1. Discuss the physiological causes of early post-operative hypoxaemia
CICMWrecks Answer
Hypoxaemia
- Hypoxaemia = Acute ↓ PaO₂ = (low partial pressure of oxygen in arterial blood)
- Normal PaO2 = 75-100mmHg
- Hypoxaemia can lead to tissue hypoxia and subsequent adverse effects
Causes of early post-operative hypoxaemia:
- Low inspired oxygen concentration
- PAO2 = PiO2 – PCO2/R + F, where PiO2 = (PATM-47mmHg) x FiO2
- FiO2 may be insufficient to meet the metabolic demands of the patient
- may been ↑due to recent surgery
- Hypoventilation
- VA = (TV – dead space) x RR
- may be due to:
- pain
- respiratory depression 2° drugs (opioids, barbiturates)
- paralysis of respiratory muscles
- damage to chest wall
- high resistance to breathing
- results in a ↓PaO2 and a ↑PaCO2
- easily reversed by increasing FiO2
- along with V/Q scatter are the major causes of post-operative hypoxaemia
- V/Q Ratio Differences
- V/Q scatter refers to areas of low ventilation compared with perfusion
- atelectasis
- V/Q mismatch refers to areas of low perfusion compared with ventilation
- severe hypotension
- PE
- V/Q scatter refers to areas of low ventilation compared with perfusion
- Shunt
- V/Q = 0
- refers to blood that enters the arterial system without going through ventilated areas of lung
- cannot be abolished by an ↑FiO2
- may be anatomical or physiological
- Increasing oxygen consumption
- may be due to:
- pyrexia (fever)
- shivering
- hypermetabolic states (thyrotoxicosis, malignant hyperthermia)
- may be due to:
- Diffusion barrier
- very minor role in healthy patients (likely to be present pre-op though)
- PaO2 usually very close to PAO2 but never quite equal
- PaO2 will decrease with blood-gas barrier thickening as it ↑ the diffusion distance between the alveolar gas and pulmonary capillary blood
- Fick’s Law of Diffusion
- Increasing Age:
- the PaO2 ↓ with ↑age from about 20 years of age
- this is due to closing capacity being greater than FRC
blood draining from these alveoli has a lower PO2
JC 2020
Examiner Comments
2010B 01: 5 (33%) of candidates passed this question.
For a good mark it was expected that candidates define hypoxaemia and, in a structured manner, discuss the physiological causes of early postoperative hypoxaemia. Candidates should always try and begin their answer with a definition of the term that is to be discussed. Many candidates invoked clinical disease related causes and not physiological. This did not score marks. Similarly, factors leading to tissue hypoxia, such as anaemia or low 2,3-dpg were not given marks. Candidates may have done so because they confused “hypoxaemia” with “hypoxia”. Better answers addressed different physiological causes of hypoxaemia, including areas of low V/Q; hypoventilation; increased oxygen consumption; loss of hypoxic pulmonary vasoconstriction and closing capacity exceeding functional residual capacity.
Syllabus: B1a2b, B1e,f,g
References: Nunn’s respiratory physiology – various sections
2. Outline the principles of compatibility testing of blood for transfusion.
CICMWrecks Answer
Blood Typing
- The purpose of blood typing is the streamlining of blood products on the basis of the presence of antiogenetic surface membrane proteins in order to minimise patient harm.
- Main Types of Antigens:
- ABO
- Rhesus
- Others:
- Kell, P, MN, Lewis
- less antigenic
ABO system:
Red blood cells possess genetically determined antigens on their cell membrane, termed
“agglutinogens”
Agglutinogens: glycoproteins, with differing terminal oligosaccharides
- H gene:
- Forms “H-antigen” by addition of fructose to membrane glycoprotein
- A gene:
- Forms “A-antigen” by extension of H-antigen with “N-acetyl-D-galactosamine”
- B gene:
- Forms “B-antigen” by extension of H-antigen with “D-galactose”
Blood Groups:
| Group | Antigen | Possess Antibody | Incidence |
|---|---|---|---|
| A | A-antigen only | Anti-B antibody | 45% |
| B | B-antigen only | Anti-A antibody | 9% |
| A/B | A and B-antigen | Lack both Anti-A and Anti-B antibodies | 3% |
| O | H-antigen only | possess anti-A and anti-B Ab’s | 43% |
Blood Group antibodies:
- Naturally-occurring Ab to A- and/or B-antigens (as anti-A and anti-B antibodies)
- IgM only
- Develop naturally after 3 months of age due to presence of Ag in bacteria/food that resemble A/B-Ag’s
Typing / Blood Group determination (Coombs)
- Red cells tested using anti-sera (Ab added to sample)
- IgM anti-A and anti-B Abs added to sample
- +ve agglutination indicates blood type
- “Reverse blood grouping” (Sample added to Ab)
- Test individual’s serum using known group A, B, and O red cells
- +ve agglutination indicates blood type
Antibody screen:
- Used to detect if an individual possesses antibodies other than those against agglutinogens A and B
- Individual’s blood is exposed to a number of red cells with known antigens, and if a reaction, an antibody is present
- A common antibody is the D antigen, or Rhesus factor
- Highly antigenic
- Present on RBC only
- Three subtypes
- Cc; Dd; Ee
- d-Ag does not exist
- Any combination of the three with a D-Ag is highly antigenic.
- Rh +ve (if D-Ag present)
Cross Matching
Major Crossmatch: Test in vitro recipient’s serum and donor’s red cell
Minor Crossmatch: In vitro serological compatibility between donor’s serum and recipient’s red cells
Major Crossmatch:
- Saline agglutination test
- To reconfirm ABO grouping
- It tests for presence of IgM (Eg. anti-A and anti-B Ab’s) in recipient serum against donor RBC
- Donor’s RBCs + saline + recipient serum
- +ve agglutination means incompatible ABO
- Indirect Coomb’s test
- To Reconfirm presence of “minor” Ab’s in recipient serum
- Two stages:
- Donor RBCs + recipient serum
- RBC will be coated if Abs present
- AHG test
- RBC washed
- “anti-human globulin” (AHG) added
- agglutination
- → recipient serum contains an Ab against donor RBC
- Donor RBCs + recipient serum
Serological testing:
All donor blood is tested for:
HIV 1 + 2
Hepatitis B
Hepatitis C
HTLV-1
Mooney / Gladwin 2016
Examiner Comments
2010B 02: 8 (53%) of candidates passed this question.
There was a reasonable knowledge of the basics of ABO antigens and antibodies by most candidates. However, many answers seemed to lack perspective of the steps performed in the laboratory to improve immunological safety of transfusion. For a good answer it was expected that candidates also discuss the basis to serological testing and antibody screening.
Syllabus: J2a, J2a2i
References: Guyton Textbook of Physiology Chp 32 and 35, Australian Red cross Transfusion Medicine manual
3. Compare and contrast Ceftriaxone and Meropenem with respect to the following:
a. Mechanism of action and spectrum (40% of marks)
b. Pharmacokinetics (30% of marks)
c. Effect of critical illness on pharmacokinetics and subsequent dosing. (30% of marks)
Examiner Comments
2010B 03: 5 (33%) of candidates passed this question.
Most candidates used a table to compare these two drugs, which was an ideal means to structure their answers to this question and a useful way to present the information required. The use of a structured format for learning pharmacokinetic data about all drugs allows more useful information in a question such as this. Candidates should also give consideration to the effects of critical illness (such as cardiac output, plasma binding and volume of distribution) affect the pharmacokinetics of drugs used in intensive care. This particular section of the question was poorly answered.
Syllabus: M2a,2d
References: Goodman and Gillman, The Pharmacological basis of therapeutics Chp 46
4. Describe the cardiovascular changes that occur in pregnancy
CICMWrecks Answer
- Pregnancy is a time of increased metabolic demand, which cardiovascular changes reflect.
- Changes begin from week 8 and ↑ to plateau at 32 weeks → return to normal 2-8 weeks post delivery
- Changes depend on stage of pregnancy
- Hormonal changes: ↑ circulating concentrations of oestrogen, progesterone, hCG
- ↑ metabolic demand esp. during labour: ~↑60% O2 consumption/ CO2 production during labour
- Mechanical effects from gravid uterus
Characteristics
- Mechanical Effects:
- Thoracic changes:
- Anatomical compression of chest
- Diaphragm pushed upwards by 4cm
- ↑ AP + transverse diameter of chest wall (2-3cm)
- placental circulation: ↓pressure, ↓resistance AV shunt
- Aortocaval compression
- Collateral blood flow via collateral paravertebral epidural veins
- Thoracic changes:
- Hormonal Changes:
- ↑ circulating concentrations of oestrogen, progesterone, hCG
- Oestrogen stimulation of RAAS
- Increased plasma volume (40% or 1~1.5L positive)
- Erythropoietin secretion
- Increased erythropoiesis and red blood cell volume (20%)
Changes in CVS
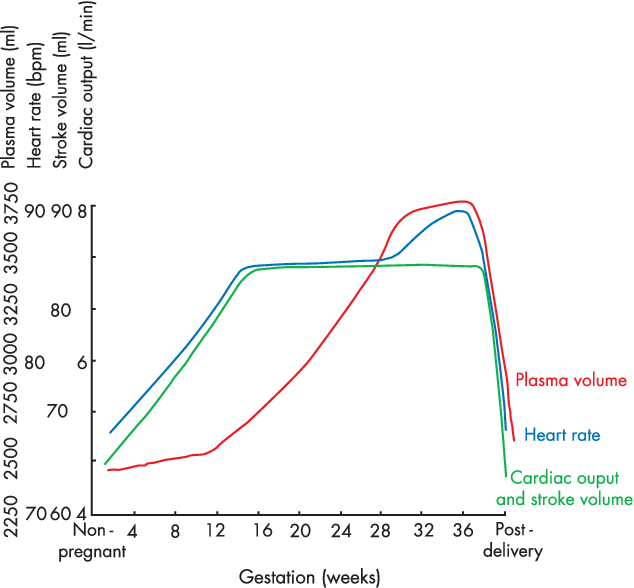
- Anaemia of pregnancy
- Disproportionate plasma volume expansion relative to erythropoiesis
- Increased cardiac output (40%)
- Increased uterine blood flow (750ml/min)
- Increased renal blood flow
- Increased HR (25% by second trimester)
- Increased SV (25% in first trimester)
- Decreased peripheral vascular resistance (30%)
- Progesterone
- Prostaglandins
- Down-regulation of α receptors
- Decreased plasma oncotic pressure (15%) → peripheral oedema
During labour
- Contraction → 300ml return to central maternal circulation
- CO increases 15%, 30% and 45% in latent, active and expulsive phases of labour respectively
- Immediately after labour CO 80% pre-labour levels due to autotransfusion due to uterine involution
- Return to non-pregnant levels 2 weeks after delivery
Sakurai / Kerr / JC 2020
Examiner Comments
2010B 04: 4 (27%) of candidates passed this question.
A structured approach to answering this question was important. The principal topics to discuss included the increased cardiac output, increased blood volume, changes in red blood cell mass and protein concentrations, decrease in blood pressure and gravitational effects of the gravid uterus on major blood vessels. Answers benefited by specific details of percentage change and reference to timing of occurrence.
Syllabus: O1,2a
References: Hemmings Foundation of Anaesthesia: Basic and Clinical Science Pg 823 – 825, Miller Anaesthesia pgs2308-2309
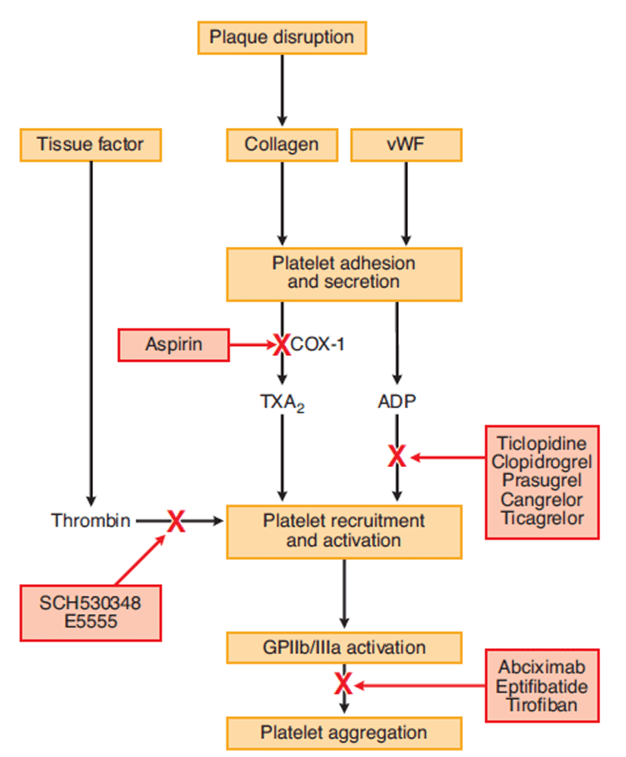
5. List the antiplatelet agents and outline their mechanisms of action, adverse effects, mode of elimination and duration of action.
CICMWrecks Answer: Anti-platelet drugs
Anti-platelet drugs
| COX Inhibitors | Aspirin | MoA | Cyclooxygenase-1 (COX-1) produces precursor to thromboxane A2 (TxA2) Aspirin irreversibly binds and inactivates |
| A/E | GI bleeding and ulceration Kidney injury Reye’s syndrome Bronchospasm In overdose – metabolic acidosis | ||
| Elimination | CYP2C19, metabolites in urine | ||
| Duration | Irreversible COX-1 inhibition → last until platelet turnover (7-10 days) | ||
| P2Y12 receptor antagonists | Clopidogrel Ticagrelor | MoA | P2Y12 is an ADP receptor, which causes platelet recruitment and activation (by inducing activation of GPIIb/IIIa) |
| A/E | Bleeding, itch | ||
| Elimination | dogrel is a prodrug, requiring activation by CYP450 Prasugrel is inactivated by CYP450 | ||
| Duration | Irreversible receptor inactivation → 7-10 days | ||
| Glycoprotein IIb/IIIa inhibitors | Abciximab Tirofiban | MoA | glycoprotein IIb/IIIa binds von Willebrand factor on damaged vascular wall, and fibrinogen to create a pro-clot with other platelets |
| AE | Higher rate of bleeding than aspirin and ADP receptor antagonists | ||
| Elimination | Variable | ||
| Duration | 6-12 hours after cessation of infusion | ||
| Phosphodiesterase inhibitors | Dipyridamole | MoA | Inhibits phosphodiesterase → increased platelet cAMP Also causes coronary vasodilation → useful in angiograms |
| AE | Bleeding, hypotension | ||
| Elimination | Mainly glucuronides in bile 5% in urine | ||
| Duration | 3 hours |
Examiner Comments
2010B 05: 10 (67%) of candidates passed this question.
Most candidates did reasonably well by including aspirin, ADP receptor blockade and glycoprotein 2b/3a blockade in their answers. The best approach to answer this type of question was to use a table with each anti-platelet agent within a column and headings for the rows such as mechanisms of action, adverse effects, mode of elimination and duration of action. Common omissions included the irreversibility of the blockade of the platelet function by many of these agents, renal toxicity and bronchospasm as side effects of aspirin, bone marrow toxicity of ADP receptor blockers, and dipyridamole as an anti-platelet agent. Some candidates classified clopidogrel as a glycoprotein 2b/3a blocker incorrectly and thought clopidogrel has a relative short duration of action on platelet function because of its half-life. Clopidogrel as a prodrug requiring activation by cytochrome P450 and hence significant potential drug interactions were not mentioned by any candidates.
Syllabus: J2a,2d
References: Goodman and Gillman, The Pharmacological basis of therapeutics Chp 55
6. Outline the physiological consequences of a tension pneumothorax. (60% of marks) Describe the anatomy relevant to the insertion of an intercostal catheter. (40% of marks)
CICMWrecks Answer: Tension Pneumothorax
- Pleura are separated by a thin layer of pleural fluid
- Surface tension keeps membranes apposed
- Balance between elastic recoil of lung tissue (natural tendency of lung to collapse) and elastic recoil of chest wall (natural tendency for chest to expand)
- Normal intrapleural pressure 2.5-6cmH2O
- When air is introduced to pleural space
- Lung not expanded by chest wall → collapse
Tension pneumothorax
- Air enters pleural space via a one-way valve in the lung
- Intrapleural pressure rises dramatically, overcoming closing capacity of one or both lungs, and may exceed right atrial pressure or even aortic pressure
Respiratory:
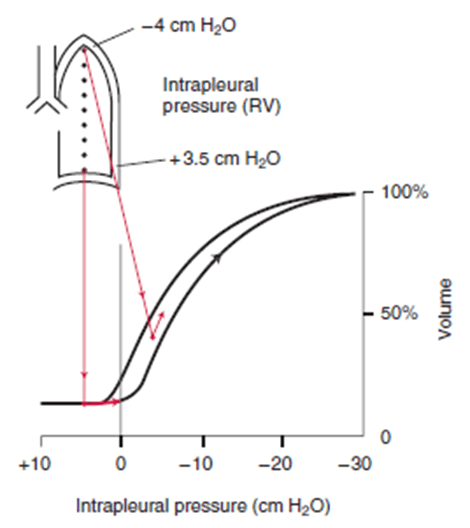
- At low and subatmospheric intrapleural pressures, lung compliance is poor → decreased ventilation → impaired gas exchange → rise in PcCO2, fall in PcO2
- If intrapleural pressure > atmospheric pressure, may exceed compressive resistance of alveoli present at low volumes
- Air moves out of lung → lung collapse
- Falling PO2 and rising PCO2 → stimulation of central and peripheral chemoreceptors → increased work of breathing (tachypnoea without much increase in TV)
V/Q
- Perfusion of lung (dependent on alveolar pressure and lung volume via effect of capillary recruitment and distension on pulmonary vascular resistance) will be maintained up until a point
- In the presence of hypoventilation → V/Q mismatch → fall in PaO2
- Lung perfusion will stop when alveolar pressure rises and lung volume drops
- However, hypoxia will still occur as a tension pneumothorax will cause V/Q mismatch in opposite lung, and may blunt cardiovascular compensation due to impaired cardiac output
Cardiovascular:
- Compression of vena cava and right atrium → decreased preload → decreased stroke volume
- Compression of aorta → increased afterload → decreased stroke volume
- The two above effects, plus hypoxia, may trigger a cardiac arrest
- Compression of ventricles → increased transmural pressure gradient → increased contractility
Mooney 2016
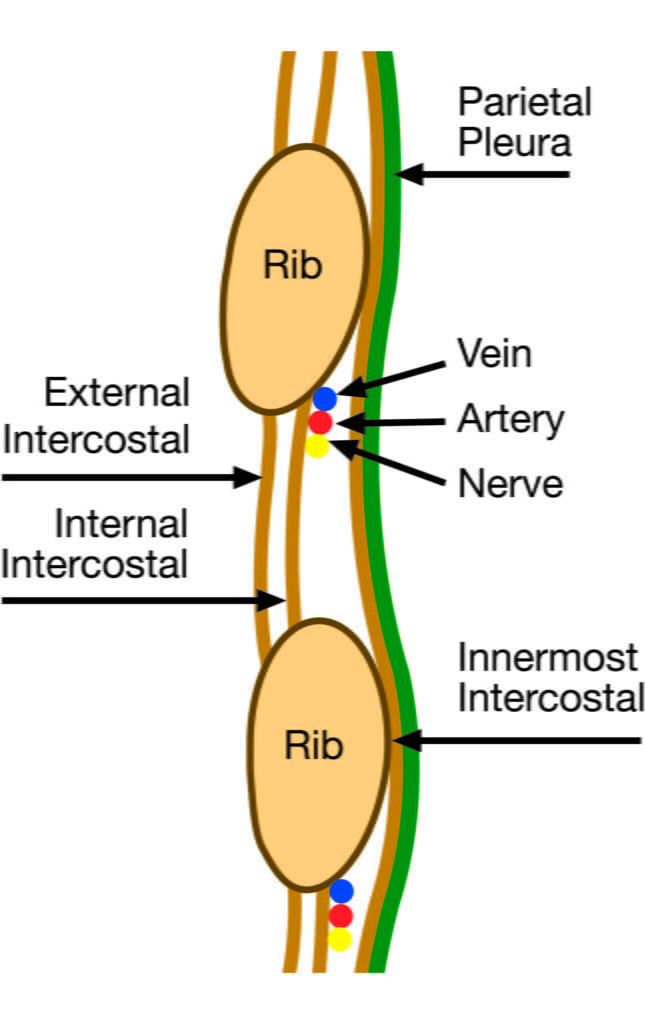
CICMWrecks Answer: Anatomy relevant to ICC Insertion
- Triangle of safety
- Lateral border of pectoralis major
- Anterior border of latissimus dorsi
- Superior to the 5th intercostal space – be aware the diaphragm (and liver/spleen) may be unexpectedly superior
- Layers (Superficial → Deep)
- Skin
- Subcutaneous connective tissue
- Fat
- Ribs and intercostal muscles
- Neurovascular bundle (intercostal artery, vein and nerves) lie in intercostal groove on infero-interior surface of the ribs
- External intercostal muscle
- Internal intercostal muscle
- Innermost intercostal muscle
- Parietal pleura
- Potential pleural space (pneumothorax)
- Visceral pleura
- Lung
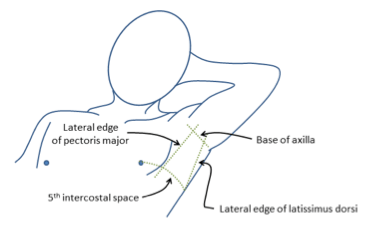
Sakurai / Mooney 2016
Examiner Comments
2010B 06: 9 (60%) of candidates passed this question.
The physiological consequences of tension pneumothorax were in general poorly described. Purely mentioning clinical features (eg distended neck veins) without an associated physiological explanation was not sufficient. Good answers described why tension pneumothorax causes hypoxaemia and hypotension. The cardiovascular mechanism for hypotension and the effect on pulmonary compliance, pulmonary vascular resistance, lung volumes, work of breathing, shunt fraction and carbon dioxide elimination should all have been described.
Many candidates used valuable time making comments about tension pneumothorax being a medical emergency requiring prompt assessment and treatment. These comments attracted no marks, as they were not sought in the question asked. Often the clinical need for insertion of a needle/cannula for decompression was mentioned, but again this attracted no marks.
Description of the anatomy relevant to the insertion of an intercostal catheter was very variable. Most candidates were able to detail the anatomical layers that the catheter had to traverse in order to gain access to the pleural space, and most explained why the catheter should enter the pleural space just above the rib. However, few accurately described where access to the pleural space should be sought and even fewer could explain why. The British Thoracic Society’s ‘safe triangle’ in the axilla and the anatomical boundaries of this were correctly described by very few candidates.
No candidate described an anterior approach through the mid clavicular line, although anatomical details of this approach would have been acceptable.
Syllabus: B1c 1 & B1b 2d
References: Nunn’s respiratory physiology 4th Ed P423, Ganong Review of Medical Physiology 22nd Ed P688
7. List the adverse properties of Propofol and Ketamine
CICMWrecks Answer
| PROPOFOL | KETAMINE | |
|---|---|---|
| Resp | – Respiratory depression – Apnoea – Decreased RR, TV, MV, FRC | – Mild respiratory stimulation – Respiratory depression if rapid IV administration – Increased secretions – Maintenance of pharygo-laryngeal reflexes > Laryngospasm – Bronchodilation > Likely Ca channel mediated via R-ketamine isomer |
| CVS | – Peripheral vasodilation – Bradycardia – Hypotension | – Indirect sympathomimetic effects → elevation of MAP > Due to inhibition of noradrenaline reuptake – Direct cardiodepressant effects |
| CNS | – Sedation – Decreased cerebral blood flow | – Increased cerebral blood flow – Increased cerebral O2 consumption – Sedation – Hallucinations – Emergence phenomena |
| GIT | – Nausea (although usually anti-emetic) | – Nausea > Due to serotonin reuptake inhibition |
| Other | – Propofol infusion syndrome > Rhabdomyolysis > Metabolic acidosis > Steatohepatitis – Injection site pain – Anaphylaxis – Leukocytosis | – Anaphylaxis – Increased muscle tone |
Examiner Comments
2010B 07: 6 (30%) of candidates passed this question.
This question was not well answered by many candidates. Candidates included advantages of these sedative / analgesic agents in their answers when they were not asked for in the question. The best approach to answer this question is to use a table listing their potential adverse properties in categories such as pharmaceutical and chemical properties, pharmacodynamic properties in different body systems, and pharmacokinetics. The common weaknesses observed included the side effects of ketamine, including its side effects on intracranial pressure, myocardial contractility and oxygen consumption, and hallucinations or delirium. Some adverse properties of propofol including bacterial contamination and pain on injection were also not well covered by many candidates. Syllabus: G2a,2a References: Goodman and Gilman The pharmacological basis of therapeutics 11th edition p350- 352
8. Outline the physiological consequences of an inability to produce insulin.
CICMWrecks Answer
Insulin
Insulin is a 51 amino acid hormone (MW 5734 D) consisting of two chains (α and β) made in the pancreatic beta cells as a folded protein. It is released in response to blood glucose levels > 5mmol/L.
Actions:
Acts by binding to the insulin receptors (α and β subunits) on the surface of target cells, causing a shape change which results in the phosphorylation of several proteins within the cell, which modulate the activity of several processes. Insulin is anabolic, increasing the storage of glucose, fatty acids, and amino acids.
Roles:
CHO:
- Stimulates GLUT 4 receptors to move to the surface of muscle and adipose tissue cells to increase glucose uptake (this does not happen in RBC, brain tissue, intestinal mucosal cells or renal tubule cells).
- Upregulates Glycogen Synthase to increase glycogen production
- Upregulates glucokinase in hepatocytes to trap glucose in cells by phosphorylating it
- Decreases gluconeogenesis and glycogenolysis (by inhibiting glucose-6-phosphatase)
Proteins:
- Upregulates amino acid uptake, enhances protein synthesis, and inhibits protein degradation in muscle and other tissues, thereby decreasing the plasma concentrations of most amino acids
Lipids:
- Upregulates Pyruvate Dehydrogenase, Lipoprotein Lipase and Fatty Acid Synthase to facilitate the breakdown of TAGs to fatty acids for uptake by adipose tissue cells
- Inhibits Hormone Sensitive lipase to decrease the hydrolysis of triglycerides stored in the adipocyte
Inability to produce insulin will therefore result in:
- Hyperglycaemia (but relative intracellular hypoglycaemia, as glucose is unable to enter cells)
- Loss of inhibition of gluconeogenesis from proteins and adipose tissue
- Reduction in protein synthesis and TAG synthesis
- Utilisation of fatty acids as an alternative fuel source à
- Adipose tissue is broken down to FFA via Hormone Sensitive lipase (which is no longer inhibited by insulin), and Acetyl CoA produced from the FFA.
- Acetyl CoA can enter the Krebs cycle or be transported to the liver for ketone body production via beta oxidation (acetoacetate, beta-hydroxybutyrate and acetone)
- Ketone bodies are distributed to the tissues, converted back to Acetyl CoA and used as fuel
- The presence of high levels of ketone bodies results in a metabolic acidosis à produces ‘air hunger’ and Kussmaul breathing in an attempt to compensate for this acidosis
- Acidosis produces hyperkalaemia (the lack of insulin-induced K entry to cells also contributes)
- Increase in plasma free fatty acids and amino acids
- Hyperglycaemia exceeds the renal tract’s ability to reabsorb glucose and a glycosuria occurs along with an osmotic diuresis of the large, osmotically active glucose molecule
- Patient becomes hypovolaemic and hyponatremic due to loss of Na through the renal tract
Examiner Comments
2010B 08: 7 (47%) of candidates passed this question.
Most candidates were able to detail how insulin allowed influx of glucose into insulin dependent cells, in combination with potassium. Good candidates were able to explain how the inability to produce insulin allowed hypovolaemia and electrolyte loss, with the ensuing tachycardia and hypotension. Few candidates mentioned insulin’s action on hormone sensitive lipase (HSL), and that deficiency of insulin leads to increased activity of this enzyme. The fact that when insulin is not produced, the liver cells carry out β-oxidation of the fatty acid (released peripherally by the action of HSL) releasing acetyl CoA which is coalesced into acetoacetic acid was mentioned by only a few. However, the fact that ketone bodies in the form of acetoacetic acid, β-hydroxybutyric acid and acetone accumulate and cause a metabolic (anion gap) acidaemia, appeared to be well known. Few, went on to describe that a compensatory respiratory alkalosis will be generated and detail how and why this occurred.
Syllabus: N2a&b, E2,F1, C1g 2b
References: Guyton and Hall Textbook of Medical Physiology 10th Ed, pg 888
9. Describe how the body defends against infection.
CICMWrecks Answer
Immune System: complex network of cells and proteins that defends the body against infection.
Physical barriers
- Skin
- Cilia
- Respiratory tract
- Acidic environment
- Stomach
Innate immunity
Recognize generic pathogenic motifs and directly attack or opsonize and phagocytose
- Defensins – perforate pathogenic membranes
- Compliment cascade – opsonize and signal for phagocytosis and directly lyse via membrane attack complex
- Innate immune cells
- Neutrophils
- Degranulate cytotoxic contents and cause oxidative damage to pathogens
- Macrophages
- Phagocytose and kill opsonized pathogens
- Dendritic cells
- Phagocytose and present antigen to cells of acquired immune system
- Natural Killer cells
- Recognize abnormal cell surface expression (e.g. lack of MHC, abnormal MHC proteins) due to intracellular pathogens (e.g. virus) and directly kill infected cells
- Neutrophils
Acquired immunity
- Pathogenic antigens presented to B cells and T cells via APCs such as dendritic cells
- Show: Specificity, Memory, Diversity, Immunological tolerance
- B cells (20%, Humoral Immune reponse)
- Produce antibody against antigen
- Antibodies opsonize and target for T cell killing, phagocytosis and compliment cascade
- B cells become memory B cells and plasma cells, primed for explosive antibody production on subsequent exposure to the same antigen
- T cells (80%, Cell-mediated immune reponse)
- Major subtypes into CD8 (Killer) T cells and CD4 (Helper) T cells
- CD8 (Cytotoxic/Killer) T cells recognize specific antigen presented to them via TCR and lyse pathogens and infected cells
- CD4 T (Helper) cells produce cytokines for adjacent B cells that recognize the same antigen, allowing B cell differenciation and antibody production
- CD28 (Regulatory) T cells dampen the immune reaction, preventing or limiting autoimmune damage.
- Memory T Cell: Reside in lymphoid tissue and induces more rapid and powerful immune response with 2nd exposure to Ag
Sakurai 2016
Examiner Comments
2010B 09: 8 (53%) of candidates passed this question.
For a good answer candidates were expected to describe physical barriers, innate and acquired immunity. Physical barriers such as skin, mucous membranes, normal flora, secretions, etc were poorly covered. Aspects of immunity such as cellular and humoral were better covered but often lacked a sufficient depth of knowledge.
Syllabus: M2a References: Guyton and Hall Textbook of Medical Physiology, Chp 33 and 34
10. Classify colloid intravenous fluids and outline the pharmacology of the hydroxyethyl starches.
Gelatins and starches are now out of clinical practice, so we will not bother answering this question
11. Compare and contrast hydrocortisone, methylprednisolone and dexamethasone.
Examiner Comments
2010B 11: 6 (40%) of candidates passed this question.
Hydrocortisone represents the main endogenous glucorticoid. Methylprednisolone and dexamethasone are synthetic glucocorticoids that all have metabolic, anti – inflammatory and immunosuppressive effects. These are commonly encountered drugs in intensive care practice, yet overall this question was poorly answered. For a good answer candidates were expected to mention the differences in their pharmacokinetics, relative glucocorticoid and mineralcorticoid effects and adverse effects. These drugs share some common features (eg all have a steroid nucleus, hepatic metabolism and renal excretion and adverse effects), features that were poorly covered by the candidates, but also expected to be mentioned for a good answer.
Syllabus: N2,2c
References: Stoelting, Pharmacology and Physiology in Anaesthetic Practice, pge 416, Peck Hill and Williams Pharmacology for Anaesthesia and Intensive Care, pg 355-8
12. Describe the renal handling of bicarbonate and the changes in urine pH along the nephron. (80% of marks) How is this affected by hypoventilation? (20% of marks)
CICMWrecks Answer
Total filtered (24×180) = 4320 mmol/day
Completely reabsorbed
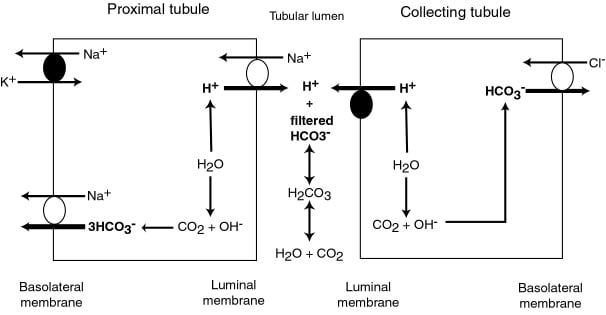
PCT (85%) / TAL (10%)
- Absorbed as CO2 after conversion by C.A. 4 on brush border
- Converted back via CA2
- Reabsorbed to peritubular capilaries via Na/HCO3 cotransporter
DCT/CCD (5%)
- Type A intercalated cells
- Secrete H via H-ATPase or H/K ATPase
- ↑ by aldosterone
- HCO3 is absorbed into peritubular capilary
- Secrete H via H-ATPase or H/K ATPase
- Type B intercalated cels
- Secrete HCO3 via luminal Cl/HCO3 exchanger
- Secrete HCO3 via luminal Cl/HCO3 exchanger
pH changes
- PCT pH = 7.4.
- Reabsorption of Bicarb → pH ~ 6.8 at descending limb of LoH
- End of DCT → pH ~ 4.4
Hypoventilation:
- ↓RR → ↑PaCO ₂ → acidosis
- Chronic Acidosis (traditional)
- → ↑ secretion of titratable acids (phosphate, Ammonia)
- → Binding and secretion of additional H
- → net ↑[HCO3] reabsorption in tubular cells (traditional model)
- Chronic Acidosis (stewart)
- ↑ ammonigenesis → ↑DCT ammonia assoc with ↑Cl secretion via luminal Cl/HCO3 exchanger → ↑SID → defense of acidosis.
Mooney 2016
Examiner Comments
2010B 12: 4 (27%) of candidates passed this question.
This question sought knowledge of an important and basic area of physiology that applies to many circumstances encountered in daily intensive care practice. For a good answer candidates were expected to mention that bicarbonate is freely filtered, its fate along the nephron and that it is not normally found in the urine. Mechanisms by how it is reabsorbed and generated along the different segments of the nephron were expected to be described in some detail. The last part of this question relating to hypoventilation was poorly answered. For a good answer candidates were expected to mention that Hypoventilation results in an increase in arterial PCO2 that readily diffuses into tubular cells resulting in increased intracellular H2CO3 and subsequently bicarbonate, that is reabsorbed, and H+ that is secreted.
Syllabus: D1 Renal Physiology, 2e, 2k
References: Ganong, Review of Medical Physiology, Ch 39 and 40
13. Define the following terms (40% of marks)
a. Saturated Vapour Pressure of Water
b. Absolute Humidity
c. Relative Humidity
d. Latent heat of vaporisation
Briefly outline how the humidity of air is altered during inspiration and expiration by the respiratory tract. (60% of marks)
CICMWrecks Answer
Humidity
Humidity
- Humidity is the concentration of water vapour present in the air.
Saturated Vapour Pressure of Water
- The water vapour pressure when the air is fully saturated.
- Depends on both pressure and Temperature
- = 47mmHg at STP
Absolute Humidity
- The amount of water vapour present in a given volume of gas (units g H2O/m3 or mgs H2O /L)
- Room air at sea level has Absolute humidity of 10g H2O/m3
- < 100% saturation
- Absolute humidity is temperature independent
- 100% saturation
- Absolute humidity is temperature dependent – due to ΔSVP fully saturated air
- at 0 °C contains 4.8 mg/L;
- at 20 °C contains 17 mg/L;
- at 37 °C contains 44 mg/L
- Absolute humidity is temperature dependent – due to ΔSVP fully saturated air
Relative Humidity
- the amount of water vapour present in the gas expressed as a percentage of the amount of water vapour that would be present if the gas were saturated with water vapour.
Latent Heat of Vaporisation
- the heat required to convert 1g of a substance from the liquid phase to the gaseous phase at a given temperature (expressed in Jg-1)
Humidification Process
INSPIRED AIR (During nose breathing)
- Air is warmed by the radiant heat from nasal blood supply.
- ↑ing temperature → ↑ SVP → ↑’s water carrying capacity
- Moisture evaporates from the epithelia → ↑ relative humidity of the inspired air to ~90%
- Mouth breathing reduces the relative humidity of inspired air to 60-70%
- At the lungs, it reaches the isothermic saturation boundary where it achieves BTPS (body temperature and pressure, saturated with water vapour) conditions.
- This usually occurs at the second generation of bronchi.
- Absolute Humidity @ Carina = 44 g H2O/m3
- Relative Humidity @ Carina = 100%
EXPIRED AIR
- Expired gas transfers heat back to the cooler trachea and nasal mucosa.
- As the saturated gas cools, it can hold less water vapour (its saturated water vapour pressure falls)
- Condensation occurs on the mucosal surfaces, where the liquid water is reabsorbed.
- Reabsorption reduces potential airway water losses from 300ml/day to ~150ml/day
- Tracheal temperature and humidity fall with an increase in respiratory rate (ie, the isothermic saturation boundary moves more away from the upper airway)
Complications of non-humidified air:
- Mucosal dehydration
- Altered ciliary function
- Inspissation of secretions
- Atelectasis and V/Q mismatching (if underlying lung disease)
- ↑ heat loss (5-10%) as the inspired gases are warmed and more H2O needs to be added as vapour
Humidification Mechanisms in ICU:
- Passive
- HME (Heat-Moisture Exchanger)
- Active
- Bubble Humidification
- Passover
- Heats water in chamber
- Evaporated water entrained by fresh gas
- Nebulisation
- Pressure and heat vapourises water
Gladwin 2016
Examiner Comments
2010B 13: 3 (20%) of candidates passed this question.
This question was poorly answered by candidates. Basic aspects, which were critical for a good answer such as definitions were often inaccurate. Terms such as ‘amount’ or ‘content’ were commonly used without provision of units, when mass or pressure was required. The importance of temperature was often not mentioned. Most candidates identified the importance of the upper airway in humidification but did not describe details of this process and failed to discuss the events occurring during expiration.
Syllabus: R2c, S2e, B1k,2d
References: Davis, Basic Physics and Measurement in Anaesthesia, pgs 145-6, Nunn’s respiratory physiology pgs 12, 19, 166-7
14. List the muscles involved in Firation and briefly describe their function.
CICMWrecks Answer
Primary groups of Muscles
| INNERVATION | FUNCTION | |
|---|---|---|
| DIAPHRAGM | ||
| Sheet of skeletal muscle Domed Inserts into lower ribs | Phrenic nerves C3, C4, C5 | Flattens on inspiration – Abdominal contents moved inferiorly → Vertical dimension of thorax increased – Ribs lift and move outwards → Circumference of thorax increased Moves up and down 1cm in tidal breathing can move 10cm in forced inspiration and expiration |
| EXTERNAL INTERCOSTAL MUSCLES | ||
| Insert into superior and inferior borders of ribs Slope inferiorly and outwardly* | Intercostal nerves Anterior rami of thoracic spinal nerves (T1 to T11) | Move the ribs superiorly and outward, increasing circumference of thorax |
| INTERNAL INTERCOSTAL MUSCLES | ||
| Insert into superior and inferior borders of ribs Slope superiorly and inwardly* | Intercostal nerves Anterior rami of thoracic spinal nerves (T1 to T11) | Move the ribs inferiorly and inward, increasing circumference of thorax Not required in quiet breathing, as expiration is passive |
*NSFW:
External Intercostal muscles: ‘hands in pockets’
Internal Intercostal muscles: ‘hands on tits’
Accessory muscles
Accesory inspiratory muscles
(not used in quiet breathing within tidal volume)
- Scalene muscles
- Elevate first two ribs
- Sternomastoids
- Raise the sternum
Accessory expiratory muscles
(not used in quiet breathing within tidal volume)
- Abdominal wall muscles
- Rectus abdominus
- Internal obliques
- External obliques
- Transversus abdominus
- Reduce abdominal cavity volume → Increase abdominal pressure → Diaphragm moves up → lungs decrease in volume
Mooney 2016
Examiner Comments
2010B 14: 5 (33%) of candidates passed this question
Most candidates correctly identified the diaphragm, intercostals, abdominals and accessory muscles as important. However, the majority of candidates omitted to mention the muscles of the larynx, pharynx and airway and thus failed to achieve a good score. Good answers included a detailed description of their function including differentiating between external and internal intercostals. Syllabus: B1b,1, 2a References: Nunn’s respiratory physiology P76 – 80 and West, Respiratory Physiology: the Essentials, pgs 93 – 95
15. Describe the physiological consequences of decreasing Functional Residual Capacity (FRC) by one litre in an adult.
CICMWrecks Answer
Functional residual capacity
- Volume of air in lungs at the end of normal tidal expiration
- Occurs at equilibrium point between the recoil of chest/diaphragm and lungs to collapse inwards
- Sum of residual volume and expiratory reserve volume
- Normal FRC 30ml/kg (approx 2.2l in 70kg male)
- Functions:
- Oxygen reservoir
- PaO2 buffer
- Prevents atelectasis
- Minimizes work of breathing
- Lung kept at most compliant segment of compliance curve
- Decreases pulmonary vascular resistance
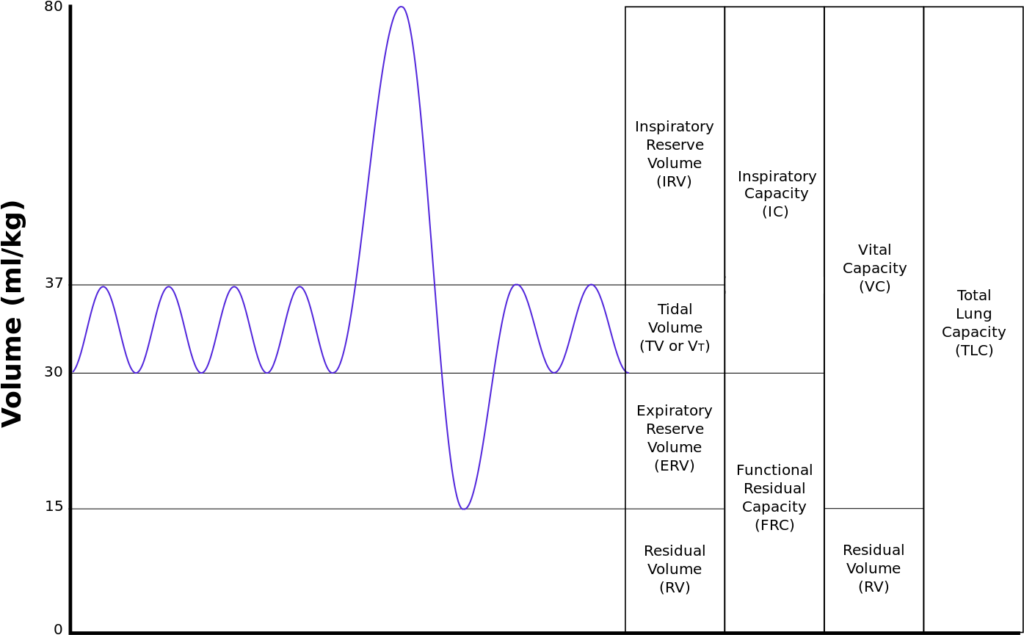
Consequence of 1L loss of FRC
- ↑Work of breathing
- FRC is the volume at the minimum work at rest
- Compliance curve shifted to a flatter portion (greater change in pressure required for change in volume)
- FRC < closing capacity → ↑dynamic air closures/↑air trapping → ↑WOB
- ↑atelectasis (↑with supine position) → ↓ lung compliance → ↑WOB
- ↑airways resistance 2° ↓caliber of airway → ↑WOB
- ↑V/Q mismatch
- 2° to dynamic airways closure, gas trapping, atelectasis
- ↑shunt
- ↓PaO2 due to inability of zone 1 to compensate for the larger zone 3
- ↑Pulmonary Vascular Resistance (PVR)
- PVR minimal at FRC
- ↓FRC → ↓radial traction on extraalveolar vessels → ↓radius of vessels → ↑PVR
- ↑PVR → ↑ RV Afterload
- ↓FRC → ↑West Zone 4 → ↑pressure on extraalveolar vessels by poorly inflated lung → ↓radius → ↓flow
- ↓O Store and Buffer capacity
- Greater variability in saturations thoughout the respiratory cycle
- More rapid desaturation with hypoxia
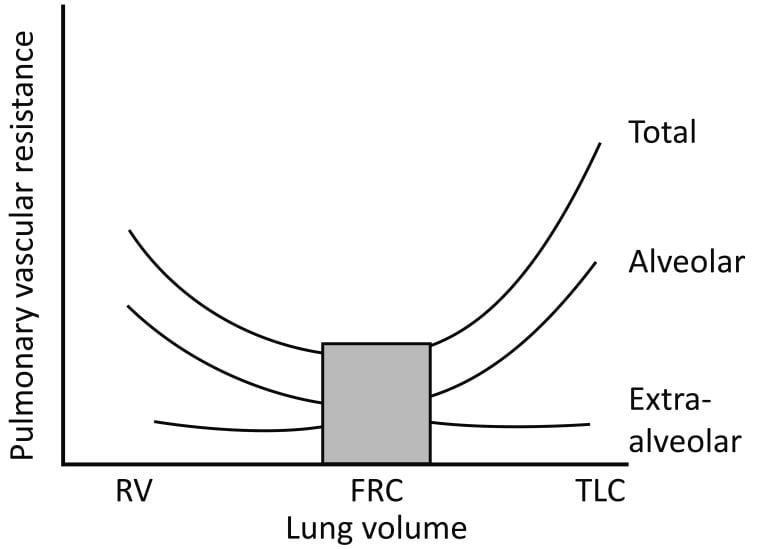
Sakurai / Gladwin / JC 2020
Examiner Comments
2010B 15: 5 (33%) of candidates passed this question.
This is core knowledge and it was expected candidates would describe physiological consequences accurately. Good answers included a definition of FRC and correct value. A number of candidates omitted this. It was also expected that candidates mention that as FRC falls, alveolar closure occurs, lung compliance decreases and airway resistance increases work of breathing increases, pulmonary vascular resistance, and thus right ventricular afterload increases. Many candidates described alveolar closure as causing increased dead space ventilation rather than altered V/Q.
Syllabus: B1e References: Nunn’s respiratory physiology, pages 51-56
16. Discuss the regulation of body potassium by the kidney.
CICMWrecks Answer
Potassium
- Predominany intracellular cation
- Total Stores: Approx 3200mmol (50mmol/kg)
- Key Functions:
- Main determinant of ICF osmolality and tonicity
- Responsible for RMP of excitable cells via Goldmann-Hodgkin-Katz due to ↑↑gK relative to other species
- Role in action potential → repolarisation phase
- Secretion of insulin and multiple other KATP dependant processes
- Regulation of IC processes (protein/glycogen synthesis)
- Involved in Na+/K+ ATPase in cell membranes
Potassium Elimination
- Faecal – 8mmol/day
- Renal – 92mmol/day (See next heading for details)
- Urinary K+ excretion = [K+ filtered by glomerulus] + [K+ secreted by CCD/LDCT] – [K+ reabsorbed by renal tubules]
- Glomerular filtration: Freely filtered = 756mmol/day (180L x 4.2mmol/L)
- PCT: 65% reabsorption
- LoH: 25~30% reabsorption
- DCT/Collecting Ducts – variable
- Determined by Aldosterone, Plasma [K+], Tubular Flow rate
- Secreted by principal cells
- Reabsorbed by intercalating cells
K+ handling within the tubular system:
- PCT (55% of filtered K+ is reabsorbed):
- K+ is reabsorbed passively via the paracellular route due to:
- Solvent drag → coupled to flow of Na+ and H2O
- [ ] gradient → created by reabsorption of H2O
- Electrical gradient → due to +ve luminal charge in late PCT
- K+ is reabsorbed passively via the paracellular route due to:
- TAL of LoH (30% of filtered K+ is reabsorbed):
- K+ is reabsorbed by 2° active transport via paracellular route → +ve charged lumen generated by ion flux created by NKCCT transporter causes K+ to be reabsorbed by diffusion down its electrical gradient
- LDCT and CCD (0 to > 15% K+ is secreted):
- Principal cells of CCD and LDCT → 2° active secretion K+ → via:
- Basolateral Na+ /K+ ATPase → (i) generates an electrochemical gradient that draws Na+ intracellularly from the tubular lumen (via the ENaC channel), and (ii) pumps K+ from peritubular capillaries into tubular cell
- The –vely charged lumen generated by influx of Na+ across ENaC channel → favours tubular secretion of K+
- o Type A intercalated cells of CCD and LDCT → 2° active reabsorption of K+ :
- H+ is produced within tubular cell by hydration of CO2 (using CA) → H+ is then exchanged for tubular K+
- Principal cells of CCD and LDCT → 2° active secretion K+ → via:
- MCD (5% of filtered K+ is reabsorbed)
Renal K+ regulation:
- Renal K+ regulation occurs MAINLY via control of K+ secretion at the distal nephron (CCD and LDCT) via the following factors:
- Circulating factors:
- Plasma [K+ ]
- ↑ [K+ ] causes ↑ K+ secretion by → (i) Directly stimulating Na+ /K+ ATPase in principal cells, and (ii) Directly stimulating Aldosterone release from adrenal cortex
- Aldosterone
- ↑ aldosterone causes ↑ K+ secretion by → ↑ production of Na+ /K+ ATPase, K+ channels and ENaC Na+ channels in the principal cells
- Plasma pH
- Alkalosis via ↓ plasma [H+ ] causes ↑ K+ secretion by → stimulating Na+ /K+ ATPase in principal cells
- Plasma [K+ ]
- Luminal factors:
- Flow of tubular fluid in DCT and CCD
- ↑ tubular fluid flow causes ↑ K+ secretion by → maintaining ↓ luminal [K+ ] (Ie. continuously washing it away) → permits passive diffusion of K+ ↓ its [ ] gradient into tubular lumen
- ↑ Na+ delivery rate to DCT and CCD → ↑ K+ secretion
- -ve lumen potential difference → ↑ K+ secretion
- Flow of tubular fluid in DCT and CCD
- Circulating factors:
JC / Gladwin / Sakurai / Bianca 2020
Examiner Comments
2010B 16: 4 (27%) of candidates passed this question
A number of candidates discussed the distribution of potassium in the body and its role in membrane potentials. This was not asked for. Common omissions were a lack of comment on glomerular filtration, a lack of detail regards mechanisms of potassium transport in various parts of the glomeruli and failing to discuss control mechanisms other than aldosterone.
Syllabus: E1,2b, D1,2f
References: Guyton and Hall Textbook of Medical Physiology, Chp 29
17. Outline the various cardiac reflexes and the mechanisms by which they maintain physiological homeostasis.
CICMWrecks Answer
Cardiac reflex: “a fast-acting reflex loop between the heart and central nervous system that contributes to regulation of cardiac function and maintenance of physiologic homeostasis”
1. Chemo receptor reflex
- Sensor
- Carotid body, central chemoreceptors
- Stimulus
- ↓in PaO2, ↑ in PaCO2
- Response
- Increased SNS stimulation
- Increased HR, SV (via increased contractility), PVR
2. Baroreceptor response (high pressure)
- Sensor
- Arterial baroreceptors
- Aortic arch (CN X)
- Carotid sinus (CN IX)
- Arterial baroreceptors
- Stimulus
- Increased MAP
- Continuous resting tone when MAP >60mmHg
- Increased MAP
- Response
- Increase SNS stimulation
- Increased HR, SV (via increased contractility), PVR
3. Baroreceptor response (low pressure)
- Sensor
- Atrial pressure sensors
- Stimulus
- Atrial filling
- Atrial contraction
- Response
- Peripheral vasodilation
- Increased HR
- Also renal and hormonal effects to decrease circulating volume
4. Bainbridge reflex
- Sensor
- Atrial stretch receptors – right atrial wall, cavoatrial jucntion
- Stimulus
- Increased atrial blood volume
- Response
- Increased HR
5. Cushing reflex
- Sensor
- Medullary sympathetic cell bodies
- Stimulus
- Compression of cell bodies with increased intracranial pressure
- Response
- Increased SNS stimulation -> increased SVR
- Reflex bradycardia
- Via reflex #3
6. Oculocardiac
- Sensor
- Via CN III
- Stimulus
- Ocular compression
- Response
- Vagal stimulation -> decreased HR
7. Bezold-Jarisch reflex
- Sensor
- Chemoreceptors and mechanoreceptors in LV wall
- Stimulus
- Noxious chemical stimuli
- Response
- Increase in parasympathetic outflow
- Bradycardia
- Peripheral vasodilation
Mooney 2016
Examiner Comments
2010B 17: 4 (27%) of candidates passed this question
This question required candidates to provide an answer that integrates their knowledge of various aspects of cardiovascular physiology. Cardiac reflexes are fast-acting reflex loops between the heart and central nervous system that contribute to regulation of cardiac function and maintenance of physiologic homeostasis. This was often overlooked by many candidates. For a good answer it was expected that at least the chemo and baroreceptor, Bainbridge (elicited by stretch receptors located in the right atrial wall and the cavoatrial junction), Cushing (result of cerebral medullary vasomotor centre ischemia), oculocardic (provoked by pressure applied to the globe of the eye or traction on the surrounding structures), Bezold-Jarisch (responds to noxious ventricular stimuli sensed by chemoreceptors and mechanoreceptors within the LV wall) reflexes be mentioned and described.
18. Outline the consequences of mild hypothermia in a patient following major surgery
CICMWrecks Answer
- Temperature
- average kinetic energy of the atoms and molecules that make up a substance
- Mild hypothermia
- Temp <35 degrees
- Interthreshold range
- the range of core temperature over which no autonomic thermoregulatory responses occur.
- Under normal conditions: 36.8 ~37.2 degrees
- Under anaesthesia
- Lower threshold decreases 2.5 degrees and upper threshold increases 1.3 degrees
- Therefore decreased ability to maintain core normothermia
Physiology
Cardiovascular system
- Decreased cardiac output
- Peripheral vasoconstriction
- Increased afterload
- Decreased diastolic relaxation
- Decreased preload
- Decreased contractility
- Increased blood viscosity
- Haemoconcentration
- Cold diuresis: Due to decreased responsiveness to ADH and shunting of peripheral blood to central circulation
- Extravasation of intravascular H2O
- Haemoconcentration
- Increased risk of myocardial ischaemia
- Peripheral vasoconstriction
- Coagulopathy
- PT and aPTT increase 9% at 34c compared to 37c
- Increase incidence of atrial arrythmias → ventricular fibrillation at severe hypothermia
- Every degree decrease in temp increases pH 0.015 and decreases pCO2 4.4%
Respiratory
- Initially tachypnoea due to sympathetic stimulation
- Decreased responsiveness to CO2
- Oxyhaemoglobin dissociation curve shifts to left → decreased tissue O2 delivery
CNS
- CMRO2 decreases 7% per 1 degree decrease in core body temperature
GI
- Decreased GI motility
Other
- Post-operative shivering increases total body O2 consumption
- Decreased enzyme activity
- Decreased temperature decreases rate constant in Arrhenius equation
- Decreasesd neutrophil degranulation
- Impaired macrophage phagocytosis
Pharmacology
Pharmacodynamics
- Arrhenius equation for rate constant K
- Where T = temperature
- Therefore as T decreases, the rate constant decreases
- Drug-Receptor interactions are slowed as temperature decreases
- Where T = temperature
Pharmacokinetics
- Decreased absorption of drugs due to delayed gut transit times
- Metabolism of drugs reduced → slow offset of sedative agents given in anaesthesia
- Decreased cardiac output → decreased renal filtration à decreased drug elimination of unchanged drugs → slow offset
- Specific drugs
- Prolonged muscle relaxation from non-depolarizing
- Opioids blunt shivering thermogenesis mechanism → decreased ability to thermoregulate
Sakurai 2016
Examiner Comments
2010B 18: 1 (7%) of candidates passed this question.
This is another very important, and not an infrequently seen, aspect of almost daily intensive care practice which was poorly understood by candidates. For a good answer candidates were expected to outline pharmacological (eg alteration in drug behaviour), physiological (eg shivering, vasoconstriction, impaired coagulation, etc) consequences. Additional points such as poor wound healing, discomfort also attracted a small number of marks. Candidates would have benefited by illustrating their answers with examples, eg prolonged recovery from anaesthesia and duration of neuromuscular blokade.
Syllabus: L2e
References: Hemmings, Foundation of Anaesthesia: Basic and Clinical Science pg 815
19. Statistics (not in current primary syllabus)
20. Outline the pharmacokinetic consequences of old age. Illustrate your answer with examples.
CICMWrecks Answer
Pharmacokinetic:
- Absorption
- Skin: Faster absorption of transdermal drugs such as Fentanyl
- GIT:
- Less gut motility may delay absorption
- Laxatives and prokinetics increase gastric emptying and reduce absorption of oral agents
- Distribution
- Less total body water and lean body mass
- water soluble drugs (digoxin) have smaller Vd
- higher central compartment concentration
- Increased fat content %
- fat soluble drugs (diazepam) have higher Vd
- Reduced albumin
- the free fraction of protein bound drugs (warfarin) is increased
- Reduced cardiac output
- reduced organ flow
- altered redistribution phase
- important in drug termination (thiopentone)
- Less total body water and lean body mass
- Metabolism
- ↓ Hepatic blood flow – high Extraction drugs (CCB) have ↓ clearance
- ↓ Enzymatic activity
Phase I > Phase II.
- Elimination
- Loss of nephron number with age reduces renal clearance
- Reduced GFR / nephrons / renal blood ow
- renal drug excretion slowed
- un-metabolised drugs (aminoglycosides) or active metabolites have prolonged activity
Pharmacodynamic:
- Increased sensitivity to sedatives, opioids, and hypnotics
- Decreased sensitivity to β-agonists and antagonists
- Decreased MAC
- Polypharmacy increases potential for drug interactions
JC 2019
Examiner Comments
2010B 20: 8 (53%) of candidates passed this question.
As the general population ages, and many elderly are admitted to intensive care units and/or encountered during intensive care ward consultations, this topic is highly relevant. Unfortunately candidate performance generally lacked sufficient depth and breadth in this area. Good answers were expected to mention changes in body compartments (eg total body water, lean body mass decrease, etc), consequences of changes in organ function (eg deteriorating glomerular filtration rate, reduced liver blood flow, etc), alterations in protein levels and binding, increased likelihood of drug interactions and the influence of disease states.
Syllabus: Generic Pharmacology III2d. References: Millers’ Anaesthesia Chp 19
21. Describe the control of gastric emptying
CICMWrecks Answer
Physiology of Gastric Emptying
Gastric emptying
- Fluids have half-time of 30mins in stomach
- Solids have half-time of 2 hours in stomach
Gastric receipt of food bolus:
- Peristaltic wave moves down the oesophagus, propelling food bolus
- Controlled by mesenteric plexus with input from vagus nerve
- Upon swallowing, the lower oesophageal sphincter and stomach relax until the oesophageal peristaltic wave has passed
- Following food bolus passage, the LOS tone increases to prevent reflux
- Food bolus reaching the stomach causes a vagal-mediated relaxation of the stomach, to accommodate gastric distension and food storage
Gastric mixing and emptying:
- Spontaneous mixing waves move down the stomach every 15-20 seconds
- Stronger waves propel gastric contents towards the pylorus (pyloric pump)
- High pyloric sphincter tone
- Allows through well-mixed liquid chyme
- Restricting gastric emptying of solids, causing mixing (retropulsion)
Control
Gastric emptying is controlled by the balance between stimulatory gastric factors and inhibitory duodenal factors
Factors promoting gastric emptying:
- Increased stomach wall stretch
- Stimulates pyloric pump
- Reduces pyloric tone
- Parasympathetic vagal stimulation
- Stimulates pyloric pump
- Gastrin
- Stimulates pyloric pump
- From G cells in antrum
- In response to presence of protein (esp. meat) in stomach
- Motilin
- Stimulates pyloric pump
- During fasting
- Stimulates pyloric pump
- From M cells in duodenum (external antigen stimulus)
- Carbohydrate: rapid emptying
Factors inhibiting gastric emptying:
- Nervous reflexes via enteric nervous system, local sympathetic trunk, and the vagal nerve to the brainstem
- in response to local conditions in the duodenum: Distension, Irritation, Acidity of chyme, Hyper- or hypo- tonicity of chyme, Breakdown products, Especially AA and FA
- Inhibit pyloric pump
- Increase pyloric sphincter tone
- Hormones (inhibit pyloric pump)
- Cholecystokinin (CCK)
- From I cells of duodenum
- In response to presence of fat and proteins
- Secretin
- From S cells of duodenum
- In response to presence of acids
- Gastric inhibitory peptide (GIP)
- From K cells of duodenum
- In response to presence of fats, proteins and carbohydrate
- Cholecystokinin (CCK)
- Other Factors :
- Sympathetic stimulus:
- Decreases contractility and reduces gastric emptying
- Dopamine:
- Decreases intragastric pressure and lower oesophageal sphincter tone
- reduces gastric emptying
- Sympathetic stimulus:
Alternate: This section can also be approached as:
- Local factors
- Gastric Factors: pump, Stomach wall distension, Amino acid
- Duodenal Factors: Type of food, stretch of wall, irritation, hyperosmolaroty, acidity, amino acid and FA content
- Neural factors
- Symptathetic
- Parasympathetic
- Hormonal factors
- Dopamine
- Gastrin
- Motilin
- Secretin
- CCK
- Somatostatin
- GIP
Mooney / Sakurai / JC 2019
Examiner Comments
2010B 21: 8 (53%) of candidates passed this question.
This was another important, relevant and essential aspect of basic physiology, for which candidates tended to lack sufficient breath and depth of the required knowledge. For a good answer candidates were expected to mention liquids empty much faster (and in an exponential fashion) than solids (which have a linear pattern), rate of emptying depends on the pressure gradient generated by the antrum against pyloric resistance. Antral pump activity is of most importance and that it is influenced by signals from both the stomach (eg distension) and the duodenum (volume, osmolarity, pH) and humoral factors (gastrin, cholecystikinin, secretin) and nervous stimulation (general parasympathetic nervous stimulation enhances gastric motility and sympathetic stimulation opposes it).
Syllabus: Q2d
References: Guyton and Hall Textbook of Medical Physiology, Chp 63, Power and Kam, Principles of Physiology for the Anaesthetists, pg 193
22. Compare and contrast the pharmacology of digoxin and amiodarone.
Examiner Comments
2010B 22: 6 (40%) of candidates passed this question.
This question required a structured approach to a comparative description of the pharmacology of two commonly used and encountered drugs in intensive care practice. Candidates who did not gain a sufficient mark, did so because of a poor knowledge of this topic, as well as a critical failure to structure their answer.
Syllabus: C2c,2b
References: Stoelting, Pharmacology and Physiology in Anaesthetic Practice pg 280 and 339,
Peck Hill and Williams, Pharmacology for Anaesthesia and Intensive Care, pgs 224, 232
23. Describe the ionic events associated with a ventricular cardiac action potential (80% of marks). Outline how the action potential relates with the mechanical events of the cardiac cycle (20 % marks).
CICMWrecks Answer
Normal Values:
- AP Duration 200-250ms
- ARP 200ms
- RRP 50ms
RMP -90
Established and maintained by:
- Na/K-Atpase
- Na/Ca antiporter
- Ca ATPase
Threshold potential -65mv
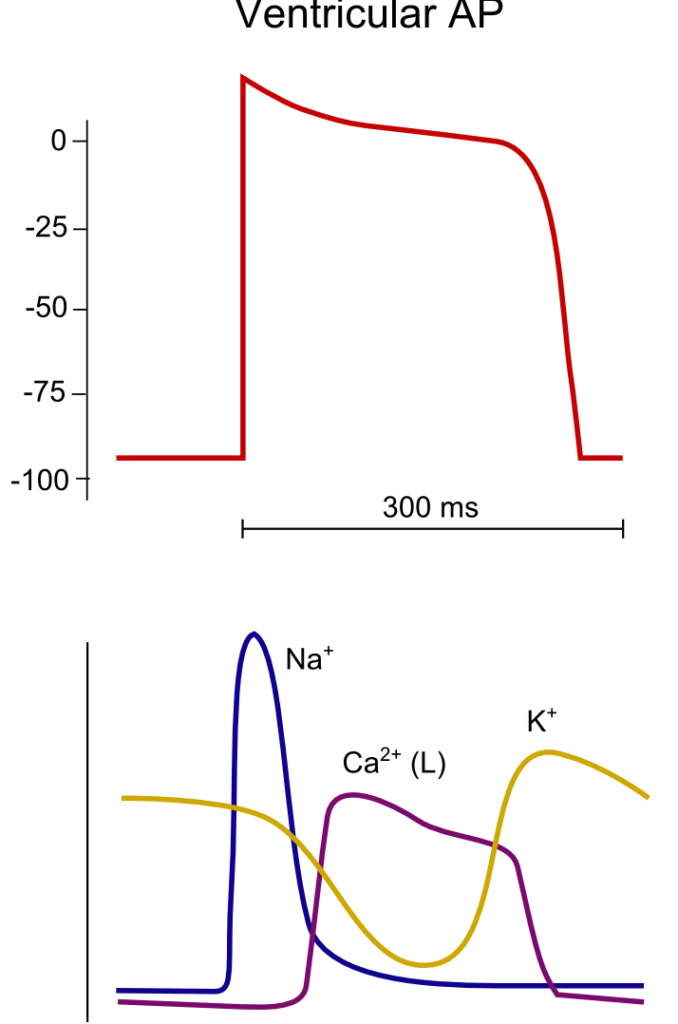
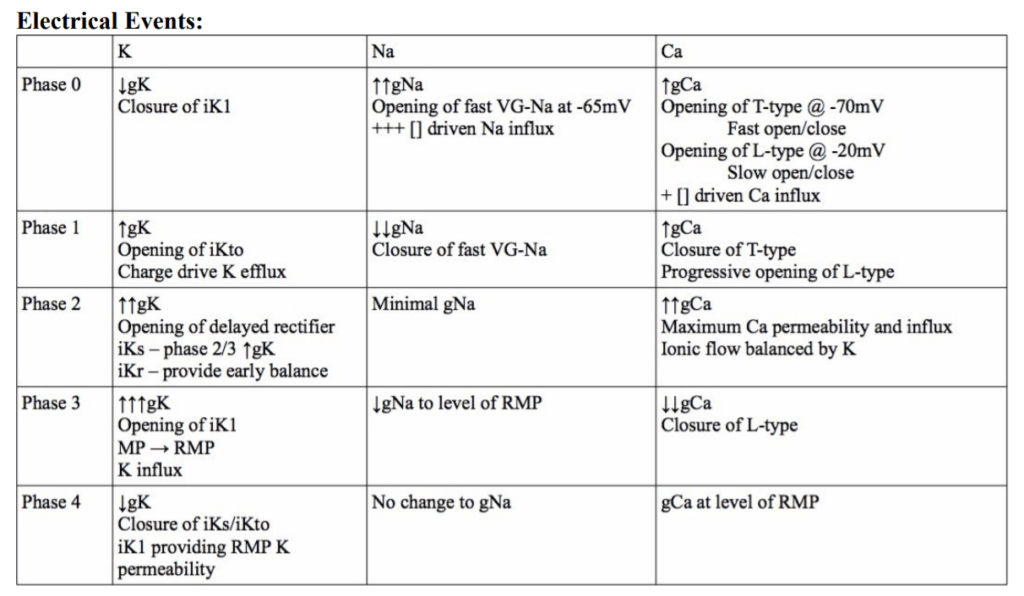
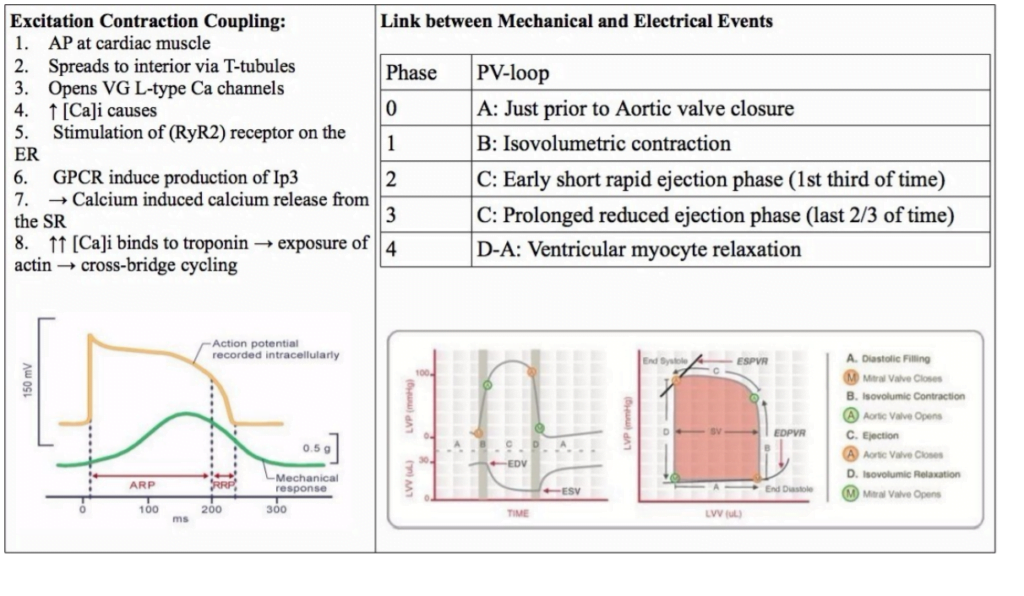
<To be updated>
Gladwin 2016
Examiner Comments
2010B 23: 4 (27%) of candidates passed this question.
To achieve a good pass in this question, candidates needed to outline the ionic events associated with Phase 0 to phase 4 of the ventricular action potential followed by a description of excitation – contraction coupling. The second part of the question was best answered using a ventricular pressure-volume loop and overlaying the phases of the ventricular action potential.
Description of the ionic events associated with the action potential phases was generally well done, but this was as far as many answers went in answering this question. Few candidates included a description of excitation-contraction coupling in there answer and few candidates considered an answer to the second part of the question. The use of illustrations helped answer this question.
Syllabus: C1b
References: Guyton and Hall Textbook of Medical Physiology, Chp 9
24. Classify antihypertensive agents by their mechanism of action, with a brief outline of each mechanism, and an example of a drug in each class.
CICMWrecks Answer
Classification of Antihypertensive Agents
- ADRENERGIC AGENTS
- Alpha antagonists – prazosin is an alpha 1 antagonist which blocks the Gp protein, reducing phospholipase C, DAG, IP3 and intracellular calcium, causing vasodilatation and thus reducing TPR and BP.
- Beta antagonists – metoprolol is a beta antagonist which blocks the GS protein and thus reduces intracellular cAMP, causing a reduction in heart rate and cardiac output
- Mixed alpha/beta antagonists – labetalol, carvedilol
- Alpha-2 agonists – clonidine, methyldopa. Act on the alpha 2 receptor to reduce central sympathetic outflow
- RAAS AGENTS
- ACE’s and ARBs block the effects of angiotensin II (namely, vasoconstriction, sympathetic stimulation, sodium and water retention via afferent/efferent arteriolar constriction and upregulation of sodium resorption along the length of the renal tubule, stimulation of aldosterone release and ADH).
- Examples include lisinopril (ACEI) and irbesartan or candesartan (ARB)
- CA CHANNEL BLOCKERS
Specifically block the L type calcium channels to reduce cardiac contractility and heart rate. In vascular endothelial cells, calcium channel blockade reduces intracellular calcium and causes vasodilatation, reducing TPR and thus BP. Three classes:- Class 1 phenylalkylamines (verapamil) – reduces HR, contractility and causes vasodilatation
- Class 2 dihydropyridines (amlodipine) – main effects are on vasodilatation
- Class 3 benzothiazepines (diltiazem) – some reduction in HR and contractility, also causes vasodilatation
- DIURETICS
There are multiple diuretics but in general they work by reducing renal sodium and hence water retention, thus reducing blood volume. Some (including frusemide) also have an effect on TPR prior to the diuretic effect occurs.- Acetazolamide works in the proximal tubule to inhibit carbonic anhydrase
- Frusemide acts in the TAL of LOH inhibiting the triple symporter
- Thiazides act in the early distal tubule inhibiting the Na/Cl symporter
- Amiloride acts in the cortical collecting duct by blocking ENaC.
- Spironolactone: Aldosterone antagonist, inhibits Na.K.ATPase activity.
- VASODILATORS
- Nitrate drugs – eg, GTN. NO activates soluble guanylate cyclase to causes vasodilatation
- Hydralazine – mechanism not entirely clear, possibly via guanylate cyclase, causes arteriolar vasodilatation
- Minoxidil – direct arteriolar dilator, possibly via K channels
- Nicorandil – K-ATP channel activator which also contains a nitrate moiety
JC 2019
Examiner Comments
2010B 24: 10 (67%) of candidates passed this question.
There are many valid lists that can be used as a template to answer this question. One such list might broadly classify antihypertensive agents into sympatholytic agents, vasodilators, calcium channel antagonists, renin-angiotensin inhibitors and diuretics. Within each of these categories are a variable number of sub classes, for example diuretics might include thiazides, loop diuretics and potassium sparing diuretics.
A good answer would include such a listing with a brief description of the mechanism of action with respect to the antihypertensive effect and the name of a typical drug that acts in the manner described. Most candidates were able to generate such a list and populate it as required by the question, thus being rewarded with good marks. Poorer answers lacked any logical classification system and were merely a random list of antihypertensive drugs and their actions.
Candidates are reminded that organisation within an answer helps in answering the question and achieving marks.
Syllabus: C2b,2e
References: Berne & Levy, Physiology, Ch 2-3
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | Basic pharm, opioid drugs |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Arterial oxygen tension, resp physio and oxygen measurement |
| G. CVS | beta blockers, basic pharm, dose response curves Cardiovascular physiology, CO, SW, V loops,Rt heart pressures and function, BP measurement |
| H. Renal | Renal circulation, blood oxygen tension in kidney, autoregulation, GFR and determinants |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | Basic pharm, opioid drugs Cerebral blood flow, relationship with PaCO2, Benzos |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | Coagulation cascade, Heparin pharm |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures | IJV cannulation, basic statistics |

Recent Comments