1. Describe the physiological consequences that follow an intravenous bolus of 50mls of 50% glucose.
CICMWrecks Answer
Immediate
50mls of 50% Glucose = bolus of 25g Glucose
- Hyperglycaemia – normal blood glucose is 4-7 when fasted, <11.1 when not fasted
- Glycosuria – resorption capacity of the renal PCT is exceeded when serum glucose >10-12mmol/l
- resorption of glucose is via co-transport with Na+
Insulin
- Secreted from
- Pancreatic beta cells in islets of Langerhans
- Mechanism of secretion
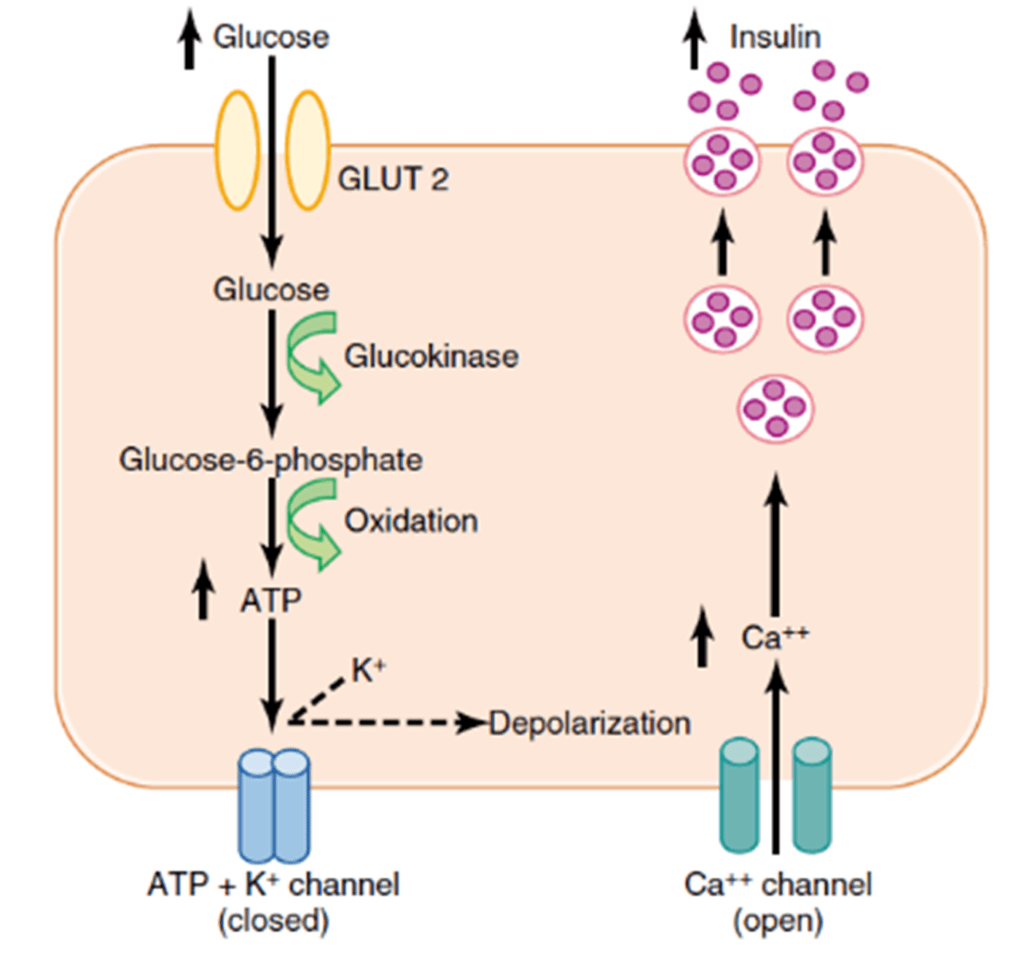
- Glucose moves into beta cells via GLUT-2 transporters
- Glucose –-(glucokinase)–> Glucose-6-phosphate –-(glycolysis)–> ATP
- ATP inhibits ATP sensitive K+ channels
- Cell depolarises → calcium influx
- Calcium stimulates exocytosis of insulin-containing vesicles
- Response
- Insulin release
- Inhibits gluconeogenesis
- Promotes muscular, adipose and peripheral cell glucose uptake
- GLUT-4 transporter
- Promotes hepatic glycogenesis
- Glucose trapping
- Hexokinase phosphorylates glucose (glucose-6-phosphate)
- Negatively charged G6P cannot cross membrane, so is trapped in hepatocyte
- Stimulates glycogen synthase
- Glucose trapping
- Inhibits glycogenolyis
- Inhibits liver phosphorylase
- Promotes FFA synthesis, fat deposition
- Inhibits lipolysis
- Promotes protein anabolism, inhibits catabolism in muscle
- Insulin release
- Fate of glucose
- Glycolysis -> pyruvate
- Pyruvate and CoA -> acetyl CoA -> enters citrate cycle
- FADH and NADH from citrate cycle -> electron transport chain
- Yield from 1 glucose is around 38 ATP
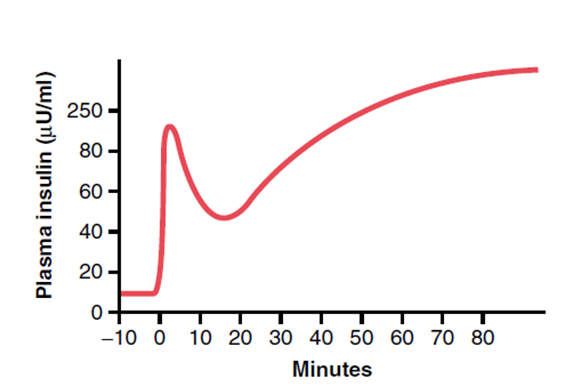
- Pattern
- Biphasic release
- Phase 1: Dumping of preformed insulin
- Phase 2: Drop-off in insulin secretion, then rise up towards a higher peak when new insulin is made
- Biphasic release
Mooney 2016
Examiner Comments
2011B 01: 14 (56%) of candidates passed this question.
It was expected answers would include a comment this would transiently increase the blood glucose and result in the stimulation of insulin production. Some detail was then required on the mechanism by which insulin production is increased and time frames over which this occurred, as well as mechanism by which glucose passes through to the urine. Other important points expected were the fate of glucose, once taken up by the cells, the inhibitory effects (and mechanisms) of insulin (ie inhibits glycogenolysis, triglyceride and protein breakdown, etc). A description of the physiological consequences of increased insulin production was required. This question has been asked before, in it’s current form. The pass rate on this occasion was higher. Candidates who did poorly did so due to a lack of detail and understanding of the topic.
2. Describe the factors contributing to inter-individual variability in drug response seen with an induction dose of an intravenous anaesthetic drug.
CICMWrecks Answer
Pharmacokinetic factors
(Amount of drug reaching the receptor)
- Absorption
- IV dose so should be 100%
- dose variability between patients
- Distribution
- Volume of distrib:
- Body mass and ratio of fat:muscle differences
- pregnancy, cardiac output states
- Protein binding:
- low albumin states, other protein bound drugs
- propofol has 98% protein binding and will be influenced
- Blood pH
- affects degree unionised which can cross lipid membranes
- eg alkalotic state with thiopentone pKa 7.6
- Volume of distrib:
- Metabolism
- Hepatic factors
- liver disease may reduce metabolism
- CYP enzymes (inhibited or upregulated) eg Ketamine
- Hepatic factors
- Excretion
- Renal impairment: onset of effect more dependent of redistribution, but can be prolonged if renally excreted
Pharmacodynamic factors
- Presence of endogenous or exogenous ligands
- Endogenous: increased catecholamines may necessitate a higher induction dose
- Exogenous:
- medications which potentiate sedation opioids, benzodiazepines, volatiles
- medications which reduce sedation – amphetamines
- Variation in the number of receptors
- Extremes of age
- Previous exposure to the drug or similar – tolerance, tachyphylaxis
Variability in the response distal to the receptor
- Pharmocogenetic factors
- idiosyncrasy
- anaphylaxis
- Pathological and physiological factors
- sepsis
- CNS function
JC 2019
Examiner Comments
2011B 02: 15 (60%) of candidates passed this question.
This was a broad question that required some structure and organisation to score well. The issues around variability in both pharmacokinetics and pharmacodynamics, of which there are many, needed be covered. For example variability due to differences in organ function, age, metabolism, body composition, were just a few of the factors expected to be mentioned.
3. Describe the role of the kidney in drug excretion and the factors affecting this (60% marks). Briefly outline how you would alter the dosing of a drug with high renal excretion in a patient with renal impairment (40% marks).
CICMWrecks Answer
Renal Drug Excretion
Due to glomerular filtration and balance of secretion and reabsorption
Glomerular Filtration
- Physiological factors
- Starling forces
- Filtration pressure ∝ (PG – PBC) – ( πG – πBC)
Where- PG = Glomerular hydrostatic pressure (60mmHg)
- PBC = Bowman’s Capsule hydrostatic pressure (18mmHg)
- πG = Glomerular oncotic pressure (32mmHg)
- πBC = Bowman’s Capsule oncotic pressure (Negligible)
- Filtration decreases
- Shock → decreased glomerular pressure
- Obstruction → increased bowman’s capsule hydrostatic pressure
- Hypoproteinaemia → hepatic failure, nephrotic syndrome
- Fick’s Law (see eqn)
- Filtration increases with decreased MW, increased concentration gradient
- Filtration decreases with increased GBM thickness (glomerulosclerosis, deposition) and loss of glomerular surface area (usually 0.8m2. may be lost with renal failure)
Fick’s Law:
- Drug factors
- Size (Due to filtration slits and podocyte foot processes)
- Particles <4nm freely filtered
- Particles >8nm excluded
- Charge
- GBM has negative charge, therefore negatively charged molecules repulsed → decreased filtration
- Protein binding
- Highly protein bound drugs inhibited from filtration due to large size and charge of proteins
- Size (Due to filtration slits and podocyte foot processes)
Secretion
- Due to specific transporters
- P-glycoprotein (Amphipathic anions)
- MPR-2 (Conjugated metabolites)
- ATP-Binding Cassette Transporters (Organic cations)
Reabsorption
- Passive reabsorption occurs in PCT and DCT
- Membrane transporters for facilitated transport are present in DCT
- Ionization
- pH of urine may lead to drug “trapping”
- Acids ionized with pH > pKa
- Bases ionized when pH < pKa
- pH of urine may lead to drug “trapping”
- Lipophilicity
- Allows permeation through phospholipid bilayers of cell membrane → reabsorption
Dose adjustment of renally excreted drugs
- According to clearance and GFR
- Loading dose = Target concentration x Vd
- Usually no alteration of loading dose required
- In ESRF, volume of distribution may be increased → loading dose may need to be increased
- Dosing rate = Target concentration x Cl
- As clearance is reduced in renal failure dosing rate requires reduction
- Maintenance dose = Dosing rate x Dosing interval
- Maintenance dose and/or the dosing interval may need to be reduced (depending on whether AUC or peak concentration of drug is required for drug effect)
- Plasma monitoring of [drug] concentration required for drugs with low therapeutic index
Dose adjustment of Gentamicin
MoA: Inhibition of bacterial protein synthesis through irreversible binding to the 30s bacterial ribosome
Has a narrow therapeutic window, hence dose optimization and therapeutic drug monitoring are crucial.
| Concentration dependant killing | Active concentration needs to be acheived (~8-10 times Minimim Inhibitory Concentration/MIC) Time above MIC does not need to be maintained | Loading dose should not be altered even in renal failure (5-7mg/kg) |
| Significant postantibiotic effect Distribution: Hydrophilic, so Distribute mainly to extra-cellular fluid. Redistribution can occurs upto 16-24 hours. | Once daily dosing sufficient without renal failure | |
| ESRF – Volume of distribution theoretically larger (but in case of Aminoglycosides, might be lower – suspected due to tissue displacement by other molecules like urea) | Loading dose adjustment in ESRF 4mg/kg | |
| Metabolism: Nil Excretion: Rapidly excreted by glomerular filtration. Hence accumulate in renal failure (t1/2 upto 30-60hrs) | Subsequent Dosing interval based on renal clearance (GFR / CrCl) Frequent dosing only increases toxicity with no improvement in efficacy. | Maintenance dosing less frequent in renal failure Dose interval based on pre-existing guidelines, and with monitoring of pre-administration levels CrCl 40 to 59 mL/minute: Administer every 36 hours CrCl 20 to 39 mL/minute: Administer every 48 hours CrCl <20 mL/minute: Monitor serum levels and redose when gentamicin level is less than 1 mg/L |
| End stage renal disease / Dialysis (t1/2 upto 100hrs) | Check level prior to dialysis, and only administer dose after dialysis (based on expert advice) | |
| Low Therapeutic index | Frequent monitoring of levels required | |
Sakurai / JC 2019
Examiner Comments
2011B 03: 13 (52%) of candidates passed this question.
It was expected candidates would expand on the role of the kidney in the excretion of drugs and metabolites. A statement that referred to the classical features of drugs that undergo renal excretion (eg polarity, lipid solubility, size and protein binding, drug metabolites) and a definition of renal clearance was expected and would have provided an excellent introduction to any answer. It was then expected that candidates would mention, in some detail, the processes of filtration and secretion (both active and passive and both proximal and distal along the renal tubules). The question also asked for factors that affect renal drug excretion, for example, a reduction in GFR or alteration in protein binding. An approach to alterations of dosing would require some consideration of assessing degree of dysfunction (GFR estimation / calculation) then an understanding that it would not impact on loading doses but would influence subsequent dosing of renal cleared drugs. Plasma monitoring provides useful information for some drugs, particularly those with a narrow therapeutic index.
Syllabus: II2d, D12h
Recommended sources: Goodman and Gilman’s the Pharmacological Basis of Therapeutics pgs10-14; Foundations of Anaesthesia Basic and Clinical Science, Hemmings pg107; Basic and Clinical Pharmacology, Katzung pgs 35, 48-49.
4. Describe the pharmacology of low molecular weight heparin (70% marks). Outline the pharmacology of hirudin (30% marks).
Examiner Comments
2011B 04: 10 (40%) of candidates passed this question.
A question that asks for information of the pharmacology should mention pharmaceutical (only briefly), pharmacokinetics and pharmacodynamics of the drug or class of drugs requested. The term “describe” requests of the candidate a greater depth of information than “outline” and both are defined in the “Notes to Candidates” document. Candidates who did well, did so because they had sufficient knowledge of this commonly used class of drugs, and could structure a well organised answer.
Syllabus: J2a 2a
Recommended sources: Rang and Dale Pharmacology, Chp 21; Goodman & Gillman The pharmacological Basis of Therapeutics Chp 54.
5. Describe the essential components of an ECG monitor (60% marks). Outline the methods employed to reduce artefact (40% marks).
CICMWrecks Answer
ECG
- 12 electrodes on the body surface to detect small potentials (0.5-2 mV) generated by the heart.
- Electrodes made of Ag/AgCl
- ECG signals detected by these electrodes
- Sent to electronic device that filters out noise and boosts the signal
- Displayed on oscilloscope
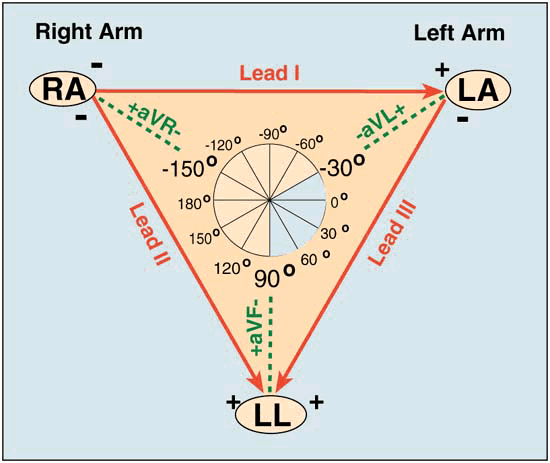
Signal Interpretation from oscilloscope readings via Enthoven’s triangle
- For orientation of the Bipolar and unipolar limb leads synthesised from the 12-lead ECG.
- Bipolar limb leads
- Lead I: RA (-) to LA (+) at 0°
- Lead II: RA (-) to LL (+) at 60°
- Lead III: LA (-) to LL (+) at 120°
- Unipolar limb leads
- aVR: RA (+) at -150°
- aVL: LA (+) at -30°
- aVF: LL (+) at 90°
- Positive deflection in a lead indicates depolarisation wave moving towards lead’s positive terminal
Calibration
- Vertical calibration
- 1 mV signal → vertical deflection of 10 mm (2x large square or 10x small squares)
- 0.1 mV per mm (or small square)
- Horizontal calibration
- paper speed is 25 mm/s → 0.04 s/mm (or small square)
Errors:
Noise and interference caused by:
- Electrical interference by any device using AC current (esp high-frequency diathermy)
- → Minimise by ECG filters, shielding of leads/cables, differential amplifers, Etc.
- Movement or shivering
- → minimised by placement over bony prominences and use of ECG filters
- High skin impedance
- → minimised by degreasing skin with EtOH and use of conducting gel with electrodes
- Incorrect electrode placement relative to heart
It looks like this Examiner reads Hemmings.
Gladwin 2016
Examiner Comments
2011B 05: 2 (8%) of candidates passed this question.
Monitoring and monitors are essential to Intensive Care practice, and is the reason why it is included in the syllabus. Unfortunately candidates have performed poorly in this question, as they have in previous measurement and monitoring questions. Future candidates need to be aware that such questions WILL get asked again.
For a good answer it was expected that mention would be made of what an ECG monitors does (ie detects and amplifies the small electrical changes on the skin that are caused when the heart muscle depolarizes), how (ie use of 2 or more electrodes, typically being made of silver or silver chloride), the type of leads (ie unipolar and bipolar, and a description of the latter), the way the signal is handled (isolation, amplification, gain, filtering) and displayed. Methods to reduce artefact and improve signal:noise ratio, should have included skin conductive measures, minimising external interference (filters, earthing), common mode signal artefact rejection, high input impedance amplification and mention of diagnostic and monitoring modes.
Syllabus: S2a
Recommended sources: Davis and Kenny pgs 160-178, also Sykes & Vickers Principles in measurement and monitoring in Anaesthesia and Intensive Care, Chps 4, 5, 6, 23.
6. Describe the physiology of the Renin and Angiotensin system.
CICMWrecks Answer
Renin-Angiotensin-Aldosterone system primarily for hormonal regulation of blood volume.
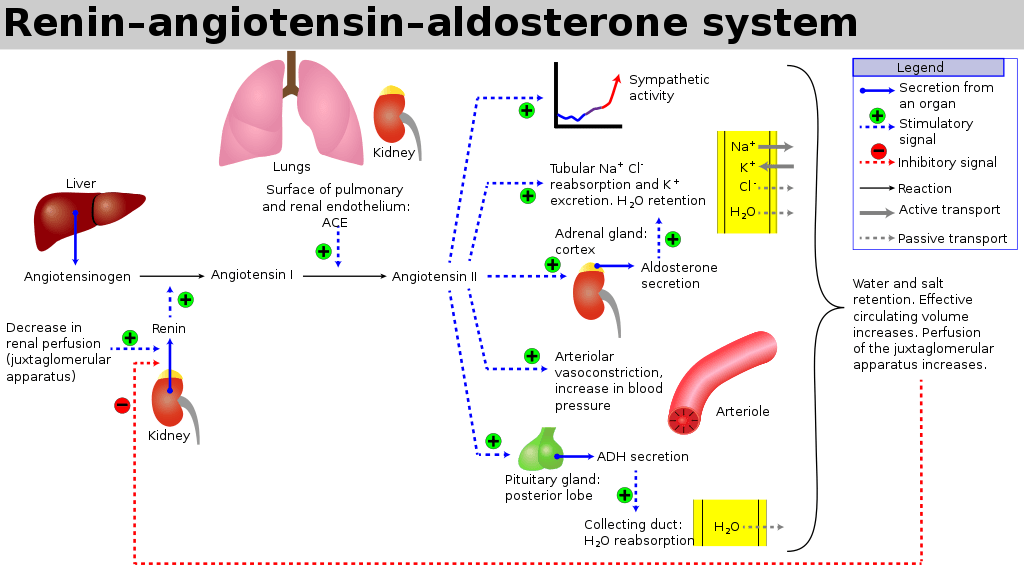
Renin
- Proteolytic enzyme
- Secreted from granula cells in juxtaglomerular apparatus
- Secretion:
- Stimulated by:
- ↓ GFR
- ↓ tubular Na/Cl at macula densa
- β1 SNS stimulation of granular cells
- ↑ prostacyclin levels
- ↓ levels of angiotensin 2 or ADH
- Inhibited by:
- ↑ renal perfusion pressure
- ↑ sodium delivery to DCT
- ↑ by ATII and ADH
- Stimulated by:
- Target:
- circulating angiotensinogen
- Effects:
- Tubular effects:
- ↑’d PCT Na/Cl reabsorption, ↑’d tubular K secretion
- Direct PCT effect
- Aldosterone release
- Volume Status effects:
- ↑’d total body water via
- ↑’d thirst (hypothal)
- ↑’d ADH secretion
- Renal Blood flow effects
- Direct: Reduced RBF and GFR (Aff>Eff)
- ↑’d renin → ↑ AT2 (see AT2 below) → ↑’d Aldosterone
- Vasoconstriction (aff=eff)
- Mesangial cell contraction
- Tubular effects:
Angiotensin 2
- Production:
- Angiotensinogen is the precursor peptide hormone produced by the liver
- Angiotensin 1 (decapeptide)
- ↑ Renin by JGA (above) → cleavage of Angiotensinogen → AT1
- No biological activity
- Angiotensin 2 (octapeptided)
- Angiotensin Converting Enzyme (ACE) in pulmonary capil endothelia
- → ↑AT2
- Angiotensin 3 (septapeptide)
- 40% of the pressor activity AT2
- 100% of the aldosterone-producing activity
- Angiotensin 4 (hexapeptide)
- Minimal biological activity
- Target:
- Mesangial cells, arteriolar SM, CNS, posterior pituitary
- AT1 and AT2 receptors
- Acts via GPCR (Gq via PLC to increase IP3 and intracellular Ca)
- Effect:
- CVS
- Vasopressor
- Resets baroreceptor control of HR at higher pressure
- Potent mitogen for smooth muscle and cardiac myocytes
- Direct positive inotrope
- CNS
- ↑’d sympathetic outflow
- ↑’d thirst
- ↑’d ADH release from posterior pituitary.
- Renal
- Negative feedback on renin release
- ↑’d aldosterone release
- ↓’d RBF and GFR
- Direct renal arteriole constriction (efferent = afferent)
- Mesangial cell contraction thus ↓’d Kf and GFR
- ↑’d sodium/chloride reabsorption in PCT
- Direct effect and via ↑’d aldosterone release
- CVS
Aldosterone
- Steroid Hormone
- Source:
- Adrenal cortex (zona glomerulosa)
- Release Stimuli:
- adrenal cortical hyperkalaemia
- ↑’d AT2
- ↑’d ACTH
- Target:
- Distal collecting duct and CCD principal cells ENaC receptors.
- Na/K ATPase co-transporters in DCT/CCD, GIT and secretory glands
- Effect:
- Sodium (↑’d plasma Na)
- Upregulates basolateral ENaC and Na/K ATPase to increase Na reabsoption
- ↑’d Na reabsorption throughout the GIT/sweat and salivary glands via Na/K ATPase
- ↑’d H2O reabsorption and ↑’d Na via solvent drag
- Potassium (↓’d plasma K)
- ↑’d K secreation at DCT/CCD principal cells (via ROMK)
- ↑’d K secretion throughtout the GIT/Salivary glands
- ↑’d intracellular K uptake
- Chloride (↑’d plasma Cl)
- ↑’d Cl uptake in distal tubule
- Acid (H) Effects
- Upregulation of H and H/K-ATPase in type A intercalated cells of CCD
- Increases H secretion (and HCO3 reabsorption)
- Sodium (↑’d plasma Na)
Gladwin 2016
Examiner Comments
2011B 06: 15 (60%) of candidates passed this question.
Renin and angiotensin are core components in the regulation of plasma volume and blood pressure regulation. It is unfortunate that many candidates presenting to this examination are not able to provide sufficient information for a pass. For a good answer, candidates were expected to mention what renin and angiotensin are and what they do, as well as briefly mention the place of angiotensin converting enzyme (converts Angiotensin I to Angiotensin II and inactivates bradykinin). Renin is a proteolytic enzyme cleaves angiotensinogen to angiotensin I, secreted by the juxtaglomerular cells of the kidney which are located in media of afferent arteriole and in close proximity to the glomerulus and the distal convoluted tubule (macula densa). Angiotensin II acts on cell surface AT1 and AT2 receptors. Major functions being to preserve of GFR & enhanced Na/H2O reabsorption in the setting of reduced renal blood flow (candidates expected to outline the mechanism by which this occurs), vasoconstriction, stimulate aldosterone secretion and increase thirst and ADH secretion. The better candidates also mentioned that it decreases sensitivity of baroreceptor reflex, increases secretion of ACTH and facilitates noradrenaline release from sympathetic nervous system as well as its fate (metabolized by blood/tissue peptidases). A good response for regulation would have been mentioning principally regulated via renin release (which in itself is influenced by renal sympathetic nervous system activity, intrarenal baroreceptors and macula densa sodium chloride delivery, ADH and intra-renal prostaglandins), negative feedback from angiotensin II.
Syllabus: N2h
Recommended sources: Textbook of medical Physiology, Guyton, Chp 26.
7. Define lung compliance (30% marks). Describe how is it measured (70% marks).
CICMWrecks Answer

Lung compliance
- Change in lung volume per unit change in transmural pressure
- Normal lung compliance ~200ml/cmH2O
- Static compliance (Cs)
- ” Compliance of lung measured when lung held at constant lung volume”
- no pressure componet due to resistance
- is a function of: Elastic recoil of the lung, Surface tension of alveoli
- Dynamic compliance (Cd)
- ” Compliance of lung measured during cycles of inspiration and expiration”
- includes the pressure required to generate flow by overcoming resistance forces
- is a function of: respiratory rate
- Dynamic Compliance usually < Static Compliance due to the degree of time dependence in the elastic behaviour of a particular lung
- Specific compliance
- Compliance per unit volume lung
- Used to compare different lungs
- Hysteresis
- any process where the future state of a system is dependent on its current and previous state
- Compliance of the lung is different in inspiration and expiration
- In dynamic compliance curves:
- Airways resistance is a function of flow rate. Flow rate (therefore resistance) is maximal at the beginning of inspiration and end-expiration.
- In static compliance curves:
- There is no resistive component. Hysteresis is due to viscous resistance of surfactant and the lung.
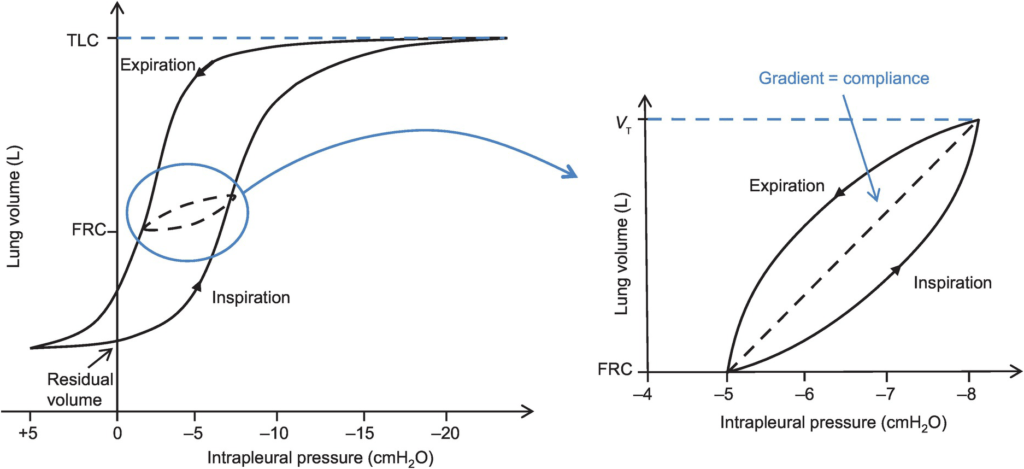
Factors Affecting Compliance
Lung Compliance
- Surfactant
- increases lung compliance
- decreases surface tension at alveolar air – water interface
- prevents small alveoli from collapsing
- accounts for most of hysteresis in intact lungs
- Lung volume
- Lung Compliance decreases at higher lung volumes
- Specific compliance (Compliance/FRC) remains constant
- Elephants have greater lung compliance than mice!
- Pulmonary blood volume
- Increased PBV decreases lung compliance
- Pulmonary venous congestion from L heart failure or mitral regurgitation
decreases lung compliance
- Bronchial smooth muscle tone
- Increased bronchial smooth muscle tone decreases compliance
- Decreased dynamic lung compliance by 50% in animal models of methacoline
challenge
- Disease
- ARDS, pneumonia decreases lung compliance
- pulmonary fibrosis → Impraired elasticity → decreases lung compliance
- Asthma, Emphysema increases lung compliance
Chest Wall Compliance
- Chest wall restriction – reduced chest wall compliance
- Obesity
- Spastic paralysis of chest wall musculature
- Ossification of costal cartilages
- Kyphosis/scoliosis
- Scarring/constriction (e.g. circumferential burns)
- Position: Prone (60% reduced compliance)
- Collagen Disorders: Increased Chest Wall Compliance
Measurement of Compliance
Dynamic Compliance
- ” Compliance of lung measured during cycles of inspiration and expiration”
- Measure:
- Measure with spirometer
- Using Volume/Pressure curve during normal rhythmic breathing at points of no flow
- Equation above then gives dynamic compliance
Static Compliance
- ” Compliance of lung measured when lung held at constant lung volume”
- Volume vs Pressure loops during inspiration and expiration demonstrate Hysteresis
- Measure:
- @ no flow in or out of the lung, AND time for pressure to equlibrate across the lung (>10sec).
- Patient exhales in steps holding the volume with open glottis
- Intrapleural pressure measured as oesophageal pressure
- Measured compliance secondary to viscoelastic properties and accounts for both short and long time-constant alveoli.
Specific Compliance
- Measure of the average compliance across all lung units
- Children = Adults ~ 0.05 cmH2O-1
Gladwin / Sakurai / JC 2020
Examiner Comments
2011B 07: 9 (36%) of candidates passed this question.
This is a core area of physiology that relates to everyday Intensive Care practice, thus it was expected that more than the observed number of candidates would have scored well. Candidates performed poorly purely from a lack of sufficient knowledge. Easy marks were to be gained purely by mentioning that compliance is defined by ∆V/∆P, the ∆P being the gradient from alveolar – intrapleural, normal values, static and dynamic compliance. Good answers would then include a mention of how static and dynamic compliance is measured (specifically how volume and pressures are measured.
Syllabus: B1d 2b
Recommended sources: Nunn Applied Respiratory Physiology, 6th Ed, Pgs 35-36
8. Explain the physiological factors that prevent gastro-oesophageal reflux.
CICMWrecks Answer
- Main barrier to reflux is the lower oesophageal sphincter (LOS).
- Sphincter pressure maintained at 15-25 mmHg > gastric pressure.
- Factor which maintain sphincter pressure are Anatomical, neurological and hormonal.
Anatomical factors which Minimse GOR:
- Functional anatomy of LOS
- Internal (Circular SM) and external sphincters (in continuity with diaphragm)
- Small portion of distal oesophagus is intra-abdominal
- ↑ Intra-abdominal pressure → ↑ distal LOS pressure → ↑ LOS barrier pressure
Neuronal factors which minimise GOR:
- Vagal (ACh) outflow
- Contracts the ”internal sphincter”
- ↑ circular SM tone → ↑ LOS pressure → ↑ LOS barrier pressure
- Phrenic nerve stimulation
- Contracts ”external sphincter” via ”pinch-cock” mechanism
- ↑ LOS pressure → ↑ LOS barrier pressure
Hormonal Factors which limit GOR:
- ↑ LOS tone → ↑ LOS barrier pressure
- Gastrin, CCK, motilin, α-adrenergic stimulation
- ↓ LOS tone → ↓ LOS barrier pressure
- Secretin, VIP, GIP, glucagon, progesterone, and prostaglandins
Gladwin 2016
Examiner Comments
2011B 08: 3 (12%) of candidates passed this question.
For a good answer candidates were expected to give a description of the lower oesophageal sphincter (the intrinsic and extrinsic sphincters and flap-valve), that it maintains a resting pressure of 15-25 mmHg above gastric pressure which prevents gastro-oesophageal reflux, which relaxes after swallowing, that the resting tone is maintained by myogenic and neurogenic mechanisms and the effects of hormones upon the sphincter (ie gastrin, motilin and α adrenergic stimulation increases and secretin, glucagon, VIP and GIP decrease tone).
Syllabus: Q1 2f
Recommended sources: Ganong Review of Medical Physiology Chp 26.
9. Define a Portal System. Describe the anatomy and function of three portal systems in the body.
CICMWrecks Answer
Portal System
- System where two separate capillary beds are supplied in consecutive series, before blood returns to the heart
Hepatic portal system
- Anatomy
- Arterial blood perfuses the GI tract (from stomach to proximal rectum) via the Coeliac trunk, superior mesenteric arteries and inferior mesenteric arteries.
- Capillary blood from GI tract drain to superior mesenteric and inferior mesenteric veins which converge to form the portal vein
- Portal vein perfuses the liver as part of the portal triad
- Hepatic venous blood drains into the inferior vena cava before returning to the heart
- Function
- Protects systemic circulation from blood from portal venous blood
- Toxins absorbed from GI tract (ammonia) metabolized by liver before reaching systemic circulation
- Pathogens encounter leukocytes (e.g. macrophages) in space of Disse in liver
- Fluctuation in blood constituents due to GI absorptions buffered by liver (e.g. glucose)
- Protects systemic circulation from blood from portal venous blood
Renal portal system
- Anatomy
- Renal arterial blood perfuses the renal cortex via afferent arterioles à glomeruli
- Glomeruli drain blood into efferent arterioles
- Efferent arteriolar vessels drain into peritubular capillary network
- Proportion of efferent arteriolar vessels form vasa recta which supply blood to renal medullary interstitium via vasa recta à drains to renal venules and veins before returning to heart
- Function
- As peritubular capillary network has lost Na and H2O to glomerular filtrate à increases reabsorptive capacity of renal tubules due to concentration gradients
Hypothalamic-Pituitary Axis
- Anatomy
- Arterioles from superior hypophysial artery (branch of circle of willis) supply hypothalamus via primary plexus à drains to portal hypophysial vessels
- Portal veins supply anterior pituitary
- Blood returns to heart via internal jugular vein
- Function
- Delivery of tropic hormones to anterior pituitary for signalling
- CRH
- TRH
- GnRH
- GRH
- PRH
- Delivery of tropic hormones to anterior pituitary for signalling
Sakurai 2016
Examiner Comments
2011B 09: 8 (32%) of candidates passed this question.
A portal system is an arrangement by which blood collected from one set of capillaries passes through a large vessel or vessels, to another set of capillaries before returning to the systemic circulation. The three portal systems are the –
1) system of blood vessels that link the hypothalamus and the anterior pituitary in the brain, which allows endocrine communication between the two structures.
2) within the liver, whereby venous blood from the GI tract drains into the superior and inferior mesenteric veins; these two vessels are then joined by the splenic vein to form the portal vein which enters the liver, drains into the hepatic sinusoids and then eventually into the hepatic veins which join the inferior vena cava, with the purpose of defending against by breaking down and metabolising most of what has been absorbed from the gastrointestinal tract (including an immunoprotective action).
3) within the kidney, whereby blood from the afferent arterioles enters the glomerulus (first capillary network), followed by the efferent arterioles, then the peritubular network (second capillary network) and eventually the venous system, with the purpose of stronger re-absorptive capacity for water from within long Loops of Henle that go deep within the renal medulla.
Syllabus: N1 2c, I2d, D1 2a
Recommended sources: Ganong Review of Medical Physiology Chps 18, 38, 29
10. Describe the pharmacology of magnesium sulphate.
Examiner Comments
2011B 10: 1 (4%) of candidates passed this question.
Magnesium is a commonly administered agent within Intensive Care practice. Candidates performed poorly because of a lack of sufficient knowledge and a failure to structure their answer. For a good answer candidates were expected to mention that Magnesium comes as an inorganic sulphate, acts as a cofactor for a vast number of reactions, can be given orally (poor absorption) as well as intravenously, renal excretion with a low threshold.
Syllabus: O2 2c
Recommended sources: Rang and Dale Pharmacology Pg 388; Katzung Basic and Clinical Pharmacology, Pg 244.
11. Outline protein metabolism, including the effects of starvation
CICMWrecks Answer
Protein Metabolism
Amino acids
- Proteins are polypeptides created from amino acids linked together by peptide linkages
- 20 amino acids present in the body in significant quantities
- Of the 20, 10 are essenstial amino acids, which cannot be synthesized within the body in sufficient quantities and must be obtained, pre-formed, from food.
- Amino acids absorbed from diet
- Absorbed by cells, especially hepatocytes
- Amino acids re-absorbed from filtrate in PCT
Protein anabolism
- Peptide linkages → covalent bonding of amino acid amine group and carboxyl group by condensation
- Proteins created in ribosomes in rough endoplasmic reticulum, according to translation of mRNA, which is itself transcribed from cell DNA
- Further post-translational processing of proteins occur in the smooth endoplasmic reticulum (e.g. glycosylation)
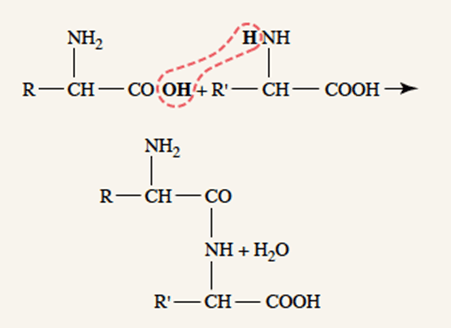
Protein storage
- After entering a cell, amino acids almost immediately converted into proteins, therefore intracellular amino acid concentration remains low
- Amino acids “stored” as proteins
- The liver stores large amounts of exchangeable proteins as rapidly available amino acid source
Protein Catabolism
- Proteins destined for degradation are “tagged” by another regulatory protein, ubiquitin, and targeted for degradation into it amino acid components by the proteosome in the cytosol
- Amino acids are recycled for formation of other proteins
- Use of proteins for energy
Deamination → Release of NH3 + NADH + H+- Ammonia released from deamination is removed from the blood by conversion to urea in the liver
- 2 x NH3 + CO2 → Urea + H2O
- Deaminated amino acids can be used in the citric acid cycle
- via Acetyl-CoA, ketogenesis or conversion to fatty acids
- Deaminated amino acids can be used for gluconeogenesis
- Ammonia released from deamination is removed from the blood by conversion to urea in the liver
Regulation of Protein Metabolism
- Insulin and growth hormone promote storage of amino acids as proteins
- Glucocorticoids promote release of amino acids from protein depots
- Testosterone promotes storage of proteins in cells (esp. muscle)
- Thyroxine → increases metabolism
- In presence of excess fat and carbohydrate → protein synthesis and storage
- In fat and carbohydrate defecit → increased protein break-down
Starvation
- Daily obligatory protein loss
- ~30g
- Except for obligatory protein loss, body utilizes carbohydrates and fats almost exclusively
- Once body carbohydrate and fat stores are depleted, amino acids are oxidized and used for energy
- ~125g protein / day
Sakurai 2016
Examiner Comments
2011B 11: 1 (4%) of candidates passed this question.
For a good answer candidates were expected to mention that there were “essential” and nonessential proteins”, that protein synthesis draws on amino acids sourced from the common amino acid pool (because there is no cellular storage of amino acids) which is the combination of amino acids derived from gut absorption of digested dietary protein and also the continuous turnover of endogenous protein, amino acids filtered via the glomerulus are reabsorbed within the renal tubule and that in health losses are minor, but increases with illness. Protein synthesis is a semicontinuous activity, the rate of which varies considerably between cells under the influence of factors neuro- endocrine factors, substrate availability and energy availability. Proteolysis occurs within nuclear and cytoplasmic proteasomes, via peptide bond disruption catalysed by proteases, which are then processed further into amino acids being then available for release into the amino acid pool or utilised immediately for protein synthesis.
In relation to starvation candidates were expected to mention that it results from a severe ongoing restriction of protein, energy and nutrient intake. That initially, glycogen stores from the liver and skeletal muscle are utilised after conversion to glucose and provide energy, thus sparing of fat and protein stores from use as an energy source for a period of less than a day, following which fats are increasingly utilised and finally protein catabolism accelerates, especially from the liver and skeletal muscle, with relative sparing of the heart and brain.
Syllabus: K2c,f
Recommended sources: Ganong Review of Medical Physiology Chp 2
12. List the functions of the liver (60% marks). Discuss the metabolism of paracetamol in toxicity and the pharmacologic management of this overdose (40% marks).
CICMWrecks Answer: Liver Functions
Liver:
- Largest abdominal solid organ
- 25 % CO at rest. approx 1,500 mls/min
- 15% of total blood volume at rest
Functions of Liver
| Filtration | |
| Immune defence | (via Kuppfer cells) against agents entering the portal circulation |
| 80% of circulating cholesterol | → bile salt |
| Biliary excretion of drugs/hormones | penicillins, amp, erythro |
| thyroxine, cortisol, estrogen | |
| calcium | |
| Immune | |
| Filtration of portal circulation | |
| Kuppfer cells | bacteria/ virus/ endotoxins/ immune complexes/ thrombin/ tumour |
| Phagocytosed, fused with lysozomes and degraded by lysosomal enzymes | |
| Antigen presentation | |
| Endotoxin neutralisation = pinocytosed | |
| Complement/CRP production | |
| Storage of metabolic substrate/fluids | |
| glycogen ~ 400g | |
| fat | |
| Fe++, B12, folate, Cu | |
| Vitamin A | |
| Blood Reservoir | |
| Metabolic | |
| CHO and intermediary metabolism | Hepatic Glucostat |
| gluconeogenesis, glycogen storage and utilisation, galactose/fructose to glucose | |
| Conversion to fat, AA’s and ketones | |
| Protein metabolism | amino acids utilisation |
| protein synthesis | |
| production of ketones | |
| deamination of fatty acids | |
| urea formation for ammonia removal | |
| plasma protein formation | |
| Fat homeostasis | metabolism (beta oxidation (rapid in hepatic cells)), synthesis and transport as lipoprotein |
| cholesterol homeostasis | |
| Endocrine | hormone synthesis & metabolism |
| Synthesis of 25 OH cholecalciferol, Metabolism of steroid hormones, Synthesis of somatomedins, Erythropoietin | |
| biotransformation | ammonia & urea cycle |
| drugs & toxins | |
| Acid-Base | Lactate metabolism |
| Synthetic functions | |
| Bile production | bile salts (incr fat absorption) |
| bilirubin (incr haem excretion) | |
| Protein synthesis | albumin 120-300mg/kg/d |
| alpha1/2 & beta globulins (transport) | |
| coagulation & fibrinolytic factors (fibrinogen, prothrombin, II, V, VII,VII, IX, X, XI, XII, XIII, antithrombin) | |
| Lymph synthesis | Up to 50% |
| Erythropoietin (10%) | |
Gladwin / JC / Bianca 2019
CICMWrecks Answer: Paracetamol Toxicity
Paracetamol Overdose
Hepatotoxicity
Toxic daily dose > 4-6 g (while lethal dose occurs at > 10-15 g (or > 300 mg/kg LBM)) → but the toxic and lethal doses are lower in “at-risk” groups:
- EtOH abuse (due to CYP450 induction (↑ toxic metabolite produced) and ↓ glutathione stores)
- Malnutrition (due to ↓ glutathione stores)
- Elderly (due to ↓ glutathione stores)
- Preexisting liver dysfunction
Mechanism:
- At therapeutic doses – “N-acetyl-p-amino-benzoquinoneimine” (highly toxic metabolite) is produced in small amounts, but is rapidly conjugated with hepatic glutathione (anti-oxidant) into a harmless metabolite
- BUT with toxic doses – Hepatic conjugation pathway is saturated and ↑↑↑ N-acetyl-p-amino-benzoquinoneimine is produced → this depletes hepatic glutathione stores, causing remaining N-acetyl-p-aminobenzoquinoneimine to then forms covalent bonds with sulphydryl groups on hepatocytes → results in centrilobular hepatic necrosis
Clinical features:
- Generally conscious and c/o N/V, epigastric pain, erythema, sweating → later developing:
- Acute haemolytic anaemia
- Develop hepatic failure (Ie. jaundice and cholestasis) after 48 hrs
- LFT/INR derangements at 3-5 days
- Fulminant hepatic failure at 3-7 days
- With severe OD, can present with hypotension/shock
Diagnosis :
Serum paracetamol levels → correlate with “nomogram” to predict likelihood of liver damage → used as a guide to dictate therapy
Treatment:
- Activated charcoal/gastric lavage→ limit paracetamol absorption
- Replace hepatic glutathione store within 12 hrs of OD → permits glucuronidation of toxic metabolite
- Oral methionine → ↑ glutathione synthesis
- IV N-acetylcysteine → hydrolysed to cysteine (which is a glutathione precursor)
- Nb. IV NAC is preferred b/c of N/V a/w toxicity (Ie. ↓ oral methionine absorption)
- IV glucose → due to risk of ↓ BGL with liver dysfunction
- Serial monitoring of LFTs and coagulation studies
- Referral to a specialist centre
Gladwin / JC / Bianca 2019
Examiner Comments
2011B 12: 5 (20%) of candidates passed this question.
A good response to this question required an ordered and well structured response. There are numerous important functions that the liver undertakes (eg bile formation, immunological, protein, lipid, glucose metabolism, storage, endocrine, etc), yet most candidates could not generate a sufficient list. In relation to paracetomol toxicity, a good response required candidates to mention that normal conjugating reactions in the liver are saturated and metabolism diverts to mixed function oxidases, which generate toxic metabolites. These in turn are inactivated by conjugation with glutathione. However when glutathione is depleted, toxic metabolites react with cellular nuclear material, thus causing liver necrosis. Toxic compound depletes sulphydril groups and also causes direct damage via lipid peroxidation. Regeneration of sulphydril groups and glutathione depends on availability of cysteine, thus the need to administer acetylcysteine.
Syllabus: I2a, G2e2c
Recommended sources: Gannong Review of Medical Physiology pg 485; Power and Kam
Principles of Physiology for the Anaesthetist pg185; Stoelting Pharmacology and Physiology in Anesthetic Practice 4th edition pg 285; Rang & Dale Pharmacology 5th Ed pg 244.
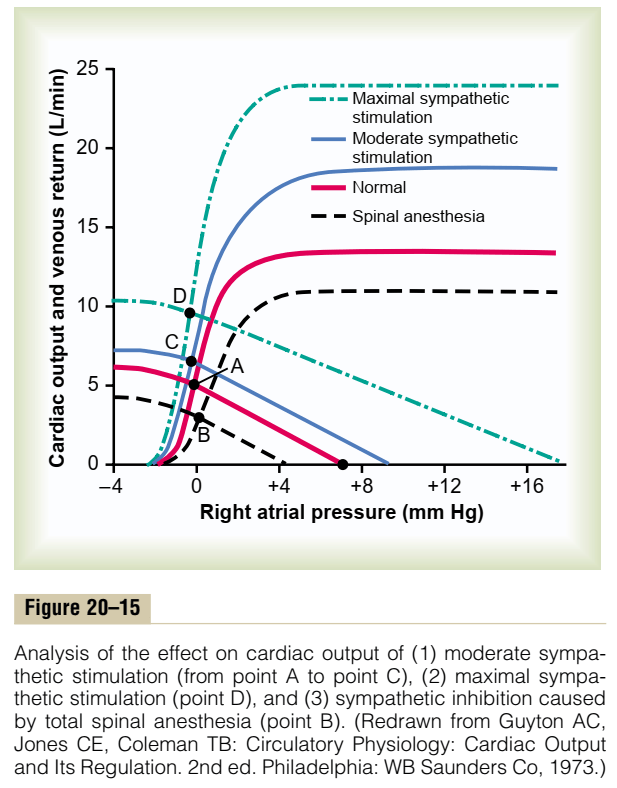
13. Discuss the regulation of cardiac output. Illustrate your answer by using a graph to describe the important physiological relationships.
CICMWrecks Answer
Cardiac output is the amount of blood pumped into the systemic circulation per unit time.
Normal Cardiac Output = 5l/min
Normal Cardiac Index = 3l/min/m2
Cardiac Output = SV x HR
Stroke Volume
SV = Amount of blood pumped into the circulation per contraction
Usually 70mls
Influenced by Preload, Afterload, Contractility
- Preload / Venous Return:
- = (MSFP – RAP) / RVR
- MSFP: Mean Systemic Filling Pressure
- Equilibration of pressures in the systemic circulation if cardiac output was abolished
- Describes the filling state of the circulation and tone of capacitance vessels
- RAP: Right Atrial Pressure
- RVR: Resistance to Venous Return
- Calibre of transmitting venous system according to Poisuille-Hagen equation (where resistance ∝ 1/r4)
- Physical obstructions to venous return
- Mechanical obstruction
- Valvular stenosis
- MSFP: Mean Systemic Filling Pressure
- Thoracic pump (negative intrathoracic pressure created during inspiration)
- Skeletal muscle pump
- One way valves
- Pump function of the ventricle
- Venous tone
- = (MSFP – RAP) / RVR
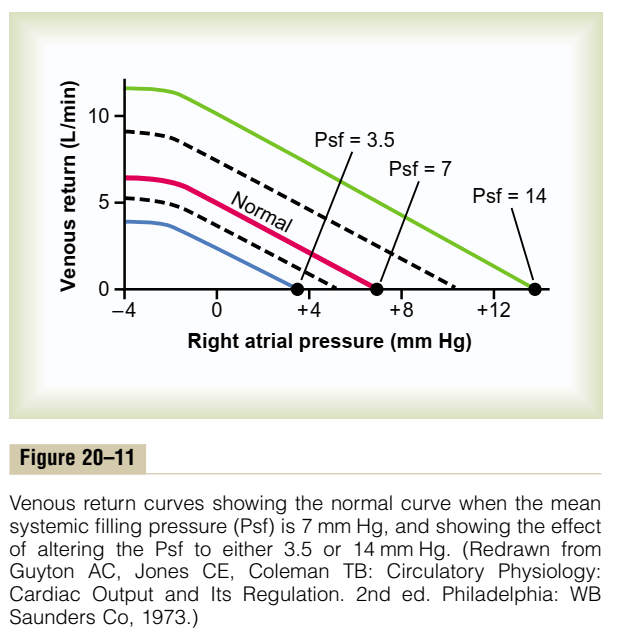
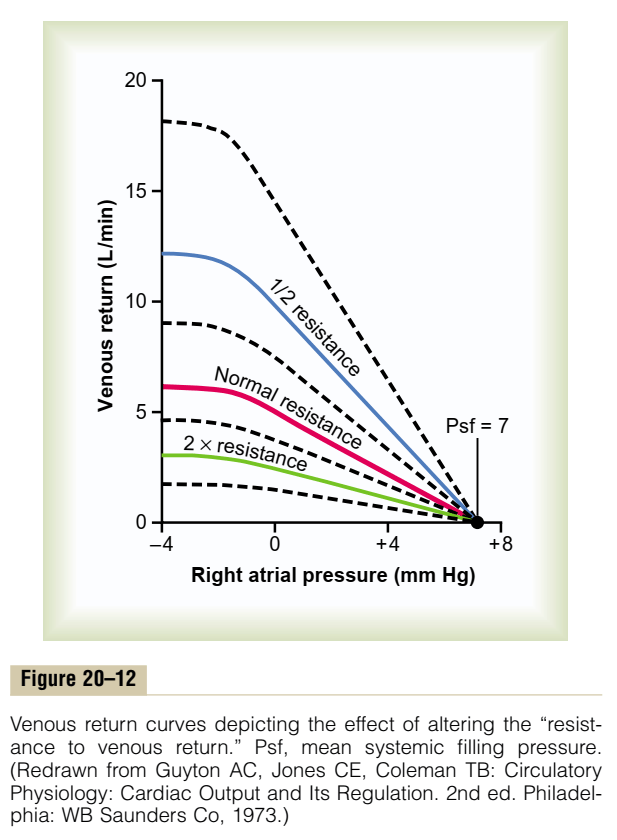
- Afterload
- Proportional to aortic pressure and ventricular size
- Inversely proportional to ventricular wall thickness
- Affected by obstruction to the LVOT
- Contractility
- Affected by adrenergic or muscarinic stimulation
- Availability of Ca
Heart Rate
- Affected by automatic rhythmicity of cell and sympathetic/muscarinic input
Cardiac Output regulated by venous return
- Ventricles have ability to adapt to various degrees of filling according to the Frank-Starling Principal
- Increasing preload increases cardiac output to a degree, before cardiac output falls
- Due to the optimal alignment of actin and myosin filaments at sarcomere length of 2.2μm
- Also due to some stretch related release of Ca2+ increasing contractility
Autonomic Regulation
- Medullary vasomotor area
- Inputs to sensory area in NTS
- Vasoconstrictor area in anterolateral upper medulla
- Sympathetic output
- Vasodilator area in anterolateral lower medulla
- Parasympathetic output
- Adrenaline and to a lesser extent noradrenaline stimulates β2 adrenoceptors
- GsPCR
- Increased cAMP due to upregulation of adenylyl cyclase
- Activation of PKA leading to phosphorylation and activation of intracellular enzymes
- Inotropy
- Increased Ca2+ release from and SR and influx from T-tubules
- Increased intracellular [Ca2+]
- Increased Ca2+ – troponin C interaction
- Increased Actin and myosin interaction due to exposure of myosin binding sites
- Increased contractility
- Chronotropy
- Inotropy
- Acetylcholine
- GiPCR → decreased cAMP and reversing the above
- Muscarinic receptor coupled K+ channels hyperpolarize the cell reducing chance for action potential
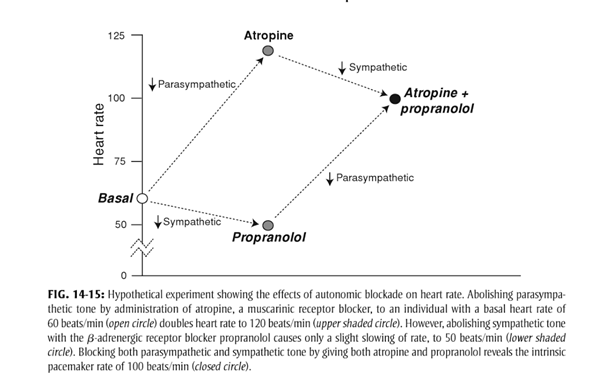
- At rest
- Parasympathetic and Sympathetic tone
- Parasympathetic > Sympathetic
- HR ~60
- + Propranolol → HR 50
- + Atropine → HR 110
- + Propranolol + Atropine → HR 100
- HR ~60
- Parasympathetic > Sympathetic
- Parasympathetic and Sympathetic tone
Reflexes
Baroreflex
- Sensor
- Aortic arch and carotid sinus stretch receptors
- Transmit via vagal nerve and glossopharyngeal nerve respectively
- Control centre
- Vasomotor area in medulla
- Increased stretch causes inhibition of sympathetic and promotion of parasympathetic output
- Effector
- Parasympathetic innervation of the heart via vagal nerve upregulated
- Sympathetic innervation via thoracic segments of the sympathetic chain
Bainbridge reflex
- Input
- Increased venous return or obstruction to atrial outflow causes atrial stretch
- Atrial stretch receptors signal to vasomotor area
- Increased sympathetic output to heart à tachycardia
Sakurai 2016
Examiner Comments
2011B 13: 11 (44%) of candidates passed this question.
Most candidates approached this question by defining cardiac output as stroke volume × heart rate and then discussing the determinants of cardiac output – preload, contractility, afterload and heart rate rather than focusing on the regulation of cardiac output. Under preload a brief description of the Frank Starling mechanism was required. Important was the concept that at rest cardiac output is controlled almost entirely by peripheral factors that determine venous return. These concepts were best illustrated by graphing vascular function (venous return vs right atrial pressure) and cardiac function (cardiac output vs right atrial pressure) curves. Then demonstrating on these curves the factors that affected preload, contractility and afterload such as changes in blood volume, sympathetic and parasympathetic stimulation and exercise as examples. Also important to demonstrate on these curves was the fact that venous return and cardiac output are equal at steady state. Most candidates tried to illustrate these cardiovascular concepts with a series of left ventricular pressure volume loops rather than use the vascular and cardiac function curves. They then went on to demonstrate via these pressure volume loops the effects of changes in preload, contractility and afterload on stroke volume. Candidates who took this approach were not penalised, if there were clear, correct diagrams with explanations indicating comprehension of these concepts. On the whole graphs were poorly drawn and were not well integrated into the answer. Some candidates also wasted time by unnecessarily describing excitation-contraction coupling and sympathetic nerve reflex pathways.
Syllabus: C1c 2a,c,e,f,g Recommended sources: Guyton Textbook of Medical Physiology 11th edition pgs 241-243.
14. Compare and contrast the pharmacology of dobutamine and milrinone
Examiner Comments
2011B 14: 14 (56%) of candidates passed this question.
Many candidates presented their information in a tabular form and this worked well as it allowed direct comparison between the two drugs. Most candidates did not mention that dobutamine was a racemic mixture of [+] and [-] isomers. Also that the [+] isomer was a potent β1 agonist and α1 antagonist, while the [-] isomer was an α1 agonist. The administration of the racemic mixture results in the overall β1 agonism responsible for its activity and also its mild β2 agonist effect. While most candidates stated that milrinone was an inodilator details on its mechanism of action as a selective phosphodiesterase type III inhibitor were on the whole vague. Within the cytoplasm of the cardiac myocyte milrinone inhibits the enzyme PDE 3 which results in the inhibition of the breakdown of cyclic AMP which in turn results in elevated cellular levels of cAMP. These elevated levels of cAMP in turn activate cAMP dependant protein kinases with a resultant increase in the influx of Ca2+ into the cell via the sarcolemma. Also uptake of Ca2+ by the sarcoplasmic reticulum is increased. The overall effect is an increase in intracellular Ca2+ which increases myocardial contractility. Milrinone also has lusitropic action, inducing left ventricular relaxation. This probably occurs as a result of the inhibition of SR membrane bound PDE3.
Milrinone also cause peripheral vasodilatation by inhibiting PDE3 in vascular smooth muscle cells which again results in elevated cAMP levels.In vascular smooth muscle cAMP normally inhibits myosin light chain kinase the enzyme that is responsible for phosphorylating smooth muscle myosin and causing muscle contraction.
Many candidates did not emphasize how very different the pharmacokinetics of these two drugs were. Milrinone has a much longer half life than dobutamine and because of its predominant renal excretion accumulates in renal failure.
Syllabus: C2d 2a
Recommended sources: Goodman & Gillman The pharmacological Basis of Therapeutics Chp 33.
15. Outline the motor and sensory pathways involved in withdrawing the lower limb from a painful stimulus.
CICMWrecks Answer
Withdrawal reflex is a spinal reflex intended to protect the body from damaging stimuli.
The upper centres are not involved.
Afferent
- Nociceptors detect the painful stimulus
- Conveyed to the dorsal horns of spinal cord by Aδ (fast) fibres and C (slow) fibres
- Through the cauda equina
- Synapse at the same spinal level, or 1-2 levels above via Lissauer’s tract
Reflex arc
- (fast response to painful stimulus)
- (outside of conscious control, but can be modulated by the cerebral cortex)
- Interneurons:
- Travel to the ipsilateral anterior horn, synapse with motor neurons
- Cross the midline to the contralateral anterior horn
- Efferent
- Motor neurons in the ipsilateral limb cause contraction of flexors, and relaxation of extensors
- Contralateral limb motor neurons cause relaxation of flexors, and contraction of extensors
- Causes withdrawal of painful limb, and shift In centre of gravity to the opposite limb
Conscious pathways
(slow response to painful stimulus)
Interneurons:
- Cross the midline via the anterior white commissure
- Ascend in the spinothalamic tract to the thalamus
- Synapse in the thalamus
- Travel to the post-central gyrus of the cerebral cortex
Efferent:
- Originate in the motor cortex (pre-central gyrus)
- Project to medulla
- 90% decussate at pyramids to innervate limbs
- Travel down the lateral corticospinal tract
- 10% continue down ipsilateral anterior corticospinal tract to innervate axial muscles
- Decussate at the target spinal level
- Synapse at the anterior horn to lower motor neurons
Mooney / Sakurai 2016
Examiner Comments
2011B 15: 5 (20%) of candidates passed this question.
A good answer required a description of the sensory pathway(s) (eg nociceptors, Aδ and C fibres, spinal dorsal horn, spinothalamic, thalamic and cortical pathways), reflex arc (nocioceptive sensory fibres synapse with spinal inter-neurons that in turn synapse with peripheral motor neurons supplying the lower limb as well as inter-neurones that also synapse with motor neurons on the contra-lateral lower limb producing a crossed extensor response), central integration and the motor pathways (fibers from the contra-lateral motor (and pre-motor) cortex pass through the posterior internal capsule forming the lateral and ventral cortico-spinal tracts, the cortico-spinal tracts pass through the anterior brainstem, the lateral tract decussating in the caudal medulla, continue to synapse with spinal motor neurons in the ipsilateral lumbosacral anterior horn cells, passage via peripheral nerves and flexor muscle stimulated, extensors inhibited, resulting in withdrawal of the limb) Common mistakes included errors in naming nerve pathways and receptors. Another error was to confuse the polysynaptic pain response pathways with the monosynaptic stretch reflex. Very few candidates mentioned feedback regulation via cerebellar input and at spinal level from muscle spindles and Golgi tendon organs
Syllabus: G12b
Recommended sources: Ganong Review of Medical Physiology Chps 11 & 16
16. Using a diagram, explain the effect of PaO2, PaCO2 and MAP (mean arterial pressure) on cerebral blood flow (60% marks). Outline the effects of propofol and ketamine on cerebral blood flow (CBF), cerebral metabolic requirement for oxygen (CMRO2), and cerebral venous oxygen saturation (40% marks).
CICMWrecks Answer: Cerebral Blood Flow
Cerebral Blood Flow
- CBF = CPP / CVR (Cerebral Perfusion Pressure / Cerebral Vascular Resistance)
- CBF 15% resting CO → ~750ml/min or 50ml/100g brain tissue/min
- Gray Matter: 75–80 mL/100 g/min – Significantly higher due to the high metabolic activity and dense synaptic connections
- White Matter: 20–30 mL/100 g/min
- Abnormal CBF
- CBF<50ml/100g/min → cellular acidosis
- CBF<40ml/100g/min → impaired protein synthesis
- CBF <30ml/100g/min → cellular oedema
- CBF <20ml/100g/min → failure of cell membrane ion pumps, loss of transmembrane electrochemical gradients
- CBF <10ml/100g/min → cell death
Determinants of CBF
- CPP
- Net pressure gradient driving blood flow through the cerebral circulation
- CPP = MAP – ICP
- MAP = CO x SVR; CO = HR x SV; SVR = MAP / CO
- ICP dependent on: brain, blood, CSF
- CVR
Regulated by 4 primary factors:
- Cerebral metabolism
- flow-metabolism coupling:
↑ metabolic demand → ↑ CBF + substrate delivery - Controlled by vasoactive metabolic mediators:
H+ ions, K, CO2, adenosine, glycolytic intermediates, NO
- flow-metabolism coupling:
- CO2 and O2
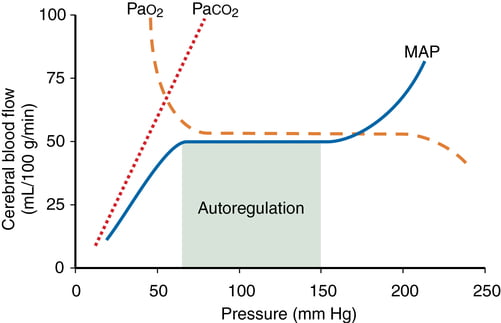
- CO2
- At normotension: relationship between PaCO2 and CBF = almost linear
- ↑ PaCO2 → cerebral arteriolar vasodilation → ↓ CVR + ↑ CBF
- 2~4% increase for every 1mmHg increase in CO2
- ↓ PaCO2 → cerebral arteriolar vasoconstriction → ↑ CVR + ↓ CBF
- Initial stimulus = ↓ brain ECF pH
- Effects regulated by: NO, prostanoids, K channels, intracellular [Ca2+]
- PaO2
- little effect at normal PaO2
- PaO2 <60mmHg → cerebral arteriolar vasodilation → ↑ CBF
- Mechanism: hypoxia acts on →
- cerebral tissue to promote release of adenosine → cerebal vasodilation
- cerebrovascular smooth muscle → hyperpolarisation → ↓ Ca2+ uptake → vasodilation
- CO2
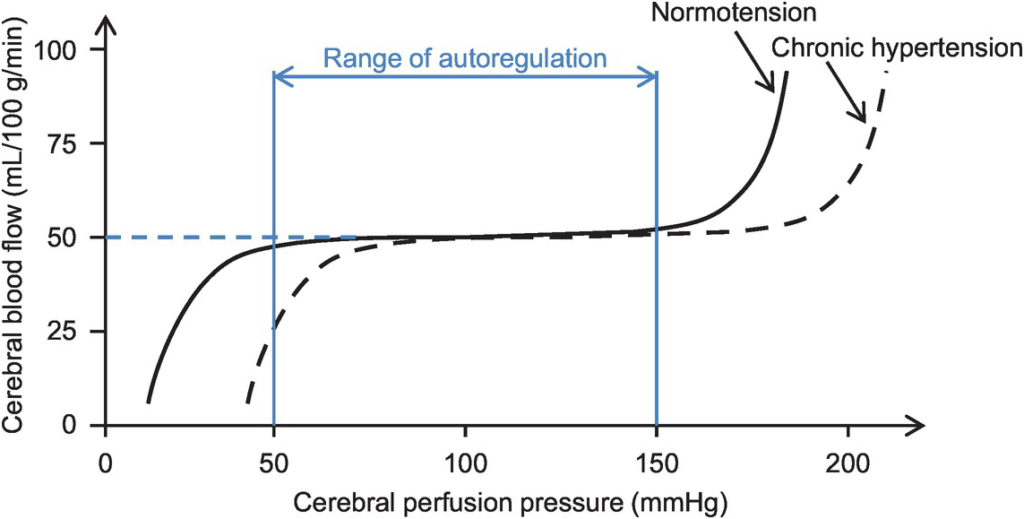
- Autoregulation
- Constant across CPP 50-150mmHg
- CPP >150mmHg: CBF µ CPP
- CPP <50mmHg: CBF <50ml/100g brain tissue/min → ischaemia
- Stimulus to autoregulation = CPP (not MAP)
- autoregulation curve R shifted in HTN; L in neonates
- Mechanism:
- Myogenic mechanism: arterioles vasoconstrict in response to ↑ wall tension + vasodilate in response to ↓ wall tension → ↓ or ↑ CVR
- May involve adenosine
- Can be impaired in SAH, tumour, stroke, head injury
- Constant across CPP 50-150mmHg
- Neurohumeral factors
- Relative lack of humoral + autonomic control on normal cerebrovascular tone
- Main action of SY nerves = vasoconstriction
- Other factors
- Blood viscosity: directly related to HCt; ↓ viscosity → ↑ CBF as per Hagen-Poiseuille law
- Temperature: ↓ CMRO2 by 7% for each ↓ 1°C in temp
- Drugs
- E.g. barbiturates ↓ cerebral metabolism
- Volatile agents → ↓ tension cerebral vascular smooth muscle → vasodilation + CBF
Kerr 2018
CICMWrecks Answer: Effect of Propofol and Ketamine
Effect of Propofol and Ketamine
| Propofol | Ketamine | |
|---|---|---|
| Action | Sedative agent | Dissociative sedative and analgesic agent |
| MoA | Effects on GABAA potentiation and Na channel blockade | Effects on NMDA receptor antagonism |
| CBF | ↓ (dose-dependant) | ↑ |
| CMRO2 | ↓ | ↑ |
| ↓ CBF ∝ ↓ CMRO2 | ↑ CBF > ↑ CMRO2 | |
| SjvO2 | minimal change | ↓ |
| doesn’t affect the autoregulatory curve of CBF and the PaCO2 response |
Kerr 2018
Examiner Comments
2011B 16: 12 (44%) of candidates passed this question.
Graphical depictions of the effect of Mean Arterial Pressure, oxygen tension and carbon dioxide tension on cerebral blood flow were common and in general accurate. Mention of factors that affected, and regulation of, the MAP vs CBF graph was expected in order to pass this question well.
The effect of propofol and ketamine on the CBF was well answered. Propofol and ketamine have an opposite effect on cerebral haemodynamic and metabolic rate. Propofol produces a dose dependent reduction in CBF with proportionate reduction in CMRO2, and thus a minimal change in cerebral venous O2 Sat. Propofol doesn’t affect the autoregulatory curve of CBF and the PaCO2 response. Ketamine produce a dose dependent increase in CBF and a mild increase in CMRO2.
Syllabus: C1f 2c, G2a,2a.
Recommended sources: Guyton Textbook of Medical Physiology Chp 61; Goodman and Gilman The pharmacological basis of therapeutics 11th edition pgs 350-352
17. Classify the calcium channel blockers and provide one example of a drug for each class (20% marks). Compare and contrast the pharmacology of nimodipine and verapamil (80% marks).
CICMWrecks Answer
Calcium Channel Blocker Classification
- Class I – Phenylalkylamines:
- Verapamil
- Reduces HR, contractility and causes vasodilatation, decrease in cardiac contractility, heart rate, and both coronary and peripheral vascular tone.
- Class II – Dihydropyridines:
- main effects are on vasodilatation
- 1st gen = nifedipine
- 2nd gen = felodipine, nimodipine
- 3rd gen = amlodipine
- Class III – Benzothiazepines:
- diltiazem
- some reduction in HR and contractility, also causes vasodilatation, mild to moderate decrease in myocardial contraction, and decreases heart rate by decreasing both the automaticity of the SA node and the rate of conduction in the AV node.
Calcium Channel Blocker Pharmacology
(Include as per the question)
Click here to Open CICMWrecks Table: Calcium Channel Blockers
Class Effects:
- Heart
- V-W Class 4 antiarrhythmic
- Decr SA node automaticity
- Decr AV node conductivity
- Vasodilation
- Negative inotropy
- reflex tachycardia
- 2) Vasculature
- Decr SVR and BP
- Decr Hypoxic pulmonary vasoconstriction
- Incr coronary and cerebral blood flow
- 3) Other
- Tocolytic
- NDMR potentiation.
Gladwin / JC 2021
Examiner Comments
2011B 17: 9 (36%) of candidates passed this question.
Most candidates were able to classify the calcium channel blockers well (Type I : Phenylalkylamines eg verapamil, Type II : Dihydropyridines eg nimodipine and Type III : Benzothiazepines eg diltiazem) However, the comparison of the pharmacology of nimodipine and verapamil was in general answered poorly. Few candidates demonstrated an organised approach to this part of the question. The two drugs’ presentation, routes of administration, indications and dosing were poorly answered considering that nimodipine in particular is used frequently in intensive care units. Mode of action was well answered, but important principles relating to pharmacokinetics (such as a basic outline of protein binding, bioavailability, and metabolism) were expected, but common omissions. More knowledge than ‘metabolism in the liver’ is required. Few candidates mentioned interactions, adverse effects, or predictable effects of over dosage of these drugs.
Syllabus: C2b 2d
Recommended sources: Peck Hill and Williams Pharmacology for Anaesthesia and Intensive care 3rd Ed pgs 252-255.
18. Outline the abnormalities in pulmonary function testing of a person with severe obstructive lung disease (40% marks). Describe the physiological changes that explain these abnormalities (60% marks).
CICMWrecks Answer
Obstructive Lung Disease
e.g. Asthma, COPD
| Pulmonary Function Test | Obstructive Lung Disease |
|---|---|
| Total lung capacity (TLC) | ↑ |
| Residual volume (RV) | ↑ |
| Forced vital capacity (FVC) | ↓ |
| Forced Expiratory Volume in 1st second (FEV1) | ↓↓ |
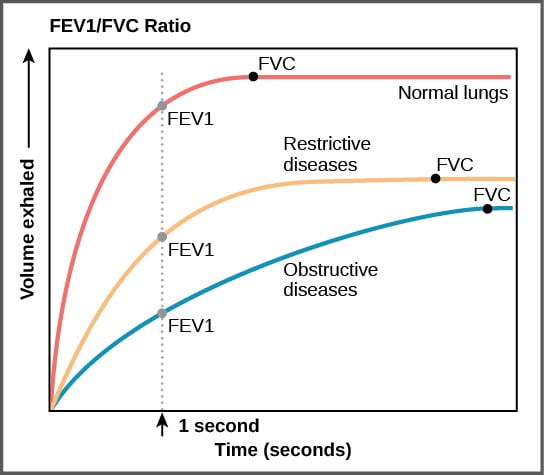
| FEV1 / FVC Ratio | ↓ , <80% |
| Maximum Voluntary Ventilation | ↓ |
| RV / TLC | ↑ |
| PEFR Peak Expiratory Flow Rate | ↓ |
| FEF 25-75% Forced expiratory flow at 25–75% of forced vital capacity | ↓ |
Physiological changes
- ↑ AWR caused by bronchial SM contraction, secretions, wall oedema, and reduced radial traction (from damaged lung parenchyma)
- Closing capacity rises towards or > FRC
- ↓ elastic potential energy is stored to generate driving pressure gradient (this is caused by ↑ lung compliance resulting from damaged lung parenchyma)
Thus
- ↓ FRMAX and ↓ steep rise in expiratory FR during effort-dependent part of the curve
- ↓ steep and “scooped” appearance of flow curve in effort-independent part of the curve due to dynamic airway compression occurring at higher lung volumes
Pulmonary function tests:
- Peak Flow
- Reduced PEF rates
- Simple spirometry:
- shows an increase in lung volumes
- decreased inspiratory capacity
- (increased FRC but not measured)
- Forced expired spirometry is diagnostic
- FEV1/FVC < 70%
- FEV1 % predicted <50%
- FEF 25 – 75% is prolonged
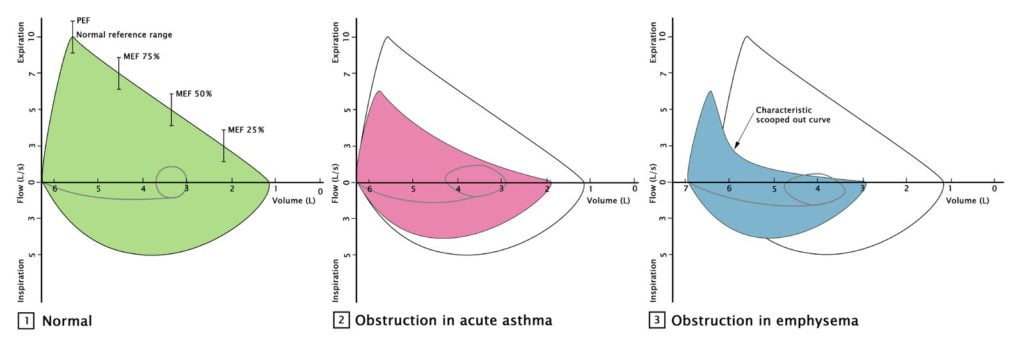
- Flow volume loops
- higher volumes
- decreased flow
- scooped effort dependent portion
https://www.wikidoc.org/index.php/File:Figure_39_03_05f.jpg
https://www.amboss.com/us/knowledge/Pulmonary_function_testing/
Gladwin 2016
Examiner Comments
2011B 18: 0 (0 %) of candidates passed this question.
The majority of candidates were able to state that obstructive lung disease resulted in a reduction in the FEV1 and the FVC. Very few gave any indication of how much these parameters might be reduced in severe obstructive lung disease or that FVC is usually only slightly reduced, whereas the FEV1 is markedly reduced and therefore so is the FEV1/FVC ratio. Candidates were also expected to indicate that peak expiratory flow rate is also reduced (by 50% or more in severe disease), that carbon monoxide diffusing capacity is also reduced, and residual volume and total lung capacity are increased. A reasonable proportion of candidates commented that the forced expiratory flow (FEF) 25-50% being reduced and this results in a ‘scalloped’ flow volume loop. Accurately reproducing a normal flow volume loop and a flow volume loop of a patient with severe obstructive lung disease on the same graph could have depicted most of the above points and scored the majority of marks available.
The second part of this question was poorly answered. Whilst the majority correctly stated that an increase in airways resistance was present, few stated what changes gave rise to this. Dynamic airway compression, was mentioned by many, but why this occurred was explained poorly. The effect of closing capacity rising above the FRC was seldom mentioned and why the FRC increased again was seldom mentioned or explained. A detailed knowledge of what is a core subject in intensive care medicine was expected.
Syllabus: B1a 2a, b; B1j 2a
Recommended sources: Respiratory Physiology: The Essentials. West 6th Ed pgs 26, 96-99, 131-142.
19. Statistics (not in current primary syllabus)
20. Describe the structure and function of platelets (50% marks). Outline the pharmacology of clopidogrel (50% marks).
CICMWrecks Answer: Platelet
PLATELET
Formation
- produced during hematopoiesis in a sub-process called thromopoiesis, or production of thrombocytes.
- Bone marrow: Common myeloid progenitor cells → promegakaryocytes → megakaryocytes
- Megakaryocytes produce protoplatelets within their cytoplasm → released in cytoplasmic extensions upon cytokine stimulus
- Protoplatelets break up into hundreds of platelets that circulate throughout the bloodstream
- The remaining nucleus of the ruptured megakaryocyte is consumed by macrophages.
- Megakaryocyte and platelet production is regulated by thrombopoietin (hormone produced by the liver and kidneys)
- Thrombopoietin stimulates differentiation of myeloid progenitor cells into megakaryocytes and causes the release of platelets.
- Thrombopoietin is regulated by a negative feedback mechanism based on platelet levels
- Each megakaryocyte produces between 5,000 and 10,000 platelets
- Altogether, around 1011 platelets are produced each day in a healthy adult
Fate
- Average lifespan of a platelet is 5 to 10 days
- Old platelets are destroyed by macrophage phagocytosis in the spleen and by Kupffer cells in the liver
- Up to 40% of platelets are stored in the spleen as a reserve, released when needed by sympathetically-induced splenic muscle contractions during severe injury.
Structure
- Small anucleated cells derived from megakaryocytes which originate from haematopoietic stem cell
- Normally 150~300 x 103 /μl
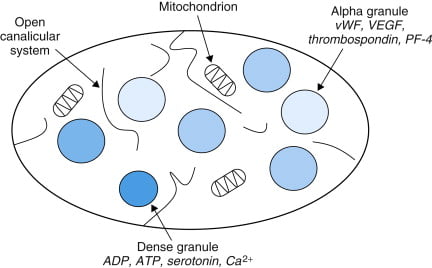
- Actin and myosin
- Remnants of the ER, SR storing calcium
- Mitochondria
- Enzyme systems → production of prostaglandins
- α granules
- Thrombin
- PDGF
- P-selectin
- Fibronectin
- vWF
- Dense granules
- ADP
- ATP
- Ca2+
- Serotonin
- Histamine
Function
- Haemostasis
- Formation of platelet plug
- Interact with collagen exposed at damaged endothelium by GPIa
- Activated by PAF, Thrombin
- Morphological change → irradiating pseudopods
- Degranulation
- Aggregation
- Expression of GPIIb/IIIa → binds fibrin and vWF
- Formation of platelet plug
- Immunomodulation
- Deployed to sites of inflammation and infection and secrete cytokines and chemokines
Sakurai / JC 2019
CICMWrecks Answer: Pharmacology of Clopidogrel
Pharmacology of Clopidogrel
Examiner Comments
2011B 20: 13 (52%) of candidates passed this question.
Platelets are small cells in blood, approximately 150,000 – 300,000 / microliter of blood with a half life of approximately 4 days. They have important membrane receptors (for collagen, vWF and fibrinogen) and intracellular contents (Actin, Myosin, Glycogen, Lysosomes, Dense granules, Alpha granules) and are involved in forming a platelet plug and clotting. Common omissions were to not give a normal platelet count or half life. Diagrams of the clotting cascade were not required.
Clopidogrel is an oral, thienopyridine class antiplatelet agent (a prodrug activated in the liver by cytochrome P450 enzymes), used to inhibit blood clots in coronary artery disease, following stent placement, peripheral vascular disease, and cerebrovascular disease. Mechanism of action is: specifically and irreversibly inhibits the P2Y12 subtype of ADP receptor, which is important in aggregation of platelets and cross-linking by the protein fibrin and thus platelet aggregation, which is inhibited when binding blocks activation of the glycoprotein IIb/IIIa pathway. Elimination half-life of about 8 hours; rapidly absorbed after oral administration; undergoes rapid hydrolysis in liver and renal excretion; 95% protein bound. Important interactions/precautions include proton pump inhibitors, phenytoin, warfarin, heparin, danaparoid, enoxaparin and various thrombolytics. Greatest risk is bleeding. The best answers had a structured approach to describing a drug.
Syllabus: J1 2f, J2 2d
Recommended sources: Ganong Review of Medical Physiology pg 485; Goodman & Gillman. The pharmacological Basis of Therapeutics Chp 54.
21. Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla.
CICMWrecks Answer
Anatomy
- Lie in the posterior wall of the abdomen – outside peritoneum (retroperitoneal)
- Each kidney approx 150g
- Medial side of each kidney = hilum
- Renal artery and vein
- Lymphatic supply
- Nerves
- Ureter
- Kidney surrounded by tough fibrous capsule
- Kidney organized into outer cortex and inner medulla
- Nephrons = functional unit of the kidney (1 million / kidney)
- Medulla
- Organised into mutliple pyramids with the base at the corticomedullary junction and apices – the papilla – at the hilum draining into the ureters
- Renal blood flow
- ~1.25L/min (24% of cardiac output or 500ml/100g tissue/min) via renal artery
- 95% blood flow to cortex, ~5% to Medulla
- Renal artery enters through hilum it divides
- → interlobar arteries
- → arcuate arteries
- → interlobular arteries
- → afferent arterioles
- → glomerular capillaries
- → efferent arterioles
- → peritubular capillaries
- Peritubular capillaries supply blood to the renal tubules
- Juxtaglomerular capillaries have specialized vasa recta which supply the LoH and is important in renal concentration of urine
- → venous system → interlobular veins → arcuate veins → interlobar veins → renal vein
Renal Medulla
- Organised into mutliple pyramids with the base at the corticomedullary junction and apices – the papilla – at the hilum draining into the ureters
- Medullary blood flow: 1~2% of total renal blood flow
- 0.6ml/g/min in inner medulla
- 2.5ml/g/min in outer medullar
- 5ml/g/min in cortex
- 20~30% nephrons are juxtaglomerular with specialized vasa recta
- Blood from post-glomerulus, therefore decreased plasma (filtered) proportion and increased viscosity
- Decreased flow
- Factors that increase filtration fraction decrease medullary flow (e.g. angiotensin II)
- All extrinsic factors which affect afferent arterioles (humoral, neural) affect medullary blood flow similar to RBF
- Oxygen supply determined by state of oxyHb dissociation curve
- Right shift favours oxygen supply
- Renal medullary pO2 = 15mmHg (compared with 50mmHg in cortex)
- Increased O2 offloading
- Renal oxygen consumption
- Increases with sodium resorption (happens mostly in medulla)
Physiology
Oxygen Delivery
CaO2 is determined by:
- CaO2 – Arterial oxygen content
- Hb – Haemoglobin
- SaO2 – Arterial Oxygen saturation
- paO2 – Arterial partial pressure of oxygen
Renal Blood Flow
- Determined by:
- Regulation:
- Renal vascular resistance maintained by interlobular arteries, afferent arterioles and efferent arterioles
- Autoregulated between 80~170mmHg
| Neural | Sympathetic | All renal vesicles richly innervated by sympathetic nerves |
| Minimal influence on GFR except in severe acute disturbances (severe haemorrhage) | ||
| Decrease blood flow due to vasoconstriction | ||
| Hormonal | Adrenaline and noradrenaline | Vasoconstrict → decrease blood flow |
| Endothelin | Vasoconstrict → decrease blood flow | |
| Angiotensin II | Constricts efferent arterioles → decrease blood flow | |
| Nitric oxide | Vasodilate → increase blood flow | |
| Prostaglandins | Vasodilate → increase blood flow |
- Starling resistors
- Increased intra-abdominal pressure will decrease blood flow in starling resistor model
- Increased intra-capsular pressure will decrease renal blood flow
Sakurai 2016
Examiner Comments
2011B 21: 1 (4%) of candidates passed this question.
A good answer required mentioning factors that affect systemic oxygen capacity and delivery (eg Hb, cardiac output, PaO2, etc), Hb-HBO2 dissociation and a description of the anatomy and regulation of renal medullary blood flow. 20 – 30% of nephrons have glomeruli deep in the cortex and long Loop of Henle that go deep into medulla. Here blood supply differs – long efferent arterioles from glomeruli into outer medulla and inner cortex that then divide into vasa recta deep in medulla. These vessels carry post glomerular blood so have less serum, mostly plasma, ie more viscous and concentrated. Factors that influence medullary blood flow include: Sympathetic stimulation – decrease (via efferent arteriolar constriction), Angiotensin II (via tubuloglomerular feedback) – decrease (via efferent arteriolar constriction), Endothelin – decrease (via efferent arteriolar constriction), Prostaglandins – increase, Bradykinin – increase, high protein meal – increase, high glucose levels – increase. Candidates performed poorly due to a lack of knowledge of the topic and/or failure to logically present their answer. Common mistakes were to give no value for renal blood flow, to discuss the function of the medulla which was not required and to not describe the factors that influence medullary blood flow.
Syllabus: B1h 2a,b; C1f 2d
Recommended sources: Guyton Medical Physiology Chp 26; Vander Renal Physiology Chps1, 5
22. Outline the mechanism of action of ampicillin, gentamicin, vancomycin and ciprofloxacin. How does resistance develop to each of these antibiotics?
Examiner Comments
2011B 22: 9 (36%) of candidates passed this question.
The question could have been answered succinctly using a table. Better answers included detail of how antibiotics inhibited enzymes, effects on ribosomal subunits, and impact on cell wall synthesis or inhibit DNA gyrase. The mechanism of resistance required an understanding of beta-lactam inhibition, gene mutation altering target enzymes and ribosomal subunits, and efflux mechanisms. Mention of Van A and Van B phenotypes was also sought.
Syllabus: M2a 2c,d
Recommended sources: Rang and Dale Pharmacology Chp 46, Katzung Chp 43, 45, 46.
23. Outline the production, release, and fate of noradHine at the sympathetic nerve terminal.
CICMWrecks Answer
Sympathetic Nervous System
- Short pre-ganglionic B fibres terminating at the paravertebral sympathetic chain → Synapse with postganglionic fibres and release Ach
- Long post-ganglionic fibres innervating target tissues → Release noradrenaline (Except sweat glands and adrenals)

Noradrenaline
- Naturally occuring catecholamine derived from catecholamine
- Direct α adrenoceptor agonist with partial β adrenoceptor agonism
- Peripheral vasoconstriction with increased MAP and diastolic pressures
Production
- Derived from tyrosine
- Tyrosine → L-DOPA (Tyrosine hydroxylase)
- L-DOPA → Dopamine (Dopa decarboxylase)
- Dopamine → Nordrenaline (Dopamine hydroxylase)
- Noradrenaline → Adrenaline (PMNT)
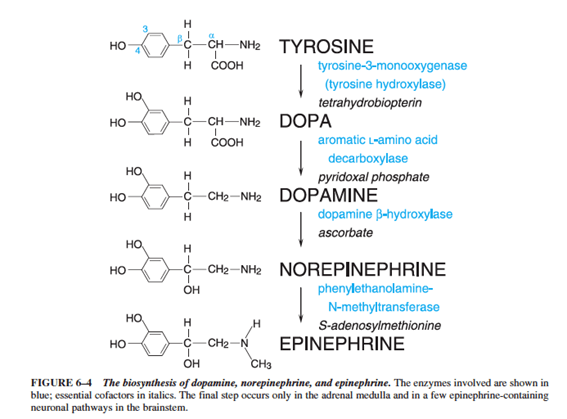
- Enzymes required for noradrenaline synthesis present widely in peripheral sympathetic neurons
- Tyrosine hydroxylase is the rate limiting step and can be upregulated by PKA phosphorylation
- Noradrenaline stored in vesicles in post-ganglionic sympathetic nerve terminals – Vesicular Monoamine Transporter (VMAT2)
Release
- On action potential arrival at sympathetic nerve terminal
- Specific mechanisms for catecholamine release are unknown, however thought to be due to increased [Ca2+] at the nerve terminal in response to action potential
- Vesicles fuse with cell membrane and contents released into the nerve-effector junction
Fate
- Noradrenaline released from nerve terminal binds to adrenergic G Protein Coupled Receptors on target tissues causing various sympathetic effects
- Noradrenaline binds to prejunctional α2 receptors preventing further noradrenaline release
- NET proteins cause re-uptake of noradrenaline into nerve terminals where they may be metabolized by monoamine oxidase (MAO) enzymes or repackaged into neurotransmitter vesicles
- Noradrenaline can diffuse away from the junctional cleft into the systemic circulation and metabolized by Catechol O-Methyl Transferase (COMT) enzymes in liver and other tissues
- Metabolites → VMA and Normetadrenaline → renal elimination
Sakurai 2016
Examiner Comments
2011B 23: 10 (40%) of candidates passed this question.
The question consisted of three parts (production, release and fate of noradrenaline). The context was the sympathetic nerve terminal. The synthesis of Noradrenaline from Tyrosine was expected. Better answers mentioned the roles of Tyrosine Hydrolase, DOPA Decarboxylase and DOPA Beta-hydroxylase. Candidates were expected to describe the storage of Noradrenaline in vesicles and Ca++-mediated exocytosis in response to an action potential. Noradrenaline binds to post-synaptic and pre-synaptic receptors. Re-uptake, metabolism by MAO and COMT, and diffusion away from the synaptic cleft should have been discussed. An accurate diagram could be used to enhance the answer.
24. Describe the pharmacology of corticosteroid drugs
Examiner Comments
2011B 24: 5 (40%) of candidates passed this question.
This question was very broad, requiring the discussion of corticosteroid pharmacology. This embraces the pharmacokinetics, pharmacodynamics and pharmaceutics of the drug class. Synthesis from cholesterol and hepatic metabolism was included in better answers. Comparison of mineralocorticoids versus glucocorticoids was expected. The mechanism of action through binding to intracellular receptors to affect protein synthesis should have been described.
Additional marks were awarded for description of indications, preparations and routes of administration. In any pharmacology question, a list of common adverse effects is important. Of particular relevance is the suppression of the adrenal axis with prolonged administration.
Syllabus: N2 2c
Recommended sources: Stoelting, Pharmacology and Physiology in Anaesthetic Practice, pg 416; Peck & Hill Pharmacology for Anaesthesia and Intensive Care, pgs 355-8.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Treatment of Asthma, pharmacology Normal pulmonary arterial pressure, drugs, NO, measurement, Pulmonary vascular resistance |
| G. CVS | Anatomy and physiology of coronary circulation, physiology of beta receptors Caridac AP, RMP, ionic mechanism, Ventricular vs SA node AP, pharamacological agents affecting pacemaker potential |
| H. Renal | |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | |
| L. Musculoskeletal | Diagram of NMJ, NM blockers, MoAction, pharmokinetic properties |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | Acute inflammatory response to tissue injury – cytokines, component, apoptosis, immune basis of rejection of allogenic organs, serotonin |
| T. Microbiology | |
| U. Endocrine | What is a hormone? Types, pit, thyroid, T3, T4, PTU |
| V. Obstetrics | Respiratory changes in pregnancy at term, ABG, foetal circulation, changes at birth |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments