1. Describe the pharmacological effects of paracetamol. Outline its toxicity and management.
CICMWrecks Answer
Pharmacological effects of Paracetamol
- Arachidonic acid ——(COX)—–>
- Prostaglandins → inflammation, sensitise spinal nerves to pain
- Prostacycline → vasodilation
- Thromboxane → platelet aggregation and activation
- Paracetamol: Weak COX-1 and COX-2 inhibitor in peripheral tissues
- Relatively selective for COX-2 → less inhibition of thromboxane production
- Possible cause for antipyretic effect:
- Inhibition of COX-3 (variant of COX-1), leading to central effects
Toxic Effects and Management
Toxicity
- Occurs in doses >4g/day
- Overdose dose lower in chronic alcoholics, elderly and children
- Paracetamol
- → glucuronidation
- → sulphation
- → N-hydroxylation to NAPQI
- In overdose, glucuronidation and sulphation pathways saturated → increased production of toxic NAPQI
- NAPQI usually conjugated with hepatic glutathione
- In overdose, glutathione exhausted → accumulation of NAPQI
- NAPQI bonds with hepatocyte sulfhydryl groups → cell death and necrosis
Therapy
- Decision to treat based on paracetamol overdose nomogram
- Serum paracetamol level, time since dose
- If within 4 hours → activated charcoal
- Hepatic failure, GI upset, renal failure (less common)
- N-acetyl-cysteine should be given
- Replenishes glutathione stores
- Directly conjugates NAPQI
- Monitoring and supportive care:
- Bilirubin
- Transaminases
- Creatinine (dialysis)
- Liver synthetic function (albumin, INR) (vitamin K, maybe FFP, but discuss with liver unit first)
- Blood sugar (glucose infusion)
- Conscious level (hepatic encephalopathy) (lactulose, intubation)
Mooney 2016
Examiner Comments
2008A 01: 2 candidates (66%) passed this question.
Main points expected for a pass included:
- Actions and mechanism of actions of paracetamol. These include inhibition of cylooxygenase (COX) activity and prevention of prostaglandin (PG) production. More marks were given for mention of a central COX 1 variant as the possible enzyme responsible for paracetamol’s central versus peripheral effects. Knowledge of the central sites of action was expected.
- Outline of toxicity. Candidates were expected to demonstrate knowledge of toxic doses, conditions enhancing toxicity (alcohol intake, chronic use etc), and the mechanism of hepatic toxicity and other organ toxicity (especially renal). A detailed list of clinical features of toxicity was not required.
- Management of toxicity. Candidates performed well in this section with good knowledge of timing, toxic doses, use of paracetamol levels and the nomogram to determine whether N-acetylcysteine should be administered. Mechanism of action of NAC was expected. Mention of monitoring (liver failure) and supportive therapy gained marks, but detailed explanations were not required
Syllabus G2e2c
Stoelting 4th edition page 285
Rand Dale 5th page 244
2. Outline the differences between heparin and enoxaparin with respect to:
a. Phamacokinetics
b. Monitoring of effect
c. Adverse effects
d. Reversal of effect
CICMWrecks Answer
Heparin
- Binds to, and stabilizes anti-thrombin 3-thrombin complex
- → Potentiates the degradation of thrombin (does not occur with LMW heparins)
- Also aids antithrombin 3 degradation of factors IX and X (major function of LMW heparins)
| HEPARIN | ENOXAPARIN | ||
|---|---|---|---|
| PK | A | Subcut or IV administration | Subcut administration |
| ~90% bioavailability SC due to tissue binding | >95% bioavailability SC – NOT bound in tissues | ||
| D | 5% protein bound | 5% protein bound | |
| Vd – 0.3l/kg | Vd – 0.3l/kg | ||
| M | Heparinases | Minimal metabolism | |
| E | Renal elimination of inactive metabolites | Renal elimination of unchanged drug Dosage adjustment required in renal failure | |
| T1/2b ~2 hours | T1/2b 4~6 hours | ||
| Monitoring | APTT, activated clotting time | Anti-Xa assay (expensive, time-consuming) | |
| Adverse Effects | Bleeding | Bleeding | |
| Heparin induced thrombocytopoenia | Less likely to develop HITS | ||
| Osteoporosis | Less likely to develop Osteoporosis | ||
| Reversal | Reversal with protamine sulfate | Partial (40%) reversal with protamine sulfate | |
Sakurai 2016
Examiner Comments
2008A 02: 1 candidate (33%) passed this question.
Candidates were expected to mention the differences between heparin and enoxaparin and to explain why these differences existed and their implications.
For example:
heparin Pharmacokinetics –
MW3000-30000. Large variability has significant effects on:
• mechanism of action (IIa, IXa, Xa)
• dosing( 2/3 molecules in dose have no active binding sites)
• bioavailability poor,T1/2 short (rapid uptake by PF4/endo cells/macrophages and
proteins of high MW components) = poor predictability and dose response both IV and SC, mandates monitoring IV
T1/2 1-2 hours: S/C 2-3 daily doses. Continuous IV – advantage is fast offset.
enoxaparin –
MW3000-5000. Inhibits Xa only.
• bioavailability (100%),longer T1/2 no uptake/protein binding
• predictable activity and dose response = decreased need for monitoring, reliable SC
• No need for IV use
T1/2 4-5 hours, 1-2 daily dosing, but no fast offset.
Better answers were in tabular format under each heading. Higher marks were awarded for mentioning of monitoring pitfalls such as heparin resistance, measuring Xa levels in a timely fashion, and the efficacy and pitfalls of protamine with each agent.
Syallabus J2 2a
Reference: Stoelting 505-511
Katzung 546-548
3. Describe the principles of measurement of arterial haemoglobin oxygen saturation using a pulse oximeter. Outline the limitations of this technique.
CICMWrecks Answer
Principles
Beer-Lambert Law
- Incidence of light is inversely proportional to the path distance and concentration of light absorbing particles within the path.
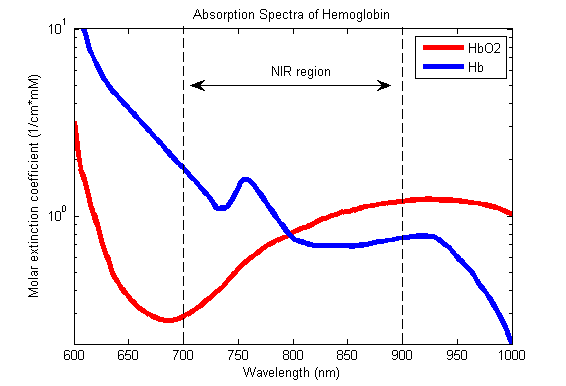
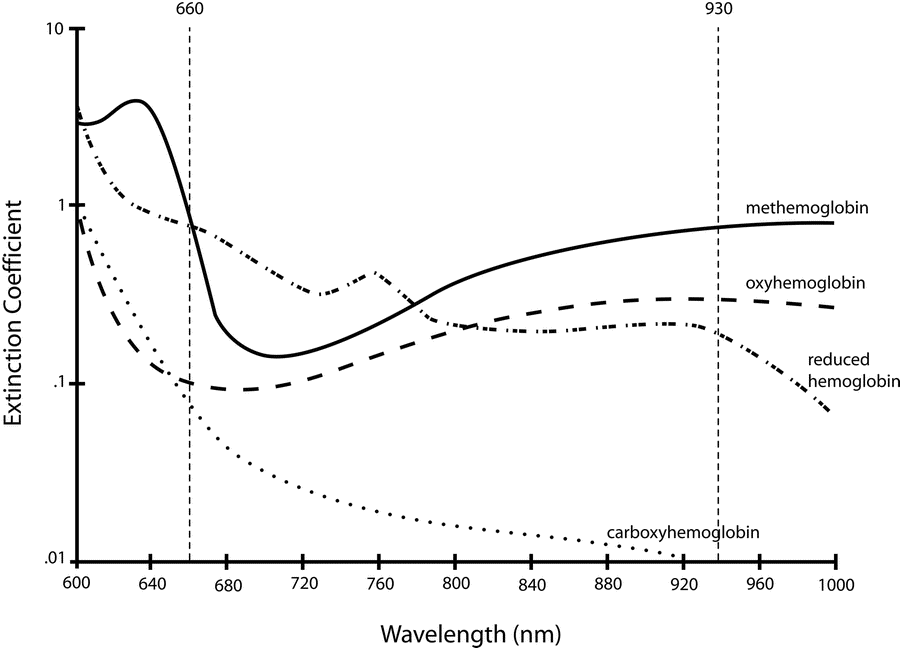
Hb absorbance at 660nm and 940nm utilized
- 660nm maximally absorbed by deoxyhaemoglobin
- Ratio of absorbance at 660nm and 940nm utilized to calculate SpO2
using healthy volunteers derived R values
Sats 100% R = 0.4
Sats 85% R = 1.0
Sats 50% R = 2
Sats 0% R = 3.4
Pulse oximeter
- Light emitter
- Produces infra-red light at 660nm and 950nm
- Light detector
- Detects light at 660nm and 950nm
- Processor
- Calculates SpO2 using the ratio of absorbance at 660nm and 950nm
- Light detected (incidence) represents light passing through both pulsatile (arterial) blood and non-pulsatile elements (venous blood and other tissues)
- Processor differentiates light incidence during maxima and minima of pulse wave and calculates SpO2 from pulsatile blood only giving SaO2
Limitations
| PATIENT FACTORS | ||
| Low or High SpO2 | Low SpO2 | Normal or High SpO2 |
| Met-Hb Sulph-Hb | Poor perfusion of finger Movement artifact Venous pulsations Fingernail polish Intravenous pigmented dyes Haemoglobinopathy Anaemia with co-existing hypoxia | Carbon Monoxide poisoning |
| EQUIPMENT FACTORS | ||
| – Ambient light interference – Poorly fitting probe – Assay calibrated using healthy volunteers only down to SpO2 80%. Unknown significance if tested SpO2 less than 80% | ||
| PHYSIOLOGICAL FACTORS | ||
| – Due to O2 dissociation curve, insensitive to changes above PaO2 80mmHg – Does not measure tissue oxygenation | ||
Absorption spectra confounded by:
- Carboxyhaemoglobin
- Absorbs 660nm, not at 940nm
- R closer to 0.4
- causes the pulse oximeter to read artificially high
- Methemoglobin
- though it absorbs 660nm light, it also absorbs 940nm light to a greater degree
- R closer to 1
- causes the SpO2 to trend towards 85%
Sakurai 2016
Examiner Comments
2008A 03: No candidates (0%) passed this question.
The main points expected for a pass included a brief description of the following:
• The system components
• The principles of light absorbance and the Beer-Lambert Law
• The differential absorbance of Hb species in red/infrared spectrum, and their use to calculate the amount of reduced and oxygenated Hb present
• LED emitting 660/940/off cycles at 450-900Hz, averages data over several cycles to eliminate ambient light, and detect pulsatile and non pulsatile elements
• Pulse added absorption of each cycle compared as ratio “R” at different wavelengths
• Calibration curve to compare “R” to SaO2 data from healthy volunteers
• The limitations of the technique including:
– quality of product, bias, precision and accuracy
– insensitivity to PaO2
– false readings and their causes
Diagrams gained marks only with sufficient labelling and explanation.
Syllabus S2f
4. Describe the factors that are important when interpreting plasma drug concentrations.
CICMWrecks Answer
FACTORS
Factors may be pharmacokinetic, Pharmacodynamic or Patient factors
1) Pharmacokinetic factors
- Absorption:
- enables an appreciation of bioavailability and variation in different routes
- heparin is an example, there is reduced absorption SC due to endothelial protein binding
- Distribution :
- highly lipid soluble drugs generally have a high volume of distribution
- plasma levels will therefore appear markedly decreased when compared to dose
- Protein Binding:
- Total plasma levels may not be reflective of active drug level
- E.g: Phenytoin and hypoalbuminemia (Low total, but enough free unbound drug)
- the pKa may be relevant as the amount ionised may be more important in determining
- effect rather than the total plasma level (ability to cross membranes)
- Effect site concentration may not be adequate inspite of plasma concentration
- (E.g Vancomycin in Ventriculitis, where high plasma levels are no guarantee of bactericidal CSF levels)
- highly lipid soluble drugs generally have a high volume of distribution
- Metabolism:
- drugs may have saturable metabolic pathways such that even at high doses the drug will
- be cleared at a fixed rate (phenytoin)
- co-administration of drugs may upregulate metabolic catalysts (e.g. CYP450s) increasing
- metabolism, or compete for substrate/catalyst decreasing metabolism
- Excretion:
- drugs that are excreted renally may have much higher plasma concentration if there is impairment or oliguria.
- Measurement related factors:
- Measurement Assay used
- Timing of sample collection
- In relation to dosing: Peak, trough, etc
- In relation to steady state concentration: Caution when interpreting before 3-5 half lives
2) Pharmacodynamic factors
- Relationship of plasma concentration to clinical drug effect:
- E.g. There is no relationship in the case of Levetiracetam
- Active Metabolites
- Plasma concentration may not correlate to clinical effect in the presence of Active metabolites
- Drug action receptor sensitivity
- action at the sensor (e.g. competitive antagonist vs non-competitive antagonist)
- Appreciation of reference values for plasma concentration levels
- To check therapeutic effect
- antimicrobials -minimal inhibitory concentration levels
- To monitor side effects
- local anaesthetic agents -CNS:CVS ratios
- To check therapeutic effect
3) Patient factors
- Age (decreased sedative concentrations required in elderly)
- Lean body mass vs toal body mass (lipid soluble versus small VD drugs)
- Renal and hepatic function (may prolong and elevate plasma concentrations)
- Pharmacogenetic factors
- abnormalities in CYP450 (e.g. variable codeine metabolism)
- abnormal plasma esterases – suxamethonium metabolism
- Individual variability in Drug Response
CLINICAL RELEVANCE
- Drug plasma concentrations are relevant when certain criteria are met:
- demonstrated relationship between drug concentration and therapeutic or toxic effect (digoxin)
- narrow therapeutic window (carbamazepine)
- variability in plasma level such that response cannot be predicted from dose alone (warfarin)
- drug produces effects, intended or unwanted, that are difficult to monitor (heparin)
- Convenient to collect, Fast and Accurate results
- Good benefit to cost
JC 2019
Examiner Comments
No candidates (0%) passed this question.
- The majority of the information required for this question is covered within the general pharmacology section of the syllabus. The main points expected for a pass were:
- Mention and discussion of pharmacokinetic factors such as drug absorption, volume of distribution, clearance, protein binding, dosing frequency and drug level sampling.
- Mention and discussion of pharmacodynamic factors such as drug sensitivity, and therapeutic range.
- Clinical relevance of a drug concentration (e.g. peak or trough level, total or free drug, etc).
- Candidates often failed to frame their answer to the question that was asked. Candidates could have made a greater use of illustrations and examples of drugs to help answer the question.
- Among other relevant listed references, candidates should seek information from within the text books – Basic and Clinical Pharmacology by B. G Katzung and Pharmacology by H. P Rang, J. M Ritter and M. M Dale.
5. Outline the physiological factors that influence cerebral blood flow.
CICMWrecks Answer: Cerebral Blood Flow
Cerebral Blood Flow
- CBF = CPP / CVR (Cerebral Perfusion Pressure / Cerebral Vascular Resistance)
- CBF 15% resting CO → ~750ml/min or 50ml/100g brain tissue/min
- Gray Matter: 75–80 mL/100 g/min – Significantly higher due to the high metabolic activity and dense synaptic connections
- White Matter: 20–30 mL/100 g/min
- Abnormal CBF
- CBF<50ml/100g/min → cellular acidosis
- CBF<40ml/100g/min → impaired protein synthesis
- CBF <30ml/100g/min → cellular oedema
- CBF <20ml/100g/min → failure of cell membrane ion pumps, loss of transmembrane electrochemical gradients
- CBF <10ml/100g/min → cell death
Determinants of CBF
- CPP
- Net pressure gradient driving blood flow through the cerebral circulation
- CPP = MAP – ICP
- MAP = CO x SVR; CO = HR x SV; SVR = MAP / CO
- ICP dependent on: brain, blood, CSF
- CVR
Regulated by 4 primary factors:
- Cerebral metabolism
- flow-metabolism coupling:
↑ metabolic demand → ↑ CBF + substrate delivery - Controlled by vasoactive metabolic mediators:
H+ ions, K, CO2, adenosine, glycolytic intermediates, NO
- flow-metabolism coupling:
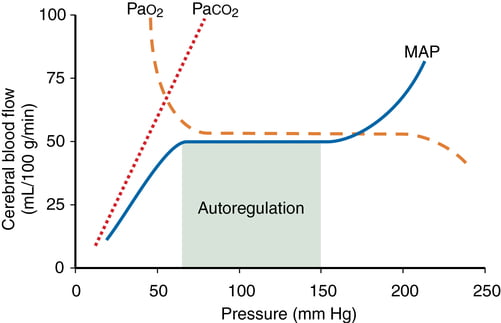
- CO2 and O2
- CO2
- At normotension: relationship between PaCO2 and CBF = almost linear
- ↑ PaCO2 → cerebral arteriolar vasodilation → ↓ CVR + ↑ CBF
- 2~4% increase for every 1mmHg increase in CO2
- ↓ PaCO2 → cerebral arteriolar vasoconstriction → ↑ CVR + ↓ CBF
- Initial stimulus = ↓ brain ECF pH
- Effects regulated by: NO, prostanoids, K channels, intracellular [Ca2+]
- PaO2
- little effect at normal PaO2
- PaO2 <60mmHg → cerebral arteriolar vasodilation → ↑ CBF
- Mechanism: hypoxia acts on →
- cerebral tissue to promote release of adenosine → cerebal vasodilation
- cerebrovascular smooth muscle → hyperpolarisation → ↓ Ca2+ uptake → vasodilation
- CO2
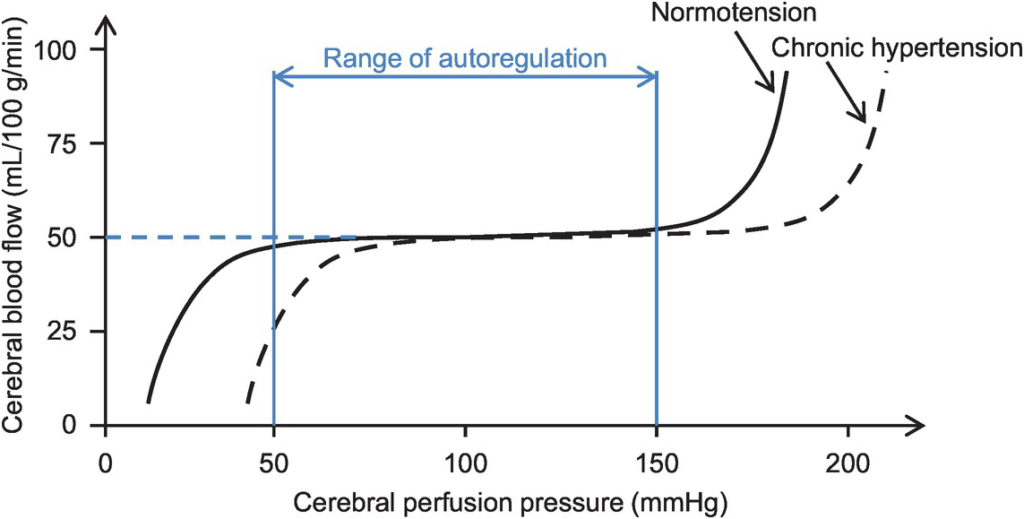
- Autoregulation
- Constant across CPP 50-150mmHg
- CPP >150mmHg: CBF µ CPP
- CPP <50mmHg: CBF <50ml/100g brain tissue/min → ischaemia
- Stimulus to autoregulation = CPP (not MAP)
- autoregulation curve R shifted in HTN; L in neonates
- Mechanism:
- Myogenic mechanism: arterioles vasoconstrict in response to ↑ wall tension + vasodilate in response to ↓ wall tension → ↓ or ↑ CVR
- May involve adenosine
- Can be impaired in SAH, tumour, stroke, head injury
- Constant across CPP 50-150mmHg
- Neurohumeral factors
- Relative lack of humoral + autonomic control on normal cerebrovascular tone
- Main action of SY nerves = vasoconstriction
- Other factors
- Blood viscosity: directly related to HCt; ↓ viscosity → ↑ CBF as per Hagen-Poiseuille law
- Temperature: ↓ CMRO2 by 7% for each ↓ 1°C in temp
- Drugs
- E.g. barbiturates ↓ cerebral metabolism
- Volatile agents → ↓ tension cerebral vascular smooth muscle → vasodilation + CBF
Kerr 2018
Examiner Comments
2008A 05: 1 candidate (33%) passed this question
The main points expected for a pass were:
• Description of the relationship of CO2; O2; MAP and Cerebral metabolism with cerebral blood flow. The use of graphs, correctly labelled, and associated free text would be an effective means of portraying this information.
• The effect of other factors such as intracranial pressure, cerebral venous pressure, vascular calibre, blood viscosity and regional blood flow differences.
Syllabus C1f2c
6. Compare the effect on arterial blood carbon dioxide and oxygen levels of ventilation / perfusion inequalities.
CICMWrecks Answer
Normal V/Q variation
- Both V and Q ↓ as you move from base to apex.
- ↓ total number of alveoli → ↓ diffusive area → ↓ V
- ↑ compression of intra-alveolar vessels → ↑ west zone 1 → ↓ Q
- V ↓’s slower than Q
- The intersection of V/Q is at rib 3, here V/Q = 1
- V/Q = 3 at the apex
- V/Q = 0.67 at bases
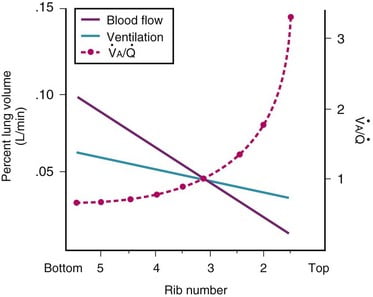
Normal Variation in V/Q causes:
- Shunt
- PACO2 and PAO2 = mixed venous blood
- PACO2 = 46mmHg
- PAO2 = 40mmHg
- Matched ventilation and perfusion
- PACO2 and PAO2 = normal blood gas for healthy patient
- PACO2 = 40mmHg
- PAO2 = 100mmHg
- Dead space
- Alveolar pressures equal that of inspired air
- PACO2 = 0mmHg
- PAO2 = 150mmHg
For PaO₂:
- Increased V/Q inequality causes:
- Mild decrease in PaO2
- At Apex
- decrease in dissolved O2
- Minimal decrease in Hb saturation due to flat part of OHDC
- Thus overall decrease in CaO2
- Base
- PAO2 = PvO2 = 40
- Addition of a small quantity of poorly oxygenated shunted blood flow (PO2 < PcO2) added to a large volume well-oxygenated PECB → small fall in CaO2 BUT a LARGER fall in PaO2
- Because carrying capacity of blood to apex is decreased there is a decrease in PaO2
For PaCO₂:
Remembering that the relationship for the Alveolar gasses:
- Increased V/Q inequality causes:
- Mild increase in PaCO2 which is rapidly compensated for due to Chemoreceptor reflexes
- Apex to base
- ↓PACO2 towards the apices of the lungs
- ↓gas transfer → ↑PaCO2
- Sensed by chemoreceptors (sensitive to ±3mmHg)
- ↑RR and Tv as per the CO2 dissociation curve
- Normal or slightly lower (after compensation) CO2
Gladwin 2016
Examiner Comments
2008A 06: No candidates (0%) passed this question.
The main points expected for a pass were:
- Range, regional pulmonary differences and gradients of V/Q ratios.
- Definitions of shunt (V/Q = 0) and dead space (V/Q = ∞).
- Explanation of why and how V/Q mismatch lowers arterial PaO2 (majority of pulmonary blood flow being from basal regions, shape of haemoglobin disassociation curve).
- Explanation of why and how V/Q mismatch lowers arterial PaCO2 (majority of pulmonary blood flow being from basal regions, predominately linear shape of CO2 disassociation curve within the physiological range of PaCO2 values).
Again, the use of illustrations would be very useful aids as part of a good answer.
Candidates often failed to frame their answer to the question that was asked and deviated to areas not directly sought after by the question. This resulted in wasted time and opportunities for marks.
Syllabus B1g
Reference Nunn 4th edition page 165-187
7. Outline the regulation of plasma calcium concentration. Outline the mechanism of action of biphosphonates for the management of hypercalcaemia.
CICMWrecks Answer
Normal Calcium: 25,000 mmol (400 mmol/kg)
Distribution:
- Readily exchangeable pool (1%) (ECF esp plasma)
- Total plasma [Ca2+] = 2.12-2.65 mmol/L
- Ionised [Ca2+] = 1.2 mmol/L
- Only the plasma free Ca is physiologically active and regulated by homeostatic mechanisms
- Two pools
- Diffusible (55%)
- 45% free/ionised
- Active form (above)
- 10% complexed
- 45% free/ionised
- Non-diffusible (45%)
- protein bound (esp to albumin)
- pH-dependent (↑ binding with ↑ pH)
- Diffusible (55%)
- Poorly exchangeable pool (99%)
- Bone/teeth (as hydroxyapatite, phosphates, carbonates)
Calcium balance
ECF and hence plasma Ca is the result of a balance between dietary intake, gastrointestinal absorption and excretion, renal excretion and exchange with bone Ca.
Normal Losses:
- Kidneys (40%) → 2.5-7.5 mmol/day
- Filtration of 250 mmol/day
- 95% reabsorbed by tubules
- PCT 65% with Na
- TAL of LoH 20%
- distal nephron 10%
- 5% excreted
- ↑ reabsorption at LoH/distal nephron
- PTH
- 1,25-dihydroxy-vitamin D
- GIT in faeces (60%) → 6-14 mmol/day
Calcium Regulation
- Calcitonin
- 32 AA peptide with 1 disulfide bond it is the hormone of Procalcitonin
- Released from Parafollicular cells (C-cells) of thyroid
- Release Stimuli: Hypercalcaemia, gastrin, beta-agonists, dopamine, oestrogen, CCK, glucagon and secretin
- Effect:
- Increases osteoblast function/Inhibits osteoclast function
- ↓intestinal calcium reabsorption
- inhibition of renal Ca and PO4 reabsorption
- Vitamin D
- Fat soluble sercosteroid from either
- diet or
- synthesis in skin of cholecalciferol from cholesterol then processed in liver and activated in PCT of the kidney
- Release stimuli
- ↑ concentration of PTH causes ↑ 1-alpha-hydroxylase activity in kidney or ↓ Ca or PO4
- Effect:
- ↑ bone release of Ca/PO4
- ↑ intestinal and renal reabsoption of Ca and PO4
- negative feedback on PTH release
- Fat soluble sercosteroid from either
- Parathyroid hormone
- Polypeptide hormone secreted from the parathyroid gland
- Release stimuli:
- ↓ Ca (primary or due to ↑ PO4) sensed by CaSR (Ca sensing receptor) in PT chief cell
- ↓ vitamin D levels
- Effect:
- ↑ Ca/PO4 reabsorption due to ↑ osteoclast activity
- ↑ Vit-D production by kidney (effect on 25 alpha hydroxylase)
- ↑ illeal Ca reabsorption
- ↑ renal reabsoption of Ca/Mg from DCT and TAL loop of henle
- ↓ PCT phosphate reabsorption
Bisphosphonates
- Indication: Hypercalcaemia
- Action:
- Analogues of pyrophosphate in which P-O-P bond is altered to P-C-P bond that is unhydralysable.
- Deposition of the analogue to the bone prevents osteoclast liberation of Calcium
Gladwin 2016
Examiner Comments
2008A 07: 1 candidate (33%) passed this question.
The main points expected for a pass were:
• The components of plasma calcium are diffusible Ca ( free and complexed ) and nondiffusible Ca (protein bound ). Only the plasma free Ca is physiologically active and regulated by homeostatic mechanisms. Plasma free Ca is also affected by plasma pH and albumin concentration.
• The distribution of Ca in the body and the fact that ECF Ca is less than 0.1% of total body Ca. ECF and hence plasma Ca is the result of a balance between dietary intake, gastrointestinal absorption and excretion, renal excretion and exchange with bone Ca.
• Tight hormonal regulation of GIT absorption, bone exchange and renal excretion mainly by parathyroid hormone and calcitriol.
Also expected were details of the actions of PTH on bone and the kidney and the actions of calcitriol on the gut and bone. No candidates described the feedback control mechanisms involving PTH and calcitriol. Additional marks were given for mention of other hormones that have a lesser effect on plasma Ca concentration.
The second part of the question on the mechanism of action of biphosphonates was poorly
answered.
Syllabus N 2i
8. Outline the role of the kidney in body water homeostasis.
CICMWrecks Answer
- Total body water = 60% body weight (approx. 42L in 70kg male)
- Intracellular fluid = 2/3 Total body water (approx. 28L)
- Extracellular fluid = 1/3 Total body water (approx. 14L)
- Plasma compartment = 1/4 Extracellular fluid (approx. 3L)
- Renal blood flow
- 1.25l/min
- Filtration fraction
- 0.2
- Glomerular filtration rate
- 180l/day
- >4x total body water
- Therefore renal water reabsorption important in H2O homeostasis
Water reabsorption
Mechanisms:
- difference in osmolality lumen : interstitial fluid. This created by reabsorption solutes (Na, Cl, glucose, AA)
- solute reabsorb actively ⇒ ↓luminal osmolality & ↑interstitial osmolality ⇒ gradient to drive water reabsorb across cells +/- tight junctions
Locations of Water Reabsorption:
PCT
- Highly permeable to water
- 65% H2O reabsorption → follows Na+ reabsorption via osmosis
LoH
- Thin descending limb
- Highly permeable to water
- 20% H2O reabsorbed
- Follows ions via osmosis
- Thick ascending limb
- Impermeable to water
- Countercurrent mechanism to increase papillary osmolarity to ~1400mosm/l
- Important for further H2O reabsorption
DCT/CD
- Variable permeability
- Controlled by aldosterone and vasopressin
If decreased total body water
- Decreased renal blood flow (beyond autoregulatory capabilities)
- Decreased glomerular filtration
- Decreased Na reaching DCT macula dense
- Release of Renin from Juxtaglomerular apparatus → Angiotensin II → Aldosterone
Angiotensin II
- Direct upregulation of proximal tubular Na reabsorption
- Efferent arteriolar vasoconstriction (as well as weak afferent arteriolar constriction) → increased filtration
- Increased thirst sensation
- Stimulates aldosterone release
- Stimulates vasopressin release
- Potentiates post-ganglionic sympathetic stimulation
Aldosterone
- Upregulates CD Na/K ATPase and inserts ENaC channels to increase Na reabsorption
- H2O follows Na via osmosis
Vasopressin (ADH)
- Stimulated by Angiotensin II, Osmoreceptors and low-volume baroreceptors
- Causes insertion of aquaporin 2 via V2 receptor stimulation
- Increased permeability of CD to H2O
- H2O reabsorbed due to high interstitial osmolarity created by counter-current mechanism and reabsorption of urea
If increased total body water
- Atrial stretch → production of natriuretic peptides (ANP, BNP)
- Leads to decreased reabsorption of Na in PCT
- Leads to decreased reabsorption of H2O
Sakurai 2016
Examiner Comments
2008A 08: 1 candidate (33%) passed this question
The main concept required was that the renal excretion of water is basic to the maintenance of constant body water conditions. This renal water excretion is controlled by multiple factors that influence glomerular filtration and tubular reabsorption. Also the kidney has mechanisms that allow it to eliminate excess water by excreting a dilute urine or to conserve water by excreting a concentrated urine.
Better answers provided details of the large GFR and the renal tubular handling of water. Also the creation of the hyperosmolar medullary interstitium by the counter current multiplier system, the special characteristics of the Loop of Henle that cause solutes to be trapped in the renal medulla and the resulting delivery of a hypoosmolar tubular fluid to the collecting ducts. Finally the variation in water permeability of the collecting ducts under the influence of ADH.
Extra marks were awarded for details on ADH including its origin, secretion, regulation and mechanism of action and the concept of electrolyte free water clearance. Some candidates confused aquaporins with vasopressin receptors. Other candidates produced long and irrelevant descriptions of the renin angiotensin system which gained no extra marks.
Syllabus D1 2f
9. Describe the physiological basis for the mechanism of action of three commonly used anticonvulsant groups. Give an example of a drug for each mechanism of action.
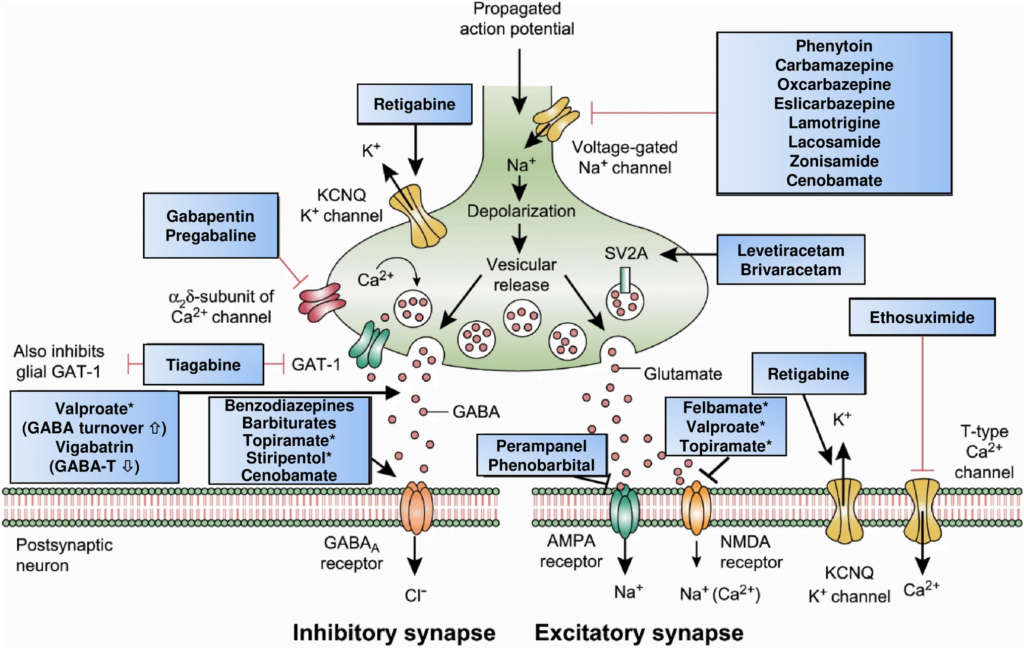
CICMWrecks Answer: Classification of Anti-Convulsants
Anticonvulsant Classification
(based on mechanism of action)
- Sodium channel blockers.
- Examples: phenytoin, carbamazepine, lamotrgine, Na valproate
- These promote the inactive state of voltage activated Na channels.
- Increased neuron refractory period to action potential generation.
- Rapid repetitive firing is diminished, spread of electrical activity to adjacent brain areas is suppressed.
- Drugs that enhance GABA mediated synaptic inhibition.
- Increases the influx of chloride ions into the cell and hyperpolarizes the neuron.
3 mechanisms:- Act on GABA receptor. Example: benzodiazepines, barbiturates.
- Inhibit GABA transporter and reduce neuronal GABA reuptake. Example: tiagabine.
- Promote GABA release. Example: gabapentin.
- Increases the influx of chloride ions into the cell and hyperpolarizes the neuron.
- Drugs that inhibit pre-synaptic Calcium channels.
- Example.: Na valproate, ?levetiracetam
- Limit activation of voltage activated Ca channel known as the T current
- Drugs that inhibit AMPA Kinate.
- Example.: Topiramate
- Limit proexcitatory action of glutamate
- Drugs that NT release.
- Example.: ?levetiracetam
- Binds to synaptic vesicle glycoprotein SV2A reducing exocytosis of synaptic vesicles
CICMWrecks Answer: Pharmacology of Gabapentin
Examiner Comments
2008A 09: 1 candidate (33%) passed this question.
The three main anticonvulsant mechanisms required were:
- Sodium channel blockers. These promote the inactive state of voltage activated Na channels. Sodium channels are unable to open for a period of time making the neuron more refractory to action potential generation. Rapid repetitive firing is diminished and spread of electrical activity to adjacent brain areas is suppressed.
Examples: phenytoin, carbamazepine, lamotrgine, Na valproate - Drugs that enhance GABA mediated synaptic inhibition. This increases the influx of chloride ions into the cell and hyperpolarizes the neuron. 3 mechanisms:
a. Act on GABA receptor. Example: benzodiazepines, barbiturates.
b. Inhibit GABA transporter and reduce neuronal GABA reuptake. Example: tiagabine.
c. Promote GABA release. Example: gabapentin. - Drugs that inhibit Calcium channels. Limit activation of voltage activated Ca channel known as the T current. Example.: Na valproate
Other mechanisms of action with examples if described earned extra marks. These included glutamate /NMDA receptor inhibition. Example: magnesium.
Syllabus G2f
10. Briefly describe the factors that influence the partial pressure of Oxygen in mixed venous blood.
CICMWrecks Answer
Mixed Venous Blood
- Mixed Venous Blood = blood taken from the pulmonary artery
- Normal mixed venous partial pressure of oxygen = 40mmHg -> 75% saturation
- Majority of oxygen in blood bound to haemoglobin
- Hb has 4 haem molecules, each can bind one oxygen molecule
- Affinity of Hb for oxygen changes with oxygen saturation, due to conformational change in the haem group on oxygen binding
- Affinity for oxygen decreased by: low pH, high CO2, high temperature, 1-3-DPG
Factors
- Complex interplay of factors which affect Venous Oxygen Partial pressure of oxygen and hence content
- Modified Fick principle: Mixed venous oxygen content = oxygen delivery – oxygen extraction
Oxygen Content
- = 20ml/100ml in arterial blood, 15ml/100ml in venous blood
- Vast majority of oxygen carried by haemoglobin, tiny dissolved portion
- Non-linear relationship between dissolved O2 and O2 content
- PO2 is the fraction of dissolved O2
- PO2 determines Sats based on Oxygen-Haemoglobin Dissociation Curve
Oxygen Delivery
= Cardiac output x Arterial oxygen content
Oxygen Demand
= Cardiac output x Arteriovenous O2 content difference
Oxygen flux
Hence SvO2 and pvO2 are surrogate markers for Oxygen flux
- O2 Delivery
- 1000ml/min in health
- Decrease in cardiac output: ↓HR, ↓SV (↓preload, ↓contractility, ↑afterload)
- Decrease in functional Hb: anaemia, carbon monoxide, congenital abnormalities of haemoglobin
- Decrease in oxygen saturation: V/Q mismatch, anatomical shunt, hypoventilation, low FiO2 (e.g. at altitude), right-shift of oxygen dissociation curve (low pH etc. – see above)
- Oxygen extraction:
- 250ml/min
- Increased by:
- increased tissue metabolism (e.g. sepsis, pregnancy, malignancy, post-operative catabolic state etc.)
- right shift of oxygen dissociation curve (see above)
Mooney / JC 2020
Examiner Comments
2008A 10: 1 candidate (33%) passed this question.
The main points candidates were expected to cover included:
- A discussion of the non-linear relationship between O2 content and partial pressure and the factors which affect this relationship. No candidate included this.
- Modification of the Fick equation as it relates mixed-venous oxygen to delivery and consumption.
- The components of delivery should have been described and use of the O2 flux equation would have been helpful. Additional marks were available for describing how these might change in physiological and pathological states.
Candidates frequently interchanged content and partial pressure, without clearly displaying how these are related. Normal values were not provided. The O2 flux equation, when included, was often written incorrectly. No consideration was given to normal variations, such as pregnancy or exercise
Reference: Nunn 5th edition pages 267 to 269, page 493
Syllabus: B1h Gas transport in the blood 2a
11. List the physiological factors that increase respiratory rate. Include an explanation of the mechanism by which each achieves this increase.
CICMWrecks Answer
Central control of ventilation
- Brainstem
- Medulla (DRG, VRG)
- Pons (Pneumotaxic, Apneustic)
- External inputs
- Sensors from lungs/airways/chemoreceptors
- Cortex
- Can override brainstem function and alter breathing patterns
- Limbic/Hypothalamic Systems
- Alters breathing patters depending on affective states (fear/anxiety/pain)
Peripheral Receptor Inputs alter respiratory rate/depth (ventilation)
- URT receptors (in nose, NP, larynx, trachea)
- mechanical/chemical stimuli
- bronchoconstriction, sneezing/coughing reflexes, and laryngeal spasms
- Joint/Muscle receptors
- limb movement (Eg. exercise) can stimulate ventilation
- Gamma-system (in muscle spindles)
- muscle spindles sense muscle elongation,
- can cause sensation of dyspnoea
- Arterial baroreceptors (in AB and CB)
- ↓ BP causes ↑ ventilation
- ↑ BP can cause ↓ ventilation (and even apnoea)
- Nociceptors
- pain causes apnoea initially, then stimulates ventilation
- Thermoreceptors
- ↑ temperature stimulates ventilation
- ↓ short deep inspiration
Hypocaponea/Hypoxia sensed by Chemoreceptors
- Central CR response
- ↓’d perfusion → ↑[H]/↓PaO2 → CR stimulation
- Afferent signal → Sinus nerve (of herring) / Vagus Nerve → Chemosensitive area of medulla
- Vasomotor area → ↑ peripheral vasoconstriction
- Respiratroy center → ↑rate and depth of respiration → ↑ venous return
- ↓’d perfusion → ↑[H]/↓PaO2 → CR stimulation
- Peripheral CR response
- PCRs found in “Glomus cells”
- ↓ PaO2 – via inhibition of O2 sensitive K-channels
- ↑ PaCO2 and/or ↓pH – via effect on pH sensitive K-channels
- Type I cells (rich in NAd, DA, ACh)
- Hypoxia causes release of NTs
- NAd/ACh – ↑ AP firing rate of AB or CB afferent fibres
- DA – Damping of type 2 cell responses
- Type II cells (rich in capillary supply)
- ↓ PaO2, ↑ PaCO2 and/or ↓pH
- ↓ IC [ATP] which leads to ↑ NT production and release
- ↑ AP firing rate of AB or CB afferent fibres
- PCRs found in “Glomus cells”
Response to:
- ↓ PaO2
- Gradual ↑ MV PaO2 < 500mmHg,
- Rapin ↑MV PaO2 < 50 mmHg
- ↑ PaCO2
- Central CRs (CCRs)
- Most (80%) of response to ↑ PaCO2 by CCRs
- Peripheral CRs
- Response to ↑ PaCO2 is < 20% of total response
- Much FASTER cf. CCR response
- Role is to match ventilation to sudden ∆ in PaCO2
- ↓ blood pH
- Sensed by PCRs in CB only
- Central CRs (CCRs)
CO₂ most important factor

- Minute ventilation is directly proportional to PaCO2 MV ↑ by 2-3 L/min per mmHg PaCO2
- Hypoxaemia has a synergistic effect on hypercapnoeic-ventilatory drive↓ PaO2 causes↑ MV for a given PaCO2,
- ↑ ∆ MV per ∆ PaCO2
O₂ Less important in normal ranges
- PaO2 plays a SMALL role in ventilatory control
- Effect of PaO2 on ventilation:At normal PaCO2PaO2 < 500 mmHg MV ↑ slowly as ↓ PaO2 decrease
- PaO2 < 50 mmHg MV ↑ drastically
- There is synergistic ↑ in ventilatory response in the presence of hypercapnoea and/or acidosis
- MV ↑ drastically when PaO2 is < 100 mmHg

Gladwin 2016
Examiner Comments
2008A 11: No candidates (0%) passed this question
The main points candidates were expected to cover included:
• A description of the central and peripheral chemoreceptors, their predominant stimuli and effect on ventilation.
• PaCO2 as the main influence on normal ventilation, the near-linear relationship to minute ventilation around the normal range, and how CO2 produces this effect.
• PaO2 and pH and their sites of action.
• Other stimuli to ventilation – exercise, pregnancy, temperature, baroreceptors.
Candidates frequently confused central and peripheral receptor activities and failed to provide any relative significance to the major factor(s). The use of a graph relating the main factors to minute ventilation would have been helpful.
Syllabus B1c 1
Reference: Nunn 6th edition 60-68, Kam 1st edition 92-98
12. Classify the commonly used inotropic agents and list their mechanisms of action.
CICMWrecks Answer
- Inotrope: Agent that alters the force of contraction of a muscle
- Inotropes can be classified in an number of ways:
- Negative or positive (the examiners did not want a discussion of negative inotropes on previous reports)
- Naturally occurring: Eg Adrenalin, Noradrenalin, Dopamine
vs Synthetic: Dobutamine, dopexamine, isoprenalin, salbutamol
Classification by Mechanism of action is most effective
Postitive Inotropes
Class 1: Increase intracellular Calcium by a variety of mechanisms → ↑force of contraction
- Direct Adrenoceptor agonists (NA, Adren):
- ↑ intracellular cAMP and ↑ Ca by Gs protein coupled mechanism
- Indirect adrenoceptor agonists (Ephedrine):
- displacing NA from vesicles into cytoplasm
- ↑’s carrier-mediated diffusion into synaptic cleft
- ↑NA release at nerve terminal → ↑’d stimulation
- Phosphodiesterase inhibitors (Theophyline, Milrinone):
- inhibit PDE → ↓breakdown of cAMP (cGMP) → effective ↑cAMP
- cAMP effects as per direct adrenoceptor agonists
- Glucagon
- Bypasses adrenergic receptor to stimulate ↑cAMP
- Na/K atpase inhibitors (digoxin):
- Inhibit Na+/K+-ATPase → ↑[Na+]i → Impair Na+/Ca2+ exchange pump → ↑[Ca2+]i
Class 2: Increase sensitivity of actin/myosin complex to calcium by action on troponin C
- Levosimendin
- ↑Ca interaction with troponin C → enhance contractility without ↑[Ca]i
- Activate KATP channels on mitochondrial membrane → protect muscle from ischaemia leading to ischaemic preconditioning
Class 3: Act via metabolic or endocrine pathways (T3, Ca, Mg)
Negative Inotropes
- Beta-blockers
- Usually β1 blocking action
- Inhibits the action of natural stimulation of Class 1 above
- Calcium-channel blockers are used for treating high blood pressure, chest pain, and irregular heart rhythm.
- ↓ intracellular Ca via action on L-type calcium channels
- nifedipine, amlodipine
- Centrally acting sympatholytics are used for treating high blood pressure.
- Act centrally on α2 receptors to decrease sympathetic tone.
- Clonidine
Gladwin 2016
Examiner Comments
2008A 12: No candidates (0%) passed this question.
Candidates could use a number of different classifications, however, were required to include all of the major groups of agents. Most made some mention of the sympathomimetics, however failed to sub-classify these, or confused catecols versus non-catecols, or naturally occurring versus synthetic agents. Other agents, such as phosphodiesterase inhibitors, calcium sensitisers, cardiac glycosides, or calcium itself received minimal attention.
Mechanisms of action required more than listing adrenergic receptor types. Some listing or discussion of the sub-cellular mechanisms was necessary. Comment about intracellular calcium being the final common end-point would have scored additional marks
Syllabus C2d 2
Reference: Stoelting 4th edition 293-320, Katzung 10th edition 121- 198, Rang and Dale 6th edition 168-187 290-291
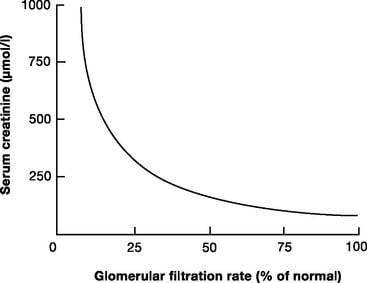
13. Describe the relationship between creatinine clearance and serum creatinine concentrations. What are the potential pitfalls in using serum creatinine concentrations to assess renal function in a critically ill patient in ICU?
CICMWrecks Answer
Clearance:
- Renal clearance = vol of plasma completely cleared of a given substance by the kidneys per unit time (ml/min)
- Involves: glomerular filtration, secretion, reabsorption, and rarely tubular metabolism
- Renal clearance = V x [U]/[P]
- V = volume of urine or urine flow rate in ml/min
- [U] = urinary concentration of substance in mg/ml
- [P] = plasma concentration of substance in mg/ml
Estimating GFR:
- GFR = renal clearance of a substance if it is:
- Freely filtered at glomerulus
- Not secreted
- Not reabsorbed
- Not synthesized
- Not metabolised
- The amount excreted in the urine = amount filtered
- i.e. [plasma] x GFR = [urine] x urine vol
- Rearrange: GFR = urine vol x [urine] / [plasma]
Creatinine
- used to approximate GFR as is more practical
- Released at a steady state from skeletal muscle cells (phosphocreatine)
- Freely filtered + not reabsorbed.
- *Small amount secreted → overestimates GFR by small amount
- Serum creatinine levels can be used as a surrogate marker of GFR
- Creatinine clearance calculation better than creatinine levels
- most accurate to collect urine and use the formula to assess creatinine clearance
- because serum creatinine it is at steady state, eGFR can be calculated by Cockroft-Gault, MDRD or CKD-Epi
- During acute changes in GFR, serum creatinine will underestimate GFR until a new steady state is reached.
Creatinine Clearance Estimation from Serum Creatinine
- CG (Cockcroft-Gault Equation): common method which has a correlation of ~0.83 with CrCl:
- CrCl = [(140−A) × W x S)] / (72 × Cr) , where:
- Cl = Clearance (mL/min), A = Age, W = Lean body Wt (kg)
- S = Sex coefficient (Male = 1, Female = 0.85), Cr = Creatinine in µmol.L-1
- CrCl = [(140−A) × W x S)] / (72 × Cr) , where:
- Alternative formulas are MDRD and CKD-EPI. These equations have two advantages over Cockcroft-Gault:
- They are better predictors of GFR
- They do not require weight, and so can be calculated by the laboratory automatically, Other required data (gender, race, age, creatinine) can be taken from hospital records.
- MDRD (Modification of Diet in Renal Disease Study): useful in estimating glomerular filtration rate (GFR) in stable chronic kidney disease
- CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): more precise formula to estimate glomerular filtrate rate (GFR) from serum creatinine and other readily available clinical parameters, especially at when actual GFR is >60 mL/min per 1.73m2
Limitations
- General limitations
- Dependent on serum creatinine, which can be highly variable. Formulas are derived from average values of dependent variables, and so will be unreliable at extremes of:
- Age
- Muscle mass
- Critically ill
- Malignancy
- Diet (High production with red meat)
- the relationship between creatinine clearance and serum creatinine is non-linear
- filtration is only one component of a complex kidney, although GFR is used as a surrogate of function
- Small amount of creatinine is secreted by proximal tubule – so Creatinine clearance is ~10-20% higher than GFR
- More severely overestimates GFR in patients with very low GFR
- Dependent on serum creatinine, which can be highly variable. Formulas are derived from average values of dependent variables, and so will be unreliable at extremes of:
- Critically ill patients
- the amount of creatinine produced varies with muscle mass, nutrition, steroid use, muscle injury
- there can be a decline of almost 50% of function before serum creatinine levels rise
- they do not indicate dynamic changes in renal function
- are modified by aggressive fluid resuscitation
JC 2019
Examiner Comments
2008A 13: No candidates (0%) passed this question.
It was expected candidates would describe that both these are surrogate measures for Glomerular filtration rate. Credit was given for clear definitions, formula and normal values (such as plasma clearance is the volume of plasma cleared per unit time). It was expected candidates could explain that serum creatinine results from a balance of creatinine produced and excreted and hence the slow response time and limitations for its use because of changes in both production and excretion.
Extra credit was given for appreciating the non linear relationship between changes in serum creatinine and creatinine clearance and that significant changes in glomerular filtration can occur before this is reflected in the serum creatinine. Comment on the problems of dilution with acute changes in fluid balance and that tubular secretion of creatinine can occur when the serum creatinine concentration is high both gained extra marks.
Syllabus D1 2
Reference Ganong 22nd edition 699-728
14. Define the terms antiseptic and disinfectant. Briefly describe the advantages and disadvantages of alcohol, chlorhexidine, glutaraldehyde and povidone iodine.
CICMWrecks Answer
- Disinfectants
- Strong chemical compounds that kill of inhibit the growth of microbes
- Antiseptic
- Disinfectants with sufficiently low toxicity to host cells, that can be used directly on skin, wounds or mucosa
Pharmacopeia - Antiseptics & Disinfectants (Level 3)
PC: Pharmaceutics, PD: Pharmacodynamics, PK: PharmacokineticsColumn Visibility: To filter out some of the drugs
Dropdown Menus: To filter out categories or items
Search bar: To search within table
Print: Print to PDF or printer. Pls remember to change to landscape if the table does not fit in portrait mode.
| CATEGORY | ITEM | ALCOHOL | CHLORHEXIDINE | IODINE | GLUTARALDEHYDE |
|---|---|---|---|---|---|
| Basics | CICM Level of Understanding | Level 3 | Level 3 | Level 3 | - |
| PD | Main Action | Likely act by denaturing proteins | Cationic biguanide -strongly adsorbs to bacterial membranes causing leakage of small particles and precipitation of cytoplasmic proteins | Oxidative damage. | Alkylation of micro-organism proteins or nucleic acids |
| PD | Mode of Action | Evaporative effects are useful when sinks with running water are not available | Resistant to inactivation by blood and organic material Low skin irritation | 1. Sporicidal 2. Cheap 3. Broad spectrum Most effective for intact skin | Used for sterilization of equipment that cannot withstand high temperatures |
| PD | Route & Doses | 1. Flammable- must be allowed to dry fully before diathermy or laser surgery 2. Corneal damage 3. Skin drying 4. Ineffective against C. Dif spores | 1. Neurotoxic 2. Delayed effect 3. No direct spore activity | 1. Hypersensitivity reactions 2. Delayed onset without residual activity 3. Stains clothes and dressings | 1. Once activated have a shelf life of 14 days 2. Failure of activity may occur due to excessive dilution or exposure to organic material (frequently reused) 3. Irritating to eyes and respiratory mucosa 4. Slow activity |
| PD | Spectrum of Activity | Onset: Rapid Duration: Lack residual action because they evaporate completely | Onset: Delayed Duration: Sustained residual activity | Onset: Iodine is bacteriocidal in 1 minute and kills spores in 15 minutes. However in povidine compounding it has a delayed onset Duration: No sustained effect | Onset: 2.4% glutaraldehyde achieve disinfection in 45 minutes |
| PD | Resistance Mechanism | - Gram positives and negatives - Acid fast bacteria are susceptible - Lipophilic viruses may be susceptible - Many fungi | - Bacteria (G+ve>G-ve) - Moderate fungal and viral activity - Inhibits spore germination | - Bacteria (G+ve and –ve and acid fast) - Sporicidal - Viruses - Fungi | - Bacteria (G+ve and –ve and acid fast) - Spores - Viruses - Fungi |
| PD | Ineffective Against: | Spores and prions Hydrophilic viruses are less susceptible | Spores and prions Hydrophilic viruses are less susceptible | Prions Hydrophilic viruses | Prions |
| Spl | SPECIAL POINTS | Optimal bacteriocidal concentration is 60-90% | Can be combined with 70% alcohol Preferred antiseptic for central venous access Not absorbed orally | Used to sterilize fiber optic endoscopes and respiratory therapy equipment |
Sakurai 2016
Examiner Comments
2008A 14: No candidates (0%) passed this question.
It was expected candidates could define and distinguish between these terms with a specific comment that disinfectants are applied to inanimate objects and antiseptics can be applied to living tissue. The advantages and disadvantages could be addressed either tabulated or discussed in point form, either was acceptable.
It was expected answers would include a comment on each agent and specifically address areas such as general spectrum of activity, speed of onset ( agents that need to dry to be effective versus those with more rapid onset), duration of effect ( residual activity), limitations of use and potential hazards. Marks were awarded for identifying Glutaraldehyde as a disinfectant (as opposed to the other antiseptic agents) and its use for cleaning equipment such as endoscopes with the precautions required for potential toxicity.
Additional credit was given for discussion of relevant facts such as the proven benefit for chlorhexidine skin preparation for central venous line insertion. Candidates are referred to several of the recommended texts which cover this area well.
15. Compare and contrast the pharmacology of sodium nitroprusside and glyceryl trinitrate for the treatment of acute hypertension
Examiner Comments
2008A 15: 2 (66%) candidates passed this question.
It was expected candidates would address specific aspects of pharmacology such as action, mechanism of action, half life and duration of effect, route of administration, potential toxicity and special precautions. These agents lend themselves to comparison and contrast as several distinct similarities and differences exist and credit was given for highlighting these. Specific comments should include that both agents result in blood vessel dilation with extra credit given for detailing the differences in the balance of arterial versus venous effects between them. For both agents the effect is mediated through nitric oxide and it was expected candidates would identify that nitroprusside releases NO spontaneously and GTN requires enzymatic degradation with the resultant effects on smooth muscle mediated via cGMP. They are both short acting agents when used intravenously and require careful titration to measured blood pressure for effect.
Extra credit was given for mentioning that routes other than IV are available for GTN (topical / oral) but not for nitroprusside. Comments on special precautions such as Nitroprusside should be protected from light and GTN given via non PVC giving sets gained additional marks. In addition to the well described adverse effects of each agent, it was expected candidates would mention the potential for cyanide toxicity with nitroprusside and extra marks were awarded for an indication of usual doses.
Syllabus C2b 2e
References Katzung 10th edition, Goodman and Gillman Chp 31 & 32
16. Describe the blood-supply to the liver.
CICMWrecks Answer
Hepatic blood flow
- 1.5l/min
- Anatomy
- Afferent via Hepatic Artery (branch of ceoliac trunk) contributing 30% and Portal Vein (confluence of inferior mesenteric, superior mesenteric and splenic veins) contributing 70% of hepatic blood flow
- Hepatic artery supplies 50% of O2 delivery
- Portal venous blood has PO2 of 80% due to mesenteric AV anastamosis
- Blood flows through low pressure hepatic sinusoidal system to ventral vein
- Efferent via Hepatic Vein → IVF
- Afferent via Hepatic Artery (branch of ceoliac trunk) contributing 30% and Portal Vein (confluence of inferior mesenteric, superior mesenteric and splenic veins) contributing 70% of hepatic blood flow
Regulation
Intrinsic
- Hepatic arterial and Portal venous pressure – Hepatic venous pressure / Hepatic vascular resistance (low)
- Autoregulation to systolic blood pressure 80mmHg
- Metabolic autoregulation
- Vasodilation in response to H+, CO2, lactate
- Semi-reciprocal relationship
- Hepatic arterial blood flow increases when protal venous flow decreases
Extrinsic
- Respiration
- Diaphragmic contraction → Decreased intrathoracic (and IVC) pressure and increased abdominal pressure → Favours hepatic blood flow
- Post-prandial
- Increased splanchnic blood flow post-prandially → Increased hepatic blood flow
- Neural
- Sympathetic
- α adrenoceptors present in portal vein AND hepatic artery
- β adrenoceptors only present in hepatic artery
- Therefore sympathetic input tends to venoconstrict the portal vein and dilate the hepatic artery
- Sympathetic
- Hormonal
- Adrenaline
- α constricts protal veins and decreases HBF
- β dilates hepatic arteries and increases HBF
- Glucagon increases hepatic blood flow
- Vasopressin decreases hepatic blood flow
- Angiotensin II decreases hepatic blood flow
- Vasoactive Intestinal Peptide (VIP) and secretin increase hepatic artery flow
- Adrenaline
Changes to drug metabolism when liver blood flow decreases:
- The two major determinants of hepatic clearance are the efficiency of drug removal from the blood and the efficiency of blood delivery to the liver.
- Efficiency of drug removal by the liver = hepatic extraction ratio
- fraction of the drug entering the liver in the blood which is irreversibly removed (extracted) during one pass of the blood through the liver.
- determined largely by:
- the free (unbound) fraction of the drug and
- the intrinsic clearance rate (the intrinsic ability of the liver to remove (metabolise) the drug in absence of restrictions imposed on drug delivery to the liver cell by blood flow or protein binding.)
- The effect of liver blood flow on hepatic clearance depends on the hepatic extraction ratio of the drug.
With decreasing hepatic blood flow, hepatic extraction ratio will increase for all drugs.
- For drugs with low intrinsic clearance:
- Hepatic extraction ratio will increase with decreasing hepatic blood flow
- Hepatic clearance will not decrease significantly with decreasing blood flow
- For drugs with high intrinsic clearance:
- Hepatic extraction ratio will increase slowly with decreasing blood flow
- Hepatic clearance will decrease in a fairly linear fashion, in proportion to hepatic blood flow
Also, Drugs which enjoy enterohepatic recirculation may have decreased half-lives due to failure of recirculation
Sakurai / JC 2019
Examiner Comments
2008A 16: No candidates (0%) passed this question.
A correct description of the vascular anatomy; the contribution and composition of hepatic artery and portal vein flow to total hepatic flow and how this is regulated would be awarded with a pass. An answer that expanded on these main points received additional marks. The interdependence of hepatic artery and portal vein flow was not appreciated by any candidate.
Either candidates knew the answer to this question or they did not. Some candidate(s) tried to guess at what the anatomy might be. This attracted no marks. Many candidates lacked sufficient knowledge to pass this question.
Syllabus I 2 d&g
17. Describe the role of the kidney in drug excretion, and the factors affecting this.
Briefly outline how you would alter the dosing of gentamicin in a patient with H impairment.
CICMWrecks Answer
Renal Drug Excretion
Due to glomerular filtration and balance of secretion and reabsorption
Glomerular Filtration
- Physiological factors
- Starling forces
- Filtration pressure ∝ (PG – PBC) – ( πG – πBC)
Where- PG = Glomerular hydrostatic pressure (60mmHg)
- PBC = Bowman’s Capsule hydrostatic pressure (18mmHg)
- πG = Glomerular oncotic pressure (32mmHg)
- πBC = Bowman’s Capsule oncotic pressure (Negligible)
- Filtration decreases
- Shock → decreased glomerular pressure
- Obstruction → increased bowman’s capsule hydrostatic pressure
- Hypoproteinaemia → hepatic failure, nephrotic syndrome
- Fick’s Law (see eqn)
- Filtration increases with decreased MW, increased concentration gradient
- Filtration decreases with increased GBM thickness (glomerulosclerosis, deposition) and loss of glomerular surface area (usually 0.8m2. may be lost with renal failure)
Fick’s Law:
- Drug factors
- Size (Due to filtration slits and podocyte foot processes)
- Particles <4nm freely filtered
- Particles >8nm excluded
- Charge
- GBM has negative charge, therefore negatively charged molecules repulsed → decreased filtration
- Protein binding
- Highly protein bound drugs inhibited from filtration due to large size and charge of proteins
- Size (Due to filtration slits and podocyte foot processes)
Secretion
- Due to specific transporters
- P-glycoprotein (Amphipathic anions)
- MPR-2 (Conjugated metabolites)
- ATP-Binding Cassette Transporters (Organic cations)
Reabsorption
- Passive reabsorption occurs in PCT and DCT
- Membrane transporters for facilitated transport are present in DCT
- Ionization
- pH of urine may lead to drug “trapping”
- Acids ionized with pH > pKa
- Bases ionized when pH < pKa
- pH of urine may lead to drug “trapping”
- Lipophilicity
- Allows permeation through phospholipid bilayers of cell membrane → reabsorption
Dose adjustment of renally excreted drugs
- According to clearance and GFR
- Loading dose = Target concentration x Vd
- Usually no alteration of loading dose required
- In ESRF, volume of distribution may be increased → loading dose may need to be increased
- Dosing rate = Target concentration x Cl
- As clearance is reduced in renal failure dosing rate requires reduction
- Maintenance dose = Dosing rate x Dosing interval
- Maintenance dose and/or the dosing interval may need to be reduced (depending on whether AUC or peak concentration of drug is required for drug effect)
- Plasma monitoring of [drug] concentration required for drugs with low therapeutic index
Dose adjustment of Gentamicin
MoA: Inhibition of bacterial protein synthesis through irreversible binding to the 30s bacterial ribosome
Has a narrow therapeutic window, hence dose optimization and therapeutic drug monitoring are crucial.
| Concentration dependant killing | Active concentration needs to be acheived (~8-10 times Minimim Inhibitory Concentration/MIC) Time above MIC does not need to be maintained | Loading dose should not be altered even in renal failure (5-7mg/kg) |
| Significant postantibiotic effect Distribution: Hydrophilic, so Distribute mainly to extra-cellular fluid. Redistribution can occurs upto 16-24 hours. | Once daily dosing sufficient without renal failure | |
| ESRF – Volume of distribution theoretically larger (but in case of Aminoglycosides, might be lower – suspected due to tissue displacement by other molecules like urea) | Loading dose adjustment in ESRF 4mg/kg | |
| Metabolism: Nil Excretion: Rapidly excreted by glomerular filtration. Hence accumulate in renal failure (t1/2 upto 30-60hrs) | Subsequent Dosing interval based on renal clearance (GFR / CrCl) Frequent dosing only increases toxicity with no improvement in efficacy. | Maintenance dosing less frequent in renal failure Dose interval based on pre-existing guidelines, and with monitoring of pre-administration levels CrCl 40 to 59 mL/minute: Administer every 36 hours CrCl 20 to 39 mL/minute: Administer every 48 hours CrCl <20 mL/minute: Monitor serum levels and redose when gentamicin level is less than 1 mg/L |
| End stage renal disease / Dialysis (t1/2 upto 100hrs) | Check level prior to dialysis, and only administer dose after dialysis (based on expert advice) | |
| Low Therapeutic index | Frequent monitoring of levels required | |
Sakurai / JC 2019
Examiner Comments
2008A 17: No candidates (0%) passed this question.
The main points candidates were expected to cover included a brief definition of renal clearance followed by a description of the drug factors that affect this (filtration, secretion and reabsorption), recognition that GFR and protein binding was important in the answer. A brief description of gentamicin kinetics that affect dosing regimens and a statement that dosing would be guided by calculated GFR and measured drug levels wold round out a good answer. Correct elaboration of the above factors were rewarded with additional marks.
Candidates failing this question submitted answers where concepts were randomly mentioned with no attempt to integrate these into a cohesive answer that demonstrated an understanding of the topic. Writing random words without examples or explanations did not demonstrate sufficient understanding to be rewarded with marks. Again, many answers lacked sufficient detail in the answer.
Syllabus – Pharmacokinetics
18. Compare and contrast the pharmacology of drugs that change the pH of gastric fluid.
CICMWrecks Answer
Mechanism of Gastric Acid Secretion
- Simulation of secretion via
- ↑Gastrin via gastrin receptors
- ↑Ach via M1 rexeptors
- Histamine via H2 Receptors
- receptors located on the basolateral membrane
- Ach and gastrin → ↑ in intracellular [Ca2+]
- H2 receptors are coupled via Gs to adenyl cyclase
- ↑ in Ca2+ and cAMP → ↑ protein kinase activity → ↑ hydrogen pump activity in the luminal membrane
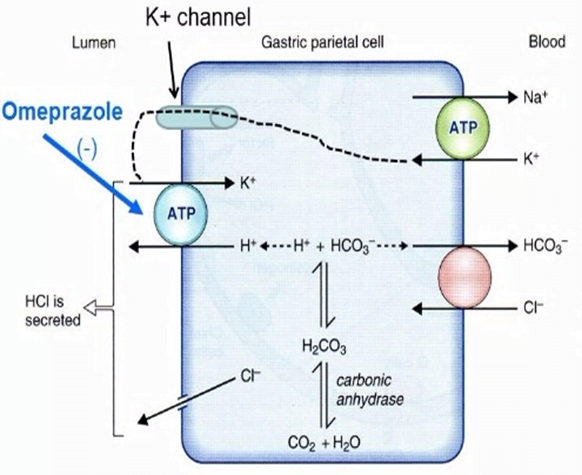
Drug Classes
- PROTON PUMP INIHBITORS (eg, pantoprazole)
- All are prodrugs
- MOA
- Converted to its active form in the low pH of stomach
- Accumulates in the parietal cells after absorption
- Binds to the H/K-ATPase anti-porter
- Inhibiting the proton pump and causing potent and long lasting suppression of basal and stimulated gastric acid secretion.
- PD:
- ↓ acidity/gastric secretions, no change emptying/LOS tone
- PK
- A:
- pKa ~4, lipophilic weak bases thus diffuse into acidic environments well (ie: parietal cell) thus attack secreting not quiesent pumps
- ~50% oral bioavailability, best on empty stomach
- D: High protein binding >90%
- M: rapid first pass metabolism
- E: t1/2 1.5hrs but irreversible H/KATPase bond lasts ~24hrs
- HISTAMINE-2 RECEPTOR ANTAGONISTS (eg; ranitidine)
- Bind to the H2 receptor on the basolateral surface of the parietal cell to reduce H2-stimulated acid secretion from the H/K ATPase on the luminal side of the cell.
- ↓ acidity/gastric secretions, no change emptying/LOS tone
- PK
- well orally absorbed, 50% first pass metabolism
- poorly protein bound 15%
- hepatic metabolism, does not induce p450
- MUSCARINIC ANTAGONISTS (eg, pirenzipine)
- Relatively specific M3 receptor antagonist which reduces the acetylcholine-mediated stimulation of the proton pump
- ANTACIDS (eg, Mylanta, Gastrogel).
- Weak bases which usually contain aluminium and/or magnesium compounds to chelate H+ ions
Gladwin 2016
Examiner Comments
2008A 18: 1 candidate (33%) passed this question.
The main points candidates were expected to mention were the major drug groups (antacids, H2 antagonists, proton pump blockers), describe their mechanism of action; briefly mention relevant pharmacokinetics and then briefly discuss the potential problems and interactions when using them. Additional credit was given for answers providing more detail. Many answers did not mention antacids or prostaglandin analogues, choosing only to discuss H2 receptor blockers and proton pump blockers. Even then, many answers included incorrect pharmacokinetic data. Drug interactions were rarely mentioned. A discussion of normal gastric acid secretion was not asked for and was not rewarded with marks.
Syllabus Q2a
19. Outline normal impulse generation and conduction in the heart. Describe the features present in a normal heart that prevent generation and conduction of arrhythmias.
CICMWrecks Answer
Normal Impulse Generation
Occurs in the sinoatrial node
- Innervated by (predominantly right) vagal nerve and sympathetic nerves
- Upslope of phase 4 due to “Funny” Na current (inward flow of Na)
- Parasympathetic (vagal) stimulation causes decrease (more negative) in the minima and shallower upward gradient of phase 4, delaying depolarization
- Sympathetic stimulation causes an increase (more positive) in the minima and steeper upward gradient of phase 4, hastening depolarization
- Action potential generated when threshold potential reached (~-50-55mV) → Opening of voltage gated Ca channels → Ca influx
- Cell begins repolarization when Ca channels close and K channels open à K efflux
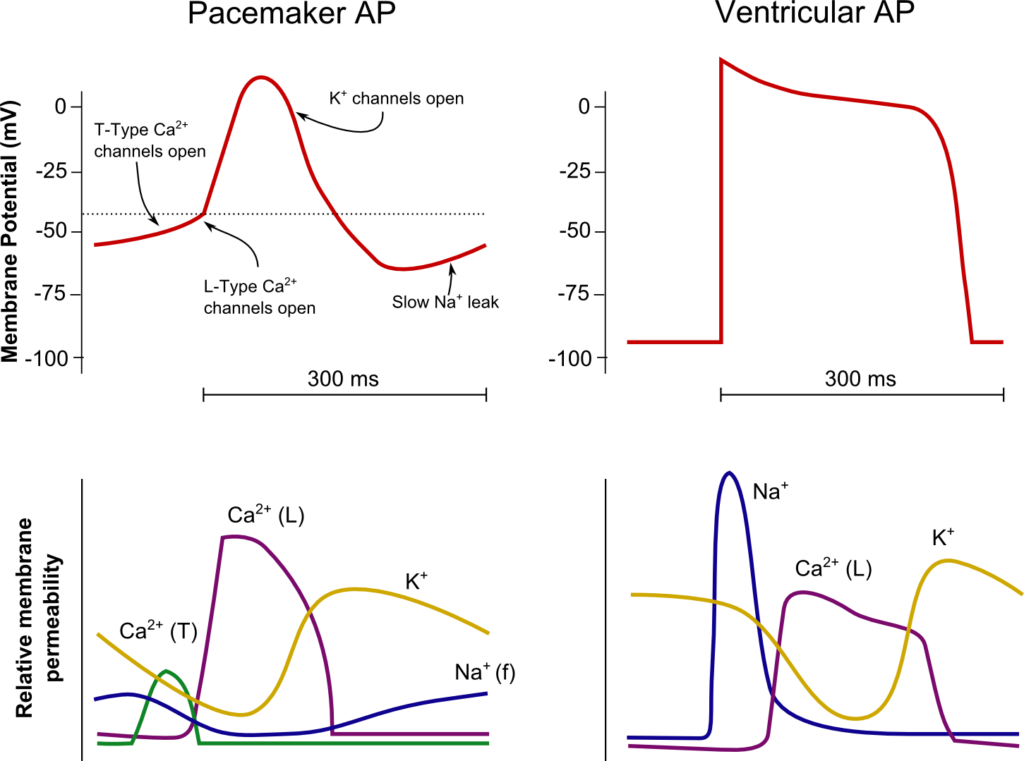
Normal Conduction
- SA node (5cm/sec) → Internodal fibres and Bachmann’s plexus (100cm/sec) → AV node (5cm/sec) → Bundle of His (100cm/sec) → Purkinje fibres (400cm/sec)
- Occurs due to fast wave action potentials (slow wave in AV node)
Ventricular AP:
- Phase 0 (Fast upstroke)
- Occurs due to opening of voltage gated Na channels (Na influx)
- Overshoot to +20mV
- Occurs due to opening of voltage gated Na channels (Na influx)
- Phase 1 (Early repolarization)
- Inactivation of Na channels and opening of K channels (K efflux) → Start of absolute refractory period
- Phase 2
- Opening of voltage gated Ca channels (Ca influx) balances K efflux
- Phase 3
- Closure of Ca channels → Repolarization
- Na channels transition to closed state (absolute refractory period transitions to relative refractory period)
- Phase 4
- Resting membrane potential (-80mV~-90mV) maintained by Na/K ATPase
Features which Prevent Arrhythmias / Conduction Abnormalities
Preventing the generation of arrhythmias
- SA nodal rate has faster intrinsic firing rate than other areas (~30bpm) causing
override inhibition of other pacemakers
Preventing the conduction of arrhythmias
- AV node prevents the conduction of impulses >220bpm
- Normal myocyte action potential has absolute (where no stimulus can initiate a new
action potential) and relative (where a greater than normal stimulus is required for initiation of a new action potential) refractory periods - Fibrous cardiac skeleton prevents conduction of action potentials other than through
the specialized conduction pathways
Sakurai 2016
Examiner Comments
2008A 19: 1 candidate (33%) passed this question.
This question required description of the SA node, its primary role and generation of the pacemaker potential and the influence of the autonomic nervous system. A diagram of the conducting pathways, highlighting specialized tissues with fast or slow conduction velocities would have been appropriate. The importance of the AV node in preventing retrograde conduction and high rates conducted to the ventricles (>220 / min) was often neglected in answers. A discussion of the Purkinje Fibres with particular reference to the absolute and relative refractory periods was essential.
Additional marks were awarded for mention of the atrial internodal pathways, conduction within the ventricles from the endocardial to epicardial surfaces and the significance of the compensatory pause in response to ectopic beats.
Syllabus C1b 2.a, b;
Reference: Cardiovascular Physiology, “Electrical Activity of the Heart” (Chapter 2), Berne and Levy.
20. Statistics (not in current primary syllabus)
21. Explain the ABO blood groups and how blood group is determined. How is blood tested for compatibility using the ABO system?
CICMWrecks Answer
Blood Typing
- The purpose of blood typing is the streamlining of blood products on the basis of the presence of antiogenetic surface membrane proteins in order to minimise patient harm.
- Main Types of Antigens:
- ABO
- Rhesus
- Others:
- Kell, P, MN, Lewis
- less antigenic
ABO system:
Red blood cells possess genetically determined antigens on their cell membrane, termed
“agglutinogens”
Agglutinogens: glycoproteins, with differing terminal oligosaccharides
- H gene:
- Forms “H-antigen” by addition of fructose to membrane glycoprotein
- A gene:
- Forms “A-antigen” by extension of H-antigen with “N-acetyl-D-galactosamine”
- B gene:
- Forms “B-antigen” by extension of H-antigen with “D-galactose”
Blood Groups:
| Group | Antigen | Possess Antibody | Incidence |
|---|---|---|---|
| A | A-antigen only | Anti-B antibody | 45% |
| B | B-antigen only | Anti-A antibody | 9% |
| A/B | A and B-antigen | Lack both Anti-A and Anti-B antibodies | 3% |
| O | H-antigen only | possess anti-A and anti-B Ab’s | 43% |
Blood Group antibodies:
- Naturally-occurring Ab to A- and/or B-antigens (as anti-A and anti-B antibodies)
- IgM only
- Develop naturally after 3 months of age due to presence of Ag in bacteria/food that resemble A/B-Ag’s
Typing / Blood Group determination (Coombs)
- Red cells tested using anti-sera (Ab added to sample)
- IgM anti-A and anti-B Abs added to sample
- +ve agglutination indicates blood type
- “Reverse blood grouping” (Sample added to Ab)
- Test individual’s serum using known group A, B, and O red cells
- +ve agglutination indicates blood type
Antibody screen:
- Used to detect if an individual possesses antibodies other than those against agglutinogens A and B
- Individual’s blood is exposed to a number of red cells with known antigens, and if a reaction, an antibody is present
- A common antibody is the D antigen, or Rhesus factor
- Highly antigenic
- Present on RBC only
- Three subtypes
- Cc; Dd; Ee
- d-Ag does not exist
- Any combination of the three with a D-Ag is highly antigenic.
- Rh +ve (if D-Ag present)
Cross Matching
Major Crossmatch: Test in vitro recipient’s serum and donor’s red cell
Minor Crossmatch: In vitro serological compatibility between donor’s serum and recipient’s red cells
Major Crossmatch:
- Saline agglutination test
- To reconfirm ABO grouping
- It tests for presence of IgM (Eg. anti-A and anti-B Ab’s) in recipient serum against donor RBC
- Donor’s RBCs + saline + recipient serum
- +ve agglutination means incompatible ABO
- Indirect Coomb’s test
- To Reconfirm presence of “minor” Ab’s in recipient serum
- Two stages:
- Donor RBCs + recipient serum
- RBC will be coated if Abs present
- AHG test
- RBC washed
- “anti-human globulin” (AHG) added
- agglutination
- → recipient serum contains an Ab against donor RBC
- Donor RBCs + recipient serum
Serological testing:
All donor blood is tested for:
HIV 1 + 2
Hepatitis B
Hepatitis C
HTLV-1
Mooney / Gladwin 2016
Examiner Comments
2008A 21: 1 candidate (33%) passed this question.
The main points candidates were expected to cover included a detailed discussion of the ABO antigens, the evolution of IgM antibodies to these antigens and the prevalence of blood groups in the general population. Answers tabulating blood groups against expected antigens and antibodies present as well as agglutination reactions to anti-sera were most effective.
Discussion of the saline agglutination test was essential and extra marks were awarded for mention of the Coombs test. Candidates were required to demonstrate an understanding of cross-matching, specifically the testing of donor red blood cells against recipient serum.
Syllabus J1 2.a
References: Review of Medical Physiology (Chapter 27), 22nd ed. Ganong, pp. 537-539.
22. Outline the factors influencing the transport of drug s across the placenta.
CICMWrecks Answer
Physiological factors
- Drug transfer via diffusion, facilitated diffusion, secondary active transport, pinocytosis (e.g. immunoglobulins)/
- Diffusion according to Fick’s Law (see eqn):
where- MW = Molecular weight
- Drugs <500da freely permeable
- Δ[Drug] = Concentration gradient between maternal anf foetal blood
- SA = Surface area for diffusion
- 16m2 compared to alveolar surface area of 60m2
- h = thickness of diffusion membrane
- 3.5 microns compared with 0.5 for alveolar diffusion membrane
- MW = Molecular weight
- Drug delivery to uterus
- Blood flow ~750ml/min (Aprrox 600ml/min to placenta)
- No autoregulatory mechanisms
- Placental transporters
- MDR1 and p-glycoprotein efflux drugs out of foetal circulation
- Placental metabolism
- P450 reactions
- Pentobarbitol metabolized by placenta
- P450 reactions
Drug factors
- Drug size
- Heparin too large to permeate (Warfarin can)
- Lipophilicity
- Highly lipophilic drugs more permeable (Fentanyl > morphine)
- Ionization
- Charged molecules less permeable (Glycopyrrolate < atropine)
- Ionization trapping
- Alfentanyl (pKa 6.5) ~90% unionized at physiological pH → more permeable
- As alfentanyl crosses into foetal circulation, becomes more ionized in lower pH → trapped in foetal circulation
- Protein binding
- Reduces permeability
Disease factors
- Inflammation of placental barrier increases permeability of drugs
Sakurai 2016
Examiner Comments
2008A 22: No candidates (0%) passed this question.
The main points candidates were expected to know included the passive and active mechanisms that regulate the transfer of drugs across placenta and the potential clinical implications of drugs use in pregnancy in order to pass this question.
Good answers to this question included examples to all the possible mechanisms that can affect the transport of drugs across placenta.
The common omissions were degree of ionisation, active transporters, placental metabolism, explanation on the interaction between protein binding and ionisation of a drug in regulating placenta transfer, and the expected molecular size or weight of a drug that affects passive placental transfer of the drug.
23. Describe the hormonal response to hypovolaemia following the acute loss of one litre of blood in an adult. Include changes that occur in the first 24 hours following the blood loss.
CICMWrecks Answer
Reduced venous return
- Reduced right atrial stretch
- Reduced atrial natriuretic peptide
ANP
- Dilates afferent arteriole, constricts efferent arteriole
- Increased glomerular capillary hydrostatic pressure
- Increased glomerular filtration
- Increased glomerular capillary hydrostatic pressure
- Inhibits glomerular capillary mesangial cell constriction
- Increases filtration coefficient by increasing surface area available for diffusion and osmosis
ADH
Reduced CO → reduced MAP → increased signalling of carotid sinus and aortic arch baroreceptors
- Stimulates release of vasopressin (ADH)
ADH
- Induces systemic vasoconstriction
- Vascular smooth muscle cell V1 receptors → IP3 second messenger → increased intracellular calcium
- Increased renal water resorption
- Distal tubule and collecting duct V2 receptors
- Via cAMP
- Expression of aquaporin 2
- Water channels
- Distal tubule and collecting duct V2 receptors
RAAS
Renin
- Release stimulated (from juxtaglomerular cells) by:
- Sympathetic nerve stimulation (β1-adrenoreceptors)
- Reduced afferent arteriolar pressure
- Reduced flow of Na+ and Cl– in macula densa
Angiotensinogen —renin–> angiotensin I —ACE–> angiotensin II —secreted from zona glomerulosa of adrenal gland —> aldosterone
Angiotensin II
- Systemic vasoconstriction
- Constrict afferent and efferent renal arterioles, constricts mesangial cells -> reduces GFR
Aldosterone
- Stimulates collecting principal cell Na/K ATPase -> sodium resorption, potassium secretion (and so indirectly causes water retention)
Others
- Stimulation of aortic and carotid baroreceptors, cardiopulmonary mechanoreceptors, chemoreceptors
- Sympathetic activation
- Release of adrenaline from adrenal medulla
- Peripheral vasodilation
- Increased inotropy, chronotropy
- Cortisol release increased
- Erythropoetin released within 24 hours after haemorrhage
Sakurai 2016
Examiner Comments
2008A 23: 2 candidates (66%) passed this question.
Candidates were expected to know the different hormonal responses to hypovolaemia The possible approach to this question can be either by explaining the hormonal response in terms of time sequence or by different hormonal systems.
Good answers to this question included how different hormonal responses are activated and mediated.
The common omissions were secretion of erythropoietin within 24 hours of haemorrhage, role of macula densa and juxtaglomercular apparatus, interactions between baroreceptors and sympathetic nervous system with the secretion of ADH, cortisol, glucagon and catecholamines.
Syllabus: C1g 2b
Reference: Kam 1st edition 156, 212, Guyton 11th edition 342,287-280
24. Draw and label a left ventricular pressure volume loop in a normal adult. List the information that can be obtained from this loop.
CICMWrecks Answer
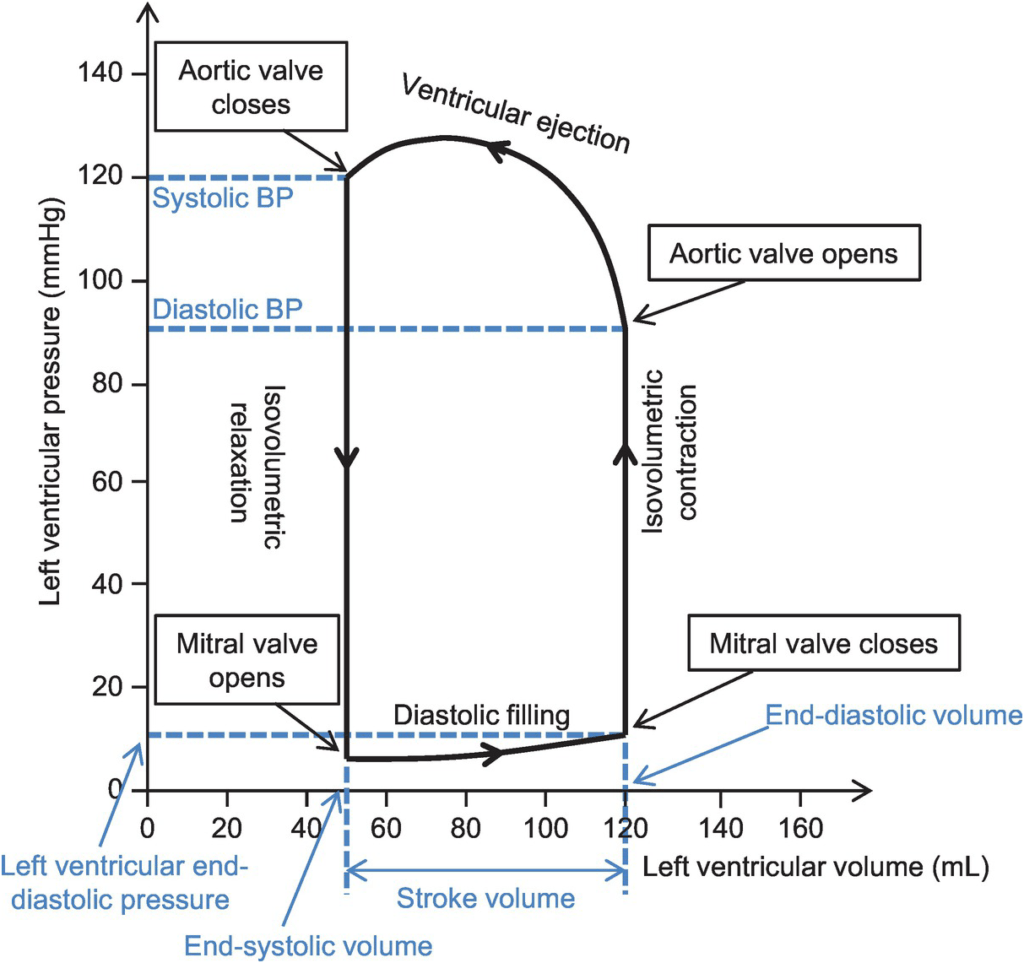
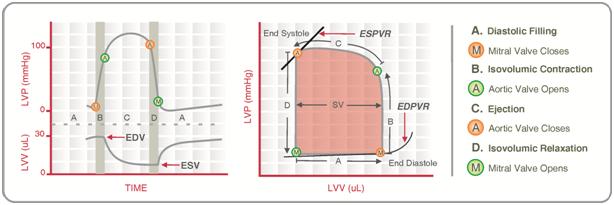
Normal P-V loop:
- Diastole
- (D) Beginning of diastole (isovolumetric ventricular relaxation)
- (A first 60% of vol) Early diastole (rapid ventricular filling – highly compliant Ventrical still relaxing)
- (A 60-90% of vol) Mid-diastole (slow ventricular filling – nearly full of blood → ↓ vent compliance)
- (A last 10% of vol) Late diastole (atrial contraction)
- Systole
- (B) Early systole (isovolumetric contraction)
- (C) Ventricular Ejection
- Early short rapid ejection phase (1st third of time)
- Prolonged reduced ejection phase (last 2/3 of time)
Derived Values:
- Stroke volume: SV = LVEDV-LVESV
- Ejection fraction as per eqn: EF = SV / EDV
- Measure of preload: LVEDV
- Measure of afterload
- Line b/w end-systolic point and locus at (LVEDV, 0)
- ↑ slope → ↑ Afterload
- Measure of contractility (ESPVR)
- End-systolic pressure volume relationship
- Slope is surrogate for contractility
- Measure of elastance (or stiffness) and compliance (or distensibility) of the LV – (EDPVR)
- End-diastolic pressure volume relationship
- Slope of the EDPVR = elastance = 1/compliance
- Peak LV pressure
- Measure of cardiac workload
- Total mechanical energy (or stroke work) (area of P-V loop)
- Heat generated by the heart during contraction
- “Diastolic work”
Gladwin 2016
Examiner Comments
2008A 24: 2 candidates (66%) passed this question.
Candidates were expected to draw and label a diagram showing the relationship between pressure and volume during the different phases of the left ventricular contraction and relaxation (or systole and diastole)
Good answers to this question consisted of a well-labelled graph with appropriate scale on both x and y-axes showing all the important events during systole and diastole of the left ventricle.
The common omissions were rapid and slow ejection phase during systole, when aortic valve closes, stroke volume, ejection fraction, end-systolic pressure volume line showing the contractility of the left ventricle.
Some candidates appeared to have confusion about which line shows contractility and which line shows left ventricular after load.
Syllabus C1c
Reference: Kam 1st edition 115-121, Guyton 11th edition 110
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | |
| G. CVS | You are considering starting a patient on captopril. Discuss the pharmacology of this drug. Name this molecule? How can we make this molecule biologically active? – Phenylethylamine – precursor of catecholamines Identify features on diagram of fetal circulation What equipment do you require to measure cardiac output via thermodilution techniques? |
| H. Renal | ABG – Metabolic alkalosis with resp compensation – Renal physiology |
| I. Body Fluids and Electrolytes | Outline the distribution of total body water |
| J. Acid Base | |
| K. Neuro | Describe the physiology of pain with respect to its mediators and pathways. |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | How are bacteria classified? |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments