response was increased mean arterial pressure. Very few candidates drew accurate grahs. Graphs required were those of the changes in intrathoracic pressure, the pulse pressure response and the heart rate response
4. Describe the pharmacology of tranexamic acid.
Examiner Comments
2013A 04:
Tranexamic acid is a drug used to reduce bleeding in trauma or surgery. It is also used for hereditary angioedema and menstrual bleeding. It is being increasingly used in critically ill patients. As a Level B listed drug within the Primary Syllabus candidates would be expected to know it in some depth. Often basic information such as mechanism of action, pharmacokinetics and adverse effects was lacking.
5. Describe the hormonal response to a meal.
CICMWrecks Answer
GIT
- Complex interplay between neuronal (ANS and ENS) and hormonal functions
Cephalic phase
- Initiation of GI secretions in preparation of a meal
- Vagal stimulation via acetylcholine
- Enterochromaffin cells cause release of histamine
- Acts on histamine receptors on gastric parietal cells
- Increase gastric HCl secretion
- G-cells → secreation of Gastrin
- Enterochromaffin cells cause release of histamine
Gastric phase
- Gastric wall stretch and peptide content in gastric lumen → stimulates G-cells in antrum → increases secretion of gastrin
- Peptide hormone
- Acts on CCK B receptors on enterochromaffin cells
- Increased histamine release → HCl secretion
- Stimulates secretion of pepsinogen by chief cells
- Increases antral pump function
Intestinal phase
- As gastric contents emptied into duodenum, low pH stimulates secretion of secretin by duodenal S cells
- Peptide hormone
- Inhibits gastric emptying
- Increases pancreatic secretion of a bicarbonate rich fluid
- Protein and FFA content in the duodenum stimulates secretion of cholecystokinin by enteroendocrine cells of the duodenum
- Peptide hormone
- Inhibits gastric emptying
- Increases pancreatic secretion of enzymes for digestion of fats and proteins
- Causes gall bladder contraction and release of bile for absorption of fats
As nutrients is absorbed from intestine
- Blood [glucose] increases → insulin secretion by pancreatic β cells
- Increases hepatic glucose uptake and glycogenesis, protein and lipid synthesis
- Increases skeletal muscle glucose uptake and glycogenesis, protein synthesis
- Increases adipocyte lipid storage
- Adipocytes secrete leptin in response to increased fat stores → suppress appetite
Long after meal
- Stomach Ghrelin cells secrete ghrelin in response to decreased gastric stretch
- Increased sensation of hunger
Sakurai 2016
Examiner Comments
2013A 05:
For a good answer candidates were expected to have an integrated knowledge of gastrointestinal physiology. Gut function is regulated by the enteric nervous system and by paracrine and endocrine hormones released by hormone secreting cells in the mucosa of the gut (enteroendocrine cells). These cells secrete hormones in response to neural innervation or in response to triggers associated with ingested food. Gut functions influenced include secretion, digestion, absorption and motility. The endocrine system also has an important role in the handling of nutrients following absorption and some mention of insulin was required.
6. Classify the oral hypoglycaemic drugs; include their mechanism of action, and their most significant side effects.
CICMWrecks Answer
| Drug class | Mechanism | Side effects |
|---|---|---|
| Biguanides (Metformin) | – Stimulates the movement of GLUT-4 receptors to the membrane of skeletal muscle and adipose tissue cells, increasing glucose uptake by those cells. – Also inhibits gluconeogenesis and glycogenolysis in the liver, and delays intestinal glucose absorption. | – Life threatening lactic acidosis may occur in the presence of renal impairment – Diarrhoea, n/v, abdominal discomfort common – Must be withheld prior to administration of iodinated IV contrast |
| Sulfonylureas (Gliclazide) | – Combine with KATP receptors on pancreatic ß cells, closing the ATP-dependent potassium channels to depolarize the cell, resulting in an influx of calcium which stimulates insulin secretion. | – Risk of hypoglycaemia – GIT disturbance – Stimulate appetite and may cause weight gain |
| Meglitinides (Repaglinide) | – Act by stimulating the same receptor as the sulfonylurea drugs but at a different side. | – Risk of hypoglycaemia – Repaglinide is a major substrate of CYP3A4 and caution should be used when administering with clarithromycin or antifungals, as may result in high plasma levels of repaglinide and consequent severe hypoglycaemia |
| Thiazolidinediones (Rosiglitazone) | – Act on the PPARg in fat cells to induce insulin sensitivity | – Associated with increased risk of peripheral limb fracture in post-menopausal women, and also some cases of diabetic maculopathy. – prone to cause severe fluid retention and precipitate heart failure, contraindicated in CCF – Contraindicated in people with known IHD. |
| Alpha- glucosidase inhibitors (Acarbose) | – Act by inhibit the enzyme that breaks down dietary complex carbs to sugar, reducing the quantity of glucose available for absorption | – Abdominal discomfort & distention, Flatulence – Elevates serum transaminases – Shouldn’t produce hypoglycaemia itself as does not promote insulin release |
| DPP-IV inhibitors (Sitagliptin) | – Inhibit the activity of the enzyme DPP-IV, which normally breaks down the gut hormones GLP-1 (glucagon-like peptide 1) and the protein GIP (gastric inhibitor peptide). GLP-1 and GIP stimulate glucose-mediated insulin release from the pancreas following an oral load of glucose. | – Monitor in renal insufficiency – May cause hypoglycaemia |
| SGLT2 inhibitors (Dapagliflozin) | – Inhibit Sodium-glucose transport protein 2 (SGLT2, which is responsible for reabsorbing glucose in kidney). – Decreased kidney reabsorption of glucose, glucosuria effect (Insulin and pancreatic b cell independent) | – May cause Hypoglycemia – May cause Severe Euglycemic Ketoacidosis, esp in post-op period – UTIs, Candidal Vulvovaginits |
JC 2019
Click to Open CICMWrecks Pharmacopeia Table: Hypoglycaemic Agents
Examiner Comments
2013A 06:
A good answer would have best been served by a tabular structure and some understanding of the information required. One system of classification of oral hypoglycaemic drugs is by their mechanism of action, or drug group e.g. Biguanides, Sulfonylureas, Thiazolidinediones, Alpha-glucosidase inhibitors, Meglitinides and Dipeptidyl peptidase (DPP) -IV inhibitors.
Click to Open CICMWrecks Pharmacopeia Table: Hypoglycaemic Agents
7. Describe how the respiratory system of a newborn differs from that of an adult.
CICMWrecks Answer
Anatomical and Mechanical Differences
- Relatively larger head
- Prominent occiput
- Short neck
- Neonates do not have dentition
- Relatively larger tongue
- Higher larynx (C3-4 in infant, C4-5 in adult)
- Larynx lies more cephalad and anteriorly
- Epiglottis is narrower, omega shaped, more floppy and relatively larger
- The narrowest part of the airway is the subglottic area at the level of the cricoid cartilage
- Infants are obligatory nasal breathers until 3-5 months of age, the narrow nasal passages can easily be obstructed by secretions
- Shorter compressible trachea
- Increased risk of laryngospasm with inappropriate instrumentation.
- The smaller the patient the harder it is to position a LMA appropriately and maintain the correct position
- More compliant tissues and reduced muscle tone
- Main carina also lies more cephalad
- Small peripheral Airways (50% are < 2 mm diameter)
- ↓↓↓ bronchial muscle → so bronchospasms uncommon and bronchodilator drugs have minimal response
- Alveoli immature, and number <10% of adult total
- Reduced mucociliary clearance
- ↓ % type I muscle fibres (highly oxidative/slow contraction) in diaphragm (25% in neonate vs 55% in adults) and IC muscles (45% vs 65%) → ↑ risk of muscle fatigue
Lung Volumes
- FRC (30 mL/kg) → same as adult (as FRC remains 40% of TLC) BUT ↓ stable and ↑ risk of atelectasis due to following reasons:
- Significant ↓ in outward recoil of CW (as rib is cartilaginous and contains very little respiratory muscles)
- Mild ↓ inward recoil
- TLC (75-80 mL/kg) → same as adult
- VC (45 mL/kg) → lower cf. adult (60 mL/kg)
- TV same as adult (7 mL/kg)
- ↑ CC due to low elastic recoil of lungs → in fact, CC > FRC such that there is small AW closure during tidal ventilation → results in gas trapping and hypoxaemia
Lung Physiology
| Variable | Neonate vs Adult |
| Oxygen consumption | 6mL/min vs 3mL/min in adult |
| O2 flux to tissues | ↑ due to: ↑ O2 carrying capacity(↑Hb, HbF, ↑MV → ↑pO2) ↑ Cardiac output |
| Minute Ventilation | 2x adult (to cope with ↑MRO2) Generated by ↑↑RR → ↑ work of breathing |
| Control of ventilation | Respiratory control less developed CO2 ventilatory response curve shifted to left O2/CO2 ventilatory response easily depressed by hypothermia Peripheral chemoreceptors immature Hering-Breuer reflex (transient apnoea lasting 5 secs evoked by gradual lung inflation) Head’s paradoxical inflation reflex (↑ inspiratory effort evoked by partial inflation of lungs) Periodic respirational (pauses lasting 5-10 sec (up to 6 x per hr during sleep) |
| Chest Wall Compliance | Neonates have ↑ chest wall compliance relative to adult → Prone to collapse on inspiration → ↓ Tv for same respiratory effort → ↑ WOB |
| Tissue Compliance | Initially neonates ↓ relative to adults until a few days of life when surfactant production is fully initiated, then → ↑ WOB in the first few days |
| V/Q matching | Significant V/Q mismatch (V/Q = 0.4) due to shunting from gas trapping/small AW closure |
| Physiological dead space | ↑ physiological dead space further necessitating ↑ MV |
| Work of Breathing | ↑ WOB overall |
Gladwin / JC 2019
Examiner Comments
2013A 07:
This question required anatomical detail relating to the upper airway and bronchial tree, which was generally answered well. The functional implications of a highly compliant chest wall in defending FRC and the relationship of FRC to closing volume was less clearly explained. Better answers mentioned the high physiological dead space, oxygen consumption and work of breathing. Additional points were awarded for discussing the immaturity of the respiratory control centre and propensity for apnoea.
Common omissions included not providing comparative adult data or a written description of how neonates differed from adults (or the significance of this). Candidates confused chest wall compliance (increased in newborns) with lung compliance (reduced in newborns but rapidly approaches normal adult values as “specific compliance”). Increased oxygen consumption necessitates increased minute ventilation (with tidal volumes equivalent to adults on a weight basis) via respiratory rate.
Functional Residual Capacity FRC (equivalent to adults) and Closing Capacity (increased relative to adults) were often confused.
Better answers provided responses often in tabular format. Discussion of cardiovascular responses and response to drugs were not requested and gained no marks.
8. Compare and contrast the mechanism of action, spectrum of activity and adverse effects of benzyl penicillin, metronidazole and clindamycin.
Examiner Comments
2013A 08:
This question asked to compare and contrast, inviting candidates to tabulate their answers.
Details concerning other elements of pharmacology (apart from mechanism, spectrum and adverse effects) were not required and did not attract marks.
There was a lack of accurate detail in answers regarding clindamycin. Spectrum of activity mentioned by candidates was often quite narrow. The gram negative and anaerobic spectrum of activity afforded by benzyl penicillin was also not mentioned by many
candidates. Adverse reactions were an opportunity to score marks – all drugs can cause
nausea, vomiting, rash and hypersensitivity phenomena – especially the antibiotics. However it is important that specific side effects for each agent are also mentioned by candidates.
Information that related to the pharmaceutics, pharmacokinetic or pharmacodynamic
properties of these drugs was not requested and did not score marks.
9. Outline the functions of the kidney.
CICMWrecks Answer
- Excretion of metabolic waste and foreign substances
- Filtration of metabolic waste products
- urea from protein
- uric acid from nucleic acids
- creatinine from muscle creatine
- Filtration of drugs
- Filtration of metabolic waste products
- Regulation of water and electrolyte balance
- Homeostatic mechanism
- 180L of fluid and other substances per day, with 99% reabsorbed along the tubule
- Concentration of urine via the countercurrent mechanism
- max 1400mOsm to reduce renal water loss
- Reabsorb K, Ca, Mg and Cl either passively or actively
- Regulation of plasma osmolality
- Plasma osmolality is altered whenever the inputs and outputs of water and solutes changes disproportionately.
- Input from the hypothalamic osmoreceptors contributes.
- Regulation of acid base balance
- Excretion of H+ ions, fixed acids, phosphate and ammonium
- Resorption of HCO3- to maintain plasma neutrality
- Regulation of blood pressure
- Production of angiotension 2 via the RAAS in response to a reduction in GFR system
- peripheral vasoconstriction
- reabsorption of sodium
- secretion of aldosterone
- → rise in MAP
- Changes in blood volume regulated by the kidney (as mentioned above) will also influence BP
- Production of angiotension 2 via the RAAS in response to a reduction in GFR system
- Endocrine function
- Vitamin D
- EPO to stimulate RBC production (in response to a reduction in pO2 the secretory cells which lie in between the cortex and medulla)
- Aldosterone and angiotensin II as regulated by the RAAS will contribute to BP management
- Prostaglandins esp PGE2 and prostacyclin – potent renal vasodilators
- Bradykinin – potent renal vasodilator
- Gluconeogenesis
- The renal cortex contains glucose-6-phosphatase and thus the ability to release glucose in times of need
Gladwin 2016
Examiner Comments
Outline type questions require a comprehensive list and a brief explanation of each major function of the kidney. This should have included water balance, electrolyte balance, endocrine function, filtration, metabolism, acid-base balance, excretion and blood pressure control.
Adequate breadth was lacking in many answers. Marks were awarded for discussion of water reabsorption at specific points along the length of the nephron and the control mechanism through Aquaporins and ADH. It was expected answers would include calcium, magnesium, glucose and amino acid handling by the kidney (as well as sodium and potassium). Better answers discussed the elimination of fixed as well as volatile acids and outlined the role of ammonia. Discussion of the endocrine functions would include EPO, ,25 dihydroxy-cholecalciferol, prostaglandin E, renin, angiotensin II and kallekrein.
Superficial responses such as, “the kidney is important in regulating volume” (or “waste” or “water” or “sodium”) were not sufficient.
10. Outline the pharmacokinetics, and mechanism of action of carvedilol and spironolactone.
Examiner Comments
2013A 10:
Carvedilol and spironolactone are common drugs used in the management of cardiac failure. They have different mechanisms of action and pharmacokinetics. Both are drugs listed in the Syllabus as Level B and thus candidates are expected to have a general understanding of their pharmacology. Many candidates gave class specific information about beta blockers rather than demonstrating an understanding of carvedilol`s particular properties. Most candidates were able to score marks by commenting upon the results of aldosterone antagonism suggesting an understanding of the physiology of this hormone but appeared to know little more about the pharmacology of spironolactone. Overall there was insufficient information provided by most candidates for both drugs.
11. Describe the oxygen cascade, from the atmosphere to the mitochondrion, in a patient breathing room air.
CICMWrecks Answer
- Fraction of O2 in atmosphere = 0.21
- Oxygen cascade:
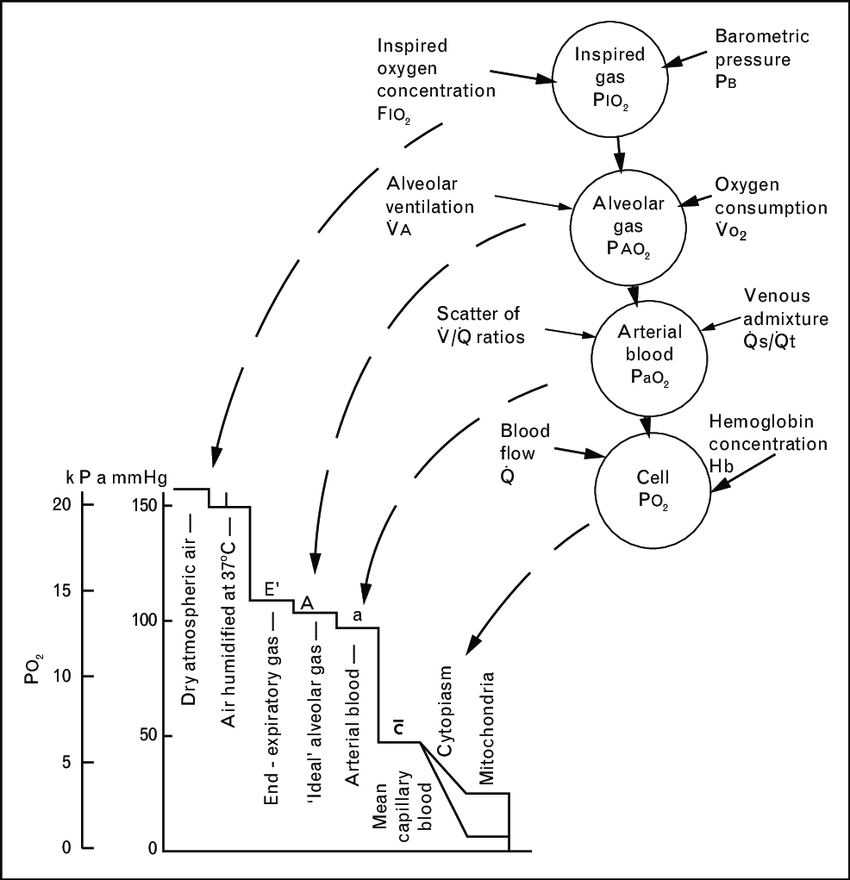
1. Oxygen in the atmosphere
- Oxygen in the atmosphere at sea-level
- Atmospheric pressure = 760mmHg
- pO2 = 760mmHg x 0.21 = 160mmHg
2. Oxygen in the bronchi
- Inspired air at sea level is humidified and saturated by H2O vapour at the isothermic saturation boundary 3cm distal to the carina
- pO2 = 0.21 (760mmHg – 47mmHg) = 150mmHg
3. Oxygen in the alveoli
- In the alveoli, inspired air is diluted with CO2 diffusing into the alveoli from the alveolar capillaries.
- pAO2 = 0.21(760mmHg – 47mmHg) – paCO2/R + f
- where, R = respiratory quotient, approx. 0.8
- f = correctional factor
- paCO2 = 40mmHg
- pAO2 = 100mmHg
- pAO2 = 0.21(760mmHg – 47mmHg) – paCO2/R + f
4. Oxygen in the arterial blood
- Oxygen diffuses from the alveoli to pulmonary capillaries (solubility coefficient 0.024) however paO2 is lower than pAO2 due to venous admixture
- Factors contributing to venous admixture
- V/Q mismatch
- Shunt
- The degree of venous admixture can be calculated by
- Oxygen in carried in blood as a predominant Hb bound portion and a minor diffused portion, governed by the oxyhaemoglobin dissociation curve
- In healthy adult, A-a gradient is no more than 15mmHg
5. Oxygen in the cytosol and mitochondria
- Oxygen diffuses from capillary blood across the plasma membrane (according to Fick’s law of diffusion) into the cell cytosol and then across the mitochondrial membranes into the mitochondria
- There is paucity of evidence to determine mitochondrial pO2 but estimated to be between 5mmHg and up to 40 mmHg in recent studies
Sakurai / Gladwin 2016
Examiner Comments
2013A 11:
This topic is a core aspect of respiratory physiology. The vast majority of the candidates could draw the oxygen cascade, but were let down by not having sufficient breadth and/or depth of information (e.g. alveolar gas equation was either omitted, inaccurate or poorly described in relation to the oxygen cascade) to describe the physiological principals at each step of the cascade.
12. Describe the physiological role, distribution and regulation of potassium (K+)
CICMWrecks Answer
Potassium
- Predominany intracellular cation
- Total Stores: Approx 3200mmol (50mmol/kg)
- Key Functions:
- Main determinant of ICF osmolality and tonicity
- Responsible for RMP of excitable cells via Goldmann-Hodgkin-Katz due to ↑↑gK relative to other species
- Role in action potential → repolarisation phase
- Secretion of insulin and multiple other KATP dependant processes
- Regulation of IC processes (protein/glycogen synthesis)
- Involved in Na+/K+ ATPase in cell membranes
Potassium balance / Determinants of Serum Potassium
- Intake
- Oral – very variable
- Approx. 50-200mmol/day
- Distribution
- Exchangable Pool
- ICF (90%): [K+] 150 mmol/L (1° ICF cation)
- ECF (2%): [K+] 3.5-5 mmol/L
- Non-exchangable Pool
- Bone (8%)
- Exchangable Pool
- Transcellular balance
| Factor | Mechanism | Effect on Serum K+ |
|---|---|---|
| Insulin | Causes intracellular shift of K+ | ↓ |
| β2 adrenergic agonism | Causes intracellular shift of K+ | ↓ |
| Aldosterone | Upregulate Na/K ATPase → Intracellular shift of K+ | ↓ |
| pH | Increased H+ (decreased pH) causes extracellular shift of K+ | ↑ |
| Plasma osmolarity | Hyperosmolar plasma initiall causes osmosis of water out of cells increasing intracellular osmolarity and [K+] → causes extracellular shift of K+ | ↑ |
| Skeletal muscle activity | Causes K+ leakage into serum | ↑ |
| Cell death | Causes K+ leakage into serum | ↑ |
- Elimination
- Faecal – 8mmol/day
- Renal – 92mmol/day (See next heading for details)
- Urinary K+ excretion = [K+ filtered by glomerulus] + [K+ secreted by CCD/LDCT] – [K+ reabsorbed by renal tubules]
- Glomerular filtration: Freely filtered = 756mmol/day (180L x 4.2mmol/L)
- PCT: 65% reabsorption
- LoH: 25~30% reabsorption
- DCT/Collecting Ducts – variable
- Determined by Aldosterone, Plasma [K+], Tubular Flow rate
- Secreted by principal cells
- Reabsorbed by intercalating cells
Renal K+ regulation:
- Renal K+ regulation occurs MAINLY via control of K+ secretion at the distal nephron (CCD and LDCT) via the following factors:
- Circulating factors:
- Plasma [K+ ]
- ↑ [K+ ] causes ↑ K+ secretion by → (i) Directly stimulating Na+ /K+ ATPase in principal cells, and (ii) Directly stimulating Aldosterone release from adrenal cortex
- Aldosterone
- ↑ aldosterone causes ↑ K+ secretion by → ↑ production of Na+ /K+ ATPase, K+ channels and ENaC Na+ channels in the principal cells
- Plasma pH
- Alkalosis via ↓ plasma [H+ ] causes ↑ K+ secretion by → stimulating Na+ /K+ ATPase in principal cells
- Plasma [K+ ]
- Luminal factors:
- Flow of tubular fluid in DCT and CCD
- ↑ tubular fluid flow causes ↑ K+ secretion by → maintaining ↓ luminal [K+ ] (Ie. continuously washing it away) → permits passive diffusion of K+ ↓ its [ ] gradient into tubular lumen
- ↑ Na+ delivery rate to DCT and CCD → ↑ K+ secretion
- -ve lumen potential difference → ↑ K+ secretion
- Flow of tubular fluid in DCT and CCD
- Circulating factors:
JC / Gladwin / Sakurai 2020
Examiner Comments
2012A 04: 0 (0%) of candidates passed.
Candidates were required to synthesize knowledge across a number of areas and have a good overview of the topic. This included the following – Insulin (acts to up-regulate Na/K ATPase activity promoting intracellular shift of potassium in adipose and muscle tissue); catecholamines (beta2 stimulation up-regulates Na/K ATPase activity promoting intracellular shift of potassium); aldosterone; pH (acidosis promotes H+/K+ exchange (via H+/K+ antiport), and reduces the activity of the Na K ATPase pump); osmolality (cellular dehydration increases intracellular K+ concentration promoting diffusion of potassium out of the cells); exercise; plasma potassium; temperature.
13. Outline the effects of critical illness on drug pharmacokinetics. Give examples.
CICMWrecks Answer
Absorption
- IV
- Unchanged
- Oral
- If GI inflammation → increased GI absorption (aminoglycosides)
- Concomittant drug administration may inhibit intestinal transporters (amiodarone inhibits p-glycoprotein) or hepatic enzymes (valproate inhibits P450 enzymes) à Variable effect on bioavailability
- If hepatic failure (i.e. ischaemic hepatopathy) → Decreased hepatic first pass effect → Increased oral bioavailability (aspirin)
- Decreased splanchnic bloodflow (due to vasopressor agents, shock) → Decreased drug absorption → Decreased bioavailability
- IM/Subcut
- Decreased peripheral blood flow due to shock, vasopressors → Delayed absorption (IM suxamethonium will delay onset)
Distribution
- Protein binding → albumin decreases as an acute phase reactant → Increased free drug fraction of protein bound drugs (propofol)
- pKa
- Acids are ionized when pH >pKa, bases ionized when pH < pKa → acid base disturbances will alter drug ionization → permeability through cell membranes (Na channel blockers are less effective in acid environments)
- Volume of distribution
- Renal failure, hepatic failure, heart failure + overzealous fluid resuscitation → increased total body water → Increased volume of distribution of drugs distributed to extracellular compartment
Metabolism
- Hepatic phase I (zone III) susceptible to ischaemia
- Decreased phase I metabolism in shock (fentanyl metabolized by CYP450 3A4)
- More global reduction of hepatic function in global hepatic failure
- Butylcholinesterase produced by liver → decreased levels in hepatic failure → decreased metabolism (suxamethonium → more drug delivery to NMJ)
Excretion
- Renal
- Shock → decreased renal perfusion pressure (although renal blood flow may actually increase in distributive shock) → decreased glomerular filtration of drugs → Decreased clearance
- Decreased plasma protein will promote glomerular filtration and filtration of free drug
- Acute tubular necrosis → decreased tubular secretion of drugs → Decreased clearance
- Biliary
- Hepatic failure → decreased biliary secretion
Mooney 2016
Examiner Comments
2013A 13:
Most candidates answered the question under the subheadings absorption, distribution, metabolism and elimination. However, they didn’t give any details of the direction or mechanism of change, often used vague statements without specifically addressing the question and failed to give examples. The impact of the shock state on different kinetic parameters including absorption from skin, tissue, muscles, enteral absorption and inhalational was often overlooked. Similarly, the consequences of changes in volume of distribution, protein binding (e.g. albumin and globulin, ionisation) was poorly understood as was alteration in liver and kidney function. Although this topic is very broad candidates were asked to only outline the details of this topic.
14. Describe the relevant anatomy for insertion of an intercostal catheter. (60% of marks) How does breathing 100% oxygen help in the resolution of a pneumothorax? (40% of marks)
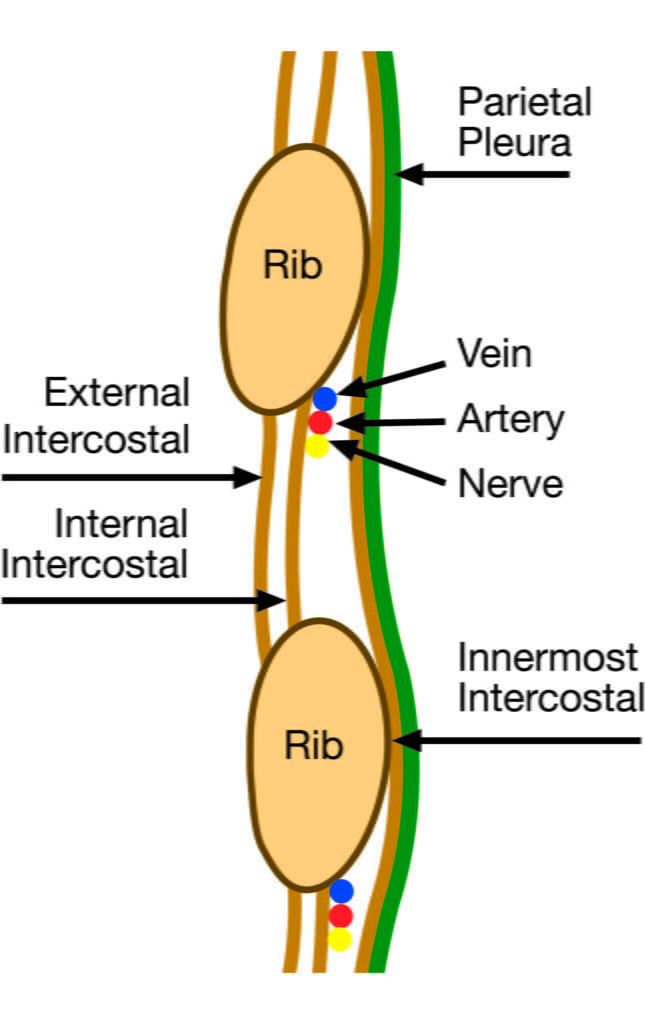
CICMWrecks Answer: Anatomy relevant to ICC Insertion
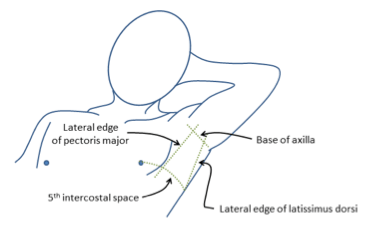
- Triangle of safety
- Lateral border of pectoralis major
- Anterior border of latissimus dorsi
- Superior to the 5th intercostal space – be aware the diaphragm (and liver/spleen) may be unexpectedly superior
- Layers (Superficial → Deep)
- Skin
- Subcutaneous connective tissue
- Fat
- Ribs and intercostal muscles
- Neurovascular bundle (intercostal artery, vein and nerves) lie in intercostal groove on infero-interior surface of the ribs
- External intercostal muscle
- Internal intercostal muscle
- Innermost intercostal muscle
- Parietal pleura
- Potential pleural space (pneumothorax)
- Visceral pleura
- Lung
Sakurai / Mooney 2016
CICMWrecks Answer: 100% O2 in management of Pneumothorax
- Relative composition of gas in pneumothorax = atmospheric gas.
- 21% O2
- 78% N2
- 1% Ar
- Partial pressures depend on pressure within pneumothorax
- Composition of alveolar gas
| Air | 100% O2 | |
|---|---|---|
| PAO2 | = 0.21(760-47) – 40/0.8 = 0.21(713) – 50 = 150 – 50 Approx. 100mmHg | = 1(760-47) – 40/0.8 = 713 – 50 Approx. 660mmHg |
| PACO2 | 40mmHg | 40mmHg |
| PAN2 | 570mmHg | 0mmHg |
- Composition of arterial gas
| Air | 100% O2 | |
|---|---|---|
| PaO2 | Approx. 100mmHg | Approx. 600mmHg |
| PaCO2 | 40mmHg | 40mmHg |
| PaN2 | 570mmHg | 0mmHg |
- Therefore, when breathing 100% O2
- Gradient for N2 reabsorption increases by approx 570mmHg
- Diffusion is proportional to partial pressure difference (Ficks Law) therefore increased reabsorption of N2
- Solubility coefficients = [Dissolved gas] / PP(Gas)
- CO2 – 0.57
- O2 – 0.024
- N2 – 0.012
- N2 requires 2x the partial pressure gradient of O2 to achieve the same amount dissolved in water
Sakurai 2016
Examiner Comments
2013A 14:
Many candidates described the technique for insertion of an intercostal catheter which was not requested. Few identified the nerve supply to the area. Most identified the “safe” triangle but failed to correctly identify its borders or draw a diagram to show anatomic landmarks. Describing the anatomy from skin to lungs, including the neurovascular bundle, was needed to pass this part of the question.
Although most knew that 100% oxygen reduce the nitrogen content in a pneumothorax and this accelerates its adsorption, but did not go on to provide any quantification of partial pressure changes in blood and in the pneumothorax bubble.
15. Describe the pharmacology of naloxone.
Examiner Comments
2013A 15:
Naloxone is a commonly used intravenous opioid antagonist, which acts as a competitive antagonist with high affinity for the mu, kappa, delta and sigma opioid receptors. It is used to ameliorate or reverse opioid effects at these sites. It has a shorter effect site and plasma half-life than most opiates so levels will fall before the opioid agonist it is being used to treat, thus a repeat dose maybe required to maintain opioid reversal. Overall candidates lacked sufficient depth of information to achieve high marks for this question.
16. Classify and describe immune hypersensitivity reactions. Give examples for each reaction.
CICMWrecks Answer
HYPERSENSITIVITY REACTIONS
| Type | Pathophysiology | Disease types |
|---|---|---|
| Type I Immediate Hypersensitivity IgE mediated | Sensitisation Plasma B cell production of IgE Mast cell proliferation → IgE binds to mast cells On re-exposure Allergenic antigen binds to mast cell (expressing IgE) → Mast cell degranulates Mast cell degranulation leads to Primary mediators: – Serotonin – Histamine Secondary mediators – Leukotrienes (SRSA) – Prostaglandins – ECFA, NCF | Anaphylaxis Atopy |
| Type II Cell Cytotoxicity IgG, IgM Mediated | Antibody attaches to antigen on target cell Complement release C5-9 “membrane attack complex” → Causes cell lysis | Blood Transfusions Goodpasteur’s syndrome Autoimmune cytopaenias |
| Type III Immune Complex IgG, IgM, IgA mediated | Circulating antibody-antigen complexes are deposited in basement membranes e.g. peritoneum, blood vessels, joints, glumeruli Complement cascade activation Attract granulocytes → inflammation | SLE Serum Sickness Necrotising vasculitis |
| Type IV Delayed hypersensitivity T-cell mediated | T cell mediated Cell mediated, as opposed to previous humoral Peak reaction 2-3 days CD4 helper T cells cause recruitment of Neutrophils Macrophages CD8 cytotoxic T cells | TB, Sarcoid Granulomatosis with polyangiitis (Wegener’s) Granulomatous vasculitis |
SRSA: “slow reacting substance” A – Mixture of Leukotrienes (LTC4, LTD4, LTE4)
ECFA: Eosinophil chemotactic factor of anaphylaxis
NCF: Neutrophil chemotactic factor of anaphylaxis
Key Points:
- “Anaphylaxis” may be:
- True anaphylaxis: a symptoms complex following exposure of a sensitised individual to an antigen, produced by a type I hypersensitivity reaction, associated with IgE mediated mast cell degranulation
- Anaphylactoid reactions: Indistinguishable from true anaphylaxis, however the immune nature of the reaction is either unknown, or not due to a type I hypersensitivity reaction, immediate generalised reaction is a better term.
Gladwin / Mooney 2016
Examiner Comments
2013A 16:
Overall, this question was well answered. Candidates were expected to classify the hypersensitivity reactions into their typical groups (Type I – IV) and to describe the mechanisms within each group. Similar such questions have been asked in previous exams. Again, those candidates who could provide some structure to their answer tended to score well.
17. Compare and contrast the mechanism of action, pharmacokinetics, and side effects of adHine, steroids, and antihistamines when used for the treatment of anaphylaxis.
CICMWrecks Answer
| Adrenaline | Steroids | Antihistamines | |
|---|---|---|---|
| An endogenous catecholamine released from the mammalian post-ganglionic sympathetic nerve terminals. | Hydrocortisone and prednisone most common in treating anaphylaxis. Pharmacokinetics of hydrocortisone will be discussed here | Range of sedating and non-sedating agents. Fexofenadine will be discussed here. | |
| PD- MoA | Stabilises mast cells to prevent degranulation Low doses: Beta agonism at (inotropy and chronotropy) and causing smooth muscle relaxation (including bronchorelaxation). Higher doses: alpha effects and causes vasoconstriction. | Work by binding to the cell nucleus and switching off the transcription of various genes which encode for inflammatory mediators such as cytokines, chemokines, adhesion molecules and arachidonic acid. They also activate anti-inflammatory genes such as MAP (mitogen activated protein) and increase the expression of beta2 receptors. | Binds to H1 receptors to block the effects of the histamine released from mast cells- namely, inhibition of vasodilatation, increased vascular permeability, and contraction of non-vascular smooth muscle |
| SE | HTN, tachycardia and arrhythmias, Metabolic (glycogenolysis, lipolysis, gluconeogenesis) and can increase BMR. Can worsen PHTN(causes pulm vasoconstriction) and glaucoma. | Single dose– minimal side effects. Repeated dosing– multiple effects on virtually every organ system. CVS– HTN. CNS– psychosis, mood disorders. GIT– gastriculcers, pancreatitis. Haem– leukocytosis. ID– increased risk of infection. MSK– osteoporosis, weakening of proximal muscles, abdominal striae, easy bruising, thin skin | May cause headache or drowsiness |
| PK A | Route- IV, IM, S/C, NEB, ETT Dose- 1mg ALS, 0.1mg anaphylaxis, titrated dose for haemodynamic instability (Start at 0.01mcg/kg/min, for alpha effects) Onset to action- seconds, duration 2 mins | Route – PO, IV, inh, neb Dose– usually 200 mg IV for anaphylaxis. May require repeat dosing. | Route – PO, BA 33% Dose – 60-240mg daily in divided doses |
| D | Does not cross the BBB 50% protein bound | 90% protein bound at low concentrations, only 60-70% bound at higher. Vd 0.3-0.5L/kg | Protein binding 70% |
| M | Taken up by the sympathetic nerve terminals and metabolised via COMT and MAO circulating Adr metabolised via COMT | Hepatic to tetrahydrocortisone | Negligible metabolism in humans. |
| E | Via urine as inactive metabolites (half-life 2 min) | Elimination half-life 1.2-1.8hrs | Terminal half-life 14-15hours |
JC 2019
Examiner Comments
2013A 17:
This question asked candidates to compare and contrast the mechanism of action, pharmacokinetics, and side effects of these drugs in the context of the treatment of anaphylaxis. Information beyond that did not score marks. A structured approach (e.g. a table) assisted in presenting the information.
18. Describe liver blood flow and its regulation.
CICMWrecks Answer
Hepatic blood flow
- 1.5l/min
- Anatomy
- Afferent via Hepatic Artery (branch of ceoliac trunk) contributing 30% and Portal Vein (confluence of inferior mesenteric, superior mesenteric and splenic veins) contributing 70% of hepatic blood flow
- Hepatic artery supplies 50% of O2 delivery
- Portal venous blood has PO2 of 80% due to mesenteric AV anastamosis
- Blood flows through low pressure hepatic sinusoidal system to ventral vein
- Efferent via Hepatic Vein → IVF
- Afferent via Hepatic Artery (branch of ceoliac trunk) contributing 30% and Portal Vein (confluence of inferior mesenteric, superior mesenteric and splenic veins) contributing 70% of hepatic blood flow
Regulation
Intrinsic
- Hepatic arterial and Portal venous pressure – Hepatic venous pressure / Hepatic vascular resistance (low)
- Autoregulation to systolic blood pressure 80mmHg
- Metabolic autoregulation
- Vasodilation in response to H+, CO2, lactate
- Semi-reciprocal relationship
- Hepatic arterial blood flow increases when protal venous flow decreases
Extrinsic
- Respiration
- Diaphragmic contraction → Decreased intrathoracic (and IVC) pressure and increased abdominal pressure → Favours hepatic blood flow
- Post-prandial
- Increased splanchnic blood flow post-prandially → Increased hepatic blood flow
- Neural
- Sympathetic
- α adrenoceptors present in portal vein AND hepatic artery
- β adrenoceptors only present in hepatic artery
- Therefore sympathetic input tends to venoconstrict the portal vein and dilate the hepatic artery
- Sympathetic
- Hormonal
- Adrenaline
- α constricts protal veins and decreases HBF
- β dilates hepatic arteries and increases HBF
- Glucagon increases hepatic blood flow
- Vasopressin decreases hepatic blood flow
- Angiotensin II decreases hepatic blood flow
- Vasoactive Intestinal Peptide (VIP) and secretin increase hepatic artery flow
- Adrenaline
Changes to drug metabolism when liver blood flow decreases:
- The two major determinants of hepatic clearance are the efficiency of drug removal from the blood and the efficiency of blood delivery to the liver.
- Efficiency of drug removal by the liver = hepatic extraction ratio
- fraction of the drug entering the liver in the blood which is irreversibly removed (extracted) during one pass of the blood through the liver.
- determined largely by:
- the free (unbound) fraction of the drug and
- the intrinsic clearance rate (the intrinsic ability of the liver to remove (metabolise) the drug in absence of restrictions imposed on drug delivery to the liver cell by blood flow or protein binding.)
- The effect of liver blood flow on hepatic clearance depends on the hepatic extraction ratio of the drug.
With decreasing hepatic blood flow, hepatic extraction ratio will increase for all drugs.
- For drugs with low intrinsic clearance:
- Hepatic extraction ratio will increase with decreasing hepatic blood flow
- Hepatic clearance will not decrease significantly with decreasing blood flow
- For drugs with high intrinsic clearance:
- Hepatic extraction ratio will increase slowly with decreasing blood flow
- Hepatic clearance will decrease in a fairly linear fashion, in proportion to hepatic blood flow
Also, Drugs which enjoy enterohepatic recirculation may have decreased half-lives due to failure of recirculation
Sakurai / JC 2019
Examiner Comments
2013A 18:
The liver has a unique dual supply – a portal venous system, and a hepatic arterial system, that floods the hepatic sinusoids and drain into the hepatic veins and then the inferior venacava. Knowledge of liver blood flow is essential for the understanding of certain pathophysiological mechanisms associated with disease and/or injury states of the liver. These two systems have unique characteristics, which most candidates seemed to have some knowledge of. A common area of weakness was the understanding/omission of the factors/mechanisms involved in the regulation of liver blood flow.
19. Describe and compare the action potentials from cardiac ventricular muscle and the sinoatrial node.
CICMWrecks Answer
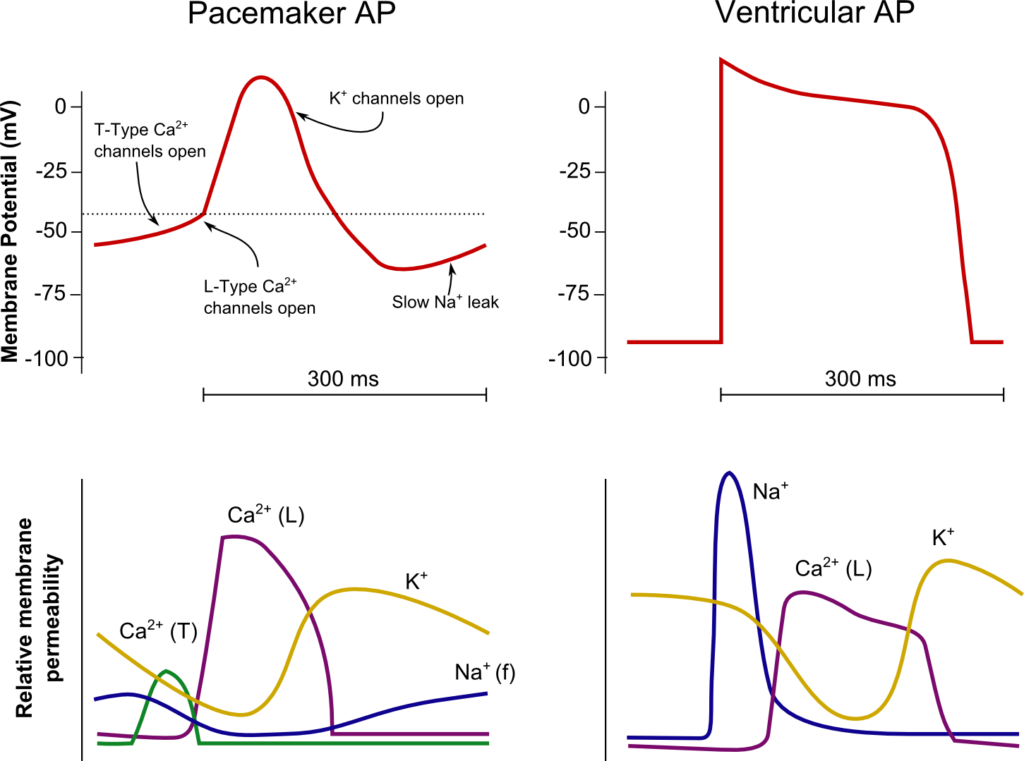
SA Node
Ventricle
Resting membrane potential
- No set RMP, however approx. -60mV
- βadrenergic stimulation causes a less negative RMP and increased slope of the initial upstroke
- muscarinic stimulation causes a more negative RMP and a decreased slope of the initial upstroke
Resting membrane potential
- Approx -90mV
Threshold potential
- Approx 40mV
Threshold potential
- Approx. -50mV
Phase 0
- Funny Na+ current allow leakage of Na+ into the the cell slowly increasing membrane potential to the threshold potential
T and L-type Ca2+ channels (voltage gated) open when threshold potential reached
- Ca2+ influx
- Depolarization
- Shallow upstroke compared to ventricular myocyte
Phase 0
- Rapid depolarization
- Opening of fast Na+ channels
- Influx of Na+
- Overshoot to +20mV
Phase 1
- Early repolarization
- Closure of fast Na+ channels and opening of K+ channels (transient outward, inward rectifier)
- Efflux of K+
Phase 2
- Plateau
- Opening of intially T-type Ca2+ channels and subsequently L-type Ca2+ channels
- Ca2+ influx balances K+ efflux
- Na+ channels in closed state à absolute refractory period
- No action potential can be generated in this state
Phase 3
K+ channels open and Ca2+ channels slowly close
- K+ efflux
- Repolarization
- No plateau phase
Phase 3
- Repolarization
- Closure of Ca2+ channels while K+ channels remain open (delayed rectifier)
- K+ efflux
- Returns potential towards RMP
- Na+ channels transition to inactive state à relative refractory period
- Another action potential can be generated with greater adequate stimulus
Phase 4
- Na+/K+ ATPase and Na+/Ca2+ ATPase maintain ionic gradients
Phase 4
- RMP
- Na+/K+ ATPase and Na+/Ca2+ ATPase maintain ionic gradients
Sakurai 2016
Examiner Comments
2013A 19:
A fundamental aspect of cardiac physiology, that overall was well answered. The majority of candidates used figures to good effect. Candidates are reminded that all figures must be correctly labelled (e.g. X and Y axis, phases of action potential, etc.). Common omissions were those that reflected an adequate depth of knowledge (e.g. some of the current flows).
20. Compare and contrast the pharmacology of frusemide and acetazolamide.
Examiner Comments
2013A 20:
Frusemide and acetazolamide are commonly used drugs and candidates were expected to have a sound knowledge of their pharmacology, including (amongst other features) their site and mode of action, renal and other organ effects, adverse effects and essential pharmacokinetics.
21. How is alveolar ventilation regulated?
CICMWrecks Answer
Respiration
- Normal RR ~ 10 breaths per minute
- Normal tidal volume ~ 500ml/kg (in 70kg male)
- Therefore normal minute ventilation approx 5l/min
Afferents / Sensors
Chemoreceptors
- Central
Located in retrotrapezoidal nucleus- Sensitive to changes in CSF [H+]
- CO2 readily diffuses across BBB and converted to H+ and HCO3– via carbonic anhydrase
- Increased [H+] (and therefore CO2) stimulated respiration
- Sensitive to changes in CSF [H+]
- Peripheral
Located in aortic bodies (innervated by vagus nerve) and carotid bodies (innervated by glossopharyngeal nerve)- Sensitive to PO2, [H+], PCO2 and blood flow
- O2 dependent K+ channels
- Respiration increases as O2 drops below 50mmHg
- O2 dependent K+ channels
- CO2
- Linear increase in respiration as CO2 increases
- Sensitive to PO2, [H+], PCO2 and blood flow
Baroreceptors:
- Located in aortic arch and carotid sinuses
- Responds to stretch
- As MAP drops → less stretch on vessel walls → increased sympathetic outflow from medullary vasomotor centre → triggers increase in respiration
- Responds to stretch
Pulmonary receptors
- Stretch receptors
- Increased stretch of pulmonary parenchyma triggers inflation reflex → inhibits inspiration to prevent overdistention
- Collapse of pulmonary parenchyma triggers deflation reflex → inhibits expiration to prevent atelectasis and loss of FRC
- J fibres
- Nociceptive mechano-chemoreceptors
- On stimulation → Bronchospasm, apnoea, bradycardia and hypotension
- Nociceptive mechano-chemoreceptors
Others
- Joint and muscle receptors stimulate ventilation
- Pain and temperature sensation can alter ventilation via the cortex and limbic system
Central control of breathing
Central input
- There is input from the hypothalamus and cortex, with the ability of the cortex to override the medulla and bring ventilation under voluntary control
Controller
- Medullary Respiratory Centre
- Dorsal respiratory group (DRG)
- Located in and adjacent to Nucleus Tractus Solitarus
- Associated with inspiration and timing
- Works as an ‘integrating centre’ with VRG
- Ventral respiratory group (VRG)
- Including Pre-Botzinger and Botzinger complex → Central Pattern Generator
- Associated with control of expiration, airway dilation and central pattern generation
- sends inhibitory impulses to Apneustic centre
- Dorsal respiratory group (DRG)
- Pontine respiratory group (PRG)
- Pneumotaxic centre
- controls both the rate and the pattern of breathing
- Sends inhibitory impulses to the inspiratory area
- Antagonist to apneustic center
- decreases tidal volume
- Apneustic centre:
- sends signals for inspiration for long and deep breaths
- controls the intensity of breathing and is inhibited by the stretch receptors of the pulmonary muscles at maximum depth of inspiration, or by signals from the pnuemotaxic center
- increases tidal volume.
- Pneumotaxic centre
- Inspiratory phase:
- Gradual ramping up of nerve activity – ↑muscle contraction
- Expiratory phase I:
- Gradual reduction of nerve activity – ↓muscle contraction
- Expiratory phase II:
- Inspiratory muscles inactive
- If increased respiratory drive, expiratory muscles are activated
Efferents
- Phrenic nerve (C3, 4, 5) → Innervates diaphragm – Main inspiratory muscle
- Spinal nerves to intercostal muscles (external intercostal → inspiration, internal intercostal → expiration)
- Brachial plexus → Pectoralis major (forced breathing inspiration)
- Accessory muscle → Sternocleidomastoid (forced breathing inspiration)
- Spinal nerves to abdominal muscles (forced expiration)
Mooney / Sakurai / JC 2020
Examiner Comments
2013A 21:
This is a core topic (syllabus Level 1) and a high level of understanding was expected. Overall candidates failed to demonstrate sufficient depth and breadth in their knowledge. A structured response considering the three basic elements underpinning the control of alveolar ventilation (the Sensors, Central integration and control and the Effectors) was core material. A detailed description of each was expected.
22. Describe the actions of endogenous vasopressin. (60% of marks) List the vasopressin analogues and their uses. (40% of marks)
Examiner Comments
2013A 22:
Overall the first section was not answered in sufficient detail. Aspects such as the antidiuretic effects of vasopressin were often overlooked. Some candidates spent more time on outlining the clinical contexts in which vasopressin is used (which did not score marks), rather than the physiology (which did score marks). DDAVP was the most common analogue mentioned, with the others often being omitted (e.g. terlipressin, ornipressin, etc.).
23. How do chemical messengers in the extracellular fluid bring about changes in cell function? Give an example of a chemical messenger for each mechanism noted.
CICMWrecks Answer
A receptor is a protein, often integral to a membrane, containing a region to which a ligand (chemical messenger) binds specifically to elicit a response.
They may be grouped into three classes based on mechanism of action:
1. Altered ion permeability (ion channels / ionotropic receptors)
Membrane spanning complexes with the potential to form a channel through the membrane.
There are three families:
- Pentameric → contain 5 membrane spanning units (eg, nicotinic Ach receptor at the NMJ which allows a Na channel to form, GABA A receptor which allows a Cl channel to form, 5HT3 receptor)
- Ionotropic glutamate → NMDA, AMPA and kainate ionotropic ligand gated ion channels. They form Na, K and (NMDA only) Ca channels when glutamate binds
- Purinergic receptors → PX1 and PX2 are activated by ATP, permeable to Na, K and Ca, and are associated with mechanosensation and pain.
2. Production of intermediate messengers (Metabotropic receptors)
Membrane bound systems that transduce a ligand gated signal presented on one side of the cell membrane into an intracellular signal transmitted by intermediate messengers.
These messengers may be:
- G proteins (most common) → binding of a chemical messenger to a G-protein coupled receptor activates the G protein, which in turn amplifies and transmits the signal to the appropriate target molecules. This can be done in several ways:
- Activation of phosplipase C (Gq), with intracellular production of DAG, IP3 and other inositol phosphates (eg; angiotensin II, noradrenaline on alpha 1 receptors, vasopressin on V1 receptors)
- Activation (Gs) or inhibition (Gi) of adenylyl cyclase, causing increased or decreased levels of intracellular cAMP (eg, noradrenaline increases cAMP via beta1 receptors, noradrenaline decreases cAMP via alpha 2 receptors)
- Increase in cGMP (eg, atrial natriuretic peptide, nitric oxide)
- Tyrosine kinase → eg, insulin activates tyrosine kinase resulting in the phosphorylation of various proteins
- Guanylyl cyclase → eg, NO, atrial natriuretic peptide
3. Regulation of gene transcription (Nuclear receptors)
- Steroids and thyroid hormones act through intracellular receptors to alter the expression of DNA and RNA, and indirectly alter the production of intracellular proteins.
JC 2019
Examiner Comments
2013A 23:
Overall answers lacked structure and depth, to what is a very fundamental topic. This topic is generally covered within the opening chapters of most physiology texts. Common errors were not answering the question, writing lists rather than describing and explaining, and poor categorisation. Candidates were expected to mention and give example for mechanisms such as hormones binding to cytoplasmic or intra-nuclear receptors, binding to transmembrane receptors coupled to G proteins, cAMP, cGMP, tyrosine kinase, etc.
24. Describe the mechanism of action, and side effects of THREE (3) classes of drugs that are used to increase uterine tone and THREE (3) classes of drugs used to decrease uterine tone.
CICMWrecks Answer
Oxytotic
(used to augment labour and ↑ uterine tone)
- Oxytocin derivatives (syntocinon, carbitocin)
- Oxytocin synthesised in hypothalamus
- Gq-PCR on SM cells
- causing increased Ca influx
- Ca induced Ca relase from SR → SM contraction
- Cause milk ejection from mamary glands, hypotension (via relaxation of SM), arrhythmias, water retention (similar structure to ADH)
- Ergot derivatives (ergometrine)
- Acts on alpha 1 and 5HT2 recepters (both Gq-PCRs) on uterine and vascular SM cells
- ↑ force and freq of contractions
- ↑ uterine tone
- contraction of cervix.
- Prostaglandins
- PGF2 alpha (Gq-PCR)
- Intra myometrial injection
- causes ↑ SM tone
- Bronchospasm if injected intravenously.
Tocolytic
(↓ uterine tone to suppress pre-term labour)
- Beta 2 agonists (salbutamol)
- β2 receptors action → ↑AC → ↑ cAMP → ↑PLC → ↓calcium → relaxation of myometrial SMCs
- Tachycardia/tremour/hypokalaemia
- Ca-channel blockers (nifedipine)
- ↓d Ca influx due to action on l-type Ca channels → ↓ uterine tone
- Inhibition of hypoxic pulmonary vasoconstriction, Impaired platlet aggregation, Headache, flushing and dizziness, Peripheral oedema
- NSAIDs (indomethacin)
- ↓ formation of PGE2 and PGF2α → ↓ uterine tone
- Can cause premature closure of ductus arteriosus, oligohydramnios, NEC and broncho-pulmonary dysplasia
Gladwin 2016
Click here for CICMWrecks Pharmacopeia Table for Oxytocics and Tocolytics
Examiner Comments
2013A 24:
Candidates often appeared to have a sufficient awareness of the choice of drugs (e.g. oxytocin analogues, ergot alkaloids, beta-receptor agonists, calcium channel blockers, etc.), but then failed to produce sufficient depth of knowledge to adequately describe their mechanisms of action in respect to uterine tone. Candidates are reminded that if asked to mention side effects, mentioning side effects of greatest relevance to intensive care (e.g. bronchospasm) in addition to the more generic side effects (e.g. rash).
Click here for CICMWrecks Pharmacopeia Table for Oxytocics and Tocolytics
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | knowledge of oxygen administration and oxygen measurement Resp physiology, Dynamic pulmonary compliance (PV loop), Expiratory flow curve |
| G. CVS | knowledge of coronary perfusion and blood pressure measurement Pressure waveforms with Rt heart, Rt atrial pressure trace (CVP) |
| H. Renal | |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | physiology of acid/base and the role of the kidney in acid/base balance, H-h equation |
| K. Neuro | knowledge of intracranial pressure and sedating drugs pain pathways and analgesia. Immediate response to a cut in the hand |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | physiology of thyroid and the thyroid hormone, anti thyroid drugs |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |




Recent Comments