1. Describe the cardiovascular changes that occur following the loss of 1000ml of blood in an adult.
CICMWrecks Answer
Distribution of water
- TBW = 60% body weight ~ 42L
- Intracellular water = 66% of TBW ~ 28L
- Extracellular water = 33% of TBW ~ 14L
- Plasma volume = 25% Extracellular water ~3.5L
- Blood volume (plasma + RBC intracellular water) = 7% of total body water ~5L
1000ml blood loss = approx 20% blood loss = Stage II shock
Cardiovascular changes
Immediate
- Decreased venous return due to loss of blood volume
- Frank-Starling Curve à Cardiac output decreased
- Systolic and mean arterial blood pressure decreases (MAP = CO x SVR)
- Baroreflex
- Decreased MAP
- Decreased stretch of aortic arch and carotid sinus baroreceptors
- Decrease firing of baroreceptors
- Decreased signalling to sensory area of medullary vasomotor centre in NTS
- Decreased inhibition of vasomotor area
- Increased sympathetic and decreased parasympathetic output
- Heart
- Increased heart rate and contractility
- Vasculature
- Vasoconstriction
- Blood diverted from peripheries to essential organ function (coronary and cerebral circulation)
- Increased diastolic blood pressure
- Vasoconstriction
- Leads to increased MAP
- Decreased MAP
- Barcroft-Edholm reflex
- If venous return severely impaired
- Mechanoreceptors in R atrium/ventricle sense collapsing of chambers in systole
- Increased vagal signal to NTS
- Bradycardia (and hypotension) in order to allow greater diastolic filling time
- If venous return severely impaired
- As all blood constituents lost → [Hb] and haematocrit unaltered
- At >10% blood volume loss, low pressure (cardiopulmonary) baroreceptors cause secretion of vasopressin from posterior pituitary
- Vasoconstriction via V1 receptor
- Renal water retention via V2 receptor (also assists in coagulation via vWF release)
Acute Changes
- Decreased intravascular hydrostatic pressure à transcellular shift of extracellular fluid into intravascular space and intracellular water into extracellular space
- Decrease in plasma osmolality (usually 275~290mOsm/L)
- Sensed by Organum Vascularis of the Lamina Terminalis (OVLT)
- Increased secretion of vasopressin from posterior pituitary
- Decreased [Hb] due to transcellular shift of water decreases blood viscosity
- Decreased resistance to laminal flow
- Promotes cardiac output
- Decrease in plasma osmolality (usually 275~290mOsm/L)
- Decreased MAP → Decreased renal perfusion pressure → Decreased glomerular filtrate (although some increase in filtration fraction to compensate)
- Decreased [Na] and [Cl] in DCT
- Activation of RAAS
- Angiotensin II
- Potent vasoconstriction
- Stimulates secretion
- ACTH
- Vasopressin
- Aldosterone
- Potentiates secretion of noradrenaline from post-ganglionic sympathetic fibres
- Stimulates thirst
- Aldosterone
- Increases cellular Na/K ATPase
- Inserts ENaC into Collecting Duct tubular cells
- Increased Na reabsorption
- Water reabsorption
- Inserts UT-A1 into Medullary Collecting Duct
- Increased Urea reabsorption
- Increased osmolarity of medullary interstitium
- Increased water reabsorption
- Decreased [Na] and [Cl] in DCT
Chronic
- Decreased DO2 to renal interstitium → activation of hypoxia inducible factors (HIF)
- Synthesis of secretion of erythropoietin (EPO)
- Stimulation of erythropoiesis by haematopoietic stem cell
- Decreased DO2 to tissues → angiogenesis
Sakurai 2016
Examiner Comments
2010A 01: 10 (100%) of candidates passed this question.
A structured approach that included mentioning that 1000mls of blood was substantial – being approximately 20% of the blood volume of a 70 kg person was required for a good answer. Candidates were expected to also include changes in systolic and diastolic blood pressure, pulse pressure, heart rate, cardiac output and the neuronal (eg sympathetic nervous system response on the various circulations) and hormonal responses (eg rennin aldosterone, Anti-Diuretic Hormone,
catecholamines, etc). Candidates were also expected to discuss differences in responses according to rate of blood loss. Flow diagram could have been used to illustrate some of these concepts.
Syllabus: C1e
References: Textbook of Medical Physiology, Guyton pg 278 – 282, Principles of Physiology for the Anaesthetist, Power & Kam pg 154
2. Describe the pharmacology of Phenytoin
Examiner Comments
2010A 02: 9 (90%) of candidates passed this question
A structured approach was expected addressing both the mechanism of action and pharmacokinetics. Candidates were expected to outline relevant mechanisms of action (such as sodium channel blockade) and how they relate to its use as an anticonvulsant agent. Additional credit was given for discussing other potential mechanisms and other uses such as pain management and antiarrhythmic properties.
Phenytoin is illustrative of several key concepts in pharmacology and mention of these was expected. Failure to address these key concepts or provide sufficient detail was a common omission. Candidates were expected to discuss that phenytoin is highly protein bound, changes from first to zero order kinetics with escalating doses and is metabolised by the cytochrome p450 enzyme system. Some discussion of the significance of these points was expected and extra credit was awarded for more detailed explanations, comments on enzyme induction and examples of drug interactions that are well known and clinically relevant. Candidates were expected to
comment on the mode of delivery and compare oral and intravenous dosing. It was expected that the need for a loading dose followed by maintenance dosing would be mentioned and extra credit was given for highlighting the potential hazards of rapid intravenous administration. Additional credit was given for mentioning the importance of a narrow therapeutic index and the need for clinical monitoring. Well organized answers such as those with an ordered list of subheadings were rewarded.
Syllabus: G2f, 2f
References: Goodman and Gilman’s the Pharmacological Basis of Therapeutics,
Chp 19
3. Outline the major clotting factors and steps in the haemostasis pathway (70% marks). Outline the mechanism of action of thrombolytics (30% marks).
CICMWrecks Answer: Haemostasis
Haemostasis Pathway
- collective term for the mechanisms that stop blood loss
- balance of pro-coagulant + anticoagulant systems
- procoagulants system: promotes coagulation → bioamplification system involving activation of clotting cascade
- anticoagulant system → regulates or inhibits coagulation
3 main components involved in haemostasis (Virchows triad)
- platelets
- endothelium
- when damaged → rapidly initiates haemostatic response
- normal function = prevent haemostasis + promote blood flow
- inhibition of platelet adhesion:
- NO + prostacyclin (PGI2)
- production of adenosine diphosphate (degrades ADP)
- anticoagulant effects:
- due to 2 endothelial membrane bound proteins:
- heparin sulphate (activates ATIII → inactivates thrombin + FXa)
- thrombomodulin: directly binds thrombin + activates protein C (inactivates FVa + VIIIa)
- Fibrinolytic effects
- Secretes tissue plasminogen activator (t-PA) → cleaves proenzyme plasminogen → form plasmin → degrades fibrin clots from endothelial cell surface (fibrinolysis)
- inhibition of platelet adhesion:
- coagulation proteins
Steps involved in haemostasis
Initiation of haemostasis.
Damaged vessel → plasma exposed to:
- Von willebrand factor (vWF): binds platelets to sub-endothelial collagen fibres
- Collagen fibres: platelets bind to collagen + become activated
- Tissue factor (TF): activates plasma coagulation proteins through extrinsic pathway → thrombin
Clot formation
3 key steps:
- Vasoconstriction
- ↓ blood flow → ↓ platelet plug washed away + ↓ blood loss
- Platelet aggregation
- Adhesion: vessel damage exposes TF, collagen, vWF → platelet GPIb-V-IX binds to subendothelial collagen via vWF
- Activation: metabolic process; adhesion triggers GPIb/IIIa activation → irreversible binding to matrix ligands; change shape + activation. Activation results in:
- Exocytosis of granules: contain: 5-HT, TXA2, ADP, PAF, vWF, fibrinogen, thrombin, Ca2+, PDGF
- Dense granules: release ADP, adrenaline, 5-HT → reinforce platelet activation
- α granules: release fibrinogen, β thromboglobulin, PAF-4, FV, vWF, PDGF, thrombospondin → mediate + reinforce platelet aggregation + adhesion
- Activation of phospholipase A2 to form TXA2
- Deformation from disc to sphere with long projections
- Promotion of coagulation cascade
- Platelet contraction (with clot contraction)
- Exocytosis of granules: contain: 5-HT, TXA2, ADP, PAF, vWF, fibrinogen, thrombin, Ca2+, PDGF
- Aggregation: activated GPIIb/IIIa mediated aggregation via fibrinogen + vWF
- Haemostatic plug: plug of degranulated platelets, fibrin mesh, leukocytes, entrapped RBCs
- Coagulation
- coagulation cascade = biological amplification system involving plasma proteins → formation of thrombin
- Classical model: intrinsic + extrinsic → common pathway
- reflects in vitro lab tests but not in vivo haemostasis
- Exrinsic pathway: activated by TF → FVII → FIIa → activates FX → start of common pathway
- Intrinsic pathway: activated by contact with -vely charged substances e.g. subendothelial collagen
- Final common pathway: FXa → converts prothrombin (FII) to thrombin (FIIa) → thrombin
- New model: cell based
- Better represents in vivo mechanism of coagulation
- Initiation phase: coagulation triggered by vessel damage → exposes plasma to TF → FV + FVII activated → activate other nearby clotting factors → formation of thrombin
- Amplification: further activation of clotting factors + platelets
- Propagation: occurs on surface of activated platelet → catalyses formation of thrombin+++
- Thrombin:
- acts on fibrinogen mesh within platelet plug → hydrolyses soluble fibrinogen → produce insoluble fibrin strands
- Activation of FXIII: forms covalent crossbridges between fibrin strands in platelet plug → stable clot
- +ve feedback loop: activates FV + FVIII → feed into cascade to produce more thrombin
- activation of protein C by thrombin-thrombomodulin complex: inhibitor of coagulation → deactivates FVa and VIIIa
Fibrinolysis
- Physiological mechanism in which the fibrin within blood clots is slowly dissolved
- Normal part of wound healing + important mechanism to keep small vessels patent
- Key points:
- Plasminogen is a b-globulin (proenzyme synthesised by the liver) → becomes interwoven into the fibrin clot as it is formed → converted to plasmin (serum protease)
- Main physiological activator of plasminogen is t-PA, expressed by endothelial cells – helps to keep the endothelial cell surface free of fibrin deposits
- Fibrin cleaved by plasmin → produces fibrin degradation products (FDPs)
- One of the FDPs is the d-dimer – cleavage product of cross-linked fibrin
- Fibrinolysis pathway can be manipulated:
- Thrombolysis eg streptokinase promotes conversion of plasminogen to plasmin → ↑ fibrinolysis
- Inhibition of fibrinolysis: eg tranexamic acid inhibits activation of plasminogen
Kerr / Bianca 2016
CICMWrecks Answer: Thrombolytic Agents
Mechanism of action of Thrombolytics
“Thrombolytic agent” → drug that enhance body’s fibrinolytic system by acting on
plasminogen activators, which ↑ conversion of plasminogen into plasmin
- Streptokinase
- Binds non-covalently to plasminogen to form a “streptokinaseplasminogen activator complex” → ↑ conversion of plasminogen to plasmin → ↑ fibrinolysis
- It is NOT fibrin-specific → causes systemic fibrinolysis
- Urokinase:
- similar to streptokinase
- Alteplase (rt-PA):
- Glycoprotein that is activated when bound to fibrin (clot) only → then selectively converts fibrin-bound plasminogen to plasmin to cause fibrinolysis
- It is fibrin-specific → causes less systemic fibrinolysis (cf. streptokinase)
Kerr / Bianca 2016
Examiner Comments
2010A 03: 8 (80%) of candidates passed this question
This question was also best answered using a structured response and illustrations.
Discussion and/or diagrams of the process of formation of temporary platelet plug and conversion to a definitive haemostatic plug after injury to the vessel wall, showing the Intrinsic, Extrinsic and Common Pathways with note of essential cofactors (tissue thromboplastin, Ca++) and fibrinolysis and clot resolution, inhibitors and controlers that prevent excessive coagulation. The latter would lead into outlining the mechanism of thrombolytics. It was expected candidates would mention such mechanisms as catalysing the formation of plasmin from plasmingoen, activation of endogenous plasminogen and direct conversion of plasminogen to plasmin.
Syllabus: J2. 1, J2. 2e
Reference: Pharmacology and Physiology in Anesthetic Practice, Stoelting pgs 510 – 511, Basic and Clinical Pharmacology, Katzung pg 380 – 383
4. Describe the underlying principles involved in the measurement of end tidal CO2 (by infrared analysis), including sources of error and interference.
CICMWrecks Answer
Principles
- Beer Lambert law: At a given wavelength, the amount of infrared radiation absorbed by gas is proportional to the concentration of gas present
- CO2 is a heteronucleic molecule, and so absorbs infra-red light
- Infrared light shone across a sample of gas
- Narrow band light emitted from infrared source
- Band frequency chosen which fits the peak absorption frequency of CO2
- 4.23micrometres
- Shone across gas, and absorbed at a detector
- Detector emits signal to analyser -> screen
- CO2 reading outputted is inversely proportional to CO2 present in sample, as per Beer-Lambert Law
- Narrow band light emitted from infrared source
- Gas can be sampled at the patient within the ventilation circuit (in-line), or in a sample of gas diverted away to a separate analysing chamber (sidestream)
Sources of error
- Sampling
- Entrainment of atmospheric gas if leak in sidestream line
- Occlusion of sidestream line causes loss of gas sampling
- Water condensation absorbs IR light -> erroneously high ETCO2
- Modern capnography includes a water trap and heater (to reduce condensation)
- Calibration
- Incorrect calibration of analyser
- Interference
- Other gases (notably N2O) have a similar absorption spectrum
- Presence may falsely elevate measured ETCO2 (esp. if infrared frequency band too broad)
- Presence of other gases causes ‘collision broadening’
- Absorption spectrum of CO2 is broadened
- Pressure
- Partial pressure, rather than percentage composition, is measured
- If pressure ↓ (e.g. by suction drawing gas into sampling chamber), erroneously low measured ETCO2
- Sampling chamber
- If too large, mixing of gas between respiratory cycles -> compression of waveform and erroneously low measured ETCO2
Mooney 2016
Examiner Comments
2010A 04: 1 (10%) of candidates passed this question
Candidates were expected to at least mention and describe the following points – absorption at the infrared spectrum; Beer-Lambert Law and it’s relevance to measurement of ETCO2; sources of error (effect of other gases, atmospheric pressure), sources of interference (gas sampling methods, heating), calibration, features of the sampling chamber that may cause error (glass construction, size), sampling rates, etc.
Answers provided by candidates lacked breadth and depth, indicated that there was generally a poor understanding of this topic. The usual mistake was to discuss the clinical reasons behind the reading not reflecting the PaCO2 rather than sources of error of the end tidal CO2. Candidates should review this topic from the references from which the answer was sought and texts included as recommended reading.
Syllabus: R2d, S2g
5. Describe the production and metabolism of lactate.
CICMWrecks Answer
Lactate is the conjugate base of lactic acid (an organic 3-C acid)
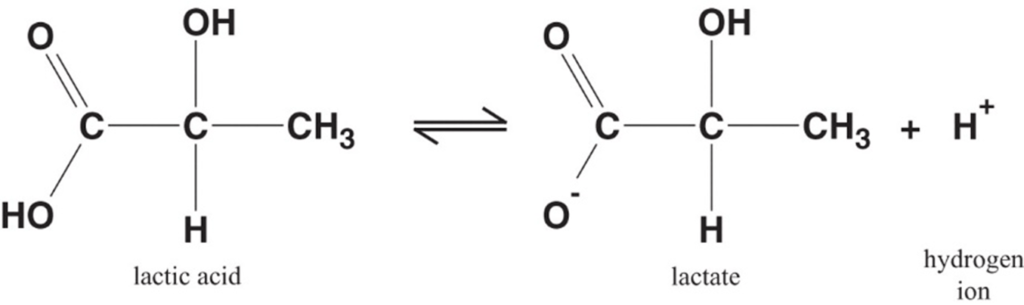
Production:
- It is produced by anaerobic metabolism of pyruvate either:
- Physiologically → in RBC (no mitochondria), renal medulla (↓ PO2), cornea/ lens (↓ PO2) → hence, normal plasma [lactate] is 0.5-2 mmol/L (and NOT zero!)
- Pathologically → reduced tissue perfusion and/or O2 delivery (Eg. shock, hypoxaemia) → thus, plasma [lactate] ↑↑↑ (> 2 mmol/L)
- Plasma [lactate] is 0.5 – 2 mM (and NOT zero) due to physiological production → it can be measured clinically as an indicator of anaerobic metabolism (Ie. ↑ anaerobic metabolism due to pathological situations lead to ↑ [lactate])
Fate of lactate:
- Persistent anaerobic metabolism (Ie. ongoing hypoxia) causes an accumulation of cellular lactate → this diffuses out of the cell into plasma along its [ ] gradient → lactate can then be:
- Used as a fuel source by the heart and brain
- Transported to the liver where it is:
- Converted back to glucose via gluconeogenesis (requires 6x ATP), which is then transported back peripherally for use → “Cori cycle”
- Converted to pyruvate intermediate → utilised locally in TCA cycle for ATP production via oxidative phosphorylation
- Resolution of hypoxia (Ie. tissue O2 tension restored) → intracellular lactate can be oxidised back to pyruvate for use in local tissue aerobic metabolism (Ie. fed into TCA cycle)
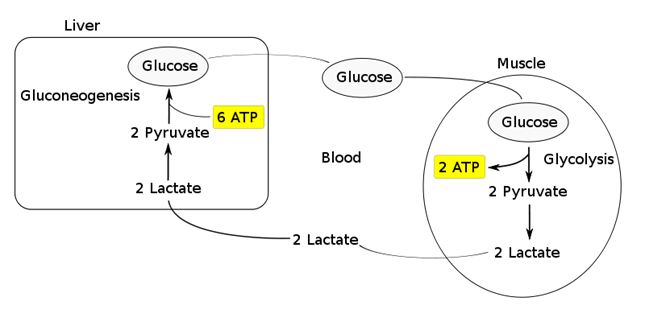
Functions of Lactate:
Lactate Sink:
- Lactate acts as a sink in heart, liver, muscle etc, allowing a period of ongoing ATP production from glycolysis when:
- cells become oxygen deplete
- Kreb’s cycle is inhibited
- Other causes of pyruvate accumulation: circulating catecholamines, exercise, sepsis or lack of mitochondria (RBCs)
Lactate Shuttle:
- Intracellular shuttle:
- Lactate may be shuttle out of:
- mitochoncrial membrane
- peroxisomes
- into cytoplasm of myocytes, neurons, astrocytes
- Ixydised by cytoplasmic LDH to pyruvate, generating NADH for energy use
- Lactate may be shuttle out of:
- Intercellular shuttle:
- Excess lactate, formed within fast-twitch fibres, is transported to other cells within the body with the oxidative capability to metabolize lactate, such as type I (slow-twitch) muscle cells, enhancing their excitability and limiting fatigue
- Furthermore, once in circulation, lactate attaches to red blood cells (RBCs) and is disassociated in the liver where inter-conversion via gluconeogenesis facilitates glucose formation, providing an alternative aerobic energy source.
Lactate as a signaling molecule:
- Redox signaling by intracellular shuttles
- Gene expression
- Increased intracellular levels of lactate can act as a signalling hormone, inducing changes in gene expression that will upregulate genes involved in lactate removal, and stimulates mitochondrial biogenesis.
- Control of lipolysis
- the shuttle regulates FFA mobilization by controlling plasma lactate levels.
- lactate functions to inhibit lipolysis in fat cells through activation of an orphan G-protein couple receptor (GPR81) that acts as a lactate sensor, inhibiting lipolysis in response to lactate
Bianca / JC 2021
Examiner Comments
2010A 05: 5 (50%) of candidates passed this question
Lactate is constantly produced from pyruvate via the enzyme lactate dehydrogenase (LDH). Lactate is produced during normal metabolism and in increased quantities during anaerobic metabolism. Candidates were expected to further describe this physiological processes.
A good answered required quantification of lactate production during normal aerobic metabolism, during anaeorobic metabolism, the pathways involved (Glucose to Pyruvate, Pyruvate to Citric Acid cycle in presence of Oxygen, Pyruvate + NADH to Lactate + NAD+ without Oxygen) associated ATP production, site of intracellular production, why red blood cells differ and lactate metabolism (eg oxidation to pyruvate by well-oxygenated muscle cells which is then directly used to fuel the citric
acid cycle conversion to glucose via the Cori cycle in the liver through the process of gluconeogenesis). Good answers illustrated the loss of energy potential with the production of lactate and discussed the situations that would lead to an inbalance between production and metabolism of lactate.
Candidates who did poorly in this question did so due to a lack of depth and breadth for this topic. For example, even though the Cori Cycle was often mentioned, it was poorly described in relation to lactate metabolism.
Syllabus: K2g
References: Textbook of Medical Physiology, Guyton Chp 67
6. List the physiological factors that increase respiratory rate. Include an explanation of the mechanism by which each achieves this increase.
CICMWrecks Answer
Central control of ventilation
- Brainstem
- Medulla (DRG, VRG)
- Pons (Pneumotaxic, Apneustic)
- External inputs
- Sensors from lungs/airways/chemoreceptors
- Cortex
- Can override brainstem function and alter breathing patterns
- Limbic/Hypothalamic Systems
- Alters breathing patters depending on affective states (fear/anxiety/pain)
Peripheral Receptor Inputs alter respiratory rate/depth (ventilation)
- URT receptors (in nose, NP, larynx, trachea)
- mechanical/chemical stimuli
- bronchoconstriction, sneezing/coughing reflexes, and laryngeal spasms
- Joint/Muscle receptors
- limb movement (Eg. exercise) can stimulate ventilation
- Gamma-system (in muscle spindles)
- muscle spindles sense muscle elongation,
- can cause sensation of dyspnoea
- Arterial baroreceptors (in AB and CB)
- ↓ BP causes ↑ ventilation
- ↑ BP can cause ↓ ventilation (and even apnoea)
- Nociceptors
- pain causes apnoea initially, then stimulates ventilation
- Thermoreceptors
- ↑ temperature stimulates ventilation
- ↓ short deep inspiration
Hypocaponea/Hypoxia sensed by Chemoreceptors
- Central CR response
- ↓’d perfusion → ↑[H]/↓PaO2 → CR stimulation
- Afferent signal → Sinus nerve (of herring) / Vagus Nerve → Chemosensitive area of medulla
- Vasomotor area → ↑ peripheral vasoconstriction
- Respiratroy center → ↑rate and depth of respiration → ↑ venous return
- ↓’d perfusion → ↑[H]/↓PaO2 → CR stimulation
- Peripheral CR response
- PCRs found in “Glomus cells”
- ↓ PaO2 – via inhibition of O2 sensitive K-channels
- ↑ PaCO2 and/or ↓pH – via effect on pH sensitive K-channels
- Type I cells (rich in NAd, DA, ACh)
- Hypoxia causes release of NTs
- NAd/ACh – ↑ AP firing rate of AB or CB afferent fibres
- DA – Damping of type 2 cell responses
- Type II cells (rich in capillary supply)
- ↓ PaO2, ↑ PaCO2 and/or ↓pH
- ↓ IC [ATP] which leads to ↑ NT production and release
- ↑ AP firing rate of AB or CB afferent fibres
- PCRs found in “Glomus cells”
Response to:
- ↓ PaO2
- Gradual ↑ MV PaO2 < 500mmHg,
- Rapin ↑MV PaO2 < 50 mmHg
- ↑ PaCO2
- Central CRs (CCRs)
- Most (80%) of response to ↑ PaCO2 by CCRs
- Peripheral CRs
- Response to ↑ PaCO2 is < 20% of total response
- Much FASTER cf. CCR response
- Role is to match ventilation to sudden ∆ in PaCO2
- ↓ blood pH
- Sensed by PCRs in CB only
- Central CRs (CCRs)
CO₂ most important factor

- Minute ventilation is directly proportional to PaCO2 MV ↑ by 2-3 L/min per mmHg PaCO2
- Hypoxaemia has a synergistic effect on hypercapnoeic-ventilatory drive↓ PaO2 causes↑ MV for a given PaCO2,
- ↑ ∆ MV per ∆ PaCO2
O₂ Less important in normal ranges
- PaO2 plays a SMALL role in ventilatory control
- Effect of PaO2 on ventilation:At normal PaCO2PaO2 < 500 mmHg MV ↑ slowly as ↓ PaO2 decrease
- PaO2 < 50 mmHg MV ↑ drastically
- There is synergistic ↑ in ventilatory response in the presence of hypercapnoea and/or acidosis
- MV ↑ drastically when PaO2 is < 100 mmHg

Gladwin 2016
Examiner Comments
2010A 06: 6 (60%) of candidates passed this question
Good candidates had a structured approach to this questions. Submitted question structures took the form of key headings (eg, PaCO2, PaO2, pH, etc) with an accompanying explanation, which included diagrams, which were often underutilised. Candidate answers that lacked any structure were more likely to have omissions and lacked sufficient depth and as a result scored fewer marks. For a good answers candidates where expected to list and explain (preferably by including diagrams) physiological factors such as PaCO2, PaO2, pH, Exercise, temperature, pregnancy and the associated receptors for each mechanism.
Syllabus: B1c 1
Reference: Nunn’s Applied Respiratory Physiology, Lumb, 6th edition 60-68
Principles of Physiology for the Anaesthetist, Power & Kam, 1st edition 92-98
7. Classify the commonly used inotropic agents and describe their mechanism of action.
CICMWrecks Answer
- Inotrope: Agent that alters the force of contraction of a muscle
- Inotropes can be classified in an number of ways:
- Negative or positive (the examiners did not want a discussion of negative inotropes on previous reports)
- Naturally occurring: Eg Adrenalin, Noradrenalin, Dopamine
vs Synthetic: Dobutamine, dopexamine, isoprenalin, salbutamol
Classification by Mechanism of action is most effective
Postitive Inotropes
Class 1: Increase intracellular Calcium by a variety of mechanisms → ↑force of contraction
- Direct Adrenoceptor agonists (NA, Adren):
- ↑ intracellular cAMP and ↑ Ca by Gs protein coupled mechanism
- Indirect adrenoceptor agonists (Ephedrine):
- displacing NA from vesicles into cytoplasm
- ↑’s carrier-mediated diffusion into synaptic cleft
- ↑NA release at nerve terminal → ↑’d stimulation
- Phosphodiesterase inhibitors (Theophyline, Milrinone):
- inhibit PDE → ↓breakdown of cAMP (cGMP) → effective ↑cAMP
- cAMP effects as per direct adrenoceptor agonists
- Glucagon
- Bypasses adrenergic receptor to stimulate ↑cAMP
- Na/K atpase inhibitors (digoxin):
- Inhibit Na+/K+-ATPase → ↑[Na+]i → Impair Na+/Ca2+ exchange pump → ↑[Ca2+]i
Class 2: Increase sensitivity of actin/myosin complex to calcium by action on troponin C
- Levosimendin
- ↑Ca interaction with troponin C → enhance contractility without ↑[Ca]i
- Activate KATP channels on mitochondrial membrane → protect muscle from ischaemia leading to ischaemic preconditioning
Class 3: Act via metabolic or endocrine pathways (T3, Ca, Mg)
Negative Inotropes
- Beta-blockers
- Usually β1 blocking action
- Inhibits the action of natural stimulation of Class 1 above
- Calcium-channel blockers are used for treating high blood pressure, chest pain, and irregular heart rhythm.
- ↓ intracellular Ca via action on L-type calcium channels
- nifedipine, amlodipine
- Centrally acting sympatholytics are used for treating high blood pressure.
- Act centrally on α2 receptors to decrease sympathetic tone.
- Clonidine
Gladwin 2016
Examiner Comments
2010A 07: 6 (60%) of candidates passed this question
This question required a classification based on chemical structure and class action. Sympathomimetics, phosphodiesterase inhibitors, calcium sensitizers and cardiac glycosides should have been mentioned. Additional detail was expected, subdividing Sympathomimetics into catecholamines (naturally occurring and synthetic), and non-catecholamines (direct and indirect acting). Further classification based on peripheral vasomotor action demonstrated greater understanding.
Better answers included diagrams illustrating the mechanism and point of action on the cardiac myocyte. Discussion of receptors, second messengers, and the role of calcium was essential.
The question was aimed at “commonly used” agents, although some marks were awarded for discussion of calcium, glucagon and other rarely used drugs.
Insufficient detail regarding mechanisms of action was a common observation.
Syllabus: C2d 2
References: Pharmacology and Physiology in Anaesthetic Practice, Stoelting 4th Ed p293-320. Basic and Clinical Pharmacology Katzung 10th Ed p121-198. Pharmacology Rang & Dale 6th Ed p168-187, 290-291
8. Describe the role of the kidney in drug excretion and the factors affecting this (80% marks). Briefly outline how you would alter the dosing of a drug with high renal excretion in a patient with renal impairment (20% marks)
CICMWrecks Answer
Renal Drug Excretion
Due to glomerular filtration and balance of secretion and reabsorption
Glomerular Filtration
- Physiological factors
- Starling forces
- Filtration pressure ∝ (PG – PBC) – ( πG – πBC)
Where- PG = Glomerular hydrostatic pressure (60mmHg)
- PBC = Bowman’s Capsule hydrostatic pressure (18mmHg)
- πG = Glomerular oncotic pressure (32mmHg)
- πBC = Bowman’s Capsule oncotic pressure (Negligible)
- Filtration decreases
- Shock → decreased glomerular pressure
- Obstruction → increased bowman’s capsule hydrostatic pressure
- Hypoproteinaemia → hepatic failure, nephrotic syndrome
- Fick’s Law (see eqn)
- Filtration increases with decreased MW, increased concentration gradient
- Filtration decreases with increased GBM thickness (glomerulosclerosis, deposition) and loss of glomerular surface area (usually 0.8m2. may be lost with renal failure)
Fick’s Law:
- Drug factors
- Size (Due to filtration slits and podocyte foot processes)
- Particles <4nm freely filtered
- Particles >8nm excluded
- Charge
- GBM has negative charge, therefore negatively charged molecules repulsed → decreased filtration
- Protein binding
- Highly protein bound drugs inhibited from filtration due to large size and charge of proteins
- Size (Due to filtration slits and podocyte foot processes)
Secretion
- Due to specific transporters
- P-glycoprotein (Amphipathic anions)
- MPR-2 (Conjugated metabolites)
- ATP-Binding Cassette Transporters (Organic cations)
Reabsorption
- Passive reabsorption occurs in PCT and DCT
- Membrane transporters for facilitated transport are present in DCT
- Ionization
- pH of urine may lead to drug “trapping”
- Acids ionized with pH > pKa
- Bases ionized when pH < pKa
- pH of urine may lead to drug “trapping”
- Lipophilicity
- Allows permeation through phospholipid bilayers of cell membrane → reabsorption
Dose adjustment of renally excreted drugs
- According to clearance and GFR
- Loading dose = Target concentration x Vd
- Usually no alteration of loading dose required
- In ESRF, volume of distribution may be increased → loading dose may need to be increased
- Dosing rate = Target concentration x Cl
- As clearance is reduced in renal failure dosing rate requires reduction
- Maintenance dose = Dosing rate x Dosing interval
- Maintenance dose and/or the dosing interval may need to be reduced (depending on whether AUC or peak concentration of drug is required for drug effect)
- Plasma monitoring of [drug] concentration required for drugs with low therapeutic index
Dose adjustment of Gentamicin
MoA: Inhibition of bacterial protein synthesis through irreversible binding to the 30s bacterial ribosome
Has a narrow therapeutic window, hence dose optimization and therapeutic drug monitoring are crucial.
| Concentration dependant killing | Active concentration needs to be acheived (~8-10 times Minimim Inhibitory Concentration/MIC) Time above MIC does not need to be maintained | Loading dose should not be altered even in renal failure (5-7mg/kg) |
| Significant postantibiotic effect Distribution: Hydrophilic, so Distribute mainly to extra-cellular fluid. Redistribution can occurs upto 16-24 hours. | Once daily dosing sufficient without renal failure | |
| ESRF – Volume of distribution theoretically larger (but in case of Aminoglycosides, might be lower – suspected due to tissue displacement by other molecules like urea) | Loading dose adjustment in ESRF 4mg/kg | |
| Metabolism: Nil Excretion: Rapidly excreted by glomerular filtration. Hence accumulate in renal failure (t1/2 upto 30-60hrs) | Subsequent Dosing interval based on renal clearance (GFR / CrCl) Frequent dosing only increases toxicity with no improvement in efficacy. | Maintenance dosing less frequent in renal failure Dose interval based on pre-existing guidelines, and with monitoring of pre-administration levels CrCl 40 to 59 mL/minute: Administer every 36 hours CrCl 20 to 39 mL/minute: Administer every 48 hours CrCl <20 mL/minute: Monitor serum levels and redose when gentamicin level is less than 1 mg/L |
| End stage renal disease / Dialysis (t1/2 upto 100hrs) | Check level prior to dialysis, and only administer dose after dialysis (based on expert advice) | |
| Low Therapeutic index | Frequent monitoring of levels required | |
Sakurai / JC 2019
Examiner Comments
2010A 08: 0 (0%) of candidates passed this question
The preponderance of marks was allocated to a discussion of renal drug excretion and factors altering this function. Detail was expected including a definition of clearance, and the balance between filtration / secretion / reabsorption in the tubules. Specific mention of GFR, molecular weight of filterable compounds, protein binding, and charge effects determining filtration at the glomerular level was anticipated. Tubular transport mechanisms for secretion and reabsorption in the proximal and distal tubule should have been included in the discussion.
Candidates needed to cover factors which alter GFR, competition for transport proteins, changes in pH on drug elimination, and disease states in answering the question.
An understanding that drug dosing should be based on estimating creatinine clearance and plasma concentration monitoring was essential. Loading dose is usually unaltered. However, maintenance dose and dosing interval need to be adjusted owing to an increased half-life. Many candidates did not emphasise the need to increase dosing interval as well as reduce maintenance dose in renal impairment.
Syllabus: II2d, D12h
References: Goodman and Gilman’s the Pharmacological Basis of Therapeutics p10-14. Foundations of Anaesthesia Basic and Clinical Science, Hemmings p107.
Basic and Clinical Pharmacology, Katzung p35, 48-49.
9. Describe the mechanism of action of drugs commonly used to treat acute severe asthma.
CICMWrecks Answer
Asthma:
- Airway obstruction that is reversible (completely or partially) either spontaneously or with treatment,
- Airway inflammation (oedema and hypersecretion)
- Increased airway responsiveness to a variety of stimuli
General Measures:
- Oxygen.
- Repeated assessment
- ABGs
Specific Pharmacology:
| Class | Example | MoA |
|---|---|---|
| Adrenergic Agonists | Salbutamol Adrenaline | Predominantly acting at the β2-adrenergic receptor Gs-PRC → ↑AC → ↑cAMP → ↑PLC activity – ↓ [Ca] via ↑ uptake and removal from cytoplasm – Active uncoupling of actin-myosin via i) Phosphorylation of MLCK ii) Phosphorylation of MLCK Phosphatase – ↑ K channel activation → hyperpolarisation → SMC relaxation Mast cell stabilisation (adrenaline) Improved mucocillary function. |
| Methyxanthines (PDE3 inhibitors) | Theophylline Aminophylline | Multiple Actions ↓ PDE acitons → ↑ cAMP in bronchial SM → sim to β2 effect Adenosine receptor antagonism → ↓ adensosine related bronchoconstriction |
| Antimuscarinics | Tiotropium, Ipratropium | M3 receptor antagonists Normally ACh stimulates M3 → Gq-PRC → ↑ DAG/IP3 → ↑ bronchiolar tone Antagonism → ↓ Vagally mediated Decreased Gq mediated effects |
| Steroids | Prednisone, Hydrocortisone | Reverse the activating effect of pro-inflammatory transcription factors → decrease inflammation – Inhibit the formation of cytokines secreted in asthma by T-lymphocytes, macrophages, and mast cells – Decreased vascular permeability – Inhibitory effect on mucus glycoprotein secretion – ? enhance B2 effects. |
| Other | Mag Sulphate | Acts as a bronchodilator by decreasing cytosolic Ca2+ concentrations. |
| Heliox | Decreased density – thus increased air flow via Hagan Pouiselle | |
| Ketamine | Bronchial smooth muscle relaxant by inhibiting Ach mediated SM constriction | |
| Sevoflurane | Direct beta agonism and inhibition of histamine release from mast cells. |
Gladwin 2016
Pharmacopeia Table
Examiner Comments
2010A 09: 7 (70%) of candidates passed this question
Answers to this question needed to address drugs that target the pathophysiology of asthma: bronchospasm, inflammation (oedema and hypersecretion), and hyperreactivity to inhaled stimuli. Not all drugs used to treat less severe forms of the disease are relevant in the critical care context.
A discussion of efficacy versus toxicity was included in better answers.
As a minimum, sympathomimetics, antimuscarinics, corticosteroids and methylxanthines should have been included. The role of inhaled Adrenaline as a B2-agonist mediating bronchodilatation and an alpha-agonist constricting the bronchial mucosa was relevant to the discussion. Ketamine and volatile anaesthetics could have been discussed as adjuncts to therapy in ventilated patients. More controversial therapies such as Magnesium and Heliox are less commonly prescribed, however marks were awarded for more comprehensive answers.
Syllabus B2a 2a
Reference: Goodman and Gilman’s the Pharmacological Basis of Therapeutics 11th Ed p717-736
10. Outline the mechanism of action of drugs commonly used to prevent stress ulceration in intensive care.
CICMWrecks Answer
- Options for preventing ulceration:
- 1) Prevent acid secretion
- 2) Eliminate acid already present
- 3) Protect mucosa from acid
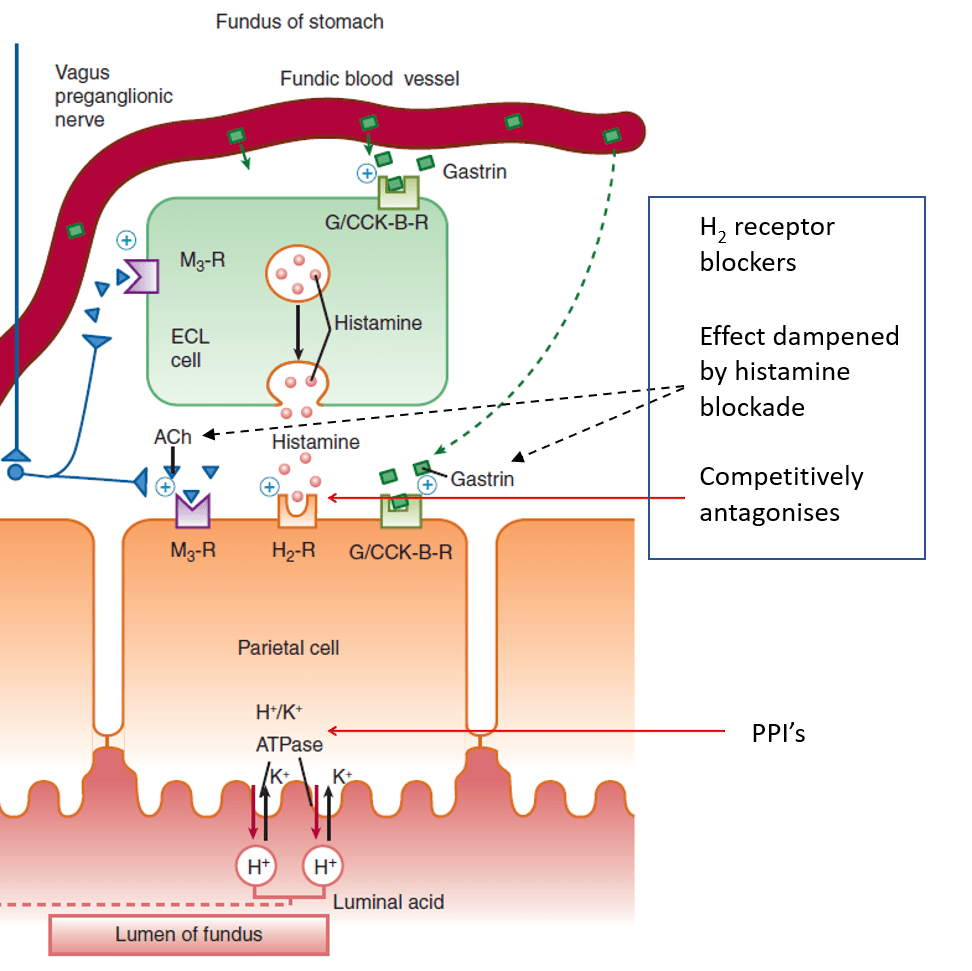
- H2-receptor antagonists:
- Competitively (and selectively) antagonise H2 receptors on parietal cells
- Dampened parietal cell response to ACh and gastrin when histamine blocked
- Proton pump inhibitors:
- Prodrug
- Intestinal absorption into parietal canaliculi
- Converted to active form
- Binds covalently to H+/K+ ATPase
- Sucralfate
- Sucrose sulphate– / aluminium+ salt, which dissociates
- Sucrose sulphate binds to positively charged proteins on damaged mucosa
- Forms a barrier, protecting them from acid
- Stimulates mucosal prostaglandin and bicarbonate secretion
- Antacids
- Weak bases, reacting with HCl to form a salt and water
- Sodium bicarbonate:
- NaHCO3 + HCl -> NaCl + H2O + CO2
- Belching, and NaCl may be an issue in CKD / CCF
- Calcium carbonate:
- CaCO3 + 2HCl -> CaCl2 + H2O + CO2
- Belching, calcium toxicity, metabolic acidosis
- Magnesium and aluminium hydroxide:
- No belching (no CO2+)
- No absorption of the metal
- Magnesium salts cause diarrhoea, aluminium salts cause constipation
Mooney 2016
Examiner Comments
2010A 10: 6 (60%) of candidates passed this question
For a good answer candidates were expected to mention the following key broad points, being there are drugs that act by decreasing acid production in the stomach, drugs that act as mucosal protectors and drugs that reduce intra gastric acidity. Based upon that candidates would be expected to mention and outline the mechanism of action of H2 receptor antagonists, H+K/=ATPase (proton pump) inhibitors, sucralfate’s mechanism of action and antacids. Candidates who structured their answer tended to provide more complete answers and score better. Candidates
who failed did so because of a lack of sufficient knowledge of the mechanism of action of the drugs.
Syllabus: Q2, 2a. b,c
References: Basic and Clinical Pharmacology Katzung 10th Ed pg 1009.
Pharmacology Rang & Dale 6th Ed p 526-7, 255, 497, 587
11. Describe a set of arterial blood gases in a pregnant woman at term and the reasons for these values.
CICMWrecks Answer
| pH | 7.41 |
| PaO2 | 102 mmHg |
| PaCO2 | 30 mmHg |
| HCO3– | 18 mmol/L |
| BE | -2~3 |
pH
- Respiratory Alkalosis → pH increases
- Complete renal compensation → pH normalizes
PO2
- Alveolar PO2 increases according to alveolar gas equation
- As PaCO2 decreases from 40 → 30mmHg
- PAO2 = 150 – 37 = 113mmHg
- However there is increased O2 consumption (15~30% above normal) in the hypermetabolic state → PaO2 ~ 102mmHg
pCO2
- Progesterone in pregnancy causes increased chemoreceptor sensitivity to CO2
- Minute ventilation increases 50%
- 25% increase in tidal volume
- 25% increase in respiratory rate
- Dead-space increases 45%
- However Vd/Vt unchanged
- CO2 production increases due to hypermetabolic state and foetal metabolism, however this is compensated by chemoreceptor sensitization
- therefore PACO2 decreases
- PACO2 approximates PaCO2 = approx 30mmHg → respiratory alkalosis
HCO3–
- Full metabolic compensation for chronic respiratory alkalosis occurs after 3 days according to the formula.
- This is due to decreased HCO3– reabsorption in the renal tubules
- In alkalosis, inhibition of renal secretion of hydrogen ions and ammonium
- glomerular bicarbonate filtration exceeds tubular acid secretion
- Excess bicarbonate is not converted to CO2 and H2O and reabsorbed → eliminated → Loss of base from system
Base Deficit
- reflects the renal loss of HCO3–
Sakurai 2016
Examiner Comments
2010A 11: 2 (20%) of candidates passed this question
For a good answer candidates were expected to describe the respiratory alkalosis and metabolic compensation associated with pregnancy. Candidates were expected to write a set of arterial blood gases showing a compensated respiratory alkalosis with a normal to slightly high P02. Values within +/- 5% of those expected, and found, in listed references would have scored candidates marks.
Candidates were then expected to mention that the PaO2 is high despite a 20% increase in oxygen consumption (relate that to increase in alveolar ventilation, decrease in PaCO2, and alveolar gas equation; PaCO2 is low due to tidal volume increase by 35%, Although anatomical dead space increases VD/VT in unchanged, despite CO2 production increased by increased basal metabolic rate; HCO3 decreases because of increased renal excretion of HCO3 due to inhibition of renal secretion of hydrogen ions and ammonium; base deficit reflects the renal loss of HCO3.
Some candidates described a low maternal P02, none commented on how the maternal P02 and PC02 enhance gas exchange to the foetus. While electrolyte values are often measured on arterial blood gas analysis they do not form part of an arterial blood gas and so comments on electrolytes gained no marks. The double Bohr and double Haldane effects were not required to answer this question. Once again, this question highlighted the importance of a structured approach to the answer, thus enhancing a candidates opportunity to cover all key areas and put their knowledge across.
Syllabus: O1, 2a References: Nunn’s Applied Respiratory Physiology, Lumb, 6th edition, Chp 14
12. Outline the classification of viruses giving examples of each class (60% marks). Describe the mechanism of action of acyclovir and oseltamivir (40% marks).
CICMWrecks Answer
Baltimore Classification system (for Viruses)
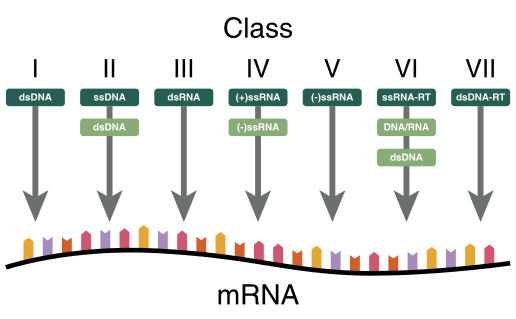
| I | dsDNA viruses | mRNA is transcribed directly from the DNA template | Adenoviruses, Herpesviruses, Poxviruses |
| II | ssDNA viruses | (+ strand or “sense”) DNA DNA is converted to double stranded form before RNA is transcribed | Parvovirus (B19) |
| III | dsRNA viruses | mRNA is transcribed from the RNA genome | Rotavirus |
| IV | (+)ssRNA viruses | (+ strand or sense) RNA Genome functions as mRNA | Picornaviruses (Entero, HepA), Flaviviruses (Dengue) |
| V | (−)ssRNA viruses | (− strand or antisense) RNA mRNA is transcribed from the RNA genome | Orthomyxoviruses (Influenza) |
| VI | ssRNA-RT viruses | (+ strand or sense) RNA with DNA intermediate in life-cycle RT makes DNA from the RNA genome DNA is then incorporated into host genome mRNA is transcribed from the incorporated DNA | Retroviruses (HIV) |
| VII | dsDNA-RT viruses | DNA with RNA intermediate in life-cycle Viral DNA is replicated through an RNA intermediate, the RNA may serve directly as mRNA or as a template to make mRNA | Hep B |
Key:
ss: Single stranded
ds: Double stranded
(-): – strand or antisense
(+): + strand or sense
RT – Reverse Transcriptase
Past Classifications (now not used):
By shape:
- filamentous
- isometric (or icosahedral)
- envelopedH
- Head and tail
By Genome Structure and Core:
- RNA / DNA
- Single-stranded / Double stranded
- Linear/Circular
- Non-segmented/Segmented
By capsid structure:
- Naked icosahedral – Hep A, Polio
- Enveloped icosahedral – EBV, HSV
- Enveloped helical – Influenza, Measles
- Naked Helical – Tobacco Mosaic virus
- Complex – Herper, Smallpox, Hep B
Mechanism of Action: Oseltamivir vs Aciclovir
| Oseltamivir | Aciclovir |
|---|---|
| Neuraminidase inhibitor (Anti-Influenza) | Viral guanosine analogue |
| Prodrug | Prodrug |
| Active metabolite inhibits viral Neuraminidase | Converted to aciclovir monophosphate by viral thymidine kinase which in turn is converted to aciclovir triphosphate by host cell |
| cleaves the sialic acid (which is found on glycoproteins on the surface of human cells that helps new virions to exit the cell) | ACV-TP is HSV-specified DNA polymerase inhibitor |
| prevents viral release by infected cells (viral shedding) | inhibits nucleic acid production, incorporates into DNA resulting in chain termination |
| Active against Influenza | Active against herpesvirus family: HSV-1,2, VZV,EBV,CMV(least) |
Click to Open Pharmacopeia: Antivirals and Antifungals (Level 2)
Mooney / JC 2020
Examiner Comments
2010A 12: 1 (10%) of candidates passed this question
Viruses are classified according to:
a) genetic material
b) mode replication
c) structural proteins (capsids)
d) presence of an envelope
Thus DNA viruses, double or single stranded DNA usually replicate in the nucleus of the host cell via polymerase, not incorporated into the host genetic material. Examples being double stranded, herpes, adenovirus, poxvirus and single stranded, parvovirus. In comparison, RNA viruses, single strand and have 2 different reproduction strategies, being RNA sense(positive) and RNA antisense(negative), an example is paramyxovirus. For retroviruses, the single stranded RNA can’t act as mRNA and is transcribed into DNA by a reverse transcriptase. This DNA is incorporated into the host DNA, so the host makes the viral RNA, for example HIV.
Candidates were also expected to briefly mention capsids and viral envelopes. In relation to the second part of the question, candidates were expected to mention that acyclovir inhibits DNA polymerase in the terminal nucleic acid chain and that oseltamivir is a neuraminidase inhibitor which prevents the budding of new viruses from the infected cells. Most candidates had very little knowledge of this area.
Syllabus: M2 2a&d 8 Reference: Medical Microbiology and Infection at a Glance, Gillespie &, Bamford pgs 58,59, Basic and Clinical Pharmacology, Katzung pgs791,815
13. Describe the physiological basis of the effects seen in the serotonin syndrome (80% marks). List the classes of drugs that may cause the serotonin syndrome (20% marks).
CICMWrecks Answer
Serotonin (5-HT or 5-Hydroxytryptophan)
- an amine neurotransmitter mainly in the CNS and GI tract
- Action on Family of receptors
- Gq-Coupled, Excitatory, ↑ DAG and IP3
- 5-HT2: CNS/PNS, GIT, Plts and Blood vessels
- Gs-Coupled, Excitatory, ↑ cAMP
- 5-HT4: CNS/PNS, GIT
- 5-HT6: CNS
- 5-HT7: CNS,GIT and Blood vessels
- Gi-Coupled, Inhibitory, ↓ cAMP
- 5-HT1: CNS and Blood Vessels
- 5-HT5: CNS
- Ligand gated Na or K, Excitatory
- 5-HT3: CNS/PNS and GIT
- Gq-Coupled, Excitatory, ↑ DAG and IP3
- Main actions
- CNS
- Inhibitory effect on pain pathways
- Inhibitory effect on higher cortical function, stabilising mood and decreasing wakefulness
- CVS:
- 5HT-2 – SMC, vasoconstriction
- Direct small positive chronotropic and inotropic effects
- GIT:
- ↑↑ tone and peristalsis in the GI tract
- CNS
Symptoms of Serotonin Syndrome
- Increased levels of serotonin in the CNS
- CNS Mediated
- hyper-reflexia
- tremor
- clonus
- skeletal muscle contraction and hyperthermia
- Peripheral effects
- hypertension
- GIT effects
- diarrhoea.
Classes of Drugs which can cause Serotonin Syndrome
- Antidepressants
- SSRI, SNRI, MAOi, TCA
- Other
- amphetamines
- pethidine, tramadol
- Methylene Blue
- Sumatriptan (5-HT1 agonist)
Gladwin 2016
Examiner Comments
2010A 13: 7 (70%) of candidates passed this question.
For a good answer candidates were expected to mention the role of serotonin (5
hydroxytryptamine) is an important neurotransmitter, a local hormone in the GIT and
involved in platelet reactions. It is formed from tryptophan and metabolised by MAO
(thus the potential effect of a combination SSRI with MAO inhibitor, or concurrent
use of several serotonin affecting drugs). Typically the serotonin syndrome is a
predictable effect of increased CNS levels of serotonin with a consequence of hyper
reflexia, tremor, clonus, skeletal muscle contraction and hyperthermia (but in
serotonin syndrome these effects are probably CNS mediated), hypertension, and
diarrhoea.
The expected list of drugs included the SSRI’s themselves, combination of SSRI’s
and MAOI’s, antidepressants (2nd generation [e.g Venlafaxine]), Tramadol (blocks
serotonin re-uptake), pethidine, fentanyl, ondansetron, sumatriptan (5-HT1 agonist).
Syllabus: M3
References: Basic and Clinical Pharmacology, Katsung pg 264 – 269
14. Describe the basic principles of ultrasound imaging including the Doppler effect.
CICMWrecks Answer
Ultrasonography Principles
- Definition
- A sound wave with a frequency > 20 kHz → higher than frequency range audible by human ear
- Used medically → typically involves frequency range of 2-15 MHz
- Piezoelectric and converse piezoelectric effect
- Change of polarization of molecules in a quartz crystal in response to
mechanical stress – Interconverts electrical and sound energy - Application of electrical field creates mechanical deformation in a crystal
- Change of polarization of molecules in a quartz crystal in response to
- US Generation: Piezoelectric crystal within probe is stimulated by electrical current to vibrate → produce sound wave
- US Detection: Sound wave reflected by medium causes same crystals to vibrate → produce electrical signal
- Piezoelectric transducers in US
- Electrical current converted into precise sound waves (1~20mHz)
- Sound waves reaches interface of two mediums of differing density (or acoustic impedance).
- Acoustic impedance = tissue density x acoustic velocity
- unique to tissue type (e.g. fat, bone, etc.)
- At interface:
- Reflection
- sound wave reflected directly back to transducer
- Amount of reflection depends of ratio of acoustic impedance (or density)
- Increased density ~ increased reflection
- Refraction
- sound wave is deflected within the medium
- based on Snell’s law
- Reflection
- Attenuation: Loss of energy or strength of an ultrasound wave as it travels through a medium. Occurs through:
- Absorption: The ultrasound wave energy is converted into heat as it interacts with the tissue.
- Reflection: The sound waves bounce off the boundaries between tissues with different acoustic impedances.
- sound wave reflected back to transducer
- Amount of reflection depends of ratio of acoustic impedance
- Increased density ~ increased reflection
- Scattering: The sound waves are deflected in various directions by irregularities in the tissue and do not reach transducer
- Refraction: bending of the sound wave as it travels from one tissue to another
- results in a change in the wave’s direction
- relationship between the angle of incidence (θi), the angle of refraction (θt), and the speeds of sound (c1 and c2) in the two media is described by Snell’s Law: sin(θi) / c1 = sin(θt) / c2
- Central Processor
- Electrical current generated by piezoelectric crystal signaled to CPU
- CPU calculates the distance between transducer and object according to
- Speed of sound (1540m/sec)
- Delay in echo return
- Information relayed to display for visualization
- Gain
- Sensitivity of CPU to signals received from transducer
- Time-Gain Compensation – selective sensitivity of CPU to different interval of sound delay
Resolution and Penetration
- Ultrasound Resolution:
- Defined as the ability to differentiate b/t structures that are closely related
- Resolution is ↑ with either:
- (i) ↑ frequency (or ↓ wavelength) of sound wave → but this ↓ tissue penetration
- (ii) ↑ amplitude of sound wave → but this ↑ artefact
- (iii) ↑ gain → but this ↑ noise
- Types of Resolution: Spatial (Axial, Lateral, Elevational), Temporal
- Spatial Resolution:
- Axial Resolution: The ability to distinguish two structures that are side-by-side and parallel to the ultrasound beam.
- Achieved with a higher frequency and shorter pulse length.
- Mathematically, it’s half the spatial pulse length.
- Lateral Resolution: The ability to distinguish two structures that are side-by-side to the ultrasound beam.
- Achieved with a narrower ultrasound beam, which is related to the width of the beam.
- Higher frequencies generally lead to narrower beams and better lateral resolution.
- Lateral resolution is roughly three times worse than axial resolution at the focal region of the beam.
- Elevational resolution (aka slice thickness resolution)
- refers to the ability to distinguish structures that are close together in the direction perpendicular to the imaging plane. (similar to lateral resolution, but in perpendicular plane)
- crucial for accurately visualizing the depth and thickness of tissues
- Temporal Resolution:
- The ability to distinguish between instantaneous events of rapidly moving structures.
- Achieved with a high frame rate.
- A higher frame rate means the ultrasound machine can capture and display more images per second, allowing for better visualization of movement.
- Axial Resolution: The ability to distinguish two structures that are side-by-side and parallel to the ultrasound beam.
- Ultrasound penetration:
- defined as the depth to which ultrasound waves can travel into tissue
- Primarily determined by the frequency of the ultrasound waves, with lower frequencies generally penetrating deeper than higher frequencies.
- Trade-off between Resolution and Penetration:
- There’s an inherent trade-off between image resolution and penetration depth.
- Higher resolution requires higher frequencies, which means less penetration, and vice versa.
Modes of Ultrasound
- A (amplitude scan): Amplitude of U/S signal plotted against time → provides information about tissue depth (BUT is no longer used)
- B (brightness): Depth recorded as bright spot (rather than a spike as in A-mode) →
amplitude of U/S signal is proportional to brightness - M (motion): B-mode plotted against time (Ie. assess heart valve movement over time)
- 2-D: Sequential B-mode across 90° (most commonly used) → requires an array of crystals
- Doppler: Uses “Doppler shift” to establish velocity of moving object which is reflecting sound waves → superimposed on 2D mode with colours representing direction of movement (red = towards, blue = away)
Doppler effect
- Change in apparent frequency of sound for an observer moving relative to its source
- Use in ultrasound
- Sound waves reflected off objects moving toward or away from transducer
(usually blood)- If object moving toward transducer, frequency appears increased – displayed as red (however this is not standardized across machines)
- If object moving away from transducer, frequency appears decreased –
displayed as blue
- Can be used to measure velocity of flow, as well as direction
- Sound waves reflected off objects moving toward or away from transducer
Sakurai 2016
Examiner Comments
2010A 14: 7 (70%) of candidates passed this question.
It was expected candidates would outline the underlying principles of ultrasound imaging (reflection, scattering, refraction, and attenuation) and discuss that the basic image is the result of reflection of the transmitted ultrasound wave. Most candidates appreciated that the amplitude of the reflected echo is a function of the acoustic mismatch of the tissues and the angle of incidence and many candidates provided details mathematical descriptions concerning these principles.
While high levels of technical details were not required the answer should include a mention of the use a piezoelectric transducer and that an ultrasound beam has 3 dimensions — Axial, Elevation and Lateral. Some comment of the modes of Display (A= Amplitude, M Time Motion, 21), etc) was expected.
Extra credit was given for answers that included details regarding limits of depth of penetration (longer wavelength penetrate deeper, but loose image quality with longer wavelengths) and the varying properties of human tissue regarding refraction and attenuation (little refraction (path deviation) in human tissue and air attenuates).
Specific comment on the Doppler Effect was required. It was expected candidates would described that it refers to the change in frequency of a sound wave reflected by a moving target and that the reflected frequency differs if moving toward or away. Correctly stating that the reflected Frequency is Higher Towards and Lower Away scored additional marks. Comments concerning obtaining the best Doppler images with lower frequencies (opposite to ultrasound) and colour Doppler attracted additional marks.
15. Discuss the important factors in exchange of gases and substrates between capillaries and tissue cells.
CICMWrecks Answer
Based on 3 main physical principles
Diffusion via Fick’s Law
- Rate of movement of solute across semi-permiable membrane J is
where
C = concentration (or partial pressure for gasses)
A = cross-sectional area
T = thickness of the membrane or distance over which diffusion takes place.
- These factors alter the rate of transfer as per the equation above.
- Valid for Most medium – small substances
- Water soluble (H2O, electrolytes, glucose, urea) via intercelular clefts
- Lipid soluble substances (O2, CO2) via endothelial cells themselves.
Starling Forces
The NET flux across the membrane is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation:
where
Jv is the trans endothelial solvent filtration volume per second
( [ Pc – Pi ] – σ [ πp – πi ] ) is the net driving force
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
κ = filtration constant = LpS = Hydraulic conductivity x Surface Area
Typically quoted values for the variables in the classic Starling equation:
| Hydrostatic pressure | Oncotic pressure |
|---|---|
| Pressure moving fluid | pressure exerted by proteins which draw water into and keep it within a compartment |
| Pc ~35 → 15mmHg (Arterial → venous) Capillary hydrostatic pressure Pressure moving fluid out of capillary | πp ~ 20mmHg Plasma oncotic pressure Pressure keeping fluid within capillary |
| Pif = 5mmHg Interstitial hydrostatic pressure Pressure moving fluid into capillary | πif ~ 0mmHg Interstitial fluid oncotic pressure Pressure keeping fluid out of capillary |
Gibbs-Donnan Effect:
“Opposing osmotic and electro-chemical gradients in the presence of a nondiffusable ion resulting in unequal distribution of the diffusable ions”
- Diffusible ions move ↓ [ ] gradient
- Because of non-diffusable ion, significant opposing electrical potential develops.
- This prevents further movement of ions, and an electrochemical equilibrium is reached.
- Because of the presence of non-diffusable ions, there is an osmotic disequilibrium.
Osmotic pressure itself can be determined from the Vant Hoff Equation
where
n = # of particles into which substance dissociates
c = [ ] (in g/L)
T = Absolute temperature
R = Universal Gas Constant (8.314 J⋅K−1⋅mol−1)
M = Molecular weight of molecules
It depends on:
- # of particles
- [ ] or molarity of the solution
- Temperature
- Inversely to the molecular weight
Active Processes
Serve to establish Starlings forces and G-D equlibrium
- Facilitated diffusion
◦ Diffusion through the membrane using a specific carrier protein to help - Active transport
◦ Movement of ions or other substances across the membrane in combination with a carrier protein
against an energy gradient.
◦ May be primary (energy derived directly from ATP)
◦ or secondary (occurs via co-transport or counter transport) - Endocytosis/Exocytosis
◦ Vesicular transport by engulfment/extrusion of particle by cellular contents
Gladwin 2016
Examiner Comments
2010A 15: 4 (40%) of candidates passed this question
Good answers were based around Fick’s Law, Starling forces and the Gibb’s Donnan effect.
It was expected that candidates would give Fick’s equation and describe the components :
Fick’s Law J = -DA dc/dx
Candidates were also expected to describe Starlings equation and the equation for Osmotic Pressure. Starling Equation:
Fluid movement = k[(Pc-Pi) – s(πp – πi)]
Osmotic pressure : sRT(Ci-Co).
Gibb’s Donnan effect and, other mechanisms of transport (filtration and pinocytosis) was also expected for a good answer.
Syllabus: A combination C1c2.d, C21 2.e, C2b2.c, C2b2.e
References: Pharmacology and Physiology in Anesthetic Practice, Stoelting pgs
294-300, 322- 325
16. Define the mechanisms of action and adverse effects of metoprolol and glyceryl trinitrate when used to manage myocardial ischaemia.
CICMWrecks Answer
Myocardial ischaemia
- Occurs when myocardial oxygen demand is not met by oxygen delivery
- Type I
- Due to atherosclerotic disease – plaque rupture, or coronary artery spasm
- Type II
- Due to increased metabolic demand of myocardium (e.g. tachycardia, hypertensive crisis)
- Type I
- Determinants of myocardial oxygen demand
- Basal metabolism of heart
- Heart rate
- Contractility
- External work
- Wall tension
| METOPROLOL | GLYCERYL TRINITRATE | |
|---|---|---|
| β1 selective adrenoceptor antagonist | Nitrate | |
| MoA | β1 adrenoceptor = GsPCR In Myocardium – Inhibition → Decreased activation of adenylyl cyclase → Decreased intracellular [cAMP] → Decreased activation of protein kinase A → Decreased phosphorylation and activation of intracellular enzymes → Decreased intracellular [Ca2+] – Decreased inotropy and decreased chronotropy → Decreased myocardial oxygen consumption → Relief of ischaemia | Nitrate groups activated by thiols to nitric oxide In vascular smooth muscle (veins > arteries) – → Diffuses intracellularly and activated guanylyl cyclase → increased intracellular [cAMP] → Decreased intracellular [Ca2+] → decreased vascular tone → venodilation and vasodilation – → Decreased preload and decreased afterload – → Decreased myocardial O2 consumption → Relief of ischaemia |
| A/E | CVS – Bradycardia – Hypotension – Arrhythmia – Decompensation of acute heart failure → Cardiogenic shock – Rebound myocardial ischaemia on abrupt cessation Resp – Bronchospasm if reactive airways disease CNS – Vivid dreams – Lethargy Other – Dyslipidaemia – Hyperglycaemia | CVS – Hypotension – Reflex tachycardia – If preload dependent cardiac output → decreased cardiac output → shock Resp – Methaemoglobin → decreased O2 delivery – Bronchodilation → V/Q mismatch CNS – Increased cerebral blood flow Other – Tolerance |
Sakurai 2016
Examiner Comments
2010A 16: 8 (80%) of candidates passed this question.
For a good answer candidates were expected to make some mention of the link between myocardial O2 demand/heart rate/contractility/ This was often overlooked, and candidates who did tended to not respond to what the question was asking, that is “when used to manage myocardial ischaemia”
Good answers had a structured response. For a good answer, candidates were expected to mention metoprolol effects of reducing left ventricular wall stress, decreased cAMP/mechanism, decreased heart rate, contractility, resultant decreased O2 demand as well as adverse effects include Bradycardia/ heart block/ hypotension/ bronchoconstriction, etc. In relation to GTN, to mention dilation via nitric oxide, predominantly venodilation, decreased venous return, LVEDP, wall stress, decreased O2 demand and increased supply via coronary vasodilation and adverse effects include hypotension/tachycardia/tolerance/headache. Candidates are reminded that if they are to use non-standard abbreviations, then those abbreviations must be defined somewhere within their answer.
17. With regard to ORAL drug dosing, describe the factors that affect the fraction of drug reaching the systemic circulation (80% marks). How may these factors be altered in a patient with shock (20% marks)?
CICMWrecks Answer
Oral Bioavailability
Absorption
- Drug factors
- Most drugs absorbed from GI via passive diffusion
- Diffusion:
- Low molecular weight substances are readily able to permeate membranes (*see below for equation)
- Charge
- Charged particles display reduced permeability across phospholipid bilayers
- pKa → pH at which 50% of the molecules are ionized
- Henderson-Hasselbach Equation pKa = pH + log (ionized/unionized)
- Acids are ionized when pH > pKa
- Bases are ionized when pH < pKa
- Hydrophilicity
- A degree of hydrophilicity is required for drug to cross aqueous layer immediately adjacent to enterocyte in intestinal lumen
- Lipophilicity
- Lipophilicity required for drug to cross phospholipid bilayer
- Pharmaceutics
- Dissolution of tablet/capsule
- Enteric coating
- Diffusion:
- Most drugs absorbed from GI via passive diffusion
- Patient factors
- Rate of gastric emptying
- Absorption surface area greater in intestines (>200m2) due to microvilli, therefore increased gastric emptying increases rate of absorption
- Blood flow to site of absorption
- Reverse transporters such as P-glycoprotein cause drug efflux from enterocytes, preventing absorption
- Inflammation → increases drug absorption
- Interaction with other co-administered drugs
- Rate of gastric emptying
Fick’s Law:
First Pass Metabolism
- Metabolism by gut bacteria (digoxin)
- Metabolism within enterocytes
- Metabolism in liver via portal circulation before reaching systemic circulation
- Secretion into bile by liver on first pass, before reaching systemic circulation
Shock
Introduction
Clinical state of acute circulatory failure with impaired oxygen utilization +/- delivery by cells leading to cellular hypoxia or dysoxia
- Hypovolaemic (Massive haemorrhage)
- Obstructive (e.g. Massive PE, tamponade)
- Cardiogenic (Acute cardiac failure)
- Distributive (Sepsis)
Effects on Bioavailability
- Drug factors will be unaltered in a patient in shock
- Patient factors
- Gastric stasis may occur leading to decreased rate of absorption
- Intestinal barrier – the integrity of the GI barrier may be decreased by inflammation or ischaemia and cell death, leading to greater absorption
- Splanchnic blood flow may be decreased leading to decreased absorption
- P-glycoprotein may be inhibited due to cell dysfunction or drug interactions (e.g. antibiotics) leading to decreased efflux → increased drug absorption
- 1st pass metabolism
- Concurrent treatment with antibiotics may alter the microbiota in the GI lumen, altering metabolism
- Hepatic dysfunction may occur due to ischaemia or haemolysis
- If hypoxia → zone 3 hepatocytes will be relatively impaired → CYP450 metabolism decreased → increased bioavailability
Sakurai 2016
Examiner Comments
6 (60%) of candidates passed this question
For a good answer candidates were expected to define bioavailability and the factors that affected a drugs oral bioavailability. This was often overlooked by candidates. For example, factors affecting absorption (Metabolism by gut flora, drug / drug interactions with in the gut, lipophilicity and hydrophilicity of the drug (drug that are markedly lipophilic or hydrophilic cross the mucus layer or villous membrane poorly), First Pass clearance and sites and mechanism of possible metabolism, to define hepatic clearance, extraction ratio (providing a formula proved helpful to many candidates) and factors that affected hepatic drug clearance. Candidates often lacked an understanding of this area, or failed to mention it. In relation to the second part of the question, candidates were expected to mention the effects of reduced absorption and altered first pass metabolism resulting in uncertain bioavailability of oral drugs.
Syllabus: Section II, 2a, b References: Pharmacology, Rang, Ritter and Dale, Chp 7. Goodman and Gilman’s the Pharmacological Basis of Therapeutics, Chp 1
18. Describe the physiology of intracranial pressure and the physiological mechanisms that limit a rise in intracranial pressure.
CICMWrecks Answer: ICP
Definition:
- ICP: hydrostatic pressure within the cranial vault
- Normal value is 5-15 mmHg
- focal ischaemia when ICP > 20 mmHg
- global ischaemia when ICP > 50mmHg
Munro-Kellie Doctrine
- The rigid and closed cranial vault forms a fixed brain volume containing
- Brain parenchyma (80%, 1400 g)
- CSF (10%; 75 mL)
- Cerebral blood and vessels (10%; 75 mL)
- Δ’s in volume of any components → Δ’s in others or and increase in ICP
CSF Production / Absorption
CSF Production:
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
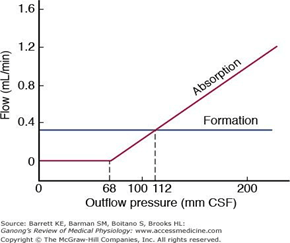
CSF Absorption:
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
Control of ICP
- ICP is regulated via volume buffering
- i.e. increase in volume of one intracranial component leads to compensatory decrease in volume of other intracranial components
- When volume buffering mechanism is exhausted → rapid increase in ICP (decompensation)
- Movement of cerebral venous blood = rapid compensation, lower capacity
- Movement of CSF = gradual compensation, larger capacity
Determinants of ICP:
- Brain
- Age / Mass
- Space occupying lesions
- Cerebral Oedema
- CSF
- CSF production
- CSF Absorption
- Cerebral Blood Volume
- Cerebral autoregulation: Flow-metabolic coupling
- Cerebral metabolic rate
- Increase in systemic blood pressure / flow
- Venous Outflow obstruction
- Vasoactive agents
- Monro-Kellie Doctrine
- Loss of above – e.g. Fractures, surgery
Compensation for Elevated ICP (Intracranial Pressure)
Early compensation
- Δ CSF distribution and flow
- CSF is displaced to spinal subarachnoid space
- ↑’d resorption rate
Late compensation
- ↑ ICP → ↓ CBF → ↓ in cerebral blood volume → cerebral ischaemia
Decompensation
- ↑ICP → ↓ in cerebral tissue volume → brain herniation
- Cushing Reflex
- Hypertension, bradycardia and abnormal breathing associated with raised ICP
- Mechanism:
- Stage 1:
- ↑ ICP → ↓ blood supply to vasomotor area → Local hypoxia/hypercarbia → ↑ SNS >> ↑ PSPS vasomotor stimulation
- ↑ TPR → ↑ MAP
- ↑ HR → ↑ CO
- → compensatory ↑CBF
- Stage 2:
- ↑ CO → Baroreceptor stimulation → ↑ Vagus nerve stimulation → Bradycardia and ↓ contractility.
- Stage 1:
Gladwin / JC 2020
Examiner Comments
2010A 18: 7 (70%) of candidates passed this question
Candidates who did well in this question used graphs to describe the various concepts, described normal physiology and covered the breadth of the topic. A good answer made mention of normal values of ICP, it’s variation with respiration and blood pressure and illustrated a trace of the ICP. An explanation of the Monroe Kelly doctrine was expected, CSF production and absorption and it’s relationship to raised ICP as well other compensatory mechanisms for a high ICP (eg displacement of CSF into spinal canal, displacement of venous blood into the jugular veins, rise in ICP leads to ischaemia if the brain. Critical ischaemia invokes the Cushing reflex. Major omissions by candidates was the use of diagrams, description of normal variation and only a superficial knowledge of compensatory mechanisms.
Syllabus: G1, 2d,g
References: Textbook of Medical Physiology, Guyton, Chp 61
19. Describe the types of dead space in the Firatory system (50% marks). Explain the consequences of increased dead space on gas exchange (50% mark)
CICMWrecks Answer
Dead space
- Ventilated lung volume in which no gas exchange occurs
- Anatomical dead space
- Dead space volume of conducting airways
- Oropharynx, nasopharynx and first 16 divisions of the tracheobronchial tree
- Approx 2ml/kg
- Measured by fowlers method (N2 washout)
- Dead space volume of conducting airways
- Alveolar dead space
- Dead space volume of parts of the respiratory airways that are ventilated but not perfused
- West Zone 1 (PA > Pa)
- Low cardiac output
- Positive pressure ventilation
- Posture
- Disease states
- Pulmonary Embolism
- West Zone 1 (PA > Pa)
- Measured by subtracting anatomical deadspace from physiological dead space
- Dead space volume of parts of the respiratory airways that are ventilated but not perfused
- Physiological dead space
- Alveolar + anatomical dead space
- Calculated from Bohr equation
- Normal arterial to end-tidal CO2 gradient is approx 5mmHg
- Increases if increased dead space
- Apparatus dead space
- Dead space contributed by artificial airway devices (endotracheal tubes, LMA, HME filters)
- Usually reduces anatomical deadspace (due to bypass of oropharynx)
Calculation / Measurement of Dead Space
Bohr’s Method
- Bohr’s Equation:
- Based on the principle that all CO2 exhaled must come from ventilated alveoli
- PĒCO2 is mixed-expired CO2 in an expired tidal breath
- Alveolar PCO2 is difficult to measure, so the Enghoff modification is used
- which assumes PACO2 = PaCO2
Fowler’s Method
Single-breath nitrogen washout test
- Single Vital Capacity Breath – 100% O2 → Exhales to Residual volume
- Expired Nitrogen concentration and volume is measured
- Plot of concentration by volume generated
- Phase I: Pure dead space. No Nitrogen present.
- Phase II: Midpoint is volume of Anatomical dead space.
- Phase III: Expired N2 plateaus
- Phase IV: Closing capacity. Sudden Increase in Nitrogen.
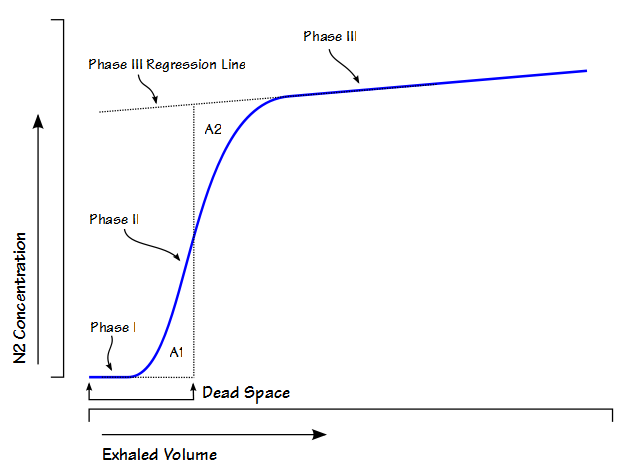
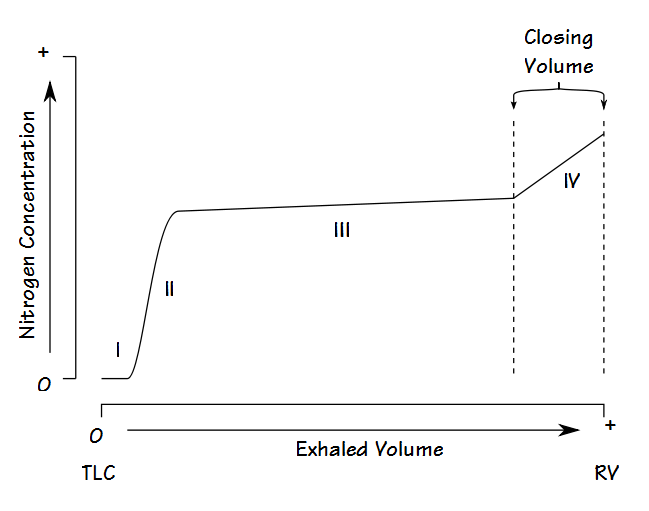
Consequences of increasing dead space
- Alveolar ventilation
- RR x (tidal volume – dead space)
- Therefore, as dead space approaches tidal volume, alveolar ventilation approaches zero, and cannot be compensated by increasing respiratory rate
- PaCO2 is inversely proportional to alveolar ventilation → PaCO2 increases as alveolar ventilation decreases
- Decreased pH
- Increased central and peripheral stimulation of ventilation → Reduction in PaCO2
- Central chemoreceptors activate medullary vasomotor centre → increased sympathetic discharge
- If PaCO2 increases, alveolar CO2 in non-dead space alveoli will increase
- PAO2 will decrease according to alveolar gas equation
Sakurai / JC 2016
Examiner Comments
2010A 19: (50%) of candidates passed this question
A definition of dead space incorporating subtypes (anatomical, apparatus, alveolar, physiological) was expected. Explanation of measurement methods attracted additional marks. Changes to End-tidal CO2 relative to PaCO2 were relevant to the question. The Bohr equation was stated and variables defined in better answers. Causes of an increased dead space should have been described including hypoperfusion and increased alveolar pressure. Increased dead space primarily results in CO2 retention, unless minute ventilation is increased commensurately. The physiological effects of
increased PaCO2, increased respiratory rate and work of breathing are central to the question. Many candidates predicted severe hypoxemia; however the alveolar gas equation was not stated to explain this observation. Hypoxemia is a relatively late effect of significant hypo-ventilation, especially if the patient is breathing supplemental O2.
Syllabus: B1e 2c Reference: Nunn’s Applied Respiratory Physiology p310-311.
Principles of Physiology for the Anaesthetist, Power & Kam p84-87 5
20. Explain the role of urea in the body.
CICMWrecks Answer
Urea
- Amino acids -> ammonia -> urea
- 30g produced per 100g of dietary protein per day
- Normal serum concentration: 3-6mmol/L
- 60% of renally filtered urea is eliminated
Important in maintaining the counter-current multiplier in the renal loop of Henle and collecting duct
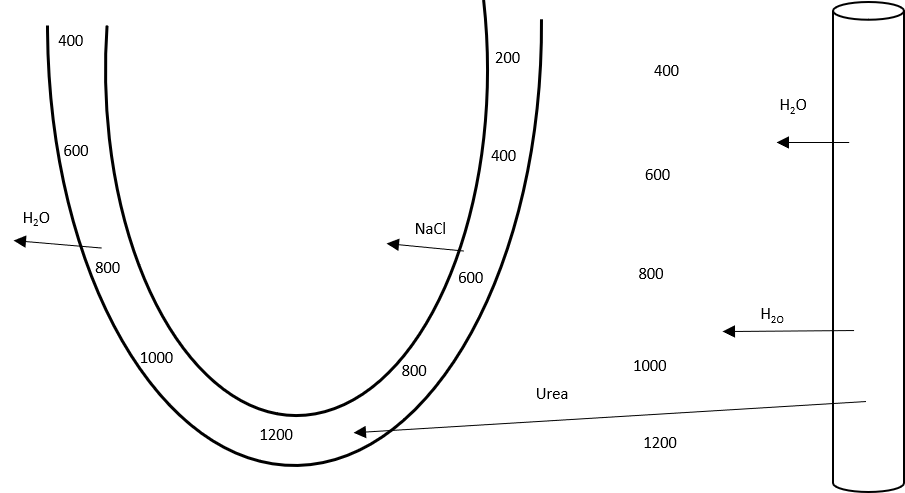
- Ascending limb – active transport of sodium and chloride out of lumen
- Increases osmolality of interstitial fluid
- Descending limb – permeable to water
- Filtrate constantly refreshed
- Leads to concentration gradient, with osmolality increasing toward the medullary interstitial fluid and tubular lumen
- In absence of vasopressin, dilute urine is excreted
- In presence of vasopressin
- Aquaporin 2 channels open in the collecting duct, allowing movement of water out of the lumen and into the interstitial fluid, particularly in medulla where osmolality is high
- Collecting duct becomes permeable to urea
- Urea increases osmolality of interstitum
- Allowing resorption of Na+ and Cl–, while maintaining a high interstitial osmolality
- Urea increases osmolality of interstitum
Mooney 2016
Examiner Comments
2010A: 1 (10%) of candidates passed this question
This question invited candidates to describe the Urea Cycle in the liver. Urea is a waste product derived from deamination of amino acids and the detoxification of NH3. Many candidates did not outline the major steps in the biochemical process.
The kidneys excrete up to 60% of the filtered urea load. The counter-current exchange mechanism in the renal medulla traps urea in the interstitium and generates a concentration gradient, essential for reabsorption of water. The handling of urea by the Loop of Henle and collecting duct, as well as the effect of ADH, should have been discussed.
Many candidates did not include sufficient detail in their answers.
Syllabus: D1 2c,g. I2a.
References: Textbook of Medical Physiology, Guyton p 795, 319.
21. Define preload and describe the determinants of preload.
CICMWrecks Answer
Definitions of Preload:
- The mean tension in the ventricular fibres or the initial myocardial fibre length prior to contraction.
- Mathematically:
- where:
- ITP = intrathoracic pressure; LVEDP = left ventricular end-diastolic pressure; LVEDR = left ventricular end-diastolic radius (at midpoint of ventricle); h = thickness of ventricle
Factors determining Preload
1. LaPlace Factors
- Extra-cardiac: End-diastolic filling pressure
- CVP
- Venous compliance
- Thoracic Blood Volume
- Total Blood Volume
- ↑ TBV → ↑MSFP → ↑ CVP → ↑ preload.
- Blood Volume distribution
- Total Blood Volume
- Venous return (VR)
- ↑VR → ↑ CVP → ↑ preload
- Afterload factors
- ↑Total peripheral resistance → ↓ VR → ↓ Preload
- Compression
- Pericardial effusion/tamponade → ↓ ventricular filling
- PEEP → ↑ ITP → ↓ VR
- Aortic pressures
- ↑ afterload (or raised aortic pressure) → ↓ SV → ↑ ESV → ↑ EDV (or ventricular preload).
- CVP
- Intra-cardiac:
- End-diastolic radius
- Ventricular and Pericardial compliance
- ↑ compliance → ↑ filling for constant pressure → ↑preload
- Atrial contraction/coordination
- ↑ atrial contraction/contractility → ↑ preload
- eg ↑ SNS stimulation / fluid bolus
- ↓ atrial contractile force/coordination in AF
- ↑ atrial contraction/contractility → ↑ preload
- HR: ↓ HR → ↑ filling time → ↑ preload
- Myocardial Wall thickness
- ↑Normal Growth → ↓ compliance and ↑ thickness (h) → ↓ Preload
- ↑Compensatory hypertrophy
- End-diastolic radius
2. Venous Return Factors (↑/↓ VR → ↑/↓ Preload)
- Venous Factors
- ↓Venomotor tone → ↓ VR
- Venous Values prevent retrograde flow → failure → ↓VR
- Pump Factors
- Skeletal Muscle pump/Respiratory pump both ↑ VR
- Inspiration
- → ↓ ITP → ↓ RAP → ↑VR
- → Diaphragmatic contraction → ↑IAP → ↑VR
- Inspiration
- Effect of ventricular contraction and relaxation
- → Systolic Rapid ejection phase → ↓RAP → ↑VR
- → Early diastole → Rapid vetricular filling (open AV valve) → ↑VR
- Skeletal Muscle pump/Respiratory pump both ↑ VR
- Posture
- Intrapericardial pressure (tamponade)
- Afterload
- ↑ Arteriolar tone → ↑ Resistance to VR → ↓ VR
3. Pathological Factors
- ↑ preload
- Ventricular systolic failure
- Outflow valve stenosis or regurgitation (AS or AR),
- Inflow valve regurgitation (MR/TR)
- ↓ preload
- Ventricular diastolic failure,
- Inflow valve stenosis (MS/TS)
Gladwin 2015
Examiner Comments
2010A 21: 1 (10%) of candidates passed this question.
A definition based on stretch of the isolated myocyte prior to contraction, and extrapolation to the human heart, was expected. Surrogate measures of preload used in clinical practice needed to be explained and related to the definition (for example, end-diastolic volume and central venous pressure). The Frank-Starling Law was relevant to discussion of the significance of preload to cardiac performance.
A diagram illustrating the interaction of important factors would have been helpful in answering this question. At a minimum, detail should have included atrial contractility, diastolic filling time, ventricular compliance, and the determinants of venous return.
Better answers included discussion of the effects of afterload, arrhythmias, and valvular pathology. A distinction between the factors determining left and right ventricular preload would have demonstrated a more sophisticated understanding of the physiology.
Syllabus: C1c, 2b,c.
Reference: Cardiovascular Physiology, Berne and Levy, p64-65.
22. Describe the physiology of vomiting.
CICMWrecks Answer
Physiology of Vomiting
Vomiting
- Involuntary, forceful and rapid expulsion of gastric contents through the mouth
Triggers
- Excessive GI tract distension
- Stimulation of CTZ
- Directly by certain drugs eg apomorphine, morphin
- Rhythmic motion of the body stimulating the vestibular labyrinth of the inner ear
- Cerebral excitation secondary to odours, pain, stress
Central control of vomiting
- Vomiting center (5HT3, NK1, Muscarinic and Histamine), near NTS
- Inputs
- Chemoreceptor trigger zone (NK1, 5HT3, Dopamine), area postrema outside BBB
- Vestibular system (Histamine and Muscarinic) via CNVIII
- GI Tract (5HT3, stretch and chemoreceptors) via vagal
- Higher centres
- Efferent arc
- via CN V,VII,IX,X,XII and spinal nerves to abdominal wall musles and diaphragm
- Inputs
| Afferent signal | Sensor | Efferent signal | Effect |
|---|---|---|---|
| Chemo/ baroreceptor input Drugs Stim from NTS Stim from GIT (5HT3) | CTZ (area postrema) | 5HT3, D2 and Opioid neurons | Direct stimulation of vomit centre in lateral reticular formation |
| Surgery/ Rhythmic motions | Labyrinths | ACh and H1 neurons | |
| Memory/ Emotions Sensory (sight, smell, taste) | Cortex | ||
| Severe painful stimuli | Pain | H1 neurones | |
| Irritation Manipulation during surgery Distension | GIT | NK1, NAdr, ACh neurons | |
| Via CNX, to NTS | Stimulation of CTZ then Vomit centre | ||
| Via 5HT3 to CTZ | CTZ to vomit centre | ||
| Manipulation of pharynx via CN9 to.. | NTS | Direct to CTZ | To vomit centre |
Vomiting act
- Antiperistalsis as the prelude to vomiting
- At the onset of vomiting, strong intrinsic contractions occur in both the duodenum and the stomach
- Partial relaxation of the lower oesophageal sphincter (LOS)
- Deep inspiration
- Raising of the hyoid bone and larynx to open the upper oesophageal sphincter
- Glottic closure
- Lifting of the soft palate to close the posterior nares
- Strong down ward contraction of diaphragm and simultaneous contraction of all the abdominal wall muscles
- Complete relaxation of the LOS
Pharmacology of Ondansetron
Click to Open CICMWrecks Table: Ondansetron
Gladwin / Sakurai 2016
Examiner Comments
2010A 22: 8 (80%) of candidates passed this question
For a good answer, candidates were expected to take the following approach and
content of information.
Triggers or initiators of vomiting include:
- Excessive distension or irritation of the upper GI tract, in particular the duodenum
- Stimulation of the chemoreceptor trigger zone (CTZ)
- Directly by certain drugs eg apomorphine, morphin
- Rhythmic motion of the body stimulating the vestibular labyrinth of the inner ear
- Cerebral excitation of vomiting by stimuli such as disquieting scenes, odors
Neuronal pathways:
Stimuli of the GI tract conveyed by vagal and sympathetic afferents to the bilateral vomiting centre within the medulla. Efferent arc from the vomiting centre via the 5th,7th,9th,10th and 12th cranial nerves, and spinal nerves to the abdominal wall muscles (and the diaphragm).
Vomiting act:
- Antiperistalsis as the prelude to vomiting
- At the onset of vomiting, strong intrinsic contractions occur in both the duodenum and the stomach
- Partial relaxation of the lower oesophageal sphincter (LOS)
- Deep breath
- Raising of the hyoid/larynx to open the upper oesophageal sphincter14
- Glottic closure
- Lifting of the soft palate to close the posterior nares
- Strong down ward contraction of diaphragm and simultaneous contraction of all the abdominal wall muscles
- Complete relaxation of the LOS
Most candidates answered this question well. Antiemetic drugs and their mechanism of action gained no marks.
Syllabus: Q1.2e
Reference: Textbook of Medical Physiology, Guyton Pg 768
23. Describe and or illustrate the anatomy relevant to the insertion of an arterial line into the femoral artery
CICMWrecks Answer
Femoral triangle (aka Scarpa’s triangle)
- The major boundaries can be recalled with the mnemonic SAIL:
- lateral border: medial border of sartorius
- medial border: medial border of adductor longus
- superior border: inguinal ligament
- floor: iliopsoas (laterally) and pectineus (medially)
- roof: skin, subcutaneous tissue, a continuation of Scarpa’s fascia, great saphenous vein (joins the femoral vein), superficial lymph nodes, fascia lata
- Contents (From lateral to medial):
- femoral nerve
- femoral sheath (thickening of the deep fascia of the thigh) which has three compartments (from lateral to medial):
- femoral artery and its branches (within the lateral compartment of the femoral sheath) and the femoral branch of the genitofemoral nerve
- femoral vein (within the intermediate compartment of the femoral sheath) and deep lymph nodes
- femoral canal (the medial compartment of the femoral sheath) which contains fat and lymph nodes (of Cloquet)
Femoral sheath
- Situated within the triangle.
- Formed from the fusion and invagination of the psoas and transversalis fascia.
- Contains femoral artery, vein and tributaries, and femoral canal
Femoral artery
Main artery of the lower limb, originates as a continuation of the external iliac artery at the level of the inguinal ligament.
Course:
- Enters the femoral triangle deep to the midpoint of the inguinal ligament (clinically the femoral pulse is usually found midway between the ASIS and pubic tubercle), lateral to the femoral vein
- Passes through the triangle and exits at its apex, entering the adductor canal (Hunter’s canal)
- Exits the adductor canal by passing through the adductor hiatus in adductor magnus (at the level of the junction between the middle and lower third of the thigh) to become the popliteal artery.
- Several branches – largest is profunda femoris, the chief artery to the thigh, which arises from the lateral aspect of femoral artery, 2-5cm below the inguinal ligament. Other branches include the superficial epigastric, superficial iliac circumflex and superficial and deep external pudendal arteries.
Relationships:
- Medial – femoral vein (upper part of the triangle)
- Lateral – femoral nerve, lumboinguinal nerve
- Superior – inguinal ligament
- Posterior – posterior part of femoral sheath, pectineal fascia, psoas major tendon, pectineus and adductor longus
- Anterior – skin, superficial fascia lata, lymph nodes, anterior part of femoral sheath
Sources: Gray’s Anatomy, Radiopedia
JC 2019
Examiner Comments
2010A 23: 3 (30%) of candidates passed this question
The femoral artery lies in the femoral triangle. Candidates were expected to describe the content of the triangle. The upper medial and lateral borders of the triangle, the anterior and the posterior relationships of the femoral artery should also be described. The femoral artery is an extension of the external iliac artery and has an important branch, the profunda femoris artery which comes off on the lateral side.
Diagrams gained marks as did mention of common anatomical landmarks to locate the artery. Candidates who failed did not have enough knowledge. Description of how to place an arterial line gained no marks.
Syllabus: C1d2g
References: Grays Anatomy pages 379,526-27,587,497
24. Describe the mechanism of action of the analgesic effect of opiates (70% marks). Explain the mechanisms by which morphine causes respiratory depression and constipation (30% marks).
CICMWrecks Answer
Mechanism of Action and Effects
- Opiates act on MOP, DOP, KOP and NOP receptors
- MOP are distributed widely:
- Cortex
- Basal ganglia
- Spinal cord – dorsal horn
- Periaqueductal grey – descending inhibition
- DOP, KOP and NOP less affected by commonly used opiates
- Produce analgesia, with different levels of other opiate effects
- Opioid receptors are GiPCR
- Inhibit Ca2+ channels
- Prevent neurotransmitter release at pre-synaptic nerve terminals
- Open K+ channels
- Hyperpolarise post-synaptic nerves, preventing signal transmission
- Inhibit Ca2+ channels
Analgesia
- Analgesia induced by inhibition of ascending pain transmission
- Sites of action
- Primary afferents
- Dorsal horn interneurons
- Descending modulatory neurons
- Inhibition of GABA-releasing inhibitory neurons
- Supraspinally
- Analgesia enhanced by centrally mediated euphoria and sedation
Respiratory depression
- MOP-mediated
- Action on medullary chemoreceptors
- Blunted increase in respiratory drive with increasing CO2
- Effect is antagonised by painful stimulus
Constipation
- Mediated by MOP, KOP and DOP receptors
- Expressed in high density throughout GIT nerve plexus
- Increased GIT tone, with subsequent loss of rhythmic peristaltic movements
Mooney 2016
Examiner Comments
2010A 24: 3 (30%) of candidates passed this question
This was a multi-part question, for which many candidates failed to apportion their time as indicated by the question. Most patients who enter Intensive Care receive opioid analgesia so candidates were expected to have detailed knowledge about the mechanics of action of opiates. For a good answer candidates were expected to mention that opiate agonists produce analgesia by binding to Mu receptors (which are G protein coupled) in the central and peripheral nervous system and spinal cord and their cellular mechanism of action eg presynaptic neurone – close voltage gated Ca channels and prevent neurotransmitter release and post synaptic neurone – hyperpolarise and inhibit post synaptic neurone. The fact that opiates also affect emotional side of pain and cause euphoria which may help with pain perception was often omitted.
Mechanism of respiratory depression is mediated via mu receptor. It occurs at normal analgesic doses, and decreases the chemosensitivity of the respiratory centre to PaCO2
Mechanism of constipation results from increased tone and decreased motility of the GIT via action on visceral smooth muscle mediated by intramural nerve plexus and all three opioid receptors.
Many candidates had poor organisation and poor knowledge of all aspects of this question.
Syllabus: G2d 2b
Reference: Pharmacology, Rang and Dale pgs 600-601, Basic and Clinical Pharmacology, Katzung pg 492
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | Dose response curve and basic pharm including ED50, Com & non Comp antagonists |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | ABG, H-H equation, Hypoxaemia, shunt, pulse oximetry |
| G. CVS | ABP trace, variation with resp, PPV, myocardial contractility, trnsducer |
| H. Renal | |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | Conc-time cure for IV bolus fentanyl, Vd, t1/2, clearance spinal cord anatomy, LA, pain and meds, ketamine |
| L. Musculoskeletal | Sacromere, length-tension relationship, muscle relaxants, malignant hyperthermia, dantrolene |
| M. ANS | |
| N. Liver | Liver functions, blood supply, drug metabolism, cirrhosis on blood flow, Frusemide |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | NS, mannitol, properties of fluids, pituitary, ADH |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |



Recent Comments