1. Explain how oxygen supply is maintained to the tissues in chronic anemia.
CICMWrecks Answer
Anaemia
- condition (acute or chronic) a/w deficiency of RBC or Hb in blood
- Low haemoglobin
- Males <140g/L
- Females <120g/L
- Eg. due to haemolysis, chronic disease, Fe-deficiency, Vitamin B12/folate deficiency, haemorrhage, etc.
Normal O2 supply to tissues
Approx 1l/min
Consequence of anaemia
- ↓ O2 content of blood (CaO2), and thus ↓ O2 delivery to tissues (DO2)
- Risk of tissue hypoxia – Increase products of anaerobic metabolism
- CO2, Lactate
- Decrease in pH as a consequence
Physiological Responses /
Compensatory Mechanisms that maintain Tissue Oxygenation
Effects on oxyhaemoglobin dissociation
- Increased PaCO2, Decreased pH and Decreased O2 cause right shift in Hb O2 dissociation curve → ↑ Tissue O2 extraction
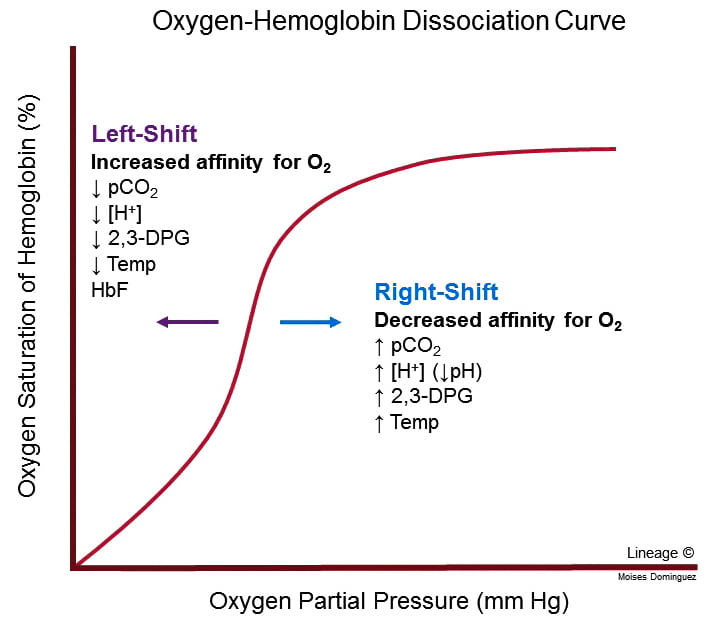
- For given PO2, Hb O2 affinity is reduced → increased offloading of O2 to tissues
- Decreased pH → Production of 2,3-DPG via Leubering-Rapaport Pathway and the enzyme BPG mutase
- Net loss of 1 ATP per 2,3-DPG generated
- Further right shift in the Oxyhaemoglobin dissociation curve
Cardiac Output Increases
- Blood flow and resistance governed by Poisuille-Hagen Equation
- Hb significant factor contributing to blood viscosity
- Anaemia → Decreased viscosity → Increased cardiac output
- Decreased viscosity → decreased resistance to venous return → increased preload → increased cardiac output
- Hb significant factor contributing to blood viscosity
- Stimulation of aortic chemoreceptors by decreased pH and increased CO2
- Increased signalling via vagus nerve NTS vasomotor sensory area → increase sympathetic output by anterolateral upper medulla → increased HR and contractility → Increased CO
Redistribution of blood flow
- To tissues where adequate O2 delivery is critical
- esp heart and brain
Respiratory effects
- Increased CO2 and decreased pH stimulates central and peripheral chemoceptors
- Medullary respiratory centre increases minute ventilation
- Increased respiratory clearance of CO2
- Alveolar CO2 ∝ arterial CO2 → increased PAO2 and hence increased PaO2
Renal compensation
- Decreased O2 delivery to renal interstitium → Hypoxia Inducible Factor (HIF) → Erythropoietin → Stimulates erythropoiesis via EPO-R JAK/STAT signalling.
#Extra:
Techniques to maintain tissue O2 balance in a patient with anaemia:
- ↑ tissue O2 supply (or DO2) by:
- ↑ C.O. → volume load to significantly ↑ C.O. as per Frank-Starling mechanism (preferably with PRBC to replace Hb also, but OK with colloids/crystalloids)
- ↑ [Hb] → replace Hb with PRBC transfusion, and support ongoing haemopoiesis with haemotinic factors (Eg. Fe, vitamin B12, folate)
- ↑ SpO2 and PaO2 → supplemental O2 (FiO2 100%)
- ↓ tissue O2 demand by:
- Sedation, paralysis and artificial ventilation → ↓ muscle MRO2 a/w activity and respiration
- Maintain normal core body temperature → avoid hypothermia and shivering 2° to hypothermia, which ↑ MRO2
- Minimal use of inotropes to maintain C.O. → avoid ↑ cardiac MRO2
CICMWrecks 2016
Examiner Comments
2007B 01: 1 candidate (14%) passed this question.
Candidates were expected to base their answers around the variables involved in the equations that describe oxygen content in blood and oxygen delivery. Although most candidates mentioned changes in haemoglobin that increase oxygen carriage, a more complete discussion of the changes that influence cardiac output and peripheral circulation was often omitted.
2. Outline the sites and mechanisms of action of diuretics. Give one example of drug acting at each site and list two side effects for each drug.
CICMWrecks Answer
Sites and Mechanisms of actions of diuretics
| Location | Drug | Mechanism of Action | Adverse Effects |
|---|---|---|---|
| PCT | Acetazolamide | Prevents the intracellular conversion of CO2 and H2O to H2CO3 → Resulting HCO3 usually reabsorbed via Na/HCO3 co transporter therefore decreased HCO3 leads to decreased Na reabsorption → decreased reabsorption of water | Metabolic acidosis Renal stones Hyponatraemia |
| LoH | Frusemide | Inhibits Na/K/2Cl transporter on luminal membrane preventing ion reabsorption → increases osmolality of luminal fluid → decreased osmotic gradient for water reabsorption | Hypochloraemic metabolic alkalosis Hyponatraemia Hypokalaemia Hypomagnesaemia |
| DCT | Thiazide | Inhibits Na/Cl transporter on the luminal membrane preventing Na and Cl reabsorption → increases osmolality in luminal fluid → decreased osmotic gradient for water reabsorption | Hypochloraemic metabolic alkalosis Hyponatraemia Hypokalaemia Dyslipidaemia Hyperglycaemia |
| CD | Spironolactone | Prevents activity of aldosterone → Decreased ENaC channels for Na reabsorption in the collecting duct and decreased Na/K ATPase activity in collecting duct → Prevents Na reabsorption and consequently reduced osmosis | Gynaecomastia Hyperkalaemia Renal stones |
| Other | Mannitol | Increases osmolality of of luminal fluid → decreases osmotic gradient for H2O reabsorption | Hypotension Tachycardia Thrombophlebitis Convulsions |
CICMWrecks 2019
Further Reading:
Examiner Comments
2007B 02: 4 candidates (57%) passed this question.
Good answers to this question were those that had a tabular format to the structure of the answer – for example column headed mechanism, sites, drug and side effects. Most common omissions were not to further describe how the different mechanisms of action of diuretics increased urine output, e.g. “disruption of the counter current multiplier system by decreasing absorption of ions from the loop of Henle into the medullary interstitium, thereby decreasing the osmolarity of the medullary interstitial fluid”. There was ofter little mention of increased urine solutes and the effect the electro chemical effect had in promoting a diuresis. Examples of drugs were well done.
3. Describe the physiological effects and principles of management of a tricyclic antidepressant overdose.
CICMWrecks Answer
TCA (e.g. amitryptilline and imipramine)
- Toxic dose of amitrytilline
- >10mg/kg
Mechanism of Action
- Act via central inhibition of noradrenaline reuptake (via inhibition of NET) and serotonin reuptake (via inhibition of SERT) but not dopamine transfer
- Also Na channel blockade
- Agonist at α1 and to a lesser extent α2 receptors
- Decreases sensitivity of muscarinic receptors
- Antagonist at H1 receptors
- Downregulates expression of GABAb and NMDA receptors
Physiological effects
CNS
- Agitation
- Seizures
- Coma and respiratory depression
CVS
- Sinus tachycardia
- Hypertension
- Na channel blockade
- Prolonged QT
- Widened QRS
- RBBB
Anticholinergic
- Dry mouth
- Dry, warm skin
- Urinary retention
- Tachycardia
Management
- Maintain airway patency
- Support ventilation
- Support organ perfusion
- Fluids, vasopressors as needed
- ABGs, blood tests, ECG
- Activated charcoal early
- However TCA rapidly absorbed
- Sodium bicarbonate
- 100mmol boluses
- Target pH >7.45
- Alkalinization promotes protein binding and limits free fraction of drug
- Alkalinization promotes dissociation of TCA and Na channel
- Consider intralipid, hypertonic saline for refractory cases
Examiner Comments
2007B 03: 1 candidate (14%) passed this question.
Good answers to this question were those that gave an accurate account of the physiological effects, e.g. inhibition of the fast sodium channels in the His-Purkinje system as well as the atrial and ventricular myocardium, decreasing conduction velocity (differential conduction inhibition of RBB being more susceptible) and increasing duration of repolarization, and the absolute refractory periods. Once having done that the rest of the answer would have flowed more easily, e.g. ECG changes and conduction disturbances. The effects of tricyclic antidepressants on Na channels and as a consequence the cardiovascular conduction abnormalities were often omitted. Anti cholinergic (e.g. slowing GIT motility) and antihistamine effects (e.g. obtundation) were often overlooked. Also frequently overlooked were basic pharmacology relevant to treatment, e.g. lipophilic, large volume of distribution, systemic acidosis reduces the extent of protein binding and increases unbound (active) drug. Additional points were available to those who not only mentioned sodium bicarbonate, but also mentioned the principles behind its use for this circumstance
4. Describe the principles of ultrasound imaging – including the Doppler Effect.
CICMWrecks Answer
Ultrasonography Principles
- Definition
- A sound wave with a frequency > 20 kHz → higher than frequency range audible by human ear
- Used medically → typically involves frequency range of 2-15 MHz
- Piezoelectric and converse piezoelectric effect
- Change of polarization of molecules in a quartz crystal in response to
mechanical stress – Interconverts electrical and sound energy - Application of electrical field creates mechanical deformation in a crystal
- Change of polarization of molecules in a quartz crystal in response to
- US Generation: Piezoelectric crystal within probe is stimulated by electrical current to vibrate → produce sound wave
- US Detection: Sound wave reflected by medium causes same crystals to vibrate → produce electrical signal
- Piezoelectric transducers in US
- Electrical current converted into precise sound waves (1~20mHz)
- Sound waves reaches interface of two mediums of differing density (or acoustic impedance).
- Acoustic impedance = tissue density x acoustic velocity
- unique to tissue type (e.g. fat, bone, etc.)
- At interface:
- Reflection
- sound wave reflected directly back to transducer
- Amount of reflection depends of ratio of acoustic impedance (or density)
- Increased density ~ increased reflection
- Refraction
- sound wave is deflected within the medium
- based on Snell’s law
- Reflection
- Attenuation: Loss of energy or strength of an ultrasound wave as it travels through a medium. Occurs through:
- Absorption: The ultrasound wave energy is converted into heat as it interacts with the tissue.
- Reflection: The sound waves bounce off the boundaries between tissues with different acoustic impedances.
- sound wave reflected back to transducer
- Amount of reflection depends of ratio of acoustic impedance
- Increased density ~ increased reflection
- Scattering: The sound waves are deflected in various directions by irregularities in the tissue and do not reach transducer
- Refraction: bending of the sound wave as it travels from one tissue to another
- results in a change in the wave’s direction
- relationship between the angle of incidence (θi), the angle of refraction (θt), and the speeds of sound (c1 and c2) in the two media is described by Snell’s Law: sin(θi) / c1 = sin(θt) / c2
- Central Processor
- Electrical current generated by piezoelectric crystal signaled to CPU
- CPU calculates the distance between transducer and object according to
- Speed of sound (1540m/sec)
- Delay in echo return
- Information relayed to display for visualization
- Gain
- Sensitivity of CPU to signals received from transducer
- Time-Gain Compensation – selective sensitivity of CPU to different interval of sound delay
Resolution and Penetration
- Ultrasound Resolution:
- Defined as the ability to differentiate b/t structures that are closely related
- Resolution is ↑ with either:
- (i) ↑ frequency (or ↓ wavelength) of sound wave → but this ↓ tissue penetration
- (ii) ↑ amplitude of sound wave → but this ↑ artefact
- (iii) ↑ gain → but this ↑ noise
- Types of Resolution: Spatial (Axial, Lateral, Elevational), Temporal
- Spatial Resolution:
- Axial Resolution: The ability to distinguish two structures that are side-by-side and parallel to the ultrasound beam.
- Achieved with a higher frequency and shorter pulse length.
- Mathematically, it’s half the spatial pulse length.
- Lateral Resolution: The ability to distinguish two structures that are side-by-side to the ultrasound beam.
- Achieved with a narrower ultrasound beam, which is related to the width of the beam.
- Higher frequencies generally lead to narrower beams and better lateral resolution.
- Lateral resolution is roughly three times worse than axial resolution at the focal region of the beam.
- Elevational resolution (aka slice thickness resolution)
- refers to the ability to distinguish structures that are close together in the direction perpendicular to the imaging plane. (similar to lateral resolution, but in perpendicular plane)
- crucial for accurately visualizing the depth and thickness of tissues
- Temporal Resolution:
- The ability to distinguish between instantaneous events of rapidly moving structures.
- Achieved with a high frame rate.
- A higher frame rate means the ultrasound machine can capture and display more images per second, allowing for better visualization of movement.
- Axial Resolution: The ability to distinguish two structures that are side-by-side and parallel to the ultrasound beam.
- Ultrasound penetration:
- defined as the depth to which ultrasound waves can travel into tissue
- Primarily determined by the frequency of the ultrasound waves, with lower frequencies generally penetrating deeper than higher frequencies.
- Trade-off between Resolution and Penetration:
- There’s an inherent trade-off between image resolution and penetration depth.
- Higher resolution requires higher frequencies, which means less penetration, and vice versa.
Modes of Ultrasound
- A (amplitude scan): Amplitude of U/S signal plotted against time → provides information about tissue depth (BUT is no longer used)
- B (brightness): Depth recorded as bright spot (rather than a spike as in A-mode) →
amplitude of U/S signal is proportional to brightness - M (motion): B-mode plotted against time (Ie. assess heart valve movement over time)
- 2-D: Sequential B-mode across 90° (most commonly used) → requires an array of crystals
- Doppler: Uses “Doppler shift” to establish velocity of moving object which is reflecting sound waves → superimposed on 2D mode with colours representing direction of movement (red = towards, blue = away)
Doppler effect
- Change in apparent frequency of sound for an observer moving relative to its source
- Use in ultrasound
- Sound waves reflected off objects moving toward or away from transducer
(usually blood)- If object moving toward transducer, frequency appears increased – displayed as red (however this is not standardized across machines)
- If object moving away from transducer, frequency appears decreased –
displayed as blue
- Can be used to measure velocity of flow, as well as direction
- Sound waves reflected off objects moving toward or away from transducer
Sakurai 2016
Examiner Comments
2007B 04: 4 (57%) candidates passed this question.
It was expected candidates would outline the underlying principles of ultrasound imaging (reflection, scattering, refraction, and attenuation) and discuss that the basic image is the result of reflection of the transmitted ultrasound wave. Most candidates appreciated that the amplitude of the reflected echo is a function of the acoustic mismatch of the tissues and the angle of incidence and many candidates provided details mathematical descriptions concerning these principles.
While high levels of technical details were not required the answer should include a mention of the use a piezoelectric transducer and that an ultrasound beam has 3 dimensions — Axial, Elevation and Lateral. Some comment of the modes of Display (A= Amplitude, M Time Motion, 21), etc) was expected.
Extra credit was given for answers that included details regarding limits of depth of penetration (longer wavelength penetrate deeper, but loose image quality with longer wavelengths) and the varying properties of human tissue regarding refraction and attenuation (little refraction (path deviation) in human tissue and air attenuates).
Specific comment on the Doppler Effect was required. It was expected candidates would described that it refers to the change in frequency of a sound wave reflected by a moving target and that the reflected frequency differs if moving toward or away. Correctly stating that the reflected Frequency is Higher Towards and Lower Away scored additional marks. Comments concerning obtaining the best Doppler images with lower frequencies (opposite to ultrasound) and colour Doppler attracted additional marks.
5. Compare and contrast the spectrum of activity and the mechanisms of microbial resistance for the following penicillins: benzyl penicillin, flucloxacillin and ampicillin.
Examiner Comments
2007B 05: 4 candidates (57%) passed this question.
It was expected candidates would specifically address both the spectrum of activity and mechanism of resistance.
Benzyl penicillin is highly active against Gram positive organisms, particulary streptococci but also effective against Meningococcus / Clostridia and other anaerobes, Listeria and is used in the treatment of syphilis (treponemma). It is readily hydrolysed by penicillinases or beta lactamases so any organisms that produce these are resistant i.e. most staphylococci.
Flucloxacillin contains a modified beta lactam ring so is not susceptible to hydrolysis by staphylococcal penicillinases, therefore the spectrum is Staphylococci not resistant to methicillin (i.e. not MRSA). Extra credit was given for comments that it won’t cover Listeria or some other organisms covered by Benzyl penicillin and it is much less active than Benzyl penicillin on organisms sensitive to both.
Ampicillin is an alpha amino benzyl penicillin (Aminopenicillin) and has a broader activity than Benzyl penicillin so covers the streptococci but aslo a variety of gram negative bacteria including some enterobacteriacae and Haemophilus influenzae. It also covers Helicobacter and Enterococci (better than Benzylpenicillin). It is destroyed by beta-lactamase.
Additional credit was given for discussion of other mechanisms of bacterial drug resistance.
6. Explain the role of the skin in maintaining body temperature.
CICMWrecks Answer
Skin
- Largest organ in body ~15% body weight
- Resting blood flow ~500ml/min in 70kg male
- Can increase 30-fold or decrease 10-fold
- Largest barrier between human body and external environment
- Composed of epidermis, dermis and subcutaneous tissue
Role of Skin
- Homeostasis
- Relatively impermeable
- Prevents fluid loss
- Only ~300ml/day lost through skin
- Immunological
- Physical barrier
- Low pH and commensal organisms
- Abundant Antigen Presenting Cells (APC) such as dendritic cells
- Sensory function
- Embedded with nerve endings
- Sensory signals to CNS and spinal reflex arcs
- Touch
- Pain
- Temperature
- Sensory signals to CNS and spinal reflex arcs
- Embedded with nerve endings
- Thermoregulation (SEE BELOW FOR DETAILS)
- Sensory
- C fibres – heat
- A δ fibres – cold
- Effector
- Heat loss
- Occurs via
- Radiation
- Conduction
- Convection
- Evaporation
- Requires transfer of core body heat to skin
- Regulated by AV shunts (glomus body)
- Occurs via
- Heat gain
- Opening of AV shunts prevents loss of core body heat to skin
- Heat loss
- Sensory
- Synthesis of cholecalciferol
- Protection against UV
Role of Skin in Thermoregulation
Sensors:
- Thermoreceptors, on free endings of sensory nerves
- Aδ fibres (myelinated, cold, sense below 38°c)
- C fibres (unmyelinated, warm, sense above 30°c)
- Anterior hypothalamus is sensitive to changes in blood temperature
Controller:
- Hypothalamus
- Maintains temperature around a set temperature
- Influenced by many factors including circadian rhythms, thyroid function and ambient temperature
Effector:
- Heat exchange is due to convection, conduction, radiation and evaporation
- Depends on the direction and size of the gradient between skin and the environment
Skin blood flow:
- Heat loss through radiation and convection
- Increased blood flow to skin increases convection and conduction (blood is warm, if temperature gradient present will lose heat to air at increased rate when more flow)
- Assuming skin temperature is below ambient temperature
- When too cold: Sympathetic outflow from hypothalamus
- Noradrenaline acts on α adrenoreceptors (abundant in skins)
- Constriction of cutaneous precapillary sphincters
- Shunting of blood through arteriovenous shunts, bypassing skin
- Constriction of cutaneous precapillary sphincters
- Noradrenaline acts on α adrenoreceptors (abundant in skins)
- When too warm: Decreased sympathetic outflow
- Cutaneous vasodilation
Sweating:
- Heat lost through evaporation and convection
- Energy of molecules in a liquid is unevenly distributed
- High energy molecules will enter gas phase, be liberated from the liquid
- Lowering the average temperature of the liquid
- Sweat glands in dermis
- Secretion of precursor fluid from the gland
- Ducts ascend through the dermis to exit from the dermis
- Pre-ganglionic sympathetic (cholinergic) innervation
- When too cold, Decreased stimulation
- Fluid moves slowly through duct
- Fluid mostly absorbed, minimal sweat
- When too hot, increased sympathetic stimulation
- Fluid moves quickly through duct
- Fluid not resorbed, lots of sweat
Galwin 2016
Examiner Comments
2007B 06: 4 candidates (57%) passed this question.
It was expected candidates would describe that the maintenance of body temperature is a balance of heat loss and heat production and outline the seies of controls that are important in this process.
An overview of the reflexes involved was expected with som ecomments on temperature receptors in skin for hot and cold, the hypothalamic integration and then the effector being the skin blood flow (vasodilation / vasoconstriction and A-V shunts).
Answers were expected to include some comment on the fact that heat exchange occurs via radiation / conduction / convection / evaporation and requires a heat gradient and that skin blood changes can facilitate or impair this exchange. Using examples such as; if body needs to loose heat there is vasodilation and sweating often added clarity. Extra credit was given for discussing swet production and the principle and the principle of how that will allow increased heat loss with further credit given for discussing the impact of humidity.
Credit was also given for mentioning piloerection, particularly if candidates went on to explain why this might be useful (even though not really applicable in man).
7. Compare and contrast the pharmacology of noradrenaline and vasopressin.
Examiner Comments
2007B 07: 5 candidates (71%) passed this question.
This was best answered using a table. The main points expected for a pass were
- Both are naturally occurring substances
- Direct acting via receptorrs
- Mechanisms by which both increase mean arterial pressure
- Metabolism
- Uses in Intensive Care, septic shock, vasodilatory shock and diabetes insipidus
- Side effects related to intense vasoconstriction and for vasopressin possible coronary ischaemia and sodium and water retention
8. Explain the role of the baroreceptors in the control of blood pressure.
CICMWrecks Answer
High Pressure Baroreceptor (HP BR)
- Carotid sinus and aortic arch receptors
- Detects > 5-10 % change in plasma volume
- ↓ Plasma volume → Increased central SNS tone and decreased PSNS tone
- Sensor:
- Stretch receptor: ↑ distension of vessel → ↑ discharge rate
- Threshold > 60mmHg → normally has baseline tone.
- Located at Carotid Sinus and Aortic Arch
- Spray like visceral nerve endings
- Transmitted by:
- Unmyelinated C-fibres (most receptors)
- Myelinated A-fibres (↑ sensitivity at lower BP)
- Individual fibres have narrow BP range
- Increased reponsiveness to pulsitile rather than continuous flow
- Afferent signal:
- Carotid sinus → Nerve of Hering → CN9
- Aortic Arch → CN10 → NTS
- Both then divide to:
- Stimulatory afferents → ↑ Medullary vagal outflow
- Inhibitory afferents → ↓ RVLM SNS outflow
- Outcome:
- ↑’d distension → ↓’d HR/Contractility/SVR
- Provides strict negative feedback to Δ’s in CO
Low Pressure Baroreceptors (LP BR)
- Location
- Located at junction of return vessels and atria, vetricular walls, pulmonary vessles
- Throughout the peripheral vasculature (esp kidney)
- Sensor:
- Stretch receptor: ↑ distension of vessel → ↑ discharge rate
- Detect > 10% decrease in plasma volume as decreased atrial stretch
- ↓ Discharge rate with
- Reduced ANP release
- Increase SNS output
- 2 types
- A receptors → fire at atrial contraction (a wave)
- B type → fire at atrial filling (v wave)
- Outcome
- ↓ PL → ↓ CO → ↓BR discharge rate
- Medulla afferents cause
- ↓ SNS (NA) and ↑PSNS (RVLM) outflow → peripheral vascular vaso and venodilation
- ↓ SNS activity to kidney → ↓ Na/H2O conservation
- ↑ SNS activity to sinus node
- Hypothalamic afferents cause
- → ↓ ADH release and ↓ Thirst
- Medulla afferents cause
- ↑ contractility/HR/SV/CO
- ↑ SVR → autotransfusion and ↑ perfusing pressure (but ↑AL)
- ↓ PL → ↓ CO → ↓BR discharge rate
Gladwin 2016
Examiner Comments
2007B 08: 5 candidates (71%) passed this question.
Good answers included the following
- description of, and types of, baroreceptors (e.g. stretch-receptors)
- their locations (e.g. walls of the aorta, carotid sinuses, the atria etc)
- the stimulus they respond to (e.g. pressure, volume)
- short term and long term responses, alteration to set points, impulse frequency / pressure
curve - a brief description of the afferent and efferent pathways and the resultant efferent effects
(e.g. alterations to heart rate, blood pressure, etc)
9. Classify the hypersensitivity reactions. Briefly describe the pathophysiological processes of each reaction. Give an example of each reaction.
CICMWrecks Answer
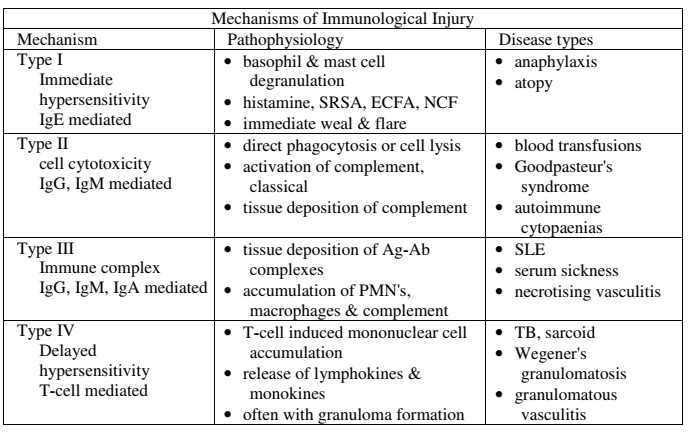
HYPERSENSITIVITY REACTIONS
| Type | Pathophysiology | Disease types |
|---|---|---|
| Type I Immediate Hypersensitivity IgE mediated | Sensitisation Plasma B cell production of IgE Mast cell proliferation → IgE binds to mast cells On re-exposure Allergenic antigen binds to mast cell (expressing IgE) → Mast cell degranulates Mast cell degranulation leads to Primary mediators: – Serotonin – Histamine Secondary mediators – Leukotrienes (SRSA) – Prostaglandins – ECFA, NCF | Anaphylaxis Atopy |
| Type II Cell Cytotoxicity IgG, IgM Mediated | Antibody attaches to antigen on target cell Complement release C5-9 “membrane attack complex” → Causes cell lysis | Blood Transfusions Goodpasteur’s syndrome Autoimmune cytopaenias |
| Type III Immune Complex IgG, IgM, IgA mediated | Circulating antibody-antigen complexes are deposited in basement membranes e.g. peritoneum, blood vessels, joints, glumeruli Complement cascade activation Attract granulocytes → inflammation | SLE Serum Sickness Necrotising vasculitis |
| Type IV Delayed hypersensitivity T-cell mediated | T cell mediated Cell mediated, as opposed to previous humoral Peak reaction 2-3 days CD4 helper T cells cause recruitment of Neutrophils Macrophages CD8 cytotoxic T cells | TB, Sarcoid Granulomatosis with polyangiitis (Wegener’s) Granulomatous vasculitis |
SRSA: “slow reacting substance” A – Mixture of Leukotrienes (LTC4, LTD4, LTE4)
ECFA: Eosinophil chemotactic factor of anaphylaxis
NCF: Neutrophil chemotactic factor of anaphylaxis
Key Points:
- “Anaphylaxis” may be:
- True anaphylaxis: a symptoms complex following exposure of a sensitised individual to an antigen, produced by a type I hypersensitivity reaction, associated with IgE mediated mast cell degranulation
- Anaphylactoid reactions: Indistinguishable from true anaphylaxis, however the immune nature of the reaction is either unknown, or not due to a type I hypersensitivity reaction, immediate generalised reaction is a better term.
Gladwin / Mooney 2016
Examiner Comments
2007B 09: 2 candidates (28%) passed this question
Key Points:
- “Anaphylaxis” may be:
- True anaphylaxis: a symptoms complex following exposure of a sensitised individual to an antigen, produced by a type I hypersensitivity reaction, associated with IgE mediated mast cell degranulation
- Anaphylactoid reactions: Indistinguishable from true anaphylaxis, however the immune nature of the reaction is either unknown, or not due to a type I hypersensitivity reaction, immediate generalised reaction is a better term. i End-organ effects, e.g. H1 and H2 receptors
10. Describe the control of gastric emptying.
CICMWrecks Answer
Physiology of Gastric Emptying
Gastric emptying
- Fluids have half-time of 30mins in stomach
- Solids have half-time of 2 hours in stomach
Gastric receipt of food bolus:
- Peristaltic wave moves down the oesophagus, propelling food bolus
- Controlled by mesenteric plexus with input from vagus nerve
- Upon swallowing, the lower oesophageal sphincter and stomach relax until the oesophageal peristaltic wave has passed
- Following food bolus passage, the LOS tone increases to prevent reflux
- Food bolus reaching the stomach causes a vagal-mediated relaxation of the stomach, to accommodate gastric distension and food storage
Gastric mixing and emptying:
- Spontaneous mixing waves move down the stomach every 15-20 seconds
- Stronger waves propel gastric contents towards the pylorus (pyloric pump)
- High pyloric sphincter tone
- Allows through well-mixed liquid chyme
- Restricting gastric emptying of solids, causing mixing (retropulsion)
Control
Gastric emptying is controlled by the balance between stimulatory gastric factors and inhibitory duodenal factors
Factors promoting gastric emptying:
- Increased stomach wall stretch
- Stimulates pyloric pump
- Reduces pyloric tone
- Parasympathetic vagal stimulation
- Stimulates pyloric pump
- Gastrin
- Stimulates pyloric pump
- From G cells in antrum
- In response to presence of protein (esp. meat) in stomach
- Motilin
- Stimulates pyloric pump
- During fasting
- Stimulates pyloric pump
- From M cells in duodenum (external antigen stimulus)
- Carbohydrate: rapid emptying
Factors inhibiting gastric emptying:
- Nervous reflexes via enteric nervous system, local sympathetic trunk, and the vagal nerve to the brainstem
- in response to local conditions in the duodenum: Distension, Irritation, Acidity of chyme, Hyper- or hypo- tonicity of chyme, Breakdown products, Especially AA and FA
- Inhibit pyloric pump
- Increase pyloric sphincter tone
- Hormones (inhibit pyloric pump)
- Cholecystokinin (CCK)
- From I cells of duodenum
- In response to presence of fat and proteins
- Secretin
- From S cells of duodenum
- In response to presence of acids
- Gastric inhibitory peptide (GIP)
- From K cells of duodenum
- In response to presence of fats, proteins and carbohydrate
- Cholecystokinin (CCK)
- Other Factors :
- Sympathetic stimulus:
- Decreases contractility and reduces gastric emptying
- Dopamine:
- Decreases intragastric pressure and lower oesophageal sphincter tone
- reduces gastric emptying
- Sympathetic stimulus:
Alternate: This section can also be approached as:
- Local factors
- Gastric Factors: pump, Stomach wall distension, Amino acid
- Duodenal Factors: Type of food, stretch of wall, irritation, hyperosmolaroty, acidity, amino acid and FA content
- Neural factors
- Symptathetic
- Parasympathetic
- Hormonal factors
- Dopamine
- Gastrin
- Motilin
- Secretin
- CCK
- Somatostatin
- GIP
Mooney / Sakurai / JC 2019
Examiner Comments
2007B 10: 2 candidate (28%) passed this question.
The main points expected for a pass were
- An appreciation that the aim of controlling gastric emptying is to present the food to the small bowel for absorption in a controlled manner
- That there are both gastric neural and hormonal mechanisms e.g. Gastric volume and the hormone gastrin
- There are duodenal neural and hormonal mechanisms e.g. composition of the chyme, secretin and cholecystokinin and the influence of duodenal distension
- Extra points were given for mentioning the effect of sympathetic stimulation and pregnancy on gastric emptying
Common problems were not enough knowledge, naming hormones but not saying what theirs action was and including drugs.
11. Describe the acid-base changes that occur in acute hypoxaemia.
CICMWrecks Answer
Hypoxaemia:
= Hypoxic Hypoxia= decreased PaO2 due to problems at the level of the lungs
Causes:
- Normal A-a Gradient
- Alveolar hypoventilation – neuromuscular disease
- Low PiO2 – FiO2 < 0.21, Barometric pressure < 760mmHg
- Raised A-a Gradient
- Diffusion defect – COPD, Pulmonary fibrosis, exercise
- V/Q Mismatch – Aging
- Right-to-left shunt – PDA, PFOs, ASDs or VSDs
- Increased O2 extraction – Sepsis
Physiological Outcome:
- ↑ local vasodilation → ↓ SVR and ↓ BP
- ↓ BP → Baroreceptors → SNS activation → ↑ HR and ↑ C.O.
- PaO2 < 50-60 mmHg
- → peripheral chemoreceptors stimulation
- → ↑ventilation
- Severe hypoxaemia
- ↓ HR and BP → shock
- ↑ CBF (regardless of PaCO2)
- Lactic Acidosis:
- Embden-meirhoff continues (glycolysis is anaerobic)
- Mitochondrial hypoxaemia → ↓d ETC (electron transport chain) activity → ↑ cytoplasmic Acetyl CoA → ↓ conversion of Pyruvate to Acetyl CoA → ↑ [Pyruvate] → ↑ shunting of pyruvate to lactate → ↓ SID → Acidosis (↑ [H])
Chemoreceptor Reflex
- Mechanism:
- ↓’d perfusion → ↑[H]/↓PaO2 → CR stimulation
- Afferent signal → Sinus nerve (of herring) / Vagus Nerve → Chemosensitive area of medulla
- Vasomotor area → ↑ peripheral vasoconstriction
- Respiratory centre → ↑rate and depth of respiration → ↑ venous return
- ↓’d perfusion → ↑[H]/↓PaO2 → CR stimulation
- Result
- Hypoxia/hypercaponea → ↑ peripheral vasosmotor tone, ↑rate and depth of respiration
- ↓ PaO2
- Gradual ↑ MV PaO2 < 500mmHg,
- Rapid ↑MV PaO2 < 50 mmHg
- ↓ PaO2
- Hypoxia/hypercaponea → ↑ peripheral vasosmotor tone, ↑rate and depth of respiration
Acid-Base changes:
- ↑[H] → ↑rate and depth of respiration → Respiratory alkalosis
- ↑ HCO3 if chronic (> 3 days)
- ↑[lactate] → ↑ Anion gap
CICMWrecks 2016
Examiner Comments
2007B 11: 1 candidate (14%) passed this question.
The main points expected for a pass were
- Definition of hypoxia
- Physiological causes of hypoxaemia
- Formation of a lactic acidosis
- Development of hypocarbia secondary to stimulation of respiration from hypoxia and acidosis
- Explanation of the decreased bicarbonate concentration and negative base excess
- Extra points were given for more detailed explanation of the lactic acidosis, commenting on the anion gap and some consequences of hypoxaemia.
A common problem was to incorrectly state the relationship between pH and hydrogen ion concentration.
12. The binding of a drug (D) to its receptor (R) is described by the equation D + R -> DR. Explain the following:
a) The ratio of koff / kon
b) The implications for a low value for the ratio
c) The term affinitiy
d) The clinical implications for a high value for affinitiy
e) Two physiological factors that affect the rate constant
CICMWrecks Answer
- The ratio of the koff / kon
- koff describes the rate constant for the reaction DR → D + R
- kon describes the rate constant for the reaction D + R → DR
- Rate constant is the rate at which a reaction will proceed in one direction if all other parameters are equal
- The ratio of the two rate constants is the dissociation constant or kd,
- The likelihood of the drug-receptor complex to dissociate into drug and receptor
- The implications for a low value for the ratio
- A low value of kd implies that the forward reaction of the drug binding to the receptor (kon) is favoured over the reverse reaction of the drug and receptors disassociating (koff)
- This may be due to high affinity between the drug and receptor
- Few molecules of drug are required to achieve a given level of receptor occupation than a drug affecting the same receptor with a high kd
- This drug is more potent
- Affinity
- Affinity is the attraction between 2 molecules in forming a complex
- High affinity bonds have stronger inter-molecular bond
- It is inversely related to the kd
- The higher the affinity, the lower the kd and vice versa
- The clinical implications for a high value of affinity
- If a drug has high affinity for a receptor, it has a lower kd than a drug that has low affinity for the same receptor
- Fewer molecules of the drug are required to achieve receptor occupancy of a certain level
- The drug is more potent
- Two physiological factors that affect the rate constant
- The rate constant is described by the Arrhenius equation
Where
k = the rate constant
A = the pre-exponential factor for the specific reaction describing the frequency of collisions in the correct orientation
Ea = the activation energy, the minimum energy required for the reaction to occur
R = Universal gas constant
T = Temperature in kelvin
As temperature increases the rate constant approaches A,
As the temperature decreases the rate constant approaches 0
Modifying the Arhenius Equation gives
Where ΔG0 = Gibb’s Free Energy, the change in enthalpy and entropy at a given temperature for the reaction
Gladwin 2016
Examiner Comments
2007B 12: 1 candidate (14%) passed this question.
The main points expected for a pass were:
- The ratio of k off/k on is the dissociation constant
- A low value indicates that less drug is required to bind to the receptors
- affinity is the reverse of dissociation constant
- Clinical application of high affinity include large effect at lower concentrations
- Physiological factors could include temperature
The main problem with this question was lack of knowlegde.
13. Briefly describe the factors that affect lung compliance.
CICMWrecks Answer

Lung compliance
- Change in lung volume per unit change in transmural pressure
- Normal lung compliance ~200ml/cmH2O
- Static compliance (Cs)
- ” Compliance of lung measured when lung held at constant lung volume”
- no pressure componet due to resistance
- is a function of: Elastic recoil of the lung, Surface tension of alveoli
- Dynamic compliance (Cd)
- ” Compliance of lung measured during cycles of inspiration and expiration”
- includes the pressure required to generate flow by overcoming resistance forces
- is a function of: respiratory rate
- Dynamic Compliance usually < Static Compliance due to the degree of time dependence in the elastic behaviour of a particular lung
- Specific compliance
- Compliance per unit volume lung
- Used to compare different lungs
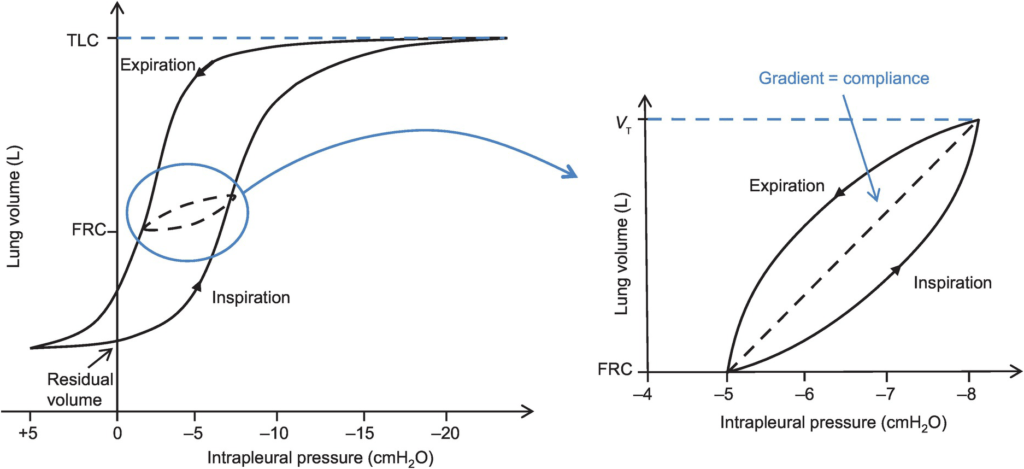
- Hysteresis
- any process where the future state of a system is dependent on its current and previous state
- Compliance of the lung is different in inspiration and expiration
- In dynamic compliance curves:
- Airways resistance is a function of flow rate. Flow rate (therefore resistance) is maximal at the beginning of inspiration and end-expiration.
- In static compliance curves:
- There is no resistive component. Hysteresis is due to viscous resistance of surfactant and the lung.
Factors Affecting Compliance
Lung Compliance
- Surfactant
- increases lung compliance
- decreases surface tension at alveolar air – water interface
- prevents small alveoli from collapsing
- accounts for most of hysteresis in intact lungs
- Lung volume
- Lung Compliance decreases at higher lung volumes
- Specific compliance (Compliance/FRC) remains constant
- Elephants have greater lung compliance than mice!
- Pulmonary blood volume
- Increased PBV decreases lung compliance
- Pulmonary venous congestion from L heart failure or mitral regurgitation
decreases lung compliance
- Bronchial smooth muscle tone
- Increased bronchial smooth muscle tone decreases compliance
- Decreased dynamic lung compliance by 50% in animal models of methacoline
challenge
- Disease
- ARDS, pneumonia decreases lung compliance
- pulmonary fibrosis → Impraired elasticity → decreases lung compliance
- Asthma, Emphysema increases lung compliance
Chest Wall Compliance
- Chest wall restriction – reduced chest wall compliance
- Obesity
- Spastic paralysis of chest wall musculature
- Ossification of costal cartilages
- Kyphosis/scoliosis
- Scarring/constriction (e.g. circumferential burns)
- Position: Prone (60% reduced compliance)
- Collagen Disorders: Increased Chest Wall Compliance
Measurement of Compliance
Dynamic Compliance
- ” Compliance of lung measured during cycles of inspiration and expiration”
- Measure:
- Measure with spirometer
- Using Volume/Pressure curve during normal rhythmic breathing at points of no flow
- Equation above then gives dynamic compliance
Static Compliance
- ” Compliance of lung measured when lung held at constant lung volume”
- Volume vs Pressure loops during inspiration and expiration demonstrate Hysteresis
- Measure:
- @ no flow in or out of the lung, AND time for pressure to equlibrate across the lung (>10sec).
- Patient exhales in steps holding the volume with open glottis
- Intrapleural pressure measured as oesophageal pressure
- Measured compliance secondary to viscoelastic properties and accounts for both short and long time-constant alveoli.
Specific Compliance
- Measure of the average compliance across all lung units
- Children = Adults ~ 0.05 cmH2O-1
Gladwin / Sakurai / JC 2020
Examiner Comments
2007B 13: 1 candidate (14%) passed this question.
Main points/concepts expected in answer.
- Surfactant:
- increases lung compliance
- decreases surface tension at alveolar air-water-interface
- prevents small alveoli from collapsing
- accounts for most of hysteresis in intact lung
- Lung elastic recoil: lung compliance changes in disease states
- Lung volume:
- lung compliance greatest around FRC
- lung compliance reduced at low and high lung volumes
- gravitational effects on regional lung compliance
- Pulmonary blood volume: Pulmonary venous congestion reduces lung compliance
- Lung size: Specific compliance = lung compliance / FRC
- Dynamic lung compliance:
- nfluenced by airways resistance
- lung compliance measured during normal breathing
- less than static lung compliance
- frequency dependence
14. Describe the term second messenger. Give an example of a drug that manifests its action via a second messenger.
CICMWrecks Answer
Second Messenger
- A downstream intracellular signalling molecule that is either released or inhibited by drug binding to its receptor.
- Examples include cAMP and cGMP.
Steps in activation of second messenger
- Ligand binding to extracellular site
- Conformation change in receptor
- Activation of intracellular domain of receptor
- Activation of enzymatic process at intracellular site effector site
- Change in second messenger concentration
- Action of second messenger on substrate
- Response
Examples:
- cAMP
- NA, ACh (M2), ACTH, ANP, Glucagon, PTH, TSH.
- Primary effector adenylyl cyclase.
- Secondary messenger cAMP.
- Secondary effector PKA
- cGMP
- ANP and NO.
- Primary effector guanylyl cyclase.
- Secondary messenger cGMP.
- Secondary effector Protein kinase G (cGMP-dependent protein kinase)
- Phosphoinositol signalling
- Noradrenaline, ACh (M1/M3). Signal transducer Gq,
- Primary effector PLC,
- Secondary messenger IP3/DAG,
- secondary effector PKC
- Arachidonic acid pathway
- Histamine.
- Primary effector phospholipase A.
- Second messenger arachidonic acid.
- Secondary effector COX/lipoxygenase.
- Tyrosine kinase pathway
- Insulin, IGF, PDGF.
- Primary effector Ras.
- Second messenger Ras-GTP.
- Secondary effector MAP3K (raf)
Gladwin 2016
Examiner Comments
2007B 14: 3 candidates (43%) passed this question.
Second messenger – Hormone/drug – receptor binding is coupled to a subsequent series of intracellular biochemical events that precipitate the ultimate hormone/drug effect.
Examples are G proteins an energy dependent process by which there is hydrolysis of G
protein-associated GTP to GDP. There are both stimulating and inhibitory proteins
which subsequently act to increase or decrease activity of the enzyme adenylyl cyclase,
resulting in increased levels of cyclic adenosine 3 ‘,5’-monophosphate ( cAMP) in the cell
which in turn activates protein kinases that phosphorylate various proteins, ion channels, and second messenger enzymes.
Also G proteins stimulate hydrolysis of phosphatidyl-inositol-4,5-bisphosphate (PIP2) generating inositol-1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol (DAG). Both systems increase intracellular calcium.
15. Compare and contrast ibuprofen and tramadol as analgesic agents in intensive care.
Examiner Comments
2007B 15: 3 candidates (43%) passed this question.
Ibuprofen – inhibition of cyclooxygenase (COX) and synthesis of prostaglandins, which are important mediators for peripheral sensitization and hyperalgesia. Act peripherally and spinal COX – non selective. Oral and PR only. Associated with a number of side effects, including decreased haemostasis, renal dysfunction, gastrointestinal hemorrhage, and effects on bone healing and osteogenesis.
Tramadol – is a synthetic opioid that exhibits weak μ-agonist activity and inhibits reuptake of serotonin and noradrenaline. Analgesic effects primarily through central mechanisms, it may exhibit peripheral local anaesthetic properties.
Tramadol is comparable in analgesic efficacy to ibuprofen. Common side effects (overall incidence of 1.6% to 6.1%) include dizziness, drowsiness, sweating, nausea, vomiting, dry mouth and headache. Tramadol should be used with caution in patients with seizures or increased intracranial pressure and in those taking monoamine oxidase inhibitors. IV and oral preparations. No bleeding, GIT and renal complications. More expensive.
Both have advantage of lack of respiratory depression, major organ toxicity, and depression of gastrointestinal motility and a low potential for abuse.
16. Describe the physiological response to hypoglycaemia.
CICMWrecks Answer
BSL normally maintained 4-7mmol/L
- maintained by –ve feeback system
- detector: islets of Langerhans cells in pancreas
- stimulus= BSL
- effector = insulin:glucagon release ratio
- aim: maintain BSL to provide substrate for obligate glucose metbaolisers (brain + RBC)
- hypoglycaemia = BSL <3mmol/L
Acute hypoglycaemia
- ↓ BSL: compensatory mechanisms not yet activated (hormonal ∆s not yet occurred) → glucagon, cortisol, GH levels minimal initial change
- early sign: hunger
- later signs: neurological impairment → confusion, agitation → progression with ↓ BSL → coma, seizures, death
| BSL (mmol.L-1) | Symptoms | Endocrine Response |
| 4.6 | Insulin secretion inhibited | |
| 3.8 | Autonomic dysfunction | Glucagon, adrenaline, and GH secretion |
| 2.8 | CNS dysfunction | Cortisol secretion |
| 2.2 | Lethargy, Coma | |
| 1.7 | Convulsions | |
| 0.6 | Permanent brain damage, Death |
Physiological consequences of acute hypoglycaemia
- SNS activation → ↑ catecholamine release
- Central: nausea, agitation, hunger
- Liver: ↑ glycogenolysis, ↑ glucose release
- Pancreas: inhibition of insulin release
- CVS: ↑ HR, ↑ SVR, peripheral shutdown, sweating
- ↓ insulin release: ↑ BSL detected by β cells of islets of Langerhans
- adipose tissue:
- ↓ glucose uptake (↓GLUT4 transporters in membrane)
- ↓ fat uptake (extracellular inhibition of LPL in endothelium)
- ↑ FFA release (↑ intracelular hormone sensitive lipase)
- muscle
- ↓ glucose uptake (↓ GLUT4)
- ↓ protein synthesis
- ↓ glucogenesis
- ↑ FFA metabolism
- liver
- ↑ glucose release (glycogenolysis)
- ↑ FFA release (some ketone body formation)
- ↑ gluconeogenesis via glycerol (fats) and lactate (RBC metabolism)
- adipose tissue:
- ↑ catabolic hormones: ↓ BSL stimulates hormonal changes which aim to ↑ BSL over sustained period
- ↑ glucagon → ↑ hepatic gluconeogenesis from aa, lactate, glycerol
- cortisol, GH → ↑ FFA release from adipose tissue
Kerr 2016
Examiner Comments
2007B 16: 4 candidates (57%) passed this question.
To pass this question, the candidate only needed to stage the range of normal blood glucose, define hypoglycaemia then give an overview of the body’s response to hypoglycaemia (control of blood glucose – sensors, integrators, effectors). Additional credit was given for a more detailed description of the various responses. Few candidates mentioned the role of the sympathetic nervous system. Much time was wasted in giving detailed descriptions of metabolic pathways to the exlusion of an overview of the body’s responses.
17. List the potential clinical uses of an alpha 2 adrenoceptor agonist. Outline the limitations of clonidine for each use. G
CICMWrecks Answer
Potential Clinical Uses of α2 adrenoreceptor agonist
| α2 Agonist Uses | Clonidine |
|---|---|
| Essential and secondary Hypertension Hypertensive Crisis | • Marked hypertensive response (α1) – Seen in infusion acutely, not common with PO • Hypertensive crisis on withdrawal (2° upregulation of NA with chronic use) following – long infusion – cessation of chronic use PO • Prolonged refractory ↓MAP 2° to prolonged elimination t½ 9-18hrs |
| Migraine | • Hypotension • Sedation • Prolonged offset |
| Chronic Pain | • Ceiling effect (partial agonist) • Dose limited by side-effects (dry-mouth, hypotension, sedation) |
| Opiate Withdrawal | • Dose limited by side-effects (dry-mouth, hypotension, sedation) |
| Spinal anaesthesia / ↓ post-op shivering | • Ceiling effect (partial agonist) • Long time to peak effect • Long elimination t½ 9-18hrs |
| ICU Sedation or Anaesthetic Augmentation (50% MAC sparing) Procedural Sedation | • Long time to peak effect (~90min IV, 3hrs PO). • Prolonged refractory ↓MAP limits use in peri-operative setting • Dexmedetomadine preferred due to shorter t½ |
| Palliation | • Treatment of symptoms of distress (intractable pain, agitation or delirium) at the end of life. • Limited by hypotension and sedation |
| Antiemesis | • Limited use due to side effects of sedation and dry mouth |
Alpha 2 Adrenoceptor Details (include some details if you have time)
- Gi-PCR (↓Adenylyl cyclase activity → ↓ cAMP → ↓ PKA activation → Effects)
- Three main types 2A, 2B and 2C vary by location.
Important Locations:
- Postsynaptic α2 receptors in CNS
- Locus coerrulus (hypnosis)
- Spinal cord (analgesia)
- Lateral Reticular Nucleus (↓SNS tone, ↑ PSNS tone)
- Presynaptic α2 receptors in Sympathetic terminals of blood vessels
- ↓Noradrenalin release (vasodilation)
Clonidine
Class: Imidazole Derivative
Administration: PO 1-2 mcg/kg oral for premed, IV (150mcg in 2mL) 1-2mcg/kg bolus, Relatively long-time to peak effect (~90min IV, 3hrs PO)
Mechanism:
- Relative selectivity α1:α2 = 1:400 (vs dexmedetomadine (1:1600))
- Stimulation of presynaptic alpha 2 receptors
- ↓’s NAd release from sympathetic nerve terminals via negative feedback mechanism.
- Hypnosis
- Hyperpolarisation of Lateral reticular nucleus → ↓ central SNS tone and ↑ central PSNS tone
- Anti-emesis
- CTZ desensitisation, ↓ Intragastric pressure, anti-sialogogue
- Analgesia (dorsal horn of the spinal cord)
- Augment endogenous opiate release
- ↓Aδ- C fibre afferent activity
- Modulates descending noradrenergic pathways (↓’d Norad and Neuropeptide Y release)
- Long term dosing → ↓ responsiveness of peripheral vessels to vasoactive substances
For Further Reading:
Click to Open CICMWrecks Table: Alpha-2 agonist agents
CICMWrecks 2021
Examiner Comments
2007B 17: 2 candidates (29%) passed this question.
Producing a list that included the following was required:
– To trat hypertension and substance withdrawal.
– To provide anxiolysis, sedation, analgesia, and sympatholysis.
A brief discussion of the abilities of clonidine in each of these areas would have rounded off a good answer.
Many candidates listed only a couple of uses of these agents, and then followed this with a comparison of clonidine and dexmedetomidine. Most answers did not include sufficient information to achieve a pass mark.
18. Describe the autoregulation of renal blood flow.
CICMWrecks Answer
Regulation of Renal Blood Flow
- Renal blood flow Autoregulated between 80~170mmHg
- Blood flow maintained by modulating resistance based on pressure
- Renal vascular resistance maintained by interlobular arteries, afferent arterioles and efferent arterioles, amenable to external regulation
- GFR approx 180l/day – Autoregulated by tubuloglomerular feedback – relatively constant in response to fluctuating renal blood flow
INTRINSIC Regulation (Autoregulation) of GFR and RBF:
- Renal blood flow (and consequently GFR) Autoregulated between MAP range of 75~170mmHg
- Blood flow maintained by modulating resistance of AFFERENT based on pressure
- Efferent arteriole is NOT involved in autoregulation!
- Autoregulation of GFR and RBF can be overridden by external influences (Eg. hormones and SNS neurons), even when renal perfusion pressure is between MAP 75-170 mmHg!
Mechanisms of Autoregulation:
- Myogenic autoregulation (Myogenic stretch response):
- In response to vascular wall stretch (due to increased intraluminal pressures), stretch dependent Ca influx occurs causing vasoconstricion of arterioles → increased resistance according to Poisuille-Hagen Equation → Decreased flow
- In response to shear stress (due to increased flow), Endothelial derived relaxation factors released (such as NO) → NO acts on guanylyl cyclase → increased cGMP → smooth muscle relaxation → arteriolar vasodilation
- Tubuloglomerular feedback (TGF)
- Negative feedback – Links the rate of GFR to concentration of salt in tubular fluid at macula densa
- Macula densa in wall of Ascending limb of loop of Henle Detects change in tubular flow (by the changing salt concentrations)
- As a consequence of decreased blood flow → GFR decreases → Tubular flow rate decreases → Increased uptake of [Na] in Ascending LoH → Reduced [Na+] and [Cl-] reaching the DCT and macula densa → Juxtaglomerular apparatus releases prostaglandins (PGE2) → vasodilation of afferent arteriole → increased resistance to glomerular blood flow
- With increased GFR → Increased tubular flow rate → Decreased [Na] uptake by the LoH → Increased [Na+] in macula densa → Juxtaglomerular apparatus secretes adenosine → Vasoconstriction of the afferent arteriole → Decreased blood flow
Note: Flow to Juxtamedullary nephrons is not autoregulated. High blood pressure increases juxtamedullary flow, increasing GFR and impairing renal concentration, resulting in a pressure diuresis.
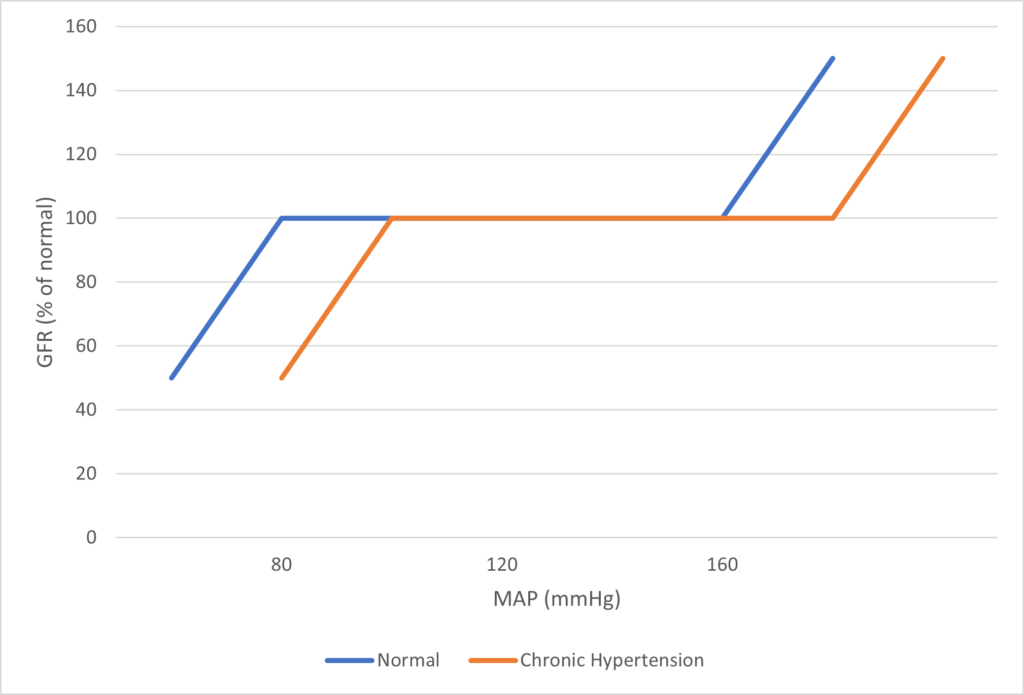
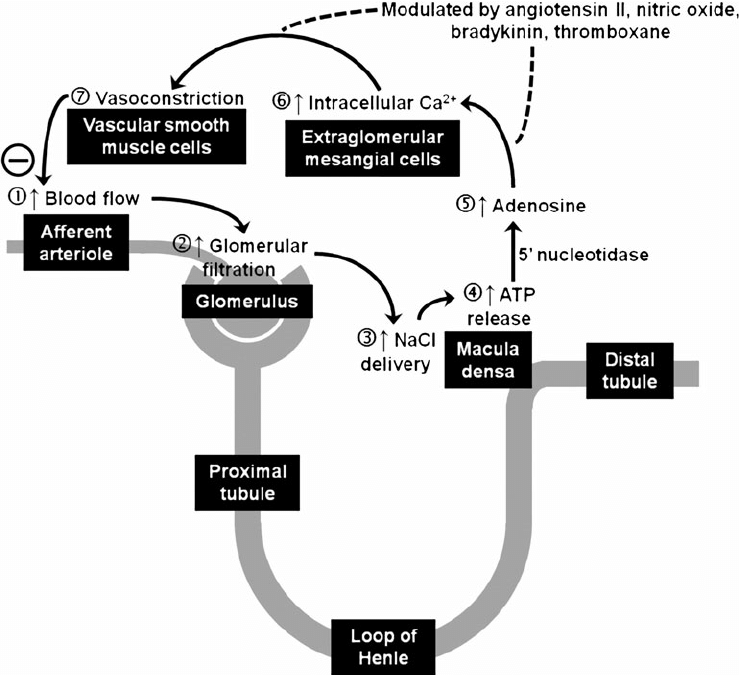
Image Source
JC / Mooney / Sakurai 2019
Examiner Comments
2007B 18: 3 candidates (43%) passed this question.
To obtain a pass candidates needed to briefly define autoregulation and state the range of MAP over which this occurs, and why it occurs, then provide a more detailed discussion about the mechanisms thought to be responsible for this.
The main site of autoregulation in the kidney is the afferent arteriole. There are two main factors that affect vascular tone in the afferent arteriloe, these are stretch-activated constriction of vessels (myogenic mechanism) and tubuloglomerular feedback (TGF).
Both of the above mechanisms are important to maintenance of near-constant blood flow. Stretch results in membrane depolarisation, increased intra-cellular concentrations of Ca and ultimately, vasoconstriction.
In tubulo-glomerular feedback, complex signals pass from the macula densa to the afferent arteriole, regulating its tone. The fundamental theme of TGF is that increased delivery of fluid and/or NaCl to the distal tubule causes vasoconstriction, thus limiting the flow (negative feedback).
The major weakness in answes was again the failure to include sufficient information to acheive a pass mark.
19. Describe the effects of a tachycardia on myocardial oxygen supply and demand in a normal heart.
CICMWrecks Answer
Coronary Blood Flow
- 80 mL/min/100 g
- or 200-250 mL/min
- 5% of CO at rest
- Can increase by 3-4 times (up to 400mL/min/100g)
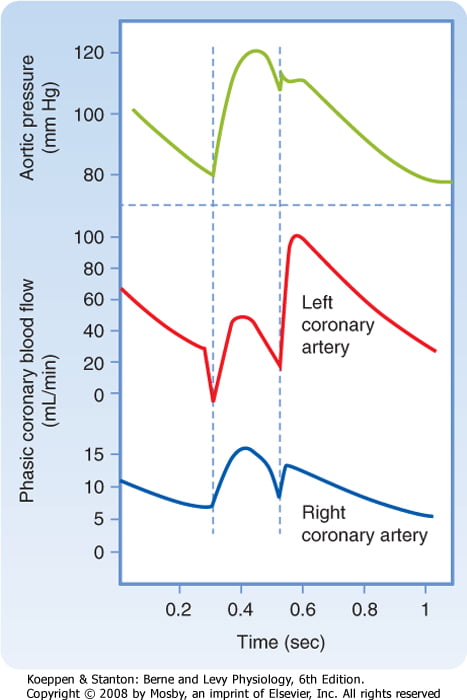
Coronary Perfusion Pressure
- ADP = Ao Diastolic Pressure
- Varies throughout the cycle and b/w ventricles
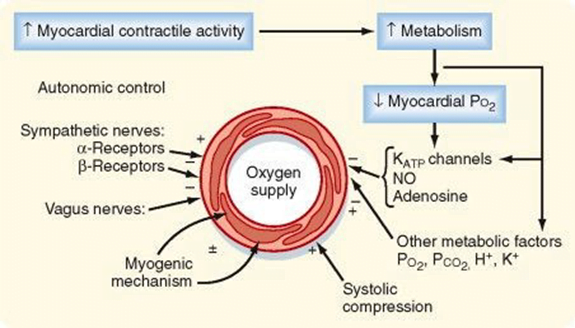
Coronary Vascular Resistance
- Physical Factors
- Extravascular compression (CPP factors)
- LCA flow stops in systloe
- RCA flow continuous throughout the cycle
- Neural and Neurohumoral Factors
- ↑ SNS tone →
- α receptor mediated vasoconstriction
- β receptor mediated vasodialtion
- ↑ force and rate of contractions → ↑ vasodialtor metabolite release
- Overall effect is dilation
- ↑ PSNS tone → KACh stimulation → mild ↓ Coronary vascular resistance
- ↑ SNS tone →
- Metabolic Factors (main)
- Vasodilatory (↑’d with ↑ HR)
- ↑ Adenosine, H, K, CO2, Lactate
- NO → GTP
- ↑ O2 demand → ↓ ATP → ↑KATP channel activation → hyperpolarisation → vasodilation
- Vasodilatory (↑’d with ↑ HR)
- Myogenic autoregulation (keep CPP 60-180 mmHg)
Determinants of myocardial O₂ demand:
- MVO₂ (ie extraction)
- High at rest (55-65%) cf. body average of 25%
- Extraction ratio can only rise by factor of < 2 to 90%
- AV Δ O₂ = 11 mL/dL
- Coronary venous O₂ content = 5 mL/dL
- Normally consumption 21 to 27 mls of O2/min.
- Determined by
- Wall tension.
- preload (EDV/EDP) and afterload (SVR)
- Contractility.
- Heart rate.
- ↑HR → ↓ supply
- ↑HR → ↑ demand (as ↑ MRO2)
- Wall tension.
CICMWrecks 2016
Examiner Comments
2007B 19: 2 candidates (29%) passed this question.
The main points expected were the determinants of myocardial oxygen supply. These include arterial oxygen content and coronary blood flow. Coronary blood flow deoends on coronary perfusion pressure and coronary vascular resistance and that most left coronary blood flow occurs in diastole.
Tachycardia reduces diastolic time and hence left coronary blood flow. In comparison blood flow in the right coronary artery is continuous both in systole and diastole and is little affected by heart rate. A correctly labelled diagram of left and right coronary blood flow attracted extra marks. Unfortunately most diagrams were inaccurate, not labelled and had no units on the axes. Systolic compression particularly reduces blood supply to the left ventricular subendocardium which is most susceptible to ischaemia. Extra marks were given for describing metabolic autoregulation, the high oxygen extraction, explaining that oxygen supply cannot be increased by increasing oxygen extraction in the coronary circulation and describing the driving pressure differences in both coronary arteries in systole and diastole.
A description of the determinants of myocardial oxygen demand was also required (e.g. left ventricular, preload, contractility, afterload, and tachycardia. This part of the question was particularly poorly answered.
20. Describe the determinants of serum potassium. Outline the consequences of acute hyperkalaemia.
CICMWrecks Answer: Potassium, K+ balance (Determinants of Serum Potassium)
Potassium
- Predominany intracellular cation
- Total Stores: Approx 3200mmol (50mmol/kg)
- Key Functions:
- Main determinant of ICF osmolality and tonicity
- Responsible for RMP of excitable cells via Goldmann-Hodgkin-Katz due to ↑↑gK relative to other species
- Role in action potential → repolarisation phase
- Secretion of insulin and multiple other KATP dependant processes
- Regulation of IC processes (protein/glycogen synthesis)
- Involved in Na+/K+ ATPase in cell membranes
Potassium balance / Determinants of Serum Potassium
- Intake
- Oral – very variable
- Approx. 50-200mmol/day
- Distribution
- Exchangable Pool
- ICF (90%): [K+] 150 mmol/L (1° ICF cation)
- ECF (2%): [K+] 3.5-5 mmol/L
- Non-exchangable Pool
- Bone (8%)
- Exchangable Pool
- Transcellular balance
| Factor | Mechanism | Effect on Serum K+ |
|---|---|---|
| Insulin | Causes intracellular shift of K+ | ↓ |
| β2 adrenergic agonism | Causes intracellular shift of K+ | ↓ |
| Aldosterone | Upregulate Na/K ATPase → Intracellular shift of K+ | ↓ |
| pH | Increased H+ (decreased pH) causes extracellular shift of K+ | ↑ |
| Plasma osmolarity | Hyperosmolar plasma initiall causes osmosis of water out of cells increasing intracellular osmolarity and [K+] → causes extracellular shift of K+ | ↑ |
| Skeletal muscle activity | Causes K+ leakage into serum | ↑ |
| Cell death | Causes K+ leakage into serum | ↑ |
- Elimination
- Faecal – 8mmol/day
- Renal – 92mmol/day (See next heading for details)
- Urinary K+ excretion = [K+ filtered by glomerulus] + [K+ secreted by CCD/LDCT] – [K+ reabsorbed by renal tubules]
- Glomerular filtration: Freely filtered = 756mmol/day (180L x 4.2mmol/L)
- PCT: 65% reabsorption
- LoH: 25~30% reabsorption
- DCT/Collecting Ducts – variable
- Determined by Aldosterone, Plasma [K+], Tubular Flow rate
- Secreted by principal cells
- Reabsorbed by intercalating cells
JC / Gladwin / Sakurai 2020
CICMWrecks Answer: Consequences of Hyperkalaemia
Serum Potassium
- Normally 3.5~5mmol/L
- Hyperkalaemia [K+] >5mmol/L
- Severe hyperkalaemia [K+] >7mmol/L
ECG Changes
- Peaked T waves at [K+] > 5.5mmol/L
- Loss of P wave at [K+] > 6.5 mmol/L
- QRS widening at [K+] > 7.5mmol/L
- VF or asystole
Consequences of Acute hyperkalaemia
- CVS
- ECG changes
- Tenting of T wave
- Flattening of P wave and increase PR interval
- Widening of QRS
- VF
- Arrythmia
- VF
- ECG changes
- CNS
- Lethargy
- Other
- Flaccid paraylis
- Muscle weakness
- Respiratory failure
JC / Sakurai 2019
Examiner Comments
2007B 20: 3 candidates (43%) passed this question.
Most candidates did not appreciate that serum potassium is a function of two variables:
1. Total body potassium
2. Distribution between the extracellular and intracellular fluid compartments.
Approximately 98% of total body potassium is intracellular due to the action of Na+/K+ ATPase. Potassium is important in the electrophysiology of excitable cells and changes in serum potassium can affect their function. Hence the importance of keeping the serium potassium within a narrow normal range.
Again most candidates did not provide the overview that serum potassium levels reflect a balance between intake, output and transcellular distribution. Normal dietary intake is highly variable. Transcellular distribution by the mechanisms of insulin and glucagol, catecholamines and Beta2 activity and acid base changes all work to rapidly restore changes in details on the long term renal regulation of serum potssium involving distal tubult potassium secretion and aldosterone and also the effect of distal tubular flow and sodium excretion.
The effects of hyperkalaemia were better described than the first part of the question. Most candidates concentrated on the cardiac effects where most marks were awarded. The effects of an increased potassium on the cardiac action potential earned extra marks. The correlation between actual serum potassium level and ECG changes is variable and depends on many factors including how acute or chronic the hyperkalaemia is.
Tratment of hyperkalaemia was mentioned by a few candidates but attracted no extra marks.
21. Compare and contrast the cardiovascular effects of an induction dose of Propofol and ketamine.
CICMWrecks Answer
| PROPOFOL | KETAMINE | |
|---|---|---|
| Dose for induction | 2-2.5mg/kg 0.5-1mg/kg in critically unwell | 0.5-1.5mg/kg |
| Autonomic nervous system | Reduction in sympathetic outflow | Increase in sympathetic outflow Centrally mediated |
| Heart rate | Blunts baroreceptor reflex → no reflex tachycardia Bradycardia due to reduced sympathetic tone | Increased due to increased sympathetic outflow |
| Contractility | Mild direct negative inotropy Secondary effect due to reduced sympathetic tone | Direct negative inotropy Increased sympathetic tone → increased overall inotropy (providing maximal sympathetic tone has not already been reached by the patient) |
| Peripheral vascular resistance | Direct vasodilation Secondary effect due to reduced sympathetic outflow | Direct vasodilation Overall, increased due to increased sympathetic tone |
| Blood pressure | Decreased | Usually increased |
| Cerebral circulation | Reduced CMRO2 Reduced cerebral blood flow | Increased CMRO2 Increased cerebral blood flow (No way are we diving in to the ICP question in this debate!) |
Examiner Comments
2007B 21: 2 candidates (29%) passed this question
The key words in this questions were “compare and contrast”, “cardiovascular effects” and “induction dose”. Some candidates described aspects of both drugs other than the cardiovascular effects but gained no marks for this. Better answers used a combination of a table plus some explanation to contrast the cardiovascular effects of the two drugs concentration on aspects such as heart rate, cardiac output, vascular resistance and blood pressure.
Many candidates were confused by the direct versus the indirect cardiovascular effects of both drugs
Propofol probably has no direct negative inotropic effect. Ketamine has a direct myocardial depressant action but this effect is overridden by the centrally mediated sympathetic action of the drug. The effect of both drugs on the baroreceptor response alone is a difficult area as there is significant interplay between the direct cardiovascular effects of the drugs and their effect on the baroreceptor reflex. Allowance was made for this in the marking. Propofol resets the baroreceptor reflex producing a slower heart rate for a given level of blood pressure. Overall both drugs depress the baroreceptor reflex.
Comparison of the effects on cerebral, coronary, renal and hepatic blood flow earned extra marks.
22. Describe the function, flow and absorption of cerebrospinal fluid.
CICMWrecks Answer
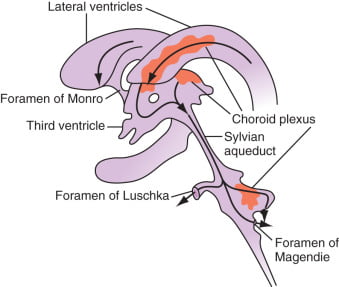
Formation / Production of CSF
- 60-70% of the CSF is formed by the choroid plexuses
- 30-40% by the cerebral vessels lining the ventricular walls
- Normal rate is 20mL/hour
- Formation independent of ventricular pressure
- Mechanism
- From Coroid Plexus by net transport of Na+, K+, Cl-, HCO3- and water, from plasma to ventricles
- Na down Conc grad
- Others down electro chem grads
Distribution / Circulation of CSF
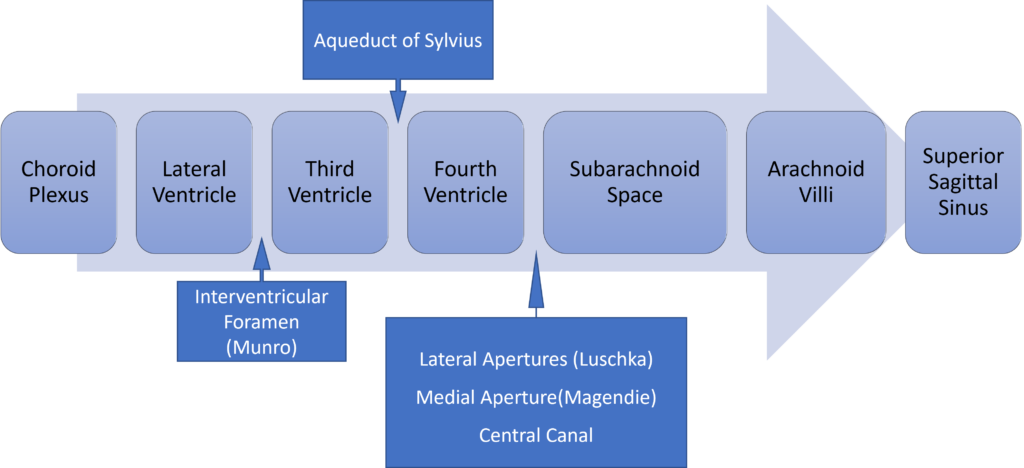
Absorption of CSF
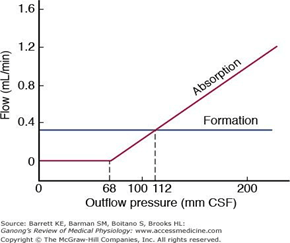
- Absorbed through the arachnoid villi into the cerebral venous sinuses
- Absorption by bulk flow, is proportional to ventricular pressure
- If pressure < 7 cmH2O, CSF absorption ceases
- Above 7cmCSF absorption is linear
- At approximately 11 cmH2O, CSF Absorption = Formation
Composition of CSF
IDENTICAL to brain ECF, but differs in several manners from plasma
Compared with plasma:
- ↑ pCO2 (50 mmHg)
- ↓ pH (7.33)
- ↓ protein content
- 0.5% of plasma; 20 mg/dL
- poor acid-base buffering capacity
- ↓ content of glucose (by 60%) and cholesterol
- ↑ [Cl-] (by 7-14%) and ↑ [Mg2+] (by 40%)
- ↓ [K+] (by 40%), ↓ [Ca2+] (by 50%) and ↓ [Pi] (by 20-30%)
- ↑ creatinine (by 25%) but ↓ urea
- IDENTICAL osmolality (295), [Na+] (145), and [HCO3] (25)
Role / Functions of CSF
- Protective role (main function)
- Water bath effect
- Attributed to the low specific gravity of CSF (1.007)
- Causes brain to be buoyant
- ↓ its effective net weight from 1400 g to 50 g
- Mechanical cushion against acceleration/deceleration forces
- Buffer ↑ ICP by CSF translocation to extracranial subarachnoid space
- Abrupt ↑ ICP buffered by translocation of CSF within the vault to extracranial compartments
- Water bath effect
- Maintains constant ionic environment conducive to neuronal electrical activity CSF
- Supply role of nutrients (Eg simple sugars, amino acids) and O2 to brain
- Excretion of toxic substances, metabolic by-products, and CO2 from brain
- “Lymph-type” function → interstitial proteins in brain ECF return to circulation by
- CSF absorption across arachnoid villi
- Acid-base regulation → due to content, CSF allows for tight respiratory control
- Endocrine transport function → transports hormones to other brain regions
Gladwin / JC 2020
Examiner Comments
2007B 22: 2 candidates (29%) passed this question.
The main points expected for a pass were:
- CSF is formed by ultra filtration and secretion
- CSF volumes and turnover
- Flow through the ventricles and subarachnoid spaces
- Absorption through the arachnoid villi
- Relationship between and absorption and pressure
This is not a question about intracranial pressure so no points were given for Munore Kellie doctrine etc, Also no points were given for the functions of CSF. Diagrams need to have the axes labelled correctly.
23. Describe the mechanisms of action of drug groups commonly used to treat acute severe asthma.
CICMWrecks Answer
Asthma:
- Airway obstruction that is reversible (completely or partially) either spontaneously or with treatment,
- Airway inflammation (oedema and hypersecretion)
- Increased airway responsiveness to a variety of stimuli
General Measures:
- Oxygen.
- Repeated assessment
- ABGs
Specific Pharmacology:
| Class | Example | MoA |
|---|---|---|
| Adrenergic Agonists | Salbutamol Adrenaline | Predominantly acting at the β2-adrenergic receptor Gs-PRC → ↑AC → ↑cAMP → ↑PLC activity – ↓ [Ca] via ↑ uptake and removal from cytoplasm – Active uncoupling of actin-myosin via i) Phosphorylation of MLCK ii) Phosphorylation of MLCK Phosphatase – ↑ K channel activation → hyperpolarisation → SMC relaxation Mast cell stabilisation (adrenaline) Improved mucocillary function. |
| Methyxanthines (PDE3 inhibitors) | Theophylline Aminophylline | Multiple Actions ↓ PDE acitons → ↑ cAMP in bronchial SM → sim to β2 effect Adenosine receptor antagonism → ↓ adensosine related bronchoconstriction |
| Antimuscarinics | Tiotropium, Ipratropium | M3 receptor antagonists Normally ACh stimulates M3 → Gq-PRC → ↑ DAG/IP3 → ↑ bronchiolar tone Antagonism → ↓ Vagally mediated Decreased Gq mediated effects |
| Steroids | Prednisone, Hydrocortisone | Reverse the activating effect of pro-inflammatory transcription factors → decrease inflammation – Inhibit the formation of cytokines secreted in asthma by T-lymphocytes, macrophages, and mast cells – Decreased vascular permeability – Inhibitory effect on mucus glycoprotein secretion – ? enhance B2 effects. |
| Other | Mag Sulphate | Acts as a bronchodilator by decreasing cytosolic Ca2+ concentrations. |
| Heliox | Decreased density – thus increased air flow via Hagan Pouiselle | |
| Ketamine | Bronchial smooth muscle relaxant by inhibiting Ach mediated SM constriction | |
| Sevoflurane | Direct beta agonism and inhibition of histamine release from mast cells. |
Gladwin 2016
Pharmacopeia Table
Examiner Comments
2007B 23: 3 candidates (43%) passed this question.
Common drugs listed were: Beta 2 agonists salbutamol and adrenaline, Steroids, Magnesium, Phosphodiesterase inhibitors.
In order to obtain marks for that class of drug the mechanism of action had to be described e.g. for theophylline; acts as a bronchodilator by inhibiting the breakdown of cyclic AMP and cyclic GMP.
There were several excellent answers to this question.
24. Explain the causes of the differences between measured end tidal and arterial partial pressures of carbon dioxide.
CICMWrecks Answer
PaCO2
- Factors affecting PaCO2
- Rate of metabolism and CO2 production
- FiCO2 (usually negligable)
- Alveolar ventilation
- Increased alveolar ventilation decreased PaCO2
End-Tidal CO2
- CO2 contained in gas at the end of tidal expiration
- Contains:
- Alveolar CO2
- High CO2 diffusion – PACO2 = PaCO2
- Only 0.7mmHg Alveolar-arterial CO2 gradient for 10% shunt
- Alveolar Dead Space CO2
- Anatomical – conducting airways
- Physiological – West zone 1 / Apex
- Varies with posture and pathology
- Alveolar CO2
- Measured by capnometer
- In-line or side-stream
- Used IR absorbance at 4.23 μm
- Side-stream: lag in CO2 detection due to increased dead space between respiratory tract and analyzer
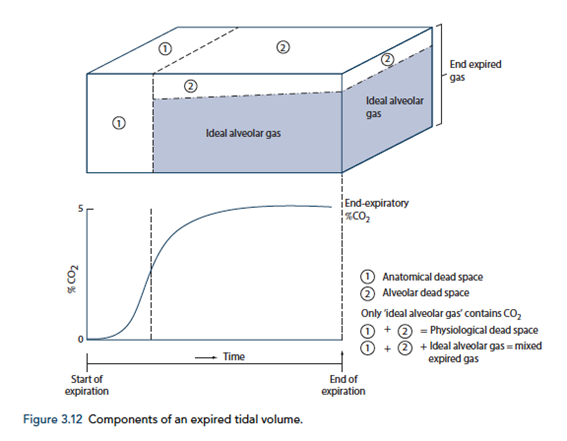
End-tidal to Arterial PaCO2 difference
- Normal ETCO2 – PaCO2 ~5mmHg
- Can be due to Artificially low ETCO2 or Artificially High PaCO2
Artificially low ETCO2
- Dead space (VD)
- Assumed PCO2 however usually low pCO2 but non 0 pCO2
- Contributes to ETCO2-PaCO2 ~5mmHg
- Pathological ↑VD
- ↑alveolar dead space (normal individuals = 0)
- Mixing of gas between perfused and non-perfused alveoli → ETCO2 < PaCO2
- Equivalent to ↑ West Zone 1
- → PE, hypotension, excess PEEP/IPPV
- COAD – poor perfusion of hyper-expanded alveoli
- ↑alveolar dead space (normal individuals = 0)
- Closing capacity (CC):
- Usually
40 CC may exceed FRC - ↑ Airways collapse/gas trapping → airways closure → ↑alveolar pCO2 within alveoli not exhaling
- Exacerbated by
- low volume ventilation close to FRC
- High FiO2 for extended periods → denitrogenation → loss of airways splinting = CC closer to FRC
- Usually
- Sampling line problems:
- Too far from trachea (ie ↑ mechanical dead space)
- Too long – unable to adequately sample as gas is trapped in line
- Air leakage/line blockage leading to measurement error with entraining of room air, loss of expired air
- Machine:
- Loss of calibration of sampling unit
- Excess H2O in water trap interfering with measurement
- Interference from other gases (N2O) artificially raises ETCO2 due to interference with IR absorber “Collision broadening”
- Incorrect timing of measurement
- Inadequate expiration time → failing to reach plateau = incomplete alveolar expiration = falsely low ETCO2
Artificially high PaCO2
- Should be measured from arterial sample
- Venous sampling will artificially ↑pCO2
- Loss of calibration of machine
Gladwin / Sakurai 2016
Examiner Comments
2007B 24: 0 candidates passed this question.
The main points for a pass were
Patient factors:
- Normal value for the difference between end tidal and arterial CO2
- Physiological factors e.g. alveolar dead space, failure to plateau of the capnograph trace
- Pathological factors e.g. cardiac arrest, air embolism, asthma
Equipment factors: Leaks, Occlusion, Samling site errors etc
A common mistake was to state that dead space caused the difference between end tidal and arterial carbon dioxide but not which type of dead space. The Bohr equation was not required.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Asthma in extremis – NMJ, NMJ blocking drugs How would you measure gas flow Causes of low PaO2 Ventilator graphs – P,V waveform |
| G. CVS | Explain physiological basis of ECG Cardiogenic shock (BP,CI,SCR given). What drugs would you use to improve BP |
| H. Renal | |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | |
| K. Neuro | |
| L. Musculoskeletal | Asthma in extremis – NMJ, NMJ blocking drugs |
| M. ANS | |
| N. Liver | Describe physiology of bilirubin production and clearance |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | Heparin as Prophylaxis against DVT after Acute Stroke – data table |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | |
| V. Obstetrics | |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments