1. Outline three (3) factors that alter the pharmacodynamic response of non-depolarising neuro-muscular blocking drugs and describe the mechanism by which they may occur.
CICMWrecks Answer
Pharmacodynamics
Prejunctional Factors:
- Electrolyte abnormalities
- Hypo/Hypercalcaemia
- ↓/↑ Prejunctional Ca entry on depoarisation → ↓/↑ ACh release
- ↓ Ca → prolongation of blockade by NDMB due to ↓ Competitive antagonism
- ↓/↑ Prejunctional Ca entry on depoarisation → ↓/↑ ACh release
- Hypo/Hyperkalaemia
- Hyper/Hypopolarisation of pre-junctional membrane
- ↓ [K] → Hyperpolarisation → prolonged effect of NDMB
- Hyper/Hypopolarisation of pre-junctional membrane
- Hypo/Hypercalcaemia
Junctional Factors:
- Presence of co-administered anticholinesterase drugs (eg neostigmine
- ↑ [Ach] in NMJ → ↑competitive antagonism of NDMB → ↓ duration of effect/delayed onset
- Effect of NDMB relates to receptor occupancy
- 95% occupancy → TOF ¼
- 90% occupancy → TOF ¾
Post-Junctional Factors:
- Thermal injury/UMN lesion/Burns
- Upregulation of foetal type junctional and extra-junctional nAChRs
- ↑ receptor numbers → greater dose required to get the same percentage of receptors occupied
- Prolonged opening compared with adult receptors → predisposition to hyperkalameia → ↑’s RMP
- ↓ Plasma cholinesterase → ↓ metabolism of mivacurium
- Upregulation of foetal type junctional and extra-junctional nAChRs
- Co-administered local anaesthetic
- Na-channel blockade →
- pre-junctional membrane stabilisation → ↓ ACh release → ↑ NDMB effect duration
- post-junctional membrane stabilisation → ↑’s receptor occupancy required to achieve excitatory post synaptic potential (EPSP) → prolonged effect of NDMB
- Na-channel blockade →
CICMWrecks 2016
Examiner Comments
2008B 01: 2 (40%) candidates passed this questions
There were a number of possible factors that candidates could have selected. Examples include drug interactions (anticholinesterases, aminoglycoside antibiotics, local anaesthetics, steroids, antiarrhythmic drugs, anticonvulsants (phenytoin), diuretics, magnesium, lithium), hypothermia / hyperthermia, acidosis, [k+], burn injury, allergic reactions, gender, altered elimination due to renal or hepatic dysfunction and disease states (adrenocortical dysfunction, myasthenia, myopathies, denervation injury). Also extremes of age and pregnancy. Areas of weakness for the candidates were failure to include sufficient factual knowledge for their selected factors, and as a result, a failure to
illustrate sufficiently the mechanisms by which the pharmacodynamic response of nondepolarising neuro-muscular blocking drugs may be affected.
Syllabus: H2a 2 (c)
Reference Text: Pharmacology and Physiology in Anaesthetic Practice / R K Stoelting
2. Statistics (not in current primary syllabus)
3. Compare and contrast the body’s bicarbonate, phosphate and protein buffer systems.
CICMWrecks Answer
Buffer
- Weakly ionised acid or base in equilibrium with its full ionised salt
- A buffer can “resist” change in pH by absorbing or releasing H+ ions
- Works best when pKa is closest to the target pH (7.4)
- Isohydric Principle
- All buffer systems which participate in defence of acid-base changes are in equilibrium with each other. There is after all only one value for [H+] at any moment. This is known as the Isohydric Principle.
Effectiveness:
- buffer pKa (most effective if pKa = pH of carrying solution)
- pH of carrying solution
- amount of buffer present
- open (physiological) vs closed (chemical) system
Buffer Systems
| Name | Location |
|---|---|
| Protein | Intra cellular |
| Haemo globin | Blood |
| Bicarb | Plasma, Interstitium, Urine (minimal) |
| Ammonia | Urine |
| Phosphate | Urine, Bone |
| CaCO3 | Bone |
Compare and Contrast:
Bicarbonate, Phosphate, Protein buffer systems
| Bicarbonate | Phosphate | Protein |
|---|---|---|
| H+ + HCO3– ⇌ H2CO3 ⇌ H2O + CO2 | H+ + HPO42- ⇌ H2PO4– | H+ + Prot– ⇌ HProt |
| Extracellular (80% of plasma buffer capacity) | Nil extracellular. Important in tubular fluid | Extracellular (20% of plasma buffer capacity) |
| poor intracellular buffers (poor membrane diffusion of H+, HCO3– (except RBC)) | Some Intracellular buffering | Major Intracellular (60~70% of total body buffering capacity occurs intracellularly and most of this is due to proteins) |
| pKa 6.1 | pKa 6.8 | pKa of histidine residues 6.8 |
| pKa far from physiological pH (7.4) However Good buffer due to abundance of substrates | pKa close to physiological pH However extracellular concentration 1.8 of bicarbonate | pKa close to physiological pH |
| Open ended system – CO2 eliminated by lungs – HCO3 eliminated renally Requires carbonic anhydrase | Becomes important buffer in renal tubules due to H2O reabsorption and consequent concetration of phosphate. | Hb important buffer in blood due to 38 histidine residues per tetramer Deoxyhaemoglobin further increases buffering capacity by increasing pKa to 7.8 |
Gladwin / Sakurai / JC 2020
Examiner Comments
2008B 03: 3 (60%) candidates passed this question
This question was generally well answered. Successful candidates illustrated their answer through the use of a table. For a good pass candidates were expected to include a definition of a buffer; mention the buffering capabilities of the differing buffering systems (bicarbonate, phosphate and protein buffers) in relation to the pKa; the area in the body where they are most effective and whether it was an open or closed system.
The protein buffer system was the least well conveyed by candidates, despite approximately 60 to 70 per cent of the total chemical buffering of the body fluids being inside the cells, and most of this from the intracellular proteins. For a good answer it was expected that candidates also mention that, except for the red blood cells, the slowness with which H+ and HCO3- move through the cell membranes often delays for several hours the maximum ability of the intracellular proteins to buffer extracellular acid-base abnormalities. In addition to the high concentration of proteins in the cells, another factor that contributes to their buffering power is the fact that the pKas of many of these protein systems are fairly close to 7.4.
Syllabus: F2b
Reference Text: Guyton Chp 30
4. Define basal metabolic rate and describe factors that influence it (70% of marks). How could you measure metabolic rate? (30% of marks)
CICMWrecks Answer
Basal Metabolic Rate
- Energy expenditure required to maintain the body’s basic homeostatic mechanisms
- Normal BMR = 40kcal/m2/hr or approx. 70kcal/hr for 70kg male
- Relative organ contribution to BMR
- Liver 30%
- Brain 20%
- Muscle 20%
- Kidney 10%
- Heart 10%
- Other 10%
Factors that affect BMR
- Prandial effects
- Metabolic rate increases post-prandially for the digestion and absorption of nutrients
- After prolonged fasting metabolic rate decreases
- Thermoregulatory effects
- Temperatures above and below the thermoneutral zone cause metabolic rate to increase to support extra thermoregulatory mechanisms
- Response to heat
- Sweating
- Vasodilatation
- Response to cold
- Shivering
- Non-shivering thermogenesis
- Vasoconstriction
- Response to heat
- Temperatures above and below the thermoneutral zone cause metabolic rate to increase to support extra thermoregulatory mechanisms
- Physical activity
- Increased muscle activity
- Exercise increases metabolic rate in order to supply ATP to sarcomeres for contraction
- Increased muscle activity
- Inflammation
- Metabolic rate increases
- Chemotaxis
- Increased oxidative bursts
- Cell proliferation and clonal expansion
- Metabolic rate increases
- Psychological stress
- Hormonal factors
- Thyroid hormones → increase metabolic rate
- Maximal thyroid hormone activity increases MR 100%
- Thyroidectomy decreases MR 50%
- Catecholamines → increase metabolic rate
- Growth hormones → increase metabolic rate ~15~20%
- Testosterone → increase metabolic rate ~10~15%
- Thyroid hormones → increase metabolic rate
- Age
- Decreases with age >20
- Sex
- Male BMR > Female BMR possibly due to testosterone effect
- Body mass
- Muscle mass accounts for significant proportion of BMR, therefore increases BMR
- Sleep
- Decreases BMR 10~20%
Measurement of BMR
- Direct Calorimetry
- Atwater Chamber
- Subject placed in insulated chamber surrounded by H2O
- Change in temperature of water proprotional to metabolic rate
- Atwater Chamber
- Indirect calorimetry
- Metabolic carts
- Measurement of O2 consumption (and CO2, Urea production) to determine Metabolic rate
- Respiratory quotient
- Carbohydrates = 1
- Fats = 0.7
- Proteins = 0.8
- Metabolic carts
Conditions required to measure BMR
- Thermoneutral zone
- Free from psychological or physical stimulus
- After a night of restful sleep
- 12 hours after last meal
- 2 hours after last exercise
Sakurai 2016
Examiner Comments
2008B 04: 3 (60%) candidates passed this question
Metabolism is all the chemical reactions in all the cells of the body. Metabolic rate is usually described in terms of rate of heat liberation. Candidates often confused basal with metabolism during activity.
A good answer was expected to outline conditions under which basal metabolic rate is measured (no food for 12 hours, after a night of restful sleep, person is at rest in reclining position for 30 minutes, all psychic or physical factors that cause excitement eliminated, at a controlled comfortable temperature), and the mention of and an explanation of the methods by which metabolic rate is measured (eg direct and indirect calorimetry). Any validated technique of determining the BMR was rewarded with marks, with oxygen consumption methods being the easiest to explain. No candidate mentioned any ‘normal’ value for the BMR.
Candidates were expected to briefly mention and explain how factors affecting metabolic rate (eg exercise, specific dynamic action of protein after a meal is ingested,, age, thyroid hormone, sympathetic stimulation, fever, gender, hormonal, climate, sleep, chronic malnutrition, trauma, inflammatory response, etc).
Questions with more than one part will have an indication of the proportion of the total mark that will be allocated to each part. It is important that candidates apportion their allocated time and content accordingly.
Syllabus: K 2a, b
Reference Text: Guyton Chp72
5. Outline the pharmacology of amiodarone.
Examiner Comments
2008B 05: 2 (40%) candidates passed this question
Successful candidates applied, a systematic approach/format to answer questions that refer to outlining pharmacology of select drugs. A number of useful mnemonics are suggested in the recommended texts for use when answering such a question. All candidates correctly stated what amiodarone is used for but most were not structured methodically and thus suffered from significant omission. Amiodarone is an important class III anti-arrhythmic (with some characteristics of all 4 Vaughan-Williams classes). For a good pass candidates were expected to actions of amiodarone (eg blocks inactivated Na channels, decreases Ca current, noncompetitive adrenergic blocking effect, blocks myocardial K channels which contributes to slowing of conduction and prolongation of refractory period in AV node, prolongs refractory period in all cardiac tissues, prolongs cardiac action potential duration) and it’s pharmacokinetics (eg bioavailability, large volume of distribution, high protein binding, complex metabolism and long elimination half life – 29 days)
Syllabus: C2c
Reference Text: Goodman and Gillman’s The Pharmacological basis of Therapeutics 11th ed 2006 and Pharmacology and Physiology in Anaesthetic Practice / Stoelting 4th ed 2006
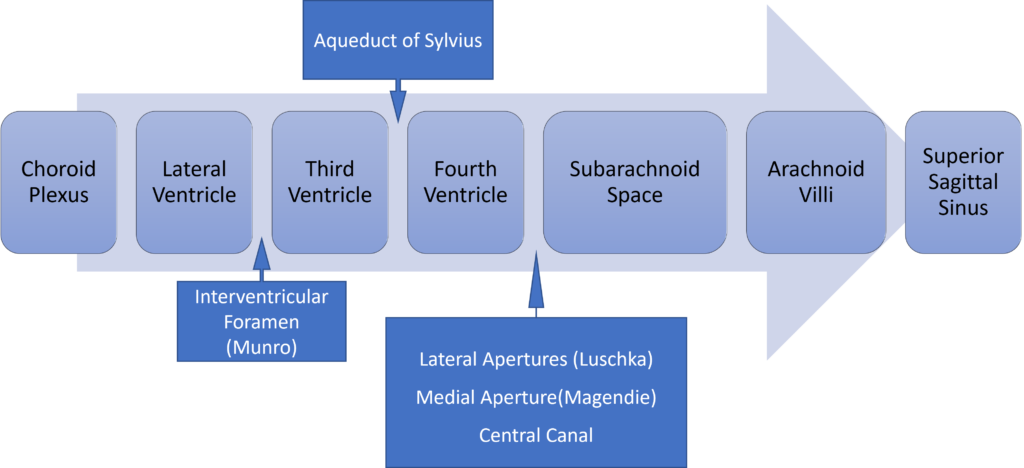
6. Describe the formation, circulation and functions of cerebrospinal fluid.
CICMWrecks Answer
Formation / Production of CSF
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
Distribution / Circulation of CSF
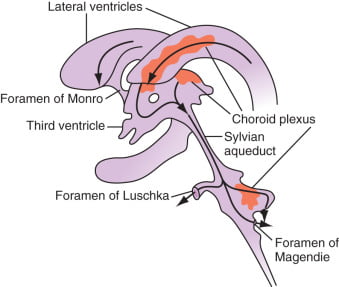
Absorption of CSF
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
Composition of CSF
IDENTICAL to brain ECF, but differs in several manners from plasma
Compared with plasma:
- ↑ pCO2 (50 mmHg)
- ↓ pH (7.33)
- ↓ protein content
- 0.5% of plasma; 20 mg/dL
- poor acid-base buffering capacity
- ↓ content of glucose (by 60%) and cholesterol
- ↑ [Cl-] (by 7-14%) and ↑ [Mg2+] (by 40%)
- ↓ [K+] (by 40%), ↓ [Ca2+] (by 50%) and ↓ [Pi] (by 20-30%)
- ↑ creatinine (by 25%) but ↓ urea
- IDENTICAL osmolality (295), [Na+] (145), and [HCO3] (25)
Role / Functions of CSF
- Protective role (main function)
- Water bath effect
- Attributed to the low specific gravity of CSF (1.007)
- Causes brain to be buoyant
- ↓ its effective net weight from 1400 g to 50 g
- Mechanical cushion against acceleration/deceleration forces
- Buffer ↑ ICP by CSF translocation to extracranial subarachnoid space
- Abrupt ↑ ICP buffered by translocation of CSF within the vault to extracranial compartments
- Water bath effect
- Maintains constant ionic environment conducive to neuronal electrical activity CSF
- Supply role of nutrients (Eg simple sugars, amino acids) and O2 to brain
- Excretion of toxic substances, metabolic by-products, and CO2 from brain
- “Lymph-type” function → interstitial proteins in brain ECF return to circulation by
- CSF absorption across arachnoid villi
- Acid-base regulation → due to content, CSF allows for tight respiratory control
- Endocrine transport function → transports hormones to other brain regions
Gladwin / JC 2020
Examiner Comments
2008B 06: 4 (80%) candidates passed this question
To achieve a pass in this question, candidates needed to state where and how CSF was formed, where it flows to after formation followed by a list of its functions. Additional credit was given for knowledge of rates of production and basic CSF composition. The fact that CSF production is constant whilst its absorption is pressure dependant was often overlooked. Thus candidates were expected to mention that there is ~ 150 ml of CSF in the adult, half within the cranium; about 60-70% of the CSF is formed by the choroid plexuses, the remaining 30-40% by the cerebral vessels lining the ventricular walls; in humans the CSF turns-over ~ 4 times/day; composition is essentially brain ECF; brain ECF normally occupies ~ 15% of brain volume; CSF flows out through the foramina of Magendie and Luschka and is absorbed through the arachnoid villi into the cerebral venous sinuses; absorption, being largely by bulk flow, is proportional to ventricular pressure [at normal pressure ~ 7.0-18.0 cmH2O (mean ~ 11), filtration = absorption, when pressure falls below ~ 7 cmH2O absorption ceases] and CSF Functions [buoyancy, constant metabolic environment, buffers CSF against rapid plasma changes in K+, Ca++, Mg++, transport of chemical messengers, sink for waste disposal].
A number of candidates embarked on long discussions of how CSF pH affects physiology to the exclusion of what was asked for in the question. Many answers did not adequately cover the three components asked for in the question.
Syllabus: CNS 2d
Reference Text: Guyton Chp 61
7. Poisoning (not in current primary syllabus)
8. Describe the clinical findings you would expect to see in a patient who underwent acute hemi-section of the spinal cord at the upper thoracic level.
CICMWrecks Answer
Spinal Cord Tracts:
(not a comparison table)
Ascending Tracts
SENSORY
Descending Tracts
MOTOR
Convey sensory info from peripheral sensors to higher centres in brain.
From posterior to anterior:
Carry motor information
- Dorsal (posterior) column:
- fine touch + proprioception + vibration
- crosses at the brain stem
- Spinocerebellar tracts (ant + post):
- Carry proprioceptive info from muscles + joints to cerebellum
- Does not cross
- Lateral spinothalamic tracts
- pain + temperature
- Crosses within 2 vertebral segments
- Anterior spinothalamic tracts
- crude touch + pressure
- Crosses within 2 vertebral segments
- Corticospinal tracts (ant + lateral) “pyramidal tracts”:
- motor function – carry axons of UMN
- crosses at the brain stem
- Relay to alpha-motor neurons (LMN) in ventralnhorn of spinal cord
- Extrapyramidal tracts:
- rubrospinal, tectospinal, vestibulospinal, olivospinal, reticulospinal
- Originate in brainstem nuclei and do not pass through medullary pyramids
- Role in control of posture + muscle tone
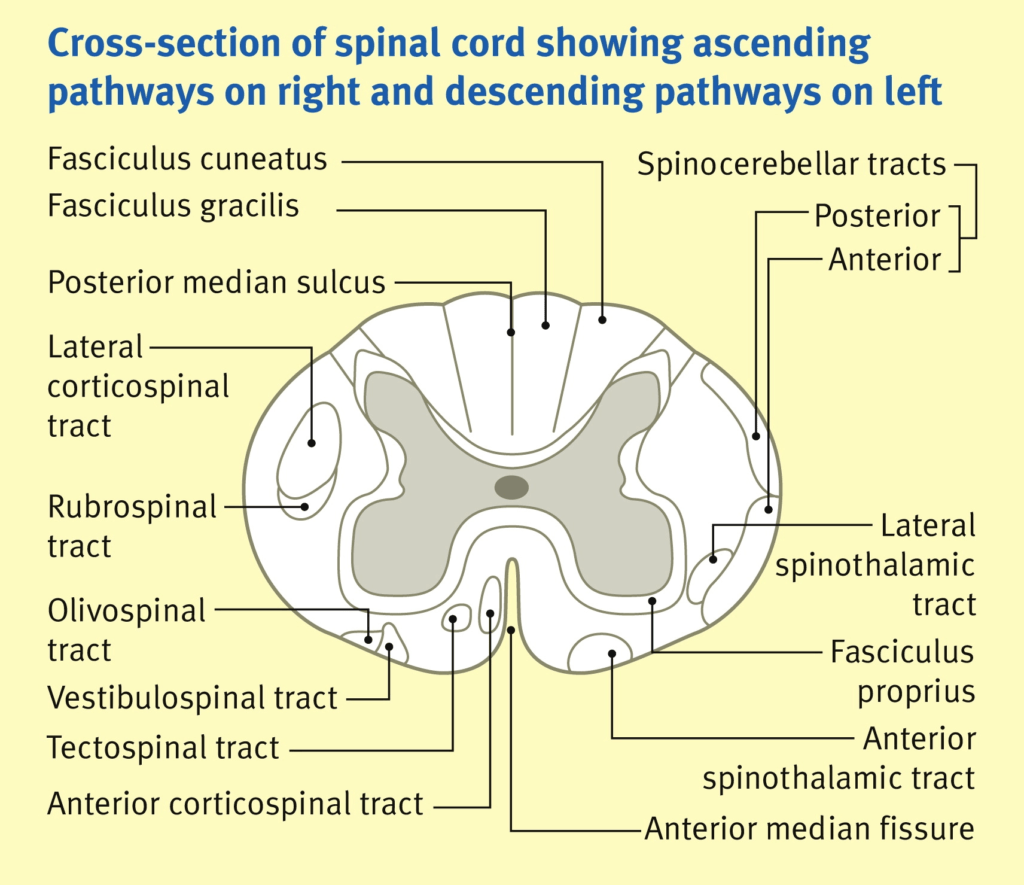
John Craven
Anaesthesia & Intensive Care Medicine.
PAIN. VOLUME 12, ISSUE 1, P26-27, JANUARY 01, 2011
Acute hemi-section of the spinal cord at the upper thoracic level
Brown-sequard syndrome: Hemisection of the cord
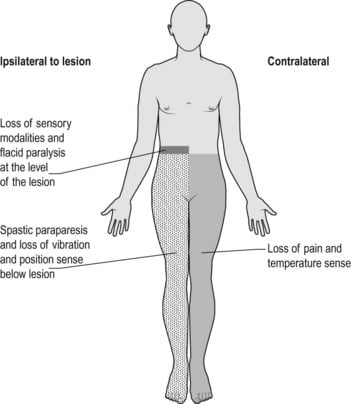
| ipsilateral loss of motor function | below level of lesion | due to interruption of descending fibres in the lateral corticospinal [pyramidal] tracts. Initially the paralysis is flaccid, later it becomes hypertonic and hyperreflexic with extensor plantar response [upper motor neurone lesion]. |
| ipsilateral loss of light touch + proprioception + vibration | below level of lesion | due to interruption of ascending fibres in the posterior [dorsal] columns |
| contralateral loss of pain + temp | below level of lesion | Interruption of ascending fibres in the crossed lateral spinothalamic tract |
| ipsilateral loss of pain + temp | at the level of the lesion | segmental anaesthesia of the dermatome due to damage of the nerve roots and anterior horn cells at this level |
Examiner Comments
2008B 08: 1 (20%) candidate passed this question
The clinical condition that results from this lesion is the so called Brown-Sequard syndrome. However only a very small proportion of points were given to mention of the latter, with the majority of points allocated to knowledge relating to spinal cord anatomy and physiology.
The expected 4 main clinical features that are associated with this lesion are –
1. There is loss of pain and temperature sensation on the contralateral side below the level of the lesion due to interruption of ascending fibres in the crossed lateral spinothalamic tract.
2. There is loss of vibration, joint position and 2 point discrimination on the ipsilateral side below the level of the lesion due to interruption of ascending fibres in the posterior [dorsal] columns.
3. There is paralysis of voluntary movement on the ipsilateral side below the level of the lesion due to interruption of descending fibres in the lateral corticospinal [pyramidal] tracts. Initially the paralysis is flaccid, later it becomes hypertonic and hyperreflexic with extensor plantar response [upper motor neurone lesion].
4. Finally there is segmental anaesthesia of the dermatome at the level of the lesion on the ipsilateral side due to damage of the nerve roots and anterior horn cells at this level.
Some candidates described the clinical features of complete section of the spinal cord which was not asked for.
Syllabus: G1 2b
Reference Text: Guyton Sections IX and XI
9. Describe the physiological consequences that follow an intravenous bolus of 50mls of 50% glucose.
CICMWrecks Answer
Immediate
50mls of 50% Glucose = bolus of 25g Glucose
- Hyperglycaemia – normal blood glucose is 4-7 when fasted, <11.1 when not fasted
- Glycosuria – resorption capacity of the renal PCT is exceeded when serum glucose >10-12mmol/l
- resorption of glucose is via co-transport with Na+
Insulin
- Secreted from
- Pancreatic beta cells in islets of Langerhans
- Mechanism of secretion
- Glucose moves into beta cells via GLUT-2 transporters
- Glucose –-(glucokinase)–> Glucose-6-phosphate –-(glycolysis)–> ATP
- ATP inhibits ATP sensitive K+ channels
- Cell depolarises → calcium influx
- Calcium stimulates exocytosis of insulin-containing vesicles
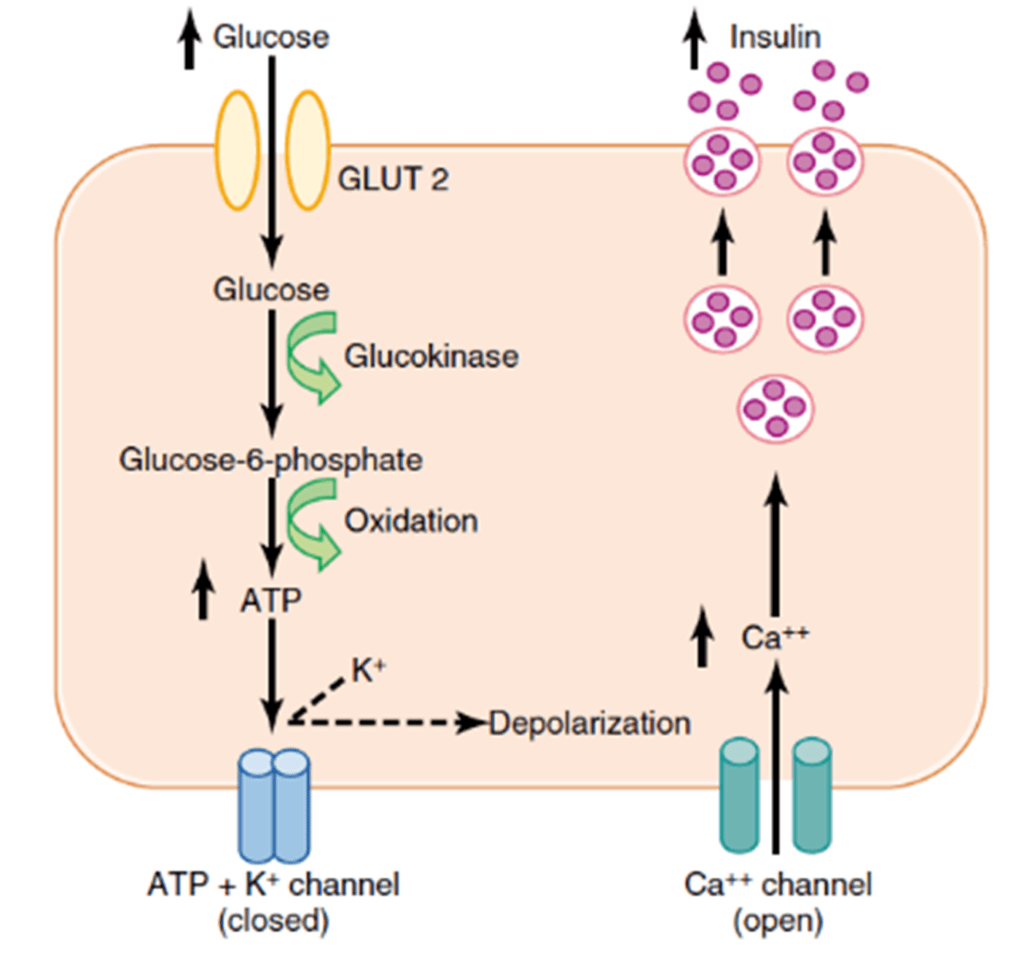
- Response
- Insulin release
- Inhibits gluconeogenesis
- Promotes muscular, adipose and peripheral cell glucose uptake
- GLUT-4 transporter
- Promotes hepatic glycogenesis
- Glucose trapping
- Hexokinase phosphorylates glucose (glucose-6-phosphate)
- Negatively charged G6P cannot cross membrane, so is trapped in hepatocyte
- Stimulates glycogen synthase
- Glucose trapping
- Inhibits glycogenolyis
- Inhibits liver phosphorylase
- Promotes FFA synthesis, fat deposition
- Inhibits lipolysis
- Promotes protein anabolism, inhibits catabolism in muscle
- Insulin release
- Fate of glucose
- Glycolysis -> pyruvate
- Pyruvate and CoA -> acetyl CoA -> enters citrate cycle
- FADH and NADH from citrate cycle -> electron transport chain
- Yield from 1 glucose is around 38 ATP
- Pattern
- Biphasic release
- Phase 1: Dumping of preformed insulin
- Phase 2: Drop-off in insulin secretion, then rise up towards a higher peak when new insulin is made
- Biphasic release
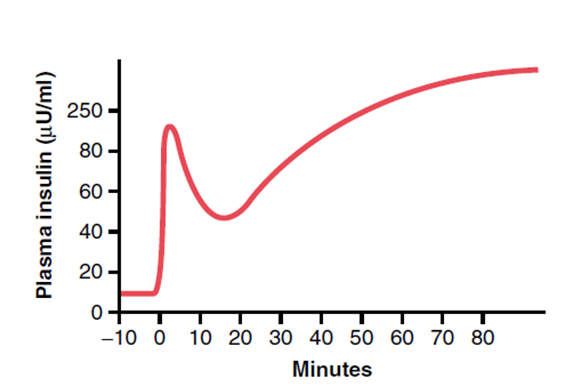
Mooney 2016
Examiner Comments
2008B 09: 1 (20%) candidate passed this question
This question was mainly about the metabolic consequences of an intravenous load of 25 grams of glucose. For a good pass the main areas that required description included: The transient glycosuria. The mechanism and time course of the biphasic release of insulin from pancreatic cells. The mechanism of the trapping of glucose within liver cells. The multiple actions of insulin which include inhibiting glycogenolysis and promoting glycgogen synthesis in the liver, promoting the uptake of glucose by muscle, fat and other cells, increasing the metabolism of glucose to fatty acids and triglycerides, inhibiting triglyceride breakdown and promoting protein synthesis. Additional marks were awarded for details of where these actions of insulin occurred and the enzymes that were stimulated or inhibited by insulin. Some candidates stated that this bolus of hypertonic glucose would produce a significant and prolonged increase in plasma osmolarity and then went on to produce detailed descriptions of the thirst and ADH mechanisms. In fact with a normal insulin response glucose is rapidly cleared from the blood
10. Outline the pharmacological properties of an ideal agent for sedating patients undergoing mechanical ventilation in intensive care (50% of marks). Describe how propofol compares to the ‘ideal’ agent (50% of marks).
CICMWrecks Answer
(Please note Midazolam was not asked in this paper)
PHARMACEUTIC
| IDEAL SEDATIVE | MIDAZOLAM Y/N | PROPOFOL Y/N |
|---|---|---|
| long shelf life | Y | Y |
| stable when drawn up and on exposure to light | Y | Y |
| water soluble | Y | N |
| no refridgeration | Y | Y |
| cheap | Y | Y |
| mixes well with other agents in the central line lumen | Y | Y |
| Bacteriostatic | Y | N |
PHARMACODYNAMIC
| IDEAL SEDATIVE | MIDAZOLAM Y/N | PROPOFOL Y/N |
|---|---|---|
| Known MOA | Y | N |
| Affects only CNS | N | N |
| No excitatory or emergence phenomenon | Y | N |
| No pain on injection | rare | N |
| Safe on Intra-arterial injection | N | N |
| No trigger for MH or porphyria | Y | N |
| Safe in pregnancy | N | Y |
| Reliable dose – effect curve with little inter- individual variation in effect | N | Y |
| Anxiolysis | Y | Y |
| Analgesic properties | N | N |
| Amnesia | Y | Y |
| No effect on cardiovascular performance | N ↓MAP, ↓SVR, ↓HR | N ↓MAP, ↓SVR, ↓HR |
| Does not depress respiratory drive | N Significant, Dose-dependent | N Mild, dose-dependent |
| Rousable sedation | Y | Y |
| Minimal side effects | N | N |
| No incidences of allergy / anaphylaxis | Y rare | Y rare |
| No idiosyncratic reactions | N | N |
| No tachyphylaxis | N | Y |
PHARMACOKINETIC
| IDEAL SEDATIVE | MIDAZOLAM Y/N | PROPOFOL Y/N |
|---|---|---|
| Low volume of distribution | Y 0.8-1.5L/kg | N 2-10 L/kg |
| rapid onset | Y 2 mins | Y 30sec |
| low protein binding | N 96% PB | N 98% PB |
| Largely unionized (high pKa) | Y pKa=6.5 (85% ionized at 7.4) | Y pKa=11 (99% unionized at 7.4) |
| Lipophilic | Y Highly lipophilic with closed ring | Y |
| inactive metabolites | N | Y |
| non-toxic metabolites | Y | Y |
| rapid clearance (context-sensitive half-life) | Y/N (CSHT 40mins at 2hrs) | Y (CSHT 20mins at 2hrs) |
| clearance not affected by either renal or hepatic dysfunction | Y | N |
| Little inter-individual variation in pharmacokinetics | N | Y |
| Availability of an antagonist | Y Flumazenil | N |
Propofol infusion syndrome:
When used at >4mg/Kg/Hr for more than 24 hours
- Metabolic acidosis,
- Lipaemia,
- rhabdomyolysis
- Cardiac failure
- arrhythmias and death
Gladwin / JC 2020
Examiner Comments
2008B 10: 4 (80%) candidates passed this question
Candidates can benefit by having a system by which they approach topics that involve a broad and general topic such as that of the pharmacology of a particular drug or ideal agent.
A good answer included the following logical subheadings:
Desirable pharmacology – long shelf life, stable when drawn up and on exposure to light, cheap, mixes well with other agents in the central line lumen. Bacteriostatic.
Desirable pharmacokinetics – Low volume of distribution, rapid clearance (context-sensitive half-life), clearance not affected by either renal or hepatic dysfunction. Little inter-individual variation in pharmacokinetics. (Availability of an antagonist).
Desirable pharmacodynamics – Affects only CNS. Reliable dose – effect curve with little inter-individual variation in effect. Anxiolysis. (Analgesic properties). No effect on cardiovascular performance. Does not depress respiratory drive.
Minimal side effects – No incidences of allergy / anaphylaxis. No idiosyncratic reactions. No tachyphylaxis.
As indicated, 50% of the marks were allocated to mentioning how well propofol reflects these properties. Mention of ‘propofol infusion syndrome’ characterised by cardiac failure which can occur when propofol is used at >4mg/Kg/Hr for more than 24 hours also attracted marks.
Syllabus: G2a 1&2
Reference Text: Pharmacology and Physiology in Anesthetic Practice / R K Stoelting
11. Describe the adult coronary circulation (50% of marks). Describe the physiological control of the coronary circulation (50% of marks).
CICMWrecks Answer
Arteries
- LCA to
- LCx to
- OM1
- OM2
- LAD has branches
- Diagonal 1 or 2
- LCx to
- RCA
- Right Ventricular
- Acute Marginal
- Posterior descending
Veins
- Great, small, middle
- Drain into the thebesian veins → ventricles directly
- Empty into the coronary sinus on the posterior wall or the RV
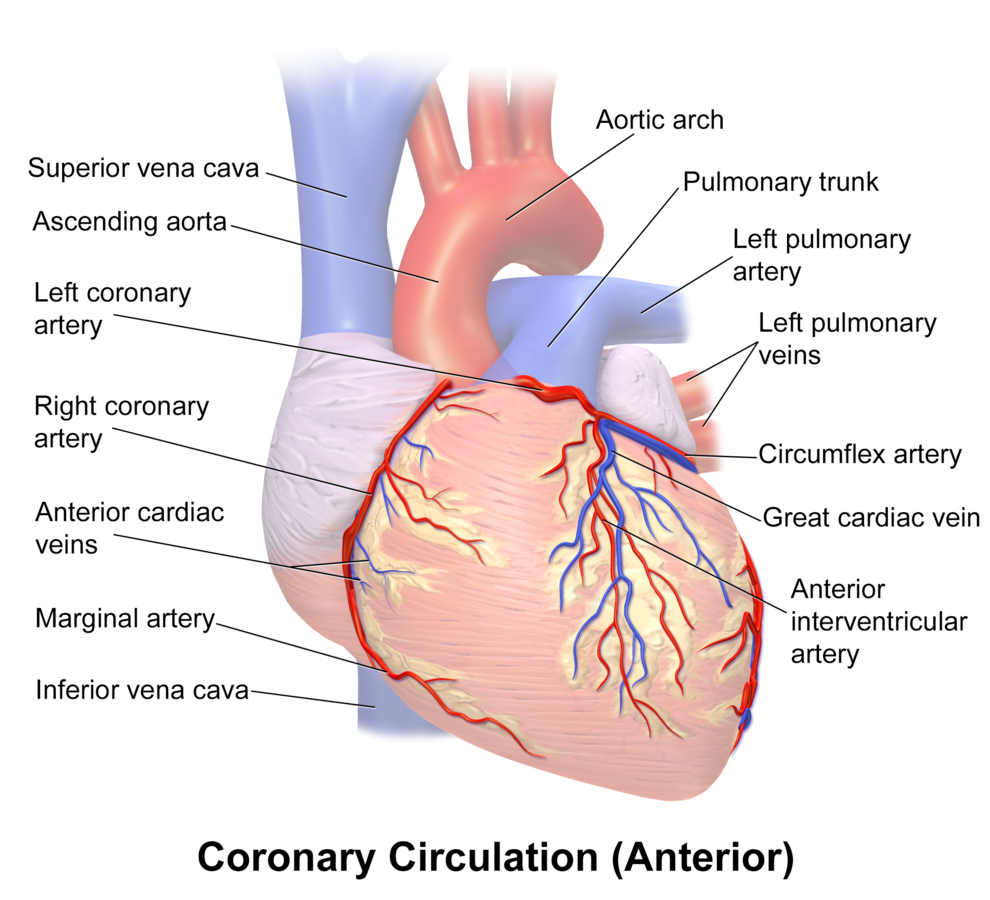
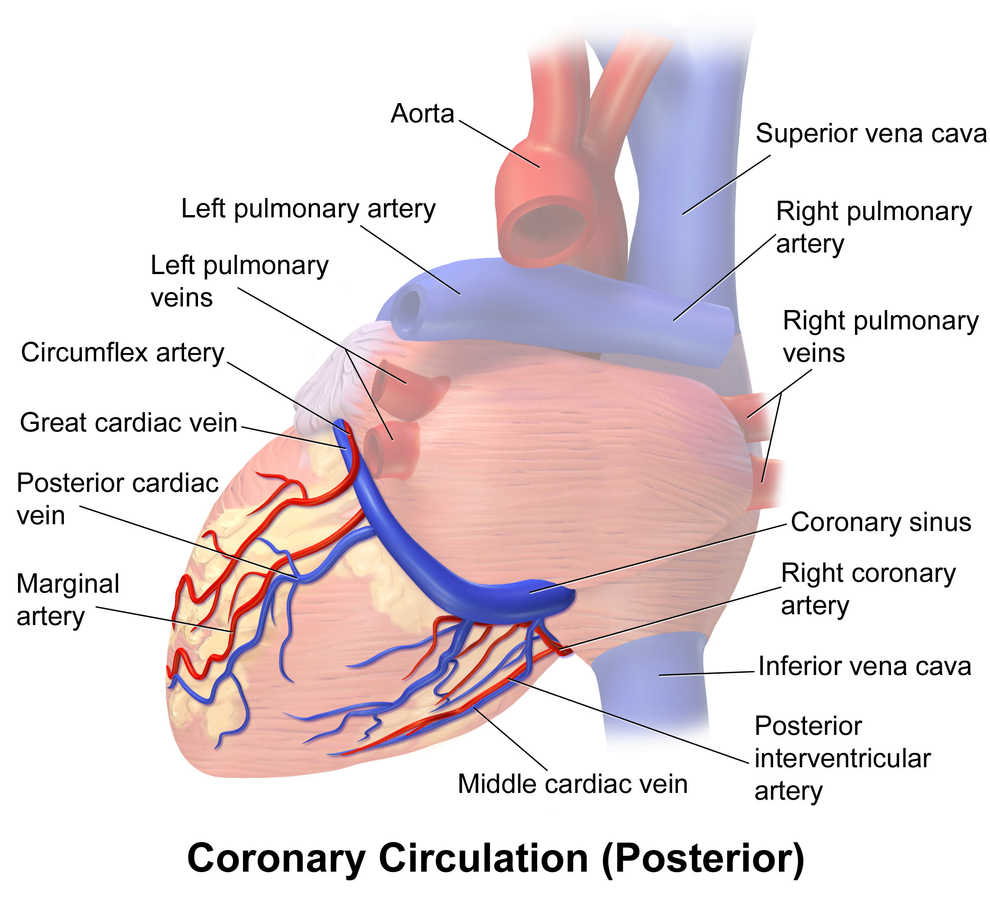
Regional Supply
| Artery | Anatomical region | ECG abnormality on occlusion |
|---|---|---|
| RCA | Down Anterior aspect of the heart, right of the pulmonary trunk Supplies RA SAN and AVN Posterior IVS | RCA occlusion causes → inferior: 2,3 aVF → true posterior: tall R in V1 |
| LCA | Left coronary artery ostia adjacent to left leaflet → Left Main → LCx and LAD Supplies LA, LV IVS AV Bundles | LCA occlusion → Lateral: 1, aVL, V1-V6 |
| LCx | LA and superior LV | anterolateral leads (1, aVL, V5 and V6) |
| LAD | RV, LV Anterior 2/3rds Interventricular Septum | Anteroseptal (V1 and V2) Anteroapical (V3 and V4) with some RCA supply. |
| Left Marginal | The continuation of the LCA after LAD branch Supplies LV laterally | Isolated Abnormalities rare V5, V6 |
| Right Marginal | The anterior branch of the RCA along the inferior border Supplies right ventricle and apex | Isolated Abnormalities rare RCA occlusion causes 2,3 aVF |
Coronary Blood Flow
- 80 mL/min/100 g
- or 200-250 mL/min
- 5% of CO at rest
- Can increase by 3-4 times (up to 400mL/min/100g)
High Extraction Ratio
High at rest (55-65%) body average of 25%
- Extraction ratio can only rise by factor of < 2 to 90%
- AV Δ O₂ = 11 mL/dL
- Coronary venous O₂ content = 5 mL/dL
- Coronary sinus SpO₂= 20mmHg
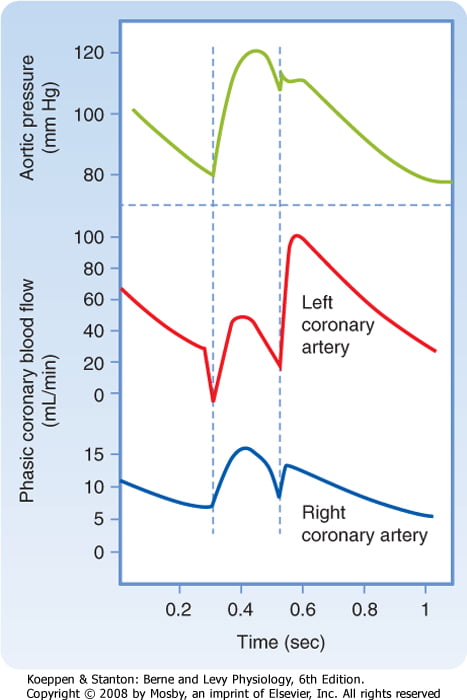
Coronary perfusion pressure (CPP)
- ADP = Ao Diastolic Prssure
- It is different throughout the cycle and b/w ventricles
Phasic Supply as per diagram.
Determinants of Coro BF
- Physical Factors
- Extravascular compression (CPP factors)
- Neural and Neurohumoral Factors
- ↑ SNS tone →
- α receptor mediated vasoconstriction
- β receptor mediated vasodialtion
- ↑ force and rate of contractions → ↑ vasodialtor metabolite release
- Overall effect is dilation
- ↑ PSNS tone → KACh stimulation → mild ↓ Coronary vascular resistance
- ↑ SNS tone →
- Metabolic Factors (main)
- Vasodilatory
- ↑ Adenosine, H, K, CO2, Lactate
- NO → GTP
- ↑ O2 demand → ↓ ATP → ↑KATP channel activation → hyperpolarisation → vasodilation
- Vasodilatory
- Myogenic autoregulation (keep CPP 60-180 mmHg)
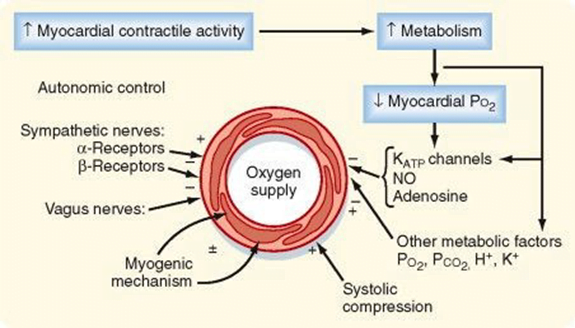
Gladwin 2016
Examiner Comments
2008B 11: 2 (40%) candidates passed this question
This question required the candidate to provide some detail why a given dose of intravenous anaesthetic administered for the purpose of induction may result in a variable response between individuals. A logical division into pharmacokinetic and pharmacodynamic factors pertaining to the drug and how these are impacted by patient physiology, citing appropriate examples, was expected. Extremes of age, pregnancy, low and high cardiac output states, sympathetic tone, body habitus and factors impacting on drug redistribution and elimination should have been addressed. Extra marks were awarded for relevant discussion of pharmacogenetics. Discussion of the “bolus effect” versus slow infusion of an induction drug, relative to these factors should have been included. Mention of drug interactions, idiosyncratic reactions and relevant pathophysiology impairing organ function reserve was appropriate. The consequences of variability in drug response in terms of over- / underdosing, haemodynamic compromise, respiratory depression, delayed recovery and allergic reactions needed emphasis to demonstrate understanding of the significance of predisposing factors.
Effective answers to this question utilised either clear headings, or a tabular format, dividing what is a large topic into discrete areas. The lack of a structured approach to this question was invariably unsuccessful.
Syllabus Ref: G2a 2.c,d,e,f,g
Suggested Reading: Pharmacology and Physiology in Anaesthetic Practice / R K Stoelting – 4th ed – 2006. Chapters 4,6.
12. Describe the concept of autoregulation as it relates to the renal circulation.
CICMWrecks Answer
Regulation of Renal Blood Flow
- Renal blood flow Autoregulated between 80~170mmHg
- Blood flow maintained by modulating resistance based on pressure
- Renal vascular resistance maintained by interlobular arteries, afferent arterioles and efferent arterioles, amenable to external regulation
- GFR approx 180l/day – Autoregulated by tubuloglomerular feedback – relatively constant in response to fluctuating renal blood flow
INTRINSIC Regulation (Autoregulation) of GFR and RBF:
- Renal blood flow (and consequently GFR) Autoregulated between MAP range of 75~170mmHg
- Blood flow maintained by modulating resistance of AFFERENT based on pressure
- Efferent arteriole is NOT involved in autoregulation!
- Autoregulation of GFR and RBF can be overridden by external influences (Eg. hormones and SNS neurons), even when renal perfusion pressure is between MAP 75-170 mmHg!
Mechanisms of Autoregulation:
- Myogenic autoregulation (Myogenic stretch response):
- In response to vascular wall stretch (due to increased intraluminal pressures), stretch dependent Ca influx occurs causing vasoconstricion of arterioles → increased resistance according to Poisuille-Hagen Equation → Decreased flow
- In response to shear stress (due to increased flow), Endothelial derived relaxation factors released (such as NO) → NO acts on guanylyl cyclase → increased cGMP → smooth muscle relaxation → arteriolar vasodilation
- Tubuloglomerular feedback (TGF)
- Negative feedback – Links the rate of GFR to concentration of salt in tubular fluid at macula densa
- Macula densa in wall of Ascending limb of loop of Henle Detects change in tubular flow (by the changing salt concentrations)
- As a consequence of decreased blood flow → GFR decreases → Tubular flow rate decreases → Increased uptake of [Na] in Ascending LoH → Reduced [Na+] and [Cl-] reaching the DCT and macula densa → Juxtaglomerular apparatus releases prostaglandins (PGE2) → vasodilation of afferent arteriole → increased resistance to glomerular blood flow
- With increased GFR → Increased tubular flow rate → Decreased [Na] uptake by the LoH → Increased [Na+] in macula densa → Juxtaglomerular apparatus secretes adenosine → Vasoconstriction of the afferent arteriole → Decreased blood flow
Note: Flow to Juxtamedullary nephrons is not autoregulated. High blood pressure increases juxtamedullary flow, increasing GFR and impairing renal concentration, resulting in a pressure diuresis.
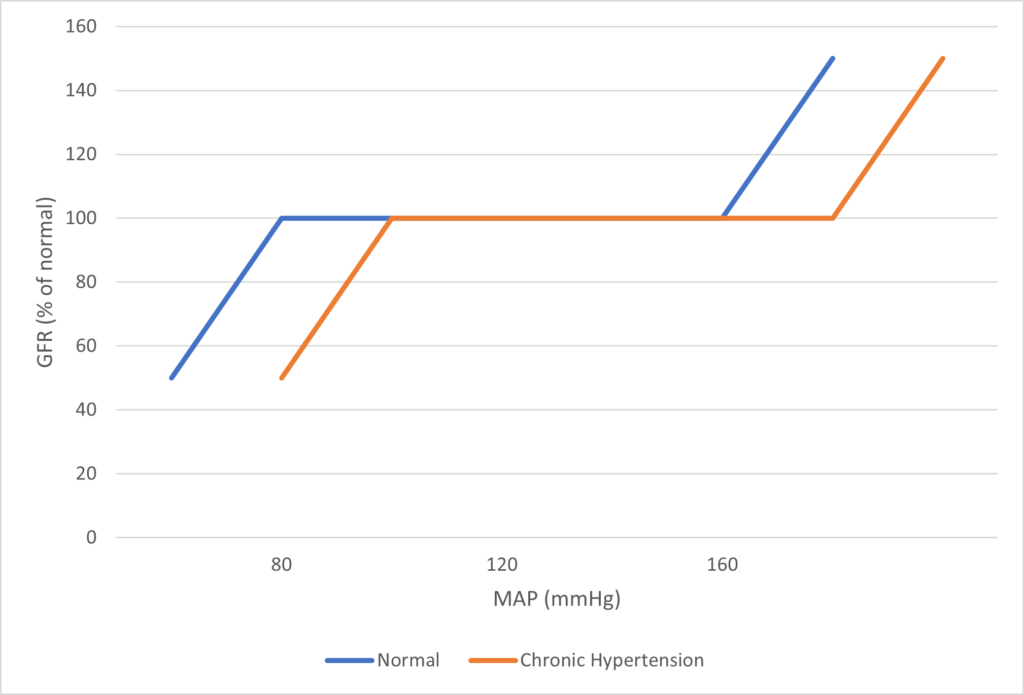
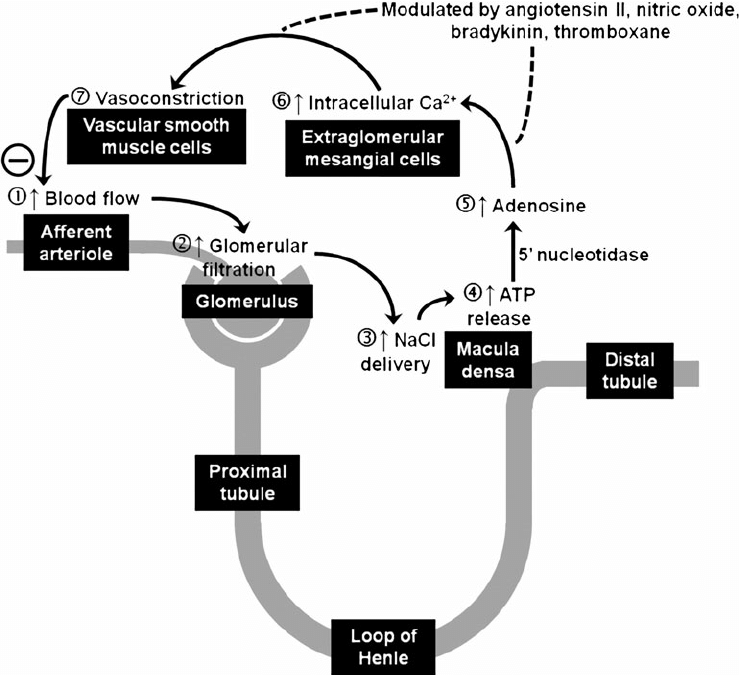
Image Source
JC / Mooney / Sakurai 2019
Examiner Comments
2008B 12 : 4 (80%) candidates passed this question
This question was concerned with a very important physiological principle and was generally well answered. A good answer included a definition of autoregulation. In relation to the renal circulation, the kidneys extract only 10% of the available O2 supply and therefore the renal blood flow is high for the purpose of filtration and not metabolic demand, renal blood flow is autoregulated to remain constant against arterial blood pressures from 75 – 160 mmHg (an illustration helps explain this concept), Tubuloglomerular Feedback (including a description of the mechanism). A discussion about other mechanisms thought to play a role is important – eg intrinsic contractile response of smooth muscle to stretch (myogenic theory of autoregulation). Vasodilator substances tend to accumulate in active tissues, and these “metabolites” (decreases in O2 tension, increased CO2 tension and decreased pH) also contribute to autoregulation (metabolic theory of autoregulation). The sympathetic nerves innervate afferent and efferent arterioles. Renal autoregulation usually overrides mild to moderate degrees of sympathetic stimulation. Strong sympathetic stimulation however will constrict renal arterioles reducing flow to 10% of normal. GFR falls to a lesser extent than renal blood flow owing to a differential effect of sympathetic stimulation constricting the efferent arteriole to a greater degree than the afferent arteriole.
Syllabus: C1f 2a, d D2i
Reference Text: Guyton Chp 26
13. Classify antiarrhythmic drugs, including their mechanisms of action, and give an example of one drug from each group.
CICMWrecks Answer
| CLASS | ACTION | ELECTRO- PHYSIOLOGY | DRUGS | THERAPEUTIC INDICATIONS |
|---|---|---|---|---|
| I. Na – Channel Blockers | ||||
| Ia | Intermediate dissociation | ↓ phase 0 ↓↓ conduction v ↑ repolarisation ↑ APD | Quinidine Disopyramide Procainamide | Atrial and ventricular arrhythmias esp. post MI |
| Ib | Fast dissociation | ↔,↓ phase 0 ↓ conduction v ↓ repolarisation ↓ APD | lignocaine phenytoin tocainide, mexilitine | Ventricular arrhythmias post MI, digoxin induced arrhythmias |
| Ic | Slow dissociation | ↓↓↓↓ phase 0 ↓↓↓↓ conduction v ± repolarisation ↔ APD | flecainide ecainide, lorcainide | Refractory arrhythmias |
| II. β – Blockers | ||||
| ↓ SA firing – ↓ rate, conduction | propranalol* atenolol, esmolol | Rate control in AF, AT, Flutter and VT | ||
| III. K – Channel Blockers | ||||
| Delay phase 3 (repol) ↑ APD ↑ ERP | amiodarone bretylium, sotalol^ | AF / Flutter termination | ||
| IV. Ca – Channel Blockers | ||||
| ↓ AV conduction ↑ PR interval ↓ rate/conduction | verapamil diltiazem | SVT and AF or Flutter | ||
| OTHERS (Some call this Class V) | ||||
| Blocks Na+/K+ ATPase → ↑Ca2+, ↓K+, ↑Ach | ↑ contractility ↓ AV conduction | Digoxin | AF rate control Heart Failure | |
| Opens K+ channels via adenosine receptors | Hyperpolarizes myocardium ↓ AV conduction ↓ SA firing | Adenosine# Ibutilide | Terminate SVT or reveal underlying rhythm in tachycardias | |
| Stimulates Na+/K+ATPase | Membrane stabilization | Magnesium | VF / Torsades de pointes | |
| Notes | * Propranalol also has Na blocking activity ^ l-sotalol has beta blocking and class III activities; d-sotalol is a pure class III agent. Commercially available sotalol is a racemic (equal part) mixture. # Amiodarone – blocks Na, Ca, K channels, and exhibits beta blockade. | |||
The effect on the myocardial action potential of each class is:
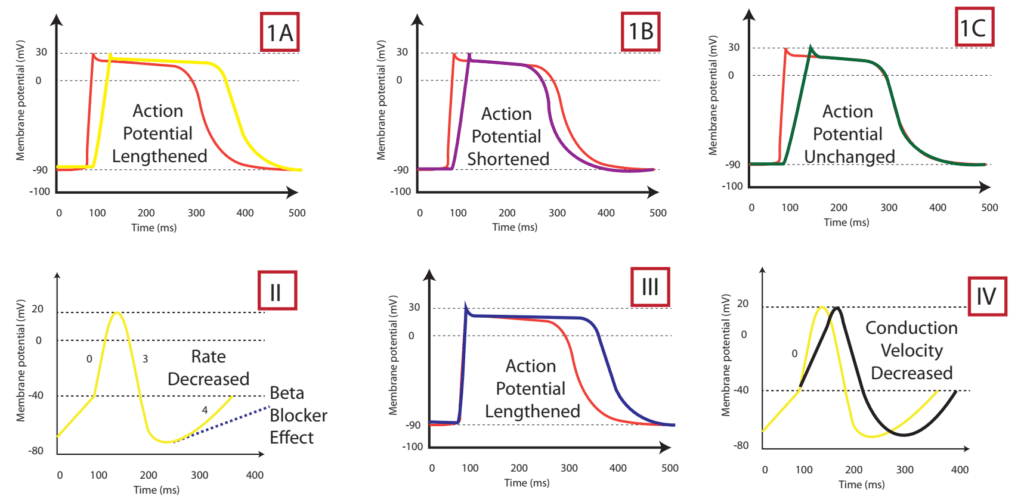
| Class Ia | Class Ib | Class Ic | Class II | Class III | Class IV | |
|---|---|---|---|---|---|---|
| Depolarisation rate (phase 0) | ↓ | ↔ or ↓ | ↓↓↓↓ | ↔ | ↔ | ↓↓↑↔± ↔ |
| Conduction velocity | ↓↓ | ↓ | ↓↓↓↓ | ↓ | ↓ | ↔ |
| Effective Refractory Period | ↑↑↑↑ | ↓ | ↑ | ↓ | ↑↑↑↑ | ↔ |
| Action potential duration | ↑ | ↓ | ↔ or ↑ | ↑ | ↑↑↑↑ | ↓ |
| Automaticity | ↓ | ↓ | ↓ | ↓ | ↓ | ↔ |
| P-R duration | ↔ | ↔ | ↑ | ↔ or ↑ | ↑ | ↔ or ↑ |
| QRS duration | ↑ | ↔ | ↑↑↑↑ | ↔ | ↑ | ↔ |
| QTc duration | ↑ | ↔ or ↓ | ↑ | ↓ | ↑↑↑↑ | ↔ |
Sotalol Effects
- l-sotalol has beta blocking and class III activities
- d-sotalol is a pure class III agent
- Commercially available sotalol is a racemic (equal part) mixture.
- Class III Effects (K – channel blocking)
- Blocking of outward K+ channels slows cardiac repolarisation, which increases the cardiac refractory period.
- Delay phase 3 (repol), ↓ AV conduction,
- ↑↑↑↑ APD, ↑↑↑↑ ERP
- ↑↑↑↑ QTc
- ↓ automaticity
- ↓ ectopy
- ↓ defibrillation energy requirement
- May ↑ inotropy
- Class II Effects (β – Blocker)
- Antagonizes β1 & β2 receptors
- ↓ SA firing – ↓ heart rate, conduction
- can prolong the QT interval.
- can cause polymorphic ventricular tachycardia (Torsades de Pointes)
- recommended to be initiated in the inpatient setting to monitor the QT interval after each dose
- can cause severe bradycardia necessitating drug discontinuation
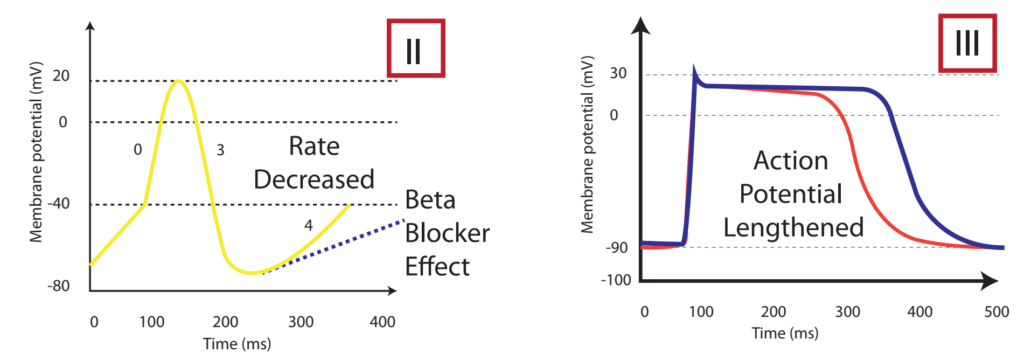
Gladwin / Sakurai / JC 2020
Examiner Comments
2008B 13: 3 (60%) candidates passed this question
This question again highlighted the importance of candidates utilising a predetermined format or structure to their questions. Well structured responses were less likely to overlook important details, which was the predominate weakness for some candidates. A table format was one useful way of displaying a good answer, for example –
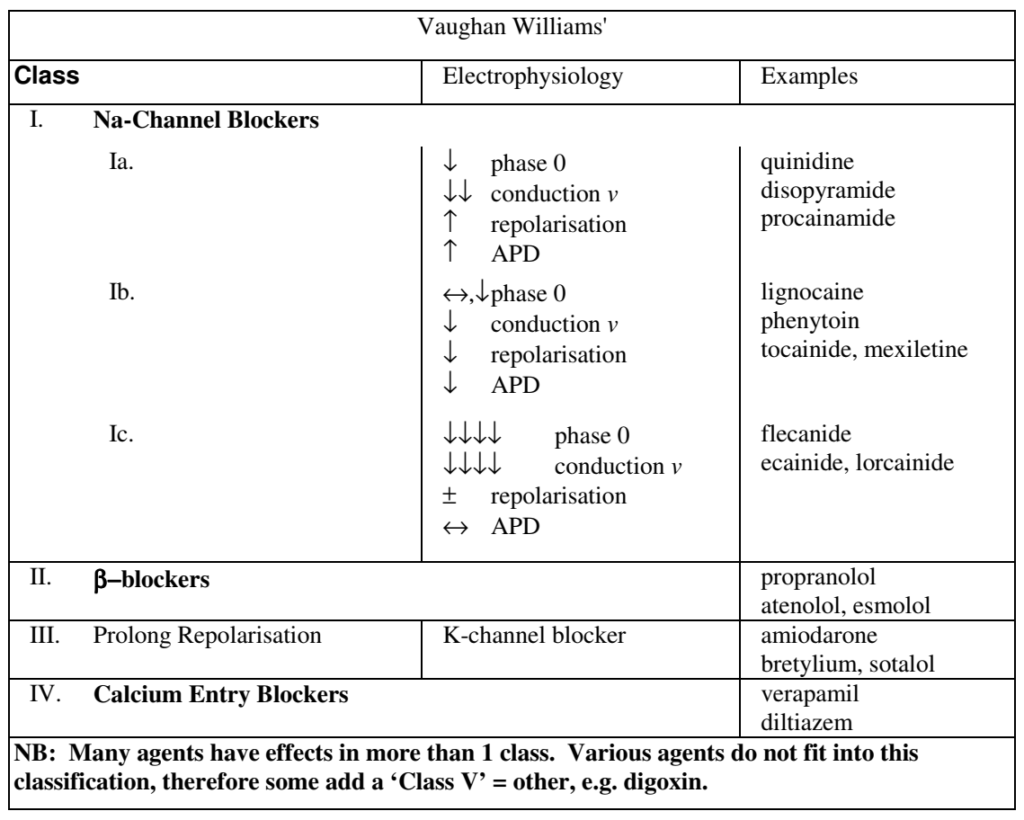
Syllabus: C2c
Reference Text: Goodman and Gillman Chp 34
14. Compare and contrast the pharmacology of midazolam and dexmedetomidine when used for sedation.
Examiner Comments
2008B 14: 1 (20%) candidate passed this question
This question was also well suited to be answered in a preset format. For example a tabular format that had headings such as mechanism of action, preparations, dosing,
pharmacokinetics, metabolism and excretion, pharmacodynamics, drug interactions and side effects.
A good answer was expected to include the following points. Under mechanism of action, mention that both drugs produce sedation by hyperpolarizing CNS nerve membranes and act on different receptors (Midazolam binds the benzodiazepine receptor and dexmedetomidine being selective for the a2 receptor). Also mention of other effects for each drug, eg anxiolytic, anticonvulsunt, analgesia, etc. A similar approach would be required for other key areas such as metabolism and excretion,(including alterations with age, organ failure, disease, etc), drug interactions, pharmacodynamics, particularly in relation to important physiological effects (eg CNS and CVS effects).
A brief summary of the similarities and differences which influence the clinical use of these agents gained more marks and showed the candidate had applied knowledge of these drugs.
The common omissions were lack of explanation of mechanism of action and failure to mention pharmacodynamic effects, drug interactions and specific advantages for each agent.
Syllabus: G2b
15. Outline the physiological consequences of hyperthyroidism in an adult.
CICMWrecks Answer
- Metabolic:
- Increased basal metabolic rate (metabolic rate increased in nearly all cells)
- Carbohydrate
- ↑absorption
- ↑gluconeogenesis
- ↑insulin secretion
- ↑cellular glucose uptake
- Lipid
- ↑absorption
- ↑resorption from adipose tissue
- → ↑plasma free fatty acids
- ↑removal of cholesterol by liver (excreted in bile)
- → ↓plasma cholesterol
- Protein
- ↑muscle catabolism
- CVS:
- +ve inotropy
- +ve chronotropy
- Vasodilation (so BP stays roughly the same)
- Resp:
- ↑VT and ↑RR
- Due to higher metabolic rate
- ↑VT and ↑RR
- Neuro:
- Lowered seizure threshold
- Anxiety
- Insomnia
- GIT:
- Increased motility
- MSK:
- ↑contractility
- Late effects of protein catabolism -> muscle wasting and weakness
Mooney 2016
Examiner Comments
2008B 15: 0 (0%) candidates passed this question
This question sought an understanding of the physiological effects of thyroid hormones. The major area of weakness for candidates was a lack of detailed understanding of the physiological actions of thyroid hormones and/or providing an answer that predominately listed clinical manifestations. A good answer would have included the following points –
- Stimulation of Carbohydrate Metabolism – all aspects of carbohydrate metabolism, including rapid uptake of glucose by the cells, enhanced glycolysis, enhanced gluconeogenesis, increased rate of absorption from the gastrointestinal tract, and increased insulin secretion
- Stimulation of Fat Metabolism – all aspects of fat metabolism are also enhanced lipids are released from fat stores and increased oxidation of free fatty acids by the cells – decreases the concentrations of cholesterol, phospholipids, and triglycerides in the plasma, even though it increases the free fatty acids
- Increased Basal Metabolic Rate – increased CMRO2
- Increased Requirement for Vitamins
- Increased vasodilatation, Cardiac Output, heart rate (not BP), contractility
- Increased Respiration – secondary to increased metabolism
- Increased Gastrointestinal Motility and secretions
- Excitatory Effects on the Central Nervous System, seizures and insomnia
- Effect on the Function of the Muscles – stimulates contractility and metabolism, but too much leads to the muscles become weakened because of excess protein catabolism. Also muscle tremor by increased reactivity of the neuronal synapses in the areas of the spinal cord that control muscle tone
- Effect on Other Endocrine Glands – . increases the rate of glucose metabolism everywhere in the body and therefore causes a corresponding need for increased insulin and glucagon secretion by the pancreas.
- Also, increase bone formation and, as a consequence, increases the need for parathyroid hormone. Thyroid hormone also increases adrenal glucocorticoid metabolism by the liver.
Syllabus: N2e
Reference Text: Guyton Chp 76
16. Classify bacteria according to the Gram stain system and the shape of the bacteria, and give two examples for each classification (40% of marks). Outline the different mechanisms of bacterial antibiotic resistance and an antibiotic for which that mechanism may apply (60% of marks).
CICMWrecks Answer: Classify Bacteria
Gram Stain
Method of differentiated bacteria according to their cell wall characteristics, using
staining with crystal violet dye
- Gram positive bacteria have a thick outer cell wall composed of peptidoglycan,
which stains positive with crystal violet dye - Gram negative bacteria have an outer cell membrane enclosing a thinner
peptidoglycan cell wall, which has decreased affinity to the crystal violet dye
Classification of bacteria
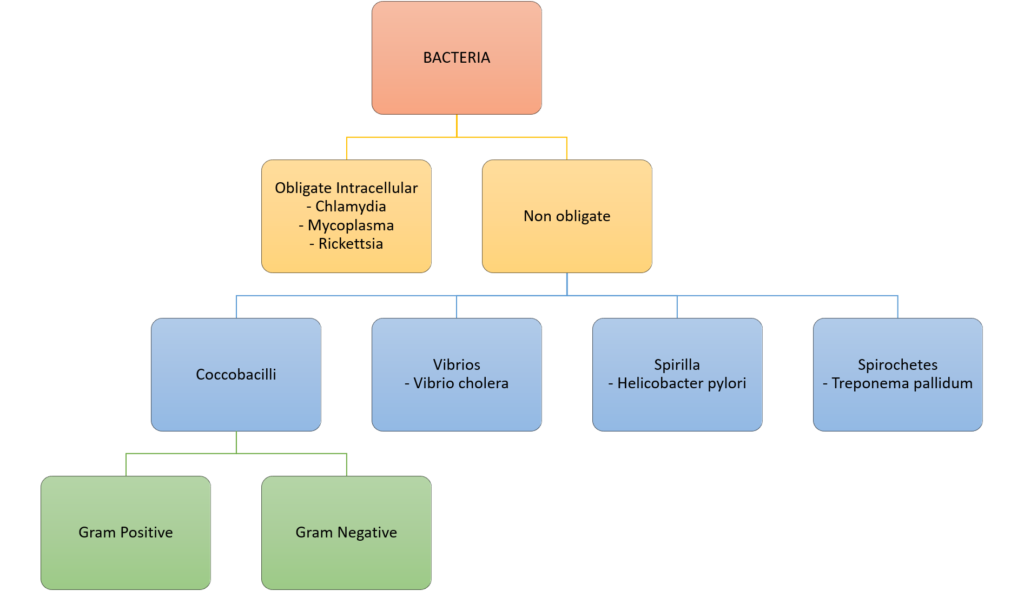
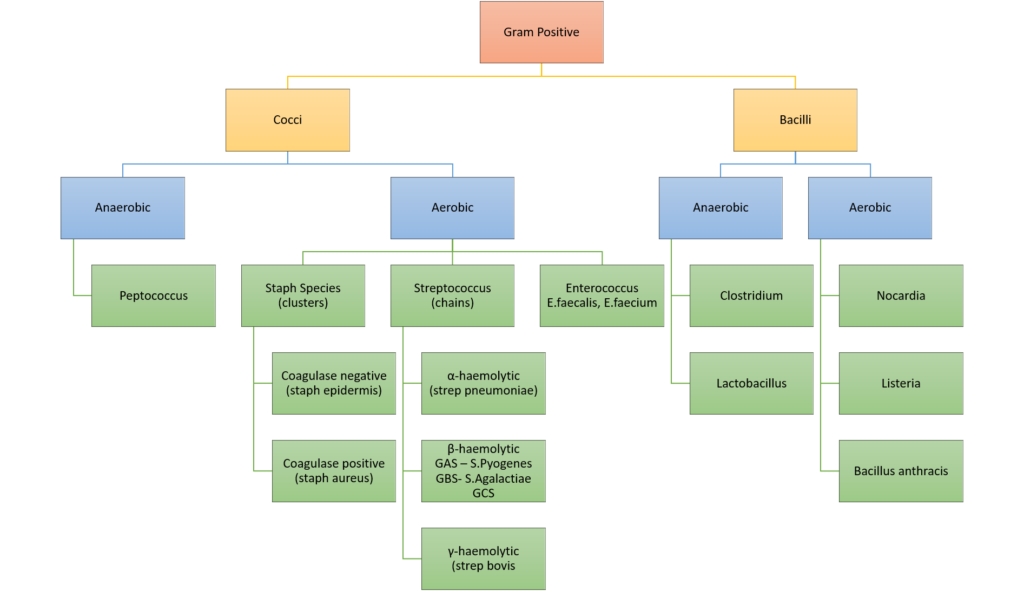
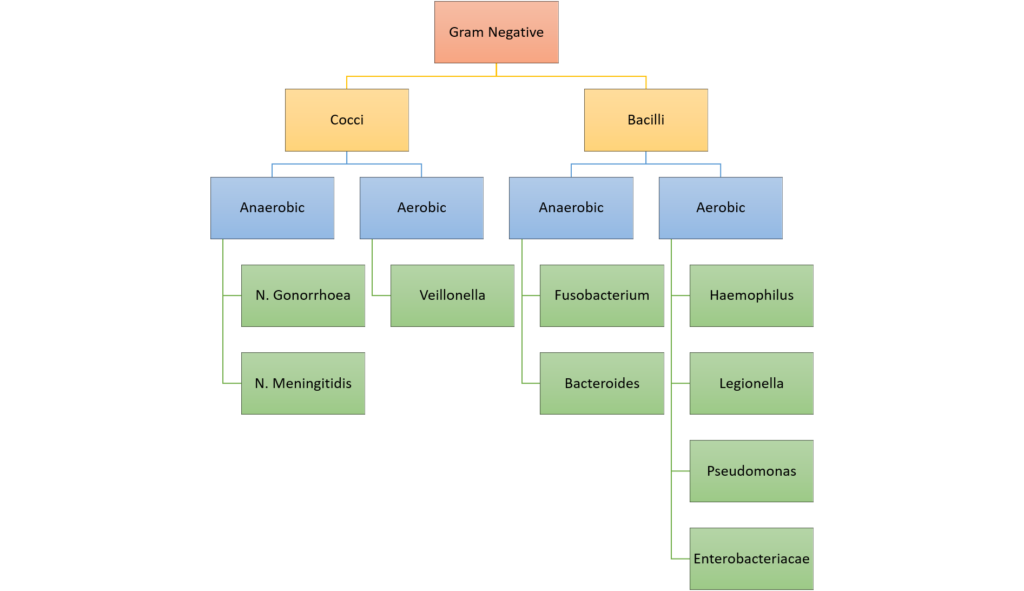
Gladwin / Sakurai / JC 2020
CICMWrecks Answer: Antibiotic resistance
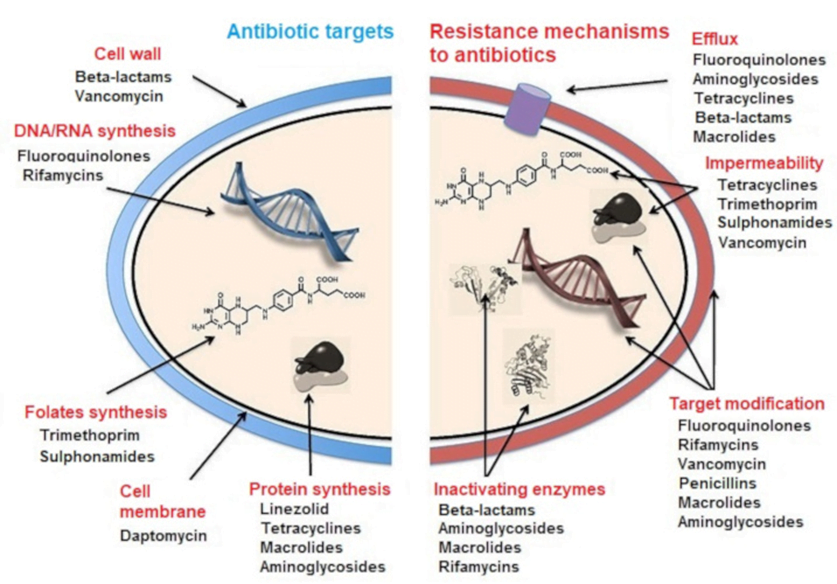
MECHANISMS OF ANTIBIOTIC RESISTANCE
- Mechanisms of Antibiotic Resistance can be classified broadly:
- Efflux Pumps
- Blocked Penetration / Alteration in access to target site
- Target Modification
- Modification of Drug or pathways
- There might be multiple resistance mechanisms at play in the same organism
| Efflux Pumps: Increased efficiency or expression of efflux pumps (inner membrane proteins). | |
| Removed from cell | Active transport of ciprofloxacin out of the bacterial cell |
| E.Coli to tetracycline. | |
| Trapped between cell wall layers | glycopeptide resistance in VRSA. |
| Blocked Penetration / Alteration in access to target site | |
| Narrowing of porin channels | Streptococcal resistance to penicillins typically occurs by reducing access to PBPs. |
| Alteration of porins in gram negative outer membranes | downregulation of Outer Membrane Proteins (eg enterobacteriaceae, pseudomonas vs penems/cephs) |
| increased selectivity of Outer Membrane Proteins (eg klebsiella outbreaks) | |
| Loss of non-essential transporter channels | Anaerobes have no oxygen-transport channel which prevents penetration by aminoglycosides (Gentamicin) |
| Reduced binding of the antibiotic | VanA and VanB vancomycin resistance involves a gene mutation leading to decreased affinity of vancomycin for the binding sites of peptidoglycan precursors |
| Changes to the DNA-binding surface of DNA supergyrase infers resistance against ciprofloxacin | |
| Target Modification | |
| Modifying the enzyme that the antibiotic inhibits | Rifampicin resistance by point mutations, insertions, or deletions in RNA polymerase gene |
| Production of an alternative enzyme for that which the antibiotic inhibits | MRSA develop or acquire the gene mecA which synthesizes an additional penicillin binding protein that enables it to continue cell wall synthesis in the presence of a beta lactam drug |
| Overproduction of the target of the antibiotic | Trimethoprim DHFR enzyme overproduction in Escherichia coli and Haemophilus influenzae. |
| Synthesis of target-protecting proteins | Ribosomal protection proteins against Tetracyclines in multiple Gram positive and gram negative bacteria |
| Modification of Drug or pathways | |
| Development of metabolic pathways to bypass site of action of antibiotic | Resistance to TMP-SMX by allowing bacteria to synthesize or absorb folic acid. |
| Enzymes produced to Metabolize the drug | β-lactamases and cephalosporinases hydrolyse β-lactam rings |
| Enzymes produced that add a chemical group to antibiotic to inhibit its activity | Aminoglycoside resistance by Staphylococcus aureus or Pseudomonas (Multiple enzymes acetyltransferase, adenyltransferase, and phosphotransferase) |
Spread of Bacterial Resistance
- Selective pressure selects for favourable mutations of resistance
- mosaic genes (from other bacteria eg strep pneumo from strep mitus) or uptake of DNA from environment
- transfer of resistant bacteria from person to person
- horizontal gene transfer;
- transduction – acquisition of bacterial DNA from a phage (a virus that propagates in bacteria);
- transformation – uptake and incorporation of free DNA released into the environment by other bacterial cells;
- conjugation – gene transfer (usually on plasmids), by direct cell-to-cell contact through a bridge.
Sources:
Microbiology Lippincott Williams & Wilkins
https://courses.lumenlearning.com/microbiology/chapter/drug-resistance/
https://www.encyclopedie-environnement.org/en/health/antibiotics-antibiotic-resistance-and-environment/
Gladwin / Sakurai / JC 2019
Examiner Comments
2008B 16: 3 (60%) candidates passed this question
This question also highlighted the importance of candidates noting the way marks were proportioned. A good answer required the following points – Classification and examples – gram-positive cocci (Staphylococcus aureus, Streptococcus pyogenes or pneumoniae or agalactiae), gram-negative cocci (Neisseria meningitidis, N. gonorrhoeae), gram-positive bacilli (Bacillus anthracis, Listeria, corynebacteria, Clostridium difficile, etc), gram-negative bacilli (Escherichia Coli or E. Coli, Proteus, Yersinia, Salmonella, Shigella, Pseudomonas aeruginosa, Klebsiella pneumoniae,
Legionella).
Mechanisms of resistance include: a) Enzyme inactivation, beta-lactamase or Extended Spectrum Beta-lactamase b) Enzyme addition: enzyme produced by bacteria that add a chemical group to the antibiotic to inhibit its activity, aminoglycoside resistance by Staphylococcus aureus or Pseudomonas. c) Impermeability, anaerobes have no oxygen dependent transport mechanism which stops the penetration of aminoglycosides into the bacteria. d) Efflux mechanisms by acquisition of an inner member protein which actively pumps antibiotics out of the cell, E.Coli becomes resistance to tetracycline by this mechanism. e) Alternative pathway to circumvent the metabolic block impose by antibiotic, Some Staphylococcus aureus are resistant to methicillin by developing or acquiring the gene mecA which produces an alternative penicillin binding protein and hence they are not
inhibited by methicillin. f) Alteration of the target site, rifampicin resistance by point mutations, insertions, or deletions in RNA polymerase gene.
Syllabus: M2a, b, c
Reference Text: Microbiology and Infection at a Glance by Gillespie & Bamford 3rd Ed, 2007 page 8-9,21.
17. Describe the respiratory changes that occur in morbid obesity.
CICMWrecks Answer
Definition
| BMI | As per LBW | |
|---|---|---|
| Normal | 15 – 25 | LBW |
| Overweight | 25 – 30 | 110% – 119% LBW |
| Obese | 30 – 35 | 120% – 199% LBW |
| Morbidly Obese | 35 – 40 | >200% LBW (or) LBW + 50-100kg |
| Super Morbidly Obese | > 40 | LBW + >100kg |
LBW(kg)
Adult males: Height (cm) – 100
Adult females: Height (cm) – 105
BMI = Weight (kg) / (Height(m))2
Respiratory Complications
- Lung Volumes
- ↓’d ERV (due to limitation of diaphragmatic excursion + Δ Compliance)
- +/- ↑’d residual volume (2nd to gas trapping or concurrent disease)
- Δ ERV is usually greater than any ↑ in RV thus
- ↓ FRC
- Can fall to < Closing capacity → right-to-left shunting + arterial hypoxemia
- ↓’d TLC
- ↓ FRC
- Lung Mechanics
- ↑’d work of breathing due to
- Altered pulmonary system compliance
- ↓’d lung compliance
- ↑ intrapulmonary blood volume
- ↓FRC to < Closing Capacity → Small airway narrowing/closure
- → ↑elastic work
- ↓’d chest wall compliance
- accumulation of fat tissue in and around the chest wall
- ↓’d lung compliance
- ↑’d airway resistance
- ↓ airway calibre
- Elevation of diaphragm during erect/head up positioning
- Altered pulmonary system compliance
- Result:
- shallow rapid pattern of breathing
- ↑ the work of breathing
- ↓’d respiratory muscle efficiency
- ↑ WOB → ↑RR → ↑ WOB → Capacity limitation
- ↑’d work of breathing due to
- Gas Exchange
- ↑’d metabolic activity of the excess fat + ↑’d workload on supportive tissues
- ↑’d O2 consumption
- ↑’d CO2 production
- ↑’d ventilation/perfusion abnormalities (especially supine/under anaesthesia)
- Limited maximum lung capacity (inability to increase ventilation in response to ↑’d demand)
- ↑’d sensitivity to respiratory depressant drugs
- ↑’d metabolic activity of the excess fat + ↑’d workload on supportive tissues
- Respiratory Control
- Obstructive sleep apnea
- defined arbitrarily: total cessation of airflow for 10 seconds or more despite continued respiratory efforts against an obstructed pharyngeal airway
- Hypopnea: a 50% reduction in airflow or a reduction sufficient to lead to >4% reduction in arterial oxygen saturation
- physiological responses to recurrent apnea
- hypoxemia and hypercapnia
- pulmonary and systemic vasoconstriction
- Secondary polycythemia and ↑ pulmonary vascular resistance
- Recurrent pulmonary vasoconstriction leads to right ventricular impairment.
- Obesity hypoventilation syndrome (chronic OSA)
- Loss of normal respiratory control during the day and daytime respiratory failure
- Altered chemoreceptor sensitivity to carbon dioxide.
- Reduction in respiratory drive leading to central apneic events (i.e. apnea without respiratory effort)
- Loss of normal respiratory control during the day and daytime respiratory failure
- Obstructive sleep apnea
- Airway Changes
- Fatty infiltration of the soft tissues of the neck →
- Difficulties maintaining airway
- Requirement for higher than normal BV mask pressures → gastric inssuflatio → ↑ aspiration risk
- Decreased FRC → ↓’d apoenic time on induction (compounded by ↑ O2 consumption)
- High risk of airway and intubation difficulties at induction of anesthesia
- Fatty infiltration of the soft tissues of the neck →
Gladwin 2016
Examiner Comments
2008B 17: 2 (40%) candidates passed this question
Obesity is an increasing problem in the broader community and in Intensive Care practice. Hence it is important candidates understand the physiological and pharmacological consequences of obesity. This question confined its scope towards obesity and the respiratory system. Major area of weakness of candidates was a lack of depth and breadth in knowledge of this topic and in applying basic physiology. A good answer required the following points –
Definition of morbid obesity (>200% ideal body weight or body mass index > 35)
Upper airway effects: fat infiltration of pharyngeal soft tissues difficult airway, prone to airway obstruction eg OSA
↑ O2 consumption and CO2 production: due to ↑ total body fat, requires ↑ cardiac output and ↑ alveolar ventilation
↓ FRC mainly via ↓ ERV: due to mass loading and splinting of diaphragm, upright obese,
closing capacity > FRC small airway closure, ↑ V/Q mismatch, ↑ venous admixture and
arterial hypoxaemia, ↓ O2 stores
↓ total respiratory system compliance: ↓ chest wall compliance , subcutaneous and intra
abdominal fat excess, ↓ lung compliance, ↑ airways resistance, ↑ work of breathing, ↓ resp
muscle efficiency
Altered ventilatory control: Obstructive sleep apnoea, Obesity hypoventilation syndrome
Syllabus: B1k B1d2k
Reference Text: Nunn’s Applied Respiratory Physiology / A B Lumb & J F Lunn – 6th ed
18. Describe the physiology of bilirubin production, metabolism and clearance (70% of marks). Outline the changes in blood and urine of the products of bilirubin metabolism with intra and post hepatobiliary disease (30% of marks).
CICMWrecks Answer
RBC Destruction
- Globin chains → AA’s
- Haem → Biliverdin, Fe2+, CO
- Biliverdin
- Haeme + Haem oxygenase → biliverdin
- Biliverdin + biliverdin reductase → bilirubin
- Free bilirubin + albumin → transported to hepatocytes
- Conjugated with glucuronide to ↑ water solubility
- Excreted to bile canniliculus
- Secreted to gut at D2 (duodenum)
- Intestinal flora hydrolyse and reduce → urobilinogen
- 3 fates:
- Gut bacteria form the dark pigment stercobilin, which is egested in the faeces
- Urobilinogen reabsorbed unchanged by the portal system and recycled by the liver
- Remainder is reabsorbed by the portal system and then excreted in the urine
- Fe2+ → oxidised to Fe3+ → recycled
- CO (only endogenous source of CO)
Hepatic Disease:
- Intrahepatic disease
- ↓’d conj billi secretion
- ↑d plasma conjugated bilirubin
- ↑ urobilinogen in stool
- ↑ urine bilirubin,
- ↓’d conj billi secretion
- Posthepatic disease (complete obstruction)
- ↑ conjugated bilirubin
- No urobilinogen in stool
- ↑ urine bilirubin
Gladwin 2016
Examiner Comments
2008B 18: 3 (60%) candidates passed this question
A good answer for this question required the description of the pathway of bilirubin production beginning with the breakdown of haemoglobin breakdown, then haem to biliverdin by biliverdin reductase, biliverdin to bilirubin, bilirubin transported to liver bound to plasma proteins, bilirubin monoglucuronide and diglucuronide by conjugation, secreted into bile. Secretion into bile is dependent on active transport and is the first to be impaired in inflammation of the liver. Bilirubis metabolized to stercobilinogen which is absorbed and excreted in urine as urobilinogen. The remaining part to this question flowed on from this point, ie Intrahepatic disease – increased conjugated bilirubin, urobilinogen and urine bilirubin, Posthepatic disease – increased conjugated bilirubin, no urobilinogen, increased urine bilirubin
Syllabus: I2c
Reference Text: Guyton Ch 70
19. Classify the hypersensitivity reactions, give an example for each reaction and describe the pathophysiological processes of each reaction.
CICMWrecks Answer
HYPERSENSITIVITY REACTIONS
| Type | Pathophysiology | Disease types |
|---|---|---|
| Type I Immediate Hypersensitivity IgE mediated | Sensitisation Plasma B cell production of IgE Mast cell proliferation → IgE binds to mast cells On re-exposure Allergenic antigen binds to mast cell (expressing IgE) → Mast cell degranulates Mast cell degranulation leads to Primary mediators: – Serotonin – Histamine Secondary mediators – Leukotrienes (SRSA) – Prostaglandins – ECFA, NCF | Anaphylaxis Atopy |
| Type II Cell Cytotoxicity IgG, IgM Mediated | Antibody attaches to antigen on target cell Complement release C5-9 “membrane attack complex” → Causes cell lysis | Blood Transfusions Goodpasteur’s syndrome Autoimmune cytopaenias |
| Type III Immune Complex IgG, IgM, IgA mediated | Circulating antibody-antigen complexes are deposited in basement membranes e.g. peritoneum, blood vessels, joints, glumeruli Complement cascade activation Attract granulocytes → inflammation | SLE Serum Sickness Necrotising vasculitis |
| Type IV Delayed hypersensitivity T-cell mediated | T cell mediated Cell mediated, as opposed to previous humoral Peak reaction 2-3 days CD4 helper T cells cause recruitment of Neutrophils Macrophages CD8 cytotoxic T cells | TB, Sarcoid Granulomatosis with polyangiitis (Wegener’s) Granulomatous vasculitis |
SRSA: “slow reacting substance” A – Mixture of Leukotrienes (LTC4, LTD4, LTE4)
ECFA: Eosinophil chemotactic factor of anaphylaxis
NCF: Neutrophil chemotactic factor of anaphylaxis
Key Points:
- “Anaphylaxis” may be:
- True anaphylaxis: a symptoms complex following exposure of a sensitised individual to an antigen, produced by a type I hypersensitivity reaction, associated with IgE mediated mast cell degranulation
- Anaphylactoid reactions: Indistinguishable from true anaphylaxis, however the immune nature of the reaction is either unknown, or not due to a type I hypersensitivity reaction, immediate generalised reaction is a better term.
Gladwin / Mooney 2016
Examiner Comments
2008B 07: 3 (60%) candidates passed this question
A tabular format is a very desirable format for a response to this question. Again candidates who struggled to provide an organised response also failed to provide sufficient and succinct detail in their answer. A good answer would have included the following features.
Syllabus: M2g
Reference Text: Goodman and Gillman Chp 64
20. Compare and contrast the pharmacology of sodium nitroprusside and glyceryl trinitrate.
Examiner Comments
2008B 20: 4 (80%) candidates passed this question.
It was expected candidates would address specific aspects of pharmacology such as action, mechanism of action, half life and duration of effect, route of administration, potential toxicity and special precautions. These agents lend themselves to comparison and contrast as several distinct similarities and differences exist and credit was given for highlighting these. Specific comments should include that both agents result in blood vessel dilation with extra credit given for detailing the differences in the balance of arterial versus venous effects between them. For both agents the effect is mediated through nitric oxide and it was expected candidates would identify that nitroprusside releases NO spontaneously and GTN requires enzymatic degradation with the resultant effects on smooth muscle mediated via cGMP. They are both short acting agents when used intravenously and require careful titration to measured blood pressure for effect.
Extra credit was given for mentioning that routes other than IV are available for GTN (topical / oral) but not for nitroprusside. Comments on special precautions such as Nitroprusside should be protected from light and GTN given via non PVC giving sets gained additional marks. In addition to the well described adverse effects of each agent, it was expected candidates would mention the potential for cyanide toxicity with nitroprusside and extra marks were awarded for an indication of usual doses.
Syllabus C2b 2e
References Katzung 10th edition, Goodman and Gillman Chp 31 & 32
21. Outline the pathophysiological basis for the use of angiotensin converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARB) in congestive cardiac failure.
CICMWrecks Answer
Angiotensinogen
- Cleaved by renin
- release from juxtaglomerular cells:
- Lowered renal arterial pressure
- Less NaCl (more about Cl–) past macula densa
- Sympathetic nervous system activation
Angiotensin I
- Converted by ACE to Angiotensin II
Angiotensin II
- Actions: Via AT1 receptors (AT2 receptors have unclear significance)
- Renal:
- Afferent and renal artery vasoconstriction (weaker)
- Efferent vasoconstriction (stronger)
- Constricts mesangial cells (decrease GFR)
- Increased sodium and water resorption
- -> Na+/H+ antiporter
- -> Decreased hydrostatic pressure in capillary (Starling force)
- Vascular:
- Widespread vaso and veno constriction
- Promotes hyperplasia
- Cardiac
- Promotes hyperplasia
- Neural:
- Directly increases noradrenaline release from presynaptic membrane
- Stimulates catecholamine release
- Promotes aldosterone release
- Degrades bradykinin
- So less NO, cAMP -> less vasodilation
Aldosterone
- Increases activity of Na+/H+ antiporter, Na+/K+ ATPase in the distal convoluted tubule and collecting duct -> water retention
- There is also an ACE independent pathway to angiotensin II formation
ACE inhibitors:
- Block ACE
- Reduce afterload
- Modulate cardiac sympathetic stimulation
- Inhibit cardiac and vascular hypertrophy
- Cause cardiac remodelling
A2RB:
- Block AT1 receptors
Mooney 2016
Further Reading:
Examiner Comments
2008B 21: 0 (0%) candidates passed this question
The renin-angiotensin system plays a central role in the pathophysiology of heart failure. Thus this question required integration of knowledge of the renin-angiotensin system and how pharmacological agents affect it in the treatment of cardiac failure. Candidates were expected to describe the pathway and the influence of these drug groups on cardiac failure and to recognise underlying basic physiological principles such as the interaction between AT1 and AT2 receptors along with awareness of production of Ang II by ACE-independent enzymes.
A good answer was expected to contain the following points: Angiotensinogen is cleaved by kidney-derived renin to form the decapeptide angiotensin I (Ang I); ACE converts Ang I to Ang II; Ang II is a potent arterial vasoconstrictor and an important mediator of Na + and water retention through its effects on glomerular filtration pressure and aldosterone secretion; Ang II potentiates neural catecholamine release, is a secretagogue for catecholamine release from the adrenal medulla, promotes vascular hyperplasia and pathologic myocardial hypertrophy.
ACE inhibitors suppress Ang II and aldosterone production, decrease sympathetic nervous system activity, and potentiate the effects of diuretics in heart failure. ACE is identical to kininase II, which degrades bradykinin and other kinins that stimulate production of NO, cyclic GMP, and vasoactive eicosanoids; these vasodilator substances seem to oppose the effects of Ang II on the growth of vascular smooth muscle and cardiac fibroblasts and on production of extracellular matrix. Thus, the increased levels of bradykinin that result from ACE inhibition may play a role in the hemodynamic and anti-remodeling effects of ACE inhibitors.
An alternative means of attenuating the haemodynamic and vascular impact of the reninangiotensin system is through inhibition of angiotensin receptors. Most of the known clinical actions of angiotensin II are mediated through the AT1 angiotensin receptor. AT1 receptor antagonists may provide more potent reduction of the effects of angiotensin II than do ACE inhibitors.
22. Describe how gas exchange is facilitated across the placenta near the end of pregnancy.
CICMWrecks Answer
Diffusion via Fick’s Law
- Flow of gas (Rate of movement of solute) across semi-permiable membrane J is
where
C = concentration (or partial pressure for gasses)
A = cross-sectional area
T = thickness of the membrane or distance over which diffusion takes place.
- These factors alter the rate of transfer as per the equation above.
- Valid for Most medium – small substances
- Water soluble (H2O, electrolytes, glucose, urea) via intercelular clefts
- Lipid soluble substances (O2, CO2) via endothelial cells themselves.
- Flow = 1.2ml of O2 mmHg-1minute-1 across entire placenta
- Surface area of placenta for diffusion = 16m2
- Thickness of placental membrane falls as pregnancy progresses
- Minimum thickness is 3.5 micrometres
Oxygen
- Mean partial pressure of oxygen (PO2) in maternal blood is considerably higher than in fetal blood
- As a consequence, oxygen readily diffuses across the placenta from maternal to fetal blood
- Despite its low PO2, fetal blood is able to transport essentially the same quantity of oxygen to tissues as maternal blood
| O2 Partial Pressure | Oxygen Content | |
|---|---|---|
| Maternal | PaO2 = 100mmHg (80-110) | CaO2 = 16.1ml/100ml |
| Fetal Umbilical Vein | PvO2 = 30mmHg (30-50) | CvO2 = 16ml/100ml |
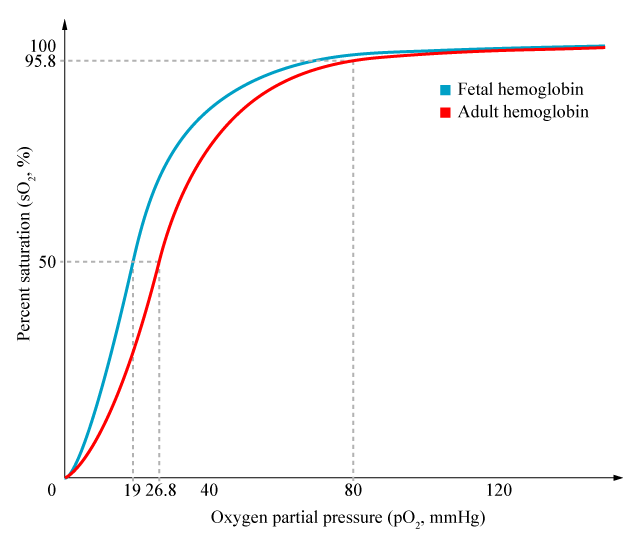
- Foetal Hb has a higher affinity for O2 than maternal Hb
- oxygen carrying capacity is improved, despite lower PaO2
- P50 is 20mmHg vs 26 in maternal
- Haemoglobin concentration in blood is higher in the foetus, increasing oxygen carriage
- 120g/L in mother
- 170g/L in foetus
- Double Bohr effect:
- Movement of CO2 from the foetal to the maternal circulation results in a simultaneous rise in maternal CO2 and fall in foetal CO2
- Rise in foetal Hb oxygen affinity, fall in maternal Hb oxygen affinity by the Bohr effect
Carbon dioxide
- Carbon dioxide is produced abundantly in the fetus
- PCO2 of fetal blood is higher than maternal blood
- Carbon dioxide therefore diffuses from fetal blood, through the placenta, into the maternal circulation, and is disposed of by expiration from the mother’s lungs.
| O2 Partial Pressure | |
|---|---|
| Maternal | PaCO2 = 32mmHg |
| Fetal Umbilical Artery | PvCO2 = 55mmHg |
- Hyperventilation in pregnancy (increased sensitivity of chemoreceptors to CO2) → Lower PaCO2
- Smaller gradient than oxygen, but oxygen has 20 times higher solubility
- Double Haldane effect:
- Simultaneous rise in foetal Oxy-Hb and fall of maternal Oxy-Hb
- Increased CO2 carrying capacity of maternal Hb, decreased in foetal Hb
Mooney / JC 2020
Examiner Comments
2008B 22: 3 (60%) candidates passed this question
Candidates were expected to cover the basic principles of gaseous diffusion across the placenta, with reference to: Both oxygen and carbon dioxide; Fick’s Law, including placental area and thickness, and relative gas tensions and solubilities; Changes in maternal and foetal blood flow approaching term; Approximate values for maternal and foetal gas tensions and content; The differences between foetal and maternal haemoglobin, quantitative and qualitative; The double-Bohr and double-Haldane effects; Relative maternal hyperventilation and it’s effects on arterial gas tensions and how these influence foetal transfer.
A good answer would also include a description of the physical arrangement of maternal sinuses and foetal capillaries; labelled dissociation curves for O2 and CO2 detailing the differences between maternal and foetal Hb; a placental gas exchange diagram, showing foetal and maternal arterial and venous gas tensions and content values.
Syllabus ref: O1 2d
Suggested Reading: Review of Medical Physiology / W F Ganong – 22nd ed Chapter 32.
Nunn’s Applied Respiratory Physiology / A B Lumb & J F Lunn – 6th ed
23. Describe the factors which contribute to inter-individual variability in drug respon seen with an induction dose of an intravenous anaesthetic drug.
CICMWrecks Answer
Pharmacokinetic factors
(Amount of drug reaching the receptor)
- Absorption
- IV dose so should be 100%
- dose variability between patients
- Distribution
- Volume of distrib:
- Body mass and ratio of fat:muscle differences
- pregnancy, cardiac output states
- Protein binding:
- low albumin states, other protein bound drugs
- propofol has 98% protein binding and will be influenced
- Blood pH
- affects degree unionised which can cross lipid membranes
- eg alkalotic state with thiopentone pKa 7.6
- Volume of distrib:
- Metabolism
- Hepatic factors
- liver disease may reduce metabolism
- CYP enzymes (inhibited or upregulated) eg Ketamine
- Hepatic factors
- Excretion
- Renal impairment: onset of effect more dependent of redistribution, but can be prolonged if renally excreted
Pharmacodynamic factors
- Presence of endogenous or exogenous ligands
- Endogenous: increased catecholamines may necessitate a higher induction dose
- Exogenous:
- medications which potentiate sedation opioids, benzodiazepines, volatiles
- medications which reduce sedation – amphetamines
- Variation in the number of receptors
- Extremes of age
- Previous exposure to the drug or similar – tolerance, tachyphylaxis
Variability in the response distal to the receptor
- Pharmocogenetic factors
- idiosyncrasy
- anaphylaxis
- Pathological and physiological factors
- sepsis
- CNS function
JC 2019
Examiner Comments
2008B 23: 2 (40%) candidates passed this question
This question required the candidate to provide some detail why a given dose of intravenous anaesthetic administered for the purpose of induction may result in a variable response between individuals. A logical division into pharmacokinetic and pharmacodynamic factors pertaining to the drug and how these are impacted by patient physiology, citing appropriate examples, was expected. Extremes of age, pregnancy, low and high cardiac output states, sympathetic tone, body habitus and factors impacting on drug redistribution and elimination should have been addressed. Extra marks were awarded for relevant discussion of pharmacogenetics. Discussion of the “bolus effect” versus slow infusion of an induction drug, relative to these factors should have been included. Mention of drug interactions, idiosyncratic reactions and relevant pathophysiology impairing organ function reserve was appropriate. The consequences of variability in drug response in terms of over- / underdosing, haemodynamic compromise, respiratory depression, delayed recovery and allergic reactions needed emphasis to demonstrate understanding of the significance of predisposing factors.
Effective answers to this question utilised either clear headings, or a tabular format, dividing what is a large topic into discrete areas. The lack of a structured approach to this question was invariably unsuccessful.
Syllabus Ref: G2a 2.c,d,e,f,g
Suggested Reading: Pharmacology and Physiology in Anaesthetic Practice / R K Stoelting –
4th ed – 2006. Chapters 4,6.
24. Describe the gravity dependent processes which affect pulmonary blood flow (70% of marks). Describe the changes that result from an acute increase in pressure in the pulmonary vessels (30% of marks).
CICMWrecks Answer
Gravity Dependent Processes
- Low pressure circuit:
- pressure drop of ~10mmHg from RV (15mmHg) to LA (5mmHg)
- PAP is just sufficient to raise blood to top of lung
- Blood flow in upright lung varies due to gravity effect (hydrostatic Pressure)
- Lung divided into vertically into 4 zones (West Zones)
- Zone 1 not present in normal lungs
- manifests in Positive pressure ventilation, Hypovolaemia, eg. haemorrhage
- Zone 4 appears in the presence of interstitial pressures or at very low lung volumes
- Zone 1 not present in normal lungs
- Normal balance between the zones varies based on:
- changes in posture
- changes in airway pressures due to anatomical changes / disease, ventilation changes
- changes in lung volume:
- At extremes pulmonary capillaries are linearly stretched and collapse as may occur with hyperinflation.
- At very low lung volumes, extra-alveolar blood vessels become compressed and flow reduces (Zone 4).
- changes in pulmonary vascular pressures related to cardiac factors or pulmonary vascular disease
- There are alternate theories explaining the heterogeneity in pulmonary perfusion and ventilation in both health and disease, which suggest that gravity has a less important role in the variation of regional distribution
- for e.g: The underlying structure of the bronchial and pulmonary vascular anatomy with non-symmetrical branching (Structural theory)
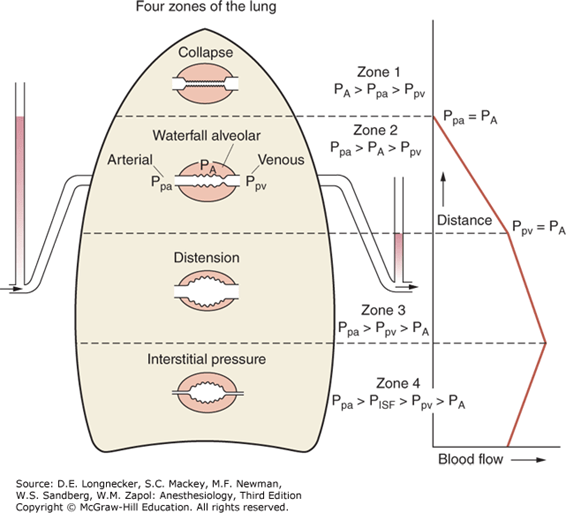
West Zone 1
- Not present in the normal lung
- Nil flow of blood to apical zones
- ↑alveolar dead space → High V/Q ratio
- Increased in:
- Hypotension, PE, Hyperinflation of lung with PPV
- Minimal change in PaO2 with increased West Zone 1 as Hb fully loaded
- Slight increase in PaCO2 as there is decreased apical perfusion, however this is compensated for by the increased RR chemoreceptor change: Minimal change in PaCO2
- Hypotension, PE, Hyperinflation of lung with PPV
West Zone 2
- Starling Resistor Mechanism
- ↑ hydrostatic P or ↓ Alveolar pressure → Pa > PA → capillary opens → flow → ↓ hydrostatic P ° to flow → Pa < PA → capillary closes
- Blood flow tends to ‘flutter’ (pulsatile flow)
- decreased in diastole, increased in systole.
- Increased in inspiration, decreased in expiration
- Present from the apex of the upright lung → 10cm above the level of the heart
West Zone 3
- Blood flow is constant due to the maintenance of arterio-venous pressure gradient
- Alveolar pressure is not a factor determining blood flow in this zone
- 10cm above the heart → bases of upright lung
- Effect of weight of lung ↓PA
- Hydrostatic pressure is maximal (~25mmHg)
- Low V/Q ratio
- Contributes to venous admixture
West Zone 4
- Interstitial pressure acts as a Starling resistor for pulmonary blood flow.
- It is seen when interstitial pressure is high (e.g due to pulmonary oedema).
- Also seen at very low lung volumes, where extra-alveolar blood vessels become compressed and flow reduces.
Effect of ↑P in pulmonary vessels
- Pulmonary circulation is low pressure circuit – ↑pressure 2° ↑PAP / PVP (LA) pressure
- Response in pulmonary vasculature is:
- Recruitment of closed capillaries
- Distension (↑caliber) of vessels
- Increased pressure delivered to the arterioles causes dilation and decreases resistance to flow, increasing flow by as much as twice what would be expected due to pressure alone
- Pulmonary arteries are approximately ½ as distensible as veins; this buffers pressure changes transmitted to the alveolar capillaries and also permits the arteries to adopt a reservoir function
- Net effect of recruitment and distension is bufering of pressure rise (↓pressure of pulmonary circulation) until these processess are exhausted or in the presence of pulmonary capillary disease
Gladwin 2016
Examiner Comments
2008B 24: 4 (80%) candidates passed this question
Most candidates quite correctly approached this from the perspective of “West’s zones of the lung. A clear description of the relationship between pulmonary arterial, venous and alveolar pressures producing the classical 3 zones was expected, along with situations which may alter the normal balance between the 3 zones, e.g. changing posture or airway pressure.
Additional points were awarded for candidates describing ‘zone 4’ or alternate theories of V/Q distribution. Whilst most candidates described recruitment and distension with respect to changing pulmonary arterial pressure, candidates were also expected to correctly state that an increase in pulmonary artery pressure is only observed when these processes are exhausted, or in the setting of pulmonary vascular disease, then describing the subsequent effects of pulmonary hypertension on the heart and circulation.
The vascular tree is distensible, that is, a change in pressure will produce a corresponding increase in dimension of the blood vessels:
Increased pressure delivered to the arterioles causes dilation and decreases resistance to flow, increasing flow by as much as twice what would be expected due to pressure alone The veins are 6 to 10 x as distensible as arteries owing to the structural differences in their respective walls. A notable exception is the pulmonary circulation where arteries are approximately ½ as distensible as veins; this buffers pressure changes transmitted to the alveolar capillaries and also permits the arteries to adopt a reservoir function. Pulmonary vascular resistance is also a function of lung volume. At extremes pulmonary capillaries are linearly stretched and collapse as may occur with hyperinflation. At very low lung volumes, extra-alveolar blood vessels become compressed and flow reduces (Zone 4). Use of appropriate graphs to illustrate some of the above points would have been desirable..
Syllabus Ref: B1i 2, B1k 2. a,c,i
Suggested Reading: Nunn’s Applied Respiratory Physiology / A B Lumb & J F Lunn – 6th ed – Chapters 7,8
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Draw Capnograph tracing Collapse Lt Lung – processes contributing to hypoxia – V/Q, shunt, PVR, Pulse oximetry |
| G. CVS | NIBP vs Invasive BP monitor Noradrenaline – Physio, Pharm, PV loop changes, effect on Myocardial oxygen consumption, work and efficiency. Dobutamine infusion on PV loop |
| H. Renal | |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | Cerebral blood flow, measurement, variations, Morphine Pharmacology of analgesic meds – Non-opioids, Opioids |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | |
| O. GIT | Prokinetics, Laxatives, Octreotide |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | Insulin – Physio, Pharm, Refeeding syndrome |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments