1. Classify commonly used inotropic agents. (40% of marks) Outline four different mechanisms of action for inotropic agents. (60% of marks)
CICMWrecks Answer
- Inotrope: Agent that alters the force of contraction of a muscle
- Inotropes can be classified in an number of ways:
- Negative or positive (the examiners did not want a discussion of negative inotropes on previous reports)
- Naturally occurring: Eg Adrenalin, Noradrenalin, Dopamine
vs Synthetic: Dobutamine, dopexamine, isoprenalin, salbutamol
Classification by Mechanism of action is most effective
Postitive Inotropes
Class 1: Increase intracellular Calcium by a variety of mechanisms → ↑force of contraction
- Direct Adrenoceptor agonists (NA, Adren):
- ↑ intracellular cAMP and ↑ Ca by Gs protein coupled mechanism
- Indirect adrenoceptor agonists (Ephedrine):
- displacing NA from vesicles into cytoplasm
- ↑’s carrier-mediated diffusion into synaptic cleft
- ↑NA release at nerve terminal → ↑’d stimulation
- Phosphodiesterase inhibitors (Theophyline, Milrinone):
- inhibit PDE → ↓breakdown of cAMP (cGMP) → effective ↑cAMP
- cAMP effects as per direct adrenoceptor agonists
- Glucagon
- Bypasses adrenergic receptor to stimulate ↑cAMP
- Na/K atpase inhibitors (digoxin):
- Inhibit Na+/K+-ATPase → ↑[Na+]i → Impair Na+/Ca2+ exchange pump → ↑[Ca2+]i
Class 2: Increase sensitivity of actin/myosin complex to calcium by action on troponin C
- Levosimendin
- ↑Ca interaction with troponin C → enhance contractility without ↑[Ca]i
- Activate KATP channels on mitochondrial membrane → protect muscle from ischaemia leading to ischaemic preconditioning
Class 3: Act via metabolic or endocrine pathways (T3, Ca, Mg)
Negative Inotropes
- Beta-blockers
- Usually β1 blocking action
- Inhibits the action of natural stimulation of Class 1 above
- Calcium-channel blockers are used for treating high blood pressure, chest pain, and irregular heart rhythm.
- ↓ intracellular Ca via action on L-type calcium channels
- nifedipine, amlodipine
- Centrally acting sympatholytics are used for treating high blood pressure.
- Act centrally on α2 receptors to decrease sympathetic tone.
- Clonidine
Gladwin 2016
Examiner Comments
2014B 01: 65% of candidates passed this question.
This question was generally well answered. The poorer answers suffered for want of a useful classification system that enabled them to separate the various drug classes
2. Describe the pharmacology of Phenytoin (75% of marks) and Levetiracetam (25% of marks).
Examiner Comments
2014B 02: 35% of candidates passed this question.
The knowledge of phenytoin was often superficial and many answers were too brief and didn’t adequately cover the required material. The knowledge around levetiracetam seemed very limited with many candidates guessing (incorrectly) what the pharmacokinetics might be. Most answers demonstrated a structured approach to this type of question.
Better answers were able to distil major issues such as the narrow therapeutic window for phenytoin or the potential clinical impact of differing ordered kinetics or altered metabolism. Candidates are reminded to read each question carefully; levetiracetam should not be confused with levosimendan.
3. Describe the physiological consequences of Positive End-Expiratory Pressure (PEEP).
CICMWrecks Answer
Positive Pressure Ventilation
- PEEP = Positive End Expiratory Pressure.
- Equivalent to a constant pressure applied throughout the respiratory cycle.
- Intrinsic PEEP = unintentional or un-measured end-expiratory hyperinflation
- Physiological effects of Positive Pressure Ventilation mostly related to increased mean airway pressure
Cardiovascular Effects
- Causes constant ↑ intrathoracic pressure (ITP) throughout respiratory cycle
- Left Heart
- On Preload
- Initially: → ↑ LV PL → ↑ CO
- Secondarily: → ↓RV output → ↓ LV PL → ↓ CO
- On Afterload
- → ↓myocardial transmural pressure → ↓LV AL → ↑CO
- PEEP > Ao diastolic pressure
- → Collapse of intrathoracic aorta → Starling resistor mechanism → ↑LV AL → ↓ CO
- PEEP < Ao diastolic pressure
- ↑ pressure gradient for flow to systemic circulation
- ↓ AL → ↓ myocardial work
- On Compliance
- diastolic buldging of septum → ↓ LV compliance → ↓LV CO
- On Preload
- Right Heart
- → ↑ Pulmonary vascular resistance → ↑ RV AL → ↓ RV output
- → ↓ Venous return → ↓ RV PL → ↓ RV output
- In the failing LV → ↑CO
- ↓ LV afterload
- ↑ pressure gradient thorax to abdomen
- ↓ transmural pressure → ↓ LV wall tension = ↓afterload
- ↓ LV preload (ie more favourable position on compliance curve)
- ↓ LV afterload
Respiratory Effects
Beneficial Effects:
- ↓ atelectasis and gas trapping
- ↑’s FRC > “closing capacity”
- Shifts position on P-V curve right, above the “closing point”
- ↑ lung compliance → shifts back to steep/compliant part of P-V curve
- ↓ intrapulmonary shunt thus improved V/Q match and ↑ PaO2
- ↓ atelectasis and gas trapping
- ↓ extravascular lung water → ↓interstitial/alveolar oedema
- ↓ AWR
- ↑ lung volume → ↑ radial traction by parenchyma → ↑ airway calibre
- ↓ work of breathing
- ↑ lung compliance = ↓ elastic work
- ↓ AWR = ↓resistance work
Negative Effects:
- ↑ V/Q mismatch
- ↑ west zone 1
- ↓ lung compliance
- shift to flat part of P-V curve
- ↑ Work of breathing (due to compliance change)
- ↑ PVR and RV afterload
- extrinsic compression of pulmonary vessels
- Barotrauma
End-Organ Effects
- Renal:
- ↓CO & ↑renal venous pressure
- → Reduced renal blood flow → Reduced GFR → Reduced urine output
- → Reduced atrial stretch and ANP release → Increased ADH → Fluid retention → Oedema
- ↓CO & ↑renal venous pressure
- Hepatic:
- Reduced hepatic blood flow due to:
- Increased CVP and decreased CO lowering the pressure gradient for hepatic flow
- May result in circulation only intermittently throughout the cardiac cycle
- Hepatocyte dysfunction
- Haematological:
- Neutrophil sequestration in the compressed pulmonary vasculature
- CNS:
- ↓VR ⇒ ↑CVP ⇒ ↑ICP
Gladwin / Mooney / JC 2020
Examiner Comments
2014B 03: 27% of candidates passed this question.
Most answers were quite brief and superficial. They simply did not cover enough of the required knowledge base to gain a pass mark. A definition of PEEP is a useful way to start this answer and this was missing in more than half the answers. Deficiencies in knowledge included even the primary respiratory and cardiovascular effects of PEEP. Many candidates incorrectly concluded that PEEP would increase afterload and decrease pulmonary vascular resistance. Some candidates provided
description of the cardiovascular effects of Valsalva, which was not part of the question. It was expected candidates would also mention physiological effects on other organ systems such as potential cerebral and renal effects.
This topic (Level 1) requires a detailed knowledge and candidates should read widely to gain the depth of understanding required. The core material is covered in texts such as Nunn’s’ Applied Respiratory Physiology and additional applied information can be found in a variety of texts such as Textbook of Critical Care by Fink et al, Irwin and Rippe’s Intensive Care Medicine or Miller’s Anaesthesia.
4. Outline the anatomy and physiology of the parasympathetic nervous system.
CICMWrecks Answer
Parasympathetic Nervous System
- The parasympathetic nervous system is one of the two main divisions of the autonomic nervous system (ANS).
- Its general function is to control homeostasis and the body’s rest-and-digest response.
- Control the body’s response while at rest. Acts as local brake
- Activates response of Rest and digest
Parasympathetic (Cranio-sacral) Efferents
- Arises from neurons in cranial (III, VII, IX, X) or sacral segments of spinal cord (S2~S4)
- Pre-ganglionic neuron
- Long fibre
- Ganglia located near or within effector organs
- Post-ganglionic neuron
- Short
- Release Acetylcholine
Cranial:
| Ganglion / Target Organ | Effect | |
|---|---|---|
| CN III | Ciliary ganglion from occulomotor nucleus | • Pupil constriction, lacrimation |
| CN VII | Chorda tympani Submaxillary ganglion | secretion of saliva |
| CN IX | Parotid gland | secretion of saliva |
| CN X | Respiratory: Pulmonary plexus | Bronchoconstriction increased mucous production |
| Cardiovascular: – SA node – AV node | ↓ inotropy (Atria > Vent) ↓↓↓ Chronotropy ↓ lusitropy (Atria > Vent) ↓↓↓ dromotropy | |
| stomach to proximal two-thirds of the transverse colon | Increased gastric motility, secretions Relaxation of pyloric sphincter |
Sacral:
| Ganglion / Target Organ | Effect | |
|---|---|---|
| 2nd, 3rd, 4th sacral segments | Hypogastric plexus – Descending colon – Rectum – Bladder – Uterus | Contracts muscular wall of rectum Relaxes internal sphincter of anus Contracts detrusor muscle |
Effector
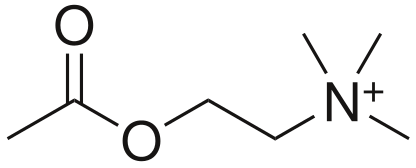
- Acetylcholine (CCOOCCNH3)
- Formed for Choline and Acetyl CoA
- Receptors
- Muscarinic
- G Protein Coupled
- Nicotinic
- Ligand gated ion channels
- Muscarinic
- Metabolism
- Acetylcholinesterase
Mooney / JC 2019
Examiner Comments
2014B 04: 0% of candidates passed this question.
Generally there was a lack of detailed knowledge, incorrect facts and at times confusion between the sympathetic and parasympathetic nervous system functions. A lack of anatomical detail was common (the origin of preganglionic cell bodies was not described clearly, and parasympathetic ganglia were not often named and located). It was expected an answer would mention the central role of Acetylcholine as a neurotransmitter at preganglionic and post ganglionic neurons in the parasympathetic system. Target organs were identified correctly but the exact action was not specified e.g. pupillary constriction vs. dilatation, GI sphincter/bladder – contraction vs.
relaxation. Detail concerning receptor physiology was not required. This is a question covering a core topic that no candidate passed. An overview of the arrangement and function of the autonomic nervous system is provided in several core physiology texts, including Ganong and Guyton.
5. Describe the effects of V/Q inequality on the partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2) in arterial blood.
CICMWrecks Answer
Normal V/Q variation
- Both V and Q ↓ as you move from base to apex.
- ↓ total number of alveoli → ↓ diffusive area → ↓ V
- ↑ compression of intra-alveolar vessels → ↑ west zone 1 → ↓ Q
- V ↓’s slower than Q
- The intersection of V/Q is at rib 3, here V/Q = 1
- V/Q = 3 at the apex
- V/Q = 0.67 at bases
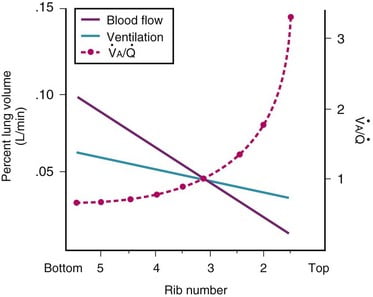
Normal Variation in V/Q causes:
- Shunt
- PACO2 and PAO2 = mixed venous blood
- PACO2 = 46mmHg
- PAO2 = 40mmHg
- Matched ventilation and perfusion
- PACO2 and PAO2 = normal blood gas for healthy patient
- PACO2 = 40mmHg
- PAO2 = 100mmHg
- Dead space
- Alveolar pressures equal that of inspired air
- PACO2 = 0mmHg
- PAO2 = 150mmHg
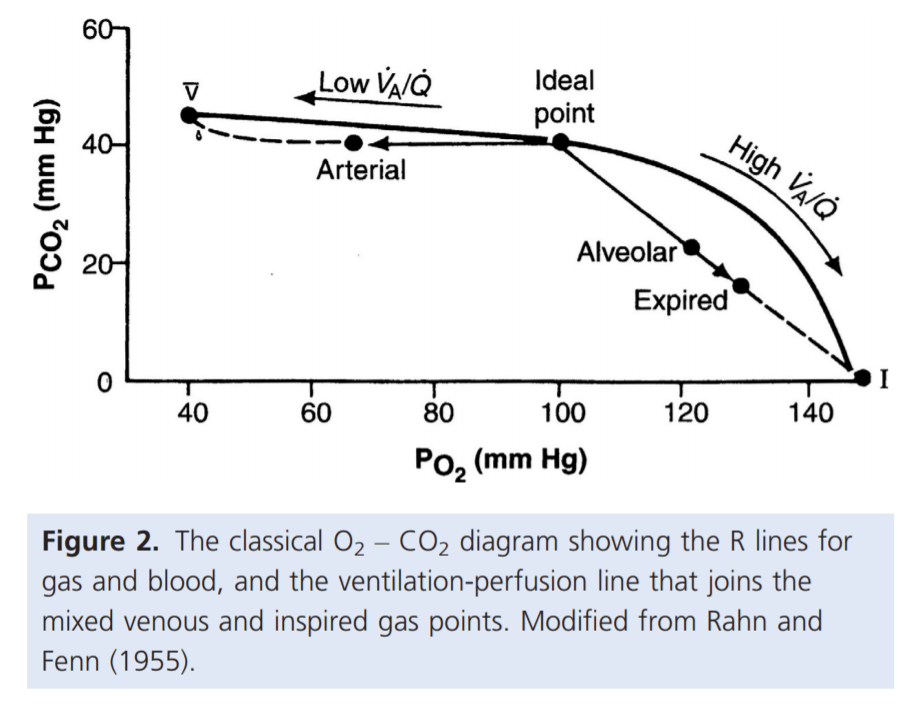
For PaO₂:
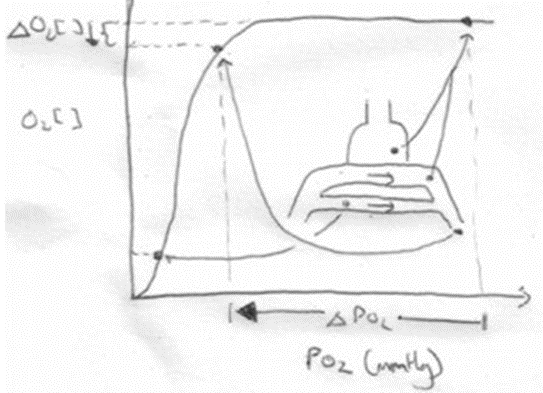
- Increased V/Q inequality causes:
- Mild decrease in PaO2
- At Apex
- decrease in dissolved O2
- Minimal decrease in Hb saturation due to flat part of OHDC
- Thus overall decrease in CaO2
- Base
- PAO2 = PvO2 = 40
- Addition of a small quantity of poorly oxygenated shunted blood flow (PO2 < PcO2) added to a large volume well-oxygenated PECB → small fall in CaO2 BUT a LARGER fall in PaO2
- Because carrying capacity of blood to apex is decreased there is a decrease in PaO2
For PaCO₂:
Remembering that the relationship for the Alveolar gasses:
- Increased V/Q inequality causes:
- Mild increase in PaCO2 which is rapidly compensated for due to Chemoreceptor reflexes
- Apex to base
- ↓PACO2 towards the apices of the lungs
- ↓gas transfer → ↑PaCO2
- Sensed by chemoreceptors (sensitive to ±3mmHg)
- ↑RR and Tv as per the CO2 dissociation curve
- Normal or slightly lower (after compensation) CO2
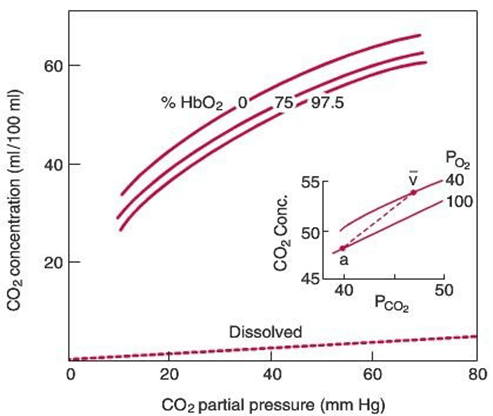
Gladwin 2016
Examiner Comments
2014B 05: 8% of candidates passed this question.
Very few candidates demonstrated understanding of this core topic. Candidates did not accurately define V/Q inequality and the physiological factors causing this phenomenon. V/Q scatter as well as true shunt (V/Q=0) and dead space (V/Q=∞) needed to be considered. The inability of high V/Q areas to compensate for low V/Q zones owing to the relatively small contribution of blood flow from these high V/Q units was not discussed. The differential effect of FiO2 on true shunt versus V/Q scatter was seldom explained. The shape of the oxy-Hb dissociation curve and CO2-dissociation curve were sometimes mentioned but their effect on arterial gas tensions not well explained.
Often graphs were reproduced inaccurately and contradictory statements made, leaving the impression that candidates did not understand the basic concepts. It is core knowledge for Intensive Care Specialists managing respiratory failure. A sophisticated knowledge based on the chapter in Nunn is a minimum standard expected for this topic.
6. List the mechanisms involved in heat production and loss by the body. (80% of marks) Define thermoneutral zone and inter-threshold range. (20% of marks)
CICMWrecks Answer
Definitions
- Thermoneutral zone:
- The range of environmental temperatures in which the metabolic heat production (and oxygen consumption) is minimal and steady and where core temperature is maintained by vasomotor activity alone.
- 25-30 C for a naked, upright man in still air
- Inter-threshold range:
- The range of core temprature over which no autonomic thermoregulatory responses occur
- Normally 0.2 -0.4 °C in a non-anaesthetized state
Mechanisms of Heat production
Total body heat production = Metabolic rate – Total external work
- Metabolism of food to form ATP
- <100% efficient
- Transfer of ATP to functional systems of cells
- <100% efficient
- Basal metabolism of cells
- Muscle contraction
- Overcoming resistance in muscles and other tissues -> friction
- Useful in shivering
- Overcoming resistance in muscles and other tissues -> friction
- Pumping blood
- Overcoming shear forces -> friction
- Breaking chemical bonds
- Flow of ions across membranes
Mechanisms of Heat loss
- Heat is lost by conduction, convection and radiation and evaporation
- The movement of warm blood from the body to skin allows heat loss to the environment
- Conduction:
- Heat transfer occurs due to direct contact to another solid body
- Comprises 3% of total heat loss
- Convection:
- Heat is lost to air via conduction
- This air then moves away, restoring the temperature gradient
- Comprises 15% of total heat loss
- Radiation:
- Heat is emitted from the skin as infrared radiation
- Comprises 60% of total heat loss
- Evaporation:
- There is a distribution of kinetic energies in liquids
- Warmer particles transition to the gas phase, lowering the average temperature of the skin
- Sweat, comprises 22% of total heat loss
Importance in sedated and intubated patient
Radiation:
- Heat loss increased
- if the patient is uncovered and surrounded by cold objects.
- by vasodilatation due to sedative drugs or cholinergics.
- by cool ambient temperature
- in Extracorporeal circuits
- Heat loss Decreased
- by vascoconstriction due to adrenergics
Conduction:
- usually a less important route
- Heat loss increased by use of cold irrigating solutions, and in extracorporeal circuits
Convection:
- Heat loss increased
- if the patient is uncovered
- by cool ambient temperature
- in Extracorporeal circuits
- Heat loss decreased
- Use of Neuromuscular blocking drugs (NMBDs can inhibit shivering, hence decreasing possible convective heat loss via bodily movement)
Evaporation:
- increased if a body cavity is opened, especially if ambient humidity is low.
- Heat loss via evaporation in the trachea and airways may be considerable if inspired gases are not humidified.
- Drugs affecting vascular tone can also alter heat loss by evaporation
Other:
- impaired temperature regulation
- peripheral (e.g. vasodilatation, shivering and impaired piloerection)
- central (central effects of drugs)
- Impairment of neurological or cardiovascular function, or deficiencies of substrate for thermogenesis → alter body’s ability to maintain thermal homeostasis
Mooney / JC 2019
Examiner Comments
2014B 06: 23% of candidates passed this question.
Many candidates did not read the question carefully and misinterpreted what was being asked. Candidates often digressed into a discussion of thermoregulation. Several candidates wrote about body’s response to cold and heat rather than mechanisms of heat production and loss as was asked.
There was confusion between mechanisms of endogenous heat production and measures to conserve heat. “Behaviour” only attracted marks in relation to voluntary muscle activity for heat production. Changing clothes or seeking a warm environment does not increase heat output by the body. Behaviour can reduce heat loss. Many candidates did not specify ambient or core body temperature.
The definitions of Thermoneutral Zone and Interthreshold Range were not clear. Knowledge generally lacked detail and this was most evident when precise definitions were asked.
7. Outline the mechanisms that potentiate the action of non-depolarising muscle relaxants and give examples.
CICMWrecks Answer
Non depolarising neuromuscular blockers
- act by non-competitive binding to the nicotinic ACh receptor on the motor end plate
- this prevents the action of ACh at the motor end plate and stops muscle excitation
- aminosteroids – rocuronium, pancuronium, vecuronium
- isoquinolones – atracurium, cisatracurium
- the pharmacodynamic response is dependent on the number of receptors occupied
- dose dependent according to the laws of mass action
- >70% to elicit a response
The factors which alter the response (number of receptors occupied) may be separated into
- pharmacodynamic factors
- pharmacokinetic factors
- pharmaceutical and drug interaction factors
Pharmacodynamic factors
- Decreased pH increases the affinity of non depolarising NMB
- Electrolyte disturbances
- Ca disturbances affect the release of ACh from the presynaptic terminal (triggered by Ca)
- hypocalcaemia potentiates blockage
- magnesium
- hypomagnesemia
- potassium
- hyperkalaemia leads to increased drug effect
- Ca disturbances affect the release of ACh from the presynaptic terminal (triggered by Ca)
Pharmacokinetic factors (affect the amount of drug reaching the receptor)
- Absorption
- dose given (higher dose – more receptors occupied)
- Distribution
- lipid soluble drugs such as vecuronium may accumulate in lipid rich tissue (esp infusion)
- Patients with decreased lipid content may lead to increased effect site conc
- Metabolism
- hepatic failure may lead to decreased metabolism of a drug – pancuronium
- deranged pH changes the metabolism of the isoquinolones which use Hoffmann elim
- Excretion
- renally excreted drugs, especially minimally metabolised vecuronium, rocuronium
Pharmaceutical/ drug interactions
- storage of the drug (isoquinolones must be stored at 4 degrees to maintain potency)
- aminoglycosides – decreased prejunctional ACh release
- volatile anaesthetics – mechanism not well understood
- frusemide – potassium effect, may interfere with cAMP and ACh release
JC 2019
Examiner Comments
2014B 07: 15% of candidates passed this question.
A structured approach would work well for this question but was often lacking. Many answers were superficial providing short lists of factors affecting NDMRs without reference to mechanism. Candidates failed to differentiate factors which potentiate NDMRs from those which inhibit the action of the drugs. Some confusion also existed confusing speed of onset kinetics with potentiation kinetics. Candidates who structured their answer into pharmacokinetic and pharmacodynamic factors generally scored better marks. Candidates who used pre-post synaptic type structure tended to omit kinetics completely from their answers. Incorrect facts were common.
8. Outline how the following tests assess coagulation: a) Prothrombin Time (PT); b) Activated Partial Thromboplastin Time (APTT); c) Activated Clotting Time (ACT); d) Thromboelastogram (TEG or ROTEM)
CICMWrecks Answer
Prothrombin Time
Activated partial prothrombin time
What is tested
Extrinsic pathway
Final pathway
Intrinsic pathway
Final pathway
Principles
- Citrate added to chelate calcium
- Sample centrifuged to give plasma
- Calcium returned at time of testing
- Tissue factor added
- Binds to VIIa, activating X
- Time until coagulation measured
- Citrate added to chelate calcium, calcium returned at time of testing
- Sample centrifuged to give plasma
- Calcium returned at time of testing
- Kaolin added
- Activates factor XII
- Cephalin added
- Provides phospholipid surface for binding of tenase and prothrombinase complexes
- Time until coagulation measured
Normal Values
11-13 seconds
30-40 seconds
Causes of Prolonged Time
- Reduction in functional VII
- Warfarin
- Liver disease
- Factor X deficiency
- DIC
Heparin
Haemophilia
DIC
Liver disease
Uses
Warfarin monitoring
Screening test for coagulopathy
Heparin monitoring
Screening test for coagulopathy
Activated clotting time
Thromboelastography
What is tested
Entire clotting cascade and platelet function
Entire clotting cascade and platelet function
Principles
- Whole, fresh blood is added to a clotting activator (e.g. kaolin)
- Time until coagulation measured
- Citrate added to chelate calcium, calcium returned at time of testing
- Sample injected into the sampling cup (containing a clotting activator)
- The cup is rotated around a pin, or the pin rotated independently
- As a clot forms, the pin’s movement is restricted by adherence to it
- This restriction is outputted graphically
Normal values
100-110 seconds
Based on test – TEG/ROTEM
Causes of prolonged time
Any coagulopathy, e.g.
• Clotting factor inhibitors e.g. warfarin, heparin
• Thrombocytopaenia or platelet inhibitors
• DIC
R time – clotting factors
α angle – fibrinogen function and preactivation
MA – platelet number and function
CLT – effectiveness of fibrinolysis
Uses
Monitoring adequacy of coagulation in cardiopulmonary bypass, ECMO, dialysis
Trauma – assess need for blood product replacement
Theatre monitoring
• Cardiac surgery
• Liver transplant
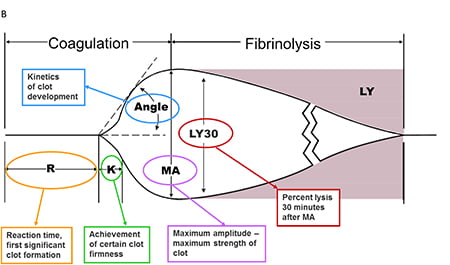
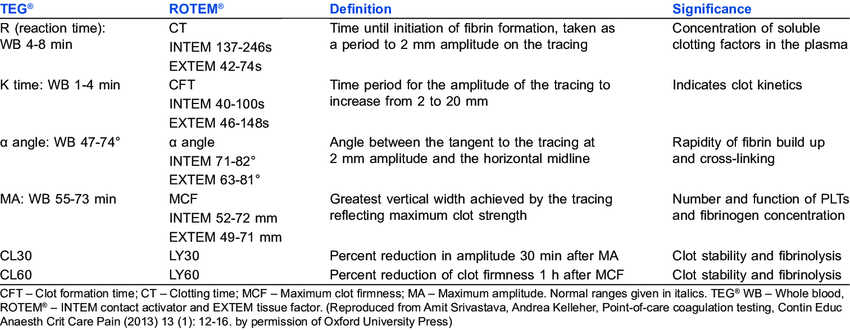
Mooney 2016
Examiner Comments
2014B 08: 0% of candidates passed this question.
It was expected candidates would cover all aspects of testing for each test listed. This would include normal, abnormal or therapeutic values, a comment on methods (either laboratory or point of care) and coagulation pathway assessment. General statements about the overall purpose of the test, collection methods, plasma vs whole blood as sample scored additional marks. Diagnoses or errors associated with abnormalities in each test would also have scored marks but were not mentioned in most answers.
Overall there was a lack of depth of knowledge and incorrect facts. Many candidates knew about TEG, but did not know details about the other tests.
9. Outline the fate of the triglyceride component of orally ingested fat.
CICMWrecks Answer
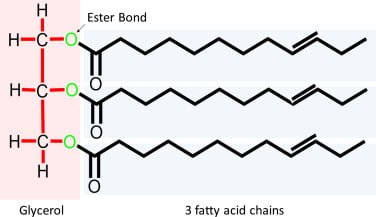
Triglyceride
- 3x long fatty acid molecules joined by glycerol
- eg. stearic/oleic/palmitic acid
Minimal Breakdown in the Upper GIT.
After exiting the stomach:
- Pancreatic lipase
- Triglycerides to FFAs and 2-monoglycerides
- Pancreatic esterase
- Cholesterol esters to cholesterol
- Actions accelerated by emulsification from bile salts
- Facilitate intestinal digestion of lipids
- aids pancreatic lipase activity
- Enhances intestinal absorption of lipids by
- emulsifying lipids
- forming “micelles” (as it is amphipathic)
- Induces intestinal motility
- Choleretic action
- bile salts → ↑ hepatocyte bile production
- Facilitate intestinal digestion of lipids
Transfer into Enterocytes by Passive diffusion as FFAs
In Enterocytes:
- Short/Medium Chain FFAs
- Diffusion into portal circulation
- Bound to albumin and trasported to liver
- Triglyceride formation
- Cholesterol
- Esterified in enterocytes
- Long Chain FFAs
- Encapsulated in phospholipids
- Cholesterol and LC FFAs combine to form TGs
- Packaged in Chylomicrons
- Exocytosed to lymphatics for transport to liver
- Cholesterol
Transport to Liver
- TAGs absorbed as glycerol, FAs, MAG, DAG
- Transported to the liver:
- Short-chain FAs (< 12 C)
- transferred directly tvia portal vein without re-esterification
- Long-chain FAs
- re-esterified to form TAGs
- packaged with cholesterol esters within Chylomicrons
- transported via lymphatics to adipocytes
- Lipids deposited in liver
- Chylomicron remnants with remaining lipids are then taken up by liver
- Short-chain FAs (< 12 C)
Fate
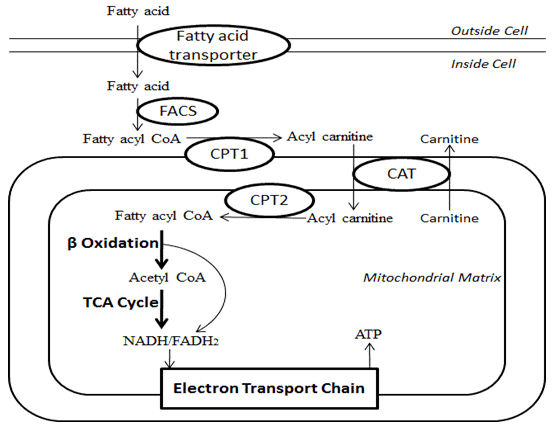
- Glycerol can be converted to Glucose by the liver
- Fatty acids transported from ECF to ICF by fatty acid transporter
- FACoA synthase in cytosol makes FACoA
- Converteted to Acyl carnitine by CPT1 and transported into cell
- Converted back to FACoA by CPT2
- Undergo β-oxidation
- Each cycle generates:
- 1 molecule of Acetyl CoA (by cleaving the 2 carbon-CoA from the whole molecule) which is used in TCA cycle
- 1 molecule of both FADH2 and NADH to be used in the Electron Transport Chain.
- These together produce up to 17 molecules of ATP
- Each cycle generates:
- Transported as chylomicrons to Adipocytes
- Degraded by Lipoprotein lipase to FFAs and Glycerol
- FFAs and Glycerol transported into Adipocyte
- Converted back to Triglycerides for storage.
- In Starvation:
- β-oxidation → ↑ acetyl-CoA → converted to HMG-CoA in liver → ketone bodies (AcAc, β-OH-butyric acid, acetone)
- used as alternate fuel source in liver, heart and brain
Gladwin 2016
Examiner Comments
2014B 09: 8% of candidates passed this question.
Triglycerides (TGs) consist of a 3 carbon glycerol backbone with 3 fatty acids attached. It was expected candidates would detail the fate from digestion and absorption, through distribution to storage and metabolism. Most candidates knew the absorptive processes very well but knowledge of TGs fate once it was packaged into a chylomicron was lacking. Some detail was expected on the passage to the liver via the portal circulation, packing and unpacking and distribution to the body. Very few candidates mentioned appropriate hormones and enzymes, target cells or ketogenesis. Some discussion was expected on the possible fate of acetyl Co A. Comment on the synthesis of lipids and the existence of essential fatty acids gained additional marks.
10. Describe the adult coronary circulation (50% of marks) and its regulation (50% of marks).
CICMWrecks Answer
Arteries
- LCA to
- LCx to
- OM1
- OM2
- LAD has branches
- Diagonal 1 or 2
- LCx to
- RCA
- Right Ventricular
- Acute Marginal
- Posterior descending
Veins
- Great, small, middle
- Drain into the thebesian veins → ventricles directly
- Empty into the coronary sinus on the posterior wall or the RV
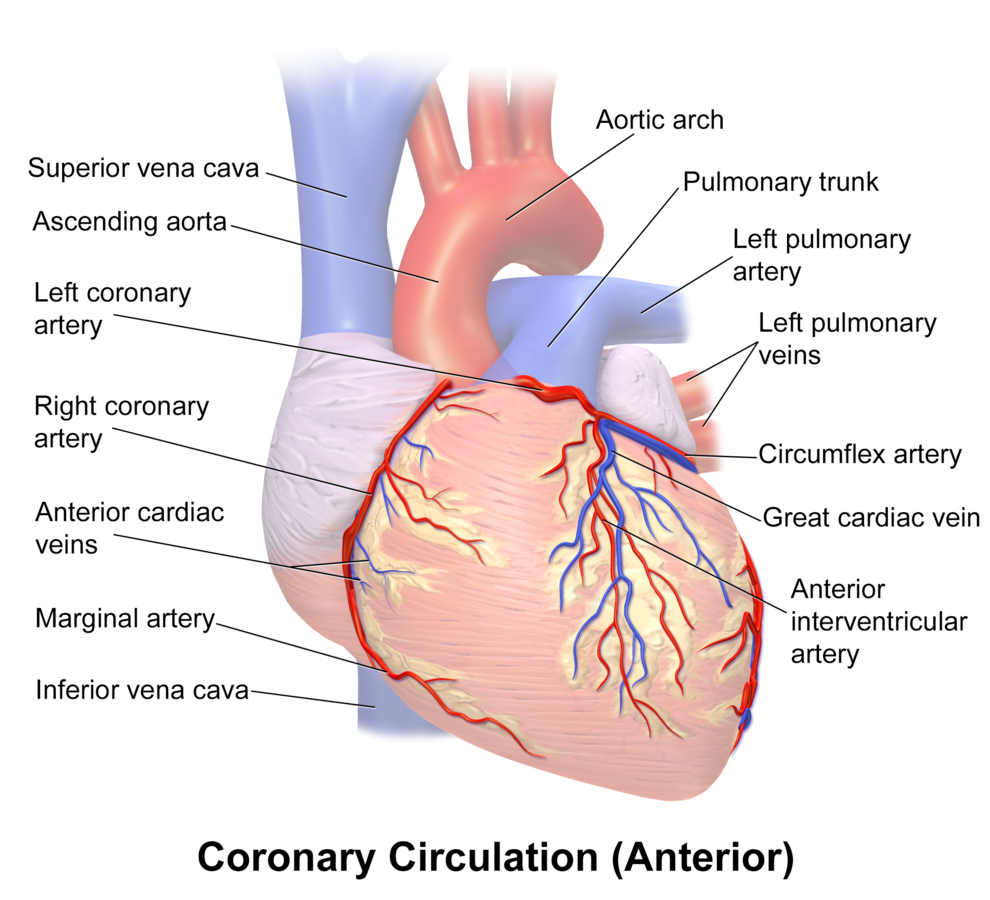
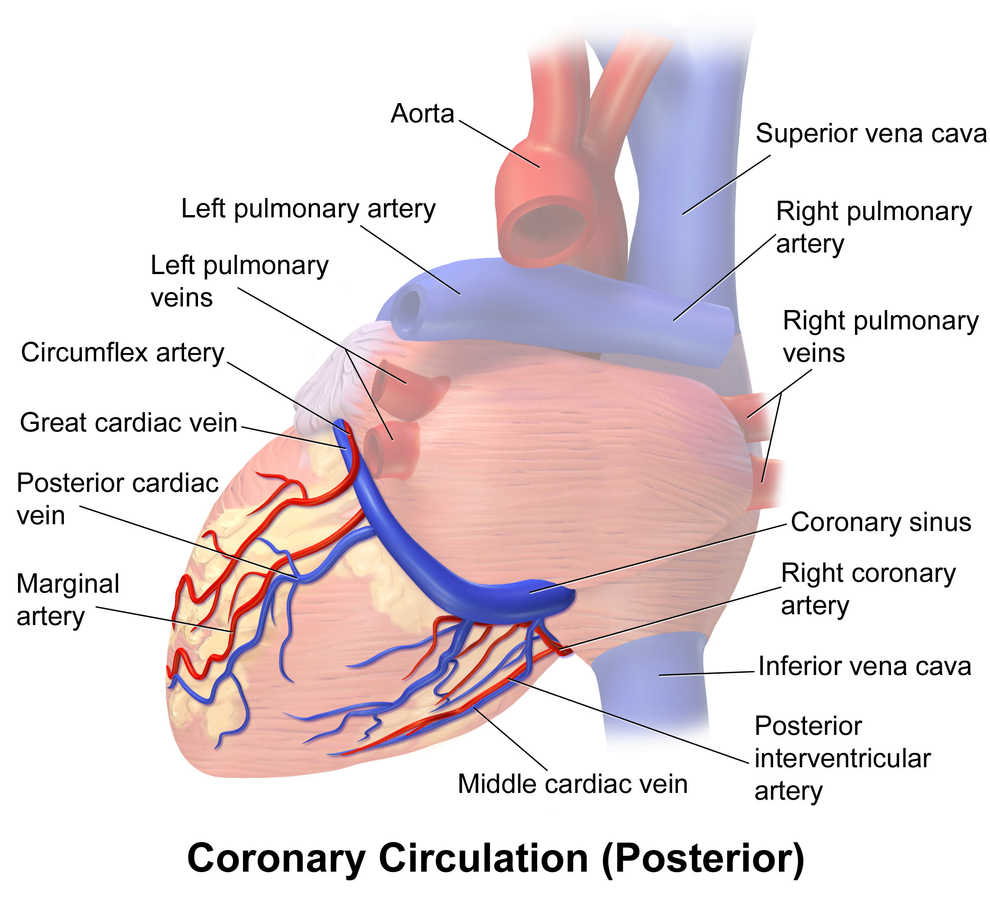
Regional Supply
| Artery | Anatomical region | ECG abnormality on occlusion |
|---|---|---|
| RCA | Down Anterior aspect of the heart, right of the pulmonary trunk Supplies RA SAN and AVN Posterior IVS | RCA occlusion causes → inferior: 2,3 aVF → true posterior: tall R in V1 |
| LCA | Left coronary artery ostia adjacent to left leaflet → Left Main → LCx and LAD Supplies LA, LV IVS AV Bundles | LCA occlusion → Lateral: 1, aVL, V1-V6 |
| LCx | LA and superior LV | anterolateral leads (1, aVL, V5 and V6) |
| LAD | RV, LV Anterior 2/3rds Interventricular Septum | Anteroseptal (V1 and V2) Anteroapical (V3 and V4) with some RCA supply. |
| Left Marginal | The continuation of the LCA after LAD branch Supplies LV laterally | Isolated Abnormalities rare V5, V6 |
| Right Marginal | The anterior branch of the RCA along the inferior border Supplies right ventricle and apex | Isolated Abnormalities rare RCA occlusion causes 2,3 aVF |
Coronary Blood Flow
- 80 mL/min/100 g
- or 200-250 mL/min
- 5% of CO at rest
- Can increase by 3-4 times (up to 400mL/min/100g)
High Extraction Ratio
High at rest (55-65%) body average of 25%
- Extraction ratio can only rise by factor of < 2 to 90%
- AV Δ O₂ = 11 mL/dL
- Coronary venous O₂ content = 5 mL/dL
- Coronary sinus SpO₂= 20mmHg
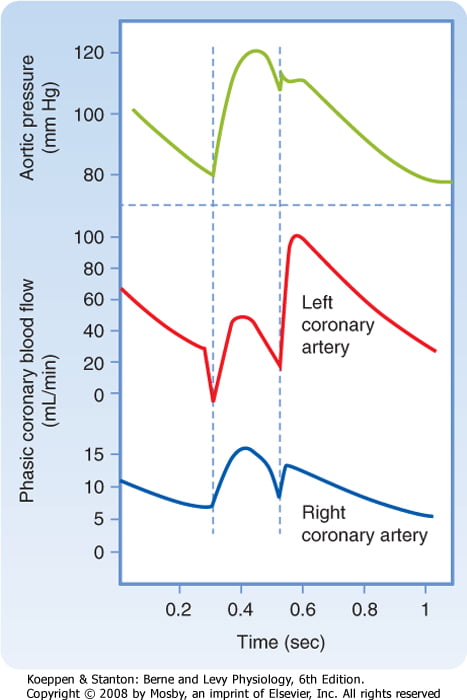
Coronary perfusion pressure (CPP)
- ADP = Ao Diastolic Prssure
- It is different throughout the cycle and b/w ventricles
Phasic Supply as per diagram.
Determinants of Coro BF
- Physical Factors
- Extravascular compression (CPP factors)
- Neural and Neurohumoral Factors
- ↑ SNS tone →
- α receptor mediated vasoconstriction
- β receptor mediated vasodialtion
- ↑ force and rate of contractions → ↑ vasodialtor metabolite release
- Overall effect is dilation
- ↑ PSNS tone → KACh stimulation → mild ↓ Coronary vascular resistance
- ↑ SNS tone →
- Metabolic Factors (main)
- Vasodilatory
- ↑ Adenosine, H, K, CO2, Lactate
- NO → GTP
- ↑ O2 demand → ↓ ATP → ↑KATP channel activation → hyperpolarisation → vasodilation
- Vasodilatory
- Myogenic autoregulation (keep CPP 60-180 mmHg)
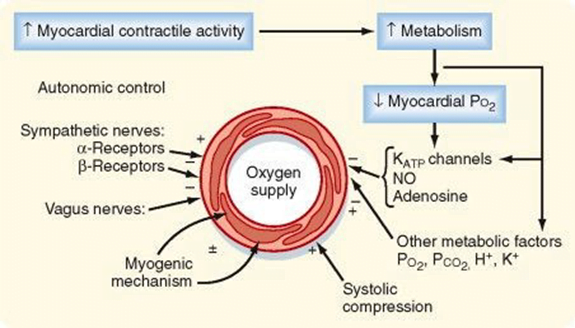
Gladwin 2016
Examiner Comments
2014B 10: 65% of candidates passed this question.
This question was generally well answered. Some candidates did not mention the factors which are peculiar to the coronary circulation and answered in a generic manner, as if for any vascular bed. The coronary circulation has a high O2-ER and flow-dependence. Many candidates seemed to lack a perspective that metabolic demand dominates control of the coronary arterial flow. The phasic nature of flow was best shown with a diagram and whilst “publication-level” graphs are not expected, the graph drawn must be factually correct and convey the principal similarities and differences. While this topic is covered in both Guyton and Ganong, additional detail can be found in the Mosby physiology monograph series: Cardiovascular Physiology, 10th Edition by Pappano and Wier,( the replacement of Berne and Levy).
11. Describe the mechanisms of action of drugs used to treat acute severe asthma and give examples.
CICMWrecks Answer
Asthma:
- Airway obstruction that is reversible (completely or partially) either spontaneously or with treatment,
- Airway inflammation (oedema and hypersecretion)
- Increased airway responsiveness to a variety of stimuli
General Measures:
- Oxygen.
- Repeated assessment
- ABGs
Specific Pharmacology:
| Class | Example | MoA |
|---|---|---|
| Adrenergic Agonists | Salbutamol Adrenaline | Predominantly acting at the β2-adrenergic receptor Gs-PRC → ↑AC → ↑cAMP → ↑PLC activity – ↓ [Ca] via ↑ uptake and removal from cytoplasm – Active uncoupling of actin-myosin via i) Phosphorylation of MLCK ii) Phosphorylation of MLCK Phosphatase – ↑ K channel activation → hyperpolarisation → SMC relaxation Mast cell stabilisation (adrenaline) Improved mucocillary function. |
| Methyxanthines (PDE3 inhibitors) | Theophylline Aminophylline | Multiple Actions ↓ PDE acitons → ↑ cAMP in bronchial SM → sim to β2 effect Adenosine receptor antagonism → ↓ adensosine related bronchoconstriction |
| Antimuscarinics | Tiotropium, Ipratropium | M3 receptor antagonists Normally ACh stimulates M3 → Gq-PRC → ↑ DAG/IP3 → ↑ bronchiolar tone Antagonism → ↓ Vagally mediated Decreased Gq mediated effects |
| Steroids | Prednisone, Hydrocortisone | Reverse the activating effect of pro-inflammatory transcription factors → decrease inflammation – Inhibit the formation of cytokines secreted in asthma by T-lymphocytes, macrophages, and mast cells – Decreased vascular permeability – Inhibitory effect on mucus glycoprotein secretion – ? enhance B2 effects. |
| Other | Mag Sulphate | Acts as a bronchodilator by decreasing cytosolic Ca2+ concentrations. |
| Heliox | Decreased density – thus increased air flow via Hagan Pouiselle | |
| Ketamine | Bronchial smooth muscle relaxant by inhibiting Ach mediated SM constriction | |
| Sevoflurane | Direct beta agonism and inhibition of histamine release from mast cells. |
Gladwin 2016
Pharmacopeia Table
Examiner Comments
2014B 11: 54% of candidates passed this question.
Asthma involves reversible bronchospasm, inflammation, and airway hyper-responsiveness to inhaled stimuli. The main classes of drugs for acute therapy include sympathomimetics, antimuscarinic agents, corticosteroids, methylxanthines and magnesium. Candidates should have a detailed knowledge of the mode of action of these mainstream drugs. Information about drugs used for prevention and for chronic asthma was not asked for and answers which provided details about long term inhaled steroids or leukotriene antagonists did not gain extra marks. This question was well covered by some candidates in a semi-table format. A structured approach worked well with details about drug class, mechanism of action and example(s).
12. Describe the role of the kidneys in the excretion of non-volatile acid.
CICMWrecks Answer
Non-volatile acid
” An acid produced from sources other than carbon dioxide, which cannot be excreted in the lungs. “
- e.g. lactate, sulphate, phosphate and ketone bodies
- 70-100mmol non-volatile acid produced per day
- 4000-5000mmol HCO3– filtered by glomerulus per day
- Renal acid-base is largely determined by handling of bicarbonate ions
- 80% of filtered bicarbonate resorbed in proximal tubule
- 20% resorbed in distal tubule and collecting duct
- Urinary Buffers (Dibasic Phosphate and ammonia) play a role
Bicarbonate Resorption
Proximal tubule
In the tubular cell:
- H+ leaves the tubular cell through the apical membrane via
- H+/Na+ antiporter (secondary active transport via Na+/K+ ATPase on basolateral membrane)
- The HCO3– re-enters the circulation via the Na/HCO3– symporter
- H+ combines with HCO3– in tubule to form H2CO3
- H2CO3 switches back to CO2 and H2O, CO2 is resorbed to turn back into the tubular cell to generate more HCO3 for resorption
More bicarbonate resorbed when:
- Higher filtered HCO3–
- Higher PaCO2
- Lower luminal flow rate
- Angiotensin 2
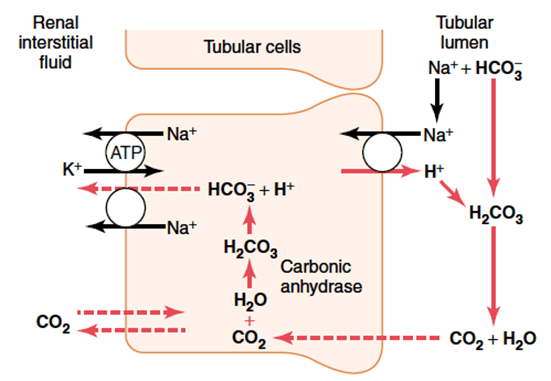
Distal convoluted tubule and collecting duct
- Similar mechanism with regards to resorption of HCO3– via secretion of H+
- Difference is that H+ is transported across the apical membrane via an H+ ATPase (active transport)
Role of Buffers
- The maximum urinary pH is 4.5
- To eliminate more protons, they must be buffered in the tubular fluid
- H+ is bound preferentially to buffers only in the presence of low tubular HCO3–
- Buffering, and so elimination of H+ without its conjugate HCO3–, requires resorption of HCO3– in the proximal convoluted tubule
Phosphate (HPO42-):
- Becomes concentrated in tubules
- pKa = 6.8, so operates effectively as a buffer at tubular pH
Ammonia
- Glutamine broken down in tubular epithelia cells to two NH4+ and two HCO3–
- Comes from hepatic amino acid metabolism
- The new HCO3– is transported back to the circulation via the Na/HCO3– symporter to re-enter the circulation
- NH4+ becomes ion trapped in the tubular fluid
- Proximal tubular fluid is not acidic enough to keep NH4+ in the tubule
- It is resorbed in the ascending limb, moves through the medulla to re-enter the collecting duct
- Here urine has a low pH, and so NH4+ can be eliminated with its proton attached
Mooney 2016
Examiner Comments
2014B 12: 27% of candidates passed this question.
This is a complex but essential area of physiology for intensive care practice. It was expected candidates would indicate that non-volatile acids are those not able to eliminated by the lungs (lactate, sulphate, phosphate and ketone bodies). The kidney plays a central role via bicarbonate (resorbing filtered bicarbonate and generating “new” bicarbonate = acid excretion). It was expected candidates could detail the processes in various parts of the renal tubules and the role or urinary buffers (dibasic phosphate and ammonia). While a number of candidates were able to provide some of the details of ion transports in the kidney, few showed understanding of the overarching concepts of the proximal bicarbonate reabsorption being necessary to allow the distal acid excretion.
13. Outline the pharmacology of amiodarone.
Examiner Comments
2014B 13: 77% of candidates passed this question.
This was a repeat question and was generally answered well. Some candidates lost marks for being too approximate on the pharmacokinetics.
14. Statistics (not in current primary syllabus)
15. Define the terms tolerance and tachyphylaxis. (20% of marks) Describe the different mechanisms by which tolerance can develop, and give examples for each. (80% of marks)
CICMWrecks Answer
Tolerance
Decreased pharmacological response of drug on repeated exposure, requiring larger subsequent doses for the same effect.
Tachyphylaxis
When tolerance occurs rapidly, in a dose-independent manner
Mechanisms
- Tolerance
- Pharmacodynamic tolerance
Persistent, high concentrations of ligand, causes receptor downregulation (decreased expression, affinity or activity)- e.g. Opioid tolerance → continual exposure leads to receptor de-coupling with the G-Protein, or decreased endocytosis-cycling leading to decreased second messenger pathway on ligand binding
- Pharmacokinetic tolerance
Persistent exposure to drug may induce expression of clearance mechanisms (induction of CYP450 enzymes in liver) leading to decreased drug reaching effect site- e.g. Ethanol tolerance → Induction of alcohol dehydrogenase reduce bioavailability of alcohol due to increased first pass metabolism
- Behavioral tolerance
Diminution of a drug-induced disruption of a goal-oriented behavior that is dependent on a learning process- e.g. Psychoactive drugs
- Pharmacodynamic tolerance
- Tachyphylaxis
- Depletion of intracellular stores of an effector, before resynthesis may occur
- e.g. Ephedrine tachyphylaxis occurs due to depletion of stored noradrenaline
- Depletion of intracellular stores of an effector, before resynthesis may occur
Sakurai 2016
Examiner Comments
2014B 15: 15% of candidates passed this question.
Tolerance is the requirement of higher doses of a drug to produce a given response. When this develops rapidly (with only a few administrations of the drug) this is termed tachyphylaxis. Various mechanisms exist by which tolerance occurs and these include cellular tolerance (e.g. neuronal adaptation to opioids or alcohol), enzyme induction and depletion of neurotransmitters. Few candidates knew a comprehensive list or had a classification system for the different types of tolerance. No candidate had a good definition of tachyphylaxis.
16. Describe baroreceptors and their role in the control of blood pressure.
CICMWrecks Answer
High Pressure Baroreceptor (HP BR)
- Carotid sinus and aortic arch receptors
- Detects > 5-10 % change in plasma volume
- ↓ Plasma volume → Increased central SNS tone and decreased PSNS tone
- Sensor:
- Stretch receptor: ↑ distension of vessel → ↑ discharge rate
- Threshold > 60mmHg → normally has baseline tone.
- Located at Carotid Sinus and Aortic Arch
- Spray like visceral nerve endings
- Transmitted by:
- Unmyelinated C-fibres (most receptors)
- Myelinated A-fibres (↑ sensitivity at lower BP)
- Individual fibres have narrow BP range
- Increased reponsiveness to pulsitile rather than continuous flow
- Afferent signal:
- Carotid sinus → Nerve of Hering → CN9
- Aortic Arch → CN10 → NTS
- Both then divide to:
- Stimulatory afferents → ↑ Medullary vagal outflow
- Inhibitory afferents → ↓ RVLM SNS outflow
- Outcome:
- ↑’d distension → ↓’d HR/Contractility/SVR
- Provides strict negative feedback to Δ’s in CO
Low Pressure Baroreceptors (LP BR)
- Location
- Located at junction of return vessels and atria, vetricular walls, pulmonary vessles
- Throughout the peripheral vasculature (esp kidney)
- Sensor:
- Stretch receptor: ↑ distension of vessel → ↑ discharge rate
- Detect > 10% decrease in plasma volume as decreased atrial stretch
- ↓ Discharge rate with
- Reduced ANP release
- Increase SNS output
- 2 types
- A receptors → fire at atrial contraction (a wave)
- B type → fire at atrial filling (v wave)
- Outcome
- ↓ PL → ↓ CO → ↓BR discharge rate
- Medulla afferents cause
- ↓ SNS (NA) and ↑PSNS (RVLM) outflow → peripheral vascular vaso and venodilation
- ↓ SNS activity to kidney → ↓ Na/H2O conservation
- ↑ SNS activity to sinus node
- Hypothalamic afferents cause
- → ↓ ADH release and ↓ Thirst
- Medulla afferents cause
- ↑ contractility/HR/SV/CO
- ↑ SVR → autotransfusion and ↑ perfusing pressure (but ↑AL)
- ↓ PL → ↓ CO → ↓BR discharge rate
Gladwin 2016
Examiner Comments
2014B 16: 62% of candidates passed this question.
This is a core topic and a detailed knowledge was expected. Baroreceptors are stretch receptors located in the walls of the heart and blood vessels and are important in the short term control of blood pressure. Those in the carotid sinus and aortic arch monitor the arterial circulation. Others, the cardiopulmonary baroreceptors, are located in the walls of the right and left atria, the pulmonary veins and the pulmonary circulation. They are all stimulated by distention and discharge at an increased rate when the pressure in these structures rises. Better answers provided some detail on the innervation for these receptors. It was expected candidates would describe that increased baroreceptor discharge inhibits the tonic discharge of sympathetic nerves and excites the vagal innervation of the heart. This results in vasodilation, venodilation, a drop in blood pressure, bradycardia and a decreased cardiac output.
Some candidates had a major misunderstanding around the purpose of “low pressure baroreceptors” with many believing that these are the ones that respond to lower blood pressures, while the “high pressure baroreceptors” respond to higher blood pressures.
17. Describe the pharmacology of oxygen.
Examiner Comments
2014B 17: 35% of candidates passed this question.
Use of a general “pharmacology” structure to answer this question would help avoid significant omissions such as only discussing pharmacokinetics or only discussing pharmacodynamics.
Oxygen has a well described list of pharmacodynamics effects that includes, cardiovascular, respiratory and central nervous system effects. Candidates’ knowledge of the pharmaceutics was limited for a routine drug. It was expected candidates would mention the potential for oxygen toxicity including a possible impact on respiratory drive in selected individuals, retrolental fibroplasia and seizures under some circumstances.
Many candidates did not answer the question asked, and instead focussed on the physiology of oxygen delivery and binding of oxygen to haemoglobin
18. Explain the similarities and differences between myoglobin and adult haemoglobin (60% of marks) and their physiologic relevance (40% of marks).
CICMWrecks Answer
The similarities and differences mean that haemoglobin is the primary means of O2 transport from the lungs to the tissues and myoglobin is the primary O2 carrying pigment of skeletal muscle and acts as local O2 reserve for times of intense muscle activity.
| Haemoglobin | Myoglobin |
|---|---|
| Function | |
| Oxygen carriage → lungs to tissues, CO₂ carriage, Acid-base buffer | Oxygen Store → for exercising muscle |
| Binds CO2, CO, NO, O2, H+ | Binds O2, tightly and firmly |
| Location | |
| in large concentrations (≈15g/L) in red blood cells that circulate throughout the blood stream. | haem containing pigment protein found in skeletal and cardiac muscle. |
| Haemoglobin is only found in blood stream following intravascular haemolysis | Myoglobin is only found in the blood stream when it is released following muscle injury → abnormal finding (↑ in AMI or rhabdomyolysis) |
| Structure | |
| structurally related. Both are globular proteins and both contain a haem moiety which binds O₂. | |
| Haem is an protoporphryin ring derivative with a central Fe2+ molecule that binds O₂ | |
| MW ≈ 65000 daltons | MW ≈ 17,700 daltons |
| Hb contains 65-70 % of total body iron | Myoglobin contains 4-5 % of total body iron |
| Globular molecule made up of four subunits, each containing a haem moiety conjugated to a polypeptide. Polypeptides collectively = globin → two pairs 2α + 2β → 4 haem moieties (Tetramer) | Myoglobin is a single-chain globular protein containing a single haem moiety (Monomer) |
| Can bind a total of 4 O2 and also exhibits cooperative affinity (each subsequent O₂ binding takes less energy → sigmoid shaped OHDC | Unlike haemoglobin it does not exhibit cooperative affinity when binding oxygen since it does not exist in a tetramer formation → its dissociation curve is a rectangular hyperbola rather than a sigmoid curve. |
| Carriage of O2 (See figure below) | |
| Sigmoid shaped dissociation curve | Rectangular hyperbole dissociation curve |
| P50 26.6mmHg, operating range 100-20mmHg | P50 2.75mmHg, operating range 5-1mmHg |
Hb has to carry oxygen from the lungs (PaO2 100) down the ‘oxygen cascade’ to the tissues. P50 suits this operating range and enables appropriate loading and unloading of O2. | Myoglobin needs to have a P50 less than Hb so it can take up O₂ from it. Myoglobin needs to be able to load and unload O₂ in the range of pO2 values that occur within the cell → it’s p50 of 2.75mmHg is well matched to the intracellular operating range of pO2 (1↔5mmHg) The myoglobin content is greatest in muscles specialized for sustained contraction → the muscle blood supply is often compressed during such contractions and myoglobin may provide O₂ when blood flow is cut off. |
| Bohr and Haldane Effect | Nil |
| Synthesis | |
| Haeme is synthesised in mitochondria and cytosol of immature RBCs, Globin is synthesized by ribosomes in cytosol. Production continues till RBCs lose their RNA after entering vasculature | Expressed solely in cardiac myocytes and oxidative skeletal muscle fibres |
| Degradation | |
| RBCs at the end of their life cycle get phagocytosed by macrophages in liver or spleen, or get hemolysed in circulation. Haemoglobin is then broken up – Haeme gets degraded into bilirubin, and the iron gets recycled. | It is released from muscle tissue by cell destruction and alteratins in permeability of muscle cell membrane. It is filtered by glomerulus and rapidly excreted by kidney. |
| Nephrotoxicity | |
| Released upon intravascular haemolysis | Released upon rhabdomyolysis Myoglobin is toxic to renal tubular epithelium and large amounts of myoglobin (eg in rhabdomyolysis) can cause renal failure. |
Examiner Comments
2014B 18: 23% of candidates passed this question.
Both are globular proteins that bind and deliver O2. Due to myoglobin containing a single globin chain its dissociation curve is hyperbolic in shape. Haemoglobin contains 4 globin chains and is a quaternary structure which exhibits cooperatively resulting in a sigmoid shaped dissociation curve. The differing dissociation curves mean that when the PO2 is high, as in the lungs, both myoglobin and haemoglobin are saturated with oxygen. However, at the lower levels of PO2 in the tissues, haemoglobin cannot bind oxygen as well as myoglobin. Myoglobin can bind the O2 released by haemoglobin, which it stores to meet the demands of muscle contraction. This means haemoglobin (with its higher p50) can offload O2 to myoglobin. Comments on the synthesis and degradation gained additional marks but were a common omission.
The physiological relevance was poorly explained. The similarities and differences mean that haemoglobin is the primary means of O2 transport from the lungs to the tissues and myoglobin is the primary O2 carrying pigment of skeletal muscle and acts as local O2 reserve for times of intense muscle activity.
19. Describe the factors that determine right and left ventricular afterload.
CICMWrecks Answer
Afterload
- Load against which the muscle exerts its contractile force (Guyton)
- It is represented by the gradient of the line connecting the end-diastolic volume, to the end-systolic point
- pressure which the ventricle has to contract against (Power & Kam)
Determinants of Afterload
Modified Laplace Equation
- where
- T represents afterload
- P represents aortic pressure
- Therefore, afterload increases as aortic (or pulmonary arterial) pressure increases
- R represents ventricular radius
- Afterload increases as ventricular radius increases
- H represents ventricular thickness
- Afterload decreases as the thickness of the ventricular wall increases (hypertrophy secondary to chronic hypertension and cardiac remodelling)
Modified Hagen-Poiseuille Equation
- Afterload is affected by resistance to cardiac output
- Afterload increased by reduced radius of systemic vasculature
- Afterload increased by increasing viscosity of blood
Resistance in parallel
- Afterload is affected by addition, or loss of large capillary networks in parallel
- Systemic vascular resistance is increased significantly by loss of placenta, with parallel vascular networks
- Pulmonary vascular resistance is decreased significantly by inflation of lung, causing creation of vast capillary network in parallel
Factors affecting Right Ventricular Afterload
- Pulmonary vascular resistance increases afterload
- Hypoxic vasoconstriction increases PVR
- Lung volumes
- Pulmonary vascular resistance minimal at FRC
- Increased pulmonary artery pressure increases RV afterload
- Left heart failure
- Critical mitral stenosis, mitral regurgitation
- Pulmonary emboli
- Right ventricular outflow tract obstruction increases afterload
- Pulmonary stenosis
- Saddle pulmonary embolism
- Right ventricular dilation
- Susceptible due to thin RV wall
- Acute PE
- RV spiral of death
- Increased intraventricular pressure decreases blood flow à ischaemia à decreased contractility à further increase in ventricular volume
- RV spiral of death
Factors affecting Left Ventricular afterload
systemic vascular resistance, aortic impedance and ventricular radius
According to La Place Equation
- Aortic pressure
- Afterload increases as aortic pressure increases (increases with hypertension)
- Ventricular radius
- Afterload increases as ventricular radius increases (increases with ventricular dilation)
- Ventricular wall thickness
- Afterload decreases and ventricular wall thickness increases (decreases with ventricular hypertrophy)
Determinants of aortic pressure indirectly affect afterload
- Aortic compliance
- Afterload decreases with increased compliance
- Arterial blood volume
- Given set compliance, afterload will increase with arterial volume
- From Modifed Poisuille-Hagen Equation
- Radius of aorta
- Autonomic vasomotor tone
- Increased intrathoracic pressure
- Physical compression
- Viscosity of blood
- Radius of aorta
- From equation MAP = CO / SVR
- Tissue flow autoregulation
- Myogenic
- Metabolic
- Tissue flow autoregulation
Other
- Left ventricular outflow tract obstruction
- Aortic stenosis
- HOCM
- Coarctation of aorta
Sakurai 2016
Examiner Comments
2014B 19: 31% of candidates passed this question.
This is a big question and required some structure to cover the material required. A Definition of afterload, factors specific to left ventricle, right ventricle and both were required. The question asked to describe and not merely list factors affecting afterload.
Marks were not given for describing pathologies rather than physiological processes that affect afterload. Candidates seemed to lack depth and understanding on this topic.
20. Describe the pharmacology of magnesium sulphate.
Examiner Comments
2014B 20: 4% of candidates passed this question.
This was a repeat question and very poorly answered for such a commonly used agent. A structured approach to describing any drugs pharmacology was often not used.
Most answers were lacking depth and detail. The questions asked the pharmacology NOT physiology of magnesium sulphate. This was best answered with a standard template addressing: Presentation, Uses, Main actions, Pharmacodynamics, Pharmacokinetics, Mode of Action, Toxicity, and any Special Points
21. Compare and contrast the pharmacology of haloperidol and diazepam.
Examiner Comments
2014B 21: 50% of candidates passed this question.
These are both commonly used agents and a tabulated format worked well. Subheadings covering the “general” pharmacology approach ensured core areas were addressed. Vague terms such as “good” or “moderate” did not allow a detailed comparison between the agents. Repetition of facts between sections such as uses, pharmacodynamics, effects and adverse effects did not gain further marks
22. Describe how the values for PaO2, PaCO2, pH and bicarbonate are determined on a blood gas sample.
CICMWrecks Answer
Blood Gas: pO2
- Measured using Clark Electrode
- Platinum cathode
- Coated in gas permeable membrane à permeable to O2
- Membrane impermeable to other oxidizing molecules
- O2 + 4e– → 2O–
2O– + 2H2O → 4OH–
- Silver chloride anode
- 4Ag → 4Ag+ + 4e–
4Ag+ + 4Cl– → 4AgCl
- 4Ag → 4Ag+ + 4e–
- Phosphate buffer with KCl
- Platinum cathode
- Cathode voltage potential to -0.65v
- In absence of O2 current = 0v
- In presence of O2, O2 diffuses through gas-permeable membrane and is reduced, absorbing 4e–
- Micro-ampmeter measures movement of electrones between anode and cathode
- The current produced is proportional to the pO2 of the test solution
- Sensitivity – related to thickness of membrane and size of cathode area
Blood Gas: pCO2
- Measured using Stowe-Severinghaus Electrode
- Modified pH electrode with outer semi-permeable membrane (Teflon or Silicon Elastic)
- CO2 diffuses into electrolyte layer
- CO2 + H2O → H2CO3 → H+ + HCO3+
- H+ diffuses across glass electrode and alter pH
- Change in pH measured
- Henderson-Hasselbach Equation used to determine pCO2
Blood Gas: pH
- Measured using Sanz Electrode
- 2 half-cells connected by KCl bridge
- Measurement half-cell contain glass membrane with layers of hydrated and non-hydrated glass, permeable or sensitive to H+
- Measurement electrode consists of Silver-Silver chloride bathed in phosphate buffer at known pH 6.84
- Reference half cell contains mercury-mercury chloride in KCl saturated solution
- Measuring electrode responds to the H+ in the sample and the difference between reference and measurement cells measured by a voltmeter or pH meter
- At 37 degrees – change in one pH unit alters voltage by 61.5mV
Blood Gas: Bicarbonate
- Calculated from pH and CO2 using Henderson-Hasselbach Equation
- Actual bicarbonate
- Aerobically drawn arterial sample
- Standard bicarbonate
- Bicarbonate level in an oxygenated plasma specimen at 37 degrees and a pCO2 of 40mmHg
Sakurai 2016
Examiner Comments
2014B 22: 23 % of candidates passed this question
A correct description of the Clarke electrode, Severinghaus and the pH electrode was expected to attain a pass. Candidates who used correct depictions of these electrodes with annotated description attracted higher marks. Most candidates didn’t comment on the temperature correction and standardization to 37 degrees. There was partial understanding on the calculation of HCO3 by most candidates. In general the question was poorly answered considering the wide spread use of blood gas analysis.
23. Describe the regulation of sodium in the body.
CICMWrecks Answer
NORMAL SODIUM
- Total Sodium (Na) = 4000 mmol (60 mmol/kg)
- Distributed:
- Bone (45%)
- ECF (50%)
- 1°ly found in ECF (major EC cation)
- ICF (5%)
- 10-15 mmol/L maintained by
- Na+/K+ ATPase
- low gNa → prevents influx of Na
- Role of Na
- Main determinant of ECF osmolality and tonicityNa/Cl ~ 90% ECF osmotic solute load
- Main determinant of ECFV
- Depolarisation in action potential 2° to ↑Na conductance
- Co-transport of substances across membranes (Eg. Glucose)
- Involved in Na+/K+ ATPase in cell membranes
- Total output =Total input
- 1-1.4 mmol/kg/day
- ~100-300 mmol/day in 70 kg adult
- 10.5 g/day
- Sodium Control – Lost via:
- Kidneys (main) ~ lose 150 mmol/day
- Filters 25000 mmol Na+/day
- 99.5% reabsorbed
- 65% PCT, 25% TAL of LoH, 5% EDCT, 4-5% LDCT and CD
- Sweat and GIT loss in faeces ~ lose 10 mmol/day (0.25 g/day each)
- Kidneys (main) ~ lose 150 mmol/day
Sodium Reabsorption in Kidney
| Location | Contribution | Mechanism |
|---|---|---|
| PCT | 65% | Secondary active transport: – Luminal Na/organic cotransporters with glucose and AAs – Luminal Na/K (NHE-3) exchanger with H from Henderson-Hasselbach intracellularly Passive transcellular – Via solvent drag passively – Down electrical gradient from positive lumenal charge |
| TAL of LoH | 25% | Secondary Active transport: – NKCCT on luminal surface – Dominant mechanism Small amount continues via Secondary active transport as per PCT Paracellular movement driven by net positive charge in lumen |
| Early DCT | 6-10% | 2° active means – Apical Na/K ATPase generates Na gradient – Basal Na/Cl symporter – No alteration in the luminal charge as electrically neutral |
| Late DCT and CD | 5-10% | – Facilitated diffusion across principle cells – Basolateral Na/K ATPase → intracellular Na deficit – Na reabsorbed from lumen via ENaC channels in principle cells up regulated by Aldosterone |
Overview of renal Na+ regulation:
- Thus, renal Na+ regulation depends on:
- Degree of glomerular filtration of Na+ → GFR (minor)
- Changes in GFR due to hyper or hypovolaemia will (indirectly) adjust sodium elimination. Increased plasma volume increases GFR, and vice versa.
- Degree of tubular reabsorption of Na+ (major)
This is the main mechanism for controlling sodium in euvolaemia.
In terms of long term Na excretion; Na reabsorbed is more impt than GFR because:- (i) GFR is heavily autoregulated
- (ii) Glomerulotubular balance blunts any major changes in Na+ excretion that would have resulted from minor changes in GFR changes that actually occurs
- Na reabsorption through GIT/sweat/salivary glands:
- Varies with diet and exercise
- Mainly action of Aldosterone via Na/K ATPase
- Degree of glomerular filtration of Na+ → GFR (minor)
- Normal values
- Na+ filtration: 140mmol/L x 180L/day = 25000mmol Na/day
- Na+ excretion: 140mmol excreted
- rest reabsorbed
- Fractional excretion = 0.5%
Na+ regulation: Control of GFR
- Intrinsic autoregulatory factors (tubuloglomerular feedback and myogenic mechanism)
- MAP has minor effect on GFR over MAP range 70-175 mmHg → BUT changes
in BP that invoke baroreceptor reflexes (BRR) can override these autoregulatory
mechanisms → alter GFR and amount of Na+ filtered
- MAP has minor effect on GFR over MAP range 70-175 mmHg → BUT changes
- Extrinsic factors: Body Na+ content (via ECFV)
- Direct renal effects – ↓ [Na+] (or ↓ ECFV) → results in ↓ GFR due to a ↓ glomerular capillary P(HYDROSTATIC) and ↑ glomerular capillary P(ONCOTIC) → ↓ GFR and Na+ filtered
- Indirect renal effects – ↓ [Na+] (or ↓ ECFV) → stimulates arterial, venous and cardiac BRR → neurohormonal response → to ↓ GFR and Na+ filtered via:
- (i) ↑ SNS and RAAS activity → cause afferent and efferent arteriolar constriction and mesangial cell contraction
- (ii) ↑ ADH → cause afferent arteriolar constriction and mesangial cell contraction
- (iii) ↓ ANP → inhibit afferent arteriolar dilation and mesangial cell relaxation
Na+ Regulation: Control of Reabsorption
- Glomerulotubular balance:
- Intrinsic autoregulatory mechanism that minimises the effect of changes in GFR on Na+ and H2O excretion
- It functions on the basis that the PCT reabsorbs a constant proportion of glomerular filtrate (65% of filtered Na+ /H2O), rather than a constant amount
- In effect – ↑ GFR = ↑ filtration of Na+/H2O = ↑ Na+/H2O reabsorption
- Mechanism:
- With ↑ GFR → large amount of plasma is filtered at the glomerulus → leads to ↑ π(ONCOTIC) of plasma in peritubular capillaries
- This results in an ↑ gradient that –
- (i) Favours tubular reabsorption, and
- (ii) Counteracts the effect of ↑ GFR on fluid leaving the PCT
- Renal interstitial hydrostatic pressure (Intrarenal physical factors)
- ↓ ECFV (and ↓ Na+) results in ↓ MAP → leads to (i) ↓ PHYDROSTATIC and (ii) ↑ πONCOTIC of peritubular capillaries → thus, ↑ Na+ (and ↑ H2O) reabsorption from tubular interstitium into peritubular capillaries
- Hormonal Influences:
- Renin
- Released by ↓ Na delevery to macula densa or β1 stimulation secondary to volume underload
- Tubular effects:
- increased PCT Na/Cl reabsorption, increased tubular K secretion
- Direct PCT effect
- Aldosterone release
- Aldosterone
- Most important regulator of Na+ reabsorption
- Alters protein translation (inducing production of tubular basolateral Na+/K+ATPase and luminal ENaC and K+channels) → causes ↑ Na+ reabsorption by DCT and Principal cells of CCD
- Increased Na reabsorption throughout the GIT/sweat and salivary glands via Na/K ATPase
- Increased H2O reabsorption and increased Na via solvent drag
- Angiotensin II
- Negative feedback on renin release
- Increased aldosterone release
- Decreased RBF and GFR
- Direct renal arteriole constriction (efferent = afferent)
- Mesangial cell contraction thus decreased Kf and GFR
- Direct stimulation of Na+ reabsorption at PCT, and
- Indirect stimulation of Na+ reabsorption via SNS, AII, and aldosterone
- SNS
- Direct stimulation of Na+ reabsorption at the PCT (α1 and β1 receptors), and
- Indirect stimulation of Na+ reabsorption via RAAS
- ADH
- → ↑ Na+ reabsorption at the CCD (principal cells) → acts synergistically with aldosterone here
- ANP
- Inhibition of Na+ reabsorption (blockage of ENaC) in the CDs
- ↓ RAAS and ↓ ADH activity
- Renin
- Other causes ↑Na reabsorb:
- Cortisol
- Oestrogen
- GH
- Thyroid hormone
- Insulin
- Dopamine
- Other cause ↓Na reabsorb:
- PGE2 inhibits NaK ATPase to reduce Na reabsorption
- Glucagon
- Progesterone
- PTH
- Renal vasoDilators:
- PGs
- Kinins
- Pressure natriuresis & diuretics
- renal compensatory mechanism that maintains long-term regulation of arterial BP by controlling the kidney’s excretory ability of Na+and H2O
- Pharmacological agents:
- Ouabain (a cardiac glycoside) inhibits NaK ATPase decreasing excretion
- Loop Diuretics → ↑ Na loss
Gladwin / Bianca / JC 2019
Examiner Comments
2014B 23: 19% of candidates passed this question.
This question was generally poorly answered. Total body sodium is regulated within 2% in normal individuals. The vast majority is contained in the extracellular compartment. While any physiological regulation involves a balance of input and output, sodium intake is essentially unregulated in humans. Output is regulated via renal, gastrointestinal and skin losses. Candidates needed to present the renal handling of Na including hormonal control and present factual knowledge about the level of absorption and GFR effects to attain a pass mark. Many candidates focused on osmolality and tonicity and some on the use of diuretics thereby not gaining marks
on regulation of sodium. Most candidates didn’t mention either the skin and GIT role in sodium balance.
- aldosterone = stimulates Na+ reabsorption at the collecting tubules
- intra renal factors such as interstitial pressure which is lowered during hypovolaemia and reduced renal perfusion, thus promoting Na+ reabsorption gradient
- sympathetic nervous system – influences interstitial pressure and increases renin production
- angiotensin II – stimulates reabsorption at proximal tubule
- Atrial Naturetic Peptide/Factor – inhibits Na+ reabsorption
- Others – dopamine, cortisol, insulin => increase Na+ reabsorption, but minor factors
Syllabus – D1, 2f
Reference – Textbook of medical Physiology, Guyton, Chp 28
24. Describe the control of gastric emptying.
CICMWrecks Answer
Physiology of Gastric Emptying
Gastric emptying
- Fluids have half-time of 30mins in stomach
- Solids have half-time of 2 hours in stomach
Gastric receipt of food bolus:
- Peristaltic wave moves down the oesophagus, propelling food bolus
- Controlled by mesenteric plexus with input from vagus nerve
- Upon swallowing, the lower oesophageal sphincter and stomach relax until the oesophageal peristaltic wave has passed
- Following food bolus passage, the LOS tone increases to prevent reflux
- Food bolus reaching the stomach causes a vagal-mediated relaxation of the stomach, to accommodate gastric distension and food storage
Gastric mixing and emptying:
- Spontaneous mixing waves move down the stomach every 15-20 seconds
- Stronger waves propel gastric contents towards the pylorus (pyloric pump)
- High pyloric sphincter tone
- Allows through well-mixed liquid chyme
- Restricting gastric emptying of solids, causing mixing (retropulsion)
Control
Gastric emptying is controlled by the balance between stimulatory gastric factors and inhibitory duodenal factors
Factors promoting gastric emptying:
- Increased stomach wall stretch
- Stimulates pyloric pump
- Reduces pyloric tone
- Parasympathetic vagal stimulation
- Stimulates pyloric pump
- Gastrin
- Stimulates pyloric pump
- From G cells in antrum
- In response to presence of protein (esp. meat) in stomach
- Motilin
- Stimulates pyloric pump
- During fasting
- Stimulates pyloric pump
- From M cells in duodenum (external antigen stimulus)
- Carbohydrate: rapid emptying
Factors inhibiting gastric emptying:
- Nervous reflexes via enteric nervous system, local sympathetic trunk, and the vagal nerve to the brainstem
- in response to local conditions in the duodenum: Distension, Irritation, Acidity of chyme, Hyper- or hypo- tonicity of chyme, Breakdown products, Especially AA and FA
- Inhibit pyloric pump
- Increase pyloric sphincter tone
- Hormones (inhibit pyloric pump)
- Cholecystokinin (CCK)
- From I cells of duodenum
- In response to presence of fat and proteins
- Secretin
- From S cells of duodenum
- In response to presence of acids
- Gastric inhibitory peptide (GIP)
- From K cells of duodenum
- In response to presence of fats, proteins and carbohydrate
- Cholecystokinin (CCK)
- Other Factors :
- Sympathetic stimulus:
- Decreases contractility and reduces gastric emptying
- Dopamine:
- Decreases intragastric pressure and lower oesophageal sphincter tone
- reduces gastric emptying
- Sympathetic stimulus:
Alternate: This section can also be approached as:
- Local factors
- Gastric Factors: pump, Stomach wall distension, Amino acid
- Duodenal Factors: Type of food, stretch of wall, irritation, hyperosmolaroty, acidity, amino acid and FA content
- Neural factors
- Symptathetic
- Parasympathetic
- Hormonal factors
- Dopamine
- Gastrin
- Motilin
- Secretin
- CCK
- Somatostatin
- GIP
Mooney / Sakurai / JC 2019
Examiner Comments
2014B 24: 23% of candidates passed this question.
An understanding of the physiology of gastric emptying has direct relevance to intensive care practice as it influences enteral feed tolerance, helps inform regarding risk of aspiration and has important pharmacologic implications.
Candidates’ answers were superficial and the role of intrinsic reflexes and local hormonal responses poorly understood and described. A simple and clear coverage is provided in Principles of Physiology for the Anaesthetist / P. Kam, I. Power – 3rd Ed.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | Dose response curves, renal clearance |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | |
| F. Respiratory | Resp physio and measurement, Capnography, dead space |
| G. CVS | Cariovascular physio and measurement, Caridac pressure waves, alterations with vasopressors, invasive BP monitoring |
| H. Renal | |
| I. Body Fluids and Electrolytes | IVF, colligative properties, anion gap, frusemide |
| J. Acid Base | |
| K. Neuro | Cerebral physio + pharm, Vasopressin Nerve action potentials, LA pharm |
| L. Musculoskeletal | |
| M. ANS | |
| N. Liver | Liver physio and blood flow, nutrition, changes with fasting and stress |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | |
| U. Endocrine | |
| V. Obstetrics | Obstetric and neonatal physiology – CV changes, gas exchange across placenta, changes with first breath, humidity |
| W. Measurement and Monitoring | |
| X. Procedures |


Recent Comments