1. Relate the surface ECG to the events of the cardiac cycle (60% of mark). Describe how the PR, 20QRS and QT intervals may be prolonged by the action of drugs.
CICMWrecks Answer
Surface ECG to events of Cardiac Cycle
ECG:
- 12 metal electrodes on chest wall
- Detect small (0.5-2mV) changes in voltage produced by the heart
- Transfer these to an oscilloscope output
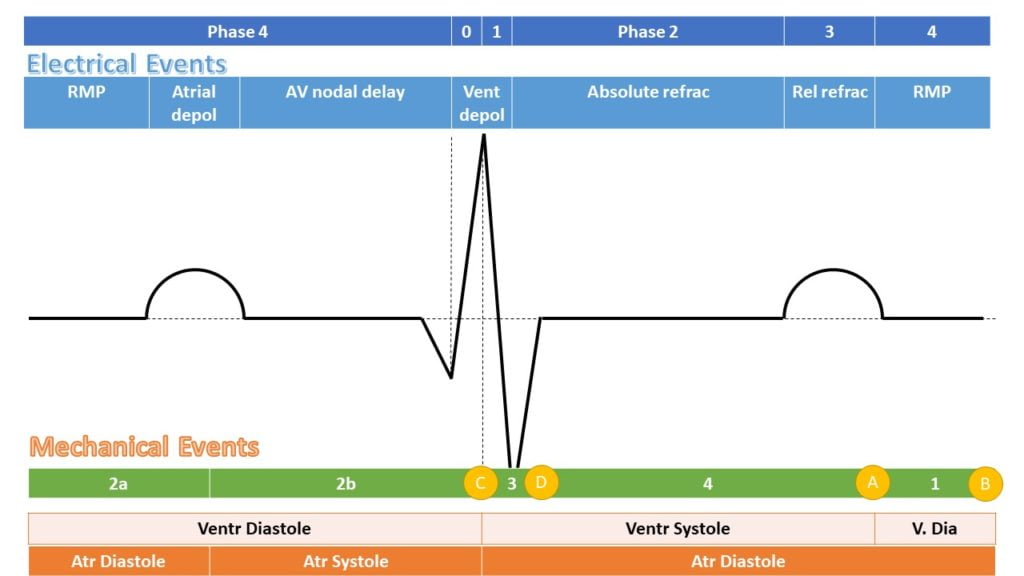
- P wave: → atrial depolarisation → atrial systole → AV valve opening
- PR interval: conduction from SA node → atrial conducting pathways → AV node (slowest) → bundle of His → LBB and RBB → Purkinje fibres
- QRS: ventricular depolarisation (endocardium → endocardium, L > R) → ventricular systole → closure of AV valves, opening of aortic and pulmonary valves
- Atrial repolarisation is low voltage, and lost within the QRS complex
- QT interval: sustained ventricular depolarisation and muscular contraction
- T wave: ventricular repolarisation (epicardium -> endocardium, L > R) -> ventricular
relaxation -> closure of aortic and pulmonary valves
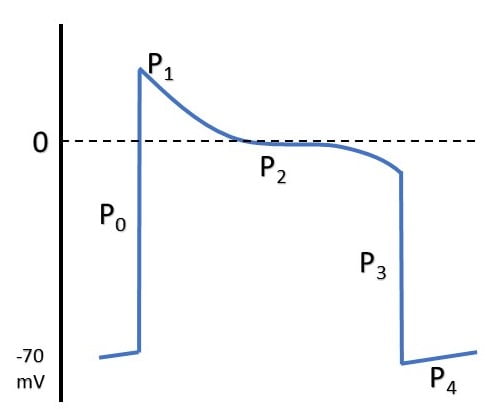
ELECTRICAL EVENTS
| P0 | Fast Na opening |
| P1 | Transient K efflux |
| P2 | Influx Na & Ca |
| P3 | Efflux K > Influx Na & Ca |
| P4 | Re-establishment of RMP |
MECHANICAL EFFECTS
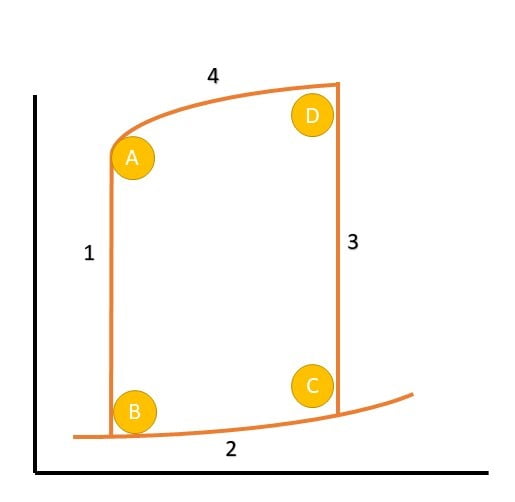
| A | Aortic Valve Closure |
| 1 | Isovolumetric relaxation |
| B | Mitral Valve Opening |
| 2a | Early Diastolic filling |
| 2b | Late Diastolic filling |
| C | Mitral Valve Closure |
| 3 | Isovolumetric Contraction |
| D | Aortic Valve Opening |
| 4 | Ventricular Ejection |
Action of Drugs
| Drug | Interval effects | Mechanism |
|---|---|---|
| VW (Vaughan-Williams) Class 1a | ↑QRS, ↑QT | Fast Na-channel blockade |
| VW Class 1b | ↓QT | |
| VW Class 1c | ↑↑QRS, ↑QT | |
| VW Class 2 | ↑PR | β blockade → negative chronotropy |
| VW Class 3 | ↑QT | K channel blockade → prolongation of RRP |
| VW Class 4 | Can ↑PR | Prolongation of ERP and RRP in pacemaker cells |
| Adenosine | ↑PR | Hyperpolarisation of myocardium via opening of K channels |
| Digoxin | ↑PR, ↓QT | Multiple effects, vagotonic at AVN |
| Amiodarone | ↑PR, ↑QRS, ↑QT, ↑RR (sinus rate) | Multiple |
| Mg | ↓QTc | Membrane stabilising. |
| TCAs | ↑QRS | Quinidine like Na-channel effect (class 1a) |
| SSRIs | ↑QT | Direct potassium blockade and downregulation of potassium → ↑ERP and ↑RRP. |
Digoxin
- ↑ RR interval (sinus rate), ↑PR interval, ↓RR interval
- Slowed AV conduction by increased vagal tone
- ACh → M2 receptors
- ↑KACh (ACh controlled K+ channels) → increased efflux of K+ → hyperpolarised
membrane - ↓If (funny current) → decreased influx of Na+ → decreased slope of phase 4
- ↓ICa(L) (long lasting) and iCa(T) (transient) → decreased efflux of calcium on partial
depolarisation → decreased slope of phase 4
Amiodarone
- ↑PR, ↑QRS, ↑QT, ↑RR (sinus rate)
- Main effect is K+ channel blockade
- Repolarisation is slowed → prolonged QT
- Some INa
- Slower phase 0 upstroke
- Slowed ventricular muscle conduction → prolonged QRS
- Some ICa
- Slower spontaneous depolarisation in SA node → decreased sinus rate
- Slowed conduction in AV node → prolonged PR
- Weakly downregulates adrenoreceptors
- Increased PR, decreased sinus rate
Mooney / Gladwin 2016
Examiner Comments
2009A 01: Pass rate: 50%
The first part of the question is best answered by a labelled and annotated diagram of the ECG with the pressure events of the cardiac cycle. Common errors included mistiming of the ECG with the pressure waveform. The second part of the question could be answered in a tabular format such as:
| Interval | Drug | Mechanism |
|---|---|---|
| PR | Digoxin | Increases refractory period of AV node probably by increased vagal activity |
| QRS | Tricyclic antidepressants | Quinidine like effect, decreasing sodium influx into cells |
| QT | SSRI’s | Malfunction of calcium ion channels |
Syllabus Cib2c, C2c2b
References: Power and Kam 2nd edition p129 – 131, Stoelting and Hillier 4th edition p403, 409, 415
2. Describe the production of carbon dioxide in the body (60% of marks). What are the physiological reasons why the PaCO2 may be high? (40% of marks)
CICMWrecks Answer
PaCO₂ is determined by the balance of production and elimination.
CO2 production:
- Normally 200ml/min (compared to average oxygen intake of 250ml/min)
- Byproduct of aerobic metabolism
- Produced in the cell mitochondria via TCA
- Entry to TCA as acetyl CoA (from CHO, FA, aa)
- TCA products
- CO2
- Reduced compounds FADH and NADH
- H+ ions
- 2 molecules of CO₂produced for each 1 molecule of ATP
- Amount of CO2 produce per molecule of substrate varies with the substrate.
- 1.5 Mole CO₂ per mole of Glucose
- Respiratory quotient
- R = CO2 production / O2 consumption
- Depends on fuel substrate
- 0.7 for fat, 1.0 for CHO
- 0.8 for typical western diet
CO2 Storage and transport
- ~ 120 litres predominantly as bicarbonate ion
- CO2 is carried in the blood in 3 forms
- dissolved (5%)
- bicarbonate (70-80%)
- carbamino compounds (20-25%).
Elimination:
- Small amount dissolved in sweat
- Most via lungs
- PaCO₂ ~ PACO₂
- PACO₂ determined by alveolar ventilation equation
Factors which ↑PaCO₂
- ↑Shunts or ↑V/Q scatter → ↑PaCO₂
- ↑ Alveolar CO2 → ↑PaCO2
- ↑ Production (VCO2)
- During vigorous exercise
- Moderate exercise doesn’t effect pCO2 and may actually result in ↓pCO2 due to ↑MV
- Hypermetabolic states (Sepsis, Fever, MH, neurolept malignant syndrome)
- Altered (ie pure carb) diet
- ↑ Barometric pressure/↓Pressure of water
- ↑ Inspired CO₂
- Determined predominantly via alveolar ventilation which depends on:
- Dead space (DS):
- PE, low CO state, ↑d PEEP → ↑West’s zone
- ↑DS → ↓Alveolar Vent → ↑Alveolar CO2 → ↑PaCO2
- Respiratory Rate: ↓RR → ↓Alveolar Vent → ↑Alveolar CO2 → ↑PaCO2
- Tidal Volume (Tv): ↓Tv → ↓Alveolar Vent → ↑Alveolar CO2 → ↑PaCO2
- Dead space (DS):
- ↑ Production (VCO2)
Gladwin 2016
Examiner Comments
2009A 02: Pass rate: 10%
The main points for a pass include a brief description of the citric acid cycle and a list of facts such as storage of CO2 (120L), production (200ml/min) and that 2 molecules of CO2 are produced for 1 molecule ATP. A statement that PaCO2 is proportional to CO2 production/alveolar ventilation would help answer the second part. An example of increased CO2 production is fever, and of decreased alveolar ventilation is increased anatomical dead space.
Syllabus B1h 2e
References: Power and Kam 2nd edition p78-79,101, Stoelting and Hillier 4th edition p790-791, Nunn 6th edition p148-156
3. Outline the factors which affect the onset, duration of action and toxicity of local anaesthetic agents.
CICMWrecks Answer
Classification of Local Anaesthetics
- Esters: Procaine, Cocaine
- Unstable in solution
- Rapid hydrolysis by plasma cholinesterases
- ↑ freq of hypersensitivity reactions due to PABA byproduct
- Amides: Lignocaine, Prilocaine, Bupivacaine, Ropivacaine
- Stable in solution
- Slower hepatic metabolism
- Low rate of hypersensitivity reactions
Factors
| Factors affecting the… | Patient Factors | Drug Factors |
|---|---|---|
| Onset | 1. Tissue pH · infected (acidic tissue) → ↑ ionised portion → ↓ ΔC → slower onset 2. Nerve diameter · ↑ diameter → ↑ surface area → faster onset 3. Nerve firing rate · ↑ firing rate → faster onset (remember mechanism of reaching internal H-gate) 4. Pregnancy · ↑ Progesterone → ↑’d sensitivity to LA | 1. Ionisation factors · PKa · Weak bases (7.6-8.9) · Only ionised fraction transfers · pH < pKa → ↑ ionisation → ↓lipid solubility → ↓ rate of onset · Higher pKa → more effective once inside axon · Alkalinisation · addition of NaHCO3 → ↑ pH of preparation · ↑ pH → ↑ unionised fraction → ↑ rate of onset 2. Lipid solubility · ↑ lipid sol → ↑ potency → ↓dose → ↓’d Δ C · ↓ rate of onset 3. MW · ↓’d MW → ↑ rate of diffusion = ↑ rate of onset 4. Diffusion distance · ↓T → ↑ rate of onset 5. Area of diffusion · ↑ A (eg large nerve axon) → ↑ rate of onset |
| Duration of Action | 1. Site of administration · ↑’d regional blood flow → ↓’d duration 2. Metabolism · Esters metabolised faster than amides → ↓’d duration | 1. Protein binding · ↑ protein binding → longer duration 2. Intrinsic vasodilator/constrictor activity · Ropivicain has intrinsic vasoconstrictor activity · ↑ vasoconstrictor activity → ↑ duration 3. Lipid Solubility · ↑ lipid solubility → ↑ potency → ↓ dose → ↓ ΔC → ↓’d diffusion rate · This ↑’s sequestration into lipid rich compartments → ↑’d duration 4. Presence of Additives · Adrenalin → vasoconstriction → ↓’d systemic absorption → ↑’d ↑duration 5. Dose · ↑ dose → ↑ duration 6. Clearance · ↓ metabolism → ↑ duration · Amides slower metabolised that esters |
| Toxicity | Site of injection ↑tox with ◦ ↑local perfusion/Cardiac output ◦ low plasma cholinesterase activity (esters) ◦ hepatic or renal failure ◦ pregnancy | CC:CNS rato (higher is safer) – Lignocaine 7, ropivacaine 4, bupivacaine 3 ↑tox with ◦ ↑Dose ◦ Type (Amide>Ester) ◦ ↑Protein binding in tissues decreases systemic absorption, ↑protein binding in CNS = ↑toxicity ◦ ↑Na channel affinity (Bupivocaine slower to dissociate than lignocaine) ◦ ↑Conc – higher conc gradient between tissue and plasma, increasing systemic absorption ◦ ↓vasoconst additives ◦ pKa – LA’s are weak bases with high pKa’s; lower pKa = more unionized = ↑systemic absorption. Lignocaine pKa 7.9 (25% unionized at pH 7.4), bupivacaine pKa 8.4, 11% unionized at 7.4 |
Note: CC/CNS ratio was required (the ratio of plasma levels at which CVS Collapse vs. Convulsions occur).
Gladwin 2016
Examiner Comments
2009A 03: Pass rate: 10%
Marks were equally divided between all three parts. Structure to the answer using a table and list of facts gained credit. Factors affecting onset would be well described by stating Ficks law of diffusion and followed with an explanation of the equation. Factors affecting duration such as protein binding, regional blood flow, metabolism and use of vasoconstrictors scored marks.
Regarding toxicity, an explanation of the CC/CNS ratio was required (the ratio of plasma levels at which CVSCollapse vs. Convulsions occur). Other factors included structure of agents, accumulation e.g. due to liver disease. A mention of features of particular agents’ toxicity such as prilocaine and methaemoglobinaemia was expected.
Syllabus G2b 2a-c
References: Peck, Hill and Williams 2nd edition p163-174
Stoelting and Hillier 4th edition p179-203
Evers and Maze p507-533
4. Explain how a normal, healthy adult regulates their body temperature (70% of marks). Explain how paracetamol exerts an antipyretic effect in a febrile patient (30% of marks).
CICMWrecks Answer: Temperature
Definitions
- Temperature – average kinetic energy of the atoms and molecules that make up a substance
- Temperature setpoint is the level at which the body attempts to maintain its temperature.
- Core body temperature:
- Refers to deep body temperature of main internal organs (in head, trunk, abdomen) →
sites where metabolic activity occur (Ie. heat production) - Kept constant at 37 +/- 0.4 °C (“Normothermia”) → displays normal variations:
- Diurnal variation – ↑ in evening (37.3 °C) and ↓ in early morning (35.8 °C)
- Menstrual variation – ↑ 0.5 °C in latter half of cycle
- Refers to deep body temperature of main internal organs (in head, trunk, abdomen) →
- Variations in core body temperature:
- Normothermia → core body temperature 37 +/- 0.4 °C
- Hypothermia → core body temperature < 36 °C
- Hyperthermia → core body temperature > 37.5 °C
- Peripheral body temperature:
- Refers to body temperature peripherally (Ie. skin, arms, legs, superficial tissues of core
sites) → sites where heat loss occur - Temperature varies widely → always LESS than core body temperature
- Refers to body temperature peripherally (Ie. skin, arms, legs, superficial tissues of core
- Regulation of body temperature is done by balancing heat loss and heat production, predominantly through behavioural mechanisms and skin
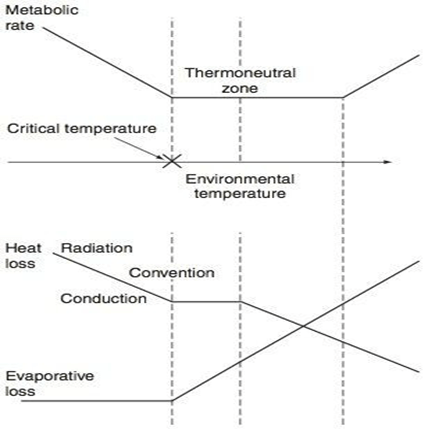
Thermoneutral Zone
- The range of environmental temperatures in which the metabolic heat production (and oxygen consumption) is minimal and steady and where core temperature is maintained by vasomotor activity alone.
- 25-30 °C for a naked, upright man in still air
Interthreshold Range
- The range of core temprature over which no autonomic thermoregulatory responses occur
- Normally 0.2 -0.4 °C in a non-anaesthetized state
Regulation of Body Temperature
Temperature sensors are central and peripheral, whilst regulation occurs centrally, and has multiple
effectors
| Cold | Warm | |
|---|---|---|
| Receptor | Bulbs of Krause | Bulbs of Ruffini |
| Afferent nerve type | via Aδ and unmyelinated C sensory fibres | Unmyelinated C fibres |
| Spinal synapse location | Rexed lamina 1,5 | Rexed lamina 1, 2 |
| Ascending tract | Lateral spinothalamic tract in anterior spinal cord | Lateral spinothalamic tract in anterior spinal cord |
| Brainstem synapse | Reticular system of medulla | Reticular system of medulla |
| Central Processor | Hypothalamus (posterior) | Hypothalamus (anterior) |
| Thresholds | No activity at 40 degrees ↑ing activity between 10-27 degrees (remember thermoneutral zone) | Activity with increasing between 30-45 °C Upper threshold limit = 46 °C |
Sensors:
- Central
- Deep tissues/viscera (Eg. in intestinal wall)
- Brain (anterior hypothalamus and extra-hypothalamic areas)
- Spinal cord
- Peripheral
- Dermis (main)
- Corneas
Central Controller:
Hypothalamus is the main body temperature regulatory centre
- Posterior hypothalamus responds to cold
- inputs from peripheral afferents
- responsible for the temperature set point
- ACh is the major neurotransmitter here.
- Anterior hypothalamus responds to heat
- both peripheral input and change in blood temperature
- Major neurotransmitters are Nad, 5HT, dopamine and prostaglandins.
Effector Mechanisms:
- Skin
- Sweating (evaporation)
- Secrete plasma-like fluid Na142, Cl- 104 (urea/K, etc v low amounts) osmolality controlled by rate of secretion and aldosterone
- loss via evaporation (latent heat of vapourisation of water)
- 1-2L in extreme exercise up to 10 L max per 25 hours.
- 0.54 kcal/gram of H2O evaporated
- Degree of evaporation determined by ambient temperature and relative humidity
- Blood Flow (radiation)
- 8 (300 mL/min) – 30% CO skin
- Vasodilation up to 30 fold increase
- Vasoconstriction up to 10 fold decrease
- vasoconstriction/dilation controlled by SNS (α1-mediated) vascular SM contraction and hypothalamic feedback
- Fat/Clothing
- Fat conducts heat 1/3 as readily as other tissues (decreased radiation)
- Clothes reduce conductive heat loss via private zone of air adjacent to skin and decreased convection air currents -50% and more if specialised
- Sweating (evaporation)
- Non-Skin
- Non-shivering thermogenesis
- Predominantly in brown fat
- Uncoupling of oxadative phosphorylation
- Increased heat gain without oxygen consumption/ATP production
- Shivering
- Increased heat gain by metabolism
- Behaviour
- For gain (exercise) or loss (submerging in water)
- Voluntary muscle contraction (Ie. ↑ activity with cold stress; ↓ with hot stress) – Affects heat production
- Body posturing (Ie. ↓ BSA with cold stress;↑ with hot stress) – Affects heat loss
- Clothing (Ie. ↑ clothing with cold stress; ↓ with hot stress) – Affects heat loss
- Appetite
- Cold stress stimulates food-induced thermogenesis → ↑ metabolic rate and heat production
- Thyroid hormone secretion
- Cold stress stimulates thyroid hormone secretion → long-term ↑ metabolic rate and heat production
- Non-shivering thermogenesis
Gladwin / JC 2019
CICMWrecks Answer: Paracetamol Antipyretic effect
Paracetamol antipyresis
- Class: It is an acetanilide derivative used as Analgesic/Antipyretic
- MOA is poorly understood
- Likely via prostaglandin synthesis inhibition, serotonergic and cannabinoid pathways.
- Inhibits COX isoenzymes (COX-1 and COX-2) in areas of low inflammation.
- Inflammation → IL1, IL6, TNF, prostaglandin formation → fever
- Antipyretic Effect
- CNS PG-E synthesis inhibition in anterior hypothalamus
Gladwin / JC 2019
Examiner Comments
2009A 04: Pass rate: 40%
Most candidates mentioned sweating, shivering, vascular response, and behavioural response to cold environment. Outlining the requirements of the temperature sensors, control processing area, and the effectors is, however, essential in order to pass this question. Most candidates did not mention where the temperature sensors are and the possible hormonal response to changes in the temperature of the environment. The interaction between interleukin-1 (and other pyrogens) and prostaglandin production in the hypothalamus was also not discussed.
Syllabus section L1.
Reference: Guyton & Hall 11th Edition page 894-901.
5. Outline the mechanism of action of drugs used to promote haemostasis.
CICMWrecks Answer
Haemostasis: the physiological processes which arrest bleeding
Drugs which reduce blood flow:
- Adrenaline: causes local vasoconstriction
- Wound glue: physical barrier to reduce bleeding
Drugs which increase the available coagulation factors:
- Desmopressin: increases plasma levels of FVIII and von Willebrand Factor, which promote formation of the platelet plug and participate in the coagulation cascade
- Vitamin K: required for the gamma-carboxylation of coagulation factors II, VII, IX and X to activate them in the clotting cascade. Also activates proteins C and S. Vitamin K may be given to reverse supratherapeutic INR associated with warfarin use; may also be given in liver disease or other situations when the INR is elevated and potential bleeding is a concern.
