1. Explain why the oxygen-haemoglobin saturation value derived by a pulse oximeter (SpO2) could be different from the measured arterial value (SaO2).
CICMWrecks Answer
Methods of Measuring oxygen saturation
Co-oximetry
- Blood gas analyzers measure arterial SaO2 by co-oximetry. (gold standard of SaO2 measurement)
- measures concentrations of oxygenated hemoglobin (oxyHb), deoxygenated hemoglobin (deoxyHb or reduced Hb), carboxyhemoglobin (COHb), and methemoglobin (MetHb) as a percentage of the total hemoglobin concentration in the blood sample
- Accurate measure of oxygen saturation: low readings indicate true hypoxia, and high readings always represent true hypoxia
- Not confused by ambient light, absence of pulsatile flow, tricuspid regurgitation, methylene blue dye.
- Uses Beer-Lambert law to detect different Hb species.
- “Incidence of light is inversely proportional to the path distance and concentration of light absorbing particles within the path”
- multi-wavelength spectrophotometry (measures the absorption of light passing through blood from several dozens of wavelengths)
Pulse oximetry
- Light emitter
- Produces infra-red light at 660nm and 950nm
- Light detector
- Detects light at 660nm and 950nm
- Processor
- Calculates SpO2 using the ratio of absorbance at 660nm and 950nm
- Light detected (incidence) represents light passing through both pulsatile (arterial) blood and non-pulsatile elements (venous blood and other tissues)
- Processor differentiates light incidence during maxima and minima of pulse wave and calculates SpO2 from pulsatile blood only giving SaO2
SpO2 different from SaO2
| PATIENT FACTORS | ||
| Low or High SpO2 | Low SpO2 | Normal or High SpO2 |
| Met-Hb Sulph-Hb | Poor perfusion of finger Movement artifact Venous pulsations Fingernail polish Intravenous pigmented dyes Haemoglobinopathy Anaemia with co-existing hypoxia | Carbon Monoxide poisoning |
| EQUIPMENT FACTORS | ||
| – Ambient light interference – Poorly fitting probe – Assay calibrated using healthy volunteers only down to SpO2 80%. Unknown significance if tested SpO2 less than 80% | ||
| PHYSIOLOGICAL FACTORS | ||
| – Due to O2 dissociation curve, insensitive to changes above PaO2 80mmHg – Does not measure tissue oxygenation | ||
Examiner Comments
2023B 01: 32% of candidates passed this question.
This question required candidates to identify that the measured arterial value (SaO2) was the gold standard to which the limitations of the pulse oximeter should be compared. A detailed description of the intrinsic and extrinsic factors of potential sources of difference of the SpO2 measurement was then expected. Intrinsic factors included wavelengths used, pulse added absorbance, derivation of the SpO2 value and time delays. Extrinsic factors where largely patient and environment related including light pollution, poor peripheral perfusion for various reasons, probe location variances and probe artefact.
2. Outline the anatomy of the cardiac ventricles including the chambers, valves and conduction elements.
CICMWrecks Answer: Anatomy (Rt vs Lt)
RIGHT
VENTRICLE
LEFT
VENTRICLE
| Pathway | |
| RA → tricuspid valve → RV → pulmonary valve → Pulmonary trunk | LA → Mitral valve → LV → Aortic valve → Aorta |
| Valves | |
| TV: 3 triangular cusps (medial anterior, inferior) attached by the base to fibrous ring of TV orifice. Papillary muscles connect trabeculae to cusps PV: 3 cusps (posterior, right anterior, left anterior) | MV: large anterior and small posterior cusp Attach to 2 large papillary muscles (anterolateral, posteromedial) by chordae tendinae AV: 3 semilunar cusps (Rt posterior, left posterior, anterior). Aortic sinuses above |
| Portions | |
| Septal portion: 1) the inflow tract: supports the tricuspid valve, Inferior aspect have ‘trabeculae carnae’ 2) Infundibuloventricular crest: muscular, lies between AV and pulmonary orifices – separates inflow and outflow 3) the outflow tract (infundibulum) – smooth walled, directed up+right towards pulm trunk | Portions: 1) Large trabeculated sinus portion with MV 2) Small smooth outflow tract that supports AV located anterior to the anterior mitral leaflet and is part of the atrioventricular (AV) septum. |
| Rest | |
| Rest of RV wall is trabecular Moderator band: muscular bundle crossing ventricular cavity from Intrerventricular septum to anterior wall – conveys right branch of AV bundle to the ventricular muscle | Wall: thick trabeculae carnae mostly. Exception of fibrous vestibule immediately below aortic orifice The free wall and apical half of the septum contain fine internal trabeculations. |
| Dimension | |
| The wall of the right ventricle is thinner than that of the left (1/3rd) Thickest at base, thinner towards apex Cavity size 85ml (=LV) | Wall 3x the size of RV Cavity size equal |
| Functions | |
| Receives deoxygenated blood from RA Pumps into pulmonary circulation | Receives oxygenated blood from LA Pumps into aorta → systemic circulation |
| Main Function | |
| maintain adequate pulmonary perfusion pressure to deliver desaturated venous blood to the gas exchange membranes of the lungs maintain low systemic venous pressure to prevent tissue and organ congestion | Maintain adequate systemic perfusion pressure to deliver oxygenated blood to tissue |
| Pressures | |
| RV Sys 15-30 | LV Sys 100-140 |
| RV Dias 4-12 | LV Dias 3-12 |
| RVEDD 10-26mm | LVEDD 36-56mm |
| RVESD 10-26mm | LVESD 20-40mm |
Sources: Last’s Anatomy, Aneskey.com, Anatomy for anaesthetists
JC 2019
CICMWrecks Answer: Coronary Circulation (Rt vs Lt)
RIGHT
VENTRICLE
LEFT
VENTRICLE
- Supply:
- Right coronary artery and its branches
- conus artery supplies the infundibulum
- acute marginal arteries supply the anterior free wall
- posterior descending artery via septal branches supply the posterior 1/3 IV septum (PDA arises from RCA in Right dominant circulation and LCx in Left dominant circulation
- the other 2/3 of the IV septum is supplied by septal branches of the left anterior descending artery
- Supply: Left Main Coronary
- LAD (supplies the free wall and most of the papillary muscles)
- Diagonals
- Septal perforators
- LCx: inferolateral LV wall
- Obtuse Marginal branches
- LAD (supplies the free wall and most of the papillary muscles)
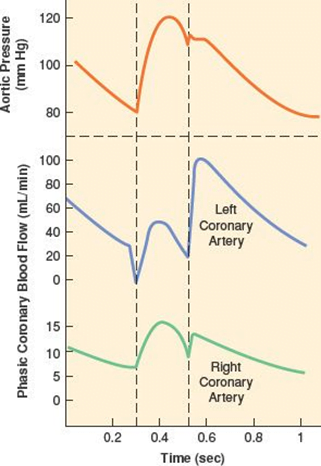
- RV Coronary blood flow
- RV pressures are low during both systole and diastole.
- flow during both systole and diastole
- LV Coronary blood flow
- The pressure inside the left ventricle is slightly higher than in the aorta during systole.
- Flow predominantly during diastole.
- Subendocardial flow ceases during systole.
Sources: Last’s Anatomy, Aneskey.com, Anatomy for anaesthetists
JC 2019
Examiner Comments
2023B 02: 11% of candidates passed this question.
This question expected an outline of the anatomy of the cardiac ventricles. Candidates that broke down the anatomy into subsections with correct and clear descriptions of each component were most successful. A potential structure to approach this question is included below;
– position, orientation, relations and characteristics of the chambers including ventricular interdepence
– internal ventricular structures including muscle type, septum, trabeculae, papillary muscules, infundibulum and moderator band
– valves and valvular rings’ position, structure and attachments
– conduction elements position and divisions
– blood and nerve supply
3. Describe the physiological mechanisms by which the kidney is able to concentrate urine.
CICMWrecks Answer
Medullary concentrating gradient
- physiological process which sets up a concentration gradient from cortex through to medulla
- allows formation of concentrated urine
- Mechanisms:
- Counter current Multiplier: creates concentrated medullary interstitium
- Counter current Exchange (vasa recta): maintains intersisital osmotic gradient
- Recycling of urea: contributes to high osmolarity of medullary interstitium
- Normal cortico-medullary gradient 300-1400mOsmol.
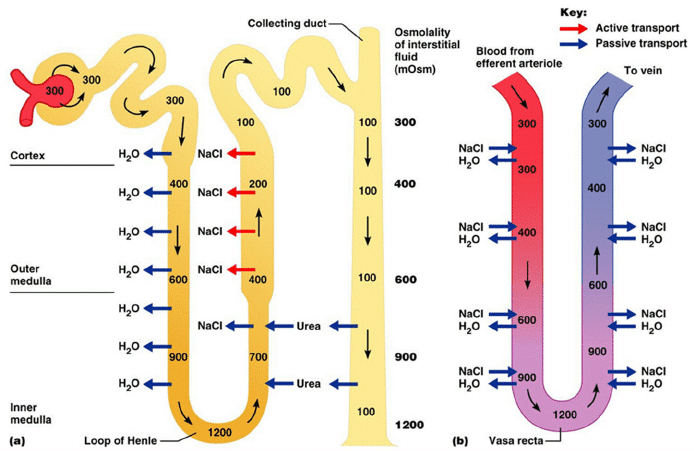
Counter-current Mechanism in Kidney
Counter Current Multiplier:
Set-up
- Descending limb LoH Permeable to water only
- Ascending limb LoH Permeable to NaCl only
- Thin passive NaCl reabs
- Thick NaCl via active NKCCT cotransport and paracellular
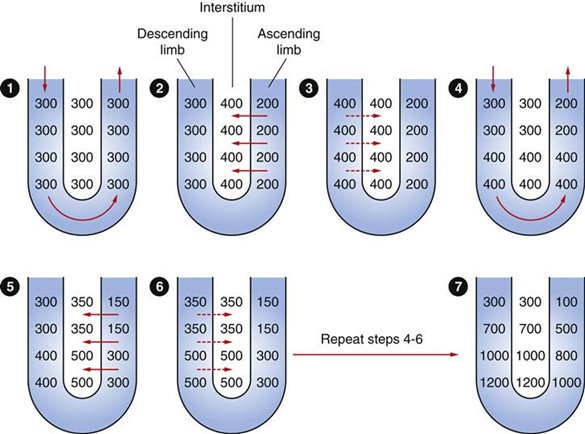
Generation
- Enters LoH osmolarity throughout = 300mOsm
- Max effort of NKCCT = 100mOsm/kg
- Tubular OSM = 200/kg
- Interstitial Osm = 400/kg
- Descending limb diffuses water down gradient
- Descend limb OSM now 400
- Process repeats (flow, pump salt, equilibrate water)
Counter Current Exchange:
- Hairpin loop arrangement of the vasa recta in the juxta-medullary nephrons.
- Provides blood flow to medullary tissue without impact of the cortico-medullary gradient.
- Relies on slow flow of blood.
- Mechanism
- On descent, water is lost from capillaries and NaCl is absorbed into capillaries increasing osmolarity
- On ascent water is reabsorbed and solute is lost
Role of Urea in Medullary Concentration Gradient
- 900mmol/day filtered
- 350-550mmol/day reabsorbed.
- Comprises 50% of osmolality (300-650 mOsm/kg)
- Freely filtered at the glomerulus
- Secreted by thin limbs of LoH down concentration gradient from medullary interstitium to tubular fluid.
- As water and Na are reabsorbed, tubular [Urea] increases
- [Urea] = >500mmol/L at collecting ducts
- Equilibrates with interstium due to slow flow rates.
- ↑ADH
- urea transporters UT1 and UT3 insertion → ↑ CD permeability to urea
- → ↑ medullary gradient (further 10% reabsorption if required)
Gladwin / JC 2020
Examiner Comments
2023B 03: 52% of candidates passed this question.
This question required the identification of the components of the kidneys function that work to concentrate urine and a detailed description of how each of these components contribute to this. These components included the role of the loop of henle and vasa recta in the establishment and maintenance of the medullary osmotic gradient, the contribution of urea and urea cycling to this gradient and the subsequent contribution of antidiuretic hormone. A focus on not how urine is formed but how it is concentrated was expected.
4. Classify the mechanisms of action of anti-convulsant drugs (30% marks).
Outline the pharmacology of gabapentin (70% marks).
CICMWrecks Answer: Classification of Anti-Convulsants
Anticonvulsant Classification
(based on mechanism of action)
- Sodium channel blockers.
- Examples: phenytoin, carbamazepine, lamotrgine, Na valproate
- These promote the inactive state of voltage activated Na channels.
- Increased neuron refractory period to action potential generation.
- Rapid repetitive firing is diminished, spread of electrical activity to adjacent brain areas is suppressed.
- Drugs that enhance GABA mediated synaptic inhibition.
- Increases the influx of chloride ions into the cell and hyperpolarizes the neuron.
3 mechanisms:- Act on GABA receptor. Example: benzodiazepines, barbiturates.
- Inhibit GABA transporter and reduce neuronal GABA reuptake. Example: tiagabine.
- Promote GABA release. Example: gabapentin.
- Increases the influx of chloride ions into the cell and hyperpolarizes the neuron.
- Drugs that inhibit pre-synaptic Calcium channels.
- Example.: Na valproate, ?levetiracetam
- Limit activation of voltage activated Ca channel known as the T current
- Drugs that inhibit AMPA Kinate.
- Example.: Topiramate
- Limit proexcitatory action of glutamate
- Drugs that NT release.
- Example.: ?levetiracetam
- Binds to synaptic vesicle glycoprotein SV2A reducing exocytosis of synaptic vesicles
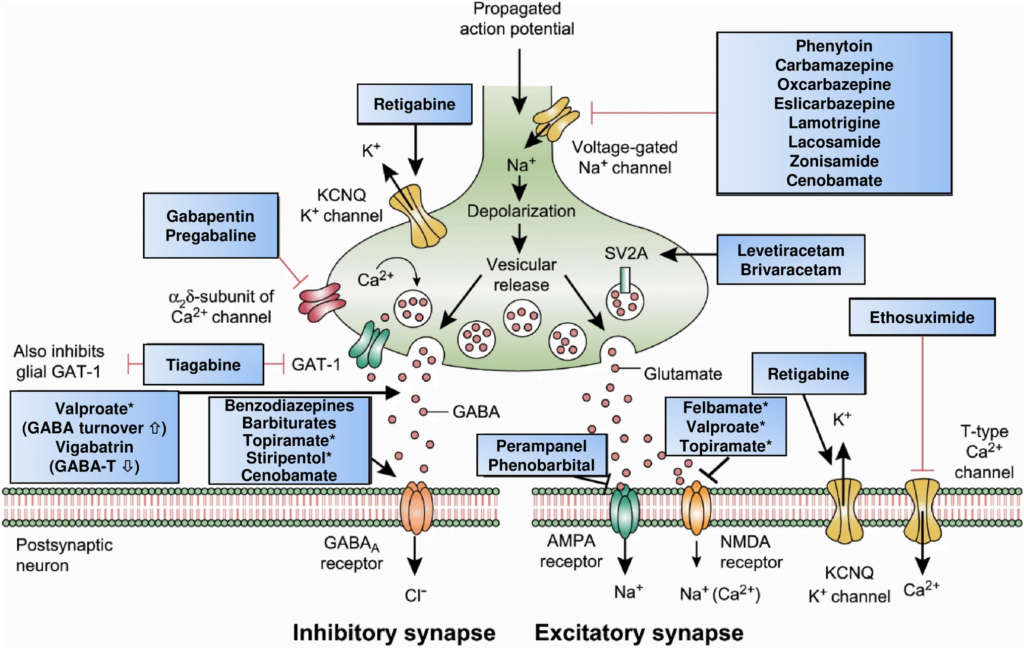
CICMWrecks Answer: Pharmacology of Gabapentin
Examiner Comments
2023B 04: 21% of candidates passed this question.
The first part of this question required candidates to correlate the mechanism of action of anti epileptic drugs with atleast one example under each section. These included GABA potentiation (Barbiturates/Tiaganbine), reduction in excitory transmission (including NMDA/AMPA antagonists) and modification of ionic conductances (sodium channel blockade-Phenytoin, Calcium channel blockadeLamotrigine, Gabapentin, activation of potassium channels- Retigabine).
The second part of this question involved an overview of gabapentin pharmacology. Gabapentin is an amino acid analogue of GABA utilised in neuropathic pain and epilepsy. There a multiple proposed mechanisms of action including Ca+ antagonism, inhibited glutamate release and anatagonism and increased GABA concentrations.. Important pharmacokinetic properties included dose dependent absorption, minimal protein binding, no metabolism with renal clearance and requirement for dose reduction in renal failure. It has largely central neurological side effects with a rare but important association with steven johnson syndrome or toxic epidermal necrolysis.
5. Outline the carbohydrate and lipid energy stores of the body (15% marks).
Outline the metabolic responses to starvation under the following headings: <24 hours; 24-72 hours; and >72 hours (85% marks).
CICMWrecks Answer
Body energy substrate reserves and utilisation
Fuel homeostasis principally regulates the needs of brain and muscle (major consumers of fuel).
brain – glucose exclusively (120g/d) until prolonged starvation (~2 days) then switches to ketones (fuel sparing)
Body energy substrate reserves:
- Glycogen stores → source of glucose (sufficient for brief period of fasting <24hrs)
- Liver (100 g; ½ day supply)
- Muscle (400 g)
- Brain (minimal; 4 mins supply)
- Fat stores (as TAG) → source of FFA and glycerol
- liver and adipose tissue
- Protein stores → source of a.a.
- muscle protein, plasma proteins, CT, enzymes
Body energy substrate utilisation:
- Brain/nervous tissue – 1°ly uses glucose for energy, but uses KBs with fasting
- Cardiac muscle – Use FFA, lactate and KB for energy
- RBC – 1°ly uses glucose for energy
- Skeletal muscle
- Uses FFA and ketone bodies for energy
- Glucose can be used for energy (esp with hyperglycaemia or anaerobi conditions (Eg. exercise)) or glycogen storage
- Adipose tissue – Uptake of glucose, glycerol and FFA → forms TAG for storage (lipogenesis); also breaks TAG into FFA/glycerol (lipolysis)
- Liver → integral role in starvation
- Control BSL by controlling glycogen stores and gluconeogenesis
- Takes up all metabolic fuel substrates → can interconvert them (Eg. gluconeogenesis, ketogenesis)
- Vital source of circulating metabolic fuel substrates
Glucose Homeostasis
| PHASE I | Well-fed state |
| PHASE II | Glycogenolysis |
| PHASE III | Gluconeogenesis |
| PHASE IV | Glucose, Ketone Body oxidation |
| PHASE V | Fatty Acid, Ketone Body oxidation |
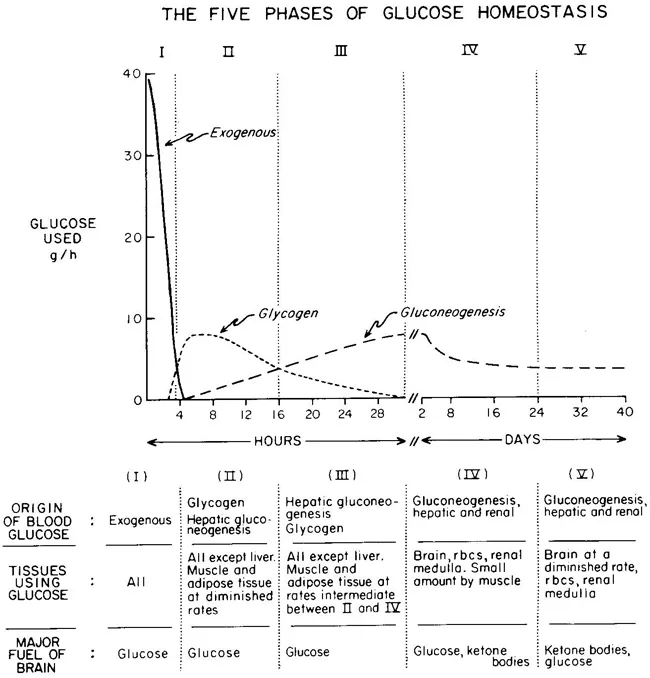
Metabolic responses to starvation
<24 hrs (early fasting)
- Initially Phase I and dietary glucose metabolized
- Phase II starts when dietary glucose exhausted
- ↓ Insulin
- Hepatic glycogenolysis maintains blood glucose
- Transient increase metabolic rate
24-72 hrs (short-term)
- Phase II starts when Glycogen stores exhaust around 20hrs
- depending on: feeding status, hepatic glycogen stores, physical activity
- ↓ Insulin ↑ Glucagon and ↑ catecholamine
- Brain and RBCs: Glucose from Hepatic Gluconeogenesis (from lactate, pyruvate, glycerol and alanine)
- Other (skeletal and cardiac muscle, kidney, liver): FFAs + glycerol (from Lipolysis / Hydrolysis of TGs)
- Metabolic rate begins to decrease after 48hrs
>72 hrs (Prolonged)
- ↓↓↓ Insulin ↑ Glucagon and ↑catecholamine
- Phase IV starts after several days of fasting
- Gluconeogenesis starts to decrease
- Ketone body accumulation increases
- Brain uses both glucose and ketone body for energy
- Further decline in metabolic rate 15-20%
- Phase V starts after prolonged fasting
- Less dependence on gluconeogenesis
- All tissues use Fatty acid and Ketone body oxidation for energy
- High ketone body conc, and glucose levels inhibit proteolysis (conservation of muscle) (protein sparing effect)
- After fat and ketone body exhausted → starvation → proteolysis of muscle
- Reduced energy expenditure
- reduced diet induced thermogenesis
- reduced weight and energy cost of movement
- decline in voluntary physical activity
Examiner Comments
2023B 05: 67% of candidates passed this question.
The first part of this question required the details of carbohydrate and lipid stores with their anatomical locations, biochemical forms, average amount of energy stored.
Under the metabolic responses to starvation, a detailed description was expected of major sources of energy production, associated biochemical processes and their transition from one process to another or one source to another over time, and the hormonal influences that govern this. A more detailed answer would also include organ specific energy utilisation under a starved state. Overall it was expected that a transition of glycogenolysis to gluconeogenesis to ketogenesis would be described. It would also be important to highlight how an initial protein conservation strategy transitions to eventual protein catabolism and how muscle glycogen, an important store of glucose is unavailable to maintain blood glucose concentrations in starvation.
6. Explain perfusion limited and diffusion limited transfer of gases in the alveolus.
CICMWrecks Answer
Fick’s law of diffusion
- Describes Diffusion through tissues
- Rate of movement of solute across semi-permiable membrane J is
where
C = concentration (or partial pressure for gasses)
A = cross-sectional area
T = thickness of the membrane or distance over which diffusion takes place.
The rate of diffusion of a gas through a tissue is:
Directly ∝:
- Surface area of the barrier
Affected by:- Parenchyma volume: Changes with Body size and in disease states
- V/Q mismatch: reduced in shunt and dead space
- Pulmonary Blood Volume: changed with vascular distension and recruitment
- Cardiac output: increased recruitment in high output states, decreased recruitment and increased V/Q mismatch in shock states
- Posture: Increased surface area while supine (compared to sitting or standing)
- Solubility of the gas
- CO2 is ~20 times as soluble as O2 in blood
- Concentration gradient (partial pressure difference)
- Rate of blood flow through lungs
- Transit time of blood across capillary (not part of Fick’s Equation) – based on HR
- alters total gas exchange across alveolus
- Can potentially change perfusion limited gas exchange to diffusion limited
- Alveolar partial pressure affected by:
- Atmospheric pressure
- Ventilation: Alveolar hypoventilation → ↑ PACO2 , ↓ PAO2
- Partial pressure in blood affected by:
- Binding of gas to protein:
- Rate of oxygenation of reduced Hb:
- Shift of oxygen dissociation curve (pH, temperature, PCO2, 2,3-DPG)
- Haematocrit
- Abnormalities of haemoglobin
- Formation of carbamino compounds
- Anaesthetic agents to plasma contents, e.g Albumin, cholesterol
- Enzymatic Action
- carbonic anhydrase (conversion of HCO3 to CO2)
- Rate of blood flow through lungs
Inversely ∝:
- Membrane thickness
- Increased thickness impedes gas exchange – Pathological states like pulmonary edema and cardiac failure
- Square root of Molecular Weight
- smaller substances diffuse more quickly
Diffusion vs. perfusion limited
Transfer of gases can be diffusion or perfusion limited dependent on the rate limiting step
Diffusion Limited
- Occurs in gases which do not reach equilibration of Pa and PA
- The rate of gas diffusion across the alveolar membrane limits its transport away from the lung
- Rate limiting step = rate of diffusion
- E.g. CO
- CO binds so avidly to Hb (250x that of O2) → virtually no CO dissolved in plasma → PaCO rises only slightly
- Even when RBC traversed entire length of pulmonary capillary, there is still substantial partial pressure difference across alveolar-capillary barrier → equilibrium of PaCO and PACO never reached
Perfusion Limited
- Characterized by complete equilibration i.e. Pa = PA
- amount of gas transferred between alveolus and capillary = dependent on amount of blood passing through the capillary
- rate limiting step = rate of blood flow
- E.g. N2O
- N2O rapidly diffuses across alveolar-capillary barrier
- Insoluble; does not bind to Hb; only carried in plasma in dissolved form
- PaN2O = PAN2O (<0.07sec); well before RBC has traversed pulmonary capillary
- ↑ diffusion rate will not ↑ blood transport away from the lungs → limiting factor = rate of blood flow / perfusion
- e.g. CO2 (ventilation limited i.e. perfusion limited in reverse)
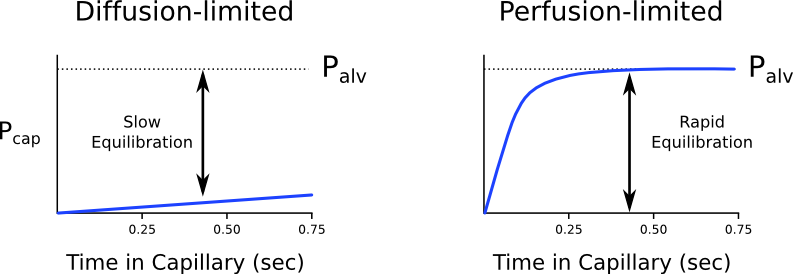
Is the transfer of O2 perfusion or diffusion limited?
- Can behave as both perfusion and diffusion limited
- O2 diffusion takes 0.25s; pulmonary capillary transit time is 0.75s
- Normal conditions
- Transfer of O2 across the alveolar capillary barrier is perfusion limited
- Equilibrium is reached between alveolar and capillary PO2 before the RBC has traversed the pulmonary capillary
- Conditions where transfer of O2 may become diffusion limited
- Disease of the alveolar capillary barrier
- Pulmonary fibrosis: thickening of alveolar-capillary barrier → ↓ rate of diffusion
- Exercise: ↑ CO → ↓ RBC transit time
- Altitude: ↓ PaO2
- Disease of the alveolar capillary barrier
Diffusion of O2 and CO2
Oxygen
- Oxygen diffusion takes ~0.25s
- Pulmonary capillary transit time is 0.75s
- Therefore, under normal conditions oxygen is a perfusion limited gas
- However, oxygen may become diffusion limited in certain circumstances:
- Alveolar-capillary barrier disease
Decreases the rate of diffusion.- Decreased surface area
- Increased thickness
- High cardiac output
Decreases pulmonary transit time. - Altitude
Decreases PAO2
- Alveolar-capillary barrier disease
Carbon Dioxide
- Carbon dioxide is ventilation limited, rather than diffusion or perfusion limited
- This is because it is:
- 20x more soluble in blood than oxygen
- Rapidly produced from bicarbonate and carbamino compounds
- Present in far greater amounts than oxygen
1.8L.kg-1 exist in the body (though 1.6L-1 of this are in bone and other relatively inaccessible compartments).
- Impairment of diffusion capacity causes type 1 respiratory failure as oxygen is affected to a much greater extent than carbon dioxide
Kerr / JC 2020
Examiner Comments
2023B 06: 48% of candidates passed this question.
This question required an explanation of the factors that influence perfusion and diffusion and a detailed description of the behaviour of specific gases including perfusion limited oxygen and carbon dioxide and diffusion limited carbon monoxide. Factors influencing perfusion include flow/cardiac output, resistance (radius and length) and viscosity whereas diffusion is influenced by the characteristics of the gas (MW and solubility), surface area of diffusing surface and the pressure/concentration gradient. Perfusion and/or diffusion limited characteristics of different gases included how quickly if at all equilibrium is reached and why this occurs with each specific gas.
7. Describe the mechanism of action, dose, pharmacokinetics and pharmacodynamics of clonidine.
Examiner Comments
2023B 07: 38% of candidates passed this question.
This question was broken down into mechanism of action, dose, PK and PD. The mechanism of action required a detailed description of its alpha agonism 200:1 affinity for alpha 2 over alpha 1 including the classification of these receptors and the downstream effects. Correct dose and/or dose ranges for oral and intravenous formulation particularly for different indications for the prescription of clonidine. Pharmacokinetic details expected the absorption, distribution, metabolism and elimination characteristics, with enough detail to demonstrate an understanding. Pharmacodynamic marking was weighted towards the significant cardiovascular, neurological and the relative absence of respiratory effects that make it desirable for use as a sedative/co-analgesic in ICU practice. A description of the these pharmacodynamic effects and why they occur was required to achieve marks in this section.
8. Compare and contrast the pharmacology of frusemide and acetazolamide.
Examiner Comments
2023B 08: 31% of candidates passed this question.
Pharmacology questions largely have a standardised structure to follow; pharmaceutics, pharmacokinetics and pharmacodynamics. However compare and contrast questions require answering in a way that highlights important similarities and differences between the drugs chosen and the impact of these differences when administered. Whilst these are both diuretics they have very different renal and non-renal effects and thus very different metabolic and electrolyte disturbances. It was expected that these points be highlighted as well as the provision of other pharmacological information in order to pass this question.
9. Outline the classification, structure and distribution of the opioid receptors (50% marks). Describe the intracellular events following opioid receptor activation (50% marks).
CICMWrecks Answer
Opioid Receptors
- Serpentine, inhibitory G protein-coupled receptors (GiPCR)
- ligands are endogenous opioids
- dynorphins, enkephalins, endorphins, endomorphins and nociceptin
- 4 types – MOR (), DOR (), KOR (), NOR
| Receptor | Subtypes | Location | Clinical Effects | ||
|---|---|---|---|---|---|
| Brain | Spinal Cord | Other | |||
| MOR (μ) | μ1, μ2, μ3 | cortex (laminae III and IV) thalamus striosomes periaqueductal gray rostral ventromedial medulla | substantia gelatinosa | peripheral sensory neurons intestinal tract | μ1: analgesia physical dependence μ2: respiratory depression miosis euphoria reduced GI motility physical dependence μ3: possible vasodilation |
| DOR (δ) | δ1, δ2 | pontine nuclei amygdala olfactory bulbs deep cortex | – | peripheral sensory neurons | Analgesia Antidepressant Convulsant Physical dependence May modulate MOR mediated respiratory depression |
| KOR (κ) | κ1, κ2, κ3 | hypothalamus periaqueductal gray claustrum | substantia gelatinosa | peripheral sensory neurons | Central analgesia anticonvulsant, depression, dissociative, hallucinogenic diuresis, miosis, dysphoria sedation, stress Visceral nociception antagonist |
| NOR (Nociceptin) | ORL1 | cortex amygdala hippocampus septal nuclei habenula hypothalamus | spinal cord | – | anxiety, depression appetite development of tolerance to μ-opioid agonists |
Opioid Receptor Structure and Activation
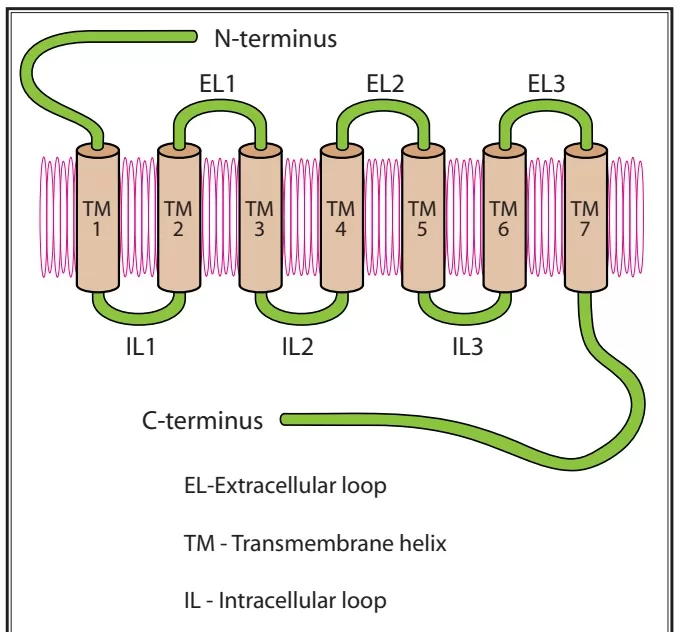
- Each receptor consists of an extracellular N-terminus, 7 transmembrane helical twists, 3 extracellular and intracellular loops, and an intracellular C-terminus
- Once the receptor is activated, it releases a portion of the G protein, which diffuses within the membrane until it reaches its target (either an enzyme or an ion channel).
- These targets alter protein phosphorylation via inhibition of cyclic AMP (cAMP) which acts as a second messenger within the cell resulting in
- the activation of protein kinases (short term effects) and
- gene transcription proteins and/or gene transcription (long term effects)
- Neurotransmitter release from neurons is normally preceded by depolarisation of the nerve terminal and Ca++ entry through voltage-sensitive Ca++ channels.
- Opioid receptors located on the presynaptic terminals of the nociceptive C-fibers and A delta fibers, when activated by an opioid agonist, will indirectly inhibit these voltage-dependent calcium channels →
- decreasing cAMP levels and blocking the release of pain neurotransmitters such as glutamate, substance P, and calcitonin gene-related peptide from the nociceptive fibers →
- resulting in analgesia
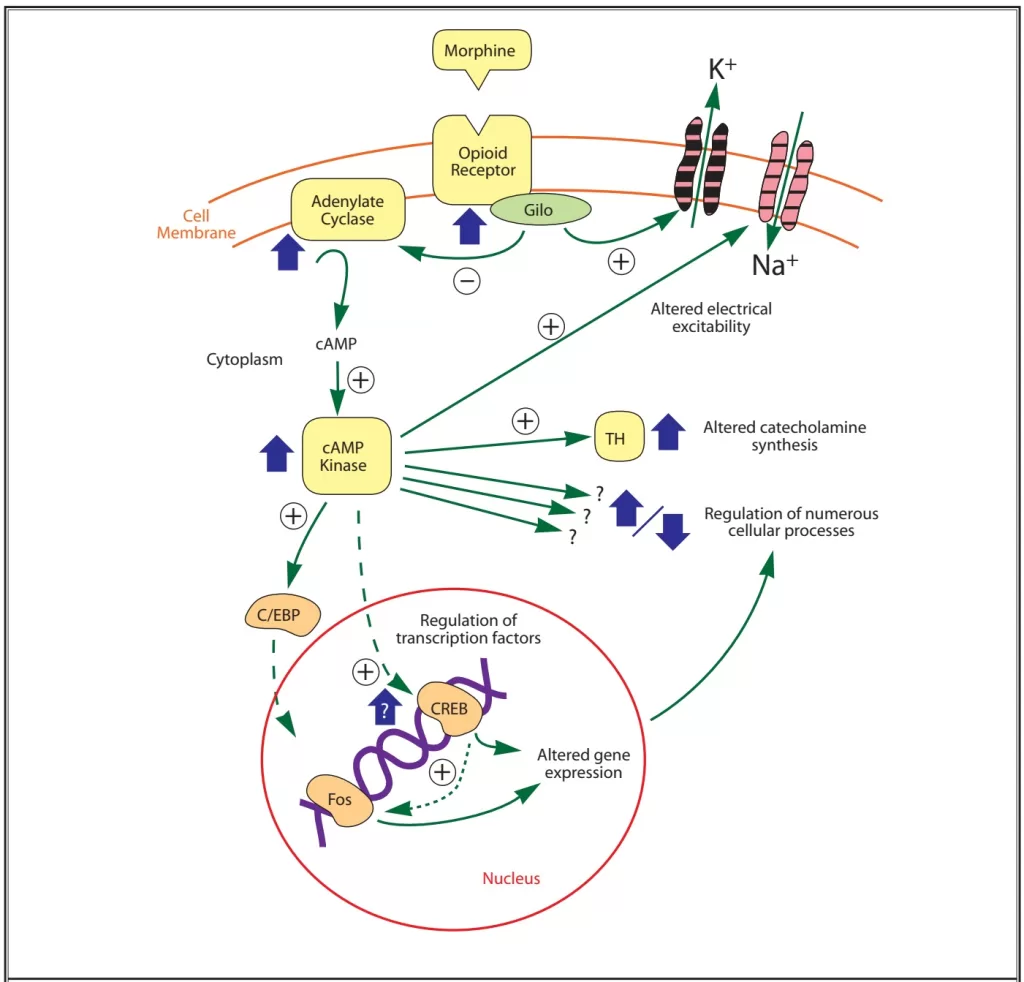
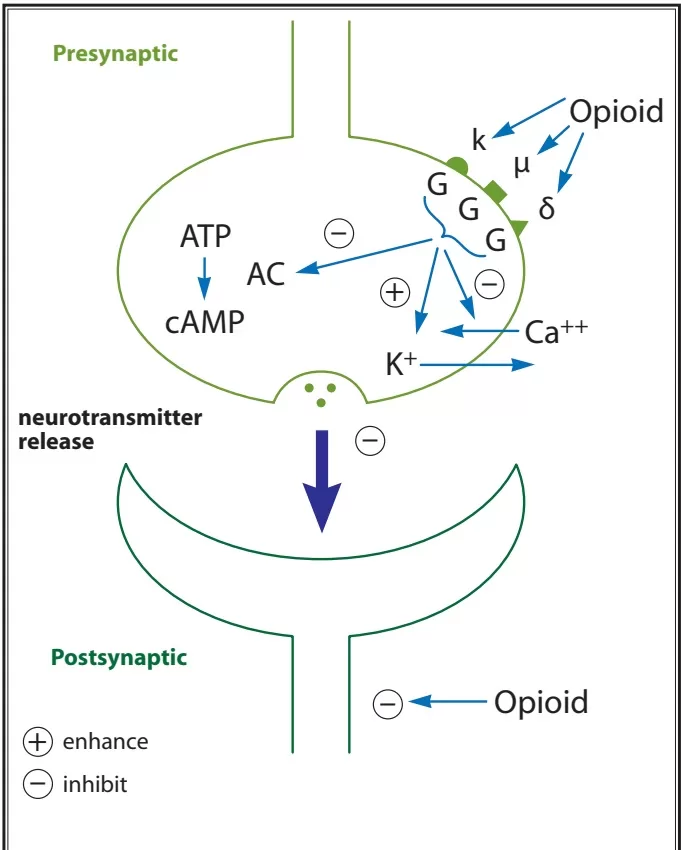
- Opiod receptor activation → Increases adenylyl cyclase, reducing cAMP
- Presynaptically inhibits voltage-gated Ca2+ channels
- Decreases Ca2+ influx
- Reduces neurotransmitter release
- Post-synaptically activates K+ channels
- Causes K+ efflux
- Leads to membrane hyperpolarisation
- Presynaptically inhibits voltage-gated Ca2+ channels
- Overall this leads to a reduction in neuronal cell excitability, resulting in reduced transmission of nociceptive impulses.
Sources: Notes,
https://www.painphysicianjournal.com/current/pdf?article=OTg3&journal=42
Examiner Comments
2023B 09: 20% of candidates passed this question.
This question was asked in a specific way to provide candidates with a template for their answer. The classification most commonly used for opioid receptors (μ(MOP), δ(DOP), k(KOP) & NOP) and a description of the important characteristics or differences between them was expected. A description of their central and peripheral distribution was required including specific central nervous system sites such as pre and post synaptic locations in the brain (ie. the periaqueductal gray, locus ceruleus and amygdala) and spinal cord (ie. primary afferent neurons in the dorsal horn).
Opioid receptors as a class are transmembrane spanning G protein receptors that have significant downstream effects including presynaptic inhibition of neurotransmitters of primary afferent neurons such as noradrenaline and substance P, and postsynaptic inhibition of membrane depolarization of dorsal horn nociceptive neurons. Specifity of detail in descriptions of these actions was expected.
10. Outline the impact of sedative agents on thermoregulation (40% marks).
Describe the physiological effects of a low body temperature (60% marks).
CICMWrecks Answer
Impact of sedative agents on Thermoregulation
3 phases:
- Phase I – 1st hour – Redistribution of heat from core → periphery
- significant decrease in core temperature from 0.5 to 1.5 C
- thermoregulatory center is depressed by anaesthesia
- Contributors:
- undressed
- skin prep
- evaporative loss
- induction → cutaneous vasodilation
- cool IVF
- cool dry ventilation of lungs
- temp drop in spinal > epidural
- Phase II – Hour 2 to 3 – Slow linear decrease in core temperature
- Increased heat loss
- anaethetic induced peripheral vasodilation
- → increased radiant & evaporative loss.
- → redistribution of heat from core to periphery
- Decreased heat production
- NMBD mean cannot shiver
- anaesthesia means cannot get clothes or eat or curl up.
- Increased heat loss
- Phase III – 3+ hours – Plateau in core temperature
- core temperature plateaus
- peripheral temperature continues to decrease.
Mechanisms of ↓ in core body temperature:
- Resetting of interthreshold range (phase II)
- ↑ width of range to 4 °C → ↓ threshold to cold by 3 °C and ↑ threshold to heat by 1° C → ↓ thermoregulatory responses to Δ in body temperature
- Caused by a central effect → GA agent interferes with normal hypothalamic function to maintain a narrow threshold range
- GA agent-induced vasodilation → redistribution of heat from central to peripheral compartments (phase I)
- Muscle paralysis → loss of shivering response and muscle activity (phase II)
- LOC and paralysis → loss of behavioural responses (phase II)
- GA-induced ↓ BMR/heat production (phase II)
- Cold gases and IVF (phase II) → temp. generally ↓ 0.25-0.5 °C/L of IVF
Physiological effects of low body temperature
| CVS | – Tachycardia initially, then progressive bradycardia with ↑ cold – ↑ cardiac arrhythmias and myocardial ischaemia due to catecholamines released from stress response – ↑ SVR and MAP due to peripheral vasoconstriction – ↓ C.O. due to direct –ve inotropic effect of cold and ↑ afterload/SVR |
| Respiratory | – Left-shift in Hb O2 dissociation curve – V/Q mismatching 2° to inhibition of hypoxic pulmonary vasoconstriction – Bronchospasms – ↓ MV (and in severe cases apnoea) – ↑ solubility of gases (incl volatiles) |
| CNS | – Altered mental state (esp ↑ drowsiness, unconsciousness and delayed awakening from GA) – ↓ CBF |
| Haematological and immunological | – Coagulopathy (due to platelet dysfunction and loss of CF enzyme function) → ↑ transfusion requirements due to bleeding – ↓ WBC activity → ↑ incidence of infections |
| Hepatic / renal | – Impaired renal function (oliguria) – Impaired hepatic metabolic function (esp ↓ drug metabolism, such as muscle relaxants) |
| Metabolic and endocrine | – Impaired wound healing (due to catabolic state and wound vasoconstriction) – ↑ protein catabolic state – ↑ stress response (steroids and catecholamine released) – Shivering → 5x ↑ general MRO2 → causes hypoxaemia (risk of myocardial and cerebral ischaemia) – In absence of shivering → general ↓ MRO2 (by up to 50%) and ↓ BMR |
| Others | – ↑ morbidity and mortality rate (due to above reasons) – Hypothermia may be beneficial during cerebral or cardiac ischaemia as it ↓ metabolic O2 requirements (provided shivering response is blunted) – ↓ anaesthetic requirements (MAC-sparing) – ↓ triggering and severity of MH |
JF / Bianca 2016
Examiner Comments
2023B 10: 66% of candidates passed this question.
The first part of the question required candidates to outline the impact of sedatives on the interthreshold range with an explanation of what this is, how heat is lost, how heat generation is impaired and the mechanism by which these occur (ie. radiation/conduction/convection via vasodilation, with absence of vasoconstriction/heat generation strategies).
The second part of the question required a systems based approach with an outline of the perturbation as a result of the low body temperature. Temperature thresholds for certain physiological effects ie. loss of consciousness or arrhythmia was also expected for an overall thorough answer to this question.
11. Describe the mechanism of action, dose, pharmacokinetics and pharmacodynamics of aminophylline.
Examiner Comments
2023B 11: 21% of candidates passed this question.
This question required a detailed description of the many mechanisms of action of aminophylline. This included PDE inhibition and the down stream pathway and its adenosine antagonist and antiinflammatory actions. Important pharmacokinetic concepts included hepatic metabolism with saturable kinetics and thus a narrow therapeutic window/index requiring need for drug monitoring and the risk of metabolic interactions with accelerated or reduced metabolism from inducers or inhibitors of the main enzyme (CYP1A2). Detailed pharacodynamic consequences on the respiratory and cardiovascular systems were prioritised as well as highlighting the neurological, cardiovascular and musculoskeletal consequences of toxicity.
12. List the effects of stimulation of adrenoreceptors on target organs and tissues (60% marks). Describe the mechanism of action and pharmacokinetics of metoprolol (40% marks).
CICMWrecks Answer
Adrenoceptor Effects
| Organ / Tissue | α1 (A, B, D) | α2 (A, B, C) | β1 | β2 | |
|---|---|---|---|---|---|
| Nervous System | CNS | ↑ locomotor activity, neurotransmission | Sedation inhibition of sympathetic flow neurotransmission | ||
| eye | iris Sm.M contracts (pupils dilate) | relaxation of ciliary muscle for far vision | |||
| Sympathetic nerve terminal | inhibit Noradrenaline release | ↑ neurotransmitter release | |||
| Cholinergic neurons and cell bodies of noradrenergic neurons | Inhibition of firing | ||||
| CVS | Myocardium | ↑ force of contraction | SA node: ↑HR Atria: ↑contractility, ↑conduction velocity A-V node, HIS, Purkinge: ↑automaticity, cond vel Ventricles: ↑contractility, conduction, velocity, automaticity, and rade of idioventriculatr pacemakers | similar effect to β1 | |
| Vascular Smooth muscle | contraction | contraction | relax | ||
| Endothelium | release of vasodilator substance | ||||
| SKELETAL MUSCLE | ↑ contractility, glycogenolysis, K+ uptake | ||||
| Smooth Muscle | GIT | relaxation | relax | ||
| sphincters (GI, bladder) | contract | relax | |||
| uterus | contract | relax | |||
| Respiratory | bronchial glands | ↓ secretion | ↑ secretion | ||
| Airway | bronchodilation | ||||
| Haem | Platelets | Aggregation granule release | |||
| GIT | Salivary gland | secretion (K+, H2O) | Amylase secretion | ||
| Jejunum | Inhibition of secretion | ||||
| Liver | Glycogenolysis, gluconeogenesis, ureogenesis | Glycogenolysis, Gluconeogenesis | |||
| motility and tone | decreased stomach, intestine | decreased | decreased | decreased | |
| Pancreas | ↓ secretion | Inhibition of insulin release | Insulin secretion | ||
| splenic capsule | contracts | relaxation | |||
| Kidney | Glucogenesis (proximal tubule) ↑ renin secretion | Inhibition of renin release | ↑ Renin secretion | ||
| Metabolic / Endocrine | Adipose tissue | Glycogenolysis | Inhibition of lipolysis | Lipolysis | |
| Posterior pituitary | ADH secretion | ||||
| Others | Eye | lacrimation | ↓ intra ocular pressure | ||
| Melanocytes | Inhibition of MSH-induced granule dispersion | ||||
β3 effects:
- Fat
- lipolysis and thermogenesis
Metoprolol Pharmacology
Examiner Comments
2023B 12: 74% of candidates passed this question.
This question required a list of effects of the stimulation of adrenoreceptors, thus detailed description of downstream effects and exact mechanisms was not required. Using a systems based structure with a subdivision into each receptor (or vice versa) meant that important GIT, GUT, endocrine and metabolic effects were not omitted. Given the need for little depth, this part of the question required breadth particularly within cardiovascular effects. Venoconstriction, dromotropy and lusitropic effects should also be covered.
The second part of the question required a detailed description of the mechanism of action and pharmacokinetics only, thus dose, pharmaceutics and pharmacodynamic information was not required. Here it would be important to elaborate on the downstream effects of blocking the beta adrenergic receptors as compared with the information required in the first part of the question.
13. Outline the distribution, clearance and physiologic functions of magnesium in the body.
CICMWrecks Answer
Normal Magnesium:
1000mmol (12mmol/kg)
Normal Distribution of magnesium
- Bone (60%)
- ICF (40% esp organs/muscles)
- Mg2+ is mainly an IC cation
- Total IC [ ] 15 mmol/L cf. 0.5 mmol/L free/ionised inECF
- ECF (< 1%)
- Plasma [ ] 0.5-1 mmol/L
- 60% free/ionised
- 33% protein-bound (esp albumin)
- 7% complexed (citrate/PO4)
- Plasma [ ] 0.5-1 mmol/L
Normal Intake:
8-20 mmol/day (min 0.5 mmol/day)
Normal Losses
- Glomerular filtration of 100 mmol/day
- 95% reabsorbed in tubules (PCT 15%, 70% LoH, 10% distal nephron)
- 5% excreted → 2.5 to 8 mmol/day
- Minimal neurohormonal regulation of Mg2+
- TMAX of Mg transporter ~ plasma [Mg]
- → ↓reabsorption with ↑ Mg
Normal Functions:
- Co-factor in enzyme reactions (as a metallo-coenzyme) eg Na+/K+ ATPase activity
- Energy storage, utilisation, transfer (via role in formation/utilisation of ATP) eg BSL homeostasis
- Protein and nucleic acid synthesis (stabilises DNA and RNA structure)
- Ca2+/K+ metabolism
- ↓ membrane excitability (eg. muscles/nerves)
- ↓ NT release at cholinergic and adrenergic synapses
- Antagonises Ca2+ activity
Gladwin 2016
Examiner Comments
2023B 13: 36% of candidates passed this question.
This question was best answered under the headings distribution, clearance and physiologic functions. Distribution involved intracellular vs extracellular concentrations, the spread amongst organ systems and state of ionisation and protein binding. Clearance of magnesium required an accurate description of its renal filtration and sites and proportion of reabsorption and secretion along the nephron. The regulatory factors and factors that influence this clearance should also be outlined. This included; Mg plasma concentrations, other cations, ECF volume and PTH. Physiologic functions should cover its role as a cofactor of metabolism and enzyme systems with some examples, the role and mechanism in the musculoskeletal system as a calcium antagonist and inhibitory action in the nervous system including the action against Ach, nerves and NMDA activity.
14. Describe the physiological factors that influence cerebral blood flow?
CICMWrecks Answer: Cerebral Blood Flow
Cerebral Blood Flow
- CBF = CPP / CVR (Cerebral Perfusion Pressure / Cerebral Vascular Resistance)
- CBF 15% resting CO → ~750ml/min or 50ml/100g brain tissue/min
- Gray Matter: 75–80 mL/100 g/min – Significantly higher due to the high metabolic activity and dense synaptic connections
- White Matter: 20–30 mL/100 g/min
- Abnormal CBF
- CBF<50ml/100g/min → cellular acidosis
- CBF<40ml/100g/min → impaired protein synthesis
- CBF <30ml/100g/min → cellular oedema
- CBF <20ml/100g/min → failure of cell membrane ion pumps, loss of transmembrane electrochemical gradients
- CBF <10ml/100g/min → cell death
Determinants of CBF
- CPP
- Net pressure gradient driving blood flow through the cerebral circulation
- CPP = MAP – ICP
- MAP = CO x SVR; CO = HR x SV; SVR = MAP / CO
- ICP dependent on: brain, blood, CSF
- CVR
Regulated by 4 primary factors:
- Cerebral metabolism
- flow-metabolism coupling:
↑ metabolic demand → ↑ CBF + substrate delivery - Controlled by vasoactive metabolic mediators:
H+ ions, K, CO2, adenosine, glycolytic intermediates, NO
- flow-metabolism coupling:
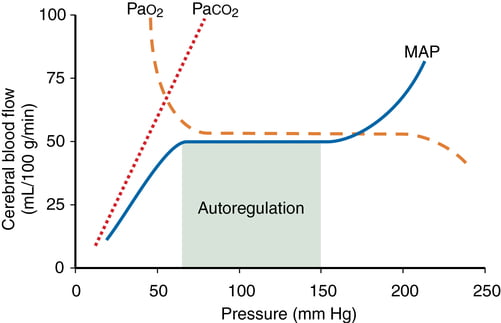
- CO2 and O2
- CO2
- At normotension: relationship between PaCO2 and CBF = almost linear
- ↑ PaCO2 → cerebral arteriolar vasodilation → ↓ CVR + ↑ CBF
- 2~4% increase for every 1mmHg increase in CO2
- ↓ PaCO2 → cerebral arteriolar vasoconstriction → ↑ CVR + ↓ CBF
- Initial stimulus = ↓ brain ECF pH
- Effects regulated by: NO, prostanoids, K channels, intracellular [Ca2+]
- PaO2
- little effect at normal PaO2
- PaO2 <60mmHg → cerebral arteriolar vasodilation → ↑ CBF
- Mechanism: hypoxia acts on →
- cerebral tissue to promote release of adenosine → cerebal vasodilation
- cerebrovascular smooth muscle → hyperpolarisation → ↓ Ca2+ uptake → vasodilation
- CO2
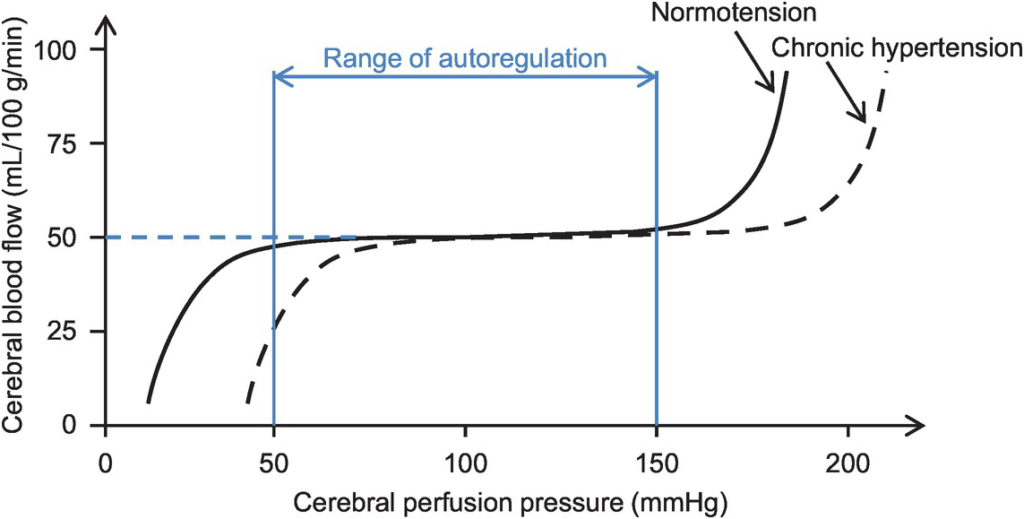
- Autoregulation
- Constant across CPP 50-150mmHg
- CPP >150mmHg: CBF µ CPP
- CPP <50mmHg: CBF <50ml/100g brain tissue/min → ischaemia
- Stimulus to autoregulation = CPP (not MAP)
- autoregulation curve R shifted in HTN; L in neonates
- Mechanism:
- Myogenic mechanism: arterioles vasoconstrict in response to ↑ wall tension + vasodilate in response to ↓ wall tension → ↓ or ↑ CVR
- May involve adenosine
- Can be impaired in SAH, tumour, stroke, head injury
- Constant across CPP 50-150mmHg
- Neurohumeral factors
- Relative lack of humoral + autonomic control on normal cerebrovascular tone
- Main action of SY nerves = vasoconstriction
- Other factors
- Blood viscosity: directly related to HCt; ↓ viscosity → ↑ CBF as per Hagen-Poiseuille law
- Temperature: ↓ CMRO2 by 7% for each ↓ 1°C in temp
- Drugs
- E.g. barbiturates ↓ cerebral metabolism
- Volatile agents → ↓ tension cerebral vascular smooth muscle → vasodilation + CBF
Kerr 2018
Examiner Comments
2023B 14: 56% of candidates passed this question.
Cerebral Blood Flow is result of CPP/CVR. The brain defends a relatively constant high blood flow via multiple auto-regulatory processes that influence cerebral vascular resistance. This question required an in detail description of the physiological factors that alter CBF. Autoregulation via the myogenic and metabolic mechanisms, the difference between grey and white matter due to metabolic variation, the role of pCO2 and O2, sympathetic nervous system and temperature was expected. A “describe” question requires not only the factors that influence CBF but how and why, so detail is required. Whilst the monroe kellie doctrine does describe changes in blood flow this is only important at extremes of intracranial pressure when these normal autoregulatory mechanisms are exceeded, as such it was not part of the answer to this question.
15. Describe the components and function of the complement system including the role, activation and control?
CICMWrecks Answer
Definition
- Proteolytic enzyme amplification cascade system; forms part of innate + acquired immune response
- Components circulate as inactive precursors
- Activation amplifies as a cascade; regulated by regulator proteins e.g. C1 esterase inhibitor
- Proteins produced by liver
- 9 complements: C1(q, r, s), C2, C3, C4, C5, C6, C7, C8, and C9
- Also B, D, H, I and properdin
Main functions
- cell lysis of bacteria (MAC)
- opsonisation → ↑phagocytosis
- release of mediators via mast cell degranulation
- local vasodilation
- neutrophil aggregation
- chemotaxis
Components (7 functional)
| Group | Factor | Function |
|---|---|---|
| Initiators | C1q, MBL | Initiators |
| Enzymatic mediators and convertase activator | C1r, C1s, MASP2, and Factor-B | proteolytic enzymes that activate other members |
| C3 convertase C5 convertase | enzymatic mediators | |
| Membrane binding components or opsonins | larger fragments such as in C3, C3b | enhances phagocytosis |
| Inflammatory mediators | Smaller fragments like in C3, C3a | Inflammatory mediators |
| Membrane attack protein | Membrane attack complex (MAC) comprises C5b, C6, C7, C8, C9 | protein of MAC inserts and creates a hole on cell memrane so cell lysis occurs |
| Complement receptor protein | CR1 | binds to complement protein and signal-specific cell function |
| Regulatory complement components | Factor I Protectin | proteins that protect the host cell against the action of complement |
Pathways
- Classical – activation by Ag-Ab complex
- system of 11 enzymes
- pathway activated by formation of Ag-ab complexes, CRP, aggregated Ig (IgM or certain IgG)
- activates C1 → reactions involving C4+C2 → formation of enzymatic complex C4b-C2a → cleaves C3a and C3b → common pathway
- Alternate – activation by lipopolysaccharides
- C3 undergoes slow, spontaneous conversion to hydrolysed C3
- Multiple steps leading to complex ith C5 convertase → activation of common pathway
- Nil ab required
- Lectin – activation by mannose on surface of parasites, candida, viruses etc
Mechanism
following activation → triggers final common pathway → C3 activation → C3b acts as C5 convertase → C5b binds C6-C9 → forms membrane attack complex → inserted into target cell membrane → leakage of cellular material → cell lysis + destruction
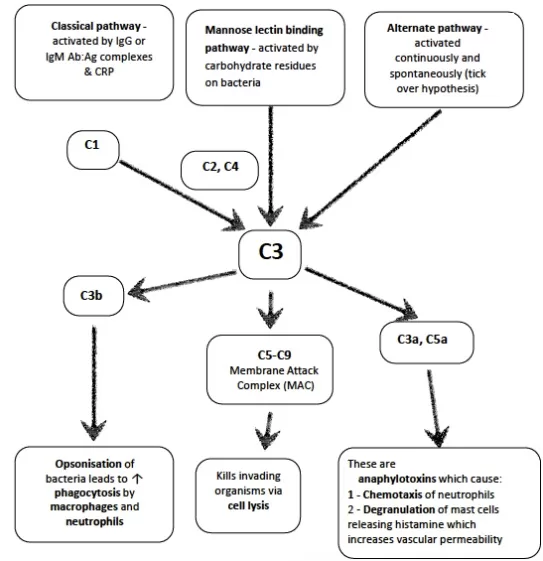
Regulation
- Half of all complement proteins serve regulatory function
- goal
- to prevent complement damage to normal host tissue (inappropriate / wrong target)
- fluid-phase activation (no target)
- deficiencies lead to excessive complement activation
- Regulatory proteins inhibit system by destabilizing activation complexes and mediating specific proteolysis of actication-derived fragments
- Complement pathways are regulated at the following critical steps
- Activation (initiation) – C1 inhibitor (C1inh) in classic and lectin pathways
- Amplification (convertase formation) – e.g. binding proteins to C3 convertases
- Membrane attack (lysis) – S-protein prevents insertion of Membrane attack complex (MAC) into membrane, CD 59 (protectin) bind C8 and C9 inhibiting final steps of Membrane attack complex
- Anaphylactoids – carboxypeptidases inactivate C3a,C4a,C5a
Sources: Kerr’s notes, biosciencenotes.com
Examiner Comments
2023B 15: 8% of candidates passed this question.
Based on the question stem answers should have been structured to address the components of the complement system as well as the role, activation and regulatory mechanisms. A description of the components should include the number and type of molecule highlighting the important combinations of complement to produce the membrane attack complex. A description regarding the role in the innate immune response against bacterial infections was then required with a detailed description regarding the many ways this is achieved including opsonisation, phagocytosis, chemotaxis, mast cell/basophil activiation, lysis of cells and clearance of immune complexes. Information about the 3 pathways of activation where expected; classic, alternate and lectin pathyway, with some detail regarding the downstream effect of each that would take into account for the amplication of the response. The cessation of complement response is largely due to the limitatations of the half lives of the particular complement glycol-proteins or presence of specific inactivators.
16. Outline the anatomy of the larynx.
CICMWrecks Answer
Cartilages
- Unpaired
- Thyroid cartilage – Level of C4~5
- Cricoid cartilage
- Epiglottis
- Paired
- Arytenoid
- Cuneiform
- Corniculate
Muscles
- Intrinsic
- Cricothyroid
- Originates in cricoid cartilage and inserts into thyroid
- Tenses vocal cords and elevates voice
- Thyroarytenoid
- Originates in thyroid cartilage and inserts into arytenoid cartilage
- Relaxes vocal cords and depresses voice
- Posterior cricoarytenoid
- Abducts the vocal cords
- Lateral cricoarytenoid
- Adducts the vocal cords
- Oblique and transverse arytenoids
- Adducts the vocal cords
- Cricothyroid
- Extrinsic
- Strap muscles
Innervation
Sensory
- Internal branch of superior laryngeal nerve
Motor
- Cricothyroid – External branch of the superior laryngeal nerve
- All other intrinsic muscles – Recurrent laryngeal nerve
Blood supply
- Superior laryngean artery (branch of external carotid)
- Inferior laryngeal artery (branch of thyrocervical trunk from subclavian artery)
Associations
- The thyroid glands lie inferolateral to the larynx (lateral to the cricoid, the isthmus is inferior to the cricoid)
- The oesophagus, anterior longitudinal ligament, cervical vertebrae lies posterior to the larynx
- The brachiocephalic trunk may arch superiorly close to the cricothyroid membrane in an anatomical variant
Sakurai 2016
Examiner Comments
2023B 16: 44% of candidates passed this question.
This question required candidates to address the following relevant to the anatomy of the larynx – it’s location and extent; relations; structure (paired and unpaired cartilages; major ligaments; intrinsic and extrinsic muscles); nerve supply (sensory and motor); and blood supply (and venous darinage). There were some marks allocated for other correct information relevant to the anatomy of the larynx (e.g. epithelium; differences in age; lymphatic drainage). This was a purely anatomy question so functions of the larynx was not required.
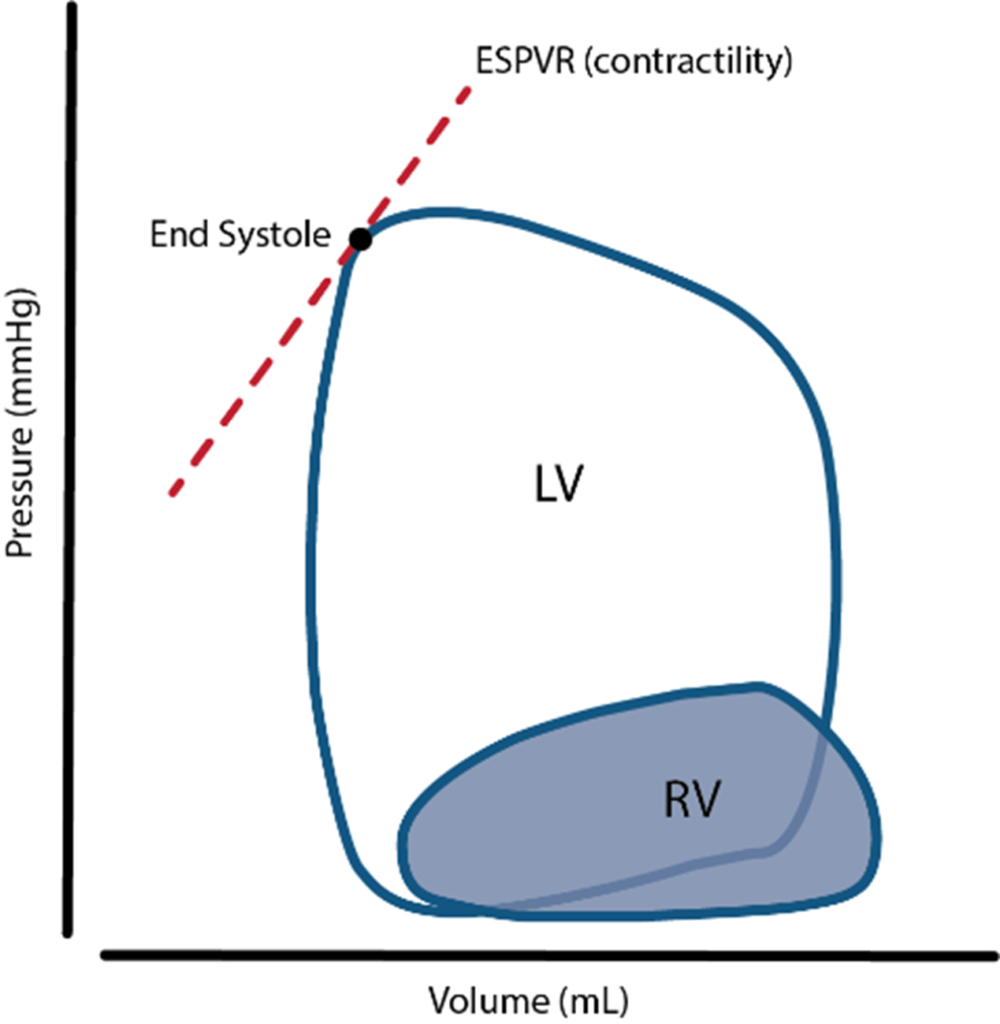
17. Describe the consequences for the left ventricle of a sudden and sustained increase in afterload.
CICMWrecks Answer
Afterload
- Load against which the muscle exerts its contractile force (Guyton)
- It is represented by the gradient of the line connecting the end-diastolic volume, to the end-systolic point
- pressure which the ventricle has to contract against (Power & Kam)
Determinants of Afterload
Modified Laplace Equation
- where
- T represents afterload
- P represents aortic pressure
- Therefore, afterload increases as aortic (or pulmonary arterial) pressure increases
- R represents ventricular radius
- Afterload increases as ventricular radius increases
- H represents ventricular thickness
- Afterload decreases as the thickness of the ventricular wall increases (hypertrophy secondary to chronic hypertension and cardiac remodelling)
Modified Hagen-Poiseuille Equation
- Afterload is affected by resistance to cardiac output
- Afterload increased by reduced radius of systemic vasculature
- Afterload increased by increasing viscosity of blood
Resistance in parallel
- Afterload is affected by addition, or loss of large capillary networks in parallel
- Systemic vascular resistance is increased significantly by loss of placenta, with parallel vascular networks
- Pulmonary vascular resistance is decreased significantly by inflation of lung, causing creation of vast capillary network in parallel
Cardiovascular Effects of a sudden increase in afterload
Effects of sudden increase in afterload can be demonstrated using a Left ventricular PV loop:
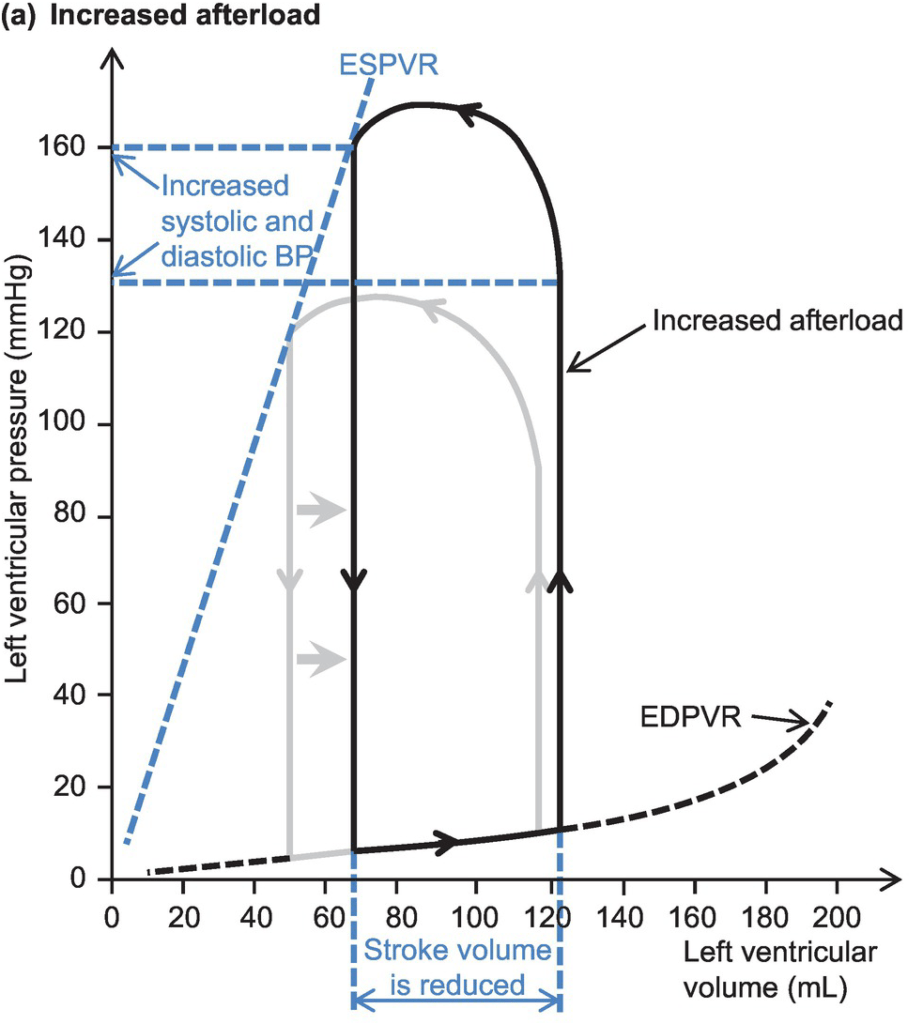
- End-Systolic Volume is increased, causing a reduction in stroke volume
- Initially, Left atrial pressure decreases
- Increased End systolic volume leads to secondary increase in end diastolic volume, hence increasing ventricular filling
- This secondary increase in preload enables the ventricle to contract with greater force (Frank-Starling mechanism) which partially offsets the reduction in stroke volume
- In patients with impaired left ventricular function, the decreased in stroke volume cannot be compensated
- ventricular end-systolic pressure: increases
- ventricular end-diastolic pressure: increases
- cardiac output (=HRxSV) – remains the same
- Initial drop in SV is compensated by increased pre-load
- In a failing heart, the drop in SV causes subsequent stimulation of baroceptors, which in turn causes increased heart rate and can potentially return cardiac output to normal
- Increase in afterload → Anrep → Small ↑Contractility to compensate
- Mechanism: ↑AL → sustained ↑stretch (prolonged isovolumetric contration) → ↑ Ca induced Ca release → ↑ contractility
- Purpose: ↑AL → ↓SV and ↑ ESV
- myocardial oxygen demand and myocardial work: Increased Afterload will increase the pressure during contraction, hence increasing MVO2 for internal work. This might be partially offset by the reduction in external work (due to decreased stroke volume) depending on the cause of the increased afterload
- coronary blood flow is autoregulated to remain normal
JC 2019
Examiner Comments
2023B 17: 7% of candidates passed this question.
This question expected a detailed description of the effect of afterload on the left ventricle. This should cover the acute effect of increased afterload on left ventricular end systolic (and diastolic) pressure and volume, contractility, work and oxygen consumption, coronary perfusion pressure and baroreceptor responses. “Sustained” implied more longer term left ventricular exposure which would include the ventricular cellular response, concentric hypertrophy and the subsequent effects on diastolic and elevations of left atrial pressure. Definitions of afterload, cardiac output equations, vascular function curves and LV/PV loops are not required if the above concepts are described in adequate detail. The use of a diagram can assist in explaining concepts however should be linked back to the question in order to demonstrate the candidates understanding of the question being asked.
18. Compare and contrast the pharmacology of Hartmann’s solution and 0.9% saline?
Examiner Comments
2023B 18: 17% of candidates passed this question.
This question asked for a comprehensive description of the components and chemical properties of each solution (including pH and calculated and measured osmolarity). A mechanistic description of the different acid base effects was expected. Marks were also allocated for the advantages and disadvantages of each fluid (for example the calcium in Hartmann’s risks causing precipitation when mixed with certain drugs and blood products). Lastly, it was expected that answers would provide situations where one fluid might be preferred over the other (for example saline to treat dehydration and metabolic alkalosis secondary to gastric losses – as in a pyloric obstruction). Descriptions of the physiological handling of each fluid after bolus or infusion was not required.
19. Describe the mechanism of action, dose, pharmacokinetics and pharmacodynamics of ceftriaxone.
Examiner Comments
2023B 19: 39% of candidates passed this question.
A structured answer under the headings of mechanism of action, dose, pharmacokinetics and pharmacodynamics worked most effectively for this question. It was expected that candidates would link the mechanism of action of Ceftriaxone (binds to PBP and inhibits final step in peptidoglycan) to its spectrum of activity. Dosing would also include indications for higher dosing, and consideration of the fact that ceftriaxone is available as an IM administration. Details on hypersensitivity (fever, nephritis, haemolytic anaemia) and consideration of C.diff infection was a main part of its pharmacodynamics. For pharmacokinetics, a structural approach is recommended, important points included excretion through both kidneys and bile and absence of liver metabolism.
20. Outline the anatomy (60% marks) and synaptic physiology (40% marks) of the vagus nerve.
CICMWrecks Answer
Vagus Nerve Anatomy
| Nuclei | Dorsal motor nucleus – sends parasympathetic fibers to the GI tract, lungs Nucleus ambiguus – sends efferent motor and parasympathetic fibers to the heart Solitary nucleus – receives special gustatory afferent from the tongue and visceral afferent fibers from organs Spinal trigeminal nucleus – receives general sensory afferent fibers from outer ear, dura of posterior cranial fossa, mucosa of larynx |
| Course | Exits brain from medulla oblangata – series of rootlets in retro-olivary groove → Travels laterally and exits skull via jugular foramen → Sensory ganglia consist of superior and inferior ganglionic swelling → Joined by cranial root of accessory nerve (CN XI) → Passes down the neck in carotid sheath (between Carotid artery and Internal Jugular Vein) → Enters thorax at base of neck → Left: travels ant to aortic arch, behind Lt main bronchus and into oesophagus → Right: travels behind oesophagus and Rt main bronchus →Both enter abdomen through oesophageal hiatus in diaphragm |
| Branches | In the jugular fossa: meningeal, auricular branches In the neck: pharyngeal, superior laryngeal, recurrent laryngeal nerves; superior cardiac branches In the thorax: inferior cardiac nerve, anterior bronchial branches, posterior bronchial branches, esophageal branches In the abdomen: gastric, celiac and hepatic branches |
| Field of innervation | General sensory afferent fibers – sensory information from larynx, auricle, external acoustic meatus, dura mater of the posterior cranial fossa General visceral afferent – information from the aortic body, esophagus, lungs, bronchi, heart, intestines Special afferent – information about taste General visceral efferent – parasympathetic division that simulates smooth muscle and glands of the pharynx, larynx, thoracic and abdominal organs. – Heart via cardiac plexus: SA node: Rt vagus. AV node, ventricles: Lt vagus. – Lungs via pulmonary plexus – Gut (from stomach to proximal to splenic flexure) via gastric plexus Branchial efferent – innervate muscles of mastication and tensor vali palatini |
| Communications | Trunk and ganglia with CN IX,XI,XII, superior sympathetic ganglion and 1st and 2nd cervical nerves Auricular branch with CN VII Pharyngeal plexus with IX and superior cervical ganglion Cardiac, pulmonary, oesophageal and gastric branches with sympathetic outflow to viscera (Superficial and deep Cardiac plexuses receives branches from cardiac nerves of vagus, recurrent laryngeal nerves and cervical ganglia of sympathetic trunk.) |
Physiology
- 75% of all parasympathetic fibres are in vagus nerve
- Pre-ganglionic fibre
- Long fibre
- Release Acetylcholine to stimulate post-ganglionic neuron at nicotinic ACh receptor
- activation is tonic
- Ganglia located near or within effector organs
- Post-ganglionic fibre
- Short
- Release Acetylcholine to stimulate muscarinic ACh receptor
Effector
- Acetylcholine (CCOOCCNH3)
- Formed for Choline and Acetyl CoA
- Receptors
- Muscarinic
- G Protein Coupled
- Nicotinic
- Ligand gated ion channels
- Muscarinic
- Metabolism
- Acetylcholinesterase
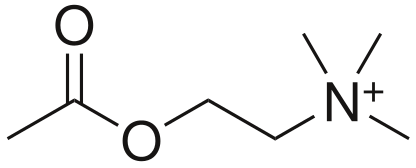
Muscarinic ACh receptor
- Known as muscarinic because muscarine also agonises this receptor
- Metabotropic, G-protein coupled
- Phosphorylate various second messengers
- Mediate slow metabolic response via second messenger cascades
- 5 subtypes: M1-M5
- M1,M3,M5 – excitatory (Gq: IP3/DAG → ↑Ca++)
- M2, M4 – Inhibitory (Gi: ↓cAMP)
- Receptor at parasympathetic postganglionic terminals
- M1: ANS
- M2: Cardiac
- M3: Lungs
| Ganglion / Target organ | Receptor | Signal | Effect |
|---|---|---|---|
| Respiratory: Pulmonary plexus | M3 (Gq) | IP3/DAG ↑Ca++ | Bronchoconstriction increased mucous production |
| Cardiovascular: – SA node – AV node | M2 (Gi) | ↓cAMP ↑ K+(G) | ↓ inotropy (Atria > Vent) ↓↓↓ Chronotropy ↓ lusitropy (Atria > Vent) ↓↓↓ dromotropy |
| stomach to proximal two-thirds of the transverse colon | M1 (Gq) | IP3/DAG ↑ Ca++ | Increased gastric motility, secretions Relaxation of pyloric sphincter |
Examiner Comments
2023B 20: 25% of candidates passed this question.
The vagus nerve anatomy was best broken down into a description of the fibers it carries (visceral, parasympathetic and somatic sensory fibres) and then origin and course from the parasympathetic, sensory and motor nuclei in the medulla as the tenth cranial nerve to its branches; the pharyngeal, cardiac, pulmonary and laryngeal branches. Pre and post-ganglionic physiology involved a detailed description of the Muscarinic Ach receptor and events. This would also include the 5 subtypes of the muscarinic receptor, with the locations and downstream effects of the M1-M3 locations being the most important to note.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Regional variability in V&Q in lung Oxygen delivery systems and measurement Work of breathing and determinants |
| G. CVS | Sympathomimetic pharmacology, Activity and metabolism of metaraminol vs adrenaline. LV P-V loop – shape, values Relationship between baroreceptor output and MAP Cardiovascular effects of PEEP |
| H. Renal | Renal handling of bicarbonate Renal handling of potassium Renal blood flow, anatomy of renal blood supply |
| I. Body Fluids and Electrolytes | |
| J. Acid Base | pH, importance, measurement |
| K. Neuro | Pupillary light reflex, anatomical pathways CSF flow from production to absorption Events that lead to perception of pain following laceration to the hand |
| L. Musculoskeletal | Events that lead to skeletal muscle contraction in reponse to motor neurone AP Skeletal myocyte: Extra and intracellular conc of Na, K, Cl |
| M. ANS | |
| N. Liver | |
| O. GIT | |
| P. Nutrition and Metabolism | Daily nutritional requirement for healthy aduly, nutrition, endocrine pharmacology |
| Q. Haematology | Strucure of platelet, haemostasis Blood type, grouping |
| R. Thermoregulation | Heat and Temperature, Temp Measurement |
| S. Immunology | |
| T. Microbiology | MoA of antibiotics with examples |
| U. Endocrine | |
| V. Obstetrics | Factors that determine rate of gas transfer across placenta |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments