1. Compare and contrast the carriage of oxygen and carbon dioxide in blood.
CICMWrecks Answer
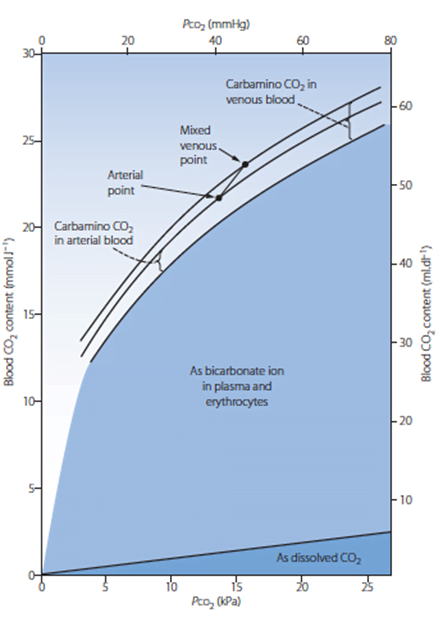
Carbon Dioxide
Total CO2 in arterial blood: 480ml/L
Total CO2 in venous blood: 520ml/L
Oxygen
CaO2 = (1.34 x [Hb] x SpO2) + 0.03 paO2
= 200ml/L
Approx 150ml/L in venous blood
Carried in 4 forms:
Carried in 2 forms:
| Dissolved CO2 CO2 has 20 times solubility compared with O2 PaCO2 ~40mmHg PvCO2 ~46mmHg | Dissolved O2 Minor component According to Henry’s law: – Dissolved O2 proportional to partial pressure of O2 in gas phase. – Solubility coefficient 0.24 PaO2 ~100mmHg PvO2 ~45mmHg |
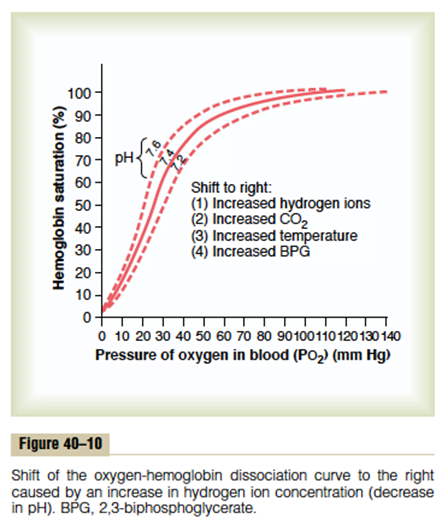
| Carbamino compounds Minor component of CO2 carriage in blood, however 30% of the A-V difference in CO2 carriage Hb is major protein for CO2 carriage Reduced Hb has 3.5x CO2 binding capacity and this is the major factor for the Haldane Effect Haldane effect Increased CO2 carrying capacity in deoxygenated blood – Due to increased buffering capacity of reduced Hb | Haemoglobin bound O2 Major component of O2 carriage According to oxyhaemoglobin dissociation curve Curve altered by pH, pCO2 and 2,3 BPG – Right shift (decreased affinity) due to increased CO2 and 2,3 BPG – Left shift (increased affinity) due to increased pH – CO2 effect on Hb is the Bohr Effect: In presence of CO2, there is decreased oxygen carrying capacity in blood |
| Bicarbonate Major component of CO2 carriage in blood | – |
| Carbonic acid Negligible | – |
Sakurai 2016
Examiner Comments
2024A 11: 55% of candidates passed this question.
A good response included normal values of the partial pressures of both oxygen and carbon dioxide including the arterial and venous contents. Methods of carriage (haemoglobin, dissolved and other forms), haemoglobin binding characterisitcs (co-operative binding and affinities); and impact on loading/off loading at tissues and lungs for both oxygen and carbon dioxide was included. Correctly labelled diagrams with a brief accompanying explanation could be used to convey some of these concepts.
2012B 01: 11 (50 %) of candidates passed.
Candidates who scored well for this question not only had a good knowledge of the topic but also displayed an organised approach to their answer through the use of a tabular format or some other structured approach. For a good answer, candidates were expected to provide information on the amount (both arterial and venous blood content, partial pressure) and form of carriage (binding to, loading and unloading from haemoglobin, dissolved, as bicarbonate, etc.) of oxygen and carbon dioxide in blood
2. Suxamethonium is a non-competitive partial agonist. Explain what is meant by this statement using definitions of the underlined terms (50% of marks). List the advantages and disadvantages of suxamethonium within Intensive Care practice (50% of marks).
CICMWrecks Answer
- Suxamethonium is the dicholine ester of succinic acid which acts as an ultrashort acting depolarising muscle relaxant.
- It is used in rapid sequence induction and to modify seizures caused by ECT.
- Mechanism of Action:
- mimics the actions of Ach by binding to the nicotinic Ach receptor and causing membrane depolarization.
- However, because its hydrolyzing enzyme is not present at the NMJ, the effect lasts longer than for Ach.
- The persistent depolarization renders the voltage sensitive Na channels inactive within 1-2 mins.
- This prevents the transmission of further APs. Initially causes fasciculations, then muscle relaxation.
- Partial agonists are drugs that bind to and activate a given receptor, but have only partial efficacy at the receptor relative to a full agonist
- A partial agonist is unable to achieve a maximal biological response following active site binding, despite increasing doses
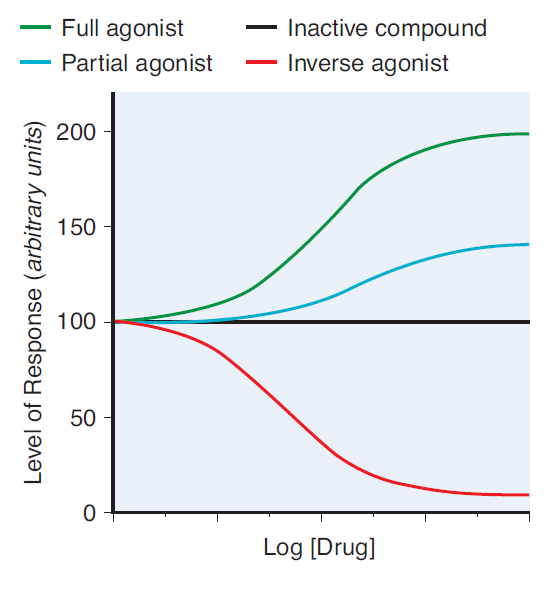
- A non-competitive antagonist binds to an allosteric (non-agonist) site on the receptor to prevent activation of the receptor
- Since it induces a lower biological response on active site binding than acetylcholine, it is a non-competitive antagonist of acetylcholine at the nicotinic receptor
- No matter how high levels of acetylcholine are at the neuromuscular junction, maximal response will not be reached
Advantages and Disadvantages of Sux in ICU
| Effect | Advantage | Disadvantage |
|---|---|---|
| Pharmaceutics | Inexpensive | Needs to be stored at 4 degrees -Shelf life only 7 days when kept in resuscitation trolley |
| Can be given peripherally | ||
| Excretion of 10% of drug unchanged in urine means active metabolites may accumulate and prolong the effects. | ||
| 0.03% of population are abnormal metabolisers resulting in prolonged apnoea | ||
| Causes fasiculations | Offset of fasiculations indicates paralysis | Muscle pains |
| Causes malignant hyperthermia | ||
| Prolonged suxemethonium apnoea | If intubation fails, prolonged apnoea | |
| Some agonism at muscarinic receptor | Second dose of suxemethonium commonly causes bradycardia | |
| Widespread neural depolarisation | Hyperkalaemia Raised intraocular pressure Raised GIT sphincter pressures | |
| Rapid onset (30-60 seconds) | Short period of time before intubating conditions achieved – Less risk of desaturation / apnoea when intubating | |
| Rapid offset (5-10 minuts) | If intubation fails, apnoea will not be prolonged | Muscle paralysis may disappear before airway secured or patient made safe |
| Metabolised by plasma cholinesterase | Metabolism not dependant on organ function | Plasma cholinesterase may be lower in some patients – myriad reasons |
Mooney / JC 2020
Examiner Comments
2018A 10: 46% of candidates passed this question.
This commonly used drug should be very well-known. The question asked for an outline, hence long explanations of various aspects of pharmacology (e.g. pseudocholinesterase deficiency) were unnecessary.
Headings should have included: advantages (e.g. rapid onset, rapid offset, short acting, IV or IM administration, not end organ dependent for metabolism, premixed, safe in pregnancy and neonates). The disadvantages section should have included the following headings: pharmaceutical, adverse drug reactions (including several potentially fatal ones), numerous contraindications, unpleasant side-effects and potential problems with repeat dosing.
2012B 02: 13 (59.1%) of candidates passed.
For a good answer candidates were expected to mention that an agonist is a drug that elicits a maximal response on binding to a receptor. A partial agonist has intrinsic affinity with only partial efficacy and hence is unable to elicit a maximal response. A competitive drug acts at the same binding site of a receptor as an endogenous ligand (e.g. acetylcholine at the neuromuscular junction) and its action therefore is surmountable with increasing concentrations of drug and how this concept relates to suxemethonium. For the remainder of the question, Candidates were expected to mention the advantages and disadvantages of suxamethonium within Intensive Care practice. Good answers included a systematic approach and use of tables and/or well organised lists.
3. Outline the anatomy and physiology of humidification during normal breathing (50% of marks). Describe the mechanisms of humidification used within Intensive Care practice (50% of marks).
CICMWrecks Answer
Humidity
Humidity
- Humidity is the concentration of water vapour present in the air.
Saturated Vapour Pressure of Water
- The water vapour pressure when the air is fully saturated.
- Depends on both pressure and Temperature
- = 47mmHg at STP
Absolute Humidity
- The amount of water vapour present in a given volume of gas (units g H2O/m3 or mgs H2O /L)
- Room air at sea level has Absolute humidity of 10g H2O/m3
- < 100% saturation
- Absolute humidity is temperature independent
- 100% saturation
- Absolute humidity is temperature dependent – due to ΔSVP fully saturated air
- at 0 °C contains 4.8 mg/L;
- at 20 °C contains 17 mg/L;
- at 37 °C contains 44 mg/L
- Absolute humidity is temperature dependent – due to ΔSVP fully saturated air
Relative Humidity
- the amount of water vapour present in the gas expressed as a percentage of the amount of water vapour that would be present if the gas were saturated with water vapour.
Latent Heat of Vaporisation
- the heat required to convert 1g of a substance from the liquid phase to the gaseous phase at a given temperature (expressed in Jg-1)
Humidification Process
INSPIRED AIR (During nose breathing)
- Air is warmed by the radiant heat from nasal blood supply.
- ↑ing temperature → ↑ SVP → ↑’s water carrying capacity
- Moisture evaporates from the epithelia → ↑ relative humidity of the inspired air to ~90%
- Mouth breathing reduces the relative humidity of inspired air to 60-70%
- At the lungs, it reaches the isothermic saturation boundary where it achieves BTPS (body temperature and pressure, saturated with water vapour) conditions.
- This usually occurs at the second generation of bronchi.
- Absolute Humidity @ Carina = 44 g H2O/m3
- Relative Humidity @ Carina = 100%
EXPIRED AIR
- Expired gas transfers heat back to the cooler trachea and nasal mucosa.
- As the saturated gas cools, it can hold less water vapour (its saturated water vapour pressure falls)
- Condensation occurs on the mucosal surfaces, where the liquid water is reabsorbed.
- Reabsorption reduces potential airway water losses from 300ml/day to ~150ml/day
- Tracheal temperature and humidity fall with an increase in respiratory rate (ie, the isothermic saturation boundary moves more away from the upper airway)
Complications of non-humidified air:
- Mucosal dehydration
- Altered ciliary function
- Inspissation of secretions
- Atelectasis and V/Q mismatching (if underlying lung disease)
- ↑ heat loss (5-10%) as the inspired gases are warmed and more H2O needs to be added as vapour
Humidification Mechanisms in ICU:
- Passive
- HME (Heat-Moisture Exchanger)
- Active
- Bubble Humidification
- Passover
- Heats water in chamber
- Evaporated water entrained by fresh gas
- Nebulisation
- Pressure and heat vapourises water
Gladwin 2016
Examiner Comments
2012B 03: 0 (0%) of candidates passed.
For a good answer candidates were expected to provide information on essential facts such as, normal inspired air has low water content, normal humidification provides for saturated water vapour 47mmHg at sea level at the alveolus, which corresponds to an absolute humidity of 44 g/m3 (or 100% relative humidity) at 37.C. Furthermore, candidates were expected to outline the basics of the anatomical features of the respiratory tract that promotes humidification e.g. lining of nose and hypopharynx dissolving warm water vapour into the dry inhaled air, the fact that the turbinates act to increase surface area and that full saturation is achieved by the time air reaches the upper trachea. For the second part of the question candidates were expected to mention, and briefly describe, the mechanisms of achieving humidification, e.g. bubble systems, heat-moister exchange filters, heated water, ultrasonic, etc. mechanisms. Candidates generally did not have sufficient knowledge of the basic conepts or a structured approach to this topic.
4. Define myocardial contractility and briefly describe dP/dT, the end systolic pressure volume (ESPV) relationship and the ejection fraction (EF).
CICMWrecks Answer
Myocardial contractility
- Ability of myocardium to contract (or myofibrils to shorten) against a given afterload, with a given preload
dP/dT
- Change in pressure / change in time
- Measure of contractile force exerted by the myocardium at the start of systole
- One of the oldest measures of global myocardial contractility
- Measured via doppler analysis of mitral regurgitant jet
- Limited by
- Requirement for MR jet
- Affected by preload, afterload and contractility
ESPVR
- Line created with a series of LV pressure volume loops where preload was varied with a fixed afterload. The point at which aortic valve closure occurred was plotted.
- This is a surrogate for contractility on the pressure-volume loop.
- Invasive and impractical
EF
- Fraction of end-diastolic volume which is ejected (stroke volume / end-diastolic volume)
- 60% in normal heart (70ml/120ml)
Sakurai 2016
Examiner Comments
2012B 04: 3 (13.6%) of candidates passed.
Contractility represents the performance of the heart at a given preload and afterload. It is the change in peak isometric force (isovolumic pressure) at a given initial fibre length (end diastolic volume). All indices of myocardial contractility are dependent on preload or afterload to a varying degree. The dP/dT is the maximum rate of change in left ventricular pressure during isovolumetric contraction, after mitral valve closes and before the aortic valve opens. It is preload dependant and afterload independent. A diagram of a pressurevolume loop is very helpful when describing the ESPV. Absence of a diagram (correctly labelled and scaled) was a weakness in many answers. Candidates were then expected to at least explain that, as preload is increased a new pressure volume loop is generated. Each new PV loop has a new end systolic point that is at a slightly higher pressure and volume than the previous end systolic point. The line connecting the end-systolic points is called the linear ESPVR. The slope of the ESPVR or Emax is used as an index of myocardial contractility. Ejection fraction is the percentage of the ventricular end diastolic volume (EDV) which is ejected with each stroke volume (SV). Ejection fraction = stroke volume/end diastolic volume X 100 (Normal range 55 to 70%). Only a minority of candidates achieved the depth of knowledge required for a Level 1 topic.
5. How does liver failure affect the pharmacology of drugs?
CICMWrecks Answer
Pharmacodynamics
- Altered receptor binding
- Postulated for relative resistance to non-depolarizing NMB in liver failure
Pharmacokinetics
- Absorption
- Portal hypertension and interstitial oedema reduce absorption of drugs
- Hepatic dysfunction
- Increased bioavailability of drugs susceptible to hepatic first pass metabolism (diazepam)
- Decreased activation of pro-drug (clopidogrel requires CYP2C19 activation)
- Distribution
- Decreased synthesis of plasma proteins
- Increases free fraction of protein bound drugs
→ Unbound drug more permeable → increased volume of distribution (amiodarone)
→ Unbound drug more active
- Increases free fraction of protein bound drugs
- Hypervolaemia secondary to renal Na+ and H2O retention
- Increased Vd for drugs distributed to extracellular volume
- Decreased pH alteres ionization of acids and bases (acids ionized when pH above pKa and bases ionized when pH below pKa)
- Decreased synthesis of plasma proteins
- Metabolism
- Decreased hepatic metabolism
- Increases T1/2b of hepatically metabolized drug → Accumulation of drug if no alteration
- Decreased butylcholinesterase production → Decreased metabolism for drugs metabolised by plasma esterases (suxamethonium – however clinically not significant for this drug) – red cell esterases remain therefore no effect for remifentanyl
- Decreased hepatic metabolism
- Elimination
- Hepatorenal syndrome → decreased renal clearance
- However renal drug clearance tends to decrease even in absence of renal impariment (drugs not hepatically converted to more water soluble metabolites)
- Biliary clearance of drug is reduced
Sakurai 2016
Examiner Comments
2012B 05: 13 (59.1%) of candidates passed.
Good answers were structured using pharmacokinetic and pharmacodynamics headings. They included some mention of changes in absorption, volume of distribution (an increase in Vd in liver failure), altered protein binding, altered metabolism and thus change in clearance, and changes in excretion (decreased biliary excretion of drugs). In respect to pharmacodynamics candidates could have mentioned increased sensitivity and prolonged action of sedative drugs, oral anticoagulants, etc. Good candidates also differentiated for acute (often hepatocellular dysfunction) and chronic liver failure (cirrhosis and changes in liver blood flow). Common problems were not using a logical structure to answer the question and stating an effect but not describing how this affected pharmacology. For example stating decreased albumin production but then not stating the consequence of this on drug distribution. Primary examination questions may often require candidates to integrate knowledge from across different sections of the syllabusor apply basic physiological or pharmacological principles.
6. Describe the structure of surfactant (25% of marks). Explain the effects of surfactant upon surface tension and lung mechanics (75% of marks)
CICMWrecks Answer
Surfactant:
- Phospholipoprotein complex
- DPPC – 40%
- Other phospholipids – 40%
- Surfactant-associated proteins – 10%
- Lipids – small amount
- DPCC and other phospholipids have a hydrophilic head, and a hydrophobic tail
- Align themselves on the interior alveolar surface
- Intermolecular forces repel each other, reducing surface tension
In an alveolus:
- Surfactant reduces surface tension, increasing compliance
- Surface tension of surfactant changes with alveolar tension
- At lower volumes, surface tension reduced to near zero
→ Prevents collapse at low volumes - At higher volumes, surface tension rises
→ Prevents overdistension - Shows hysteresis
- At lower volumes, surface tension reduced to near zero
- Surface tension draws fluid out of peri-alveolar capillaries
- Reduced surface tension
→ reduces hydrostatic pressure in capillaries
→ prevents transudate
- Reduced surface tension
Mooney 2016
Examiner Comments
2012B 06: 8 (36.4%) of candidates passed.
The answer required a description of surfactant composition (phospholipids 85%, neutral lipids 5%, and proteins 10%). Phospholipid dipalmitoylphosphatidylcholine is the main surface active component. It was expected candidates would provide a description of the arrangement of the phospholipids with the hydrophilic head in the aqueous phase and the hydrophobic tail in the airspace of the alveolus. The effects of surfactant required an explanation of surface tension and how this affects alveoli. One good way to explain this was describing how La Place’s law would affect alveoli with, and without, surfactant. As the alveoli decrease in size, the surfactant molecules are pushed together and exert a greater surface tension lowering effect. Surfactant is also important in the lung elastic recoil and hysteresis and for alveolar stability preventing collapse and thereby improving lung compliance and decreasing work of breathing. Surfactant also helps oppose the starling forces in the lung and keep fluid from being drawn into the alveoli. Candidates often misunderstood La Place’s law and did not explain how surfactant decreases surface tension.
7. Describe the anatomy of the antecubital fossa and peripheral veins of the upper arm relevant to a peripherally inserted central venous catheter (PICC).
CICMWrecks Answer
Antecubital fossa – a triangular view on the anterior aspect of the elbow
Boundaries
- Medial – lateral border of pronator teres from the medial epicondyle
- Lateral – medial border of brachioradialis from the lateral supraepicondylar ridge of the humerus
- Superior – an imaginary horizontal line connecting the medial and lateral humeral epicondyles
- Inferior – the apex is directed inferiorly and is formed by the meeting point of the medial and lateral boundaries
- Deep (floor) – brachialis (proximal) and supinator (distal) muscles
- Superficial (roof) – skin, superficial fascia (containing the medial cubital vein, lateral and medial cutaneous nerves of
- the forearm, deep fascia (reinforced by the bicipital aponeurosis)
Contents (from medial to lateral)
- Median nerve – leaves the fossa between the two heads of pronator teres
- Brachial artery – bifurcates into the radial and ulnar arteries at the apex of the fossa
- Biceps tendon
- Radial nerve – not strictly in the fossa but is in the vicinity, passing underneath brachioradialis
Venous anatomy in the superficial fascia of the fossa
(from medial to lateral)
- Basilic vein
- commences in the medial forearm, joined by the medial cubital vein at the level of the ACF and then perforates the brachial fascia above the medial epicondyle, joining the brachial veins to form the axillary vein.
- Favoured for PICC cannulation because it is usually of substantial size, with predictable anatomy and is easily viewed with USS.
- Medial cubital vein (variable anatomy)
- Cephalic vein
- commences in the lateral forearm and continues up the lateral aspect of the arm before it enters the deltopectoral groove and empties into the axillary vein.
- Less used for PICC insertion because the presence of the claviopectoral fascia superiorly causes it to enter the axillary vein at an acute angle, which is often difficult for the catheter to negotiate.
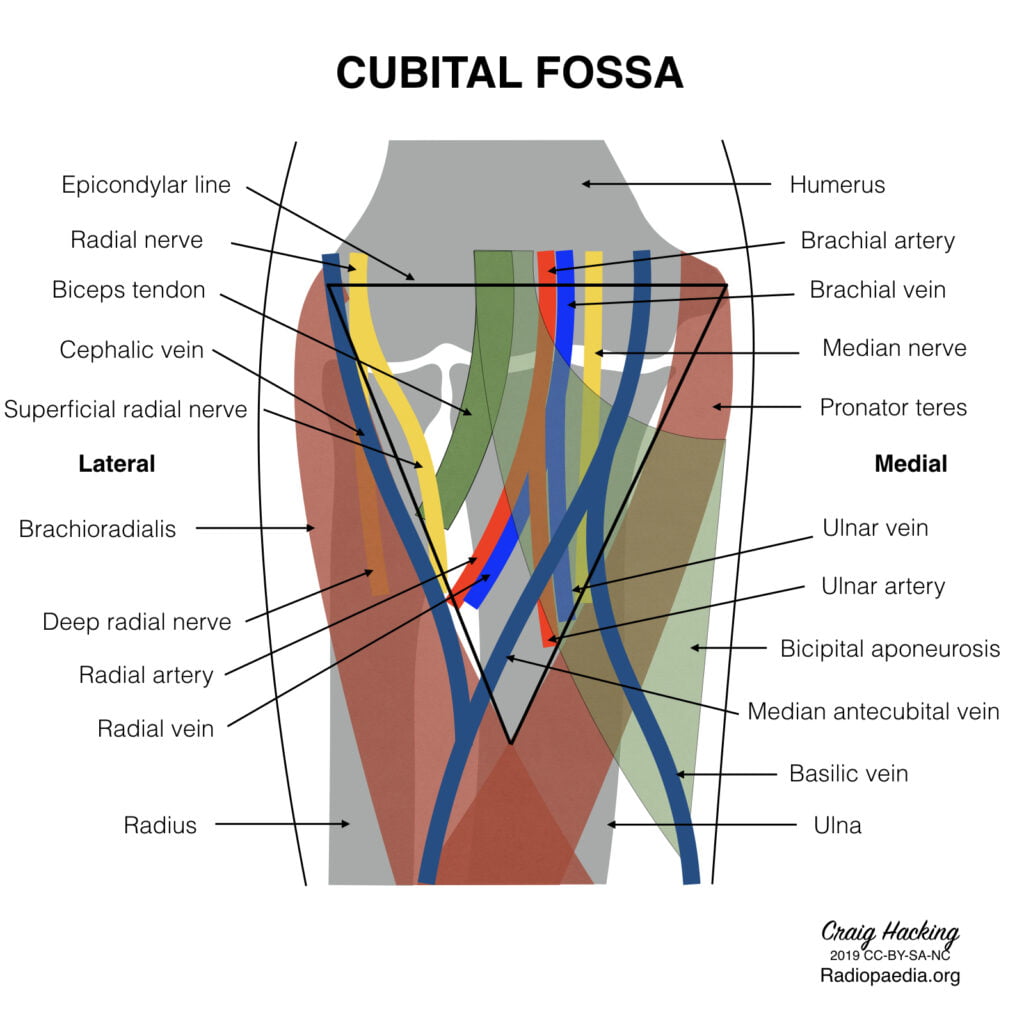
Gladwin 2016
Examiner Comments
2012B 07: 5 (22.7%) of candidates passed
Knowledge of anatomy of the areas of the body where common procedures are performed in the intensive care unit is essential. Defining the question by giving the boundaries and contents of the antecubital fossa along with a diagram illustrating the arrangement of the veins would have constituted a pass. The course of the basilic vein and an explanation of why it is favoured over the cephalic vein (presence of the clavipectoral fascia which provides an acute angle for the catheter to negotiate along with a valve frequently located at the junction) was important applied anatomy relevant to PICC line insertion. Transposition of medial and lateral structures was a common error in answers to this question.
8. Describe the physiology of gastric emptying (80% of marks). Outline the gastrointestinal effects of erythromycin (20% of marks)
CICMWrecks Answer: Gastric Emptying
Physiology of Gastric Emptying
Gastric emptying
- Fluids have half-time of 30mins in stomach
- Solids have half-time of 2 hours in stomach
Gastric receipt of food bolus:
- Peristaltic wave moves down the oesophagus, propelling food bolus
- Controlled by mesenteric plexus with input from vagus nerve
- Upon swallowing, the lower oesophageal sphincter and stomach relax until the oesophageal peristaltic wave has passed
- Following food bolus passage, the LOS tone increases to prevent reflux
- Food bolus reaching the stomach causes a vagal-mediated relaxation of the stomach, to accommodate gastric distension and food storage
Gastric mixing and emptying:
- Spontaneous mixing waves move down the stomach every 15-20 seconds
- Stronger waves propel gastric contents towards the pylorus (pyloric pump)
- High pyloric sphincter tone
- Allows through well-mixed liquid chyme
- Restricting gastric emptying of solids, causing mixing (retropulsion)
Control
Gastric emptying is controlled by the balance between stimulatory gastric factors and inhibitory duodenal factors
Factors promoting gastric emptying:
- Increased stomach wall stretch
- Stimulates pyloric pump
- Reduces pyloric tone
- Parasympathetic vagal stimulation
- Stimulates pyloric pump
- Gastrin
- Stimulates pyloric pump
- From G cells in antrum
- In response to presence of protein (esp. meat) in stomach
- Motilin
- Stimulates pyloric pump
- During fasting
- Stimulates pyloric pump
- From M cells in duodenum (external antigen stimulus)
- Carbohydrate: rapid emptying
Factors inhibiting gastric emptying:
- Nervous reflexes via enteric nervous system, local sympathetic trunk, and the vagal nerve to the brainstem
- in response to local conditions in the duodenum: Distension, Irritation, Acidity of chyme, Hyper- or hypo- tonicity of chyme, Breakdown products, Especially AA and FA
- Inhibit pyloric pump
- Increase pyloric sphincter tone
- Hormones (inhibit pyloric pump)
- Cholecystokinin (CCK)
- From I cells of duodenum
- In response to presence of fat and proteins
- Secretin
- From S cells of duodenum
- In response to presence of acids
- Gastric inhibitory peptide (GIP)
- From K cells of duodenum
- In response to presence of fats, proteins and carbohydrate
- Cholecystokinin (CCK)
- Other Factors :
- Sympathetic stimulus:
- Decreases contractility and reduces gastric emptying
- Dopamine:
- Decreases intragastric pressure and lower oesophageal sphincter tone
- reduces gastric emptying
- Sympathetic stimulus:
Alternate: This section can also be approached as:
- Local factors
- Gastric Factors: pump, Stomach wall distension, Amino acid
- Duodenal Factors: Type of food, stretch of wall, irritation, hyperosmolaroty, acidity, amino acid and FA content
- Neural factors
- Symptathetic
- Parasympathetic
- Hormonal factors
- Dopamine
- Gastrin
- Motilin
- Secretin
- CCK
- Somatostatin
- GIP
Mooney / Sakurai / JC 2019
CICMWrecks Answer: GI Effects of Erythromycin
Erythromycin
- Action:
- Agonist at motilin receptors on GI muscle
- Enchances motilin release from M cells in duodenum
- Promotes onset, frequency and duration of migrating motor complex
- Increases lower oesophageal tone
- May eliminate normal gut flora
Examiner Comments
2012B 08: 9 (41%) of candidates passed.
This question was best answered by using a classification system, or systematic approach to gastric emptying. Receptive relaxation (triggered by movement of food through the pharynx and oesophagus), vagally mediated relaxation of fundus and upper body of stomach, the pyloric pump (being intense peristalsis in lower body of the stomach that results in stomach emptying) and the pyloric sphincter (a circular muscle that allows water and fluids to easily pass through but restricts solids until it is mixed in chime to almost fluid consistency).
Candidates were also expected to mention regulatory factors e.g. food volume through myenteric reflexes / gastrin stimulatory motor effects and enhanced pyloric pump, acidity and osmolality of chyme in duodenum, presence of breakdown products of protein and fat through enteric nervous system, sympathetic and parasympathetic nervous systems and hormones such as cholecystokinin, secretin and gastric inhibitory peptide. Erythromycin is a commonly used prokinetic and some knowledge of effects was expected (e.g. the fact that it stimulates motilin receptors on GI smooth muscle and promote onset, frequency and duration of migrating motor complex, from stomach and spreading caudally thus increasing gastric emptying).
9. Classify the anti-arrhythmic drugs using the Vaughan-Williams classification (30% of marks). Compare and contrast the electrophysiological effects of Class 1 anti-arrhythmic drugs (70% of marks).
CICMWrecks Answer
| CLASS | ACTION | ELECTRO- PHYSIOLOGY | DRUGS | THERAPEUTIC INDICATIONS |
|---|---|---|---|---|
| I. Na – Channel Blockers | ||||
| Ia | Intermediate dissociation | ↓ phase 0 ↓↓ conduction v ↑ repolarisation ↑ APD | Quinidine Disopyramide Procainamide | Atrial and ventricular arrhythmias esp. post MI |
| Ib | Fast dissociation | ↔,↓ phase 0 ↓ conduction v ↓ repolarisation ↓ APD | lignocaine phenytoin tocainide, mexilitine | Ventricular arrhythmias post MI, digoxin induced arrhythmias |
| Ic | Slow dissociation | ↓↓↓↓ phase 0 ↓↓↓↓ conduction v ± repolarisation ↔ APD | flecainide ecainide, lorcainide | Refractory arrhythmias |
| II. β – Blockers | ||||
| ↓ SA firing – ↓ rate, conduction | propranalol* atenolol, esmolol | Rate control in AF, AT, Flutter and VT | ||
| III. K – Channel Blockers | ||||
| Delay phase 3 (repol) ↑ APD ↑ ERP | amiodarone bretylium, sotalol^ | AF / Flutter termination | ||
| IV. Ca – Channel Blockers | ||||
| ↓ AV conduction ↑ PR interval ↓ rate/conduction | verapamil diltiazem | SVT and AF or Flutter | ||
| OTHERS (Some call this Class V) | ||||
| Blocks Na+/K+ ATPase → ↑Ca2+, ↓K+, ↑Ach | ↑ contractility ↓ AV conduction | Digoxin | AF rate control Heart Failure | |
| Opens K+ channels via adenosine receptors | Hyperpolarizes myocardium ↓ AV conduction ↓ SA firing | Adenosine# Ibutilide | Terminate SVT or reveal underlying rhythm in tachycardias | |
| Stimulates Na+/K+ATPase | Membrane stabilization | Magnesium | VF / Torsades de pointes | |
| Notes | * Propranalol also has Na blocking activity ^ l-sotalol has beta blocking and class III activities; d-sotalol is a pure class III agent. Commercially available sotalol is a racemic (equal part) mixture. # Amiodarone – blocks Na, Ca, K channels, and exhibits beta blockade. | |||
The effect on the myocardial action potential of each class is:
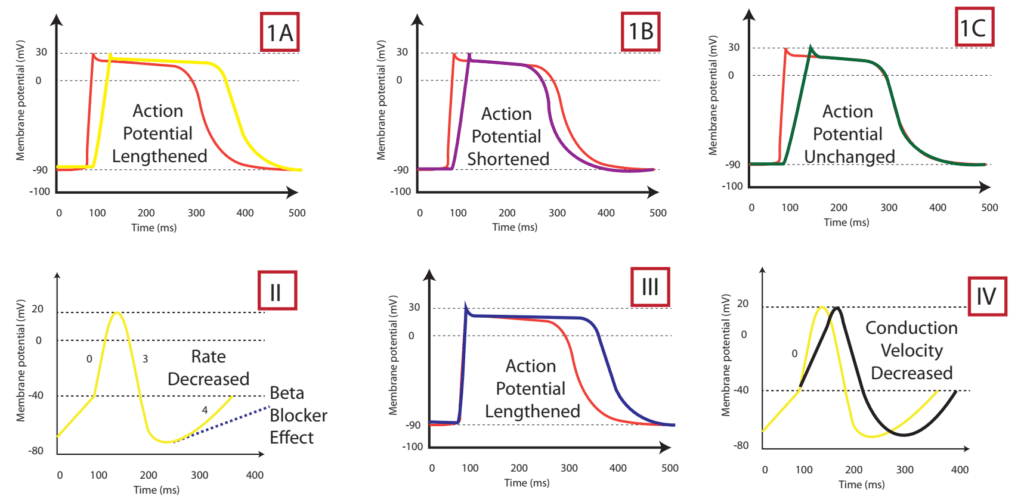
| Class Ia | Class Ib | Class Ic | Class II | Class III | Class IV | |
|---|---|---|---|---|---|---|
| Depolarisation rate (phase 0) | ↓ | ↔ or ↓ | ↓↓↓↓ | ↔ | ↔ | ↓↓↑↔± ↔ |
| Conduction velocity | ↓↓ | ↓ | ↓↓↓↓ | ↓ | ↓ | ↔ |
| Effective Refractory Period | ↑↑↑↑ | ↓ | ↑ | ↓ | ↑↑↑↑ | ↔ |
| Action potential duration | ↑ | ↓ | ↔ or ↑ | ↑ | ↑↑↑↑ | ↓ |
| Automaticity | ↓ | ↓ | ↓ | ↓ | ↓ | ↔ |
| P-R duration | ↔ | ↔ | ↑ | ↔ or ↑ | ↑ | ↔ or ↑ |
| QRS duration | ↑ | ↔ | ↑↑↑↑ | ↔ | ↑ | ↔ |
| QTc duration | ↑ | ↔ or ↓ | ↑ | ↓ | ↑↑↑↑ | ↔ |
Sotalol Effects
- l-sotalol has beta blocking and class III activities
- d-sotalol is a pure class III agent
- Commercially available sotalol is a racemic (equal part) mixture.
- Class III Effects (K – channel blocking)
- Blocking of outward K+ channels slows cardiac repolarisation, which increases the cardiac refractory period.
- Delay phase 3 (repol), ↓ AV conduction,
- ↑↑↑↑ APD, ↑↑↑↑ ERP
- ↑↑↑↑ QTc
- ↓ automaticity
- ↓ ectopy
- ↓ defibrillation energy requirement
- May ↑ inotropy
- Class II Effects (β – Blocker)
- Antagonizes β1 & β2 receptors
- ↓ SA firing – ↓ heart rate, conduction
- can prolong the QT interval.
- can cause polymorphic ventricular tachycardia (Torsades de Pointes)
- recommended to be initiated in the inpatient setting to monitor the QT interval after each dose
- can cause severe bradycardia necessitating drug discontinuation
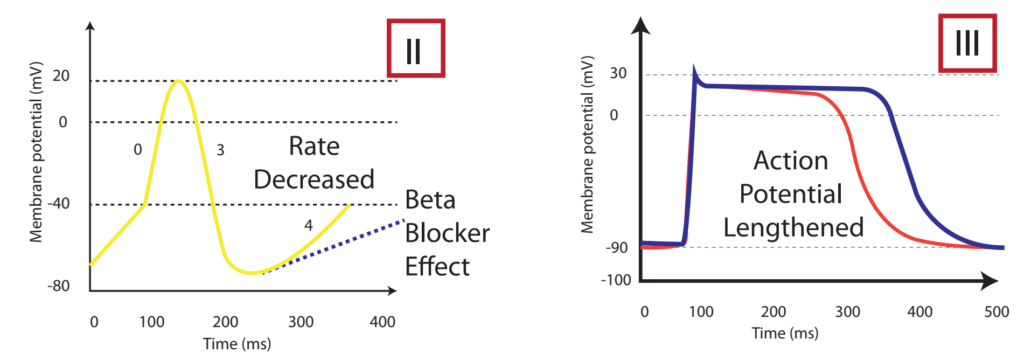
Gladwin / Sakurai / JC 2020
Examiner Comments
2012B 09: 16 (72.7%) of candidates passed.
Most candidates displayed a basic knowledge of the Vaughan-Williams classification and
gave an example of each class. The remainder of the question lent itself very well to a
tabular format. Better answers included the effect on the action potential (diagrams were
useful here), channel dissociation kinetics (this was frequently omitted) and examples from each class of drug. There is an excellent table in Stoelting which answers this question nicely. Marks were not awarded for clinical effects. Overall, this question was generally well.
10. Describe transport mechanisms across cell membranes. Give an example of each.
CICMWrecks Answer
Transmembrane Transport Mechanisms
| Mechanism | Energy Expenditure | Electrochemical gradient | Example | ||||
|---|---|---|---|---|---|---|---|
| Diffusion | Passive diffusion | Molecule crosses a membrane to which it is permeable by diffusion | No | With | Carbon dioxide across vascular endothelium | ||
| Facilitated diffusion | Molecule crosses a membrane via a channel, without energy expenditure | No | With | Potassium across excitable cell membranes, via rectifier channels | |||
| Active transport | Primary | Primary active transport | Molecule crosses a membrane via a channel, with energy expenditure (ATP) | Yes | Against | 3Na+ /2K+ ATPase | |
| Secondary | Symport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Co-transported) | Not directly | May be Passive or Active based on gradient of 2nd | sodium and an amino acid | ||
| Antiport | Molecule crosses membrane against its electrochemical gradient, with the energy being provided by the transport of another molecule (Anti-transported) | Not directly | May be Passive or Active based on gradient of 2nd | Na+ / H+ antiporter in proximal convoluted tubule | |||
| Ligand-gated ion channel | Binding of a ligand causes conformational change in membrane channel, allowing movement of ion across membrane | No | With | Nicotinic acetylcholine receptor. ACh as ligand, Na+ and K + as ions | |||
| Exocytosis | Substance packed in vesicle, moves to cell membrane, two membranes merge -> substance exits cell | Yes | Usually against | Exocytosis of ACh by presynaptic neuron | |||
| Endocytosis | Cell membrane extends to engulf a substance or object, which is then contained in the cell within a vesicle | Yes | Can be with, against or refer to a macroscopic object | Phagocytosis of bacteria by macrophage | |||
JC / Mooney 2019
Examiner Comments
2012B 10: 11 (50%) of candidates passed.
Candidates were able to list types of transport across cell membranes but frequently described them incorrectly or gave an incorrect example. In a number of answers, there was confusion between facilitated diffusion and secondary active transport. Though diagrams were not required, several Candidates used a diagram of the cell very effectively to illustrate the mechanisms of transport across the membrane. For a good answer, some mention and description of exocytosis, endocytosis, ion channels, facilitated diffusion, passive diffusion, primary and secondary active transport was expected.
11. Discuss the pharmacokinetic factors that affect drug half-life.
CICMWrecks Answer
Definitions
Half life: Time taken for a reduction in one half the total ammount of drug from the body.
Half time: Time taken for a reduction in one half the plasma concentration of a drug
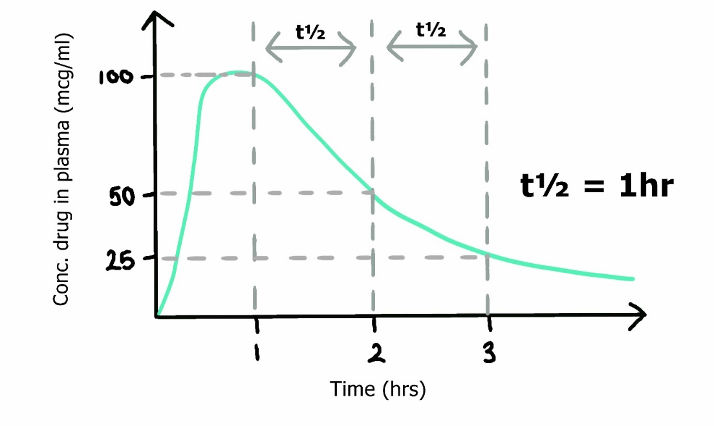
Simplified equation for half life
- Where
- Vd is volume of distribution of a drug
- Cl is its clearance
- ln(2), the natural log of 2, can be approximated as 0.693
Pharmacokinetic factors
Absorption
- Minimal impact on T1/2
- If initial plasma concentration saturates its metabolic pathway, clearance will become zero-order and rate of elimination will be independent of drug concentration
Distribution
- In the multi-compartment pharmacokinetic model,
- Drug initially entering the central (plasma) compartment will redistribute to other compartments (tissues), decreasing the amount remaining in the central compartment.
- As Vd increases, the ammount redistributing to other tissues increases → increased distribution away from central compartment → T1/2α (Distribution half time) decreases.
- This reduction in plasma concentration will decrease the concentration of drug reaching its metabolic organs (unless plasma metabolism) and prolong the elimination half-life.
- Drugs with high volumes of distribution display context-sensitive half time
- Protein binding of drug increases half life
- Prevents glomerular filtration
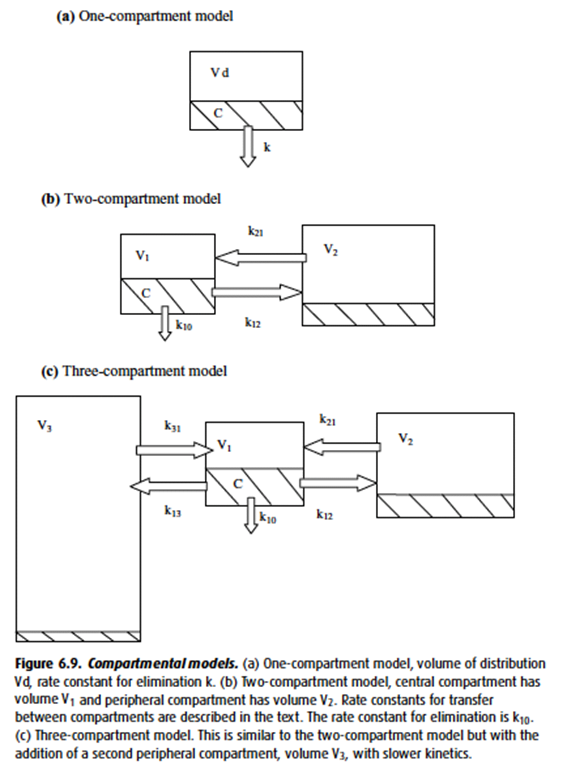
Metabolism and elimination
- Clearance is the volume of plasma completely cleared of drug per unit time
- Total body clearance is a sum of renal clearance (via metabolism or elimination), hepatic clearance (via metabolism, biliary secretion) and other mechanisms (such as lung metabolism, plasma cholinesterases etc.)
- As drug metabolism and elimination increases, T1/2β decreases
Zero-order kinetics
- As elimination of drug is independent of drug concentration, half life is not constant and progressively shorten by half
- E.g. 100mg of drug in body eliminated at 10mg/hr hour
- Half life from 100mg (to 50mg) would be 5 hours
- Subsequent half life (from 50mg to 25mg) would be 2.5 hours etc.
- E.g. 100mg of drug in body eliminated at 10mg/hr hour
Sakurai 2016
Examiner Comments
2012B 11: 10 (45.4%) of candidates passed.
Half-life (t½) is the time required to change the amount of a drug in the body by one-half during elimination. Candidates were expected to discuss the two main factors which affect drug half-life, namely volume of distribution and clearance. Marks were awarded for the formula (t½ = 0.693 x Vd /CL), the factors which affect the volume of distribution and drug clearance but not for a discussion of factors affecting drug absorption.
12. Describe the blood brain barrier (50% of marks). What characteristics does a drug need to effectively penetrate the blood brain barrier? (50% of marks)
CICMWrecks Answer
Blood brain barrier
- Anatomical and chemical partition separating the intravascular space from CNS interstitial space
Structure
- Mechanical barrier
- Endothelial cells
- Tight junctions between cells formed by membrain proteins (e.g. occludin) prevents paracellular flow
- Lack fenestrations
- Lack transcellular pathways such as vescicles
- Selective transport proteins (e.g. GLUT, various amino acid transporters)
- Pericytes embedded in basement membrane
- Astrocyte end feet
- Supportive role for endothelium
- Aquaporin regulation
- Endothelial cells
- Physiological barrier
- Enzymatic inactivation
- Enzymtes within endothelial cells metabolise substances absorbed from capillary lumen
- Monoamine oxidase
- Cholinesterase
- Aminopeptidase and endopeptidase
- Enzymtes within endothelial cells metabolise substances absorbed from capillary lumen
- Efflux pumps
- Substances that are absorbed across the luminal capillary membrane may be pumped back into capillary lumen by efflux pumps
- P-Glycoprotein
- ABC-Transporter
- Substances that are absorbed across the luminal capillary membrane may be pumped back into capillary lumen by efflux pumps
- Enzymatic inactivation
- Areas of brain outside BBB
- Subfornical organ
- Organum Vasculosum Lamina Terminalis
- Pituitary
- Area postrema
Function
- Regulate uptake of nutrients and electrolytes into brain
- Regulate migration of leukocytes and inflammatory responses in the brain
- Buffer brain parenchyma and interstitium from fluctuations in blood
- Prevent toxins and pathogens entering brain
- Some substances (such as water) pass BBB readily. These are characterised by:
- Small molecular weight
- Lipophilic (thiopentone)
- Uncharged (e.g. atropine vs. glycopyrollate)
- Poorly protein bound
Characteristics of drugs to penetrate BBB
- Size
- According to Graham’s Law – Diffusion inversely proportional to square root of molecular weight
- Lipophilicity
- Highly lipid soluble compounds can permeate phospholipid bilayers with relative ease (fentanyl > morphine)
- Charge
- Unionized molecules can permeate with relative ease (alfentanil > fentanyl)
- Not metabolized within BBB
- No active efflux mechanism
- Protein binding
- Protein bound drug permeate poorly
Sakurai 2016
Examiner Comments
2012B 12: 9 (41%) of candidates passed.
The BBB is the separation of the blood from the brain extracellular fluid and serves to maintain consistent internal environment in the brain and protect the brain from large harmful substances and microorganisms. Most answers displayed some knowledge of the structure of the BBB but many answers did not include its function. Better answers included substances to which the BBB is permeable, how permeability changes with age and a mention of the circumventricular organs and their significance (i.e. are outside the BBB). Most candidates correctly identified the characteristics of drugs that cross the BBB. Marks were also allocated for giving examples.
13. Describe the effects of obesity on drug pharmacology (70% of marks). Give examples of drugs that illustrate those effects (30% of marks).
CICMWrecks Answer
Pharmacodynamics
- Receptor resistance
- Insulin resistance secondary to obesity
Pharmacokinetics
Absorption
- Increased cardiac output
→ Delayed onset of action of inhalational agents (volatile anaesthetic agents) - Decreased subcutaneous absorption due to tissue binding (heparin)
- Failure of IM administration due to increased depth (adrenaline is anaphylaxis)
Distribution
- Increased total body fat
- Increased volume of distribution of lipophillic drugs
- Barbiturates, benzodiazepines → should be administered according to actual weight
- Lipid insoluble drugs should be dosed according to ideal weight
- Increased volume of distribution of lipophillic drugs
- Decreased fractional proportion of total body water
- Increased blood volume and cardiac output
- May require increased loading dose for therapeutic effect (thiopentone)
- Displacement of protein bound drug due by fatty acids (propofol)
Metabolism
- Decreased hepatic function due to non-alcoholic steatohepatitis
- Increased T1/2b of drugs metabolized by liver
- Increased plasma and tissue esterases
- Increased metabolism of suxamethonium, remifentanyl
Excretion
- Increased cardiac output
→ Increased renal blood flow
→ Increased GFR
→ Increased tubular secretion
→ Increased renal clearance - Increased T1/2b due to accumulation of lipid soluble drugs in fat, and slow redristribution into central component
- Propofol, volatile anaesthetics
Sakurai 2016
Examiner Comments
2012B 13: 8 (36.4%) of candidates passed
This question could be approached by describing the effects of obesity on drug distribution, binding and elimination. Candidates that took this approach generally did better than those with a less structured approach. With obesity, fat body mass increases relative to the increase in lean body mass leading to an increased volume of distribution particularly for highly lipid soluble drugs, e.g. midazolam. However, the dosing of non-lipid soluble drugs, e.g. non-depolarising muscle relaxants, should be based on ideal body weight. An increase in blood volume and cardiac output associated with obesity may require an increased loading dose to achieve a therapeutic effect, e.g. thiopentone. Plasma protein binding of drugs may be decreased due to an increased binding of lipids to plasma proteins, resulting in an increased free fraction of drug. A reduction in plasma protein concentration due to an increase in acute phase proteins may also result in decreased plasma protein drug binding and increased free fraction of drug. Pseudocholinesterase levels are increased in obesity and therefore the dose of suxamethonium should be based on total body weight. Plasma and tissue esterase levels are increased resulting in the increased clearance of drugs by these enzymes e.g. remifentanil. Hepatic clearance is usually normal but may be impaired in liver disease caused by obesity. Renal clearance is usually increased due to increased body weight, increased renal blood flow and increased glomerular filtration rate. Renal clearance may be impaired in renal disease caused by obesity related diseases, e.g. diabetes. Insulin doses may be increased due to peripheral insulin resistance in type 2 diabetes caused by obesity. Most answers were deficient in examples of drugs to illustrate the effects of obesity on drug pharmacology
14. Statistics (not in current primary syllabus)
15. Outline the respiratory and cardiovascular consequences of an acute complete spinal cord transection at C6.
CICMWrecks Answer
Cardiovascular
Innervation
- ANS
- Afferents
- Visceral afferent nerves from the heart via the vagal nerve to the NTS
- Baroreceptors via vagal (aortic arch) and glossopharyngeal (carotid sinus)
- Sympathetic system
- Efferents to the heart from the post-ganglionic sympathetic fibres synapsing from T2~5 pre-ganglionic sympathetic fibres
- Positive inotropy, chronotropy, lusitropy and dromotropy
- Efferents to vascular smooth muscle via pre-ganglionic fibres from the thoracolumbar spinal roots synapsing on the sympathetic chain
- Parasympathetic system
- Efferents from the vagal nerve
- Negative inotropy, chronotropy, lusitropy and dromotropy
- Unknown significance of muscarinic innervation of the vasculature
Effects of transection at C6
- Immediate
- Sympathetic stimulation from catecholamine release from adrenal medulla
- Hypertension
- Tachycardia
- Sympathetic stimulation from catecholamine release from adrenal medulla
- Acute
- Afferents from heart and baroreceptors in tact
- Central processing of vasomotor centre in tact
- Efferent sympathetic innervation to heart will be lost
- Resting sympathetic tone of heart lost
- HR will decrease from ~60 to 50bpm
- Baroreceptor response to hypotension will be lost
- No reflex tachycardia or positive inotropy
- Resting sympathetic tone of heart lost
- Efferent sympathetic innervation to vasculature will be lost
- Resting sympathetic tone of blood vessels lost → vaso and venodilatation → decreased MAP and decreased venous return
- Decreased cardiac output
- Decreased baroreflex vasoconstriction in response to hypotension
- Resting sympathetic tone of blood vessels lost → vaso and venodilatation → decreased MAP and decreased venous return
- Efferent parasympathetic innervation in tact (via vagus nerve).
- Parasympathetic tone of resting heart rate will remain
- Reflex negative inotropy and chronotropy in response to hypertension will remain
Respiratory
Innervation
- Afferents from central and peripheral chemoreceptors
- Meduallary respiratory centre
- Sympathetic
- Unknown innervation and significance although β2 adrenoceptors are present within respiratory system
- Postulated response to circulating catecholamine from adrenal medulla
- Parasympathetic
- Vagal innervation of respiratory system causing bronchoconstriction and mucous secretion
- Motor innervation of respiratory muscles
- Phrenic nerve – C3~5
- Diaphragm
- Thoracic spinal nerve roots
- Intercostal muscles
- External (inspiration)
- Internal (expiration)
- Innermost
- Intercostal muscles
- Accessory muscles of respiration
- Accessory nerve innervation of sternocleidomastoid and trapezius
- Brachial plexus
- Pec. major/minor
- Nerve to subclavius
- Serratus anterior
- Thoracic spinal roots
- External, internal, innermost intercostal muscles
- Abdominal wall muscles
- Phrenic nerve – C3~5
Effect of C6 transection
- Sympathetic
- Decreased adrenaline secretion from adrenal medulla → decreased circulating adrenaline → unopposed muscarinic stimulation of lung → increased likelihood of bronchoconstriction
- Vasodilation leading to pooling of blood in pulmonary circulation → decreased lung compliance
- Parasympathetic
- Innervation will remain → bronchoconstriction
- Motor innervation
- Phrenic nerve function in tact
- Diaphragm – Tidal inspiration will largely remain
- Intercostal muscle function lost
- External – Loss of elevation of ribs to increase AP diameter – required in inspiration
- Internal – Loss of lowering of ribs to decrease AP diameter – required in expiration
- Accessory muscles
- Variable effects
- Sternocleidomastoid and trapezius function in tact (forced inspiration)
- Scalenus anterior, medius, posterior in tact (forced inspiration)
- Pec. major and minor in tact (forced inspiration)
- Abdominal muscle function lost
- Required in forced expiration, cough
- Variable effects
- Loss of expiratory reserve volume
- Decreased vital capacity
- Phrenic nerve function in tact
Sakurai 2016
Examiner Comments
2012B 15: 9 (41%) of candidates passed.
The main respiratory consequences of an acute C6 transection include the effects on the inspiratory muscles, the expiratory muscles, lung volumes, effects of changes in posture and effects on gas exchange. Sparing of the phrenic nerve, the main muscle of inspiration (C3 – 5), but paralysis of the external intercostal muscles innervated by thoracic nerve roots results in paradoxical inward movement of the chest wall on inspiration. Paralysis of all the expiratory muscles including the internal intercostal muscles innervated by thoracic nerve roots and the abdominal wall muscles, which are innervated by lower thoric and lumbar nerves. Many candidates did not mention these muscles or their innervation in their answers. While expiration is normally passive these muscles are required for manoeuvres involving forced exhalation like coughing. Forced expiratory lung volumes (FEV1 and FVC) are reduced. Work of breathing is increased. Static lung volumes reveal a restrictive lung defect with most lung volumes decreased but in particular expiratory reserve volume (ERV) is significantly reduced. The reduction in FRC leads to airway closure, atelectasis and pathologic low V/Q and shunt and hence hypoxemia. These mechanisms can result in significant hypoxemia but were not described by many candidates. The second part of the question concerning the cardiovascular consequences of C6 transection was better answered. Areas that required mention in this section included the early massive sympathetic outflow and hypertension via the release of catecholamines from the adrenal medulla. Neurogenic shock is also seen due to interruption of the sympathetic outflow and impaired reflex vasoconstriction secondary to hypotension of any cause. Finally the loss of sympathetic innervation of the heart (T1-T4) results in unopposed parasympathetic cardiac stimulation and bradycardia and bradyarrthymias.
16. List the constituents of plasma and the functions of plasma proteins.
CICMWrecks Answer
Plasma
- Plasma is the liquid (non-cellular) component of blood, in which the red blood cells, white blood cells, and platelets are suspended.
- 93% water, 6% proteins and 1% other solutes
- Makes up 60% of blood volume
- Makes up 18% of extracellular fluid (or 5% of bodyweight)
Plasma Constituents
- Water
- Proteins
- Albumin (60%)
- Globulins (35%)
- α1 (α1 anti trypsin, α1-fetoprotein, serum amyloid A)
- α2 (haptoglobin, ceruloplasmin, Protein C, thyroxin-binding globulin)
- β (transferrin, plasminogen, β2 microglobulin, C-reactive protein)
- γ (immunoglobulins)
- Fibrinogen (4%)
- Regulatory proteins (<1%)
- Coagulation factors
- Complement proteins
- Other Solutes:
- Nutrients – vitamins, glucose
- Gases – oxygen, CO2, nitrogen
- Hormones
- Electrolytes – Na, K, Cl, Mg
- Lipids and other products of metabolism – urea, creatinine, nitrogenous wastes
Functions of Plasma Proteins
- Maintenance of fluid compartments/oncotic pressure
- starling forces, gibbs-donnan control bulk flow.
- Carrier/transport functions
- transferrin → Fe2+; albumin → free fatty acids.
- Role in acid-base balance and CO2 transport
- buffer e.g. carbamino compounds.
- Immunity
- antibodies e.g. IgG.
- Proteolytic enzymes
- complement, kinins, coagulation, fibrinolytic system.
- Metabolism of xenobiotics
- e.g. plasma cholinesterase.
- Anticoagulant proteins
- vWF; antithrombin III.
Functions of Albumin
- Osmotic pressure
- supplies 80% of total plasma Colloid Osmotic Pressure
- retards fluid efflux from plasma and oedema formation
- Transport and metabolism functions
- Transports thyroid hormones
- Transports other hormones, in particular, ones that are fat-soluble
- Transports fatty acids (“free” fatty acids) to the liver and to myocytes for utilization of energy
- Transports unconjugated bilirubin
- Transports many drugs; serum albumin levels can affect the half-life of drugs
- Extra-cellular acid-base buffer
- Detoxification
- solubilizes bilirubin and neutralizes its toxic effects
- Anti-oxidant effects
- Blocks Cu2+-mediated LDL oxidation
- Blocks Free-radical-mediated haemolysis
- anticoagulant effect
- protein store – for signalling molecules and nitric oxide
- Immunomodulation
- Other
- Competitively binds calcium ions (Ca2+)
- Prevents photodegradation of folic acid
- marker of an inflammatory state
- prevents apoptosis of proximal renal tubular cells
- stimulates proliferation of proximal renal tubular cells
JC / Gladwin 2020
Examiner Comments
2012B 16: 15 (68.2%) of candidates passed.
This question was generally well answered. The constituents of plasma include water, electrolytes, glucose, liver enzymes, urea, creatinine, uric acid, dissolved gases and proteins. Plasma does not contain any cells. The proteins in plasma are albumin, globulins and fibrinogen. The globulins include alpha 1 and 2 and beta globulins and gamma globulins. Examples of α1- Globulins are: α1-fetoprotein, α1-protease inhibitor and prothrombin. Examples of α2-Globulins include: ceruloplasmin, haptoglobin, α2-macroglobulin and thyroxin-binding globulin. Examples of β-Globulins are: C-reactive protein, β2-microglobulin and transferrin. Examples of δ-Globulins are the immunoglobulins , IgG, IgA, IgM, etc. There are many more other globulins including the coagulation factors, the complement system and lipoproteins. The functions of plasma proteins include oncotic pressure, transport/carrier function, role in acid base balance (buffering, CO2 transport) and proteolytic systems such as complement, kinins, coagulation and fibrinolysis. More functions include the immune response, enzyme activity eg pseudocholinesterase, metabolism i.e. plasma proteins can be broken down and contribute amino acids to the amino acid pool and a role in thermoregulation. Many answers were deficient in details on the plasma proteins and their functions. The question asked to “list” the constituents, so the level of detail required to score marks reflected this and should have been achievable in the allocated timeframe.
17. Classify the 5HT receptors and give examples of pharmacological agents that affect them (60% of marks). Outline the pharmacology of ondansetron (40% of marks).
CICMWrecks Answer: 5HT receptors and agents
5HT Receptors
5-HT receptor subtypes comprise the largest known neurotransmitter-receptor family.
- 4 5-HT receptor families recognized
- All members of GPCR superfamily except 5HT3
- 5HT3 is a ligand gated ion channel that conducts Na and K
- Overall effects
- CNS
- Vasoconstriction
- Increased inotropy and chronotropy
- Resp
- Bronchial smooth muscle constriction
- CNS
- Sleep-wake cycle
- Temperature regulation
- Appetite
- Mood
- Impulsivity
- Anxiety and Depression
- Sexual behaviour
- CNS
| Mechanism | Sites of Action | Effect | Drugs | |
|---|---|---|---|---|
| 5HT1 | – GiPCR – Some subtypes also act as ligand gated ion channels | Inhibitory autoreceptor on serotonergic cells Endothelium | – Inhibition of serotonin secretion – Endothelial No release | Agonist – Sumitriptan (migraine) – Ergotamine – LSD |
| 5HT2 | Gq or G11PCR | CNS – Pre-frontal – Parietal – Somatosensory – Choroid plexus GI Tract Platelets | – Platelet aggregation and vasoconstriction | Agonist – LSD Antagonist – Clozapine – Risperidone |
| 5HT3 | Ligand gated Na and K channel | CNS – Area Postrema (CTZ) – NTS (Vomiting centre) GI Tract – Parasympathetic nerve terminals | Emesis | Antagonist Ondansetron |
| 5HT4 | GsPCR | CNS GI Tract – Myenteric plexus – Secretory cells | GI peristalsis and secretion |
- Additional receptors cloned
- 5HT5~7 (GsPCR) Unknown clinical relenvance
- Other drugs with effects on serotonergic system
- Increase serotonin release
- MDMA
- Decrease serotonin reuptake
- Tramadol
- 1R2R(+) Tramadol
- SSRI
- Tricyclic Antidepressants
- Tramadol
- Decrease serotonin metabolism
- MAO Inhibitors
- Increase serotonin release
Mooney 2016
CICMWrecks Answer: Pharmacology of Ondansetron
Pharmacology of Ondansetron
Examiner Comments
2012B 17: 10 (45.4%) of candidates passed.
The 5HT (5 Hydroxytryptamine or serotonin) receptor is a monoamine neurotransmitter synthesized from tryptophan, and is an important receptor in the body.. It is found in the CNS, gastrointestinal tract, platelets and mast cells. There are 7 main receptor subtypes (GProtein coupled are 5HT1, 5HT2, 5HT4 and 5HT7 and ligand-gated ion channel 5HT3). Drugs may affect them by acting on serotonergic transmission (degradation inhibitors – MAOI e.g. selegiline, storage inhibitors- amphetamine, reuptake inhibitors – SSRI’s or tricyclic antidepressants), serotonin agonists (selective-5HT1B, 5HT1D e.g. triptans for migraine, non-selective, e.g. ergotamine), serotonin antagonists (ketanserin, clozapine, ondansetron).
Ondansetron is a commonly used anti-emetic. Candidates were expected to mention, that it is a selective antagonist at the 5HT3 receptor centrally and peripherally. To outline “pharmacology” it was also expected that answers would mention that it comes in a variety of formulations and to outline its fundamental pharmacokinetic properties.
18. List the properties of the ideal inotrope (50% of marks). How does adHine compare with Fect to these ideal properties? (50% of marks)
CICMWrecks Answer
An Ideal Inotrope is pharmaceutically suitable, has beneficial pharmacodynamic properties, has an excellent pharmacokientic profile.
PHARMACEUTIC
| IDEAL INOTROPE | ADRENALINE |
|---|---|
| Non-toxic | Y |
| Cost effective | Y |
| Stable preparation | Y |
| Compatible with other drugs | Y |
| Peripherally deliverable | Y |
| Multiple preparations | Y |
| Stable in all solutions | N (Oxidises to adenochrome in alkaline solutions, turns pink) |
PHARMACODYNAMIC
| IDEAL INOTROPE | ADRENALINE |
|---|---|
| Increases contractility | Y |
| Increases mean arterial pressure, Maintenance of diastolic blood pressure | Y |
| Increases cardiac output | Y |
| Improves regional perfusion, without pharmacodynamic costs | N |
| No increase in myocardial oxygen consumption | N (increases myocardial oxygen consumption) |
| Avoidance of tachycardia, Non-arrhythmogenic | N (causes tachyarrhythmias) |
| Suitable in pregnancy and paediatric populations | Y |
| No Adverse effects | N (hyperglycaemia, lactic acid production, worsens pulm HTN, Peripheral necrosis) |
PHARMACOKINETIC
| IDEAL INOTROPE | ADRENALINE |
|---|---|
| Does not develop tolerance | N |
| Titratable | Y |
| Rapid onset, Rapid termination of action | Y |
| Metabolised independent of liver and kidney function | N (some hepatic metabolism) |
| Doesn’t require concentration monitoring | Y |
JC 2019
Examiner Comments
2012B 18: 19 (86.4%) of candidates passed.
Inotropes are drugs that increase the force and velocity of myocardial contraction resulting in increased contractility and stroke volume and hence cardiac output. Good answers were those that adopted a systematic approach, such as providing a coherent list of ideal properties that included pharmaceutical, pharmacokinetic and pharmacodynamics characteristics, and then contrasted adrenaline against that list. The area less well covered was that of those aspects of adrenaline that made it less than an ideal inotrope, e.g. it increases myocardial oxygen consumption, causes tachyarrhythmias, tolerance may develop, hyperglycaemia, lactic acid production, etc.
19. Describe the changes that occur in the plasma with renal dysfunction.
CICMWrecks Answer
Water
- Hypervolaemia may occur if oliguric renal failure
Electrolytes
- Renal failure can retard the kidneys ability to eliminate excess potassium
- Hyperkalaemia
- Sodium relatively preserved
- Acid-Base balance
- Approx. 80mEq/day of non-volatile acids require elimination daily
- Renal failure leads to progressive acidosis due to loss of buffering ability and limiting urinary pH of 4.5
- Loss of renal compensation of respiratory acid/base disturbances
- Approx. 80mEq/day of non-volatile acids require elimination daily
- Renal conversion of 25-hydroxy Vit.D to 1,25,-dihydroxy Vit D promotes GI reabsorption of Ca à lost in renal dysfunction leading to hypocalcaemia and hypophosphataemia
Proteins
- Damage to the glomerular basement membrane (Goodpasture’s Disease, other causes of nephrotic syndrome) can lead to renal elimination of protein and loss of plasma proteins due to increased glomerular filtration
- The kidney’s eliminate nitrogenous waste products of protein metabolism (urea), and uricaemia occurs in renal failure
Other
- Creatinine
- Metabolite of creatine phosphate, generated at constant rate, and eliminated renally. Increases in renal failure
- Only after 75% loss of functional nephrons
- Decreased production of Erythropoietin
- Parathyroid hormone increased due to hypocalcaemia
Sakurai 2016
Examiner Comments
2012B 19: 9 (41%) of candidates passed.
A good answer required an integrated knowledge of various aspects of basic physiology. Most often there was a lack of breadth and/or depth of knowledge (e.g. mention that plasma creatinine increases, but failure to mention that it only increases after substantial (>75%) loss of nephron function). It was expected that some mention of changes in electrolytes (e.g. Na+, K+, Ca2+), HCO3, PO4, hormones (1, 25 vitamin D, erythropoietin), proteins, etc. be included.
20. What are drug enantiomers? (20% of marks).
Explain the clinical relevance of enantiomerism (60% of marks).
Give a clinically relevant example (20% of marks).
CICMWrecks Answer
Enantiomers
- Molecules which have the same bond structure but are mirror images of each other.
- These molecules rotate light in opposite directions:
- (S)-(+)-naproxen is used to treat arthritis pain
- (R)-(–)-naproxen causes liver poisoning with no analgesic effect.
- (S)-(+)-warfarin, metabolised by CYP2C9, 3-5 times more potent
- (R)-(–)-warfarin, metabolised by CYP2C19, CYP1A2, CYP3A4
- These are isomers which share pharmachemical properties (boiling point, density…) and chemical formula but different 3-D structural arrangement.
- If action depends on pharacochemical properties then it is shared between enantomes
- If action depends on binding to specific sites then it can be markedly different between enantomers.
Enantomers are a subclass of Sterioisomers which are molecules with the same bond structure, different 3D arrangement.
- Sterioisomers have a singel chiral center and can be either:
- Geometric
- Optical or enantomeric
- Diastereoisomers have more than one chiral center and can be either:
- Geometric
- Optical non-enantomeric
Gladwin 2016
Examiner Comments
2012B 09: (41%) of candidates passed.
Enantiomers refer to isomeric molecules with centres of asymmetry in 3 dimensions that are mirror images of each other but not superimposable. Enantiomers may be distinguished by the direction in which polarised light is rotated. Interactions involving weak drug-receptor bonds feature a dependence upon recognition of shape, i.e. stereochemical structure is often important. Frequently one enantiomer may bind to a given receptor more avidly than the other, thus pharmacodynamics, pharmacokinetics and toxicity may vary between enantiomers. Many drugs are supplied as racemic mixtures, the components of which have different activity. Clinically relevant examples that candidates could have mentioned, included bupivacaine, ropivacaine, ketamine and carvedilol.
21. Describe the physiological consequences of a progressive rise in blood carbon dioxide levels.
CICMWrecks Answer
Normal Ranges
- Normal 35 < PaCO₂< 45mmHg
- Moderate hypercaponea 45 < PaCO₂< 80mmHg
- Severe hypercaponea 80mmHg < PaCO₂
Sensors
| Central Chemoreceptor (CCR) | Peripheral Chemoreceptor (PCR) | |
|---|---|---|
| Location | Below the ventral surface of the medulla Within the BBB | In Glomus cells of: • Carotid bodies: At the bifurcation of the common carotid arteries • Aortic bodies: Above and below the aortic arch. |
| Mechanism | CO2 rapidly diffuses into CSF • Converted to H+ by carbonic anhydrased • Δ ECF [H+] closes pH sensitive K-channels • ↑ ECF [H+] stimulates ↑ ventilation • CSF has: – Low CSF protein and HCO3 (c.f. plasma) – Poor buffering capacity – Thus ΔCSF pH per ΔPaCO2 is GREATER than that of blood | Type 2 cells: • ↓ PaO2, ↑ PaCO2 and/or ↓pH • ↓ IC [ATP] which leads to ↑ NT production and release • ↑ AP firing rate of AB or CB afferent fibres |
| Contribution to ↑MV | 80% | 20% |
| Response Speed | Fast | Relatively fast compared with CCR |
Physiological effects of Hypercaponea
Moderate 45mmHg < PaCO₂<100mmHg
- Resp
- CO₂ strongest determinant of ↑MV (2-3 L/min per mmHg)
- Hypoxaemia has a synergistic effect on hypercapnoeic-ventilatory drive
- CO₂ is a potent pulmonary vasoconstrictor → ↑ pulmonary vascular resistance
- CVS
- Direct depression of cardiac muscle and vascular SM
- Peripheral vasodilation → ↓ SVR and ↑ regional BF
- ↓ heart rate/contractility
- ↑ RV Afterload due to effect on PVR
- ↑ circulating catecholamines
- Direct depression of cardiac muscle and vascular SM
- Neuro
- ↑ CBF (4% per mmHg ↑PaCO2) → ↑ ICP
- Haeme
- Right shift in OHDC
Severe (PaCO₂ >100mmHg)
- SNS stimulation
- hyperkinetic circulation →
- ↑ C.O., ↑ BP and arrhythmias
- ↑ cardiac MRO2 demand
- ↓ cardiac blood supply
- “CO2 nacrosis”
- PaCO2 > 80 mmHg → ↓ CSF pH
- Disorientation, confusion, headache, unconsciousness
- Hypoxia due to displacement of O2 from alveoli (as per Alveolar gas equation) → further ↑MV as above
- Respiratory acidosis →
- ↑ PaCO2 overwhelms buffering capacity of blood
- ↑ serum K+ and Ca2+ (stewart)
- ↑PaCO2 can cause anuria
Gladwin 2016
Examiner Comments
2012B 21: 5 (22.7%) of candidates passed.
Candidates were expected to present a mechanistic description the neuro-cellular events following a rise in PaCO2 such as changes in H+ in CSF, stimulation of central and peripheral chemoreceptors and neural pathways that lead to stimulation of respiratory centre. A systematic approach to the question with in-depth details of direction and magnitude of physiologic changes were required. Most candidates presented graphs of cerebral blood flow and tidal volume changes with increasing PaCO2. Common omissions included other important points, such as the cardiovascular and respiratory effects of rising CO2 and the rightward shift of oxygen haemoglobin curve.
22. Describe the factors that increase the risk of systemic toxicity of the amide local anaesthetics
CICMWrecks Answer
Local anaesthetic
- Lipophilic group attached to ionized group via ester linkage (ester local anaesthetic) or amide group (amide local anaesthetic agents
- MoA
- Binds to and blocks volatage-gated Na channel on the inner surface of the cell membrane, inhibiting depolarization
- Increases threshold for depolarization
- Decreases impulse conduction
- Action potential abolished
- Higher affinity for open or inactive Na channels
- Hypercalcaemia stabilizes membrane (Na channels in rested state) reducing efficacy of local anaesthetics
- Hyperkalaemia depolarizes membrane (Na channels in open or inactive state) increasing the efficacty of local anaesthetics
Structure-Activity Relationship
- Ficks law of diffusion
- Membrane permeation
- Lipophilicity
- Small molecular weight
- Protein binding
- Can be displaced
- Membrane permeation
Toxicity
- CNS
- Paraesthesia in lips and tongue
- Convulsions
- Sedation
- Coma
- CVS
- Arrhythmia
Factors affecting local anaesthetic toxicity
Pharmacokinetics
A
- Dose
- Lignocaine 3~5mg/kg without adrenaline
- Ropivacaine 3mg/kg
- Bupivacaine 3mg/kg
- Route of administration
- IV
- Highly vascularized tissues
- Use of adrenaline decreases toxicity risk
D
- Lipophilicity
- pKa – Most LAs 7.9~8.4
- Low fraction of unionized drug
- pKa – Most LAs 7.9~8.4
- Size of drug
- Protein binding
- If high, less likely to cross BBB
- However if reaches cardiac myocytes, more likely to bind → prolonged effects
M and E
- Amide anaesthetic longer duration. Hepatic vs. ester metabolism
- Both renally excreted
Patient factors
- pH – will affect ionization state with acidosis reducing LA permeation, however once across the membrane, increased ionization increases LA binding to channel and effect
- Hepatic function
- Decreased metabolism à prolonged effect
- Decreased pasma protein à increased free fraction à increased effect
- Renal function
- Decreased GFR à decreased clearance à prolonged effect
Sakurai 2016
Examiner Comments
2012B 22: 4 (18.2%) of candidates passed.
The amide group of local anaesthetics consist of lignocaine, prilocaine, ropivacaine and bupivacaine. The systemic toxicity primarily relates to toxic plasma levels and the factors that influence this. The main factors expected can be categorized under drug factors (including kinetics), patient factors, site of injection and external factors. Many candidates omitted important details such as pKA, lipid solubility and addition of vasoconstrictors. For example, absorption is affected by drug pKA, (thecloser to physiological pH the more rapid the absorption), use of vasoconstrictors and the drugs own vasoactive properties, site of injection (intercostal>epidural>brachial plexus>subcutaneous infiltration). Distribution is dependent on physicochemical properties of the amide. The rate of metabolism, mechanism of action (bupivacaine, in comparison to lignocaine has stronger binding to inactivated resting sodium channels and a slower rate of dissociation) and external factors (e.g. systemic acidosis) are other factors that should have been mentioned, and expanded upon with relevant detail.
23. Outline the mechanisms that control regional skeletal muscle blood flow.
CICMWrecks Answer
- Normal blood flow to skeletal muscle ~1l/min
- Can increase 20-fold during exercise
Local factors
Acute (Autoregulation)
- Myogenic
- Stretch of vessel walls causes constriction due to stretch mediated Ca2+ release
- Prominent in small arterioles throughout body
- Prevents excessive increases in blood flow in response to pressure
- Sheer stress on vessels due to increased flow causes release of Endothelial Derived Relaxing Factors such as Nitric Oxide which dilates the vessel, increasing vessel diameter and reducing sheer stress
- Stretch of vessel walls causes constriction due to stretch mediated Ca2+ release
- Metabolic
- Increased concentration of metabolic byproducts such as CO2, lactate, adenosine and H+, K+, cause upstream vasodilation
- This diffuse through the interstitium to upstream pre-capillary sphincters, arterioles and meta-arterioles
- Due to alteration of vessel radius, flow increases, according to the Poisuille-Hagen Equation
- Conversely, presence of excess nutrients (and O2) leads to vasoconstriction and decreased flow
- Increased concentration of metabolic byproducts such as CO2, lactate, adenosine and H+, K+, cause upstream vasodilation
- Reactive hyperaemia
- During muscle contraction, interstitial pressure increases compressing blood vessels and decreasing flow
- On restoration of flow to the tissue, flow can increase 4~7 fold due to buildup of metabolites and autoregulatory mechanisms
- During muscle contraction, interstitial pressure increases compressing blood vessels and decreasing flow
- Active hyperaemia
- During exercise, due to the consumption of nutrients and production of metabolites, blood flow increases due to autoregulation
Long term
- Arterioles and vessels increase in size and number due to increased metabolic requirements of a tissue and chronic hypoxia
- Important factors
- VEGF
- Angiogenin
- Important factors
Neural factors
- Sympathetic
- Sympathetic α adrenergic stimulation causes vasoconstriction (integral in baroreceptor response)
- Resting sympathetic tone contributes significantly to peripheral vascular resistance
- Sympathetic cholinergic vasodilator supply under cortical control
- Sympathetic α adrenergic stimulation causes vasoconstriction (integral in baroreceptor response)
Hormonal factors
- β2 mediated vasodilation in response to adrenaline
- α1 mediated vasocontriction at higher concentrations
Sakurai 2016
Examiner Comments
2012B 23: 4 (18.2%) of candidates passed.
Candidates were expected to present the cellular mechanisms underlying the control of skeletal muscle blood flow. Many candidates correctly identified a role for sympathetic nervous system, metabolic (e.g. vasodilator metabolites such as CO2, H+, K+, lactate and adenosine), vasoactive substances released by endothelium (nitric oxide, prostacyclin, endothelin 1, etc.) and autoregulatory control but failed to present any details of direction and magnitude of control. Better answers also mentioned humeral (e.g. catecholamines, vasopressin, ANP, angiotensin II, histamine, serotonin, etc.) or myogenic control (i.e. when the pressure within a smooth muscle blood vessel is suddenly increased, the vascular smooth muscle is stretched.
24. Compare and contrast peptide and steroid hormones. Give four examples of each.
CICMWrecks Answer
| Peptide Hormone | Steroid Hormone | |
|---|---|---|
| Example | Anterior Pituitary Hormones (FLAGTOP) FSH, LH, ACTH, GH, TSH, Oxytocin, Prolactin | Cortisol, Aldosterone, Oestrogen, Progesterone, Testosterone |
| Structure | Multiple amino acid residues (3-200) Charged surface structure | Lipid soluble Sterol Ring structure |
| Precursors | Amino acids Cleaved from prehormones to prohormones to hormones | Cholesterol |
| Synthesis | Transcription from DNA/RNA | |
| Storage | Secretory Vesicles | Minimal storage Normally mobilised from cholesterol esters in cytoplasm as required |
| Transport | Dissolved in Plasma | Bound to plasma proteins |
| Target | Cell surface receptors | Cytoplasmic and nucleic receptors which results in effectors in the nucleus |
| MOA | Via action of 2nd messenger systems | Bind to DNA and intracellular pathway mRNA and alter transcription |
| Onset | Rapid | Slow |
| Offset | Rapid | Prolonged |
Gladwin 2016
Examiner Comments
2012B 24: 3 (13.7%) of candidates passed.
Most candidates used a table format to present their answer. Most also were able to give examples of peptides and steroids hormone. A common omission was an explanation of the differences in mechanism of action and different mediators. Better answers were able to compare and contrast aspects such as structure, synthesis, precursor, storage, transport and kinetics. Examples of peptide hormones include the anterior and posterior pituitary hormones, Parathyroid hormone, calcitonin, etc. Examples of steroid hormones are the adrenocortical hormones (e.g. glucocorticoid, mineralocorticoids, androgens), sex hormones and 1, 25 di OH vitamin D.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Hypoxia vs hypoxaemia, clark electrode, physiological mechanisms of hypoxaemia, asthma, bronchodilators |
| G. CVS | Calcium , Verapamil |
| H. Renal | |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | Cerebral blood flow, regulation, propofol Sedatives and effect on Resp and CNS, Midaz vs haloperidol, term – “neuroleptic”, EEG |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | Liver, portal triad in acinus, liver blood flow, regulation, handling of amoonia, urea cycle, laxatives |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | blood products and transfusion, storage, TXA |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | |
| V. Obstetrics | ABG for pregnant woman at term. Resp changes, drug transfer across placenta, syntocine, ergotamine |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments