SAQs
1. Describe and compare the action potentials from cardiac ventricular muscle cells and the sino-atrial node.
CICMWrecks Answer
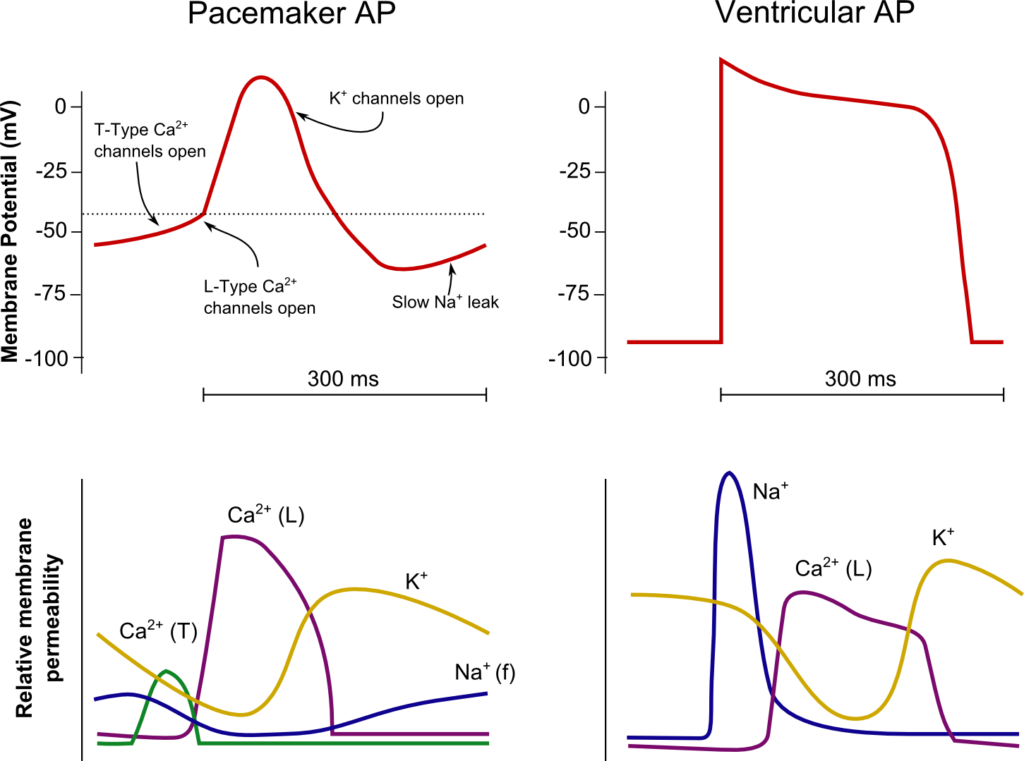
SA Node
Ventricle
Resting membrane potential
- No set RMP, however approx. -60mV
- βadrenergic stimulation causes a less negative RMP and increased slope of the initial upstroke
- muscarinic stimulation causes a more negative RMP and a decreased slope of the initial upstroke
Resting membrane potential
- Approx -90mV
Threshold potential
- Approx 40mV
Threshold potential
- Approx. -50mV
Phase 0
- Funny Na+ current allow leakage of Na+ into the the cell slowly increasing membrane potential to the threshold potential
T and L-type Ca2+ channels (voltage gated) open when threshold potential reached
- Ca2+ influx
- Depolarization
- Shallow upstroke compared to ventricular myocyte
Phase 0
- Rapid depolarization
- Opening of fast Na+ channels
- Influx of Na+
- Overshoot to +20mV
Phase 1
- Early repolarization
- Closure of fast Na+ channels and opening of K+ channels (transient outward, inward rectifier)
- Efflux of K+
Phase 2
- Plateau
- Opening of intially T-type Ca2+ channels and subsequently L-type Ca2+ channels
- Ca2+ influx balances K+ efflux
- Na+ channels in closed state à absolute refractory period
- No action potential can be generated in this state
Phase 3
K+ channels open and Ca2+ channels slowly close
- K+ efflux
- Repolarization
- No plateau phase
Phase 3
- Repolarization
- Closure of Ca2+ channels while K+ channels remain open (delayed rectifier)
- K+ efflux
- Returns potential towards RMP
- Na+ channels transition to inactive state à relative refractory period
- Another action potential can be generated with greater adequate stimulus
Phase 4
- Na+/K+ ATPase and Na+/Ca2+ ATPase maintain ionic gradients
Phase 4
- RMP
- Na+/K+ ATPase and Na+/Ca2+ ATPase maintain ionic gradients
Sakurai 2016
Examiner Comments
2020B 01: 72% of candidates passed this question.
This question details an aspect of cardiac physiology which is well described in multiple texts. Comprehensive answers included both a detailed description of each action potential and a comparison highlighting and explaining any pertinent differences. The question lends itself to well-drawn, appropriately labelled diagrams and further explanations expressed in a tabular form. Better answers included a comparison table with points of comparison such as the relevant RMP, threshold value, overshoot value, duration, conduction velocity, automaticity, ion movements for each phase (including the direction of movement) providing a useful structure to the table. Incorrect numbering of the phases (0 – 4) and incorrect values for essential information (such as resting membrane potential) detracted from some responses.
2. Define functional residual capacity (10% marks).
Outline the functions of the functional residual capacity (70% marks) and the factors affecting it (20% marks).
CICMWrecks Answer
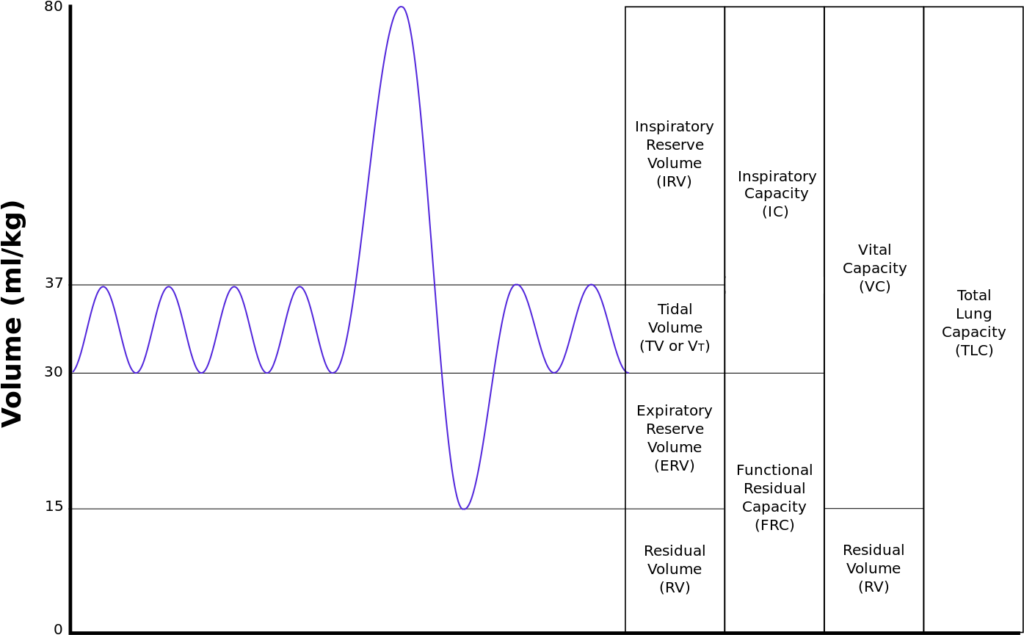
Functional residual capacity
- Volume of air in lungs at the end of normal tidal expiration
- Occurs at equilibrium point between the recoil of chest/diaphragm and lungs to collapse inwards
- Sum of residual volume and expiratory reserve volume
- Normal FRC 30ml/kg (approx 2.2l in 70kg male)
Function of FRC
- Oxygen reservoir
- Normally 270mls O2 → can be expanded to 1.8l if breathing FiO2 100%
- PaO2 buffer
- Ensures no fluctuation in PaO2 between inspiration and expiration
- Prevents atelectasis
- Normally FRC above closing capacity
- Decreases V/Q mismatch
- Minimizes work of breathing
- Minimizes airway collapse → resistance decreased
- Minimizes alveolar atelectasis → decreased work required to re-inflate alveoli
- Lung kept at most compliant segment of compliance curve
- Decreases pulmonary vascular resistance
- Extra-alveolar and alveolar capillaries kept open
- Minimizes hypoxic vasoconstriction
Factors Affecting FRC
| Factors which increase FRC | Factors which reduce FRC |
|---|---|
| Age | Impaired Lung and chest wall compliance |
| Acute asthma | Increased Intra-abdominal pressure |
| Emphysema | Supine position (by 20%) |
| PEEP Extrinsic Intrinsic (gas trapping) | Anaesthesia (by 20) |
| Upright and Prone position | Obesity |
Sakurai / Gladwin / JC 2020
Examiner Comments
2020B 02: 79% of candidates passed this question.
This question was in two parts with the percentage of marks allocated an indication of the relevant time or detail expected per part. The second part of the question also contained two distinct headings which should have been used in the answer. As an outline question, dot points with a brief explanation of each point were expected. Most candidates drew diagrams, few of which added value. For a diagram to add value it should be accurate, have labelled axes, a scale with numerical values and units. As a general rule, diagrams should also be explained and help to illustrate or relate to a written point.
For factors affecting FRC, to score full marks, it should be clearly stated if the factor causes an increase or decrease in FRC. This topic is well covered in the recommended respiratory texts.
3. Describe the pharmacology of hydrocortisone
Examiner Comments
2020B 03: 69% of candidates passed this question.
Hydrocortisone is a level 1 drug in the syllabus. Most answers were well structured, many used key headings. In general, detailed information specific to hydrocortisone was lacking. Answers that focused on the mechanism of action, pharmacodynamic effects and pharmacokinetics effects which were detailed and accurate scored well. It was expected that significant detail be included in the sections with relevance to clinical practice for example, the mechanism of action and pharmacodynamic effects including the side effect profile. An indication/appreciation of the timelines of such was also represented in the marking template.
4. Outline the role of the liver in the metabolism of fat (⅓ marks), carbohydrate (⅓ marks) and proteins (⅓ marks)
CICMWrecks Answer
Fat
Anabolic Role
Synthesis or lipoproteins for transport of lipids from dietary FFA’s
- Chylomicrons
- LDL/HDLs
Lipogenesis via citrate in the TCA:
- Citrate leaves mitochondria and is converted back to Acetyl-CoA
- Acetyl-CoA goes to Malonyl-CoA (conversion ↑d by insulin)
- Fatty acids: via fatty acyl-CoA
- Malonyl-CoA → Fatty-Acyl CoA in the reverse of B-oxidation and creates Triglycerides
- Cholesterol: via HMG-CoA
- blocked by statins
Catabolic Role
Β-oxidation:
- FACoA synthase in cytosol makes FACoA
- FACoA → Acyl carnitine by CPT1 and transported into cell
- Acyl carnitine → FACoA by CPT2
- Undergoes B oxidation
- 1 Acetyl CoA (by cleaving the 2 carbon-CoA from the whole molecule) → Used in TCA cycle
- 1 FADH2 + 1 NADH to be used in the Electron Transport Chain.
- → up to 17 molecules of ATP
Carbohydrate
Anabolic Role
Glucostat Function
- Gluconeogenesis from pyruvate derived from
- Complex polysaccharides (fructose and galactose)
- Glycogen
- Gluconeogenesis
- lactate, pyruvate, glycerol (from TAGs), glucogenic a.a’s (esp Ala and Glu)
- Pentose phosphate shunt
- Glycerol (but not FFAs)
- Glycogen synthesis
Catabolic Role
Glycolysis:
- Primary function
- For generation of pyruvate + 2 ATP
- Pyruvate then utilised to form
- fat, AA’s and ketones
- Lactate
Glycogenolysis:
- From G6P.
Protein
Anabolic Role
Generation of functional proteins in serum:
- Albumin
- Fibrinogen
- Coagulation proteins
- Regulatory proteins
- Coagulation factors
- Complement proteins
- Globulins
- α1 (α1 anti-trypsin, α1-fetoprotein)
- α2 (haptoglobulin),
- β (transferrin)
Amino acids and nucleosides from α-ketogluterate in the TCA
Haeme from Succinyl-CoA in the TCA
Catabolic Role
- Breakdown of proteins to form
- ketones as energy source (liver, heart and brain)
- free amino acids for addition to the AA pool.
- Amino acids utilisation for energy or protein synthesis
- Ammonia metabolism and recycling
- Deamination of fatty acids
- Urea formation for ammonia removal Part of the urea cycle takes place in liver
Gladwin 2016
Examiner Comments
2020B 04: 54% of candidates passed this question.
This question relates to basic hepatic physiology and is well described in the recommended texts.
The mark allocation and division of time was indicated in the question. Better answers used the categorisation in the question as an answer structure. Many candidates gave a good description of beta oxidation, the formation of Acetyl Co A and ketone synthesis. A description of the synthesis of cholesterol, phospholipids, lipoproteins and fatty acid synthesis from proteins and carbohydrates mainly using glycogen, glucose and lactate also received marks. Candidates seem to have a better understanding of fat and glucose metabolism than protein metabolism. Higher scoring candidates appreciated the anabolic and catabolic processes of each component.
5. Describe the anatomy (70% marks) and effects (30% marks) of the sympathetic nervous system.
CICMWrecks Answer
Anatomy of SNS
- Posterior hypothalamus is the main site of sympathetic nervous outflow
- Receives input from cardiovascular centres of medulla and pons
- Sympathetic innervation from Sympathetic trunks
- Paired bundle of sympathetic neurons run lateral from vertebral bodies from T1 to L2
- Consists of two neurons in series
- Short Pre-ganglionic neuron → Sympathetic ganglion → Long Post-ganglionic neuron
Origin
- Origin: Preganglionic fibres originate in the grey matter of the spinal cord lateral horn
- Nerve Fibres and Pathway: Leave the spinal cord through ventral roots (with spinal nerves)
- Leave spinal nerves, travel as white rami communicantes (short myelinated B fibres)
- Meet with the ganglia of the sympathetic trunk
- Synapse with post-ganglionic neurons
- Neurotransmitter: Preganglionic neurotransmitter is acetylcholine
- Receptor: nicotinic receptors
Sympathetic trunk:
4 parts
- Cervical part
- Head
- Neck
- Thorax
- Thoracic part (T1-T5)
- Aortic plexus
- Pulmonary plexus
- Cardiac plexus
- Thoracic splanchnic nerves
- Lumbar sympathetic ganglia
- Coeliac plexus
- Pelvic part
- Hypogastric plexus
- Pelvic plexus
Post-ganglionic
- Nerve Fibres and Pathway: Post-ganglionic neurons leave the ganglia as grey rami communicantes
- Long unmyelinated C fibres
- Rejoin the spinal nerves to travel to target organs
- Neurotransmitter: Postganglionic neurotransmitter is noradrenaline
- (acetylcholine in muscles, sweat glands and hair follicles)
- Receptor: Adrenergic alpha and beta receptors on target organs/vessels
Exception is adrenal medullary outflow:
- Modified post-ganglionic cells release adrenaline into circulation
- Innervated directly by pre-ganglion sympathetic fibres
Effects of SNS
| Function | Control the body’s response during perceived threat. Diffuse physiological accelerator |
| Activates response of | Fight-or-flight |
| General Body Response | Body speeds up, tenses up, becomes more alert. Functions not critical to survival shut down. |
| Cardiovascular System | ↑↑↑ Chronotropy, ↑↑↑ inotropy, ↑↑↑ lusiotropy, ↑↑ dromotropy |
| Vasculature | Constriction |
| Pulmonary System | Bronchial tubes dilate |
| Musculoskeletal System | Sweating, contraction, lipolysis |
| Pupils | Dilate |
| Gastrointestinal System | Decreased salivation and GIT motility, increased sphincter tone, gluconeogenesis |
| Salivary Glands | Saliva production decreases |
| Endocrine | Adrenaline and noradrenaline release |
| GU | Detrusor relaxation, sphincter contraction, ↑ uterine tone |
Mooney / JC 2020
Examiner Comments
2020B 05: 51% of candidates passed this question.
Most candidates had a suitable structure to their answers, those without a clear organisation of thought tended to gain fewer marks. In many cases incorrect information or limited detail, particularly around the anatomical organisation prevented higher marks.
6. Classify the oral hypoglycaemic drugs (20% marks); include their mechanism of action (40% marks) and their most significant side effects (40% marks).
CICMWrecks Answer
| Drug class | Mechanism | Side effects |
|---|---|---|
| Biguanides (Metformin) | – Stimulates the movement of GLUT-4 receptors to the membrane of skeletal muscle and adipose tissue cells, increasing glucose uptake by those cells. – Also inhibits gluconeogenesis and glycogenolysis in the liver, and delays intestinal glucose absorption. | – Life threatening lactic acidosis may occur in the presence of renal impairment – Diarrhoea, n/v, abdominal discomfort common – Must be withheld prior to administration of iodinated IV contrast |
| Sulfonylureas (Gliclazide) | – Combine with KATP receptors on pancreatic ß cells, closing the ATP-dependent potassium channels to depolarize the cell, resulting in an influx of calcium which stimulates insulin secretion. | – Risk of hypoglycaemia – GIT disturbance – Stimulate appetite and may cause weight gain |
| Meglitinides (Repaglinide) | – Act by stimulating the same receptor as the sulfonylurea drugs but at a different side. | – Risk of hypoglycaemia – Repaglinide is a major substrate of CYP3A4 and caution should be used when administering with clarithromycin or antifungals, as may result in high plasma levels of repaglinide and consequent severe hypoglycaemia |
| Thiazolidinediones (Rosiglitazone) | – Act on the PPARg in fat cells to induce insulin sensitivity | – Associated with increased risk of peripheral limb fracture in post-menopausal women, and also some cases of diabetic maculopathy. – prone to cause severe fluid retention and precipitate heart failure, contraindicated in CCF – Contraindicated in people with known IHD. |
| Alpha- glucosidase inhibitors (Acarbose) | – Act by inhibit the enzyme that breaks down dietary complex carbs to sugar, reducing the quantity of glucose available for absorption | – Abdominal discomfort & distention, Flatulence – Elevates serum transaminases – Shouldn’t produce hypoglycaemia itself as does not promote insulin release |
| DPP-IV inhibitors (Sitagliptin) | – Inhibit the activity of the enzyme DPP-IV, which normally breaks down the gut hormones GLP-1 (glucagon-like peptide 1) and the protein GIP (gastric inhibitor peptide). GLP-1 and GIP stimulate glucose-mediated insulin release from the pancreas following an oral load of glucose. | – Monitor in renal insufficiency – May cause hypoglycaemia |
| SGLT2 inhibitors (Dapagliflozin) | – Inhibit Sodium-glucose transport protein 2 (SGLT2, which is responsible for reabsorbing glucose in kidney). – Decreased kidney reabsorption of glucose, glucosuria effect (Insulin and pancreatic b cell independent) | – May cause Hypoglycemia – May cause Severe Euglycemic Ketoacidosis, esp in post-op period – UTIs, Candidal Vulvovaginits |
JC 2019
Click to Open CICMWrecks Pharmacopeia Table: Hypoglycaemic Agents
Examiner Comments
2020B 06: 37% of candidates passed this question.
High scoring answers most often started with a strong and logical structure and focused on the requested categories of information. Many candidates gave good answers across the wide range of drugs. Several candidates could have scored more highly by giving more correct information on biguanides and sulphonylureas,
Click to Open CICMWrecks Pharmacopeia Table: Hypoglycaemic Agents
7. Compare and contrast external ventricular drains and intraparenchymal fibreoptic pressure monitors.
CICMWrecks Answer
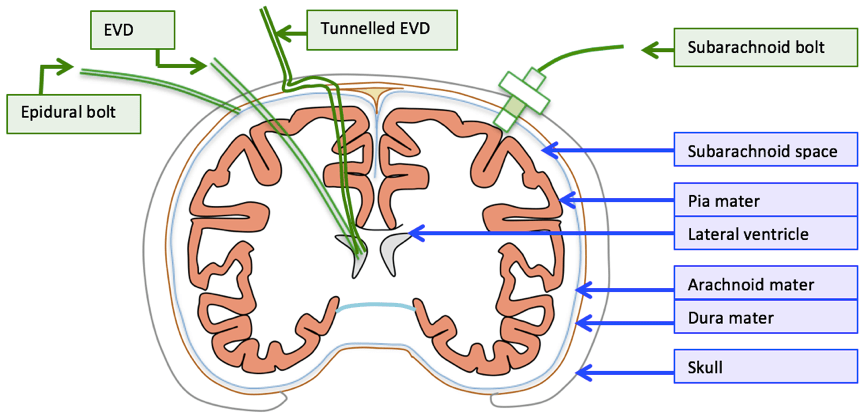
| EVD | Intraparenchymal fiberoptic | |
| Description | • A flexible plastic catheter placed inside the lateral ventricles. • Can be either unilateral or bilateral placement • Gold standard for ICP monitoring | A think catheter inserted into parenchyma of the brain |
| Indication | • Hydrocephalus • Post surgical/trauma intracranial haemorrhage • Meningitis | same |
| Location of tip | Lateral ventricles | Below the dura – usually a few cms |
| Component | • Plastic catheter • Wheatstone bridge • Fluid filled non-compressible tubing • Pressurized fluid bag • Drainage bag • Monitor | • Microsensor – intracranial • Fiber Optic cable • Monitor |
| Principle | Pressure is transmitted to a Wheatstone bridge transducer via fluid filled non-compressible tubing | Changes in ICP move a displaceable mirror at the tip of the sensor to alter the intensity of light reflected back along the fiber optic cable |
| calibration | Able to be zero’d post insertion | Cannot be zero’d post insertion |
| Sources of error | • Migration of catheter • Blockage • Incorrect leveling to tragus • Damping and resonance | • “Drift” • Increased inaccuracy >72hrs • Only measures ICP locally |
| Advantage | • Has diagnostic values (CSF sampling, elevated ICP, new haemorrhage) as well as therapeutic values (drainage of excessive CSF, administration of medication) • Global measurement of ICP • Can be converted to cerebral shunt – long term treatment • Cheap | • Less expertise required for insertion • Less traumatic • Able to be placed in patients with small collapsed ventricles • Less infection risk • No risk of blockage |
| Disadvantage | • More skill required for insertion • More traumatic compared to bolt • Increased risk of ventriculitis • May be blocked • Complications: bleeding, malplacement, obstruction, migration, infection | • Only local ICP is measured • No therapeutic value: CSF cannot be drained • Less accurate due to drift and unable to be calibrated post insertion • Expensive |
Guo 2021
Examiner Comments
2020B 07: 22% of candidates passed this question.
This question is ideally suited to a tabular format, where candidates are expected to highlight the significant similarities and differences as well as why a certain monitor may be chosen in preference to another rather than compile two lists written next to each other. To score well in this question, a statement of what could be measured (ICP: global vs local), a description of the measurement principles, along with other measurement related information like calibration and sources of error was required. Also sought was information regarding anatomical placement (e.g., lateral ventricle for EVD) and method of placement. Furthermore, a comparison with each other (e.g., higher infection/bleeding risk with EVD, greater risk of trauma due to size and insertion, expertise to insert, cost, therapeutic benefit, risk of blocking) was required for completion. Candidates who structured these elements into advantages and disadvantages were generally able to elucidate this information and score better.
8. Describe the cough reflex.
CICMWrecks Answer
Afferent
- In response to chemical, mechanical or noxious stimuli in larynx, trachea, carina or bronchi
- Signal transduced via internal laryngeal nerve (vagus nerve)
Central processor
- Medullary respiratory centre
- No single “cough centre” isolated
Efferent
Co-ordinated action of inspiratory muscles, pharyngeal muscles, and expiratory muscles
- Inspiratory phase
- Inhalation of a volume of air sufficient for cough generation (phrenic nerve and intercostal nerves)
- Compressive phase
- Closure of the glottis (recurrent laryngeal nerve)
- Activation of expiratory muscle against closed glottis
- Generation of transient increase in pressure of 300mmHg in thorax arterial blood and CSF
- Expulsive phase
- Opening of glottis due to posterio-cricoarytenoid muscle activation
- High velocity flow generated in bronchi, trachea and larynx
- “Choke point” – high velocity flow causes turbulant flow → increased shear stress between gas and airway lining → drag and expulsion of irritant
Intrinsic muscles of the larynx
- Cricothyroid
- Upper lip of cricoid cartilage → Inferior aspect of thyroid cartilage
- Innervated by superior laryngeal nerve
- Tenses vocal cords – elevates vocal tone
- Thyroarytenoid
- Anterior inner surface of thyroid à arytenoid cartilage
- Innervated by inferior laryngeal nerve
- Relaxes vocal cords – depresses vocal tone
- Posterior cricoarytenoid
- Posterior aspect of cricoid cartilage → arytenoid cartilage
- Innervated by inferior laryngeal nerve
- Abducts vocal cords
- Lateral cricoarytenoid
- Lateral aspect of cricoid cartilage → arytenoid cartilage
- Innervated by inferior laryngeal nerve
- Adducts vocal cords
- Transverse and Oblique arytenoids
- Spans the arytenoid cartilages
- Innervated by the inferior laryngeal nerve
- Adducts vocal cords
Sakurai 2016
Examiner Comments
2020B 08: 62% of candidates passed this question.
Overall, this question was reasonably well answered. Those that performed well had suitably detailed knowledge and structured their responses which generally included a definition and purpose of the reflex as well as the identification and a description of the afferent, integrator/controller, and efferent limbs of the reflex. This structure allowed a logical platform for the elucidation of the detail required in the answer, including types of stimulus, receptors, nerves (for both limbs of the reflex) and the muscles used in the phasic response to be clearly articulated
9. Outline the daily nutritional requirements, including electrolytes, for a normal 70 kg adult.
CICMWrecks Answer
MACRONUTRIENTS
CHNOPS (carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulphur)
Provide bulk energy
- Carbohydrates: compounds made up of types of sugar.
- monosaccharides (such as glucose and fructose)
- disaccharides (such as sucrose and lactose)
- oligosaccharides
- polysaccharides (such as starch, glycogen, and cellulose).
- Proteins: are organic compounds that consist of amino acids joined by peptide bonds.
- Essential (cannot be manufactured in the body: phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine) and non-essential
- Fats: glycerin molecule with three fatty acids attached.
- Essential (alpha-linolenic acid (ω-3) and linoleic acid (ω -6)) and non-essential
- Saturated and Unsaturated
| Biomolecule | Kilocalories per 1 gram |
|---|---|
| Protein | 4 |
| Carbohydrate | 4 |
| Fat | 9 |
MICRONUTRIENTS
Support metabolism
- Dietary minerals: exogenous chemical elements indispensable for life
- Vitamins: organic molecules essential for an organism that are not classified as amino acids or fatty acids. They commonly function as enzymatic cofactors, metabolic regulators, or antioxidants.
Suggested daily nutritional requirements in humans
| Requirement | 70 kg Adult | |
|---|---|---|
| ENERGY REQUIREMENTS | ||
| Calories | 25kcal/kg | 1750kcal |
| Energy | 100kJ/kg | 7000kJ |
| MACRONUTRIENTS | ||
| Carbohydrate | 4g/kg | 280g |
| Protein | 1.5g/kg | 105g |
| Fat | 1g/kg | 70g |
| WATER AND ELECTROLYTES | ||
| H2O | 30 mL/kg | 2100ml |
| Na+ | 2mmol/kg | 140mmol |
| K+ | 1mmol/kg | 70mmol |
| Ca2+ | 0.1mmol/kg | 7mmol |
| Mg2+ | 0.1mmol/kg | 7mmol |
| PO4 | 0.1mmol/kg | 7mmol |
| VITAMINS | |
| Water sol B complex folate vitamin C vitamin B12 | |
| Fat soluble ADEK | |
| TRACE ELEMENTS | |
| Fe | 10mg |
| Zn | 15mg |
| Cu | 3mg |
| iodine | 150μg |
| manganese | 5mg |
| chromium | 200μg |
| selenium | 200μg |
Please include common sources of specific nutrients – This is best done based on individual candidate’s method of presentation
JC 2020
Examiner Comments
2020B 09: 40% of candidates passed this question.
This topic is well covered in the recommended physiology textbooks. Many answers unfortunately simply listed the various components without providing sufficient detail; outline questions require some context around the key points as opposed to just a list. Most candidates had a good estimate for the basal energy requirements of a resting adult. Good candidates were able to outline the g/kg daily protein requirements and the distribution of remaining energy intake between carbohydrates and lipids and included how this may change during periods of stress. They also stated the energy derived per gram of each of those food groups. Few candidates mentioned the need to include essential amino acids. Similarly, with fat intake, few candidates mentioned the need for essential fatty acids. A definition of “vitamin” would have received credit. Most candidates were able to classify vitamins as water soluble or fat soluble. Most candidates mentioned trace elements (with an abbreviated list) and mentioned bone minerals. A daily intake requirement for Na and K was expected, though not for bone minerals or trace elements.
10. Describe the pharmacology of suxamethonium.
Examiner Comments
2020B 10: 63% of candidates passed this question.
This was a level 1 pharmacology question, and it represents core knowledge. The mechanism of action of suxamethonium and the interactions at the neuromuscular junction as well as pharmaceutics were areas that often required further detail. Few candidates mentioned the effects of suxamethonium on the autonomic nervous system. Another common omission related to the factors that reduce plasma cholinesterase activity beyond genetic deficiency (such as liver disease, renal failure, thyrotoxicosis). Pleasingly, there was generally a good understanding of role, dosing, side effect profile, pharmacokinetics and of special situations and limitations of use pertinent to this drug.
11. Describe the changes in the circulatory system that occur during exercise.
CICMWrecks Answer
Definition:
Exercise = Strenuous physical activity where there is increased metabolic activity leading to increased requirement for energy and oxygen
CVS changes to accommodate and compensate for such increase in metabolism
Normal parameters at rest:
| Cardiac output | C.O. | 5L/min |
| Heart rate | HR | 60-100 bpm |
| Systemic vascular resistance | SVR | 1600 dynes.s.cm-5 |
| Stroke volume | SV | 70mls |
| Blood pressure | BP | 120/80 |
| Central venous pressure | CVP | 0-5mmHg |
Changes to CVS parameters:
| Cardiac output | ↑ | Can increase up to 4-5 folds CO plateaus at near-maximal workload |
| HR | ↑ | Max HR = 220-age Increases and plateaus at max |
| SV | ↑→↓ | Initially increases, then decreases due to diminishing diastolic time |
| Contractility | ↑ | Increased HR → increased contractility (treppe effect)Activation of sympathetic nervous system → increased contractility |
| CVP | ↑ | Increases |
| Systolic BP | ↑ | Increases |
| Diastolic BP | ↓ | Decreases |
| MAP | ↑ | Increases slightly |
| Pulse pressure | ↑ | Widens |
| PVR | ↑ | Decreases overall |
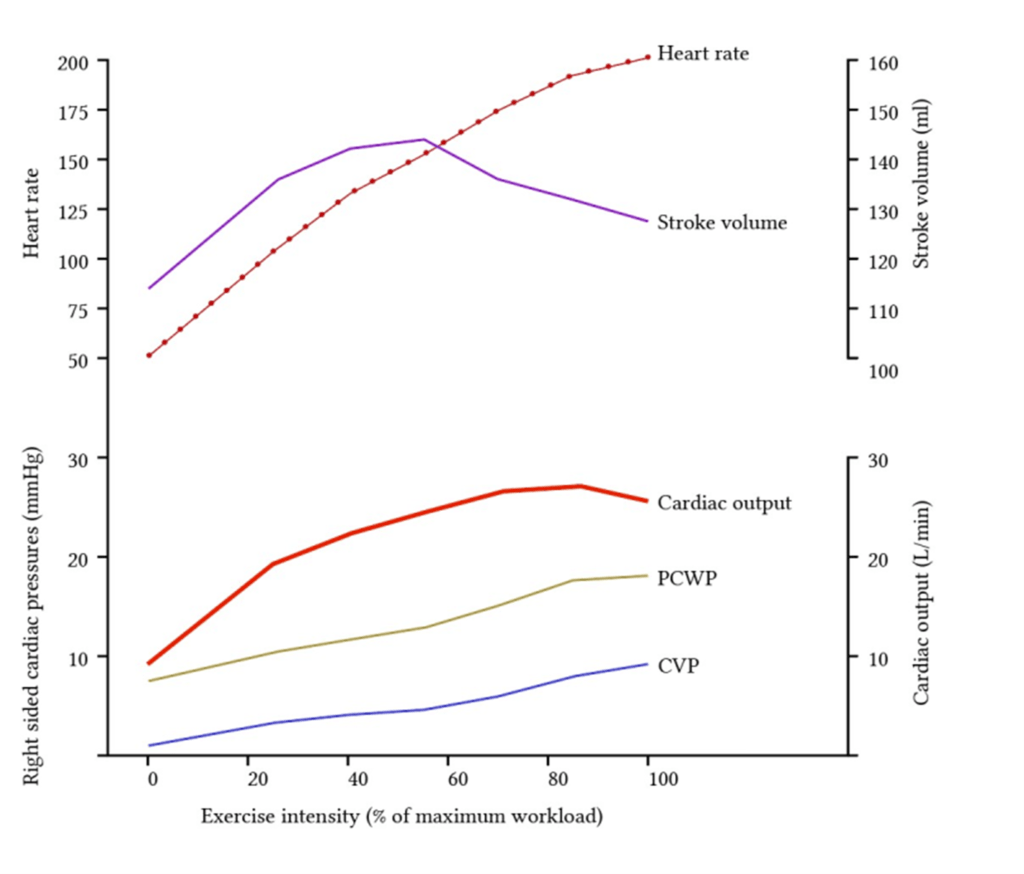
Redistribution of blood flow
- Increased muscle activity -> increased o2 demand
- Regional vasodilation
- Increased local concentration of metabolites ie Co2, lactate, K+
- Vasoactive mediator released by endothelium ie NO, ATP
- Sympathetic stimulation -> B2 adrenoceptor activation
- Overall: decrease in vascular resistance to muscles -> increased blood flow
- Corresponding vasoconstriction of viscera and skin
Increase in cardiac output
- Cardiac output can increase up to 0L/min
- Due to both increase in HR and SV and decreased afterload
- With increasing workload HR increases but SV decreases due to dec diastolic time
Guo 2021
Examiner Comments
2020B 11: 22% of candidates passed this question.
This is an applied physiology question. Better answers categorised the changes in some manner and included a measure of the degree of change as applicable (e.g., what increases, what decreases and what may stay the same). The question was to describe the changes so that the detail behind the mechanisms enabling these changes to occur was expected (e.g., neurohumoral, local factors). Marks were also awarded for any regional variation that occurs.
12. Describe the physiology (50% marks) and pharmacology (50% marks) of albumin.
CICMWrecks Answer
Physiology of Albumin
| ALBUMIN | |
| DEFINITION | A naturally occurring protein found in blood plasma. Recombinant human albumin is a product that is manufactured to be structurally equivalent to native human serum albumin. |
| CLASSIFICATION | 6 subfamilies – vit D binding proteins occupy family 1-2 |
| SYNTHESIS | In liver |
| Synthesized as preproalbumin → N-terminal peptide removed → proalbumin → cleared in Golgi vesicle → albumin | |
| FACTORS AFFECTING SYNTHESIS | Nutrition |
| Liver function | |
| infection /sepsis/inflammation | |
| DISTRIBUTION | 40% intravascular space 60% extravascular space |
| The balance between plasma and interstitial space is established at varying rates with respect to the two subcompartments of the extravascular albumin pool | |
| Factors affecting distribution: HTN, CCF, trauma, exercise, DM, Burns, CPB | |
| BREAKDOWN | Broken down by cysteine protease |
| Elimination half life – 16hrs | |
| FUNCTIONS OF ALBUMIN | |
| Osmotic pressure | supplies 80% of total plasma Colloid Osmotic Pressure |
| retards fluid efflux from plasma and oedema formation | |
| Transport and metabolism functions | Transports thyroid hormones |
| Transports other hormones, in particular, ones that are fat-soluble | |
| Transports fatty acids (“free”) to the liver and to myocytes for utilization of energy | |
| Transports unconjugated bilirubin | |
| Transports many drugs; serum albumin levels can affect the half-life of drugs | |
| Buffer | Extra-cellular acid-base buffer |
| Detoxification | solubilizes bilirubin and neutralizes its toxic effects |
| Anti-oxidant effects | Blocks Cu2+-mediated LDL oxidation |
| Blocks Free-radical-mediated haemolysis | |
| Anticoagulant effect | |
| Protein store | for signalling molecules and nitric oxide |
| Immunomodulation | |
| Other | Competitively binds calcium ions (Ca2+) |
| Prevents photodegradation of folic acid | |
| marker of an inflammatory state | |
| prevents apoptosis of proximal renal tubular cells | |
| stimulates proliferation of proximal renal tubular cells | |
| MANUFACTURING PROCESS | |
| Source | From Aus-sourced plasma Pooled from thousands of donations (minimises the infection risk) |
| Whole plasma is fractionated to prepare Albumin | |
| Fractionation | Physical separation (by precipitation) |
| Chromatography (separates molecules based on chemical and physical properties | |
| Removal of pathogens by: – Pasteurisation (vapour heat 60C for 10hrs) – Use of solvents and detergents – Exposure to low pH conditions – Nanofiltration | |
| Partitioning | allows purification of immunoglobulins, clotting factors and albumin from plasma |
| Storage | stored in secure & monitored environments |
Pharmacology of Albumin
CICMWrecks Table – Albumin (Click to Open)
Guo / JC 2021
Examiner Comments
2020B 12: 19% of candidates passed this question.
The question required an equal treatment of the physiology and pharmacology of albumin. The physiology discussion needed to include synthesis, factors affecting synthesis, distribution in the body (including the proportion divided between the plasma and interstitial space), functions, breakdown, and elimination half-life. Discussion of the pharmacology should have included available preparations (4% and 20% Albumin) and pharmaceutics, distribution, elimination (both the protein and crystalloid components), mechanism of action to expand the plasma compartment, longevity in the plasma compartment, indications, and adverse effects. Oedema, circulatory overload, immunological reactions, and relative contraindication in brain injury were important to mention. There was some confusion regarding the infectious risks of albumin. An outline of the manufacturing process from donated plasma and pasteurisation was expected.
13. Describe the anatomical (20% marks) and physiological (80% marks) features of the pulmonary circulation.
CICMWrecks Answer
Anatomy
- Thin walled arteries and veins, contains little smooth muscle → easily distorted by lung expansion
- Pulmonary arteries (deoxygenated blood) -> pulmonary capillaries (gas exchange) → pulmonary veins (oxygenated blood)
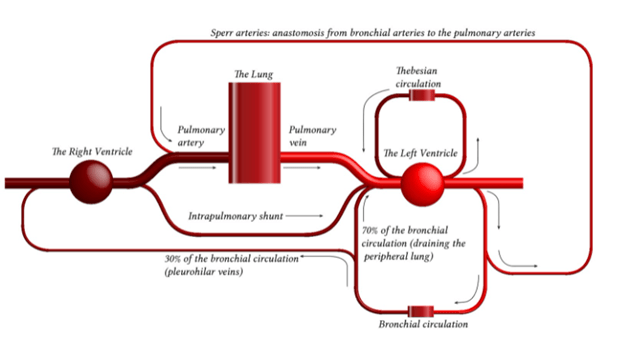
- Pulmonary capillaries are exposed to alveolar pressure and not supported by solid tissue → prone to collapse
- Contains 10% of circulating blood volume – 500mls
Physiology
- General:
- Low pressure 25/8 MPAP 15mmHg
- Low resistance 20-120 dynes.sec.cm-5
- Flow: total of cardiac output 5L/min
- Pulmonary blood volume
- ~500mls of blood – blood reservoir
- Varies over course of the respiratory and cardiac cycle and in response to gravity
- Varies in response to changes in intrathoracic pressure
- Pulmonary blood pressure
- Normal PA systolic pressure = 18-25 mmHg
- Normal PA diastolic pressure = 8-15 mmHg
- Normal mean pulmonary arterial pressure = 9-16 mmHg
- Capillary pressure is 8-10 mmHg
- Venous pressure 6-12 mmHg
- Resistance
- The resistance in the pulmonary circulation can be calculated as follows:
- PVR = 80 × (mPAP- PAOP)/CO
- The normal value for PVR is 100-200 dynes/sec/cm-5
- Approximately 1/10th of systemic circulation
- Regulation of blood flow/resistance
- West zones
- In zone 1, PA > Pa > Pv No flow of blood
- In zone 2, Pa > PA > Pv Resistance to flow is determined by alveolar pressure (Starling resistor effect)
- In zone 3, Pa > Pv > PA Resistance to flow is determined by venous pressure Venous pooling causes increased distension of pulmonary capillaries
- In zone 4 Low lung volume causes narrowing of extra-alveolar vessels
- Hypoxia – pulmonary hypoxic vasoconstriction
- Lung volumes
- West zones
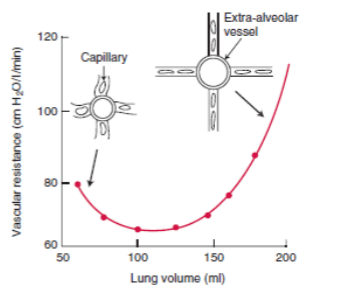
- Regional distribution
- Distension of partially collapsed capillaries
- Recruitment of completely collapsed capillaries
- Local mediators: NO, prostacycline
- Function
- Gas exchange
- Filtration of clots and debris
- Immunological – pulmonary macrophages, IgA production
- Metabolic – metabolism of drugs, removal of proteases
- Endocrine – source of ACE
Guo 2021
Examiner Comments
2020B 13: 25% of candidates passed this question.
The examiners consider that an understanding of the pulmonary circulation is core area of the syllabus. In general, the anatomy section was better answered than the physiological features. As well as a description of the gross anatomy of the pulmonary circulation tracking it from the pulmonary valve to the left atrium, some mention of the microscopic anatomy was required (e.g., that the pulmonary arteries are thin walled with little smooth muscle).
For the second part of the question, a breadth of knowledge was required. Candidates were expected to address the following physiological features of the pulmonary circulation: volume, pressure, resistance, regulation and regional distribution and function. Marks were apportioned to each section, so it was important to write something on each section. Focussing on one section in detail (e.g., a very detailed description of West’s Zones) usually came at the expense of missing one or more of the other sections, most commonly the functions of the pulmonary circulation. Indeed, candidates that scored well provided information on each section and for the functions of the pulmonary circulation mentioned more than gas exchange.
14. Describe the anatomy of the larynx.
CICMWrecks Answer
Cartilages
- Unpaired
- Thyroid cartilage – Level of C4~5
- Cricoid cartilage
- Epiglottis
- Paired
- Arytenoid
- Cuneiform
- Corniculate
Muscles
- Intrinsic
- Cricothyroid
- Originates in cricoid cartilage and inserts into thyroid
- Tenses vocal cords and elevates voice
- Thyroarytenoid
- Originates in thyroid cartilage and inserts into arytenoid cartilage
- Relaxes vocal cords and depresses voice
- Posterior cricoarytenoid
- Abducts the vocal cords
- Lateral cricoarytenoid
- Adducts the vocal cords
- Oblique and transverse arytenoids
- Adducts the vocal cords
- Cricothyroid
- Extrinsic
- Strap muscles
Innervation
Sensory
- Internal branch of superior laryngeal nerve
Motor
- Cricothyroid – External branch of the superior laryngeal nerve
- All other intrinsic muscles – Recurrent laryngeal nerve
Blood supply
- Superior laryngean artery (branch of external carotid)
- Inferior laryngeal artery (branch of thyrocervical trunk from subclavian artery)
Associations
- The thyroid glands lie inferolateral to the larynx (lateral to the cricoid, the isthmus is inferior to the cricoid)
- The oesophagus, anterior longitudinal ligament, cervical vertebrae lies posterior to the larynx
- The brachiocephalic trunk may arch superiorly close to the cricothyroid membrane in an anatomical variant
Sakurai 2016
Examiner Comments
2020B 14: 40% of candidates passed this question.
For this question, candidates were expected to address the location of the larynx, its relations, the cartilages (single and paired), ligaments, muscles (intrinsic and extrinsic), innervation (sensory and muscular) and blood supply (including venous drainage). Marks were apportioned to each section, so whilst some detail was required, breadth of knowledge was also important. Most candidates had a grasp of the gross anatomy, the main relations and at least the innervation provided by the recurrent laryngeal nerve. However, an understanding of the functional anatomy of the cartilages was not always apparent. It should be noted that not every single muscle needed to be named (especially for the extrinsic muscles), but an understanding of the major muscle groups should have been included.
15. Compare and contrast the pharmacology of dobutamine and levosimendan.
Examiner Comments
2020B 15: 41% of candidates passed this question.
The objective of this question was that candidates relay a detailed knowledge of both drugs with respect to their individual pharmacology highlighting the important clinical aspects of each drug (e.g., mechanism of action, metabolism, duration of effect). Then an integration of this knowledge was in the contrast section where the better candidates highlighted features of the drug that would influence when or why one may use it with respect to the second agent. Tabular answers of the pharmacology of each drug without any integration or comparison scored less well. A detailed knowledge of both agents was expected to score well.
16. Describe the formation of gastric acid (50% marks) and the regulation of gastric acid secretion (50% marks).
CICMWrecks Answer
Volume and composition of gastric juice:
- 2-2.5 L/day of gastric juice is produced
- It contains:
- H2O and electrolytes (> 99.5%)
- Gastric juice is slightly hyperosmotic (325 mOsm/L) with ↑ H+ (150-170 mmol/L), ↑ Cl- (190 mmol/L), and ↑ K+ (10 mmol/L), but ↓ Na+ (2-4 mmol/L) → cf. plasma
- It is also more acidic (pH 1-1.5) → due to ↑ H+ content
- Solid material (< 0.5%)
- Digestive enzymes – Pepsin, Gastric lipase, Gastric amylase
- Mucous in alkaline fluid (HCO3 – -rich)
- Intrinsic factor
- H2O and electrolytes (> 99.5%)
Gastric Acid Secretion:
Parietal cells contain an H+-K+ ATPase (exchange) pump – GPCR – cAMP dependent
- This pump is activated in response to increased levels of intracellular Ca2+ from stimulation by:
- ACh
- Histamine (H2)
- Gastrin
- Inhibited by:
- Low gastric pH
- Somatostatin
Process:
- H+ is produced by action of carbonic anhydrase on CO2 and water, with ‘waste’ HCO3– removed from the cell in exchange for Cl–.
- Respiratory quotient of the stomach may become negative due to consumption of CO2
- HCO3– :
- transported out of the basolateral membrane in exchange for chloride.
- Outflow into blood results in a slight elevation of blood pH known as the “alkaline tide”. This process serves to maintain intracellular pH in the parietal cell.
- Chloride and potassium ions are transported into the lumen of the cannaliculus by conductance channels, and such is necessary for secretion of acid.
- Hydrogen ion is pumped out of the cell, into the lumen, in exchange for potassium through the action of the proton pump; potassium is thus effectively recycled.
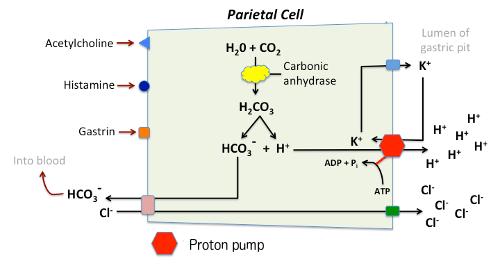
Regulation of gastric juice secretion:
- Cephalic phase → 50% of gastric juice secretions
- Initiated by thought, sight, taste and smell of food
- Mediated via vagal (ACh) outflow
- Gastric phase → ~ 50% of gastric juice secretions (prolonged secretion at a slower rate)
- Initiated by entry of food into stomach
- Mediated by – (i) Local and vago-vagal reflexes → due to distension of body (of stomach), and (ii) Gastrin release from G-cells → due to antral distension
- Intestinal phase → < 1% gastric juice secretion
- Initiated by chyme entering duodenum
- Mediated by enterogastric neural (ANS/ENS) and hormonal (CCK, VIP, GIP, Etc.) reflexes
JC 2019
Examiner Comments
2020B 16: 26% of candidates passed this question.
The is question was divided into two sections offering equal marks. The first section required a description of the generation and transport of both H+ and Cl into the stomach lumen by the parietal cell. The contributions of basolateral and luminal ion channels, the role of carbonic anhydrase and accurate description of the net flux was expected for full marks. The second section required comments on the roles of neural and endocrine regulation. Increased acid secretion via acetylcholine (via muscarinic M3), histamine (via H2) and gastrin were expected as was reduced secretion via secretin and somatostatin. Better responses were able to combine and integrate these into cephalic, gastric, and intestinal phases. The nature and function of other gastric secretions and the role of pharmacologic agents was not asked for and therefore not awarded any marks.
17. Describe the pharmacology of inhaled nitric oxide (NO).
Examiner Comments
2020B 17: 24% of candidates passed this question.
Nitric Oxide (NO) is an inorganic colourless and odourless gas presented in cylinders containing 100/800 ppm of NO and nitrogen. Many candidates mentioned oxygen instead of nitrogen. The exposure of NO to oxygen is minimized to reduce formation of nitrogen dioxide and free radicals. Hence it is administered in inspiratory limb close to the endotracheal tube. Many candidates did not mention the contraindications/caution for NO use. Candidates generally did well in mentioning the impact on improving V/Q mismatch by promoting vasodilatation only in the ventilated alveoli and reducing RV afterload. Many candidates did not mention the extra cardio-respiratory effects. The expected adverse effects of NO were nitrogen dioxide related pulmonary toxicity, methemoglobinemia and rebound pulmonary hypertension on abrupt cessation. Pharmacokinetics of NO carried a significant proportion of marks. It was expected that the answers would involve mention of location of delivery of NO in inspiratory limb and reason behind it, the high lipid solubility and diffusion, the dose (5-20ppm), very short half-life of < 5 seconds and combination with oxyhemoglobin to produce methaemoglobin and nitrate. The main metabolite is nitrate which is excreted in urine.
18. Define afterload (10% marks) and describe the physiological factors that may affect afterload on the left ventricle (90% marks).
CICMWrecks Answer
Afterload
- Load against which the muscle exerts its contractile force (Guyton)
- It is represented by the gradient of the line connecting the end-diastolic volume, to the end-systolic point
- pressure which the ventricle has to contract against (Power & Kam)
Determinants of Afterload
Modified Laplace Equation
- where
- T represents afterload
- P represents aortic pressure
- Therefore, afterload increases as aortic (or pulmonary arterial) pressure increases
- R represents ventricular radius
- Afterload increases as ventricular radius increases
- H represents ventricular thickness
- Afterload decreases as the thickness of the ventricular wall increases (hypertrophy secondary to chronic hypertension and cardiac remodelling)
Modified Hagen-Poiseuille Equation
- Afterload is affected by resistance to cardiac output
- Afterload increased by reduced radius of systemic vasculature
- Afterload increased by increasing viscosity of blood
Resistance in parallel
- Afterload is affected by addition, or loss of large capillary networks in parallel
- Systemic vascular resistance is increased significantly by loss of placenta, with parallel vascular networks
- Pulmonary vascular resistance is decreased significantly by inflation of lung, causing creation of vast capillary network in parallel
Factors affecting Right Ventricular Afterload
- Pulmonary vascular resistance increases afterload
- Hypoxic vasoconstriction increases PVR
- Lung volumes
- Pulmonary vascular resistance minimal at FRC
- Increased pulmonary artery pressure increases RV afterload
- Left heart failure
- Critical mitral stenosis, mitral regurgitation
- Pulmonary emboli
- Right ventricular outflow tract obstruction increases afterload
- Pulmonary stenosis
- Saddle pulmonary embolism
- Right ventricular dilation
- Susceptible due to thin RV wall
- Acute PE
- RV spiral of death
- Increased intraventricular pressure decreases blood flow à ischaemia à decreased contractility à further increase in ventricular volume
- RV spiral of death
Factors affecting Left Ventricular afterload
systemic vascular resistance, aortic impedance and ventricular radius
According to La Place Equation
- Aortic pressure
- Afterload increases as aortic pressure increases (increases with hypertension)
- Ventricular radius
- Afterload increases as ventricular radius increases (increases with ventricular dilation)
- Ventricular wall thickness
- Afterload decreases and ventricular wall thickness increases (decreases with ventricular hypertrophy)
Determinants of aortic pressure indirectly affect afterload
- Aortic compliance
- Afterload decreases with increased compliance
- Arterial blood volume
- Given set compliance, afterload will increase with arterial volume
- From Modifed Poisuille-Hagen Equation
- Radius of aorta
- Autonomic vasomotor tone
- Increased intrathoracic pressure
- Physical compression
- Viscosity of blood
- Radius of aorta
- From equation MAP = CO / SVR
- Tissue flow autoregulation
- Myogenic
- Metabolic
- Tissue flow autoregulation
Other
- Left ventricular outflow tract obstruction
- Aortic stenosis
- HOCM
- Coarctation of aorta
Sakurai 2016
Examiner Comments
2020B 18: 53% of candidates passed this question.
Afterload can be defined as factors resisting ventricular ejection and contributing to myocardial wall stress during systole. Most answers utilised the law of Laplace to expand upon factors affecting ventricular wall tension. Systemic vascular resistance was commonly mentioned, but less frequently defined. Aortic and left ventricular outflow tract impedance were commonly referred to. Effects of preload and neurohumoral stimuli were less well outlined. Description of factors affecting right ventricular afterload and depictions of left ventricular pressure volume loops earned no extra marks unless directly referenced to the question.
19. Explain how the kidney handles an acid load.
CICMWrecks Answer
Non-volatile acid
” An acid produced from sources other than carbon dioxide, which cannot be excreted in the lungs. “
- e.g. lactate, sulphate, phosphate and ketone bodies
- 70-100mmol non-volatile acid produced per day
- 4000-5000mmol HCO3– filtered by glomerulus per day
- Renal acid-base is largely determined by handling of bicarbonate ions
- 80% of filtered bicarbonate resorbed in proximal tubule
- 20% resorbed in distal tubule and collecting duct
- Urinary Buffers (Dibasic Phosphate and ammonia) play a role
Bicarbonate Resorption
Proximal tubule
In the tubular cell:
- H+ leaves the tubular cell through the apical membrane via
- H+/Na+ antiporter (secondary active transport via Na+/K+ ATPase on basolateral membrane)
- The HCO3– re-enters the circulation via the Na/HCO3– symporter
- H+ combines with HCO3– in tubule to form H2CO3
- H2CO3 switches back to CO2 and H2O, CO2 is resorbed to turn back into the tubular cell to generate more HCO3 for resorption
More bicarbonate resorbed when:
- Higher filtered HCO3–
- Higher PaCO2
- Lower luminal flow rate
- Angiotensin 2
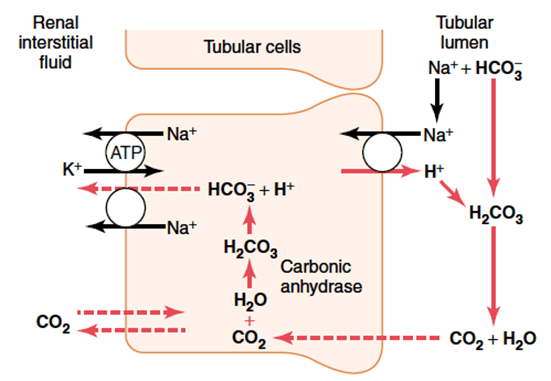
Distal convoluted tubule and collecting duct
- Similar mechanism with regards to resorption of HCO3– via secretion of H+
- Difference is that H+ is transported across the apical membrane via an H+ ATPase (active transport)
Role of Buffers
- The maximum urinary pH is 4.5
- To eliminate more protons, they must be buffered in the tubular fluid
- H+ is bound preferentially to buffers only in the presence of low tubular HCO3–
- Buffering, and so elimination of H+ without its conjugate HCO3–, requires resorption of HCO3– in the proximal convoluted tubule
Phosphate (HPO42-):
- Becomes concentrated in tubules
- pKa = 6.8, so operates effectively as a buffer at tubular pH
Ammonia
- Glutamine broken down in tubular epithelia cells to two NH4+ and two HCO3–
- Comes from hepatic amino acid metabolism
- The new HCO3– is transported back to the circulation via the Na/HCO3– symporter to re-enter the circulation
- NH4+ becomes ion trapped in the tubular fluid
- Proximal tubular fluid is not acidic enough to keep NH4+ in the tubule
- It is resorbed in the ascending limb, moves through the medulla to re-enter the collecting duct
- Here urine has a low pH, and so NH4+ can be eliminated with its proton attached
Mooney 2016
Examiner Comments
2020B 19: 51% of candidates passed this question.
This question required candidates to understand the renal response to an acid load. It was expected that candidates would answer with regard to recycling of bicarbonate in the proximal tubule, excretion of titratable acid via the phosphate buffer system and generation of ammonium and its role in acid secretion. Many candidates had a good understanding of the bicarbonate system but used this to explain the secretion of new acid.
20. Describe the pharmacology of intravenous sodium nitroprusside.
Examiner Comments
2020B 20: 49% of candidates passed this question.
This was a straightforward pharmacology question relating to a relatively common and archetypal intensive care medication. The structure of the question was well handled by most of the candidates; easily falling into the classic pharmaceutics, pharmacokinetic and pharmacodynamics framework. Many candidates had a superficial knowledge of the presentation and formulation of the drug, aside from its light sensitivity. Better answers detailed the drug according to the above-mentioned framework but also accurately highlighted specific points relevant to the ICU practise such as the metabolic handling of sodium nitroprusside and relating this to the consequences of the various metabolic products.
VIVAs
| A. Pharmaceutics | |
| B. Pharmacokinetics | |
| C. Pharmacodynamics | |
| D. Variability in Drug Response | |
| E. Cellular Physiology | Movement of solute across semi-permeable membrane Movement of solute across semi-permeable membrane |
| F. Respiratory | Elderly: gas exchange, physiology, pharmacology Factors that affect respiratory rate Oxygen content, transport Oxygen cascade Compliance |
| G. CVS | AP of pacemaker cell, anti-arrhythmic agents, ECG Systemic vs pulmonary circulation Coronary blood flow regulation Structure of left ventricle, beta-blockers CVP, Fluctiations in right atrial pressure |
| H. Renal | Bowman’s capsule, renal anatomy and physiology |
| I. Body Fluids and Electrolytes | Water content 70kg male, distribution, regulation 1L blood loss over 10 minutes |
| J. Acid Base | ABG Interpretation (pH 7.3 pO2 400 pCO2 30 HCO3 14 BE -8 Na 140 K 4 Cl 120) Henderson Hasselbach equation, Albumin |
| K. Neuro | Noxious stimulus: sudden sharp cut to hand Cerebral blood flow, receptors Benzos: Midazolam, effects Cranial neve anatomy, physiology of brain stem reflexes, pupillary reflex GABA signalling, Propofol Opioid receptors, pharmacology |
| L. Musculoskeletal | Smooth muscle, G proteins |
| M. ANS | |
| N. Liver | Functions of liver, biliary metabolism |
| O. GIT | |
| P. Nutrition and Metabolism | |
| Q. Haematology | Platelet production, anti-platelet drugs Cell-based model, initial phase of coagulation Classic intrinsic pathway, coagulation |
| R. Thermoregulation | |
| S. Immunology | |
| T. Microbiology | Bacteria gram stain, antibiotics Aminoglycoside pharmacology, Gentamicin MoA |
| U. Endocrine | Thyroid physiology, fate of dietary iodine, beta-blockers |
| V. Obstetrics | Uterine blood flow at term, percentage to placenta |
| W. Measurement and Monitoring | |
| X. Procedures |

Recent Comments