- Prothrombinex: powder for injection containing 500IU each of FII, IX and X, low levels of FV and VII, 25mg of antithrombin which participate in the coagulation cascade as normal
- Biostate: Human derived FVIII/VWF complex which can participate in the coagulation cascade
- Other factor concentrates
Drugs which reduce clot breakdown:
- Tranexamic acid: acts by inhibiting the binding of plasminogen and plasmin to fibrin, to inhibit fibrinolysis of a formed clot
- Aprotinin: a naturally occurring proteolytic enzyme inhibitor acting on trypsin, plasmin and tissue kallikrein. It inhibits the fibrinolytic activity of the streptokinase-plasminogen complex and decreases activation of the clotting cascade.
- Aminocaproic acid: Binds competitively to plasminogen, blocks the binding of plasminogen to fibrin and subsequent conversion to plasmin, resulting in inhibition of fibrinolysis
- Oestrogen: inhibits Protein C (naturally occurring regulator of haemostasis)
Drugs which reverse anticoagulants:
- Vitamin K: as above
- Protamine sulfate: binds to heparin in circulation to produce a stable inactive complex for removal by reticuloendothelial system. Useful in cases of bleeding associated with heparin use.
- Idarucizumab: specific reversal agent for dabigatran
- Andexanet Alfa: Recombinant protein that inactivates direct factor Xa inhibitors like Rivaroxaban as well as antithrombin activated by LMWH or Fondaparinux
JC 2019
Examiner Comments
2009A 05: Pass rate: 10%
Most candidates mentioned factor VIIa, Vitamin K, and desmopressin in their answers.
Outlining the mechanism of action of the drugs used is essential in order to pass this question. The answers may include which coagulation factors are affected by warfarin and Vitamin K, the mechanism by which desmopressin promotes haemostasis and the multiple effects of Aprotinin. Topical treatment (e.g. adrenaline, glue) and drugs such as protamine, oestrogen, and tranexamic acid were common omissions.
Syllabus J 2a 2
Reference: Stoelting and Hillier 4th edition page 449 and 607.
6. Describe the pharmacology of oxygen.
Examiner Comments
2009A 06: Pass rate: 20%
Oxygen can be regarded as a ‘drug’ and the best answer will describe oxygen with such a perspective in mind. A good answer would require good understanding and integration of knowledge from different parts of the syllabus.
Most candidates mentioned the oxygen is an odourless and colourless gas and its effects on pulmonary pressure and atelectasis. Common omissions included the clinical uses oxygen other than reversing hypoxia, pharmaceutic properties of oxygen, pharmacodynamic response of different body systems (CVS, CNS, RESP) to hyperoxia, and pharmacokinetics of oxygen including distribution & transfer of oxygen between body systems and the metabolism of oxygen.
Syllabus: B1f, B1h, B1i, B2a, C1f, O1.
Reference: Nunn’s applied respiratory physiology.
7. Define afterload (10% of mark). Describe the factors that can affect left ventricular afterload (90% of mark).
CICMWrecks Answer
Afterload
- Load against which the muscle exerts its contractile force (Guyton)
- It is represented by the gradient of the line connecting the end-diastolic volume, to the end-systolic point
- pressure which the ventricle has to contract against (Power & Kam)
Determinants of Afterload
Modified Laplace Equation
- where
- T represents afterload
- P represents aortic pressure
- Therefore, afterload increases as aortic (or pulmonary arterial) pressure increases
- R represents ventricular radius
- Afterload increases as ventricular radius increases
- H represents ventricular thickness
- Afterload decreases as the thickness of the ventricular wall increases (hypertrophy secondary to chronic hypertension and cardiac remodelling)
Modified Hagen-Poiseuille Equation
- Afterload is affected by resistance to cardiac output
- Afterload increased by reduced radius of systemic vasculature
- Afterload increased by increasing viscosity of blood
Resistance in parallel
- Afterload is affected by addition, or loss of large capillary networks in parallel
- Systemic vascular resistance is increased significantly by loss of placenta, with parallel vascular networks
- Pulmonary vascular resistance is decreased significantly by inflation of lung, causing creation of vast capillary network in parallel
Factors affecting Right Ventricular Afterload
- Pulmonary vascular resistance increases afterload
- Hypoxic vasoconstriction increases PVR
- Lung volumes
- Pulmonary vascular resistance minimal at FRC
- Increased pulmonary artery pressure increases RV afterload
- Left heart failure
- Critical mitral stenosis, mitral regurgitation
- Pulmonary emboli
- Right ventricular outflow tract obstruction increases afterload
- Pulmonary stenosis
- Saddle pulmonary embolism
- Right ventricular dilation
- Susceptible due to thin RV wall
- Acute PE
- RV spiral of death
- Increased intraventricular pressure decreases blood flow à ischaemia à decreased contractility à further increase in ventricular volume
- RV spiral of death
Factors affecting Left Ventricular afterload
systemic vascular resistance, aortic impedance and ventricular radius
According to La Place Equation
- Aortic pressure
- Afterload increases as aortic pressure increases (increases with hypertension)
- Ventricular radius
- Afterload increases as ventricular radius increases (increases with ventricular dilation)
- Ventricular wall thickness
- Afterload decreases and ventricular wall thickness increases (decreases with ventricular hypertrophy)
Determinants of aortic pressure indirectly affect afterload
- Aortic compliance
- Afterload decreases with increased compliance
- Arterial blood volume
- Given set compliance, afterload will increase with arterial volume
- From Modifed Poisuille-Hagen Equation
- Radius of aorta
- Autonomic vasomotor tone
- Increased intrathoracic pressure
- Physical compression
- Viscosity of blood
- Radius of aorta
- From equation MAP = CO / SVR
- Tissue flow autoregulation
- Myogenic
- Metabolic
- Tissue flow autoregulation
Other
- Left ventricular outflow tract obstruction
- Aortic stenosis
- HOCM
- Coarctation of aorta
Sakurai 2016
Examiner Comments
2009A 07: Pass rate: 20%
Many definitions of afterload were accepted. The main factors affecting left ventricular afterload are systemic vascular resistance, aortic impedance and ventricular radius. Other factors include blood viscosity and positive intrathoracic pressure. Good answers expanded on the points above. Candidates who failed this question did not have enough facts.
Syllabus C1c C2c
Reference: Bray 4th edition p 342-344 and p 360-361
8. Outline the anatomy relevant to performing a percutaneous tracheostomy.
CICMWrecks Answer
Tracheostomy – insertion of a tube through the anterior portion of the neck into the trachea to facilitate ventilation
Trachea Course:
- Larynx connects to the superior part of the trachea at C6 into the thorax and terminates at the level of the sternal angle, where it divides into the right and left mainstem bronchi.
- Initially anterior, then moves posteriorly as it descends to move behind the sternal notch
Tracheal Structure:
- A fibrocartilaginous tube 10cm long, approx 5cm in neck
- Supported by incomplete cartilaginous tracheal rings, which keep the trachea patent.
- The tracheal rings are joined by fibroelastic tissue.
- They are deficient posteriorly where the trachea lies anterior to the oesophagus; the posterior gap is spanned by the involuntary smooth trachealis muscle
Relationships:
- Lateral – carotid sheaths (common carotid arteries, vagus and internal jugular veins), thyroid lobes, inferior thyroid arteries, recurrent laryngeal nerves
- Inferior to the isthmus of the thyroid gland are the inferior thyroid veins
- Posterior – oesophagus, vertebral column
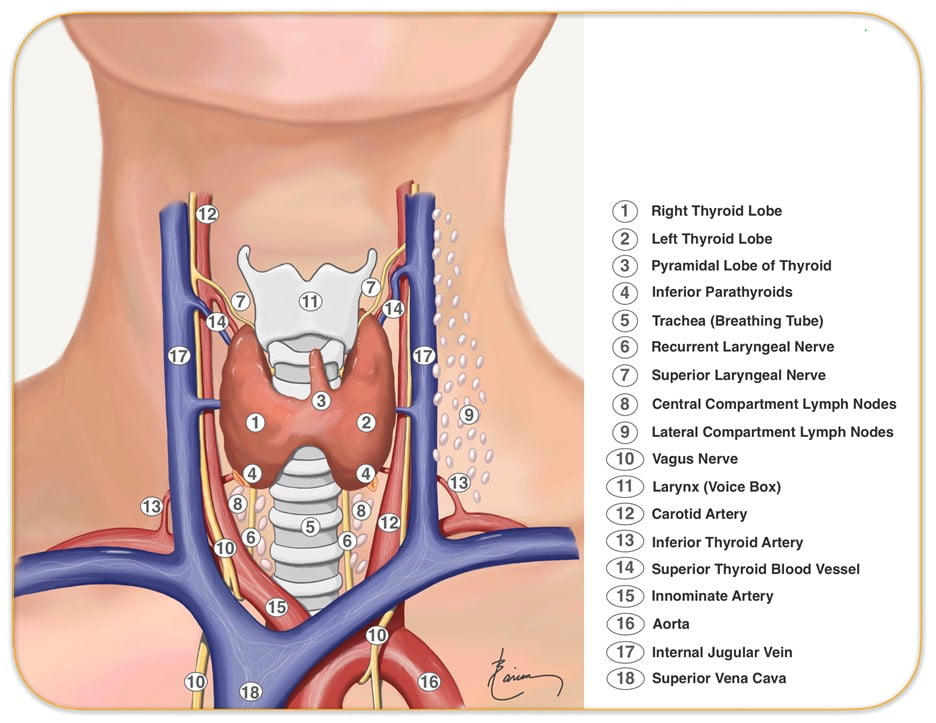
Relevant surface anatomy (in midline of neck):
- Hyoid bone (at level of C3)
- Thyroid cartilage
- Cricothyroid membrane
- Cricoid cartilage (at level of C6)
- Thyroid gland
- Sternohyoid muscle just lateral to midline structures, overlies sternothyroid and thyrohyoid muscles
Layers of dissection in tracheostomy:
- Skin
- Subcutaneous tissue
- Fat
- Pretracheal fascia (superficial and deep)
- Passage through the fibroelastic tissue in between the 1st and 2nd rings (common in perc trache) or 2nd /3rd or 3rd/4th (surgical trache)
- Trachea
Gladwin 2016
Examiner Comments
2009A 08: Pass rate: 20%
Surface landmarks, anatomy of the trachea and its important relationships with the thyroid, carotid sheath and oesophagus were required to pass this question. Several candidates described the procedure of percutaneous tracheostomy in great detail. Descriptions of the procedure gained no marks.
Syllabus B1b2g
Reference: Anatomy for the Anaesthetist, Ellis and Feldman.
9. Explain the causes of the difference between measured end tidal and arterial partial pressures of carbon dioxide.
CICMWrecks Answer
PaCO2
- Factors affecting PaCO2
- Rate of metabolism and CO2 production
- FiCO2 (usually negligable)
- Alveolar ventilation
- Increased alveolar ventilation decreased PaCO2
End-Tidal CO2
- CO2 contained in gas at the end of tidal expiration
- Contains:
- Alveolar CO2
- High CO2 diffusion – PACO2 = PaCO2
- Only 0.7mmHg Alveolar-arterial CO2 gradient for 10% shunt
- Alveolar Dead Space CO2
- Anatomical – conducting airways
- Physiological – West zone 1 / Apex
- Varies with posture and pathology
- Alveolar CO2
- Measured by capnometer
- In-line or side-stream
- Used IR absorbance at 4.23 μm
- Side-stream: lag in CO2 detection due to increased dead space between respiratory tract and analyzer
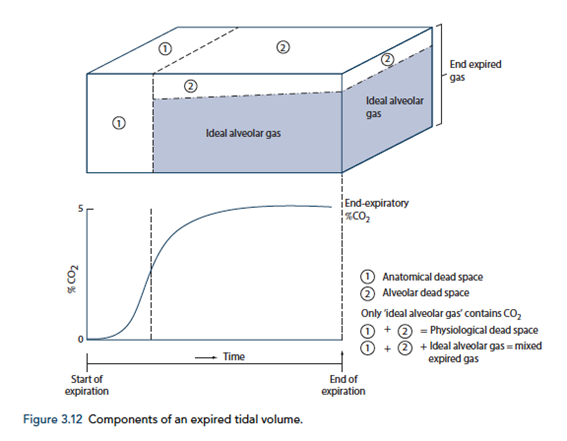
End-tidal to Arterial PaCO2 difference
- Normal ETCO2 – PaCO2 ~5mmHg
- Can be due to Artificially low ETCO2 or Artificially High PaCO2
Artificially low ETCO2
- Dead space (VD)
- Assumed PCO2 however usually low pCO2 but non 0 pCO2
- Contributes to ETCO2-PaCO2 ~5mmHg
- Pathological ↑VD
- ↑alveolar dead space (normal individuals = 0)
- Mixing of gas between perfused and non-perfused alveoli → ETCO2 < PaCO2
- Equivalent to ↑ West Zone 1
- → PE, hypotension, excess PEEP/IPPV
- COAD – poor perfusion of hyper-expanded alveoli
- ↑alveolar dead space (normal individuals = 0)
- Closing capacity (CC):
- Usually 40 CC may exceed FRC
- ↑ Airways collapse/gas trapping → airways closure → ↑alveolar pCO2 within alveoli not exhaling
- Exacerbated by
- low volume ventilation close to FRC
- High FiO2 for extended periods → denitrogenation → loss of airways splinting = CC closer to FRC
- Usually 40 CC may exceed FRC
- Sampling line problems:
- Too far from trachea (ie ↑ mechanical dead space)
- Too long – unable to adequately sample as gas is trapped in line
- Air leakage/line blockage leading to measurement error with entraining of room air, loss of expired air
- Machine:
- Loss of calibration of sampling unit
- Excess H2O in water trap interfering with measurement
- Interference from other gases (N2O) artificially raises ETCO2 due to interference with IR absorber “Collision broadening”
- Incorrect timing of measurement
- Inadequate expiration time → failing to reach plateau = incomplete alveolar expiration = falsely low ETCO2
Artificially high PaCO2
- Should be measured from arterial sample
- Venous sampling will artificially ↑pCO2
- Loss of calibration of machine
Gladwin / Sakurai 2016
Examiner Comments
2009A 09: Pass rate: 40%
Good answers were in tabular format.
The antidepressant action is similar for each agent. Initial increase in 5HT and NA, followed in 2-3 weeks by a down regulation or change in efficiency of 5HT transmission. The agents produce elevated neurotransmitters via different mechanisms, either reuptake blockade or enzyme inhibition. MAOIs can be competitive or non-competitive. Mention of the different neurotransmitters affected by each agent was required.
A description of significant side effects at therapeutic doses, and in overdose was expected with explanations provided. These should have included – the anticholinergic effects and cardiotoxicity of TCAs, postural hypotension, the catecholamine, pethidine and tyramine related complications of MAOIs, and serotonin syndrome with SSRI/MAOI use and or overdose. More marks were gained for mention that side effect profiles can be beneficial e.g. analgesic properties of TCAs, sedation with TCAs/ SSRIs and energizing benefits of SSRIs/SNRIs. SSRI’s safety and efficacy have markedly reduced the use of MAOIs and to a lesser extent TCA’s..
Syllabus G2f2d
Reference: Stoelting p 398-407, Katzung p 476-487.
10. Describe the calculations involved in determining the loading dose and maintenance dose for an intravenous infusion (50% of marks). What factors may affect these values in the critically ill (50% of marks)?
CICMWrecks Answer
Continuous IV Infusion
- Volume of distribution
- Apparent volume into which a drug disperses in order to produce the observed plasma conc
- The physicochemical properties of a drug influence Vd
- lipid solubility – highly lipid soluble drugs have larger Vd
- charge characteristics – highly charged drugs have smaller volumes of distribution
- tissue binding may result in increased Vd
- pathology – renal and hepatic disease leads to increased Vd due to fluid changes
- Plasma concentration
- is the amount of drug/volume within which it is diluted
- Clearance
- is the volume of plasma from which the drug is cleared from per unit time (usually ml/min)
- it is also the dose/area under the curve
- Loading dose (mg)
- aims to achieve a required plasma concentration
- therefore = desired peak concentration (mg/L) × clearance (L/hr)
- (divided by bioavailability if not IV)
- Maintenance dose (mg/hr)
- aims to achieve a constant plasma concentration range
- is equal to the elimination of the drug
- Is desired peak concentration (mg/L) × clearance (L/hr)
Factors which affect loading dose and maintenance dose in critically ill
| Effects of critical illness | Impact on loading dose and maintenance dose |
| VOLUME OF DISTRIBUTION | |
| Increased Vd (due to fluid overload) | Increased loading dose and maintenance dose or dose rate |
| Decreased Vd (due to hypovolaemia) | Decreased loading dose and maintenance dose or dose rate |
| CLEARANCE | |
| Decreased renal clearance (due to decreased renal blood flow or renal parenchymal damage) | Decreased maintenance dose or dose rate; also possibly increased dosing interval. Loading dose could remain unchanged |
| Increased renal clearance (hyperdynamic states, eg. early sepsis) | Increased maintenance dose or dose rate; also possibly dencreased dosing interval Loading dose could remain unchanged |
| Decreased hepatic clearance (decreased hepatic blood flow or inhibited liver enzyme function) | Decreased maintenance dose or dose rate; also possibly increased dosing interval. IV loading dose could remain unchanged Oral loading dose would need to be decreased to accommodate for the decreased first pass metabolism |
| Increased hepatic clearance (increased hepatic blood flow or hepatic parenchymal clearance) | Increased maintenance dose or dose rate; also possibly decreased dosing interval. IV loading dose could remain unchanged Oral loading dose would need to be increased to accommodate for the increased first pass metabolism |
| BIOAVAILABILITY | |
| Decreased protein binding (due to lower levels of protein) | Increased free unbound fraction of the drug, which gives rise to increased clearance and increased drug effect. |
| Decreased gut absorption (due to decreased splanchnic blood flow and/or decreased peristalsis) | Variable and inconsistent absorption of an otherwise correctly calculated oral loading dose |
| Competition for protein binding (eg. where bilirubin competes for albumin binding sites) | Increased free unbound fraction of the drug |
Examples of drugs illustrating an understanding of pharmacokinetics
- Theophylline: a drug which is dosed every half-life (300mg every 8 hours), which is equivalent to a dose rate of 37.5mg/hr, which is in turn equivalent to an infusion rate of 37.5mg/hr.
- Phenobarbitone: a drug with a vast volume of distribution, where the loading dose would be massive and toxic
- Morphine: a drug which is poorly orally bioavailable; an example of how the oral loading dose is affected by bioavailability
- Phenytoin: a drug which is highly protein-bound, and which is highly affected by the low plasma albumin associated with critical illness (thus, with low total drug levels the levels of free unbound drug may still be therapeutic)
- Corrected Phenytoin = Measured Phenytoin Level / ( (adjustment x albumin) + 0.1)
- Adjustment = 0.2; In patients with Creatinine Clearance < 20, adjustment = 0.1.
- Gentamicin: a drug which is cleared rapidly by the kidneys, a clearance which is significantly affected by poor renal function. It is an example of how the loading or maintenance dose should remain unchanged; instead the dosing interval should be extended.
- Vancomycin and β-lactams: examples of drugs which are subject to increased renal clearance in the context of hyperdynamic circulatory states, for example in early sepsis.
Source: Deranged Physiology
JC 2019
Examiner Comments
2009A 10: Pass rate: 30%
Main points for a pass included the equations for determining the loading and maintenance doses. Points were awarded for explaining the rationale for giving a loading dose and for relevant diagrams.
Answers to the second part of the question often lacked detail. Candidates should have mentioned alterations in volume of distribution, plasma proteins, renal & hepatic function. Examples of drugs illustrating an understanding of pharmacokinetics attracted extra marks.
Syllabus II 2 f
Reference: Rang Ritter Dale p120-123
11. Describe the control of cerebral blood flow.
CICMWrecks Answer: Cerebral Blood Flow
Cerebral Blood Flow
- CBF = CPP / CVR (Cerebral Perfusion Pressure / Cerebral Vascular Resistance)
- CBF 15% resting CO → ~750ml/min or 50ml/100g brain tissue/min
- Gray Matter: 75–80 mL/100 g/min – Significantly higher due to the high metabolic activity and dense synaptic connections
- White Matter: 20–30 mL/100 g/min
- Abnormal CBF
- CBF<50ml/100g/min → cellular acidosis
- CBF<40ml/100g/min → impaired protein synthesis
- CBF <30ml/100g/min → cellular oedema
- CBF <20ml/100g/min → failure of cell membrane ion pumps, loss of transmembrane electrochemical gradients
- CBF <10ml/100g/min → cell death
Determinants of CBF
- CPP
- Net pressure gradient driving blood flow through the cerebral circulation
- CPP = MAP – ICP
- MAP = CO x SVR; CO = HR x SV; SVR = MAP / CO
- ICP dependent on: brain, blood, CSF
- CVR
Regulated by 4 primary factors:
- Cerebral metabolism
- flow-metabolism coupling:
↑ metabolic demand → ↑ CBF + substrate delivery - Controlled by vasoactive metabolic mediators:
H+ ions, K, CO2, adenosine, glycolytic intermediates, NO
- flow-metabolism coupling:
- CO2 and O2
- CO2
- At normotension: relationship between PaCO2 and CBF = almost linear
- ↑ PaCO2 → cerebral arteriolar vasodilation → ↓ CVR + ↑ CBF
- 2~4% increase for every 1mmHg increase in CO2
- ↓ PaCO2 → cerebral arteriolar vasoconstriction → ↑ CVR + ↓ CBF
- Initial stimulus = ↓ brain ECF pH
- Effects regulated by: NO, prostanoids, K channels, intracellular [Ca2+]
- PaO2
- little effect at normal PaO2
- PaO2 <60mmHg → cerebral arteriolar vasodilation → ↑ CBF
- Mechanism: hypoxia acts on →
- cerebral tissue to promote release of adenosine → cerebal vasodilation
- cerebrovascular smooth muscle → hyperpolarisation → ↓ Ca2+ uptake → vasodilation
- CO2
- Autoregulation
- Constant across CPP 50-150mmHg
- CPP >150mmHg: CBF µ CPP
- CPP <50mmHg: CBF <50ml/100g brain tissue/min → ischaemia
- Stimulus to autoregulation = CPP (not MAP)
- autoregulation curve R shifted in HTN; L in neonates
- Mechanism:
- Myogenic mechanism: arterioles vasoconstrict in response to ↑ wall tension + vasodilate in response to ↓ wall tension → ↓ or ↑ CVR
- May involve adenosine
- Can be impaired in SAH, tumour, stroke, head injury
- Constant across CPP 50-150mmHg
- Neurohumeral factors
- Relative lack of humoral + autonomic control on normal cerebrovascular tone
- Main action of SY nerves = vasoconstriction
- Other factors
- Blood viscosity: directly related to HCt; ↓ viscosity → ↑ CBF as per Hagen-Poiseuille law
- Temperature: ↓ CMRO2 by 7% for each ↓ 1°C in temp
- Drugs
- E.g. barbiturates ↓ cerebral metabolism
- Volatile agents → ↓ tension cerebral vascular smooth muscle → vasodilation + CBF
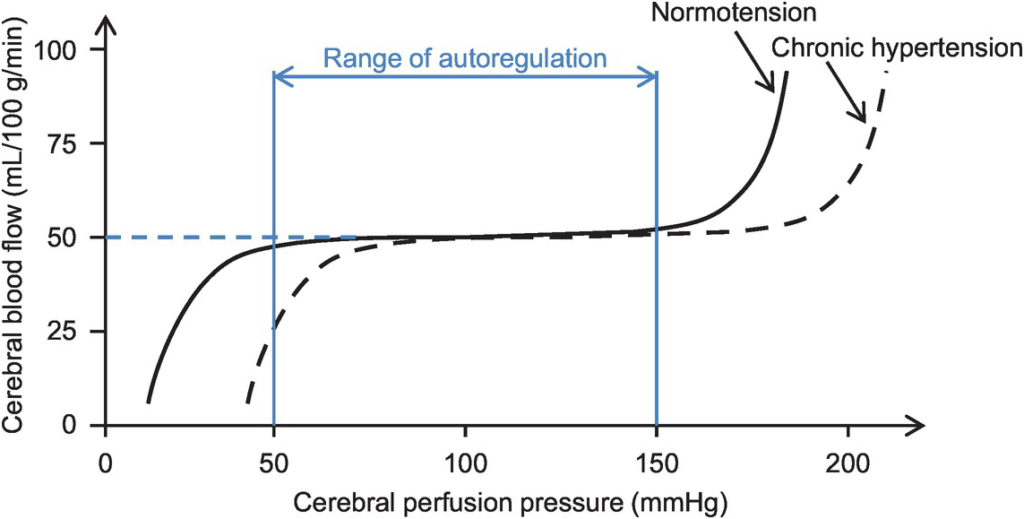
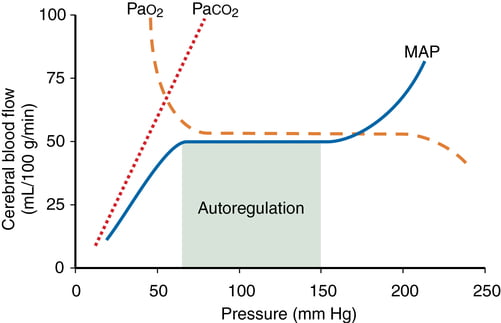
Kerr 2018
Examiner Comments
2009A 11: Pass rate: 50%
Good answers included an equation and then explored the various components of the equation. Main points for a pass included pressure and metabolic autoregulation and the various factors that affect cerebral vascular resistance. Graphs were a useful way to answer this question but were generally underutilised. Several candidates wrote about the Monro-Kellie doctrine which was not directly relevant to the question.
Syllabus C1d2a
Reference: Power and Kam 1st edition p 42-43, Guyton and Hall 11th edition p 761-3
12. Outline the pharmacology of an opioid injected into the spinal intrathecal space.
CICMWrecks Answer
Pharmacokinetic Effects
- D: Dependent on degree of lipid solubility. Fentanyl has > systemic absorption than Morphine. More likely to cause systemic opoid effects
- M: Minimal metabolic effects occur in the intrathecal space
- E: Clearance from the intrathecal space is via the arachnoid villi.
Pharmacodynamic Effects and Side-effects
Secondary to:
- Local Effects
- Analgesia
- MOP in spinal cord → ↓pain transmission primary afferent neurons
- Orthostatic hypotension
- 2° direct SNS blockade at sympathetic chain → vasodilation → venous pooling
- ↓ temperature
- Inhibition of shivering
- Analgesia
- Cephalic migration:
- Depends primarily on the lipid solubility of the drug
- Sedation
- Direct effect on μ-receptors in reticular formation
- Confusion
- Anticholinergic (pre-synaptic blockade of ACh release)
- Pruritis
- Interaction with μ-receptors in trigeminal nucleus
- CNS Excitation
- In overdose → myoclonic jerks
- Due to bulk flow to brainstem/basal ganglia
- ↓MV
- Direct opioid effect depressing ventilatory centre of brainstem
- ↑PaCO2 due to right-shift in ventilation v PaCO2 curve
- N&V
- Systemic Absorption
- ↓gastric emptying, ↓gut motility
- ↓MAP
- Mast cell degranulation → histamine release → vasodilation (rare with fentanyl, ↑with morphine)
Gladwin 2016
Examiner Comments
2009A 12: Pass rate: 30%
Though it would be unusual for patients to receive spinal opioids whilst they are in intensive care, the complications of spinal opioids are not an uncommon reason for admission to intensive care thus it is important candidates understand their pharmacology. Answers generally lacked structure. Outlining pharmacology should include pharmacokinetics, pharmacodynamics and side effects (both common and dangerous). An explanation of the effect of lipid solubility was expected. Following a structure will ensure a more complete answer.
Syllabus G2d2e
Reference: Neural Blockade. 3rd edition Cousins and Bridenbaugh
13. Outline the factors that determine the composition and volume of glomerular filtrate in a normal person.
CICMWrecks Answer
GFR
GFR:
- “The amount of glomerular ultrafiltrate formed divided by the time of filtration”
- Normal value is approx 125 mL/min or 180 L/day
- Renal blood flow 1.25l/min in 70kg male → Filtration fraction 0.2
Functional anatomy:
3 Distinct Layers:
- glomerular capillary endothelium
- highly specialised endothelium with fenestrations to ↓ filter thickness
- prevents cellular components of blood from coming into contact with BM
- glomerular BM
- made of CT; -vely charged
- acts as filter
- bowmans epithelial cells (podocytes)
- epithelial cells with foot processes → large SA
- negatively charged
- maintain BM + phagocytic functions
Direct GFR Determinants
The GFR (Net flux across the membrane) is the balance of hydrostatic pressure and oncotic pressure, as defined by the Classic Starling Equation.
where
Kf = Filtration coefficient
P = hydrostatic pressure
π = oncotic pressure
σ = Staverman’s reflection coefficient ie. Permeability of membrane to protein
- Normally NFP = 17mmHg
- Tubular oncotic pressure = zero throughout
- GC oncotic pressure varies from 21 to 33 mmHg as filtrate is removed.
- Thus less GFR produced at distal end of tubule.
| Afferent end of Glomerular Capillary (mmHg) | Efferent end of Glomerular Capillary (mmHg) | |
|---|---|---|
| PGC | 60 | 58 |
| PT | 15 | 15 |
| πGC | 21 | 33 |
| NFP | 24 | 10 |
| Kf | Filtration coefficient | = LpS = Hydraulic conductivity x Surface Area Glomerular surface area = 0.8m2 • Altered by Mesangial cell contraction (see circulating factors e.g. Angiotensin II → ↓SA → ↓GFR) Patency of the normal capillary wall structure (ie in tubular dysfunction Kf ↑’s ↑GFR) |
| PGC | hydrostatic pressure in capillary | relates to • RBF which is autoregulated for MAP 70-170mmHg • relative afferent/efferent arteriolar tone Affected by • Catecholamines • Local autoregulation -> Myogenic -> Tubuloglomerular feedback -> Hormones (see below) |
| PT | hydrostatic pressure in tubule | relates to • obstruction to urinary flow (usually pathological) • ↑PT → ↓ GFR (ie post renal obstructiion causing renal failure) |
| πGC | oncotic pressure in capillary | relates to • plasma protein concentration (incr in dehydration, decreased in heart failure) • ↑Systemic plasma oncotic pressure → ↑πGC → ↓GFR • ↓Renal plasma flow → ↑πGC → ↓GFR |
| πT | oncotic pressure in tubule | • usually zero, but can increase in renal failure/proteinuria • ↑’d πT → ↑GFR |
| σ | Staverman’s reflection coefficient | • Permeability of membrane to protein usually 1 (no protein leak) • can decrease with nephritis/proteinuria |
Factors influencing GFR
Circulating Factors
- Prostaglandins
- PGI2 and PGE2 → vasodilates → ↑ renal blood flow → ↑ GFR
- ↓ PGE2, PGI2 and NO levels → inhibits afferent arteriolar vasodilation → ↓ GFR
- Long term → ↓ renin release → ↓ ATII-induced efferent arteriolar vasoconstriction → ↓ GFR
- Noradrenaline / Adrenaline
- constrict renal afferent and efferent arterioles → ↓ renal blood flow and reducing filtration → ↓GFR
- Constrict mesangial cells to → ↓ GFR.
- Mesangial cell tone:
- Angiotensin 2, Endothelin, vasopressin → ↓ glomerular surface area and GFR
- ANP, PGE2, Dopamine and cAMP → all increase GFR
Solute factors
- Size
- < 7 kDa (Eg. glucose, ions, urea, H2O) filtered freely,
- > 70 kDa (Eg. albumin) are not filtered;
- neutral particles < 4 mm diameter filtered freely, > 8 mm are excluded
- Charge
- Negatively charge (Anionic) particles repulsed
- Cationic substances filtered more readily
- Protein binding
- Albumin excluded
Disease Factors
- Filtration decreases
- Shock → decreased glomerular pressure
- Obstruction → increased bowman’s capsule hydrostatic pressure
- Hypoproteinaemia → hepatic failure, nephrotic syndrome
JC / Gladwin / Sakurai / Kerr 2020
Examiner Comments
2009A 13: Pass rate: 30%
The volume and composition of the glomerular filtrate are best explained by referring to the equation: Glomerular Filtration = Kf x net filtration pressure. Then describing the factors that affect each part of the equation e.g. hydrostatic pressure, oncotic pressure, factors that influence the filtration coefficient e.g. surface area
Glomerular filtrate is an ultrafiltrate of plasma and factors that affect the passage of proteins and other molecules should be discussed Extra marks were given for an explanation of filtration fraction and short facts about GFR in paediatric patients and the elderly.
No marks were given for any discussion of drugs.
Syllabus D2b
Reference; Power and Kam 1st edition p 197-199
14. Statistics (not in current primary syllabus)
15. Outline the physiology of excitation and conduction in nerve axons (60% of marks). List the factors which delay axonal conduction (40% of the marks).
CICMWrecks Answer
Resting Membrane Potential
- Defined as the steady state potential difference (measured in mV) that exists across the cell membrane when the cell is in an unexcited state
- RMP of different excitable tissues varies → due to differing ionic permeability of the
membrane (Ie. opening states of membrane ion channels) in the respective tissues at rest
Myelinated axon: -70 mV
Skeletal muscle cell: – 80 mV
Ventricular myocyte: – 90 mV
Cardiac pacemaker cell: – 60 mV
How is the membrane potential produced?
RMP is generated by uneven distribution of charged particles (i.e. ions and proteins) across the cell membrane 2o to:
- Semi permeable membrane / selective membrane permeability to different ions
At rest CM is:- Slightly permeable to Na: Na channels closed
- Very permeable to K: open K+ leak channels → K down conc gradient from ICP to ECF
- Variable permeability to Cl based on cell type
- Different ionic concentrations of ICF and ECF
- Na+: 140mmol/L ECF; 20mmol/L ICF
- K+: 150mmol/L ICF; 5mmol/L ECF
- Na/K ATPase: 3Na+ out for 2K+ in. Consequences:
- Osmotic effect: ↑ ECF [Na+] balances osmotic effect of ↑intracellular conc of –vely charged protein
- Electrogenic effect: cell interior hyperpolarised
- Gibbs Donnan effect
- Minor contribution to RMP
- unequal distribution of large –vely charged protein impermeable to CM → affects distribution of other diffusible ions (K, Cl) and hence RMP by ~-10mV
Principles
Principles
- Nernst equation
- Nerst potential: voltage difference generated by EC gradient of an ion across CM (assuming complete permeability) i.e. contribution that a single ion makes to RMP
- Calculated from valency, conc difference across membrane, and temp
- The ion with ↑ membrane permeability → Nerst potential has ↑ contribution to total RMP
- Nernst applied:
- RMP has ↑ K permeability → net efflux of +vely charged K down conc gradient
- drives membrane potential towards Nernst potential for K+
- RMP ↓ permeability to Na+ ions
- Therefore: measured neuronal RMP (-70mM) = close to Nernst potential for K+
- Goldman –Hodgkin-Katz equation
- considers all ionic permeabilities and concentrations → RMP more precisely quantified
- Gibbs Donnan effect
Action Potentials
“Action Potential” (AP) → large rapid change in membrane potential that occurs in excitable cells
- AP = electrical response of neurons and other excitable tissues during which membrane potential rapidly ↑ and ↓
- All or nothing phenomenon
- Allow rapid signalling within excitable cells over long distances
- AP results from brief ↑ in membrane conductance to Na+, followed by slower ↑ in membrane conductance to K+
- Key parameters
- RMP -70mV
- Threshold potential -55mV
- Peak potential (depolarisation) +20-40mV
- Duration of AP 1-2ms
Events of an AP:
- Phase 1 – threshold potential: depolarisation stimulus reaches neuron → CM reaches -55mV → activation of voltage gated Na+ channels → Na+ influx > K+ efflux
- Phase 2 – AP: rapid influx of Na → further depolarisation → +ve feedback → rapid upstroke → drives membrane potential to Nernst potential for Na of ~+50mV → peak potential +30mV
- Phase 3 – repolarisation: AP never reaches theoretical max (+50mV) due to 2 events:
- Inactivation of voltage gated Na+
channels → ↓ membrane permeability to Na+ - Delayed activation of voltage gated K+
channels: ↑ membrane K+ permeability → K+
efflux → membrane potential driven back towards Nernst for K+ of ~-90mV - Membrane potential briefly more –ve than RMP = “after hyperpolarisation” – due to gradual closure of voltage-gated K+
channels
- Inactivation of voltage gated Na+
- Phase 4 – restoration of RMP
- -70mV maintained by: Na/K ATPase (EC gradient) + Na/K pump
Saltatory conduction
- Saltatory conduction: propagation of AP along myelinated axons, whereby wave of depolarisation “jumps” from one rode of Ranvier to the next
- Mechanism of saltatory conduction
- Depolarisation of a node → influx of Na ions → creating a sink (area of –ve charge at the surface)
- +ve charge on nodes ahead flows into sink → ↓ polarity inside the membrane → AP → propagating current activates fast Na channels → wave of
depolarisation down axon - minimal electrical signal degradation as axon is insulated by myelin sheath
- AP reaches next node of Ranvier → continues down myelinated fibre
- Nerve impulses appear to rapidly jump from one node to the next
Factors affecting Velocity of conduction
Velocity of conduction dependent on several factors:
- Nerve fibre myelination: AP propogation slow in Unmyelinated fibres
- Axon diameter: ↓ diameter → ↑ resistance to flow → ↓ conduction velocity
- Transmembrane resistance: ↓ resistance → ↑ loss of current flow → ↓ conduction
- Membrane capacitance: ↑ capacitance → longer to alter polarity → ↓ speed of propagation.
- Temperature: ↓ temp → ↓ rate Na+ channel opening → ↓ velocity
- Hyponatremia: ↓ Na+ conc gradient → ↓ velocity
- Hypermagnesemia: → ↓ ACh release → ↓ velocity
- acidosis → ↓ velocity
Bianca / Kerr 2016
Examiner Comments
2009A 15: Pass rate: 30%
The following points were expected to be outlined in this question
1. The resting membrane potential (RMP) and its physiological basis
2. How the RMP changes rapidly after a stimulus e.g. electrical or chemical and reaches a threshold potential and an all or none action potential results
3. The ionic basis of the action potential
4. How the action potential is propagated
5. Factors that delay axonal conduction such as fibre size, myelination and electrolyte abnormalities e.g. hypermagnesaemia.
Syllabus G2a
Reference: Power and Kam 1st edition p 6-9
16. Outline the role of platelets in blood clotting following an injury to a blood vessel.
CICMWrecks Answer
Platelet adhesion:
- Vessel wall subendothelium is exposed
- Platelets bind to subendothelium via
- GpIa directly to collagen
- GpIIa/IX via vWF attached to subendothelial microfibrils
Platelet activation:
- Collagen binding or presence of thrombin
- Thromboxane A2 produced by COX-1
- Granules degranulate
- Dense granules release ADP and serotonin
- ADP binds to ADP receptors on external membrane
- Increased expression of GpIIa-IIIb
- Platelet also changes in morphology → long pseudopods
Platelet aggregation:
- ADP and thromboxane A2 released from the first binding platelets
- Aggregation and activation of further platelets
- ADP → increased expression of GpIIa-IIIb
- Fibrinogen linkage via GpIIaIIIb
- Formation of a platelet plug
- Thrombin (from clotting cascade) converts the fibrinogen into fibrin → stable plug
Factors increasing aggregation: Platelet activating factor
Factors inhibiting aggregation: Nitric oxide (via eNOS), Prostacycline (PGI2)
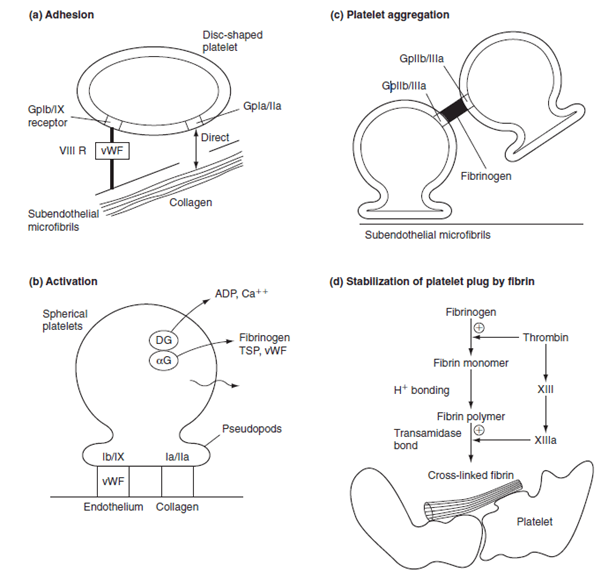
Mooney 2016
Examiner Comments
2009A 16: Pass rate: 40%
The main points expected in this answer were descriptions of platelet activation following exposure to collagen, platelet adhesion to the endothelium and ADP release and platelet aggregation secondary to activation of the GP11b/111a, COX-1 and other agents e.g. prostaglandin E2
Factors that interacted with platelet receptors e.g. platelet activating factor which increase aggregation and factors that inhibited platelet activation e.g. Prostaglandin I2 and nitric oxide gained marks.
17. Describe the physiological consequences of positive end expiratory pressure.
CICMWrecks Answer
Positive Pressure Ventilation
- PEEP = Positive End Expiratory Pressure.
- Equivalent to a constant pressure applied throughout the respiratory cycle.
- Intrinsic PEEP = unintentional or un-measured end-expiratory hyperinflation
- Physiological effects of Positive Pressure Ventilation mostly related to increased mean airway pressure
Cardiovascular Effects
- Causes constant ↑ intrathoracic pressure (ITP) throughout respiratory cycle
- Left Heart
- On Preload
- Initially: → ↑ LV PL → ↑ CO
- Secondarily: → ↓RV output → ↓ LV PL → ↓ CO
- On Afterload
- → ↓myocardial transmural pressure → ↓LV AL → ↑CO
- PEEP > Ao diastolic pressure
- → Collapse of intrathoracic aorta → Starling resistor mechanism → ↑LV AL → ↓ CO
- PEEP < Ao diastolic pressure
- ↑ pressure gradient for flow to systemic circulation
- ↓ AL → ↓ myocardial work
- On Compliance
- diastolic buldging of septum → ↓ LV compliance → ↓LV CO
- On Preload
- Right Heart
- → ↑ Pulmonary vascular resistance → ↑ RV AL → ↓ RV output
- → ↓ Venous return → ↓ RV PL → ↓ RV output
- In the failing LV → ↑CO
- ↓ LV afterload
- ↑ pressure gradient thorax to abdomen
- ↓ transmural pressure → ↓ LV wall tension = ↓afterload
- ↓ LV preload (ie more favourable position on compliance curve)
- ↓ LV afterload
Respiratory Effects
Beneficial Effects:
- ↓ atelectasis and gas trapping
- ↑’s FRC > “closing capacity”
- Shifts position on P-V curve right, above the “closing point”
- ↑ lung compliance → shifts back to steep/compliant part of P-V curve
- ↓ intrapulmonary shunt thus improved V/Q match and ↑ PaO2
- ↓ atelectasis and gas trapping
- ↓ extravascular lung water → ↓interstitial/alveolar oedema
- ↓ AWR
- ↑ lung volume → ↑ radial traction by parenchyma → ↑ airway calibre
- ↓ work of breathing
- ↑ lung compliance = ↓ elastic work
- ↓ AWR = ↓resistance work
Negative Effects:
- ↑ V/Q mismatch
- ↑ west zone 1
- ↓ lung compliance
- shift to flat part of P-V curve
- ↑ Work of breathing (due to compliance change)
- ↑ PVR and RV afterload
- extrinsic compression of pulmonary vessels
- Barotrauma
End-Organ Effects
- Renal:
- ↓CO & ↑renal venous pressure
- → Reduced renal blood flow → Reduced GFR → Reduced urine output
- → Reduced atrial stretch and ANP release → Increased ADH → Fluid retention → Oedema
- ↓CO & ↑renal venous pressure
- Hepatic:
- Reduced hepatic blood flow due to:
- Increased CVP and decreased CO lowering the pressure gradient for hepatic flow
- May result in circulation only intermittently throughout the cardiac cycle
- Hepatocyte dysfunction
- Haematological:
- Neutrophil sequestration in the compressed pulmonary vasculature
- CNS:
- ↓VR ⇒ ↑CVP ⇒ ↑ICP
Gladwin / Mooney / JC 2020
Examiner Comments
2009A 17: Pass rate: 40%
Points required included a definition of PEEP, both intrinsic and extrinsic.
The important physiological consequences that need to be discussed are respiratory including increased FRC, increased compliance and decreased work of breathing.
Cardiovascular consequences include decreased venous return and subsequently decreased cardiac output and an increased pulmonary vascular resistance.
Renal consequences include decreased renal blood flow and increased ADH
Effects on intra-abdominal pressure, hepatic blood flow and the beneficial effects in cardiac failure earned marks.
Syllabus B1k.2a
Reference: Nunn 6th edition p. 431.9
18. Describe the factors that affect airway resistance.
CICMWrecks Answer
Airway Resistance
- Resistance is the impedence to flow.
- Normal Airways Resistance (AWR):
- Adult: ~2 cmH2O/L/s
- Newborn: ~20 cmH2O/L/s – declines markedly
- Main Site of AWR:
- Mid-sized bronchi 7th/8th generation
- comparatively smaller cross-sectional area
- (note that in neonates, a greater proportion of Raw comes from the smaller peripheral airways)
- In the airway flow can be laminar or turbulent
- Flow depends on Depends on Reynolds number
where
Re is Reynold’s number
r is radius
ρ is density
v is velocity
η is viscosity
Laminar Flow
- Ordered flow occuring in concentric layers within a tube
- Flow in the center is fastest and flow in the most peripheral layer is the slowest
- Resistance to laminar flow obeys the Poisuille-Hagen Equation
where
R is vessel resistance
η is viscosity
L is length of vessel
r is radius of vessel
- Increases in viscosity of gas, or length of the tube increase resistance
- Increases in radius of the tube, decreases resistance by a power of 4
Turbulent Flow
- In turbulent flow, due to the disorganized flow and increased likelihood of friction
with the static airway wall, resistance is markedly increased. - Resistance to turbulent flow obeys the following equation
where
R is Resistance to flow
ρ is density
l is length of vessel
r is radius of vessel
- Increases in density of gas, or length of the tube increase resistance
- Increases in radius of the tube, decreases resistance by a power of 5
Factors affecting Airway Resistance
- Physics factors (see above):
- Location in the airway (see above): Mid-sized bronchi are the location of greatest airway resistance, resistance progressively declines with successive airway generations
- Flow:
- Laminar Flow vs turbulent flow
- Depends on Reynolds number factors
- density more important than dynamic viscosity, velocity (flow rate) as Main Site is Turbulent.
- Flow rate:
- Flow related airway collapse
- Airways beyond generation 11 have no structural rigidity
- High flows can reverse transmural pressure gradient and cause airway collapse
- Flow related airway collapse
- Newborn: ~20 cmH2O/L/s – declines markedly with age to ~2 cmH2O/L/s
- Radius changes
- Muscular control of airway diameter
- Neural:
- Parasympathetics important in bronchomotor tone → airway constriction via ACh and muscarinic receptors
- Sympathetic system virtually absent in lung
- Hormonal:
- Although no symppathetic innervation, abundance of β2 adrenoceptors → airway dilation
- Neural:
- Smooth mm tone:
- (↓r) bronchospasm, Musc antag (PSNS), LTs, PGF2-alpha
- (↑r) β2-agonists, adrenaline neb, SNS
- ↓intramural radius:
- oedema, ↑mucous, wall hypertrophy
- External compression:
- tumour, haemorrhage, PTX
- dynamic airways compression with forced expiration
- Muscular control of airway diameter
- Lung Volume
- ↑ lung vol →
- ↑ radial traction → ↓ AWR
- ↑ -ve intrapleural → ↑ patency of small airways
- ↓ lung vol → ↑ AWR
- CPAP or PEEP → ↑ FRC → reduces flow-related airway collapse at low lung volumes
- ↑ lung vol →
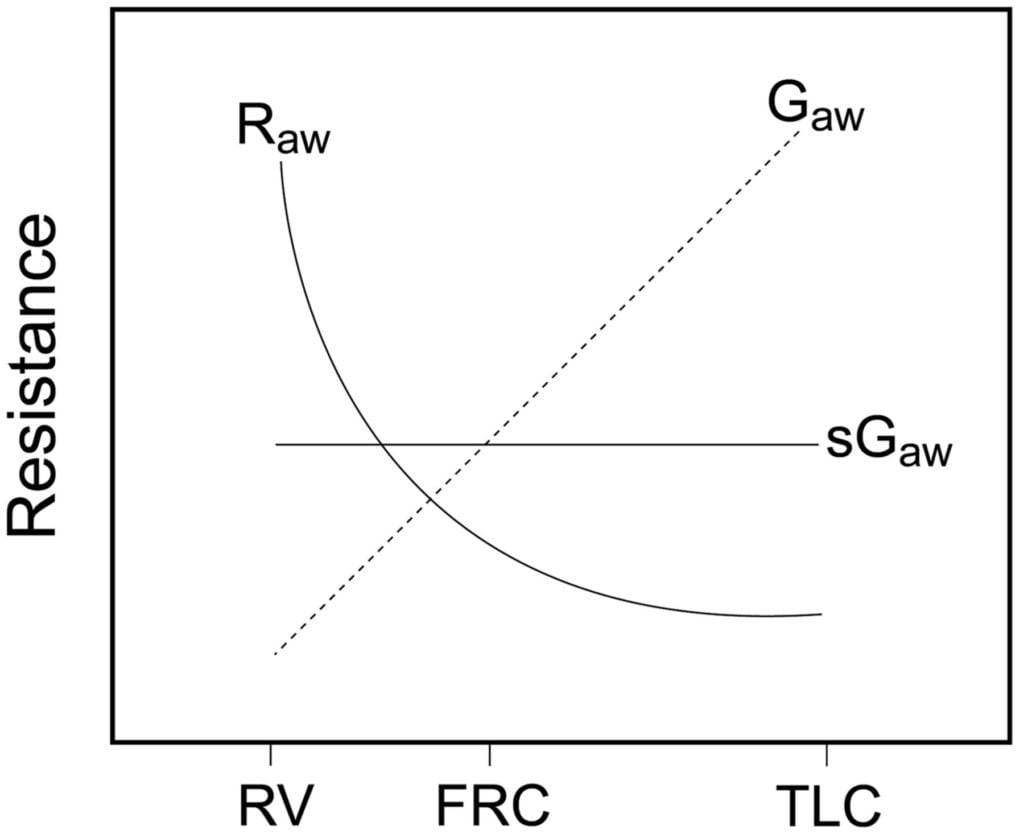
Relationship between Lung volume, Airway Resistance (R) , reciprocal of resistance (Conductance G, specific conductance sG)
Notice that only sGaw is independent of lung volume
Measurement of Airway Resistance
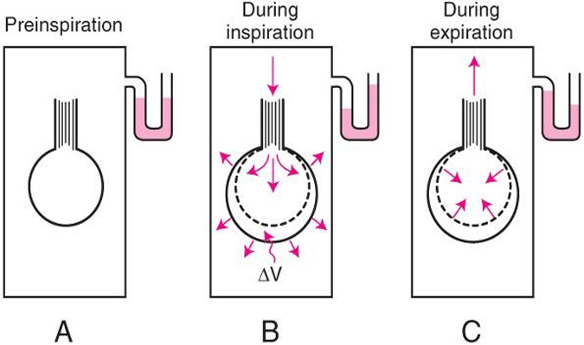
Body Plethysmography:
- Q measured with flow meter
- Lung Volume is measured with Plethysmography
- ∆P via Plethysmography and Boyle’s Law
- (A) box pressure is atmospheric
- (B) inspiration
- ∆V allows for ∆P measurement for given Q since
- PV = constant
- Then AWR can be calculated from 1
Interrupter Technique (direct from AWR eqn)
- Method
- Manometer distal to shutter
- Used to measure mouth and alveolar pressure
- Flow during inspiration or expiration in interrupted for 50–100 ms repeatedly throughout the respiratory cycle.
- Measure
- Q – flow rate (measured before interruption)
- P2 – mouth pressure (measured before interruption)
- P1 – pressure in alveoli (measured at the end of the interruption at te level of the mouth after equilibration)
- Apply Ohm’s Law (AWR equation above).
- Adequate for normal lungs not diseased.
Gladwin / Sakurai 2016
Examiner Comments
2009A 18: Pass rate: 30%
Important factors to be discussed in this answer were anatomical site, laminar versus turbulent flow, airway calibre and factors that affect it such as oedema and sympathetic tone. The effect of lung volume on airway resistance is usefully described in a diagram. The differences in infants earned extra marks
Syllabus B1d, 2h
Reference: Nunn 6th edition p39-47.
19. Describe the functions of the gastric secretions.
CICMWrecks Answer
Functions of Gastric Secretions: Gastric Juice
1. Gastric Acid
- Activation of pepsin (from pepsinogen I) → protein digestion
- Facilitates protein digestion by ↓ pH alone
- Defence against micro-organisms
- Facilitates iron absorption in duodenum
- Stimulates biliary/pancreatic juice secretion (via duodenal CCK/secretin)
- –ve feedback on further HCl secretion
2. Pepsinogen
- Pepsinogen undergoes autocatalytic cleavage by acidic pH of stomach → forms pepsin → proteolytic enzyme that aids protein digestion
3. Intrinsic Factor
- glycoprotein that facilitates Vitamin B12 (cobalamin) absorption
4. Mucous in alkaline-rich fluid
- Forms “gastric mucosal barrier” → keeps H+ out of mucosa (prevents autodigestion by HCl → ulceration) and Na+ in it (maintains potential difference across mucosal surface)
- Lubricates food
- Traps bacteria
5. Gastric Lipase and Amylase
- Aid digestion of fats and CHO (minor role only)
Functions of Gastric Secretions: Non-Gastric Juice
1. Gastrin
- ↑ gastric HCl secretion (1500x more potent vs. histamine) → stimulates parietal cells directly and indirectly (via histamine from ECL cells)
- ↑ pepsinogen secretion from chief cells
- +ve trophic effect on small intestinal/colonic mucosa and parietal cell mass
- ↑ gastric and intestinal motility
- ↑ LOS contraction (preventing reflux)
- ↑ GB contractions and pancreatic secretions
2. Histamine
- MAJOR stimulus for gastric HCl secretion → acts via H2R (Gs) on parietal cells
3. Somatostain
- Potent inhibitor of gastric acid secretion (acts directly on parietal cells and indirectly by inhibiting gastrin release from G-cells)
- Inhibiting secretion of most GI hormones (Eg. gastrin, VIP, GIP, secretin)
- Inhibits pancreatic exocrine secretion
- Inhibits gastric motility (including ↓ gastric emptying rate)
- Inhibits gallbladder contraction
- Inhibits intestinal absorption of nutrients
JC 2019
Examiner Comments
2009A 19: Pass rate: 40%
Candidates were expected to list and briefly define the role of the various substances produced and secreted by the stomach. These included the hormones gastrin and somatostatin, the enzymes pepsin, lipase and gelatinase, the electrolytes Na+, K+ and HCO3- , HCl and water, prostaglandins and mucus, and intrinsic factor. For example: HCl secreted by parietal cells to produce a very acidic environment pH 1-3.5. This optimizes proteolytic activity of pepsin, has a direct proteolytic role, aids ferric iron conversion to the more soluble ferrous ion, and is important for bactericidal activity and innate immunity. It also stimulates pancreatic and biliary secretions.
Good answers divided the functions into digestive, hormonal, mucosal protection, immunity etc. Marks were not gained for mention of the secretions of other GIT organs.
SyllabusQ12b
Reference: Guyton and Hall 11th edition p791-799
20. Describe the pharmacological basis of the management of organophosphate poisoning.
CICMWrecks Answer
Organophosphate posisoning
SOURCE – insecticides, exposure to nerve agents (most commonly seen in farming settings)
PATHOPHYSIOLOGY
- Organophosphates form a covalent bond with acetylcholinesterase, inhibiting the metabolism of acetylcholine.
- This results in high levels of Ach at the neuromuscular junction (both nicotinic and muscarinic receptors), causing a cholinergic crisis
SYMPTOMS
- SLUDGE (salivation, urination, defaecation, gastrointestinal motility, emesis)
- CNS – confusion, anxiety, tremors, seizures
- CVS – bradycardia, hypotension
- RESP – respiratory impairment due to muscle weakness, difficulty clearing secretions
MANAGEMENT
- Remove the patient from the source of poisoning
- PPE for staff – mask, goggles, neoprene gloves and gowns rather than latex as hydrocarbons can penetrate latex
- ABC’s and resuscitation as required
- Decontamination –
- activated charcoal / gastric lavage / whole bowel irrigation unlikely to be effective due to rapid absorption of organophosphates.
- Clothes should be removed and discarded and the patient bathed with soap and water to remove organophosphates from skin.
- Irrigate the eyes if ocular exposure has occurred.
- Symptom control
- Anti muscarinic agents –
- atropine in large doses will reduce the antimuscarinic effects by competitively antagonizing Ach at the receptor.
- ETG suggests 1.2-3mg boluses, aiming SBP>80mmHg, HR >80bpm and absence of wheeze, doubling the dose every 5 mins until atropinisation is achieved, followed by an infusion.
- Glycopyrrolate can be used to reduce secretions post initial atropinisation, however does not cross the BBB so will have no effect on the central cholinergic effects
- There is no antinicotinic agent so supportive care only for muscle weakness.
- Benzodiazepines for seizure control
- Anti muscarinic agents –
- Definitive treatment
- Cholinesterase regenerator (an oxime such as pralidoxime) may reactive AchE and prevent the organophosphate-acetylocholinesterase complex ageing and becoming an irreversible bond if given within 48 hrs
Examiner Comments
2009A 20: Pass rate: 40%
Organophosphates (OGP) bind irreversibly to acetyl cholinesterase. They produce a cholinergic crisis and muscle paralysis due to excess Acetyl choline (ACh) at all muscarinic and nicotinic receptors.
Candidates were required to discuss the pharmacology relevant to treating OGP poisoning, including active decontamination/staff protection due to high lipid solubility, use of antimuscarinics with central and peripheral action to treat cholinergic symptoms, supportive therapy for muscle weakness (there is no antinicotinic agent available which does not exacerbate muscle weakness), and finally the use of the cholinesterase regenerator, Pralidoxime, which may prevent the OGP-AChE complex ageing and becoming an irreversible bond if given in a timely fashion.
Good answers included a discussion of the mechanism of action of the therapeutic agents, the time course of therapy, the large doses/infusions of atropine required and the titration of therapy to reversal of muscarinic effects. Long lists of signs and symptoms were not required to pass this question.
Syllabus H2b2c
Reference: Rang Dale Ritter 6th edition p 164-166, Katzung 10th edition p 116-117, 968.
21. Compare and contrast the mechanism of action and side effects of tricyclic antidepressants, selective serotonin reuptake inhibitors and monoamine oxidase inhibitors.
CICMWrecks Answer
| TRICYCLIC | SSRI | SNRI (Not in this qn) | MAOI | |
|---|---|---|---|---|
| E.g. | Amitriptyline | Fluoxetine | Venlafaxine | Phenylzine |
| PK | Well-absorbed PO with high first pass. Highly protein bound. Very lipid soluble. Hepatic phase 1 and 2 metabolism Renal excretion T1/2= 30-45 hours | 50:50 racemic mixture Well absorbed Hepatic metabolism Does require dose adjustment in renal failure for metabolites | High first pass- F=45% Large Vd with much lower PB (30%) Hepatic 2D6 metabolism to desvenlafaxine (active) Renal elimination. T1/2= 10 hours | Incomplete data available Renal elimination of metabolites Duration depends on time taken for MAO synthesis |
| MoA | Competitively inhibit neuronal uptake (uptake 1) of noradrenaline and serotonin, thereby increasing their concentrations in the synapse Additional: 1. Antimuscarinic 2. Antihistamine 3. Alpha-adrenoreceptor antagonism | Inhibition of SERT transporter increases synaptic 5-HT. No effect on NET | Inhibition of serotonin re-uptake (SERT) and noradrenaline re-uptake (NET). Low affinity for NET at low doses. Little muscarinic, H1 or alpha1 antagonism effects | Irreversible MAO A and B inhibition leading to accumulation of: 1. Noradrenaline 2. Serotonin 3. Dopamine |
| A/E | 1. Initially increase suicidality in youth 2. CNS- sedation (H1), seizures 3. Anti-Ach: dry mouth, constipation, urinary retention, blurred vision 4. CVS- postural hypotension | Serotonin A/E (shared by all AD): – Insomnia/somnolence – N/V/D – Sexual- low libido, impotence – Initial suicidality | 1. Serotonin A/E 2. Norad- HTN, tachycardia | 1. Serotonin 2. Histamine 3. Anticholinergic 4. Anti-alpha 5. Adrenergic |
| OD | 1. CVS – Sinus tachy – QT prolongation – QRS prolongation → VT/VF (Sodium channel blockade) – RBBB – Labile BP 2. CNS – Excitation and seizures then depression 3. Mydriasis, hyperthermia Treatment: 1. Benzos for seizures 2. Hyperventilation and sodium bicarbonate if QRS prolonged 3. Avoid inotropes if possible | 1. Serotonin Syndrome | 1. Serotonin syndrome 2. Cardiotoxic | 1. Serotonin syndrome Interacts with other antidepressants, foods high in tyramine (cheese) and sympathomimetic |
Ruan 2020
Examiner Comments
2009A 21: Pass rate: 40%
Good answers were in tabular format.
The antidepressant action is similar for each agent. Initial increase in 5HT and NA, followed in 2-3 weeks by a down regulation or change in efficiency of 5HT transmission. The agents produce elevated neurotransmitters via different mechanisms, either reuptake blockade or enzyme inhibition. MAOIs can be competitive or non-competitive. Mention of the different neurotransmitters affected by each agent was required.
A description of significant side effects at therapeutic doses, and in overdose was expected with explanations provided. These should have included – the anticholinergic effects and cardiotoxicity of TCAs, postural hypotension, the catecholamine, pethidine and tyramine related complications of MAOIs, and serotonin syndrome with SSRI/MAOI use and or overdose. More marks were gained for mention that side effect profiles can be beneficial e.g. analgesic properties of TCAs, sedation with TCAs/ SSRIs and energizing benefits of SSRIs/SNRIs. SSRI’s safety and efficacy have markedly reduced the use of MAOIs and to a lesser extent TCA’s..
Syllabus G2f2d
Reference: Stoelting p 398-407, Katzung p 476-487.
22. Outline the mechanism of action of drugs used to control raised intracranial pressure.
CICMWrecks Answer
Intro
ICP: hydrostatic pressure within the cranial vault
- Normal value is 5-15 mmHg
- focal ischaemia when ICP > 20 mmHg
- global ischaemia when ICP > 50mmHg
Munro-Kellie
- The rigid and closed cranial vault forms a fixed brain volume containing
- Brain parenchyma (80%, 1400 g)
- CSF (10%; 75 mL)
- Cerebral blood and vessels (10%; 75 mL)
- Δ’s in volume of any components → Δ’s in others
Drugs Control:
Volume of Brain Parenchyma
- Osmotic Agents
- Mannitol (Response Augmented by concurrent use of Loop Diuretics
- Hypertonic Saline
Changes in Cerebral Blood Flow (CBF)
- ↑ CBF from ↑
- CO (thus HR and SV)
- Inotropes
- SVR
- Vasopressors
- CO (thus HR and SV)
- ↓ CBF from ↑
- radius (thus peripheral metab (esp adenosine), incr CO2, decreased O2, vagal stimulation)
- Induction agents (thiopental) → ↓ CMRO2 → ↓ CO2 production → ↓ CBF → ↓ Volume
- Other ↓CMRO2 agents
- Anticonvulsants (phenytoin, levitiractam) → ↓CMRO2 and ↓ Temperature
- Analgesic (opiods etc)
- Sedatives (benzodiazepine)
- Antipyretics should be instituted to reduce fever which will increase metabolic demands
- viscosity
- ↑ Free water excretion (frusemide + mannitol)
- radius (thus peripheral metab (esp adenosine), incr CO2, decreased O2, vagal stimulation)
Volume of CSF
- Acetazolamide (Carbonic Anhydrase Inhibitor): ↓ CSF production
Gladwin 2016
Examiner Comments
2009A 22: Pass rate: 20%
The answers to this question were generally not broad enough. Only one or two drugs were discussed rather than the range of drugs used in this situation. Discussion should have included benzodiazepines, intravenous induction agents, opioid narcotics, diuretics including loop diuretics and mannitol. Hypertonic saline also gained marks. Generally the discussions on the drugs mentioned were done well.
Some candidates discussed the physiological control of intracranial pressure which was not required and gained no marks.
Syllabus G2g, G2a2a E2a2a
23. Describe the mechanism of action, antibacterial spectrum and pharmacokinetics of aminoglycosides.
CICMWrecks Answer: Aminoglycosides
AMINOGLYCOSIDES
Mechanism of Action
- Permeates into the bacterial cell down electronegative gradient (negative interior) via O2 dependent process
- Can be retarded by acidic, anaerobic environments (abscess)
- Inhibits bacterial 30S ribosomal subunit
- Inhibits bacterial protein synthesis
- Inhibition of bacterial growth (bacteriostatic) → bacteriocidal
- Synergism with cell wall inhibitors (β lactam antibiotics)
- Penicillins inhibit peptidoglycan synthesis → increased permeability to aminoglycosides → inhibition of protein synthesis (including penicillin binding protein) → Further increase in cell permeability → death
- Displays concentration dependent killing and post-antibiotic effect
- Peak plasma aminoglycoside >8 MIC required
- Large AUC not required
Antimicrobial Spectrum
- Strong activity against aerobic gram negative bacteria
- Effective against gram positives esp. with β lactam synergism
- Ineffective against obligate anaerobes
Pharmacokinetics
- Absorption
- Poor GI absorption, UNLESS inflammation or ulceration
- Distribution
- Vd approximating extracellular fluid
- Poorly protein bound
- Poor CNS, ocular penetrance
- Metabolism
- Minimal metabolism
- Elimination
- Renal clearance via filtration
- T1/2b 2~3 hours
- Can be removed by dialysis
Examiner Comments
2009A 23: Pass rate: 50%
This answer was generally well done. Most answers showed good understanding of mechanism of action. The antibacterial spectrum and pharmacokinetics were done less well.
Clinical observation guiding your study would help in answering this question, such as renal dosing, monitoring drug levels and situations in which aminoglycosides are used.
Syllabus M2 2d
Reference: Katzung 10th edition p 755-762
24. Explain the factors which influence the transfer of drugs across the placenta to the foetus.
CICMWrecks Answer
Physiological factors
- Drug transfer via diffusion, facilitated diffusion, secondary active transport, pinocytosis (e.g. immunoglobulins)/
- Diffusion according to Fick’s Law (see eqn):
where- MW = Molecular weight
- Drugs <500da freely permeable
- Δ[Drug] = Concentration gradient between maternal anf foetal blood
- SA = Surface area for diffusion
- 16m2 compared to alveolar surface area of 60m2
- h = thickness of diffusion membrane
- 3.5 microns compared with 0.5 for alveolar diffusion membrane
- MW = Molecular weight
- Drug delivery to uterus
- Blood flow ~750ml/min (Aprrox 600ml/min to placenta)
- No autoregulatory mechanisms
- Placental transporters
- MDR1 and p-glycoprotein efflux drugs out of foetal circulation
- Placental metabolism
- P450 reactions
- Pentobarbitol metabolized by placenta
- P450 reactions
Drug factors
- Drug size
- Heparin too large to permeate (Warfarin can)
- Lipophilicity
- Highly lipophilic drugs more permeable (Fentanyl > morphine)
- Ionization
- Charged molecules less permeable (Glycopyrrolate < atropine)
- Ionization trapping
- Alfentanyl (pKa 6.5) ~90% unionized at physiological pH → more permeable
- As alfentanyl crosses into foetal circulation, becomes more ionized in lower pH → trapped in foetal circulation
- Protein binding
- Reduces permeability
Disease factors
- Inflammation of placental barrier increases permeability of drugs
Sakurai 2016
Examiner Comments
2009A 24: Pass rate: 30%
Good answers showed an understanding of diffusion, the influence on transfer of lipid solubility, molecular size, degree of ionisation and protein binding. They also made reference to placental transporters and placental metabolism. Extra points were scored for mentioning that the real concern is teratogenicity to the foetus.
Syllabus O2 2d
Reference: Katzung 10th edition p 971-973.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | pharmacokinetics in: Alcohol, Ageing |
| D. Variability in Drug Response | |
| E. Cellular Physiology | discuss cell membrane physiology and electrolyte disturbances. |
| F. Respiratory | Patient with PE. Calculate the A-a gradient. |
| G. CVS | action potential of the ventricle, sinus node and the atrioventricular node. It will also assess knowledge of anti-arrhythmic drugs |
| H. Renal | |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | Red Blood Cells and the Clark electrode |
| R. Thermoregulation | |
| S. Immunology | Outline the body’s defence mechanisms against infection |
| T. Microbiology | How do bacteria differ from the majority of normal human cells (eukaryotes)? Antimicrobial agents. |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures | US image of Carotid, IJV – how can you distinguish the two |

Recent Comments