1. Outline the physiological detection and response to an acute decrease in PaO2
CICMWrecks Answer
Hypoxaemia
Hypoxaemia = Acute ↓ PaO₂ = (low partial pressure of oxygen in arterial blood)
Overall response: Hypoxaemia → ↑ peripheral vasosmotor tone, ↑rate and depth of respiration
Local response to ↓ PaO₂:
- ↑ local vasodilation → ↓ SVR and ↓ BP
- ↓ BP → Baroreceptors → SNS activation → ↑ HR and ↑ C.O.
- PaO2 < 50-60 mmHg
- → peripheral chemoreceptors stimulation (see below)
- → ↑ventilation
- If ↓ PaO₂ is Gradual ↑ MV PaO2 < 500mmHg,
- If ↓ PaO₂ is Rapid ↑MV PaO2 < 50 mmHg
- Severe hypoxaemia
- ↓ HR and BP → shock
- ↑ CBF (regardless of PaCO2)
Chemoreceptor (CR) Reflex:
Two types of CR:
- Central CR
- CR location: Below the ventral surface of the medulla
- Stimuli: Δ ECF [H+] whereby ↑ ECF [H+] stimulates ↑ ventilation
- Rapid Response:
- CO2 rapidly diffuses into CSF
- Low CSF protein and HCO3 (c.f. plasma)
- Poor buffering capacity
- ΔCSF pH per ΔPaCO2 is GREATER than that of blood
- Peripheral CR
- CR location:
- “Carotid bodies” (more important)
- at bifurcation point of common carotid arteries
- supplied by CN IX
- “Aortic bodies” (less important)
- above and below the aortic arch
- supplied by CN X
- “Carotid bodies” (more important)
- Composition:
- PCRs found in “Glomus cells”
- ↓ PaO2 – via inhibition of O2 sensitive K-channels
- ↑ PaCO2 and/or ↓pH – via effect on pH sensitive K-channels
- Type I cells (rich in NAd, DA, ACh)
- Hypoxia causes release of NTs
- NAd/ACh – ↑ AP firing rate of AB or CB afferent fibres
- DA – Damping of type 2 cell responses
- Hypoxia causes release of NTs
- Type II cells (rich in capillary supply)
- ↓ PaO2, ↑ PaCO2 and/or ↓pH
- ↓ IC [ATP] which leads to ↑ NT production and release
- ↑ AP firing rate of AB or CB afferent fibres
- PCRs found in “Glomus cells”
- Response:
- ↓’d perfusion → ↑[H]/↓PaO2 → CR stimulation
- Afferent signal → Sinus nerve (of herring) / Vagus Nerve → Chemosensitive area of medulla
- Vasomotor area → ↑ peripheral vasoconstriction
- Respiratroy center → ↑rate and depth of respiration → ↑ venous return
- CR location:
Chronic Response
- Adrenal medullary hypoxia → EPO
- ↑ erythropoiesis (chronic)
Gladwin 2016
Examiner Comments
2012A 01: 0 (0%) of candidates passed.
Candidates were expected to focus on the physiology (including mechanisms) involved with detection of hypoxia (e.g. peripheral chemoreceptor stimulation) as well as response. Examples of response included sympathetic stimulation, the respiratory centre and organ specific (e.g. cardiac, CNS, blood, cellular, etc) responses. This topic is well covered within a number of fundamental texts, in particular, Nunn’s Respiratory Physiology. In general candidates’ answers were not “broad” enough, lacked detail and were too focused on chemoreceptors only.
2. Outline the physiological consequences of therapeutic hypothermia at 32 degrees Celsius
CICMWrecks Answer
Hypothermia:
- Core body temperature < 35 degrees
- Mild: 32-35
- Moderate: 28-32
- Severe: < 28
Effects via system:
- Endocrine and metabolic consequences
- ↓ carbohydrate metabolism and hyperglycaemia
- ↓ drug metabolism and clearance
- ↑ protein catabolic state
- Impaired wound healing (due to catabolic state and wound vasoconstriction)
- ↑ stress response (steroids and catecholamine released)
- Altered Metabolism
- Shivering
- 5x ↑ general MRO2
- ↑hypoxaemia → risk of myocardial and cerebral ischaemia
- In absence of shivering
- general ↓MRO2 (by up to 50%)
- ↓BMR
- Shivering
- Haematological consequences
- ↑ hematocrit and blood viscosity
- Neutropenia and thrombocytopenia
- Coagulopathy and platelet dysfunction
- Respiratory consequences
- ↓ RR and medullary sensitivity to CO2 → ↓ MV (and in severe cases apnoea)
- Left-shift in Hb O2 dissociation curve
- Acid-base changes: alkalosis and hypocapnea
- Rise of pH with falling body temperature
- Fall of PaCO2 with falling body temperature
- Effects on gas solubility
- ↑ oxygen solubility and O2-haemoglobin affinity
- ↑ solubility of gases (incl volatiles)
- V/Q mismatching 2° to inhibition of hypoxic pulmonary vasoconstriction
- Bronchospasm and alterations to deadspace
- Cardiovascular consequences
- ↓ C.O. due to direct –ve inotropic effect of cold and ↑ afterload/SVR
- Tachycardia initially, then progressive bradycardia with ↑ cold
- QT prolongation and the J wave
- Arrhythmias – classically AF and VF
- Resistance to defibrillation
- ↑ SVR and MAP due to peripheral vasoconstriction
- Renal consequences
- “Cold diuresis” due to ↓ vasopressin synthesis
- Central nervous system effects
- Confusion and ↓ level of consciousness
- Shivering
- ↑ seizure threshold
- ↓ CBF
- Immunological consequences
- ↓ granulocyte and monocyte activity
Gladwin 2016
Examiner Comments
2012A 02: 0 (0%) of candidates passed.
It is well documented and often stressed that the Primary Exam is focused upon the basic sciences that underpin clinical Intensive Care. It will examine a candidates understanding of, for example, the physiology associated with a clinical circumstance. Many candidates discussed why we use therapeutic hypothermia after cardiac arrest rather than outline the physiological consequences of hypothermia at 32 degrees Celsius, for which they would not have scored any marks. A good answer was expected to outline changes in metabolism as well as specific organ responses such as cardiovascular (e.g. bradycardia; vasoconstriction; decreased cardiac output, etc), respiratory (decreased minute volume; haemoglobin-oxygen dissociation curve moves left; increased anatomical dead space; diminished HPV; increased pulmonary vascular resistance, etc), renal (e.g. diuresis, changes to GFR, etc) as well as other organs. Again candidates failed to synthesize a coherent and detailed answer.
3. Describe the pharmacology of phenytoin
Examiner Comments
2012A 03: 6 (60%) of candidates passed.
Many candidates scored well in this question by using a standardised approach such as: Pharmacoceutics, Pharmacokinetics and Pharmacodynamics. This topic is well covered in the reference texts and a high degree of content was expected.
4. Describe the factors that affect the flux of potassium across the cell membrane
CICMWrecks Answer
Potassium
- Predominany intracellular cation
- Total Stores: Approx 3200mmol (50mmol/kg)
- Key Functions:
- Main determinant of ICF osmolality and tonicity
- Responsible for RMP of excitable cells via Goldmann-Hodgkin-Katz due to ↑↑gK relative to other species
- Role in action potential → repolarisation phase
- Secretion of insulin and multiple other KATP dependant processes
- Regulation of IC processes (protein/glycogen synthesis)
- Involved in Na+/K+ ATPase in cell membranes
Potassium flux is dictated by
- Fick’s law of diffusion
- D proportional to Δ [K] → ↑/↓ ECF K → ↑/↓ diffusion
2. Nernst Equation
where
E is the equilibrium potential for the ion
R is the gas constant (8.314 J.K-1.mol-1 )
T is the temperature in Kelvin
F is Faraday’s Constant
z is the ionic valency (e.g. +2 for Mg2+, -1 for Cl–)
EK = -90 mV
ENa = +55mV
ECl = -65mV
- Temperature via the nernst equation → ↑ temperature → more negative V → increase potassium permeability → ↑ intracellular potassium
3. Gibbs-donnan
- In the presence of non-diffusable species the relative osmolarity drives flow of diffusable species.
- ↑ intracellular osmolarity or ↓ extracellular osmolarity → potassium efflux
4. Homeostatic processess
- Na/K ATPase
- ↑ intracellular K
- β adrenergic stimulation → ↑ Na/K ATPase activity
- Insulin release → ↑ Na/K ATPase activity
- ↑ intracellular K
- Acidosis:
- ↑ H/K antiporter activity →
- ↑ extracellular potassium
- Utilisation of intracellular protein buffer
- ↑ H/K antiporter activity →
- Hormonal factors
- Insulin → ↑ Na/K ATPase activity → ↑ intracellular K
- Aldosterone → ↑ Na/K ATPase activity → ↑ intracellular K
- Activity of ATP sensitive potassium channels
- Glucose (eg in pancreatic cells) → Diffuses into cells → ↑ ATP/ADP ratio → closure of ATP sensitive K channels → ↓ ECF K
- Gluagon → direct increase of cAMP production → ↓ ATP → ↑ gK → ↓ intracellular K
- Burns and UMN lesions
- upregulation of foetal/extrajunctional form of nAChRs
- Prolonged opening relative to foetal form → ↑ potassium efflux on depolarisation
Gladwin 2016
Examiner Comments
2012A 04: 0 (0%) of candidates passed.
Candidates were required to synthesize knowledge across a number of areas and have a good overview of the topic. This included the following – Insulin (acts to up-regulate Na/K ATPase activity promoting intracellular shift of potassium in adipose and muscle tissue); catecholamines (beta2 stimulation up-regulates Na/K ATPase activity promoting intracellular shift of potassium); aldosterone; pH (acidosis promotes H+/K+ exchange (via H+/K+ antiport), and reduces the activity of the Na K ATPase pump); osmolality (cellular dehydration increases intracellular K+ concentration promoting diffusion of potassium out of the cells); exercise; plasma potassium; temperature.
5. Compare and contrast the pharmacology of dexmedetomidine and propofol.
Examiner Comments
2012A 05: 7 (70%) of candidates passed.
A basic and fundamental pharmacology question which required candidates to present their answer in a coherent fashion (a table worked best) as well as demonstrate sufficient knowledge. The majority of candidates did so, and so scored well. Candidates tended to struggle with the pharmacokinetic properties of these drugs.
6. Describe the carriage of carbon dioxide (CO2) in blood
CICMWrecks Answer
CO2
- End-product of aerobic metabolism
- Produced almost entirely in mitochondria
- Normal production approx. 200ml/min
- Total CO2 in blood
- Venous 52mL/dL
- Arterial 48mL/dL
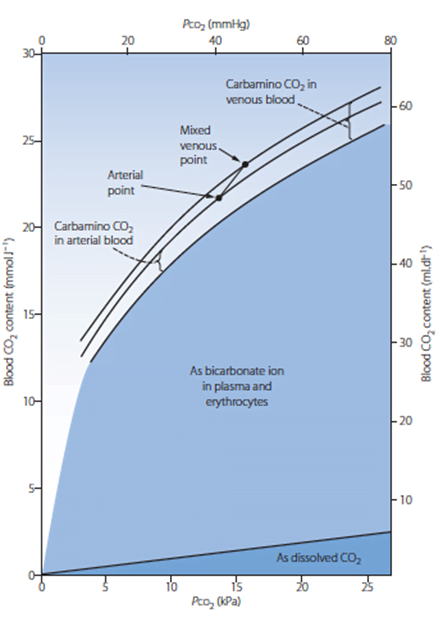
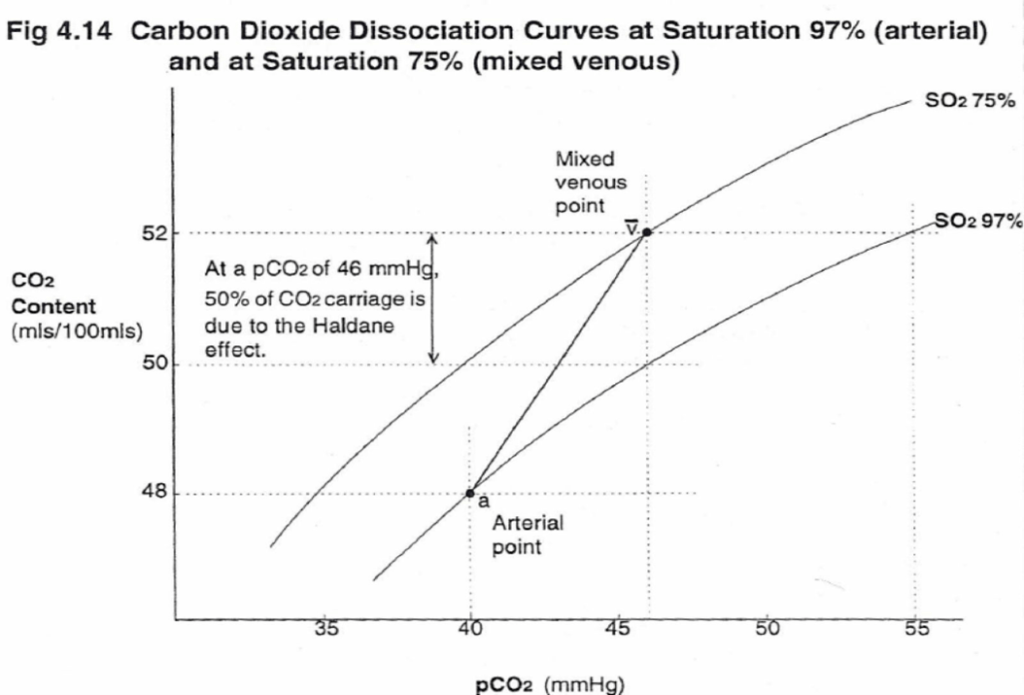
CO2 Carriage in 4 forms:
| Arterial % | % of A-V diff | Details | |
|---|---|---|---|
| 1. Bicarbonate | 90 | 60 | Main form of CO2 delivery in blood Via Hendersen-hasselbach: CO2 + H2O ⇔ H2CO3 ⇔ H+ + HCO3– Process 1. CO2 diffuses into RBCs 2. Carbonic anhydrase converts it to Bicarb 3. Hydrogen ions are buffered by binding to Hb molecules inside RBCs (30% Haldane.) Higher percentage in venous blood as: • Deoxygenated pKa 8.2 • Oxygenated pKa 6.6 • decreased pH = greater increase in [A-]/[HA] of DeoxyHb rel to OxyHb thus greater ability to accept protons 4. Bicarb is transferred out of cell in exchange for Cl ion by an exchange transporter called Band 3. (Hamburger Shift) |
| 2. Carbamino compounds | 5 | 30 | CO2 able to bind amino end of proteins Hb largely responsible as in much greater concentrations than any other protein (15 g/dL vs 7 g/dL) in serum CO2 combines with terminal amine to form carbamic acid dissosciates → at physiological pH to carbamate Affinity of Hb for CO2 increases in venous blood via Conformational change (70% Haldane): • Unbinding of O2 causes a conformational change in the beta chain • CO2 binding site at the N-terminus moves 1nm • Affinity for CO2 is increased. • Deoxy-Hb is 2-3x better at forming carbamino compounds than oxy-Hb |
| 3. Dissolved | 5 | 10 | CO2 ~20 x more soluble than O2 thus via Henry’s Law more CO2 is dissolved PvCO2 ~46mmHg (PaCO2 40mmHg) |
| 4. Carbonic Acid | <1% | <1% | Carried as dissolved H2CO3 Conversion to CO2 via Hendersen-Hasselbach equation above has pKa 6.1 At physiological pH Carbonic acid is ~96% dissociated Reaction is catalysed by Carbonic Anhydrase Not present in plasma, present in high concentrations in erythrocytes and pulmonary capilaries |
Gladwin / Sakurai 2016
Examiner Comments
2012A 06: 8 (80%) of candidates passed.
For a good answer candidates were expected to mention values for CO2 content in blood as well as the various ways it is carried (e.g. dissolved, as bicarbonate, combined with haemoglobin, etc) and a description of these modes. Wherever possible candidates are encouraged to illustrate their answer, in particular if those illustrations are core knowledge. Candidates who didn’t, were not penalised if they were still able to provide the required responses. However, candidates who did appeared to better synthesize a response. Candidates are reminded to include, and know, what are the appropriate units for any values they mention.
7. Compare and contrast the characteristics of B and T lymphocytes.
CICMWrecks Answer
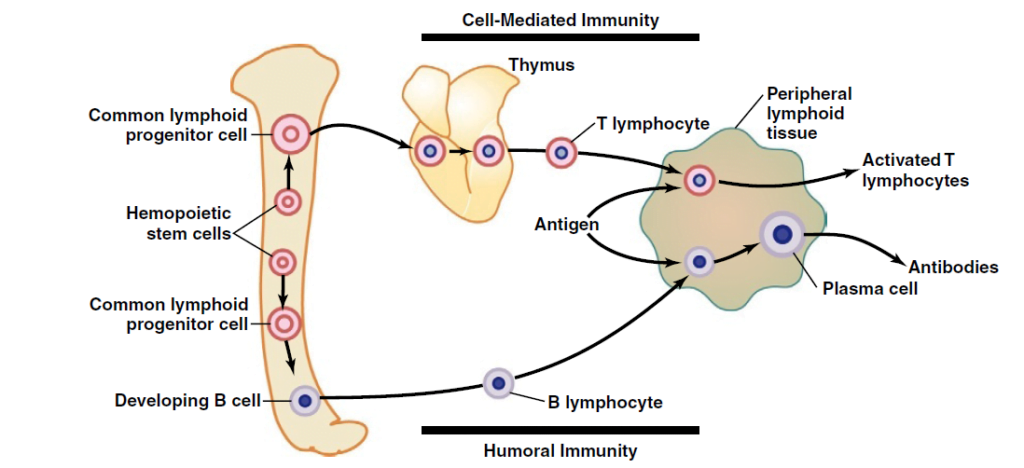
Lymphocytes are responsible for acquired immunity
Antigens on foreign material stimulate proliferation and immune response
- In T and B cells, there a millions of different clones which express different antigen binding site
- Each lymphocyte clone is highly specific for a particular antigen, and can only be activated by it
| T cell | B cell | |
|---|---|---|
| Cell mediated Immunity | Humoral Immunity | |
| Origin | Haemopoeitic stem cells in bone marrow → common lymphoid progenitor cell | Haemopoeitic stem cells in bone marrow → common lymphoid progenitor cell |
| Site of differentiation | Thymus | Liver in mid-foetal Bone marrow in late-foetal and after birth |
| Site of storage | Lymph (thymus, spleen, submucosa of GIT, bone marrow, lymph glands) | Lymph (thymus, spleen, submucosa of GIT, bone marrow, lymph glands) |
| Receptor | T cell receptor Only respond to antigens bound to antigen-presenting cell (macrophage) | B Cell receptor (Immunoglobulin molecules) Can bind to antigens directly |
| Mechanism of activation | Antigen binds to major histocompatability complex on 1) macrophages 2) B-lymphocytes 3) dendritic cells Antigen bound to MHC is sensed by surface receptor | Activated following T-cell activation |
| Type of immunity | Cell-mediated Cytotoxic T cells bind to pathogen (via antigen) → Release perforins → Punch holes in cell membrane → Also release cytotoxics into these holes | Humoral Antibody can cause: Precipitation Lysis Agglutination Neutralisation Antibody-antigen complex activates complement cascade → pathogen damage → recruitment of phagocytes and local inflammation |
| Derived cells | Helper T cells Encourage proliferation of cytotoxic and suppressor T cells Stimulate B-cell proliferation Attract and activate macrophages Cytotoxic T cells Suppressor T cells Suppress both cytotoxic and helper T cells Memory T cells Circulate until reactivation | Plasma cells Produce IgG antibodies and release them into lymph → blood Memory B cells Propogate and circulate to all lymph tissue, ready for reactivation |
| Function | produce antibodies that can bind to and neutralize extracellular pathogens or toxins | recognize and eliminate intracellular pathogens, such as viruses, or help other immune cells to do so |
| Lifespan of memory cells | Many years | 6-12 months (as B memory cells can reactivate them) |
Mooney 2016
Examiner Comments
2012A 07: 4 (40%) of candidates passed.
A structured response was required for this question. Candidates were expected to mention the origin of the cells, sites of differentiation (foetal liver, lymph nodes, bone marrow in early childhood for T lymphocytes and thymus for B lymphocytes), antigen receptors and what they bind with (highly specific IgM surface antibodies that bind with extracellualr antigens for T lymphocytes and surface receptors that bind to antigen presenting cells for B lymphocytes), type of activation cell, type of immunity (humoral for T lymphocytes, cell mediated for B lymphocytes), their functions and life span (T lymphocytes much shorter than B lymphocytes. Although some candidates found this question challenging, of those who passed it, most achieved a high score.
8. Compare and contrast adrenaline and levosimendan
Examiner Comments
2012A 08: 6 (60%) of candidates passed.
A basic and fundamental question which required candidates to present their answer in a coherent fashion (a table worked best), as well as demonstrate sufficient knowledge. The majority of candidates did so, and so scored well. Candidates tended to struggle most with levosimendan. Candidates also confused the use of the terms “elimination” and “metabolism”, often using them interchangeably.
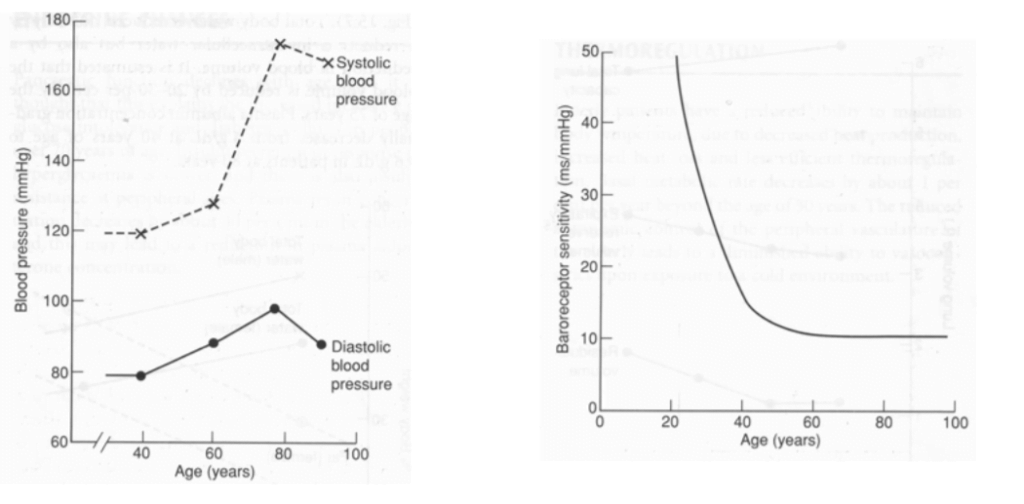
9. Describe the changes to cardiovascular physiology in a healthy elderly person.
CICMWrecks Answer
Ageing = physiological time-dependent process which results in ↓ cellular function + ↓ reserve
- Heart
- ↑ fibrous infiltration of myocardium → ↓ ventricular compliance (esp LV > RV)
- ↓ # myocytes but compensated by hypertrophy → ↑ myocardial wall thickness (concentric) → ↓ ventricular compliance (esp LV > RV)
- ↑ fibrous infiltration of endocardium → ↓ ventricular compliaince (esp LV > RV)
- Calcification of heart valves → valvular incompetence (stenosis/regurgitation)
- Conducting system (Ie. SAN, AVN, bundle of His, Purkinje):
- ↑ fibrous/fatty infiltration
- ↑ loss of pacemaker cells
- ↓ # and sensitivity of β receptors within pacemaker cells
- Peripheral vasculature:
- ↓ elasticity of large arteries due to thickening and calcification of vessel wall
| Change with ageing | Reason | |
|---|---|---|
| HR | Resting HR → unchanged ↓ maximum HR (Max. HR = 220 – age) ↑ arrhythmias (AF, AV heart block, VEBs, BBB) | Conduction system (SAN, AVN, bundle of His, Purkinje) → fibrous/fatty infiltration and loss of pacemaker cells ↓ # and sensitivity of β receptor of pacemaker cells |
| SV | ↓ resting SV ↓ maximum SV | ↓ ventricular compliance (2° to fibrous infiltration of myocardium/endocardium, and ventricular hypertrophy) Valvular disease |
| CO | ↓ due to preconditioning (Ie. sedentary lifestyle) or age-related disease Unchanged in “healthy” subjects Nb. ↑ C.O. occurs mainly by ↑ SV (preload) via Frank-Starling mechanism (rather than ↑ HR) | ↓ HR – see above ↓ preload (↓ SV) – see above ↑ afterload (↑ SVR) – see below ↑ reliance on “atrial kick” (30% of C.O. vs 5% in adults) → AF/↑HR not well tolerated |
| BP | ↑ MAP and SBP DBP ↑ slightly (until age 60), then ↓ ↑ pulse pressure (due to ↑ SBP > DBP) | ↓ elasticity of large arteries due to thickening and calcification of vessel wall |
| Pulmonary circulation | ↑ PAP a/w ↑ PVR | |
| Control of CVS | ↓ maximum exercise tolerance (due to age-related ↓ maximum HR, SV, C.O.) Impaired BRR → postural hypotension ↓ β receptor response of CVS to catecholamines (↓ # or affinity of receptor, ↓post-receptor signalling, ↓ myocardial contractile response with stimulation) ↑ vagal tone |
Bianca 2016
Examiner Comments
2012A 09: 1 (10%) of candidates passed.
It is clearly stipulated in the syllabus that candidates would be expected to understand physiology as it applies at the extremes of age. In the past, questions have been asked relating to foetal and neonatal physiology as well as for the elderly. This question was poorly answered as candidates lacked a detailed and coherent knowledge of this topic. For a good answer candidates were expected to at least mention the effects on the heart, e.g. increases in size due to concentric ventricular hypertrophy (LVH), hypertrophy of myocytes but a decrease in the number of myocytes, cardiac output decreases, the increase in cardiac output in response to severe exertion is attenuated, ventricular filling is particularly dependent on diastolic relaxation which is impaired, greater contribution of atrial contraction, increase in left ventricular afterload, effects on vasculature, e.g. intima and media thickening result in less distensibility, effects on endothelial function, e.g. nitric oxide release is decreased, effects upon autonomic and integrated responses, e.g. decline in receptor numbers, down regulation of post-receptor signalling and decreased receptor density and impaired baroreceptor reflexes.
10. Describe the anatomy of the neck and trachea relevant to the insertion of a tracheostomy
CICMWrecks Answer
Tracheostomy – insertion of a tube through the anterior portion of the neck into the trachea to facilitate ventilation
Trachea Course:
- Larynx connects to the superior part of the trachea at C6 into the thorax and terminates at the level of the sternal angle, where it divides into the right and left mainstem bronchi.
- Initially anterior, then moves posteriorly as it descends to move behind the sternal notch
Tracheal Structure:
- A fibrocartilaginous tube 10cm long, approx 5cm in neck
- Supported by incomplete cartilaginous tracheal rings, which keep the trachea patent.
- The tracheal rings are joined by fibroelastic tissue.
- They are deficient posteriorly where the trachea lies anterior to the oesophagus; the posterior gap is spanned by the involuntary smooth trachealis muscle
Relationships:
- Lateral – carotid sheaths (common carotid arteries, vagus and internal jugular veins), thyroid lobes, inferior thyroid arteries, recurrent laryngeal nerves
- Inferior to the isthmus of the thyroid gland are the inferior thyroid veins
- Posterior – oesophagus, vertebral column
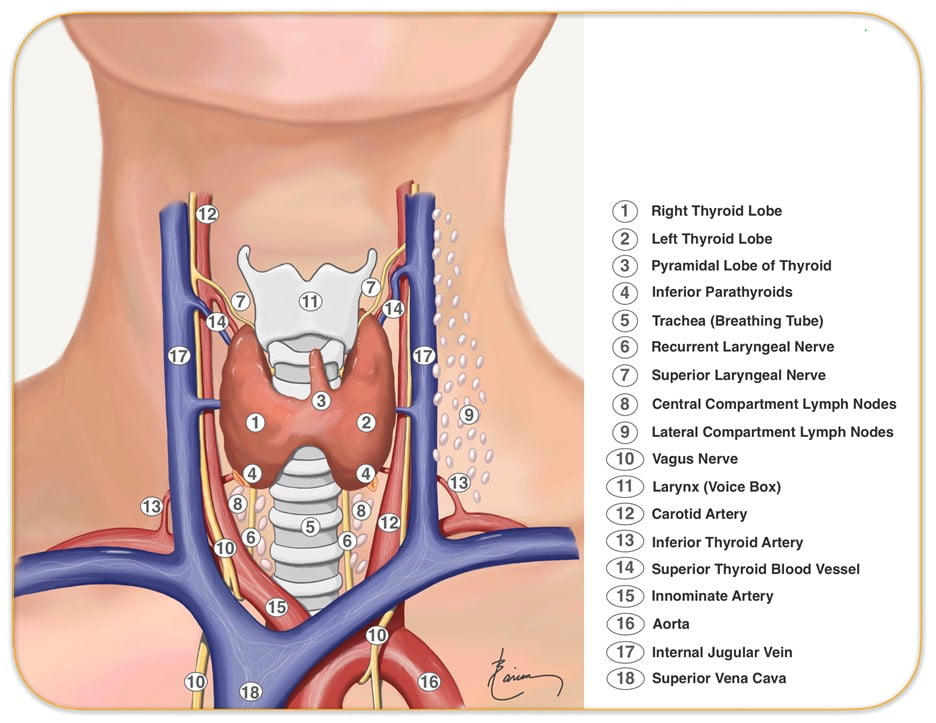
Relevant surface anatomy (in midline of neck):
- Hyoid bone (at level of C3)
- Thyroid cartilage
- Cricothyroid membrane
- Cricoid cartilage (at level of C6)
- Thyroid gland
- Sternohyoid muscle just lateral to midline structures, overlies sternothyroid and thyrohyoid muscles
Layers of dissection in tracheostomy:
- Skin
- Subcutaneous tissue
- Fat
- Pretracheal fascia (superficial and deep)
- Passage through the fibroelastic tissue in between the 1st and 2nd rings (common in perc trache) or 2nd /3rd or 3rd/4th (surgical trache)
- Trachea
Gladwin 2016
Examiner Comments
2012A 10: 4 (40%) of candidates passed.
For a good answer candidates were expected to mention surface anatomy of the anterior of the neck from the superior to inferior aspects (e.g. hyoid bone, thyroid cartilage,
cricothyroid ligament, cricoid cartilage and thyroid gland with sternohyoid muscle just lateral to the midline structures, the pathway of the trachea from anterior at level of larynx to more posterior as it enters the chest behind the sternal notch, nature of the tracheal rings (C shaped cartilages (first cartilage is bigger than the others in the cervical trachea) joined vertically by fibro-elastic tissue and connected posteriorly by the trachealis muscle, layers of dissection for tracheostomy (e.g. skin, subcutaneous tissue, fat, pre-tracheal fascia (superficial and deep), trachea and the relationship of thyroid to the trachea and surrounding vessels. There were some good answers amongst the successful candidates, whereas those who failed to pass this question did so because of a lack of detailed knowledge and relational anatomy. Candidates were not asked, and thus did not receive marks for, describing how to perform a tracheostomy.
11. Compare and contrast renal and hepatic blood flow and its regulation
CICMWrecks Answer
Blood flow
| Renal | Hepatic | |
|---|---|---|
| Blood Flow | 1.25l/min Highest blood flow per tissue mass | 1.5l/min Highest blood flow per organ |
| Afferent | Renal artery (from aorta) | – Hepatic artery (30%) from coeliac trunk – Portal vein (70%) from SMV, IMV and splenic veins – Hepatic artery supplies 50% of O¬2 |
| Course | Portal system → Afferent arteriole → glomerulus → efferent arteriole → peritubular capillary (inc. vasa recta) → renal vein | Via portal triad → hepatic sinusoids → central vein → hepatic vein |
| Efferent | Renal vein to IVC | Hepatic vein to IVC |
| Liver has blood reservoir function – 400ml |
Regulation
| Renal | Hepatic | |
|---|---|---|
| Hepatic arterial tone above sBP >80mmHg | ||
| Local | Myogenic mechanism – Stretch of afferent arterioles causes contraction decreasing blood flow Tubuloglomerular feedback – Na and Cl are sensed by the macula densa causing adenosine release – Adenosine constricts the afferent arteriole reducing renal blood flow | Myogenic autoregulation – Stretch of arterioles causes contraction decreasing blood flow Semi-reciprocal relationship between portal venous and hepatic arterial blood flow – Decreased blood flow via portal vein increases hepatic arterial blood flow |
| External – Hormonal | RAAS – Juxtaglomerular apparatus secretes renin in response to decreased tubular [Na] and [Cl], as well as to β stimulation – Angiotensin II constricts both afferent and efferent arterioles reducing renal blood flow | Adrenaline – Constricts portal vein and dilates hepatic artery – increasing hepatic artery contribution to blood flow Angiotensin II – Vasoconstricts both portal vein and hepatic artery decreasing hepatic artery blood flow Vasoactive intestinal peptide and secretin – Increase hepatic artery blood flow Glucagon – Increased hepatic blood flow |
| External -Neural | – Sympathetic stimulation constricts the renal afferent and efferent arterioles reducing renal blood flow | – Sympathetic (α adrenergic) stimulation by noradrenaline causes portal venous constriction and decreased hepatic blood flow |
| External -Starling resistors | – Increased intra-abdominal pressure will decrease blood flow in starling resistor model – Increased intra-capsular pressure will decrease renal blood flow | Respiration – Inspiration decreases hepatic venous blood flow |
| External – dietary | High blood amino acid/ glucose level – vasodilation and increased renal blood flow | Post-prandial – Increased splanchnic blood flow – increased portal venous blood flow |
Examiner Comments
2012A 11: 7 (70%) of candidates passed.
Another fundamental physiology topic that required candidates to understand, and synthesize knowledge from multiple areas. Generally well done with some very good answers. A tabular format worked well. Candidates were expected to mention values for renal and hepatic flow (total flow, % of cardiac output and oxygen consumption), basic anatomical comparisons, distribution (e.g. renal cortex 95% , renal medulla 5%,), two capillary beds (glomerular and peritubular) for renal, and hepatic triad and sinusoids for the liver, function (filtration for renal blood flow and metabolic activity for hepatic) and regulatory mechanisms for each (e.g. myogenic, autonomic, metabolic and humoral for both and tubuloglomerular feedback for renal).
12. Outline the pharmacology of vancomycin
Examiner Comments
2012A 12: 6 (60%) of candidates passed.
A basic and fundamental pharmacology question which required candidates to present their answer in a coherent fashion, as well as demonstrate sufficient knowledge. Candidates were expected to mention spectrum and mechanism of action, pharmacokinetics (including dose, distribution, elimination, etc) and adverse effects, activity profile e.g. time-dependent, antimicrobial activity depends on the duration that the serum drug concentration exceeds the minimum inhibitory concentration (MIC) of the target organism and not concentration dependence.
13. Draw (include labels and normal values) a normal spirometry trace in a young adult (20% of marks). Describe the changes seen in the spirometry, and the respiratory system overall, in pregnancy at term (80% of marks).
CICMWrecks Answer
Normal Spirometry trace
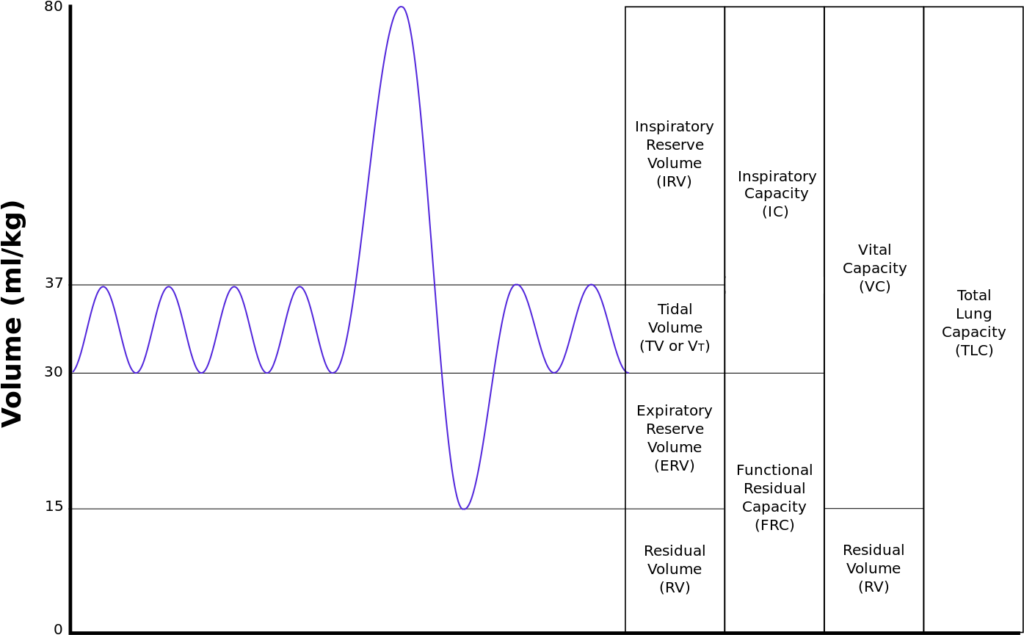
| Volumes | per kg | 70kg Adult |
|---|---|---|
| Inspiratory reserve volume | 45ml/kg | 3 L |
| Tidal volume | 7ml/kg | 500 ml |
| Expiratory reserve volume | 15ml/kg | 1.1 L |
| Residual volume | 15ml/kg | 1.1 L |
| Total Lung Capacity | 82ml/kg | 5.7 L |
| Vital Capacity | 67ml/kg | 4.6 L |
| Functional Residual Capacity | 30ml/kg | 2.2 L |
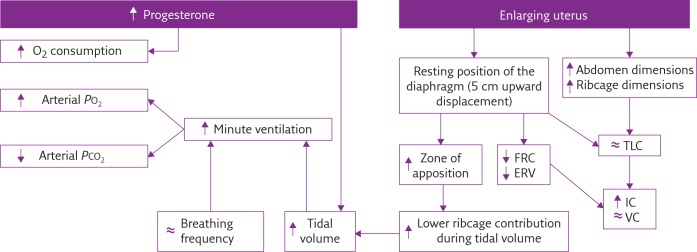
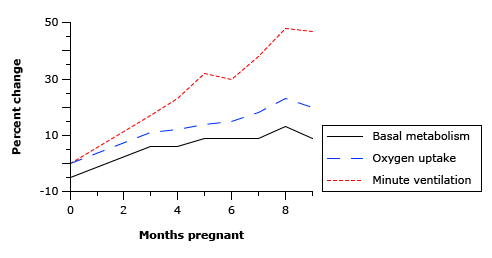
In Pregnancy
Anatomical
- Diaphragm ascends ~4cm due to foetus
- AP and lateral diameters of thorax increase 2~3cm due to relaxin effect on chest
wall - Thoracic circumference increases 5~7cm (AP and lateral diameters increased 2~3cm)
- Airway dilatation → decreased resistance (35%) and increased deadspace (45%)
- Increased cardiac output, intravascular volume, and decreased oncotic pressure
- Increased plasma volume → increased pulmonary blood volume → decreased compliance
- Venous engorgement → Upper airway oedema
Mechanical
- Lung volume changes occur from 20 weeks
- TLV decreases 5% from baseline
- FRC decreased 20% due to both ERV and RV
- Significant postural change in FRC of 70%
- Progesterone → increased sensitivity to CO2 → Minute ventilation increases 50%
- TV increases (25%)
- RR increases
- Results in hypocarbia and compensated respiratory alkalosis
- pCO2 ~ 26-32mmHg
- HCO3 ~ 18-20mmol/l
- Further increase in MV during contractions
- Inspiratory capacity increased by 10%
- Closing capacity unchanged
- Increased O2 consumption during labour (60%)
After birth
- FRC and RV return to normal within 48 hours
- TV returns to normal within 5 days
Sakurai 2016
Examiner Comments
2012A 13: 5 (50%) of candidates passed.
This question was generally handled well. Significant variation exists in normal values between textbooks, and examiners took account of this variation. Some candidates failed to provide any normal values or gave values not in accord with the traces they provided. Some candidates confused spirometry (volume/time) with flow volume loops or even a forced expiratory volume trace. These scored no marks. The second part was not well answered by some candidates with failure to describe changes – or even contradicting correctly graphed changes (for example the reduction in residual volume with pregnancy described as increased). Memorising curves needs to be accompanied by understanding. No marks were awarded for detailed discussion of the Haldane effect, as this was not asked. Candidates are reminded to read and answer the question.
14. What is an adverse drug reaction? Classify (with drug examples) the different types.
CICMWrecks Answer
Adverse Drug Reaction: An unwanted, unintended or harmful reaction as a consequence of drug administration
| Explanation | Example | |
|---|---|---|
| A. Augmented | • Predictable • Related to pharmacological action of drug • Common | Hypoglycaemia from insulin |
| B. Bizarre | • Unpredicatable • Not related to pharmacological action of drug • Uncommon | Anaphylaxis to penicillin |
| C. Chronic | Related to cumulative dose | Suppression of hypothalamic-pituitary-adrenal axis by chronic corticosteroid use |
| D. Delayed | • Becomes apparent some time after use of drug • Uncommon | Carcinogenesis Teratogenesis |
| E. Withdrawal | Occurs on withdrawal of drug treatment | • Opioid withdrawal • Angina from β blocker withdrawal |
| F. Failure | Unexpected failure of drug therapy | Clopidogrel in non-metabolizers |
Sakurai 2016
Examiner Comments
2012A 14: 1 (10%) of candidates passed.
This question was very poorly answered. Textbooks give different classifications so some largesse was allowed, but both immunologic and physicochemical (kinetic and dynamic) were expected. Marks were allocated for both type and an example – some candidates failed to provide examples or listed a drug side effect without explanation. Some candidates were ‘creative’ with classification systems but where appropriate examples were given marks were awarded. No marks were gained for detailed descriptions of LD50 and therapeutic ratios.
15. Briefly outline the production and fate of Red Blood Cells (RBC) (40% of marks). Describe the breakdown of haemoglobin (Hb) (60% of marks).
CICMWrecks Answer
RBC
Role
- Oxygen transport
- Carbon dioxide transport
- Bound to N-terminal of globin → carbamino-Hb
- Buffering function
- Imidazole group on Hb Histadine residues
- Iron storage
- 65-70% of total iron stores
Normal Values
- 40-50% of the blood volume
- Usual value 4-5 x 1012/L
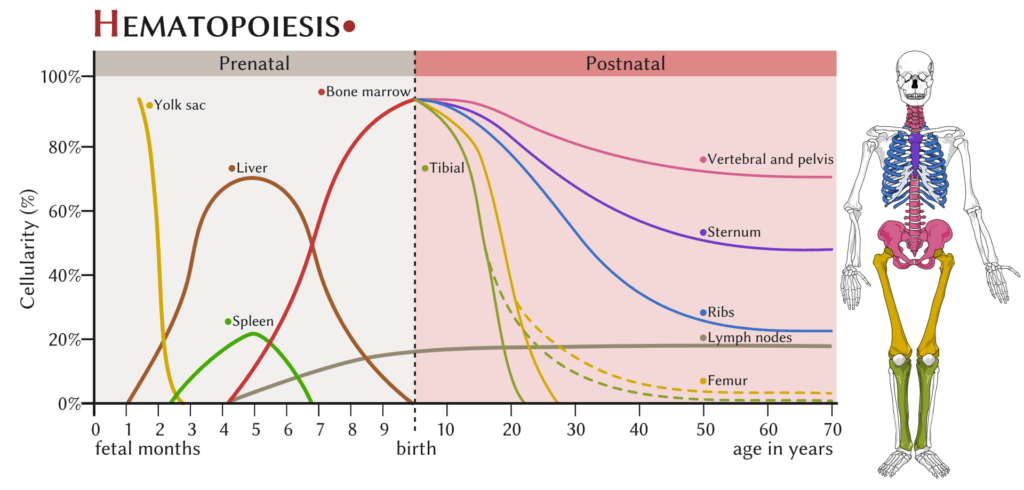
RBC Production
- 3-4w embryionic → Mesenchymal stem cells in yolk sac
- 6w-7mo
- → Liver (and spleen to lesser extent)
- continues until birth
- 7mo onwards:
- Bone marrow
- Proximal ends of femur/humerus
- fat replacement of long bone marrow occurs by age 18-20 y.o.
- Central skeleton (vertebrae, pelvis, ribs, sternum and skull)
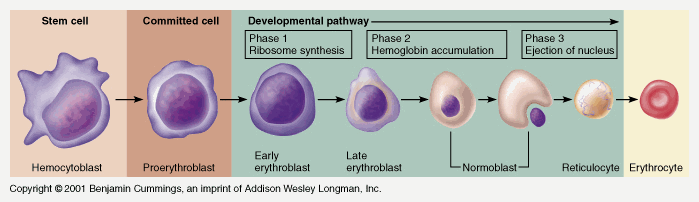
Takes 7 days
- Proerythroblast propagates
- From proerythroblast to reticulocyte
- Concentration of haemoglobin increases
- Nucleus disappears
- Endoplasmic reticulum disappears
- Reticulocyte
- Ribosomes
- Mitochondria
- Reticulocyte released into circulation
- Matures to RBC in 1-2 days
EPO increases rate of differentiation
Produced in the kidney (in response to low PO2)
RBC Destruction
Globin chains → aa’s
Haem → Biliverdin + Fe + CO
- Biliverdin
- Haeme + Haem oxygenase → biliverdin
- Biliverdin + biliverdin reductase → bilirubin
- Free bilirubin + albumin → transported to hepatocytes
- Conjugated with glucuronide to ↑ water solubility
- Excreted to bile canniliculus
- Secreted to gut at D2 (duodenum)
- Intestinal flora hydrolyse and reduce → urobilinogen
- 3 fates:
- 1) Gut bacteria form the dark pigment stercobilin, which is egested in the faeces
- 2) Urobilinogen reabsorbed unchanged by the portal system and recycled by the liver
- 3) Remainder is reabsorbed by the portal system and then excreted in the urine
- Fe2+ → oxidised to Fe3+ → recycled
- CO (only endogenous source of CO)
Gladwin 2016
Examiner Comments
2012A 15: 6 (60%) of candidates passed.
The production and fate of red blood cells was well known to most candidates. Marrow production and its change with development, the sequence of haematogeny and RBC lifespan were well known. Some candidates failed to mention the role of erythropoietin and its stimulus by oxygen tension. The breakdown of haemoglobin caused much confusion. Globin (protein), Fe and haem (porphyrin ring) were all expected to be considered separately – many candidates omitted one or all. The steps were often confused as was the nature of transfer in blood, conjugation and release into bile. Conversion to stercobilinogen or urobilinogen (with reabsorption from the gut and excretion in urine) caused similar confusion. No marks were awarded for discussion of bile salt metabolism from cholesterol (not haem) or the differences between direct and indirect acting bilirubin.
16. Describe the biochemical abnormalities, and the mechanisms by which they arise, that may be observed in a patient who is taking frusemide.
CICMWrecks Answer
Frusemide:
- Loop Diuretic
- MOA:
- Inhibition of Na/K/2Cl symporter TAL of LoH.
- ↓ reabsorption of Na, K, Cl.
- Less positive luminal charge
Biochemical Abnormalities with Frusemide
| Abnormality | Mechanism |
|---|---|
| Hyponatraemia | Blockade of Na/K/2Cl symporter – ↓ trans cellular Na reabsorption – loss of luminal positive charge → ↓ paracellular Na reabsorption |
| Hypokalaemia | Blockade of Na/K/2Cl symporter – loss of paracellular reabsorption of K |
| Hypochloraemia | Blockade of Na/K/2Cl symporter |
| Hypomagnesaemia/hypocalcaemia | loss of luminal positive charge → ↓ paracellular reabsorption of Mg and Ca |
| Hyperuricaemia | ↓ECF → ↑renin → ↑aldosterone → ↓ Urea secretion and ↑ tubular reabsorption of urea |
| Hyperglycaemia | ↓ glycolysis in RBCs ↓ sensitivity of insulin-induced skeletal muscle uptake |
| Metabolic Alkalosis | ↓ plasma chloride → ↑SID → alkalosis |
Gladwin 2016
Examiner Comments
2012A 16: 3 (30%) of candidates passed.
This was a relatively straightforward question with marks available for listing the abnormality and then discussing its origin. Many candidates simply listed an abnormality or confused the direction of electrolyte change. Few candidates went beyond hypokalaemia, hyponatraemia and hypochloraemia. Several candidates gave confused answers as to the mechanism(s) or drew pictures of a tubule with directional arrows for electrolytes with inadequate explanation. Some candidates simply ran out of time and wrote very little – this is a pity as a list would have generated marks. Candidates are reminded to practise the exams to time and attempt all questions.
17. Define afterload and describe the physiological factors that may affect afterload
CICMWrecks Answer
Afterload
- Load against which the muscle exerts its contractile force (Guyton)
- It is represented by the gradient of the line connecting the end-diastolic volume, to the end-systolic point
- pressure which the ventricle has to contract against (Power & Kam)
Determinants of Afterload
Modified Laplace Equation
- where
- T represents afterload
- P represents aortic pressure
- Therefore, afterload increases as aortic (or pulmonary arterial) pressure increases
- R represents ventricular radius
- Afterload increases as ventricular radius increases
- H represents ventricular thickness
- Afterload decreases as the thickness of the ventricular wall increases (hypertrophy secondary to chronic hypertension and cardiac remodelling)
Modified Hagen-Poiseuille Equation
- Afterload is affected by resistance to cardiac output
- Afterload increased by reduced radius of systemic vasculature
- Afterload increased by increasing viscosity of blood
Resistance in parallel
- Afterload is affected by addition, or loss of large capillary networks in parallel
- Systemic vascular resistance is increased significantly by loss of placenta, with parallel vascular networks
- Pulmonary vascular resistance is decreased significantly by inflation of lung, causing creation of vast capillary network in parallel
Factors affecting Right Ventricular Afterload
- Pulmonary vascular resistance increases afterload
- Hypoxic vasoconstriction increases PVR
- Lung volumes
- Pulmonary vascular resistance minimal at FRC
- Increased pulmonary artery pressure increases RV afterload
- Left heart failure
- Critical mitral stenosis, mitral regurgitation
- Pulmonary emboli
- Right ventricular outflow tract obstruction increases afterload
- Pulmonary stenosis
- Saddle pulmonary embolism
- Right ventricular dilation
- Susceptible due to thin RV wall
- Acute PE
- RV spiral of death
- Increased intraventricular pressure decreases blood flow à ischaemia à decreased contractility à further increase in ventricular volume
- RV spiral of death
Factors affecting Left Ventricular afterload
systemic vascular resistance, aortic impedance and ventricular radius
According to La Place Equation
- Aortic pressure
- Afterload increases as aortic pressure increases (increases with hypertension)
- Ventricular radius
- Afterload increases as ventricular radius increases (increases with ventricular dilation)
- Ventricular wall thickness
- Afterload decreases and ventricular wall thickness increases (decreases with ventricular hypertrophy)
Determinants of aortic pressure indirectly affect afterload
- Aortic compliance
- Afterload decreases with increased compliance
- Arterial blood volume
- Given set compliance, afterload will increase with arterial volume
- From Modifed Poisuille-Hagen Equation
- Radius of aorta
- Autonomic vasomotor tone
- Increased intrathoracic pressure
- Physical compression
- Viscosity of blood
- Radius of aorta
- From equation MAP = CO / SVR
- Tissue flow autoregulation
- Myogenic
- Metabolic
- Tissue flow autoregulation
Other
- Left ventricular outflow tract obstruction
- Aortic stenosis
- HOCM
- Coarctation of aorta
Sakurai 2016
Examiner Comments
2012A 17: 6 (60%) of candidates passed
Definitions for afterload vary slightly amongst common physiology textbooks, and candidates were expected to mention any one commonly accepted definition. Essentially afterload is the resistance to ventricular ejection – the “load” that the heart must eject blood against and is related to ventricular wall stress (Law of Laplace, T=Pt.r/u). Candidates were expected to mention aortic valve and systemic vascular resistance, aortic impedance, blood viscosity, intathoracic pressure and relationship of ventricular radius and volume.
Candidates generally did well, but few substantially good answers, with a lack of detail being the biggest limiting factor.
18. Describe the processes of excitation and contraction within a smooth muscle cell (60% of marks). Briefly outline the mechanism by which nitric oxide affects smooth muscle cell activity (40% of marks)
CICMWrecks Answer: Smooth Muscle
Excitation Contraction Coupling in SM
- Motor neuron depolarisation
- ACh vesicular release
- Multiple MEPs → End plate potential
- Muscle AP propagates via T-tubles
- Opens L-type Ca channels
- Influx of EC Ca → ↑ IC [Ca]
- Variable amount of Sarcoplamic reticula in SM
- Can have some ↑ IC [Ca] via Ca induced Ca release.
- Ca binds Calmodulin → Ca-Calmodulin complex
- Ca-Calmod → ↑ MLCK activity
- → Phosphorylation of Myosin Light Chain (MLC)
- → Activation of Myosin ATPase
- Cross-bridge cycling.
Cross Bridge Cycling
- “Flexed” Myosin without ATP binds to Actin.
- Binding of ATP to Myosin causes release of Actin/Myosin complex and extension of the head
- In the presence of Ca-Calmod complex
- Actin binding sites are open and binding of myosin/Actin/ATP complex is formed
- Hydrolysis of ATP to ADP + P in myosin head by Myosin ATPase
- → conformational change “initial flexing” the myosin head and releasing phosphate
- Release of ADP further flexes Myosin head
- In presence of Ca return to step 2.
Relaxation
- Ca removed from cell
- Ca ATPase
- Ca2+/Na+ antiport
- Myosin Light Chain Phosphatase
- dephosphorylates MLC → inhibition of myosin ATPase
Gladwin 2016
CICMWrecks Answer: Effect of NO on Smooth Muscle
Action of Nitric Oxide
- Produced:
- Endothelium
- L-arginine via reaction with the enzyme nitric oxide synthase
- 3 forms (iNOS, nNOS and eNOS)
- Action:
- Diffuses into vascular SMCs
- Binds to Guanylate Cyclase
- ↑ intracellular cGMP
- ↓’s Ca entry into the cell
- Activates K+ channels → hyperpolarization and relaxation
- ↑ cGMP-dependent protein kinase action
- Activates myosin light chain phosphatase
- Dephosphorylates myosin light chains
- Smooth muscle relaxation.
Gladwin 2016
Examiner Comments
2012A 18: 2 (20%) of candidates passed.
Insufficient breadth and depth of knowledge limited candidates’ performance to this question. Candidates were expected to mention mechanisms of muscle cell membrane activation (e.g. Ca2+ channel mediated action potential, Pacemaker potential, etc), sources of rise in intracellular Ca2+, intracellular Ca2+ binding to calmodulin in cytoplasm Ca2+calmodulin complex binding to, and activation of myosin light chain kinase, energy dependent myosin cross bridges and cycling and mechanism of smooth muscle relaxation.
Nitric Oxide (NO) activates guanylyl cyclise which in turn catalyzes the dephosphorylation of GTP to cyclicGMP which in turn induces smooth muscle relaxation, and the various mechanisms by which this occurs.
19. Outline the distribution, clearance and physiologic functions of magnesium in the body
CICMWrecks Answer
Normal Magnesium:
1000mmol (12mmol/kg)
Normal Distribution of magnesium
- Bone (60%)
- ICF (40% esp organs/muscles)
- Mg2+ is mainly an IC cation
- Total IC [ ] 15 mmol/L cf. 0.5 mmol/L free/ionised inECF
- ECF (< 1%)
- Plasma [ ] 0.5-1 mmol/L
- 60% free/ionised
- 33% protein-bound (esp albumin)
- 7% complexed (citrate/PO4)
- Plasma [ ] 0.5-1 mmol/L
Normal Intake:
8-20 mmol/day (min 0.5 mmol/day)
Normal Losses
- Glomerular filtration of 100 mmol/day
- 95% reabsorbed in tubules (PCT 15%, 70% LoH, 10% distal nephron)
- 5% excreted → 2.5 to 8 mmol/day
- Minimal neurohormonal regulation of Mg2+
- TMAX of Mg transporter ~ plasma [Mg]
- → ↓reabsorption with ↑ Mg
Normal Functions:
- Co-factor in enzyme reactions (as a metallo-coenzyme) eg Na+/K+ ATPase activity
- Energy storage, utilisation, transfer (via role in formation/utilisation of ATP) eg BSL homeostasis
- Protein and nucleic acid synthesis (stabilises DNA and RNA structure)
- Ca2+/K+ metabolism
- ↓ membrane excitability (eg. muscles/nerves)
- ↓ NT release at cholinergic and adrenergic synapses
- Antagonises Ca2+ activity
Gladwin 2016
Examiner Comments
2012A 19: 2 (20%) of candidates passed.
Insufficient breadth and depth of knowledge limited candidates’ performance to this question. Candidates were expected to mention normal plasma (0.7 – 1.1 mmol/l) and intracellular (20mmol/l) levels, distribution (approximately 50% of total body magnesium is in bone & 20% in skeletal muscles), clearance (almost solely renal, approaches GFR, renal threshold set at just above normal serum Mg concentration, below which get almost complete reabsorption), activity (e.g. co-factor in metabolism), effects on muscles (reduces muscle excitability, inhibits excitation-contraction coupling, reduced contractility / weakness / depressed reflexes), effects on nerves (e.g. reduces nerve excitability, blocks NMDA receptors), systemic and coronary vasodilation and inhibits platelet function.
20. List the physiological factors affecting the diffusion of oxygen across the alveolar membrane (70% of marks). Describe how this changes with exercise (30% of marks).
CICMWrecks Answer
- Diffusion capacity:
- “The volume of gas which will diffuse through a membrane each minute for a partial pressure difference of 1mmHg (ml/min/mmHg)”
- Normal diffusion capacity for oxygen (DLO2): 20ml/min/mmHg
- Fick equation:
- Thickness of membrane (normal: 0.3μm)
- Surface area of membrane (normal: 50-100m2)
- Age, posture, degree of inflation (1 mark for any)
- Partial pressure gradient across membrane
- Rate of blood flow through lungs
- (no points for mentioning diffusion constant of gas – question relates to only oxygen)
- Rate of oxygenation of reduced Hb
- Shift of oxygen dissociation curve (pH, temperature, PCO2, 2,3-DPG)
- Haematocrit
- Abnormalities of haemoglobin
In exercise
- DLO2 increases to 30-60ml/min/mmHg
- Increased VT and RR
- Increased alveolar ventilation → partial pressure gradient of O2 across capillary increases (significant as flow of erythrocytes through pulmonary vasculature likely to increase)
- Recruitment of dormant pulmonary capillaries (increased surface area for diffusion)
- due to: Increased lung inflation, Increased pulmonary arterial systolic pressure
- Perfusion increases from 21ml/min/mmHg up to 65ml/min/mmHg
- Better matching of ventilation-perfusion
Mooney 2016
Examiner Comments
2012A 20: 5 (50%) of candidates passed.
The first section of this question was generally well done, but few really good marks, whereas the second section was poorly understood. The diffusing capacity is defined as the volume of gas that will diffuse through the membrane each minute for a partial pressure difference of 1mmHg. Factors affecting the rate of gas diffusion through the respiratory membrane include those related to the Fick equation (e.g. thickness of the membrane, surface area of the membrane, diffusion coefficient of the gas in the substance of the membrane, partial pressure difference of the gas between the two sides of the membrane, gas’s solubility), rate of combination of oxygen with reduced haemoglobin and factors affecting surface area (e.g. lung volume, age, posture). During strenuous exercise the oxygen diffusion is increased as pulmonary blood flow is greatly increased and there is opening up of previously dormant pulmonary capillaries increasing the surface area of blood into which oxygen can diffuse. Also, alveolar ventilation increases and there is better matching of ventilation and perfusion increases from 21ml/min/mmHg up to 65ml/min/mmHg.
21. Statistics (not in current primary syllabus)
22. Outline the importance of the citric acid cycle in metabolism
CICMWrecks Answer
The Citric Acid Cycle
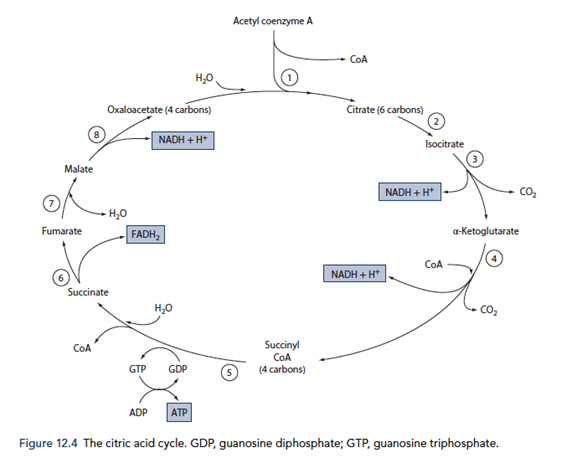
Energy production
- Required for the total metabolism of glucose, for the production of approx. 38 ATP (Compared to 8 ATP production from metabolism of glucose to pyruvate)
- Also used in the metabolism of fats, ketone bodies and amino acids via Acetyl CoA
- 12 ATP generated for each Acetyl CoA molecule
- Occurs within the mitochondrial matrix
- Pyruvate generated in glycolysis is converted to Acetyl CoA by pyruvate dehydrogenase releasing 1 NADH per pyruvate molecule (leading to the generation of 6 ATP equvalents per glucose molecule)
- Acetyl CoA condensed with oxaloacetate to for citric acid and in a number of subsequent steps forms 1GTP, 3NADH and 1FADH2 (Total of 24 ATP equivalents per glucose molecule)
- The NADH and FADH2 generated in the citric acid cycle is used to generate ATP with O2 as the terminal electron carrier to form water.
- Therefore O2 is required for the citric acid cycle to continue
- The electron transport chain
- Inner mitochondrial membrane
- Series of 4 cytochrome enzymes that pump H+ across into intermembrane space as electrons are transferred from NADH and FADH2
- NAD and FAD regenerated in the process replenishing substrates for the citric acid cycle
- Proton gradient used to generate ATP from ADP and Pi
- CO2 in generated in the citric acid cycle
Note: Erythrocytes lack mitochondria and therefore are unable to utilize the citric acid cycle for energy production (Therefore can only utilize glucose)
Biosynthesis
- Components of the citric acid cycle are used for biosynthesis of fuels
- Malate and oxaloacetate – used in gluconeogenesis
- Citrate – used in fatty acid and cholesterol generation
- Oxaloacetate and αketoglutarate – used in amino acid synthesis
- Succinyl CoA – used in porphyrin synthesis
- Oxaloacetate – Porphyrins
Sakurai 2016
Examiner Comments
2012A 22: 2 (20%) of candidates passed.
Only an outline of the importance of the citric acid cycle (CAC) was expected, and not a detailed understanding. Candidates struggled to appreciate its importance. For a good answer candidates were expected to mention that the CAC has two main functions: energy production and biosynthesis. Some understanding of the overall reaction ( AcCoA + 3NAD+ + FAD + GDP + Pi + 2H2O 2CO2 + 3NADH + FADH2 + GTP +CoA ), the link between oxidation of metabolic fuel (carbohydrates, lipids and protein) and aerobic energy production (oxidative phosphorylation), that Acetyl CoA is final common product of these oxidations (Glucose via glycolysis (cytoplasm), Free fatty acids via β oxidation (mitochondrial matrix) and Amino acids – glucogenic and ketogenic reactions), that CAC is first stage of aerobic respiration, requires oxygen to proceed, that the CAC
together with oxidative phosphorylation accounts for 2/3 to 3/4 of ATP generated from fuel oxidation and anabolic involvement, whereby several biosynthetic pathways utilize CAC intermediates, e.g. Glucose biosynthesis (gluconeogenesis) via malate then oxaloacetate, fatty acid and cholesterol biosynthesis via citrate + CoA AcCoA, Amino acid biosynthesis via reductive amination and transamination reactions, Porphyrin (organic component of haeme) via succinyl CoA and Purine and pyrimidine biosynthesis – precursors of nucleotide bases for DNA and RNA.
23. Classify antiemetic drugs and give an example from each group (60% of marks). Outline the gastrointestinal effects of metoclopramide (40% of marks).
CICMWrecks Answer: Anti-emetics
Anti-emetic Agents
| CLASS | EXAMPLES | MECHANISM OF ACTION |
|---|---|---|
| Anticholinergics | Hyoscine Atropine | – M1-Ach-R antagonism (NTS, CTZ, VC) – Small antihistamine and D2 antagonist effects |
| Antihistamines (H1) | Cyclizine Promethazine | – H1 antagonism- VC, vestibular nucleus and CTZ – Anti-muscarinic effects- NTS, CTZ, vomit center – D2 antagonism (GIT, CTZ) |
| 5-HT3 Antagonists | Ondansetron | – Peripheral in GIT – Central at VC and CTZ |
| Dopamine Antagonists | 1. Phenothiazines – Prochlorperazine (stemetil) 2. Butyrophenones – Droperidol, domperidone 3. Benzamides – Metoclopramide | – Decreased sensitivity of visceral afferents to vomit center – Central D2 blockade increased threshold at CTZ Other effects: – Inhibition of 5-HT3 – Anti-H1 effects |
| Steroids | Dexamethasone | – Proposed to act centrally to inhibit prostaglandin synthesis and inhibit endorphin receptors |
| Miscellaneous | 1. Propofol 2. Benzos 3. Cannabinoids 4. NK1- Receptor antagonists (aprepitant) | – Propofol and BZD- GABAergic inhibition of VC – Cannabinoids- Direct CTZ and VC inhibition – NK1 antagonists inhibit VC |
CICMWrecks Answer: GI Effects of Metoclopramide
Gastrointestinal effects of Metoclopramide
- Selective stimulation of gastric muscarinic receptors
- acts primarily by augmenting release of ACh and perhaps by inhibition of 5-HT release
- Coordinated gastric, pyloric and duodenal activity contraction (accelerates gastric emptying)
- Increased amplitude and frequency of Longitudinal muscle contraction
- Lowers threshold for peristaltic reflex to occur
- Reduces intestinal muscle fatigue
- Direct action on smooth muscle to increase tone (including increased LOS)
- relaxes the pyloric sphincter
Examiner Comments
2012A 23: 4 (40%) of candidates passed.
Antiemetics, as a topic has been frequently asked, in various formats in the past. Candidates who performed well had a good depth and breadth of knowledge as well as sufficient integration of knowledge to be able to classify and understand the basis to the various classifications. Essentially it was expected that candidates mention the classifications of Anticholinergics, Antihistamines, Anti 5HT, Antidopaminergics (benzamides, butyrophenones, phenothiazines), Steroids and other agents with known antiemetic activity (e.g. propofol, benzodiazepines, etc). In relation to metoclopramide it was expected that candidates would mention that it lowers pressure threshold for occurrence of intestinal peristaltic reflex, reduces intestinal muscle fatigue, enhances frequency and amplitude of longitudinal muscle contraction, coordinates gastric, pyloric and duodenal activity to improve GI motility, mechanism of action appears to depend on intramural cholinergic neuron, acts primarily by augmenting release of ACh and perhaps by inhibition of 5-HT release, increases lower oesophageal sphincter pressure, relaxes the pyloric sphincter and antagonize the inhibitory neurotransmitter, dopamine.
24. Describe the potential causes, and effects, of resonance and damping on an invasive arterial blood pressure trace.
CICMWrecks Answer
Definitions
Natural frequency: the frequency at which a system oscillates when not subjected to a continuous or repeated external force.
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of a periodically applied force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts.
Resonant frequency: The frequency of a dynamic system at which the amplitude of response to an oscillating force is a relative maximum is known as a resonant frequency (OR) A natural frequency of vibration determined by the physical parameters of the vibrating object.
Damping: the property of a system that diminishes resonance.
Critical Damping: Occurs when the damping coefficient (DC or γ gamma) is equal to the undamped resonant frequency of the oscillator.
Optimum Damping: Level of damping which provides a compromise between the speed of the system with its accuracy
Undamped simple harmonic oscillator
- obeys hooke’s law
- motion is sinusoidal in time
- demonstrates a single resonant frequency.
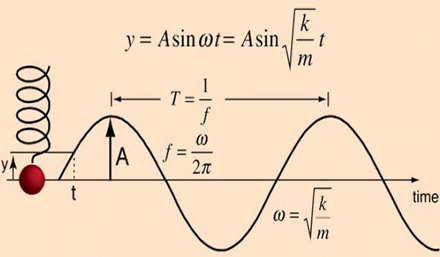
Resonance
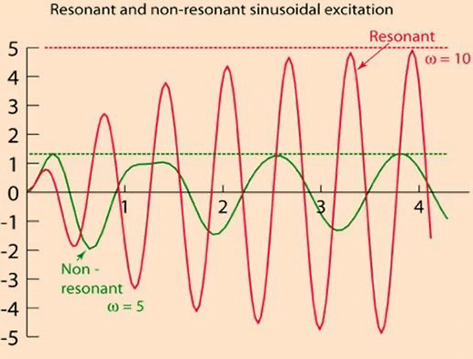
Invasive arterial blood pressure is a driven harmonic oscillator
- An external force (pulsations of the heart) adds to the oscillations of the system (the tubing/transducer etc).
- If the frequency of the driving force is similar to, or a harmonic of, the natural frequency of the system then the result is amplification
Arterial waveform is a complex summation of multiple simple sinusoidal waveforms (a fourier series)
- The frequency of the arterial wave (i.e., the pulse rate) is known as the natural or fundamental frequency
- The sine waves used to reproduce it must have a frequency that is a multiple (or harmonic) of the fundamental frequency
- Increasing the number of harmonics allows better reproduction of high-frequency components, such as a steep systolic upstroke
In most clinical systems
- Natural resonant frequency is 10 to 15 Hz
- Primary frequency of the arterial waveform (the heart rate is 60 to 120 bpm or 1 to 2 Hz)
- However the higher frequency components of the more complex arterial waveform are closer to the natural resonant frequency and are amplified to a larger degree.
To minimize the potential of amplification and improve the dynamic accuracy of the real arterial pressure, system should have resonant frequency >> than 6-8 times frequency of the system being measured:
- Should have noncompliant (i.e. stiff) tubing
- Total mass of liquid in the system should also be minimized (shorter/thinner tubes)
- air bubbles or blood clots or occlusion should be eliminated
Damping
the property of a system that diminishes resonance.
- Damping:
- decreases the magnitude of the oscillations
- allows the system to come to rest at a new value.
- Degree of damping determines the rate at which the system achieves that new value.
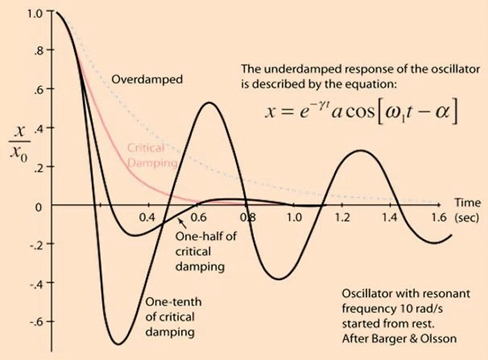
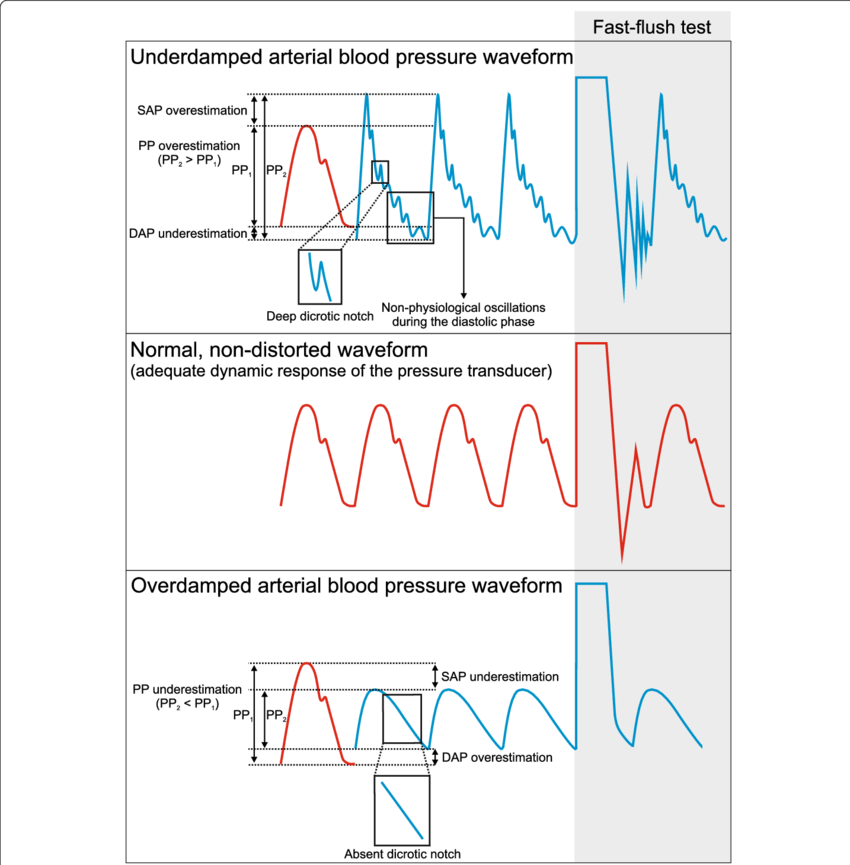
System can be:
- Underdamped (DC < 0.7):
- Reaches the zero position quickly
- Oscillates around it
- Causes falsely ↑ SBP and falsely ↓ DBP
- Critically damped (DC = 1):
- Quickest approach to zero amplitude for a damped oscillator without overshoot.
- Occurs when the damping coefficient (DC or γ gamma) is equal to the undamped resonant frequency of the oscillator.
- Optimal damping (DC = 0.64):
- Provides a compromise between the speed of the system with its accuracy
- Minimises overshoot of oscillations, phase and amplitude distortion, and provides maximal frequency response
- Overdamped (DC > 1):
- The approach to zero is slower
- Very slow to respond
- Causes falsely ↓ SBP, falsely ↑ DBP and loss of fine details of waveform (Eg. dichrotic notch)
Bedside Flush Test:
- The degree of damping in an invasive arterial line system can be determined by the bedside flush test
- Briefly open the continuous flush device to produce a square wave
Gladwin / Sakurai / JC 2020
Examiner Comments
2012A 24: 3 (30%) of candidates passed
For a good answer candidates were expected to mention that the arterial pressure waveform is made up of many different sine waves (as determined by Fourier Analysis) with each sine wave having a specific frequency. Every system has its own natural oscillatory frequency, or resonant frequency. The pressure measuring system has a resonant frequency at which oscillations occur, and if this is less than 40 Hz, it falls within the range of frequencies present in the blood pressure waveform and oscillations may produce a sine wave which is superimposed on the blood pressure wave form. The resonant frequency can be increased by using a short, wide, stiff catheter. In respect to damping, some damping is inherent in any system and acts to slow down the rate of change of signal between the patient and pressure transducer. Mention of causes of damping and the optimal damping coefficient (0.677) were expected. An under-damped system is one whereby resonance occurs causing the signal to oscillate and overshoot (damping factor <0.7) and an overdamped is one whereby the signal takes a long time to reach equilibrium but will not overshoot. It may not reach equilibrium in time for a true reading to be given (damping factor >1.0). Both resonance and damping can alter the measured systolic and diastolic values but the mean pressure is not affected
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | Thiopentone, conc vs time curves, Vd and factors, elimination, half life |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Hb saturation of newborn breathing air, Fetal Hb diss cureve, fetal/newboen circulation, changes at birth, normal values of newborn vital signs Vascular anatomy of blood flow in lungs, venous admixture, shunts, systemic vs pum circ, drugs to treat pulm HTN |
| G. CVS | beta blockers, basic pharm, dose response curves, PV loop of cardiac cycle, changes in loop following administration of esmolol and labetalol |
| H. Renal | |
| I. Body Fluids and Electrolytes | NS, osmol, colligative properties, anion gap, diuretics |
| J. Acid Base | |
| K. Neuro | Cerebral blood flow, grey vs white matter blood flow, EEG related to blood flow, factors affecting CBF Thiopentone, conc vs time curves, Vd and factors, elimination, half life |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | Components of blood, Blood groups, antigens, changes to blood stored over time, process of group and screen |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | Classify bacteria, antibiotics, gentamicin |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments