Syllabus (Fourth Edition, 2023)
Topics
i. Explain the Frank-Starling mechanism and its relationship to excitation-contraction coupling.
ii. Explain the measurement of central venous pressure, the components of its waveform and the factors that determine its magnitude.
iii. Define the components and determinants of cardiac output including the effects of positive pressure ventilation.
iv. Describe myocardial oxygen demand and supply and the conditions that may alter each.
v. Describe and explain cardiac output curves, vascular function curves and their correlation.
vi. Describe the pressure-volume relationships of the ventricles and their clinical applications.
vii. Describe the cardiac reflexes.
Topics not covered in previous SAQs
i. Explain the Frank-Starling mechanism and its relationship to excitation-contraction coupling.
v. Describe and explain cardiac output curves, vascular function curves and their correlation.
Learning Objectives for the First Part Examination in Intensive Care Medicine
- This will ensure that trainees, tutors, and examiners can work from a common base.
- All examination questions are based around this Syllabus.
- These learning objectives are designed to outline the minimum level of understanding required for each topic.
- The accompanying texts are recommended on the basis that the material contained within them provides sufficient information for trainees to meet the learning objectives.
- Trainees are strongly encouraged to explore the existing and evolving body of knowledge of the Basic Sciences as they apply to Intensive Care Medicine by reading widely.
- For all sections of the syllabus an understanding of normal physiology and physiology at extremes of age, obesity, pregnancy (including foetal) and disease (particularly critical illness) is expected.
- Similarly, for pharmacology, trainees are expected to understand a drug’s pharmacology in these contexts.
- An understanding of potential toxicity and relevant antidotes is also expected.
Definitions
Throughout the document specific wording has been used under the required abilities to indicate the level of knowledge and understanding expected and a glossary of these terms is provided.
Definitions
| Calculate | Work out or estimate using mathematical principles. |
| Classify | Divide into categories; organise, arrange. |
| Compare and contrast | Examine similarities and differences. |
| Define | Give the precise meaning. |
| Describe | Give a detailed account of. |
| Explain | Make plain. |
| Interpret | Explain the meaning or significance. |
| Outline | Provide a summary of the important points. |
| Relate | Show a connection between. |
| Understand | Appreciate the details of; comprehend. |
SAQs
i. Explain the Frank-Starling mechanism and its relationship to excitation-contraction coupling.
ii. Explain the measurement of central venous pressure, the components of its waveform and the factors that determine its magnitude.
2021A 05
Outline the factors that determine central venous pressure (60% marks) and explain how it is measured (40% marks).
2019B 06
Outline the factors that determine central venous pressure and explain how it is measured.
CICMWrecks Answer
CVP
- Central venous pressure (CVP) is mean vena caval or right atrial pressure
- In the absence of tricuspid stenosis, equals right ventricular end-diastolic pressure.
- expressed in mmHg or cmH2O (1.36 cm water = 1.0 mm Hg)
- Normal 0-6mmHg in spontaneously breathing non-ventilated patient
- Venous Return, VR = (MSFP – RAP or CVP) / Resistance to venous return
- CVP is a major determinant of RV filling pressure (RV Preload)
- This regulates stroke volume through Frank-Starling mechanism
- CVP increased in disorders that increase Rt sided diastolic pressures
- left heart disease, lung disease, primary pulmonary hypertension, and pulmonic stenosis
- CVP is determined by an interplay of various factors
- Mainly influenced by volume of blood and compliance of central compartment
Factors affecting the measured CVP:
- Central venous blood volume (Inc volume à inc flow à inc CVP)
- Venous return/cardiac output
- Vena cava compression (Obesity/Valsalva/pregnancy/inc IAbd pressure – dec VR à dec CVP)
- Total blood volume
- Regional vascular tone
- Venous return/cardiac output
- Compliance of central compartment (inc Comp à inc return and venous pressure)
- Vascular tone
- Right ventricular compliance
- Myocardial disease
- Pericardial disease
- Tamponade
- Tricuspid valve disease
- Stenosis (inc mean CVP)
- Regurgitation (transient inc CVP)
- Cardiac rhythm (dec CVP with no atrial contraction, inc CVP when RA contracting against closed TV)
- Junctional rhythm
- AF
- A-V dissociation
- Reference level of transducer (inc or dec)
- Positioning of patient
- Intrathoracic pressure (inc pressure à inc CVP)
- Respiration (Inspiration makes intrathoracic pressure more negativeà increases CVP)
- Intermittent positive pressure ventilation (IPPV)
- Positive end-expiratory pressure (PEEP)
- Tension pneumothorax
Measurement
- Invasive:
- by inserting central venous catheter into Internal Jugular or Subclavian Vein
- Non-Invasive:
- Height of JVP provides visual estimate
- peripheral vein collapse on the dorsum of the hand or antecubital fossae
- Bedside ECHO and doppler techniques: IJV, Hepatic veins, IVC assessment
Invasive Measurement:

Complications of CVP Monitoring
- Mechanical complications:
- Arterial puncture and cannulation, Hematoma, Hemothorax, Surrounding nerve injuries, Pneumothorax, Embolization of broken catheter or guide wire, Air embolus, Arrhythmias, Lymphatic system injury
- Infectious complications:
- Sepsis, Endocarditis
- Thrombotic complications:
- Venous thrombosis, Pulmonary embolism
CVP Waveform
(not required for this answer)
| Mechanical | ECG | Clinical | |
|---|---|---|---|
| a wave | Atrial contraction | Starts just after p wave ends | Closely parallels increase in RVEDP |
| z point | Just before closure of TV (just at onset of c wave) | Coincides with middle of QRS | Good indicator of RVEDP esp when a waves not visible (AF) |
| c wave | Closure of tricuspid valve Crest – bulging of TV | Correlates with end of QRS complex | |
| x descent | Fall in intra atrial pressure during atrial relaxation | Before T wave | |
| v wave | Passive atrial filling Crest – opening of TV | Usually after t wave | |
| y descent | Decreased atrial pressure as RA empties into RV | Before p wave |
Changes in waveform:
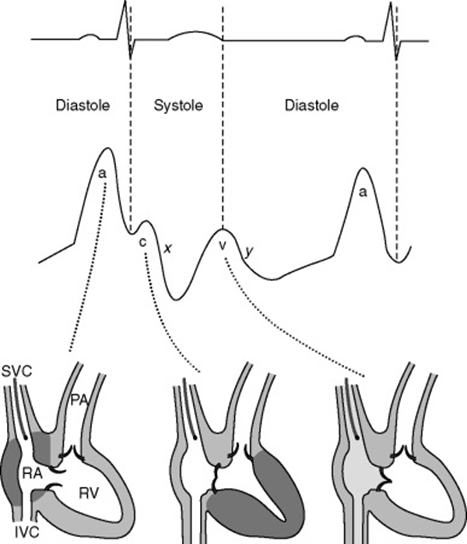
| AF | a wave lost, c wave more prominent. If coarse AF, fibrillation waves may be visible |
| A-V dissociation / Junctional rhythm | tall canon a waves (due to atrial contraction against closed Tricuspid valve) |
| TR | large fused c-v wave (due to blood ejected back during ventricular systole) |
| TS | attenuated a-wave and y-descent (increased pressure required to overcome TS) |
| RV compliance decreased | a wave accentuated |
| Pericardial constriction | short steep y-descent |
| Cardiac tamponade | CVP monophasic with a single x-descent |
Sources:
Daniel Saddawi-Konefka, Critical Care Secrets
Shay McGuinness Cardiothoracic Critical Care 2007
Richard E. Klabunde, CVphysiology.com
T. Smith, Central Venous Pressure: Uses and Limitations
JC 2019
Examiner Comments
2021A 05: 57% of candidates passed this question.
This question examined a core area of cardiac physiology and measurement. Considering this, candidates overall, scored poorly in this section. There was a common misunderstanding around the relationship between cardiac output and CVP. A decrease in cardiac output (e.g. due to either decreased stroke volume or heart rate) will cause an increase in CVP as blood backs up in the venous circulation, increasing venous volume as less blood moves through to the arterial circulation; the resultant increase in thoracic volume increases central venous pressure. Several candidates confused the direction of their arrows, for example “increased right atrial compliance increases CVP”. Double negatives were used by several candidates which then resulted in the incorrect relationship described. (e.g., “arrow down compliance and arrow down CVP”). The measurement section should have included an explanation of the components of an invasive pressure monitoring system relevant to the measurement of CVP.
2019B 06: 18% of candidates passed this question.
It was expected that answers include central venous blood volume, central venous vascular compliance, intrathoracic pressure and tricuspid valvular function. Good answers outlined how each of these factors determine CVP and whether it was increased or decreased. Many candidates incorrectly described the effect of venous return.
iii. Define the components and determinants of cardiac output including the effects of positive pressure ventilation.
2021B 03
Discuss the physiological determinants of cardiac output.
2011B 13
Discuss the regulation of cardiac output. Illustrate your answer by using a graph to describe the important physiological relationships.
CICMWrecks Answer
Cardiac output is the amount of blood pumped into the systemic circulation per unit time.
Normal Cardiac Output = 5l/min
Normal Cardiac Index = 3l/min/m2
Cardiac Output = SV x HR
Stroke Volume
SV = Amount of blood pumped into the circulation per contraction
Usually 70mls
Influenced by Preload, Afterload, Contractility
- Preload / Venous Return:
- = (MSFP – RAP) / RVR
- MSFP: Mean Systemic Filling Pressure
- Equilibration of pressures in the systemic circulation if cardiac output was abolished
- Describes the filling state of the circulation and tone of capacitance vessels
- RAP: Right Atrial Pressure
- RVR: Resistance to Venous Return
- Calibre of transmitting venous system according to Poisuille-Hagen equation (where resistance ∝ 1/r4)
- Physical obstructions to venous return
- Mechanical obstruction
- Valvular stenosis
- MSFP: Mean Systemic Filling Pressure
- Thoracic pump (negative intrathoracic pressure created during inspiration)
- Skeletal muscle pump
- One way valves
- Pump function of the ventricle
- Venous tone
- = (MSFP – RAP) / RVR
- Afterload
- Proportional to aortic pressure and ventricular size
- Inversely proportional to ventricular wall thickness
- Affected by obstruction to the LVOT
- Contractility
- Affected by adrenergic or muscarinic stimulation
- Availability of Ca
Heart Rate
- Affected by automatic rhythmicity of cell and sympathetic/muscarinic input
Cardiac Output regulated by venous return
- Ventricles have ability to adapt to various degrees of filling according to the Frank-Starling Principal
- Increasing preload increases cardiac output to a degree, before cardiac output falls
- Due to the optimal alignment of actin and myosin filaments at sarcomere length of 2.2μm
- Also due to some stretch related release of Ca2+ increasing contractility
Autonomic Regulation
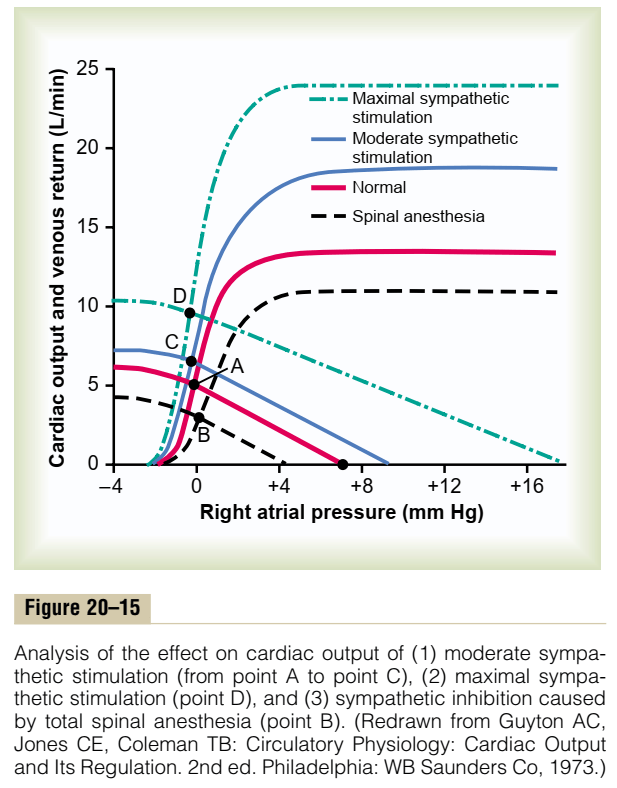
- Medullary vasomotor area
- Inputs to sensory area in NTS
- Vasoconstrictor area in anterolateral upper medulla
- Sympathetic output
- Vasodilator area in anterolateral lower medulla
- Parasympathetic output
- Adrenaline and to a lesser extent noradrenaline stimulates β2 adrenoceptors
- GsPCR
- Increased cAMP due to upregulation of adenylyl cyclase
- Activation of PKA leading to phosphorylation and activation of intracellular enzymes
- Inotropy
- Increased Ca2+ release from and SR and influx from T-tubules
- Increased intracellular [Ca2+]
- Increased Ca2+ – troponin C interaction
- Increased Actin and myosin interaction due to exposure of myosin binding sites
- Increased contractility
- Chronotropy
- Inotropy
- Acetylcholine
- GiPCR → decreased cAMP and reversing the above
- Muscarinic receptor coupled K+ channels hyperpolarize the cell reducing chance for action potential
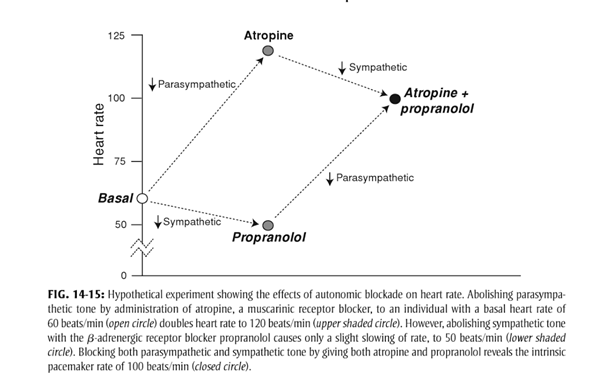
- At rest
- Parasympathetic and Sympathetic tone
- Parasympathetic > Sympathetic
- HR ~60
- + Propranolol → HR 50
- + Atropine → HR 110
- + Propranolol + Atropine → HR 100
- HR ~60
- Parasympathetic > Sympathetic
- Parasympathetic and Sympathetic tone
Reflexes
Baroreflex
- Sensor
- Aortic arch and carotid sinus stretch receptors
- Transmit via vagal nerve and glossopharyngeal nerve respectively
- Control centre
- Vasomotor area in medulla
- Increased stretch causes inhibition of sympathetic and promotion of parasympathetic output
- Effector
- Parasympathetic innervation of the heart via vagal nerve upregulated
- Sympathetic innervation via thoracic segments of the sympathetic chain
Bainbridge reflex
- Input
- Increased venous return or obstruction to atrial outflow causes atrial stretch
- Atrial stretch receptors signal to vasomotor area
- Increased sympathetic output to heart à tachycardia
Sakurai 2016
Examiner Comments
2021B 03: 65% of candidates passed this question.
Although the pass rate for this question was reasonably high the examiners commented on a lack of detailed knowledge within most answers for such a core component of our daily practice. Several candidates failed to provide a normal value and only few provided anything other than 5l/min. There was a general lack of detail, and at times, some confusion about the Frank Starling effect. Most candidates outlined the major determinants of stroke volume, although many were light on the
determinants of each or incorporated incorrect facts. Several candidates did not mention HR as a determinant of CO.
2011B 13: 11 (44%) of candidates passed this question.
Most candidates approached this question by defining cardiac output as stroke volume × heart rate and then discussing the determinants of cardiac output – preload, contractility, afterload and heart rate rather than focusing on the regulation of cardiac output. Under preload a brief description of the Frank Starling mechanism was required. Important was the concept that at rest cardiac output is controlled almost entirely by peripheral factors that determine venous return. These concepts were best illustrated by graphing vascular function (venous return vs right atrial pressure) and cardiac function (cardiac output vs right atrial pressure) curves. Then demonstrating on these curves the factors that affected preload, contractility and afterload such as changes in blood volume, sympathetic and parasympathetic stimulation and exercise as examples. Also important to demonstrate on these curves was the fact that venous return and cardiac output are equal at steady state. Most candidates tried to illustrate these cardiovascular concepts with a series of left ventricular pressure volume loops rather than use the vascular and cardiac function curves. They then went on to demonstrate via these pressure volume loops the effects of changes in preload, contractility and afterload on stroke volume. Candidates who took this approach were not penalised, if there were clear, correct diagrams with explanations indicating comprehension of these concepts. On the whole graphs were poorly drawn and were not well integrated into the answer. Some candidates also wasted time by unnecessarily describing excitation-contraction coupling and sympathetic nerve reflex pathways.
Syllabus: C1c 2a,c,e,f,g Recommended sources: Guyton Textbook of Medical Physiology 11th edition pgs 241-243.
2018B 18
Describe the factors affecting left ventricular function.
CICMWrecks Answer
LV Systolic Function
LV systolic function is a function of its contractility.
Contractility = the change in force generated independent of preload
Factors affecting Contractility
Contractility is primarily dependent on intracellular Ca2+. Determinants include:
- Metabolic:
- substrate supply – glucose, fat, protein
- metabolic/electrolyte homeostasis
- hormones – thyroid, insulin/glucagon
- Physiological:
- integrity of myofilaments
- co-ordinated depolarisation
- functional muscle mass
- autonomic tone – sympathetic and parasympathetic
- Drugs: Digoxin, b agonists, PDE3 inhibitors
- Minor increases in contractility occur as an intrinsic response to:
- increased afterload
- Anrep Effect: Autoregulation method in which myocardial contractility increases with afterload.
- increased heart rate
- Bowditch Effect: Autoregulation method by which myocardial tension increases with an increase in heart rate. Also known as the Treppe phenomenon, Treppe effect or staircase effect .
- (Contractility improves at faster heart rates. This is because the myocardium does not have time to remove calcium, so it accumulates intracellularly.)
- increased afterload
- Disease
- Ischaemia / Coronary Blood flow
Reduced ATP production secondary to hypoxia, which impairs sarcoplasmic reticulum Ca2+ function. Further exacerbated by intracellular acidosis from anaerobic metabolism. - Heart Failure
Impaired contractility reserve, i.e. minimal increase in contractility with sympathetic stimulation.- Reduced peak Ca2+and sarcoplasmic reticulum uptake of Ca2+
- Ischaemia / Coronary Blood flow
LV Diastolic Function
LV diastolic function is determined by its compliance. LV compliance is primarily determined by myocardial characteristics and load.
Factors affecting LV diastolic function:
- Normal HR and rhythm
- LV systolic function
- Wall thickness
- Chamber geometry
- Duration, rate and extent of myocyte relaxation
- LV untwisting and elastic recoil
- Magnitude of diastolic suction
- LA-LV pressure gradient
- Passive elastic properties of LV myocardium
- Viscoelastic effects (rapid LV filling and atrial systole)
- LA structure and function
- Mitral valve structure and function
- Pulmonary venous blood flow
- Pericardial restraint
- RV loading conditions and function
- Ventricular interdependence
- Coronary blood flow and vascular engorgement
- Compression by mediastinal masses
JC 2019
Examiner Comments
2018B 18: 12% of candidates passed this question.
Candidates often misinterpreted the question and described determinants of cardiac output. The answer should have focussed on factors affecting/contributing to normal LV function – not pathological states. Some answers showed a lack of appreciation that normal left ventricular function is afterload independent, due to compensatory reflexes. Answers needed to consider intrinsic and extrinsic factors affecting LV function – the latter (e.g. SNS, PSNS, hormones, drugs) was often left out. Answers needed to consider both systolic and diastolic function. An excellent answer included physiological phenomena such as the Treppe effect, Anrep effect and baroreceptor and chemoreceptor reflexes. Mention of normal conduction and pacing as well as blood supply limited by diastole scored additional marks.
2011A 08
Describe the factors that affect the output of the right ventricle
CICMWrecks Answer
Intracardiac
Extracardiac
HR
- ↓HR → ↑ diastolic filling → ↑ preload → ↑ CO
- Arrhythmia (eg AF) → loss of atrial kick
- less important in RV, can contribute as much as 30% of EDV
- SNS stimulation → ↑ HR → ↓ Diastolic filling time → ↓ Preload → ↓ RV output
Preload
PL = [(RVEDP – ITP) RVEDR]/2h
- h – thickness of the right ventricle
- Less than LV → ↑ PL for equivlant end diastolic pressure (EDP) and end diastolic radius (EDR) compared with the left
- RVEDR – Right ventricular end diastolic radius
- Ventricular wall compliance
- Ventricular interdependence → coordinated, simultaneous contraction of ventricles limits the excursion of intraventricular septum → ↓ventricular compliance
- Ventricular wall compliance
- ITP – Intrathoracic pressure
Mostly RVEDP factors
- Central venous pressure
- Venous compliance
- ↑ venomotor tone → ↑ CVP → ↑ preload → ↑ RV output
- Venous compliance
- Thoracic venous blood volume
- Total blood volume (TBV)
- ↑ TBV → ↑MSFP → ↑ CVP → ↑ preload → ↑ PL
- Total blood volume (TBV)
- Venous return
- VR = (MSFP – RAP)/(SVR)
- ↑VR → ↑ CVP → ↑ preload
- Pulmonary Artrial pressure (PAP)
- ↑ afterload (or PAP) → ↓ SV → ↑ ESV → ↑ EDV (or ventricular preload) → ↑ RV output
- External compression
- Pericardial effusion/tamponade → ↓ ventricular filling
- PEEP → ↑ ITP → ↓ VR
Afterload
AL = [(RVESP – ITP) RVESR]/2h
- RVESR (Vetricular Systolic Radius_
- End-diastolic radius
- Transmural pressure (RVESP – ITP)
- Intraventricular pressure (RVESP)
- Outflow tract obstruction
- Intraventricular pressure (RVESP)
- Myocardial wall thickness
- Thin wall → ↑ compliance → ↓ AL
Transmural pressure (RVESP – ITP)
- Extra-cardiac pressure (ITP)
- ↑ ITP → ↓ Δ P → ↓ AL
- Pulmonary Arterial pressure
- Pulmonary vascular resistance (PVR) = (8 η L)/(π r^4)
- ↑PEEP → ↓vessel radius → ↑AL
- Position (Erect > PVR cf Supine)
- Pulmonary vascular resistance (PVR) = (8 η L)/(π r^4)
Contractility
- Afterload
- “Anrep Effect”
- Preload
- Initial myocyte stretch via Frank-starling
- Heart rate
- “Bowditch effect” or “Treppe effect”
- ANS (main factor)
- Circulating catecholamines – Augments SNS adrenergic effect
Gladwin / Sakurai 2016
Examiner Comments
2011A 08: 6 (50%) of candidates passed this question.
An approach that covered the main determinants of right ventricular cardiac output including heart rate, right ventricular preload, contractility, afterload and the relationship with left ventricular output, ventricular interdependence, and the respiratory system would have provided the framework for a good answer. Some candidates used this approach but described more features of left ventricular than right ventricular output. The observation that the right ventricle is relatively thin
walled and its output is very sensitive to changes in right ventricular preload and afterload particularly was central to this question. The unique shape of the right ventricle and its contraction characteristics involving ventricular interdependence were rarely mentioned. Also details on right ventricular afterload and the importance of factors affecting pulmonary vascular resistance were lacking in most answers.
Syllabus: C1c
Recommended sources: Review of Medical Physiology, Ganong, Chps 31 and 33, Textbook of Medical Physiology, Guyton & Hall Chp 9 and 20
2015B 15 – 2010A 21
Define cardiac preload and describe its determinants.
CICMWrecks Answer
Definitions of Preload:
- The mean tension in the ventricular fibres or the initial myocardial fibre length prior to contraction.
- Mathematically:
- where:
- ITP = intrathoracic pressure; LVEDP = left ventricular end-diastolic pressure; LVEDR = left ventricular end-diastolic radius (at midpoint of ventricle); h = thickness of ventricle
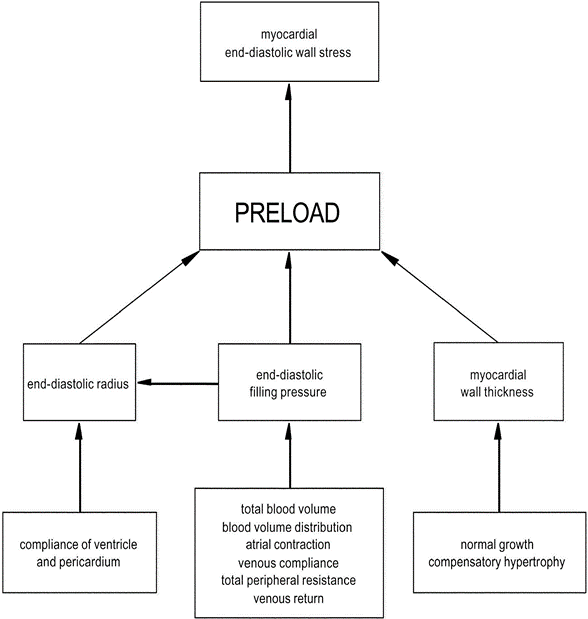
Factors determining Preload
1. LaPlace Factors
- Extra-cardiac: End-diastolic filling pressure
- CVP
- Venous compliance
- Thoracic Blood Volume
- Total Blood Volume
- ↑ TBV → ↑MSFP → ↑ CVP → ↑ preload.
- Blood Volume distribution
- Total Blood Volume
- Venous return (VR)
- ↑VR → ↑ CVP → ↑ preload
- Afterload factors
- ↑Total peripheral resistance → ↓ VR → ↓ Preload
- Compression
- Pericardial effusion/tamponade → ↓ ventricular filling
- PEEP → ↑ ITP → ↓ VR
- Aortic pressures
- ↑ afterload (or raised aortic pressure) → ↓ SV → ↑ ESV → ↑ EDV (or ventricular preload).
- CVP
- Intra-cardiac:
- End-diastolic radius
- Ventricular and Pericardial compliance
- ↑ compliance → ↑ filling for constant pressure → ↑preload
- Atrial contraction/coordination
- ↑ atrial contraction/contractility → ↑ preload
- eg ↑ SNS stimulation / fluid bolus
- ↓ atrial contractile force/coordination in AF
- ↑ atrial contraction/contractility → ↑ preload
- HR: ↓ HR → ↑ filling time → ↑ preload
- Myocardial Wall thickness
- ↑Normal Growth → ↓ compliance and ↑ thickness (h) → ↓ Preload
- ↑Compensatory hypertrophy
- End-diastolic radius
2. Venous Return Factors (↑/↓ VR → ↑/↓ Preload)
- Venous Factors
- ↓Venomotor tone → ↓ VR
- Venous Values prevent retrograde flow → failure → ↓VR
- Pump Factors
- Skeletal Muscle pump/Respiratory pump both ↑ VR
- Inspiration
- → ↓ ITP → ↓ RAP → ↑VR
- → Diaphragmatic contraction → ↑IAP → ↑VR
- Inspiration
- Effect of ventricular contraction and relaxation
- → Systolic Rapid ejection phase → ↓RAP → ↑VR
- → Early diastole → Rapid vetricular filling (open AV valve) → ↑VR
- Skeletal Muscle pump/Respiratory pump both ↑ VR
- Posture
- Intrapericardial pressure (tamponade)
- Afterload
- ↑ Arteriolar tone → ↑ Resistance to VR → ↓ VR
3. Pathological Factors
- ↑ preload
- Ventricular systolic failure
- Outflow valve stenosis or regurgitation (AS or AR),
- Inflow valve regurgitation (MR/TR)
- ↓ preload
- Ventricular diastolic failure,
- Inflow valve stenosis (MS/TS)
Gladwin 2015
Examiner Comments
2015B 15: 29% of candidates passed this question.
This question required synthesis and application of knowledge derived from multiple sources rather than regurgitation of a published list in a text. Many candidates failed to recognise that venous return is not the only determinant of preload. Most candidates failed to discuss determinants of venous return. Factors such as contractility, afterload or chamber filling and emptying can all impact preload. In addition to listing determinants the question required an explanation of their relationship with preload (e.g. the direction of change).
A discussion about the determinants of cardiac output was not asked for as did not score marks.
2010A 21: 1 (10%) of candidates passed this question.
A definition based on stretch of the isolated myocyte prior to contraction, and extrapolation to the human heart, was expected. Surrogate measures of preload used in clinical practice needed to be explained and related to the definition (for example, end-diastolic volume and central venous pressure). The Frank-Starling Law was relevant to discussion of the significance of preload to cardiac performance.
A diagram illustrating the interaction of important factors would have been helpful in answering this question. At a minimum, detail should have included atrial contractility, diastolic filling time, ventricular compliance, and the determinants of venous return.
Better answers included discussion of the effects of afterload, arrhythmias, and valvular pathology. A distinction between the factors determining left and right ventricular preload would have demonstrated a more sophisticated understanding of the physiology.
Syllabus: C1c, 2b,c.
Reference: Cardiovascular Physiology, Berne and Levy, p64-65.
2020A 19 – 2018B 06
Outline the determinants of venous return to the heart.
CICMWrecks Answer
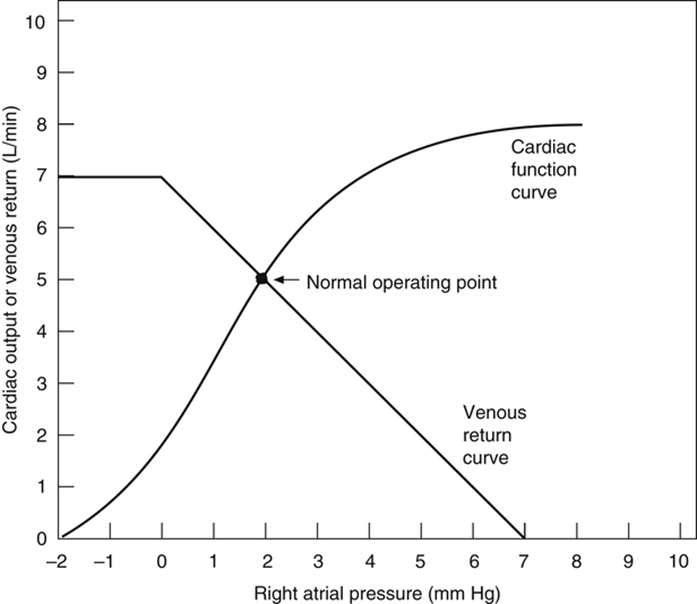
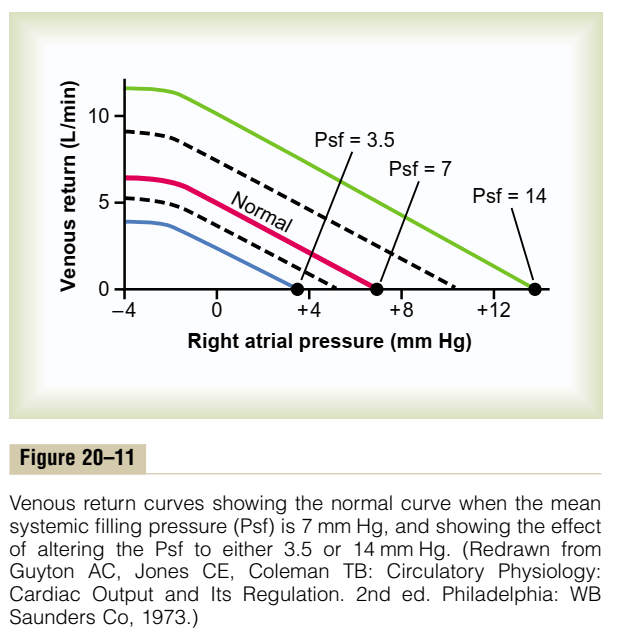
Vascular function (venous return) curve shows the effect of increases in venous return or CO (independent variable) on RA
pressure (dependent variable)
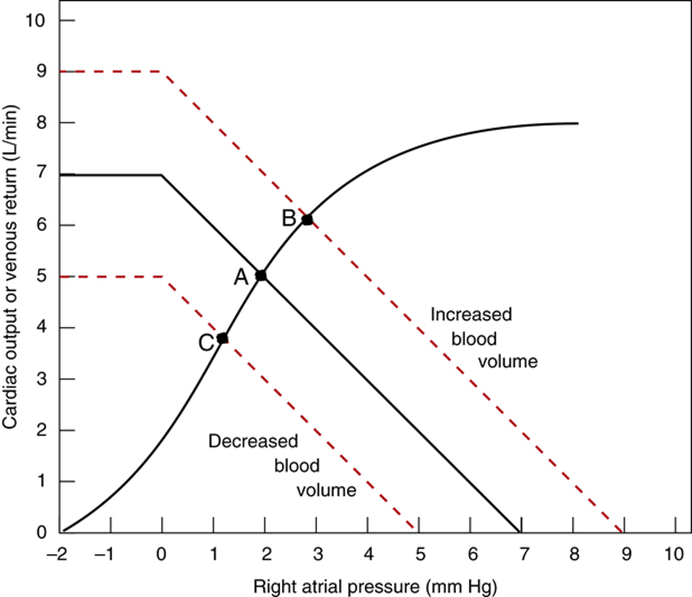
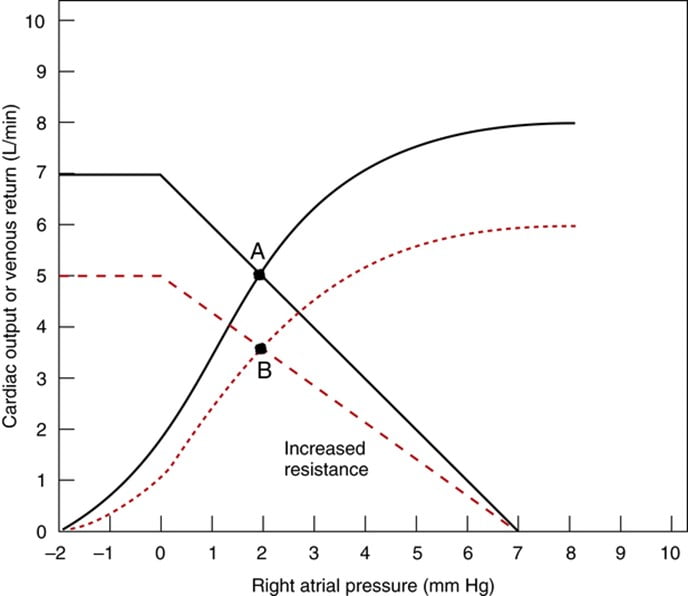
Factors that determine venous return back to the heart
The factors that influence VR are captured in 2 formulae:
The three variables that influence venous return are:
- Mean Systemic Filling pressure (MSFP)
- MSFP is the theoretical pressure present in the systemic circulation at equilibrium
- MFSP is the main driving pressure moving blood back towards the RA – It is the MAIN factor determining venous return (and thus CO)
- MSFP can be used to assess the degree of filling of systemic circulation: It is normally 7 mmHg (0-20 mmHg)
- Two factors influence MSFP:
- Blood volume ↑ → ↑ MSFP
- Venomotor tone (venous capacitance) ↑ → ↑ MSFP
- RA pressure (RAP)
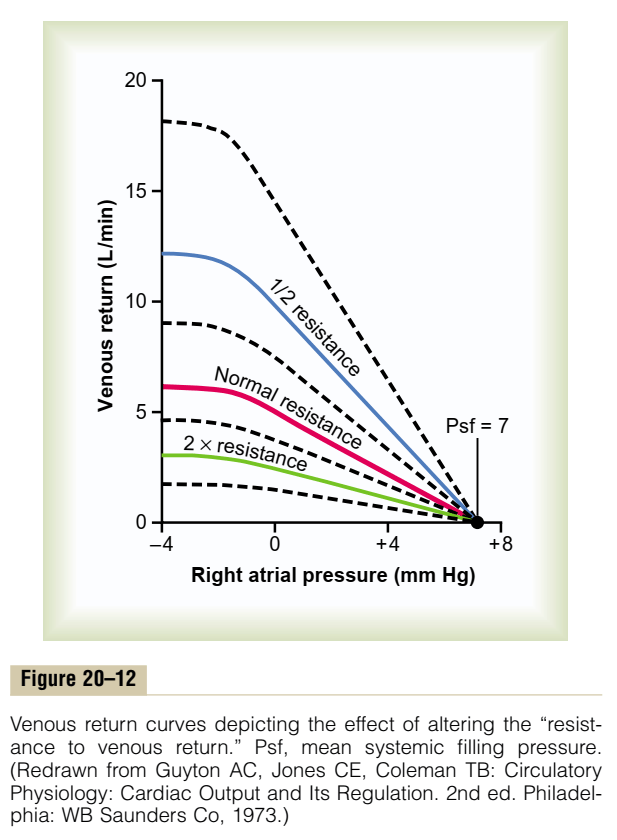
- Resistance to venous return (RVR)
Therefore, the factors that affect venous return influence one or more of the aforementioned variables:
- Blood volume
- Blood volume is proportional to MSFP and thus venous return
- Venomotor tone
- ↑ venomotor tone (Eg. due to SNS activity) ↓ vein compliance and capacity → ↑ MSFP → ↑ venous return
- ↓ venomotor tone (Eg. due to SAB) ↑ vein compliance and capacity → ↓ MSFP → ↓ venous return
- This has a greater effect on venous return when
- (i) venous pressures are normal, and
- (ii) veins are circular (not collapsed and contain large volumes of blood)
- Venous valves
- Veins have one-way valves that prevent retrograde flow
- Skeletal muscle pump
- Alternating contraction and relaxation of limb skeletal muscle forces blood out of the veins towards the heart (thus, increasing MSFP)
- During contraction, veins compress to expel blood towards the heart, then during relaxation, veins distend and fill with blood
- With exercise, this pump function enhances net venous return
- Respiratory pump
- During inspiration, venous return is ↑ due to
- (i) ↓ RAP (associated with fall in PINTRAPLEURAL), and
- (ii) ↑ IAP (due to diaphragmatic contraction)
- During expiration, the effects are reversed
- Note that if RAP is < 0 mmHg, the respiratory pump will NOT have any effect on venous return as the thoracic veins would have collapsed at subatmospheric pressures
- During inspiration, venous return is ↑ due to
- Posture
- When going from supine to erect, there is ↓ venous return due to venous pooling in the lower extremities
- Normally, there is reflex vasoconstriction to prevent this BUT this reflex is delayed and less effective in the elderly
- Effect of ventricular contraction and relaxation
- During rapid ejection phase of ventricular systole, atrial pressure falls sharply (to zero or –ve values) as the ventricle contraction pulls the atrioventricular fibrous ring downwards and increases the atrial volume – This causes net blood flow into atria and increases venous return
- During early diastole, ventricles fill rapidly causing both ventricular and atrial pressures to decline – This facilitate blood flow into atria and increases venous return
- Intrapericardial pressure
- ↑ intrapericardial pressure (Eg. tamponade) can ↑ RAP, thus impeding venous return
- Afterload
- Changes in the dimension of the resistance vessels (arterioles) has a small effect on MSFP as only 2% of blood volume is in arterioles (cf. venous capacitance vessels) – Instead, it has an impact on “Resistance to venous return”
- ↓ afterload (such as decreased SVR due to arteriolar vasodilation) → ↓ RVR → ↑ VR
- ↑ afterload (such as increased SVR due to arteriolar vasoconstriction) ↑ RVR → ↓ VR
Bianca 2020
Examiner Comments
2020A 19: 67% of candidates passed this question.
The factors that influence VR are captured in 2 formulae; VR = CO, and VR = (MSFP-RAP) / Venous Resistance. Candidates that used these as the backbone structure of their answer scored well. Quite a few candidates failed to consider factors that affect left heart CO also effect VR.
Recognising that CO does = VR appeared to elude some candidates.
2018B 06: 31% of candidates passed this question.
Answers should have included a description of the need for a pressure gradient for flow and a discussion on right atrial pressure, mean systemic filling pressure and resistance to blood flow. The discussion of each of these factors included definitions, normal values, factors affecting them and the direction of change on venous return. Diagrams were not essential, but their use assisted some candidates in explaining the effects of RAP on venous return
2020B 18
Define afterload (10% marks) and describe the physiological factors that may affect afterload on the left ventricle (90% marks).
2014B 19
Describe the factors that determine right and left ventricular afterload
2012A 17
Define afterload and describe the physiological factors that may affect afterload
2009A 07
Define afterload (10% of mark). Describe the factors that can affect left ventricular afterload (90% of mark).
CICMWrecks Answer
Afterload
- Load against which the muscle exerts its contractile force (Guyton)
- It is represented by the gradient of the line connecting the end-diastolic volume, to the end-systolic point
- pressure which the ventricle has to contract against (Power & Kam)
Determinants of Afterload
Modified Laplace Equation
- where
- T represents afterload
- P represents aortic pressure
- Therefore, afterload increases as aortic (or pulmonary arterial) pressure increases
- R represents ventricular radius
- Afterload increases as ventricular radius increases
- H represents ventricular thickness
- Afterload decreases as the thickness of the ventricular wall increases (hypertrophy secondary to chronic hypertension and cardiac remodelling)
Modified Hagen-Poiseuille Equation
- Afterload is affected by resistance to cardiac output
- Afterload increased by reduced radius of systemic vasculature
- Afterload increased by increasing viscosity of blood
Resistance in parallel
- Afterload is affected by addition, or loss of large capillary networks in parallel
- Systemic vascular resistance is increased significantly by loss of placenta, with parallel vascular networks
- Pulmonary vascular resistance is decreased significantly by inflation of lung, causing creation of vast capillary network in parallel
Factors affecting Right Ventricular Afterload
- Pulmonary vascular resistance increases afterload
- Hypoxic vasoconstriction increases PVR
- Lung volumes
- Pulmonary vascular resistance minimal at FRC
- Increased pulmonary artery pressure increases RV afterload
- Left heart failure
- Critical mitral stenosis, mitral regurgitation
- Pulmonary emboli
- Right ventricular outflow tract obstruction increases afterload
- Pulmonary stenosis
- Saddle pulmonary embolism
- Right ventricular dilation
- Susceptible due to thin RV wall
- Acute PE
- RV spiral of death
- Increased intraventricular pressure decreases blood flow à ischaemia à decreased contractility à further increase in ventricular volume
- RV spiral of death
Factors affecting Left Ventricular afterload
systemic vascular resistance, aortic impedance and ventricular radius
According to La Place Equation
- Aortic pressure
- Afterload increases as aortic pressure increases (increases with hypertension)
- Ventricular radius
- Afterload increases as ventricular radius increases (increases with ventricular dilation)
- Ventricular wall thickness
- Afterload decreases and ventricular wall thickness increases (decreases with ventricular hypertrophy)
Determinants of aortic pressure indirectly affect afterload
- Aortic compliance
- Afterload decreases with increased compliance
- Arterial blood volume
- Given set compliance, afterload will increase with arterial volume
- From Modifed Poisuille-Hagen Equation
- Radius of aorta
- Autonomic vasomotor tone
- Increased intrathoracic pressure
- Physical compression
- Viscosity of blood
- Radius of aorta
- From equation MAP = CO / SVR
- Tissue flow autoregulation
- Myogenic
- Metabolic
- Tissue flow autoregulation
Other
- Left ventricular outflow tract obstruction
- Aortic stenosis
- HOCM
- Coarctation of aorta
Sakurai 2016
Examiner Comments
2020B 18: 53% of candidates passed this question.
Afterload can be defined as factors resisting ventricular ejection and contributing to myocardial wall stress during systole. Most answers utilised the law of Laplace to expand upon factors affecting ventricular wall tension. Systemic vascular resistance was commonly mentioned, but less frequently defined. Aortic and left ventricular outflow tract impedance were commonly referred to. Effects of preload and neurohumoral stimuli were less well outlined. Description of factors affecting right ventricular afterload and depictions of left ventricular pressure volume loops earned no extra marks unless directly referenced to the question.
2014B 19: 31% of candidates passed this question.
This is a big question and required some structure to cover the material required. A Definition of afterload, factors specific to left ventricle, right ventricle and both were required. The question asked to describe and not merely list factors affecting afterload.
Marks were not given for describing pathologies rather than physiological processes that affect afterload. Candidates seemed to lack depth and understanding on this topic.
2012A 17: 6 (60%) of candidates passed
Definitions for afterload vary slightly amongst common physiology textbooks, and candidates were expected to mention any one commonly accepted definition. Essentially afterload is the resistance to ventricular ejection – the “load” that the heart must eject blood against and is related to ventricular wall stress (Law of Laplace, T=Pt.r/u). Candidates were expected to mention aortic valve and systemic vascular resistance, aortic impedance, blood viscosity, intathoracic pressure and relationship of ventricular radius and volume.
Candidates generally did well, but few substantially good answers, with a lack of detail being the biggest limiting factor.
2009A 07: Pass rate: 20%
Many definitions of afterload were accepted. The main factors affecting left ventricular afterload are systemic vascular resistance, aortic impedance and ventricular radius. Other factors include blood viscosity and positive intrathoracic pressure. Good answers expanded on the points above. Candidates who failed this question did not have enough facts.
Syllabus C1c C2c
Reference: Bray 4th edition p 342-344 and p 360-361
2024A 03
The systemic vascular resistance is suddenly increased, describe the consequences for the otherwise healthy left ventricle.
2023B 17
Describe the consequences for the left ventricle of a sudden and sustained increase in afterload.
2016A 13
Describe the cardiovascular effects of a sudden increase in afterload
CICMWrecks Answer
Afterload
- Load against which the muscle exerts its contractile force (Guyton)
- It is represented by the gradient of the line connecting the end-diastolic volume, to the end-systolic point
- pressure which the ventricle has to contract against (Power & Kam)
Determinants of Afterload
Modified Laplace Equation
- where
- T represents afterload
- P represents aortic pressure
- Therefore, afterload increases as aortic (or pulmonary arterial) pressure increases
- R represents ventricular radius
- Afterload increases as ventricular radius increases
- H represents ventricular thickness
- Afterload decreases as the thickness of the ventricular wall increases (hypertrophy secondary to chronic hypertension and cardiac remodelling)
Modified Hagen-Poiseuille Equation
- Afterload is affected by resistance to cardiac output
- Afterload increased by reduced radius of systemic vasculature
- Afterload increased by increasing viscosity of blood
Resistance in parallel
- Afterload is affected by addition, or loss of large capillary networks in parallel
- Systemic vascular resistance is increased significantly by loss of placenta, with parallel vascular networks
- Pulmonary vascular resistance is decreased significantly by inflation of lung, causing creation of vast capillary network in parallel
Cardiovascular Effects of a sudden increase in afterload
Effects of sudden increase in afterload can be demonstrated using a Left ventricular PV loop:
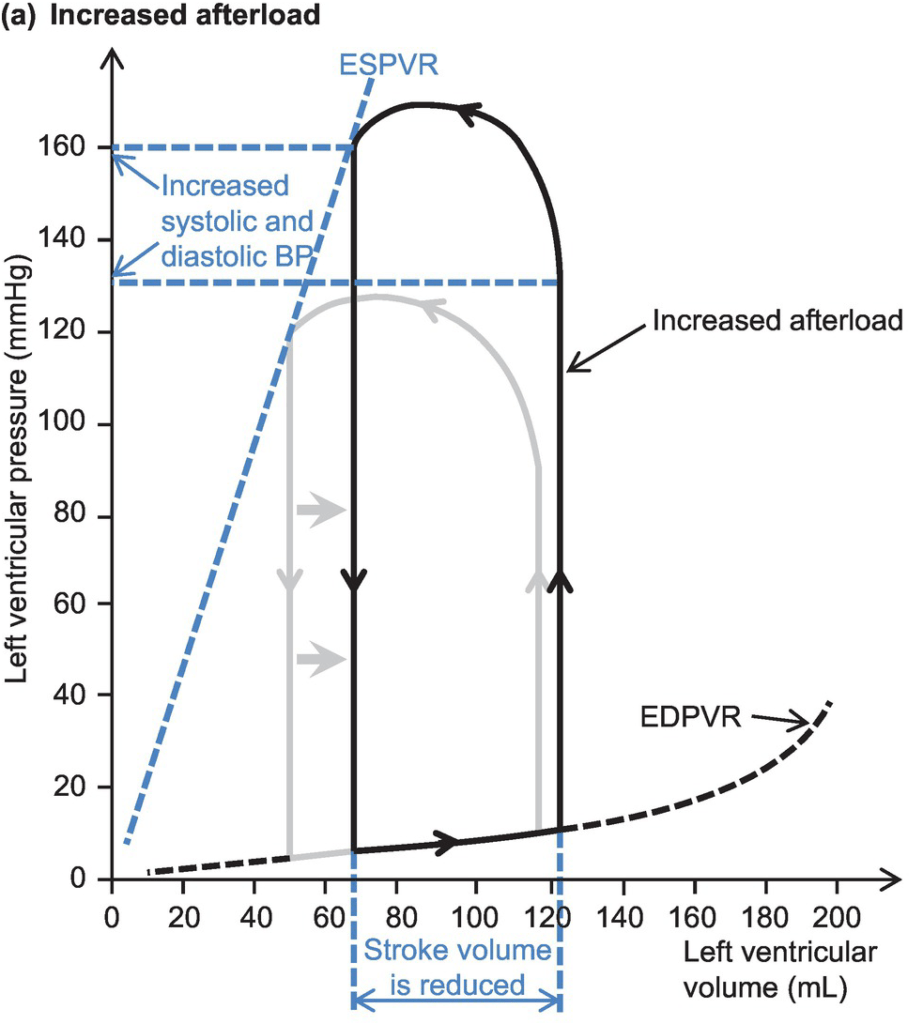
- End-Systolic Volume is increased, causing a reduction in stroke volume
- Initially, Left atrial pressure decreases
- Increased End systolic volume leads to secondary increase in end diastolic volume, hence increasing ventricular filling
- This secondary increase in preload enables the ventricle to contract with greater force (Frank-Starling mechanism) which partially offsets the reduction in stroke volume
- In patients with impaired left ventricular function, the decreased in stroke volume cannot be compensated
- ventricular end-systolic pressure: increases
- ventricular end-diastolic pressure: increases
- cardiac output (=HRxSV) – remains the same
- Initial drop in SV is compensated by increased pre-load
- In a failing heart, the drop in SV causes subsequent stimulation of baroceptors, which in turn causes increased heart rate and can potentially return cardiac output to normal
- Increase in afterload → Anrep → Small ↑Contractility to compensate
- Mechanism: ↑AL → sustained ↑stretch (prolonged isovolumetric contration) → ↑ Ca induced Ca release → ↑ contractility
- Purpose: ↑AL → ↓SV and ↑ ESV
- myocardial oxygen demand and myocardial work: Increased Afterload will increase the pressure during contraction, hence increasing MVO2 for internal work. This might be partially offset by the reduction in external work (due to decreased stroke volume) depending on the cause of the increased afterload
- coronary blood flow is autoregulated to remain normal
JC 2019
Examiner Comments
2024A 03: 11% of candidates passed this question.
This question was specific to the left ventricle only. Good answers provided information on immediate changes in LV Volumes and Pressures and how compensatory mechanisms largely within the LV itself eventually lead to restoration of stroke volume. Marks were allocated for a description of the Frank Starling mechanism, baroreceptor reflex and anrep effect. Good answers also discussed the effect of increased SVR on LV myocardial work and oxygen consumption and coronary perfusion. Common errors included focusing on definitions and determinants of SVR and providing LV PV loops without relating this to the situation of an increased SVR or without adequately labelling or explaining what they were demonstrating with the graph. This question required the integration of a number of physiological principles and is challenging.
2023B 17: 7% of candidates passed this question.
This question expected a detailed description of the effect of afterload on the left ventricle. This should cover the acute effect of increased afterload on left ventricular end systolic (and diastolic) pressure and volume, contractility, work and oxygen consumption, coronary perfusion pressure and baroreceptor responses. “Sustained” implied more longer term left ventricular exposure which would include the ventricular cellular response, concentric hypertrophy and the subsequent effects on diastolic and elevations of left atrial pressure. Definitions of afterload, cardiac output equations, vascular function curves and LV/PV loops are not required if the above concepts are described in adequate detail. The use of a diagram can assist in explaining concepts however should be linked back to the question in order to demonstrate the candidates understanding of the question being asked.
2016A 13: 21% of candidates passed this question.
It was expected the answer would start with a definition of afterload and then proceeded to indicate what effects this increase in afterload would have on ventricular end-systolic pressure, ventricular end-diastolic pressure, left atrial pressure, cardiac output, myocardial oxygen demand and myocardial work, coronary blood flow and systemic blood pressure.
Most candidates who failed to pass this question submitted answers that were just too brief, only including a small subset of the material required. Very few candidates included any mention of myocardial oxygen demand or myocardial work or the impact upon the cardiac output. A number of candidates included a detailed description of the Sympathetic Nervous System and the Renin-Angiotensin system, material which was not asked for. There were quite a number of incorrect perceptions about what effect a sudden increase in afterload would have on the systemic blood pressure. Candidates who mentioned the baroreceptor response and the stretch receptor response where rewarded with additional credit.
2012B 04
Define myocardial contractility and briefly describe dP/dT, the end systolic pressure volume (ESPV) relationship and the ejection fraction (EF).
CICMWrecks Answer
Myocardial contractility
- Ability of myocardium to contract (or myofibrils to shorten) against a given afterload, with a given preload
dP/dT
- Change in pressure / change in time
- Measure of contractile force exerted by the myocardium at the start of systole
- One of the oldest measures of global myocardial contractility
- Measured via doppler analysis of mitral regurgitant jet
- Limited by
- Requirement for MR jet
- Affected by preload, afterload and contractility
ESPVR
- Line created with a series of LV pressure volume loops where preload was varied with a fixed afterload. The point at which aortic valve closure occurred was plotted.
- This is a surrogate for contractility on the pressure-volume loop.
- Invasive and impractical
EF
- Fraction of end-diastolic volume which is ejected (stroke volume / end-diastolic volume)
- 60% in normal heart (70ml/120ml)
Sakurai 2016
Examiner Comments
2012B 04: 3 (13.6%) of candidates passed.
Contractility represents the performance of the heart at a given preload and afterload. It is the change in peak isometric force (isovolumic pressure) at a given initial fibre length (end diastolic volume). All indices of myocardial contractility are dependent on preload or afterload to a varying degree. The dP/dT is the maximum rate of change in left ventricular pressure during isovolumetric contraction, after mitral valve closes and before the aortic valve opens. It is preload dependant and afterload independent. A diagram of a pressurevolume loop is very helpful when describing the ESPV. Absence of a diagram (correctly labelled and scaled) was a weakness in many answers. Candidates were then expected to at least explain that, as preload is increased a new pressure volume loop is generated. Each new PV loop has a new end systolic point that is at a slightly higher pressure and volume than the previous end systolic point. The line connecting the end-systolic points is called the linear ESPVR. The slope of the ESPVR or Emax is used as an index of myocardial contractility. Ejection fraction is the percentage of the ventricular end diastolic volume (EDV) which is ejected with each stroke volume (SV). Ejection fraction = stroke volume/end diastolic volume X 100 (Normal range 55 to 70%). Only a minority of candidates achieved the depth of knowledge required for a Level 1 topic.
iv. Describe myocardial oxygen demand and supply and the conditions that may alter each.
2016B 07
Compare and contrast the supply and demand of oxygen for the right and left ventricle.
CICMWrecks Answer
Myocardial O2 Supply
RIGHT VENTRICLE
LEFT VENTRICLE
- Supply:
- Right coronary artery and its branches
- conus artery supplies the infundibulum
- acute marginal arteries supply the anterior free wall
- posterior descending artery via septal branches supply the posterior 1/3 IV septum (PDA arises from RCA in Right dominant circulation and LCx in Left dominant circulation
- the other 2/3 of the IV septum is supplied by septal branches of the left anterior descending artery
- Supply: Left Main Coronary
- LAD (supplies the free wall and most of the papillary muscles)
- Diagonals
- Septal perforators
- LCx: inferolateral LV wall
- Obtuse Marginal branches
- LAD (supplies the free wall and most of the papillary muscles)
- RV Coronary blood flow
- RV pressures are low during both systole and diastole.
- flow during both systole and diastole
- LV Coronary blood flow
- The pressure inside the left ventricle is slightly higher than in the aorta during systole.
- Flow predominantly during diastole.
- Subendocardial flow ceases during systole.
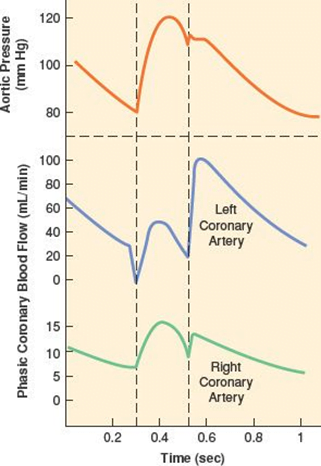
Myocardial O2 Demand
Determinants of myocardial O₂ demand:
- MVO₂ (ie extraction)
- High at rest (55-65%) cf. body average of 25%
- Extraction ratio can only rise by factor of < 2 to 90%
- AV Δ O₂ = 11 mL/dL
- Coronary venous O₂ content = 5 mL/dL
- Normally consumption 21 to 27 mls of O2/min.
- Determined by
- Wall tension.
- preload (EDV/EDP) and afterload (SVR)
- Contractility.
- Heart rate.
- ↑HR → ↓ supply
- ↑HR → ↑ demand (as ↑ MRO2)
- Wall tension.
RIGHT VENTRICLE
LEFT VENTRICLE
| Pumps against less resistance (PVR) → less systolic pressure → less O2 demand may be compromised during acute or chronic increases in right ventricle afterload resulting from pulmonary arterial hypertension | Pumps against higher afterload, thus more demand |
| ability to downregulate its metabolic demand during coronary hypoperfusion and thereby maintain contractile function and energy stores | |
| Activation of SNS causes relatively small changes in O2 demand | Activation of SNS causes more pronounced increase in O2 demand |
Examiner Comments
2016B 07: 29% of candidates passed this question.
An integrated answer to supply and demand of oxygen was expected, as a comparison between the right and left ventricles. Many candidates concentrated on differences not similarities. Myocardial oxygen demand was in general poorly described.
About 85 – 90% of oxygen demand is for internal work (major determinants wall tension 30 – 40%, heart rate 15 – 25%, myocardial contractility 10 – 15%, basal metabolism 25%). 10 – 15% of oxygen demand for external work or pressure volume work, determined by MPAP x CO.
It was expected answers would comment on the phasic nature of coronary blood flow which differs between left and right and the consequence of this to subendocardial oxygen supply during systole usually. Coronary blood flow is affected by coronary perfusion pressure (determined by aortic pressure and RV pressure) & coronary vascular resistance (determined by autoregulation, metabolic factors, humoral factors, nervous control interacting with local endothelial factors)
Generally, coronary blood flow is tightly coupled to oxygen demand/consumption due to high basal oxygen consumption (8 – 10 ml/ min/100g) and high oxygen extraction ratio (75%). Better answers noted that oxygen supply can only be increased to cope with increased demand only by increased coronary blood flow.
2007B 19
Describe the effects of a tachycardia on myocardial oxygen supply and demand in a normal heart.
CICMWrecks Answer
Coronary Blood Flow
- 80 mL/min/100 g
- or 200-250 mL/min
- 5% of CO at rest
- Can increase by 3-4 times (up to 400mL/min/100g)
Coronary Perfusion Pressure
- ADP = Ao Diastolic Pressure
- Varies throughout the cycle and b/w ventricles
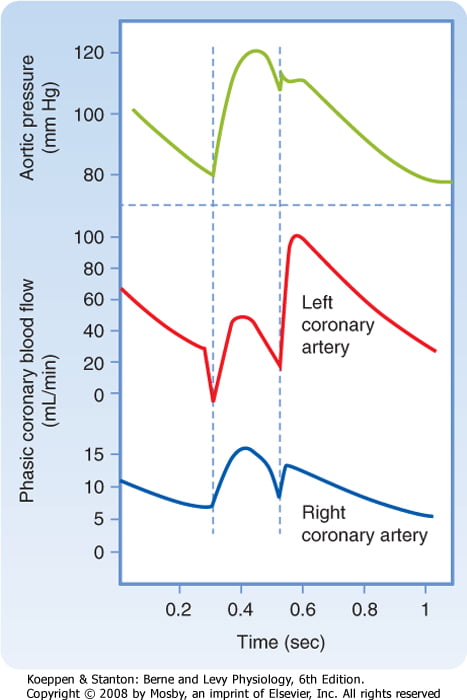
Coronary Vascular Resistance
- Physical Factors
- Extravascular compression (CPP factors)
- LCA flow stops in systloe
- RCA flow continuous throughout the cycle
- Neural and Neurohumoral Factors
- ↑ SNS tone →
- α receptor mediated vasoconstriction
- β receptor mediated vasodialtion
- ↑ force and rate of contractions → ↑ vasodialtor metabolite release
- Overall effect is dilation
- ↑ PSNS tone → KACh stimulation → mild ↓ Coronary vascular resistance
- ↑ SNS tone →
- Metabolic Factors (main)
- Vasodilatory (↑’d with ↑ HR)
- ↑ Adenosine, H, K, CO2, Lactate
- NO → GTP
- ↑ O2 demand → ↓ ATP → ↑KATP channel activation → hyperpolarisation → vasodilation
- Vasodilatory (↑’d with ↑ HR)
- Myogenic autoregulation (keep CPP 60-180 mmHg)
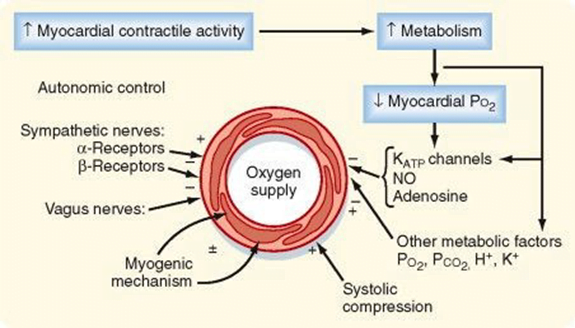
Determinants of myocardial O₂ demand:
- MVO₂ (ie extraction)
- High at rest (55-65%) cf. body average of 25%
- Extraction ratio can only rise by factor of < 2 to 90%
- AV Δ O₂ = 11 mL/dL
- Coronary venous O₂ content = 5 mL/dL
- Normally consumption 21 to 27 mls of O2/min.
- Determined by
- Wall tension.
- preload (EDV/EDP) and afterload (SVR)
- Contractility.
- Heart rate.
- ↑HR → ↓ supply
- ↑HR → ↑ demand (as ↑ MRO2)
- Wall tension.
CICMWrecks 2016
Examiner Comments
2007B 19: 2 candidates (29%) passed this question.
The main points expected were the determinants of myocardial oxygen supply. These include arterial oxygen content and coronary blood flow. Coronary blood flow deoends on coronary perfusion pressure and coronary vascular resistance and that most left coronary blood flow occurs in diastole.
Tachycardia reduces diastolic time and hence left coronary blood flow. In comparison blood flow in the right coronary artery is continuous both in systole and diastole and is little affected by heart rate. A correctly labelled diagram of left and right coronary blood flow attracted extra marks. Unfortunately most diagrams were inaccurate, not labelled and had no units on the axes. Systolic compression particularly reduces blood supply to the left ventricular subendocardium which is most susceptible to ischaemia. Extra marks were given for describing metabolic autoregulation, the high oxygen extraction, explaining that oxygen supply cannot be increased by increasing oxygen extraction in the coronary circulation and describing the driving pressure differences in both coronary arteries in systole and diastole.
A description of the determinants of myocardial oxygen demand was also required (e.g. left ventricular, preload, contractility, afterload, and tachycardia. This part of the question was particularly poorly answered.
v. Describe and explain cardiac output curves, vascular function curves and their correlation.
vi. Describe the pressure-volume relationships of the ventricles and their clinical applications.
2017A 23 – 2008A 24
Draw and label a left ventricular pressure volume loop in a normal adult (40% of marks).
List the information that can be obtained from this loop (60% of marks).
CICMWrecks Answer
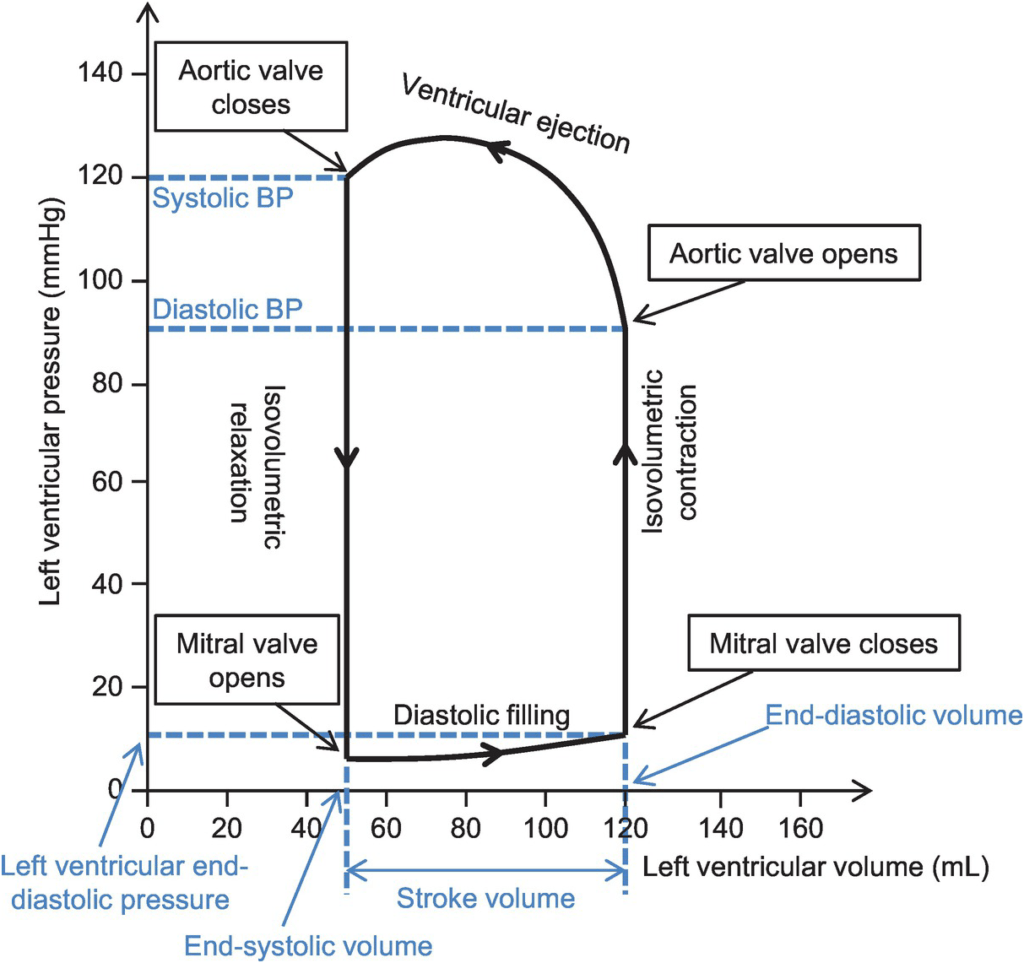
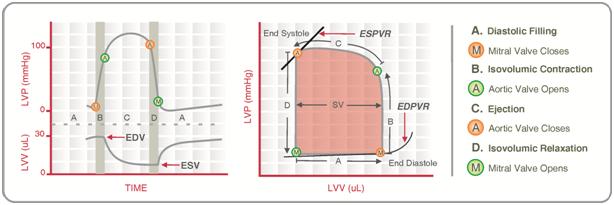
Normal P-V loop:
- Diastole
- (D) Beginning of diastole (isovolumetric ventricular relaxation)
- (A first 60% of vol) Early diastole (rapid ventricular filling – highly compliant Ventrical still relaxing)
- (A 60-90% of vol) Mid-diastole (slow ventricular filling – nearly full of blood → ↓ vent compliance)
- (A last 10% of vol) Late diastole (atrial contraction)
- Systole
- (B) Early systole (isovolumetric contraction)
- (C) Ventricular Ejection
- Early short rapid ejection phase (1st third of time)
- Prolonged reduced ejection phase (last 2/3 of time)
Derived Values:
- Stroke volume: SV = LVEDV-LVESV
- Ejection fraction as per eqn: EF = SV / EDV
- Measure of preload: LVEDV
- Measure of afterload
- Line b/w end-systolic point and locus at (LVEDV, 0)
- ↑ slope → ↑ Afterload
- Measure of contractility (ESPVR)
- End-systolic pressure volume relationship
- Slope is surrogate for contractility
- Measure of elastance (or stiffness) and compliance (or distensibility) of the LV – (EDPVR)
- End-diastolic pressure volume relationship
- Slope of the EDPVR = elastance = 1/compliance
- Peak LV pressure
- Measure of cardiac workload
- Total mechanical energy (or stroke work) (area of P-V loop)
- Heat generated by the heart during contraction
- “Diastolic work”
Gladwin 2016
Examiner Comments
2017A 23: 65% of candidates passed this question.
Many candidates lost marks for poor quality diagrams with inaccurate labelling. An accurate
diagram was required. Many answers lacked sufficient detail regarding contractility and
afterload.
2008A 24: 2 candidates (66%) passed this question.
Candidates were expected to draw and label a diagram showing the relationship between pressure and volume during the different phases of the left ventricular contraction and relaxation (or systole and diastole)
Good answers to this question consisted of a well-labelled graph with appropriate scale on both x and y-axes showing all the important events during systole and diastole of the left ventricle.
The common omissions were rapid and slow ejection phase during systole, when aortic valve closes, stroke volume, ejection fraction, end-systolic pressure volume line showing the contractility of the left ventricle.
Some candidates appeared to have confusion about which line shows contractility and which line shows left ventricular after load.
Syllabus C1c
Reference: Kam 1st edition 115-121, Guyton 11th edition 110
vii. Describe the cardiac reflexes.
2013B 02
Describe the various rapidly acting cardiac reflexes that influence cardiac function and the mechanisms by which they act.
2010B 17
Outline the various cardiac reflexes and the mechanisms by which they maintain physiological homeostasis.
CICMWrecks Answer
Cardiac reflex: “a fast-acting reflex loop between the heart and central nervous system that contributes to regulation of cardiac function and maintenance of physiologic homeostasis”
1. Chemo receptor reflex
- Sensor
- Carotid body, central chemoreceptors
- Stimulus
- ↓in PaO2, ↑ in PaCO2
- Response
- Increased SNS stimulation
- Increased HR, SV (via increased contractility), PVR
2. Baroreceptor response (high pressure)
- Sensor
- Arterial baroreceptors
- Aortic arch (CN X)
- Carotid sinus (CN IX)
- Arterial baroreceptors
- Stimulus
- Increased MAP
- Continuous resting tone when MAP >60mmHg
- Increased MAP
- Response
- Increase SNS stimulation
- Increased HR, SV (via increased contractility), PVR
3. Baroreceptor response (low pressure)
- Sensor
- Atrial pressure sensors
- Stimulus
- Atrial filling
- Atrial contraction
- Response
- Peripheral vasodilation
- Increased HR
- Also renal and hormonal effects to decrease circulating volume
4. Bainbridge reflex
- Sensor
- Atrial stretch receptors – right atrial wall, cavoatrial jucntion
- Stimulus
- Increased atrial blood volume
- Response
- Increased HR
5. Cushing reflex
- Sensor
- Medullary sympathetic cell bodies
- Stimulus
- Compression of cell bodies with increased intracranial pressure
- Response
- Increased SNS stimulation -> increased SVR
- Reflex bradycardia
- Via reflex #3
6. Oculocardiac
- Sensor
- Via CN III
- Stimulus
- Ocular compression
- Response
- Vagal stimulation -> decreased HR
7. Bezold-Jarisch reflex
- Sensor
- Chemoreceptors and mechanoreceptors in LV wall
- Stimulus
- Noxious chemical stimuli
- Response
- Increase in parasympathetic outflow
- Bradycardia
- Peripheral vasodilation
Mooney 2016
Examiner Comments
2013B 02: 6 candidates passed (22.2%).
Cardiac reflexes are fast-acting reflex loops between the heart and central nervous system that contribute to regulation of cardiac function and maintenance of physiologic homeostasis. It was expected candidates would include within their answer a mention of the stimulus and how it is sensed, the reflex arc and the resultant effect. Thus candidates could have mentioned the Baroreceptor Reflex/Carotid Sinus Reflex, Chemoreceptor, Bainbridge, Cushing, Oculocardiac and Bezold-Jarisch (involves response to ventricular stimuli, sensed by receptors within the LV wall that trigger vagal afferent type C fibers and the resultant triad of hypotension, bradycardia, and coronary artery dilatation) reflexes.
2010B 17: 4 (27%) of candidates passed this question
This question required candidates to provide an answer that integrates their knowledge of various aspects of cardiovascular physiology. Cardiac reflexes are fast-acting reflex loops between the heart and central nervous system that contribute to regulation of cardiac function and maintenance of physiologic homeostasis. This was often overlooked by many candidates. For a good answer it was expected that at least the chemo and baroreceptor, Bainbridge (elicited by stretch receptors located in the right atrial wall and the cavoatrial junction), Cushing (result of cerebral medullary vasomotor centre ischemia), oculocardic (provoked by pressure applied to the globe of the eye or traction on the surrounding structures), Bezold-Jarisch (responds to noxious ventricular stimuli sensed by chemoreceptors and mechanoreceptors within the LV wall) reflexes be mentioned and described.

Recent Comments