1. Explain the mechanisms by which a bacterium may become resistant to an antimicrobial agent and provide an example (organism and antimicrobial) of each mechanism. (60% of marks) How is antimicrobial resistance spread? (40% of marks)
CICMWrecks Answer: Antibiotic resistance
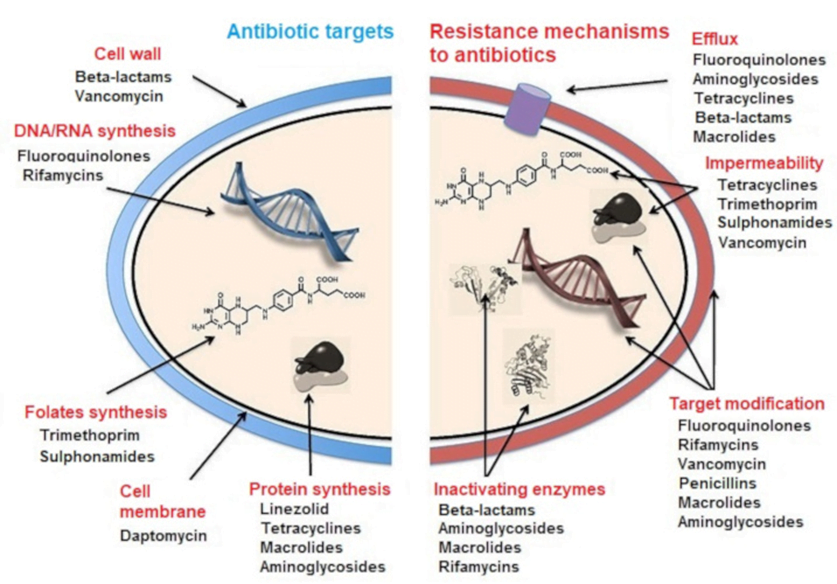
MECHANISMS OF ANTIBIOTIC RESISTANCE
- Mechanisms of Antibiotic Resistance can be classified broadly:
- Efflux Pumps
- Blocked Penetration / Alteration in access to target site
- Target Modification
- Modification of Drug or pathways
- There might be multiple resistance mechanisms at play in the same organism
| Efflux Pumps: Increased efficiency or expression of efflux pumps (inner membrane proteins). | |
| Removed from cell | Active transport of ciprofloxacin out of the bacterial cell |
| E.Coli to tetracycline. | |
| Trapped between cell wall layers | glycopeptide resistance in VRSA. |
| Blocked Penetration / Alteration in access to target site | |
| Narrowing of porin channels | Streptococcal resistance to penicillins typically occurs by reducing access to PBPs. |
| Alteration of porins in gram negative outer membranes | downregulation of Outer Membrane Proteins (eg enterobacteriaceae, pseudomonas vs penems/cephs) |
| increased selectivity of Outer Membrane Proteins (eg klebsiella outbreaks) | |
| Loss of non-essential transporter channels | Anaerobes have no oxygen-transport channel which prevents penetration by aminoglycosides (Gentamicin) |
| Reduced binding of the antibiotic | VanA and VanB vancomycin resistance involves a gene mutation leading to decreased affinity of vancomycin for the binding sites of peptidoglycan precursors |
| Changes to the DNA-binding surface of DNA supergyrase infers resistance against ciprofloxacin | |
| Target Modification | |
| Modifying the enzyme that the antibiotic inhibits | Rifampicin resistance by point mutations, insertions, or deletions in RNA polymerase gene |
| Production of an alternative enzyme for that which the antibiotic inhibits | MRSA develop or acquire the gene mecA which synthesizes an additional penicillin binding protein that enables it to continue cell wall synthesis in the presence of a beta lactam drug |
| Overproduction of the target of the antibiotic | Trimethoprim DHFR enzyme overproduction in Escherichia coli and Haemophilus influenzae. |
| Synthesis of target-protecting proteins | Ribosomal protection proteins against Tetracyclines in multiple Gram positive and gram negative bacteria |
| Modification of Drug or pathways | |
| Development of metabolic pathways to bypass site of action of antibiotic | Resistance to TMP-SMX by allowing bacteria to synthesize or absorb folic acid. |
| Enzymes produced to Metabolize the drug | β-lactamases and cephalosporinases hydrolyse β-lactam rings |
| Enzymes produced that add a chemical group to antibiotic to inhibit its activity | Aminoglycoside resistance by Staphylococcus aureus or Pseudomonas (Multiple enzymes acetyltransferase, adenyltransferase, and phosphotransferase) |
Spread of Bacterial Resistance
- Selective pressure selects for favourable mutations of resistance
- mosaic genes (from other bacteria eg strep pneumo from strep mitus) or uptake of DNA from environment
- transfer of resistant bacteria from person to person
- horizontal gene transfer;
- transduction – acquisition of bacterial DNA from a phage (a virus that propagates in bacteria);
- transformation – uptake and incorporation of free DNA released into the environment by other bacterial cells;
- conjugation – gene transfer (usually on plasmids), by direct cell-to-cell contact through a bridge.
Sources:
Microbiology Lippincott Williams & Wilkins
https://courses.lumenlearning.com/microbiology/chapter/drug-resistance/
https://www.encyclopedie-environnement.org/en/health/antibiotics-antibiotic-resistance-and-environment/
Gladwin / Sakurai / JC 2019
Examiner Comments
2009B 01: 6 (67%) of candidates passed this question.
Candidates were expected to explain and provide an example of an antimicrobial agent for mechanisms such as enzyme inactivation of the antimicrobial, alteration of antimicrobial binding/target sites, reduction of antimicrobial drug uptake or active efflux of drug by the bacteria and alteration in enzymatic pathways. Use of a table aided candidates expressing this information. Examples of the methods of spread of antibiotic resistance in bacteria that were expected were: transfer of resistant bacteria from person to person; horizontal gene transfer; transduction – acquisition of bacterial DNA from a phage (a virus that propagates in bacteria); transformation – uptake and incorporation of free DNA released into the environment by other bacterial cells; conjugation – gene transfer (usually on plasmids), by direct cell-to-cell contact through a bridge.
Most candidates could name various mechanisms of resistance but many failed to gain marks as they did not provide an example of an organism and antimicrobial. The second part of the question concerning the spread of resistance was generally done quite well.
Syllabus – M2a2c
Reference: Pharmacology, Rang and Dale Ch 46, Goodman & Gillman, Ch42
2. Describe the physiological consequences and responses after an acute haemorrhage of 2.0 litres in a healthy 70kg adult if there is no immediate fluid resuscitation.
CICMWrecks Answer
Normal circulating Volume ~ 5L
2L Haemorrhage ~ 40% loss without resuscitation – Major Haemorrhage
Immediate:
Baroreceptor Response
- HP BR
- Stretch receptor: ↑ distension of vessel → ↑ discharge rate
- Threshold > 60mmHg → normally has baseline tone.
- Located at Carotid Sinus and Aortic Arch
- Carotid sinus and aortic arch receptors
- Detects > 5-10 % change in plasma volume
- ↓ Plasma volume → Increased central SNS tone and decreased PSNS tone
- ↑’d distension → ↓’d HR/Contractility/SVR
- Provides strict negative feedback to Δ’s in CO
- LP BR
- Located at junction of return vessels and atria, vetricular walls, pulmonary vessles
- Throughout the peripheral vasculature (esp kidney)
- Detect > 10% decrease in plasma volume as decreased atrial stretch
- Reduced ANP release
- Increase SNS output
- 2 types
- A receptors → fire at atrial contraction (a wave)
- B type → fire at atrial filling (v wave)
- 1. Medulla afferents cause
- ↓ SNS (NA) and ↑PSNS (RVLM) outflow → peripheral vascular vaso and venodilation
- ↓ SNS activity to kidney → ↓ Na/H2O conservation
- ↑ SNS activity to sinus node
- 2. Hypothalamic afferents cause
- → ↓ ADH release and ↓ Thirst
- Outcome
- ↓ PL → ↓ CO → ↓BR discharge rate
- → ↓ PSNS medullary outflow
- → ↑ RVLM outflow
- ↑ contractility/HR
- ↑ SVR → autotransfusion and ↑ perfusing pressure (but ↑AL)
- ↓ PL → ↓ CO → ↓BR discharge rate
Chemoreceptor response
- Peripheral CR
- “Carotid bodies” (more important)
- at bifurcation point of common carotid arteries
- supplied by CN IX
- “Aortic bodies” (less important)
- above and below the aortic arch
- supplied by CN X
- PCRs found in “Glomus cells”
- ↓ PaO2 – via inhibition of O2 sensitive K-channels
- ↑ PaCO2 and/or ↓pH – via effect on pH sensitive K-channels
- Type I cells (rich in NAd, DA, ACh)
- Hypoxia causes release of NTs
- NAd/ACh – ↑ AP firing rate of AB or CB afferent fibres
- DA – Damping of type 2 cell responses
- Type II cells (rich in capillary supply)
- ↓ PaO2, ↑ PaCO2 and/or ↓pH
- ↓ IC [ATP] which leads to ↑ NT production and release
- ↑ AP firing rate of AB or CB afferent fibres
- CCRs located Below the ventral surface of the medulla
- ↑ ECF [H+] stimulates ↑ ventilation
- CO2 rapidly diffuses into CSF
- Low CSF protein and HCO3 (c.f. plasma)
- Poor buffering capacity
- ΔCSF pH per ΔPaCO2 is GREATER than that of blood
- Cause
- ↑’s SVR due to
- ↓ hypothalamic perfusion pressure
- ↓ Hct
- ↑’d RR → ↑ venous return
- ↑ ADH secretion (intermediate term response)
- ↑’s SVR due to
Intermediate term response
- Chemoreceptor stimulation (↓’d perfusion → ↑pCO₂:pO₂)
- ↑ ADH secretion
- ↑’d RAAS activation
- ↓ renal perfusion → ↑ renin → ↑AT2 and Aldosterone
- ↑ADH → ↑Na/H2O retention and vasoconstriction
- ↓Hydrostatic pressure
- altered starlings forces
- autotransfusion of 0.25mL/kg/min ~ 1L/hr
Long Term Response
- ↑ RAAS → ↑ Na/H2O retention
- Osmoreceptor response
- Anterior hypothalamic receptors
- Detect 1% decrease from normal (280-300mOsm/kg)
- Threshold 280mOsm/kg
- Promotes release of ADH from posterior pituitary
- ↑thirst
- EPO release
- From adrenal medulla → ↑ RBC mass
- ↑ hepatic plasma protein synthesis
Gladwin 2016
Examiner Comments
2009B 02: 5 (56%) of candidates passed this question.
To adequately answer this question, candidates must be able to demonstrate that they recognised this to be a major haemorrhage. When a weight and a volume are supplied it is expected the percentage blood loss would be calculated and the shock graded or the haemorrhage at least described as severe. Often the consequences were omitted.
Consequences were best described in organ systems e.g. CV, renal, metabolic. Many candidates failed to mention the patient would be hypotensive and tachycardic.
A good answer should include mention, and provide explanations, of the mechanisms for the
following compensatory responses:
• Activation of both baroreceptors and chemoreceptors and their consequences
• The sympathetic nervous system response
• Fluid shifts
• Renal effects – most candidates mentioned the urine output would be decreased but did not provide a mechanism for this
Endocrine effects, eg secretion and actions of ADH, ACTH/Cortisol
Syllabus – C1e1
Reference: Textbook of Medical Physiology, Guyton and Hall, Foundations of Anaesthesia:
Basic Clinical Science, Hemmings and Hopkins
3. Describe the factors that influence the speed of ONSET of neuromuscular blockade.
CICMWrecks Answer
Drug Factors
- Dose: Higher dose (eg. multiples of ED95) = faster onset.
- Plasma clearance: Postulated that drugs with faster clearance (eg. sux) have faster onset.
- Potency:
- Inverse log relationship between potency and speed of onset (non-depolarisers).
- Low potency = bigger dose needed (more molecules), so higher conc gradient between plasma and site of action = more rapid onset.
- Depolarisers vs non-depolarisers.
Patient Factors
- Rate of injection: faster rate = faster onset.
- Site of injection: Central vein (more rapid distribution)> peripheral vein > IM
- Cardiac output and muscle blood flow: Higher CO and muscle flow = faster delivery to site of action.
- Priming: small dose of non-depolariser as a premed can partially block NMJ, followed by intubating dose. This decreases time from intubating dose to intubation conditions.
- Disease states: eg. myasthenia gravis (fewer ACh receptors, so needs more sux, but about 10% dose of non-depolariser).
Other factors
- Muscle group: Fastest affected = small, rapidly moving muscle groups (eye, digits), with trunk/abdo muscles last affected.
- Recovery is in reverse order: diaphragm/intercostals recover first.
- Onset more rapid in muscle groups relevant to intubation: laryngeal, masseters. Slower in monitored muscles (eg.adductor pollicus).
- Probably due to muscle blood flow.
- Smaller volume of distribution due to blood loss can augment potency ie: speed of onset(Stoelting)
Drugs:
eg. ephedrine can increase speed of rocuronium onset. Inhalationals/aminoglycosides.
JC 2019
Examiner Comments
2009B 03: 0 (0%) of candidates passed this question.
The better candidates had an organised approach to their answer. For example factors could be broadly classified as pharmacokinetic and pharmacodynamic factors or patient and drug factors. For example, pharmacokinetic factors could include dose, the use of a priming dose, patient’s volume status, cardiac output, muscle group and skeletal muscle blood flow. Pharmacodynamic factors expected were those of mechanism of blockade, receptor affinity, agent potency, neuromuscular disorders, age, drug interactions and electrolyte disorders. Many answers listed factors which affect the duration of neuromuscular blockade instead of the onset. Marks were not allocated for information provided by candidates that did not address the question asked. It is important that candidates provide an answer specific to the question asked. Many candidates applied Fick’s Law inappropriately. Lipid solubility and pKa are not relevant as these drugs do not cross the nerve membrane.
Syllabus – H2a, 2c
Reference: Pharmacology and Physiology in clinical practice, Stoelting Pg 186, Foundations
of Anaesthesia: Basic clinical Science, Hemmings and Hopkins ( 2nd ed)
4. Describe the body’s mechanisms for regulating blood glucose.
CICMWrecks Answer
General
- Normal BSL 4-6mmol/L
- Tight BSL control important as:
- ↓ BSL: disrupt normal function of brain, retina, gonads (obligate glucose users)
- ↑ BSL: ↑ osmolality, osmotic load on kidneys → diuresis + cellular dehydration, loss of electolytes/ substrate → tissue damage
BSL control via feedback mechanism
- Sensors: pancreatic islets of Langerhans
- Central regulator: lateral (feeding) and ventromedial (satiety) centres of hypothalamus
- Effectors:
- Behavioural (feeding)
- Hormonal: insulin vs. glucoagon balance (act on liver, muscle, adipocytes)
- Renal (excretion)
- Modulated by: catecholamines, cortisol, thyroid homrones
- short term regulation: via secretion or inhibition of insulin + glucagon from pancreatic islets
- long term: neuronal mechanisms (SNS activation) + hormones (cortisol, GH)
Sensors
- Pancreatic beta cells → sense ↑ BSL
- Secrete insulin in biphasic pattern: initial rapid ↑ → prolonged slow ↑
- 1st phase of insulin secretion: ↑ BSL → glucose enters via GLUT2 → converted to pyruvate → enters TCA → generate ATP → inhibit ATP sensitive K channel → ↓ K efflux → depolarisation → open voltage gated Ca2+ channels → exocytosis of insulin granules
- 2nd phase of insulin secretion: glutamate produced as by-product of TCA → maturation of other insulin granules
- Pancreatic alpha cells → sense ↓ BSL
- ↑ glucagon release
Effectors
- insulin: secreted in response to ↑ BSL → following effects to ↓ BSL
- ↑ GLUT4 insertion into cell membrane → ↑ glucose uptake into cells esp. muscle + fat
- ↑ glycogen synthesis
- ↑ glucose utilisation + ↑ fat and protein synthesis
- ↓ glycogenolysis / ↓ gluconeogenesis
- glucagon: secreted in reponse to ↓BSL → following effects to ↑ BSL
- ↑ glycogenolysis / ↑ gluconeogenesis
- minimal effect on adipose tissue and muscle
- Adrenaline: stimulated by ↓ BSL, stress
- Inhibit insulin
- Liver: ↓ glycogenesis, ↑ glucose release, ↑ KB
- Fat: ↑ FFA release, ↓ glucose uptake
- Muscle: ↓ glucose uptake, ↑ FFA metabolism
- Sustained ↓ BSL stimulates GH + cortisol release
- ↓ glucose utilisation + ↑ fat utilisation → limiting further ↓ BSL
- ↓ protein synthesis / ↑ aa release / ↑ FFA metabolism
- Neuronal Mechanisms:
- Hypothalamus directly stimulated by hypoglycaemia → ↑ SYNS activity → adrenaline release → stimulates hepatic glucose release
Kerr 2016
Examiner Comments
2009B 04: 4 (44%) of candidates passed this question.
This question sought a candidate’s knowledge of the basic science underpinning a topical area of clinical Intensive Care practice. Candidates were expected to mention normal values of blood glucose and detail the relevant humoral (dominant) and neural (sympathetic) factors.
These included mention of, description of and mechanisms for the stimulation/inhibition and actions of humoral factors such as insulin, glucagon, cortisol, etc as the specifically apply to blood glucose control, neural stimulation and the liver’s role as a glucostatic organ.
Good candidates would demonstrate a structured response to their answer.
Syllabus – N12b
Reference: Textbook of Medical Physiology 11th ed Guyton and Hall, Ch. 78
5. Outline the kinetic characteristics and the mode of action of digoxin. (75% of marks) List the cardiovascular effects of digoxin (25% of marks).
Examiner Comments
2009B 05: 0 (0%) of candidates passed this question
The Syllabus for the Primary examination describes an outline to be “Provide a summary of the important points.” Thus candidates were expected to briefly mention the fundamental pharmacokinetic characteristics (eg highly lipid soluble, well absorbed from small intestine, oral bioavailability of 60 – 90%, protein binding of 20 – 30%, volume of distribution, half life, etc) and mode of action. This was poorly done and candidates’ answers often lacked structure.
The question outlines the distribution of marks, being 25% for listing cardiovascular effects. Thus candidates were expected to broadly list the important cardiovascular effects relating to mechanical (eg increase intensity of myocardial contraction, direct venous and arteriolar constriction, etc) and electrical ( increase phase 4 slope & automaticity, hyperpolarization, shortening of atrial action potentials, decrease AV conduction velocity and prolong AV refractory period, increase PR & QT intervals, dose and baseline autonomic activity dependent actions, etc).
6. Define venous admixture and list its causes. (50% of marks) How is it diagnosed and how is it quantified? (50% of marks)
CICMWrecks Answer: Venous Admixture
Venous admixture
“Theoretical volume of mixed venous blood added to pulmonary end-capillary blood to produce the observed CaO2 “
Causes of venous admixture
Shunt
- Physiological
- Thebesian vessels – drain venous blood from coronary circulation directly into cardiac chambers (including L ventricle)
- Bronchial circulation – Fracture of blood from bronchial circulation drains into pulmonary veins
- Pathological
Blood from pulmonary capillaries supplying unventilated alveoli (V/Q = 0)- Obstruction within bronchial tree
- Atelectasis
- Usually mitigated by hypoxic pulmonary vasoconstriction
V/Q mismatch
- Variation in ventilation and perfusion within the lung
- V/Q > 1 – Areas of lung with high relative ventilation to perfusion
- West Zone 1
- Lung apices
- In pathology
- Positive pressure ventilation
- Shock and hypoperfusion
- PE
- V/Q < 1 – Areas of lung with low relative ventilation to perfusion
- Lung bases
- West zone 4
- In pathology: Left heart failure, Pulmonary oedema
- V/Q > 1 – Areas of lung with high relative ventilation to perfusion
- Areas of high V/Q cannot compensate for areas of low V/Q due to the shape of the oxyHb dissociation curve and the sharp desaturation of deoxygenated blood. Also there may be decreased flow through the highly ventilated areas of lung
- V/Q mismatch in pathology
- Obstructive lung diseases: Asthma, COPD
- Restrictive lung diseases: ILD, ARDS
Sakurai 2016
CICMWrecks Answer: Diagnosis and Quantification
Diagnosis of venous admixture
Estimated using A-a gradient using alveolar gas equation
R – Respiratory quotient, usually 0.8
Normal A-a gradient = 2.5 + (0.21 x Age)
Quantification of venous admixture
Shunt equation
Where
- Qt = Total cardiac output
- Qns = Flow through non-shunt areas
- Qs = Flow through shunt
- CaO2 = arterial content of O2
- CcO2 = pulmonary end-capillary O2 (presumed 100% SpO2)
- CmvO2 = mixed venous content of O2
- Blood sampled from R atrium
V/A quantification
- MIGET (Multiple inert gas elimination technique)
- IV injection of a volume of liquid with known quantities of 6 inert gases
- V/Q calculated from the elimination spectra of these gases
- PET
- Xe133 gas and Tc99 labelled RBC
- MRI
Sakurai 2016
Examiner Comments
2009B 06: 5 (56%) of candidates passed this question
This question related to an area of basic respiratory physiology. A good answer necessitated the precise meaning of venous admixture, being that amount of mixed venous blood which would have to be added to ideal pulmonary end-capillary blood to explain the observed pulmonary end-capillary to arterial PO2 difference. Diagnosis required the candidate to mention, a demonstrated increased in the Aa-DO2, what are normal values. For quantification mention and description of the shunt equation was required.
Candidates lacked a definition for venous admixture, were inaccurate with their description of the shunt equation and often overlooked mentioning calculation of arterial and mixed venous blood oxygen content.
7. Compare and contrast the anatomy of the upper airway of a term newborn with that of an adult.
CICMWrecks Answer
Miller’s 5 Differences
| Newborn | Adult |
|---|---|
| Disproportionately large tongue that complicates laryngoscopy No dentition | Smaller tongue |
| Larynx is more cephaled (Cricoid at C4) | Larynx more caudal (Cricoid at C6) |
| Epiglottis is shaped differently (U shaped), being short, stubby, omega shaped, and angled over the laryngeal inlet | Epiglottis is longer and stiffer |
| Vocal cords are angled | |
| Larynx is funnel shaped, the narrowest portion occurring at the cricoid cartilage Oedema due to trauma may rapidly cause airway obstruction. |
Other Differences
| Airway | Narrower airways increase the resistance and work of breathing Airway more oedematous due to hormones from mother (oestrogen/progesterone) | Larger airways, less resistance and WOB |
| Nose | Obligate nose breathers Nasal obstruction may significantly impair respiration. | |
| Head | Proportionally enlarged head and occiput Optimal intubating position is neutral rather than ramped. | Head is relatively smaller |
| Neck | Less muscular and more mobile neck Proportionally short neck Favours airway obstruction when flexed. Less effective pharyngeal dilators | Decreased mobility and more likely to have arthritis and pathological issues More effective pharyngeal dilator muscles |
| Trachea | Intrathoracic trachea is also shorter May be only 4cm long, so there is little margin for error in tube placement. | |
| Bronchi | Angles of the bronchi take off is similar making left sided intubation as likely as right | Accidental intubation more likely to be right sided |
| Other differences | Increased physiological dead space due to large head 3.3 ml/kg | Decreased PDS 2 ml/kg |
JC / Gladwin 2020
Examiner Comments
2009B 07: 2 (22%) of candidates passed this question.
Good answers to this question directly compared the anatomical differences using two columns: one for the newborn and the other for the adult airway. They further divided the differences anatomically into mouth/naso and oro pharynx, the glottis (and epiglottis), the larynx, and the trachea (main carina and main bronchi).
Common omissions were failure to mention that neonates do not have dentition, have more compliant tissues and reduced muscle tone, that the neonates’ larynx lies more cephalad and anteriorly, is covered by a large floppy epiglottis and that the main carina also lies more cephalad.
Few candidates were able to list more than 4 differences between the anatomy of the two airways and none mentioned the possibility of disease affecting the neck in the adult.
Syllabus – P2e
Reference: Anatomy for anaesthetists and T K Oh Chp95, Anatomy at a glance.
8. Describe the blood brain barrier. (50% of marks) What characteristics does a drug need to effectively penetrate into the central nervous system? (50% of marks)
CICMWrecks Answer
Blood brain barrier
- Anatomical and chemical partition separating the intravascular space from CNS interstitial space
Structure
- Mechanical barrier
- Endothelial cells
- Tight junctions between cells formed by membrain proteins (e.g. occludin) prevents paracellular flow
- Lack fenestrations
- Lack transcellular pathways such as vescicles
- Selective transport proteins (e.g. GLUT, various amino acid transporters)
- Pericytes embedded in basement membrane
- Astrocyte end feet
- Supportive role for endothelium
- Aquaporin regulation
- Endothelial cells
- Physiological barrier
- Enzymatic inactivation
- Enzymtes within endothelial cells metabolise substances absorbed from capillary lumen
- Monoamine oxidase
- Cholinesterase
- Aminopeptidase and endopeptidase
- Enzymtes within endothelial cells metabolise substances absorbed from capillary lumen
- Efflux pumps
- Substances that are absorbed across the luminal capillary membrane may be pumped back into capillary lumen by efflux pumps
- P-Glycoprotein
- ABC-Transporter
- Substances that are absorbed across the luminal capillary membrane may be pumped back into capillary lumen by efflux pumps
- Enzymatic inactivation
- Areas of brain outside BBB
- Subfornical organ
- Organum Vasculosum Lamina Terminalis
- Pituitary
- Area postrema
Function
- Regulate uptake of nutrients and electrolytes into brain
- Regulate migration of leukocytes and inflammatory responses in the brain
- Buffer brain parenchyma and interstitium from fluctuations in blood
- Prevent toxins and pathogens entering brain
- Some substances (such as water) pass BBB readily. These are characterised by:
- Small molecular weight
- Lipophilic (thiopentone)
- Uncharged (e.g. atropine vs. glycopyrollate)
- Poorly protein bound
Characteristics of drugs to penetrate BBB
- Size
- According to Graham’s Law – Diffusion inversely proportional to square root of molecular weight
- Lipophilicity
- Highly lipid soluble compounds can permeate phospholipid bilayers with relative ease (fentanyl > morphine)
- Charge
- Unionized molecules can permeate with relative ease (alfentanil > fentanyl)
- Not metabolized within BBB
- No active efflux mechanism
- Protein binding
- Protein bound drug permeate poorly
Sakurai 2016
Examiner Comments
2009B 08: 2 (22%) of candidates passed this question.
Candidates were expected to state the purpose of the blood brain barrier (BBB), define what constituted the BBB, what its function is, and what parts of the brain lie outside of this barrier (and why). Further candidates should have mentioned what substances cross the BBB, and how this is achieved.
Common omissions included not mentioning the presence of astrocyte foot processes in addition to the tight junctions between the capillary endothelial cells, the presence of active pumps for sugars amines, and some ions, and what parts of the brain lay outside of the BBB.
Characteristics of a drug that will penetrate the BBB included low molecular weight, good lipid solubility, a low volume of distribution, low potency and low protein binding. Also expected was a discussion of Fick’s Law and the drug features that would allow a high concentration gradient in the cerebral blood vessels to be achieved and a high diffusion coefficient and mention of drugs that resemble natural ligands for active transport mechanisms.
Syllabus – G12d G2a
References: Pharmacology, Rang and Dale 6th edition page 476, Principles of physiology for the anaesthetist, Kam page39-40, Pharmacology and Physiology in Anaesthetic Practice, Stoelting, Pg 681, Textbook of medical physiology, Guyton and Hal11th edition page 766. Foundations of Anesthesia. Hemmings and Hopkins. 2nd edition.
9. A six (6) month old child is diagnosed with a gastric outlet obstruction. Investigations reveal a metabolic alkalosis and a urine pH of 5. Describe the physiological basis of these results.
CICMWrecks Answer
Paradoxical aciduria – Formation of acidic urine in face of alkalosis
Gastric outlet obstruction
- Large volume projectile vomiting
- Hypovolaemia → Contraction alkalosis
- Cl– loss → increased SID → metabolic alkalosis
Compensation
- Respiratory compensation of metabolic alkalosis
- Decreased plasma [H+] causes hypoventilation → CO2 increases
- PaCO2 = 0.7 [HCO3–] + 20
- Renal compensation – Renal handling of HCO3
- PCT
- 85% reabsorbed in PCT via H2O and CO2 (due to carbonic anhydrase)
- In PCT cell, H2O and CO2 reform HCO3– and H+ (via CA)
- H+ secreted into tubular lumen via H+ ATPase
- Increased plasma CO2 due to repiratory compensation of alkalosis, increases intracellular CO2 and causes increased absorption of HCO3 → inhibits HCO3 elimination
- LoH
- 10% reabsorbed
- DCT
- Type B intercalated cells secrete HCO3–
- Via Pendrin antiporter (Secretes HCO3– in exchange for Cl-) on luminal membrane. H+ ATPase on basolateral membrane
- HCO3– eliminated and Cl– reabsorbed leading to decreased SID andcompensation
- However, due to hypochloridaemia from hyperemesis, this mechanism is retarded
- PCT
- Effects due to volume contraction, stress
- RAAS system activated by decreased Na+ and Cl– reaching DCT and macula densa.
- JGA secretes renin → angiotensin → aldosterone
- Aldosterone effect on kidney
- DCT
- Increased Na reabsorption due to upregulation of basolateral Na/K ATPase and luminal ENaC
- Increased Na/K ATPase activity causes increased K+ allowing K secretion → This leads to hypokalaemia
- DCT
- Defense of total body K+
- Total body K+ approx 50mmol/kg.
- K+ in glomerular filtrate reabsorbed in the PCT (65%) and LoH (30%) with remaining DCT accounting for secretion or reabsorption depending on body balance
- With hypokalaemia, K+ reabsorbed by intercalated cells of DCT via H+/K+ antiporter
- Leads to H+ secretion into DCT/Collecting ducts
Sakurai 2016
Examiner Comments
2009B 09: 3 (33%) of candidates passed this question.
Candidates were expected to identify what compounds are lost during vomiting associated with gastric outlet obstruction and that intravascular volume depletion is likely. Furthermore the candidate was then expected to explain that overall the child’s body will defend volume – then tonicity – then acid base status, in this order. An outline of the major physiological defences of volume status (e.g. the renin-angiotensin-aldosterone system), and how these will perpetuate a metabolic alkalosis (prevent the kidney from clearing the excess bicarbonate in the form of an
alkaline urine) was then expected.
A common omission was to not appreciate that the preservation of volume (sodium or chloride) by the kidneys conflicts with the physiological mechanisms that would allow bicarbonate loss through the kidneys’ tubules. Restoration of normovolaemia would allow an ‘alkaline tide’ or bicarbonate loss by the kidneys (alkaline urine production).
Syllabus – G12d G2a
References: Power and Kam. P247-266
10. Describe the changes in inspired and alveolar oxygen partial pressure with increasing altitude. (20% of marks)
Outline the respiratory physiological responses to altitude. (80% of marks)
CICMWrecks Answer
Changes in Inspired and Alveolar PO2
PAO₂ used as surrogate for PaO2
Alveolar PO2 can be described by the alveolar gas equation
Where
- FiO2 is the fraction of inspired O2
- 0.21 when breathing room air
- PATM is the atmospheric pressure which changes with altitude
- 760mmHg at sea-level
- 560mmHg in pressurized cabin of commercial aircraft (610mmHg in Boeing 787)
- Approx. 240mmHg at the summit of Everest
- SVPH2O is the saturated vapour pressure of water (47mmHg)
- ↓’s with ↑ing altitude → small ↑ in PAO₂
- PaCO2 is the arterial partial pressure of CO2
- Respiratory exchange ratio
- P is a correction factor
High Altitudes
- With ascension in altitude, PATM decreases therefore, PAO2 will decrease
- A-VO₂ gradient is lower
- Rate of O₂ dissolving in blood ↓’s at altitude → ↓ Diffusion by Fick
- PvO2 sits in the lower portion of the OHDC → ↓O₂ Affinity → ↑PaO2 to get same HbO2 carriage
- CaO2 rises much slower and may become diffusion limited with any exertion
Physiological Response to Altitude
Serum
- If there is no change in the diffusion of O2 PaO2 will similarly decrease proportionally
- Tissues will have a greater reliance on anaerobic metabolism, with an increase production of lactate, and decrease in pH.
Control of ventilation
- Peripheral chemoreceptors in the aortic bodies, sense changes in paO2, paCO2, [H+].
- Central chemoreceptors in the retrotrapezoidal nucleus (RTN) in the medulla sense changes in paCO2, once CO2 diffuses across the BBB and converts to H+ via carbonic anhydrase.
- Both signal to the medullary respiratory centre leading to an increase in ventilation (RR and tidal volume) via ventral group CPG
- There is an increase sensitivity of the respiratory centre to pO2 that is poorly understood
- Ventilation is more sensitive to changes in pCO2, usually maintained within 35~45mmHg.
- Response to hypoxia occurs once paO2 drops below 50~60mmHg and occurs in 3 stages
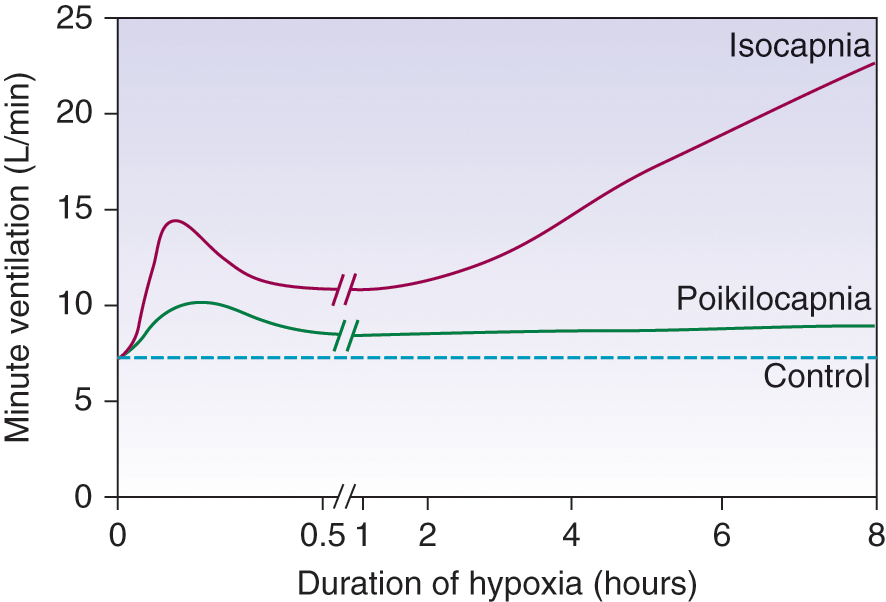
Three Phases:
- Acute Hypoxic Response
- ↑ altitude causes hypoxaemia
- ↓barometric pressure → ↓PiO2 → ↓PAO2 → ↓ PaO2
- ↓ PaO2 stimulates Peripheral Chemo Receptors (in CB and AB)
- ↑ MV significantly
- ↑ altitude causes hypoxaemia
- Hypoxic ventilatory decline (HVD)
- ↑ MV then ↓ PaCO2 causing
- ↓ brain ECF [H+] → ↓Central CR stimulation (in medulla) (→ response curve reset to left)
- limits any further ↑ MV due to hypoxaemia
- Ventilatory response to sustained hypoxia
- Brain ECF [H+] returns to normal (3 days)
- HCO3 rapidly equilibrates across the BBB
- hypoxic ventilatory drive is restored
- allows further ↑ MV to occur
↑ MV causes
- ↑venous return → ↑ CO → ↑ pulmonary Blood volume
- May ↑/↓ V/Q matching → ↑/↓ PaO₂
- ↓ pulmonary compliance → ↑WOB
- ↑ risk of diffusion limitation on exercise
Other Important Physiological Changes
- Hypoxia → ↑2,3DPG → ↓ HbO₂ affinity → ↑delivery
- Hypoxia Inducible Factors in the kidneys upregulate production of EPO, increasing O2 carriage.
- Alkalosis → ↓ammoniogesis → ↓HCO3 reabsorption → ↑ effective bicarb secretion which tends to return pH to normal
- High-Altitude Pulmonary Oedema may occur
- Poorly understood, however throught to be due, in part, to hypoxic pulmonary vasoconstriction
- This inhibits diffusion of O2 across the alveolar membrane, increasing the A-a gradient
Sakurai / Gladwin 2016
Examiner Comments
2009B 10: 0 (0%) of candidates passed this question.
Any description of the changes in inspired and alveolar PO2 with altitude required the description of, and an understanding of, the equations for calculation of PiO2
(PiO2=0.21(BMP-47)mmHg or 0.21(BMP-6.3)kPa) and PAO2 (PAO2 =PiO2 – PaCO2/R).
It was important to mention alteration in atmospheric pressure (BMP) with altitude and that saturated vapour pressure (SVP) is constant for the same body temperature.
A good answer to the second part of the question (respiratory physiological responses to altitude) was one that had some structure, eg responses divided into acute and chronic, respiratory, renal, haematological, cardiovascular and CNS categories, and relevant detail for each category. Candidates were expected to at least mention, but not restrict themselves to the following: acute responses, eg carotid/aortic body chemoreceptor stimulation hyperventilation, proportional to reduced BMP and hypoxia, limitations to hypocarbia (due to alterations in CSF pH) etc: physiological responses to early and chronic acclimatisation (eg renal HCO3-excretion, respiratory centre CO2 response curve resets to left, altered peripheral chemoreceptor sensitivity, 2,3, DPG levels and related right. Shift in HbO2 curve, alterations in pulmonary diffusing capacity due to increased alveolar surface area and pulmonary blood volume, etc).
It is important that current and future candidates use the examination reports to guide their learning. Thus it is essential that candidates not just read the feedback provided to each exam question but also refer to the reference(s) supplied below and other relevant sources they find to be useful.
Syllabus – B1k, 2i
Reference – Nunn’s Respiratory Physiology, Ch 17 and 5.
Power and Kam 432-4.
11. Describe and / or illustrate the anatomy relevant for the insertion of a central line into the left subclavian vein using any of the available techniques.
CICMWrecks Answer:
Origin
- Continuation of axillary vein
- Lateral border of 1st rib
Course
- Follows subclavian artery
- Deep to clavicle
- Superior to 1st rib
Termination
- Deep to sternoclavicular joint at medial border of scalenus anterior
- Joints internal jugular vein to form bilateral brachiocephalic veins vein on left
Relations
- Anterior
- Clavicle, subclavius
- Posterior
- Subclavian artery runs deep/posterior (separated by scalenus anterior)
- Internal mammary artery is posterior medially
- Phrenic nerve is posterior
- Superior
- Skin, superficial aponeurosis
- Inferior
- Apex of lung and 1st rib
- Medial
- Brachiocephalic trunk, thoracic duct and trachea and vagal trunks
- Lateral
- Inferior trunk of brachial plexus
Surface Anatomy
- Clavicle
- Deltopectoral groove
- 2 heads of sternocleidomastoid
- Suprasternal notch
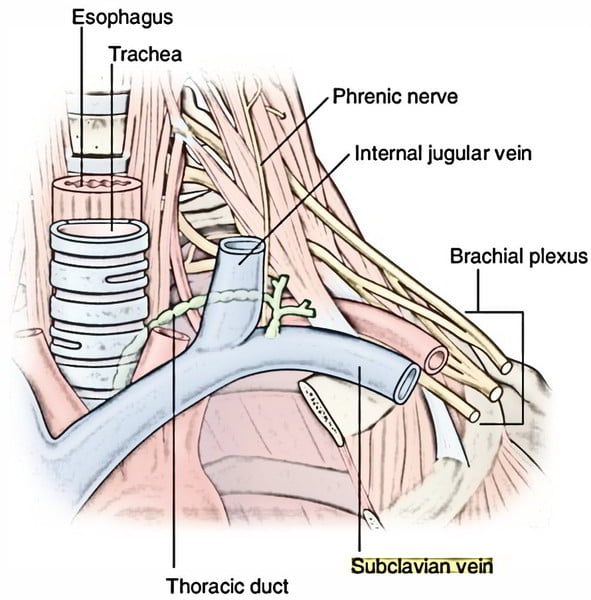
Sakurai 2016
Examiner Comments
2009B 11: 0 (0%) of candidates passed this question.
There are a few areas of anatomy that are essential for Intensivist to have a high level of knowledge of. The subclavian vein is one of them. Unfortunately the extent of knowledge displayed by the candidates was very shallow. A good answer required as a minimum, a clear description of the course of the vein. This included the following facts – it is a continuation of the axillary vein, it’s relationship to the first rib, to the medial border of the scalenus anterior, the fact that it arches up, medial, then down and that it joins the internal jugular vein (behind the subclavian joint, anatomical relationships (eg Anterior – clavicle, subclavius muscle, Posterior – first rib, apex lung superiorly, first intercostal space inferiorly, Superior – subclavian artery, thoracic duct medially, phrenic nerve, lower trunk brachial plexus laterally and Medial – brachiocephalic artery, trachea, vagal trunk, thoracic duct and oesophagus posteriorly) and surface anatomy (related to the various available techniques (eg the infraclavicular and supraclavicular approach)
Syllabus – C1d, 2g
Reference: Anatomy for Anaesthetists, Ellis and Feldman
12. Outline the pharmacology of noradrenaline.
Examiner Comments
2009B 12: 2 (22%) of candidates passed this question.
Candidates should expect that questions relating to “the pharmacology of ……” are likely to be common. Thus candidates should have prepared structured approach for any such question. For example, one that includes predefined major categories such as pharmacodynamics and pharmacokinetics and sub-categories such as mechanism of action, absorption, preparations, bioavailability, volume of distribution, metabolism, elimination, adverse effect, clinical indications, precautions/interactions, etc. and the information relevant to each category. Failure to take a structured approach to such questions, as was observed amongst some candidates within this exam, risks omission of vital facts (and not gaining marks) and errors. Noradrenaline is such a common drug within intensive care practice and so candidates would be expected to know it in great detail. There are many references for it, such as the ones listed below.
Syllabus – G3a, 2b
References – Goodman and Gillman Chp 10 and Katzung.
13. Outline the ideal properties of a colloid intravenous fluid. (25% marks) Compare and contrast Gelatins, Hydroxyethyl starch and 4 % Albumin solutions. (75% marks)
Gelatins and starches are now out of clinical practice, so we will not bother answering this question
14. Classify antiemetic drugs and describe their mechanism of action.
CICMWrecks Answer
Anti-emetic Agents
| CLASS | EXAMPLES | MECHANISM OF ACTION |
|---|---|---|
| Anticholinergics | Hyoscine Atropine | – M1-Ach-R antagonism (NTS, CTZ, VC) – Small antihistamine and D2 antagonist effects |
| Antihistamines (H1) | Cyclizine Promethazine | – H1 antagonism- VC, vestibular nucleus and CTZ – Anti-muscarinic effects- NTS, CTZ, vomit center – D2 antagonism (GIT, CTZ) |
| 5-HT3 Antagonists | Ondansetron | – Peripheral in GIT – Central at VC and CTZ |
| Dopamine Antagonists | 1. Phenothiazines – Prochlorperazine (stemetil) 2. Butyrophenones – Droperidol, domperidone 3. Benzamides – Metoclopramide | – Decreased sensitivity of visceral afferents to vomit center – Central D2 blockade increased threshold at CTZ Other effects: – Inhibition of 5-HT3 – Anti-H1 effects |
| Steroids | Dexamethasone | – Proposed to act centrally to inhibit prostaglandin synthesis and inhibit endorphin receptors |
| Miscellaneous | 1. Propofol 2. Benzos 3. Cannabinoids 4. NK1- Receptor antagonists (aprepitant) | – Propofol and BZD- GABAergic inhibition of VC – Cannabinoids- Direct CTZ and VC inhibition – NK1 antagonists inhibit VC |
Examiner Comments
2009B 14: 3 (33%) of candidates passed this question.
Antiemetics can be classified by their receptor actions including, dopamine antagonists, serotonin antagonists, antihistamines, anticholinergics, antimotilinic etc or by their drug type – butyrophenones, phenothiazines, benzamides etc. Marks were divided between each class, and for “other/novel” antiemetics – such as, steroids, GABA agonists, alpha 2 agonists, mu receptor antagonists, canabinoids, propofol and NK1 receptor antagonists.
Candidates were expected to mention the drug type, receptor interaction, the location of the receptors and the pathways inhibited by the drug – central CTZ, vestibular nuclei, solitary tract nucleus, vagal afferents or GIT motility and secretions. Most drugs work at one or more of these sites and marks were awarded for mention of this. Commonest errors were omission of adequate detail in each class. Antihistamines, anticholinergics and “others/novel” were frequently omitted.
Syllabus – Q12e and Q2b2a
Reference: Chp 37 Goodman and Gillman
15. Describe the mechanism of actions and duration of effect of drugs used to lower potassium in hyperkalaemia.
CICMWrecks Answer
Serum Potassium
- Normally 3.5~5mmol/L
- Hyperkalaemia [K+] >5mmol/L
- Severe hyperkalaemia [K+] >7mmol/L
Management
Drugs that eliminate body K+
- Loop diuretics e.g. frusemide
- 40mg IV
- Inhibition of the Na+/K+/2Cl- cotransporter on luminal membrane of the thick ascending Loop of Henle
- This causes loss of normal positive charge in lumen, leading to the loss of paracellular resorption of K+
- Net result is K+ excretion
- Elimination half-life 45-9 mins
- Cation exchange resins e.g. sodium polystyrene sulfonate
- 15~45gm
- Not absorbed in GI tract
- Prevents potassium reabsorption in the GI tract (predominantly in the colon where K+ secretion occurs)
- Onset of action: 1 hr PR, 4-6hrs PO
- Duration: variable
- No effect acutely
Drugs causing increased intracellular uptake of K+
- Insulin/glucose
- 10 units IV (with 50ml 50% dextrose)
- Insulin receptor agonism → transcellular shift of K from extracellular to intracellular space via upregulation of Na/K ATPase → transient reduction in serum [K+]
- Onset: 20-30 mins
- Duration: 2-6 hours (prolonged if i.v. infusion)
- Sodium bicarbonate
- Alkalosis increases the activity of the Na+/K+ ATPase pump, increasing K+ uptake
- Onset: 30-60 mins
- Duration: 2-3 hours
- β2 agonists e.g. salbutamol
- 5~20mg via nebulizer, repeated (or 6-12 puffs via MDI, repeated)
- β2 agonism → increased intracellular [cAMP] → transcellular shift of K from extracellular to intracellular space → transient reduction in serum [K+]
- Onset: 30 mins
- Duration: 2-3 hours
- I.V. fluid
- Causes haemodilution
- Can increase renal excretion of K+ by increasing renal perfusion and increasing urine output
Other Treatments in Hyperkalaemia:
- Calcium (No effect on K+ level)
- 10ml 10% CaCl (6.8mmol) or 10% CaGluconate (2.2mmol)
- Immediate onset
- Stabilizes cardiac membrane by reducing threshold potential
- Dialysis
- via continous Veno-Venous Dialysis or intermittent haemodialysis
- Dialysis fluid adjusted to create large concentration gradient for potassium
extraction via dialysis of filtration
JC / Sakurai 2019
Examiner Comments
2009B 15: 5 (55%) of candidates passed this question.
A good answer to this question required the collation of knowledge from broad range of areas, ie drug activity. Marks were divided between each of the following: 8.4%NaHCO3-, insulin/glucose, K+ exchange resin, frusemide/loop diuretics, Beta2 agonists and K+ free fluid rehydration/dilution. Candidates often overlooked the fact that only loop diuretics and K+ exchange resins lower total body K+ content, whilst the others induce an intracellular K+ shift which is not sustained and do not directly result in body elimination of K+. Intravenous fluid rehydration lowers total body K+ if there is a resulting diuresis.
Most candidates just passed this question. Common omissions included NaHCO3, loop diuretics and beta 2 agonists. No candidate mentioned rehydration/dilution. Calcium does not lower serum potassium.
16. Discuss the factors affecting pulmonary vascular resistance.
CICMWrecks Answer
- Vascular resistance is the resistance to blood flow for a given pressure gradient across the vessels
- Pulmonary vasculature is a high capacitance, low pressure system
- Pulmonary artery pressures: 25/8 (MAP 15)
- Pulmonary Vascular Resistance (PVR)
- ~ 1/10th of SVR
- ~ 0.25-1.6 mmHg/L/min (or) 20-120 dynes.Sec.Cm-5
- Factors Affecting Pulmonary Vascular Resistance:
- Recruitment and Distension
- Gravity
- Autonomic Innervation
- Volume
- Hypoxia, Hypercapnoea, Acidosis
- Local Mediators
1. Recruitment and distension:
- At normal pressure and flows, most capillaries are either partially or completely collapsed
- At higher pressures, decrease in resistance due to
- Distension of partially collapsed capillaries
- Recruitment of completely collapsed capillaries
2. Gravity
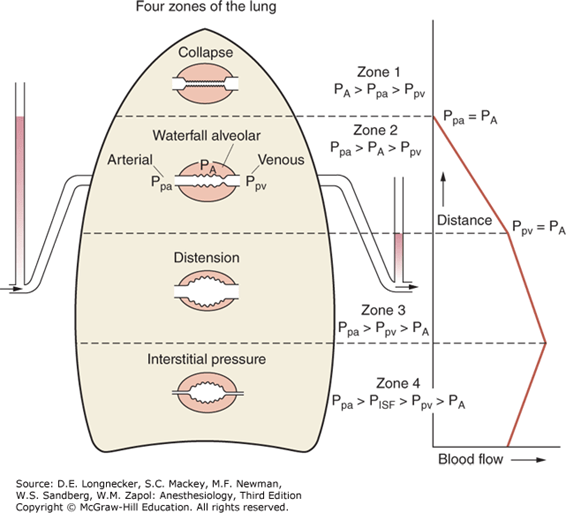
- Divided into West Zones based on relationship between Alveolar pressure (PA) Arterial pressure (Pa) Venous pressure (Pv) Interstitial pressure (Pi)
- West Zones 1,2,3: Due to changes in hydrostatic pressure when pumping to top of lung vs. bottom of lung
| West Zone | Pressure Relationships | Physiology | Location |
|---|---|---|---|
| Zone 1 | PA > Pa > Pv | No flow of blood, as arterial pressure completely opposed by alveolar pressure | not seen in normal lung |
| Zone 2 | Pa > PA > Pv | Resistance to flow is determined by alveolar pressure (Starling resistor effect) | about 3cm above the heart |
| Zone 3 | Pa > Pv > PA | Resistance to flow is determined by venous pressure Venous pooling causes increased distension of pulmonary capillaries | majority of healthy lung |
| Zone 4 | Pa > Pi > Pv > PA | Low lung volume causes narrowing of extra-alveolar vessels | Lung bases at low lung volume or in pulmonary edema |
3. Autonomic innervation:
- Sympathetic innervation from sympathetic trunk
- Noradrenaline
- α1 (vasoconstriction) and β (vasodilation) receptors
- α effects dominate (vasoconstricton)
- Parasympathetic innervation from vagus nerve
- Acetylcholine (M3 receptors)
- Cause vasodilation
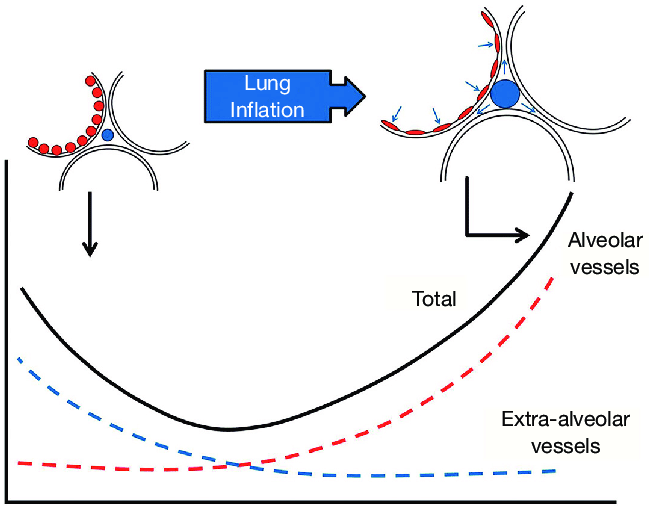
4. Volume
- Extra-alveolar vessels are stretched laterally by increased volumes
- Compressed by low volumes
- Pulmonary capillaries are stretched longitudinally by increased volumes, decreasing
radius -> more resistance - PVR is a balance of these two effects
- PVR is lowest at functional residual capacity
5. Hypoxia, Hypercapnoea, Acidosis
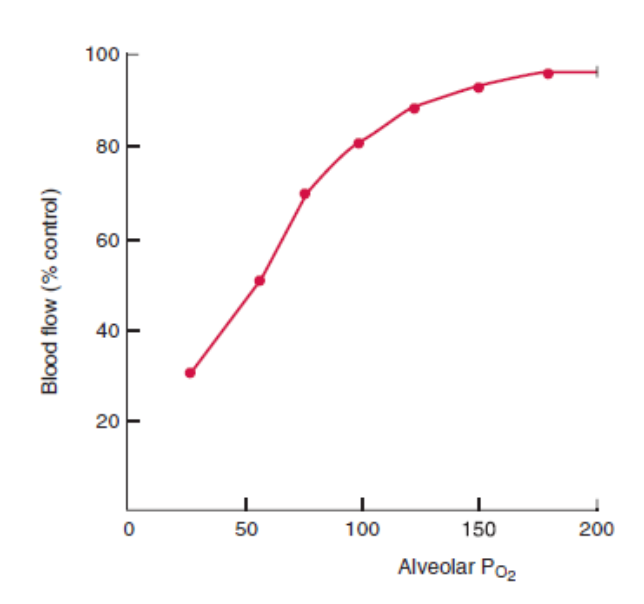
- Hypoxia:
- Alveolar (not arteriolar) hypoxia causes local vasoconstriction of pulmonary vessels
- This limits perfusion of underventilated lung regions, reducing shunt
- There is a similar, but less potent, effect of hypercapnoea and acidosis on vascular tone, reducing perfusion of hypoventilated regions
6. Local mediators:
- Dilators:
- Nitric oxide
- Prostacycline
- Isoprenaline
- Constrictors:
- Histamine
- Serotonin
- Endothelins
Mooney 2016
Examiner Comments
2009B 16: 3 (33%) of candidates passed this question.
A good answer to this question required a discussion that focused on the following key
points-
- Recruitment and distension of pulmonary capillaries. Helps limit pulmonary vascular resistance as pressure and flow increase.
- Lung volume. Lung inflation is thought to have a dual effect, expanding large vessels by traction while compressing smaller vessels and resistance is dependent upon lung volume, being lowest at approx FRC.
- Gravity. Perfusion is distributed down a vertical gradient in the lung, reflecting the balance between intra-alveolar pressure and the distending vascular pressure. (West’s Zones)
- Oxygen. Hypoxia causes pulmonary vasoconstriction. Diverts blood away from hypoxic regions of the lung.
- Hypercapnia and acidosis vasoconstriction
- Autonomic innervation. Alpha -adrenergic stimulation – vasoconstrictor, Beta-adrenergic stimulation – vasodilatation, Parasympathetic stimulation – Vasodilatation
- Local mediators. Vasoconstrictors (Serotonin, histamine, endothelins) and Vasodilators (NO, prostacyclin, isoprenaline)
The majority of these points could be efficiently explained through the use of graphs/figures,
all of which are commonly found in the majority of physiology books that include respiratory
physiology. Those candidates who failed to do so were also those who scored fewer points
with this question.
17. Explain the difference and the clinical relevance, between zero and first order pharmacokinetics. (60% marks) Give an example that is relevant to intensive care practice. (40% marks)
CICMWrecks Answer
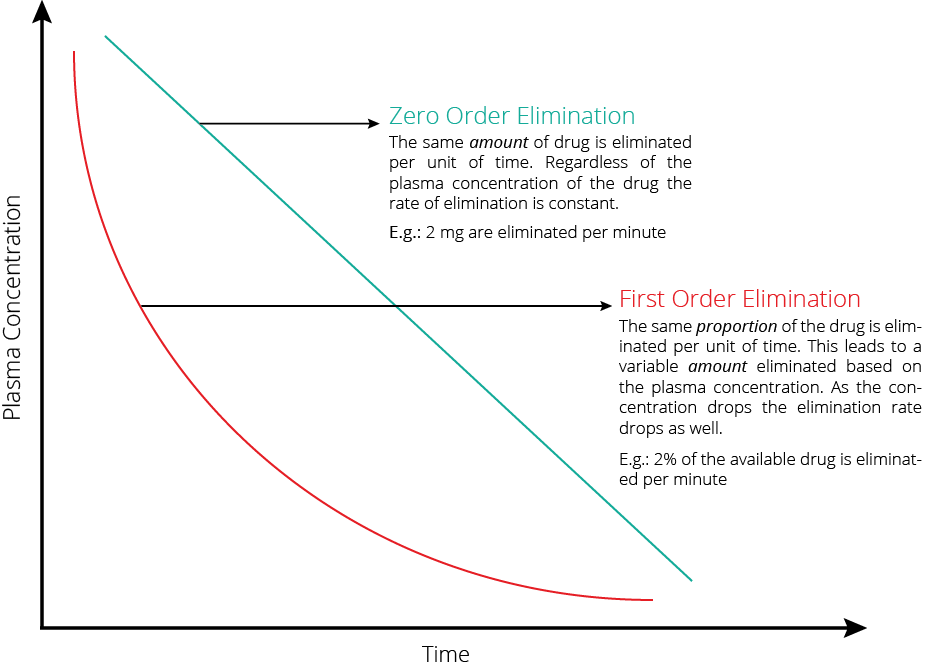
| Zero order kinetics | First order kinetics | |
|---|---|---|
| Definition | Rate of elimination = dP/dT = constant | dC/dt = kC |
| Elimination = constant | Rate of Elimination = Cl x C | |
| Explanation | • Elimination of a drug from the body is saturable. • The rate of change of concentration with time is constant (v) | • Rate of Elimination of a drug from the body is proportional to the concentration. • Elimination is equal to the area under the curve of the concentration versus time curve after dose of drug is administered. |
| Example | Phenytoin when plasma [ ] > 10 μg/mL Other examples include • Ethanol • Aspirin | Phenytoin when plasma [ ] > 10 μg/mL |
| Implication | • Small changes in doses or adjustment of other medications effecting pheytoin metabolism • Narrow therapeutic index, and zero order kinetics and therapeutic drug monitoring is important | Elimination is dependent on blood flow (ie high extraction ratio) |
Phenytoin or Aspirin
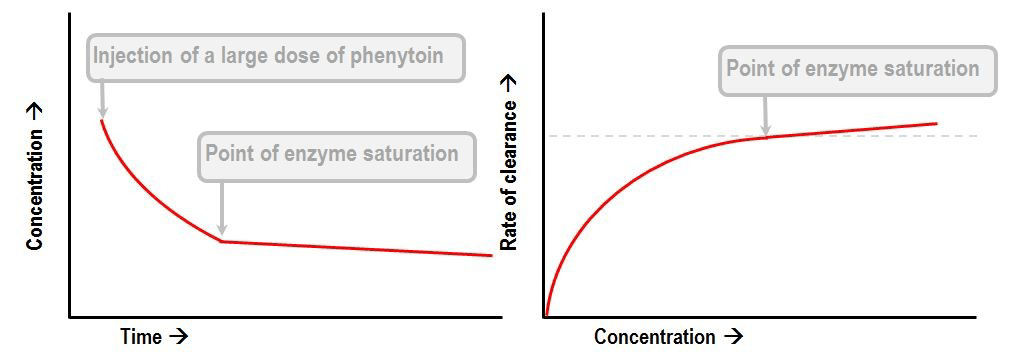
- Display Michaelis-Menten kinetics
- Zero order at high concentration when enzyme saturate
- First order at low concentrations
- Thus highly variable plasma concentrations
- Where
- S is the substrate concentraton
- Vmax is the maximum elimination capacity
- Km is the drug concentration at which the rate of elimination is half of the maximal rate.
Gladwin 2016
Examiner Comments
2009B 17: 2 (22%) of candidates passed this question
Candidates should expect that questions relating to “the pharmacology of ……” are likely to be common. Thus candidates should have prepared structured approach for any such question. For example, one that includes predefined major categories such as pharmacodynamics and pharmacokinetics and sub-categories such as mechanism of action, absorption, preparations, bioavailability, volume of distribution, metabolism, elimination, adverse effect, clinical indications, precautions/interactions, etc. and the information relevant to each category. Failure to take a structured approach to such questions, as was observed amongst some candidates within this exam, risks omission of vital facts (and not gaining marks) and errors. Noradrenaline is such a common drug within intensive care practice and so candidates would be expected to know it in great detail. There are many references for it, such as the ones listed below.
Syllabus – G3a, 2b
References – Goodman and Gillman Chp 10 and Katzung.
18. Outline the physiology of, and factors which regulate levels of angiotensin.
CICMWrecks Answer
Angiotensinogen is a peptide hormone produced by the liver
- Angiotensin 1 (decapeptide)
- ↑ Renin by JGA → cleavage of Angiotensinogen → AT1
- No biological activity
- Angiotensin 2 (octapeptided)
- ACE in pulmonary capil endothelia
- → ↑AT2
- Angiotensin 3 (septapeptide)
- 40% of the pressor activity AT2
- 100% of the aldosterone-producing activity
- Angiotensin 4 (hexapeptide)
- Minimal biological activity
Actions of AT2 include:
- Acts via GPCR (Gq via PLC to increase IP3 and intracellular Ca)
- CVS
- Vasopressor
- Resets baroreceptor control of HR at higher pressure
- Potent mitogen for smooth muscle and cardiac myocytes
- Direct positive inotrope
- CNS
- ↑ sympathetic outflow
- ↑ thirst
- ↑ ADH release from posterior pituitary.
- Renal
- Negative feedback on renin release
- ↑ aldosterone release
- ↓ RBF and GFR
- Direct renal arteriole constriction (efferent = afferent)
- Mesangial cell contraction thus ↓ Kf and GFR
- ↑ sodium/chloride reabsorption in PCT
- Direct effect and via ↑ aldosterone release
Factors which alter renin release cause a corresponding change in Angiotensin 2
- Stimulation of renin secretion (↑ renin → ↑ angiotensin 2)
- β-1 agonism
- ↓ renal perfusion pressure
- ↓ sodium delivery to DCT
- Prostaglandins
- Inhibition or renin secretion (↓ renin → ↓ angiotensin 2):
- ↑ renal perfusion pressure (via afferent arteriolar dilatation)
- ↑ sodium delivery to DCT
- ↑ by angiotensin II (negative feedback) and vasopressin
Gladwin 2016
Examiner Comments
2009B 18: 4 (44%) of candidates passed this question.
For a good answer it was expected that candidates would mention the relationship of angiotensinogen, angiotensin I, renin and Angiotensin Converting Enzyme to angiotensin II production, the actions and fate of angiotensin II and factors that regulate angiotensin II. It was expected that candidates mention angiotensin acts as a potent vasoconstrictor, stimulates aldosterone secretion, facilitates noradrenaline release, preferentially vasoconstricts Efferent arteriole in nephron, preserving GFR in low perfusion states, increases renal tubular Na + reabsorption and increases secretion of vasopressin and ACTH.
Regulation of Angiotensin could be separated into factors increasing angiotensin levels (eg prostaglandins, low K+ levels, Sympathetic stimulation, ↓ Na+ delivery at distal tubule, any factor contributing to reduced renal blood flow (eg hyovolaemia, cardiac failure, renal artery stenosis) and factors reducing angiotensin levels (eg hypervolaemia, afferent arteriolar dilatation and vasopressin).
Syllabus – N1 (h), C2b (f)
Reference: Textbook of medical Physiology, Guyton, Chp 26, Goodman and Gillman, Chp 30
19. Define the following terms (40%)
a. Saturated Vapour Pressure of Water
b. Absolute Humidity
c. Relative Humidity
d. Latent heat of vaporisation
Briefly outline how the humidity of air is altered during inspiration and expiration by the Firatory tract (60%)
CICMWrecks Answer
Humidity
Humidity
- Humidity is the concentration of water vapour present in the air.
Saturated Vapour Pressure of Water
- The water vapour pressure when the air is fully saturated.
- Depends on both pressure and Temperature
- = 47mmHg at STP
Absolute Humidity
- The amount of water vapour present in a given volume of gas (units g H2O/m3 or mgs H2O /L)
- Room air at sea level has Absolute humidity of 10g H2O/m3
- < 100% saturation
- Absolute humidity is temperature independent
- 100% saturation
- Absolute humidity is temperature dependent – due to ΔSVP fully saturated air
- at 0 °C contains 4.8 mg/L;
- at 20 °C contains 17 mg/L;
- at 37 °C contains 44 mg/L
- Absolute humidity is temperature dependent – due to ΔSVP fully saturated air
Relative Humidity
- the amount of water vapour present in the gas expressed as a percentage of the amount of water vapour that would be present if the gas were saturated with water vapour.
Latent Heat of Vaporisation
- the heat required to convert 1g of a substance from the liquid phase to the gaseous phase at a given temperature (expressed in Jg-1)
Humidification Process
INSPIRED AIR (During nose breathing)
- Air is warmed by the radiant heat from nasal blood supply.
- ↑ing temperature → ↑ SVP → ↑’s water carrying capacity
- Moisture evaporates from the epithelia → ↑ relative humidity of the inspired air to ~90%
- Mouth breathing reduces the relative humidity of inspired air to 60-70%
- At the lungs, it reaches the isothermic saturation boundary where it achieves BTPS (body temperature and pressure, saturated with water vapour) conditions.
- This usually occurs at the second generation of bronchi.
- Absolute Humidity @ Carina = 44 g H2O/m3
- Relative Humidity @ Carina = 100%
EXPIRED AIR
- Expired gas transfers heat back to the cooler trachea and nasal mucosa.
- As the saturated gas cools, it can hold less water vapour (its saturated water vapour pressure falls)
- Condensation occurs on the mucosal surfaces, where the liquid water is reabsorbed.
- Reabsorption reduces potential airway water losses from 300ml/day to ~150ml/day
- Tracheal temperature and humidity fall with an increase in respiratory rate (ie, the isothermic saturation boundary moves more away from the upper airway)
Complications of non-humidified air:
- Mucosal dehydration
- Altered ciliary function
- Inspissation of secretions
- Atelectasis and V/Q mismatching (if underlying lung disease)
- ↑ heat loss (5-10%) as the inspired gases are warmed and more H2O needs to be added as vapour
Humidification Mechanisms in ICU:
- Passive
- HME (Heat-Moisture Exchanger)
- Active
- Bubble Humidification
- Passover
- Heats water in chamber
- Evaporated water entrained by fresh gas
- Nebulisation
- Pressure and heat vapourises water
Gladwin 2016
Examiner Comments
2009B 19: 1 (11%) of candidates passed this question
It is essential that candidates read and respond to the question asked of them. The first part of the question required simple, accurate definitions, which the majority of candidates were unable to provide. Marks were awarded for the definition only. Descriptions of measurement, potential effects on oxygenation, etc. were not asked and gained no marks.
The core of the second part required an outline of the exchange of heat and moisture through the upper airways and bronchial tree, culminating in fully saturated gas at core body temperature by level of the 2nd generation bronchi. Whilst the question asked ‘humidity’ and not temperature, correct definitions in the first part would have dictated a joint outline of both. The effects of surface area, the nasal turbinates, mucosal secretion and blood flow were all relevant. The contribution to insensible moisture and heat loss should have been mentioned. No candidate considered the effects of respiratory rate, mouth versus nose breathing, or dry medical gases versus room air.
Syllabus – B1k2d
Reference: Nunn pages15 and 26
20. Compare and contrast the pharmacology of heparin and enoxaparin.
Examiner Comments
2009B 20: 8 (89%) of candidates passed this question.
The vast majority of candidates chose to answer this question in tabular format and in doing so were easily able to consolidate a high scoring answer. This also allowed clear
identification of important differences, such as molecular weight, mechanism of action, halflife, dose-interval, monitoring, elimination, reversal of effect, the influence of renal
impairment and potential side-effects.
One exception to this approach would be clinical indications, where these show marked similarity. Candidates should consider writing similarities once whilst incorporating both drugs so as use the time allocated more efficiently.
Syllabus – J2 2a
Reference: Pharmacology and Physiology in Anaesthetic practice, Stoelting 505-511 Basic
and Clinical Pharmacology, Katzung 546-548
21. List the hormones secreted by the pituitary gland. (30% marks) Outline the physiological factors that control secretion of hormones from the posterior pituitary. (70% marks)
CICMWrecks Answer
Pituitary
- HPA describes complex feedback loops between these endocrine organs
- Shortloop feedback: -ve feedback from pituitary on the hypothalamus e.g. thyroxin inhibiting TSH release
- Long-loop feedback: -ve feedback from pituitary target gland (e.g. thyroid, adrenal, gonads) on the hypothalamus e.g. cortisol inhibiting CRH (as well as ACTH) release
- Pituitary hormones
- Anterior pituitary
- Secretes 6 hormones in response to hypothalamic endocrine stimulus
- Stimulating hormones:
- Act at another gland
- Includes: ACTH, TSH, FSH, LH
- Directly acting hormones
- Include: GH, prolactin
- Posterior pituitary
- ADH
- Oxytocin
- Anterior pituitary
Control of secretion of hormones from the posterior pituitary
ADH:
- Nonapeptide (9 a.a.) synthesized 1°ly in cell body of SON (some also in PVN) of the
hypothalamus → transported to posterior pituitary via infundibulum where it is stored - It is secreted in response to:
- ↑ plasma osmolality (Major determinant)
- Detected by osmoreceptors (in anterior hypothalamus near SON/PVN)
- Very sensitive (detects 1% change in osmolality) → threshold for ADH
release is 280 mosm/kg (slightly less than normal plasma osmolality) →
steep linear rise > 290 mosm/kg
- Non-osmotic stimuli:
- Haemodynamic changes
- ↓ PV → ↓ MAP that is sensed by baroreceptors (mainly lowpressure BR in atrium) → cause ↑ ADH release
- ↓ sensitivity cf. osmotic stimuli (detects 5-10% change in PV)
- BUT very potent → overrides osmoreceptors (in terms of control
of ADH secretion) when there are LARGE changes in PV!!!
- ↑ AII
- Pain
- Nausea/vomiting (powerful stimuli)
- Exercise
- Drugs (↑ release – morphine, nicotine, barbiturate; ↓ release – EtOH)
- Haemodynamic changes
- ↑ plasma osmolality (Major determinant)
- Effects:
- V1 receptor (GPCR via Gq → activates PLC to ↑ IP3 → ↑ IC [Ca2+] → smooth muscle contraction) → this causes:
- Contraction of vascular SM cells (potent vasoconstrictor effect) → ↑ TPR and MAP
- Renal afferent arteriolar constriction and contraction of renal mesangial cells → ↓ GFR/RBF
- Platelet aggregation and degranulation
- V2 receptor (GPCR via Gs → activates AC to ↑ cAMP → activates PKA) → this causes:
- Upregulates insertion of apical membrane AQP2 (stored in vesicles) in principal cells of CCD and MCD → ↑ H2O permeability → ↑ H2O reabsorption into hypertonic medullary interstitium (across BLM AQP3 and 4) → causes ↓ plasma osmolality (and ↑ urine concentration)
- Upregulates “urea transporters” in principal cells of inner MCD → ↑ permeability to urea → ↑ urea absorption to maintain ↑ medullary osmolality (strengthens CCM)
- ↑ Na+ reabsorption and K+
secretion by principal cells of CCD - ↑ CF VIII release by vascular endothelium
- Other effects:
- CNS → promotes memory, learning, attention and concentration
- ACTH release form anterior pituitary gland
- V1 receptor (GPCR via Gq → activates PLC to ↑ IP3 → ↑ IC [Ca2+] → smooth muscle contraction) → this causes:
- ADH’s effect is very short-lived → short t ½ ~ 20 mins (rapidly inactivated by tissue peptidases → excreted by liver and kidney)
Oxytocin
- Nonapeptide (9 a.a.) synthesised 1°ly in cell body of PVN (some also in SON) of the hypothalamus → transported via infundibulum to be stored in posterior pituitary gland
- Control of secretion – (i) ↑ release in response to → cholinergic stimulation, (ii) ↓ release in response to → β-adrenergic activity, EtOH, enkephalins
- Effects:
- Ejection of milk – Somatic touch stimulation of nipple (Ie. suckling) stimulates “let-down reflex” → cause oxytocin release to induce contraction of myoepithelium of lactating mammary glands → milk secretion
- Myometrial contraction of pregnant uterus → during late pregnancy, a neuroendocrine reflex loop causes ↑ both oxytocin secretion and oxytocin receptor population → role in inducing labour/delivery
- Uterine secretion/contractions during coitus → facilitates propulsion of semen to fallopian tubes
- Various behavioural effects
Bianca / Kerr 2016
Full Pituitary Hormone Table
| Location | Hormone | Action | Stimulated by: | Inhibited by: |
|---|---|---|---|---|
| Anterior pituitary | ACTH | Short chain peptide Stimulates cortisol release from zona fasiculata | CRH | Cortisol |
| TSH | Glycoprotein Stimulates synthesis + release of T3 + T4 | TRH | T3 | |
| FSH | Glycoprotein gonadotropin Females: stimulates oestrogen synthesis + ovarian follicle development Males: stimulates sperm maturation | GnRH | Sex steroids | |
| LH | Glycoprotein gonadotropin Females: rapid ↑ stimulates ovulation + corpus luteum development Males: stimulates testosterone synthesis | |||
| GH | Long chain peptide released in pulsatile fashion Anabolic effects: directly stimulates lipolysis → ↑FFA Indirectly stimulates IGF-1 release → promoting cell growth + development | GHRH High with exercise, hypoglycaemia, stress | Somatostatin IGF-1 | |
| Prolactin | Long chain peptide breast development during gestation + lactation post delivery | |||
| Posterior pituitary | ADH | Short chain peptide Acts on: – V1 R in vascular smooth muscle → vasoconstriction – V2 R in CD (↑water reabsorption) + endothelium (↑vWF FVIII release) – V3 R in pituitary → stimulate ACTH release | Hypothalamic neural stimulus | |
| Oxytocin | Short chain peptide; structurally similar to ADH Causes: uterine contraction, let down reflex, psychological, bonding |
Kerr 2016
Examiner Comments
2009B 21: 4 (44%) of candidates passed this question.
Once again candidates are reminded to read and answer the question presented to them as well as take into consideration the proportion of marks allocated. The expectation from the first section of the question was for candidates to only “list” the hormones secreted by the pituitary gland. The main hormones expected were ACTH, TSH, GH, FSH, LH and prolactin anteriorly; and, ADH (vasopressin) and oxytocin posteriorly.
Unfortunately, a number of candidates confused which hormones come from which region and therefore were unable to score marks in the second part. The question clearly asked for factors controlling secretion of hormones from the posterior pituitary, therefore, detailed descriptions of the mechanism of action of ADH in the kidney, or of hormones arising from the anterior pituitary did not relate to the question and were not allocated marks.
With respect to ADH, most marks were gained by candidates who described not only osmolality and plasma volume, but the relative sensitivity of the pituitary to these and the pre-potent nature of volume over osmolality; also, the influence of other hormonal axes involved in plasma volume regulation. Marks were also available for other inputs including pain, stress, exercise, etc. A brief description of factors influencing oxytocin was also expected.
Syllabus – N12d
Reference: Gannong p383, Guyton p359
22. Describe how the kidney handles sodium. (50 marks) What factors influence urinary sodium excretion (50 marks)
CICMWrecks Answer
NORMAL SODIUM
- Total Sodium (Na) = 4000 mmol (60 mmol/kg)
- Distributed:
- Bone (45%)
- ECF (50%)
- 1°ly found in ECF (major EC cation)
- ICF (5%)
- 10-15 mmol/L maintained by
- Na+/K+ ATPase
- low gNa → prevents influx of Na
- Role of Na
- Main determinant of ECF osmolality and tonicityNa/Cl ~ 90% ECF osmotic solute load
- Main determinant of ECFV
- Depolarisation in action potential 2° to ↑Na conductance
- Co-transport of substances across membranes (Eg. Glucose)
- Involved in Na+/K+ ATPase in cell membranes
- Total output =Total input
- 1-1.4 mmol/kg/day
- ~100-300 mmol/day in 70 kg adult
- 10.5 g/day
- Sodium Control – Lost via:
- Kidneys (main) ~ lose 150 mmol/day
- Filters 25000 mmol Na+/day
- 99.5% reabsorbed
- 65% PCT, 25% TAL of LoH, 5% EDCT, 4-5% LDCT and CD
- Sweat and GIT loss in faeces ~ lose 10 mmol/day (0.25 g/day each)
- Kidneys (main) ~ lose 150 mmol/day
Sodium Reabsorption in Kidney
| Location | Contribution | Mechanism |
|---|---|---|
| PCT | 65% | Secondary active transport: – Luminal Na/organic cotransporters with glucose and AAs – Luminal Na/K (NHE-3) exchanger with H from Henderson-Hasselbach intracellularly Passive transcellular – Via solvent drag passively – Down electrical gradient from positive lumenal charge |
| TAL of LoH | 25% | Secondary Active transport: – NKCCT on luminal surface – Dominant mechanism Small amount continues via Secondary active transport as per PCT Paracellular movement driven by net positive charge in lumen |
| Early DCT | 6-10% | 2° active means – Apical Na/K ATPase generates Na gradient – Basal Na/Cl symporter – No alteration in the luminal charge as electrically neutral |
| Late DCT and CD | 5-10% | – Facilitated diffusion across principle cells – Basolateral Na/K ATPase → intracellular Na deficit – Na reabsorbed from lumen via ENaC channels in principle cells up regulated by Aldosterone |
Overview of renal Na+ regulation:
- Thus, renal Na+ regulation depends on:
- Degree of glomerular filtration of Na+ → GFR (minor)
- Changes in GFR due to hyper or hypovolaemia will (indirectly) adjust sodium elimination. Increased plasma volume increases GFR, and vice versa.
- Degree of tubular reabsorption of Na+ (major)
This is the main mechanism for controlling sodium in euvolaemia.
In terms of long term Na excretion; Na reabsorbed is more impt than GFR because:- (i) GFR is heavily autoregulated
- (ii) Glomerulotubular balance blunts any major changes in Na+ excretion that would have resulted from minor changes in GFR changes that actually occurs
- Na reabsorption through GIT/sweat/salivary glands:
- Varies with diet and exercise
- Mainly action of Aldosterone via Na/K ATPase
- Degree of glomerular filtration of Na+ → GFR (minor)
- Normal values
- Na+ filtration: 140mmol/L x 180L/day = 25000mmol Na/day
- Na+ excretion: 140mmol excreted
- rest reabsorbed
- Fractional excretion = 0.5%
Na+ regulation: Control of GFR
- Intrinsic autoregulatory factors (tubuloglomerular feedback and myogenic mechanism)
- MAP has minor effect on GFR over MAP range 70-175 mmHg → BUT changes
in BP that invoke baroreceptor reflexes (BRR) can override these autoregulatory
mechanisms → alter GFR and amount of Na+ filtered
- MAP has minor effect on GFR over MAP range 70-175 mmHg → BUT changes
- Extrinsic factors: Body Na+ content (via ECFV)
- Direct renal effects – ↓ [Na+] (or ↓ ECFV) → results in ↓ GFR due to a ↓ glomerular capillary P(HYDROSTATIC) and ↑ glomerular capillary P(ONCOTIC) → ↓ GFR and Na+ filtered
- Indirect renal effects – ↓ [Na+] (or ↓ ECFV) → stimulates arterial, venous and cardiac BRR → neurohormonal response → to ↓ GFR and Na+ filtered via:
- (i) ↑ SNS and RAAS activity → cause afferent and efferent arteriolar constriction and mesangial cell contraction
- (ii) ↑ ADH → cause afferent arteriolar constriction and mesangial cell contraction
- (iii) ↓ ANP → inhibit afferent arteriolar dilation and mesangial cell relaxation
Na+ Regulation: Control of Reabsorption
- Glomerulotubular balance:
- Intrinsic autoregulatory mechanism that minimises the effect of changes in GFR on Na+ and H2O excretion
- It functions on the basis that the PCT reabsorbs a constant proportion of glomerular filtrate (65% of filtered Na+ /H2O), rather than a constant amount
- In effect – ↑ GFR = ↑ filtration of Na+/H2O = ↑ Na+/H2O reabsorption
- Mechanism:
- With ↑ GFR → large amount of plasma is filtered at the glomerulus → leads to ↑ π(ONCOTIC) of plasma in peritubular capillaries
- This results in an ↑ gradient that –
- (i) Favours tubular reabsorption, and
- (ii) Counteracts the effect of ↑ GFR on fluid leaving the PCT
- Renal interstitial hydrostatic pressure (Intrarenal physical factors)
- ↓ ECFV (and ↓ Na+) results in ↓ MAP → leads to (i) ↓ PHYDROSTATIC and (ii) ↑ πONCOTIC of peritubular capillaries → thus, ↑ Na+ (and ↑ H2O) reabsorption from tubular interstitium into peritubular capillaries
- Hormonal Influences:
- Renin
- Released by ↓ Na delevery to macula densa or β1 stimulation secondary to volume underload
- Tubular effects:
- increased PCT Na/Cl reabsorption, increased tubular K secretion
- Direct PCT effect
- Aldosterone release
- Aldosterone
- Most important regulator of Na+ reabsorption
- Alters protein translation (inducing production of tubular basolateral Na+/K+ATPase and luminal ENaC and K+channels) → causes ↑ Na+ reabsorption by DCT and Principal cells of CCD
- Increased Na reabsorption throughout the GIT/sweat and salivary glands via Na/K ATPase
- Increased H2O reabsorption and increased Na via solvent drag
- Angiotensin II
- Negative feedback on renin release
- Increased aldosterone release
- Decreased RBF and GFR
- Direct renal arteriole constriction (efferent = afferent)
- Mesangial cell contraction thus decreased Kf and GFR
- Direct stimulation of Na+ reabsorption at PCT, and
- Indirect stimulation of Na+ reabsorption via SNS, AII, and aldosterone
- SNS
- Direct stimulation of Na+ reabsorption at the PCT (α1 and β1 receptors), and
- Indirect stimulation of Na+ reabsorption via RAAS
- ADH
- → ↑ Na+ reabsorption at the CCD (principal cells) → acts synergistically with aldosterone here
- ANP
- Inhibition of Na+ reabsorption (blockage of ENaC) in the CDs
- ↓ RAAS and ↓ ADH activity
- Renin
- Other causes ↑Na reabsorb:
- Cortisol
- Oestrogen
- GH
- Thyroid hormone
- Insulin
- Dopamine
- Other cause ↓Na reabsorb:
- PGE2 inhibits NaK ATPase to reduce Na reabsorption
- Glucagon
- Progesterone
- PTH
- Renal vasoDilators:
- PGs
- Kinins
- Pressure natriuresis & diuretics
- renal compensatory mechanism that maintains long-term regulation of arterial BP by controlling the kidney’s excretory ability of Na+and H2O
- Pharmacological agents:
- Ouabain (a cardiac glycoside) inhibits NaK ATPase decreasing excretion
- Loop Diuretics → ↑ Na loss
Gladwin / Bianca / JC 2019
Examiner Comments
2009B 22: 2 (22%) of candidates passed this question.
Candidates were essentially expected to describe the fate of sodium as t passes through the kidney from filtration at the glomerulus to ending up in the urine. Essentially, most Na+ is reabsorbed at the proximal tubule (65%), reabsorption being down an electrochemical gradient (inside cell negative and low Na+ concentration) which is maintained by active Na+/K+ ATPase activity at the basolateral membrane. Then Loop of Henle reabsorbs a further 15% of filtered Na+. At descending limb – no Na+ reabsorption. At ascending limb, thick segment – active process as per proximal tubule, but mostly coupled to K+ and Cl- and paracellular diffusion through tight junctions. Finally the distal convoluted tubule and collecting ducts, a further almost 20% reabsorbed – leaving < 1%
Factors influencing loss are
- aldosterone = stimulates Na+ reabsorption at the collecting tubules
- intra renal factors such as interstitial pressure which is lowered during hypovolaemia and reduced renal perfusion, thus promoting Na+ reabsorption gradient
- sympathetic nervous system – influences interstitial pressure and increases renin production
- angiotensin II – stimulates reabsorption at proximal tubule
- Atrial Naturetic Peptide/Factor – inhibits Na+ reabsorption
- Others – dopamine, cortisol, insulin => increase Na+ reabsorption, but minor factors
Syllabus – D1, 2f
Reference – Textbook of medical Physiology, Guyton, Chp 28
23. Outline the principal functions of the Liver, and give examples.
CICMWrecks Answer: Liver Functions
Liver:
- Largest abdominal solid organ
- 25 % CO at rest. approx 1,500 mls/min
- 15% of total blood volume at rest
Functions of Liver
| Filtration | |
| Immune defence | (via Kuppfer cells) against agents entering the portal circulation |
| 80% of circulating cholesterol | → bile salt |
| Biliary excretion of drugs/hormones | penicillins, amp, erythro |
| thyroxine, cortisol, estrogen | |
| calcium | |
| Immune | |
| Filtration of portal circulation | |
| Kuppfer cells | bacteria/ virus/ endotoxins/ immune complexes/ thrombin/ tumour |
| Phagocytosed, fused with lysozomes and degraded by lysosomal enzymes | |
| Antigen presentation | |
| Endotoxin neutralisation = pinocytosed | |
| Complement/CRP production | |
| Storage of metabolic substrate/fluids | |
| glycogen ~ 400g | |
| fat | |
| Fe++, B12, folate, Cu | |
| Vitamin A | |
| Blood Reservoir | |
| Metabolic | |
| CHO and intermediary metabolism | Hepatic Glucostat |
| gluconeogenesis, glycogen storage and utilisation, galactose/fructose to glucose | |
| Conversion to fat, AA’s and ketones | |
| Protein metabolism | amino acids utilisation |
| protein synthesis | |
| production of ketones | |
| deamination of fatty acids | |
| urea formation for ammonia removal | |
| plasma protein formation | |
| Fat homeostasis | metabolism (beta oxidation (rapid in hepatic cells)), synthesis and transport as lipoprotein |
| cholesterol homeostasis | |
| Endocrine | hormone synthesis & metabolism |
| Synthesis of 25 OH cholecalciferol, Metabolism of steroid hormones, Synthesis of somatomedins, Erythropoietin | |
| biotransformation | ammonia & urea cycle |
| drugs & toxins | |
| Acid-Base | Lactate metabolism |
| Synthetic functions | |
| Bile production | bile salts (incr fat absorption) |
| bilirubin (incr haem excretion) | |
| Protein synthesis | albumin 120-300mg/kg/d |
| alpha1/2 & beta globulins (transport) | |
| coagulation & fibrinolytic factors (fibrinogen, prothrombin, II, V, VII,VII, IX, X, XI, XII, XIII, antithrombin) | |
| Lymph synthesis | Up to 50% |
| Erythropoietin (10%) | |
Gladwin / JC / Bianca 2019
CICMWrecks Answer: Paracetamol Toxicity
Paracetamol Overdose
Hepatotoxicity
Toxic daily dose > 4-6 g (while lethal dose occurs at > 10-15 g (or > 300 mg/kg LBM)) → but the toxic and lethal doses are lower in “at-risk” groups:
- EtOH abuse (due to CYP450 induction (↑ toxic metabolite produced) and ↓ glutathione stores)
- Malnutrition (due to ↓ glutathione stores)
- Elderly (due to ↓ glutathione stores)
- Preexisting liver dysfunction
Mechanism:
- At therapeutic doses – “N-acetyl-p-amino-benzoquinoneimine” (highly toxic metabolite) is produced in small amounts, but is rapidly conjugated with hepatic glutathione (anti-oxidant) into a harmless metabolite
- BUT with toxic doses – Hepatic conjugation pathway is saturated and ↑↑↑ N-acetyl-p-amino-benzoquinoneimine is produced → this depletes hepatic glutathione stores, causing remaining N-acetyl-p-aminobenzoquinoneimine to then forms covalent bonds with sulphydryl groups on hepatocytes → results in centrilobular hepatic necrosis
Clinical features:
- Generally conscious and c/o N/V, epigastric pain, erythema, sweating → later developing:
- Acute haemolytic anaemia
- Develop hepatic failure (Ie. jaundice and cholestasis) after 48 hrs
- LFT/INR derangements at 3-5 days
- Fulminant hepatic failure at 3-7 days
- With severe OD, can present with hypotension/shock
Diagnosis :
Serum paracetamol levels → correlate with “nomogram” to predict likelihood of liver damage → used as a guide to dictate therapy
Treatment:
- Activated charcoal/gastric lavage→ limit paracetamol absorption
- Replace hepatic glutathione store within 12 hrs of OD → permits glucuronidation of toxic metabolite
- Oral methionine → ↑ glutathione synthesis
- IV N-acetylcysteine → hydrolysed to cysteine (which is a glutathione precursor)
- Nb. IV NAC is preferred b/c of N/V a/w toxicity (Ie. ↓ oral methionine absorption)
- IV glucose → due to risk of ↓ BGL with liver dysfunction
- Serial monitoring of LFTs and coagulation studies
- Referral to a specialist centre
Gladwin / JC / Bianca 2019
Examiner Comments
2009B 23: 4 (44%) of candidates passed this question.
For a good answer candidates were expected to at least mention the following —
Formation and secretion of bile
Carbohydrate metabolism (Glycogen synthesis and breakdown, Gluconeogensis)
Lipid metabolism (Fatty acid oxidation, Synthesis of cholesterol and phospholipids, Production of ketoacids)
Protein metabolism Breakdown
Metabolism of toxins, drugs (Phase I reactions – oxidation, reduction, hydrolysis, Phase II reactions- conjugation/glucuronidation)
Storage (Vitamins B12, A, D 2, Iron as ferritin, Glycogen)
Immunity
Endocrine (Synthesis of 25 OH cholecalciferol, Metabolism of steroid hormones, Synthesis of somatomedins, Erythropoietin)
Miscellaneous (Acid base role – lactate metabolism, Blood store)
Syllabus – I2a
Reference: Gannong p485, Power and Kam p185
24. Describe how gas exchange is influenced across the placenta near the term of pregnancy.
CICMWrecks Answer
Diffusion via Fick’s Law
- Flow of gas (Rate of movement of solute) across semi-permiable membrane J is
where
C = concentration (or partial pressure for gasses)
A = cross-sectional area
T = thickness of the membrane or distance over which diffusion takes place.
- These factors alter the rate of transfer as per the equation above.
- Valid for Most medium – small substances
- Water soluble (H2O, electrolytes, glucose, urea) via intercelular clefts
- Lipid soluble substances (O2, CO2) via endothelial cells themselves.
- Flow = 1.2ml of O2 mmHg-1minute-1 across entire placenta
- Surface area of placenta for diffusion = 16m2
- Thickness of placental membrane falls as pregnancy progresses
- Minimum thickness is 3.5 micrometres
Oxygen
- Mean partial pressure of oxygen (PO2) in maternal blood is considerably higher than in fetal blood
- As a consequence, oxygen readily diffuses across the placenta from maternal to fetal blood
- Despite its low PO2, fetal blood is able to transport essentially the same quantity of oxygen to tissues as maternal blood
| O2 Partial Pressure | Oxygen Content | |
|---|---|---|
| Maternal | PaO2 = 100mmHg (80-110) | CaO2 = 16.1ml/100ml |
| Fetal Umbilical Vein | PvO2 = 30mmHg (30-50) | CvO2 = 16ml/100ml |
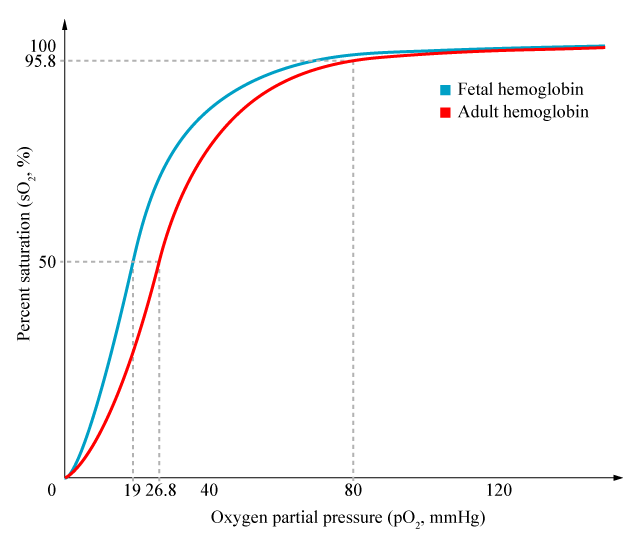
- Foetal Hb has a higher affinity for O2 than maternal Hb
- oxygen carrying capacity is improved, despite lower PaO2
- P50 is 20mmHg vs 26 in maternal
- Haemoglobin concentration in blood is higher in the foetus, increasing oxygen carriage
- 120g/L in mother
- 170g/L in foetus
- Double Bohr effect:
- Movement of CO2 from the foetal to the maternal circulation results in a simultaneous rise in maternal CO2 and fall in foetal CO2
- Rise in foetal Hb oxygen affinity, fall in maternal Hb oxygen affinity by the Bohr effect
Carbon dioxide
- Carbon dioxide is produced abundantly in the fetus
- PCO2 of fetal blood is higher than maternal blood
- Carbon dioxide therefore diffuses from fetal blood, through the placenta, into the maternal circulation, and is disposed of by expiration from the mother’s lungs.
| O2 Partial Pressure | |
|---|---|
| Maternal | PaCO2 = 32mmHg |
| Fetal Umbilical Artery | PvCO2 = 55mmHg |
- Hyperventilation in pregnancy (increased sensitivity of chemoreceptors to CO2) → Lower PaCO2
- Smaller gradient than oxygen, but oxygen has 20 times higher solubility
- Double Haldane effect:
- Simultaneous rise in foetal Oxy-Hb and fall of maternal Oxy-Hb
- Increased CO2 carrying capacity of maternal Hb, decreased in foetal Hb
Mooney / JC 2020
Examiner Comments
2009B 24: (11%) of candidates passed this question.
For a good answer candidates were expected to account how the placenta allows for effective diffusion to occur. Good answers incorporated the parameters within the Fick equation (eg. area of placental gas exchange, pressure of gas in maternal sinuses (PO2 = 50 mmHg), pressure of gas in umbilical vein (PO2 = 30 mmHg ). Also expected was an explanation of the role of foetal haemoglobin, the double Bohr and double Haldane effects. These were commonly omitted.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Curve – Pulmonary compliance, Surfactant, Ventilator waveforms |
| G. CVS | Diuretics, Vasoactive agents Cardiovascular physiology, draw ECG tracing + Statistics |
| H. Renal | Nephron – physiology, blood supply Urinalysis, Ketone, Urobiilinogen, Specific gravity |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | Cerebral blood flow, measurement, regulation, Opioids and their CNS effects |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | temperature control and measurement |
| S. Immunology | Allergic reactions, tratment, common agents in ICU |
| T. Microbiology | |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments