1. Describe the physiological consequences of breathing 100% oxygen at sea level.
CICMWrecks Answer
Therefore, owing to equation (2) and the haemoglobin dissociation curve, at PaO2 > 100mmHg there is:
Only a minimal increase in haemoglobin saturation
Only a minimal increase in dissolved oxygen concentration
Oxygen stores
Functional residual capacity = lung volume with no active insp. or exp. effort
= volume when equilibrium between chest wall recoil and lung elastic recoil
If FiO2 increased → more oxygen in FRC (not exhaled with tidal volume)
→ Able to tolerate a period of hyperventilation
Absorption atelectasis
- Nitrogen is an inert gas, comprising ~78% of room air
- As it is biologically inert, it is in equilibrium throughout body tissues
- There is no concentration gradient to cause absorption from the lungs
- If FiO2 increased
- PiO2 of O2 increases
- PaN2 decreases as a result
- Moves out of alveoli down its concentration gradient
- ∴Total alveolar pressure falls -> alveolar collapse
- Clinically significant when FiO2 > 50%
Hypoxic vasoconstriction
- In under ventilated lungs portions, there is adaptive pulmonary vasoconstriction, reducing V/Q mismatching
- If FiO2 increased
- Under ventilated lung portions have a higher PaO2
- V/Q mismatching -> venous admixture, ↓ pulmonary vein PAO2
- Under ventilated lung portions have a higher PaO2
Haldane effect
Deoxygenated haemoglobin has a higher affinity for CO2 than oxygenated Hb
If SaO2 is artificially raised, lower CO2 carrying capacity -> build-up of CO2 in tissues
Oxygen radicals
- A radical is a molecule with an unpaired electron outside of an electron shell
- Highly reactive, cause tissue damage
- Important reactive oxygen species (ROS): Superoxide radical (O2–); peroxide radical (O22-); hydroxide radical (•OH)
- ROS can usually be eliminated by cellular antioxidant defence mechanisms
- When FiO2 > 50%
- Defense mechanisms overwhelmed
- Paranchymal injury
- Tracheobronchitis
- Pulmonary capillary endothelial damage -> pulmonary oedema + fibrosis
Mooney 2016
Examiner Comments
2011A 01: 6 (60%) of candidates passed
The question related to physiological changes occurring when FiO2=1. Many candidates focused on the toxic effects of oxygen, which were often incorrect (CNS symptoms will not occur at one atmosphere). Candidates simply lacked knowledge, those that did have some understanding failed to provide adequate detail (ie. it was occasionally mentioned oxygen stores are increased but not the mechanism by which or extent to which stores are increased). In addition, it was expected that candidates would outline and describe the mechanism behind the changes in PaO2in arterial and mixed venous blood, shift in CO2 ventilation, hypoxic pulmonary vasoconstriction as well as pulmonary toxic effects.
2. Outline the principal anatomical features of the diaphragm that are important to its function.
CICMWrecks Answer
Anatomy of the Diaphragm
- Insertion
- Anterior
- Xiphoid process
- Costal cartilage of ribs 6~12
- Posterior
- Lateral arcuate ligament (overlies quadratus lumborum)
- Medial arcuate ligament (overlies psoas major)
- Right and left crux (blends with anterior longitudinal ligament from vertebral bodies L1~3 on right, L1~2 on left)
- Median arcuate ligament (between right and left crux, overlying aorta)
- Central tendon – blends with pericardium superiorly and fibrous capsule of liver inferiorly
- Anterior
- Openings
- Caval orifice (T8) – Within central tendon – Passage of the IVC
- Oesophageal hiatus (T10) – Within sling of right crura – Passage of oesophagus and vagus nerve
- Aortic hiatus (T12) – Posterior to median arcuate ligament between right and left crura – Passage of aorta, azygous vein and thoracic duct
- Innervation
- Motor – Phrenic nerve (C3~5)
- Sensory – Phrenic and inferior intercostal nerves
- Blood supply
- Branches of the lower intercostal arteries, superior phrenic arteries
- Inferior phrenic artery (branch of aorta) from inferior surface
Function of the Diaphragm
- On inspiration
- Diaphragm contracts
- Dome of diaphragm flattens and descends
- Pushes lower thoracic ribs outwards
- Increases AP diameter and superior-inferior distance of thorax → increased thoracic volume → Negative intrathoracic pressure → inspiration of air
- Caval orifice dilates → increases venous return (Liver compressed squeezing hepatic blood reservoir, increased intraabdominal pressure due to descending diaphragm)
- Aortic hiatus constricts → prevents arterial blood regurgitation
- Oesophageal hiatus constricts → aids lower oesophageal sphincter and prevents regurgitation of gastric contents
- Diaphragm contracts
- On expiration
- Diaphragm relaxes
- Elastic recoil of lung causes deflation and expiration
- Diaphragm relaxes
Sakurai 2016
Examiner Comments
2011A 02: 3 (25%) of candidates passed this question.
Most candidates had a basic knowledge of diaphragmatic function however were uncertain of anatomy and rarely related the two. Candidates were expected to describe the attachments of the diaphragm, openings, nerve supply, actions, including it’s role upon the oesophageal sphincter
Syllabus: B1b 2c
Recommended sources: Anatomy for Anaesthetists, Ellis and Feldman, pages 317 – 323
3. Compare and contrast the pharmacology of intravenously administered atropine and glycopyrrolate
Examiner Comments
2011A 13: 6 (50%) of candidates passed this question.
Most candidates exhibited a structural approach with reasonable understanding of the pharmacology of atropine although there was a lack of precision (anticholinergic is correct, competitive muscarinic antagonist is more precise). Some answers did not contrast glycopyrrolate adequately. The phrase ‘hepatic metabolism, renal excretion’ needed to be accompanied with detail if marks were to be awarded.
4. Outline the role of calcium in the body (70% of marks). Outline the differences between calcium chloride and calcium gluconate solutions (30% of marks).
CICMWrecks Answer
Calcium
- Total body stores of calcium = 25,000mmol (400mmol/kg)
- Distribution
- 99% in poorly exchangeable pool (bone, teeth)
- 1% in readily exchangeable pool, majority of this is plasma
- 2.15-2.65mmol/l in plasma
- Absorbed from gut (increased with calcitriol)
- Homeostasis maintained
- Short-term by distal tubular resorption, under control of PTH and calcitriol
- Long-term by bone osteoclast activity, influenced by PTH, calcitriol and calcitonin
Role of Calcium
- Intracellular:
- Myocyte excitation-contraction coupling
- Required for cross bridge cycling
- Regulates mitotic function
- Intracellular second messenger for enzyme activation
- Cell membrane:
- Flow of calcium is responsible for automaticity in SA and AV nodes
- Calcium channels are involved in membrane excitation
- Extracellular:
- Forms part of the mineral matrix giving bone and teeth strength
- Involved in fibrinolysis
- Factor IV in the clotting cascade, required for binding of other clotting factors to phospholipids
- Required for the complement cascade
Ca Gluconate vs Ca Chloride
| Calcium gluconate | Calcium chloride | |
|---|---|---|
| Chemical structure | 2 gluconate ions 1 Ca2+ ions | 2 chloride ions 1 Ca2+ ions |
| Dose | 10ml of 10% | 10ml of 10% |
| Potency | 2.2mmol in 10ml | 6.8mmol in 10ml |
| pH | 6.0-8.2 | 5.5-7.5 |
| Difference in adverse effects | Somewhat irritant to veins | Very irritable to veins |
| Administration | Can be safely given through peripheral IV cannulae | Best through CVC Can use in PIVC in resuscitation |
Mooney 2016
Examiner Comments
2011A 04: 4 (33%) of candidates passed this question
The question sought an understanding of the diverse roles of calcium. Some candidates spent considerable time in details of one or two roles. Limited marks were awarded for demonstrating knowledge of calcium distribution & homeostasis. Few candidates had a good understanding of the differences between calcium chloride and gluconate.
5. Outline the process of digestion and absorption of dietary carbohydrate.
CICMWrecks Answer
Introduction
Carbohydrate
- Equal proportions of carbon and H20
- C:H:O = 1:2:1
Starch
- Polysaccharide which functions as a carbohydrate store in green plants.
- Range in structure from linear to highly branched.
Dietary Forms
- Polysaccharides
- Plant sources: Amylopectin, amylose
- Animal souces: Glycogen
- Disaccharides
- Lactose and Sucrose
- Monosacchaerides
- Glucose and Fructose
Absorption and digestion: Takes place throughout GIT
- Conversion of Starches and Glycogen to Di and monosaccharides
- sucrose: Glucose-Fructose (α-glycosidic)
- maltose: Glucose-Glucose (α-glycosidic)
- lactose: Glucose-Galactose (β-glycosidic)
- Initial breakdown in Upper GIT
- Salivary alpha-amylase in mouth
- Action terminated by acidic pH of stomach.
- Salivary alpha-amylase in mouth
- On exiting the stomach
- Pancreatic alpha-amylase on brush border
- Maltose by Maltase
- Maltotriose
- Glucose Polymers
- alpha-limit dextrans
- Lactase
- Sucrase
- Pancreatic alpha-amylase on brush border
- Absorption in intestine (maximal rate = 120g/hr)
- Galactose (C4)
- Pentoses (C5)
- Rapidly absorbed
- Hexoses (C6)
- Rapidly absorbed
- Glucose:
- Secondary active transport
- Basal Na/K atpase
- 20% Cotransport of Glucose/Na from lumen via SGLUT (shares with Galactose)
- 80% Passive diffusion down concentration gradient.
- Apically Via Glut2 to portal circulation
- Fructose (C6)
- Carrier mediated facilitated diffusion via GLUT5
Transported to the liver via the portal circulation
- 10% stored as glycogen
- 40% converted to TAGs as fat stores
- 50% used in glycolysis (forms ATP for other liver functions)
Gladwin 2016
Examiner Comments
2011A 05: 2 (17%) of candidates passed this question.
A number of candidates had absolutely no understanding of this subject. Some candidates had a basic understanding of either digestion or absorption but few demonstrated knowledge of both processes. Inaccuracies were common with many discussing the role of the gastric acid & enzymes in carbohydrate digestion. Most forgot to mention simple dietary carbohydrates. For a good answer candidates were expected to outline the forms of dietary carbohydrates, gastro-intestinal enzyme action and mechanism of absorption
Syllabus: Q1, 2c
Recommended sources: Review of Medical Physiology, Ganong, Chp 27
6. Describe the pharmacology of suxamethonium
Examiner Comments
2011A 06: 7 (58%) of candidates passed this question.
Most candidates presented a structured answer and demonstrated reasonable understanding of the pharmacology of suxamethonium. This is however a core subject and candidates should be able to answer this question in depth.
Syllabus: H2a, 2c
Recommended sources: Basic and Clinical Pharmacology, Katzung, Chp 27
7. Briefly describe the factors that affect the partial pressure of carbon dioxide in mixed venous blood.
CICMWrecks Answer
Mixed venous blood
- Mixture of all venous blood from all tissue capillary beds.
- Sampled from pulmonary artery
- In reality, omits venous blood from physiological shunt
PCO2
- Small portion of CO2 carriage in blood – approx. 5%
- The rest being HCO3 (approx. 90%), Carbamino compounds (approx. 5%), and carbonic acid (negligable)
- Constitutes 10% of A-V difference in CO2 carriage
- The rest being HCO3 (approx. 60%) and Carbamino compounds (approx. 30%)
- Obeys Henry’s Law
- Mass of dissolved gas is proportional to its partial pressure
- Solubility coefficient of CO2 ~0.54
- Obeys Dalton’s Law
- Sum of all partial pressures equals the environmental atmospheric pressure
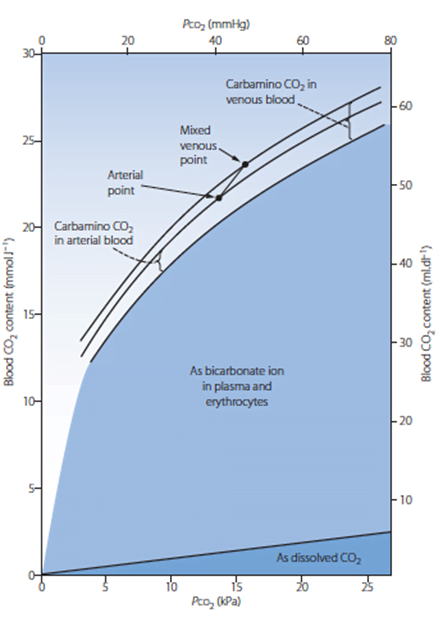
PMVCO2
- Sum of PaCO2 and dissolved portion of CO2 produced
- PaCO2
- In ideal system equal to PACO2 – 10% shunt only increases CO2 by 0.7mmHg
- PACO2 = (CO2 delivery to lung)/(Alveolar ventilation)
- Alveolar ventilation = (Vt – Vd) x RR
- Vt = Tidal volume – Approx. 7ml/kg
- Vd = Dead space volume
- Anatomical deadspace = first 16 divisions of airway – approx 2ml/kg
- Alveolar deadspace – Volume of non-perfused lung
- Increases with
- West zone 1
- PE
- Decreased lung perfusion
- Decreased cardiac output
- Positive pressure ventilation
- Upright posture
- Increases with
- RR – Regulated by medullary respiratory centre
- Chemoreceptor
- Peripheral
- Sensitive to decreased pO2, decreased pH, decreased blood flow and increased pCO2
- Increases respiratory rate
- Central
- Sensitive to CO2 via conversion to [H+]
- Increases respiratory rate
- Peripheral
- Baroreceptor
- Decreased stretch of aortic and carotid baroreceptors increases respiratory rate
- Pulmonary receptors
- J fibres
- Stimulation causes apnoea, bronchoconstriction, bradycardia and hypotention
- Stretch receptors
- Inflation reflex – Inhibits inspiration on lung inflation
- Deflation reflex – Inhibits expiration on lung deflation
- J fibres
- Chemoreceptor
- CO2 production
- Occurs predominantly in mitochondria
- From metabolism of glucose
- Glucose + 6O2 → 6CO2 + 6H2O
- From
- BMR – 40kcal/hr/m2
- Increased muscle activity
- Post-prandial metabolism
- Thyroid hormones increase metabolism
- Catecholamines increase metabolism
- Disease states
- Fever
- Malignant hyperthermia
- Tourniquet
- Relative perfusion of tissues
- Blood from highly metabolic tissues contributes relatively greater amounts of CO2 per weight
- Blood from less metabolic tissues contributes relatively less CO2 per weight
- From metabolism of glucose
- Occurs predominantly in mitochondria
Sakurai 2016
Examiner Comments
2011A 07: 1 (8%) of candidates passed this question.
Candidates were expected to provide a definition of important terms such as mixed venous. Many candidates provided much information about the partial pressure of carbon dioxide in arterial blood without discussing the factors which alter the mixed venous pressure.
Partial pressure of CO2 in mixed venous blood depends on the CO2 content of the mixed venous blood, which in turn represents a balance between CO2 production in the tissues and the CO2 content in arterial blood. Good answers demonstrated an understanding of this and provided relevant details about these aspects. The partial pressure of CO2 is related to the CO2 content by the CO2 dissociation curve, the position of which is determined by the state of oxygenation of haemoglobin, the Haldane effect. CO2 production is related to aerobic metabolism in cells and total production is defined by the metabolic rate. Examples of increased and decreased CO2 production gained additional marks. The partial pressure of CO2 in mixed venous blood is related to the partial pressure or content of CO2 in arterial blood.
This is determined mainly by alveolar ventilation under the control of chemoreceptorsand the brainstem respiratory centre.
Syllabus: B1h, 2c
Recommended sources: Applied Respiratory Physiology, Nunn 5th edition, Chp 10 pages 222 to 239
8. Describe the factors that affect the output of the right ventricle
CICMWrecks Answer
Intracardiac
Extracardiac
HR
- ↓HR → ↑ diastolic filling → ↑ preload → ↑ CO
- Arrhythmia (eg AF) → loss of atrial kick
- less important in RV, can contribute as much as 30% of EDV
- SNS stimulation → ↑ HR → ↓ Diastolic filling time → ↓ Preload → ↓ RV output
Preload
PL = [(RVEDP – ITP) RVEDR]/2h
- h – thickness of the right ventricle
- Less than LV → ↑ PL for equivlant end diastolic pressure (EDP) and end diastolic radius (EDR) compared with the left
- RVEDR – Right ventricular end diastolic radius
- Ventricular wall compliance
- Ventricular interdependence → coordinated, simultaneous contraction of ventricles limits the excursion of intraventricular septum → ↓ventricular compliance
- Ventricular wall compliance
- ITP – Intrathoracic pressure
Mostly RVEDP factors
- Central venous pressure
- Venous compliance
- ↑ venomotor tone → ↑ CVP → ↑ preload → ↑ RV output
- Venous compliance
- Thoracic venous blood volume
- Total blood volume (TBV)
- ↑ TBV → ↑MSFP → ↑ CVP → ↑ preload → ↑ PL
- Total blood volume (TBV)
- Venous return
- VR = (MSFP – RAP)/(SVR)
- ↑VR → ↑ CVP → ↑ preload
- Pulmonary Artrial pressure (PAP)
- ↑ afterload (or PAP) → ↓ SV → ↑ ESV → ↑ EDV (or ventricular preload) → ↑ RV output
- External compression
- Pericardial effusion/tamponade → ↓ ventricular filling
- PEEP → ↑ ITP → ↓ VR
Afterload
AL = [(RVESP – ITP) RVESR]/2h
- RVESR (Vetricular Systolic Radius_
- End-diastolic radius
- Transmural pressure (RVESP – ITP)
- Intraventricular pressure (RVESP)
- Outflow tract obstruction
- Intraventricular pressure (RVESP)
- Myocardial wall thickness
- Thin wall → ↑ compliance → ↓ AL
Transmural pressure (RVESP – ITP)
- Extra-cardiac pressure (ITP)
- ↑ ITP → ↓ Δ P → ↓ AL
- Pulmonary Arterial pressure
- Pulmonary vascular resistance (PVR) = (8 η L)/(π r^4)
- ↑PEEP → ↓vessel radius → ↑AL
- Position (Erect > PVR cf Supine)
- Pulmonary vascular resistance (PVR) = (8 η L)/(π r^4)
Contractility
- Afterload
- “Anrep Effect”
- Preload
- Initial myocyte stretch via Frank-starling
- Heart rate
- “Bowditch effect” or “Treppe effect”
- ANS (main factor)
- Circulating catecholamines – Augments SNS adrenergic effect
Gladwin / Sakurai 2016
Examiner Comments
2011A 08: 6 (50%) of candidates passed this question.
An approach that covered the main determinants of right ventricular cardiac output including heart rate, right ventricular preload, contractility, afterload and the relationship with left ventricular output, ventricular interdependence, and the respiratory system would have provided the framework for a good answer. Some candidates used this approach but described more features of left ventricular than right ventricular output. The observation that the right ventricle is relatively thin
walled and its output is very sensitive to changes in right ventricular preload and afterload particularly was central to this question. The unique shape of the right ventricle and its contraction characteristics involving ventricular interdependence were rarely mentioned. Also details on right ventricular afterload and the importance of factors affecting pulmonary vascular resistance were lacking in most answers.
Syllabus: C1c
Recommended sources: Review of Medical Physiology, Ganong, Chps 31 and 33, Textbook of Medical Physiology, Guyton & Hall Chp 9 and 20
9. Describe how the kidney maintains the medullary concentration gradient.
CICMWrecks Answer
Medullary concentrating gradient
- physiological process which sets up a concentration gradient from cortex through to medulla
- allows formation of concentrated urine
- Mechanisms:
- Counter current Multiplier: creates concentrated medullary interstitium
- Counter current Exchange (vasa recta): maintains intersisital osmotic gradient
- Recycling of urea: contributes to high osmolarity of medullary interstitium
- Normal cortico-medullary gradient 300-1400mOsmol.
Counter-current Mechanism in Kidney
Counter Current Multiplier:
Set-up
- Descending limb LoH Permeable to water only
- Ascending limb LoH Permeable to NaCl only
- Thin passive NaCl reabs
- Thick NaCl via active NKCCT cotransport and paracellular
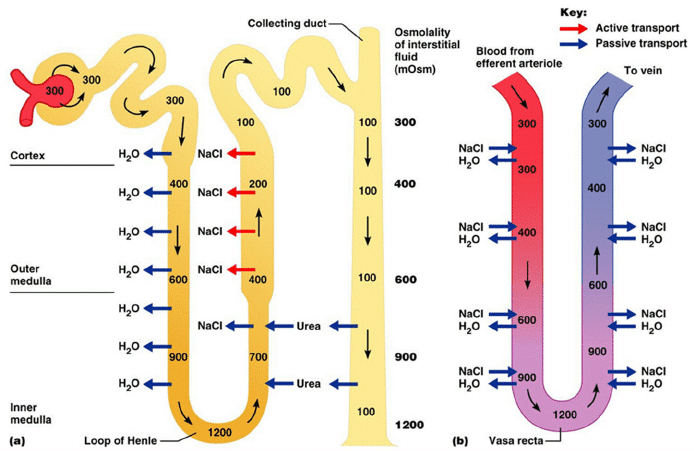
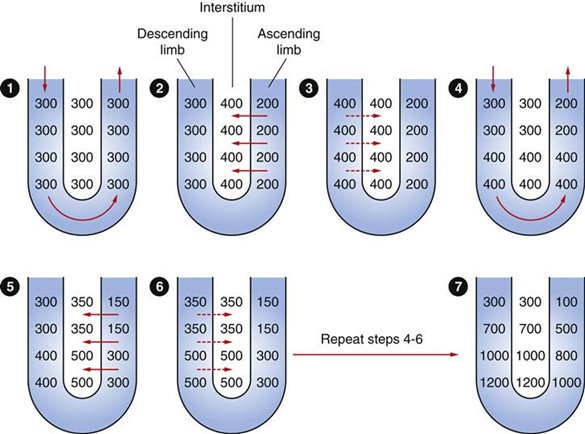
Generation
- Enters LoH osmolarity throughout = 300mOsm
- Max effort of NKCCT = 100mOsm/kg
- Tubular OSM = 200/kg
- Interstitial Osm = 400/kg
- Descending limb diffuses water down gradient
- Descend limb OSM now 400
- Process repeats (flow, pump salt, equilibrate water)
Counter Current Exchange:
- Hairpin loop arrangement of the vasa recta in the juxta-medullary nephrons.
- Provides blood flow to medullary tissue without impact of the cortico-medullary gradient.
- Relies on slow flow of blood.
- Mechanism
- On descent, water is lost from capillaries and NaCl is absorbed into capillaries increasing osmolarity
- On ascent water is reabsorbed and solute is lost
Role of Urea in Medullary Concentration Gradient
- 900mmol/day filtered
- 350-550mmol/day reabsorbed.
- Comprises 50% of osmolality (300-650 mOsm/kg)
- Freely filtered at the glomerulus
- Secreted by thin limbs of LoH down concentration gradient from medullary interstitium to tubular fluid.
- As water and Na are reabsorbed, tubular [Urea] increases
- [Urea] = >500mmol/L at collecting ducts
- Equilibrates with interstium due to slow flow rates.
- ↑ADH
- urea transporters UT1 and UT3 insertion → ↑ CD permeability to urea
- → ↑ medullary gradient (further 10% reabsorption if required)
Gladwin / JC 2020
Examiner Comments
2011A 09: 4 (33%) of candidates passed this question.
A useful introduction could include a definition of medullary concentration gradient and its function. The answer was expected to describe the roles of sodium, chloride and urea in the countercurrent multiplier and the features of the vasa recta countercurrent exchange system.
The 3 main areas that needed to be addressed to pass this question included:
[1] The loops of Henle with their water permeable descending limbs and water impermeable ascending limbs, which actively remove solutes from the tubular lumen. The counter current multiplier system.
[2] The vasa recta which run parallel to the loops of Henle and are permeable to water and solute and have low flow. This allows the medullary concentration gradient to be maintained. The counter current exchange mechanism.
[3] The role of urea which is concentrated in the medulla by mechanisms which involve changes in permeability to urea in different regions of the tubules partly influenced by the effects of antidiuretic hormone.
Some candidates elected to draw the loop of Henle and vasa recta together with the movement of various solutes and water and answer the question from it.
Unfortunately mistakes in these diagrams only confused their answers further.
Syllabus: Section D1, 2c
Recommended sources: Principles of Physiology for the Anaesthetist, Power and Kam, page 234
10. Discuss the bacteriocidal activity, and toxicity, of gentamicin
CICMWrecks Answer: Gentamicin (Limited)
GENTAMICIN
Gentamicin
- Covers a wide range of gram negative enterobacteria and has gram positive cover that includes staph and some streptococci.
- No anaerobic activity but is synergistic with beta lactams and vancomycin.
- Used for infections of the GUT, GIT, respiratory tract, skin and soft tissues, neutropenic sepsis, CNS infections, and surgical prophylaxis
Mechanism
- Bactericidal antibiotic, which inhibits the bacterial 30S ribosomal subunit
- This impairs transcription and/or induces misreading of the mRNA, impairing protein synthesis
- diffuse across outer membranes via porins
- actively transported by oxygen dependent process across cell membrane to cytoplasm
- low O2 and extracellular pH prevent this process
Toxicity
- Gentamicin is not metabolized in humans
- It is excreted in the urine unchanged
- The presence of transport molecules in the epithelial cells of the proximal and distal tubule and the cortical collecting ducts allows gentamicin to accumulate within the cytosol of these cells.
- In the cytosol, gentamicin acts on the endoplasmic reticulum, impairing protein synthesis, and on the mitochondria to impair ATP production, increasing oxidative stress via the production of free radicals and superoxides. It also acts on lysosomes to impair protease degradation, causing further cell damage
- Independent of the cellular damage, gentamicin also inhibits some of the epithelial cell transport processes. Tubular damage may then partially or totally obstruct the lumen, causing further disruption of the nephron resorptive processes à hence the rise in plasma creatinine and fall in eGFR
- The accumulation of drug within the cell means that the toxic effects can continue long after plasma drug levels have declined
- Ototoxicity may be caused through similar processes, via accumulation of the drug in the inner ear perilymph, where it disrupts mitochondrial protein synthesis and promotes formation of free radicals within the hair cells
- Nephrotoxicity usually reverses with cessation of drug, however ototoxicity may be permanent
- Gentamicin may also cause muscle weakness due to impairment of prejunctional release of ACh, use with caution with NMDR and in myasthenia gravis.
Examiner Comments
2011A 10:
The first part of the question on bactericidal activity of gentamicin was better answered than the second part on its toxicity. Details on the cellular mechanisms of bactericidal action and toxicity were lacking in most answers.
Most candidates did not appreciate that gentamicin is avidly accumulated and retained by proximal renal tubular cells in concentrations many times higher than the plasma concentration. Also these high tubular cell concentrations of gentamicin are maintained long after the plasma concentrations have fallen to very low levels, thus enhancing its toxic effects. Gentamicin has multiple toxic effects within the tubular cell including adverse effects on protein synthesis, translation and folding, impairment of mitochondrial function and production of reactive oxygen species and damage to the nucleus.
Syllabus: M2a, 2d. Recommended sources: Pharmacological Basis of Therapeutics, Goodman and Gillman, Chp 45 and page 1162
11. Outline the influence of pregnancy upon drug pharmacokinetics
CICMWrecks Answer
Pharmacokinetics:
Absorption:
- ↓ Oral absorption
- ↑ N/V
- ↓ gastric emptying during labour
- ↓ gastric motility 2° to intestinal compression
- ↑ gastric absorption
- ↓intestinal absorption due to ↓ intestinal blood flow
- ↑ IM/SC/transdermal absorption
- ↑ skin blood flow
- ↑ C.O. (by 30-40%)
- ↓ SVR
- ↑ skin blood flow
- IV (↑ onset)
- Neuraxial
- ↓ epidural space 2° to EDVs
- ↓ spinal and epidural doses
- Inhalational
- Progesterone-mediated ↑ MV (by 50-70%)
- ↑ FA/FI ratio (= uptake
Distribution:
- ↑ VD (↑ TBW/ECF and fat)
- ↑ TBW/ECF (by 50%) (important for polar/ionized drugs)
- ↑ body fat % (important for lipid soluble drugs)
- ↓ plasma protein 2° to dilutional effect
- ↓ albumin →
- ↑ free % of acidic drugs (Eg. STP, propofol)
- ↓ dose required
- ↑ transplacental transfer of drug.
- ↓ A1AGP (by 30%) →
- ↑ free % of basic drugs (Eg. LA, β blockers)
- ↓ dose required
- ↑ transplacental transfer of drug
- ↓ albumin →
- Ionisation (mild ↑pH alters ionisation based on pKa)
- ↑ MV = mild respiratory alkalosis
- ↑ transplacental transfer of basic drugs as they will have ↑ % in unionized form
- (Base in base is less ionised)
- ↑ transplacental transfer of basic drugs as they will have ↑ % in unionized form
- ↑ ion trapping in more acidotic foetal circulation
- ↑ MV = mild respiratory alkalosis
Metabolism:
- Progesterone:oestrogen ratio
- Progesterone → induces hepatic enzymes
- Oestrogen → inhibits hepatic enzymes
- ↓ plasma cholinesterase (30%)
- Placenta metabolises some drugs
- Foetal liver has functioning CYP450
- Can metabolise drugs
- But requires transfer back to maternal circ for conjugation
Excretion:
- ↑ RBF/GFR (50%)
- ↑ clearance/↓ elimination t1⁄2 of water-soluble drugs
- ↑ MV/↓FRC
- ↑ washout of volatile agents
Pharmacodynamics:
- Decreased MAC – Increased sensitivity to volatile anaesthetics
- Increased LA sensitivity due to decreased α1-glycoprotein
- Increased sensitivity to IV anaesthetics
Gladwin / JC 2019
Examiner Comments
2011A 11: 4 (33%) of candidates passed this question.
Answers framed around the structure of absorption, distribution, metabolism and excretion performed better. An approach based on the physiologic changes of pregnancy performed less well because important areas of pharmacokinetics were omitted. The effects of pregnancy on oral absorption should have included a discussion of gastrointestinal motility, nausea and vomiting and gut blood flow. Absorption from sites other than the gastrointestinal tract, such as skin, lung and the epidural space and the effect of pregnancy on these should have been mentioned. Many answers were vague on the effects of increases in total body water and plasma volume and cardiac output and changes in plasma protein binding on the distribution of drugs. Most answers did not provide enough specific examples. The effect of pregnancy hormones on liver enzyme activity were mentioned by few.
Syllabus: Generic Pharmacology III 2d
Recommended sources: Foundations of Anaesthesia: Basic clinical Science.
Hemmings and Hopkins, and Anaesthesia, Miller.
12. Describe the principles, and limitations, of the measurement of cardiac output using an indicator dilution technique
CICMWrecks Answer
Cardiac Output
Cardiac output = Volume of blood ejected by the heart per unit time (minute)
HR determined by autonomic input
- Resting HR approx 60
- Sympathetic blockade causes HR to slow to 50
- Sympathetic output to heart governed by the medullary vasomotor area
- Parasympathetic blockade causes HR to hasten to 110
- Both sympathetic and parasympathetic blockade causes HR to hasten to 100
- Electrolytes
- Drugs
- Sympathetic blockade causes HR to slow to 50
SV = Amount of blood ejected by the heart per contraction
Governed by:
- Preload
- Tension applied to myocyte immediately prior to contraction
- Starling relationship
- Optimal sarcomere length ~2.2um
- Over-stretch inhibited by fibrous pericardium
- Preload governed by
- Blood volume and MSFP (increases preload)
- RAP (decreases preload)
- Resistance to venous return (decreases preload)
- Negative intrathoracic pressure (aids in venous return)
- Musculovenous pumps (aids in venous return)
- One-way venous valves (aids in venous return)
- Starling relationship
- Tension applied to myocyte immediately prior to contraction
- Afterload
- Load against which the ventricle must exert its contractile force
- According to Laplace law
- Afterload ∝ (Aortic pressure x ventricular radius) / thickness of ventricular wall
- Other factors affecting afterload
- Resistance
- Viscosity inversely proportional to flow
- As viscosity decreases (e.g. Hb decrease), output increases
- Flow is proportional to vessel radius to the 4th power
- Viscosity inversely proportional to flow
- Resistance
- Contractility
- Intrinsic ability of myocardium to contract at given preload and afterload
- Governed
- Sympathetic increases contractility
- Parasympathetic decreases contractility
- Other hormones
- Electrolytes
Measurement
Cardiac output measurement can be performed:
- Invasively
- Pulmonary Artery Catheter
- Thermodilution
- Fick Principle
- Indicator Dilution Technique
- TOE
- Arterial waveform analysis
- PiCCO
- FloTrak/Vigileo
- Acumen/Hemosphere
- Pulmonary Artery Catheter
- Non-invasively
- TTE
- MRI
- Thoracic impedance
Thermodilution
Indicator Dilution (Stewart-Hamilton)
Introduction
Invasive
Thermodilution remains the gold standard of cardiac output measurement.
Invasive
Equipment
Pulmonary Artery Catheter
- A proximal port at the RA/SVC
- A temperature probe at the tip: Typically a silicon oxide thermistor.
- A balloon at the tip: To float it into position.
- A distal (PA) port is required for measuring PAP and the PCWP, but is not required for CO calculation
Can also be done using other devices like PiCCO
- dyes: indocyanine green, isobestic or radioactive isotope.
- Catheter for injection
- Arterial Sampling catheter
- Measurement device
Method / Theoretical Basis
- Catheter passed into right atrium
- Known quantity of fluid of known temperature injected into right atrium
- Second catheter in pulmonary artery
- Measured change in temperature
- Change in temperature over time graphed
- Return of temperature to baseline extrapolated to zero to account for
recirculation - Average change in temperature for duration of curve calculated
- CO = temperature of indicator / (average change in temp x duration of curve)
Method:
- rapidly inject 10mL of dye into venous circullation in RA.
- indicator mixes quickly with cardiac contents.
- during next few beats, blood-indicator mixture is measured by continual sampling from proximal artery in arm for one complete circulation (30 seconds).
- mean concentration of mixture for one circulation determined.
Q = amount of indicator injected / indicator concentration over time.
Assumptions:
- retention of indicator
- complete mixing
- constant flow rate
Advantages / Disadvantages / Errors
Advantages:
- Simple
- No toxicity
- Repeated measurements possible
Disadvantages / Errors:
- Natural variability (reduced by using mean of 3-5 measurements)
- Issues with incorrect volume and temperature of injectate
- Improper positioning of PAC and complications associated with insertion and use
- Tricuspid regurgitation
Results in retrograde ejection of injectate back past the valve. - Arrhythmia
Advantages:
- rapid Q determination
Disadvantages:
- need to continuously withdraw arterial blood to plot the concentration curve.
- dye can build up.
- intracardiac shunt will effect recirculation.
Sakurai / JC 2019
Examiner Comments
2011A 12: 7 (58%) of candidates passed this question.
Most candidates chose to describe the thermodilution technique of cardiac output measurement. Descriptions of other techniques and indicators such as dye dilution using indocyanine green were acceptable alternatives.
Better answers included a description of the Fick Principle and the fact that it is based on the law of conservation of matter. For thermodilution, heat lost from the blood = heat gained from the injectate. Also required were an accurate description of the technique, a description of the indicator-time curve and errors encountered in the technique. For thermodilution these included the requirement for a Swan Ganz catheter, nature and temperature of the injectate, temperature measurement using a thermistor in the pulmonary artery and an appreciation that it is the curve of a
decrease in temperature versus time that is being analysed.
Syllabus: S2c
Recommended sources: Anaesthesia, Miller, Chp 40
13. Relate the surface electrocardiogram (ECG) to the events of the cardiac cycle (60% of marks). Briefly describe the mechanism of the effects of digoxin, and the mechanism of the effects of amiodarone, on the ECG (40% of marks)
CICMWrecks Answer
Surface ECG to events of Cardiac Cycle
ECG:
- 12 metal electrodes on chest wall
- Detect small (0.5-2mV) changes in voltage produced by the heart
- Transfer these to an oscilloscope output
- P wave: → atrial depolarisation → atrial systole → AV valve opening
- PR interval: conduction from SA node → atrial conducting pathways → AV node (slowest) → bundle of His → LBB and RBB → Purkinje fibres
- QRS: ventricular depolarisation (endocardium → endocardium, L > R) → ventricular systole → closure of AV valves, opening of aortic and pulmonary valves
- Atrial repolarisation is low voltage, and lost within the QRS complex
- QT interval: sustained ventricular depolarisation and muscular contraction
- T wave: ventricular repolarisation (epicardium -> endocardium, L > R) -> ventricular
relaxation -> closure of aortic and pulmonary valves
ELECTRICAL EVENTS
| P0 | Fast Na opening |
| P1 | Transient K efflux |
| P2 | Influx Na & Ca |
| P3 | Efflux K > Influx Na & Ca |
| P4 | Re-establishment of RMP |
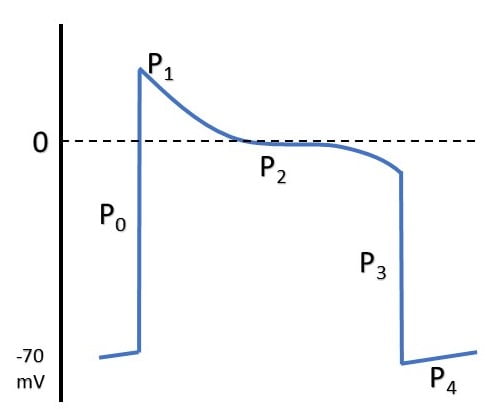
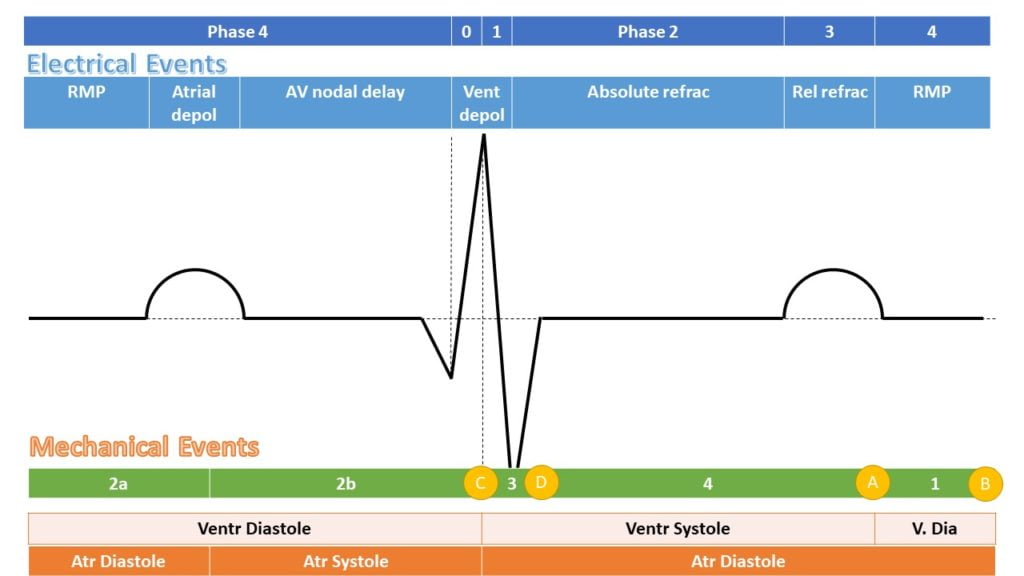
MECHANICAL EFFECTS
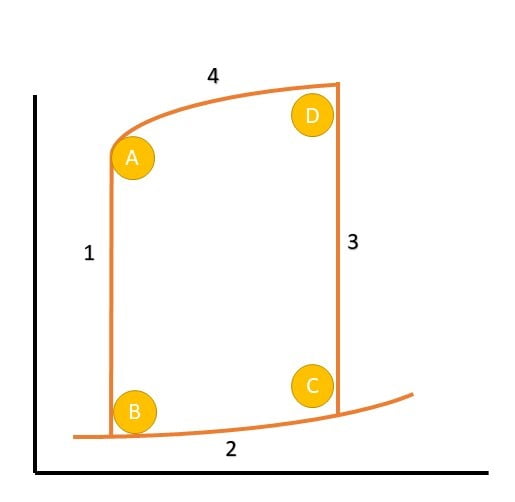
| A | Aortic Valve Closure |
| 1 | Isovolumetric relaxation |
| B | Mitral Valve Opening |
| 2a | Early Diastolic filling |
| 2b | Late Diastolic filling |
| C | Mitral Valve Closure |
| 3 | Isovolumetric Contraction |
| D | Aortic Valve Opening |
| 4 | Ventricular Ejection |
Action of Drugs
| Drug | Interval effects | Mechanism |
|---|---|---|
| VW (Vaughan-Williams) Class 1a | ↑QRS, ↑QT | Fast Na-channel blockade |
| VW Class 1b | ↓QT | |
| VW Class 1c | ↑↑QRS, ↑QT | |
| VW Class 2 | ↑PR | β blockade → negative chronotropy |
| VW Class 3 | ↑QT | K channel blockade → prolongation of RRP |
| VW Class 4 | Can ↑PR | Prolongation of ERP and RRP in pacemaker cells |
| Adenosine | ↑PR | Hyperpolarisation of myocardium via opening of K channels |
| Digoxin | ↑PR, ↓QT | Multiple effects, vagotonic at AVN |
| Amiodarone | ↑PR, ↑QRS, ↑QT, ↑RR (sinus rate) | Multiple |
| Mg | ↓QTc | Membrane stabilising. |
| TCAs | ↑QRS | Quinidine like Na-channel effect (class 1a) |
| SSRIs | ↑QT | Direct potassium blockade and downregulation of potassium → ↑ERP and ↑RRP. |
Digoxin
- ↑ RR interval (sinus rate), ↑PR interval, ↓RR interval
- Slowed AV conduction by increased vagal tone
- ACh → M2 receptors
- ↑KACh (ACh controlled K+ channels) → increased efflux of K+ → hyperpolarised
membrane - ↓If (funny current) → decreased influx of Na+ → decreased slope of phase 4
- ↓ICa(L) (long lasting) and iCa(T) (transient) → decreased efflux of calcium on partial
depolarisation → decreased slope of phase 4
Amiodarone
- ↑PR, ↑QRS, ↑QT, ↑RR (sinus rate)
- Main effect is K+ channel blockade
- Repolarisation is slowed → prolonged QT
- Some INa
- Slower phase 0 upstroke
- Slowed ventricular muscle conduction → prolonged QRS
- Some ICa
- Slower spontaneous depolarisation in SA node → decreased sinus rate
- Slowed conduction in AV node → prolonged PR
- Weakly downregulates adrenoreceptors
- Increased PR, decreased sinus rate
Mooney / Gladwin 2016
Examiner Comments
2011A 13: 8 (66%) of candidates passed this question.
Candidates were expected to provide sufficient detail in answers. Extra marks were awarded for diagrams relating the ECG accurately to pressure events during the cardiac cycle. Time intervals, units of measurement and clear labels were essential for diagrams.
Mechanisms pertaining to ion flux and ion channels needed to be specifically explained. Discussion of mechanisms needed to be accurate and relevant to the effect on the ECG. For example, better answers noted that AV conduction was depressed by Digoxin, predominantly due to an increase in Vagal tone
14. Describe the mechanism of action, and adverse effects, of pulmonary vasodilators that are administered via the inhalational route.
Examiner Comments
2011A 14: 1 (8%) of candidates passed this question.
Many candidates neglected to include oxygen which is also a drug with significant pulmonary vasodilating properties. Accurate detail concerning the receptor and second messenger effects of drugs was expected. The importance of V/Q matching and reduction in systemic effects via inhalational administration needed to be stated.
Better answers included discussion of serious adverse effects such as methaemoglobinaemia, acute lung injury, systemic hypotension, rebound phenomena and heart failure.
Syllabus: B2a, 2b,c,d,e
Recommended sources: Basic and Clinical Pharmacology, Katzung, Chp 18, 19
15. Statistics (not in current primary syllabus)
16. Compare and contrast the pharmacology of morphine, fentanyl and remifentanil.
Examiner Comments
2011A 16: 2 (17%) of candidates passed this question.
The question asked for a comparison of the pharmacology (pharmacokinetics and pharmacodynamics) of three commonly used opiates. Better answers made use of a well constructed table with headings including chemistry, protein binding, lipid solubility, half-lives, context sensitive half-time, volume of distribution, metabolism, active metabolites, oral bioavailability, and clearance. A distinction should have been clearly drawn between onset, peak, and duration of effect. CNS stimulant effects as well as depressant effects, were expected to be listed.
Syllabus: G2d, 2d
Recommended sources: Anaesthesia, Miller Chp 11 and Pharmacological Basis of Therapeutics, Goodman and Gillman, Chp 21
17. Outline the physiological processes that occur in a blood vessel after venipuncture (80% of marks). How are these altered by the administration of aspirin (20% of marks)?
CICMWrecks Answer
Cell-based model of coagulation
Initiation
- Small amounts of Factor VII, X and prothrombin leak into interstitium from intravascular space
- Factor VII interacts with Tissue Factor leading to activation of extrinsic pathway
- Activation of Factor X
- Small amounts of prothrombin converted to thrombin
Amplification
- At onset of tissue damage and vessel disruption
- Platelets exposed to thrombin generated in initiation phase, together with collagen via GPIa
- Platelet activation and release of α and dense granules, and morphological change
- Factor XII activated, leading to consequent activation of factors XI and IX
- Formation of Xase complex on platelet surface
- Amplified activation of factor X
Propagation phase
- Increased activated factor X causes “thrombin burst”
- Fibrinogen activated to fibrin
- Further propagation of platelet activation and aggregation
Modulation of coagulation response
- Circulating proteins C and protein S bind and inactivate factors V and VIII
- Antithrombin 3 binds to thrombin, as well factors IX and X and inhibit these
- Thrombomodulin on intact endothelium binds thrombin → anti-coagulant effect and increased protein C activation
- Plasminogen converted to plasmin → degradation of fibrin and therefore thrombolysis
Effect of aspirin
- Non-selective COX inhibition
- At low doses
- Irreversibly inhibits platelet COX in the portal circulation → inhibits platelet production of thromboxane (TXA)
- TXA stored in α granules and augments the activation of surrounding platelets, therefore aspirin reduces platelet activation in response to coagulation cascade
- At high doses
- Aspirin overcomes hepatic first pass metabolism and escapes to systemic circulation
- Inhibits systemic COX → inhibits endothelial production of prostacyclin
- Prostacyclin inhibits platelet aggregation and adherence, therefore increases clot formation and strength
Sakurai 2016
Examiner Comments
2011A 17: 8 (66%) of candidates passed this question.
The question was answered well overall. Better answers included detail of the platelet receptor and mediator interactions. Discussion of the role of the platelet in providing a phospholipid surface to enable the formation of the activated Xa complex was expected. Modulation of the coagulation cascade and prevention of clot propagation via protein C, nitric oxide, thrombomodulin and fibrinolysis was important to note in a comprehensive answer. The pharmacodynamic action of aspirin was generally understood.
Syllabus: J1,2c and J2, 2d
Recommended sources: Basic and Clinical Pharmacology, Katzung, Chp 34, 36
18. Explain the physiological processes involved in the development of tissue interstitial oedema.
CICMWrecks Answer
Interstitial oedema occurs due to increased permeation of fluid from intravascular to interstitial spaces, and inability of the lymphatics to reabsorb this additional fluid
Starling Forces
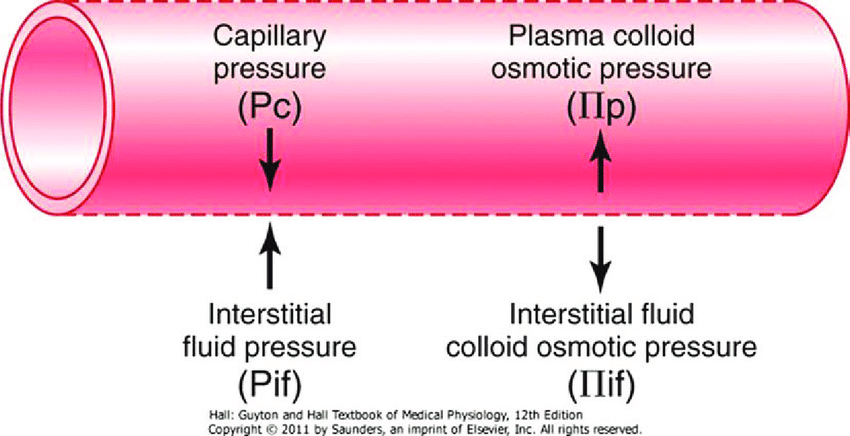
- The NET flux across the membrane is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation:
where
Jv is the trans endothelial solvent filtration volume per second
( [ Pc – Pi ] – σ [ πp – πi ] ) is the net driving force
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
κ = filtration constant = LpS = Hydraulic conductivity x Surface Area
- Typically quoted values for the variables in the classic Starling equation:
| Hydrostatic pressure | Oncotic pressure |
|---|---|
| Pressure moving fluid | pressure exerted by proteins which draw water into and keep it within a compartment |
| Pc ~35 → 15mmHg (Arterial → venous) Capillary hydrostatic pressure Pressure moving fluid out of capillary | πp ~ 20mmHg Plasma oncotic pressure Pressure keeping fluid within capillary |
| Pif = 5mmHg Interstitial hydrostatic pressure Pressure moving fluid into capillary | πif ~ 0mmHg Interstitial fluid oncotic pressure Pressure keeping fluid out of capillary |
- In general,
- at the arterial end of capillary NFP is positive (filtration) +10mmHg
- At the venous end NFP is negative (absorption) -10mmHg
- Approx. 24L fluid filtered / day
- 85% reabsorbed into capillaries
- Rest reabsorbed via lymphatics (~3.5L/day) = Net fluid loss from filtration
Tissue Interstitial Oedema
| Increased κ promotes oedema | – Inflammation |
| Increased Pc promotes oedema | Increased resistance to venous return – Diastolic HF – Increased R atrial pressures – Venous obstruction (DVT, mass) – Loss of one way valves – Loss of veno-muscular pump (in bed-bound patients) |
| Increased intravascular volume – Activation of RAAS in CHF, cirrhosis, nephrotic syndrome – Crystalloid administration in ICU | |
| Gravity and posture – Lower limbs if standing – Sacrum if supine | |
| Decreased πc promotes oedema | – Hepatic failure and decreased plasma proteins – Nephrotic syndrome |
| Endothelial glycocalyx – damage promotes oedema | – Glycoprotein layer on inner surface of capillaries which sequester plasma proteins |
| Decreased lymphatic flow promotes oedema | Obstruction – Compression – Tumour – Infection – filariasis |
| Increased Pi inhibits oedema | – Compression stockings – Deep sea diving, increased atmospheric pressure |
Sakurai / JC 2020
Examiner Comments
2011A 18: 2 (17%) of candidates passed this question.
The question required an accurate statement of Starling’s Equation, including the filtration and reflection co-efficients, and definitions of terms. Marks were awarded for numerical values pertaining to hydrostatic and oncotic pressure gradients and net filtration in a 24 hour period.
A satisfactory answer explained the factors which cause imbalance in Starling’s relationship including; precapillary vasodilation, increased venous pressures, gravity/ posture, fall in plasma protein concentration, changes to capillary permeability and lymphatic obstruction.
Syllabus: E1
Recommended sources: Review of Medical Physiology, Ganong, Chp 23 and other sections
19. Explain the role of haemoglobin as a buffer
CICMWrecks Answer – Haemoglobin as a buffer
Buffer
- Weakly ionised acid or base in equilibrium with its full ionised salt
- A buffer can “resist” change in pH by absorbing or releasing H+ ions
- Works best when pKa is closest to the target pH (7.4)
- Isohydric Principle
- All buffer systems which participate in defence of acid-base changes are in equilibrium with each other. There is after all only one value for [H+] at any moment. This is known as the Isohydric Principle.
Haemoglobin (Blood)
- Protein buffering system
- Important intracellular buffer in RBC
- Exists as weak acid – HHb and potassium salt KHb.
- Hb a pKa of 8.2 in deoxy form and 6.6 in oxyHb
- Buffering capacity due to imidazole residues on 38 histidine residues on Hb molecule
- pKa of residues 6.8 à close to physiological pH
- Also important in extracellular buffering (following bicarbonate buffer system)
- Due to fast equilibration of HCO3– (Hamburger Shift)
- Band3 Transporter (HCO3–/Cl– antiporter)
- Allows carbonic anhydrase reaction to take place by limiting build-up of HCO3– (la chatelier principle)
- Formed in erythrocytes as a tetramer of 4 subunits
- Hb ~ 6 times the number of histadine (38) residues compared with albumin. (3-6x buffering capacity)
- Hb is in much greater concentrations than any other protein (15 g/dL vs 7 g/dL)
- For each mmol OxyHb, 0.7mmol H+ is buffered and 0.7mmol of CO2 can enter circulation without a change in pH
- Available at high concentrations in RBC
- Isohydric exchange
- the buffer system (HHbO2-HbO2-) is converted to another more effective buffer (HHb-Hb-) exactly at the site where an increased buffering capacity is required
- Deoxyhaemoblobin is a much more effective buffer
- oxygen unloading increases the amount of deoxyhaemoglobin and this better buffer is produced at exactly the place where additional H+ are being produced because of bicarbonate production for CO2 transport in the red cells.
Gladwin / Sakurai / JC 2020
Examiner Comments
2011A 19: 4 (33%) of candidates passed this question.
To pass this question, the candidate only needed to define a buffer (weakly ionised acid or base in equilibrium with its full ionised salt), what it does, then discuss how Haemoglobin functions in this capacity. In that regard, brief review of how CO2 is buffered, the role of haemoglobin histidine residues, buffering capacity of oxy haemoglobin and deoxy haemoglobin and how this contributes to the Haldane effect would have rounded out a very good answer.
Additional credit was given for an understanding that histidine contains an imidazole group and how these groups are effective as a buffer.
Few candidates mentioned that haemoglobin was quantitatively significant and no candidate mentioned that it is the primary buffer for CO2. Many answers were quite brief and did not explore the subject matter asked. Syllabus: B1h, 2c, 2b and Section F
Recommended sources: Nunn’s Applied Respiratory Physiology, Lumb, page 228 to 230
20. Describe how previous immunisation protects against subsequent infection.
CICMWrecks Answer
Definitions
Immune System: complex network of cells and proteins that defends the body against infection. They can be classified as:
- Physical barries
- Innate Immunity: Recognize generic pathogenic motifs and directly attack or opsonize and phagocytose
- Acquired immunity: Pathogenic antigens presented to B cells and T cells via APCs such as dendritic cells
Immunization: A process by which a person becomes protected against a disease through vaccination. This term is often used interchangeably with vaccination or inoculation.
Vaccine: A product that stimulates a person’s immune system to produce immunity to a specific disease, protecting the person from that disease.
Immunization:
- Passive
- Active
- Passive Immunization:
- transfer of preformed antibodies to an unimmunized individual.
- develop temporary immunity to the particular organism or toxin
- Once these preformed antibodies have been destroyed, the individual would no longer have immunity to this microorganism or toxin.
- e.g: Natural: passage of maternal antibodies through the placenta to the fetus
- e.g: Artificial: administration of pooled human immune gamma globulin and antivenin
- Active immunization:
- occurs with the exposure of an unimmunized individual to a pathogenic agent
- Immune system begins the process of developing immunity to this agent
- produces long-term immunity due to the stimulation of the individual’s immune system.
Stimulation of Immunity by vaccines:
Detection:
- by innate immune system, and/or B-cells
- recognize epitopes on antigens
Primary Response to vaccine administration:
- Innate Immune System activation:
- Opsonize or bind to agent → aid in engulfment by Antigen-presenting cells (macrophages or monocytes) → insert processed antigen + MHC protein onto its surface
- Complex presented to Adaptive Immune System:
- Viral: Antigen + MHC I complex → presented to CD8 cell → trigger cell mediated immunity
- Bacterial or parasitic: Antigen + MHC II protein → presented to CD4 cell → trigger antibody- mediated immunity
- Memory T-cells and B-cells formed → undergo clonal selection following infection, which increases antigen-binding affinity
Subsequent Infection: 2° immune response
- Subsequent exposure to Ag
- memory T cells rapidly proliferate into active helper and cytotoxic T cells specific to that antigen
- memory B cells rapidly produce antibodies to neutralize the pathogen.
- more rapid, prolonged and powerful immune response
Types of vaccines:
- Live attenuated vaccines: produce a strong cellular and antibody responses and typically produce long-term immunity with only one to two doses: MMR, Typhoid
- Inactivated vaccines: Influenza
- Toxoid: Tetanus, diphteria
- Subunit vaccines: Hepatitis B
- Conjugate vaccine: Haemophilus Influenza B vaccine
- Outer membrane vesicle vaccine: B-Meningococcal
- Heterotypic/Heterologous: BCG
- Viral vector: Ebola
- RNA: Pfizer–BioNTech COVID-19 vaccine
JC 2020
Examiner Comments
2011A 20: 1 (8%) of candidates passed this question.
Providing a statement about what vaccines do followed by some detail about the processes involved in triggering a response and the nature of that response in both Innate immunity and acquired immunity would have achieved a good pass. Many candidates failed to adequately describe the nature of the primary and the secondary response to antigen exposure. The fact that previous immunisation enabled a brisk secondary response was recognised by most candidates but that this was largely due to the proliferation of IgG antibody producing B lymphocytes and effector T cells was not appreciated. Many answers simply did not include sufficient information to achieve a pass mark.
Syllabus: M2i
Recommended sources: Review of Medical Physiology, Ganong, Chp 3
21. Briefly describe the cardiovascular events that occur during ventricular diastole.
CICMWrecks Answer
Diastole in the cardiac cycle corresponds with cardiac myocyte relaxation, and includes periods of isovolumetric relaxation and ventricular filling. It lasts approximately 430ms.
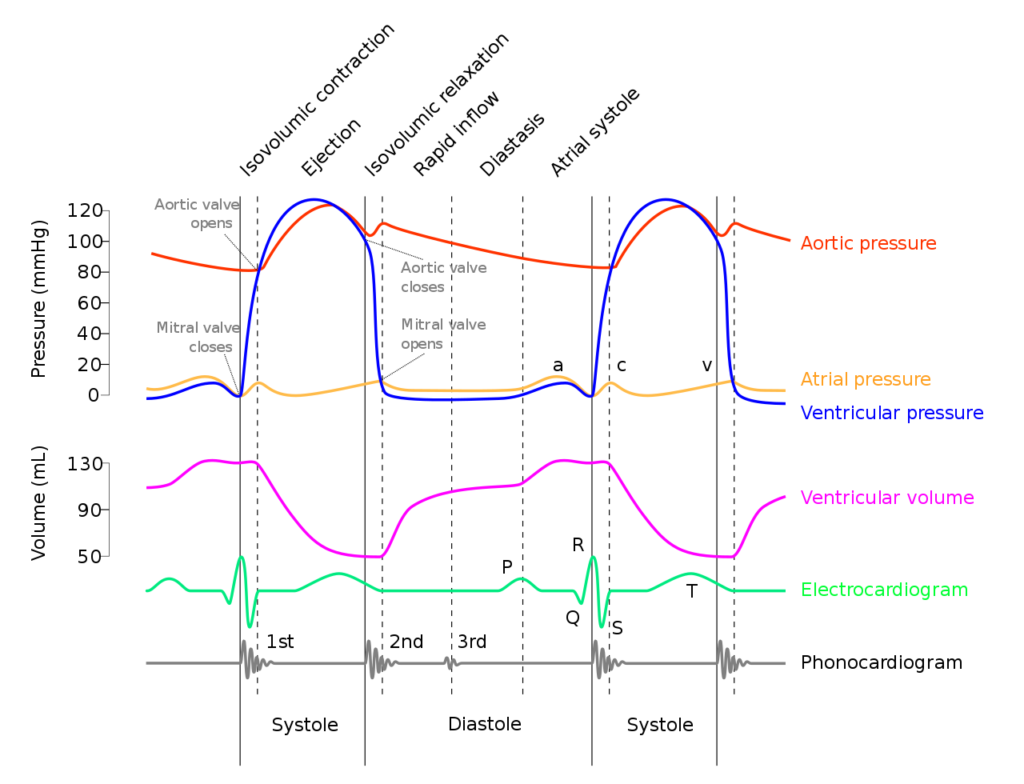
Isovolumetric relaxation
- As aortic pressure overcomes ventricular pressure, and the inertia of forward flow of blood out of the LVOT, the aortic valve closes
- The ventricular myocytes relax, while both the mitral (or tricuspid) and aortic (or pulmonary valves) remain closed
- The volume of blood within the ventricle remains constant (LV End-Systolic Volume ~50mls) , however ventricular pressure decreases from 120mmHg to close to 0mmHg (or 20 to 0mmHg in R ventricle
Ventricular filling
- Passive phase
- As ventricular pressure decreases below the atrial pressure, the mitral (or tricuspid) valve opens allowing forward flow of blood from the atria to the ventricles leading to a progressive increase in ventricular volume and pressure.
- The first two-thirds of the ventricular filling is due to passive filling
- Active phase
- 20% of ventricular filling is provided by the atrial kick during the final quarter of ventricular filling resulting in a slight increase in ventricular pressure
- Ventricular pressure overcomes atrial pressure and the mitral (or tricuspid) valves close
Electrochemical events
- The cardiac action potential is tied to contraction via excitation-contraction mechanism.
- Diastole corresponds to phase IV where voltage gated L and T-type Ca channels close, ceasing Ca influx, and ongoing K efflux repolarizes the myocyte.
- ECG
- The T wave corresponds with ventricular repolarization and occurs immediately prior to ventricular diastole.
- The QRS complex corresponds with ventricular depolarization and occurs immediately prior to ventricular systole
- Diastole corresponds with the terminal part of the T wave to immediately after the QRS complex
- Electrical current through cardiac cycles
- During depolarization, current runs from the base of the heart to the apex (corresponding to flow down bundle of His) the just before the end of depolarization, the direction of flow reverses (corresponding to flow down Purkinje fibres)
- During repolarization, the order reverses, from Purkinje system → Bundle of His
Coronary artery perfusion
- Due to the high ventricular pressures impeding blood flow during systole in a starling-resistor model, left ventricular perfusion (or right ventricular perfusion during pressure-overloaded states) occurs predominantly during diastole.
- As right ventricular pressures are significantly lower than aortic pressures even during systole, perfusion continues (in a normal right ventricle)
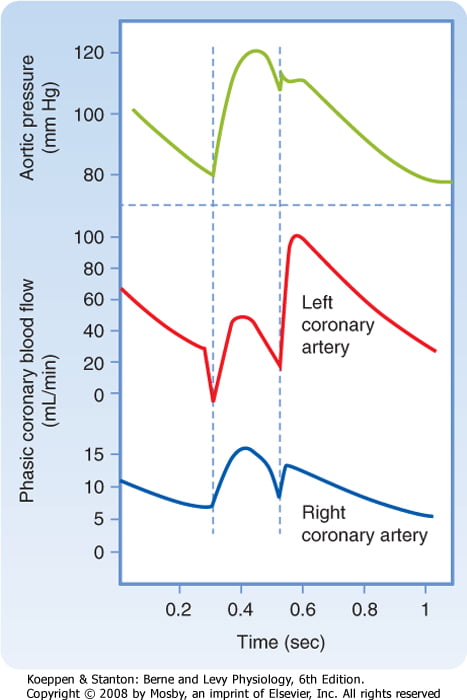
Sakurai 2016
Examiner Comments
2011A 21: 1 (8%) of candidates passed this question.
One possible way to answer this question is to offer a definition of the diastolic period then to split the events up for description into mechanical events, ECG events and electrical/ionic events.
Few candidates defined the diastolic period, and whilst many talked about opening and closing of valves, there was generally a poor understanding of the sequence of events whereby the left ventricle comes to be filled with blood. The better answers included a description of the ionic events that occurred at the various stages of diastole. Many answers lacked any reference to the ECG events in diastole.
The major weakness in answers was again the failure to include sufficient information to achieve a pass mark. This was probably as a result of the lack of a systematic approach when answering a question of this nature.
Syllabus: C1b, 2d,e and C1c, 2e,f
Recommended sources: Textbook of Medical Physiology, Guyton & Hall, Chp 9 – 11 and Review of Medical Physiology, Ganong, Chp 31
22. Compare and contrast the pharmacology of drugs that alter the pH of gastric fluid
CICMWrecks Answer
Mechanism of Gastric Acid Secretion
- Simulation of secretion via
- ↑Gastrin via gastrin receptors
- ↑Ach via M1 rexeptors
- Histamine via H2 Receptors
- receptors located on the basolateral membrane
- Ach and gastrin → ↑ in intracellular [Ca2+]
- H2 receptors are coupled via Gs to adenyl cyclase
- ↑ in Ca2+ and cAMP → ↑ protein kinase activity → ↑ hydrogen pump activity in the luminal membrane
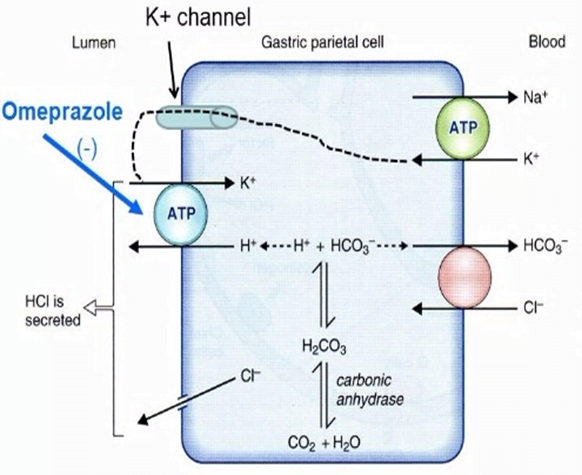
Drug Classes
- PROTON PUMP INIHBITORS (eg, pantoprazole)
- All are prodrugs
- MOA
- Converted to its active form in the low pH of stomach
- Accumulates in the parietal cells after absorption
- Binds to the H/K-ATPase anti-porter
- Inhibiting the proton pump and causing potent and long lasting suppression of basal and stimulated gastric acid secretion.
- PD:
- ↓ acidity/gastric secretions, no change emptying/LOS tone
- PK
- A:
- pKa ~4, lipophilic weak bases thus diffuse into acidic environments well (ie: parietal cell) thus attack secreting not quiesent pumps
- ~50% oral bioavailability, best on empty stomach
- D: High protein binding >90%
- M: rapid first pass metabolism
- E: t1/2 1.5hrs but irreversible H/KATPase bond lasts ~24hrs
- HISTAMINE-2 RECEPTOR ANTAGONISTS (eg; ranitidine)
- Bind to the H2 receptor on the basolateral surface of the parietal cell to reduce H2-stimulated acid secretion from the H/K ATPase on the luminal side of the cell.
- ↓ acidity/gastric secretions, no change emptying/LOS tone
- PK
- well orally absorbed, 50% first pass metabolism
- poorly protein bound 15%
- hepatic metabolism, does not induce p450
- MUSCARINIC ANTAGONISTS (eg, pirenzipine)
- Relatively specific M3 receptor antagonist which reduces the acetylcholine-mediated stimulation of the proton pump
- ANTACIDS (eg, Mylanta, Gastrogel).
- Weak bases which usually contain aluminium and/or magnesium compounds to chelate H+ ions
Gladwin 2016
Examiner Comments
2011A 22: 2 (17%) of candidates passed this question.
Moderately well answered overall, however many candidates lacked a systematic approach to their comparison of the pharmacology of drugs that alter the pH of gastric fluid. Few candidates discussed pharmacokinetics in sufficient detail, with only a very limited discussion comparing the absorption, metabolism and elimination of even common drugs. Relevant information such as bioavailability, duration of effect, and available formulations with dosing was often lacking. Similarly, little attention was given to important drug interactions. Many candidates included drugs which are used for gastric problems or mucosal protection, but do not specifically influence gastric pH e.g. sucrulfate. Some candidates gave unnecessarily detailed accounts of the physiology of gastric fluid production and the acid-base mechanisms involved. All candidates provided details of H2 blockers and PPIs, but often did not list representative examples or compare the effects on basal versus stimulated acid secretion. Many candidates also discussed antacids, but did not indicate their
mechanisms of action properly and did not outline potential adverse effects. Some candidates included prostaglandin analogues and anticholinergic drugs for completeness and were able to indicate their roles in affecting gastric acid secretion.
Syllabus: Q2a 2b,c
Recommended sources: Basic and Clinical Pharmacology, Katzung, Chp 62
23. Compare and contrast the pharmacology of Noradrenaline and Vasopressin
Examiner Comments
2011A 23: 6 (50%) of candidates passed this question.
A straightforward question that was reasonably answered, with most candidates using a methodical tabular approach to explaining the differences in pharmacology between noradrenaline and vasopressin. The benefits of adhering to a wellorganised system of columns showing direct comparisons of the various relevant drug characteristics was clear, with candidates who chose this approach covering most of the necessary information in a clear and comprehensive manner. Some candidates managed to provide a great deal of relevant detail within the allocated time as would be expected in this relatively uncomplicated question. Simple definitions were often lacking and failing to provide this basic introductory information resulted in lower marks for this question. While most candidates were able to discuss the effects of each drug on the cardiovascular system, not as many were able to give outline other physiological effects in sufficient detail. For example, many candidates made little mention of important renal, metabolic and haematological effects. While some candidates discussed pharmacokinetics well, many provided only a very superficial outline of this aspect. The important area of adverse reactions could also have been covered in greater detail.
Syllabus: C2d, 2a and N2, 2f
Recommended sources: Basic and Clinical Pharmacology, Katzung, Chp 9 and 37
24. Describe the PHYSICAL PRINCIPLES that are involved in the flow of blood through a dialysis circuit, and, in the movement of solutes across a dialysis membrane.
CICMWrecks Answer
Dialysis
- “process whereby the composition of a solution (patient’s blood) is altered by exposure to a second solution (dialysate) through a semi-permeable membrane”
- involves transfer of H2O and LMWT solutes across the membrane between the solutions via diffusion, osmosis and solvent drag
- Indications:
Failure of normal renal functions, i.e.:- Acid-base imbalance
- Electrolyte derangement – Particularly hyperkalaemia.
- Intoxications
- Overload
- Ureamia
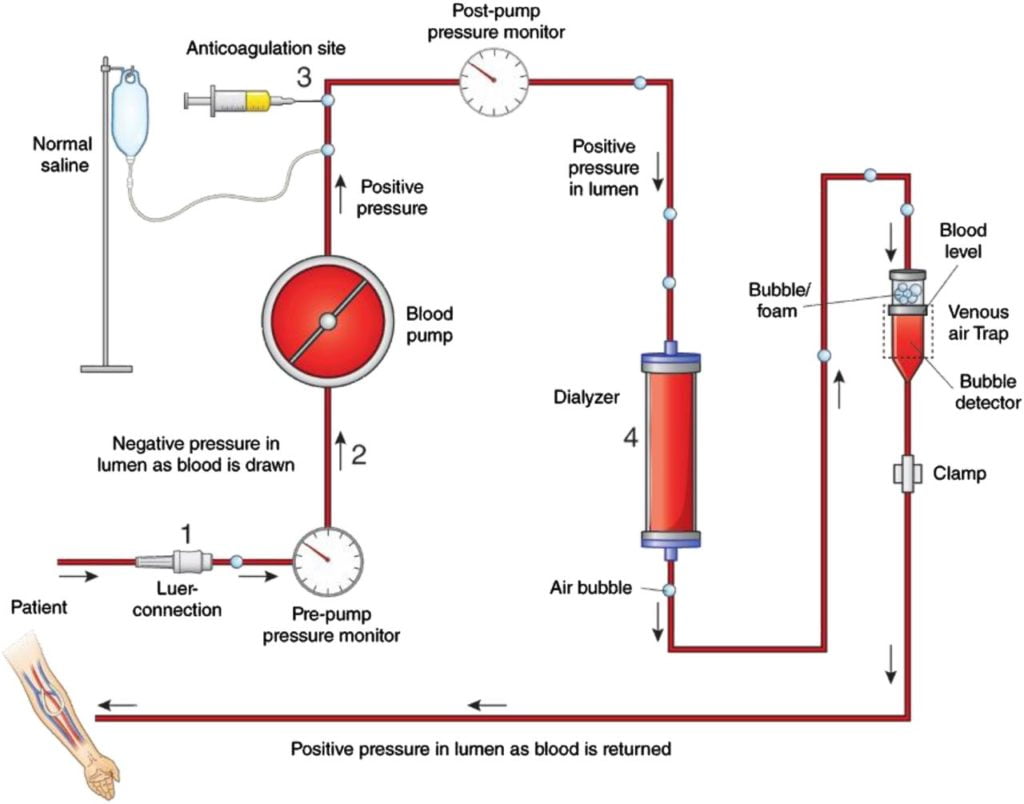
Physical principles related to blood flow:
where, Q = Flow
ΔP = pressure gradient
r = radius
η = Viscosity
l = length
- In a dialysis circuit, blood travels from the patient to a dialysis machine where it passes through a dialyser, allowing movement of fluid and plasma solutes across the membrane in either direction.
- Flow across the circuit is determined by:
- Set flow rate of centrifugal dialysis pump
- parts of the circuit:
- access port of vascular access (catheter / fistula / graft)
- from patient (pre-membrane)
- across membrane (trans-membrane)
- to patient (post-membrane)
- return port of vascular access (catheter / fistula / graft)
- Assuming that machine delivers the flow which is set and the flow remains laminar and constant, the pressure across the various components varies based on the resistance.
- The pressure gradient across the circuit depends upon:
- characteristics of patient:
- Blood viscosity
- hematocrit
- Blood protein and lipid content
- Temperature changes changes flow and viscosity
- Fåhræus–Lindqvist effect: Viscosity reduces in smaller diameter – because erythrocytes move over to the centre of the vessel/circuit, leaving only plasma near the wall of the vessel.
- variable resistance during breathing or movement
- collapsing vessels due to decreased vessel size / hypovolemia
- antivoagulation used
- Blood viscosity
- characteristics of vascular access:
- gauge and length of access
- resistance from physical obstruction
- resistance position
- resistance from clots, fibrin
- characetristics of circuit:
- Age of circuit, presence of clots, fibrin
- radius
- length (usually constant ~3.5m)
- surface coating of circuit
- anticoagulation used
- characteristics of membrane:
- resistance worsens with clot, fibrin, lipid deposition
- characteristics of patient:
- In some cases, resistance increases significantly, flow changes to turbulent, and dialysis machine may not be able to acheive set blood flow.
(This usually activates some sort of alarm and will require quick troubleshooting) - As flow is set, the pressure across the various components varies based on the resistance
Physical Mechanisms of fluid and solute removal
- Fluid removal Mechanisms:
- Osmosis:
- the movement of a pure solvent such as water, through a differentially permeable membrane, from a solution that has a lower solute (particle) concentration to one that has a higher solute concentration
- The rate of osmosis depends on the concentration of solute, the temperature of the solution, the electrical charge of the solute and the difference between the osmotic pressures exerted by the solutions.
- Movement across the membrane continues until the concentrations of the solutions equalise.
- Ultrafiltration
- the movement of fluid through a membrane caused by a pressure gradient (hydrostatic or osmotic pressure)
- Uses both positive and negative pressure:
- Positive pressure: pressure exerted by the blood flowing through the dialyzer, Results from blood being pushed by blood pump
- Negative pressure: pressure applied to the dialysate side by the machine
- Pulls excess fluid from blood compartment to dialysate compartment
- Solute removal mechanisms:
- Diffusion
- the movement of solutes from a high to a low solute concentration across a semipermeable membrane
- a concentration gradient is necessary for diffusion to occur
- it removes all small molecules
- the rate of diffusion is dependant on
- surface area of filter
- ratio of dialysate flow to blood flow
- size of the molecules
- Convection:
- The movement of solutes with a water-flow “solvent drag” across a membrane during osmosis or ultrafiltration
- Important for movement of small solute (urea, creatinine).
- Adsorption:
- when the molecules (solutes) adhere to the surface or interior of the membrane.
- with the movement of fluid across the membrane, if no fluid is moving then adsorption can not occur.
- in 2 manners:
- surface adsorption where the molecules are too large to permeate and migrate through the membrane; however they can adhere to the membrane.
- bulk adsorption occurs within the whole membrane where molecules can permeate it.
- Molecules that can be effectively adsorbed include:
- B2 microglobulin
- Cytokines
- Coagulation factors
- Anaphylatoxins
- The rate of movement of solute across the dialyzing membrane depends on
- the concentration gradient of the solute between the two solutions,
- the permeability of the membrane to the solute,
- the surface area of the membrane, and
- the length of time that the blood and fluid remain in contact with the membrane
- Concentration gradient:
- The concentration gradient is developed by using dialysate fluid with low concentration of solutes that are usually cleared by the kidney (eg; Na, K, PO4, urea, creatinine), in order to create a steep concentration gradient to encourage movement of these solutes from the blood to the dialysate.
- The movement of blood and dialysate in different directions ensures this concentration gradient does not reach equilibrium and reduce further solute exchange.
JC 2019
Examiner Comments
2011A 24: 2 (17%) of candidates passed this question.
This question required candidates to describe the physical principles of blood flow through a dialysis circuit and the movement of solute across a dialysis membrane. While most candidates were able to allude to important factors contributing to the flow of a fluid through a hollow tube, few did so in a systematic way and only some provided relevant formulae showing the relationship between pressure, fluid viscosity and tube resistance. A short discussion proceeding to flesh out the factors that determine blood viscosity, circuit pressures and practical examples was expected.
Some candidates discussed convective processes extensively, which was not required in this question focussed on dialysis. Most candidates were able to describe the physical chemistry involved in diffusion across a semipermeable membrane in basic terms, however few provided sufficient details of these important principles.
Very few candidates went on to properly discuss electrochemical forces affecting solute and water movement across a membrane or the factors that influence the performance of dialytic therapies in practical application.
Syllabus: A2c, R2e, D1, 2b,c
Recommended sources: Basic Physics and Measurement in anaesthesia, Davis and Kenny, various sections. Also Review of Medical Physiology, Ganong, chp 2, 32
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | Oral drug absorption, basic pharm |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Resp – flow volume loop, dynamic airways compression, alveolar elastic recoil pressire |
| G. CVS | Pharm and Physio of peripheral circ, SNiP, Endothelium and secretions (prostacyclin, EDRF, endothelin), Vasomotor cenre |
| H. Renal | Renal function, hyperkalaemia, cardiac consequences (Action Potential, ECG) |
| I. Body Fluids and Electrolytes | Hartmann’s, colloid vs crystalloid, semipermeable membrane |
| J. Acid Base | |
| K. Neuro | ICP, CBF, control mechanisms, drugs |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | Body temp control, diurnal variation, thermoneutral zone, differences with neonates, newborns, children, drugs |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | Glucose regulation, hypoglycamic drugs, insulin, T4 vs T3 |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments